ABSTRACT
CD4+Foxp3+ regulatory T cells (Tregs) in the tumor microenvironment restrain antitumor immunity, resulting in tumor aggression and poor survival in hepatocellular carcinoma (HCC). CD8+CD122+ Tregs have been previously shown to be more potent in immunosuppression than are CD4+Foxp3+ Tregs. Previous studies have demonstrated that resveratrol exerts its anti-cancer effects by downregulating CD4+Foxp3+ and M2-like macrophages, two key immunoregulatory cells that maintain the immunosuppressive tumor microenvironment. In this study, we found that resveratrol inhibited the tumor growth in a subcutaneous Hepa1-6 HCC model and decreased the frequency of CD8+CD122+ Tregs in the tumor as well as lymph nodes and spleen of the tumor-bearing mice. It also increased the percentage of IFN-γ-expressing CD8+ T cells in the tumor and peripheral lymphoid organs. The antitumor effects of resveratrol were partially reversed by the adoptive transfer of exogenous CD8+CD122+ Tregs into the tumor-bearing mice. Meanwhile, resveratrol treatment downregulated immunosuppressive cytokines, including TGF-β1 and interleukin-10, in the tumor while elevating antitumor cytokines, TNF-α and IFN-γ. It also inhibited the activation of STAT3 signaling in the tumor. As expected, resveratrol reduced the percentage of M2-like macrophages in the mice. Importantly, resveratrol suppressed orthotopic H22 tumor growth and decreased the frequency of CD8+CD122+ Tregs and M2-like macrophages in the tumor-bearing mice. Furthermore, our studies showed that resveratrol, at non-cytotoxic concentrations, inhibited CD8+CD122+ Treg differentiation from CD8+CD122− T cells in vitro. Thus, our studies unveiled a new immune mechanism underlying the immunosuppressive tumor microenvironment and demonstrated that resveratrol could help reverse it by diminishing CD8+CD122+ Tregs.
KEYWORDS: Resveratrol, regulatory T cells, tumor-associated macrophages, tumor microenvironment, hepatocellular carcinoma
Introduction
The importance of the tumor immune microenvironment in solid tumors has been well recognized in recent years. The major types of non-tumoral cells that establish the tumor microenvironment are cancer-associated fibroblasts, endothelial cells and immune cells, including macrophages and T cells.1 Macrophages can polarize into two functional phenotypes, classically activated M1 and alternatively activated M2.2 Tumor-associated macrophages (TAMs) are abundant in the neoplastic microenvironment, and are mostly of M2 phenotype, thus supporting tumor progression.3 On the other hand, CD4+CD25+Foxp3+ Tregs also contribute to the immunosuppressive tumor microenvironment4 while CD8+ Tregs are emerging as an important subset of Tregs recently.5 Since the pioneering work by Suzuki’s group provided the first evidence that CD8+CD122+ T cells maintain T-cell homeostasis,6 mounting evidence has shown that CD8+CD122+ T cells are also Tregs that suppress conventional T cell responses5,7–10 and antitumor immunity.11–13 Although the exact mechanisms underlying the immunosuppression by CD8+CD122+ Tregs are still largely unknown, IL-10 and Fas/FasL death pathway have been demonstrated to be involved.5,8,9,14 In the context of tumor, CD4+CD25+Foxp3+ Tregs have been extensively studied, whereas there are only few studies on CD8+CD122+ Tregs, although it has been demonstrated that elimination of CD8+CD122+ Tregs can enhance antitumor immunity.11–13
Hepatocellular carcinoma (HCC) is one of the most common cancers that is ranked the fifth in prevalence and third in mortality.15 It has been demonstrated that tumor-infiltrating M2 macrophages are enriched16 in HCC. Establishment of M2 macrophage population is also associated with poor prognosis in HCC.17 On the other hand, CD4+Foxp3+ Tregs in tumors are significantly increased in HCC patients, and upregulation of Tregs is associated with a reduction in effector CD8+ T cells in tumors and a worse prognosis for the patients.18 Given that CD8+CD122+ Tregs are more potent in the suppression of T cell proliferation than CD4+CD25+ Tregs,9 we propose that diminishing CD8+CD122+ Tregs may be a strategy for HCC immunotherapy.
Resveratrol (trans-3,4ʹ,5-trihydroxystilbene), originally isolated from the roots of white hellebore, is also found in several dietary sources, such as grapes, berries, and peanuts.19 Resveratrol exerts a variety of biological effects, including antioxidant and anti-inflammatory properties.19 Resveratrol also exhibits multiple antitumor activities, including suppression of tumor cell proliferation, induction of tumor cell apoptosis, increases in chemosensitization of tumor cells, and anticancer activity as a caloric restriction mimetic.20,21 It has recently become evident that resveratrol can regulate the tumor microenvironment. Resveratrol has been reported to inhibit CD4+Foxp3+ Tregs in different tumor models,22–25 while its role in TAMs/M2 polarization has been preliminarily explored.26,27 Although researchers have explored the effects of resveratrol on HCC,28–31 it remains unclear how resveratrol impacts the tumor microenvironment. Here, we found that resveratrol suppressed HCC progression in a subcutaneous tumor mouse model, reduced CD8+CD122+ Tregs and TAMs (M2 macrophages), elevated IFN-γ-expressing CD8+ T cells in the tumor, and downregulated inhibitory cytokines while increasing effector cytokines in the tumor. Its effects on tumor growth, reduction in CD8+CD122+ Tregs and TAMs, and the reversal of immunosuppressive tumor microenvironment at the cytokine level were also confirmed in an orthotopic HCC mouse model.
Materials and methods
Animals
C57BL/6 and BALB/c mice (6–8 weeks old, body weight 20 ± 2 g) were purchased from Experimental Animal Center of Guangdong Province (Guangzhou, China). All mice were housed in a specific pathogen-free animal facility with a controlled condition. All animal experiments were approved by the Institutional Animal Care and Use Committee of Guangdong Provincial Academy of Chinese Medical Sciences.
Chemical and cell lines
Resveratrol was purchased from Med Chem Express (MCE, USA). Mouse HCC cell lines Hepa1-6 and H22 were provided by China Infrastructure of Cell Lines and Resources (Beijing, China).
HCC tumor models and treatments
To establish subcutaneous HCC tumor models, each C57BL/6 mouse was injected with 5 × 106 Hepa1-6 cells in 100 μl PBS on the right flank. Seven days after tumor cell inoculation, mice were randomly grouped and treated with resveratrol (50 mg/kg body weight) in 0.5% of carboxymethylcellulose-sodium salt (CMC-Na, also used as vehicle) through oral gavage daily. Mice were weighed, while tumor volume was monitored every three days by caliper measurement and calculated by length× width2/2. Mice were sacrificed for various experiments 3 weeks after differential treatment because the tumors in some mice reached 1.5 cm in diameter 4 weeks after the treatment, which is against our animal ethics.
To establish an orthotopic HCC tumor model, 5 × 106 H22 cells in 100 μl PBS was subcutaneously injected to the right flank of BALB/c mouse. The tumor was peeled and cut into around 1 mm3 pieces when its diameter reached about 1 cm, and then one piece of tumor was implanted into the left lobe of the liver in recipient BALB/c mice under anesthesia. Five days after tumor implantation, mice were grouped, weighed and treated with resveratrol as described above. Three weeks after tumor implantation, mice were sacrificed for various experiments.
Isolation of immune cells from blood, spleen, draining lymph nodes (LNs), or abdominal cavity
At the end of treatment, blood from tumor-bearing mice was collected and lysed with ammonium chloride–potassium (ACK) lysing buffer (Boster, China) for 5 minutes to generate cell suspensions. Spleens and LNs were mechanically homogenized in Dulbecco’s phosphate-buffered saline (DPBS), lysed with ACK and then passed through 70 μm cell strainer (BD Falcon). Peritoneal cavity washes (PCW) were extracted from mice that were intraperitoneally injected with 5 ml DPBS and gently kneaded for several times.
Preparation of tumor single-cell suspension and isolation of tumor-infiltrating lymphocytes (TILs)
Tumors were finely chopped and digested with 1 mg/mL collagenase type V (Sigma) for 1 h at 37°C. After incubation, digested tissues were filtered using a 70 μm cell strainer. The harvested single-cell suspension was incubated with relevant antibody cocktails for flow cytometric assays or utilized to isolate TILs using Mouse Tumor-Infiltrating Lymphocyte Isolation Kit (Solarbio).
Flow cytometric assays
Single-cell suspensions derived from the tumor, LN, spleen, blood or abdominal cavity were harvested and stained with fluorochrome-conjugated antibodies. However, the tumor single-cell suspensions were stained with Fixable Viability Stain 510 (Thermo Fisher Scientific) prior to antibody staining to distinguish between living and dead cells. To analyze M2 macrophages, cells were stained with anti-CD45 PerCP/Cy5.5 (BioLegend), F4/80 APC (BioLegend), CD11b PE (BioLegend) and CD206 PE/CY7 (eBioscience) antibodies. To detect CD8+CD122+ Tregs, cells were stained with anti-CD45 PerCP/Cy5.5, CD8 APC/eFluor780 (eBioscience) and CD122 PE or isotypes (BioLegend) antibodies. To measure IFN-γ+ T cells, 1 × 106 cells were incubated in 96-well plates in the presence of PMA (50 ng/ml, MultiSciences) and Ionomycin (1 μg/ml, MultiSciences) for 6 h at 37°C. Monensin (5 μg/ml, MultiSciences) was added 2 h after the addition of PMA/Ionomycin. Cells were first stained with anti-CD45 PerCP/Cy5.5 and CD8 APC/eFluor780, fixed in 1% paraformaldehyde, permeabilized and then stained with anti-IFN-γ APC (eBioscience). Cells finally were analyzed through FACSCalibur (BD Biosciences).
ELISA
For blood cytokine assays, murine blood was centrifuged at 2000 rpm for 20 min at room temperature, and then serum was harvested and analyzed using ELISA kits for TNF-α, IFN-γ, TGF-β1 and IL-10 (MultiSciences). For tumor cytokine assays, tumors were weighed, finely chopped, mixed with DPBS with PMSF protease inhibitor (90 μl per 10 mg of tumor tissue), and homogenized mechanically. The supernatant was harvested after centrifugation at 5000 g for 10 min at 4°C, and the concentration of the protein was measured using a BCA protein assay kit (Thermo Fisher Scientific). Finally, the absorbance was read at 450 nm via a spectrophotometer (Thermo Fisher Scientific).
Immunohistochemistry and immunofluorescence
Formalin-fixed and paraffin-embedded tumor samples were cut into 3.5-µm sections, deparaffinized, and antigen-retrieved using sodium citrate solution (0.01 M, PH: 6.0). Then, the sections were processed for antibody staining for immunohistochemistry (IHC) or immunofluorescence (IF). For IHC, slides were incubated with primary anti-PCNA (1:250, sc-56, Santa Cruz), anti-Foxp3 (1:100, 12,653, Cell Signaling Technology) or anti-CD8 Ab (1:200, ab203035, Abcam), and then secondary antibody HPR anti-mouse or rabbit IgG (Maxim). The slides were colored with 3, 3ʹ-Diaminobenzidine (DAB, Sigma-Aldrich) and counterstained by hematoxylin. For IF, anti-CD31 (1:250, sc-376,764, Santa Cruz), anti-CD206 (1:1000, ab64693, Abcam) and anti-F4/80 (1:250, sc-377,009, Santa Cruz) were used as primary Abs while anti-mouse IgG (H + L) (1:1000, 4408, Cell Signaling Technology) and anti-rabbit IgG (H + L) (1:1000, 4413, Cell Signaling Technology) were used as secondary Abs. The sections finally were mounted using DAPI-Fluoromount-G clear mounting agents (Southern Biotech, Birmingham, UK) and images were taken using fluorescence microscopy (Nikon, Japan). Positive cells were quantified using Image J software and expressed as the mean of the percentage of positive cells ± SD with 10 randomly selected fields per sample.
TUNEL assays
TUNEL assays were performed using a TUNEL Apoptosis Detection Kit (Keygentech, China) for the detection of apoptotic tumor cells in the paraffin-embedded tumor tissue. Briefly, upon permeabilized with proteinase K, the slides were treated sequentially with biotin-11-dUTP, terminal deoxynucleotidyl transferase and streptavidin-fluorescein, and finally stained with DAPI-fluoromount-G clear mounting agents. The slides were monitored using fluorescence microscopy. The fluorescence signal was quantified using Image J software, with 10 randomly selected microscopic fields per sample.
Adoptive transfer of CD8+ CD122+ Tregs
Cells were isolated from the spleen of naïve C57BL/6 mice, stained with fluorochrome-conjugated anti-CD8-APC/eFluor780 and anti-CD122-PE antibodies, and then sorted for CD8+CD122+ Tregs via FACSAria III (BD Biosciences). The establishment of tumor models and treatment with resveratrol were described above. Three days after the start of resveratrol therapy, 1 × 106 CD8+CD122+ Tregs per mouse were injected intravenously through the tail vein.
Cytotoxicity assays
Cytotoxicity was detected using Cell Counting Kit-8 (CCK-8) assays (MedChemExpress). Briefly, 20 µL of CCK-8 was added to each well and incubated at 37°C for 2 h. The absorbance was measured by a microplate spectrophotometer (Thermo Fisher Scientific, USA) at the wavelength of 450 nm. Viability of control sample without resveratrol was set as 100%.
Induction and inhibition of CD8+CD122+ Tregs in vitro
CD8+ CD122-T cells were FACS-sorted from C57BL/6 mice, cultured in 96-well plates (5 × 105 cells/well) in complete RPMI-1640 medium containing 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin, and stimulated with anti-CD3/anti-CD28 mAbs (2.5 µg/ml) plus IL-2 (10 ng/ml, Peprotech) in the presence or absence of IL-15 (50 ng/ml, Peprotech) and different concentrations (0, 10, 20 and 40 µM, respectively) of resveratrol for 4 days. The frequency of CD8+ CD122+ Tregs was determined via FACS analysis.
Western blot analysis
Cells or tumor tissues were lysed in RIPA lyzing buffer (Beyotime, China), followed by centrifugation at 12,000 rpm and 4◦C for 10 min. The supernatant was harvested and the concentration of protein was measured using a BCA protein assay kit (Thermo Fisher Scientific). The protein samples were electrophoresed in 10% SDS-PAGE gel and electro-transferred to a PVDF membrane. After blocking in TBST containing 5% (w/v) BSA, the membrane was incubated with rabbit anti-STAT3, anti-p-STAT3, or anti-GADPH (1:1000, all from Cell Signaling Technology) antibody at 4°C overnight. The membrane was then incubated with HPR-conjugated goat anti-rabbit IgG (1:2000, Cell Signaling Technology) for 1 h at room temperature. Finally, blots were detected by a Bio-Rad Gel imaging system and analyzed using Image J software.
Statistical analysis
Data were presented as the mean ± SD and analyzed using GraphPad Prism 6 (GraphPad Software, USA). Statistical comparisons between two groups were performed using a two-tailed Student’s t-test. Statistically significant difference was defined as P < .05.
Results
Resveratrol inhibits the growth of hepatocellular carcinoma in a subcutaneous Hepa1-6 tumor model of mice
To evaluate the antitumor efficacy of resveratrol on HCC, we established a subcutaneous Hepa1-6 HCC model of mice and monitored the tumor growth and body weight during the intervention in immune-competent C57BL/6 mice. The treatment was terminated after 3 weeks of oral gavage with resveratrol daily. Based on TumGrowth,32 a web tool to analyze tumor growth curves, we found that there were no obvious differences in murine body weight gain between vehicle and resveratrol-treated groups (Figure 1a), but the tumor growth was significantly inhibited (Figure 1b and 1c) by resveratrol. Immunohistochemical analysis revealed that resveratrol significantly inhibited the tumor cell proliferation, as judged by a decrease in expression of the proliferating cell nuclear antigen (PCNA) (Figure 1d). The TUNEL assays showed that apoptotic cells were significantly increased in the tumor of mice treated with resveratrol compared to that of control vehicle mice (Figure 1e). Moreover, immunofluorescence was used to quantify CD31 expression, a well-established marker for endothelial cells.33 We found that resveratrol reduced the percentage of CD31+ cells and microvessel density (Figure 1f). These findings indicate that antitumor activity of resveratrol may result from inhibiting cell proliferation/vascularization or inducing apoptosis in the syngeneic Hepa1-6 tumor models.
Figure 1.
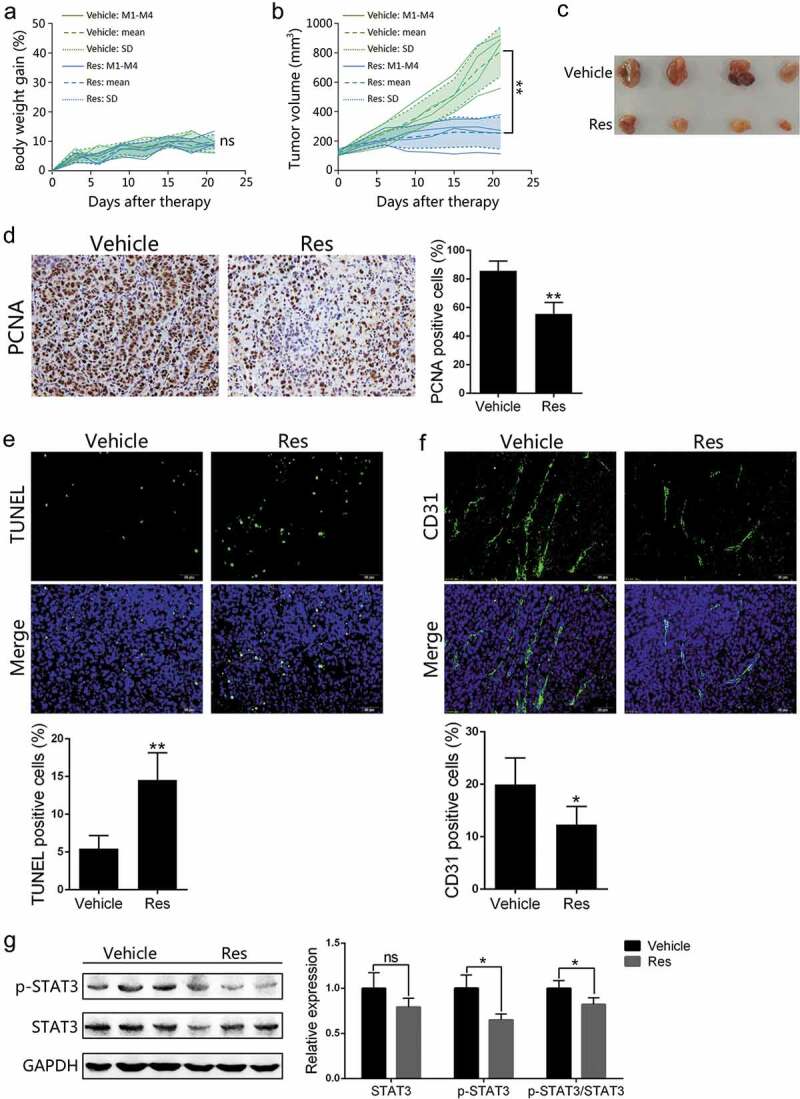
Resveratrol inhibits the growth of subcutaneous Hepa1-6 tumors in C57BL/6 mice. Individual C57BL/6 mice were injected subcutaneously with 5 × 106 Hepa1-6 cells. One week after implantation, mice were treated with vehicle or resveratrol for 21 days. Body weight gains (a) and tumor volumes (b) were measured every three days during the treatment. The solid line represents the value of a single mouse, the long dotted line and the short dotted line represent the mean and SD, respectively, pooled from 4 mice. Images of tumors at the end of the treatment are also shown (c). Paraffin sections were prepared for immunohistochemical staining using anti-PCNA antibody (d), TUNEL staining (e) or immunofluorescence staining using anti-CD31 antibody (f), and images were taken using microscopy (200×) (n = 4 mice/group). Phosphorylated-STAT3 (p-STAT3) and total STAT3 expression levels in the tumor tissue derived from resveratrol- or vehicle-treated mice (n = 3 mice/group) were measured via Western blotting (g). Representative images from each experiment are shown. Data are expressed as mean ± SD from a representative of three independent experiments (M, mice; Res, resveratrol; ns, nonsignificant; two-tailed t-test: *p < 0.05, **p < 0.01 vs. Vehicle)
Resveratrol treatment suppresses the activation of STAT3
Activation of STAT3 signaling in tumors plays a pivotal role in tumor growth and it counteracts antitumor immune responses in cancer immunotherapy.34 Given that resveratrol suppressed STAT3 activation in myeloma cells,35 and that the progression of HCC was inhibited by resveratrol treatment in our studies (Figure 1b and 1c), we examined the p-STAT3/STAT3 expression in Hepa1-6 tumors through western blotting. Our results demonstrated that p-STAT3 level was obviously downregulated by resveratrol while it also reduced the ratio of p-STAT3 vs. total STAT3 (Figure 1g).
Resveratrol decreases Tregs in Hepa1-6 tumor-bearing mice
It has been reported that resveratrol can treat breast cancer,23 lymphoma24 and melanoma25 through inhibition of CD4+Foxp3+ Tregs. In our HCC models, we also found that resveratrol decreased the frequency of CD4+Foxp3+ Tregs in tumor tissue and peripheral lymphoid organs (Fig. S1). Given the negative impacts of CD8+CD122+ Tregs on tumor immunity has been recognized recently, we determined the effects of resveratrol on CD8+CD122+ Tregs via FACS analysis. The gating strategy is shown in the supplementary figure (Fig. S2-S3). Twenty-one days after resveratrol treatment, we found an increase in CD8+ T cells within CD45+ leukocytes in the tumors in resveratrol-treated group compared with vehicle group, while the proportion of CD122+ T cells within CD8+ T cell population was significantly decreased by resveratrol (Figure 2a). The percentages of CD8+CD122+ Tregs in spleens and draining lymph nodes were also determined. As shown in Figure 2b, resveratrol treatment lowered the percentages of CD8+CD122+ Tregs in both spleen and lymph nodes compared to vehicle group.
Figure 2.
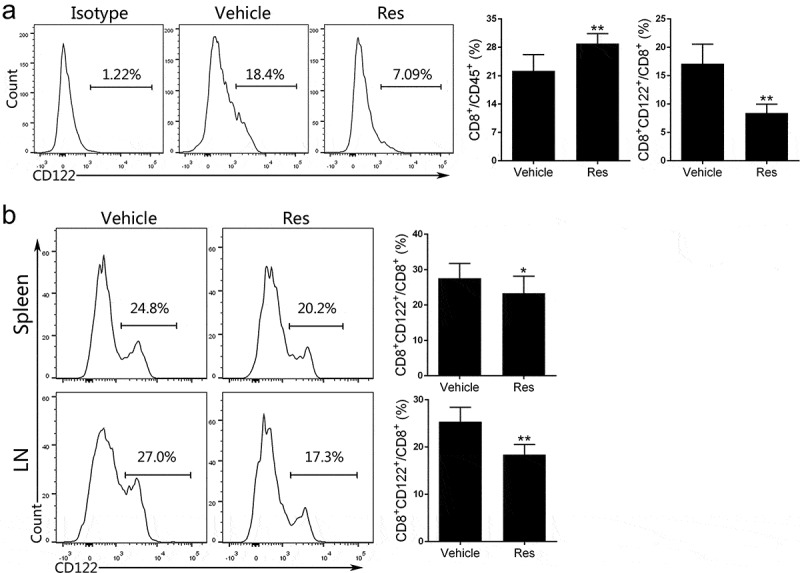
Resveratrol treatment reduces the frequency of CD8+ CD122+ Tregs in Hepa1-6 tumor-bearing mice. (a) Single-cell suspensions isolated from tumor tissue 21 days after the treatment with resveratrol were prepared, stained with anti-CD45, CD8, and CD122 antibodies and analyzed via FACS. (b) Spleen and draining lymph node (LN) cells were isolated from the tumor-bearing mice. The frequencies of CD8+CD122+ Tregs were also analyzed through FACS. Data are presented as mean ± SD (Res, resveratrol; n = 9 mice/group pooled from three separate experiments; two-tailed t-test: *p < 0.05 and **p < 0.01 compared to Vehicle). One representative of three separate experiments is shown
Resveratrol also reduces the M2-like macrophages in Hepa1-6 tumor-bearing hosts
We then determined the effects of resveratrol on TAMs. As shown in Figure 3a, the percentage of TAMs (CD11b+F4/80+CD206+ M2 macrophages) in the tumor, as analyzed by FACS, was obviously decreased in resveratrol-treated group compared with the vehicle control. Meanwhile, similar results were also observed by immunofluorescence analysis of F4/80+CD206+ M2 macrophages in the tumor tissue (Figure 3b). Furthermore, cells from spleen and peritoneal cavity washes (PCW) in resveratrol- and vehicle-treated mice were also analyzed by FACS. The results showed that M2 macrophages from both spleen and PCW were reduced in resveratrol-treated group (Figure 3c). The gating strategy is shown in the supplementary figure (Fig. S4). Taken together, these findings suggest that resveratrol can reduce protumoral populations of CD8+CD122+ Tregs and M2-type TAMs.
Figure 3.
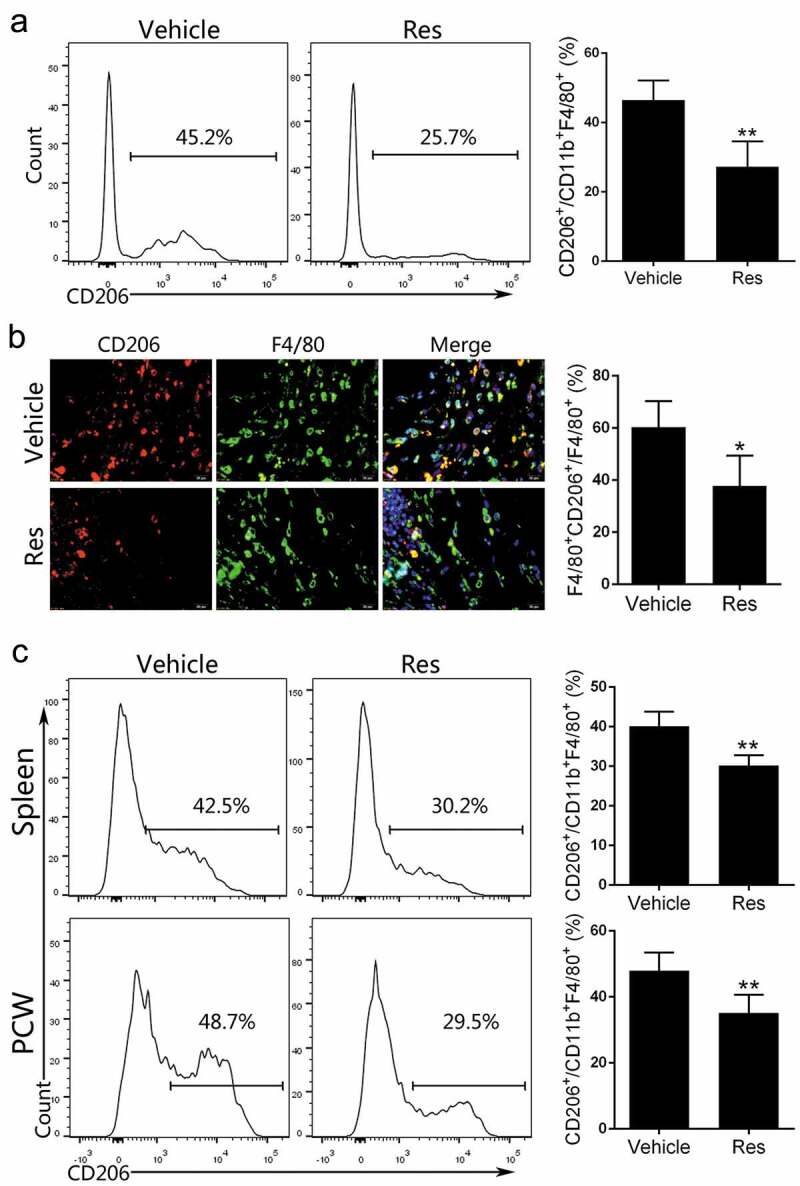
Resveratrol treatment decreases the percentages of M2-type macrophages in Hepa1-6 tumor-bearing mice. (a) Single-cell suspensions isolated from tumor tissue of mice 21 days after treatment with resveratrol were prepared, stained with anti-CD45, F4/80, CD11b and CD206 antibodies and analyzed via FACS. (b) Immunofluorescence analysis (400×) of M2-type macrophages stained with anti-F4/80 and CD206 antibodies in the tumor tissue. (c) Peritoneal cavity washes (PCW) and spleen cells were isolated from the tumor-bearing mice and the frequencies of F4/80+ CD11b+ CD206+ M2-type macrophages were enumerated by FACS analysis. Data of column graphs are presented as mean ± SD (n = 4 mice/group; two-tailed t-test: *p < 0.05 and **p < 0.01 compared to Vehicle). One representative of three separate experiments is shown
Resveratrol augments IFN-γ-positive CD8+ T cells in Hepa1-6 tumor-bearing mice
IFN-γ is an important effector cytokine secreted by CTLs to eliminate tumors.36 37 To further examine that resveratrol would enhance antitumor immunity, the expression of IFN-γ by CD8+ T cells was analyzed by FACS. Tumor-infiltrating lymphocytes (TILs) were isolated from tumors on day 21, incubated with PMA/Ionomycin and monensin, and stained with anti-CD8 and anti-IFN-γ antibodies. The gating strategy is shown in the supplementary figure (Fig. S5). Results showed that the percentage of IFN-γ-expressing CD8+ T cells in tumors was significantly increased by resveratrol treatment (Figure 4). Similarly, the percentage of CD8+IFN-γ+ T cells from both spleen and lymph nodes was elevated in resveratrol-treated group compared to vehicle group (Figure 4).
Figure 4.
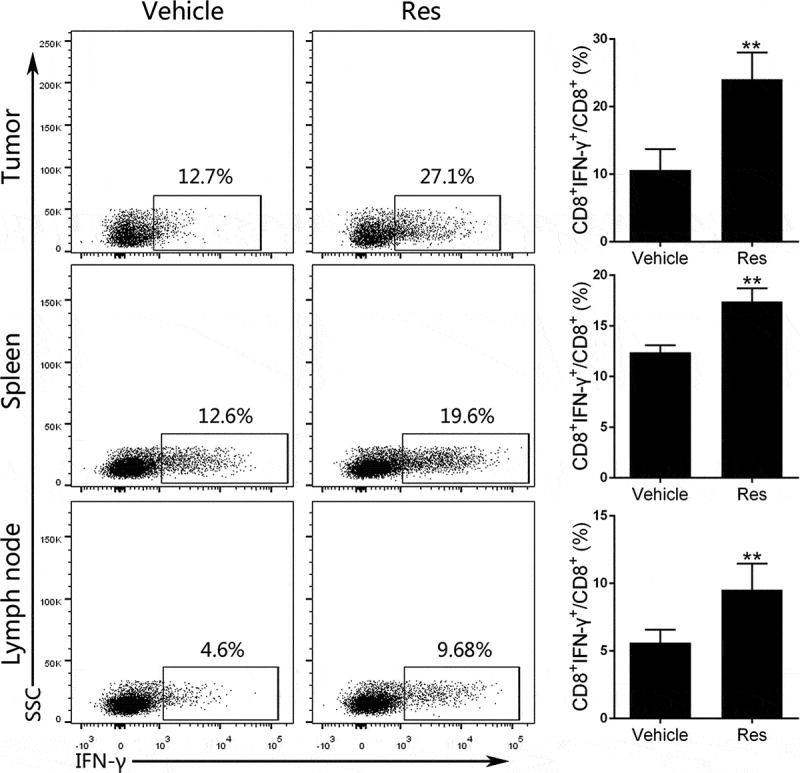
Resveratrol treatment increases IFN-γ-expressing CD8+ T cells in the Hepa1-6 tumor-bearing mice. Tumor-infiltrating lymphocytes, spleen and draining lymph node (LN) cells were isolated from tumor-bearing mice of resveratrol- or vehicle-treated group on day 21. Then 1 × 106 cells were incubated in the presence of PMA/Ionomycin and monensin as described in the methods. The percentages of CD8+IFN-γ+ T cells from tumors, spleens or lymph nodes were enumerated by FACS analysis after intracellular staining of IFN-γ. Gating strategy for dot plots was indicated in supplemental materials. Data are shown as mean ± SD from a representative of three independent experiments (Res, resveratrol; n = 4–5 mice/group; two-tailed t-test: **p < 0.01 compared to Vehicle)
Adoptive transfer of CD8+CD122+ Tregs promotes tumor growth in C57BL/6 mice
As our results showed that resveratrol reduced CD8+CD122+ Tregs in the tumors (Figure 2) and increased IFN-γ expression in CD8+ T cells (Figure 4), we speculated that resveratrol-induced antitumor immunity is associated with the reduction of CD8+CD122+ Tregs in tumor-bearing mice. To confirm this finding, external CD8+CD122+ Tregs were adoptively transferred to Hepa1-6 tumor-bearing mice that were treated with resveratrol (Figure 5a). Compared to the group treated with resveratrol alone (Res), the adoptive transfer of CD8+CD122+ Tregs plus resveratrol (Res+Treg) promoted the tumor growth (Figure 5b-5c). Then, we detected CD8+CD122+ Tregs in tumor-bearing mice following their adoptive transfer. Results showed that in tumors, the percentage of CD8+CD122+ Tregs in Res+Treg group was significantly increased compared to Res alone group, albeit still lower than that in Vehicle group (Figure 5d). Similarly, the frequencies of CD8+CD122+ Tregs from blood and draining LNs, but not spleen, in Res+Treg group were also elevated when compared with Res group (Figure 5e), indicating that the transferred Tregs have likely remained in the tumor, blood and LNs. Immunohistochemical analysis of tumor tissue with anti-PCNA antibody and TUNEL assays confirmed that the transfer of CD8+CD122+ Tregs promoted the tumor cell proliferation but reduced apoptosis (Figure 5f). Results suggest that the antitumor activity of resveratrol is, at least in part, due to a reduction in CD8+CD122+ Tregs.
Figure 5.
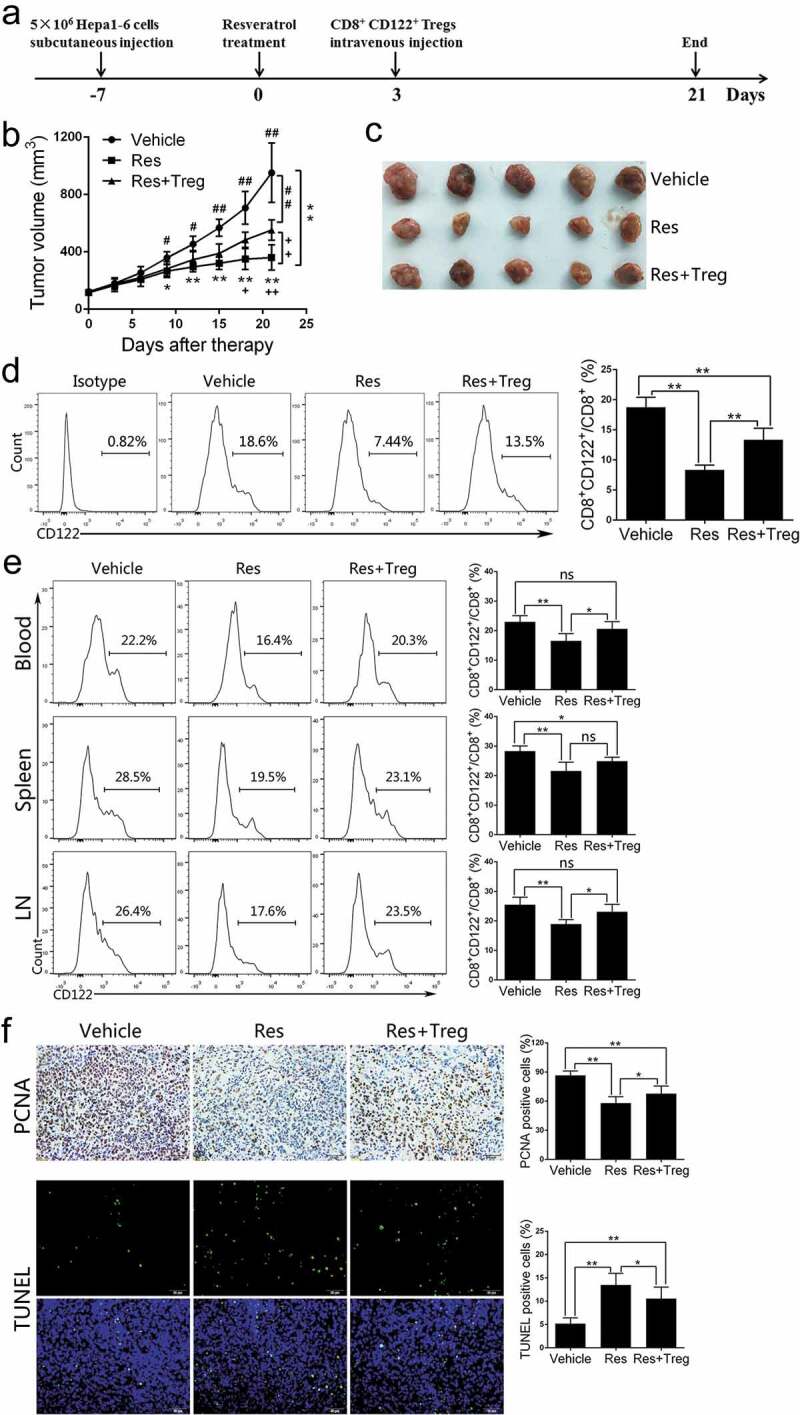
Adoptive transfer of CD8+CD122+ Tregs partially diminishes the effects of resveratrol on Hepa1-6 tumor growth. The tumor models and resveratrol treatment were described in the methods, while 1 × 106 CD8+CD122+ Tregs were injected intravenously 3 days after the start of resveratrol therapy. (a) Treatment schedule for Hepa1-6 tumor model. (b) Tumor growth curves over time. Significant difference is indicated as “*” between Vehicle and Res group, “+” between Res and Res+Treg group, and “#” between Vehicle and Res+Treg group (*p < 0.05, **p < 0.01; +p < 0.05, ++p < 0.01; #p < 0.05, ##p < 0.01). (c) Representative images of the tumors at the end of treatment. (d) Single-cell suspensions isolated from tumor tissue at the end of treatment were prepared, stained with anti-CD45, CD8, and CD122 antibodies and analyzed via FACS. (e) Blood, spleen and draining lymph node (LN) cells were isolated from the tumor-bearing mice. The frequencies of CD8+CD122+ Tregs (d and e) were analyzed via FACS. (f) Paraffin sections were prepared for immunohistochemical staining using anti-PCNA antibody or fluorescence staining for TUNEL. Data are shown as mean ± SD (n = 4–6 mice/group; two-tailed t-test: *p < 0.05 and **p < 0.01). One of three separate experiments is shown
Resveratrol alters cytokine expression in the Hepa1-6 tumor-bearing mice
To further analyze the effects of resveratrol on the immunosuppressive tumor microenvironment, we then monitored cytokine levels using ELISA. As shown in Figure 6, resveratrol significantly increased the level of cytokines TNF-α and IFN-γ in both the tumor and blood while reducing and IL-10 level in the tumor. In the blood, IL-10 was not detectable. Resveratrol also lowered TGF-β1 level in both the tumor and blood. Importantly, the adoptive transfer of CD8+CD122+ Tregs decreased IFN-γ, but not TNF-α, level compared to Res alone while increasing both TGF-β1 and IL-10 levels in the tumor. In the blood, Treg transfer reduced IFN-γ but increased TGF-β1 level compared to Res alone. These results indicate that treatment with resveratrol leads to a reversal of the immunosuppressive tumor microenvironment at the level of cytokines in HCC.
Figure 6.
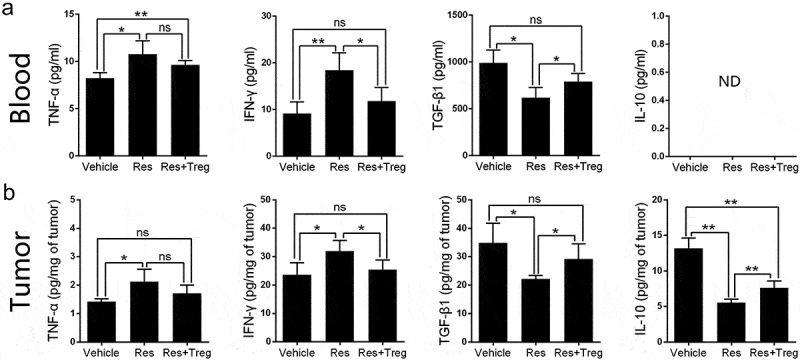
Resveratrol treatment alters the level of cytokines in the tumor-bearing mice. Cytokine levels of TNF-α, IFN-γ, TGF-β1 and IL-10 in blood (a) or tumors (b) of tumor-bearing mice at the end of treatment (day 21) were measured by ELISA. Some mice received 1 × 106 CD8+CD122+ Tregs on day 3. Data are shown as mean ± SD (ns, nonsignificant; ND, not detectable; n = 4 mice/group; two-tailed t-test: *p < 0.05 and **p < 0.01). One of three separate experiments is shown
Resveratrol inhibits tumor growth and downregulates CD8+CD122+ Tregs and M2-like macrophages in an orthotopic HCC mouse model
To further validate our findings, we established an orthotopic tumor model with another mouse HCC cell line H22 in BALB/c mice, which were treated with resveratrol. As shown in Figure 7a-7c, the orthotopic tumor growth was also inhibited by resveratrol therapy in this model. Then, we checked the changes of the corresponding immunoregulatory cells and cytokines. FACS analysis of tumor-infiltrating cells revealed a decrease of CD8+CD122+ Tregs within CD8+ T cells, but an increase in CD8+ T cells within CD45+ leukocyte population in Res group compared with Vehicle group (Figure 7d). Resveratrol treatment also reduced the percentage of CD8+CD122+ Tregs in the blood, spleen and LNs (Figure 7e). Furthermore, the frequency of CD206+ M2-like macrophages in the tumor, spleen and peritoneal cavity washes (PCW) was also decreased by resveratrol (Figure 7f). Finally, the levels of cytokines TNF-α, IFN-γ, TGF-β1 and IL-10 in the tumors and blood were detected via ELISA. We found that the level of TNF-α or IFN-γ in blood was increased while that of TGF-β1 was decreased by resveratrol treatment. Resveratrol also increased IFN-γ but reduced TGF-β1 and IL-10 levels in the tumor (Figure 7g). Taken together, our results in the orthotopic HCC mouse models strengthened our findings that resveratrol exerts antitumor effects through reducing CD8+CD122+ Tregs and M2-like macrophages.
Figure 7.
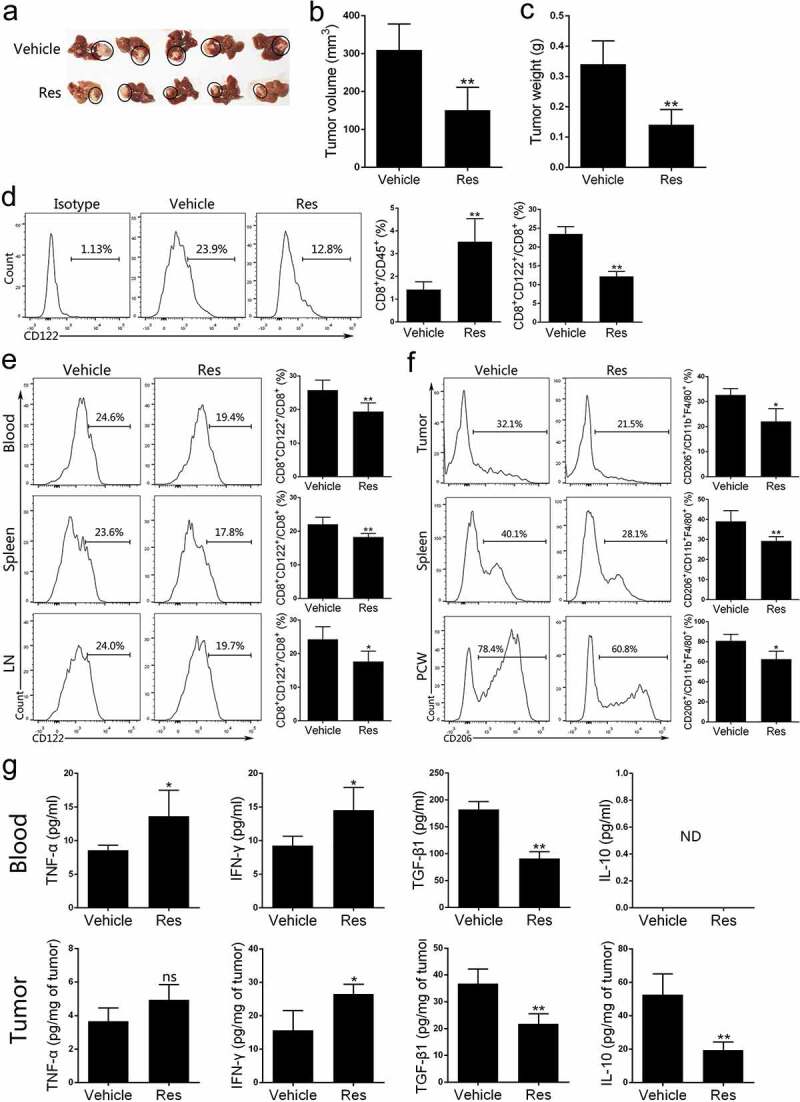
Resveratrol inhibits the growth of orthotopic H22 tumors in BALB/c mice and decreases the percentage of CD8+CD122+ Tregs and M2 macrophages in the tumor-bearing mice. 21 days after orthotopic implantation of H22 tumors in the liver, images of the tumors are shown (a). Then tumors were peeled from liver while their volumes (b) and weights (c) were measured. (d) Single-cell suspensions isolated from tumor tissue at the end of treatment with or without resveratrol were prepared, stained with anti-CD45, CD8, and CD122 antibodies and analyzed via FACS. (e) Blood, spleen and draining lymph node (LN) cells were isolated from tumor-bearing mice and the frequencies of CD8+CD122+ Tregs were analyzed through FACS. (f) Single-cell suspensions of tumors, peritoneal cavity washes (PCW) and spleen from tumor-bearing mice at the end of treatment were prepared, stained with anti-CD45, F4/80, CD11b and CD206 antibodies and analyzed by FACS for F4/80+ CD11b+ CD206+ M2-type macrophages. (g) Cytokine levels of TNF-α, IFN-γ, TGF-β1 and IL-10 in blood or tumors of tumor-bearing mice treated with resveratrol were measured via ELISA. Data are shown as mean ± SD from one representative of three independent experiments (ns, nonsignificant; ND, not detectable; n = 4–6 mice/group; two-tailed t-test: *p < 0.05 and **p < 0.01 vs. Vehicle)
Resveratrol inhibits CD8+CD122+ Treg generation in vitro
Since we found that resveratrol reduced CD8+CD122+ Tregs in vivo, we further determined whether it would do so in vitro. CD8+CD122− T cells were purified from C57BL/6 mice and then co-stimulated with anti-CD3/CD28 mAbs in the presence of IL-2 and/or IL-15 for 4 days to induce CD8+CD122+ Tregs. Two concentrations of resveratrol, 10 and 20 μM, respectively, were tested to generate no cytotoxicity (Figure 8a) and thus were used for Treg induction. The results showed that compared to groups without resveratrol, 10 μM resveratrol partially reduced the frequency of CD8+CD122+ Tregs, while resveratrol, at 20 μM, further decreased their percentage (Figure 8b and 8c). These results indicate that non-cytotoxic concentrations of resveratrol can inhibit the generation of CD8+CD122+ Tregs from CD8+CD122− T cells in vitro.
Figure 8.
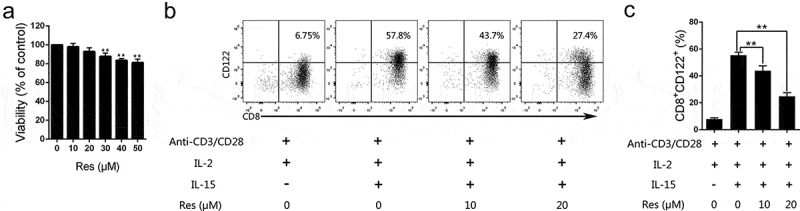
Resveratrol inhibits the generation of CD8+CD122+ Tregs invitro.
(A) CD8+CD122− T cells were FACS-sorted from the spleen of naïve mice and then cultured in the presence of anti-CD3/CD28 mAbs, IL-2 and different concentrations of resveratrol for 4 days. Cytotoxicity was performed using CCK-8 assays. (B and C) CD8+CD122− T cells were cultured in the presence or absence of anti-CD3/CD28 mAbs, IL-2, IL-15 and/or resveratrol as indicated for 4 days. The frequencies of CD8+CD122+ Tregs were detected via FACS analysis. Representative dot plots (B) or quantification data (C) are also shown. Data are presented as mean ± SD (Two-tailed t-test: **p < .01). One representative of three separate experiments is shown.
Discussion
Immunoregulatory cells, such as TAMs and Tregs in the tumor microenvironment, impair the antitumor activity of CTLs by secreting immunosuppressive cytokines TGF-β1 and IL-10.37 Therefore, in order to achieve sufficient efficacy of antitumor immunotherapy, it is necessary to reduce the impacts of these immunosuppressive cells on the tumor microenvironment. Although the capacity of resveratrol to prevent cancer development has been known for many years, its underlying mechanisms remain to be fully elucidated. The antitumor effects of resveratrol on HCC have been reported by some researchers, with a focus on inhibition of tumor cell proliferation, induction of cell apoptosis, an increase in chemosensitization of tumor cells as a caloric restriction mimetic, and chemopreventive effects.21,28–31 In this study, we emphasized the effects of resveratrol on the tumor microenvironment in HCC, especially tumor-promoting regulatory immune cells.
First, we confirmed that resveratrol, at moderate doses (50 mg/kg), effectively inhibited the progression of HCC in a mouse model, which was reflected by the inhibition of cell proliferation (PCNA) and tumor angiogenesis (CD31) as well as induction of cell apoptosis (TUNEL). To further explore the effects of resveratrol on tumor microenvironment, we examined immune cells in the tumor tissue. We found that two subsets of immunosuppressive cells, CD8+CD122+ Tregs and TAMs (M2 macrophages), were reduced, while IFN-γ-expressing cytotoxic effector CD8+ T cells were increased by resveratrol treatment. Next, we verified that the similar changes occurred at the level of effector or immunosuppressive cytokines. The level of TGF-β1 and IL-10 was reduced, while that of TNF-α and IFN-γ was increased in the tumor microenvironment by resveratrol treatment. These results indicated that the antitumor effects of resveratrol on HCC were caused not only by inhibiting cell proliferation and inducing apoptosis of tumor cells but also in part by reversing the immunosuppressive tumor microenvironment. Tumor cells have been shown to produce TGF-β, which in turn facilitates the M2 polarization of macrophages in the tumor microenvironment.34,38 It has been reported that resveratrol inhibits the production of TGF-β and thus alters downstream cell functions mediated by TGF-β.39–42 Thus, we propose that the effects of resveratrol on the polarization of macrophages are probably related to TGF-β signaling pathway.
A main finding in this study is that the frequency of IFN-γ-expressing CD8+ T cells was increased while that of CD8+CD122+ Tregs was decreased in the tumors by resveratrol. CD8+CD122+ Tregs were reported to be more potent in suppressing allograft rejection and regulating vaccine-induced expansion and survival of tumor-specific T cells than CD4+Foxp3+ Tregs.9,11 In this study, the antitumor effects of resveratrol were partially offset when we reintroduced the exogenous CD8+CD122+ Tregs into the tumor-bearing mice treated with resveratrol, confirming that one of the mechanisms by which resveratrol exerts antitumor effects is a reduction in CD8+CD122+ Tregs. The decrease in CD8+CD122+ Tregs in tumors caused by resveratrol treatment likely promoted the expansion of cytotoxic CD8+IFNγ+ T cells. Our results were consistent with a recent study showing that anti-CD122 mAb treatment resulted in a increase in cytolytic intratumoral CD8+ T cells and a decrease in granulocytic myeloid-derived suppressor cells (G-MDSCs) in the tumor microenvironment of colon cancer.13 In addition, we found that CD8+CD122+ Tregs in the blood and second lymphoid organs of the tumor-bearing mice were also reduced by resveratrol, suggesting that resveratrol has a systemic impact on CD8+CD122+ Treg generation. Previous studies demonstrated that IL-10 was a key cytokine produced by CD8+CD122+ Tregs to sustain their suppressive function, and that CD8+CD122+ Tregs produced more IL-10 than did CD4+Foxp3+ Tregs.5,9,43 In present study, resveratrol decreased IL-10 level in the tumor while this effect was reversed by the adoptive transfer of CD8+CD122+ Tregs, indicating that the decrease in IL-10 expression may be attributed to a reduction in CD8+CD122+ Tregs. Thus, we have provided the first evidence that resveratrol downregulates CD8+CD122+ Tregs. These findings suggest that resveratrol can be used not only as an antitumor monotherapy, but also as an immunotherapeutic combination regimen, such as its combination with a chemotherapy, thereby exerting better therapeutic effects.21
We found that resveratrol reduced both CD8+CD122+ Tregs and M2 macrophages in the tumor-bearing mice, likely due to its cytotoxicity or immunoregulatory activities. Some researchers have reported that resveratrol, at low doses, plays an immunomodulatory role.23,24 In this study, doses of 50 mg/kg, which was commonly used by others, had no obvious effects on mouse general health. One may argue that a reduction in CD8+CD122+ Tregs by resveratrol could be due to its cytotoxicity. In order to rule out this possibility, inhibition assays in vitro at a cellular level were conducted. The concentrations of 10 μM and 20 μM, which were non-cytotoxic to CD8+CD122− T cells, still reduced CD8+CD122+ Tregs, confirming that non-cytotoxic doses of resveratrol can inhibit CD8+CD122+ Treg generation. Another mechanism underlying the antitumor effects of resveratrol could be its regulation of PD-L1 expression in the tumor since previous studies have demonstrated that it reduces PDL1 expression in cancer cells and interferes with PDL1 dimerization and glycosylation.44,45 Thus, resveratrol has a broad impact on antitumor immunity.
High STAT3 expression has been detected in HCC samples and associated with tumor invasiveness and poor prognosis.46 Resveratrol was reported to be a potent inhibitor of STAT3 phosphorylation,35 which specifically induced apoptosis of malignant cells expressing activated STAT3.47 Inhibition of STAT3 signaling in tumor cells reversed the immunosuppressive tumor microenvironment48,49 and resulted in enhanced antitumor effects. This information drove us to detect the expression of STAT3/p-STAT3 in HCC. As expected, STAT3 phosphorylation was indeed decreased by resveratrol. The constitutive activation of STAT3 has been observed not only in tumor cells but also in tumor-infiltrating inflammatory cells, such as TAMs,50 so we speculate that the decreased activation of STAT3 may be partially due to the reduction in TAMs by resveratrol.
In conclusion, using both subcutaneous and orthotopic HCC mouse models, we revealed that resveratrol effectively suppressed HCC progression by inhibiting CD8+CD122+ Treg generation, TAMs/M2 macrophage polarization and activation of STAT3 pathway while increasing IFN-γ-expressing effector CD8+ T cells in the tumor-bearing mice. Further, resveratrol also suppressed CD8+CD122+ Treg induction in vitro. Thus, resveratrol improved the immunosuppressive tumor microenvironment by down-regulating CD8+CD122+ Tregs and M2-like macrophages. Based on our results, the antitumor effects of resveratrol result from not only its cytotoxicity to cancer cells but also its immunomodulatory activity, which favors CTLs in the tumor microenvironment, suggesting that resveratrol may be more effective in the treatment of solid tumors when used in combination with other immunotherapeutic drugs.
Supplementary Material
Acknowledgments
This study was supported by the funds from Natural Science Foundation of Guangdong Province (2019A1515110741, 2018A030310530 and 2018A030313256) and Innovation and Enhancement Research Program of Guangzhou University of Chinese Medicine (2018KQNCX050).
Funding Statement
This work was supported by the Natural Science Foundation of Guangdong Province [2019A1515110741]; Natural Science Foundation of Guangdong Province [2018A030310530 and 2018A030313256]; Innovation and Enhancement Research Program of Guangzhou University of Chinese Medicine [2018KQNCX050].
Abbreviation
- HCC
hepatocellular carcinoma
- LN
lymph nodes
- STAT3
activation of signal transducers and activators of transcription 3
- p-STAT3
phosphorylated STAT3
- TAM
tumor-associated macrophage
- TIL
tumor-infiltrating lymphocyte
- Treg
regulatory T cell
Disclosure of interest
The authors of this manuscript have no interests to declare.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Han Y, Jo H, Cho JH, Dhanasekaran DN, Song YS.. Resveratrol as a tumor-suppressive nutraceutical modulating tumor microenvironment and malignant behaviors of cancer. Int J Mol Sci. 2019;20:925. doi: 10.3390/ijms20040925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–13. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–527. doi: 10.1053/j.gastro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paluskievicz CM, Cao X, Abdi R, Zheng P, Liu Y, Bromberg JS. T regulatory cells and priming the suppressive tumor microenvironment. Front Immunol. 2019;10:2453. doi: 10.3389/fimmu.2019.02453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endharti AT, Rifa IM, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, Takeda K, Isobe KI, Suzuki H. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 6.Rifa’i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200(9):1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endharti AT, Okuno Y, Shi Z, Misawa N, Toyokuni S, Ito M,Isobe K-I, Suzuki H. CD8 + CD122 + regulatory T cells (Tregs) and CD4 + tregs cooperatively prevent and cure CD4 + Cell-Induced Colitis. J Immunol. 2011;186(1):41–52. doi: 10.4049/jimmunol.1000800. [DOI] [PubMed] [Google Scholar]
- 8.Akane K, Kojima S, Mak TW, Shiku H, Suzuki H. CD8+CD122+CD49dlow regulatory T cells maintain T-cell homeostasis by killing activated T cells via Fas/FasL-mediated cytotoxicity. Proc Natl Acad Sci U S A. 2016;113:2460–2465. doi: 10.1073/pnas.1525098113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai Z, Zhang S, Xie Q, Wu S, Su J, Li S, Xu Y, Li XC. Natural CD8+CD122+ T cells are more potent in suppression of allograft rejection than CD4+CD25+ regulatory T cells. Am J Transplant. 2014;14(1):39–48. doi: 10.1111/ajt.12515. [DOI] [PubMed] [Google Scholar]
- 10.Molloy MJ, Zhang W, Usherwood EJ. Suppressive CD8+ T cells arise in the absence of CD4 help and compromise control of persistent virus. J Immunol. 2011;186:6218–6226. doi: 10.4049/jimmunol.1003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang LX, Li Y, Yang G, Pang PY, Haley D, Walker EB. CD122+CD8+ Treg suppress vaccine-induced antitumor immune responses in lymphodepleted mice. Eur J Immunol. 2010;40:1375–1385. doi: 10.1002/eji.200839210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu P, Steel JC, Zhang M, Morris JC, Waitz R, Fasso M, Allison JP, Waldmann TA. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci U S A. 2012;109(16):6187–6192. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villarreal DO, Allegrezza MJ, Smith MA, Chin D, Luistro LL, Snyder LA. Targeting of CD122 enhances antitumor immunity by altering the tumor immune environment. Oncotarget. 2017;8(65):109151–109160. doi: 10.18632/oncotarget.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16(24):6019–6028. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24(1):1–17. doi: 10.1016/j.soc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Sakai Y, Honda M, Fujinaga H, Tatsumi I, Mizukoshi E, Nakamoto Y, Kaneko S. Common transcriptional signature of tumor-infiltrating mononuclear inflammatory cells and peripheral blood mononuclear cells in hepatocellular carcinoma patients. Cancer Res. 2008;68(24):10267–10279. doi: 10.1158/0008-5472.CAN-08-0911. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125:1640–1648. doi: 10.1002/ijc.24556. [DOI] [PubMed] [Google Scholar]
- 18.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 19.Piotrowska H, Kucinska M, Murias M. Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat Res. 2012;750(1):60–82. doi: 10.1016/j.mrrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, et al. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017;18:2589. doi: 10.3390/ijms18122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, Levesque S, Castoldi F, Jacquelot N, Yamazaki T, et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell. 2016;30(1):147–160. doi: 10.1016/j.ccell.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Yang S, Liao W, Xiong Y. Modification of antitumor immunity and tumor microenvironment by resveratrol in mouse renal tumor model. Cell Biochem Biophys. 2015;72(2):617–625. doi: 10.1007/s12013-015-0513-z. [DOI] [PubMed] [Google Scholar]
- 23.Lee-Chang C, Bodogai M, Martin-Montalvo A, Wejksza K, Sanghvi M, Moaddel R, de Cabo R, Biragyn A. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol. 2013;191(8):4141–4151. doi: 10.4049/jimmunol.1300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Paik JH, Cho D, Cho JA, Kim CW. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int Immunopharmacol. 2008;8:542–547. doi: 10.1016/j.intimp.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Guan H, Singh NP, Singh UP, Nagarkatti PS, Nagarkatti M. Resveratrol prevents endothelial cells injury in high-dose interleukin-2 therapy against melanoma. PLoS One. 2012;7:e35650. doi: 10.1371/journal.pone.0035650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun L, Chen B, Jiang R, Li J, Wang B. Resveratrol inhibits lung cancer growth by suppressing M2-like polarization of tumor associated macrophages. Cell Immunol. 2017;311:86–93. doi: 10.1016/j.cellimm.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee S, Hussaini R, White R, Atwi D, Fried A, Sampat S, Piao L, Pan Q, Banerjee P. TriCurin, a synergistic formulation of curcumin, resveratrol, and epicatechin gallate, repolarizes tumor-associated macrophages and triggers an immune response to cause suppression of HPV plus tumors. Cancer Immunol Immun. 2018;67:761–774. doi: 10.1007/s00262-018-2130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao F, Deng G, Liu W, Zhou K, Li M. Resveratrol suppresses human hepatocellular carcinoma via targeting HGF-c-Met signaling pathway. Oncol Rep. 2017;37(2):1203–1211. doi: 10.3892/or.2017.5347. [DOI] [PubMed] [Google Scholar]
- 29.Chai R, Fu H, Zheng Z, Liu T, Ji S, Li G. Resveratrol inhibits proliferation and migration through SIRT1 mediated posttranslational modification of PI3K/AKT signaling in hepatocellular carcinoma cells. Mol Med Rep. 2017;16:8037–8044. doi: 10.3892/mmr.2017.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B, Yin X, Sui S. Resveratrol inhibited the progression of human hepatocellular carcinoma by inducing autophagy via regulating p53 and the phosphoinositide 3kinase/protein kinase B pathway. Oncol Rep. 2018;40:2758–2765. [DOI] [PubMed] [Google Scholar]
- 31.Bishayee A, Dhir N. Resveratrol-mediated chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis: inhibition of cell proliferation and induction of apoptosis. Chem Biol Interact. 2009;179:131–144. doi: 10.1016/j.cbi.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Enot DP, Vacchelli E, Jacquelot N, Zitvogel L, Kroemer G. TumGrowth: an open-access web tool for the statistical analysis of tumor growth curves. Oncoimmunology. 2018;7:1462431. doi: 10.1080/2162402X.2018.1462431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 34.Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 36.Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002;99:3280–3285. doi: 10.1182/blood.V99.9.3280. [DOI] [PubMed] [Google Scholar]
- 37.Zhao F, Korangy F, Greten TF. Cellular immune suppressor mechanisms in patients with hepatocellular carcinoma. Dig Dis. 2012;30(5):477–482. doi: 10.1159/000341695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, Liang X, Ma C. Tim-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages. Gut. 2015;64:1593–1604. doi: 10.1136/gutjnl-2014-307671. [DOI] [PubMed] [Google Scholar]
- 39.Tiwari V, Chopra K. Resveratrol prevents alcohol-induced cognitive deficits and brain damage by blocking inflammatory signaling and cell death cascade in neonatal rat brain. J Neurochem. 2011;117:678–690. [DOI] [PubMed] [Google Scholar]
- 40.Rahal K, Schmiedlin-Ren P, Adler J, Dhanani M, Sultani V, Rittershaus AC, Reingold L, Zhu J, McKenna BJ, Christman GM, et al. Resveratrol has antiinflammatory and antifibrotic effects in the peptidoglycan-polysaccharide rat model of Crohn’s disease. Inflamm Bowel Dis. 2012;18:613–623. doi: 10.1002/ibd.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suenaga F, Hatsushika K, Takano S, Ando T, Ohnuma Y, Ogawa H, Nakao A. A possible link between resveratrol and TGF-beta: resveratrol induction of TGF-beta expression and signaling. FEBS Lett. 2008;582:586–590. doi: 10.1016/j.febslet.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 42.Serrero G, Lu R. Effect of resveratrol on the expression of autocrine growth modulators in human breast cancer cells. Antioxid Redox Signal. 2001;3:969–979. doi: 10.1089/152308601317203512. [DOI] [PubMed] [Google Scholar]
- 43.Rifa’i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe KI, Suzuki H. CD8(+)CD122(+) regulatory T cells recognize activated T cells via conventional MHC class I-alpha beta TCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20:937–947. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- 44.Lin CC, Chin YT, Shih YJ, Chen YR, Chung YY, Lin CY, Hsiung CN, Whang-Peng J, Lee SY, Lin HY, et al. Resveratrol antagonizes thyroid hormone-induced expression of checkpoint and proliferative genes in oral cancer cells. J Dent Sci. 2019;14:255–262. doi: 10.1016/j.jds.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verdura S, Cuyas E, Cortada E, Brunet J, Lopez-Bonet E, Martin-Castillo B, Bosch-Barrera J, Encinar JA, Menendez JA. Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity. Aging (Albany NY). 2020;12(1):8–34. doi: 10.18632/aging.102646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, Carter B, Turkson J, Jove R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther. 2006;5(3):621–629. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- 48.Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(-) stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121:2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G, Liu A, Wang TC, Yang CS. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res (Phila). 2012;5(2):205–215. doi: 10.1158/1940-6207.CAPR-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S,Niu G, Kay H, Mulé J, Kerr WG, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11(12):1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


