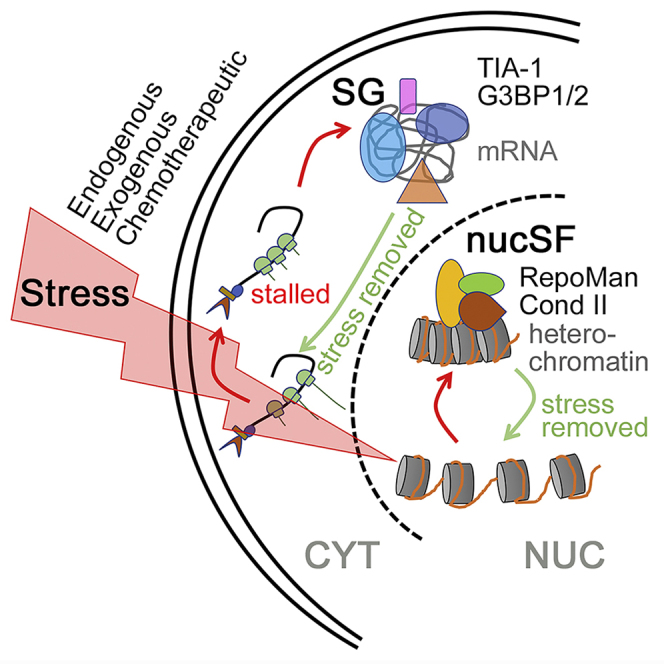
Summary
Stress adaptation is exploited by cancer cells to survive and proliferate under adverse conditions. Survival pathways induced by stress are thus highly promising therapeutic targets. One key pathway involves formation of cytoplasmic stress granules, which regulate the location, stability, and translation of specific mRNAs. Here, we describe a transcriptional stress response that is triggered by similar stressors and characterized by accumulation of RepoMan (cell division cycle associated 2) at nuclear stress foci (nucSF). Formation of these structures is reversible, and they are distinct from known nuclear organelles and stress bodies. Immunofluorescence analysis revealed accumulation of heterochromatic markers, and increased association of RepoMan with the adenylate cyclase 2 (ADCY2) gene locus in stressed cells accompanied reduced levels of ADCY2 mRNA and protein. Quantitative comparison of the RepoMan interactome in stressed vs. unstressed cells identified condensin II as a nucSF factor, suggesting their functional association in the establishment and/or maintenance of these facultative heterochromatic domains.
Subject Areas: Proteomics, Optical Imaging, Cell Biology, Molecular Biology
Graphical Abstract
Highlights
-
•
RepoMan and condensin II accumulate at nuclear foci in stressed cells
-
•
nucSF are triggered by stresses that induce canonical cytoplasmic stress granules
-
•
nucSF are sites of accumulation of heterochromatic markers
-
•
RepoMan shows increased association with the ADCY2 gene locus in arsenite-stressed cells
Proteomics; Optical Imaging; Cell Biology; Molecular Biology
Introduction
In the face of adverse environmental conditions, cells activate an adaptive signaling pathway termed the integrated stress response (Pakos-Zebrucka et al., 2016). Stress-specific activation of one of four monitoring kinases (PERK, PKR, HRI, GCN2) that phosphorylate the eukaryotic translation initiation factor eIF2α on Ser51 triggers cytoplasmic events that promote survival and restore cellular homeostasis. Phosphorylation shifts the balance of protein synthesis from active translation initiation to ribosomal runoff, and subsequent accumulation of stalled polysomes promotes formation of stress granules (SGs). These dynamic RNA/protein structures serve as triage sites that regulate the location, stability, and translation of specific mRNAs (Protter and Parker, 2016). A general reduction in global translation conserves energy and prevents accumulation of misfolded proteins, while selective translation of genes that promote cell survival and recovery is maintained (Lu et al., 2004). SGs also sequester key protein components, such as the TORC1 kinase that coordinates nutrient availability with cell growth (Takahara and Maeda, 2012). Upon removal of stress, eIF2α is dephosphorylated, restoring normal protein synthesis and promoting SG disassembly (Lu et al., 2004). The microenvironment of solid tumors is often characterized by conditions that induce SG formation, such as low nutrient availability and a hypoxic core, and SG and SG-like foci have been detected in tumors of various origins. These include breast carcinoma and glioma (Baguet et al., 2007; Moeller et al., 2004; Somasekharan et al., 2015; Vilas-Boas et al., 2016). There is also increasing evidence that SGs, by contributing to re-programming of gene expression, promote tumor progression and development of therapeutic resistance (Anderson et al., 2015; Eisinger-Mathason et al., 2008; Grabocka and Bar-Sagi, 2016; Mahboubi and Stochaj, 2014; Somasekharan et al., 2015).
This study has uncovered a novel nuclear stress response that involves the rapid and reversible formation of heterochromatic domains in response to treatments that induce cytoplasmic SGs. Nuclear stress foci (nucSF) were first detected as an unexpected accumulation of the protein RepoMan within discrete nuclear foci in response to hypoxia. We originally identified RepoMan (cell division cycle associated 2 [Walker, 2001]) as a PP1 phosphatase regulatory subunit that recruits the catalytic subunit to chromatin-associated substrates (recruits PP1 onto mitotic chromatin at anaphase) (Trinkle-Mulcahy et al., 2006). Subsequent work linked the RepoMan/PP1 complex to regulation of key mitotic events that include chromosome segregation (Vagnarelli et al., 2006; Wurzenberger et al., 2012), chromosomal Aurora B kinase targeting (Qian et al., 2013, 2011), and nuclear envelope reassembly (Qian et al., 2011; Vagnarelli et al., 2011). Equally important non-mitotic roles were suggested by its persistence on chromatin during interphase and the cell cycle–independent apoptosis observed upon siRNA-mediated knockdown (Trinkle-Mulcahy et al., 2006). RepoMan/PP1 was shown to counteract the phosphorylation-mediated activation of ataxia-telangiectasia mutated, a key upstream signaling kinase in DNA damage response. As a consequence, excess RepoMan desensitizes cells to DNA damage (Peng et al., 2010), which can decrease genomic stability by allowing mutations to accumulate. Consistent with a pro-survival role, overexpression of RepoMan promotes proliferation of colorectal cancer and lung cancer adenocarcinoma cells in culture (Feng et al., 2019; Shi et al., 2017) while knockdown sensitizes oral squamous cell carcinoma cells to DNA damage-induced apoptosis (Uchida et al., 2013).
Increasing evidence points to RepoMan as a strong prognostic indicator that is elevated in numerous human cancers. RepoMan mRNA transcripts are overexpressed in late-stage cancers, with increased levels observed in breast, ovary, lung, and colon cancer tissues (Feng et al., 2019; Peng et al., 2010; Shi et al., 2017), as well as in oral squamous cell carcinoma and malignant breast cancer cell lines (Peng et al., 2010; Uchida et al., 2013; Vagnarelli, 2014). It is part of a cohort of 18 signature genes associated with malignant melanoma progression (Ryu et al., 2007) and one of two top-ranked genes correlated with metastatic behavior of synovial sarcomas (Lagarde et al., 2013). RepoMan is among the top scoring genes upregulated in aggressive stage 4 neuroblastoma tumors (Krasnoselsky et al., 2005), and high expression correlates with poor prognosis (Vagnarelli, 2014). RepoMan is also highly upregulated in tumor-infiltrating microglia in glioma-bearing brains (Gieryng et al., 2017), suggesting contribution to tumor immunity mechanisms that reprogram non-tumor cells within the tumor microenvironment.
More recently, RepoMan was shown to participate in heterochromatin formation in early G1, counteracting histone phosphorylation at mitotic exit to permit recruitment of heterochromatin proteins and accumulation of repressive histone marks (de Castro et al., 2017). We thus propose that nucSF represent facultative heterochromatin domains that function to repress specific genes as part of the cellular stress response. Association of RepoMan with these regions may explain its link to later stage cancers and poor prognosis and suggests that the nucSF pathway could be exploited to target cells that have developed therapeutic resistance through stress adaption.
Results and Discussion
Exogenous and Endogenous Cellular Stressors Induce Formation of nucSF in Parallel with Cytoplasmic SGs
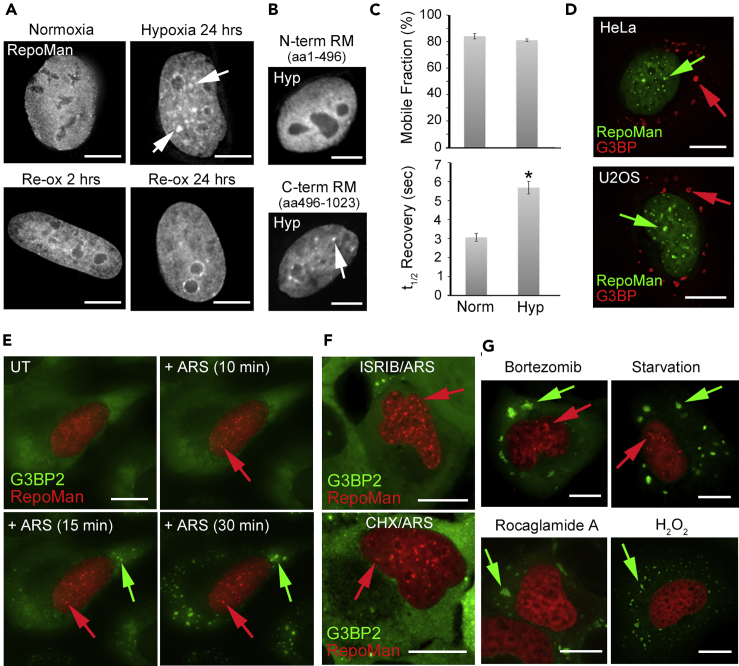
Given that most solid tumors contain gradients of hypoxia and cells in hypoxic regions are the most aggressive and resistant to radiation and chemotherapy (Wilson and Hay, 2011), we were interested in testing whether or not excess RepoMan could further blunt the reduced DNA damage response observed in hypoxic cells. A surprising observation was that growth of cells in hypoxic conditions (1% O2 for 24 hr) induced accumulation of RepoMan in multiple discrete nuclear foci (Figure 1A). These foci largely disassemble within 24 hr of re-oxygenation. Recruitment of RepoMan to nucSF requires its C-terminal half (Figure 1B), which governs association with chromatin (Prévost et al., 2013) (Vagnarelli et al., 2011). Fluorescence recovery after photobleaching (FRAP) experiments revealed a significantly reduced turnover rate for nucSF-associated green fluorescent protein (GFP)-tagged RepoMan, with no change in the overall mobile fraction (Figure 1C). We hypothesized that they may represent regions of more highly condensed chromatin. Senescence-associated heterochromatic foci induced by oncogenic stress and linked to suppression of pro-proliferative genes (Aird and Zhang, 2013; Narita et al., 2003) have been previously described; however, formation of nucSF (which are smaller and more numerous) is rapid and reversible, and cells continue to proliferate after recovery.
Figure 1.
Exogenous and Endogenous Stressors Trigger Formation of nucSF in Parallel with Cytoplasmic SGs
(A) GFP-RepoMan transiently expressed in HeLa cells exposed to hypoxic (1% O2) conditions for 24 hr showed accumulation in multiple bright nuclear foci (arrows). These foci were largely resolved 24 hr after re-oxygenation (Re-ox).
(B) Hypoxia (Hyp; 24 hr) triggered accumulation of the C-terminal half (aa 496–1023) but not the N-terminal half (aa 1–496) of RepoMan at nuclear foci (arrow).
(C) FRAP analysis of GFP-RepoMan at hypoxia-induced nucSF revealed a significant decrease in turnover rate with no change in the overall mobile fraction (mean ± SE for >3 biological replicates; ∗p = 2.3 × 10−6 as determined by a paired Student's t-test).
(D) GFP-RepoMan accumulates at nucSF (green arrows) in both HeLa and U2OS cells treated with 0.5 mM sodium arsenite for 30 min. The cells were fixed and stained with anti-G3BP to confirm formation of cytoplasmic SGs (red arrows).
(E) Live imaging of U2OS cells stably expressing GFP-G3BP2 and transiently expressing mCherry-RepoMan. NucSF (red arrows) and SGs (green arrows) were detected as early as 10 and 15 min, respectively, following addition of 0.5 mM arsenite. See Video S1.
(F) Inclusion of ISRIB (200 nM) or pre-treatment with CHX (50 μg/mL, 2 hr) selectively blocked SG but not nucSF formation in arsenite-treated cells.
(G) Treatment with the proteasome inhibitor bortezomib (25 μM, 4 hr) and overnight glucose/serum starvation induced both SGs and nucSF, while treatment with the eIF4A inhibitor Rocaglamide (1 μM, 1 hr) or hydrogen peroxide (H2O2; 1 mM, 2 hr) selectively induced only SGs.
See Video S2 for the full Rocaglamide A time course. Scale bars are 5 μm.
We screened a panel of 16 different cellular stressors that included DNA damaging agents, transcription and replication inhibitors, ER stress inducers, heat and osmotic shock, and nutrient starvation. Formation of nucSF was induced by a subset of stressors that, along with hypoxia, have also been shown to trigger formation of cytoplasmic SGs (Table S1; for review see (Mahboubi and Stochaj, 2017) (Protter and Parker, 2016)). These include sodium arsenite (Mahboubi et al., 2013), proteasome inhibition with MG132 (Mazroui et al., 2007) or the chemotherapeutic bortezomib (Fournier et al., 2010), osmotic stress (Dewey et al., 2011), heat shock (Mahboubi et al., 2013), and nutrient/serum starvation (Reineke et al., 2018). Of significance is the fact that hypoxia, arsenite, and the proteasome inhibitor bortezomib have all been linked to promotion of therapy resistance(Fournier et al., 2010; Ganapathy et al., 2014; Wilson and Hay, 2011).
Figure 1D demonstrates formation of SGs and nucSF in HeLa and U2OS cells treated for 30 min with 0.5 mM arsenite. Arsenite-induced formation of SGs and nucSF was observed in additional cancer cell lines (MCF7, HCT116, SW480/620, SHSY5Y, NTERA-2), the virally transformed HEK293 cell line, and hTERT-immortalized retinal pigmental epithelial cells, suggesting that it is a conserved stress response (Table S2, Figure S2). Live imaging revealed that formation of arsenite-induced nucSF and SGs occurs on a similar timescale in U2OS cells (Figure 1E; Video S1).
Formation of SGs and nucSF in response to arsenite could be uncoupled by stressing cells in the presence of the small molecule integrated stress response inhibitor (ISRIB) (Figure 1F), which reverses the effects of eIF2α phosphorylation on translation and SG assembly (Sidrauski et al., 2015). Pre-treatment with cycloheximide, which stabilizes polysomes and prevents SG assembly (Kedersha et al., 2000), also selectively blocks SG but not nucSF formation (Figure 1F). Similarly, although the mechanism remains unclear, treatment with arsenite at a lower temperature inhibits SG formation (Wheeler et al., 2016), but does not affect nucSF formation (Figure S1N).
Stressors that induce SGs in a phospho-eIF2α-independent manner did not induce nucSF (Figures 1G and S1). These include H2O2, which induces SGs that are compositionally distinct from canonical SGs and requires remodeling of the cap-binding eIF4E complex (Emara et al., 2012), and Rocaglamide A, which interferes with the RNA helicase eIF4A to induce formation of granules that are positive for core SG markers but negative for poly(A) mRNAs (Aulas et al., 2017) (Figure 1G; Video S2). Having observed that more extreme heat stress (45°C for 1 hr vs 30 min) was required to induce nucSF compared to SGs (Figures S1K–S1L), we extended the incubation time for Rocaglamide A from 1 to 6 hr but still did not see nucSF formation (Figure S1M). SG assembly can also be induced by overexpression of several SG-associated proteins, including TIA-1, which promotes nucleation and aggregation. In this case, nucSF assembly is not induced (Figures S1O–S1P). Taken together, these results suggest that the cellular and nuclear stress pathways are triggered by a shared kinase-mediated signaling event that is either upstream or related to activation of the eIF2α stress kinases. This will be tested systematically in future work using a panel of inhibitors of various stress-related kinases.
It is also likely that nucSF, like SGs, have stress-specific properties. Although nucSF were induced by the osmotic stressors sorbitol and NaCl, for example, they were fewer in number and clustered at the nucleolar periphery (Figures S1I–S1J). NucSF induced by extreme heat shock showed this nucleolar clustering to an even greater extent (Figure S1L), which might represent a more dramatic reorganization of the chromatin landscape.
Importantly, heat shock– and arsenite-induced nucSF are distinct from the heat shock–induced nuclear stress bodies that accumulate mRNA processing factors and are marked by accumulation of heat shock factor 1 ([Jolly et al., 1999]; Figures S1L and S3H). And although RepoMan plays a role in DNA damage sensing, nucSF do not show accumulation of γH2AX, which is a marker for sites of DNA double-strand breaks (Figure S3G [Mah et al., 2010]). NucSF are also distinct from previously described membraneless nuclear bodies that include promyelocytic leukemia protein (PML) bodies, Cajal bodies, splicing speckles, and paraspeckles (Figures S3A–S3D (Sleeman and Trinkle-Mulcahy, 2014). These bodies are primarily enriched in RNA, whereas nucSF are sites of increased DNA but not RNA staining (Figures S1E–S1F). Chromatin insulator bodies are aggregates of chromatin insulator proteins that have been observed to form in response to osmotic stress in Drosophila nuclei (Schoborg et al., 2013). Their function is thought to be sequestration of insulator proteins away from DNA, with release triggered when cells are returned to isotonicity. Although they have not been observed in human cells, we stained arsenite-stressed cells with antibodies that recognized human CCCTC-binding factor (hCTCF), the human ortholog of Drosophila CTCF (dCTCF), to confirm that this factor does not show accumulation at nucSF (Figure S3I).
nucSF Are Sites of Accumulation of Heterochromatic Markers
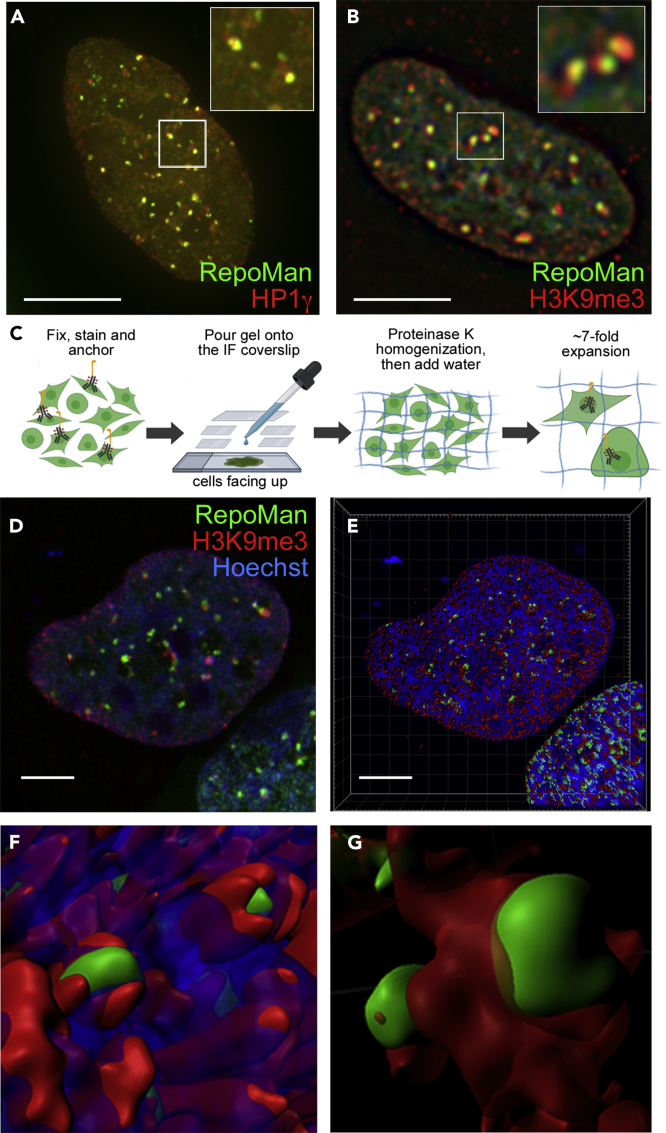
To determine whether nucSF represent regions of more highly condensed chromatin, we subjected arsenite-stressed cells to immunofluorescence analysis and confirmed accumulation of the heterochromatin markers HP1γ and tri-methylated lysine residue 9 of histone H3 (H3K9me3) at these regions (Figures 2A and 2B). Having noted partial overlap of the H3K9me3 and RepoMan signals (Figure 2B; see inset), we set out to define their spatial relationship at the nanoscale level using expansion microscopy (ExM)-based super-resolution imaging. ExM is a sample preparation technique in which fixed and stained cells are embedded in a dense cross-linked network of swellable polyelectrolyte hydrogel (Truckenbrodt et al., 2019). Addition of water isotropically expands the sample up to 10-fold, increasing the physical distance between biomolecules (Figure 2C). ExM was recently used to demonstrate functional subdomains in cytoplasmic SGs (Cirillo et al., 2020). Although structures can already be resolved at the sub-diffraction level (<200 nm) using a conventional microscope, we paired ExM (expansion factor calculated as ∼7-fold; Figure S4A) with Airyscan imaging on an LSM880 confocal microscope to achieve a further 1.7x increase in resolution (Huff, 2015). We used ExM/Airyscan imaging and 3D volume rendering to demonstrate that H3K9me3 staining surrounds and spreads out from smaller RepoMan foci at nucSF (Figures 2D–2G). This suggests that RepoMan may play a role in nucSF nucleation and/or spreading and that individual nucSF may mediate repression of multiple genes.
Figure 2.
NucSF are Sites of Accumulation of Heterochromatic Markers
(A and B) U2OS cells transiently expressing GFP-RepoMan (green) and treated with 0.5 mM arsenite for 30 min were fixed and stained with anti-HP1γ (A) or anti-H3K9me3 (B) and Alexa Fluor 555 secondary antibodies (red).
(C) Simplified model of ExM protocol. The expansion factor averaged 7.1x (see Figure S4).
(D) Airyscan image of an expanded arsenite-stressed U2OS cell expressing GFP-RepoMan and fixed and stained with anti-H3K9me3-AlexaFluor555 and Hoechst 33342.
(E) 3D volume rendering of the data set in (D).
(F and G) Enlarged insets (F and G) show RepoMan (green) relative to H3K9me3 (red) and DNA (blue). Scale bars are 5 μm.
Arsenite Induces Increased Association of RepoMan with the ADCY2 Gene Locus
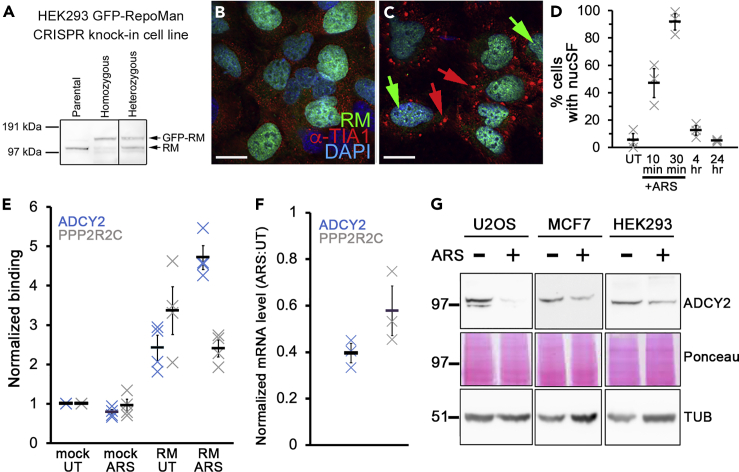
Analysis of endogenous RepoMan has been limited by the lack of specific and reliable antibodies (de Castro et al., 2017; Prévost et al., 2013). We used CRISPR/Cas9 gene editing to fuse GFP to the N-terminus of endogenous RepoMan in HEK293 cells, confirming its shift in molecular mass by Western blot analysis and its expected subcellular localization by live imaging (Figures 3A and 3B). We also confirmed accumulation of endogenous GFP-tagged RepoMan at nucSF in response to arsenite treatment, using anti-TIA1 counter staining (Kedersha and Anderson, 2007) to mark SGs (Figure 3C). After 30 min of arsenite treatment, >90% of cells were positive for nucSF (>6 foci per cell; Figure 3D). These structures were fully disassembled 24 hr after removal of the stress.
Figure 3.
Arsenite Stress Induces Increased Association of RepoMan with the ADCY2 Gene Locus
(A) Western blot analysis of HEK293/GFP-RepoMan knock-in cell lysates with anti-RepoMan to assess the shift in molecular mass in heterozygous (single allele knock-in) and homozygous (double allele knock-in) cells.
(B and C) To assess their response to acute arsenite treatment (0.5 mM for 30 min), homozygous cells were fixed following no treatment (B) or arsenite treatment (C) and stained with anti-TIA1 (red) and DAPI (blue). Green arrows indicate nucSF and red arrows point to SGs. Scale bars are 5 μm.
(D) Time course of arsenite-induced nucSF formation and disassembly in HEK293 GFP-RepoMan CRISPR knock-in cells. For each time point, multiple fields of view were imaged and cells scored for nucSF formation (>6 foci per cell). Results are shown for 3 independent experiments, with a total of 167–262 cells counted for each time point (mean ± SE indicated by black bar).
(E) ChIP-qPCR was used to compare binding of GFP-RepoMan to the ADCY2 (blue X) and PPP2R2C (gray X) gene loci in the knock-in cell line (untreated vs. 0.5 mM arsenite for 30 min). Mock immunoprecipitations (IPs) from untreated (UT) and arsenite-treated (ARS) lysates were included as controls, and all data normalized to the mock UT values. The experiment was repeated twice, with 2 technical replicates each time. Black bars indicate mean ± standard deviation (SD).
(F) RNA isolated from untreated vs. arsenite-stressed U2OS cells was subjected to RT-qPCR analysis. Values were normalized to GAPDH (see Figure S4 for confirmation that its cellular levels do not change with arsenite stress). Mean ± standard error (SE) is indicated for 3 biological replicates.
(G) Lysates from arsenite-stressed vs. untreated U2OS, MCF7, and HEK293 cells were subjected to Western blot analysis with anti-ADCY2.
Ponceau staining is shown for the corresponding region on the blot, and alpha-tubulin was stained as an additional loading control (TUB; see Figure S4 for confirmation that its cellular levels do not change with arsenite stress).
We optimized GFP-RepoMan chromatin immunoprecipitation (ChIP) using this HEK293 knock-in cell line and confirmed association with two gene loci identified in a previously published in vitro screen to be bound by RepoMan: ADCY2 (adenylate cyclase) and PPP2R2C (PP2A regulatory subunit) (de Castro et al., 2017). ChIP-quantitative polymerase chain reaction (ChIP-qPCR) revealed increased association of RepoMan with the ADCY2 gene locus in response to arsenite treatment (Figure 3E), and reverse transcription PCR (RT-PCR) confirmed a >two-fold reduction in ADCY2 mRNA levels in arsenite-stressed cells (Figure 3F). Consistent with this, ADCY2 protein levels were shown by Western blot analysis to be reduced in arsenite-stressed U2OS, MCF7, and HEK293 cells (Figure 3G). Adenylate cyclase catalyzes production of cAMP from ATP. This second messenger plays a key role in regulation of cell proliferation, and upregulation of cAMP has been proposed as a cancer therapy approach (Chen et al., 1998; Fajardo et al., 2014; Li et al., 2016). Notably, adenylate cyclase was identified as one of the most highly downregulated proteins following long-term exposure of human embryonic carcinoma cells to low levels of arsenite (Das et al., 2011). Future experiments will utilize ChIP-sequencing (ChIP-seq) (Nakato and Sakata, 2020) and/or CUT&Tag (Kaya-Okur et al., 2019) approaches to identify additional nucSF target genes and determine whether, like SGs, there are stress-specific differences.
Condensin II Accumulates at nucSF and Associates with RepoMan in Arsenite-Stressed Cells
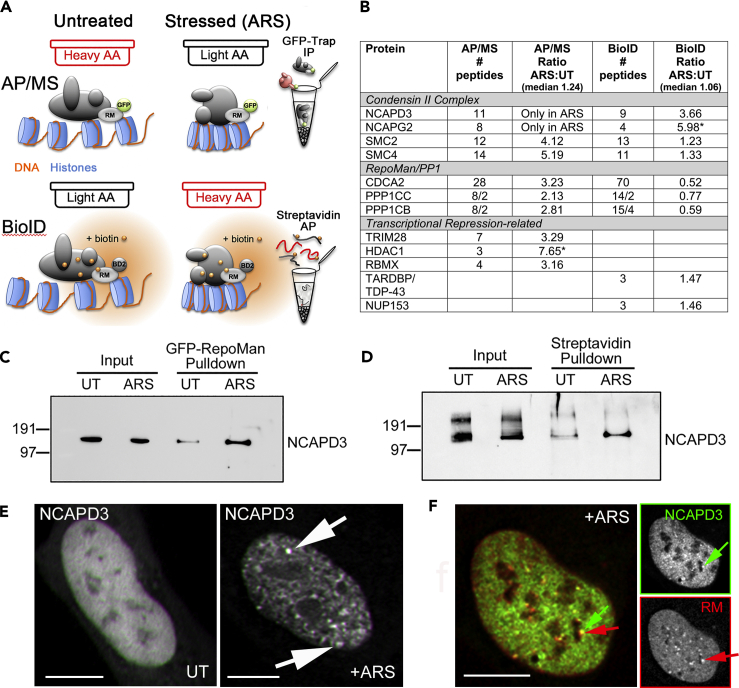
To identify other factors that localize to nucSF, we compared the interactome of RepoMan in arsenite-stressed vs. untreated cells using two complementary strategies: (1) affinity purification/mass spectrometry (AP/MS), which identifies proteins that co-precipitate with affinity-purified bait protein, and (2) BioID, in which a biotin ligase fused to the bait protein drives biotinylation of proximal proteins for capture on a streptavidin affinity matrix and identification by MS (Figure 4A). Both incorporated SILAC (stable isotope labeling by amino acids [AAs] in culture) metabolic labeling to facilitate the robust and reliable identification of bona fide enriched factors above background contaminants (Trinkle-Mulcahy, 2012). The AP/MS experiment was performed using the GFP-RepoMan knock-in HEK293 cell line and high affinity GFP-Trap_A resin (Trinkle-Mulcahy et al., 2008), with endogenous GFP-RepoMan captured from untreated cells labeled with heavy AAs and arsenite-stressed cells (0.5 mM for 30 min) labeled with light AA. The BioID experiment was carried out in lentiviral-transduced U2OS cells expressing RepoMan fused to the smaller and more active BioID2 biotin ligase from A. aeolicus (Kim et al., 2016). The labeling strategy was reversed for this experiment, with biotin was added for 2 hr in the presence (heavy AA) or absence (light AA) of 0.1 mM arsenite. We also used a lower dose of arsenite for a longer incubation time to ensure efficient biotinylation and confirmed nucSF formation by staining fixed cells with fluorophore-tagged streptavidin (Figure S4D). Proteins that showed increased association with RepoMan in arsenite-stressed cells were readily identified by their increased light:heavy (AP/MS) or heavy:light (BioID) ratios (Table S3).
Figure 4.
Condensin II Accumulates at nucSF and Associates with RepoMan in Arsenite-Stressed Cells
(A) Diagram detailing the complementary SILAC-based quantitative AP/MS and BioID strategies used to compare the interactome of RepoMan in arsenite-stressed vs untreated cells. For AP/MS, untreated cells were labeled with heavy (H) media and arsenite-treated cells with light (L) media. Endogenous GFP-tagged RepoMan in the HEK293 knock-in cell line was immunoprecipitated using the GFP-Trap_A affinity resin and associated proteins identified by MS analysis. For BioID, the labeling strategy was flipped so that untreated cells were labeled with light media and arsenite-treated cells with heavy media. BioID2-tagged RepoMan was lentivirally transduced in U2OS cells and biotinylated proteins captured on streptavidin affinity resin and identified by MS analysis. ARS:UT ratios were determined for all proteins identified (L:H for AP/MS, (H)L for BioID).
(B) The table highlights overlapping and novel hits that showed increased association with RepoMan in single replicates of the internally controlled complementary screens (full data sets provided as Table S3). The number of peptides detected for each protein is listed, as well as its ratio ARS:UT (with the median ratio for the experiment listed for comparison). Note that in the AP/MS experiment NCAPD3 and NCAPG2 peptides were only detected in the L (+ARS) form. Asterisk (∗) indicates that MaxQuant did not calculate a SILAC ratio for the protein, so enrichment was estimated by comparing the summed L vs. H peptide intensities.
(C) AP/Western blot validation of the increased association of endogenous NCAPD3 with endogenous GFP-tagged RepoMan in arsenite-stressed HEK293 knock-in cells.
(D) BioID/Western blot validation of increased biotinylation of endogenous NCAPD3 by transduced BioID2-RepoMan in U2OS cells. Both blots were probed with anti-NCAPD3.
(E) Live imaging of U2OS cells stably expressing GFP-NCAPD3, either untreated (left panel) or treated with 0.5 mM arsenite for 30 min (arrows indicate stress-induced nuclear foci).
(F) Live imaging of U2OS cells stably expressing GFP-NCAPD3 (green) and transiently expressing mCherry-RepoMan (red), treated with 0.5 mM arsenite for 30 min to induce nucSF (red arrow) at which NCAPD3 (green arrow) accumulates. The inset panels show the individual NCAPD3 (top panel) and RepoMan (bottom panel) signals. Scale bars are 5 μm.
Overlapping hits, some of which are highlighted in the table in Figure 4B, include the condensin II complex members NCAPD3, NCAPG2, SMC2, and SMC4 (Uhlmann, 2016). In contrast to condensin I, condensin II is found in the nucleus throughout the cell cycle and has been proposed to play a role in establishment of chromatin architecture (Rosin et al., 2018). Increased association of NCADP3 with RepoMan in arsenite-stressed cells was validated by AP/Western blot (Figure 4C) and BioID/Western blot (Figure 4D) analysis. We further confirmed recruitment of NCAPD3 to nucSF with arsenite treatment (Figure 4E), where it shows overlap with the RepoMan signal (Figure 4F). The subnuclear localization of NCAPD3 does not change in cells in which SGs are induced by TIA-1 overexpression (Figure S1P). Having previously shown that condensin II and the RepoMan/PP1 complex co-operate in the regulation of chromosome architecture during mitosis (Vagnarelli et al., 2006), we propose that they may also play a co-operative role in the regulation of nucSF formation and/or function in stressed cells. The observation of arsenite-induced NCAPD3 accumulation in nuclear foci in cells treated with RepoMan siRNA suggests that RepoMan may not be required for its recruitment to nucSF (Figure S4E); however, a more comprehensive analysis is required to determine whether these foci are functionally comparable to RepoMan-containing nucSF. RNAi-mediated RepoMan knockdown also triggers apoptotic pathways and widescale cell death that may confound the results (Trinkle-Mulcahy et al., 2006). Moving forward, we will take advantage of the temporal resolution of acute strategies such as auxin-inducible degradation (Li et al., 2019) to functionally dissect the contributions of RepoMan and NCAPD3 to this novel stress pathway.
With stress adaptation posing a significant challenge to the efficient treatment of cancer, characterization of cellular pro-survival pathways triggered by stress is essential for the identification of new molecular targets for both primary and adjuvant therapies. Factors involved in formation of cytoplasmic SGs are attractive candidates and have received significant attention recently. What these studies did not appreciate, however, is the nuclear stress pathway that we now know occurs in parallel with SG formation in response to similar endogenous and exogenous stressors. These findings thus open up an entirely new avenue of research.
Limitations of the Study
Molecular dissection of the contributions of RepoMan and condensin II to the formation/function of nucSF is complicated by their key roles in other cellular pathways, such as cell cycle regulation and DNA damage response. This limits the usefulness of traditional knockdown/knockout approaches and necessitates optimization of more rapid protein depletion methods that can be implemented upstream of acute cellular stress.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Laura Trinkle-Mulcahy (ltrinkle@uottawa.ca).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
The published article includes all data sets generated or analyzed during this study.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank colleagues in the Trinkle lab (Michèle Prévost, Jennifer Law, Sarah Ooi, and Virja Mehta) for their contributions and helpful discussions and Dr. Ryan Russell for advice and guidance with CRISPR/Cas9 gene editing. We also thank Lawrence Puente at the Ottawa Hospital Research Institute Proteomics Core Facility and Chloë van Oostende-Triplet and Skye Greene at the Cell Biology and Image Acquisition Core Facility for technical support. pSpCas9(BB)-2A-Puro (PX459) V2.0 was a gift from Feng Zhang (Addgene plasmid # 62988; http://n2t.net/addgene:62988; RRID:Addgene_62988) and myc-BioID2-MCS was a gift from Kyle Roux (Addgene plasmid # 74223; http://n2t.net/addgene:74223; RRID:Addgene_74223). This work was funded by the Cancer Research Society (20478) and Cancer Research Society/uOttawa Alliance (23484). T.D. was funded by a Natural Sciences and Engineering Research Council of Canada Undergraduate Student Research Award.
Author Contributions
T.D., A.G.L., and C.G.P. carried out methodology and investigation; D.C. carried out investigation and validation; C.S.A. and V.R. carried out investigation; M.B. provided methodology and resources; and L.T.M. was responsible for visualization, methodology, supervision, formal analysis, and writing.
Declaration of Interests
The authors declare no competing interests.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101664.
Supplemental Information
References
- Aird K.M., Zhang R. Detection of senescence-associated heterochromatin foci (SAHF) In: Galluzzi L., Vitale I., Kepp O., Kroemer G., editors. Cell Senescence: Methods and Protocols, Methods in Molecular Biology. Humana Press; 2013. pp. 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Kedersha N., Ivanov P. Stress granules, P-bodies and cancer. Biochim. Biophys. Acta. 2015;1849:861–870. doi: 10.1016/j.bbagrm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulas A., Fay M.M., Lyons S.M., Achorn C.A., Kedersha N., Anderson P., Ivanov P. Stress-specific differences in assembly and composition of stress granules and related foci. J. Cell Sci. 2017;130:927–937. doi: 10.1242/jcs.199240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguet A., Degot S., Cougot N., Bertrand E., Chenard M.-P., Wendling C., Kessler P., Hir H.L., Rio M.-C., Tomasetto C. The exon-junction-complex-component metastatic lymph node 51 functions in stress-granule assembly. J. Cell Sci. 2007;120:2774–2784. doi: 10.1242/jcs.009225. [DOI] [PubMed] [Google Scholar]
- Chen T.C., Hinton D.R., Zidovetzki R., Hofman F.M. Up-regulation of the cAMP/PKA pathway inhibits proliferation, induces differentiation, and leads to apoptosis in malignant gliomas. Lab. Invest. 1998;78:165–174. [PubMed] [Google Scholar]
- Cirillo L., Cieren A., Barbieri S., Khong A., Schwager F., Parker R., Gotta M. UBAP2L forms distinct cores that act in nucleating stress granules upstream of G3BP1. Curr. Biol. 2020;30:698–707.e6. doi: 10.1016/j.cub.2019.12.020. [DOI] [PubMed] [Google Scholar]
- Das N.D., Park J.H., Jung K.H., Lee H.T., Park K.S., Choi M.R., Chai Y.G. Sodium arsenite dependent protein expression analysis on human embryonic carcinoma (NCCIT) cell line. Toxicol. Lett. 2011;207:149–158. doi: 10.1016/j.toxlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- de Castro I.J., Budzak J., Di Giacinto M.L., Ligammari L., Gokhan E., Spanos C., Moralli D., Richardson C., de las Heras J.I., Salatino S. Repo-Man/PP1 regulates heterochromatin formation in interphase. Nat. Commun. 2017;8:1–16. doi: 10.1038/ncomms14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey C.M., Cenik B., Sephton C.F., Dries D.R., Mayer P., Good S.K., Johnson B.A., Herz J., Yu G. TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol. Cell. Biol. 2011;31:1098–1108. doi: 10.1128/MCB.01279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger-Mathason T.S.K., Andrade J., Groehler A.L., Clark D.E., Muratore-Schroeder T.L., Pasic L., Smith J.A., Shabanowitz J., Hunt D.F., Macara I.G., Lannigan D.A. Codependent functions of RSK2 and the apoptosis-promoting factor TIA-1 in stress granule assembly and cell survival. Mol. Cell. 2008;31:722–736. doi: 10.1016/j.molcel.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara M.M., Fujimura K., Sciaranghella D., Ivanova V., Ivanov P., Anderson P. Hydrogen peroxide induces stress granule formation independent of eIF2α phosphorylation. Biochem. Biophys. Res. Commun. 2012;423:763–769. doi: 10.1016/j.bbrc.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo A.M., Piazza G.A., Tinsley H.N. The role of cyclic nucleotide signaling pathways in cancer: targets for prevention and treatment. Cancers. 2014;6:436–458. doi: 10.3390/cancers6010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Qian W., Zhang Y., Peng W., Li J., Gu Q., Ji D., Zhang Z., Wang Q., Zhang D., Sun Y. CDCA2 promotes the proliferation of colorectal cancer cells by activating the AKT/CCND1 pathway in vitro and in vivo. BMC Cancer. 2019;19:576. doi: 10.1186/s12885-019-5793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M.-J., Gareau C., Mazroui R. The chemotherapeutic agent bortezomib induces the formation of stress granules. Cancer Cell Int. 2010;10:12. doi: 10.1186/1475-2867-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy S., Xiao S., Seo S.-J., Lall R., Yang M., Xu T., Su H., Shadfan M., Ha C.S., Yuan Z.-M. Low-dose arsenic induces chemotherapy protection via p53/NF-κB-mediated metabolic regulation. Oncogene. 2014;33:1359–1366. doi: 10.1038/onc.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieryng A., Pszczolkowska D., Bocian K., Dabrowski M., Rajan W.D., Kloss M., Mieczkowski J., Kaminska B. Immune microenvironment of experimental rat C6 gliomas resembles human glioblastomas. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-17752-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabocka E., Bar-Sagi D. Mutant KRAS enhances tumor cell fitness by upregulating stress granules. Cell. 2016;167:1803–1813.e12. doi: 10.1016/j.cell.2016.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff J. The Airyscan detector from ZEISS: confocal imaging with improved signal-to-noise ratio and super-resolution. Nat. Methods. 2015;12 i–ii. [Google Scholar]
- Jolly C., Usson Y., Morimoto R.I. Rapid and reversible relocalization of heat shock factor 1 within seconds to nuclear stress granules. Proc. Natl. Acad. Sci. U S A. 1999;96:6769–6774. doi: 10.1073/pnas.96.12.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya-Okur H.S., Wu S.J., Codomo C.A., Pledger E.S., Bryson T.D., Henikoff J.G., Ahmad K., Henikoff S. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 2019;10:1930. doi: 10.1038/s41467-019-09982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N., Anderson P. Methods in Enzymology, Translation Initiation: Cell Biology, High-Throughput Methods, and Chemical-Based Approaches. Academic Press; 2007. Mammalian stress granules and processing bodies; pp. 61–81. [Google Scholar]
- Kedersha N., Cho M.R., Li W., Yacono P.W., Chen S., Gilks N., Golan D.E., Anderson P. Dynamic shuttling of tia-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 2000;151:1257–1268. doi: 10.1083/jcb.151.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.I., Jensen S.C., Noble K.A., Kc B., Roux K.H., Motamedchaboki K., Roux K.J. An improved smaller biotin ligase for BioID proximity labeling. MBoC. 2016;27:1188–1196. doi: 10.1091/mbc.E15-12-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnoselsky A.L., Whiteford C.C., Wei J.S., Bilke S., Westermann F., Chen Q.-R., Khan J. Altered expression of cell cycle genes distinguishes aggressive neuroblastoma. Oncogene. 2005;24:1533–1541. doi: 10.1038/sj.onc.1208341. [DOI] [PubMed] [Google Scholar]
- Lagarde P., Przybyl J., Brulard C., Pérot G., Pierron G., Delattre O., Sciot R., Wozniak A., Schöffski P., Terrier P. Chromosome instability accounts for reverse metastatic outcomes of pediatric and adult synovial sarcomas. JCO. 2013;31:608–615. doi: 10.1200/JCO.2012.46.0147. [DOI] [PubMed] [Google Scholar]
- Li K., Zhang H., Qiu J., Lin Y., Liang J., Xiao X., Fu L., Wang F., Cai J., Tan Y. Activation of cyclic adenosine monophosphate pathway increases the sensitivity of cancer cells to the oncolytic virus M1. Mol. Ther. 2016;24:156–165. doi: 10.1038/mt.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Prasanna X., Salo V.T., Vattulainen I., Ikonen E. An efficient auxin-inducible degron system with low basal degradation in human cells. Nat. Methods. 2019;16:866–869. doi: 10.1038/s41592-019-0512-x. [DOI] [PubMed] [Google Scholar]
- Lu P.D., Harding H.P., Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah L.-J., El-Osta A., Karagiannis T.C. γH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- Mahboubi H., Kodiha M., Stochaj U. Automated detection and quantification of granular cell compartments. Microsc. Microanal. 2013;19:617–628. doi: 10.1017/S1431927613000159. [DOI] [PubMed] [Google Scholar]
- Mahboubi H., Stochaj U. Cytoplasmic stress granules: dynamic modulators of cell signaling and disease. Biochim. Biophys. Acta. 2017;1863:884–895. doi: 10.1016/j.bbadis.2016.12.022. [DOI] [PubMed] [Google Scholar]
- Mahboubi H., Stochaj U. Nucleoli and stress granules: connecting distant relatives. Traffic. 2014;15:1179–1193. doi: 10.1111/tra.12191. [DOI] [PubMed] [Google Scholar]
- Mazroui R., Di Marco S., Kaufman R.J., Gallouzi I.-E. Inhibition of the ubiquitin-proteasome system induces stress granule formation. MBoC. 2007;18:2603–2618. doi: 10.1091/mbc.E06-12-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller B.J., Cao Y., Li C.Y., Dewhirst M.W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Nakato R., Sakata T. Methods for ChIP-seq analysis: a practical workflow and advanced applications. Methods. 2020;S1046-2023 doi: 10.1016/j.ymeth.2020.03.005. S1046-2023(20)30059-1. [DOI] [PubMed] [Google Scholar]
- Narita Masashi, Nuñez S., Heard E., Narita Masako, Lin A.W., Hearn S.A., Spector D.L., Hannon G.J., Lowe S.W. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- Pakos-Zebrucka K., Koryga I., Mnich K., Ljujic M., Samali A., Gorman A.M. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng A., Lewellyn A.L., Schiemann W.P., Maller J.L. Repo-man controls a protein phosphatase 1-dependent threshold for DNA damage checkpoint activation. Curr. Biol. 2010;20:387–396. doi: 10.1016/j.cub.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévost M., Chamousset D., Nasa I., Freele E., Morrice N., Moorhead G., Trinkle-Mulcahy L. Quantitative fragmentome mapping reveals novel, domain-specific partners for the modular protein RepoMan (recruits PP1 onto mitotic chromatin at anaphase) Mol. Cell Proteomics. 2013;12:1468–1486. doi: 10.1074/mcp.M112.023291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protter D.S.W., Parker R. Principles and properties of stress granules. Trends Cell Biol. 2016;26:668–679. doi: 10.1016/j.tcb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J., Beullens M., Lesage B., Bollen M. Aurora B defines its own chromosomal targeting by opposing the recruitment of the phosphatase scaffold repo-man. Curr. Biol. 2013;23:1136–1143. doi: 10.1016/j.cub.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Qian J., Lesage B., Beullens M., Van Eynde A., Bollen M. PP1/Repo-Man dephosphorylates mitotic histone H3 at T3 and regulates chromosomal Aurora B targeting. Curr. Biol. 2011;21:766–773. doi: 10.1016/j.cub.2011.03.047. [DOI] [PubMed] [Google Scholar]
- Reineke L.C., Cheema S.A., Dubrulle J., Neilson J.R. Chronic starvation induces noncanonical pro-death stress granules. J. Cell Sci. 2018;131:jcs220244. doi: 10.1242/jcs.220244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin L.F., Nguyen S.C., Joyce E.F. Condensin II drives large-scale folding and spatial partitioning of interphase chromosomes in Drosophila nuclei. PLoS Genet. 2018;14:e1007393. doi: 10.1371/journal.pgen.1007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu B., Kim D.S., Deluca A.M., Alani R.M. Comprehensive expression profiling of tumor cell lines identifies molecular signatures of melanoma progression. PLoS One. 2007;2:e594. doi: 10.1371/journal.pone.0000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoborg T., Rickels R., Barrios J., Labrador M. Chromatin insulator bodies are nuclear structures that form in response to osmotic stress and cell death. J. Cell Biol. 2013;202:261–276. doi: 10.1083/jcb.201304181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Zhang C., Wu Y., Wang X., Sun Q., Sun J., Xia W., Dong G., Wang A., Jiang F., Xu L. CDCA2 promotes lung adenocarcinoma cell proliferation and predicts poor survival in lung adenocarcinoma patients. Oncotarget. 2017;8:19768–19779. doi: 10.18632/oncotarget.15519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C., McGeachy A.M., Ingolia N.T., Walter P. The small molecule ISRIB reverses the effects of eIF2α phosphorylation on translation and stress granule assembly. Elife. 2015;4:e05033. doi: 10.7554/eLife.05033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman J.E., Trinkle-Mulcahy L. Nuclear bodies: new insights into assembly/dynamics and disease relevance. Curr. Opin. Cell Biol. 2014;28:76–83. doi: 10.1016/j.ceb.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Somasekharan S.P., El-Naggar A., Leprivier G., Cheng H., Hajee S., Grunewald T.G.P., Zhang F., Ng T., Delattre O., Evdokimova V. YB-1 regulates stress granule formation and tumor progression by translationally activating G3BP1. J. Cell Biol. 2015;208:913–929. doi: 10.1083/jcb.201411047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara T., Maeda T. Transient sequestration of TORC1 into stress granules during heat stress. Mol. Cell. 2012;47:242–252. doi: 10.1016/j.molcel.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L. Resolving protein interactions and complexes by affinity purification followed by label-based quantitative mass spectrometry. Proteomics. 2012;12:1623–1638. doi: 10.1002/pmic.201100438. [DOI] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L., Andersen J., Lam Y.W., Moorhead G., Mann M., Lamond A.I. Repo-Man recruits PP1 gamma to chromatin and is essential for cell viability. J. Cell Biol. 2006;172:679–692. doi: 10.1083/jcb.200508154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L., Boulon S., Lam Y.W., Urcia R., Boisvert F.-M., Vandermoere F., Morrice N.A., Swift S., Rothbauer U., Leonhardt H., Lamond A. Identifying specific protein interaction partners using quantitative mass spectrometry and bead proteomes. J. Cell Biol. 2008;183:223–239. doi: 10.1083/jcb.200805092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truckenbrodt S., Sommer C., Rizzoli S.O., Danzl J.G. A practical guide to optimization in X10 expansion microscopy. Nat. Protoc. 2019;14:832–863. doi: 10.1038/s41596-018-0117-3. [DOI] [PubMed] [Google Scholar]
- Uchida F., Uzawa K., Kasamatsu A., Takatori H., Sakamoto Y., Ogawara K., Shiiba M., Bukawa H., Tanzawa H. Overexpression of CDCA2 in human squamous cell carcinoma: correlation with prevention of G1 phase Arrest and apoptosis. PLoS One. 2013;8:e56381. doi: 10.1371/journal.pone.0056381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann F. SMC complexes: from DNA to chromosomes. Nat. Rev. Mol. Cell Biol. 2016;17:399–412. doi: 10.1038/nrm.2016.30. [DOI] [PubMed] [Google Scholar]
- Vagnarelli P. Repo-Man at the intersection of chromatin remodelling, DNA repair, nuclear envelope organization, and cancer progression. In: Schirmer E.C., de las Heras J.I., editors. Cancer Biology and the Nuclear Envelope: Recent Advances May Elucidate Past Paradoxes, Advances in Experimental Medicine and Biology. Springer; 2014. pp. 401–414. [DOI] [PubMed] [Google Scholar]
- Vagnarelli P., Hudson D.F., Ribeiro S.A., Trinkle-Mulcahy L., Spence J.M., Lai F., Farr C.J., Lamond A.I., Earnshaw W.C. Condensin and Repo-Man-PP1 co-operate in the regulation of chromosome architecture during mitosis. Nat. Cell Biol. 2006;8:1133–1142. doi: 10.1038/ncb1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnarelli P., Ribeiro S., Sennels L., Sanchez-Pulido L., de Lima Alves F., Verheyen T., Kelly D.A., Ponting C.P., Rappsilber J., Earnshaw W.C. Repo-Man coordinates chromosomal reorganization with nuclear envelope reassembly during mitotic exit. Dev. Cell. 2011;21:328–342. doi: 10.1016/j.devcel.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilas-Boas F.de A.S., da Silva A.M., de Sousa L.P., Lima K.M., Vago J.P., Bittencourt L.F.F., Dantas A.E., Gomes D.A., Vilela M.C., Teixeira M.M., Barcelos L.S. Impairment of stress granule assembly via inhibition of the eIF2alpha phosphorylation sensitizes glioma cells to chemotherapeutic agents. J. Neurooncol. 2016;127:253–260. doi: 10.1007/s11060-015-2043-3. [DOI] [PubMed] [Google Scholar]
- Walker M.G. Drug target discovery by gene expression analysis cell cycle genes. Curr. Cancer Drug Targets. 2001;1:73–83. doi: 10.2174/1568009013334241. [DOI] [PubMed] [Google Scholar]
- Wheeler J.R., Matheny T., Jain S., Abrisch R., Parker R. Distinct stages in stress granule assembly and disassembly. Elife. 2016;5:e18413. doi: 10.7554/eLife.18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W.R., Hay M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- Wurzenberger C., Held M., Lampson M.A., Poser I., Hyman A.A., Gerlich D.W. Sds22 and Repo-Man stabilize chromosome segregation by counteracting Aurora B on anaphase kinetochores. J. Cell Biol. 2012;198:173–183. doi: 10.1083/jcb.201112112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all data sets generated or analyzed during this study.