Abstract
Objective
We conducted a narrative review to investigate whether antidepressant therapy, including the use of selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) or the use of supportive drugs (i.e., citicoline or choline alfoscerate) as a substitute for antidepressant therapy, reduces depression in patients with cerebrovascular diseases.
Methods
A systematic search of the PubMed and Web of Science databases was performed, including review articles and other studies to identify additional citations. Only 4 of 1566 publications met the inclusion/exclusion criteria and were selected.
Results
Studies showed that post-stroke depression (PSD) could be treated with antidepressant therapy, as well as supportive drugs such as citicoline or choline alfoscerate, which may have antidepressant effects.
Conclusions
The findings support the efficacy of citicoline as a treatment for depression. Studies aimed to discover the characteristics of these psychostimulants in relation to PSD treatment should be performed.
Keywords: Antidepressants, cerebrovascular disease, stroke, selective serotonin reuptake inhibitor, serotonin-norepinephrine reuptake inhibitor, citicoline, choline
Introduction
Worldwide, cerebrovascular diseases are the second leading cause of death and the third leading cause of disability.1 Incidence rates for stroke in people aged 55 to 64 years range between 10 and 20 per 10,000 individuals, increasing to 200 per 10,000 individuals in the elderly population.2 Motor and cognitive impairment resulting from stroke significantly interferes with the quality of life of patients,3 also affecting behavioral and emotional domains.4
Post-stroke depression (PSD) is a mood disorder characterized by depression and anhedonia caused by stroke. A recent meta-analysis reported that approximately 30% of stroke survivors suffer from PSD within 5 years.5 The depressive symptoms gradually increase during the first 6 months, ease slightly at approximately 12 months, and worsen again during the second year after the stroke. Patients with PSD usually present a wide range of symptoms such as sleep disturbance, fatigue, weight changes, and apathy, which lengthen the hospital stay and decrease participation in rehabilitation programs, resulting in reduced functional improvement.6 Thus, the patient’s tendency to become socially isolated can affect the recovery of cognitive functions such as memory, language, attention, understanding, and calculation, leading to further cognitive impairment7,8 Antidepressant treatments have shown positive results in the PSD population.9 These drugs stimulate neurogenesis and influence the plasticity of the new neurons generated.10 Such benefits can be extended to mood symptoms, thus encouraging motor, cognitive, and executive recovery.11
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are frequently used to treat depressive symptoms. SSRIs are considered the first-choice treatment because their side effects are generally well-tolerated.12 In contrast, SNRIs are associated with more side-effects such as irritability, insomnia, nausea, vomiting, and cardiovascular responses.13
Citicoline is an endogenous nucleotide compound that participates in the biochemical process of phosphatidylcholine synthesis and is one of the most abundant cell membrane lipids in human and animal tissues.14 Citicoline participates in neuronal processes by enhancing brain functions, as well as reducing cognitive deficits and improving memory performance. Therefore, citicoline has been successfully used as a neuroprotective agent to prevent neuronal aging and improve memory,15 in addition to slowing the degeneration of neurological diseases and glaucoma.16,17 Citicoline increases norepinephrine and dopamine levels in the central nervous system by acting on brain metabolism and neurotransmitter modulation, leading to an improved depressive profile.18 Although the effects of citicoline and choline supplementation on recovery in stroke survivors have been reported in the literature,19 few studies have investigated the use of these drugs to treat PSD without an antidepressant therapy (i.e., SSRIs or SNRIs).
This narrative review investigates the effects of pharmacological treatments for depression (i.e., SSRIs and SNRIs) in the post-stroke population. We also examined the role of citicoline and choline alfoscerate as adjuvant antidepressant treatments.
Methods
Search strategy
Studies in the literature were identified by searching the PubMed and Web of Science databases. The search combined the following terms using logical boolean operators (i.e., AND, OR), as approtiate: “Post stroke depression”, “Depression post cerebrovascular disease”, “Depression and mild cognitive impairment in cerebrovascular disease”, “SSRI”, “SNRI”, “Citicoline”, and “Choline”. The same queries were used when searching both databases.
Inclusion and exclusion criteria
Studies that satisfied the following criteria were included in this review:
The study population included cerebrovascular patients;
The effects of antidepressant therapy were investigated in patients with PSD;
Pharmacological treatments included either SSRIs and SNRIs or citicoline and choline;
Written in English.
Studies that met the following criteria were excluded:
Studies conducted on animals rather than humans;
The aim was not focused on using either SSRIs, SNRIs, or citicoline to treat PSD;
The study was a dissertation, commentary, letter, or editorial.
Systematic integrative and narrative reviews were included. We only considered articles published from 2002 to 2018.
Study selection
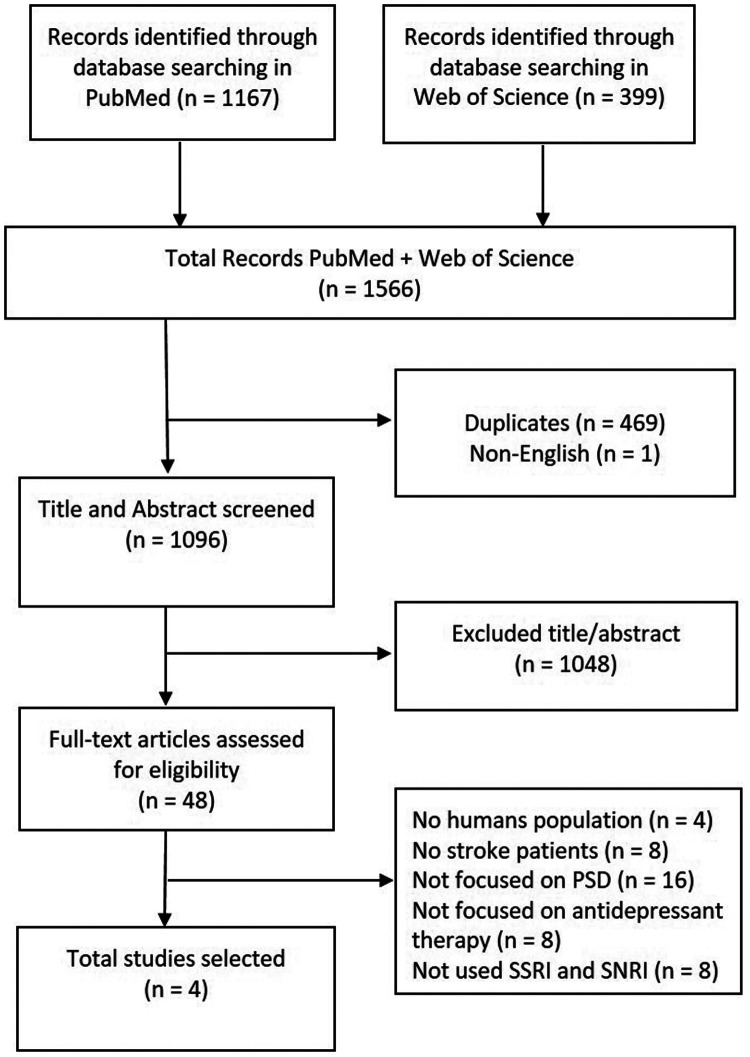
The procedure for study selection included three phases. First, articles not written in English and duplicates were removed. Second, the titles and abstracts were screened for relevance to the specific pharmacological treatment of PSD. Third, the full text of all potential articles was evaluated in depth (Figure 1).
Figure 1.
Study selection procedure.
Results
We identified 1566 studies: 1167 articles from PubMed and 399 articles from the Web of Science. However, only 4 of these 1566 studies were selected (Figure 1).
The articles included 883 patients with diagnoses of stroke and depression. As reported in Table 1, one article20 highlighted the antidepressant effects of SSRIs and SNRIs, two studies21,22 examined, respectively, the role of duloxetine (i.e., an SNRI) and the use of citalopram (i.e., an SSRI) compared with cognitive behavioral therapy and rehabilitation. The last selected article18 investigated the role of citicoline in depressive treatment. There were no studies concerning the effects of choline on PSD. Four measures of depression were identified (Table 2), and one study20 used a standardized form with the patient's data and rehabilitative information.
Table 1.
Characteristics of the selected studies.
| Reference | Aim | Measures | Socio-demographic characteristics | Setting | Conclusion |
|---|---|---|---|---|---|
| Engelter et al., 2012 [20] | To observe the frequency and determinants of the use of PESR | Standardized form with patient data and rehabilitation information | 464 patients (244 men, 220 women), aged 72 ± 12.4 years Diagnoses: 364 ischemic stroke, 75 intracerebral hemorrhage, 25 subarachnoid hemorrhage | Hospital stroke rehabilitation | More than half of the patients received agents that potentially enhanced recovery in routine clinical treatment, especially SSRIs, SNRIs, levodopa, and acetylcholinesterase inhibitors. SSRIs and SNRIs were predominately used to treat accompanying depression. Additionally, 159 patients received PESR, which was primarily used to treat aphasia and paresis (the principal agent was levodopa). |
| Zhang et al., 2013 [21] | To evaluate whether duloxetine (an SNRI) could help to prevent PSD | Hamilton Depression Scale, National Institute of Stroke Scale, Mini-Mental State Examination, Chinese version of the Activities of Daily Living Scale, Short Form 36 Health Survey Questionnaire | 95 patients: controls aged 64.7 ± 10.1 years, patients treated with duloxetine aged 64.1 ± 10.9 years26 men and 22 women in the control group, 27 men and 20 women in the duloxetine groupDiagnosis: ischemic stroke | Rehabilitation | Duloxetine decreased the incidence of PSD and promoted rehabilitation, cognitive functions, and quality of life. |
| Gao et al., 2017 [22] | To evaluate the appropriate therapy for post-ischemic stroke depression at different times after stroke | Beck Depression Inventory, 17-item Hamilton Depression Scale, Bech–Rafaelsen Melancholia Scale, Udvalg for Kliniske Undersogelser Side-Effect Rating Scale, Functional Independence Measure scale | Group A: 91 patients, 48 men, mean age 67.2 ± 9.6 years; Group B: 91 patients, 46 men, mean age 66.0 ± 7.3 years; Group C: 92 patients, 48 men, mean age 64.9 ± 8.0 years; Diagnosis: ischemic stroke without history of depression | Outpatient clinic | Similar effects between citalopram or cognitive behavioral therapy and rehabilitative treatment alone for early-onset post-ischemic stroke depression; rehabilitation and citalopram are suitable for delayed onset post-ischemic stroke depression; rehabilitation and cognitive behavioral therapy are more effective than rehabilitative treatment alone for late-onset post-ischemic stroke depression |
| Roohi-Azizi et al., 2017 [18] | To evaluate the effectiveness of citicoline as an adjuvant therapy in major depression | Hamilton Depression Rating Scale | 50 patients (35 women, 15 men), aged 18–50 years. Diagnosis: stroke with major depressive disorder | Hospital rehabilitation | Citicoline could be considered as an effective adjuvant to citalopram in the treatment of major depressive disorder. |
SSRI selective serotonin reuptake inhibitor, SNRI serotonin-norepinephrine reuptake inhibitor, PSD post-stroke depression, PESR pharmacological enhancement in stroke rehabilitation.
Table 2.
Description of the depression measures used in the selected studies.
| Measure | Domains | Items | Scales | Focus |
|---|---|---|---|---|
| HAM-D (Hamilton M, J Neurol Neurosurg Psychiatry 1960) [43] | 6 domains: anxiety/somatization, weight, cognitive disorders, diurnal variations, slowing down, sleep disorders | 21 items | 5-, 4- or 3-point scale | To assess the severity of depressive symptoms |
| HAMD17 (Hamilton M, J Neurol Neurosurg Psychiatry 1960) [43] | Brief version of the HAM-D | 17 items | 5- or 3-point scale | To assess the severity of depressive symptoms (Derived from the HAM-D) |
| BDI (Beck AT et al., Arch Gen Psychiatry 1961) [44] | 21 domains: mood, pessimism, sense of failure, self-dissatisfaction, guilt, punishment, self-dislike, self-accusation, suicidal ideas, crying, irritability, social withdrawal, indecisiveness, body image change, work difficulty, insomnia, fatigability, loss of appetite, weight loss, somatic preoccupation, and loss of libido | 21 items | 4-point scale | To assess the severity of depressive symptoms in cognitive domains |
| MES (Bech P., Acta Psychiatr Scand 1980) [45] | 11 domains: depressed mood, tiredness, work and interests, concentration difficulties, sleep disturbances, psychic anxiety, emotional introversion, worthless and guilt, suicidal thoughts, decreased verbal activity, and decreased motor activity | 11 items | 5-point scale | To assess depression severity and measure change in depressive states during treatment |
HAM-D Hamilton Depression Scale.
Efficacy of antidepressant treatment for PSD
Engelter et al.20 conducted a prospective, multicenter, exploratory study. They observed the frequency and determinants of using pharmacological enhancement in stroke rehabilitation (PESR). PESR involves the use of agents that could improve the post-stroke recovery to support regular rehabilitative therapies. Their sample included 464 patients, of whom 257 had taken agents that could potentially enhance recovery. The most commonly used drugs were SSRIs, SNRIs, levodopa, and acetylcholinesterase inhibitors. SSRIs and SNRIs were predominately used to improve depressive symptoms, whereas levodopa was exclusively used to improve motor rehabilitation in the absence of an otherwise established indication.
Zhang et al.,21 in their prospective cohort study, enrolled 95 ischemic stroke patients without symptoms of depression. With the aim of assessing the effectiveness of duloxetine in preventing PSD, patients were randomly divided into two groups: 47 received duloxetine for 12 weeks as medication for depression, in addition to the routine therapy for ischemic stroke, while 48 controls only received the routine therapy without any antidepressants. Follow-up observations were performed for 24 weeks by evaluating depression, cognitive and neurological function, rehabilitation, and the quality of life of patients. Their findings indicated that the use of duloxetine decreased the incidence of PSD and accelerated functional and cognitive recovery by improving the patient’s quality of life. Gao et al.22 conducted a randomized, controlled trial on 274 patients with ischemic stroke without a history of depression. By comparing three interventions (placebo vs SSRIs vs cognitive behavioral therapy without antidepressants), they aimed to assess the appropriate treatments for PSD at different times after the acute event. Thus, patients were enrolled at different times after discharge, and all underwent rehabilitation. No significant differences were observed among the three treatments in patients enrolled up to 3 months after discharge. In contrast, among patients recruited at 6 months, the group receiving SSRIs showed improvement, whereas among patients recruited at 9 months, an improvement was observed in the group receiving cognitive behavioral therapy. Moreover, the group of patients taking SSRIs showed significantly greater rates of adverse events, without significant differences among time periods of recruitment. A subgroup analysis was also performed according to PSD onset. The results showed significant effects of SSRI treatment when the onset of PSD was delayed (3 to 6 months after stroke) or late (more than 6 months after stroke), while no effects were observed among treatments for early-onset PSD (the first 3 months after a stroke, including hospitalization). Their hypothesis was that effects of antidepressants are not evident in the early phase because the recovery of function itself reduces depression.
Roohi-Azizi et al.,18 in their randomized trial, evaluated the effectiveness of citicoline as an adjuvant therapy for major depression. They enrolled 50 patients with major depressive disorder who were previously treated with an SSRI and allocated them into two groups: one group receiving citicoline and one placebo group. Depression was evaluated at baseline and after 2, 4, and 6 weeks. The results showed a significantly greater improvement of the citicoline group compared with the placebo group at all assessment times. This effect was accompanied by a significantly greater rate of remission in the citicoline group. These results support the consideration of citicoline as an effective adjuvant therapy in the treatment of major depressive disorder.
Discussion
Almost one-third of stroke survivors suffer from depression, especially during rehabilitation.23 Indeed, a decrease in motivation reduces compliance and increases resistance during rehabilitative treatments. Because depression interferes with functional recovery, early recognition of depressive symptoms is important to improve functional recovery.24 Although depressive symptoms appear in the first month after the stroke, tending to become chronic over time,4 the onset of depression depends on the interaction of different factors, such as the severity of stroke and disability, cognitive impairment, personality, general vascular damage, and psychosocial and genetic factors.25 Hence, it is essential to define new guidelines for early detection and management of PSD.
Indeed, it is important to plan a pharmacological therapy for recovery of a patient’s deficits, being careful to monitor the patient's response. Recently, the American Heart Association Stroke Council published a scientific statement concluding that antidepressant medications may be effective in treating PSD, although further research is needed to determine the optimal timing, threshold, and medications for treatment.26 In this review, we considered studies reporting effects of antidepressant drugs such as SSRIs and SNRIs in PSD treatment. We found that SSRIs are the first-choice medication, acting as inhibitors of serotonin reuptake, and are generally well-tolerated.27 SSRIs are involved in complex signaling cascades, which result in an increase in neurogenesis that stimulates axonal sprouting and promotes the development of new synapses.28 A recent trial reported lower rates of PSD occurrence together with a significant improvement in motor functions in subjects taking SSRIs compared with placebo controls, even when the statistical model controlled for the reduction in depression.29 These findings raised the question of whether depression prevents motor recovery or whether SSRIs may affect neuroplasticity and motor recovery.26 The benefits of SSRIs on factors such as motor recovery, cognitive/executive functioning, and partial or full independence in daily life are associated with an improvement in quality of life.30 However, further research is needed to determine how to improve quality of life in individuals with or at risk for PSD.
Another type of medication used to treat PSD is SNRIs (which are often compared with SSRIs in adult patients with depression). However, SNRIs should be cautiously used because of their side effects.13 Notably, the findings of a recent meta-analysis including 12 randomized controlled trials (n = 1121) suggested a beneficial effect of antidepressants on remission and responses (measured as a >50% reduction in mood scores), reporting a higher rate of adverse events, especially central nervous system and gastrointestinal side effects, in subjects who received the active medication compared with those who received the placebo.31 Indeed, norepinephrine can increase sympathetic nervous system activity, including tachycardia and hypertension, which is considered an important risk factor for cardiovascular and cerebrovascular events.32 Overall, both types of drugs exhibited long-term benefits, but considering the complexity of post-stroke outcomes, some psychostimulants could be useful to support antidepressant therapy.33
Recently, trials of alternative nondrug therapies for PSD to alleviate the adverse effects and drug dependence caused by antidepressants have been conducted, including methods such as acupuncture34 and the use of citicoline and choline alfoscerate. Thus, we also searched for studies assessing the efficacy of citicoline and choline alfoscerate not only as adjuvant therapies, but also considering their potential antidepressant effects. However, we found few studies focused on this topic. Notably, most of these studies were conducted on animals, showing that citicoline can be used as an adjuvant drug to reduce neuronal degeneration and increase neurotransmitter levels (i.e., dopamine, norepinephrine, and serotonin) to improve the management of certain neurodegenerative disorders.35,36 Other studies have evaluated effects on cognition and dementia, showing only marginal effects on depression.37 However, the relationship between depression and deterioration of cognitive functions (concentration, attention, or memory) is well known. Indeed, citicoline acts on neurotransmitters with functional neuroprotective and neuro-restorative effects on brain damage, improving cognitive impairment.33 In addition, we found studies indicating that citicoline administration can improve mood disorders in patients with bipolar depression.38,39
The use of citicoline in elderly depressed patients has been shown to reduce the risk of side effects caused by traditional antidepressants, decreasing the period of hospitalization.40 According to Secades,41 this psychostimulant also improves motor functions during the rehabilitation phase after stroke, especially in the early stage and over the first 6 months, to preserve neurocognitive functions. Similarly, Jadavji et al.19 observed a neuroprotective role of choline after stroke, reporting its antioxidant activity and mechanisms that enhance neuroplasticity. Sharp et al.42 suggested the importance of analyzing the impact of the cholinergic system on possible post-ischemic neuroprotective effects.
The main limitation of this study is the limited amount of evidence from pooled data, especially concerning studies evaluating the effectiveness of adjuvant therapy based on the use of citicoline and choline in support of antidepressant therapy. Indeed, only one study was included in our analysis.18 In their study, Roohi-Azizi et al. showed how citicoline could be considered an effective adjuvant to citalopram in the treatment of depression. This interesting result leads us to speculate the efficacy of these psychostimulants in influencing, facilitating, and accelerating patient recovery. However, the characteristics of citicoline and choline alfoscerate in relation to depression treatment should be further investigated, especially in stroke patients. Another limitation was the different assessments for depression used in the selected studies. In particular, one study measured the level of depression with a self-reported survey,20 and patients could have underestimated or overestimated their symptoms. Future long-term intervention studies for PSD should investigate the action of new psychostimulant drugs on depressive symptoms to evaluate a possible improvement in functional recovery.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Francesco Corallo https://orcid.org/0000-0003-4862-3832
Marcella Di Cara https://orcid.org/0000-0002-4275-4340
References
- 1.World Health Organization. https://www.who.int/healthinfo/global_burden_disease/estimates/en/ (last accessed 9 July 2020)
- 2.Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry 2015; 173: 221–231. [DOI] [PubMed] [Google Scholar]
- 3.Lo Buono V, Corallo F, Bramanti P, et al. Coping strategies and health-related quality of life after stroke. J Health Psychol 2017; 22: 16–28. [DOI] [PubMed] [Google Scholar]
- 4.Espárrago Llorcaa G, Castilla-Guerrab L, Fernández Morenoc MC, et al. Post-stroke depression: an update. Neurología 2015; 30: 23–31. [DOI] [PubMed] [Google Scholar]
- 5.Tu J, Wang LX, Wen HF, et al. The association of different types of cerebral infarction with post-stroke depression and cognitive impairment. Medicine (Baltimore) 2018; 97: e10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugawara N, Metoki N, Hagii J, et al. Effect of depressive symptoms on the length of hospital stay among patients hospitalized for acute stroke in Japan. Neuropsychiatr Dis Treat 2015; 11: 2551–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillen R, Tennen H, McKee T, et al. Depressive symptoms and history of depression predict rehabilitation efficiency in stroke patients. Arch Phys Med Rehabil 2001; 82: 1645–1649. [DOI] [PubMed] [Google Scholar]
- 8.Serrano S, Domingo J, Rodríguez-Garcia E, et al . Frequency of cognitive impairment without dementia in patients with stroke: a two-year follow-up study. Stroke 2007; 38: 105–110. [DOI] [PubMed] [Google Scholar]
- 9.Deng L, Sun X, Qiu S, et al. Interventions for management of post-stroke depression: a Bayesian network meta-analysis of 23 randomized controlled trials. Sci Rep 2017; 7: 16466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paolucci S. Advances in antidepressants for treating post-stroke depression. Expert Opin Pharmacother 2017; 18: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Ling S, Yang Y, et al. Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuro Endocrinol Lett 2014; 35: 104–109. [PubMed] [Google Scholar]
- 12.Mortensen JK, Andersen G. Safety of selective serotonin reuptake inhibitor treatment in recovering stroke patients. Expert Opin Drug Saf 2015; 14: 911–919. [DOI] [PubMed] [Google Scholar]
- 13.Lee YC, Lin CH, Lin MS, et al. Comparison of the effects of serotonin-norepinephrine reuptake inhibitors versus selective serotonin reuptake inhibitors on cerebrovascular events. J Clin Psychiatry 2016; 77: e1–e7. [DOI] [PubMed] [Google Scholar]
- 14.Fagone P, Jackowski S. Phosphatidylcholine and the CDP-choline cycle. Biochim Biophys Acta 2013; 1831: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gareri P, Castagna A, Cotroneo AM, et al. The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives. Clin Interv Aging 2015; 10: 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberhardt R, Birbamer G, Gerstenbrand F, et al . Citicoline in the treatment of Parkinson’s disease. Clin Ther 1990; 12: 489–495. [PubMed] [Google Scholar]
- 17.Ottobelli L, Manni GL, Centofanti M, et al. Citicoline oral solution in glaucoma: is there a role in slowing disease progression? Ophthalmologica 2013; 229: 219–226. [DOI] [PubMed] [Google Scholar]
- 18.Roohi-Azizi M, Arabzadeh S, Amidfar M, et al. Citicoline combination therapy for major depressive disorder: a randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol 2017; 40: 1–5. [DOI] [PubMed] [Google Scholar]
- 19.Jadavji NM, Emmerson JT, MacFarlane AJ, et al. B-vitamin and choline supplementation increases neuroplasticity and recovery after stroke. Neurobiol Dis 2017; 103: 89–100. [DOI] [PubMed] [Google Scholar]
- 20.Engelter ST, Urscheler N, Baronti F, et al. Frequency and determinants of using pharmacological enhancement in the clinical practice of in-hospital stroke rehabilitation. Eur Neurol 2012; 68: 28–33. [DOI] [PubMed] [Google Scholar]
- 21.Zhang LS, Hu XY, Yao LY, et al. Prophylactic effects of duloxetine on post-stroke depression symptoms: an open single-blind trial. Eur Neurol 2013; 69: 336–343. [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Lin M, Zhao J, et al. Different interventions for post-ischaemic stroke depression in different time periods: a single-blind randomized controlled trial with stratification by time after stroke. Clin Rehabil 2017; 31: 71–81. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava A, Taly AB, Gupta A, et al. Post-stroke depression: prevalence and relationship with disability in chronic stroke survivors. Ann Indian Acad Neurol 2010; 13: 123–127. doi: 10.4103/0972-2327.64643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Towfighi A, Ovbiagele B, El Husseini N, et al. Poststroke depression: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48: e30–e43. [DOI] [PubMed] [Google Scholar]
- 25.Göthe F, Enache D, Wahlund LO, et al. Cerebrovascular diseases and depression: epidemiology, mechanisms and treatment. Panminerva Med 2012; 54: 161–170. [PubMed] [Google Scholar]
- 26.Provinciali L, Coccia M. Post-stroke and vascular depression: a critical review. Neurol Sci 2002; 22: 417–428. [DOI] [PubMed] [Google Scholar]
- 27.Turner-Stokes L, Hassan N. Depression after stroke: a review of the evidence base to inform the development of an integrated care pathway. Part 2: Treatment alternatives. Clin Rehabil 2002; 16: 248–260. [DOI] [PubMed] [Google Scholar]
- 28.Lee EJ, Oh MS, Kim JS, et al. Serotonin transporter gene polymorphisms may be associated with poststroke neurological recovery after escitalopram use. J Neurol Neurosurg Psychiatry 2018; 89: 271–276. [DOI] [PubMed] [Google Scholar]
- 29.Chollet F, Cramer SC, Stinear C, et al. Pharmacological therapies in post stroke recovery: recommendations for future clinical trials. J Neurol 2014; 261: 1461–1468. [DOI] [PubMed] [Google Scholar]
- 30.Loubinoux I, Kronenberg G, Endres M, et al. Post‐stroke depression: mechanisms, translation and therapy. J Cell Mol Med 2012; 16: 1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hackett ML, Anderson CS, House A, et al. Interventions for treating depression after stroke. Cochrane Database Syst Rev 2008: CD003437. [DOI] [PubMed] [Google Scholar]
- 32.Leong C, Alessi-Severini S, Enns MW, et al. Cerebrovascular, cardiovascular, and mortality events in new users of selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors: a propensity score-matched population-based study. J Clin Psychopharmacol 2017; 37: 332–340. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y, Xiang Y, Yang Y, et al. Depression after minor stroke: prevalence and predictors. J Psychosom Res 2015; 79: 143–147. [DOI] [PubMed] [Google Scholar]
- 34.Lu H, Li M, Zhang B, et al. Efficacy and mechanism of acupuncture for ischemic poststroke depression: study protocol for a multicenter single-blinded randomized sham-controlled trial. Medicine (Baltimore) 2019; 98: e14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yıldırım T, Eylen A, Lule S, et al. Poloxamer-188 and citicoline provide neuronal membrane integrity and protect membrane stability in cortical spreading depression. Int J Neurosci 2015; 125: 941–946. [DOI] [PubMed] [Google Scholar]
- 36.Roohi-Azizi M, Torkaman-Boutorabi A, Akhondzadeh S, et al. Influence of citicoline on citaloram-induced antidepressant activity in depressive-like symptoms in male mice. Physiol Behav 2018; 195: 151–157. [DOI] [PubMed] [Google Scholar]
- 37.Piamonte BLC, Espiritu AI, Anlacan VMM. Effects of citicoline as an adjunct treatment for Alzheimer's disease: a systematic review [published online ahead of print, 2020 Jun 6]. J Alzheimers Dis 2020; 76: 725–732. [DOI] [PubMed] [Google Scholar]
- 38.Brown ES, Gabrielson B. A randomized, double-blind, placebo-controlled trial of citicoline for bipolar and unipolar depression and methamphetamine dependence. J Affect Disord 2012; 143: 257–260. [DOI] [PubMed] [Google Scholar]
- 39.Yoon SJ, Lyoo IK, Haws C, et al. Decreased glutamate/glutamine levels may mediate cytidine’s efficacy in treating bipolar depression: a longitudinal proton magnetic resonance spectroscopy study. Neuropsychopharmacology 2009; 34: 1810–1818. [DOI] [PubMed] [Google Scholar]
- 40.Kalyn Y, Gavrilova S, Safarova T, et al. Comparative efficacy and safety of antidepressant mono- and multimodal therapy with citicoline in elderly patients with depression in psychogeriatric unit. Eur Psychiatry 2016; 33: S470. [Google Scholar]
- 41.Secades JJ. Probably role of citicoline in stroke rehabilitation: review of the literature. Rev Neurol 2012; 54: 173–179. [PubMed] [Google Scholar]
- 42.Sharp SI, Francis PT, Elliott MS, et al. Choline acetyltransferase activity in vascular dementia and stroke. Dement Geriatr Cogn Disord 2009; 28: 233–238. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23(1): 56–62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beck AT, Ward CH, Mendelson M, et al. An Inventory for Measuring Depression. Arch Gen Psychiatry 1961; 4(6): 561–571. doi:10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 45.Bech P, andRafaelsen OJ.. The use of rating scales exemplified by a comparison of the Hamilton and the Bech‐Rafaelsen Melancholia Scale. Acta Psychiatrica Scandinavica 1980; 62: 128–132. doi:10.1111/j.1600-0447.1980.tb07683.x [Google Scholar]