Abstract
Aim
The purpose of this study was to investigate the psychological and behavioural effects of animal‐assisted therapy on cognitive function, emotional state, problematic behaviours, and activities of daily living among older adults with dementia.
Methods
A nonequivalent control group pretest and post‐test study design was used in this study. Twenty‐eight participants—14 in the intervention group and 14 in the control group—were recruited from two hospitals in Gyeonggi Province, South Korea, between February and April 2015. The intervention group received two 60‐min sessions of animal‐assisted therapy weekly for 8 weeks, while the control group received conventional care. The cognitive function, emotional state (mood, depression), activities of daily living, and problematic behaviours of the two groups were compared at three points (before the study, at week 4, and at week 8).
Results
The results showed significant group‐by‐time interactions of cognitive function (P < 0.001), mood status (P < 0.001), depressive symptoms (P < 0.01), degrees of activities of daily living (P < 0.001), and problematic behaviours (P < 0.001). There were no significant group differences, but significant time differences were observed in cognitive function (P < 0.001), mood status (P < 0.05), degrees of activities of daily living (P < 0.01), and problematic behaviours (P < 0.05).
Conclusion
The findings of the study suggest the adoption of animal‐assisted therapy in the daily care of older adults with dementia for improving their psychological and behavioural problems.
Keywords: animal‐assisted therapy, dementia, intervention study, older adults, pet therapy
INTRODUCTION
Dementia causes numerous physical and psychological symptoms that result in the loss of independence and human dignity.1 The main symptoms of dementia are depression, anxiety, psychomotor agitation, aggressiveness, shouting, wandering, hyperactivity, apathy, delusion, and hallucination.1 These behavioural and psychological symptoms significantly decrease the quality of life of those with dementia and their families while increasing hospital admissions and their families' burden of care.2, 3 Many drugs used for dementia alleviate problematic behaviours but not the underlying causes.4 Long‐term use of these drugs causes side‐effects and increases mortality.5, 6 Therefore, non‐pharmacological interventions to improve cognitive function, behavioral symptoms, and psychological symptoms should be considered before any pharmacological intervention, which requires close monitoring for side effects.
Researchers have reported on the development and efficacy of various non‐pharmacological interventions, such as music, art, cultural activities, recreation, physical activities, aromatherapy, and massage.7, 8, 9, 10, 11 Animal‐assisted therapy (AAT) programmes using live animals have been reported to be effective in enhancing the quality of life of patients with dementia, but AAT has been relatively less explored than other non‐pharmacological therapies.12 AAT is a professional, complementary alternative therapy using animals that meet specific standards established by animal‐mediated psychological counsellors.13 The therapy utilizes the solidarity between human and animals to enhance humans' bodily, social, emotion, and cognitive functions.13, 14 The programmes have been effective at enhancing self‐efficacy, promoting socialization, improving cognition, increasing physical activity, and relieving irritability, depression, and problematic behaviours.13, 14, 15
The two studies on AAT conducted in Korea used pet insects for older adults and for mentally disordered children.16, 17 The findings supported the advantages of the therapy for improving depression and cognitive functions in older adults and improving children's emotional health.16, 17 Although the therapy had a small to medium effect, it was cost‐effective and safe relative to other therapies.16
The purpose of the present study was to investigate the effects of an AAT programme using dogs, a familiar domestic pet in Korea, on cognitive function, emotional status, problematic behaviours, and activities of daily living (ADL) in older adults with moderate dementia. The programme used live dogs managed by an animal‐mediated psychological counsellor. This study is significant because it is the first to employ dogs for AAT in older Korean adults and to explore the effects of this type of therapy.
METHODS
Study design and participants
A nonequivalent control group pretest and post‐test design was used in this study. Attachment theory served as the basis for this study.18 Animals, especially pets, are often regarded as family members who provide a sense of security and support.19 Companion animals have been shown to offer psychological health benefits, such as reducing anxiety and providing emotional support.20 Based on this attachment theory, the study variables were determined: the independent variable was participation in the AAT programme, and the dependent variables were levels of cognitive function, emotional condition (mood, depression), ADL, and problematic behaviours (Fig. 1).
Figure 1.
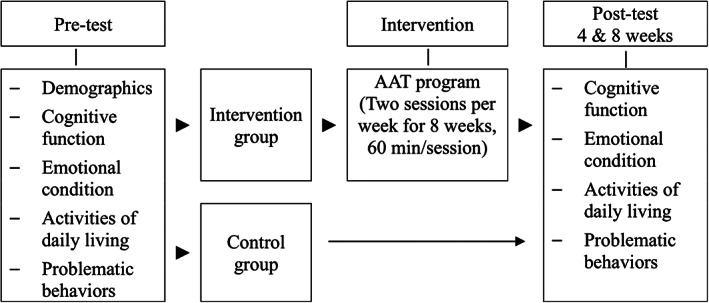
Research design of the study. The intervention group received animal‐assisted therapy (AAT) programme for 8 weeks. Participants from both intervention and control groups were assessed for their cognitive function, emotional state (mood, depression), activities of daily living, and problematic behaviours.
The study participants were adults who were older than 65 years of age and had been diagnosed with dementia. The participants were recruited from two hospitals in Yeoju, Gyeonggi Province, South Korea, and those who met the inclusion criteria were enrolled in the study. The inclusion criteria were as follows: (i) a score <10–19 on the Korean version of the Mini‐Mental Status Examination (MMSE‐K); (ii) the ability to read, hear, and communicate; (iii) no neurological or psychological diagnosis other than dementia; (iv) no allergy to dog fur; and (v) consent to participate in the study. Informed consent was obtained from either the patients or their caregivers. To minimize the risk of treatment contamination, the study employed the cluster randomization method. The coin‐toss method was used to randomly assign one hospital to the intervention group and the other to the control group. The project investigator enrolled the participants with the collaborating physicians at each hospital.
The sample size of 14 for each group was calculated using G*Power (3.1.9.2)21 based on a power of 0.80, alpha level of 0.05, and effect size of 0.25. To address possible dropouts, 18 participants were recruited for each group. Four participants from the intervention group dropped out of the study because of unexpected falls, hospital discharge, and pneumonia, and four participants from the control group dropped out because of hospital discharge, pneumonia, and sepsis. Data from 28 participants (14 from each group) were analyzed.
Intervention
The intervention group participated in an AAT programme consisting of two 60‐min sessions weekly for 8 weeks. The programme was developed by the study's primary investigator, an animal‐assisted psychotherapist, and a professor from the Graduate School of Health & Complementary Medicine at the Wonkwang University. In previous studies, programme length ranged from 3 days to 12 weeks,22, 23, 24 but based on the participating hospitals' availability, we set the length of this study at 8 weeks. The effects of the intervention were assessed at weeks 4 and 8.
The 16 AAT sessions were grouped into three stages: the introductory, developmental, and final stages. In the introductory stage, the relationships between the therapy dogs and the study participants were built by introducing the dogs and encouraging the participants to hug and name them. The aims of the developmental stage were to enhance interaction, emotional stability, ADL, and cognitive function and to reduce problematic behaviour as the participants became more familiar with the therapy dogs, trimmed their fur, trained and walked them, and talked about feelings with them. The purpose of the final stage was to minimize the psychological problems that the participants might experience after separation from the therapy dogs after completing AAT. The activities in this stage included taking pictures, recalling, giving home‐made snacks to the therapy dogs, making photo albums, awarding mock diplomas, and holding a final party (Table 1).
Table 1.
Components of animal‐assisted therapy programme
| Phase | Purpose | Contents | Session | Duration(min) |
|---|---|---|---|---|
| Introduction | To prevent infection and form affinity |
Hand washing Greeting |
1–16 | 10 |
| Main programme | To establish relationships with assistance dogs |
Introducing assistance dogs Making name tags for the dogs |
1–2 | 40 |
|
To enhance interaction and emotional stability To improve cognitive function To decrease behavioural problems, depressed feelings To improve physical functions To improve sensibility |
Memorizing the name of assistance dogs Grooming the assistance dogs Training assistance dogs Answering to quiz (contents from previous class) Sharing your feelings Ball playing with the dogs Speed gaming with the dogs Making snacks for the dogs Taking a walk with the dogs |
3–12 | ||
| To form trust and enhance emotional stability |
Spending enough time with the dogs Making photo album with the dogs Awarding certificates for participating in the therapy Enjoying farewell party |
15–16 | ||
| Closing |
To prevent infection To close the session |
Sharing thoughts about the session with participants Explaining next session Hands washing |
1–16 | 10 |
The AAT program team consisted of the project investigator, three animal mediation psychologists, two hospital social workers and administrators, one research assistant, and one pet partner. The animal mediation psychologist performed the overall management of the AAT programme and kept observational logs of the participants. The pet partner was responsible for the overall management of the AAT programme and the vaccination and hygiene management of the therapy dogs. The hospital administrators videotaped the sessions, while the social workers and research assistants helped ensure the participants' safety, for example, by assisting those using walkers or wheelchairs to move and use the restrooms. The AAT programme was conducted in a space large enough for the intervention group and therapy dogs (i.e. 99 m2).
The selection criteria for the therapeutic dog were as follows: (i) free of all veterinary infectious diseases; (ii) no abnormal findings in comprehensive health check‐ups; (iii) completion of all necessary vaccinations; (iv) moderate grades on aptitude and technical tests; and (v) participation in previous therapy programmes. The pet partners handled preparation and management of the therapy dogs, which were bathed and sanitized at least 24 h before the programme.
Instruments
The first outcomes of the study were cognitive function, emotional state, depression, ADL, and problem behaviours. To assess cognitive function, the Korean version of the Mini‐Mental Status Examination, a modified version of Mini‐Mental Status Examination, was used.25 The tool collectively measures orientation, memory, attention, calculation ability, language understanding, and judgement. The tool has seven categories: time orientation (5 points), place orientation (5 points), memory registration (3 points), memory recall (3 points), attention and calculation ability (5 points), language (7 points), and understanding and comprehension ability (2 points). Scores range between 0 and 30 points. Scores of more than 24 points are defined as definitively normal, 20–23 as suspected cognitive dysfunction and 19 or less as definitive cognitive impairment. In this study, the reliability Cronbach's α for reliability was 0.84.
Emotional state was inferred by assessing the participants' mood state and degree of depression. Mood state was measured using the Faces Rating Scale developed by Wong and Baker.26 The facial scale consists of six pictures showing facial expressions from a smiling face (most comfortable face) to a crying face (most unpleasant face). The scale ranges from 0 (‘I am feeling good’) to 5 (‘I am not feeling good’). Lower scores indicate better moods. The reliability of the instrument in this study had a Cronbach's α of 0.84.
Depression was measured using the Korean version of the Cornell Scale for Depression in Dementia (CSDD), which was translated by the Korean Association of Dementia.27 This 19‐item has a 3‐point Likert scale generating scores of 0–38 points. Scores of 8 or higher indicate a state of depression, and higher scores indicate higher levels of depression. The Cronbach's α for the instrument's reliability in this study was 0.91.
ADL were assessed using the physical function items in the hospital's inpatient evaluation tool. The items cover 10 daily activities: dressing and undressing, face washing, brushing teeth, taking a bath, having a meal, changing positions, sitting up, changing seats, getting out of a room, and using the bathroom. The scores range from 0 (full independence) to 4 (requiring full assistance), with lower scores indicating greater independence. The Cronbach's α for the reliability of this instrument in this study was 0.96.
A problem behaviour assessment tool was used to measure the degrees of behavioural problems in older adults with dementia.28 This 25‐item tool has six domains: aggression (six items), nervousness (six items), resistance to nursing (five items), physical symptoms (three items), neurological symptoms (three items), and psychiatric symptoms (two items). The frequency of problematic behaviours over the course of a week was observed, and 0 points were given if no behaviours occurred, one point for rarely, second points for often (2–3 times), and three points for always (more than three times). The scores range from 0 to 75, and higher scores indicate higher frequency of problematic behaviours. This tool had a Cronbach's α 0.88 for this study.
Data collection and analysis
Data collection took place from February to April 2015. After the Institutional Review Board (MC14FASI0118) granted approval, the purpose of the study was explained to the directors of the two hospitals with which the research team members were affiliated. To increase the reliability of the study, one hospital was assigned to the intervention group, and the other the control group. Agreement to participate in the study was secured by the hospital directors, nursing department, and the doctors of potential participants. Written informed consent was provided by the patients and their caregivers. The participants' cognitive function, emotional status, ADL, and problematic behaviour were assessed at weeks 4 and week 8 of the study. The three research assistants, who were fourth‐year nursing students, received training in correct usage of the measurement tools and conducted the data collection without knowing which hospitals were assigned to the intervention and control groups.
The study data were analysed using SPSS ver. 21.0. (IBM Corp., Armonk, NY) Chi–test and t‐test were used to verify the homogeneity between the two groups. Repeated measures analysis of variance (anova) was performed to evaluate the effects of the AAT programme. Effect sizes for anovas are reported as partial omega effect size (ω p 2 ) calculation.29 The value over 0.01 indicates small, over 0.059 indicates medium, and over 0.138 indicates large effect size.30
RESULTS
Homogeneity verification
The intervention and control groups showed no significant differences in their general characteristics, such as age, sex, admission period, hospitalization period, and experience of having pets. Cognitive function, emotional status, and ADL measured before the intervention showed significant differences between the two groups (Table 2).
Table 2.
Baseline Information Between Animal‐assisted Therapy Group and Control group
| Characteristics | AAT group (M ± SE or n%) | Control group (M ± SE or n%) | t / X 2 | P | |
|---|---|---|---|---|---|
| Age(year) | 82.3 ± 1.8 | 82.1 ± 1.2 | 0.89 | 0.382 | |
| Gender | Male | 10 (71.4) | 12 (85.7) | 0.84 | 0.648 |
| Female | 4 (28.6) | 2 (14.3) | |||
| Length of hospitalization (year) | 2.64 ± 0.3 | 2.57 ± 0.3 | 0.15 | 0.382 | |
| Experience of keeping pets (Yes) | Yes | 11 (78.6) | 11 (78.6) | 0.0 | >0.999 |
| No | 3 (21.4) | 3 (21.4) | |||
| Cognitive function | MMSE‐K (score) | 14.60 ± 1.6 | 14.95 ± 2.5 | 0.08 | 0.932 |
| Emotion state | FRS(score) | 2.42 ± 1.0 | 2.35 ± 1.0 | 0.18 | 0.853 |
| CSDD(score) | 9.64 ± 4.9 | 9.35 ± 0.1 | 0.16 | 0.869 | |
| ADL(score) | 12.50 ± 8.8 | 12.30 ± 7.3 | 0.07 | 0.945 | |
| MBPC(score) | 8.14 ± 4.2 | 8.21 ± 3.1 | 0.05 | 0.960 | |
Note. AAT, Animal‐assisted Therapy; M, Mean; SE, Standard Error; MMSE‐K, Korean version of Mini Mental Status Examination; FRS, Faces Rating Scale; CSDD, Cornell Scale of Depression in Dementia; ADL, Activities of Daily Living; MBPC, Memory & Behavior Problems Checklist.
Cognitive function
For cognitive function, the intervention group scored 14.64 points before the study, 14.71 points at week 4, and 15.50 points at week 8, while the control group scored 14.57 points initially, 14.50 points at week 4, and 14.42 points at week 8. There was a significant interaction between the groups and time of measurement for cognitive function (P < 0.001). Although the differences between the groups were not significant (P = 0.587), there were significant differences observed by time of measurement (P < 0.001). The effect size was small for group (ω p 2 = 0.03), large for time (ω p 2 = 0.41), and large for group and time interaction (ω p 2 = 0.39) (Table 3).
Table 3.
Changes in study variables between animal‐assisted therapy group and control group
| Variable | Group | Baseline (M ± SE) | 4 Weeks (M ± SE) | 8 Weeks (M ± SE) | Source | F | p | ω p 2 |
|---|---|---|---|---|---|---|---|---|
| MMSE‐K |
AAT Control |
14.64 ± 0.45 14.57 ± 0.69 |
14.71 ± 0.43 14.50 ± 0.66 |
15.50 ± 0.45 14.42 ± 0.67 |
G T G*T |
0.30 8.22 14.15 |
0.587 <0.001 <0.001 |
0.03 0.41 0.39 |
| FRS |
AAT Control |
2.42 ± 0.27 2.35 ± 0.26 |
2.14 ± 0.25 2.42 ± 0.27 |
1.57 ± 0.17 2.57 ± 0.17 |
G T G*T |
1.77 4.68 13.00 |
0.194 0.014 <0.001 |
0.07 0.30 0.36 |
| CSDD |
AAT Control |
9.64 ± 1.32 9.35 ± 1.09 |
7.92 ± 4.06 10.92 ± 1.57 |
6.35 ± 4.06 10.71 ± 1.27 |
G T G*T |
2.04 1.81 8.94 |
0.165 0.184 0.002 |
0.09 0.07 0.27 |
| ADL |
AAT Control |
12.57 ± 2.35 12.28 ± 1.97 |
11.28 ± 2.33 13.07 ± 2.15 |
9.07 ± 2.10 13.42 ± 2.07 |
G T G*T |
0.4 11.23 39.38 |
0.529 0.001 <0.001 |
0.04 0.50 0.65 |
| MBPC |
AAT Control |
8.14 ± 1.13 8.21 ± 0.85 |
7.85 ± 1.08 8.50 ± 0.91 |
7.07 ± 0.96 8.64 ± 0.89 |
G T G*T |
0.30 4.40 18.31 |
0.586 0.042 <0.001 |
0.03 0.25 0.45 |
Note. AAT, Animal‐assisted Therapy; G, Group; T, Time; G*T, Group*Time; M, Mean; SE, Standard error; MMSE‐K, Korean version of Mini Mental Status Examination; FRS, Faces Rating Scale; CSDD, Cornell Scale of Depression in Dementia; ADL, Activities of Daily Living; MBPC, Memory & Behavior Problems Checklist.
Emotional state
Mood
On the facial expression scale, the intervention group scored 2.42 points initially, 2.14 points at week 4, and 1.57 points at week 8, while the control group scored 2.35 points initially, 2.42 points at week 4, and 2.57 points at week 8. There was a significant interaction between groups and time of measurement for mood state. There were no significant differences between the groups (P = 0.194), but significant differences were observed between the measurement time points (P < 0.014). The effect size was medium for group (ω p 2 = 0.07), large for time (ω p 2 = 0.30), and large for group and time interaction (ω p 2 = 0.36) (Table 3).
In addition to using the scale, the research team observed several cues during the AAT. Participants who engaged in little or no spontaneous conversations during the first session smiled more as the sessions proceeded. During the emotion handling component of the seventh session, most participants chose the cards for ‘happy’ and ‘I love you,’ showing improved mood state. The interactions among the participants also improved as they were observed conversing about their past pets while smiling and showing affection to and hugging the therapy dogs.
Depression
On the CSDD, the intervention group scored 9.64 points initially, decreasing to 7.92 points at week 4 and 6.35 points at week 8. The control group scored 9.35 points initially, 10.92 points at week 4, and 10.71 points at week 8. There was a significant interaction between groups and time of measurement for the depressive symptoms (P < 0.002). There were no significant differences between the groups (P = 0.165) or by time of measurement (P = 0.184). The effect size was medium for group (ω p 2 = 0.09), medium for time (ω p 2 = 0.07), and large for group and time interaction (ω p 2 = 0.27) (Table 3).
Activities of daily living
For ADL, the intervention group scored 12.57 points initially, 11.28 points at week 4, and 9.07 points at week 8, while the control group scored 12.28 points initially, 13.07 points at week 4, and 13.42 points at week 8. There was a significant interaction between groups and time of measurement (P < 0.001). There were no significant differences between the groups (P = 0.529), but there were significant differences by time of measurement (P = 0.001). The effect size was small for group (ω p 2 = 0.04), large for time (ω p 2 = 0.50), and large for group and time interaction (ω p 2 = 0.65) (Table 3).
Problematic behaviours
For occurrence of problematic behaviours, the intervention group scored 8.14 points initially, 7.85 points at week 4, and 7.07 points at week 8, while the control group scored 8.21 points initially, 8.50 points at week 4, and 8.64 points at week 8. There was a significant interaction between the groups and time of measurement for problematic behaviours (P < 0.001). There were no significant differences between the groups (P = 0.586), but there were significant differences by time of measurement (P = 0.042) The effect size was small for group (ω p 2 = 0.03), small for time (ω p 2 = 0.25), and large for group and time interaction (ω p 2 = 0.45) (Table 3).
DISCUSSION
This study assessed the efficacy of an 8‐week AAT programme at improving cognitive function, emotional state, ADL, and problematic behaviour in older adults with dementia. Based on the study findings, four suggestions were made for clinical practice.
First, when using AAT for improving cognitive function, a longer therapy period is necessary. The results showed significant interaction between groups and time of measurement for cognitive function and significant interaction by time of measurement. We intended to observe the effects of the AAT program at week 4 by measuring changes in cognitive function. There was a difference by time of measurement, but after analysis with a corrected P‐value using the Bonferroni method, there was no significant effect at week 4. Considering that previous studies showed improved depressive symptoms after 6 weeks of one‐hour AAT sessions for older adults,24 a 4‐week period does not seem sufficient to result in improvements.
Second, AAT can be considered an effective therapy for enhancing emotional status. In this study, AAT intervention resulted in positive effects in terms of improving the mood state of older adults with dementia. In addition, by analysing the observational logs and video‐recordings of the 16 sessions, the research team could conclude that the interactions between the therapy dogs and participants in the intervention group increased throughout the sessions. Most participants smiled and hugged the therapy dogs and even laughed out loud when the dogs followed instructions (e.g., shake, sit). These findings supported previous studies that reported that AAT had positive effects, such as improving older adults' cognitive functions and overall quality of life,31 relieving social isolation and boredom,32 and reducing anxiety.20
Moreover, the depressive symptoms of the participants in this study significantly decreased, supporting an earlier study that reported an AAT programme alleviated depression in older adults with dementia.33 The participants' nurses and caregivers in this study reported that, even on days when AAT was not scheduled, the participants experienced better moods and expressed interest in the programme, calling the names of the therapy dogs and asking when they were coming. Depression was believed to be lessened because therapy dogs readily understand the participants' body language and provide an enjoyable time, forming a rapport with the participants through appropriate reactions. Thus, AAT programmes are expected to ultimately improve the quality of life of older adults by reducing anxiety and depression, which are often seen in older adults with dementia.32 These findings are consistent with a previous study implementing AAT on persons with Alzheimer's disease in Portland.34 The participants reported statistically significant improvements in feelings of excitement and interest.34
Third, AAT provides physical benefits for older adults. The ADL of the intervention group were enhanced, possibly as a result of increased physical activity, such as feeding, combing, hugging, and walking the dogs. The findings were similar in a previous study exploring the physical benefits of AAT in a critical care setting, where the space for physical activities is limited.35 Studies conducted in Brazil36 and Canada37 examining the physical effects of ATT have also shown improvement in motor dysfunction, balance, gait pattern, and walking speed.
Fourth, AAT can be considered for the nonpharmacological management of problematic behaviours in older adults with dementia. The findings of the study which showed the lowering of occurrence of problematic behaviours in the intervention group supported previous research reporting that AAT decreased behavioural problems, aggression, and irritability in older adults with dementia.13, 31 Analysis of the videos and observation logs throughout the 16 AAT sessions revealed that the participants' indifference toward their surroundings, a major problematic behavior in older adults with dementia, decreased. Participants with little emotional expression started to show interest in the therapy dogs, voluntarily communicated, and actively participated in the programme by calling the dog's names and hugging them. Most participants showed affection to the therapy dogs and found security through physical contact with them. Therefore, it can be concluded that the AAT programme was an effective method to reduce problematic behaviours among older adults with dementia. Similar results were obtained in prior studies showing that AAT reduces agitated behaviours in persons with Alzheimer's disease.34 Since pharmacological management for problematic behaviours often has numerous side effects, AAT should be considered a good alternative treatment.
Conclusion
Based on the study findings, we concluded that the 8‐week AAT programme was effective at improving cognitive function, emotional state, ADL, and problematic behaviours among older adults with moderate dementia. However, the study has several limitations. First, the study is limited by its small sample size. Although the sample size was sufficient for the power of the study, further studies with larger sample sizes are necessary to improve the generalizability of the study's findings. Second, the study is limited by its analytical method. Performing post hoc tests with anova could have specified between which time points (i.e. initial, 4‐week, or 8‐week) the study's variables significantly differed. Third, the study poses a risk of response bias. Considering the participants of this study were older adults with dementia, some of the responses could have been inaccurate. By including more subjective and objective data, the study could have enhanced its validity.
When older adults have contact with pets under safe circumstances, including protection against allergies and infections, not only their quality of life improves, but the psychological, physical, and economic burdens of their supporting families are also lessened. Based on the positive effects observed in this study, future studies to validate, specify (e.g., intervention period, human–animal ratio), and diversify (e.g., degrees of dementia, clinical settings) the AAT programme are urged. We expect this study to serve as baseline data for future AAT studies and suggest AAT as a possible intervention in caring for older adults with dementia.
COMPLIANCE WITH ETHICAL STANDARDS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (XC17QEDI0080) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
DATA SHARING AND DATA ACCESSIBILITY
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
ACKNOWLEDGMENTS
The authors would like to thank the medical staffs from the two hospitals for their assistance in data collection.
Disclosure: The authors have no conflicts of interest to report and received no funding for this study.
This study was conducted at Yeoju Geriatric Hospital (Yeoju, South Korea) and CandO Hospital (Yeoju, South Korea)
References
- 1. Jutkowitz E, Brasure M, Fuchs E et al Care‐delivery interventions to manage agitation and aggression in dementia nursing home and assisted living residents: a systematic review and meta‐analysis. J Am Geriatr Soc 2016; 64: 477–488. [DOI] [PubMed] [Google Scholar]
- 2. Perales J, Cosco TD, Stephan BCM, Haro JM, Brayne C. Health‐related quality‐of‐life instruments for Alzheimer's disease and mixed dementia. Int Psychogeriatr 2013; 25: 691–706. [DOI] [PubMed] [Google Scholar]
- 3. Papastavrou E, Kalokerinou A, Papacostas SS, Tsangari H, Sourtzi P. Caring for a relative with dementia: family caregiver burden. J Adv Nurs 2007; 58: 446–457. [DOI] [PubMed] [Google Scholar]
- 4. Thompson Coon J, Abbott R, Rogers M et al Interventions to reduce inappropriate prescribing of antipsychotic medications in people with dementia resident in care homes: a systematic review. J Am Med Dir Assoc 2014; 15: 706–718. [DOI] [PubMed] [Google Scholar]
- 5. Tjia J, Rothman MR, Kiely DK et al Daily medication use in nursing home residents with advanced dementia. J Am Geriatr Soc 2010; 58: 880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cadwell S, Dearmon V, VandeWaa EA. Reducing falls in residents with dementia by reducing psychotropic medication use: does it work? J Nurse Pract. 2017;13:e191–e194. [Google Scholar]
- 7. Petrovsky D, Cacchione PZ, George M. Review of the effect of music interventions on symptoms of anxiety and depression in older adults with mild dementia. Int Psychogeriatr 2015; 27: 1661–1670. [DOI] [PubMed] [Google Scholar]
- 8. Camic PM, Tischler V, Pearman CH. Viewing and making art together: a multi‐session art‐gallery‐based intervention for people with dementia and their carers. Aging Ment Health 2014; 18: 161–168. [DOI] [PubMed] [Google Scholar]
- 9. Li DM, Li XX. The effect of folk recreation program in improving symptoms: a study of Chinese elder dementia patients. Int J Geriatr Psychiatry 2017; 32: 901–908. [DOI] [PubMed] [Google Scholar]
- 10. Blankevoort CG, Van Heuvelen MJG, Boersma F, Luning H, De Jong J, Scherder EJA. Review of effects of physical activity on strength, balance, mobility and ADL performance in elderly subjects with dementia. Dement Geriatr Cogn Disord 2010; 30: 392–402. [DOI] [PubMed] [Google Scholar]
- 11. Yoshiyama K, Arita H, Suzuki J. The effect of aroma hand massage therapy for people with dementia. J Altern Complement Med 2015; 21: 759–765. [DOI] [PubMed] [Google Scholar]
- 12. Flynn R, Roach P. Animal‐assisted therapy in dementia care: a critical appraisal of evidence. J Dementia Care 2014; 22: 32–36. [Google Scholar]
- 13. Nordgren L, Engström G. Animal‐assisted intervention in dementia: effects on quality of life. Clin Nurs Res 2014; 23: 7–19. [DOI] [PubMed] [Google Scholar]
- 14. Mossello E, Ridolfi A, Mello AM et al Animal‐assisted activity and emotional status of patients with Alzheimer's disease in day care. Int Psychogeriatr 2011; 23: 899–905. [DOI] [PubMed] [Google Scholar]
- 15. Sellers DM. The evaluation of an animal assisted therapy intervention for elders with dementia in long‐term care. Act Adapt Aging 2006; 30: 61–77. [Google Scholar]
- 16. Ko HJ, Youn CH, Kim SH, Kim SY. Effect of pet insects on the psychological health of community‐dwelling elderly people: a single‐blinded, randomized. Contr Trial Gerontol 2016; 62: 200–209. [DOI] [PubMed] [Google Scholar]
- 17. Jun YS, Bae SM, Shin TY et al Effects of an insect‐mediated mental healthcare program for mentally disordered children. Entom Res 2016; 46: 85–90. [Google Scholar]
- 18. Geist TS. Conceptual framework for animal assisted therapy. Child Adolesc Social Work J 2011; 28: 243–256. [Google Scholar]
- 19. Compitus K. Traumatic pet loss and the integration of attachment‐based animal assisted therapy. J Psychoth Integr 2019; 29: 119–131. [Google Scholar]
- 20. Meehan M, Massavelli B, Pachana N. Using attachment theory and social support theory to examine and measure pets as sources of social support and attachment figures. Anthrozoos 2017; 30: 273–289. [Google Scholar]
- 21. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2017; 39:175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 22. Colombo G, Buono MD, Smania K, Raviola R, De Leo D. Pet therapy and institutionalized elderly: a study on 144 cognitively unimpaired subjects. Arch Gerontol Geriatr 2006; 42: 207–216. [DOI] [PubMed] [Google Scholar]
- 23. Lynch CE, Magann EF, Barringer SN et al Pet therapy program for antepartum high‐risk pregnancies: a pilot study. J Perinatol 2014; 34: 816–818. [DOI] [PubMed] [Google Scholar]
- 24. Stasi MF, Amati D, Costa C et al Pet‐therapy: a trial for institutionalized frail elderly patients. Arch Gerontol Geriatr 2004; 38: 407–412. [DOI] [PubMed] [Google Scholar]
- 25. Lee JY, Dong Woo L, Cho SJ et al Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal cognitive assessment. J Geriatr Psychiatry Neurol 2008; 21: 104–110. [DOI] [PubMed] [Google Scholar]
- 26. Ware LJ, Epps CD, Herr K, Packard A. Evaluation of the revised faces pain scale, verbal descriptor scale, numeric rating scale, and Iowa pain thermometer in older minority adults. Pain Manag Nurs 2006; 7: 117–125. [DOI] [PubMed] [Google Scholar]
- 27. Lim HK, Hong SC, Won WY, Hahn C, Lee CU. Reliability and validity of the Korean version of the Cornell scale for depression in dementia. Psychiatry Investig 2013; 10: 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim K. Development of an assessment tool of problematic behaviors for institutionalized old people with dementia. (Unpublished doctoral dissertation). In press, 2003.
- 29. Olejnik S, Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol Methods 2003; 8: 434–447. [DOI] [PubMed] [Google Scholar]
- 30. Finch WH, French BF. A comparison of methods for estimating confidence intervals for omega‐squared effect size. Educ Psychol Meas 2012; 72: 68–77. [Google Scholar]
- 31. Moretti F, De Ronchi D, Bernabei V et al Pet therapy in elderly patients with mental illness. Psychogeriatrics 2011; 11: 125–129. [DOI] [PubMed] [Google Scholar]
- 32. Cherniack EP, Cherniack AR. The benefit of pets and animal‐assisted therapy to the health of older individuals. Curr Gerontol Geriatr Res 2014; 2014: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walsh PG, Mertin PG, Verlander DF, Pollard CF. The effects of a ‘pets as therapy’ dog on persons with dementia in a psychiatric ward. Aust Occup Ther J 1995; 42: 161–166. [Google Scholar]
- 34. Richeson NE. Effects of animal‐assisted therapy on agitated behaviors and social interactions of older adults with dementia. Am J Alzheimers Dis Other Demen 2003; 18: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rugari SM, Hunter CL, Carswell BM. Animal‐assisted therapy and activities in the critical care setting. Nurs Crit Care 2017; 12: 32–40. [Google Scholar]
- 36. Beinotti F, Correia N, Christofoletti G, Borges G. Use of hippotherapy in gait training for hemiparetic post‐stroke. Arq Neuropsiquiatr 2010; 68: 908–913. [DOI] [PubMed] [Google Scholar]
- 37. Rondeau L, Corriveau H, Bier N, Camden C, Champagne N, Dion C. Effectiveness of a rehabilitation dog in fostering gait retraining for adults with a recent stroke: a multiple single‐case study. NeuroRehabilitation 2010; 27: 155–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


