Abstract
The present review focuses on the multi-faceted effects of curcumin on the neurobiology glioblastoma multiforme (GBM), with a special emphasis on autophagy (ATG)-dependent molecular pathways activated by such a natural polyphenol. This is consistent with the effects of curcumin in a variety of experimental models of neurodegeneration, where the molecular events partially overlap with GBM. In fact, curcumin broadly affects various signaling pathways, which are similarly affected in cell degeneration and cell differentiation. The antitumoral effects of curcumin include growth inhibition, cell cycle arrest, anti-migration and anti-invasion, as well as chemo- and radio-sensitizing activity. Remarkably, most of these effects rely on mammalian target of rapamycin (mTOR)-dependent ATG induction. In addition, curcumin targets undifferentiated and highly tumorigenic GBM cancer stem cells (GSCs). When rescuing ATG with curcumin, the tumorigenic feature of GSCs is suppressed, thus counteracting GBM establishment and growth. It is noteworthy that targeting GSCs may also help overcome therapeutic resistance and reduce tumor relapse, which may lead to a significant improvement of GBM prognosis. The present review focuses on the multi-faceted effects of curcumin on GBM neurobiology, which represents an extension to its neuroprotective efficacy.
Keywords: curcuma longa, natural polyphenols, neuroprotection, anti-cancer effects, glioblastoma stem-like cells, autophagy
1. Introduction
Curcumin is a natural polyphenol extracted from the rhizome of Curcuma longa; it is also known as turmeric [1] and was introduced to Europe in the 14th century as a culinary spice [2]. This natural compound has been used for centuries in the traditional Indian Ayurvedic and Chinese medicine for treating respiratory and liver disorders, infections, allergies, and rheumatisms [3]. Nowadays, curcumin is widely used due to its multiple biological activities, encompassing anti-inflammatory, anti-oxidant, scavenging reactive oxidative species (ROS), regulation of mitochondrial homeostasis, stem cell modulation, and neurogenesis [4]. Hence, curcumin may be useful in a wide range of human diseases, encompassing cardiovascular [5,6], inflammatory [7,8], and metabolic disorders [9,10,11].
In particular, in the last decades, phytochemicals, and especially curcumin, have gathered increasing interest in both experimental and pre-clinical studies for their beneficial effects on the central nervous system (CNS). Among dietary polyphenolic compounds, curcumin appears as a useful agent for adjunct therapy in a variety of neurogenerative disorders (NDDs) [4,12]. In fact, curcumin appears to exert multiple neuroprotective effects through its strong anti-oxidant, anti-inflammatory, and anti-protein aggregation properties [13]. Moreover, increasing evidence argues for curcumin’s anti-proliferative, anti-invasive, anti-angiogenic, and chemo-preventive potential, which suggest a potential usefulness in neoplasms [14]. In line with this, numerous studies demonstrated the chemo- and radio-sensitizing effects of curcumin in various cancers, including glioblastoma multiforme (GBM) [15,16,17,18,19].
The pleiotropic therapeutic activities of curcumin rely on its ability to influence various signaling pathways [20]. In fact, curcumin operates in multiple ways by modulating the activity of kinases and transcription factors as well as by regulating the expression of genes involved in cell survival and malignant transformation [20]. The ability of curcumin to target different pathways is bound to its unique and complex chemical structure. In fact, this natural polyphenol, also known as diferuloylmethane (1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-hepadiene-3,5-dione; C21H20O6), possesses three reactive functional groups, namely 1,3-diketone moiety and two phenolic groups. The latter are key for curcumin’s multiple biological activities [21], which render this natural bioactive compound an attractive and flexible therapeutic option that was tested in experimental models of neurodegeneration and glioblastoma multiforme (GBM).
It is remarkable that the beneficial effects induced by curcumin in GBM are tightly associated with neuroprotective activities in a variety of neurological insults. Within this frame, the onset and progression of GBM is considered for many instances as reminiscent of an accelerated variant of neurodegeneration. This is key to comprehend the common background based on mammalian target of rapamycin (mTOR), which is a ubiquitously expressed serine-threonine kinase, which controls major processes such as cell growth, proliferation, metabolism, and protein degradation pathways such as autophagy (ATG). Remarkably, mTOR dysfunction and related ATG alterations are described in a variety of neurodegenerative conditions while being powerfully manifest in GBM [22,23].
A brief overview of the wide range of neuroprotective effects of curcumin in neurodegeneration is provided here to link such an activity to curcumin-induced anti-cancer effects in GBM. Such an approach allows discussing the multi-faceted effects of curcumin on GBM neurobiology. Thus, we provide evidence on how curcumin inhibits GBM growth and infiltration through the modulation of ATG-dependent pathways. In this respect, we specifically focus on how curcumin-activated ATG in turn modulates stem-like properties of GBM cancer stem cell (GSCs), while forcing them to undergo differentiation, thus limiting GBM infiltration and volume growth. Novel insights into the mechanisms by which curcumin impacts GSCs tumorigenicity, and thus a GBM malignant phenotype, will contribute to developing novel therapeutic strategies while improving GBM poor prognosis. The last part of the review is focused on the potential of curcumin as a disease-modifier of GBM neurobiology beyond its effect as an ATG inducer.
2. Pleiotropic Effects of Curcumin in the CNS: from Neuroprotection to Anti-Cancer Activities
Among a number of phytochemicals, curcumin has been widely investigated in experimental and clinical studies for its potential benefits to counteract oxidative stress, mitochondrial damage, synaptic dysfunction, neuro-inflammation, and the accumulation of aggregate-prone proteins, which are implicated in various NDDs, including Parkinson’s disease (PD) and Alzheimer’s disease (AD). Far from being independent, these effects converge into common metabolic pathways, which appear to be ubiquitously altered in most NDDs. This is independent of etiology and neuroanatomical site-specificity. This is the case of mTOR-dependent alterations of the ATG pathway, which appears as a recurring feature in NDDs [23,24,25].
Within this frame, it is remarkable that the beneficial effects of curcumin including anti-oxidant, anti-inflammatory, mitochondrial protection, and anti-amyloidogenic activities are bound to the modulation of mTOR-dependent ATG. In fact, by targeting the ATG pathway, curcumin breaks a vicious cycle, which is initiated by ATG defects and impaired proteostasis. This is worsened by protein misfolding, oxidative stress, and neuro-inflammation. All these events further boost the neurodegeneration cascade. A large body of evidence converges in demonstrating that curcumin supplementation confers neuroprotection by rescuing ATG. For instance, curcumin counteracts 6-OHDA-induced cell death in SH-SY5Y cells by enhancing the ATG-dependent clearance of damaged mitochondria and removal of excess ROS [26]. Curcumin also counteracts mitochondrial damage by enhancing mitochondria ATG (i.e., mitophagy), which selectively removes damaged and/or aged mitochondria [27]. As already demonstrated for physical exercise or caloric restriction, which can induce beneficial adaptations for metabolic homeostasis by ameliorating mitochondrial function [28], also, phytochemicals were shown to enhance mitochondrial function, which, in turn, is bound to the ATG-dependent orchestration of mitochondrial dynamics [29]. Furthermore, curcumin-dependent ATG activation suppresses the inflammatory response following neurological insults [30,31]. For instance, curcumin-treated transgenic AD mice show a marked decrease in pro-inflammatory cytokines (i.e., IL-1β, TNF-α, IL-6) levels and microglial activation, which are associated with brain injury and inflammatory processes [32,33]. In contrast, ATG inhibition abrogates the beneficial effects of curcumin [31]. Again, anti-fibrillogenic effects of curcumin in counteracting the aggregation of proteins such as tau, amyloid-beta, and α-synuclein, which occur in NDDs, rely on the very same mTOR inhibition, which in turn produces ATG activation [33,34,35]. For instance, treatment with curcumin reduces the pathological accumulation of mutated (A53T) α-synuclein in dopamine (DA)-containing SH-SY5Y cells through downregulation of the mTOR/p70S6K pathway and thus ATG recovery [36]. Similarly, curcumin counteracts the aggregation of prion protein [37], which besides classic prion disorders is a manifest of neurotoxicant-induced models of PD [38,39]. Since curcumin produces a strong activation of ATG, the potential beneficial effects of such a compound were tested in various ATG deficiencies. This is the case of GBM, which due to a marked mTOR up-regulation, features an occlusion of the ATG pathway. Thus, in further studies, the ability of curcumin in mediating neuroprotective effects through ATG induction took a center stage in cancer research, and particularly in GBM.
According to the World Health Organization (WHO), GBM is the most aggressive and lethal primary brain tumor [40,41]. In fact, despite current advances in neurosurgery and radio- and chemotherapy, the prognosis is still dismal [42]. The biology of this neoplasm is complex. In particular, GBM carries numerous genetic and molecular alterations leading to aberrant signaling pathways that contribute to GBM pathogenesis [43,44]. Therefore, developing therapeutic approaches targeting multiple oncogenic signaling aberrations associated with GBM needs articulated compounds.
Curcumin is reported to produce anti-proliferative, anti-migration, and pro-apoptotic effects in experimental models of GBM [45,46,47,48] (Table 1).
Table 1.
In vitro anti-tumor effects of curcumin on glioblastoma multiforme.
| Cell Line (s) | Dose (s) | Molecular Target (s) | Effect (s) | Reference |
|---|---|---|---|---|
| T98G *, U87MG *, T67 *, C6 ‡ | 25–50 μmol/L | AP-1, NFκB | Reduces cell survival; suppresses chemotherapy resistance | [44] |
| U251 * | 10 μM | p53, ING4, p21 WAF-1/CIP-1 | Inhibits cell growth, induces G2/M cell cycle arrest | [46] |
| A172 *, MZ-18 *, MZ-54 §, MZ-256 §, MZ-304 § | 10 μM, 20 μM and 50 μM | JAK/STAT3 | Inhibits cell proliferation, migration, and invasion | [47] |
| U251 *, SNB19 * | 10 μM, 15 μM | pAkt, p57, Skp2 | Inhibits cell proliferation, migration, and invasion, induces cell cycle arrest and apoptosis | [48] |
| U87MG* | 25 μM, 50 μM | NFκB, IAPs, Smac/Diablo, Bax, Bcl-2, caspase-3 | Decreases cell viability and induces apoptosis | [49] |
| U87 *, U251 * | 2.5 μM (IC50 25 μM) |
STAT3, MAPK IAP, ROS, | Decreases cell viability, inhibits proliferation, sphere-forming ability, and colony-forming potential of glioblastoma stem cells | [50] |
| SU-2 *, SU-3 * | 2 μM | GFAP, Tuj1, Olig2, βIIItubulin, LC3 | Induces ATG and differentiation, while inhibiting self-renewal in glioma-initiating cells (GICs) | [51] |
| U87 * | 10 µM/L, 20 µM/L | STAT3 | Inhibits cell migration and invasion | [52] |
| A172 * | 10 μM | Atg5, Beclin-1 | Induces ATG | [53] |
| U87-MG *, U373-MG * | 20 μM, 40 μM | Akt/mTOR/p70S6K, ERK1/2 | Induces G2/M arrest, inhibits cell growth, induces ATG | [54] |
| U-87MG *, GL261 †, F98 ‡, C6 ‡ | 25 μM | Atg5, Atg7, Beclin-1, LC3A/B, p62, PI3K/Akt/mTOR | Induces ATG | [55] |
| U87 *, U373 *, U138MG *, C6 ‡ | 7.5 μM, 10 μM and 15 μM (IC50 19–28 μM) |
PI3K/Akt, NFκB, caspase-3 | Induces G2/M cell cycle arrest, inhibits cell proliferation | [56] |
| U251 *, U87 * | 10 μM, 20 μM and 40 μM | p-Akt, p-mTOR, PTEN, p53 | Inhibits cell proliferation, migration, and invasion, while inducing apoptosis | [57] |
| SNB19 *, A1207 * | 10 μM, 15 μM and 20 μM | PI3K/Akt, Notch1, NEDD4 | Inhibits cell proliferation, induces cell cycle arrest, inhibits cell migration and invasion | [58] |
* Human glioma cell lines; § Human recurrent GBM cell lines; † Mouse glioma cell lines; ‡ Rat glioma cell lines.
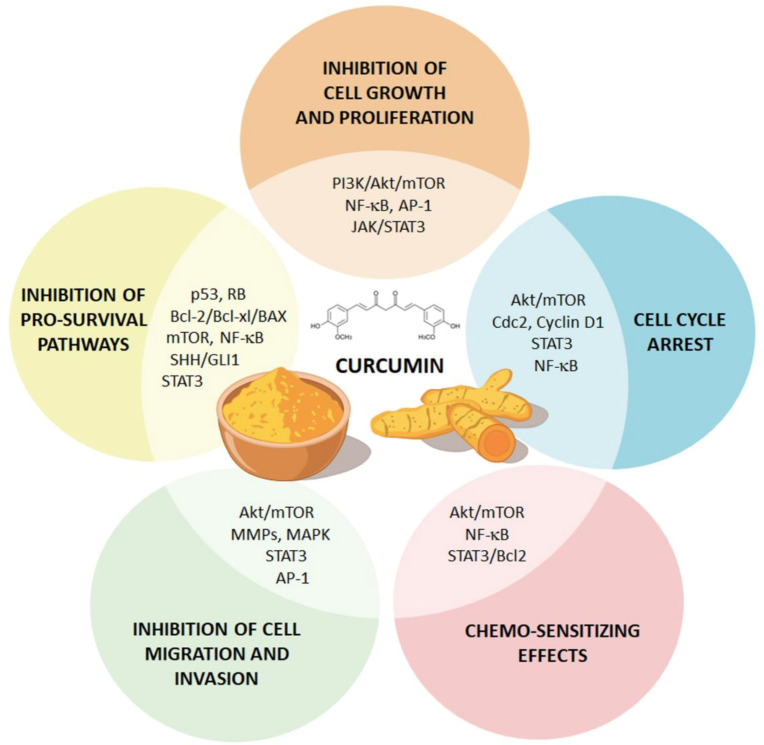
In fact, curcumin modulates the core-signaling pathways of GBM neurobiology, including the nuclear factor κB (NF-κB), activator protein-1 (AP-1), Janus kinase/signal transducers and activators of transcription (JAK/STAT), TP53, and RB, as well as mitogen-activated protein kinase/extracellular-signal-regulated kinase (MAPK/ERK) and phosphoinositide 3-kinases/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) and ATG pathways [44] (Figure 1).
Figure 1.
Curcumin modulates major glioblastoma multiforme (GBM)-associated signaling pathways. The cartoon summarizes the major effects of curcumin on GBM cells. In fact, curcumin was shown to broadly affect core-signaling pathways of GBM neurobiology. For instance, curcumin suppresses tumor growth by inhibiting tumor-promoting pathways (i.e., nuclear factor κB (NF-kB), phosphoinositide 3-kinases/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR), Janus kinase/signal transducers and activators of transcription (JAK/STAT3) and mitogen-activated protein kinase (MAPK) pathways), while up-regulating major tumor-suppressing (i.e., p53 and p21, and caspase).
Consistent with in vitro studies, beneficial effects of curcumin have been reported in several in vivo models of GBM. These encompass the inhibition of cell proliferation and matrix metalloproteinases (MMP)-dependent cell migration and invasion, thus resulting in decreased tumor volume along with increased survival time [49,59,60] (Table 2).
Table 2.
In vivo anti-tumor effects of curcumin on glioblastoma multiforme.
| Model (s) | Cell line (s) and Injection Site | Dose (s) | Effect (s) | Reference |
|---|---|---|---|---|
| Intracranial xenograft | U-87; caudate-putamen | i.p. injection (120 mg/kg) | Increases survival of curcumin-treated mice | [59] |
| Intracranial xenograft | SU-2 and SU-3; caudate nucleus | i.p. injection (300 mg/kg) | Increases survival of curcumin-treated mice | [51] |
| Subcutaneous injection | U87MG; right flank | Intratumoral injection (100 mg/kg) | Inhibits tumor growth and induces ATG | [54] |
| Intracranial xenograft | C6; striatum | i.p. injection (50 mg/kg) | Inhibits tumor growth | [56] |
| Subcutaneous injection | U87; flank | i.p. injection (60 mg/kg) | Decreases tumor volume | [57] |
It is noteworthy that curcumin recently emerges as a promising adjunct therapy against GBM owing to its potential to target GSCs [50,51,52,61,62,63,64,65,66]. This represents a sub-population of cancer cells endowed with stem-like features, such as increased self-renewal, pluripotency, and clonogenic potential [22,39]. Notably, GSCs are responsible for GBM initiation, progression, tumor re-growth after surgical resection, and thus patient relapse [67]. GSCs are resistant to standard treatments [68], which contribute to an inauspicious prognosis for GBM patients. Therefore, strategies aimed at eradicating these cells hold great promises for developing novel therapeutic approaches against GBM.
Among various pathways, which sustain GSCs’ oncogenic properties and metabolism, mTOR-dependent ATG plays a seminal role. While finely tuned mTOR signaling is essential for normal CNS development, GSCs take advantage of an improper mTOR activity to fuel tumor growth and infiltration [69]. In fact, these cells feature a robust up-regulation of mTOR, and thus a marked ATG suppression, which relates to key biological properties, such as increased self-renewal, marked proliferation, and invasion. Hence, depressed ATG is a culprit in contributing to GSCs’ self-renewal and invasion within neighboring tissues. As a proof of concept, low levels of ATG are detected at baseline levels in GMB from patients in vivo, ex vivo, and in patients’ cell cultures [22,70,71,72,73]. The same occurs in experimental models including GBM xenografts and GBM cell lines [69,72,74,75,76,77]. In experimental settings, rescuing ATG associates with an inhibition of cell proliferation and invasion [52,69,72,74,77,78,79].
In this regard, various studies have shown that curcumin exerts anti-cancer activity by inhibiting mTOR signaling, which among various effects activates the ATG pathway [53,54,64,80,81,82]. Curcumin induces ATG trough the modulation of several upstream regulators of the mTOR pathway, such as the adenosine monophosphate-activated protein kinase (AMPK), the phosphatase and tensin homolog (PTEN)/Akt, the IκB kinase β (IKKβ), and the neural precursor cell expressed developmentally down-regulated protein 4 (NEDD4) [83,84,85]. Independently from mTOR upstream molecules, disruption of the mTOR–raptor interaction is another proposed mechanism of curcumin-mediated ATG activation [86]. In the next section, we provide an overview of the experimental studies centered on curcumin-mediated ATG induction as a strategy to combat GSCs in GBM.
3. Curcumin Suppresses GSCs’ Tumorigenicity through ATG Induction
Compelling evidence indicates that curcumin and curcuminoids counteract GBM malignant phenotype and aggressiveness by targeting ATG within GSCs. In this way, curcumin modulates GSC cell biology, including proliferation, viability, migration, and invasion [55,87,88].
Several in vitro studies reported that curcumin effectively prevents GBM cell proliferation through the perturbation of the mTOR pathway. For instance, Aoki et al. (2007) [81] demonstrate that curcumin induces G2/M cell cycle arrest through the induction of mTOR-dependent ATG in two human malignant glioma cell lines, namely U87-MG and U373-MG. This was evidenced by an increase in LC3 immunoblotting, enhanced red fluorescence using flow cytometry after acridine orange (AO) staining, and the ultrastructure of ATG vacuoles. Conversely, administration of the ATG inhibitor 3-methyladenine (3-MA) or a recombinant full-length human active Akt1 protein (rAkt1) suppresses the anti-tumor effects of curcumin [81]. Similar results were documented by Maiti et al. (2019) [56] in both mouse (GL261) and rat (F98). In these species, the levels of ATG markers within glial tumor cells (i.e., Atg5, Atg7, Beclin-1, LC3A/B, and p62) and the number of ATG vacuoles were increased following curcumin administration [56]. It is noteworthy that levels of PI3Kp85, p-PI3Kp85, total Akt, p-Akt, mTOR, and p-mTOR were decreased following curcumin administration. This indicates that this natural polyphenol significantly activates the mTOR-dependent ATG pathway [56]. This is in line with findings of Zanotto-Filho and colleagues (2011) [49,89], who found that curcumin inhibits the constitutive activation of the PI3K/Akt/mTOR pathway. This significantly reduces in vitro GBM cell viability. Intriguingly, curcumin does not modify the phenotype of health astrocytes, suggesting that this natural compound selectively targets glioma cells [49,89]. A curcumin-mediated increase in ATG levels occurs along with the down-regulation of cell survival markers such as Bcl-2 and the cytoprotective mitochondrial protein bcl-x. This is likely to depend on the cytostatic/anti-proliferative effect of curcumin on GBM cells [49,56,89].
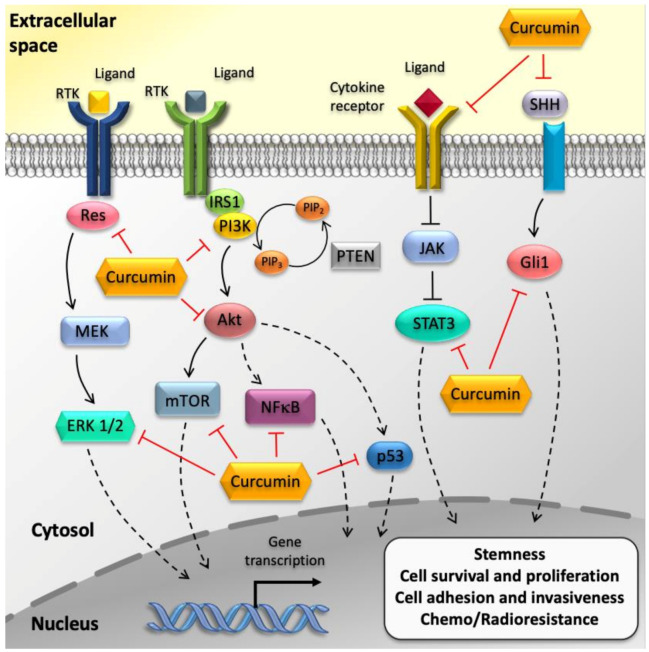
Enhancing ATG in GSCs following curcumin administration produces multiple effects, well beyond a mere arrest of the cell cycle. For instance, curcumin suppresses the tumorigenic stem-like features and the invasiveness of GSCs by down-regulating Akt/mTOR activity (Figure 2). This is remarkable, since ATG defect contributes to the desensitization of GSCs to normal differentiation cues, and GSC also increased the invasive potential and therapeutic resistance [69,82,90].
Figure 2.
Effects of curcumin on GBM cancer stem cells (GSCs). The cartoon summarizes the major effects of curcumin on GSCs. In particular, curcumin was shown to decrease malignant characteristics of GSCs by targeting core-signaling pathways such as PI3K/Akt/mTOR, JAK/STAT3, and MAPK pathways.
In line with this, Zhuang et al. (2012) [52] provided evidence that curcumin suppresses GSC stem-like features, while triggering the ATG-dependent differentiation of GSCs both in vitro and in vivo [52]. In detail, the up-regulation of neural markers (i.e., βIII-tubulin, Tuj1, Olig2) and the marked reduction of GSC self-renewal and clonogenic ability occurs concomitantly with ATG induction following curcumin administration. Such an ATG-dependent pro-differentiating effect was replicated in vivo in nude mice bearing intracranial GBM xenografts. In fact, tumor sections obtained from curcumin-treated mice feature a remarkable increase in the number of LC3 immunofluorescent puncta and autophagosomes at transmission electron microscope. Notably, all these effects were accompanied by a marked reduction of tumor burden and increased mice survival. Consistently with the in vitro data, the dual effect of curcumin on GSC stemness and differentiation in xenograft tumors were reversed following treatment with 3-MA [52].
One of the major factors contributing to GBM malignancy is the highly invasive potential of GSCs. Unlike non-stem tumor cells, GSCs easily migrate and infiltrate within the surrounding healthy brain parenchyma [57]. It is known that hyper-activation of the Akt/mTOR pathway sustains the extremely invasive phenotype of GSCs, while mTOR inhibition down-regulates both mRNA, protein levels, and the activity of the matrix metalloproteinases (MMPs) MMP-9 and MMP-2, which promote tumor invasion through extracellular matrix degradation [69]. As a proof of concept, mTOR-dependent ATG induction following curcumin administration significantly impairs GSCs migration in vitro, as well as their ability to invade the brain parenchyma in vivo [49,58,64]. For instance, Zhang et al. (2016) [64] reported that curcumin-loaded nanoparticles (Cur/LDH NPs) significantly reduce cell migration and invasion in a A172 glioma cell line. These effects were associated with mTOR-dependent ATG stimulation. In fact, the number of ATG vacuoles and expression levels of ATG markers (LC3A/B, Atg5-Atg12) were increased in A172 cells exposed to Cur/LDH NPs [64]. Again, curcumin-induced down-regulation of the oncogenic protein NEDD4 impairs the migration of highly invasive SNB19 and A1207 GBM cell lines [91]. Notably, the NEDD4 is an E3-ubiquitin ligase, which promotes ubiquitin-mediated PTEN degradation, and thus PI3K/Akt activation and cell proliferation [91,92], and it is frequently overexpressed in various cancers, including GBM [92]. Collectively, these data confirm that the effects of curcumin on GSCs are tightly bound to ATG induction, and they further strengthen the notion that the ATG-dependent differentiation, migration arrest, and occluded invasion induce by curcumin may be promising in the adjunct therapeutic of GBM.
In line with this, curcumin derivatives exert beneficial effects on GBM due to their potential to suppress the tumorigenic features of GSCs [63,65,93,94]. Increasing evidence demonstrates that these synthetic compounds decrease cancer stem cell-like phenotype as well as invasive potential by targeting upstream regulators of the mTOR signaling in GSCs. For instance, hydrazinobenzoylcurcumin (HBC) suppresses self-renewal ability, cell migration, and invasion by down-regulating the Ca2+/CaM-dependent protein kinase II (CaMKII)/c-Met axis, which, in turn, modulates the expression of stemness markers through the activation of Akt/mTOR signaling [65].
Furthermore, curcumin sensitizes GSCs to several chemo- and radio-therapeutic agents due to its ability to modulate different cell signaling, beyond ATG [18,45,89,95,96,97,98]. In fact, when administered in combination with currently used therapies, curcumin further potentiates their anti-tumor activity against GBM [19,99,100,101]. For instance, curcumin enhances temozolomide (TMZ)-induced cytotoxicity by disputing the Akt/mTOR pathway in U87MG cell lines, thereby overcoming GBM therapeutic resistance [18]. Conversely, co-treatment with ATG inhibitors (i.e., 3-MA, hydroxychloroquine (HCQ), or LY294002) attenuates the antitumor effects of curcumin on human GBM cells, leading to an increased resistance to antitumor agents [80].
Finally, it is worth mentioning that mTOR alterations and defective ATG are also bound to several non-tumoral cells, which support tumor growth and mediate GBM relapse and infiltration [69]. These represent the micro-environment that borders tumor growth and promotes GSCs proliferation. This is the case of endothelial cells (ECs), which reinforce GSCs stem-like phenotype through the mTOR pathway [69,102]. Remarkably, curcumin markedly decreases in vitro tube formation and cell migration of rat brain capillary endothelial cells (RBE4) as well as in vivo blood vessel formation in mice bearing GBM xenografts, thus supporting an anti-angiogenic activity of curcumin in GBM [60]. Likewise, curcumin targets GBM-associated microglia, which is key in GSC immune evasion. In fact, these immune cells are recruited by GSCs through mTOR-dependent extracellular vesicle (EVs) release [103]. This process is quite powerful in the natural course of GBM and it consists of the release of GSC-derived EVs, which harbor diffusible pro-oncogenic cargoes. Once released within the extracellular milieu, these tumor-promoting EVs disseminate as paracrine factors to induce phenotypic and epigenetic modifications in glioma-associated stromal cells. In this way, GSCs actively recruit different non-tumorigenic stromal cells, which, in turn, participate in tumor micro-environment (TME) adaptive remodeling and immune modulation, which foster tumor growth and progression [103]. At the molecular level, recent publications revealed a potential role of mTOR-dependent ATG in modulating EV-mediated intercellular communication within GBM TME. In fact, there is emerging evidence that ATG and EV release are tightly intermingled and reciprocally regulated [104,105,106]. Hence, it is not surprising that mTOR hyper-activation and subsequent ATG suppression may profoundly alter the EV-dependent release of cytosolic cargoes, which intensely occurs in GBM. This is in line with the observation that GSCs secrete significant amounts of EVs enriched in proteins, mRNAs, and miRNAs, which, in turn, enable an unconventional mechanism of EV-mediated disease spreading [107,108]. Within this frame, EV-dependent cell-to-cell communication has been implicated in GSC-driven immune escape. Intriguing reports demonstrate that GSC-derived EVs act on monocytes, inducing a shift toward an immune-suppressive, tumor-supporting M2 macrophage phenotype, which in turn fosters tumor invasion and progression [103,109,110]. Remarkably, a recent in vivo study revealed that curcumin induces the repolarization of tumor-supporting M2-like microglia/macrophages toward the tumoricidal M1-like phenotype and intra-GBM recruitment of activated natural killer (NK) cells [111]. Notably, these effects were associated with a suppression of GSC and decreased tumor volume [111]. Although the specific molecular mechanisms have yet to be explored, novel insights on how curcumin-mediated ATG induction can reshape the GBM tumor micro-environment represents a novel field worth being deeply investigated.
4. Effects of Curcumin on GBM beyond ATG Induction
In the last decades, curcumin received considerable interest in anti-cancer research, and especially in GBM, due to its versatile bioactivity via the modulation of several pathways, beyond ATG. In fact, at the molecular level, curcumin exerts pleiotropic effects by influencing a number of cellular signaling pathways, which are involved in tumor initiation, growth, and progression [112]. For instance, curcumin suppresses tumor growth by inhibiting tumor-promoting pathways, such as the NF-kB, Wnt/β-catenin, Notch signaling, as well as the JAK/STAT3 and MAPK pathways [45,101,113,114,115]. At the same time, curcumin up-regulates major tumor-suppressing proteins, namely p53, p21, and caspase 3 [46,116,117].
4.1. Curcumin Suppresses GBM Cell Proliferation and Survival via Inhibition of NF-κB and AP-1 Pathways
NF-κB is a transcription factor, which regulates the gene expression of several target genes involved in major cellular and biological processes, such as proliferation, survival immune response, and inflammation [118]. NF-κB is constitutively over-expressed in GBM [119], and its aberrant activation is linked to de-regulated, tumor-promoting EGFR and PI3K/Akt/mTOR signaling [120,121]. Similarly, the constitutive over-activation of c-Jun N-terminal kinase (JNK)/AP-1 transcription factor is associated with GBM growth, infiltration, and therapeutic resistance [45]. In fact, AP-1 is an upstream modulator of MMPs gene expression, thereby regulating GBM invasive potential [122].
In vitro studies, using both human (T98G, U87MG, T67, U373) and rat (C6) GBM cell lines, demonstrated that curcumin and curcuminoids (i.e., demethoxycurcumin (DMC)) reduce cell survival through the suppression of both NF-κB and AP-1 signaling activation. This prevents the constitutive activation of Akt and JNK [45,100,101,123,124]. At the same time, the curcumin-dependent inhibition of AP-1 signaling suppresses MMPs transcription, thus markedly repressing GBM cells invasive potential [122]. These results are in line with research reports demonstrating that the curcumin-dependent suppression of MMP-9 promoter activity occurs via the inhibition of NF-κB and AP-1 DNA binding activities [125]. Furthermore, the inhibition of NF-κB signaling with curcumin potentiates the anti-tumor effects against GBM of different therapeutic agents such as the alkylating agent nimustine hydrochloride (ACNU) [101] and the microtubule-stabilizing agent paclitaxel (PTX) [100].
4.2. Curcumin Induces GBM Cell Cycle Arrest through the Modulation TP53 and RB Pathways
Compelling evidence demonstrates that curcumin exerts anti-proliferative effects on glioma cells by modulating TP53/MDM2/MDM4/p14ARF and RB1/CDK4/p16INK4A signaling. These latter represent two main cell cycle regulating pathways, which are often impaired in GBM [44]. Remarkably, curcumin significantly inhibits GBM cell growth and proliferation via the suppression of cell cycle progression in different human glioma cell lines [19,47,81,113,126,127]. For instance, Liu et al. (2007) [47] demonstrate that curcumin induces G2/M cell cycle arrest in a p53-dependent manner. In fact, p53 protein levels are increased in curcumin-treated U251 glioma cells, followed by the induction of CDK inhibitor/cell-cycle regulator p21 and tumor suppressor ING4, thus resulting in cell cycle arrest [47]. Similarly, treatment with curcumin up-regulates p53 and p21 expression while suppressing the cdc2 and RB pathways in DBRTG glioma cells [113]. Moreover, another study in U87MG cells shows that curcumin induces GBM cell-cycle arrest through the up-regulation of p21, along with cyclin D1 down-regulation. Remarkably, this occurs independently from p53 function, since it relies on the activation of Egr-1 transcription factor via ERK and JNK/MAPK/Elk signaling cascades. In fact, curcumin-induced p21 transcription is abolished in human U87MG cells transfected with Egr-1 siRNA [117]. In the attempt to unravel the molecular mechanisms underlying curcumin-mediated growth inhibition, a recent paper reported that U251-treated cells are arrested in the G2/M phase by increased expression of the tumor suppressor death-associated protein kinase 1 (DAPK1) [127]. Interestingly, this effect is accompanied by the inhibition of NF-κB and STAT3 pathways, along with caspase 3 activation. In fact, siRNA-mediated knock-down of DAPK1 attenuates curcumin-induced inhibition of NF-κB and STAT3 while preventing caspase 3-mediated apoptosis [127].
4.3. Curcumin Hampers GBM Aggressiveness through the Modulation of The JAK/STAT Pathway
A recent report demonstrates that curcumin hampers GBM aggressiveness in vitro by inhibiting the JAK/STAT3 pathway [128]. In fact, curcumin potently suppresses GBM cell proliferation through the modulation of the JAK/STAT3 pathway both in GBM human primary and recurrent cell lines [48]. In detail, the anti-proliferative effect was associated, at least in part, with a reduction of STAT3 intracellular levels, resulting in the reduced transcription of cell cycle regulating gene c-Myc and proliferation marker Ki-67 [48].
In addition to anti-proliferative effects, curcumin-mediated inhibition of JAK/STAT3 signaling strongly correlates with a marked suppression of GBM cell migration and invasive potential [48]. Similarly, DMC and TMZ synergistically inactivate JAK/STAT3 signaling in human GBM cells lines, which accounts for a significant inhibition of cell proliferation and concomitant increase in apoptosis [62].
4.4. Curcumin Induces Pro-Apoptotic Pathways in GBM Cells
Among the pleiotropic activities exerted by curcumin, the anti-proliferative effects of this natural polyphenol rely, at least in part, on the activation of pro-apoptotic pathways. In fact, several studies demonstrate that curcumin-induced G2/M cell cycle arrest frequently occurs along with an induction of caspase-mediated cell death [46,123,127,129]. Remarkably, curcumin induces apoptosis via a caspase-dependent pathway in human GBM cells [46,130]. In fact, treatment with curcumin increases the expression pro-apoptotic proteins, namely caspase-3, caspase-7, caspase-8, and caspase-9, which initiate and execute the apoptotic cascade [46,130]. Although the molecular mechanisms are yet to be explored, curcumin triggers several pro-apoptotic effects, beyond an increase in caspase activity. In fact, curcumin induces DNA fragmentation as well as a cleavage of poly(ADP-ribose) polymerase (PARP-1) nuclear enzyme in human glioma CHME cells [131]. Furthermore, a loss of mitochondrial membrane potential and ROS production are increased in curcumin-treated cells compared with controls [131]. This suggests indeed the induction of the apoptotic cascade. At the same time, curcumin exerts anti-GBM activity by suppressing anti-apoptotic signals [45,126], as confirmed by an increased BAX:BCL2 ratio in several human GBM cell lines [50,130,131]. It is noteworthy that these effects are related, at least in part, to the inhibition of sonic hedgehog (SHH)/glioma-associated oncogene homolog 1 (GLI1) signaling, resulting in down-regulation of the downstream oncoprotein Bcl-2 [59].
5. Concluding Remarks
Extensive research in the past two decades demonstrates the beneficial effects of phytochemicals in general, and especially curcumin, in a wide range of human diseases, encompassing NDDs and brain tumors, and particularly GBM. In fact, curcumin supplementation provides neuroprotection due to its anti-oxidant, anti-inflammatory and, anti-protein aggregation properties, which are tightly bound to ATG modulation. Furthermore, mounting evidence demonstrates that curcumin is capable of targeting undifferentiated and highly tumorigenic cancer stem cells, and especially GCSs, through the modulation of the mTOR-dependent ATG pathway. Notably, mTOR hyper-activation and defective ATG are both implicated in the maintenance of GSCs’ oncogenic properties and metabolism. Conversely, rescuing ATG with curcumin suppresses the tumorigenic features of GSCs, thus counteracting GBM establishment and growth. Since curcumin influences various aberrant signaling pathways associated with GBM, and especially mTOR-dependent ATG, it should be further exploited as a potential adjunct therapy for GBM standard treatments.
Outstanding Questions
Even though the hydrophobic nature, low water solubility, and physicochemical stability may limit its bioavailability [132], increasing evidence demonstrates that curcumin is able to cross the blood-brain barrier, thus yielding therapeutic benefits within the CNS [133]. However, extensive efforts are currently devoted to develop novel delivery systems (i.e., nanoparticles, liposomes, micellar systems, combination with piperine) [134,135,136] as well as curcumin derivatives [137,138], thus possibly overcoming these obstacles, while improving curcumin’s efficacy.
Author Contributions
Writing—original draft preparation, L.R. and F.F.; writing—review, editing, and art-work, L.R., F.B. and C.L.B.; conceptualization, L.R., A.F. and F.F.; supervision, A.F. and F.F.; funding acquisition, F.B., C.L.B., G.L. and F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by a grant from Ministero della Salute (Ricerca Corrente 2020).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prasad S., Aggarwal B.B. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In: Benzie I.F.F., Wachtel-Galor S., editors. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. CRC Press/Taylor & Francis; Boca Raton, FL, USA: 2011. [PubMed] [Google Scholar]
- 2.Aggarwal B.B., Sundaram C., Malani N., Ichikawa H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 3.Ammon H.P., Wahl M.A. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 4.Limanaqi F., Biagioni F., Busceti C.L., Ryskalin L., Polzella M., Frati A., Fornai F. Phytochemicals Bridging Autophagy Induction and Alpha-Synuclein Degradation in Parkinsonism. Int. J. Mol. Sci. 2019;20:3274. doi: 10.3390/ijms20133274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miriyala S., Panchatcharam M., Rengarajulu P. Cardioprotective effects of curcumin. Adv. Exp. Med. Biol. 2007;595:359–377. doi: 10.1007/978-0-387-46401-5_16. [DOI] [PubMed] [Google Scholar]
- 6.Jagtap S., Meganathan K., Wagh V., Winkler J., Hescheler J., Sachinidis A. Chemoprotective mechanism of the natural compounds, epigallocatechin-3-O-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr. Med. Chem. 2009;16:1451–1462. doi: 10.2174/092986709787909578. [DOI] [PubMed] [Google Scholar]
- 7.Funk J.L., Oyarzo J.N., Frye J.B., Chen G., Lantz R.C., Jolad S.D., Solyom A.M., Timmermann B.N. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J. Nat. Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal B.B., Sung B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Alappat L., Awad A.B. Curcumin and obesity: Evidence and mechanisms. Nutr. Rev. 2010;68:729–738. doi: 10.1111/j.1753-4887.2010.00341.x. [DOI] [PubMed] [Google Scholar]
- 10.Singh R., Sharma P. Hepatoprotective Effect of Curcumin on Lindane-induced Oxidative Stress in Male Wistar Rats. Toxicol. Int. 2011;18:124–129. doi: 10.4103/0971-6580.84264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellegrini C., Fornai M., Antonioli L., Blandizzi C., Calderone V. Phytochemicals as Novel Therapeutic Strategies for NLRP3 Inflammasome-Related Neurological, Metabolic, and Inflammatory Diseases. Int. J. Mol. Sci. 2019;20:2876. doi: 10.3390/ijms20122876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darvesh A.S., Carroll R.T., Bishayee A., Novotny N.A., Geldenhuys W.J., Van der Schyf C.J. Curcumin and neurodegenerative diseases: A perspective. Expert Opin. Investig. Drugs. 2012;21:1123–1140. doi: 10.1517/13543784.2012.693479. [DOI] [PubMed] [Google Scholar]
- 13.Maiti P., Dunbar G.L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2018;19:1637. doi: 10.3390/ijms19061637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal B.B., Shishodia S., Takada Y., Banerjee S., Newman R.A., Bueso-Ramos C.E., Price J.E. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 16.Li M., Zhang Z., Hill D.L., Wang H., Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 17.Kunnumakkara A.B., Guha S., Krishnan S., Diagaradjane P., Gelovani J., Aggarwal B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 18.Yin H., Zhou Y., Wen C., Zhou C., Zhang W., Hu X., Wang L., You C., Shao J. Curcumin sensitizes glioblastoma to temozolomide by simultaneously generating ROS and disrupting AKT/mTOR signaling. Oncol. Rep. 2014;32:1610–1616. doi: 10.3892/or.2014.3342. [DOI] [PubMed] [Google Scholar]
- 19.Qian Y., Ma J., Guo X., Sun J., Yu Y., Cao B., Zhang L., Ding X., Huang J., Shao J.F. Curcumin enhances the radiosensitivity of U87 cells by inducing DUSP-2 up-regulation. Cell. Physiol. Biochem. 2015;35:1381–1393. doi: 10.1159/000373959. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S.C., Prasad S., Kim J.H., Patchva S., Webb L.J., Priyadarsini I.K., Aggarwal B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011;28:1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priyadarsini K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013;19:2093–2100. doi: 10.2174/138161213805289228. [DOI] [PubMed] [Google Scholar]
- 22.Ryskalin L., Limanaqi F., Biagioni F., Frati A., Esposito V., Calierno M.T., Lenzi P., Fornai F. The emerging role of m-TOR up-regulation in brain Astrocytoma. Histol. Histopathol. 2017;32:413–431. doi: 10.14670/HH-11-835. [DOI] [PubMed] [Google Scholar]
- 23.Ryskalin L., Lazzeri G., Flaibani M., Biagioni F., Gambardella S., Frati A., Fornai F. mTOR-Dependent Cell Proliferation in the Brain. Biomed Res. Int. 2017;2017:7082696. doi: 10.1155/2017/7082696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenzi P., Lazzeri G., Biagioni F., Busceti C.L., Gambardella S., Salvetti A., Fornai F. The Autophagoproteasome a Novel Cell Clearing Organelle in Baseline and Stimulated Conditions. Front. Neuroanat. 2016;10:78. doi: 10.3389/fnana.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazzeri G., Biagioni F., Fulceri F., Busceti C.L., Scavuzzo M.C., Ippolito C., Salvetti A., Lenzi P., Fornai F. mTOR Modulates Methamphetamine-Induced Toxicity through Cell Clearing Systems. Oxid. Med. Cell. Longev. 2018;2018:6124745. doi: 10.1155/2018/6124745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhuang X.X., Wang S.F., Tan Y., Song J.X., Zhu Z., Wang Z.Y., Wu M.Y., Cai C.Z., Huang Z.J., Tan J.Q., et al. Pharmacological enhancement of TFEB-mediated autophagy alleviated neuronal death in oxidative stress-induced Parkinson’s disease models. Cell Death Dis. 2020;11:128. doi: 10.1038/s41419-020-2322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W., Xu J. Curcumin Attenuates Cerebral Ischemia-reperfusion Injury Through Regulating Mitophagy and Preserving Mitochondrial Function. Curr. Neurovasc. Res. 2020;17:113–122. doi: 10.2174/1567202617666200225122620. [DOI] [PubMed] [Google Scholar]
- 28.Toti L., Bartalucci A., Ferrucci M., Fulceri F., Lazzeri G., Lenzi P., Soldani P., Gobbi P., La Torre A., Gesi M. High-intensity exercise training induces morphological and biochemical changes in skeletal muscles. Biol. Sport. 2013;30:301–309. doi: 10.5604/20831862.1077557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davinelli S., De Stefani D., De Vivo I., Scapagnini G. Polyphenols as Caloric Restriction Mimetics Regulating Mitochondrial Biogenesis and Mitophagy. Trends Endocrinol. Metab. 2020;31:536–550. doi: 10.1016/j.tem.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Drion C.M., van Scheppingen J., Arena A., Geijtenbeek K.W., Kooijman L., van Vliet E.A., Aronica E., Gorter J.A. Effects of rapamycin and curcumin on inflammation and oxidative stress in vitro and in vivo—in search of potential anti-epileptogenic strategies for temporal lobe epilepsy. J. NeuroInflamm. 2018;15:212. doi: 10.1186/s12974-018-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W., Yao S., Li H., Meng Z., Sun X. Curcumin promotes functional recovery and inhibits neuronal apoptosis after spinal cord injury through the modulation of autophagy. J. Spinal Cord Med. 2019:1–9. doi: 10.1080/10790268.2019.1616147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim G.P., Chu T., Yang F., Beech W., Frautschy S.A., Cole G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin L., Li C., Zhang D., Yuan M., Chen C.H., Li M. Synergic Effects of Berberine and Curcumin on Improving Cognitive Function in an Alzheimer’s Disease Mouse Model. Neurochem. Res. 2020;45:1130–1141. doi: 10.1007/s11064-020-02992-6. [DOI] [PubMed] [Google Scholar]
- 34.Maiti P., Rossignol J., Dunbar G.L. Curcumin Modulates Molecular Chaperones and Autophagy-Lysosomal Pathways In Vitro after Exposure to Aβ42. J. Alzheimers Dis. Parkinsonism. 2017;7:299. [Google Scholar]
- 35.Sharma N., Nehru B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology. 2018;26:349–360. doi: 10.1007/s10787-017-0402-8. [DOI] [PubMed] [Google Scholar]
- 36.Jiang T.F., Zhang Y.J., Zhou H.Y., Wang H.M., Tian L.P., Liu J., Ding J.Q., Chen S.D. Curcumin ameliorates the neurodegenerative pathology in A53T α-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J. Neuroimmune Pharmacol. 2013;8:356–369. doi: 10.1007/s11481-012-9431-7. [DOI] [PubMed] [Google Scholar]
- 37.Lin C.F., Yu K.H., Jheng C.P., Chung R., Lee C.I. Curcumin reduces amyloid fibrillation of prion protein and decreases reactive oxidative stress. Pathogens. 2013;2:506–519. doi: 10.3390/pathogens2030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrucci M., Ryskalin L., Biagioni F., Gambardella S., Busceti C.L., Falleni A., Lazzeri G., Fornai F. Methamphetamine increases Prion Protein and induces dopamine-dependent expression of protease resistant PrPsc. Arch. Ital. Biol. 2017;155:81–97. doi: 10.12871/000398292017129. [DOI] [PubMed] [Google Scholar]
- 39.Ryskalin L., Busceti C.L., Biagioni F., Limanaqi F., Familiari P., Frati A., Fornai F. Prion Protein in Glioblastoma Multiforme. Int. J. Mol. Sci. 2019;20:5107. doi: 10.3390/ijms20205107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louis N., Perry A., Reifenberge R.G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 41.Ostrom Q.T., Gittleman H., Farah P., Ondracek A., Chen Y., Wolinsky Y., Stroup N.E., Kruchko C., Barnholtz-Sloan J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15:ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koshy M., Villano J.L., Dolecek T.A., Howard A., Mahmood U., Chmura S.J., Weichselbaum R.R., McCarthy B.J. Improved survival time trends of glioblastoma using the SEER 17 population-based registries. Neuro Oncol. 2012;107:207–212. doi: 10.1007/s11060-011-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Meir E.G., Hadjipanayis C.G., Norden A.D., Shu H.K., Wen P.Y., Olson J.J. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA Cancer J. Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao H., Lebrun D.G., Yang J., Zhu V.F., Li M. Deregulated signaling pathways in glioblastoma multiforme: Molecular mechanisms and therapeutic targets. Cancer Invest. 2012;30:48–56. doi: 10.3109/07357907.2011.630050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhandapani K., Mahesh V., Brann D. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J. Neurochem. 2007;102:522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 46.Huang T., Tsai T., Hsu C., Hsu Y. Curcuminoids suppress the growth and induce apoptosis through caspase-3-dependent pathways in glioblastoma multiforme (GBM) 8401 cells. J. Agric. Food Chem. 2010;58:10639–10645. doi: 10.1021/jf1016303. [DOI] [PubMed] [Google Scholar]
- 47.Liu E., Wu J., Cao W., Zhang J., Liu W., Jiang X., Zhang X. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J. Neurooncol. 2007;85:263–270. doi: 10.1007/s11060-007-9421-4. [DOI] [PubMed] [Google Scholar]
- 48.Senft C., Polacin M., Priester M., Seifert V., Kogel D., Weissenberger J. The nontoxic natural compound Curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer. 2010;10:491. doi: 10.1186/1471-2407-10-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanotto-Filho A., Braganhol E., Edelweiss M.I., Behr G.A., Zanin R., Schröder R., Simões-Pires A., Battastini A.M., Moreira J.C. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J. Nutr. Biochem. 2012;23:591–601. doi: 10.1016/j.jnutbio.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Karmakar S., Banik N.L., Ray S.K. Curcumin suppressed anti-apoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells. Neurochem. Res. 2007;32:2103–2113. doi: 10.1007/s11064-007-9376-z. [DOI] [PubMed] [Google Scholar]
- 51.Gersey Z.C., Rodriguez G.A., Barbarite E., Sanchez A., Walters W.M., Ohaeto K.C., Komotar R.J., Graham R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer. 2017;17:99. doi: 10.1186/s12885-017-3058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhuang W., Long L., Zheng B., Ji W., Yang N., Zhang Q., Liang Z. Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci. 2012;103:684–690. doi: 10.1111/j.1349-7006.2011.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shakeri A., Cicero A.F.G., Panahi Y., Mohajeri M., Sahebkar A. Curcumin: A naturally occurring autophagy modulator. J. Cell. Physiol. 2019;234:5643–5654. doi: 10.1002/jcp.27404. [DOI] [PubMed] [Google Scholar]
- 54.Shinojima N., Yokoyama T., Kondo Y., Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2007;3:635–637. doi: 10.4161/auto.4916. [DOI] [PubMed] [Google Scholar]
- 55.Sahab-Negah S., Ariakia F., Jalili-Nik M., Afshari A.R., Salehi S., Samini F., Rajabzadeh G., Gorji A. Curcumin Loaded in Niosomal Nanoparticles Improved the Anti-tumor Effects of Free Curcumin on Glioblastoma Stem-like Cells: An In Vitro Study. Mol. Neurobiol. 2020;57:3391–3411. doi: 10.1007/s12035-020-01922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maiti P., Scott J., Sengupta D., Al-Gharaibeh A., Dunbar G.L. Curcumin and Solid Lipid Curcumin Particles Induce Autophagy, but Inhibit Mitophagy and the PI3K-Akt/mTOR Pathway in Cultured Glioblastoma Cells. Int. J. Mol. Sci. 2019;20:399. doi: 10.3390/ijms20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng L., Wu Q., Guryanova O.A., Huang Z., Huang Q., Rich J.N., Bao S. Elevated invasive potential of glioblastoma stem cells. Biochem. Biophys. Res. Commun. 2011;406:643–648. doi: 10.1016/j.bbrc.2011.02.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z., Liu F., Liao W., Yu L., Hu Z., Li M., Xia H. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression. Arch Biochem. Biophys. 2020;689:108412. doi: 10.1016/j.abb.2020.108412. [DOI] [PubMed] [Google Scholar]
- 59.Du W.Z., Feng Y., Wang X.F., Piao X.Y., Cui Y.Q., Chen L.C., Lei X.H., Sun X., Liu X., Wang H.B., et al. Curcumin suppresses malignant glioma cells growth and induces apoptosis by inhibition of SHH/GLI1 signaling pathway in vitro and vivo. CNS Neurosci. Ther. 2013;19:926–936. doi: 10.1111/cns.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perry M.C., Demeule M., Régina A., Moumdjian R., Béliveau R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol. Nutr. Food Res. 2010;54:1192–1201. doi: 10.1002/mnfr.200900277. [DOI] [PubMed] [Google Scholar]
- 61.Park K.S., Yoon S.Y., Park S.H., Hwang J.H. Anti-migration and anti-invasion effects of curcumin via suppression of Fascin expression in glioblastoma cells. Brain Tumor Res. Treat. 2019;7:16–24. doi: 10.14791/btrt.2019.7.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi L., Sun G. Low-dose DMC significantly enhances the effect of TMZ on glioma cells by targeting multiple signaling pathways both in vivo and in vitro. Neuromol. Med. 2015;17:431–442. doi: 10.1007/s12017-015-8372-8. [DOI] [PubMed] [Google Scholar]
- 63.Leng L., Zhong X., Sun G., Qiu W., Shi L. Demethoxycurcumin was superior to temozolomide in the inhibition of the growth of glioblastoma stem cells in vivo. Tumour Biol. 2016 doi: 10.1007/s13277-016-5399-x. [DOI] [PubMed] [Google Scholar]
- 64.Zhang H., Zhu Y., Sun X., He X., Wang M., Wang Z., Wang Q., Zhu R., Wang S. Curcumin-Loaded Layered Double Hydroxide Nanoparticles-Induced Autophagy for Reducing Glioma Cell Migration and Invasion. J. Biomed. Nanotechnol. 2016;12:2051–2062. doi: 10.1166/jbn.2016.2291. [DOI] [PubMed] [Google Scholar]
- 65.Shin H.J., Lee S., Jung H.J. A curcumin derivative hydrazinobenzoylcurcumin suppresses stem-like features of glioblastoma cells by targeting Ca2+/calmodulin-dependent protein kinase II. J. Cell. Biochem. 2019;120:6741–6752. doi: 10.1002/jcb.27972. [DOI] [PubMed] [Google Scholar]
- 66.Sansalone L., Veliz E.A., Myrthil N.G., Stathias V., Walters W., Torrens I.I., Schürer S.C., Vanni S., Leblanc R.M., Graham R.M. Novel Curcumin Inspired Bis-Chalcone Promotes Endoplasmic Reticulum Stress and Glioblastoma Neurosphere Cell Death. Cancers. 2019;11:357. doi: 10.3390/cancers11030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corsaro A., Bajetto A., Thellung S., Begani G., Villa V., Nizzari M., Pattarozzi A., Solari A., Gatti M., Pagano A., et al. Cellular prion protein controls stem cell-like properties of human glioblastoma tumor-initiating cells. Oncotarget. 2016;7:38638–38657. doi: 10.18632/oncotarget.9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qazi M.A., Vora P., Venugopal C., Sidhu S.S., Moffat J., Swanton C., Singh S.K. Intratumoral heterogeneity: Pathways to treatment resistance and relapse in human glioblastoma. Ann. Oncol. 2017;28:1448–1456. doi: 10.1093/annonc/mdx169. [DOI] [PubMed] [Google Scholar]
- 69.Ryskalin L., Gaglione A., Limanaqi F., Biagioni F., Familiari P., Frati A., Esposito V., Fornai F. The Autophagy Status of Cancer Stem Cells in Gliobastoma Multiforme: From Cancer Promotion to Therapeutic Strategies. Int. J. Mol. Sci. 2019;20:3824. doi: 10.3390/ijms20153824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang X., Bai H.M., Chen L., Li B., Lu Y.C. Reduced expression of LC3B-II and Beclin 1 in glioblastoma multiforme indicates a down-regulated autophagic capacity that relates to the progression of astrocytic tumors. J. Clin. Neurosci. 2010;17:1515–1519. doi: 10.1016/j.jocn.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 71.Li X.Y., Zhang L.Q., Zhang X.G., Li X., Ren Y.B., Ma X.Y., Li X.G., Wang L.X. Association between AKT/mTOR signalling pathway and malignancy grade of human gliomas. J. Neurooncol. 2011;103:453–458. doi: 10.1007/s11060-010-0424-1. [DOI] [PubMed] [Google Scholar]
- 72.Arcella F., Biagioni M., Oliva A., Bucci D., Frati A., Esposito V., Cantore G., Giangaspero F., Fornai F. Rapamycin inhibits the growth of glioblastoma. Brain Res. 2013;1495:37–51. doi: 10.1016/j.brainres.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 73.Shukla S., Patric I.R., Patil V., Shwetha S.D., Hegde A.S., Chandramouli B.A., Arivazhagan A., Santosh V., Somasundaram K. Methylation silencing of ULK2, an autophagy gene, is essential for astrocyte transformation and tumor growth. J. Biol. Chem. 2014;289:22306–22318. doi: 10.1074/jbc.M114.567032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwamaru A., Kondo Y., Iwado E., Aoki H., Fujiwara K., Yokoyama T., Mills G.B., Kondo S. Silencing mammalian target of rapamycin signaling by small interfering RNA enhances rapamycin-induced autophagy in malignant glioma cells. Oncogene. 2007;26:1840–1851. doi: 10.1038/sj.onc.1209992. [DOI] [PubMed] [Google Scholar]
- 75.Jiang H., White E.J., Conrad C., Gomez-Manzano C., Fueyo J. Autophagy pathways in glioblastoma. Methods Enzymol. 2009;453:273–286. doi: 10.1016/S0076-6879(08)04013-5. [DOI] [PubMed] [Google Scholar]
- 76.Zhao Y., Huang Q., Yang J., Lou M., Wang A., Dong J., Qin Z., Zhang T. Autophagy impairment inhibits differentiation of glioma stem/progenitor cells. Brain Res. 2010;1313:250–258. doi: 10.1016/j.brainres.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Ferrucci M., Biagioni F., Lenzi P., Gambardella S., Ferese R., Calierno M.T., Falleni A., Grimaldi A., Frati A., Esposito V., et al. Rapamycin promotes differentiation increasing βIII-tubulin, NeuN, and NeuroD while suppressing nestin expression in glioblastoma cells. Oncotarget. 2017;8:29574–29599. doi: 10.18632/oncotarget.15906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang H., Gomez-Manzano C., Aoki H., Alonso M.M., Kondo S., McCormick F., Xu J., Kondo Y., Bekele B.N., Colman H., et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: Role of autophagic cell death. J. Natl. Cancer Inst. 2007;99:1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 79.Liu R., Li J., Zhang T., Zou L., Chen Y., Wang K., Lei Y., Yuan K., Li Y., Lan J., et al. Itraconazole suppresses the growth of glioblastoma through induction of autophagy: Involvement of abnormal cholesterol trafficking. Autophagy. 2014;10:1241–1255. doi: 10.4161/auto.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee J.E., Yoon S.S., Moon E.Y. Curcumin-Induced Autophagy Augments Its Antitumor Effect against A172 Human Glioblastoma Cells. Biomol. Ther. (Seoul) 2019;27:484–491. doi: 10.4062/biomolther.2019.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aoki H., Takada Y., Kondo S., Sawaya R., Aggarwal B.B., Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of Akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 82.Zhuang W., Li B., Long L., Chen L., Huang Q., Liang Z. Induction of autophagy promotes differentiation of glioma-initiating cells and their radiosensitivity. Int. J. Cancer. 2011;129:2720–2731. doi: 10.1002/ijc.25975. [DOI] [PubMed] [Google Scholar]
- 83.Bharti A.C., Donato N., Singh S., Aggarwal B.B. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 84.Xiao K., Jiang J., Guan C., Dong C., Wang G., Bai L., Sun J., Hu C., Bai C. Curcumin induces autophagy via activating the AMPK signaling pathway in lung adenocarcinoma cells. J. Pharmacol. Sci. 2013;123:102–109. doi: 10.1254/jphs.13085FP. [DOI] [PubMed] [Google Scholar]
- 85.Su J., Zhou X., Yin X., Wang L., Zhao Z., Hou Y., Zheng N., Xia J., Wang Z. The effects of curcumin on proliferation, apoptosis, invasion, and NEDD4 expression in pancreatic cancer. Biochem. Pharmacol. 2017;140:28–40. doi: 10.1016/j.bcp.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 86.Beevers C.S., Chen L., Liu L., Luo Y., Webster N.J., Huang S. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res. 2009;69:1000–1008. doi: 10.1158/0008-5472.CAN-08-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fong D., Yeh A., Naftalovich R., Choi T.H., Chan M.M. Curcumin inhibits the side population (SP) phenotype of the rat C6 glioma cell line: Towards targeting of cancer stem cells with phytochemicals. Cancer Lett. 2010;293:65–72. doi: 10.1016/j.canlet.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y., Ying X., Xu H., Yan H., Li X., Tang H. The functional curcumin liposomes induce apoptosis in C6 glioblastoma cells and C6 glioblastoma stem cells in vitro and in animals. Int. J. Nanomed. 2017;12:1369–1384. doi: 10.2147/IJN.S124276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zanotto-Filho A., Coradini K., Braganhol E., Schroder R., de Oliveira C.M., Simoes-Pires A., Battastini A.M.O., Pohlmann A.R., Guterres S.S., Forcelini C.M., et al. Curcumin-loaded lipid-core nanocapsules as a strategy to improve pharmacological efficacy of curcumin in glioma treatment. Eur. J. Pharm. Biopharm. 2013;83:156–167. doi: 10.1016/j.ejpb.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 90.Tao Z., Li T., Ma H., Yang Y., Zhang C., Hai L., Liu P., Yuan F., Li J., Yi L., et al. Autophagy suppresses self-renewal ability and tumorigenicity of glioma-initiating cells and promotes Notch1 degradation. Cell Death Dis. 2018;9:1063. doi: 10.1038/s41419-018-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X., Deng J., Yuan J., Tang X., Wang Y., Chen H., Liu Y., Zhou L. Curcumin exerts its tumor suppressive function via inhibition of NEDD4 oncoprotein in glioma cancer cells. Int. J. Oncol. 2017;51:467–477. doi: 10.3892/ijo.2017.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X., Trotman L.C., Koppie T., Alimonti A., Chen Z., Gao Z., Wang J., Erdjument-Bromage H., Tempst P., Cordon-Cardo C., et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi L., Fei X., Wang Z. Demethoxycurcumin was prior to temozolomide on inhibiting proliferation and induced apoptosis of glioblastoma stem cells. Tumour Biol. 2015;36:7107–7119. doi: 10.1007/s13277-015-3427-x. [DOI] [PubMed] [Google Scholar]
- 94.Lim K.J., Bisht S., Bar E.E., Maitra A., Eberhart C.G. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol. Ther. 2011;11:464–473. doi: 10.4161/cbt.11.5.14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhuang W., Qin Z., Liang Z. The role of autophagy in sensitizing malignant glioma cells to radiation therapy. Acta Biochim. Biophys. Sin. 2009;41:341–351. doi: 10.1093/abbs/gmp028. [DOI] [PubMed] [Google Scholar]
- 96.Li H., Chen L., Li J.J., Zhou Q., Huang A., Liu W.W., Wang K., Gao L., Qi S.T., Lu Y.T. miR-519a enhances chemosensitivity and promotes autophagy in glioblastoma by targeting STAT3/Bcl2 signaling pathway. J. Hematol. Oncol. 2018;11:70. doi: 10.1186/s13045-018-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang X., Peng L., Liu A., Ji J., Zhao L., Zhai G. The enhanced effect of tetrahydrocurcumin on radiosensitivity of glioma cells. J. Pharm. Pharmacol. 2018;70:749–759. doi: 10.1111/jphp.12891. [DOI] [PubMed] [Google Scholar]
- 98.Castonguay A., Doucet C., Juhas M., Maysinger D. New ruthenium(II)-letrozole complexes as anticancer therapeutics. J. Med. Chem. 2012;55:8799–8806. doi: 10.1021/jm301103y. [DOI] [PubMed] [Google Scholar]
- 99.Ramachandran C., Nair S.M., Escalon E., Melnick S.J. Potentiation of etoposide and temozolomide cytotoxicity by curcumin and turmeric force™ in brain tumor cell lines. J. Complement. Integr. Med. 2012;9:20. doi: 10.1515/1553-3840.1614. [DOI] [PubMed] [Google Scholar]
- 100.Fratantonio D., Molonia M.S., Bashllari R., Muscara C., Ferlazzo G., Costa G., Saija A., Cimino F., Speciale A. Curcumin potentiates the antitumor activity of Paclitaxel in rat glioma C6 cells. Phytomedicine. 2019;55:23–30. doi: 10.1016/j.phymed.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 101.Zhao J., Zhu J., Lv X., Xing J., Liu S., Chen C., Xu Y. Curcumin potentiates the potent antitumor activity of ACNU against glioblastoma by suppressing the PI3K/AKT and NF-kappaB/COX-2 signaling pathways. OncoTargets Ther. 2017;10:5471–5482. doi: 10.2147/OTT.S149708. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Galan-Moya E.M., Le Guelte A., Lima Fernandes E., Thirant C., Dwyer J., Bidere N., Couraud P.O., Scott M.G., Junier M.P., Chneiweiss H., et al. Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO Rep. 2011;12:470–476. doi: 10.1038/embor.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ryskalin L., Biagioni F., Lenzi P., Frati A., Fornai F. mTOR Modulates Intercellular Signals for Enlargement and Infiltration in Glioblastoma Multiforme. Cancers. 2020;12:2486. doi: 10.3390/cancers12092486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poehler A.-M., Xiang W., Spitzer P., May V.E.L., Meixner H., Rockenstein E., Chutna O., Outeiro T.F., Winkler J., Masliah E., et al. Autophagy modulates SNCA/α-synuclein release, thereby generating a hostile microenvironment. Autophagy. 2014;10:2171–2192. doi: 10.4161/auto.36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu J., Camfield R., Gorski S.M. The interplay between exosomes and autophagy-partners in crime. J. Cell Sci. 2018;131:jcs215210. doi: 10.1242/jcs.215210. [DOI] [PubMed] [Google Scholar]
- 106.Oshima M., Seki T., Kurauchi Y., Hisatsune A., Katsuki H. Reciprocal Regulation of Chaperone-Mediated Autophagy/Microautophagy and Exosome Release. Biol. Pharm. Bull. 2019;42:1394–1401. doi: 10.1248/bpb.b19-00316. [DOI] [PubMed] [Google Scholar]
- 107.Skog J., Wurdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakano I., Garnier D., Minata M., Rak J. Extracellular vesicles in the biology of brain tumour stem cells--Implications for inter-cellular communication, therapy and biomarker development. Semin. Cell Dev. Biol. 2015;40:17–26. doi: 10.1016/j.semcdb.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 109.De Vrij J., Maas S.L., Kwappenberg K.M., Schnoor R., Kleijn A., Dekker L., Luider T.M., de Witte L.D., Litjens M., van Strien M.E., et al. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int. J. Cancer. 2015;137:1630–1642. doi: 10.1002/ijc.29521. [DOI] [PubMed] [Google Scholar]
- 110.Gabrusiewicz K., Li X., Wei J., Hashimoto Y., Marisetty A.L., Ott M., Wang F., Hawke D., Yu J., Healy L.M., et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology. 2018;7:e1412909. doi: 10.1080/2162402X.2017.1412909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mukherjee S., Baidoo J.N.E., Sampat S., Mancuso A., David L., Cohen L.S., Zhou S., Banerjee P. Liposomal TriCurin, A Synergistic Combination of Curcumin, Epicatechin Gallate and Resveratrol, Repolarizes Tumor-Associated Microglia/Macrophages, and Eliminates Glioblastoma (GBM) and GBM Stem Cells. Molecules. 2018;23:201. doi: 10.3390/molecules23010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aggarwal B.B., Kumar A., Bharti A. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 113.Su C., Wang M., Chiu T. The anti-cancer efficacy of curcumin scrutinized through core signaling pathways in glioblastoma. Int. J. Mol. Med. 2010;26:217–224. doi: 10.3892/ijmm_00000455. [DOI] [PubMed] [Google Scholar]
- 114.Chen Q.G., Zhou W., Han T., Du S.Q., Li Z.H., Zhang Z., Shan G.Y., Kong C.Z. MiR-378 suppresses prostate cancer cell growth through downregulation of MAPK1 in vitro and in vivo. Tumour Biol. 2016;37:2095–2103. doi: 10.1007/s13277-015-3996-8. [DOI] [PubMed] [Google Scholar]
- 115.Kuo C.L., Wu S.Y., Ip S.W., Wu P.P., Yu C.S., Yang J.S., Chen P.Y., Wu S.H., Chung J.G. Apoptotic death in curcumin-treated NPC-TW human nasopharyngeal carcinoma cells is mediated through the ROS, mitochondrial depolarization and caspase-3-dependent signaling responses. Int. J. Oncol. 2011;39:319–328. doi: 10.3892/ijo.2011.1057. [DOI] [PubMed] [Google Scholar]
- 116.Liontas A., Yeger H. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res. 2004;24:987–998. [PubMed] [Google Scholar]
- 117.Choi B., Kim C., Bae Y., Lim Y., Lee Y., Shin S. p21 Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: Role of early growth response-1 expression. Cancer Res. 2008;68:1369–1377. doi: 10.1158/0008-5472.CAN-07-5222. [DOI] [PubMed] [Google Scholar]
- 118.Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang H., Wang H., Zhang W., Huang H.J., Liao W.S., Fuller G.N. Analysis of the activation status of Akt, NFkappaB, and Stat3 in human diffuse gliomas. Lab Invest. 2004;84:941–951. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 120.Dan H.C., Cooper M.J., Cogswell P.C., Duncan J.A., Ting J.P., Baldwin A.S. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Puliyappadamba V.T., Hatanpaa K.J., Chakraborty S., Habib A.A. The role of NF-κB in the pathogenesis of glioma. Mol. Cell. Oncol. 2014;1:e963478. doi: 10.4161/23723548.2014.963478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim S.Y., Jung S.H., Kim H.S. Curcumin is a potent broad spectrum inhibitor of matrix metalloproteinase gene expression in human astroglioma cells. Biochem. Biophys. Res. Commun. 2005;337:510–516. doi: 10.1016/j.bbrc.2005.09.079. [DOI] [PubMed] [Google Scholar]
- 123.Kumar R., Lal N., Nemaysh V., Luthra P.M. Demethoxycurcumin mediated targeting of MnSOD leading to activation of apoptotic pathway and inhibition of Akt/NF-kappaB survival signalling in human glioma U87 MG cells. Toxicol. Appl. Pharmacol. 2018;345:75–93. doi: 10.1016/j.taap.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 124.Hesari A., Rezaei M., Rezaei M., Dashtiahangar M., Fathi M., Rad J.G., Momeni F., Avan A., Ghasemi F. Effect of curcumin on glioblastoma cells. J. Cell. Physiol. 2019;234:10281–10288. doi: 10.1002/jcp.27933. [DOI] [PubMed] [Google Scholar]
- 125.Woo M.S., Jung S.H., Kim S.Y., Hyun J.W., Ko K.H., Kim W.K., Kim H.S. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem. Biophys. Res. Commun. 2005;335:1017–1025. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- 126.Luthra P., Kumar R., Prakash A. Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis in human glioma U87 cells. Biochem. Biophys. Res. Commun. 2009;384:420–425. doi: 10.1016/j.bbrc.2009.04.149. [DOI] [PubMed] [Google Scholar]
- 127.Wu B., Yao H., Wang S., Xu R. DAPK1 modulates a curcumin-induced G2/M arrest and apoptosis by regulating STAT3, NF-κB, and caspase-3 activation. Biochem. Biophys. Res. Commun. 2013;434:75–80. doi: 10.1016/j.bbrc.2013.03.063. [DOI] [PubMed] [Google Scholar]
- 128.Weissenberger J., Priester M., Bernreuther C., Rakel S., Glatzel M., Seifert V., Kogel D. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin. Cancer Res. 2010;16:5781–5795. doi: 10.1158/1078-0432.CCR-10-0446. [DOI] [PubMed] [Google Scholar]
- 129.Nagai S., Kurimoto M., Washiyama K., Hirashima Y., Kumanishi T., Endo S. Inhibition of cellular proliferation and induction of apoptosis by curcumin in human malignant astrocytoma cell lines. J. Neurooncol. 2005;74:105–111. doi: 10.1007/s11060-004-5757-1. [DOI] [PubMed] [Google Scholar]
- 130.Khaw A.K., Hande M.P., Kalthur G., Hande M.P. Curcumin inhibits telomerase and induces telomere shortening and apoptosis in brain tumor cells. J. Cell. Biochem. 2013;114:1257–1270. doi: 10.1002/jcb.24466. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y., Tu L., Zhou X., Li B. Curcumin-Mediated Induction of Apoptosis in Human Glioma CHME Cells. Med. Sci. Monit. Basic Res. 2018;24:216–224. doi: 10.12659/MSMBR.912313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Prasad S., Tyagi A.K., Aggarwal B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014;46:2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dei Cas M., Ghidoni R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients. 2019;11:2147. doi: 10.3390/nu11092147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tsai Y.-M., Chien C.-F., Lin L.-C., Tsai T.-H. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int. J. Pharm. 2011;416:331–338. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 135.Zhao M., Zhao M., Fu C., Yu Y., Fu A. Targeted therapy of intracranial glioma model mice with curcumin nanoliposomes. Int. J. Nanomed. 2018;13:1601–1610. doi: 10.2147/IJN.S157019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dutzmann S., Schiborr C., Kocher A., Pilatus U., Hattingen E., Weissenberger J., Gessler F., Quick-Weller J., Franz K., Seifert V., et al. Intratumoral concentrations and effects of orally administered micellar curcuminoids in glioblastoma patients. Nutr. Cancer. 2016;68:943–948. doi: 10.1080/01635581.2016.1187281. [DOI] [PubMed] [Google Scholar]
- 137.Jayaprakasha G., Rao L.J., Sakariah K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006;98:720–724. doi: 10.1016/j.foodchem.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 138.Huang T.Y., Hsu C.W., Chang W.C., Wang M.Y., Wu J.F., Hsu Y.C. Demethoxycurcumin Retards Cell Growth and Induces Apoptosis in Human Brain Malignant Glioma GBM 8401 Cells. Evid. Based Complement Altern. Med. 2012;2012:396573. doi: 10.1155/2012/396573. [DOI] [PMC free article] [PubMed] [Google Scholar]