Abstract
Neuropathic pain (NP) resulting from injury or disease of the somatosensory system is a common, debilitating chronic disorder that significantly undermines quality of life. Preclinical and clinical studies have considerably advanced our understanding of the myriad peripheral and central changes in neuronal and non-neuronal cells associated with persistent pain states. Disappointingly, advances in clinical therapies for NP have not paralleled the substantial advances in basic science. Most drugs currently used for the treatment of NP bind to receptors that are widely expressed in the peripheral and central nervous systems, and hence are frequently associated with adverse effects. Therefore, identifying key targets involved in the initiation and maintenance of NP in the periphery, with guidance from meticulous clinical evidence, merits renewed focus. We consider the relative importance of peripheral and central mechanisms and present several lines of clinical and preclinical evidence supporting the tenet that spontaneous and ectopic activity in primary afferent neurons plays a critical role in NP. Several potential peripheral “pain generator” sites have been identified, and defining their role in NP states of different etiologies is important for targeted therapy. Hyperexcitability of peripheral neurons may represent a “low-hanging target” in the development of safe therapies for a subset of patients with NP. We summarize work from our group and others that identifies potential peripheral drug targets, including peripheral opioid receptors, involved in the modulation of NP. In future drug development efforts, therapies directed at peripheral mechanisms may offer potential advantages over current CNS-penetrating drugs, including minimal adverse effects.
1. Introduction
Neuropathic pain (NP) results from injury to or disease of the somatosensory system. It is estimated that approximately 7–10% of the population and 25–30% of individuals with chronic pain suffer from NP [22,144,151]. The burning, stinging, shooting, dysesthetic sensations associated with NP can be excruciating, markedly detract from quality of life, and lead to depression and even suicide. Most drugs currently in use for NP bind to receptors that are widely expressed throughout the central nervous system (CNS), and hence are frequently associated with dose-limiting adverse effects (sedation, dizziness, cognitive dysfunction), addiction, and abuse. The limited efficacy of the NP drugs may have also contributed, in part, to the increased use of opioids for treating this chronic pain disorder. Therefore, developing new treatments for NP with minimal CNS-related adverse effects is a high research priority [111,123,126].
Since the initial neurophysiologic evidence, nearly four decades ago, for an increase in excitability of CNS neurons after peripheral tissue injury in an animal model [160], much has been learned about the peripheral and central changes associated with persistent pain states, particularly NP. A myriad of associated functional, structural, and molecular changes in neurons, glial cells, and other non-neuronal cells (e.g., macrophages) from the periphery to the brain have been characterized in several preclinical models (Figure 1, for review see references [9,16,26,65,77]. Regrettably, advances in clinical therapies for NP have not paralleled the significant advances in basic science [21,137]. Anticonvulsants, such as gabapentin and pregabalin, and antidepressants, such as nortriptyline and duloxetine, remain the first-line drugs for the treatment of NP [36,51]. Not including reformulations, the proportion of new drugs for pain treatment entering phase 1 clinical trials (relative to all new drugs) declined between 2000–2002 and 2013–2015 [64]. Moreover, only 11% of new drugs for pain advanced from Phase 2 to Phase 3 trials, and drug development for pain swung from cultivating new molecular entities to primarily reformulating old drugs. Bridging this ongoing gap between advances in mechanistic research and the discovery of safe and effective novel clinical therapies, the “valley of death” in drug development, may warrant a renewed focus on identifying key targets involved in the initiation and maintenance of NP with guidance from meticulous accrual of clinical evidence.
Figure 1.
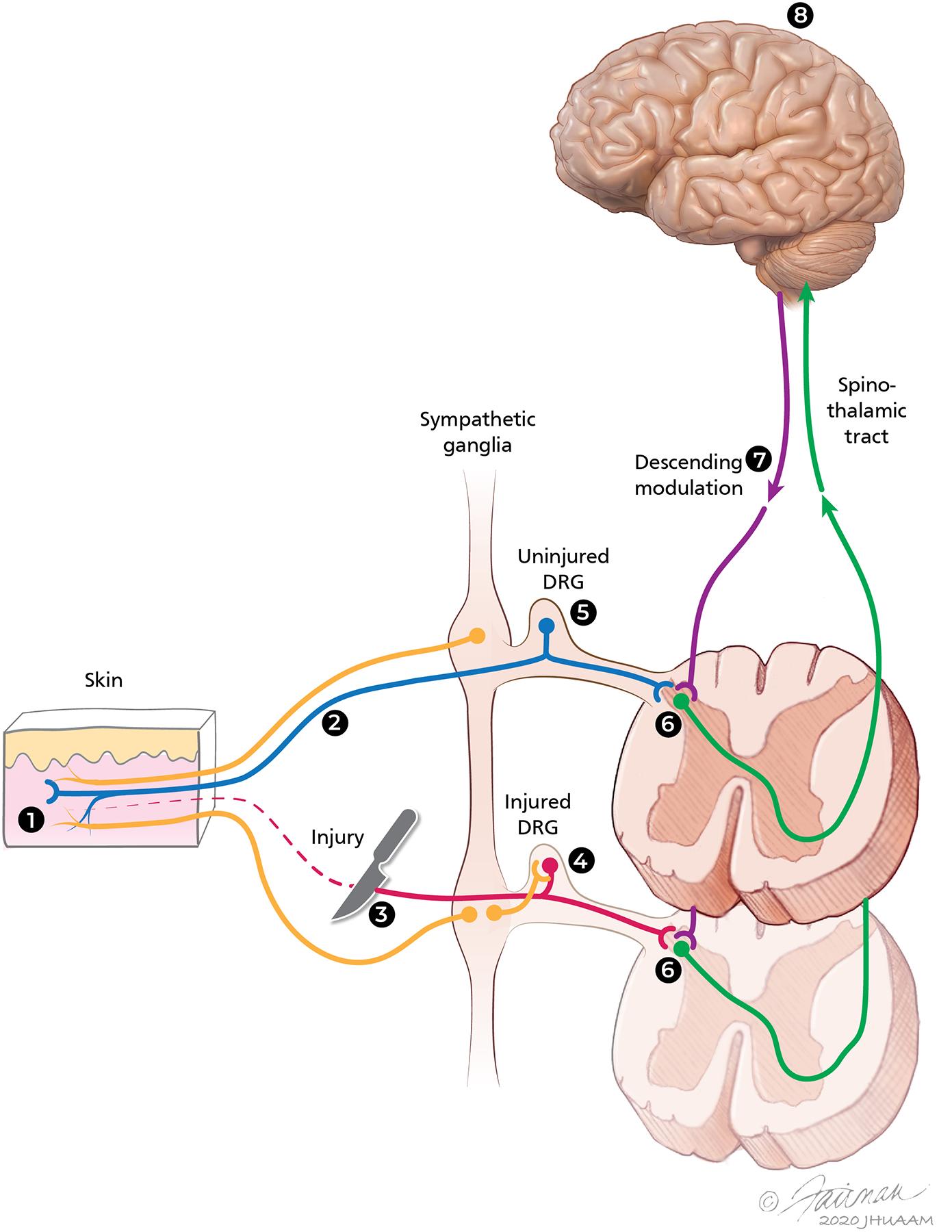
Sites along the peripheral and central nervous systems where pathophysiologic changes may contribute to the mechanisms of neuropathic pain. Different sites where physiologic and/or anatomic changes have been described in various models of neuropathic pain are shown: (1) Primary afferent nociceptors, (2) Axons in peripheral nerves, (3) Site of nerve injury, (4) Dorsal root ganglion (DRG) at the level of nerve injury, (5) Adjacent uninjured DRG, (6) Spinal dorsal horn at the level of injury and at adjacent levels, and (7) Descending modulatory systems: decrease of descending inhibition and/or increase of descending facilitation systems may contribute to the enhanced responsiveness of dorsal horn neurons. (8) Central mechanisms involve various sites in the brain, particularly in central neuropathic pain states. Post-ganglionic sympathetic efferent fibers that may influence the activity of neurons at the level of the injured DRG and enhance the responses of nociceptive afferents in the periphery are also shown.
Human and preclinical studies in the latter part of the last century shed considerable light on the mechanisms that contribute to peripheral and central sensitization after acute tissue injuries, such as a burn and surgical wound. Fortunately, in most cases, the increased sensitivity of peripheral and central neurons that develops after such injuries is short-lived. Considerable advances have also been made in the treatment of patients with acute pain. However, the management of certain chronic pain conditions, particularly NP, remains a challenge.
2. Peripheral and central sensitization: Insights from models of acute pain
Broadly speaking there are two potential sources of persistent pain. One is the peripheral nervous system (PNS) and the second encompasses the CNS from the spinal cord to the brain. One possibility is that persistent pain may be driven primarily by events in the PNS. An alternative view is that, although pain may be initiated by events in the periphery, pain becomes centralized in association with CNS hypersensitivity, a mechanism termed central sensitization. The concept of centralized pain is now commonly invoked to account for pain that is difficult to treat. If the basis of ongoing pain is centralized, addressing a peripheral source of pain may be futile. Here, we consider the relative importance of peripheral and central mechanisms and the clinical implications of these insights.
Both peripheral and central sensitization play a role in pain signaling after acute injury. Within moments after a burn injury, nociceptors sensitize to heat stimuli [96,112]. Sensitization is manifest by a lowered threshold and an increased response to suprathreshold stimuli. In addition, nociceptors develop spontaneous activity. Sensitization corresponds to the psychophysical changes, namely, a lowered pain threshold, increased pain to suprathreshold stimuli, and ongoing (spontaneous) stimulus-independent pain. These changes, all at the site of the injury, are termed primary hyperalgesia. The changes in pain sensibility, however, extend beyond the primary zone. The responses to mechanical stimuli are augmented in a discreet area that surrounds the initial area of injury (Figure 2). The augmented pain sensibility in the surrounding zone is termed secondary hyperalgesia.
Figure 2.
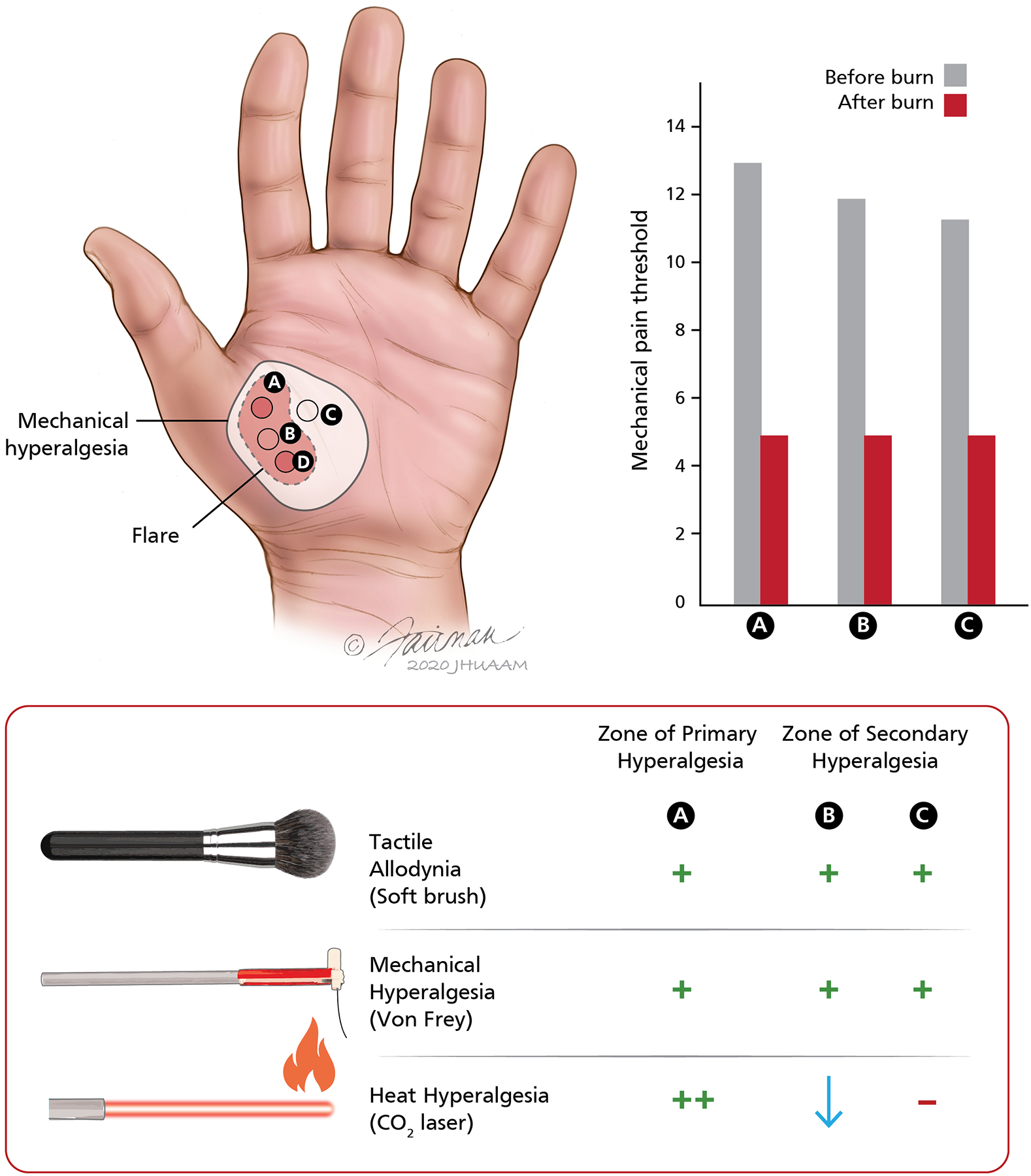
Evidence for different mechanisms of primary and secondary hyperalgesia after cutaneous burn injuries in human subjects. (Top left) The illustration shows the relative location of the stimulus sites on the glabrous skin of the hand and the area of flare and mechanical hyperalgesia that develops after cutaneous burn injuries at sites A and D. The heat injury consisted of two burns (53°C, 30 s) applied over an area 7.5 mm in diameter separated (center to center) by a 2 cm interval. The area of mechanical hyperalgesia was larger than the flare region, and mechanical hyperalgesia persisted even after the flare disappeared. Mechanical thresholds for pain, assessed by von Frey hairs, and ratings of pain to controlled laser thermal stimuli (41–49°C, 10 stimuli at 1°C increments) were recorded at sites A, B, and C before and after the burns at sites A and D. (Top right) Mean pain thresholds to mechanical stimuli at the primary site (site A) and secondary hyperalgesia sites (B, C) before and after the burns at A and D. The mechanical pain threshold was significantly reduced after the cutaneous injury and was similar at the regions of primary and secondary hyperalgesia. (Bottom) Schematic representation of sensory changes in the zones of primary and secondary hyperalgesia. In the region of primary hyperalgesia (site A), allodynia to brush stimuli and hyperalgesia to von Frey hairs were observed. Pain ratings to heat stimuli were increased more than four-fold at this site (thermal hyperalgesia). Although pain in response to mechanical stimuli was enhanced at both secondary hyperalgesia sites (B, C), the pain to thermal stimuli was either unchanged (site C) or decreased (site B). Thus, thermal hyperalgesia was observed only at the injury site, whereas hypoalgesia to heat was observed at the site between the two burn injuries. Adapted from Raja et al. [115].
In initial electrophysiological studies, heat hyperalgesia in the primary zone appeared adequately explained by the corresponding sensitization of the nociceptors [25,73,81,112,142]. However, the nociceptors in the surrounding zone do not sensitize to heat or mechanical stimuli [79,146]. One of the salient findings in the zone of secondary hyperalgesia is the development of pain to stroking stimuli, termed dynamic allodynia. Given that the nociceptors which innervate the region next to the injury do not sensitize, the conclusion is that the sensory changes are due to central sensitization, an enhanced sensitivity of central neurons that signal pain to peripheral afferent inputs [161,162].
Importantly, psychophysical and microneurographic studies of secondary hyperalgesia in human subjects have provided additional evidence for central sensitization. We studied the effects of a burn injury applied to human subjects in two locations separated by several millimeters on the thenar eminence of the hand. Sensory testing revealed hyperalgesia to heat and mechanical stimuli in the burn injury site’s zone of primary hyperalgesia (Figure 2) [115]. However, in the zone between the two burn injuries, the zone of secondary hyperalgesia, there was a dissociated sensory change. Strikingly that area exhibited hyperalgesia to mechanical stimuli but hypo-algesia (reduced painfulness compared to normal) to heat. The dissociated sensory change cannot be explained by peripheral sensitization and therefore must result from central mechanisms. Torebjork and colleagues [143] placed microelectrodes into sensory nerves of human subjects and stimulated A-fibers that innervate the zone of secondary hyperalgesia. Stimulation of these afferents normally evokes a tactile non-painful sensation. However, this same stimulation after injection of capsaicin evoked pain, signifying a central sensitization to the inputs of A fibers, including low-threshold A-β fibers.
What we have learned so far is that, as a result of central sensitization, neural activity in tactile mechanosensitive afferents manifests as pain to stroking stimuli. A logical next question is whether central sensitization also occurs to the inputs of nociceptive afferents. An important discovery highlighted by the work of LaMotte and colleagues [80] is that mechanical hyperalgesia is characterized not only by stroking pain (dynamic allodynia) but also punctate hyperalgesia (also referred to as static hyperalgesia). Punctate hyperalgesia, in contrast to allodynia, is evidenced by augmented pain to a normally painful von Frey hair stimulus. It persists longer after injury and is less dependent on ongoing input of sensitized nociceptors in the region of injury. In contrast, allodynia mediated by A-β fibers reverses when painful inputs from the zone of primary hyperalgesia are blocked [7]. Punctate hyperalgesia in human subjects was studied after intradermal injection of capsaicin. Marked secondary hyperalgesia was evident for many hours after injection. A blockade of A-fiber conduction, which presumably affects A-fiber nociceptors, reduced punctate hyperalgesia [173].
In a study of patients with NP, we demonstrated that the switch to A-β fiber-mediated pain signaling is manifest in chronic pain as well [27]. In 15 of 17 patients with NP caused by peripheral nerve injury, an acute and selective block of A-β function affecting mechanosensitive afferents that normally signal tactile sensations led to a marked reduction or elimination of allodynia. The selective conduction block studies, as well as additional lines of evidence from local anesthetic block and latency to pain detection, indicated that the allodynia in NP could be explained only by a signaling mechanism that involved the rapidly conducting mechanosensitive A-β fibers.
Therefore, hyperalgesia and allodynia after acute injury likely entail three mechanisms: 1) sensitization of primary afferent nociceptors; 2) central sensitization to the inputs of nociceptive primary afferents; and 3) a switch in the messaging of tactile low-threshold A-fibers such that their inputs acquire the capacity to signal pain. Studies in subjects with NP suggest that mechanisms similar to that observed in acute injury may also be involved.
3. Neuropathic pain: role of peripheral mechanisms in the maintenance of ongoing pain and central sensitization
3.1. Is input from the periphery critical in the maintenance of NP?
A fundamental question regarding chronic NP caused by disease or injury to the PNS is whether it is maintained by peripheral input or, alternatively, by autonomous, central (spinal or supraspinal) generators, that is, does chronic pain become “centralized”? This question is not merely one of academic interest but one that is likely to have profound clinical consequence [93,102]. The answer determines the tissue(s) that should be the primary target for therapies and directs future drug development efforts [118,119].
Spontaneous activity in sensory afferent neurons is considered to be the primary neurophysiologic correlate for symptoms associated with NP, such as spontaneous pain, paresthesia, and dysesthesia [40,147]. We present several lines of clinical and preclinical evidence to support the postulate that spontaneous and ectopic activity in primary afferent neurons represents a “low-hanging target” in the development of safe therapies for a subset of patients with NP. Although the focus of our review is on neuronal hyperactivity, it is worth recognizing that these neurons have complex interactions with glia, immune cells, keratinocytes, and other non-neuronal cells that may play a role in sensitization of the primary afferent neurons and generation of spontaneous activity. Recent reports suggest that peripheral nerve injury induces the expression of colony-stimulating factor 1 (CSF1) in injured DRG neurons. The axonal transport and release of CSF-1 in the spinal dorsal horn induces microglial proliferation and upregulation of pain-related genes, which contribute to NP [57]. Peripheral nerve injury also induces proliferation of macrophages both at the injury site and around injured sensory neurons in DRG. Interestingly, Shepherd et al. [129] reported that angiotensin II receptor-expressing macrophages at the site of injury play a critical role in NP. However, a more recent study concluded that macrophages in the DRG, but not at the peripheral nerve injury site, trigger a reciprocal interaction with sensory neurons and contribute to the initiation and persistence of NP [171]. Thus, it is likely worthwhile to study drugs that selectively target peripheral sites, such as primary afferent neurons, or non-neuronal substrates in the PNS, such as satellite glial cells in the DRG and macrophages in sensory ganglia or at the injury site.
3.1.1. Clinical evidence
Several studies in recent years have shown that peripheral local anesthetic blocks of nerves that innervate the painful region alleviate ongoing pain and hypersensitivity in a proportion of subjects with NP of varying etiologies [23,61,152]. If a peripheral local anesthetic nerve block relieves the pain, then the presumption is that the driver for pain is in the periphery. Hence, these studies imply that peripheral neuronal hyperexcitability amplifies pain signaling and may contribute to the maintenance of unremitting pain symptoms, such as spontaneous pain, tactile allodynia, and thermal hyperalgesia, in a subset of patients.
Nerve injury and peripheral neuropathic pain:
Haroutounian and colleagues [61] reported the effects of a peripheral nerve block with lidocaine and an intravenous infusion of lidocaine on spontaneous pain in patients who had chronic NP associated with traumatic peripheral nerve injury or distal symmetric polyneuropathy (DSP). Similar to other reports [32,54,75,97], the peripheral local anesthetic nerve block induced complete relief of ongoing spontaneous pain in both patient groups. Intravenous lidocaine infusion, however, had only a small effect on nerve injury-induced spontaneous pain, whereas it produced more than 50% pain relief in the majority of patients with DSP. This disparity may suggest that different peripheral generator sites contribute to the pain signal in peripheral nerve injury and DSP. This report, as well as earlier observations by Gracely et al. [54] that local anesthetic block of painful foci in patients with reflex sympathetic dystrophy not only reduced pain but also abolished touch-evoked allodynia outside the anesthetic area, suggests that both spontaneous pain and central sensitization in chronic NP may be maintained by input from the periphery. The beneficial effects of topical lidocaine and high-concentration (8%) capsaicin in a subset of patients with postherpetic neuralgia and other localized peripheral NP states further supports the postulate that peripheral neuronal hyperexcitability is a key factor in the maintenance of the chronic pain state in these subjects [12,95,121,122]. For reviews, see also [18,39].
Postamputation pain:
Additional evidence for the critical role of peripheral afferent activity stems from studies in subjects with postamputation pain. In a study by Chabal et al. [32] phantom limb pain (PLP) was attenuated by perineuronal application of lidocaine. Years later, Birbaumer et al. [17] showed that anesthesia of the affected limb with a brachial plexus block relieved PLP. The plexus block not only blocked PLP in 50% of the patients studied, but also led to a reversal of amputation-induced cortical reorganization [17]. It has been argued that a peripheral pain generator proximal to the site of the axillary brachial plexus (i.e., dorsal root ganglion [DRG]) might contribute to the failure of local anesthetic block in those who do not obtain pain relief. To investigate whether the DRG plays a crucial role in maintaining PLP, Vaso et al. [152] performed intrathecal and intraforaminal anesthetic blocks in lower limb amputees with PLP. Seventy-five percent (21 of 28) of patients subjected to the intraforaminal lidocaine block experienced full pain relief, and six experienced a substantial (75–90%) reduction in pain. A more recent double-blind randomized, placebo-controlled study further supports the role of peripheral afferent input in postamputation pain. Buch et al. [23] showed that PLP intensity in amputees was significantly reduced after lidocaine block of peripheral nerves, compared with that after saline injection.
Post-stroke pain:
Most studies that have examined a role for afferent neuronal hyperexcitability have been carried out in subjects with NP of peripheral etiology. Pain resulting from CNS disease is generally attributed to a loss of inhibitory neuronal mechanisms and a disinhibition of somatosensory neurons, resulting in autonomous ectopic nociceptive activity at CNS sites. Interestingly, in a recent small study of patients with central post-stroke pain in an extremity, ultrasound-guided peripheral nerve blocks with lidocaine completely abolished pain in 7 of 8 subjects and provided >50% pain relief in the other participant [60]. This intriguing observation suggests that, even in central NP states, the ongoing pain may be partly dependent on afferent input from the painful region in the periphery.
Results from the studies described above suggest that activity in primary afferent nerve fibers and/or DRG (i.e., peripheral hypersensitivity) plays an important role in the maintenance of chronic NP. Hyperexcitable primary afferent neurons may enhance excitatory neurotransmitter release in the spinal cord, producing a prolonged state of CNS neuronal hyperexcitability, and may also contribute to the maintenance of complex activity-dependent plasticity in the CNS. Despite having pain for prolonged periods, the patients apparently did not develop a central, autonomous “pain generator.” Although in many NP states, the central changes may be insufficient to maintain spontaneous pain by themselves, central functional and structural changes may lead to amplification of the incoming peripheral input. In such cases, even “weak” input from primary afferents that does not normally result in pain becomes sufficient to produce painful sensations. Thus, the clinical studies presented do not negate a role for central mechanisms in spontaneous pain. The studies in patients with peripheral NP caused by neuropathy or peripheral nerve injury imply that the pain generator is localized in the peripheral distal axon, whereas the studies in amputees suggest that the primary pain generator may be localized at the DRG.
3.1.2. Preclinical evidence
The role of nociceptive afferent neurons in the transduction and transmission of noxious information from the periphery to the CNS has been well characterized [146]. Early events soon after the nerve injury, such as injury discharge and spontaneous activity (SA), may be critical to trigger subsequent neurophysiologic and chemical changes that lead to the development of NP [53,164,165]. In animal models of peripheral nerve injury or neuropathies of various etiologies, SA in primary afferents may originate from the injury site [4–6,19,24,68].
SA has been observed in uninjured, unmyelinated afferents after spinal nerve ligation [4]. This SA may be induced in peripheral target tissue where partial denervation leads to upregulation of trophic factors that in turn increase neuronal excitability. Alternatively, the inflammatory milieu accompanying Wallerian degeneration of neighboring axons may produce SA along the axon of uninjured afferents. Recent studies also indicate that dysfunction of ion channels Nav1.8, TRPA1, and hyperpolarization-activated cyclic nucleotide-gated (HCN) channel 2 in axons of nociceptive afferents may play a central role in the pathogenesis of painful diabetic neuropathy [8,49,50,136,149]. Several studies suggest that DRG neurons may become spontaneously active after nerve injury. Therefore, they may represent an important pain generator site after nerve injury and provide a useful therapeutic target [24,35,87,89,135,172]. DRG neurons may develop ectopic discharges and increased excitability for a prolonged period after nerve injury [89,153], or nerve root compression [103], possibly driven by both external (e.g., inflammation) and intrinsic factors (e.g., changes in gene expression to injury, regeneration response). Immuno-histochemical and patch-clamp electrophysiologic recordings from dissociated human DRG cells isolated from subjects with radicular pain or cancer-related NP demonstrated an unpregulation of Nav1.7 channels and the presence of SA in neuronal soma (Figure 3) [86]. The importance of the DRG as a generator site was highlighted in a recent study from Yatziv and Devor [169] that used an L5 spinal nerve ligation model. When injected in the vicinity of the L5 DRG, a low concentration of lidocaine that did not block conduction or cause sensory-motor deficits did relieve mechanical allodynia. The same concentration of lidocaine administered in the region of the neuroma or adjacent L4 DRG was not effective at decreasing mechanical allodynia. The authors concluded that ectopic activity in injured DRGs, rather than originating in the neuroma and in neighboring uninjured afferents, contributes primarily to NP. They opined that continuous infusion of a low dose of local anesthetic in the vicinity of the injured DRG could be a potential interventional strategy for relieving NP with an increased benefit/risk ratio.
Figure 3.
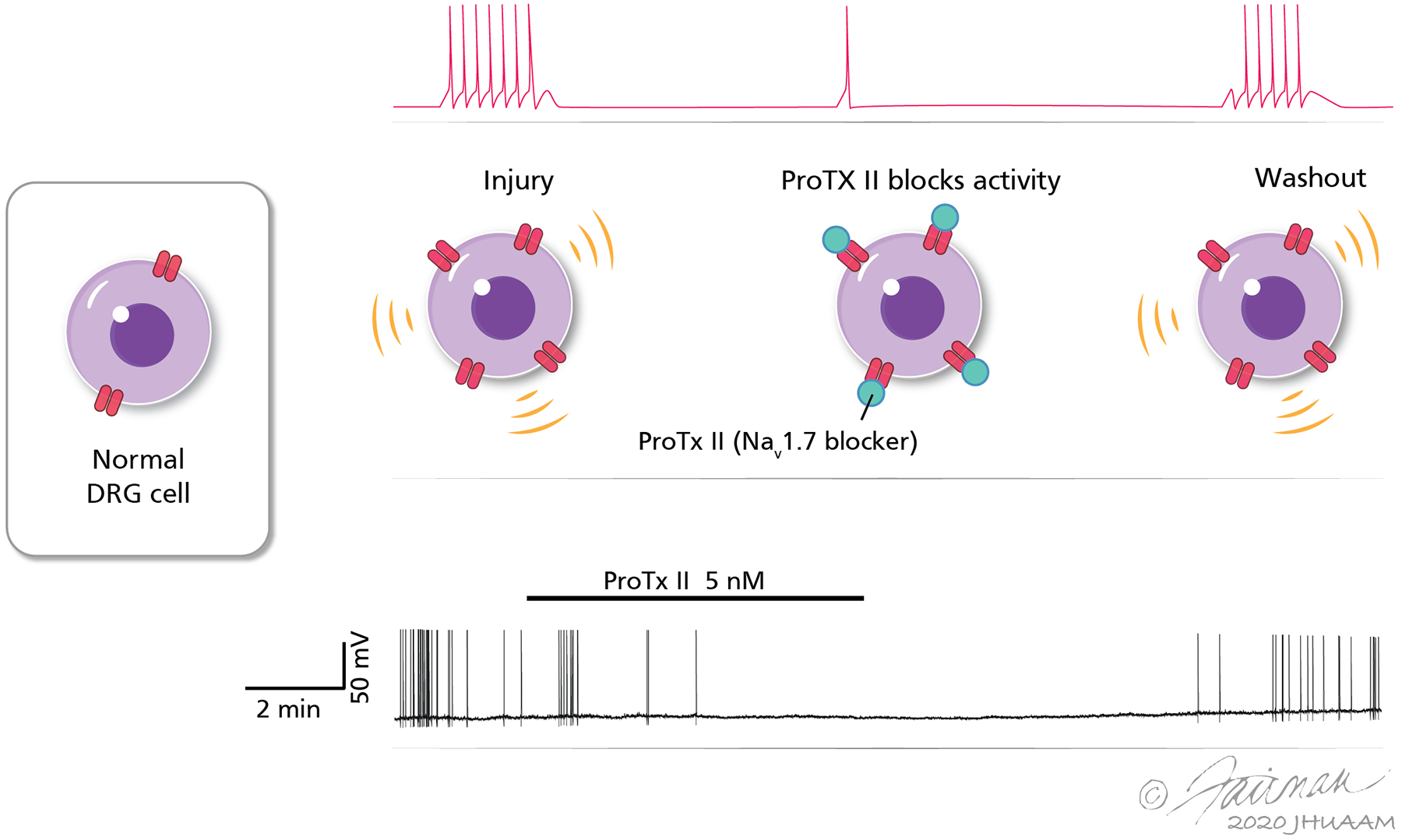
Sodium channels (Nav1.7) expressed in human dorsal root ganglion (DRG) neurons and satellite cells contribute to ectopic spontaneous activity. The top panel shows a schematic representation of increased Nav1.7 ion channel expression in DRG cells, based on immunohistochemical studies. The expression of Nav1.7 was significantly higher in DRG cells with spontaneous activity (SA) than in those without SA. Selective blockade of the Na channel results in suppression of SA. The bottom panel shows a representative recording of a DRG neuron from a patient with cancer-related neuropathic pain shows that SA is suppressed by bath application of the selective Nav1.7 blocker Pro-TX II. Activity recovers after wash-out of the drug from the bath. SA was observed only in human DRG neurons collected from dermatomes where patients had a history of radiating pain and/or radiographic evidence of nerve root compression. Adapted from Li Y et al. [86], courtesy Drs. Y. Li and P.M. Dougherty.
The development of peripheral neural hyperexcitability appears not to be restricted to lesions of the PNS and may also contribute to central NP after spinal cord injury. In an isolated skin–nerve preparation, Carlton et al. [30] showed that contusive spinal cord injury enhanced background activity in peripheral fibers of nociceptors, suggesting that SA can be generated in or near the peripheral terminals of nociceptors. DRG neurons below the level of spinal cord injury also develop chronic hyperexcitability and increased SA that can last for months. The DRG neurons that exhibited SA were mostly nociceptors either responding to capsaicin or binding isolectin B4 [15,30,59,63,156].
The evidence presented above indicates that pain generators can develop at several sites along the peripheral neuraxis. Potential sites that have been suggested include the nerve fiber endings in skin and deeper tissues, dying back distal axon terminals in metabolic and HIV neuropathies [110,114], the distal cut end of an injured nerve, focal sites of demyelination along diseased or partially injured nerves, and the DRG at and adjacent to the level of injury (Figure 4). Thus, the critical site of SA initiation may differ based on the disease pathophysiology. Additional studies are needed to determine the best target for a specific NP state.
Figure 4.
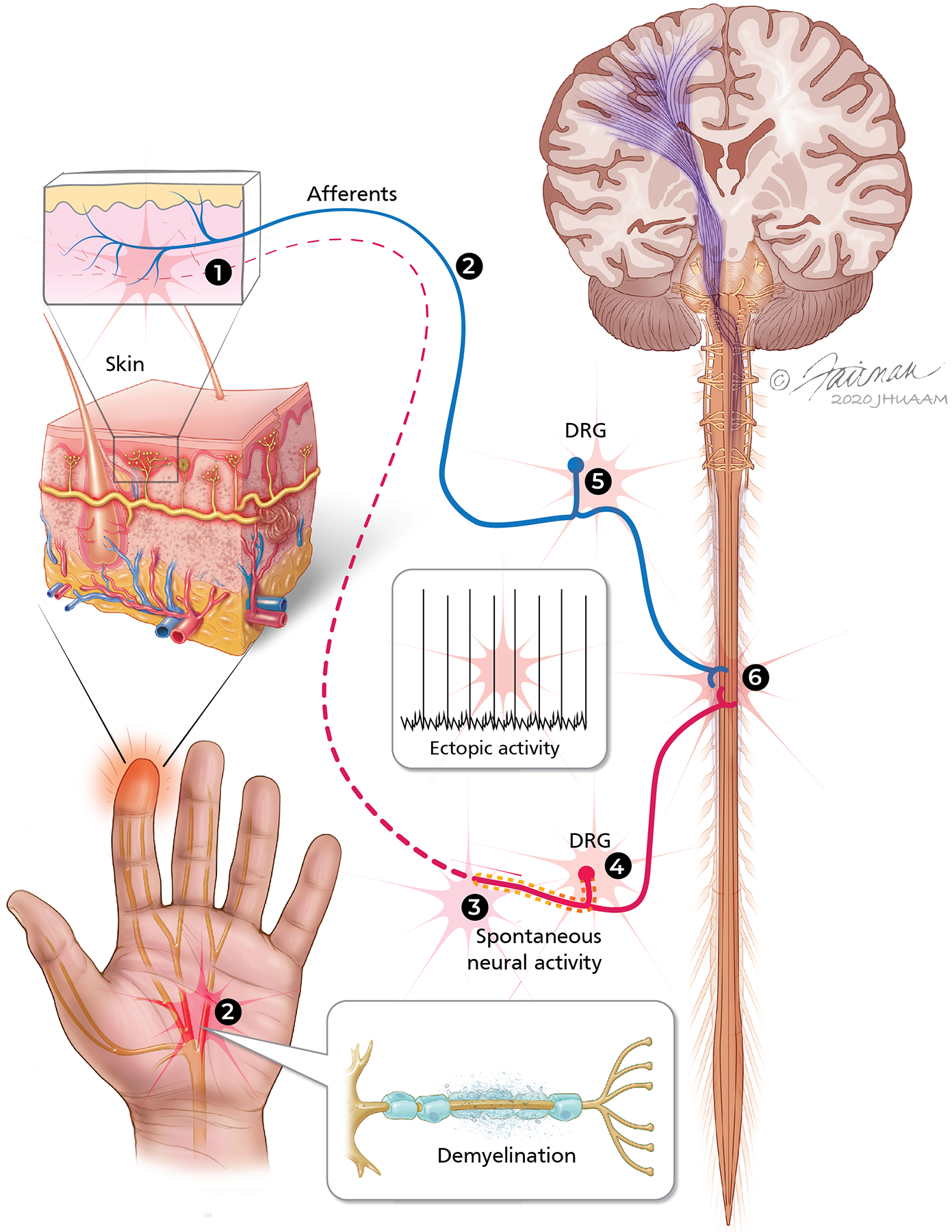
Sites in the peripheral nervous system where spontaneous and ectopic activity and hyperexcitability may represent potential targets for developing neuropathic pain therapeutic strategies. (1) In the periphery, hyperactivity in nociceptive afferent fibers may develop from several mechanisms. Wallerian degeneration of the distal part of the injured nerve exposes axons from uninjured portions of the nerve to a milieu of cytokines and growth factors that sensitize nociceptors and cause sprouting of terminals. In addition, afferent terminals dying back from the epidermal innervation may be potential sites of ectopic activity in idiopathic, toxic, and HIV neuropathies. (2) Localized axon demyelination in peripheral nerves may represent sites of ectopic activity in metabolic neuropathies. (3) Ectopic activity and sensitivity to mechanical stimuli develop at the site of nerve injury/neuroma. (4) Spontaneous neural activity develops in dorsal root ganglia (DRG) at the injury level owing to dysregulation of ion channels, loss of trophic support from the periphery, and changes in gene regulation. (5) Increased trophic factors transported from the periphery may cause hyperactivity in the uninjured DRG owing to altered expression of different molecules.
3.2. What type of primary afferent neurons drive spontaneous pain in NP?
3.2.1. Clinical evidence
The types of primary afferent nerve fibers that drive spontaneous pain in chronic pain patients is a subject of considerable debate. Microneurographic studies of activity from nociceptive afferents in humans and whole-cell patch clamp recordings of dissociated DRG neurons from patients treated for NP have revealed spontaneous activity and hyperexcitability in unmyelinated fibers. This hyperexcitability has been found in patients with NP conditions as diverse as phantom limb pain, trigeminal neuralgia, diabetic neuropathy, and erythromelalgia [14,20,71,104,108,128]. Unfortunately, because of technical limitations (low signal-to-noise ratio, ongoing electrical stimulation), the rate of the spontaneous discharge activity is difficult to determine in microneurography studies. Although selective A-fiber block resolved touch-evoked allodynia in patients with chronic NP [27], it did not reduce spontaneous pain, which was presumably mediated by C-fiber input [75].
3.2.2. Preclinical evidence
Because ongoing pain is challenging to measure in animal models, its mechanisms have been less well investigated than evoked pain. In animal models of peripheral nerve injury, the observed discharge rates of spontaneous activity in unmyelinated afferent fibers is low and varies substantially (0.01–5 Hz) [1,42,43,163], but such input may be responsible for pain behavior if the input is amplified in the CNS. Indeed, the level of spontaneous activity in C-fibers correlates with spontaneous pain behavior [43]. Multiple animal studies have also revealed spontaneous activity in injured myelinated nerve fibers [19,70,89,153,154]. It has been proposed that this activity can contribute to NP [29,40] because injured large fibers undergo a phenotypic switch [100,158] whereby they begin to synthesize substance P, which is normally expressed in nociceptive afferents. By this switch, input from large myelinated fibers may indeed gain access to spinal horn neurons involved in processing nociceptive input. Studies have suggested that activation of TRPV1-expressing DRG neurons may play an important role in spontaneous pain [69,106]. Resiniferatoxin, an ultra-potent and selective TRPV1 agonist, activates and desensitizes the TRPV1 receptor, resulting in a selective decrease in excitability of TRPV1-expressing neurons [69,106]. In a recent study, we showed that intraperitoneal injection of resiniferatoxin abolished heat hyperalgesia and reduced the spontaneous activity in spinal dorsal horn neurons of nerve-injured rats [139].
3.3. Does the sympathetic nervous system drive spontaneous activity?
Based on the clinical presentation of some patients (spontaneous pain and allodynia together with trophic changes in skin, bones, and nails; autonomic dysregulation with alterations in blood flow; and sweating), researchers proposed decades ago that the sympathetic nervous system may play a role in injury-induced chronic pain. Earlier, such clinical cases were commonly termed causalgia, reflex sympathetic dystrophy, or “sympathetically maintained pain.” Depending on the absence or presence of a nerve injury, these clinical syndromes are now classified as complex regional pain syndrome (CRPS) type I and type II, respectively.
3.3.1. Clinical evidence
Local anesthetic sympathetic blocks are widely used for the treatment of CRPS pain when involvement of the sympathetic nervous system is suspected. Clinical observations and case reports suggest that onset of pain relief occurs within minutes of block application, even in patients who have been experiencing pain for a prolonged period. In addition to the reduction in spontaneous pain, mechanical allodynia and hyperalgesia are also significantly reduced [38,94,145]. In agreement with findings from sympathetic block experiments, maneuvers that increase sympathetic activity (by, for example, whole body cooling or arousal) increase spontaneous pain [13,46] and areas of mechanical allodynia/hyperalgesia.[13] As the sympathetic paravertebral ganglia are situated outside the CNS and part of the PNS, these findings suggest that the interaction between the afferent and efferent systems must be in the periphery. Blocking the cervical or lumbar sympathetic chain with local anesthetics is a somewhat invasive procedure and is prone to false-positive and false-negative results from blockade of adjacent somatic afferent fibers or incorrect placement of the needle. As an alternative to blocking the sympathetic chain with local anesthetics, we and others have used intravenous infusion of the α-adrenergic antagonist phentolamine to block sympathetic effects [10,116]. Intravenous phentolamine and local anesthetic sympathetic block had equal effects in patients suspected of having sympathetically maintained pain, and the pain-relieving effects lasted longer than what would have been expected from the pharmacokinetics of phentolamine [116]. Like the anesthetic sympathetic block, intravenous phentolamine attenuated both spontaneous and stimulus-evoked pain [10,116]. The findings from these studies indicate that, in some NP patients, activity within the sympathetic nervous system drives somatic afferents, and that this coupling is within the PNS. Although it may occur anywhere in the PNS, experimental evidence suggests that coupling occurs in peripheral target tissues (e.g., skin), the site of nerve injury, and DRG (Figure 1).
Multiple lines of evidence suggest that an interaction between sympathetic efferent and somatic afferent fibers can occur in the skin. Application of norepinephrine into the skin of patients whose pain and allodynia were relieved after surgical sympathectomy or anesthetic sympathetic block, rekindled spontaneous pain and signs of mechanical hyperalgesia [3,141,155]. Topical, cutaneous application of clonidine (an α2-agonist) relieved signs of mechanical and cold hyperalgesia in the treated area, presumably by activating α2-receptors on peripheral sympathetic terminals and thereby reducing norepinephrine release. Furthermore, injection of norepinephrine or phenylephrine (an α1-agonist) into the treated site produced pain and rekindled signs of hyperalgesia [37]. Notably, α1-adrenoreceptors are upregulated in the epidermis and on dermal nerve fibers in some patients with CRPS-II [45,47]. Microneurographic recordings in one patient with sympathetically maintained pain showed that mechano-insensitive C-fibers were activated by arousal stimuli and injection of norepinephrine [67]. However, a larger study that used microneurography did not find such evidence in patients with CRPS-I or CRPS-II [28]. Sympathetic-sensory coupling at the injury site has been tested in patients with postamputation stump pain. Perineuronal injection of epinephrine close to a peripheral nerve neuroma produced intense pain [33]. Similarly, injection of norepinephrine close to the neuroma induced a dose-dependent increase in pain that was partially blocked by pretreatment with phentolamine [88].
3.3.2. Preclinical evidence
Evidence for sympathetic-sensory coupling at the skin is supported by the observation that stimulation of the sympathetic chain induces activity in cutaneous nociceptive afferents and adrenergic sensitivity after different types of nerve injury [124]. After chronic constriction injury of the saphenous nerve, polymodal C-fibers developed adrenergic sensitivity [74], and after a lumbar spinal nerve ligation in nonhuman primates, uninjured cutaneous C-fibers developed spontaneous activity and were activated by α1- and α2-agonists [4]. Partial ligation of the sciatic nerve led to upregulation of α1-adrenoreceptors in dermal nerve fibers [45], and sympathetic efferent fibers were shown to sprout into the upper dermis [98,170], closely associated with sprouting peptidergic nerve fibers after peripheral nerve injury [170]. The possibility that adrenergic sensitivity develops at the injury site is supported by observations that injured afferent fibers with spontaneous activity are activated by adrenergic agents administered into the blood stream supplying the injured nerve [76], systemic injection of adrenergic agents [34,41], stimulation of the sympathetic chain [41,58,66], or application of norepinephrine to the injury site [41,58,66,159,167,168].
Basket-like structures around large and medium sized DRG neurons formed by sprouting sympathetic nerve fibers after peripheral nerve injury [82,92,117] may represent an anatomical substrate for sympathetic-sensory coupling in the DRG. Sprouting sympathetic fibers have also been observed in human DRGs that were surgically removed from patients for the treatment of pain [130]. In animal models, inhibition of sympathetic sprouting partially inhibited NP behavior in animals [85,117]. Stimulation of sympathetic efferents and systemic administration or topical application of adrenergic agents activates DRG neurons in various models of NP [24,34,167]. In vitro, norepinephrine depolarized acutely dissociated, small to medium sized DRG neurons harvested from animals with peripheral nerve injury [113]. Activation of sympathetic sprouts led to enhanced activity in large and medium sized DRG neurons, an effect that was abolished by blocking sympathetic transmission [166].
Combined together, substantial clinical and basic science evidence supports the concept that sympathetic-somatosensory coupling after nerve injury may account for pain and hyperalgesia in some patients with NP. Most of the clinical evidence, however, remains anecdotal, and placebo-controlled trials are needed to evaluate the long-term effects of local anesthetic sympathetic blocks [105]. Preclinical studies are lacking advanced behavioral test paradigms (e.g., CPP) to investigate the role of the sympathetic nervous system in animal models of NP. These gaps may be challenging to fill, but the clinical need to clarify the role of the sympathetic nervous system in pain should justify such research efforts.
3.4. Advantages of targeting peripheral afferent neurons for neuropathic pain treatment
As discussed earlier, both clinical and experimental studies provide mounting evidence that peripheral neuronal mechanisms contribute to central sensitization in individuals with NP. Targeting peripheral mechanisms has several potential advantages as compared to CNS-penetrating drugs for the relief of pain. First, drugs that act on peripheral afferent fibers and soma of DRG neurons will inhibit “pain at its source” and act at sites before the pain signals diverge over multiple CNS pathways [111,134,157]. Second, interrupting pain signals in the periphery will not only disrupt the initiation of pain, but may also inhibit afferent inputs to the CNS, potentially reversing central sensitization [26,52]. Third, a subpopulation of DRG neurons may be involved in NP. Targeting only those would preserve protective pain sensation and other sensations such as touch and proprioception. Finally and importantly, peripherally restricted analgesics are likely to have minimal off-target effects in the CNS and hence fewer side effects, fewer drug interactions, and a better safety profile [125,133]. DRGs are not protected by a blood-nerve barrier because the endothelium of their supply vessels lacks tight junctions. Therefore, peripherally acting drugs can access their targets on DRG neurons and inhibit peripheral pain generators.
3.5. Potential peripheral drug targets involved in the modulation of NP
G-protein-coupled receptors (GPCRs) located at the cell membrane have been used frequently as drug targets for a variety of pharmacotherapies, including those for NP [109]. GPCRs such as opioid, cannabinoid, α-adrenergic, adenosine receptor subtype 1, and some Mrgprs (C, D) expressed on afferent fibers and DRG neurons participate in modulation of pain transmission [44,55,84] and have been suggested as attractive targets for the development of new NP treatments. Many of these receptors modulate pain through actions at the central terminals of DRG neurons in association with functions such as inhibition of neurotransmitter release. Because the central terminals of DRG neurons are located within the blood-brain barrier and cannot be accessed by peripherally restricted drugs, their roles in NP inhibition will not be reviewed here. Ligand-gated channels (e.g., TRPV1, TRPA1, P2Xs), voltage-gated ion channels (e.g., sodium, calcium, and potassium channels), and HCN ion channels have also been shown to play important roles in multiple animal NP models. In particular, Nav1.7 is important to inherited human pain phenotypes, and its expression is increased in the DRG of patients with NP conditions such as chemotherapy-induced peripheral neuropathy [86]. Some of these TRPV, Nav, HCN2, and MrgprC (human MrgprX1) targets, by virtue of their restricted distribution in nociceptive neurons, represent compelling pain-specific targets for pharmacologic NP therapy [48,157].
3.6. Peripheral opioid and cannabinoid receptors as targets for inhibition of NP
The lack of effective therapies for NP and the increasing morbidity and death associated with systemic opioids highlights the need for new treatment strategies. Important families of GPCRs, such as mu-opioid receptors (MORs), delta-opioid receptors (DORs), kappa-opioid receptors (KORs), and cannabinoid type 1 receptors (CB1Rs), are expressed on DRG neurons and may modulate the function of nociceptive neurons. Over the last decade, we and others have examined whether peripheral opioid and cannabinoid receptors may be suitable targets for inhibition of NP [2,31,72,78,90,120,127]. Here, we provide a brief summary of our studies that support peripheral MORs as a potential target for the relief of NP.
3.6.1. Inhibition of evoked hypersensitivity and ongoing NP behavior by peripherally restricted opioids
Our studies and those of others suggest that mu-opioid agonists act at peripheral sites to decrease persistent pain. Such results provide proof of concept for the development of peripherally acting drugs that will reduce primary afferent activity without CNS adverse effects, such as dependence and addiction [90]. To examine whether inhibition of the peripheral MORs effectively alleviates NP-related behavior in rats, we used peripherally acting MOR-preferring agonists such as loperamide hydrochloride and dermorphin [d-Arg2, Lys4] (1–4) amide (DALDA). DALDA, derived from the natural heptapeptide MOR agonist dermorphin, is highly hydrophilic and exhibits restrictive penetration into the CNS after systemic administration. Both drugs dose-dependently reversed mechanical and heat hypersensitivities in nerve-injured rats when administered systemically [56,140]. These effects were blocked by systemic pretreatment with a peripherally acting MOR antagonist, methyl-naltrexone. DALDA-treated rats did not exhibit motor deficits or locomotor impairment, suggesting that it does not induce central side effects.
Ongoing and paroxysmal spontaneous pain are common in NP patients, but the underlying mechanisms and treatment strategies may differ from those for evoked sensory hypersensitivity [11,69,150]. Behavioral assays in animals have primarily relied on reflex responses that do not always correlate with analgesic efficacy in humans [69,99,107,138,148]. Therefore, the conditioned place preference (CPP) test was developed to examine inhibitory effects of drugs on ongoing pain. The CPP is based on the assumption that pain relief is rewarding and would motivate animals in pain to prefer a context associated with reduced pain. A recent study in which we used complementary approaches, including CPP, wheel running experiments, spontaneous neuronal activity in dorsal horn neurons, and high-throughput calcium imaging of DRG neurons, showed that peripherally acting mu-opioids DALDA and loperamide attenuate ongoing NP and spontaneous neuronal activity in nerve-injured rats [139]. Using the CPP assay, we showed that neuropathic, but not naive animals, exhibit a preference for the environment associated with drug treatment. Methylnaltrexone blocked DALDA–induced place preference in neuropathic rats. DALDA also improved wheel running performance in nerve-injured mice. Voluntary wheel running may be indicative of wellbeing, and changes in this activity may constitute an objective way to measure the overall impact of aversive state in injured rodents.
3.6.2. Mechanisms of action
Electrophysiologic and molecular biologic studies help us to elucidate the site of action and cellular mechanisms by which peripherally acting MOR agonists inhibit NP. In cultured DRG neurons, DALDA inhibited the capsaicin-induced increase in [Ca2+] more than the β-alanine–induced increase in [Ca2+]; capsaicin and β-alanine activate subpopulations of neurons involved in the signaling of heat and mechanical pain, respectively [140]. Using high-throughput in vivo calcium imaging, we showed that topical ganglionic application of DALDA reduced the numbers of small-diameter DRG neurons activated by test stimulation after nerve injury. In vivo electrophysiologic recording of spinal wide-dynamic range (WDR) neurons showed that systemic DALDA decreased spontaneous firing rates and inhibited the C-fiber–mediated, but not A-fiber–mediated, response of WDR neurons in neuropathic rats. This neuronal inhibitory effect of DALDA was also blocked by methylnaltrexone pretreatment [139].
3.6.3. Perspectives of targeting peripheral MORs for inhibition of NP
As described earlier, peripherally acting mu-opioids attenuate both evoked pain and ongoing pain-related behavior and its neurophysiologic correlates in animal models of NP. MOR expression in uninjured peripheral nerves and DRG may increase after nerve injury and serve as a target to increase the effectiveness of peripherally acting MOR agonists [83]. Thus, peripheral MORs may be suitable targets or a potential alternative to current opioid therapy for the treatment of NP. Additionally, they are likely to be associated with minimal adverse effects.
The potential limitations and associated adverse effects of peripheral opioid analgesia, including constipation, itch, and tolerance, need to be considered. Like CNS-penetrating opioids, peripherally acting opioids may also induce tolerance development after repeated administration. The inhibition of mechanical hypersensitivity by both loperamide and DALDA decreased after repeated systemic and intraplantar administration [62,139]. How the prolonged use of peripherally acting opioids affects motor function and NP-related comorbidities (e.g., anxiety and depression) also needs to be determined. Future studies are needed to address limitations that may affect the clinical application of peripherally restricted opioids for NP management. A recent meta-analysis of preclinical studies indicated that the systemic administration of cannabinoids had opioid-sparing effects [101]. Whether peripheral opioids and cannabinoid agonists exhibit similar synergistic interactions is being investigated.
It is encouraging that the pharmaceutical industry is examining the clinical utility of a new class of opioid molecules that have low blood-brain barrier permeability and hence a decreased propensity for euphoria, abuse, and addiction (Nektar, NKTR-181). In Phase 3 clinical trials it showed efficacy for chronic low back pain, but the drug has not been approved by the U.S. Food and Drug Administration. A novel peripherally acting MOR agonist, NFEPP, was recently designed to exploit the pathologic conformation dynamics of MOR-ligand interactions. The initial report established the feasibility of preferentially targeting the pathologic conformation of MORs in the PNS by using a pH-sensitive binding agonist that produced “injury-restricted inhibition” of inflammatory and postoperative pain in rodents [132]. It did not cause respiratory depression, sedation, or constipation and did not have addiction potential. In a subsequent report, the intravenous administration of NFEPP also reduced mechanical and thermal hyperalgesia in a chronic constriction injury model of NP. The anti-hyperalgesic effects of this opioid agonist were fully reversed by the peripherally restricted opioid receptor antagonist naloxone methiodide when it was injected at the nerve injury site, indicating a peripheral site of action [120]. In the chronic constriction injury model, doses of opioid and TRPV1 antagonist SB366791 that were ineffective when administered separately diminished heat and mechanical sensitivity when co-injected at the nerve injury site or into the injured paw [78].
Recent findings about peripherally acting CB1 and KOR agonists as potential pain modulators support the notion that primary afferent hyperexcitability in NP can be modulated in the periphery. Several studies indicate a modulatory effect of the endocannabinoid system in NP (for review see Maldonado et al. [91]). In the periphery, CB1 receptors are present in nociceptive terminals and the DRG, whereas CB2 receptors are located in immune cells and keratinocytes. Selective deletion of CB1 in peripheral neurons intensified NP behavior and decreased the analgesic efficacy of systemic cannabinoid agonists [2]. A recent study also suggested similar promise with targeting peripheral KORs, which are expressed in subpopulations of primary sensory neurons, including peptidergic neurons. Importantly, peripherally restricted KOR agonists attenuated both pain and itch behaviors and inhibited neurogenic inflammation and nociceptor sensitization [131].
4. Opportunities and Challenges in Developing New Therapies for the Management of NP
Nerve injuries and neuropathies lead to complex neurophysiologic and neurochemical changes in the peripheral nerve and DRG. Several lines of evidence indicate that chronic NP is maintained by spontaneous or ectopic activity in primary afferent nerve fibers and/or DRG cells. Although we are optimistic that a better understanding of the peripheral mechanisms in various neuropathic pain states make possible the development of safer peripherally acting analgesics, potential challenges remain. Novel drugs that target peripheral sites must have minimal access to the CNS even when administered systemically and should not interfere with normal protective sensations. In addition, the safety of blocking the peripheral target would need to be carefully evaluated to avoid unexpected adverse effects, such as the hyperpyrexia seen with TRPV1 antagonists and the rapidly progressive osteoarthritis and osteonecrosis reported with anti-nerve growth factor therapy. Alternatively, minimally invasive techniques for administering the drugs in the vicinity of the “pain generator” would need to be developed. Although there is substantial clinical and basic science evidence for sympathetic-somatosensory coupling after nerve injury that may account for pain and hyperalgesia, we still lack high-quality clinical evidence for the potential long-term beneficial effects of sympathetic blockade. The holy grail in providing safe, personalized medicine will be to establish paradigms to detect the likely peripheral pain generator site in a given patient and develop therapies that effectively suppress that activity without affecting normal sensations.
Acknowledgements:
We are grateful to Claire F. Levine, MS, ELS (scientific editor, Department of Anesthesiology/CCM, Johns Hopkins University), for her assistance in editing the manuscript and Jennifer E. Fairman, MA, MPS, CMI, FAMI (Associate Professor, Assistant Director of Production, Art As Applied to Medicine, Johns Hopkins University), for the illustrations.
Disclosure of funding: This commentary was supported mainly by grants from the National Institutes of Health (Bethesda, Maryland, USA): NS26363 (S.N.R.), AR070875 (M.R.), NS110598 (Y.G.), and NS70814 (Y.G.).. The authors have no other conflicts of interest related to the manuscript.
REFERENCES
- [1].Acosta C, Djouhri L, Watkins R, Berry C, Bromage K, Lawson SN. TREK2 expressed selectively in IB4-binding C-fiber nociceptors hyperpolarizes their membrane potentials and limits spontaneous pain. J Neurosci 2014;34:1494–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 2007;10:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ali Z, Raja SN, Wesselmann U, Fuchs PN, Meyer RA, Campbell JN. Intradermal injection of norepinephrine evokes pain in patients with sympathetically maintained pain. Pain 2000;88:161–8. [DOI] [PubMed] [Google Scholar]
- [4].Ali Z, Ringkamp M, Hartke TV, Chien HF, Flavahan NA, Campbell JN, Meyer RA. Uninjured C-fiber nociceptors develop spontaneous activity and alpha-adrenergic sensitivity following L6 spinal nerve ligation in monkey. J Neurophysiol 1999;81:455–66. [DOI] [PubMed] [Google Scholar]
- [5].Amir R, Devor M. Axonal cross-excitation in nerve-end neuromas: comparison of A- and C-fibers. J Neurophysiol 1992;68:1160–6. [DOI] [PubMed] [Google Scholar]
- [6].Amir R, Devor M. Ongoing activity in neuroma afferents bearing retrograde sprouts. Brain Res 1993;630:283–8. [DOI] [PubMed] [Google Scholar]
- [7].Andersen OK, Gracely RH, Arendt-Nielsen L. Facilitation of the human nociceptive reflex by stimulation of A beta-fibres in a secondary hyperalgesic area sustained by nociceptive input from the primary hyperalgesic area. Acta Physiol Scand 1995;155:87–97. [DOI] [PubMed] [Google Scholar]
- [8].Andersson DA, Gentry C, Light E, Vastani N, Vallortigara J, Bierhaus A, Fleming T, Bevan S. Methylglyoxal evokes pain by stimulating TRPA1. PLoS One 2013;8:e77986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Apkarian AV, Bushnell MC, Schweinhardt P. Representation of pain in the brain In: McMahon SB, Koltzenburg M, Tracey I, Turk D, editors. Wall and Melzack’s Textbook of Pain. Philadelphia, PA: Elsevier Saunders, 2013. pp. 111–128. [Google Scholar]
- [10].Arner S Intravenous phentolamine test: diagnostic and prognostic use in reflex sympathetic dystrophy. Pain 1991;46:17–22. [DOI] [PubMed] [Google Scholar]
- [11].Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–19. [DOI] [PubMed] [Google Scholar]
- [12].Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of 5% lidocaine (lignocaine) medicated plaster in comparison with pregabalin in patients with postherpetic neuralgia and diabetic polyneuropathy: interim analysis from an open-label, two-stage adaptive, randomized, controlled trial. Clin Drug Investig 2009;29:231–41. [DOI] [PubMed] [Google Scholar]
- [13].Baron R, Schattschneider J, Binder A, Siebrecht D, Wasner G. Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: a case-control study. Lancet 2002;359:1655–60. [DOI] [PubMed] [Google Scholar]
- [14].Baumann TK, Burchiel KJ. A method for intraoperative microneurographic recording of unitary activity in the trigeminal ganglion of patients with trigeminal neuralgia. J Neurosci Methods 2004;132:19–24. [DOI] [PubMed] [Google Scholar]
- [15].Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Grill RJ, Carlton SM, Walters ET. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci 2010;30:14870–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Beggs S, Salter MW. Microglia: critical mediators of pain hypersensitivity after peripheral nerve injury In: McMahon SB, Koltzenburg M, Tracey I, Turk D, editors. Wall and Melzack’s Textbook of Pain. Philadelphia, PA: Elsevier Saunders, 2013. pp. 68–76. [Google Scholar]
- [17].Birbaumer N, Lutzenberger W, Montoya P, Larbig W, Unertl K, Topfner S, Grodd W, Taub E, Flor H. Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci 1997;17:5503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Blair HA. Capsaicin 8% dermal patch: a review in peripheral neuropathic pain. Drugs 2018;78:1489–500. [DOI] [PubMed] [Google Scholar]
- [19].Blumberg H, Janig W. Discharge pattern of afferent fibers from a neuroma. Pain 1984;20:335–53. [DOI] [PubMed] [Google Scholar]
- [20].Bostock H, Campero M, Serra J, Ochoa JL. Temperature-dependent double spikes in C-nociceptors of neuropathic pain patients. Brain 2005;128:2154–63. [DOI] [PubMed] [Google Scholar]
- [21].Bouhassira D, Attal N. The multiple challenges of neuropathic pain. Neurosci Lett 2019;702:6–10. [DOI] [PubMed] [Google Scholar]
- [22].Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136:380–7. [DOI] [PubMed] [Google Scholar]
- [23].Buch NS, Ahlburg P, Haroutounian S, Andersen NT, Finnerup NB, Nikolajsen L. The role of afferent input in postamputation pain: a randomized, double-blind, placebo-controlled crossover study. Pain 2019;160:1622–33. [DOI] [PubMed] [Google Scholar]
- [24].Burchiel KJ. Spontaneous impulse generation in normal and denervated dorsal root ganglia: sensitivity to alpha-adrenergic stimulation and hypoxia. Exp Neurol 1984;85:257–72. [DOI] [PubMed] [Google Scholar]
- [25].Campbell JN, Khan AA, Meyer RA, Raja SN. Responses to heat of C-fiber nociceptors in monkey are altered by injury in the receptive field but not by adjacent injury. Pain 1988;32:327–32. [DOI] [PubMed] [Google Scholar]
- [26].Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006;52:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain 1988;32:89–94. [DOI] [PubMed] [Google Scholar]
- [28].Campero M, Bostock H, Baumann TK, Ochoa JL. A search for activation of C nociceptors by sympathetic fibers in complex regional pain syndrome. Clin Neurophysiol 2010;121:1072–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Campero M, Serra J, Marchettini P, Ochoa JL. Ectopic impulse generation and autoexcitation in single myelinated afferent fibers in patients with peripheral neuropathy and positive sensory symptoms. Muscle Nerve 1998;21:1661–7. [DOI] [PubMed] [Google Scholar]
- [30].Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 2009;147:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ceredig RA, Pierre F, Doridot S, Alduntzin U, Salvat E, Yalcin I, Gaveriaux-Ruff C, Barrot M, Massotte D. Peripheral delta opioid receptors mediate duloxetine antiallodynic effect in a mouse model of neuropathic pain. Eur J Neurosci 2018;48:2231–46. [DOI] [PubMed] [Google Scholar]
- [32].Chabal C, Jacobson L, Russell LC, Burchiel KJ. Pain responses to perineuromal injection of normal saline, gallamine, and lidocaine in humans. Pain 1989;36:321–5. [DOI] [PubMed] [Google Scholar]
- [33].Chabal C, Jacobson L, Russell LC, Burchiel KJ. Pain response to perineuromal injection of normal saline, epinephrine, and lidocaine in humans. Pain 1992;49:9–12. [DOI] [PubMed] [Google Scholar]
- [34].Chen Y, Michaelis M, Janig W, Devor M. Adrenoreceptor subtype mediating sympathetic-sensory coupling in injured sensory neurons. J Neurophysiol 1996;76:3721–30. [DOI] [PubMed] [Google Scholar]
- [35].Chung JM, Chung K. Importance of hyperexcitability of DRG neurons in neuropathic pain. Pain Pract 2002;2:87–97. [DOI] [PubMed] [Google Scholar]
- [36].Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN. Neuropathic pain. Nat Rev Dis Primers 2017;3:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Davis KD, Treede RD, Raja SN, Meyer RA, Campbell JN. Topical application of clonidine relieves hyperalgesia in patients with sympathetically maintained pain. Pain 1991;47:309–17. [DOI] [PubMed] [Google Scholar]
- [38].Dellemijn PL, Fields HL, Allen RR, McKay WR, Rowbotham MC. The interpretation of pain relief and sensory changes following sympathetic blockade. Brain 1994;117 (Pt 6):1475–87. [DOI] [PubMed] [Google Scholar]
- [39].Derry S, Wiffen PJ, Moore RA, Quinlan J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst Rev 2014;CD010958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Devor M Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 2009;196:115–28. [DOI] [PubMed] [Google Scholar]
- [41].Devor M, Janig W. Activation of myelinated afferents ending in a neuroma by stimulation of the sympathetic supply in the rat. Neurosci Lett 1981;24:43–7. [DOI] [PubMed] [Google Scholar]
- [42].Djouhri L, Fang X, Koutsikou S, Lawson SN. Partial nerve injury induces electrophysiological changes in conducting (uninjured) nociceptive and nonnociceptive DRG neurons: Possible relationships to aspects of peripheral neuropathic pain and paresthesias. Pain 2012;153:1824–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 2006;26:1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 2001;106:619–32. [DOI] [PubMed] [Google Scholar]
- [45].Drummond PD, Drummond ES, Dawson LF, Mitchell V, Finch PM, Vaughan CW, Phillips JK. Upregulation of alpha1-adrenoceptors on cutaneous nerve fibres after partial sciatic nerve ligation and in complex regional pain syndrome type II. Pain 2014;155:606–16. [DOI] [PubMed] [Google Scholar]
- [46].Drummond PD, Finch PM, Skipworth S, Blockey P. Pain increases during sympathetic arousal in patients with complex regional pain syndrome. Neurology 2001;57:1296–303. [DOI] [PubMed] [Google Scholar]
- [47].Drummond PD, Morellini N, Finch PM, Birklein F, Knudsen LF. Complex regional pain syndrome: intradermal injection of phenylephrine evokes pain and hyperalgesia in a subgroup of patients with upregulated alpha1-adrenoceptors on dermal nerves. Pain 2018;159:2296–305. [DOI] [PubMed] [Google Scholar]
- [48].Emery EC, Luiz AP, Wood JN. Nav1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opin Ther Targets 2016;20:975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 2011;333:1462–6. [DOI] [PubMed] [Google Scholar]
- [50].Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017;93:1296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc 2015;90:532–45. [DOI] [PubMed] [Google Scholar]
- [53].Govrin-Lippmann R, Devor M. Ongoing activity in severed nerves: source and variation with time. Brain Res 1978;159:406–10. [DOI] [PubMed] [Google Scholar]
- [54].Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain 1992;51:175–94. [DOI] [PubMed] [Google Scholar]
- [55].Grazzini E, Puma C, Roy MO, Yu XH, O’Donnell D, Schmidt R, Dautrey S, Ducharme J, Perkins M, Panetta R, Laird JM, Ahmad S, Lembo PM. Sensory neuron-specific receptor activation elicits central and peripheral nociceptive effects in rats. Proc Natl Acad Sci U S A 2004;101:7175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, Meyer RA, Raja SN. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain 2008;138:318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 2016;19:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferents in chronically lesioned nerves by adrenaline and excitation of sympathetic efferents in the cat. Neurosci Lett 1987;82:35–40. [DOI] [PubMed] [Google Scholar]
- [59].Hains BC, Waxman SG. Sodium channel expression and the molecular pathophysiology of pain after SCI. Prog Brain Res 2007;161:195–203. [DOI] [PubMed] [Google Scholar]
- [60].Haroutounian S, Ford AL, Frey K, Nikolajsen L, Finnerup NB, Neiner A, Kharasch ED, Karlsson P, Bottros MM. How central is central poststroke pain? The role of afferent input in poststroke neuropathic pain: a prospective, open-label pilot study. Pain 2018;159:1317–24. [DOI] [PubMed] [Google Scholar]
- [61].Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrom JB, Jensen TS. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain 2014;155:1272–9. [DOI] [PubMed] [Google Scholar]
- [62].He SQ, Yang F, Perez FM, Xu Q, Shechter R, Cheong YK, Carteret AF, Dong X, Sweitzer SM, Raja SN, Guan Y. Tolerance develops to the antiallodynic effects of the peripherally acting opioid loperamide hydrochloride in nerve-injured rats. Pain 2013;154:2477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev 2009;60:202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hwang TJ, Sinha MS, Dave CV, Kesselheim AS. Prescription opioid epidemic and trends in the clinical development of new pain medications. Mayo Clin Proc 2019;94:2437–43. [DOI] [PubMed] [Google Scholar]
- [65].Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 2018;19:138–52. [DOI] [PubMed] [Google Scholar]
- [66].Janig W Activation of afferent fibers ending in an old neuroma by sympathetic stimulation in the rat. Neurosci Lett 1990;111:309–14. [DOI] [PubMed] [Google Scholar]
- [67].Jorum E, Orstavik K, Schmidt R, Namer B, Carr RW, Kvarstein G, Hilliges M, Handwerker H, Torebjork E, Schmelz M. Catecholamine-induced excitation of nociceptors in sympathetically maintained pain. Pain 2007;127:296–301. [DOI] [PubMed] [Google Scholar]
- [68].Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol 1992;68:734–44. [DOI] [PubMed] [Google Scholar]
- [69].King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain 2011;152:1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kirillova I, Teliban A, Gorodetskaya N, Grossmann L, Bartsch F, Rausch VH, Struck M, Tode J, Baron R, Janig W. Effect of local and intravenous lidocaine on ongoing activity in injured afferent nerve fibers. Pain 2011;152:1562–71. [DOI] [PubMed] [Google Scholar]
- [71].Kleggetveit IP, Namer B, Schmidt R, Helas T, Ruckel M, Orstavik K, Schmelz M, Jorum E. High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain 2012;153:2040–7. [DOI] [PubMed] [Google Scholar]
- [72].Klein AH, Mohammad HK, Ali R, Peper B, Wilson SP, Raja SN, Ringkamp M, Sweitzer S. Overexpression of micro-opioid receptors in peripheral afferents, but not in combination with enkephalin, decreases neuropathic pain behavior and enhances opioid analgesia in mouse. Anesthesiology 2018;128:967–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kocher L, Anton F, Reeh PW, Handwerker HO. The effect of carrageenan-induced inflammation on the sensitivity of unmyelinated skin nociceptors in the rat. Pain 1987;29:363–73. [DOI] [PubMed] [Google Scholar]
- [74].Koltzenburg M, Kees S, Budweiser S, Ochs G, Toyka KV. The properties of unmyelinated nociceptive afferents change in a painful chronic constriction neuropathy In: Gebhart GF, Hammond DL, Jensen TS, editors. Progress in pain Research and Management. Seattle: IASP Press, 1994. pp. 511–522. [Google Scholar]
- [75].Koltzenburg M, Torebjork HE, Wahren LK. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain 1994;117 (Pt 3):579–91. [DOI] [PubMed] [Google Scholar]
- [76].Korenman EM, Devor M. Ectopic adrenergic sensitivity in damaged peripheral nerve axons in the rat. Exp Neurol 1981;72:63–81. [DOI] [PubMed] [Google Scholar]
- [77].Kuner R Central mechanisms of pathological pain. Nat Med 2010;16:1258–66. [DOI] [PubMed] [Google Scholar]
- [78].Labuz D, Spahn V, Celik MO, Machelska H. Opioids and TRPV1 in the peripheral control of neuropathic pain--Defining a target site in the injured nerve. Neuropharmacology 2016;101:330–40. [DOI] [PubMed] [Google Scholar]
- [79].LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol 1992;448:749–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol 1991;66:190–211. [DOI] [PubMed] [Google Scholar]
- [81].LaMotte RH, Thalhammer JG, Torebjork HE, Robinson CJ. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci 1982;2:765–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lee BH, Yoon YW, Chung K, Chung JM. Comparison of sympathetic sprouting in sensory ganglia in three animal models of neuropathic pain. Exp Brain Res 1998;120:432–8. [DOI] [PubMed] [Google Scholar]
- [83].Lee CY, Perez FM, Wang W, Guan X, Zhao X, Fisher JL, Guan Y, Sweitzer SM, Raja SN, Tao YX. Dynamic temporal and spatial regulation of mu opioid receptor expression in primary afferent neurons following spinal nerve injury. Eur J Pain 2011;15:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lembo PM, Grazzini E, Groblewski T, O’Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, et al. Proenkephalin A gene products activate a new family of sensory neuron--specific GPCRs. Nat Neurosci 2002;5:201–9. [DOI] [PubMed] [Google Scholar]
- [85].Li JY, Xie W, Strong JA, Guo QL, Zhang JM. Mechanical hypersensitivity, sympathetic sprouting, and glial activation are attenuated by local injection of corticosteroid near the lumbar ganglion in a rat model of neuropathic pain. Reg Anesth Pain Med 2011;36:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Li Y, North RY, Rhines LD, Tatsui CE, Rao G, Edwards DD, Cassidy RM, Harrison DS, Johansson CA, Zhang H, Dougherty PM. DRG voltage-gated sodium channel 1.7 is upregulated in paclitaxel-induced neuropathy in rats and in humans with neuropathic pain. J Neurosci 2018;38:1124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Li Y, Tatsui CE, Rhines LD, North RY, Harrison DS, Cassidy RM, Johansson CA, Kosturakis AK, Edwards DD, Zhang H, Dougherty PM. Dorsal root ganglion neurons become hyperexcitable and increase expression of voltage-gated T-type calcium channels (Cav3.2) in paclitaxel-induced peripheral neuropathy. Pain 2017;158:417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lin EE, Horasek S, Agarwal S, Wu CL, Raja SN. Local administration of norepinephrine in the stump evokes dose-dependent pain in amputees. Clin J Pain 2006;22:482–6. [DOI] [PubMed] [Google Scholar]
- [89].Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain 2000;85:503–21. [DOI] [PubMed] [Google Scholar]
- [90].Liu S, Huang Q, He S, Chen Z, Gao X, Ma D, Duan W, Ford N, Yang F, Chen X, Raja SN, Hao D, Guan Y. Dermorphin [D-Arg2, Lys4] (1–4) amide inhibits below-level heat hypersensitivity in mice after contusive thoracic spinal cord injury. Pain 2019;160:2710–23. [DOI] [PubMed] [Google Scholar]
- [91].Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain 2016;157 Suppl 1:S23–S32. [DOI] [PubMed] [Google Scholar]
- [92].McLachlan EM, Janig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature 1993;363:543–6. [DOI] [PubMed] [Google Scholar]
- [93].Meacham K, Shepherd A, Mohapatra DP, Haroutounian S. Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Headache Rep 2017;21:28. [DOI] [PubMed] [Google Scholar]
- [94].Meier PM, Zurakowski D, Berde CB, Sethna NF. Lumbar sympathetic blockade in children with complex regional pain syndromes: a double blind placebo-controlled crossover trial. Anesthesiology 2009;111:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Meier T, Wasner G, Faust M, Kuntzer T, Ochsner F, Hueppe M, Bogousslavsky J, Baron R. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain 2003;106:151–8. [DOI] [PubMed] [Google Scholar]
- [96].Meyer RA, Campbell JN. Myelinated nociceptive afferents account for the hyperalgesia that follows a burn to the hand. Science 1981;213:1527–9. [DOI] [PubMed] [Google Scholar]
- [97].Miclescu A, Schmelz M, Gordh T. Differential analgesic effects of subanesthetic concentrations of lidocaine on spontaneous and evoked pain in human painful neuroma: A randomized, double blind study. Scand J Pain 2015;8:37–44. [DOI] [PubMed] [Google Scholar]
- [98].Nascimento FP, Magnussen C, Yousefpour N, Ribeiro-da-Silva A. Sympathetic fibre sprouting in the skin contributes to pain-related behaviour in spared nerve injury and cuff models of neuropathic pain. Mol Pain 2015;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A 2012;109:20709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 1996;384:360–4. [DOI] [PubMed] [Google Scholar]
- [101].Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, Murnion B, Lintzeris N, Khor KE, Farrell M, Smith A, Le FB. Opioid-sparing effect of cannabinoids: a systematic review and meta-analysis. Neuropsychopharmacology 2017;42:1752–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].North RY, Lazaro TT, Dougherty PM. Ectopic spontaneous afferant activity and neuropathic pain. Neurosurgery 2018;65:49–54. [DOI] [PubMed] [Google Scholar]
- [103].North RY, Li Y, Ray P, Rhines LD, Tatsui CE, Rao G, Johansson CA, Zhang H, Kim YH, Zhang B, Dussor G, Kim TH, Price TJ, Dougherty PM. Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain 2019;142:1215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nystrom B, Hagbarth KE. Microelectrode recordings from transected nerves in amputees with phantom limb pain. Neurosci Lett 1981;27:211–6. [DOI] [PubMed] [Google Scholar]
- [105].O’Connell NE, Wand BM, Gibson W, Carr DB, Birklein F, Stanton TR. Local anaesthetic sympathetic blockade for complex regional pain syndrome. Cochrane Database Syst Rev 2016;7:CD004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain 2011;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, Ossipov MH, Xie J, Dussor GO, King T, Porreca F. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain 2012;153:924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Orstavik K, Weidner C, Schmidt R, Schmelz M, Hilliges M, Jorum E, Handwerker H, Torebjork E. Pathological C-fibres in patients with a chronic painful condition. Brain 2003;126:567–78. [DOI] [PubMed] [Google Scholar]
- [109].Pan HL, Wu ZZ, Zhou HY, Chen SR, Zhang HM, Li DP. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol Ther 2008;117:141–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst 2001;6:21–7. [DOI] [PubMed] [Google Scholar]
- [111].Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov 2009;8:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Perl ER, Kumazawa T, Lynn B, Kenins P. Sensitization of high threshold receptors with unmyelinated (C) afferent fibers. Prog Brain Res 1976;43:263–77. [DOI] [PubMed] [Google Scholar]
- [113].Petersen M, Zhang J, Zhang JM, LaMotte RH. Abnormal spontaneous activity and responses to norepinephrine in dissociated dorsal root ganglion cells after chronic nerve constriction. Pain 1996;67:391–7. [DOI] [PubMed] [Google Scholar]
- [114].Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain 2004;127:1606–15. [DOI] [PubMed] [Google Scholar]
- [115].Raja SN, Campbell JN, Meyer RA. Evidence for different mechanisms of primary and secondary hyperalgesia following heat injury to the glabrous skin. Brain 1984;107 (Pt 4):1179–88. [DOI] [PubMed] [Google Scholar]
- [116].Raja SN, Treede RD, Davis KD, Campbell JN. Systemic alpha-adrenergic blockade with phentolamine: a diagnostic test for sympathetically maintained pain. Anesthesiology 1991;74:691–8. [DOI] [PubMed] [Google Scholar]
- [117].Ramer MS, Bisby MA. Adrenergic innervation of rat sensory ganglia following proximal or distal painful sciatic neuropathy: distinct mechanisms revealed by anti-NGF treatment. Eur J Neurosci 1999;11:837–46. [DOI] [PubMed] [Google Scholar]
- [118].Richards N, McMahon SB. Targeting novel peripheral mediators for the treatment of chronic pain. Br J Anaesth 2013;111:46–51. [DOI] [PubMed] [Google Scholar]
- [119].Ringkamp M, Raja SN. A sore spot: central or peripheral generation of chronic neuropathic spontaneous pain? Pain 2014;155:1189–91. [DOI] [PubMed] [Google Scholar]
- [120].Rodriguez-Gaztelumendi A, Spahn V, Labuz D, Machelska H, Stein C. Analgesic effects of a novel pH-dependent mu-opioid receptor agonist in models of neuropathic and abdominal pain. Pain 2018;159:2277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rowbotham MC, Davies PS, Fields HL. Topical lidocaine gel relieves postherpetic neuralgia. Ann Neurol 1995;37:246–53. [DOI] [PubMed] [Google Scholar]
- [122].Rowbotham MC, Davies PS, Verkempinck C, Galer BS. Lidocaine patch: double-blind controlled study of a new treatment method for post-herpetic neuralgia. Pain 1996;65:39–44. [DOI] [PubMed] [Google Scholar]
- [123].Salat K, Kowalczyk P, Gryzlo B, Jakubowska A, Kulig K. New investigational drugs for the treatment of neuropathic pain. Expert Opin Investig Drugs 2014;23:1093–104. [DOI] [PubMed] [Google Scholar]
- [124].Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science 1991;251:1608–10. [DOI] [PubMed] [Google Scholar]
- [125].Sawynok J, Liu J. Contributions of peripheral, spinal, and supraspinal actions to analgesia. Eur J Pharmacol 2014;734:114–21. [DOI] [PubMed] [Google Scholar]
- [126].Schaible HG. Emerging concepts of pain therapy based on neuronal mechanisms. Handb Exp Pharmacol 2015;227:1–14. [DOI] [PubMed] [Google Scholar]
- [127].Seltzman HH, Shiner C, Hirt EE, Gilliam AF, Thomas BF, Maitra R, Snyder R, Black SL, Patel PR, Mulpuri Y, Spigelman I. Peripherally selective cannabinoid 1 receptor (CB1R) agonists for the treatment of neuropathic pain. J Med Chem 2016;59:7525–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Serra J, Bostock H, Sola R, Aleu J, Garcia E, Cokic B, Navarro X, Quiles C. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain 2012;153:42–55. [DOI] [PubMed] [Google Scholar]
- [129].Shepherd AJ, Mickle AD, Golden JP, Mack MR, Halabi CM, de Kloet AD, Samineni VK, Kim BS, Krause EG, Gereau RW, Mohapatra DP. Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc Natl Acad Sci U S A 2018;115:E8057–E8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Shinder V, Govrin-Lippmann R, Cohen S, Belenky M, Ilin P, Fried K, Wilkinson HA, Devor M. Structural basis of sympathetic-sensory coupling in rat and human dorsal root ganglia following peripheral nerve injury. J Neurocytol 1999;28:743–61. [DOI] [PubMed] [Google Scholar]
- [131].Snyder LM, Chiang MC, Loeza-Alcocer E, Omori Y, Hachisuka J, Sheahan TD, Gale JR, Adelman PC, Sypek EI, Fulton SA, et al. Kappa opioid receptor distribution and function in primary afferents. Neuron 2018;99:1274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Spahn V, Del VG, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, Durmaz V, Sabri P, Reidelbach M, Machelska H, Weber M, Stein C. A nontoxic pain killer designed by modeling of pathological receptor conformations. Science 2017;355:966–9. [DOI] [PubMed] [Google Scholar]
- [133].Stanos SP, Galluzzi KE. Topical therapies in the management of chronic pain. Postgrad Med 2013;125:25–33. [DOI] [PubMed] [Google Scholar]
- [134].Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, Spencer RH. Peripheral mechanisms of pain and analgesia. Brain Res Rev 2009;60:90–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Study RE, Kral MG. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain 1996;65:235–42. [DOI] [PubMed] [Google Scholar]
- [136].Sun W, Miao B, Wang XC, Duan JH, Wang WT, Kuang F, Xie RG, Xing JL, Xu H, Song XJ, Luo C, Hu SJ. Reduced conduction failure of the main axon of polymodal nociceptive C-fibres contributes to painful diabetic neuropathy in rats. Brain 2012;135:359–75. [DOI] [PubMed] [Google Scholar]
- [137].Taneja A, Della PO, Danhof M. Challenges in translational drug research in neuropathic and inflammatory pain: the prerequisites for a new paradigm. Eur J Clin Pharmacol 2017;73:1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Tappe-Theodor A, Kuner R. Studying ongoing and spontaneous pain in rodents--challenges and opportunities. Eur J Neurosci 2014;39:1881–90. [DOI] [PubMed] [Google Scholar]
- [139].Tiwari V, Anderson M, Yang F, Tiwari V, Zheng Q, He SQ, Zhang T, Shu B, Chen X, Grenald SA, Stephens KE, Chen Z, Dong X, Raja SN, Guan Y. Peripherally acting μ-opioid receptor agonists attenuate ongoing pain-associated behavior and spontaneous neuronal activity after nerve injury in rats. Anesthesiology 2018;128:1220–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Tiwari V, Yang F, He SQ, Shechter R, Zhang C, Shu B, Zhang T, Tiwari V, Wang Y, Dong X, Guan Y, Raja SN. Activation of peripheral μ-opioid receptors by dermorphin [D-Arg2, Lys4] (1–4) amide leads to modality-preferred inhibition of neuropathic pain. Anesthesiology 2016;124:706–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Torebjork E, Wahren L, Wallin G, Hallin R, Koltzenburg M. Noradrenaline-evoked pain in neuralgia. Pain 1995;63:11–20. [DOI] [PubMed] [Google Scholar]
- [142].Torebjork HE, LaMotte RH, Robinson CJ. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: simultaneous recordings in humans of sensory judgments of pain and evoked responses in nociceptors with C-fibers. J Neurophysiol 1984;51:325–39. [DOI] [PubMed] [Google Scholar]
- [143].Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol 1992;448:765–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain 2006;7:281–9. [DOI] [PubMed] [Google Scholar]
- [145].Treede RD, Davis KD, Campbell JN, Raja SN. The plasticity of cutaneous hyperalgesia during sympathetic ganglion blockade in patients with neuropathic pain. Brain 1992;115 (Pt 2):607–21. [DOI] [PubMed] [Google Scholar]
- [146].Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol 1992;38:397–421. [DOI] [PubMed] [Google Scholar]
- [147].Truini A A review of neuropathic pain: from diagnostic tests to mechanisms. Pain Ther 2017;6:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Truini A, Garcia-Larrea L, Cruccu G. Reappraising neuropathic pain in humans--how symptoms help disclose mechanisms. Nat Rev Neurol 2013;9:572–82. [DOI] [PubMed] [Google Scholar]
- [149].Tsantoulas C, Lainez S, Wong S, Mehta I, Vilar B, McNaughton PA. Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy. Sci Transl Med 2017;9:eaam6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 2011;152:990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].van HO, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014;155:654–62. [DOI] [PubMed] [Google Scholar]
- [152].Vaso A, Adahan HM, Gjika A, Zahaj S, Zhurda T, Vyshka G, Devor M. Peripheral nervous system origin of phantom limb pain. Pain 2014;155:1384–91. [DOI] [PubMed] [Google Scholar]
- [153].Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain 1983;17:321–39. [DOI] [PubMed] [Google Scholar]
- [154].Wall PD, Gutnick M. Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp Neurol 1974;43:580–93. [DOI] [PubMed] [Google Scholar]
- [155].Wallin G, Torebjork E, Hallin R. Preliminary observations on the pathophysiology of hyperalgesia in the causalgic pain syndrome In: Zotterman Y, editor. Sensory Functions of the Skin of Primates with Special Reference to Man. Elmsford, NY: Pergamon Press, 1976. pp. 489–502. [Google Scholar]
- [156].Walters ET. Neuroinflammatory contributions to pain after SCI: roles for central glial mechanisms and nociceptor-mediated host defense. Exp Neurol 2014;258:48–61. [DOI] [PubMed] [Google Scholar]
- [157].Waxman SG, Zamponi GW. Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci 2014;17:153–63. [DOI] [PubMed] [Google Scholar]
- [158].Weissner W, Winterson BJ, Stuart-Tilley A, Devor M, Bove GM. Time course of substance P expression in dorsal root ganglia following complete spinal nerve transection. J Comp Neurol 2006;497:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Welk E, Leah JD, Zimmermann M. Characteristics of A- and C-fibers ending in a sensory nerve neuroma in the rat. J Neurophysiol 1990;63:759–66. [DOI] [PubMed] [Google Scholar]
- [160].Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983;306:686–8. [DOI] [PubMed] [Google Scholar]
- [161].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000;288:1765–9. [DOI] [PubMed] [Google Scholar]
- [163].Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci 2001;21:RC140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Xie W, Strong JA, Meij JT, Zhang JM, Yu L. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain 2005;116:243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Xie W, Strong JA, Zhang JM. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience 2009;160:847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Xie W, Strong JA, Zhang JM. Increased excitability and spontaneous activity of rat sensory neurons following in vitro stimulation of sympathetic fiber sprouts in the isolated dorsal root ganglion. Pain 2010;151:447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Xie Y, Zhang J, Petersen M, LaMotte RH. Functional changes in dorsal root ganglion cells after chronic nerve constriction in the rat. J Neurophysiol 1995;73:1811–20. [DOI] [PubMed] [Google Scholar]
- [168].Xie YK, Xiao WH. Electrophysiological evidence for hyperalgesia in the peripheral neuropathy. Sci China B 1990;33:663–72. [PubMed] [Google Scholar]
- [169].Yatziv SL, Devor M. Suppression of neuropathic pain by selective silencing of dorsal root ganglion ectopia using nonblocking concentrations of lidocaine. Pain 2019;160:2105–14. [DOI] [PubMed] [Google Scholar]
- [170].Yen LD, Bennett GJ, Ribeiro-da-Silva A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J Comp Neurol 2006;495:679–90. [DOI] [PubMed] [Google Scholar]
- [171].Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 2020;11:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol 1999;82:3359–66. [DOI] [PubMed] [Google Scholar]
- [173].Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain 1999;122 (Pt 12):2245–57. [DOI] [PubMed] [Google Scholar]


