Abstract
Amyotrophic lateral sclerosis (ALS) is a rapidly disabling and fatal neurodegenerative disease. Due to insufficient disease-modifying treatments, there is an unmet and urgent need for elucidating disease mechanisms that occur early and represent common triggers in both familial and sporadic ALS. Emerging evidence suggests that impaired DNA damage response contributes to age-related somatic accumulation of genomic instability and can trigger or accelerate ALS pathological manifestations. In this review, we summarize and discuss recent studies indicating a direct link between DNA damage response and ALS. Further mechanistic understanding of the role genomic instability is playing in ALS disease pathophysiology will be critical for discovering new therapeutic avenues.
Keywords: amyotrophic lateral sclerosis, DNA damage response, Frontotemporal dementia, Genome instability, Neurodegeneration, Repeat expansion
Amyotrophic lateral sclerosis (ALS) is a lethal degenerative motor neuron disease with a median survival of 2–4 years after diagnosis [1] and no available effective treatment [2]. Caused by loss of motor neurons in the motor cortex, brain stem and spinal cord, the worldwide annual incidence of ALS is approximately 1 per 50,000 live births and is expected to exponentially increase in the next 20 years [3]. Since it leads to severe disability with high fatality rate, there is an extensive socioeconomic burden alongside the unmet medical need [1,4,5]. Most likely, as for most neurodegenerative diseases, one of the reasons for the slow progression in the development of novel therapies in ALS is the fact that the underlying neurodegeneration may start decades before clinical diagnosis [6–10]. Thus, a better understanding of the disease mechanisms that appear early and represent common triggers in both familial (fALS) and sporadic (sALS) forms of ALS is required as to inform on early diagnostic/prognostic markers and therapies.
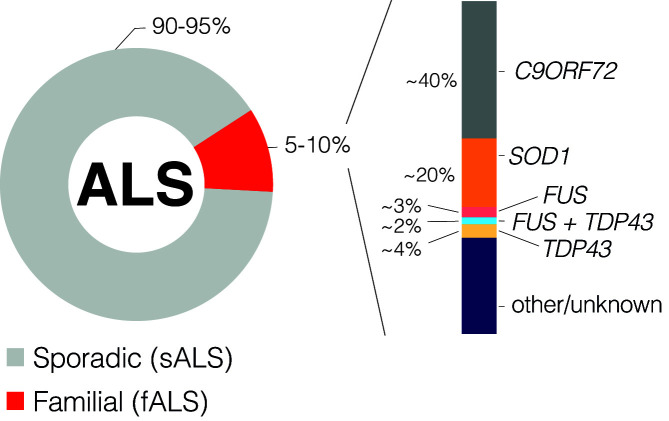
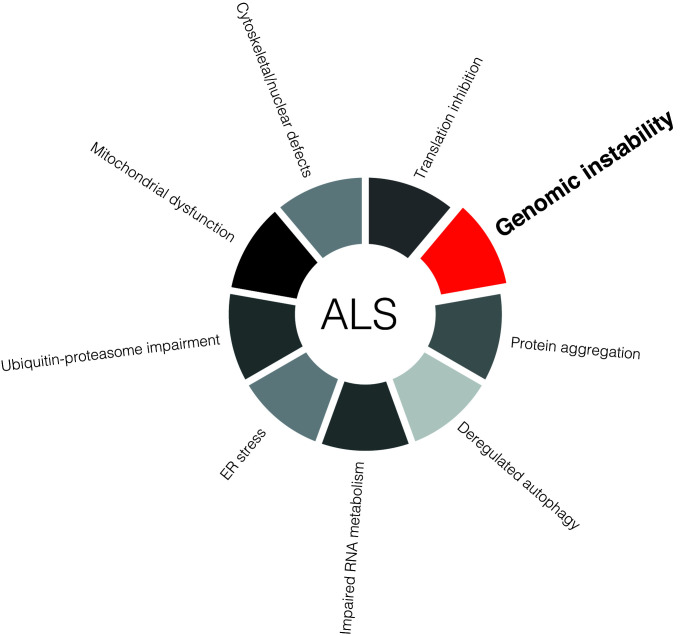
Although ALS is a mainly sporadic disease (90–95% of patients) [11], attention has been focused on the 5–10% of patients that have fALS where a gene-disease association can be made (Figure 1). Currently around 30 genes associated with fALS have been identified [12–16] and while a common molecular mechanism remains uncertain, recent evidence suggests that accumulation of genomic instability (GIN) – via impaired DNA damage recognition or defective DNA repair – is one of the hallmarks of ALS (Figure 2 and Table 1) [17].
Figure 1. ALS patient stratification.
Although some genetic heterogeneity is observed across the world, literature suggests these are the approximate proportions of ALS patients with mutations in the represented genes. Table 1 highlights other genetic contributors and their links to DDR.
Figure 2. Molecular hallmarks of ALS.
Current evidence suggests several underlying etiological factors in ALS. Genomic instability, caused by defective DNA damage signalling or DNA repair, toxic DNA repair, impaired clearance of endogenous genotoxic stressors (i.e. ROS), or due to imbalanced chromatin structure states, could be a unifying pathophysiological characteristic of the disease.
Table 1. DDR associated mutations in ALS.
| Gene | DDR link |
|---|---|
| TDP-43 | TAR DNA-binding protein 43; ALS-linked mutations [145]; impairs DDR in ALS [34] |
| FUS/TLS | Fused in sarcoma; ALS-linked mutation [79,146]; impairs DDR in ALS [147] |
| HNRNP | Heterogeneous nuclear ribonucleoprotein; modifies TDP43 [148,149]; associated with DDR [150]; hnRNP L recruits 53BP1 and BRCA1 in cancer [151]; hnRNP F,H, and K are related to ALS [152] and p53 recruitment [153] |
| HNRNPA1 | ALS linked mutations [154]; telomere protection and telomerase activation [155]; regulated by TDP43 [156] |
| HNRNPA2/B1 | ALS linked mutations [154] |
| SARM1 | Sterile alpha and TIR motif containing 1; ALS linked mutations [157]; SARM1 deletion suppresses TDP43-linked ALS [158] |
| PFN1 | Profilin-1; mutated PFN1 aggregates and shifts TDP43 from nuclei to cytoplasm in ALS [159] |
| UBQLN2 | Ubiquilin-2; ALS-linked mutations [160]; interacts with TDP43 [161] |
| CCNF | Cyclin F; ALS-linked mutations [162]; increases ubiquitinated TDP43 [162] |
| ERBB4 | Erb-B2 Receptor Tyrosine Kinase 4; interacts with TDP43 [163]; regulates p53-dependent DDR [164]; interacts with KAP1 for DDR [165]; activates p53 and p21 [166] |
| SIGMAR1 | Sigma nonopioid intracellular receptor 1; interacts with TDP43 [167] |
| GLE1 | RNA export mediator; ALS-linked mutations [168]; interacts with TDP43 [169]; GLE1 deletion increases phosphorylated H2AX, decreases BRCA1 and FANCD2 and increases ATR resulting in delayed DDR [170] |
| SOD1 | Superoxide dismutase; ALS-linked mutations [33]; protects DNA from oxidative stress damage in ALS [171] |
| DAO | D-amino acid oxidase; ROS production [172] |
| KIAA1563/ALS2 | Alsin; ALS-linked mutations [154]; increases ROS in ALS [173]; regulates autophagy [174] |
| C9ORF72 | Induces DNA damage in ALS [175] |
| SETX | Senataxin; encodes a DNA/RNA helicase protein involved in DDR and RNA production in ALS4 (Juvenile ALS) [176,177] |
| ATXN2 | Ataxin-2; ALS-linked mutations [178]; R-loop suppressor [65]; affects R-loop in ALS [179] |
| VCP | Valosin-containing protein; ALS-linked mutations [180]; facilitates 53BP1 recruitment for DSB repair [17,181]; causes p62 accumulation in ALS [182] |
| NEK1 | NIMA-Related Kinase 1; mutation induces DNA damage in ALS and impairs ATM-mediated DDR [183] |
| C21ORF2 | NEK1 interactor; involved in HR repair [48,157] |
| MATR3 | Matrin-3; activated by ATM and involved in the early stage of the DSB response [184] |
| SQSTM1 /p62 | Sequestosome-1; inhibits nuclear RNF168; an E3 ligase essential for histone H2A ubiquitination and DDR [185] |
| TBK1 | TANK-binding kinase 1; ALS-linked mutations [157]; an inducer of type-1 interferons; major role in autophagy and mitophagy [186]; cGAS/Sting/TBK1/IRF3 regulates p21 maintaining chromosomal stability [138] |
| ELP3 | Elongator complex protein 3; ALS-linked mutations [154]; binds to PCNA; linked to DNA replication and repair [187] |
| TIA1 | T-cell intracellular antigen 1; affects DDR; binds to p53 mRNA and controls p53 expression [188]; promotes phase separation and alters SG dynamics in ALS [15] |
Without excluding the importance and relevance of other molecular mechanisms that have been extensively covered by others [18–21], in this review we will examine the evidence revealing a role for the DNA damage response (DDR) in ALS, by discussing some of the particular genes, proteins and cellular processes implicated at the intersection of the DDR and ALS.
Sources of genomic instability and connection to ALS
DDR is starting to be recognized as a unifying mechanism in neurodegenerative disorders [22]. DNA damage can arise from both endogenous and exogenous sources and if not repaired will lead to the accumulation of GIN and ensuing pathologies [23]. To enable normal neuronal functions and survival, the DDR encompasses complex mechanisms that recognize DNA damage and signal for DNA damage repair [22,24]. Increasing evidence indicates that mature neurons are highly dependent on accurate DDR and that DNA damage accumulation accelerates in the normal human brain particularly after 40 years of age [25]. With ageing, there is thus an even greater requirement for DDR, and failure to deal with GIN accumulation will eventually lead to increased neuronal loss. Paradoxically, the DDR is known to change and deteriorate with age [26]. GIN arises from the buildup of lesions, such as base modifications, abasic sites, single- or double-stranded breaks (SSBs; DSBs) [27,28]. DSBs are particularly deleterious and, if left unrepaired, are detrimental to cell survival. DSBs are repaired by homologous recombination (HR) and non-homologous end joining (NHEJ). Carried out exclusively in cells that are in S- or G2-phase of the cell cycle, HR is the preferential DSB repair pathway as it is a relatively error-free process. Because under physiological conditions neurons are outside of the replicative cell cycle in the quiescent G0-phase, even though error-prone, NHEJ is the primary repair pathway for DSBs. That being said, recent evidence suggests that in addition to classical NHEJ, neurons could employ transcription-coupled repair mechanisms utilizing mRNA as a template for homology directed repair [29].
Since mature neurons are post-mitotic non-replicating cells that are difficult to replace [30,31], unsanctioned neuronal loss will lead to neurodegeneration. Concomitantly, ageing also brings other imbalances that can accelerate such DDR-related processes [32].
In ALS, the endogenous sources contributing to deleterious accumulation of GIN are from both impaired removal of reactive metabolic genotoxins (i.e. reactive oxygen species; ROS) that can overwhelm DDR [33] and from the incapability to recognize or repair DNA damage [34,35]. Although in this review, we are focusing on endogenous sources of DNA damage, one must keep in mind the geographical heterogeneity of ALS that cannot be explained by genetic risk factors alone [12,36]. Thus, future research should consider environmental genotoxic influences that might also play a role in both sALS and fALS.
SOD1 and DNA damage in ALS
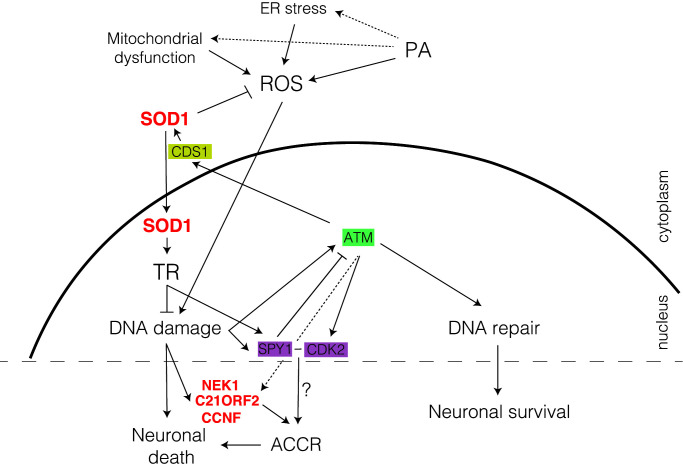
Superoxide dismutase 1 (SOD1) is a free radical scavenging enzyme that in the cytoplasm catalyzes the conversion of superoxide anions formed during mitochondrial respiration into hydrogen peroxide [37] and protects motor neurons – which are particularly prone to the toxic effects of mutant and dysfunctional SOD1 – against oxidative damage and neurodegeneration [33]. In both sALS and fALS, SOD1-induced neuronal toxicity occurs through gain-of-function mutations (Figure 1) [33,38] that lead to accumulation of injuries produced from the unscheduled free radical attack on pyrimidine and purine bases [39,40]. Secondarily, in both sALS and fALS, SOD1 can be secreted as monomers into the extracellular space leading to cell death [41]. Unexpectedly, recent data show that independent from its catalytic function, SOD1 performs additional roles in the nucleus. In response to elevated ROS, in an ataxia-telangiectasia mutated (ATM; a core DDR gene [42]) dependent manner, CDP-diacylglycerol synthase 1 (CDS1) kinase phosphorylates SOD1 at S60 and S99 promoting rapid SOD1 translocation to the nucleus where it regulates the expression of a large set of genes involved in oxidative stress defence and DDR [43]. Furthermore, nuclear SOD1 increases SpeedyA1 (SPY1) expression promoting cell survival and inhibiting damage-induced apoptosis. In ALS, pathologic SOD1–G93A cannot translocate to the nucleus and exercise its protective role via SPY1 regulation [44]. SPY1 is a nuclear protein that controls the transition between G1- and S-phases of the cell cycle via checkpoint-dependent kinase 2 (CDK2) activation [45]. In neurons, re-entry into cell cycle (CCR) is partly controlled by ATM, is atypical, and leads to neuronal death [30,46,47]. The observation that SOD1 can influence such decisions will require further investigation especially since other fALS genes, such as NEK1, C21ORF2 and CCNF are also involved in cell cycle progression [48–51], suggesting CCR should be considered in ALS pathology.
Thus, SOD1 protection against DNA damage accumulation is bi-modal, with the first tier of defence being executed in the cytoplasm through ROS scavenging, and the second in the nucleus where it controls the expression of DDR-related genes (Figure 3).
Figure 3. SOD1 plays a dual role in DDR.
SOD1 nuclear translocation is ATM/CDS1-dependent. Once SOD1 enters the nucleus, it activates transcription (TR) of many genes that are involved in DDR or ROS defence. SOD1 regulates SPY1 expression, which activates CDK2, a G1- to S-phases check point. Other cell cycle regulation (CCR) gene mutations (e.g. NEK1, C21ORF2 and CCNF) are implicated in defective DDR, suggesting a role for atypical cell cycle re-entry (ACCR) in ALS. Mutated genes identified in ALS (red), homologous recombination (HR; green), atypical cell cycle checkpoint (AACR; purple) and ROS regulation (yellow). Dotted arrows are proposed, yet not completely proven, interactions.
TDP43 mislocalization impairs DDR
Transactivation response DNA-binding protein 43 (TDP43) is a highly conserved nuclear protein that acts as transcription and splicing regulator as well as scaffold for nuclear bodies [52]. While in normal conditions TDP43 is primarily localized in the nucleus, in disease states it gets trapped in insoluble cytoplasmic inclusions (stress granules; SG; see Figure 4a) [53,54]. Although mutated TDP43 accounts for only approximately 4% of fALS cases (Figure 1), TDP43-SG accumulation is a pathology characteristic for ∼95% of all ALS and ∼50% of frontotemporal dementia (FTD) cases [21,55–57], as well as a secondary pathology in other neurodegenerative diseases, including Alzheimer’s [58], Parkinson’s [59] and Huntington’s diseases [60,61].
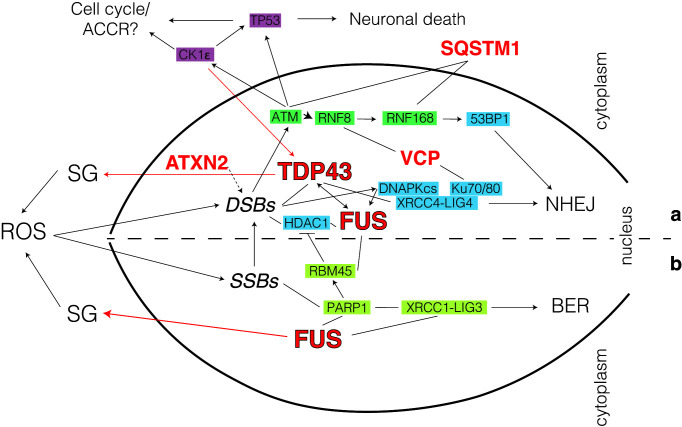
Figure 4. Role for TDP43 and FUS in maintaining genome stability in ALS.
Pathway choice is directed by the balance between TDP43 and FUS interaction at break sites. Simplified model for the role of TDP43 (a) and FUS (b) in DDR. Mutated genes identified in ALS (red), atypical cell cycle checkpoint (AACR; purple), non-homologous end-joining (NHEJ; blue), homologous recombination (HR; green) and base excision repair (BER; chartreuse). Dotted arrows are proposed, yet not completely proven, interactions.
Pathologic TDP43 mislocalization activates the mitochondrial unfolded protein response [62], elevates ROS levels and affects cytoplasmic-nuclear trafficking, eventually leading to increased neuronal stress and subsequent cell death [63,64]. Associated with such stress, GIN accumulation was described in sALS and fALS patients as well as in model organisms with orthologous TDP43 loss-of-function [34]. In addition, TDP43 cytoplasmic retention can be aggravated by other factors such as ataxin 2 (ATXN2), itself associated with DDR processes [65], thereby further increasing the risk of developing ALS [66]. Furthermore, TDP43 mislocalization and GIN accumulation maintain a vicious cycle via casein kinase 1ε (CK1ε) that has been shown to promote cytoplasmic accumulation of TDP43 [67]. Together with other CK1 isoforms, CK1ε is activated upon GIN build-up and controls several cellular processes linked to DNA damage signalling and repair, including apoptosis and cell cycle checkpoint control (Figure 4a) [68].
Although initially the connection between TDP43 dysfunction and the accumulation of GIN in ALS was thought to be a secondary feature, recent evidence shows that neuronal TDP43 plays an important direct role in DDR by controlling the nuclear recruitment of the XRCC4-DNA ligase 4 (LIG4) complex, critical for DSB repair via NHEJ [63]. In ALS/FTD, TDP43 nuclear exclusion incapacitates the transport of XRCC4/LIG4 leading to abortive NHEJ with consequent accumulation of toxic DSBs. The involvement of DSB repair in ALS/FTD is further substantiated by the observation that other proteins mutated in fALS such as valosin-containing protein (VCP)/p97 and sequestosome 1(SQSTM1)/p62 are linked to NHEJ [69,70]. VCP has been shown to directly interact with the canonical NHEJ proteins Ku70/80 [69] as well as with ring finger proteins (RNF) 8/168 [71] to balance DNA repair pathway choice and promote cell survival. This process is done in close correlation with SQSTM1/p62 that via interactions with ATM, RAD50 and RNF168 also regulates the choice between HR and NHEJ in favour of the latter [70]. The TDP43/XRCC4 direct connection is somewhat unexpected as replicating cells have less of a requirement for TDP43 in NHEJ (Figure 4a). This should prompt a more detailed analysis of these pathways in neurons where the relationship between different DDR components might be rewired. Further studies will be required to look, for example, at the interplay between TDP43 and other NHEJ proteins, such as PAXX and XRCC4-like factor (XLF) [72] or the SHIELDIN complex [73]. Additionally, given its RNA-binding capabilities, TDP43 has been implicated in impeding DNA:RNA hybrids (R-loops) formation [17,74]. This places TDP43 squarely in the middle of both DSB repair and the transcriptional stress that neurons endure.
FUS-mediated solid-to-liquid phase transition promotes DDR in ALS
Fused in sarcoma (FUS) is a nuclear ribonucleoprotein involved in a variety of cellular functions including transcription, protein translation and RNA splicing and transport [75,76]. Initially studied for its roles in cancer [77,78], it was later discovered that around 5% of fALS and 1% of sALS cases are associated with FUS mutations (Figure 1) [79].
Following oxidative damage, in a poly(ADP-ribose) polymerase (PARP1)-dependent manner, FUS facilitates the recruitment of XRCC1/LIG3 to SSBs and enhances LIG3 ligation activity thus promoting base excision repair (BER; Figure 4b) [80–83]. These interactions are, at least, partly based on the ability of FUS to rapidly traffic to the nucleus, as mutations in the nuclear localization sequence induce FUS aggregation, genomic instability, and consecutive neurodegeneration [84]. Additionally, in ATM and DNA-PKcs-dependent manner, FUS is involved in DSB repair by directly controlling the recruitment of histone deacetylases 1 (HDAC1) to chromatin [35]. The involvement of FUS in HDAC1 recruitment and activation is bimodal. Firstly, following DSB induction, FUS recruitment of HDAC1 promotes deacetylation and activation of NHEJ [85]. Secondly, in a PARP-dependent manner, FUS interacts with RNA-binding motif protein 45 (RBM45) and prevents excessive recruitment of HDAC1 [86].
These data build a model in which FUS controls the choice between SSB repair and DSB repair pathways in healthy neurons. Further research will be required to specifically understand the connection between FUS and TDP43 in DDR as well as the requirement of HR versus microhomology mediated end-joining (alternative NHEJ; MMEJ). In some patients, ALS is evidenced to manifest on the basis of oligogenic rather than monogenic alterations, with summative effects from several DDR pathologies [87], as indicated in Figure 4.
C9ORF72 repeat expansion and impaired DDR in ALS
Nucleotide repeat expansion (NRE) disorders encompass more than 20 human genetic diseases, most of which affect the nervous system, that arise from an expansion of a particularly unstable tandem of 3–12 DNA bases [88,89]. The deleterious effects of these NREs depend on the location of the repeat within the affected gene, its sequence, as well as the size of the repeat. C9ORF72 ALS/FTD is caused by the expansion of a hexanucleotide GGGGCC (G4C2) track in the first intron of the C9ORF72 gene [90].
G4C2–NREs are pathogenic through several non‐exclusive mechanisms that can all influence the accumulation of DNA damage lesions and affect their repair (Figure 5A). Initially, transcription over G4C2 tracks is problematic and will lead to accumulation of R-loops [91,92] and accumulation of toxic DNA secondary structures, hairpins and G-quadruplexes, which require DDR to be resolved [93,94]. Intriguingly, mutations in the R-loop processing factors senataxin (SETX) and HNRNPD also lead to fALS [95,96]. Subsequently or in parallel, transcripts containing repeats can form RNA repeat expansion (RRE) foci that will bind and sequester various RNA‐binding proteins such as TDP43, FUS, nucleophosmin (NPM1) or AP endonuclease (APE1), potentially altering their localization and DDR functions [91,97–105]. Finally, the G4C2–NREs are non‐AUG (RAN) translated into dipeptide repeats (DPR)‐containing proteins (poly-GR; -GP; -GA; -PR; -PA) that form inclusions throughout the brain of patients with ALS/FTD [106–109] and can lead to endoplasmic reticulum (ER) stress, mitochondrial dysfunction with ROS accumulation [110] and sequestration of DDR proteins [111,112]. Moreover, DPRs can accumulate at the nuclear membrane and the nuclear pore complex (NPC) to promote nuclear membrane abnormalities (NMA), impaired nuclear-cytoplasmic transport [113–115] and imbalanced chromatin states [116]. Furthermore, in a vicious feedback loop, the expanded G4C2 can interfere with the transcription and translation of the C9ORF72 mRNA thus leading to decreased autophagy and further accumulation of DPRs [117].
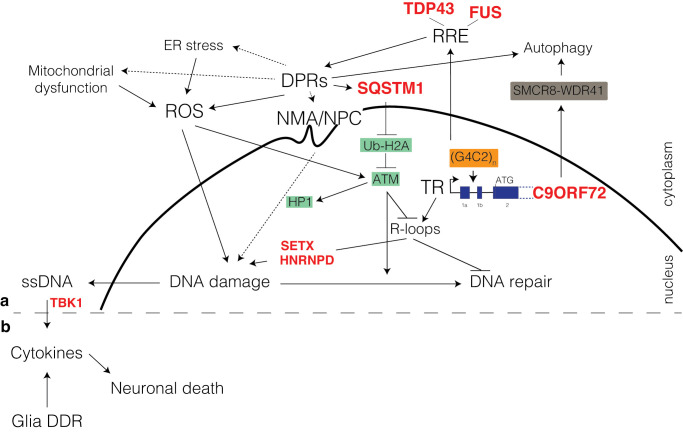
Figure 5. DDR defects in ALS with C9orf72 mutations.
G4C2–NREs in the first intron of the C9ORF72 gene increases RRE, which impairs DDR through binding to RNA-binding proteins. Transcription over G4C2–NREs leads to R-loop formation and subsequent DNA damage accumulation. RAN translation produced DPRs that can increase ROS, induce nuclear membrane alterations (NMA) and may potentially sequester DDR proteins. NMA include structural and functional disturbances at the nuclear pore complexes (NPC) involving transport receptors. Abnormal nucleo-cytoplasmic transport of both RNA and proteins at NPC has been suggested to be, either related to molecule sequestrations by DPR and RRE or in parallel with other factors, a strong C9ORF72 disease modifier. G4C2–NREs also decreases C9ORF72 expression, which impairs autophagy and exacerbates DPRs accumulation. Mutated genes identified in ALS (red), homologous recombination (HR; green) and autophagy (brown). Dotted arrows are proposed, yet not completely proven, interactions.
Consequence of these pathologic mechanisms, C9ORF72 ALS/FTD patients show increased GIN accumulation both in the brain [118] and spinal cord [97] where presence of DDR markers can be detected. One of the clearest evidences for a direct DDR deficiency in ALS comes from the observation that expressing RREs and/or DPRs results in elevated R-loop levels and DSBs build-up in rat neurons, human cells and C9ORF72 ALS patient spinal cord tissues. This is as a result of the incapability of C9ORF72-ALS neurons to mount a suitable DDR signalling cascade which occurs due to defective ATM-mediated signalling that arises as a consequence of SQSTM1/p62 accumulation and impaired H2A ubiquitylation (Figure 5A). Most likely due to this ATM signalling problem, NHEJ seems to be up-regulated to toxic levels that can be rescued in fly models via Ku (NHEJ), APEX1 (BER) or ERCC1 (interstrand cross-link DNA repair) dysregulation [119]. Although more information is needed to understand where the NHEJ or other DNA repair dependent toxicity is coming from, such observation would be in line with similar mechanisms present in ATM deficient replicating cells [120].
Another important link between G4C2 expansion and DDR is the observation that DPR accumulation leads to imbalanced chromatin states with impact on DNA repair [121]. Poly-PR, for example, specifically binds DNA at heterochromatin, evicts HP1α and causes abnormal histone H3 methylation leading to altered chromatin structure and NMA [116]. In response to endogenous DNA damage, to activate DDR, HP1α is phosphorylated by ATM [122], while H3K9me3 is required to activate the acetyltransferase activity of TIP60 [123]. Moreover, DPR accumulation at the nuclear membrane can lead to nuclear membrane rupture with subsequent GIN [124,125] as well as bi-directional transport defects at the NPC resulting in impaired shuttling of RNA and proteins. Such transport disturbances might interfere with factors involved in DDR and DNA repair, further feeding a vicious circle of DNA damage with insufficient repair [57,84,126]. These mechanisms might also influence the onset of age-related ALS, as perturbed nucleo-cytoplasmic cargo delivery is itself a feature of the CNS ageing process [127]. Thus, because the NPC has been shown to play important roles in DNA repair and the organization of genome architecture [128] while in response to DNA damage chromatin undergoes dramatic genome-wide changes that are at the heart of DDR [129], further scrutiny will be required to apprehend the relationship between nuclear DPR accumulation at specific nuclear structures (i.e. NPC), imbalanced chromatin states and their link to DDR in ALS.
Neuroinflammation and DDR in ALS
It must be highlighted that neurons do not live in isolation, and neurodegeneration is associated with microglial reactivity and activation of innate immune responses. Neuroinflammation is a common characteristic of ALS and comprises the stimulation of microglia, astrocytes and inflammatory T cells [130]. Upon activation, these cells secrete proinflammatory cytokines, such as tumour necrosis factor α, interferon γ, and interleukin 1β [131,132]. Typically, the innate immune response is activated by the presence of foreign cytoplasmic DNA via activation of cyclic GMP–AMP synthetase, (cGAS), and the cyclic dinucleotide receptor, stimulator of interferon genes (STING) [133]. Recently, more attention is being given to the link between accumulating GIN, the subsequent leakage of DNA in the cytoplasm and the activation of the cGAS-STING cascade [134]. In this model, ALS-GIN accumulating neurons can amass increasing amounts of single-stranded DNA (ssDNA) in the cytoplasm and promote neuroinflammation with production of cytokines and subsequent neuronal death (Figure 5B). Interestingly, haploinsufficiency in the STING activating kinase TANK-binding kinase (TBK1) [135] is associated with fALS and FTD [136,137] (Figure 5B). Within this pathway, TBK1 is important for several functions, including maintenance of chromosomal stability [138]. A functional cGAS/STING pathway is also known to be required for normal chromosomal segregation in cancer cells via a p21-dependent mechanism modulating G2/M transition [138]. The putative genome surveillance role in post-mitotic non-replicating cells is less clear.
Neuroinflammation with subsequent neurodegeneration can also result from a glia autonomous problem in dealing with DDR. Mutant human TDP43 expressed specifically in Drosophila glial cells causes DNA damage, elevated replication of retrotransposable elements (RTE) [139], and Gypsy endogenous retroviruses [140] and apoptosis in the nearby neurons. During their replication, the expression of RTE cDNA can lead to genome instability and accumulation of DSBs [141]. These studies highlight that TDP43 mutations in glial cells promote ALS progression, at least partly through impaired DDR signalling. Among glia, aberrant astrocyte function has also been implicated in ALS pathology which has been discussed extensively by others and merits further research [142–144]. Further studies will be required to better understand the relationship between DDR and neuroinflammation in ALS/FTD.
Conclusion
ALS is one of the most common adult-onset neurodegenerative disorders. Currently, ALS is fatal and incurable with patients expected to survive ∼2–4 years after diagnosis, revealing an urgent need for effective therapeutic strategies. Proof of DNA damage accumulation and DNA repair deficiency in both ALS initiation and progression is amassing, highlighting the fact that genomic instability is a hallmark of disease pathogenesis. Shedding light on the specific DDR mechanisms at play has important therapeutic potential.
Summary
Genomic instability is a hallmark of both sporadic and familial ALS with many ALS genes involved in recognition or repair of DNA damage.
Outside of the nucleus SOD1 works to impede ROS accumulation and in the nucleus to influence DNA damage response via transcription regulation.
TDP43 and FUS work mainly to balance the pathway choice between SSB repair and DSB repair.
Expansion of a repeated G4C2 track in the C9ORF72 gene leads to impaired ATM signalling.
Genomic instability may be a starting point for neuroinflammation in ALS.
Acknowledgements
We thank all G.B. laboratory members as well as Dr. Andras Lakatos and Dr. Kornelia Szebenyi for discussions.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- APE1
AP endonuclease 1
- ATM
ataxia-telangiectasia mutated
- ATXN2
Ataxin 2
- BER
base excision repair
- CCR
cell cycle re-entry
- CDK2
checkpoint-dependent kinase 2
- CDS1
CSP-diacylglycerol synthase 1
- CK1ε
casein kinase 1ε
- DDR
DNA damage response
- DRP
dipeptide-repeat proteins
- DSB
DNA double-strand break
- ER
endoplasmic reticulum
- fALS
familial ALS
- FTD
frontotemporal dementia
- FUS
fused in sarcoma
- G4C2
hexanucleotide GGGGCC
- GIN
genomic instability
- HDAC1
histone deacetylases 1
- HNRNPD
heterogeneous nuclear ribonucleoprotein D
- HR
homologous recombination
- LIG4
ligase 4
- MMEJ
microhomology mediated end-joining
- NEK1
NIMA-related kinase 1
- NHEJ
nonhomologous end-joining
- NMA
nuclear membrane abnormalities
- NPM1
Nucleophosmin 1
- NRE
nucleotide repeat expansion
- PARP1
Poly (ADP-ribose) polymerase 1
- RAN
Non‐AUG; AUG is a start codon
- RBM45
RNA-binding motif protein 45
- RNF
Ring finger protein
- ROS
reactive oxygen species
- RRE
RNA repeat expansion
- RTE
retrotransposable elements
- sALS
Sporadic ALS
- SETX
Senataxin
- SG
stress granules
- SOD1
superoxide dismutase 1
- SPY1
SpeedyA1
- SQSTM1
Sequestosome 1
- SSB
single-stranded break
- ssDNA
single-standed DNA
- STING
stimulator of interferon genes
- TBK1
TANK-binding kinase 1
- TDP43
transactivation response DNA-binding protein 43
- VCP
Valosin-containing protein
- XLF
XRCC4-like factor
Competing Interests
G.B. is a co-founder and consultant for Adrestia Therapeutics Ltd. The remaining authors declare no competing interests.
Funding
Research in G.B. laboratory is supported by the UK Dementia Research Institute which receives its funding from UK DRI Ltd., funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK. Additional funds to the G.B. laboratory are coming from Open Targets [grant number OTAR2072]; CHDI Foundation and a Evelyn Trust Grant. A.M.H is funded by Open Targets. A.J.C. is funded by the Cambridge Trust Vice-Chancellor’s and Masonic Charitable Foundation PhD Prize Studentship.
Open Access
Open access for this article was enabled by the participation of University of Cambridge in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
Author Contribution
G.B. conceptualized the review. G.B. and Y.S. wrote the review with help from A.J.C. and A.M.H. A.J.C. and A.M.H. implemented the response to reviewers with help from G.B. and Y.S.
References
- 1.del Aguila M.A., Longstreth W.T., McGuire V., Koepsell T.D. and van Belle G. (2003) Prognosis in amyotrophic lateral sclerosis: A population-based study. Neurology 60, 813–819 [DOI] [PubMed] [Google Scholar]
- 2.Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W. et al. (2017) Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 3, 17071 10.1038/nrdp.2017.71 [DOI] [PubMed] [Google Scholar]
- 3.Arthur K.C., Calvo A., Price T.R., Geiger J.T., Chiò A. and Traynor B.J. (2016) Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat. Commun. 7, 12408 10.1038/ncomms12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Bastida J., Perestelo-Pérez L., Montón-Alvarez F., Serrano-Aguilar P. and Alfonso-Sanchez J.L. (2009) Social economic costs and health-related quality of life in patients with amyotrophic lateral sclerosis in Spain. Amyotroph Lateral Scler Off. Publ. World Fed Neurol. Res Group Mot. Neuron. Dis. 10, 237–243 [DOI] [PubMed] [Google Scholar]
- 5.Schepelmann K., Winter Y., Spottke A.E., Claus D., Grothe C., Schröder R. et al. (2009) Socioeconomic burden of amyotrophic lateral sclerosis, myasthenia gravis and facioscapulohumeral muscular dystrophy. J. Neurol. 257, 15–23 10.1007/s00415-009-5256-6 [DOI] [PubMed] [Google Scholar]
- 6.Kordower J.H., Olanow C.W., Dodiya H.B., Chu Y., Beach T.G., Adler C.H. et al. (2013) Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain J. Neurol. 136, 2419–2431 10.1093/brain/awt192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrag A., Horsfall L., Walters K., Noyce A. and Petersen I. (2015) Prediagnostic presentations of Parkinson's disease in primary care: a case-control study. Lancet Neurol. 14, 57–64 10.1016/S1474-4422(14)70287-X [DOI] [PubMed] [Google Scholar]
- 8.Jack C.R., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. et al. (2013) Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabrizi S.J., Reilmann R., Roos R.A., Durr A., Leavitt B., Owen G. et al. (2012) Potential endpoints for clinical trials in premanifest and early Huntington's disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol. 11, 42–53 10.1016/S1474-4422(11)70263-0 [DOI] [PubMed] [Google Scholar]
- 10.Eisen A., Kiernan M., Mitsumoto H. and Swash M. (2014) Amyotrophic lateral sclerosis: a long preclinical period? J. Neurol. Neurosurg. Psychiatry 85, 1232–1238 10.1136/jnnp-2013-307135 [DOI] [PubMed] [Google Scholar]
- 11.Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W. et al. (2017) Correction: Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 3, 17085 10.1038/nrdp.2017.85 [DOI] [PubMed] [Google Scholar]
- 12.van Es M.A., Hardiman O., Chio A., Al-Chalabi A., Pasterkamp R.J., Veldink J.H. et al. (2017) Amyotrophic lateral sclerosis. Lancet 390, 2084–2098 10.1016/S0140-6736(17)31287-4 [DOI] [PubMed] [Google Scholar]
- 13.Renton A.E., Chiò A. and Traynor B.J. (2013) State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 17, 17–23 10.1038/nn.3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith B.N., Topp S.D., Fallini C., Shibata H., Chen H.-J., Troakes C. et al. (2017) Mutations in the vesicular trafficking protein annexin A11 are associated with amyotrophic lateral sclerosis. Sci. Transl. Med. 9, eaad9157 10.1126/scitranslmed.aad9157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackenzie I.R., Nicholson A.M., Sarkar M., Messing J., Purice M.D., Pottier C. et al. (2017) TIA1 Mutations in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia Promote Phase Separation and Alter Stress Granule Dynamics. Neuron 95, 808.e9–816.e9 10.1016/j.neuron.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper-Knock J., Moll T., Ramesh T., Castelli L., Beer A., Robins H. et al. (2019) Mutations in the Glycosyltransferase Domain of GLT8D1 Are Associated with Familial Amyotrophic Lateral Sclerosis. Cell Rep. 26, 2298.e5–2306.e5 10.1016/j.celrep.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker C. and El-Khamisy S.F. (2018) Perturbed autophagy and DNA repair converge to promote neurodegeneration in amyotrophic lateral sclerosis and dementia. Brain 141, 1247–1262 10.1093/brain/awy076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balendra R. and Isaacs A.M. (2018) C9orf72-mediated ALS and FTD: multiple pathways to disease. Nat. Rev. Neurol. 14, 544–558 10.1038/s41582-018-0047-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hetz C. and Saxena S. (2017) ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 13, 477–491 10.1038/nrneurol.2017.99 [DOI] [PubMed] [Google Scholar]
- 20.Paez-Colasante X., Figueroa-Romero C., Sakowski S.A., Goutman S.A. and Feldman E.L. (2015) Amyotrophic lateral sclerosis: mechanisms and therapeutics in the epigenomic era. Nat. Rev. Neurol. 11, 266–279 10.1038/nrneurol.2015.57 [DOI] [PubMed] [Google Scholar]
- 21.Ling S.-C., Polymenidou M. and Cleveland D.W. (2013) Converging Mechanisms in ALS and FTD: Disrupted RNA and Protein Homeostasis. Neuron 79, 416–438 10.1016/j.neuron.2013.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madabhushi R., Pan L. and Tsai L.-H. (2014) DNA Damage and Its Links to Neurodegeneration. Neuron 83, 266–282 10.1016/j.neuron.2014.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson S.P. and Bartek J. (2009) The DNA-damage response in human biology and disease. Nature 461, 1071–1078 10.1038/nature08467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maiuri T., Suart C.E., Hung C.L.K., Graham K.J., Bazan C.A.B. and Truant R. (2019) DNA Damage Repair in Huntington’s Disease and Other Neurodegenerative Diseases. Neurotherapeutics 16, 948–956 10.1007/s13311-019-00768-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu T., Pan Y., Kao S.-Y., Li C., Kohane I., Chan J. et al. (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891 10.1038/nature02661 [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K., Ikuno Y., Kakeya Y., Ikeno S., Taniura H., Kurono M. et al. (2019) Age-related dysfunction of the DNA damage response in intestinal stem cells. Inflamm. Regen. 39, 8 10.1186/s41232-019-0096-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackford A.N. and Jackson S.P. (2017) ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 66, 801–817 10.1016/j.molcel.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 28.Ciccia A. and Elledge S.J. (2010) The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204 10.1016/j.molcel.2010.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welty S., Teng Y., Liang Z., Zhao W., Sanders L.H., Greenamyre J.T. et al. (2017) RAD52 is required for RNA-templated recombination repair in post-mitotic neurons. J. Biol. Chem. 293, 1353–1362 10.1074/jbc.M117.808402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frade J.M. and Ovejero-Benito M.C. (2015) Neuronal cell cycle: the neuron itself and its circumstances. Cell Cycle 14, 712–720 10.1080/15384101.2015.1004937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Anda F.C., Madabhushi R., Rei D., Meng J., Gräff J., Durak O. et al. (2016) Cortical neurons gradually attain a post-mitotic state. Cell Res. 26, 1033–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou Y., Dan X., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L. et al. (2019) Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 10.1038/s41582-019-0244-7 [DOI] [PubMed] [Google Scholar]
- 33.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A. et al. (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 10.1038/362059a0 [DOI] [PubMed] [Google Scholar]
- 34.Mitra J., Guerrero E.N., Hegde P.M., Liachko N.F., Wang H., Vasquez V. et al. (2019) Motor neuron disease-associated loss of nuclear TDP-43 is linked to DNA double-strand break repair defects. Proc. Natl. Acad. Sci. 116, 4696–4705 10.1073/pnas.1818415116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W.-Y., Pan L., Su S.C., Quinn E.J., Sasaki M., Jimenez J.C. et al. (2013) Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat. Neurosci. 16, 1383–1391 10.1038/nn.3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiernan M.C., Vucic S., Cheah B.C., Turner M.R., Eisen A., Hardiman O. et al. (2011) Amyotrophic lateral sclerosis. Lancet 377, 942–955 10.1016/S0140-6736(10)61156-7 [DOI] [PubMed] [Google Scholar]
- 37.Fridovich I. (1997) Superoxide Anion Radical (O·̄ 2), Superoxide Dismutases, and Related Matters. J. Biol. Chem. 272, 18515–18517 10.1074/jbc.272.30.18515 [DOI] [PubMed] [Google Scholar]
- 38.Gagliardi S., Cova E., Davin A., Guareschi S., Abel K., Alvisi E. et al. (2010) SOD1 mRNA expression in sporadic amyotrophic lateral sclerosis. Neurobiol. Dis. 39, 198–203 10.1016/j.nbd.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 39.Canugovi C., Misiak M., Ferrarelli L.K., Croteau D.L. and Bohr V.A. (2013) The role of DNA repair in brain related disease pathology. DNA Repair (Amst.) 12, 578–587 10.1016/j.dnarep.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slupphaug G., Kavli B. and Krokan H.E. (2003) The interacting pathways for prevention and repair of oxidative DNA damage. Mutat. Res. Fundam. Mol. Mech. Mutagen. 531, 231–251 10.1016/j.mrfmmm.2003.06.002 [DOI] [PubMed] [Google Scholar]
- 41.Kabashi E., Valdmanis P.N., Dion P. and Rouleau G.A. (2007) Oxidized/misfolded superoxide dismutase-1: the cause of all amyotrophic lateral sclerosis? Ann. Neurol. 62, 553–559 10.1002/ana.21319 [DOI] [PubMed] [Google Scholar]
- 42.Shiloh Y. and Ziv Y. (2013) The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 14, 197–210 10.1038/nrm3546 [DOI] [PubMed] [Google Scholar]
- 43.Tsang C.K., Liu Y., Thomas J., Zhang Y. and Zheng X.F.S. (2014) Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 5, 3446 10.1038/ncomms4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X.-D., Zhu M.-W., Shan D., Wang S.-Y., Yin X., Yang Y.-Q. et al. (2019) Spy1, a unique cell cycle regulator, alters viability in ALS motor neurons and cell lines in response to mutant SOD1-induced DNA damage. DNA Repair (Amst.) 74, 51–62 10.1016/j.dnarep.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 45.Porter L.A., Kong-Beltran M. and Donoghue D.J. (2003) Spy1 Interacts with p27 Kip1 to Allow G 1 /S Progression. Mol. Biol. Cell 14, 3664–3674 10.1091/mbc.e02-12-0820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye W. and Blain S.W. (2010) S phase entry causes homocysteine-induced death while ataxia telangiectasia and Rad3 related protein functions anti-apoptotically to protect neurons. Brain J. Neurol. 133, 2295–2312 10.1093/brain/awq139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kruman I.I., Wersto R.P., Cardozo-Pelaez F., Smilenov L., Chan S.L., Chrest F.J. et al. (2004) Cell Cycle Activation Linked to Neuronal Cell Death Initiated by DNA Damage. Neuron 41, 549–561 10.1016/S0896-6273(04)00017-0 [DOI] [PubMed] [Google Scholar]
- 48.Fang X., Lin H., Wang X., Zuo Q., Qin J. and Zhang P. (2015) The NEK1 interactor, C21ORF2, is required for efficient DNA damage repair. Acta Bioch. Bioph. Sin. 47, 834–841 10.1093/abbs/gmv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelegrini A.L., Moura D.J., Brenner B.L., Ledur P.F., Maques G.P., Henriques J.A.P. et al. (2010) Nek1 silencing slows down DNA repair and blocks DNA damage-induced cell cycle arrest. Mutagenesis 25, 447–454 10.1093/mutage/geq026 [DOI] [PubMed] [Google Scholar]
- 50.Wang Z., Liu P., Inuzuka H. and Wei W. (2014) Roles of F-box proteins in cancer. Nat. Rev. Cancer 14, 233–247 10.1038/nrc3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai C., Richman R. and Elledge S.J. (1994) Human cyclin F. EMBO J. 13, 6087–6098 10.1002/j.1460-2075.1994.tb06955.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen-Plotkin A.S., Lee V.M.-Y. and Trojanowski J.Q. (2010) TAR DNA-binding protein 43 in neurodegenerative disease. Nat. Rev. Neurol. 6, 211–220 10.1038/nrneurol.2010.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winton M.J., Igaz L.M., Wong M.M., Kwong L.K., Trojanowski J.Q. and Lee V.M.-Y. (2008) Disturbance of Nuclear and Cytoplasmic TAR DNA-binding Protein (TDP-43) Induces Disease-like Redistribution, Sequestration, and Aggregate Formation. J. Biol. Chem. 283, 13302–13309 10.1074/jbc.M800342200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Igaz L.M., Kwong L.K., Xu Y., Truax A.C., Uryu K., Neumann M. et al. (2008) Enrichment of C-Terminal Fragments in TAR DNA-Binding Protein-43 Cytoplasmic Inclusions in Brain but not in Spinal Cord of Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Am. J. Pathol. 173, 182–194 10.2353/ajpath.2008.080003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lagier-Tourenne C. and Cleveland D.W. (2009) Rethinking ALS: the FUS about TDP-43. Cell 136, 1001–1004 10.1016/j.cell.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smethurst P., Sidle K.C.L. and Hardy J. (2015) Review: Prion-like mechanisms of transactive response DNA binding protein of 43 kDa (TDP-43) in amyotrophic lateral sclerosis (ALS). Neuropath. Appl. Neurol. 41, 578–597 10.1111/nan.12206 [DOI] [PubMed] [Google Scholar]
- 57.Hergesheimer R.C., Chami A.A., de Assis D.R., Vourc'h P., Andres C.R., Corcia P. et al. (2019) The debated toxic role of aggregated TDP-43 in amyotrophic lateral sclerosis: a resolution in sight? Brain 142, 1176–1194 10.1093/brain/awz078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masters C.L., Simms G., Weinman N.A., Multhaup G., McDonald B.L. and Beyreuther K. (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. 82, 4245–4249 10.1073/pnas.82.12.4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spillantini M.G., Schmidt M.L., Lee V.M.-Y., Trojanowski J.Q., Jakes R. and Goedert M. (1997) α-Synuclein in Lewy bodies. Nature 388, 839–840 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- 60.Davies S.W., Turmaine M., Cozens B.A., DiFiglia M., Sharp A.H., Ross C.A. et al. (1997) Formation of Neuronal Intranuclear Inclusions Underlies the Neurological Dysfunction in Mice Transgenic for the HD Mutation. Cell 90, 537–548 10.1016/S0092-8674(00)80513-9 [DOI] [PubMed] [Google Scholar]
- 61.DiFiglia M., Sapp E., Chase K.O., Davies S.W., Bates G.P., Vonsattel J.P. et al. (1997) Aggregation of Huntingtin in Neuronal Intranuclear Inclusions and Dystrophic Neurites in Brain. Science 277, 1990–1993 10.1126/science.277.5334.1990 [DOI] [PubMed] [Google Scholar]
- 62.Wang P., Deng J., Dong J., Liu J., Bigio E.H., Mesulam M. et al. (2019) TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLoS Genet. 15, e1007947 10.1371/journal.pgen.1007947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guerrero E.N., Mitra J., Wang H., Rangaswamy S., Hegde P.M., Basu P. et al. (2019) Amyotrophic lateral sclerosis-associated TDP-43 mutation Q331K prevents nuclear translocation of XRCC4-DNA ligase 4 complex and is linked to genome damage-mediated neuronal apoptosis. Hum. Mol. Genet. 28, 2459–2476 10.1093/hmg/ddz062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang W., Wang L., Lu J., Siedlak S.L., Fujioka H., Liang J. et al. (2016) The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat. Med. 22, 869–878 10.1038/nm.4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abraham K.J., Chan J.N.Y., Salvi J.S., Ho B., Hall A., Vidya E. et al. (2016) Intersection of calorie restriction and magnesium in the suppression of genome-destabilizing RNA-DNA hybrids. Nucleic Acids Res. 44, 8870–8884 10.1093/nar/gkw752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elden A.C., Kim H.-J., Hart M.P., Chen-Plotkin A.S., Johnson B.S., Fang X. et al. (2010) Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466, 1069–1075 10.1038/nature09320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu J., Hu W., Tan X., Qu S., Chu D., Gong C.-X. et al. (2019) Elevation of casein kinase 1ε associated with TDP-43 and tau pathologies in Alzheimer's disease. Brain Pathol. Zurich Switz 30, 283–297 10.1111/bpa.12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knippschild U., Krüger M., Richter J., Xu P., García-Reyes B., Peifer C. et al. (2014) The CK1 Family: Contribution to Cellular Stress Response and Its Role in Carcinogenesis. Front. Oncol. 4, 96 10.3389/fonc.2014.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van den Boom J., Wolf M., Weimann L., Schulze N., Li F., Kaschani F. et al. (2016) VCP/p97 Extracts Sterically Trapped Ku70/80 Rings from DNA in Double-Strand Break Repair. Mol. Cell 64, 189–198 10.1016/j.molcel.2016.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hewitt G., Carroll B., Sarallah R., Correia-Melo C., Ogrodnik M., Nelson G. et al. (2016) SQSTM1/p62 mediates crosstalk between autophagy and the UPS in DNA repair. Autophagy 12, 1917–1930 10.1080/15548627.2016.1210368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh A.N., Oehler J., Torrecilla I., Kilgas S., Li S., Vaz B. et al. (2019) The p97-Ataxin 3 complex regulates homeostasis of the DNA damage response E3 ubiquitin ligase RNF 8. EMBO J. 38, e102361 10.15252/embj.2019102361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balmus G., Barros A.C., Wijnhoven P.W.G., Lescale C., Hasse H.L., Boroviak K. et al. (2016) Synthetic lethality between PAXX and XLF in mammalian development. Gene. Dev. 30, 2152–2157 10.1101/gad.290510.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dev H., Chiang T.-W.W., Lescale C., de Krijger I., Martin A.G., Pilger D. et al. (2018) Shieldin complex promotes DNA end-joining and counters homologous recombination in BRCA1-null cells. Nat. Cell Biol. 20, 954–965 10.1038/s41556-018-0140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hill S.J., Mordes D.A., Cameron L.A., Neuberg D.S., Landini S., Eggan K. et al. (2016) Two familial ALS proteins function in prevention/repair of transcription-associated DNA damage. Proc. Natl. Acad. Sci. 113, E7701–E7709 10.1073/pnas.1611673113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ratti A. and Buratti E. (2016) Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J. Neurochem. 138, 95–111 10.1111/jnc.13625 [DOI] [PubMed] [Google Scholar]
- 76.Guerrero E.N., Wang H., Mitra J., Hegde P.M., Stowell S.E., Liachko N.F. et al. (2016) TDP-43/FUS in motor neuron disease: Complexity and challenges. Prog. Neurobiol. 145–146, 78–97 10.1016/j.pneurobio.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crozat A., Åman P., Mandahl N. and Ron D. (1993) Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature 363, 640–644 10.1038/363640a0 [DOI] [PubMed] [Google Scholar]
- 78.Rabbitts T.H., Forster A., Larson R. and Nathan P. (1993) Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nat. Genet. 4, 175–180 10.1038/ng0693-175 [DOI] [PubMed] [Google Scholar]
- 79.Vance C., Rogelj B., Hortobagyi T., Vos K.J.D., Nishimura A.L., Sreedharan J. et al. (2009) Mutations in FUS, an RNA Processing Protein, Cause Familial Amyotrophic Lateral Sclerosis Type 6. Science 323, 1208–1211 10.1126/science.1165942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel A., Lee H.O., Jawerth L., Maharana S., Jahnel M., Hein M.Y. et al. (2015) A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 162, 1066–1077 10.1016/j.cell.2015.07.047 [DOI] [PubMed] [Google Scholar]
- 81.Mastrocola A.S., Kim S.H., Trinh A.T., Rodenkirch L.A. and Tibbetts R.S. (2013) The RNA-binding protein fused in sarcoma (FUS) functions downstream of poly(ADP-ribose) polymerase (PARP) in response to DNA damage. J. Biol. Chem. 288, 24731–24741 10.1074/jbc.M113.497974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H., Rangaswamy S., Kodavati M., Mitra J., Guo W., Guerrero E.N. et al. (2019) RT2 PCR array screening reveals distinct perturbations in DNA damage response signaling in FUS-associated motor neuron disease. Mol. Brain 12, 103 10.1186/s13041-019-0526-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H., Guo W., Mitra J., Hegde P.M., Vandoorne T., Eckelmann B.J. et al. (2018) Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in Amyotrophic Lateral Sclerosis. Nat. Commun. 9, 3683 10.1038/s41467-018-06111-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naumann M., Pal A., Goswami A., Lojewski X., Japtok J., Vehlow A. et al. (2018) Impaired DNA damage response signaling by FUS-NLS mutations leads to neurodegeneration and FUS aggregate formation. Nat. Commun. 9, 335 10.1038/s41467-017-02299-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller K.M., Tjeertes J.V., Coates J., Legube G., Polo S.E., Britton S. et al. (2010) Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat. Struct. Mol. Biol. 17, 1144–1151 10.1038/nsmb.1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gong J., Huang M., Wang F., Ma X., Liu H., Tu Y. et al. (2017) RBM45 competes with HDAC1 for binding to FUS in response to DNA damage. Nucleic Acids Res. 45, 12862–12876 10.1093/nar/gkx1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuuluvainen L., Kaivola K., Mönkäre S., Laaksovirta H., Jokela M., Udd B. et al. (2019) Oligogenic basis of sporadic ALS: The example of SOD1 p.Ala90Val mutation. Neurol. Genet. 5, e335 10.1212/NXG.0000000000000335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paulson H. (2018) Handbook of Clinical Neurology. Handb Clin. Neurol. 147, 105–123 10.1016/B978-0-444-63233-3.00009-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao X.-N. and Usdin K. (2015) The Repeat Expansion Diseases: The dark side of DNA repair. DNA Repair (Amst.) 32, 96–105 10.1016/j.dnarep.2015.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mossevelde S.V., van der Zee J., Cruts M. and Broeckhoven C.V. (2017) Relationship between C9orf72 repeat size and clinical phenotype. Curr. Opin. Genet. Dev. 44, 117–124 10.1016/j.gde.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 91.Haeusler A.R., Donnelly C.J., Periz G., Simko E.A.J., Shaw P.G., Kim M.-S. et al. (2014) C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature 507, 195–200 10.1038/nature13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reddy K., Schmidt M.H.M., Geist J.M., Thakkar N.P., Panigrahi G.B., Wang Y.-H. et al. (2014) Processing of double-R-loops in (CAG)·(CTG) and C9orf72 (GGGGCC)·(GGCCCC) repeats causes instability. Nucleic Acids Res. 42, 10473–10487 10.1093/nar/gku658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fratta P., Mizielinska S., Nicoll A.J., Zloh M., Fisher E.M.C., Parkinson G. et al. (2012) C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms RNA G-quadruplexes. Sci. Rep.-U.K. 2, 1016 10.1038/srep01016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reddy K., Zamiri B., Stanley S.Y.R., Macgregor R.B. and Pearson C.E. (2013) The disease-associated r(GGGGCC)n repeat from the C9orf72 gene forms tract length-dependent uni- and multimolecular RNA G-quadruplex structures. J. Biol. Chem. 288, 9860–9866 10.1074/jbc.C113.452532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bennett C.L., Dastidar S.G., Ling S.-C., Malik B., Ashe T., Wadhwa M. et al. (2018) Senataxin mutations elicit motor neuron degeneration phenotypes and yield TDP-43 mislocalization in ALS4 mice and human patients. Acta Neuropathol. 136, 425–443 10.1007/s00401-018-1852-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taylor J.P., Brown R.H. and Cleveland D.W. (2016) Decoding ALS: from genes to mechanism. Nature 539, 197–206 10.1038/nature20413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farg M.A., Konopka A., Soo K.Y., Ito D. and Atkin J.D. (2017) The DNA damage response (DDR) is induced by the C9orf72 repeat expansion in Amyotrophic Lateral Sclerosis. Hum. Mol. Genet. 26, 2882–2896 10.1093/hmg/ddx170 [DOI] [PubMed] [Google Scholar]
- 98.Almeida S., Gascon E., Tran H., Chou H.J., Gendron T.F., Degroot S. et al. (2013) Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathol. 126, 385–399 10.1007/s00401-013-1149-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donnelly C.J., Zhang P.-W., Pham J.T., Haeusler A.R., Heusler A.R., Mistry N.A. et al. (2013) RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron 80, 415–428 10.1016/j.neuron.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lagier-Tourenne C., Baughn M., Rigo F., Sun S., Liu P., Li H.-R. et al. (2013) Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc. Natl. Acad. Sci. U.S.A. 110, E4530–E4539 10.1073/pnas.1318835110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee Y.-B., Chen H.-J., Peres J.N., Gomez-Deza J., Attig J., Stalekar M. et al. (2013) Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep. 5, 1178–1186 10.1016/j.celrep.2013.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mizielinska S., Lashley T., Norona F.E., Clayton E.L., Ridler C.E., Fratta P. et al. (2013) C9orf72 frontotemporal lobar degeneration is characterised by frequent neuronal sense and antisense RNA foci. Acta Neuropathol. 126, 845–857 10.1007/s00401-013-1200-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cooper-Knock J., Walsh M.J., Higginbottom A., Highley J.R., Dickman M.J., Edbauer D. et al. (2014) Sequestration of multiple RNA recognition motif-containing proteins by C9orf72 repeat expansions. Brain 137, 2040–2051 10.1093/brain/awu120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Conlon E.G., Lu L., Sharma A., Yamazaki T., Tang T., Shneider N.A. et al. (2016) The C9ORF72 GGGGCC expansion forms RNA G-quadruplex inclusions and sequesters hnRNP H to disrupt splicing in ALS brains. Elife 5, e17820 10.7554/eLife.17820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Conlon E.G., Fagegaltier D., Agius P., Davis-Porada J., Gregory J., Hubbard I. et al. (2018) Unexpected similarities between C9ORF72 and sporadic forms of ALS/FTD suggest a common disease mechanism. Elife 7, e37754 10.7554/eLife.37754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ash P.E.A., Bieniek K.F., Gendron T.F., Caulfield T., Lin W.-L., Dejesus-Hernandez M. et al. (2013) Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77, 639–646 10.1016/j.neuron.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gendron T.F., Bieniek K.F., Zhang Y.-J., Jansen-West K., Ash P.E.A., Caulfield T. et al. (2013) Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 126, 829–844 10.1007/s00401-013-1192-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mori K., Weng S.-M., Arzberger T., May S., Rentzsch K., Kremmer E. et al. (2013) The C9orf72 GGGGCC Repeat Is Translated into Aggregating Dipeptide-Repeat Proteins in FTLD/ALS. Science 339, 1335–1338 10.1126/science.1232927 [DOI] [PubMed] [Google Scholar]
- 109.Zu T., Liu Y., Banez-Coronel M., Reid T., Pletnikova O., Lewis J. et al. (2013) RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc. Natl. Acad. Sci. 110, E4968–E4977 10.1073/pnas.1315438110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lopez-Gonzalez R., Lu Y., Gendron T.F., Karydas A., Tran H., Yang D. et al. (2016) Poly(GR) in C9ORF72-Related ALS/FTD Compromises Mitochondrial Function and Increases Oxidative Stress and DNA Damage in iPSC-Derived Motor Neurons. Neuron 92, 383–391 10.1016/j.neuron.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Solomon D.A., Stepto A., Au W.H., Adachi Y., Diaper D.C., Hall R. et al. (2018) A feedback loop between dipeptide-repeat protein, TDP-43 and karyopherin-α mediates C9orf72-related neurodegeneration. Brain 141, 2908–2924 10.1093/brain/awy241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nihei Y., Mori K., Werner G., Arzberger T., Degeneration GC for FL, Alliance BBB et al. (2019) Poly-glycine-alanine exacerbates C9orf72 repeat expansion-mediated DNA damage via sequestration of phosphorylated ATM and loss of nuclear hnRNPA3. Acta Neuropathol. 139, 99–118 10.1007/s00401-019-02082-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Freibaum B.D., Lu Y., Lopez-Gonzalez R., Kim N.C., Almeida S., Lee K.-H. et al. (2015) GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 525, 129–133 10.1038/nature14974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jovičić A., Mertens J., Boeynaems S., Bogaert E., Chai N., Yamada S.B. et al. (2015) Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat. Neurosci. 18, 1226–1229 10.1038/nn.4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang C.-Z., Spektor A., Cornils H., Francis J.M., Jackson E.K., Liu S. et al. (2015) Chromothripsis from DNA damage in micronuclei. Nature 522, 179–184 10.1038/nature14493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y.-J., Guo L., Gonzales P.K., Gendron T.F., Wu Y., Jansen-West K. et al. (2019) Heterochromatin anomalies and double-stranded RNA accumulation underlie C9orf72 poly(PR) toxicity. Science 363, eaav2606 10.1126/science.aav2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boivin M., Pfister V., Gaucherot A., Ruffenach F., Negroni L., Sellier C. et al. (2020) Reduced autophagy upon C9ORF72 loss synergizes with dipeptide repeat protein toxicity in G4C2 repeat expansion disorders. EMBO J. 39, e100574 10.15252/embj.2018100574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Walker C., Herranz-Martin S., Karyka E., Liao C., Lewis K., Elsayed W. et al. (2017) C9orf72 expansion disrupts ATM-mediated chromosomal break repair. Nat. Neurosci. 20, 1225–1235 10.1038/nn.4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lopez-Gonzalez R., Yang D., Pribadi M., Kim T.S., Krishnan G., Choi S.Y. et al. (2019) Partial inhibition of the overactivated Ku80-dependent DNA repair pathway rescues neurodegeneration in C9ORF72 -ALS/FTD. Proc. Natl. Acad. Sci. 116, 9628–9633 10.1073/pnas.1901313116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Balmus G., Pilger D., Coates J., Demir M., Sczaniecka-Clift M., Barros A.C. et al. (2019) ATM orchestrates the DNA-damage response to counter toxic non-homologous end-joining at broken replication forks. Nat. Commun. 10, 87 10.1038/s41467-018-07729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kramer N.J., Haney M.S., Morgens D.W., Jovičić A., Couthouis J., Li A. et al. (2018) CRISPR-Cas9 screens in human cells and primary neurons identify modifiers of C9ORF72 dipeptide-repeat-protein toxicity. Nat. Genet. 50, 603–612 10.1038/s41588-018-0070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dinant C. and Luijsterburg M.S. (2009) The Emerging Role of HP1 in the DNA Damage Response. Mol. Cell. Biol. 29, 6335–6340 10.1128/MCB.01048-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun Y., Jiang X., Xu Y., Ayrapetov M.K., Moreau L.A., Whetstine J.R. et al. (2009) Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat. Cell Biol. 11, 1376–1382 10.1038/ncb1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Earle A.J., Kirby T.J., Fedorchak G.R., Isermann P., Patel J., Iruvanti S. et al. (2019) Mutant lamins cause nuclear envelope rupture and DNA damage in skeletal muscle cells. Nat. Mater. 19, 464–473 10.1038/s41563-019-0563-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Frost B., Bardai F.H. and Feany M.B. (2016) Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Curr. Biol. 26, 129–136 10.1016/j.cub.2015.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhong Y., Wang J., Henderson M.J., Yang P., Hagen B.M., Siddique T. et al. (2017) Nuclear export of misfolded SOD1 mediated by a normally buried NES-like sequence reduces proteotoxicity in the nucleus. ELife e23759 10.7554/eLife.23759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.D'Angelo M.A., Raices M., Panowski S.H. and Hetzer M.W. (2009) Age-Dependent Deterioration of Nuclear Pore Complexes Causes a Loss of Nuclear Integrity in Postmitotic Cells. Cell 136, 284–295 10.1016/j.cell.2008.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bukata L., Parker S.L. and D'Angelo M.A. (2013) Nuclear pore complexes in the maintenance of genome integrity. Curr. Opin. Cell Biol. 25, 378–386 10.1016/j.ceb.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 129.Hauer M.H. and Gasser S.M. (2017) Chromatin and nucleosome dynamics in DNA damage and repair. Gene. Dev. 31, 2204–2221 10.1101/gad.307702.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Philips T. and Robberecht W. (2011) Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 10, 253–263 10.1016/S1474-4422(11)70015-1 [DOI] [PubMed] [Google Scholar]
- 131.Hanisch U.-K. and Kettenmann H. (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394 10.1038/nn1997 [DOI] [PubMed] [Google Scholar]
- 132.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A. and Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 10.1016/j.it.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 133.Sun L., Wu J., Du F., Chen X. and Chen Z.J. (2012) Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 339, 786–791 10.1126/science.1232458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li T. and Chen Z.J. (2018) The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 215, 1287–1299 10.1084/jem.20180139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tanaka Y. and Chen Z.J. (2012) STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 5, ra20 10.1126/scisignal.2002521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pottier C., Bieniek K.F., Finch N., van de Vorst M., Baker M., Perkersen R. et al. (2015) Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol. 130, 77–92 10.1007/s00401-015-1436-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Freischmidt A., Wieland T., Richter B., Ruf W., Schaeffer V., Müller K. et al. (2015) Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat. Neurosci. 18, 631–636 10.1038/nn.4000 [DOI] [PubMed] [Google Scholar]
- 138.Basit A., Cho M.-G., Kim E.-Y., Kwon D., Kang S.-J. and Lee J.-H. (2020) The cGAS/STING/TBK1/IRF3 innate immunity pathway maintains chromosomal stability through regulation of p21 levels. Exp. Mol. Med. 52, 643–657 10.1038/s12276-020-0416-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Krug L., Chatterjee N., Borges-Monroy R., Hearn S., Liao W.-W., Morrill K. et al. (2017) Retrotransposon activation contributes to neurodegeneration in a Drosophila TDP-43 model of ALS. PLos Genet. 13, e1006635 10.1371/journal.pgen.1006635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chang Y.-H. and Dubnau J. (2019) The Gypsy Endogenous Retrovirus Drives Non-Cell-Autonomous Propagation in a Drosophila TDP-43 Model of Neurodegeneration. Curr. Biol. Cb 29, 3135.e4–3152.e4 10.1016/j.cub.2019.07.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wallace N.A., Belancio V.P. and Deininger P.L. (2008) L1 mobile element expression causes multiple types of toxicity. Gene 419, 75–81 10.1016/j.gene.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yamanaka K. and Komine O. (2018) The multi-dimensional roles of astrocytes in ALS. Neurosci. Res. 126, 31–38 10.1016/j.neures.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 143.Rostalski H., Leskelä S., Huber N., Katisko K., Cajanus A., Solje E. et al. (2019) Astrocytes and Microglia as Potential Contributors to the Pathogenesis of C9orf72 Repeat Expansion-Associated FTLD and ALS. Front. Neurosci.-Switz 13, 486 10.3389/fnins.2019.00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pehar M., Harlan B.A., Killoy K.M. and Vargas M.R. (2018) Role and Therapeutic Potential of Astrocytes in Amyotrophic Lateral Sclerosis. Curr. Pharm. Design 23, 10.2174/1381612823666170622095802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sreedharan J., Blair I.P., Tripathi V.B., Hu X., Vance C., Rogelj B. et al. (2008) TDP-43 Mutations in Familial and Sporadic Amyotrophic Lateral Sclerosis. Science 319, 1668–1672 10.1126/science.1154584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kwiatkowski T.J., Bosco D.A., LeClerc A.L., Tamrazian E., Vanderburg C.R., Russ C. et al. (2009) Mutations in the FUS/TLS Gene on Chromosome 16 Cause Familial Amyotrophic Lateral Sclerosis. Science 323, 1205–1208 10.1126/science.1166066 [DOI] [PubMed] [Google Scholar]
- 147.Wang H. and Hegde M.L. (2019) New Mechanisms of DNA Repair Defects in Fused in Sarcoma-Associated Neurodegeneration: Stage Set for DNA Repair-Based Therapeutics? J. Exp. Neurosci. 13, 1–5 10.1177/1179069519856358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Appocher C., Mohagheghi F., Cappelli S., Stuani C., Romano M., Feiguin F. et al. (2017) Major hnRNP proteins act as general TDP-43 functional modifiers both in Drosophila and human neuronal cells. Nucleic Acids Res. 45, gkx477 10.1093/nar/gkx477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gittings L.M., Foti S.C., Benson B.C., Gami-Patel P., Isaacs A.M. and Lashley T. (2019) Heterogeneous nuclear ribonucleoproteins R and Q accumulate in pathological inclusions in FTLD-FUS. Acta Neuropathol. Commun. 7, 18 10.1186/s40478-019-0673-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haley B., Paunesku T., Protić M. and Woloschak G.E. (2009) Response of heterogeneous ribonuclear proteins (hnRNP) to ionising radiation and their involvement in DNA damage repair. Int. J. Radiat. Biol. 85, 643–655 10.1080/09553000903009548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu W., Lei L., Xie X., Huang L., Cui Q., Dang T. et al. (2019) Heterogeneous nuclear ribonucleoprotein L facilitates recruitment of 53BP1 and BRCA1 at the DNA break sites induced by oxaliplatin in colorectal cancer. Cell Death. Dis. 10, 550 10.1038/s41419-019-1784-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Geuens T., Bouhy D. and Timmerman V. (2016) The hnRNP family: insights into their role in health and disease. Hum. Genet. 135, 851–867 10.1007/s00439-016-1683-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Decorsière A., Cayrel A., Vagner S. and Millevoi S. (2011) Essential role for the interaction between hnRNP H/F and a G quadruplex in maintaining p53 pre-mRNA 3′-end processing and function during DNA damage. Gene Dev. 25, 220–225 10.1101/gad.607011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chia R., Chiò A. and Traynor B.J. (2018) Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol. 17, 94–102 10.1016/S1474-4422(17)30401-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sui J., Lin Y.-F., Xu K., Lee K.-J., Wang D. and Chen B.P.C. (2015) DNA-PKcs phosphorylates hnRNP-A1 to facilitate the RPA-to-POT1 switch and telomere capping after replication. Nucleic Acids Res. 43, 5971–5983 10.1093/nar/gkv539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Deshaies J.-E., Shkreta L., Moszczynski A.J., Sidibé H., Semmler S., Fouillen A. et al. (2018) TDP-43 regulates the alternative splicing of hnRNP A1 to yield an aggregation-prone variant in amyotrophic lateral sclerosis. Brain 141, 1320–1333 10.1093/brain/awy062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Registry P., Group S., Registry S., Consortium F.S., Consortium S., Group N.S. et al. (2016) Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat. Genet. 48, 1043–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.White M.A., Lin Z., Kim E., Henstridge C.M., Altamira E.P., Hunt C.K. et al. (2019) Sarm1 deletion suppresses TDP-43-linked motor neuron degeneration and cortical spine loss. Acta Neuropathol. Commun. 7, 166 10.1186/s40478-019-0800-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Matsukawa K., Hashimoto T., Matsumoto T., Ihara R., Chihara T., Miura M. et al. (2016) Familial Amyotrophic Lateral Sclerosis-linked Mutations in Profilin 1 Exacerbate TDP-43-induced Degeneration in the Retina of Drosophila melanogaster through an Increase in the Cytoplasmic Localization of TDP-43. J. Biol. Chem. 291, 23464–23476 10.1074/jbc.M116.729152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deng H.-X., Chen W., Hong S.-T., Boycott K.M., Gorrie G.H., Siddique N. et al. (2011) Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 477, 211–215 10.1038/nature10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Renaud L., Picher-Martel V., Codron P. and Julien J.-P. (2019) Key role of UBQLN2 in pathogenesis of amyotrophic lateral sclerosis and frontotemporal dementia. Acta Neuropathol. Commun. 7, 103 10.1186/s40478-019-0758-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Williams K.L., Topp S., Yang S., Smith B., Fifita J.A., Warraich S.T. et al. (2016) CCNF mutations in amyotrophic lateral sclerosis and frontotemporal dementia. Nat. Commun. 7, 11253 10.1038/ncomms11253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Takahashi Y., Uchino A., Shioya A., Sano T., Matsumoto C., Numata‐Uematsu Y. et al. (2019) Altered immunoreactivity of ErbB4, a causative gene product for ALS19, in the spinal cord of patients with sporadic ALS. Neuropathology 39, 268–278 10.1111/neup.12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Icli B., Bharti A., Pentassuglia L., Peng X. and Sawyer D.B. (2012) ErbB4 localization to cardiac myocyte nuclei, and its role in myocyte DNA damage response. Biochem. Bioph. Res. Commun. 418, 116–121 10.1016/j.bbrc.2011.12.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gilmore-Hebert M., Ramabhadran R. and Stern D.F. (2010) Interactions of ErbB4 and Kap1 Connect the Growth Factor and DNA Damage Response Pathways. Mol. Cancer Res. 8, 1388–1398 10.1158/1541-7786.MCR-10-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Arasada R.R. and Carpenter G. (2005) Secretase-dependent Tyrosine Phosphorylation of Mdm2 by the ErbB-4 Intracellular Domain Fragment. J. Biol. Chem. 280, 30783–30787 10.1074/jbc.M506057200 [DOI] [PubMed] [Google Scholar]
- 167.Fukunaga K., Shinoda Y. and Tagashira H. (2015) The role of SIGMAR1 gene mutation and mitochondrial dysfunction in amyotrophic lateral sclerosis. J. Pharmacol. Sci. 127, 36–41 10.1016/j.jphs.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 168.Kaneb H.M., Folkmann A.W., Belzil V.V., Jao L.-E., Leblond C.S., Girard S.L. et al. (2015) Deleterious mutations in the essential mRNA metabolism factor, hGle1, in amyotrophic lateral sclerosis. Hum. Mol. Genet. 24, 1363–1373 10.1093/hmg/ddu545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Aditi X.X.X., Glass L., Dawson T.R. and Wente S.R. (2016) An amyotrophic lateral sclerosis-linked mutation in GLE1 alters the cellular pool of human Gle1 functional isoforms. Adv. Biological Regul. 62, 25–36 10.1016/j.jbior.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Okamura M., Yamanaka Y., Shigemoto M., Kitadani Y., Kobayashi Y., Kambe T. et al. (2018) Depletion of mRNA export regulator DBP5/DDX19, GLE1 or IPPK that is a key enzyme for the production of IP6, resulting in differentially altered cytoplasmic mRNA expression and specific cell defect. PLoS ONE 13, e0197165 10.1371/journal.pone.0197165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bordoni M., Pansarasa O., Dell'Orco M., Crippa V., Gagliardi S., Sproviero D. et al. (2019) Nuclear Phospho-SOD1 Protects DNA from Oxidative Stress Damage in Amyotrophic Lateral Sclerosis. J. Clin. Med. 8, 729 10.3390/jcm8050729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kondori N.R., Paul P., Robbins J.P., Liu K., Hildyard J.C.W., Wells D.J. et al. (2018) Focus on the Role of D-serine and D-amino Acid Oxidase in Amyotrophic Lateral Sclerosis/Motor Neuron Disease (ALS). Front. Mol. Biosci. 5, 8 10.3389/fmolb.2018.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cai H., Lin X., Xie C., Laird F.M., Lai C., Wen H. et al. (2005) Loss of ALS2 Function Is Insufficient to Trigger Motor Neuron Degeneration in Knock-Out Mice But Predisposes Neurons to Oxidative Stress. J. Neurosci. 25, 7567–7574 10.1523/JNEUROSCI.1645-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gautam M., Jara J.H., Sekerkova G., Yasvoina M.V., Martina M. and Özdinler P.H. (2016) Absence of alsin function leads to corticospinal motor neuron vulnerability via novel disease mechanisms. Hum. Mol. Genet. 25, 1074–1087 10.1093/hmg/ddv631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Konopka A. and Atkin J. (2018) The Emerging Role of DNA Damage in the Pathogenesis of the C9orf72 Repeat Expansion in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 19, 3137 10.3390/ijms19103137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tripolszki K., Török D., Goudenège D., Farkas K., Sulák A., Török N. et al. (2017) High‐throughput sequencing revealed a novel SETX mutation in a Hungarian patient with amyotrophic lateral sclerosis. Brain Behav. 7, e00669 10.1002/brb3.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen Y.-Z., Bennett C.L., Huynh H.M., Blair I.P., Puls I., Irobi J. et al. (2004) DNA/RNA Helicase Gene Mutations in a Form of Juvenile Amyotrophic Lateral Sclerosis (ALS4). Am. J. Hum Genet. 74, 1128–1135 10.1086/421054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sproviero W., Shatunov A., Stahl D., Shoai M., van Rheenen W., Jones A.R. et al. (2017) ATXN2 trinucleotide repeat length correlates with risk of ALS. Neurobiol. Aging 51, 178.e1–178.e9 10.1016/j.neurobiolaging.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhao D.Y., Gish G., Braunschweig U., Li Y., Ni Z., Schmitges F.W. et al. (2016) SMN and symmetric arginine dimethylation of RNA polymerase II C-terminal domain control termination. Nature 529, 48–53 10.1038/nature16469 [DOI] [PubMed] [Google Scholar]
- 180.Koppers M., van Blitterswijk M.M., Vlam L., Rowicka P.A., van Vught P.W.J., Groen E.J.N. et al. (2012) VCP mutations in familial and sporadic amyotrophic lateral sclerosis. Neurobiol. Aging 33, 837.e7–837.e13 10.1016/j.neurobiolaging.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 181.Acs K., Luijsterburg M.S., Ackermann L., Salomons F.A., Hoppe T. and Dantuma N.P. (2011) The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat. Struct. Mol. Biol. 18, 1345–1350 10.1038/nsmb.2188 [DOI] [PubMed] [Google Scholar]
- 182.Ayaki T., Ito H., Fukushima H., Inoue T., Kondo T., Ikemoto A. et al. (2014) Immunoreactivity of valosin-containing protein in sporadic amyotrophic lateral sclerosis and in a case of its novel mutant. Acta Neuropathol. Commun. 2, 172 10.1186/s40478-014-0172-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Higelin J., Catanese A., Semelink-Sedlacek L.L., Oeztuerk S., Lutz A.-K., Bausinger J. et al. (2018) NEK1 loss-of-function mutation induces DNA damage accumulation in ALS patient-derived motoneurons. Stem Cell Res. 30, 150–162 10.1016/j.scr.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 184.Salton M., Lerenthal Y., Wang S.-Y., Chen D.J. and Shiloh Y. (2010) Involvement of Matrin 3 and SFPQ/NONO in the DNA damage response. Cell Cycle 9, 1568–1576 10.4161/cc.9.8.11298 [DOI] [PubMed] [Google Scholar]
- 185.Wang Y., Zhu W.-G. and Zhao Y. (2016) Autophagy substrate SQSTM1/p62 regulates chromatin ubiquitination during the DNA damage response. Autophagy 13, 212–213 10.1080/15548627.2016.1245262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oakes J.A., Davies M.C. and Collins M.O. (2017) TBK1: a new player in ALS linking autophagy and neuroinflammation. Mol. Brain 10, 5 10.1186/s13041-017-0287-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li Q., Fazly A.M., Zhou H., Huang S., Zhang Z. and Stillman B. (2009) The Elongator Complex Interacts with PCNA and Modulates Transcriptional Silencing and Sensitivity to DNA Damage Agents. PLos Genet. 5, e1000684 10.1371/journal.pgen.1000684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Díaz-Muñoz M.D., Kiselev V.Y.U., Novère N.L., Curk T., Ule J. and Turner M. (2017) Tia1 dependent regulation of mRNA subcellular location and translation controls p53 expression in B cells. Nat. Commun. 8, 530 10.1038/s41467-017-00454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]