Abstract
Background
Breast cancer has a high incidence and increasing mortality in Southern Brazil. The present study evaluated clinical and sociodemographic characteristics, and their association with overall survival in a private cancer center.
Methods
1113 breast cancer patients were included in this study. The association between survival and clinicopathological and sociodemographic characteristics was analyzed using Cox regression and Kaplan-Meyer curves.
Results
Median age at diagnosis was 52 years (SD 13.5). Most patients were diagnosed in stages 0 and I (62.7%), while only 1.3% had stage IV disease. Five- and 10-year overall survival were 93.5% and 83.8%, respectively. According to multivariate analysis, age at diagnosis (HR 1.05; CI95 1.03–1.06), staging (stage III: HR 4.04; CI95 1.34–12.19; stage IV: HR 9.61; CI95 2.17–42.50), high KI67 (HR 5.46; CI95 1.27–23.32) and distant recurrence (HR 7.28; CI95 4.79–11.06) were significantly associated with survival. Smoking status, years of education, BMI, and tumor biological status were not significantly associated with mortality.
Conclusions
This cohort of Brazilian patients, who received timely and appropriate treatment, achieved outcomes that are comparable to those from high income countries. Breast cancer mortality seems dependent on the quality of health care available to patients.
Keywords: Breast cancer, Cancer survival, Mortality
Highlights
-
•
High breast cancer mortality in Brazil might be associated with the type of health care that is available to women.
-
•
Staging, intermediate/high KI67, distant recurrence and increasing age at diagnosis were associated with higher risk of death.
-
•
Brazilian patients who receive adequate and timely treatment may achieve outcomes similar to those of high-income countries.
-
•
The high breast cancer mortality in Brazil seems dependent on the health care system available to cancer patients.
1. Introduction
Brazil is the sixth most populated country in the world with over 200 million people [1], corresponding to approximately one third of the population in Latin America [2]. Breast cancer is the leading cause of cancer death among women in this upper middle-income nation [3]. A recent study [4] has shown a trend toward increased mortality from breast cancer in young women from 1996 to 2013 – from 12.1 to 15.7 per 100,000 women – with marked differences among the five major Brazilian regions. The highest average mortality rate in the study period was recorded in the Southern region (16.4 per 100,000 women). Porto Alegre, capital of the southernmost state Rio Grande do Sul, is the Brazilian city with the highest incidence of breast cancer (crude rate estimated at 114.25/100,000 for 2018) [5].
Despite this trend, little data are available regarding the characteristics of Brazilian women with breast cancer and the aspects associated with survival [3,4]. Cecilio et al. [3] pointed out that the testing of molecular markers of breast cancer in Brazil is often not standardized or absent, limiting the amount and quality of the available information and complicating the design of health policies, prevention actions, and targeted treatments.
Previous studies have suggested that the high breast cancer mortality in Brazil might be associated with the type of health care that is available to women [6,7]. A large nationwide study including 3142 women showed that women treated at the public healthcare system (69% of the study population) had significantly longer time from diagnosis to treatment, poorer outcomes, and lower disease-free survival [8], perhaps because they were diagnosed with more advanced disease. While universal care is provided by the Brazilian public health care system (the Unified Health System, SUS), private healthcare has shown progressive growth since the 1990s, reaching approximately 25% of the population in 2016, which corresponds to roughly 50 million people relying on private insurance for their health care needs [7].
Given this scenario, the present study aims to describe the clinical and sociodemographic characteristics as well as survival in 1113 women diagnosed, treated, and followed-up over a 20-year period at a center of excellence in breast cancer treatment (Núcleo Mama Moinhos, NMM) established in a private non-profit hospital Porto Alegre, state capital of Rio Grande do Sul.
2. Methods
This is a retrospective cohort study including all women with breast cancer who were treated and followed-up at NMM from January 1995 to December 2017. All cases had centrally reviewed histological confirmation. NMM includes a comprehensive database with demographic and clinical information on patients, with systematic updates through clinical visits or telephone calls. The NMM protocol for management of patients involves diagnosis, treatment, and clinical follow-up according to current American Society of Clinical Oncology (ASCO) guidelines. Diagnostic procedures comprise triple assessment with clinical examination and staging, imaging with mammography and breast ultrasonography, and an image-guided core needle biopsy for histological diagnosis, with assessment of tumor grade and immunohistochemical status by a single, local laboratory.
For the present cohort, after pathology reports became available, women were informed of their diagnosis and treatment plans were outlined. Initial treatment typically involved surgery, chemotherapy, radiotherapy, or palliative care when indicated. Patients received timely and adequate treatment, that is: treatment started no more than 2–3 weeks after diagnosis and patients received all treatments based on international guidelines. After completed treatment, quarterly follow-up visits were scheduled for the first 3 years, semiannual from year 4 to year 5, and annual thereafter.
All data were obtained by review of NMM electronic records. Patients were contacted over the phone to determine survival status if they had not been to a follow-up visit in the previous 12 months. All-cause mortality was chosen as an outcome indicator to avoid the bias inherent in the determination of causes of death in retrospective studies.
The study protocol was approved by the institutional review board of Hospital Moinhos de Vento. Since the analysis was based on medical records, individual participant consent was not required.
2.1. Setting
NMM is housed in a large non-profit hospital providing care to private insured patients only. Patients treated at NMM come mainly from Porto Alegre (population 1.5 million) but also from other towns in the state or adjacent states where high-quality specialized oncologic care is not available. NMM has a formal multidisciplinary team case management and weekly tumor boards for discussion of all breast cancer cases.
2.2. Primary outcome and prognostic indicators
The primary outcome measure was overall survival, from diagnosis to death. Women known to be alive in December 2017 (last data collection) were censored. The association among survival and the following variables was evaluated: age at diagnosis, disease stage at diagnosis, length of education, smoking status, body mass index (BMI, kg/m2), menopausal status, hormone replacement therapy, family history of breast cancer, tumor characteristics, type of treatment, date of detection of regional/distant metastasis, date of death and cause of death.
2.3. Statistical analysis
Descriptive analysis was used to characterize the study population. Categorical variables were summarized using absolute frequencies and percentages, while continuous variables were analyzed using means and standard deviation (SD). Additionally, age of diagnosis was categorized, with the cutoff point set at the age 50. To estimate survival curves, the Kaplan-Meier estimator was used, and the curves were compared with the log rank test. For the multivariate analysis, we used the Cox regression model using p < 0.2 for selecting covariates for the model, obtaining hazard ratios (HR) and 95% confidence intervals [CI]. Statistical analysis was performed using R software, version 3.6.0. P values were considered statistically significant at <0.05.
3. Results
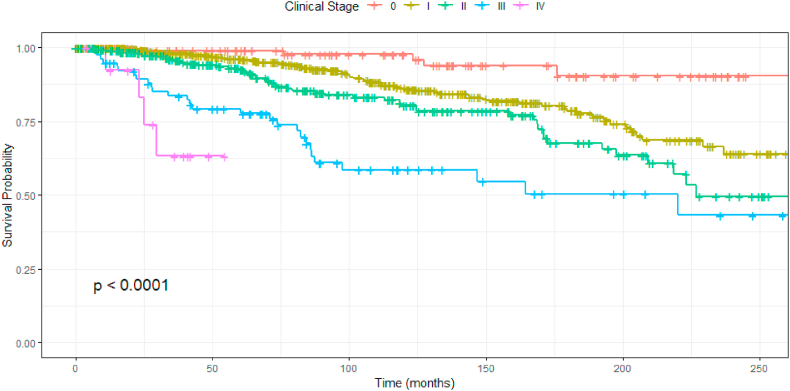
A total of 1113 patients were included in this study. The median follow-up was 84 months. During the 20-year follow-up, 168 women died from breast cancer (15%). Five-, 10- and 20-year overall survival were 93.5%, 83.8% and 60.4%, respectively. Table 1 describes all baseline characteristics of the cohort. Median age at diagnosis was 52 years (SD 13.5). The majority of patients had BMI <30 kg/m (84.5%) and were never smokers (76%). Most patients were postmenopausal (64%) and, from these, 40% had received hormone replacement therapy. Most breast cancers were luminal (76.6%), 19.5% were HER2-positive and 14.4% were triple-negative. The majority of patients was diagnosed in clinical stages 0 and I (62.7%), while only 1.3% were diagnosed in stage IV. Fig. 1 shows overall survival according to stage of breast cancer.
Table 1.
Clinicopathological and demographic characteristics of patients diagnosed with breast cancer.
| Patient characteristics | |
|---|---|
| Median age at diagnosis (n = 1113) | 52 years (SD 13.5) |
| BMI (n = 1082) | |
| <25 | 488 (45.1%) |
| 25–30 | 427 (39.4%) |
| >30 | 167 (15.4%) |
| Education (n = 986) | |
| University degree | 538 (53.8%) |
| 9–12 years of education | 320 (32%) |
| Up to 8 years of education | 142 (14.2%) |
| Smoking (n = 1056) | |
| No | 802 (76%) |
| Smoker/former smoker | 254 (24%) |
| Postmenopausal (n = 1110) | 710 (64%) |
| Hormone replacement therapy | 285 (40.1%) |
| Family history of breast cancer (n = 1000) | 180 (18%) |
| Clinical staging at diagnosis (n = 1032) | |
| Stage 0 | 127 (12.1%) |
| Stage I | 530 (50.6%) |
| Stage II | 297 (28.3%) |
| Stage III | 78 (7.4%) |
| Stage IV | 14 (1.3%) |
| HER2 status (n = 933) | |
| Positive | 182 (19.5%) |
| ER-positive (n = 1105) | 848 (76.7%) |
| PR-positive (n = 1104) | 751 (68%) |
| Biological subtype (n = 933) | |
| Luminal | 617 (66.1%) |
| Triple-negative | 134 (14.4%) |
| Her2+/HR- | 75 (8%) |
| Her2+/HR+ | 107 (11.5%) |
| Histology (n = 1069) | |
| Invasive ductal carcinoma | 909 (85%) |
| Invasive lobular carcinoma | 28 (2.6%) |
| In situ ductal carcinoma | 132 (12.4%) |
| KI67 (n = 909) | |
| Negative | 28 (3.1%) |
| Low | 447 (49.1%) |
| Intermediate | 175 (19.3%) |
| High | 259 (28.5%) |
Fig. 1.
Kaplan-Meier estimate of overall survival among patients according to breast cancer stage.
Table 2 shows treatment characteristics of the patients. Most patients underwent breast conservative surgery (51.1%). Chemotherapy was administered in the neoadjuvant setting in 12.4% of patients and in the adjuvant setting in 41.7%; 64.5% of patients underwent radiation therapy. Table 3 shows 5-, 10 and 20-year survival for curable breast cancer according to stage.
Table 2.
Treatment characteristics of breast cancer patients.
| Treatment | n (%) |
|---|---|
| Mastectomy | 522/1113 (47%) |
| Breast conservative surgery | 569/1113 (51.1%) |
| Adjuvant chemotherapy | 463/1109 (41.7%) |
| Neoadjuvant chemotherapy | 137/1109 (12.4%) |
| Radiotherapy | 715/1109 (64.5%) |
Table 3.
Survival according to breast cancer stage.
| Stage | 5 years | 10 years | 20 years |
|---|---|---|---|
| 0 | 99.0% | 97.7% | 90.4% |
| I | 96.1% | 86.3% | 64.0% |
| IIA | 92.4% | 80.9% | 54.4% |
| IIB | 92.7% | 79.7% | 42.2% |
| III | 79.3% | 58.5% | 43.2% |
∗For stage IV, median survival was 63.3% in 30 months.
In the univariate analysis, the variables associated with survival were age at diagnosis, clinical staging, BMI, lower level of education, positive Ki67 and distant recurrence. According to multivariate analysis (Table 4), age at diagnosis (HR 1.05; CI95 1.03–1.06), staging (stage III: HR 4.04; CI95 1.34–12.19; stage IV: HR 9.61; CI95 2.17–42.50), high KI67 (HR 5.46; CI95 1.27–23.32) and distant recurrence (HR 7.28; CI95 4.79–11.06) were significantly associated with survival. None of the other variables were associated with mortality, including smoking status, years of education, BMI, and tumor biological status.
Table 4.
Multivariate analysis for risk of mortality.
| Variable | Hazard ratio (CI) | P value |
|---|---|---|
| Pathological staging | ||
| I | 1.45 (0.51–4.16) | 0.47 |
| II | 2.83 (0.99–8.10) | 0.05 |
| III | 4.04 (1.34–12.19) | 0.01 |
| IV | 9.61 (2.17–42.50) | 0.002 |
| Age at diagnosis | 1.05 (1.03–1.06) | <0.001 |
| KI67 | ||
| Negative | 1.00 | |
| Low | 3.60 (0.85–15.12) | 0.07 |
| Intermediate/high | 5.46 (1.27–23.32) | 0.02 |
| Distant recurrence | ||
| Yes | 7.28 (4.79–11.06) | <0.001 |
4. Discussion
The overall survival found in this study was similar to those reported in England and Wales, where breast cancer survival has been reported to have increased from 82% in 1971–1972 to 96% in 2010–2011 [9]. In the U.S., the 5-year relative survival in women with localized breast cancer is 98.9% [10]. The present results suggest that patients who receive adequate and timely treatment in a comprehensive cancer center may achieve outcomes similar to those seen in developed countries. To the best of our knowledge, this is the largest breast cancer cohort with an up to 20-year follow-up in Brazil. It is important to highlight that all improvements of diagnosis and treatment of breast cancer were implemented in our cohort; for example, 100% of patients with HER2-positive breast cancer received HER2-blockade in the adjuvant setting from the year of 2005, when the large adjuvant trials with trastuzumab were published [11,12].
Differences in breast cancer survival have been attributed to disparities in the access to diagnosis and treatment [13,14]. In South Africa, Cubasch et al. [15] reported a 3-year survival of 84% for early stage breast cancer and 62% for late stage at diagnosis. They concluded that survival was suboptimal in part because of delays in diagnosis and treatment. Previous studies have suggested an association between the type of care provided and breast cancer mortality in Brazil [8], with women treated in the public system facing significantly longer time from diagnosis to treatment, poorer outcomes, and lower disease-free survival [16]. Post-relapse survival was also significantly worse in public healthcare patients, suggesting that type of coverage and care received in public and private settings may play an important role. Baseline data from the Amazona III study, which is prospectively following almost 3000 Brazilian patients with breast cancer, showed that patients diagnosed in the public have more advanced disease when compared to patients treated in the Brazilian private system [17]. A survey with breast cancer patients in England showed that a substantial proportion of ethnic differences in treatment may reflect a high concentration of ethnic minority patients in low-performing practices [18]. Regarding mortality in young patients, Carioli et al. showed that all countries in Americas and Australasia showed declining trends in mortality in young women (20–49 years), except Brazil, Chile, Venezuela and the Philippines [19]. There are more studies discussing this subject. Hortobagyi et al. showed that differences in survival among developed and developing countries was related to the lack of mammographic screening, late stage at diagnosis, poor access to care and substandard treatment regimens in developing countries [20].
In the present study multivariate analysis showed that staging, intermediate/high KI67, presence of distant recurrence, and increasing age at diagnosis were associated with higher risk of death in breast cancer patients. A previous study performed in Southern Brazil also found that staging was the variable with the strongest association with 10-year survival in 170 women with breast cancer [21]. The 10-year overall survival in that cohort was 83.1%, which is similar to the one found in the present study.
Limitations of the present study include its retrospective nature which generated missing data, regardless of the efforts made to update the database. Moreover, the percentage of stage IV patients was only 1.3%, which is much lower than in the literature (around 5%). This may contribute to the favorable outcome of the study cohort. Conversely, the results are strengthened by the inclusion of women diagnosed, treated, and followed-up at one single center, with centrally reviewed pathologic reports, providing high-quality cancer care.
Due to the retrospective nature of our cohort we could not collect reliable data on breast cancer-specific mortality. We know, from the literature, that the accuracy of cancer specific mortality depends on identifying the correct cause of death [22]. Therefore, we preferred not to report it, in order to avoid bias. We reported all-cause mortality knowing that it varies widely by demographic factors such as age and race and by social class and may exhibit geographic and temporal trends [23]. However, as we analyzed a very homogeneous population with large access to good health system and with similar lifestyle, we considered all-cause mortality more reliable for our retrospective cohort.
Major results regarding treatment-related outcomes are expected in the next decade from the Amazona III study established in January 2016 with 2950 women from 24 research centers across the country [17].
5. Conclusion
Our study showed that Brazilian patients who receive adequate and timely treatment in a comprehensive cancer center may achieve outcomes that are similar to those of high-income countries. The high breast cancer mortality in Brazil seems dependent on the health care system available to cancer patients.
Ethical approval
The study protocol was approved by the institutional review board of Hospital Moinhos de Vento. Since the analysis was based on medical records, individual participant consent was not required.
Declaration of competing interest
MC: nothing to declare.IC: nothing to declare.NAB: nothing to declare.RAR: nothing to declare.YA: nothing to declare.LGJ: nothing to declare.NN: nothing to declare.DDR: Consultant for: Roche, Novartis, AstraZeneca, Lilly, GSK, Sanofi, Libbs, Eisai, Pfizer, Dr Reddy’s, Teva, United Medical. Research funding from: Amgen, Roche, GSK, L’Òreal. Expert testimony for: Roche, Novartis, Pfizer, AstraZeneca, Lilly. Travel, Accommodations, Expenses: Roche, Novartis, Lilly, Amgen.
Acknowledgments
A special thank to Marina Bessel and Luana Giongo Pedrotti for all institucional statistical support and to all Medical residents and Research fellows that contributed to collecting data since 2003.
References
- 1.Instituto Brasileiro de Geografia e Estatística . 2018. Populações. Projeção da população do Brasil e das Unidades da Federação.https://ww2.ibge.gov.br/apps/populacao/projecao/ Feb 7, 2018 2018. [Google Scholar]
- 2.Economic commission for Latin America and the caribbean (ECLAC), demographic observatory. CEPAL; Santiago: 2016. [Google Scholar]
- 3.Cecilio A.P., Takakura E.T., Jumes J.J., Dos Santos J.W., Herrera A.C., Victorino V.J., Panis C. vol. 7. Dove Med Press; 2015. Breast cancer in Brazil: epidemiology and treatment challenges, Breast Cancer; pp. 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha-Brischiliari S.C., Oliveira R.R., Andrade L., Brischiliari A., Gravena A.A., Carvalho M.D., Pelloso S.M. The rise in mortality from breast cancer in young women: trend analysis in Brazil. PloS One. 2017;12(1) doi: 10.1371/journal.pone.0168950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Instituto Nacional de Câncer José Alencar Gomes da Silva, Estimativa . Rio Grande do Sul e Porto Alegre; 2018. Incidência de câncer no Brasil.http://www.inca.gov.br/estimativa/2018/rio-grande-sul-porto-alegre.asp 2018. [Google Scholar]
- 6.Malta D.C., Stopa S.R., Pereira C.A., Szwarcwald C.L., Oliveira M., Reis A.C. Private health care coverage in the Brazilian population, according to the 2013 Brazilian national health survey. Ciência Saúde Coletiva. 2017;22(1):179–190. doi: 10.1590/1413-81232017221.16782015. [DOI] [PubMed] [Google Scholar]
- 7.Agência Nacional de Saúde Suplementar . 2018. Informações em saúde suplementar.http://www.ans.gov.br/perfil-do-setor/dados-e-indicadores-do-setor 2018. [Google Scholar]
- 8.Liedke P.E., Finkelstein D.M., Szymonifka J., Barrios C.H., Chavarri-Guerra Y., Bines J., Vasconcelos C., Simon S.D., Goss P.E. Outcomes of breast cancer in Brazil related to health care coverage: a retrospective cohort study. Cancer Epidemiol Biomark Prev. 2014;23(1):126–133. doi: 10.1158/1055-9965.EPI-13-0693. [DOI] [PubMed] [Google Scholar]
- 9.Cancer Research Uk Breast cancer survival trends over time. 2017. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/survival#heading-Two
- 10.National Cancer Institute Cancer stat facts: female breast cancer. 2017. https://seer.cancer.gov/statfacts/html/breast.html
- 11.Piccart-Gebhart M.J., Procter M., Leyland-Jones B. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 12.Romond E.H., Perez E.A., Bryant J. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 13.Justo N., Wilking N., Jonsson B., Luciani S., Cazap E. A review of breast cancer care and outcomes in Latin America. Oncol. 2013;18(3):248–256. doi: 10.1634/theoncologist.2012-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman M.P., Quaresma M., Berrino F., Lutz J.M., De Angelis R., Capocaccia R., Baili P., Rachet B., Gatta G., Hakulinen T., Micheli A., Sant M., Weir H.K., Elwood J.M., Tsukuma H., Koifman S., Ga E.S., Francisci S., Santaquilani M., Verdecchia A., Storm H.H., Young J.L., C W. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9(8):730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 15.Cubasch H., Dickens C., Joffe M., Duarte R., Murugan N., Tsai Chih M., Moodley K., Sharma V., Ayeni O., Jacobson J.S., Neugut A.I., McCormack V., Ruff P. Breast cancer survival in Soweto, Johannesburg, South Africa: a receptor-defined cohort of women diagnosed from 2009 to 11. Cancer Epidemiol. 2018;52:120–127. doi: 10.1016/j.canep.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon S.D., Bines J., Werutsky G. Characteristics and prognosis of stage I-III breast cancer subtypes in Brazil: the AMAZONA retrospective cohort study. Breast. 2019;44:113–119. doi: 10.1016/j.breast.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Rosa D.D., Bines J., Werutsky G. Breast Cancer Res Treat; 2020. The impact of sociodemographic factors and health insurance coverage in the diagnosis and clinicopathological characteristics of breast cancer in Brazil: AMAZONA III study (GBECAM 0115) [published online ahead of print, 2020 Jul 29] [DOI] [PubMed] [Google Scholar]
- 18.Lyratzopoulos G., Elliott M., Barbiere J.M., Henderson A., Staetsky L., Paddison C. Understanding ethnic and other socio-demographic differences in patient experience of primary care: evidence from the English general practice patient survey. BMJ Qual Saf. 2012;21(1):21–29. doi: 10.1136/bmjqs-2011-000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carioli G., Malvezzi M., Rodriguez T., Bertuccio P., Negri E., La Vecchia C. Trends and predictions to 2020 in breast cancer mortality: Americas and Australasia. Breast. 2018;37:163–169. doi: 10.1016/j.breast.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Hortobagyi G.N., de la Garza Salazar J., Pritchard K. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Canc. 2005;6(5):391–401. doi: 10.3816/cbc.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 21.Hofelmann D.A., Anjos J.C., Ayala A.L. [Survival for ten years and prognostic factors for women with breast cancer in Joinville in the State of Santa Catarina, Brazil] Ciência Saúde Coletiva. 2014;19(6):1813–1824. doi: 10.1590/1413-81232014196.03062013. [DOI] [PubMed] [Google Scholar]
- 22.Penston J. Should we use total mortality rather than cancer specific mortality to judge cancer screening programmes? Yes. BMJ. 2011;343:d6395. doi: 10.1136/bmj.d6395. [DOI] [PubMed] [Google Scholar]
- 23.Dignam J.J., Huang L., Ries L., Reichman M., Mariotto A., Feuer E. Estimating breast cancer-specific and other-cause mortality in clinical trial and population-based cancer registry cohorts. Cancer. 2009;115(22):5272–5283. doi: 10.1002/cncr.24617. [DOI] [PMC free article] [PubMed] [Google Scholar]