Abstract
Background & Aims:
Deep remission, based on clinical remission and evidence of healing during endoscopic evaluation, are goals of medical treatments for Crohn’s disease (CD). We investigated whether histologic healing is associated with outcomes of patients with CD ileitis.
Methods:
We performed a retrospective study of 101 patients with CD (52% male) isolated to the terminal ileum who had a colonoscopy between September 2005 and June 2015. Our analysis included patients in clinical remission at colonoscopy who had biopsies collected from colon and ileum. The ileum was evaluated for endoscopic healing (no ulceration) and histologic evidence of healing (no active inflammation, erosions, ulceration, or neutrophil infiltration). We compared times of clinical relapse-free survival, medication escalation, corticosteroid use, or hospitalization secondary to disease activity between patients with and without histological and endoscopic healing, followed for a median 21 months. We identified factors associated with survival using Kaplan Meier analysis and Cox proportional hazard model.
Results:
At ileo-colonoscopy, 63% of patients had endoscopic healing and 55% had histologic evidence of healing. The level of agreement between endoscopic and histologic activity was fair (62%, K=0.2250, P=.0064). Forty-two patients had clinical relapse, 45 had medication escalation, 30 required corticosteroids, and 17 were hospitalized (3 required surgery). On multivariate analysis, only histologic healing was associated with decreased risk of clinical relapse (hazard ratio [HR], 2.05; 95% CI, 1.07–3.94; P=.031), medication escalation (HR, 2.17; 95% CI, 1.2–3.96; P=.011), and corticosteroid use (HR, 2.44; 95% CI, 1.17–5.09; P=.018). No factors were associated with hospitalization.
Conclusions:
In patients with ileal CD in clinical remission, histologic healing but not endoscopic healing is associated with decreased risk of clinical relapse, medication escalation, or corticosteroid use.
Keywords: inflammatory bowel disease, mucosal healing, histology, histopathology, prognostic factor
INTRODUCTION
In patients with Crohn’s disease (CD), persistent inflammation leads to bowel damage. Cumulative bowel damage, described as progressing from inflammatory to stricturing and then to a penetrating phenotype, may predict long-term disability, and can lead to linical symptoms and need for surgery.1
Historically, treatment of CD aimed to control clinical symptoms. However, clinical symptoms poorly correlate with endoscopic mucosal disease activity, and resolution of symptoms alone fails to alter this natural progression of CD and cumulative bowel damage.2 Mucosal healing, on the other hand, is associated with better long-term outcomes; patients who achieve mucosal healing have lower rates of hospitalization and surgery, and are less likely to have a clinical flare on follow-up.3–6 Therefore, ‘deep remission’, defined as clinical remission and endoscopic healing without bowel ulceration has emerged as the recommended goal of treatment therapy.7, 8
Histological inflammation may persist in the setting of mucosal healing. In UC, histological inflammation is a stronger predictor of clinical flares, corticosteroids use and hospitalization for disease activity than mucosal healing.9, 10 In addition, histological normalization has been associated with improved long-term clinical outcomes when compared to histological quiescence.11 This has led to suggestions that histological assessment in UC should be used as an adjunct to mucosal disease assessment in standard care.11, 12
The role of histological assessment in CD has been poorly explored. Despite the recent appreciation for the value of endoscopic assessment in CD, there is little evidence as to whether microscopic examination of the mucosa adds any further prognostic information. Histological healing is achievable in CD13, 14 but, due to the patchy nature of the disease, it is often felt that histological assessment is subject to too much biopsy bias and is too difficult to study.15 In addition, there is currently no consensus on the use of a specific scoring system when assessing histological changes in CD.
The aims of this study were to explore whether histological healing (supplementary Table 1) provides any further prognostic information in regard to clinical outcomes, hospitalization and medication escalation in patients with ileal CD when compared to endoscopic healing (EH) alone.
METHODS
An exploratory retrospective case-control study of patients with CD limited to the terminal ileum was performed and was approved by the Institutional Review Board (IRB13–1063). All patients who underwent colonoscopy for CD at the University of Chicago between September 2005 and June 2015 were identified. For patients with a confirmed diagnosis of CD based on clinical and histological information, the electronic medical record was then reviewed. Inclusion criteria comprised a colonoscopy to the terminal ileum, biopsies of both the colon and terminal ileum with disease limited to the terminal ileum both macroscopically and microscopically throughout the duration of their disease, clinical remission at the time of the colonoscopy and ≥6 months of clinical follow-up following colonoscopy. Patients with inadequate documentation, inflammation present in the colon, undergone a colectomy, or confirmed Clostridioides difficile infection at the time of follow-up colonoscopy were excluded.
Medical Records Abstraction
Endoscopy reports were retrieved through an electronic documentation system (Provation, Minneapolis, MN). Demographic, clinical, histologic and biochemical data were collected from the electronic medical record system (EPIC, Wisconsin, USA), including date of disease onset, disease duration, smoking history, CD phenotype according to Montreal classification (B1–inflammatory, B2–stricturing, B3–penetrative), disease location (ileal disease only included), and previous and current use of anti-inflammatory agents and/or immunosuppressant therapy (steroids, immunomodulators, anti-TNF agents) at the time colonoscopy.
Endoscopic Assessment
An academic IBD expert gastroenterologist with minimum of 5 years’ experience performed all endoscopies, during which endoscopic photographs were obtained from each bowel segment, with targeted photos of the areas of endoscopic activity. As per the inclusion criteria, patients could not have evidence of past or present colonic CD. Consistent with recent large clinical trials and the STRIDE guidelines, endoscopic healing (EH) was defined as the presence of no mucosal ulceration including aphthae12,16, 17, which was confirmed by both the endoscopic report and photographic evidence.
Histologic Assessment
Within the unit, mucosal biopsies from both the ileum and colon are routinely taken targeting the area of most significant mucosal inflammation. Patients were excluded if there was histological evidence of CD in the colon.
The histopathology reports from all diagnostic, screening and surveillance endoscopic biopsies contained in the patient’s electronic medical records were reviewed. Two pathologists (JRT and JH) who specialize in gastrointestinal histology and whose agreement has been previously described,18 routinely assessed all biopsies and reported the worst affected area. There are several histological scoring schemes that have been utilized in CD, however none of these have been validated.19–21
In the absence of a validated histological grading score in CD, histological healing was assessed using a modified ileal global histological disease activity score (iGHAS).14 The iGHAS is the most commonly used histological index in CD and assesses and scores two features of chronicity (architectural changes and infiltration of mononuclear cells in the lamina propria) and five features of activity (epithelial damage, polymorphonuclear cells in the lamina propria, polymorphonuclear cells in the epithelium, presence of erosions and/or ulcers, epithelial granuloma). For the purposes of the current study, histologic assessment was dichotomized to ‘histologic healing’, where none of the features of activity above were present, and ‘histologic activity’, where one or more of the features were present. Severity of inflammation was not scored, as stated in Supplementary Table 1.
Assessment of Clinical Outcomes
At every patient clinic visit at the University of Chicago, the Harvey Bradshaw Index (HBI) is calculated.22 All patients who were in clinical remission at the time of the colonoscopy, defined as an HBI ≤ 4, and who had ≥ 6 months of follow-up were included. Clinical relapse-free survival, medication-escalation-free survival, corticosteroid-free survival and hospitalization-free survival were calculated and defined as time from colonoscopy to event. Clinical relapse was defined at clinical follow-up as HBI > 4 that resulted in alteration or addition of medical therapy, hospitalization or surgery. Escalation of medication was defined as need for a course of corticosteroid or change in medication maintenance including change of biologic agent or escalation of dose, addition or change of immunomodulator in combination therapy for CD, or escalation from immunomodulator to biologic agent. Corticosteroid use was defined as the requirement for an increase dosage or new course of oral corticosteroids including budesonide for active CD symptoms. Hospitalization was recorded if required for disease activity or refractory disease including need for surgery.
Statistical Analysis
Continuous variables were summarized using medians and interquartile ranges (IQR). Categorical variables were expressed as the percentage and number of cohort. Cohen’s kappa coefficient (κ) was calculated to measure agreement between endoscopic and histological activity.
Kaplan-Meier analyses were performed to compare clinical relapse-free survival, medication escalation-free survival, corticosteroid-free survival and hospitalization-free survival in those with and without EH and with and without histological healing. Univariate and multivariate Cox proportional hazard regression analysis was performed to identify predictors of clinical relapse, medication escalation, corticosteroid use and hospitalization. All variables with p-values of less than or equal to 0.10 on univariate analysis were retained and integrated into the multivariable models. A two-sided p-value of 0.05 or less was considered statistically significant. All data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
Patients
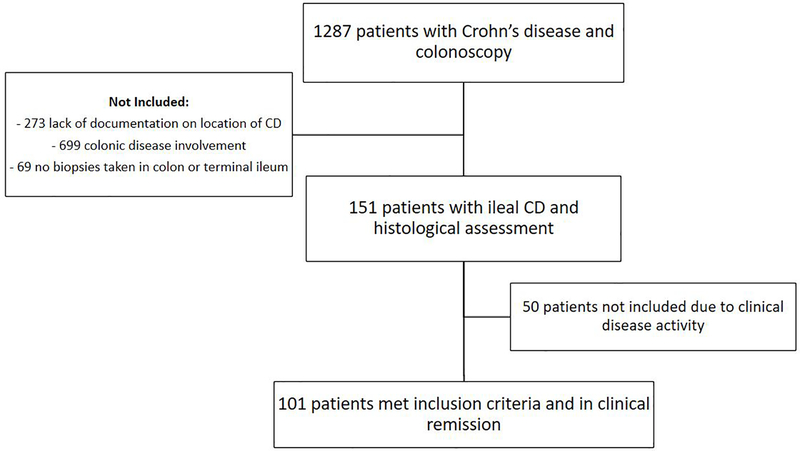
Of 1287 patients with documented CD and a colonoscopy, 150 fulfilled entry criteria with disease limited to the terminal ileum, normal colonoscopic biopsies and evaluable biopsies from the ileum. Of these, 101 were in clinical remission at the time of colonoscopy and were included in the study (Figure 1).
Figure 1:
Flow chart of patients included in the study
The patients’ demographics and clinical characteristics are shown in Table 1. 52% of patients were male with median age of diagnosis of 25 years old and median duration of disease at time of colonoscopy of 12 years. 86% of patients had two or more sets of biopsies taken of the terminal ileum. At colonoscopy, 64 patients (63%) had EH and 55% had achieved histological healing. The level of agreement between endoscopic activity and histological activity was fair at 62% (κ=0.2250; p=0.011).
Table 1:
Clinical Characteristics at Baseline
| Baseline characteristics (N = 101) | Patients with histological healing n=56 | Patients without histological healing N=45 |
|---|---|---|
| Median (IQR) or Percentage (n) | ||
| Age at diagnosis of CD (years) | 24 (16–31) | 27 (19–34) |
| Gender (male) | 48% (n=27) | 58% (n=26) |
| Duration of disease (years) | 14 (9–25) | 9 (3–19) |
| Disease phenotype | ||
| B1 (inflammatory) | 14% (n=8) | 38% (n=17) |
| B2 (stricturing) | 61% (n=34) | 40% (n=18) |
| B3 (penetrating) | 25% (n=14) | 22% (n=10) |
| Medications at time of colonoscopy: | ||
| Oral prednisolone/budesonide | 20% (n=11) | 18% (n=8) |
| 5-amino-salcylic acid | 9% (n=5) | 11% (n=5) |
| 6-mercaptopurine/azathioprine | 45% (n=25) | 38% (n=17) |
| Methotrexate | 11% (n=6) | 9% (n=4) |
| Anti-tumor necrosis factor | 32% (n=18) | 31% (n=14) |
| Ustekinumab | 2% (n=1) | 0% (n=0) |
| Vedolizumab | 7% (n=4) | 4% (n=2) |
| Endoscopic healing | 73% (n=41) | 51% (n=23) |
Clinical Relapse-free Survival
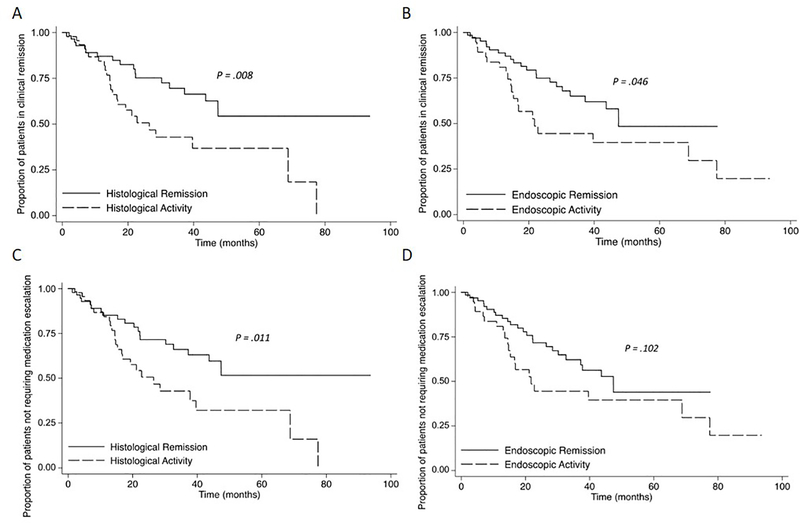
Median follow-up time was 21 (IQR 12–40) months. Clinical relapse occurred in 42% (n=42) of patients after median time of 16 (IQR 7–26) months. As shown in Figure 2, patients with EH and histological healing were less likely to experience clinical relapse (Figure 2a–b). By univariate analysis, no other factors were associated with improved clinical relapse-free survival (Table 2). By multivariable analysis, only histological activity remained associated with clinical-relapse free survival with HR 2.05 (1.07, 3.94; p=0.031; Table 2). EH did not independently predict a lower rate of clinical relapse.
Figure 2:
Kaplan-Meier analysis of effect of endoscopic and histological activity on (A) Clinical relapse-free survival versus histological healing (B) Clinical relapse-free survival versus endoscopic healing (C) Medication escalation-free survival versus histological healing (D) Medication escalation -free survival versus endoscopic healing
Table 2:
Univariable predictors of clinical outcome measures in patients with Crohn’s Disease
| Variable | Clinical Relapse n=42† HR (95% CI), p-value | Medication escalation n=45† HR (95% CI), p-value | Corticosteroid Requirement n=32† HR (95% CI), p-value | Hospitalization for severe disease n=17† HR (95% CI), p-value |
|---|---|---|---|---|
| Age diagnosis of CD (years) | 0.98 (0.95, 1.01), p=0.196 | 0.98 (0.95,1.00), p=0.127 | 0.98 (0.95, 1.01), p=0.204 | 0.98 (0.94,1.03), p=0.463 |
| Sex (male) | 0.66 (0.36, 1.23), p=0.197 | 0.60(0.32,1.09), p=0.095 | 0.65 (0.32, 1.32), p=0.239 | 0.49 (0.18, 1.36), p=0.171 |
| Disease duration (years from diagnosis to colonoscopy) | 0.99 (0.97, 1.02), p=0.569 | 0.99 (0.97,1.01), p=0.656 | 0.99 (0.96, 1.02), p=0.637 | 1.00 (0.96, 1.04), p=0.980 |
| Penetrative vs Inflammatory (B2vsB1) | 0.78 (0.39, 1.58), p=0.493 | 0.89 (0.44,1.76), p=0.729 | 1.08 (0.45, 2.59), p=0.860 | 1.95 (0.43, 8.90), p=0.391 |
| Stricturing vs Inflammatory (B3vsB1) | 0.44 (0.16, 1.20), p=0.111 | 0.45(0.17, 1.22), p=0.120 | 0.62 (0.19, 1.99), p=0.422 | 1.06 (0.17, 6.56), p=0.947 |
| Maintenance therapy | ||||
| - 5-ASA | 1.21 (0.48, 3.11), p =0.682 | 1.13(0.44, 2.88), p=0.794 | 0.90 (0.27, 2.97), p=0.861 | 1.08 0.24, 4.76), p=0.923 |
| - Immunomodulator | 0.66 (0.35, 1.25), p=0.202 | 0.73(0.39, 1.36), p=0.327 | 0.62 (0.30, 1.27), p=0.193 | 0.41 (0.13, 1.25), p=0.117 |
| On oral corticosteroids at endoscopy | 1.50 (.73, 3.05), p=0.267 | 1.20(0.47, 3.05), p=0.703 | 1.34 (0.47, 3.88), p=0.578 | 1.29 (0.29, 5.72), p=0.737 |
| Ongoing histological activity | 2.31 (1.24, 4.31), p=0.008* | 2.17(1.20, 3.96),p=0.011* | 2.36 (1.16, 4.81), p=0.018* | 1.27 (0.47, 3.43), p=0.636 |
| Ongoing endoscopic activity | 1.87 (1.01, 3.45), p=0.046* | 1.64(0.91,2.99), p=0.102 | 1.39 (0.68, 2.85), p=0.369 | 0.97 (0.35, 2.67), p=0.951 |
Cox regression univariate analyses presented.
p Value of ≤0.05 considered significant and marked by*.
5ASA, 5-aminosalicylic acid.
Medication Escalation-Free Survival
The medication regimen was escalated in 45% (n=45) of patients, two of whom were in clinical remission, but had moderate or severe endoscopic disease activity on colonoscopy. Thirty-two patients required a course of oral corticosteroids (budesonide or prednisolone), 19 required a new biologic agent, 1 had escalation of biologic agent dosing and 6 patients had an immunomodulator added to their biologic therapy. Patients with histological healing were less likely to have medication escalation (Figure 2c). EH was not significantly associated with a lower rate of medication escalation. (Figure 2d) The only factor associated with improved medication escalation-free survival was the achievement of histological healing compared to histological activity with HR 2.17 (1.20, 3.96; p=0.011) on univariate analysis and HR 2.08 (1.14, 3.80; p=0.017) on multivariable analysis (Table 2).
Corticosteroid-free survival
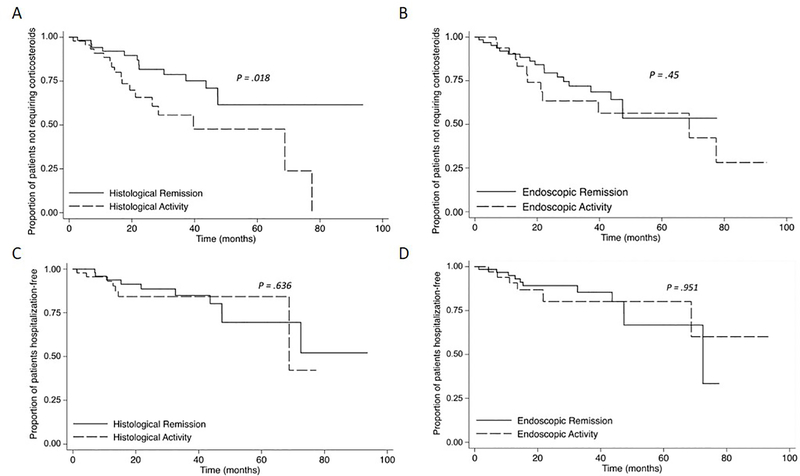
Patients with histological healing, but not EH, were less likely to have a requirement for salvage therapy with corticosteroids (Figure 3a–b). By univariate and multivariable analyses, the only factor associated with corticosteroid-free survival was the achievement of histological healing compared to histological activity (HR 2.44 (1.17, 5.09), p=0.018; Table 2).
Figure 3:
Kaplan-Meier analysis of effect of endoscopic and histological activity on (A) Corticosteroid-free survival versus histological healing (B) Corticosteroid-free survival versus endoscopic healing (C) Hospitalization-free survival versus histological healing (D) Hospitalization -free survival versus endoscopic healing
Hospitalization-free survival
17% (n=17) of patients were hospitalized and 3 patients proceeded to ileocecectomy. Due to small numbers, predictors of surgery could not be analyzed. No factor was associated with hospitalization-free survival. EH and histological healing did not protect from hospitalization on follow-up (See figure 3c–d).
DISCUSSION
Recently there has been increased emphasis placed on objective markers of disease activity. In CD particularly, the association with clinical symptoms and bowel damage is poor.3 This may, in part, explain why, despite the advent of many new therapies, the natural history of the disease has, up until recently, barely changed.23 Recently, expert consensus has stated that the target of treatment for CD should be EH, as defined by lack of ulceration, in order to attempt to prevent ongoing bowel damage.12 Unlike UC, where histological healing has been defined as an adjunct to EH24 and the combination of both has been proposed as a new endpoint of interest25, the role of histology in CD, beyond diagnosis, is poorly defined. In the current exploratory study, the prognostic value of histology in patients with CD restricted to the ileum has clearly shown that histological remission is associated with superior clinical-relapse free survival and reduced need for medication escalation and corticosteroids. Moreover, the results indicate the poor performance of EH alone as a prognostic predictor.
As in UC, this study demonstrates that there is a disparity between EH and histological remission. We found that the level of agreement between endoscopic activity and histological activity was only fair at 63 % (k=0.2250). Microscopic inflammation persisted in 36% of those who achieved EH. This is similar to previous reports of persistent microscopic inflammation in the setting of EH of between 25–37% 8, 26 and emphasizes the need to consider histologic outcomes separate to endoscopic measures of remission in CD.
Several studies have demonstrated the effect of medical therapy on histologic healing in patients with CD; azathioprine, methotrexate and the biologics can all result in histological healing.13, 14, 16, 17, 27 A sub-analysis of 13 patients from the ACCENT 1 study established that histological improvement after 54 weeks of infliximab was associated with a consistent decrease in the expression of inflammatory markers including CD68 and gelatinase B in the colonic mucosa.28 The relationship of histology to chance of relapse was explored in a study of 46 patients with Crohn’s colitis undergoing surveillance colonoscopy and histology and/or active mucosal disease did not predict chance of relapse. 20 This study also found similar lack of association in patients with UC. However, a recent paper of 62 CD patients in clinical remission demonstrated that histological inflammation was strongly associated with an increased risk of clinical flares within 1–2 years and that endoscopic activity alone did not predict clinical flares on follow-up. 19 Two recent studies have also specifically examined the link between histological remission and outcomes in ulcerative colitis and found that histological disease activity was linked to an increased chance of clinical relapse. Bryant et al10 demonstrated that histological remission predicted reduced corticosteroid use and episodes of acute severe colitis requiring hospitalization in a cohort of 91 patients with UC over a period of 6 years. In a large cohort of 310 patients from Chicago, histological normalization was also found to be achievable and this predicted a lower chance of clinical relapse over the ensuing 16 months.11 Due to such studies, routine histological assessment is now recommended in UC. 12 This exploratory study on ileal CD demonstrates that histological assessment in CD patients is also clinically relevant, despite the patchy nature of the disease. Therefore, we recommend that routine ileal biopsies be obtained when patients with terminal ileal disease are being assessed.
As we strive to achieve deeper markers of disease control, histological healing may emerge as a treatment target in CD. This aspiration, however, raises several issues. Dichotomously scoring the histopathology of individual biopsies as healed versus inflamed using the criteria applied in the current study should be relatively easy, as opposed to scoring the severity of inflammation. It is the patchy nature of inflammation in CD with the implications around what should be defined as ‘healed’ that provides the uncertainty and controversy. Studies of where the biopsies should be taken and how many are needed to confidently make such a decision are required so a validated and reproducible histological index can be established. Despite this, the results of the current study have clearly demonstrated that the goal of gaining meaningful prognostic information from assessing histological healing in the terminal ileum is achievable.
Even if these guidelines existed, it is as yet unclear if it is even possible to achieve histologic healing in the majority of patients or whether treating our patients more aggressively with medical therapy will improve rates of histological healing. Hence, while histologic healing might provide prognostic information, it cannot, at this stage, be recommended as a target upon which therapeutic decisions in patients with ileal disease can be made. While those who have a healed ileum have a better clinical outlook, whether this group who achieve this level of “deeper remission” need less stringent follow-up or monitoring, or are less likely to have disease that progresses to a stricturing or penetrating phenotype requires further study.
CD causes chronic transmural inflammation of the gastrointestinal tract. There is evidence that patients who achieve transmural healing also have more favorable clinical outcomes on follow-up compared to patients who achieve EH alone.29 It is unclear if transmural inflammation persists in the setting of histologic healing as demonstrated on mucosal biopsies or if achieving the potentially even deeper target of transmural healing could result in further improvement in clinical outcome compared to histologic healing alone but this should be looked at in future research.
There are several limitations to this study. First, this is a retrospective analysis with a relatively small sample size and there may be inaccuracies in data collection that affect results. The extensive experience of the involved clinicians and the overlapping data sources (electronic records, endoscopy records and pathology reports) aim to minimize this limitation, but those patients in prolonged clinical remission may not be included due to not having had a colonoscopy or endoscopic biopsies. Secondly, the generalizability of the data is also uncertain; this is a single-center study based in a tertiary hospital setting where experts in the area of IBD manage patients. Thirdly, the findings are currently applicable only to patients with ileal CD. The patchiness of the disease make histologic studies in patients with colonic or ileo-colonic disease challenging. In the same way, however, the restriction to terminal ileal disease was a strength of the current study as it aimed to examine a concept with clearly positive results. It provides the impetus to expand the work to more extensive disease to determine whether normalization of histology has prognostic value as it does in ileal disease and UC. Fourthly, even though the current histologic scale was not assessing severity of inflammation, but rather the normality of biopsies, its application and reproducibility has not undergone independent validation. Because there are currently no validated histological indices in Crohn’s disease, we focused on the absence of an active inflammatory infiltrate to represent the absence of histologic activity. The presence of acute inflammation, which is of clinical significance, is simple and reproducible, and is the outcome that has been reported to improve following biologic treatment in previous CD trials.14, 16, 17 Fifthly, the use of clinical remission as an inclusion criterion for this study also has its own limitations. Patients with both histologic and EH may have clinical symptoms, so not all patients with EH would be included in this study. In addition, it is unclear what percentage of patients had an inflammatory relapse as the outcomes analyzed were clinical relapse and need for steroids or medication escalation, which may have occurred in some patients who had worsening symptoms despite no increase in inflammatory burden. Finally, it is noted that biomarker assessment at time of colonoscopy would strengthen this study and that future prospective studies should include calprotectin, C-reactive protein assessment and perhaps intestinal ultrasound to determine how they compare to histological and endoscopic assessment alone.
In conclusion, we have demonstrated in patients with CD restricted to the terminal ileum the potential for histological healing to act as a prognostic marker. It is associated with improved clinical outcomes, less chance of clinical relapse and decreased need for medication escalation. We propose that histological assessment of healing should be part of endoscopic assessment in CD. However, there is a clear need for standardized and validated histological indices in CD and the prognostic value of their application to CD affecting the colon and rectum require evaluation.
Supplementary Material
Need to Know.
Background:
Deep remission, based on clinical remission and evidence of healing on endoscopic evaluation are goals of medical treatments for Crohn’s disease (CD).
Findings:
In patients with ileal CD in clinical remission, histologic healing, but not endoscopic healing, indicates that patients have decreased risk of clinical relapse, medication escalation, or corticosteroid use.
Implications for patient care:
Patients in remission from CD should be evaluated for histologic evidence of healing.
Acknowledgments
Financial/Grant Support: Funded in part by the “Digestive Disease Research Core Center of the University of Chicago (DK42086)”. Britt Christensen receives support through an “Australian Government Research Training Program Scholarship”.
Abbreviations:
- EH
Endoscopic Healing
- CD
Crohn’s disease
- HBI
Harvey Bradshaw Index
Footnotes
Competing Interests/Disclosures: Dr Christensen reports personal fees from Gilead, Novartis, Janssen, Takeda, Pfizer, and Abbvie, grants from Janssen, grants from Janssen and Ferring Pharmaceuticals; Dr Gibson reports personal fees from Allergan, Janssen, MSD, Pfizer, Anatara, Atmo Biosciences, Immunic Therapeutics, Novozymes, and Takeda, and grants from MSD; Dr Rubin reports personal fees from Abbvie, Abgenomics, Allergan Inc, Boehringer Ingelheim Ltd, Bristol-Myers Squibb, Celgene Corp/Syneos, Check-cap, Dizal Pharmaceuticals GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences, GlaxoSmithKline Group, Janssen Pharmaceuticals, Lilly, Narrow River Mgmt, Pfizer, Prometheus Laboratories, Reistone, Shire, Takeda, Techlab, Inc, and grants from Abbvie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, Takeda. The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lemann score. Inflamm Bowel Dis 2011;17:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002;8:244–50. [DOI] [PubMed] [Google Scholar]
- 3.Solem CA, Loftus EV Jr., Tremaine WJ, et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis 2005;11:707–12. [DOI] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Diamond RH, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc 2006;63:433–42; quiz 464. [DOI] [PubMed] [Google Scholar]
- 5.Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009;15:1295–301. [DOI] [PubMed] [Google Scholar]
- 6.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology 2004;126:402–13. [DOI] [PubMed] [Google Scholar]
- 7.Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:414–22 e5. [DOI] [PubMed] [Google Scholar]
- 8.Molander P, Sipponen T, Kemppainen H, et al. Achievement of deep remission during scheduled maintenance therapy with TNFalpha-blocking agents in IBD. J Crohns Colitis 2013;7:730–5. [DOI] [PubMed] [Google Scholar]
- 9.Riley SA, Mani V, Goodman MJ, et al. Microscopic activity in ulcerative colitis: what does it mean? Gut 1991;32:174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016;65:408–14. [DOI] [PubMed] [Google Scholar]
- 11.Christensen B, Hanauer SB, Erlich J, et al. Histologic Normalization Occurs in Ulcerative Colitis and Is Associated With Improved Clinical Outcomes. Clin Gastroenterol Hepatol 2017;15:1557–1564 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 13.Mantzaris GJ, Christidou A, Sfakianakis M, et al. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn’s disease. Inflamm Bowel Dis 2009;15:375–82. [DOI] [PubMed] [Google Scholar]
- 14.D’Haens G, Van Deventer S, Van Hogezand R, et al. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn’s disease: A European multicenter trial. Gastroenterology 1999;116:1029–34. [DOI] [PubMed] [Google Scholar]
- 15.Bryant RV, Winer S, Travis SP, et al. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis 2014;8:1582–97. [DOI] [PubMed] [Google Scholar]
- 16.Li K, Friedman JR, Chan D, et al. Effects of Ustekinumab on Histologic Disease Activity in Patients With Crohn’s Disease. Gastroenterology 2019. [DOI] [PubMed] [Google Scholar]
- 17.Danese S, Sandborn WJ, Colombel JF, et al. Endoscopic, Radiologic, and Histologic Healing With Vedolizumab in Patients With Active Crohn’s Disease. Gastroenterology 2019. [DOI] [PubMed] [Google Scholar]
- 18.Rubin DT, Huo D, Kinnucan JA, et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol 2013;11:1601–8 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan GT, Melton SD, Spechler SJ, et al. Clinical Implications of Histologic Abnormalities in Ileocolonic Biopsies of Patients With Crohn’s Disease in Remission. J Clin Gastroenterol 2017;51:43–48. [DOI] [PubMed] [Google Scholar]
- 20.Baars JE, Nuij VJ, Oldenburg B, et al. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis 2012;18:1634–40. [DOI] [PubMed] [Google Scholar]
- 21.Naini BV, Cortina G. A histopathologic scoring system as a tool for standardized reporting of chronic (ileo)colitis and independent risk assessment for inflammatory bowel disease. Hum Pathol 2012;43:2187–96. [DOI] [PubMed] [Google Scholar]
- 22.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 23.Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis 2007;13:481–9. [DOI] [PubMed] [Google Scholar]
- 24.Vuitton L, Peyrin-Biroulet L, Colombel JF, et al. Defining endoscopic response and remission in ulcerative colitis clinical trials: an international consensus. Aliment Pharmacol Ther 2017;45:801–813. [DOI] [PubMed] [Google Scholar]
- 25.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 26.Korelitz BI, Sommers SC. Response to drug therapy in Crohn’s disease: evaluation by rectal biopsy and mucosal cell counts. J Clin Gastroenterol 1984;6:123–7. [DOI] [PubMed] [Google Scholar]
- 27.Laharie D, Reffet A, Belleannee G, et al. Mucosal healing with methotrexate in Crohn’s disease: a prospective comparative study with azathioprine and infliximab. Aliment Pharmacol Ther 2011;33:714–21. [DOI] [PubMed] [Google Scholar]
- 28.Geboes K, Rutgeerts P, Opdenakker G, et al. Endoscopic and histologic evidence of persistent mucosal healing and correlation with clinical improvement following sustained infliximab treatment for Crohn’s disease. Curr Med Res Opin 2005;21:1741–54. [DOI] [PubMed] [Google Scholar]
- 29.Serban ED. Treat-to-target in Crohn’s disease: Will transmural healing become a therapeutic endpoint? World J Clin Cases 2018;6:501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.