Summary
Regulatory T cells (Treg) are suppressor cells that control self-reactive and excessive effector conventional T helper cell (Tconv) responses. Breakdown of the balance between Tregs and Tconvs is a hallmark of autoimmune and inflammatory diseases. Interleukin-2 (IL-2) is a growth factor for both populations and subtle leverage to restore the healthy immune balance in IL-2 therapy. By using a mechanistic mathematical model, we introduced an adaptive control strategy to design the minimal therapeutic IL-2 dosage required to increase and stabilize Treg population and restrict inflammatory response. This adaptive protocol allows for dose adjustments based on the feedback of the immune kinetics of the patient. Our simulation results showed that a minimal Treg population was required to restrict the transient side effect of IL-2 injections on the effector Tconv response. In silico results suggested that a combination of IL-2 and adoptive Treg transfer therapies can limit this side effect.
Subject Areas: Biological Sciences, Immunology, Mathematical Bioscience
Graphical Abstract
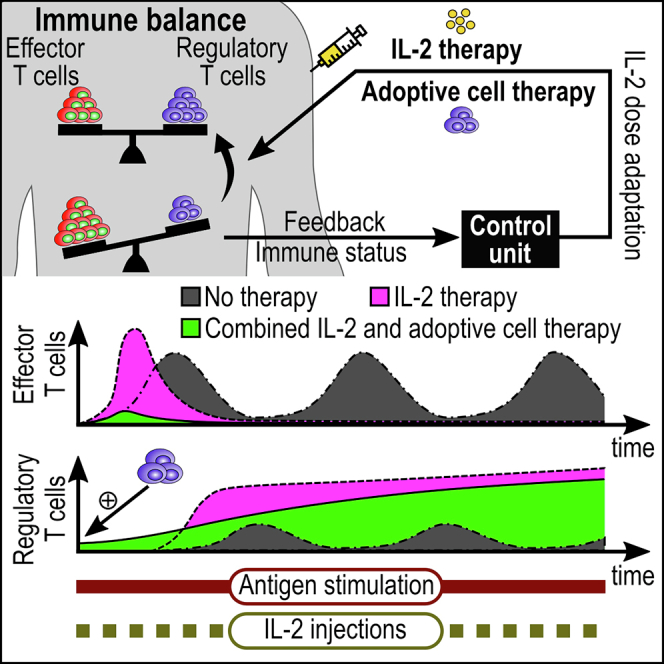
Highlights
-
•
An adaptive dosing strategy for IL-2 therapy is introduced and analyzed in silico
-
•
IL-2 injections can be tuned to increase and stabilize regulatory T-cell numbers
-
•
Immunosuppressive IL-2 therapy may transiently exacerbate effector T-cell responses
-
•
Combined IL-2 and adoptive regulatory T-cell therapy can safely limit inflammation
Biological Sciences; Immunology; Mathematical Bioscience
Introduction
Among numerous factors that control immunological tolerance, the balance between regulatory T cells (Treg) and conventional T helper cells (Tconv) is indispensable. By suppressing Tconv through various mechanisms (Sakaguchi et al., 2009), Tregs can control magnitude and duration of inflammatory responses in order to maintain healthy immune homeostasis and protect the host from immune-mediated pathology. Manipulation of homeostasis and interplay of Treg and Tconv is a therapeutic approach in the context of autoimmunity, transplantation, and cancer where inflammation works against the patient. In cancer, enhanced Treg homeostasis is deleterious, while quantitative and qualitative defects in the Treg compartment are implicated in multiple autoimmune diseases in humans and mice (Noack and Miossec, 2014).
In the absence of foreign antigens (Ags), the homeostatic number of Tregs and Tconvs is under control of homeostatic proliferation and thymic export. Activation of both, Treg and Tconv, via their T-cell receptor (TCR) is stimulated by antigen-presenting cells (APCs) (Thornton and Shevach, 1998; Takahashi et al., 1998). Interleukin-2 (IL-2), a monomeric glycoprotein, is characterized as a proinflammatory cytokine that is predominantly produced by activated Tconvs, but not Tregs. IL-2 acts as an autocrine T-cell growth factor and is necessary for the survival of Tregs and proliferation of both Tregs and Tconvs (Liao et al., 2013; Barron et al., 2010; Malek, 2003; Scheffold et al., 2005). Given that the biological factors impacting survival, activation, and proliferation of Tregs and Tconvs are mostly shared, immunotherapeutic perturbations using such factors impact both populations and demand caution. For example, with the premise that IL-2 stimulates the effector T-cell population, high doses of IL-2 administration were extensively used in patients with cancer, despite poor safety profile due to side effects (Rosenberg, 2014). The therapeutic outcome was only partially successful, and the unintended impact of IL-2 on Treg expansion, at least in part, may explain the failure of this therapy in some patients with cancer (Ahmadzadeh and Rosenberg, 2006; Zhang et al., 2005; Lemoine et al., 2009; Weiss et al., 2010).
Benefits of low-dose IL-2 administration were shown in patients with hepatitis C virus-induced vasculitis (Saadoun et al., 2011), type 1 diabetes (Hartemann et al., 2013), and chronic graft-versus-host disease (Koreth et al., 2011), which resulted in significant Treg proliferation and resolving the deficiency of Treg numbers in the context of autoimmune and alloimmune inflammatory diseases. Such results motivated the search for optimal doses and frequencies of IL-2 administration (Hartemann et al., 2013; von Spee-Mayer et al., 2016; He et al., 2016).
Due to the subtle role of IL-2 in regulating both effector and suppressor arms of the immune system, IL-2 therapy could be a double-edged sword and result in unintended adverse outcomes. In this study, by employing a mechanistic mathematical model of T-cell responses, we conducted an in silico analysis of IL-2 therapy as an approach to increase and stabilize the size of either T-cell subset. We introduced a feedback control scheme to calculate a time-resolved adaptive IL-2 dosing for each individual in silico patient. This scheme is based on the “impulsive zone model predictive control (iZMPC)” algorithm (Rivadeneira et al., 2015, 2017). The adaptive algorithm calculates proper IL-2 doses at each injection episode based on (1) feedback from the current status of the patient's immune response, (2) prediction of how the immune response progresses according to the mathematical model for a limited time horizon, and (3) predefined desired range and constraints for the immune variables (Tconvs, Tregs, and IL-2). We provided qualitative results as a proof of principle for our methodology in the context of transplantation as a use case scenario. In this scenario, restricting acute/chronic effector responses against the graft by increasing and stabilizing Treg numbers is the main goal. The adaptive IL-2 dosing algorithm is general and can be employed in other inflammatory contexts, such as cancer where increasing the number of effector T cells is desired.
Mathematical Framework
Dynamical Model of T-cell Responses
We described the dynamic interplay of activated Tconv (T) and Treg (R) populations under the influence of IL-2 (I) as depicted in Figure 1 by the following set of ordinary differential equations (Khailaie et al., 2013):
| (Equation 1) |
where N and denote naïve T cells and resting Tregs, respectively, and follow
| (Equation 2) |
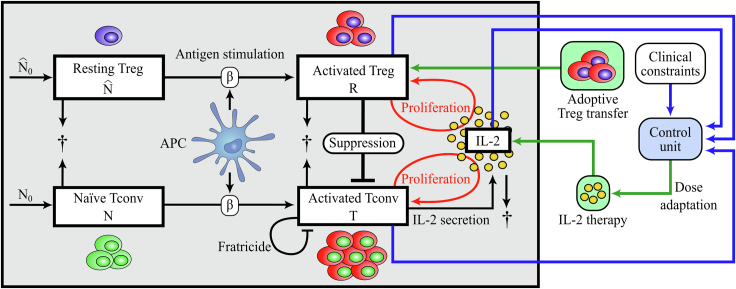
Figure 1.
Scheme of the T Cell Response Model and Adaptive Dosing IL-2 Therapy
IL-2 concentration and the population of two T-cell subsets, Tconvs and Tregs, are the immune variables considered in the mathematical model (1). Naïve Tconvs and resting Tregs originated from thymic selection are under homeostatic turnover in the periphery. Upon Ag stimulation provided by APCs, naïve Tconvs and resting Tregs become activated. In contrast to activated Tconvs, activated Tregs do not secrete IL-2, but both activated populations proliferate in dependence on the presence of IL-2. Activated Tregs suppress activated Tconvs in a cell contact–dependent and cytokine-driven manner. In contrast to Tregs, activated Tconvs undergo Fas-induced apoptosis by interacting with each other (fratricide). All cells undergo natural cell death and IL-2 is degraded. In the context of IL-2 therapy, the control unit provides the next optimal IL-2 dose according to a feedback from the current status of the immune variables. The control unit calculates the IL-2 dose that is needed to keep T-cell numbers and systemic IL-2 concentration in a predefined range (clinical constraints). Adoptive Treg transfer is the therapeutic process of increasing Treg numbers in the immune system by transiently transferring Tregs to the individual.
The definition and values of parameters are given in Table 1.
Table 1.
Parameters of the T cell Response Model
| Parameter | Value | Description | Dimension |
|---|---|---|---|
| a | 0.4 | Proliferation rate of activated Tconvs | molecule−1time−1 |
| b | 0.1 | Natural death rate of activated Tconvs and Tregs | time−1 |
| c | Fratricide death rate of activated Tconvs | cell−1time−1 | |
| d | 0.01 | IL-2 secretion rate by activated Tconvs | molecule cell−1time−1 |
| e | 0.01 | IL-2 consumption rate by activated Tconvs and Tregs | cell−1time−1 |
| f | 1 | IL-2 decay rate | time−1 |
| g | 0.1 | Natural death rate of naïve Tconvs and resting Tregs | time−1 |
| β | 0.05 | Ag stimulation of naïve Tconvs and resting Tregs | time−1 |
| γ | 0.1 | Treg-mediated suppression rate | cell−1time−1 |
| ε | 0.6 | Ratio of proliferation rates of Tregs to Tconvs | – |
| λ | 0.006 | Ratio of renewal rates of resting Tregs to naïve Tconvs | – |
| 4 | Renewal rate of naïve Tconvs | cell time−1 | |
| Renewal rate of resting Tregs | cell time−1 |
We assume that activation of Tconvs and Tregs in the presence of sufficiently strong Ag stimulation (large β) occurs much faster than other processes such as proliferation. Then, the variables N and have faster dynamics compared to T, R and I, and can be treated in the quasi-steady-state approximation by replacing N and with and , respectively.
The biological grounds for the choice of the model and underlying assumptions are explained in (Khailaie et al., 2013) and summarized in Figure 1. The model was constructed by starting from a minimal version that captures T-cell proliferation. Further complexities were added hierarchically to the model in order to adjust the resulting qualitative behavior to the biological observations. For example, the process of fratricide was added to resolve the problem of unlimited proliferation of activated Tconvs, and Tregs were added to shut down the immune response after the elimination of the antigen. To enforce the required model behavior, several constraints were imposed on the parameter ranges which were derived from the parametric stability analysis. Since such constraints are in the form of parameter ranges, the choice of parameter values that results in qualitative similarities to the experimentally observed T-cell kinetics is not unique. The values of the parameters in Table 1 were not informed by a quantitative data set and were chosen arbitrarily with respect to the parametric constraints obtained in (Khailaie et al., 2013). Therefore, numerically obtained variables and times are given on an arbitrary scale, and the results shall be interpreted qualitatively.
Mathematical Description of IL-2 Therapy
After intravenous administration of a drug, instantaneous jumps are observed in the drug concentration in plasma and the target organ (Yang, 2001). In the framework of mathematical modeling, systems with discontinuities in their dynamics can be categorized as impulsive systems and allow for application of control engineering design tools (Sopasakis et al., 2015; Rivadeneira et al., 2015, 2016, 2017; González et al., 2017; Fontes and Pereira, 2012; Magni et al., 2007; Bahremand et al., 2019; Montaseri et al., 2018, 2020; Rivadeneira and Moog, 2012). Among these methods, model predictive control (MPC) techniques have been widely used (Sopasakis et al., 2015; Rivadeneira et al., 2015, 2016, 2017; González et al., 2017; Fontes and Pereira, 2012; Magni et al., 2007; Bahremand et al., 2019) due to their ability to consider systemic constraints, which confine the dynamics of the system variables or the external control input. In the context of pharmacodynamics, the control input is the administered drug.
MPC algorithms follow a receding horizon strategy to construct a feedback control law, here, the amount of the administered drug. At each administration time, system variables are measured and provided to the MPC algorithm. Given the horizon parameter N, MPC predicts the system dynamics for the next N steps using the current measurement as the initial value of the dynamics. Then, it calculates the next N optimal doses by solving a constrained optimization problem aiming at steering the system dynamics to the desired and predefined equilibrium points. After calculation of the N optimal doses, only the first dose is kept and administered. This process is repeated at each next drug administration time point. In this study, we employed the iZMPC algorithm (Rivadeneira et al., 2015, 2017), an advanced version of MPC, in which system dynamics is moved toward a desired equilibrium set or space (instead to an equilibrium point) no matter which point inside the set.
Suppose an IL-2 administration scheme where IL-2 doses are sequentially injected intravenously at time intervals , where i is an increasing sequence of positive integers. We assume equidistant IL-2 injection times, i.e., . For , variables , , and follow Equation (1). At the moment of IL-2 injection (), we assume a sudden change in the amount of IL-2, i.e.
| (Equation 3) |
where denotes the time instance after , is Dirac delta function, , and is the dose of IL-2 injection. Considering the mathematical description of T-cell responses under IL-2 therapy (Figure 1), the problem of finding adaptive doses is formulated as the calculation of .
By employing iZMPC, the optimal IL-2 doses are obtained by repeatedly solving a constrained optimization problem at each injection time using the current measurement of the system dynamics T, R, and I. During the treatment, the amount of T, R, and I should be kept in the allowed physiological ranges (). To have a successful treatment, system dynamics should remain in the therapeutic target window (). In addition, the injected dose should be confined to a range predefined by the safety or toxicology limitations. The physiological and therapeutic ranges, as well as the safety considerations, are implemented in the iZMPC, and the optimal dose is calculated if the optimization problem is feasible. Infeasible cases can be solved by enlarging the constraining ranges or reducing the drug administration time interval. For more details about the iZMPC algorithm and the inclusion of design constraints, see the Supplemental Information.
Results
Dynamics of T-cell Responses under Chronic Ag Stimulation
The T-cell response model (1) that was constructed based on the basic principles of the balance between effector and suppressor cells is sufficient to represent different immune regimes such as sub-critical, acute, oscillatory, and over-suppressive immune responses depending on the level and dynamics of the Ag stimulation (Khailaie et al., 2013). Herein, we analyze the effect of IL-2 therapy in a scenario where Ag stimulation is persistent, leading to a chronic activation of T cells and the interplay of activated Tregs and Tconvs (the term “activated” is omitted hereinafter). We describe a scenario without therapy where the T-cell response model (1) is stimulated with a sufficiently high chronic Ag stimulation (a high constant value for β) such that a substantial proliferative Tconv response is initiated. It is assumed that the model starts from a healthy initial condition, i.e. all cells are in a non-activated state. This scenario could represent the immune challenge in transplantation where the Ag stimulation starts and stays chronic after the surgery.
In the absence of IL-2 therapy, the interplay of Tregs and Tconvs results in an oscillatory response (limit cycle) in all variables (see Figure 2). The initial (acute) response of Tconvs consists of an initial rise that results from the activation of naïve Tconvs by Ag stimulation as well as the proliferation associated with the secreted IL-2. Tregs that are activated with the same Ag stimulation cannot efficiently proliferate and suppress Tconvs until the concentration of IL-2 secreted by Tconvs rises. When Tregs proliferate, the number of Tconvs declines due to the direct suppression as well as IL-2 consumption by Tregs. The delay between the peaks of Tconvs and Tregs in the model results from the dependence of Tregs on IL-2 for proliferation and their inability to secrete this growth factor. The persistent stimulation of T cells with Ag (constant β) is responsible for the re-initiation of another Tconv response after having been suppressed by Tregs. This type of oscillatory response reflects a relapse-and-remission form of autoimmune diseases due to chronic stimulatory factors.
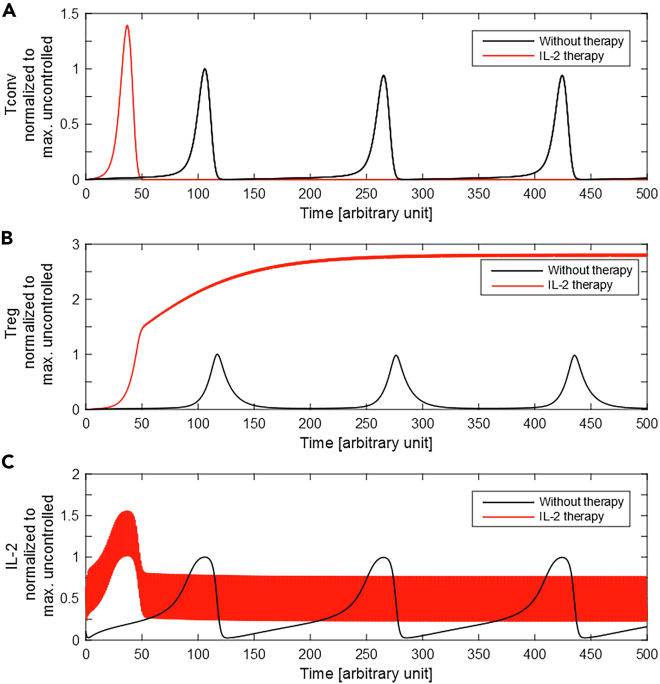
Figure 2.
Immune Response with and without IL-2 Therapy
The T-cell response model, Equation (1), was numerically solved in the presence (red) and absence (black) of IL-2 injections. The values of (A) Tconvs, (B) Tregs, and (C) IL-2 concentration were normalized to their maximum value in the absence of IL-2 injections. For IL-2 therapy, constant doses of 0.5 (arbitrary unit) are administered every unit of time (). The initial conditions are set to zero.
IL-2 Therapy Alone Cannot Avoid Acute Tconv Response but Controls Further Relapses
A fixed-dose of IL-2 injections was employed at the time of Ag stimulation within equidistant intervals. The time evolution of the system variables was obtained in the presence and absence of IL-2 injection (Figure 2). With IL-2 therapy, the initial proliferative response of Tconvs starts earlier and reaches a higher peak than without therapy (see Figure 2A). The continuation of IL-2 injections keeps the number of Tregs high and prevents re-initiation of Tconv responses.
The undesired consequence of IL-2 therapy in our simulation scenario is the stronger and earlier acute Tconv response. This effect results from the augmentation of external IL-2, raising the total systemic IL-2 concentration to a higher value than without the therapy. However, once the number of Tregs increases to a sufficient level, IL-2 injections can maintain a Treg population sufficient to suppress Tconvs. The suppression relies on the continuation of IL-2 injections for as long as the Ag stimulation persists.
Restricting Acute Tconv Responses with Adoptive Treg Transfer
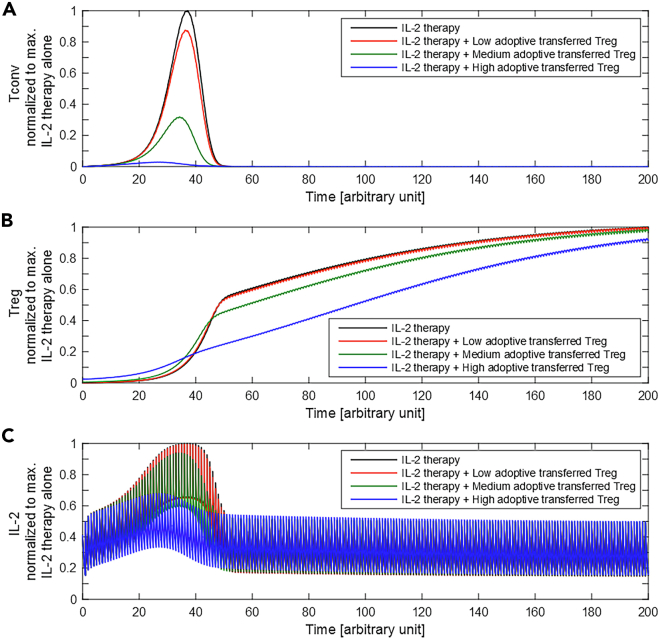
According to the T-cell response model, the IL-2 therapy may control re-initiation of the Tconv response after transiently boosting the first Tconv response. This initial boosting is due to the absence of sufficient Tregs in the early time points needed to suppress Tconvs and compete for IL-2. Therefore, in the context of transplantation where suppression of the acute Tconv response is a necessity for graft accommodation, IL-2 therapy alone may not be a safe immune suppressive strategy. One solution to restrict the rise of the Tconv population in the early episodes of IL-2 injection is to raise the initial number of activated Tregs by adoptive Treg transfer. This combined strategy is implemented in the numerical simulation by assuming a nonzero initial value for Tregs (i.e. ). Simulations with different levels of transferred Tregs were performed to observe the quantitative impact of the therapy on the peak of Tconv responses. A higher amount of adoptively transferred Tregs resulted in a larger reduction of the Tconv peak (Figure 3A) and, consequently, a reduced peak of the systemic IL-2 concentration due to less IL-2 secretion (Figure 3B). After the IL-2 and Tconv peak, all variables converged to a similar range as determined by the IL-2 dose alone. The simulations differ only in the initial Treg value, and therefore, only a transient impact is induced. The speed of convergence decreases with more adoptively transferred Tregs due to less secreted IL-2 by Tconvs and, thus, lower systemic IL-2 concentrations.
Figure 3.
IL-2 Therapy Combined with Adoptive Treg Transfer
Equation (1) was solved for the cases of IL-2 therapy alone (black) and in combination with different levels of adoptive Tregs transfer at (colors). The values of (A) Tconvs, (B) Tregs, and (C) IL-2 concentration were normalized to their maximum value for the case of IL-2 therapy alone. The administration frequency is 1. Low, medium, and high adoptive Tregs correspond to initial values of R of 0.01, 0.04, and 0.5, respectively.
These results suggest that the combined strategy of IL-2 therapy with an one-time adoptive transfer of Tregs is able to restrict the undesirable effect on Tconv proliferation and the systemic increase of IL-2.
Fixed versus Adaptive IL-2 Dosing
So far, IL-2 doses were fixed irrespective of the state of the immune response. In other words, no information of the state of the system was used, and therefore, the knowledge about the interplay between variables of the system is fully neglected. We addressed the possibility of adapting IL-2 doses automatically, taking into account the state of the T-cell response at the time of each IL-2 injection as well as predefined constraints on the immune variables.
We casted the problem of adaptive IL-2 dosing in the framework of feedback control systems in order to profit from advanced tools in the control engineering discipline. First, at each IL-2 injection, measurements of immune variables (T, R, and I) are needed. These measurements are used as an input to the control unit for calculation of the best IL-2 dose with the iZMPC algorithm, as described in the Supplemental Information. Figure 1 shows the scheme of the closed loop feedback system between the immune variables and the control unit.
The calculated IL-2 dose is applied to the system at (via Equation (3)). At the next IL-2 injection (i.e. ), the same calculation is repeated. This procedure continues until the variables of the system are steered in the desired ranges (predefined constraints). Thus, the IL-2 doses are optimal, in the sense that the control unit proposes the minimum IL-2 dose sufficient to force and keep the variables of the system within the target ranges. The variable IL-2 doses reflect the adaptation of IL-2 doses to the behavior of the system and take advantage of our knowledge about the immune response as captured in Equation (1).
In Figure 4, the behavior of the immune variables with fixed versus adaptive dose IL-2 therapy, each combined with adoptive Treg transfer, is compared. While adaptive doses reduced the peak response of Tconvs, the steady-state value of Tconvs settled at a higher level and Tregs at a lower level (Figures 4A and 4B). The advantage of the adaptive method becomes evident by noting the kinetics of systemic IL-2 at the time of the Tconv peak response as well as the dose of injected IL-2 (Figures 4C and 4D). The control unit reduced the IL-2 dose when the contribution of Tconvs to IL-2 secretion is increased, as well as at later time points when the Treg population is stabilized. This is also reflected in the lower amplitude of IL-2 oscillations.
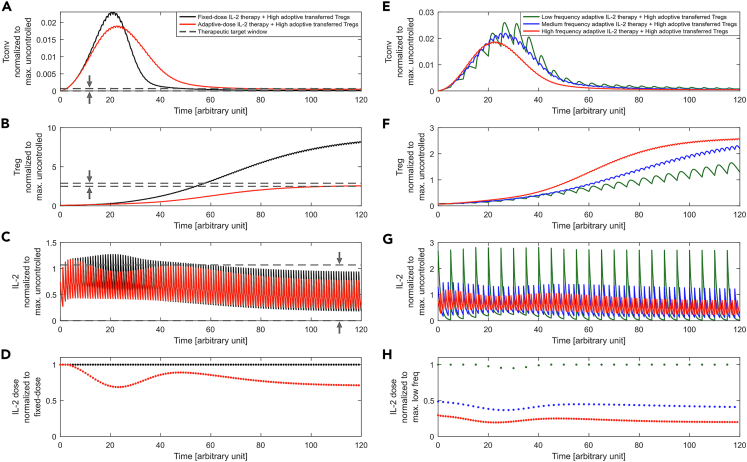
Figure 4.
Adaptive IL-2 Dosing Strategy
The kinetics of (A) Tconvs, (B) Tregs, (C) IL-2, and (D) IL-2 dose is shown for fixed (black) and adaptive dose IL-2 therapy (red), each applied at every time unit () and combined with adoptive Treg transfer (). The physiological range was and the therapeutic target window was . IL-2 doses were constrained to . For the fixed-dose therapy, the maximum allowed dose (i.e., 0.7) was administered. The calculated adaptive doses successfully enforced variables to the therapeutic target window (horizontal dashed lines).
The kinetics of (E) Tconvs, (F) Tregs, (G) IL-2, and (H) IL-2 dose is shown for different IL-2 injection frequencies δ of 1, 2, and 5 time units, corresponding to high, medium, and low frequency, respectively. The control unit selects the corresponding suitable doses in each case. The value of Tconvs, Tregs, and IL-2 is normalized to its maximum value for the uncontrolled case (without IL-2 therapy, Figure 2, black curves). For low frequencies, higher doses are required. Therefore, the maximum allowed IL-2 dose was increased to 2.5 to make the optimization problem feasible. The IL-2 dose is normalized to the dose in (D) fixed-dose value and (H) maximum dose value in low frequency.
These results suggest that the proposed strategy of IL-2 dosing is able to limit systemic IL-2 levels by adaptively reducing the dose when external IL-2 is not needed.
IL-2 Therapy: Frequency versus Dose
In addition to the dose, the frequency of IL-2 injections (δ) is a free parameter for designing the therapy. To evaluate the impact of the IL-2 injection frequency on the dynamics of T cells, the frequency was altered and adaptive IL-2 dosing was calculated (see Figures 4E–4H). In order to control the Tconv response with low frequency IL-2 injections, higher doses of IL-2 are needed. This results in a higher peak of systemic IL-2 concentration.
Discussion
Feedback control design provides a level of robustness in achieving objectives and constraints in uncertain and complex conditions. It has been successfully applied in engineering applications within multiple aspects of human daily life. Physiology of the human body itself contains many feedback control loops to regulate processes under uncertain and stochastic environmental conditions (Stone et al., 2015). Feedback control concepts are less common in medicine and therapeutic design. Therapeutic interventions based on feedback allow for adaptation to the unforeseen and unavoidable disturbing factors that are imposed to an individual. Using measurements prior to each therapeutic intervention and evaluating a mathematical model-based prediction of system state trajectory allows for informed adjustments of the therapy. This approach would lead to a more robust therapeutic outcome than a protocol-based treatment which is typically based on a one-fits-all approach. In this study, a feedback control scheme inspired from the control engineering field was assessed in silico for IL-2 therapy with the aim to regulate T-cell responses. The control scheme was applied to a mathematical model of T-cell responses that relies on established principles of Tconv and Treg activation, proliferation, and regulation (Khailaie et al., 2013). The model was used in the parametric regime that an oscillatory response (limit cycle) of Tconvs, Tregs, and IL-2 was resulted from constant exposure to Ag stimulation. We showed that by sequential administration of exogenous IL-2, the oscillatory response of cell populations and systemic IL-2 concentration could be removed but with a side effect of a stronger first peak. Elimination of oscillatory response resulted from increase and stabilization of Tregs (see Figure 2). Furthermore, we showed that by providing additional Tregs at the beginning of the Ag stimulation, the side effect of exogenous IL-2 administration could be highly restricted (see Figure 3).
In the context of immune tolerance induction/breakdown, antigen-specific interventions are typically desired. Such interventions require targeting a particular subset of Tconv and Treg clones with high specificity to the antigen. However, IL-2 therapy is an antigen-nonspecific immune intervention that influences all specificities of T cells and also targets both Tconvs and Tregs. Therefore, violation of tolerable concentration of IL-2 could cause significant dose-related morbidity, such as in application to cancer (Kammula et al., 1998). According to our in silico results, a fixed IL-2 dose ignoring the contribution of the endogenously secreted IL-2 would lead to an unwanted increased systemic IL-2 concentration (Figure 2C). The adaptive IL-2 dosing scheme could limit this side effect by taking into account the measured IL-2 concentration prior to injection episodes, as well as enforcing the system to a confined range of IL-2 concentration determined by clinical constraints (Figure 4C).
The presented methodology is general and can be adapted to different design requirements, such as increasing the Tconv number that would be beneficial in cancer applications. In this study, we targeted tolerance induction with the objective of increasing and stabilizing Treg numbers with specificity to an antigen, such as graft-specific antigen in transplantation. Tconvs and Tregs are both activated by their TCR recognizing the specific antigen. However, once Tregs are activated, their suppressive function is antigen nonspecific and could suppress Tconv responses against unrelated antigens (Thornton and Shevach, 2000). This bears the risk of unwanted tolerance induction against pathogenic agents (Brunstein et al., 2013). Therefore, the converging number of Tregs upon long-term IL-2 treatment and its impact on immunity against other antigens is a therapeutic design concern. In the presented adaptive control scheme, the therapeutic target window of variables can be imposed to the control unit reflecting such clinical constraints (Figure 1). As an example, we showed that by confining the Treg numbers to a specific range, even a lower number of Tregs compared to the fixed-dose IL-2 therapy ensures a similar extent of Tconv suppression during the acute phase (compare fixed- and adaptive-dose therapies in Figures 4A and 4B). Therefore, the proposed control scheme has the flexibility to enforce such clinical constraints into the therapy design (IL-2 dosing).
IL-2 therapy as an antigen-nonspecific approach is often used in conjunction with the antigen-specific therapies of adoptive (Tconvs/Tregs) cell transfer with the aim to sustain the vitality and efficacy of the transferred cells (McDonald-Hyman et al., 2016; Yee et al., 2002). The frequency of antigen-specific cells in patients, in particular of Tregs, is typically low, and ex vivo expansion protocols are required to increase the cell number. In the context of transplantation, our in silico results showed that the peak of Tconv response is inversely related to the number of adoptively transferred Tregs that was initially provided to the system (Figure 3A), suggesting that increasing the number of adoptively transferred Tregs specific to the graft antigens increases the chance of graft accommodation. However, prolonged in vitro expansion of endogenous antigen-specific Tregs is shown to impair their suppressive function (Wang et al., 2011). Using chimeric antigen receptors (CARs) to change the specificity of T cells (Zhang et al., 2018) is a promising method to construct a sufficient number of antigen-specific cells. Our adaptive control scheme can incorporate CAR Tregs and then be employed to optimize the IL-2 therapy with the aim to regulate and stabilize their number after transfer to the patient. Note that the adaptive control design is not directly linked to the amount of adoptively transferred cells, as it just temporarily changes the initial condition of the system and influences the transient immune response. However, the designed IL-2 dosing changes accordingly to enforce the transient T-cell response to the predefined physiological ranges of the system variables (clinical constraints). The long-term state of the system is only dictated by long-term therapies.
To evaluate the proposed control strategy, we imposed the condition of a persistent time-invariant Ag stimulation for the T-cell response model. This resulted in an oscillatory behavior of T-cell populations. In reality, Ag stimulation is dynamic and impacted by T-cell responses, resulting in a typical rise and fall of the pathogen load observed in viral or bacterial infections. The oscillatory behavior can be interpreted as a worst-case scenario where the extensive proliferative response is recurrent. The immune response against antigens typically involves T cells with different levels of specificity which may result in different kinetics of cell proliferation. The employed mathematical model does not represent such diversity of T cells and is limited to representing a monoclonal T-cell response. The impact of the IL-2 therapy on polyclonal or concurrent T-cell responses needs further investigations.
We provided qualitative in silico results for an adaptive control scheme in IL-2 therapy, as a proof of principle to motivate further investigations in this direction. There are more and more promising results from IL-2 therapies in experimental studies and clinical trials, which call for an interdisciplinary approach to bring the presented methodology to a quantitative level and pave the way for ultimate incorporation and validation in translational studies and clinical trials.
Limitations of the Study
Despite attractive benefits of using the presented adaptive IL-2 dosing methodology, its application for an individual patient is challenged by different sources of uncertainty that requires further investigation. The performance of the control design in action relies on the accuracy of the mathematical representation of the system. The mathematical model that we used here contains a low degree of freedom, which simplifies parameter inference from experimental and clinical data. In principle, there is a trade-off between model complexity and parameter identifiability. On the one hand, increasing the model complexity leads to a better representation of the multiple interactions existing in real T-cell responses and, thus, increases the control performance. On the other hand, most experimental and clinical measurements are limited by the accessibility of the immune variables, as well as the availability of biomarkers, which may not directly or uniquely be linked to the considered immune variables in the model. This limitation leads to unidentifiability and poor individualization of the model parameters for the patient and, consequently, weakens the control performance. Another source of uncertainty is the environmental or internal disturbing factors that are imposed on the patient during the therapy, such as infections. Due to the nature of feedback control design, the adaptive therapy is calculated according to the current state of the system, and therefore, the therapy would be robust to such disturbing factors. However, such an infection might interfere with the T-cell dynamics of the system, which is not reflected in the current model.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Sahamoddin Khailaie (khailaie@theoretical-biology.de).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
All data produced or analyzed for this study are included in the published article.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
S.K. was supported by the German Federal Ministry of Education and Research within the Measures for the Establishment of Systems Medicine, project SYSIMIT (BMBF eMed project SYSIMIT, FKZ: 01ZX1308B) and by the Helmholtz Association, Zukunftsthema Immunology and Inflammation (ZT-0027). The authors would like to thank Sebastian Binder for revising the manuscript.
Author Contributions
S.K. designed the study. G.M. and S.K. proposed the adaptive control scheme. G.M. developed the adaptive control methodology and performed the simulations. M.H.H. supervised the study. All authors contributed to the interpretation of the results and writing the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101663.
Contributor Information
Sahamoddin Khailaie, Email: khailaie@theoretical-biology.de.
Michael Meyer-Hermann, Email: mmh@theoretical-biology.de.
Supplemental Information
References
- Ahmadzadeh M., Rosenberg S.A. IL-2 administration increases CD4+ CD25hi Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahremand S., Ko H.S., Balouchzadeh R., Lee H.F., Park S., Kwon G. Neural network-based model predictive control for type 1 diabetic rats on artificial pancreas system. Med. Biol. Eng. Comput. 2019;57:177–191. doi: 10.1007/s11517-018-1872-6. [DOI] [PubMed] [Google Scholar]
- Barron L., Dooms H., Hoyer K.K., Kuswanto W., Hofmann J., O’Gorman W.E., Abbas A.K. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J. Immunol. 2010;185:6426–6430. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein C., Blazar B., Miller J., Cao Q., Hippen K., McKenna D., Curtsinger J., McGlave P., Wagner J. Adoptive transfer of umbilical cord blood-derived regulatory T cells and early viral reactivation. Biol. Blood Marrow Transpl. 2013;19:1271–1273. doi: 10.1016/j.bbmt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes F.A., Pereira F.L. Model predictive control of impulsive dynamical systems. IFAC Proc. Volumes. 2012;45:305–310. [Google Scholar]
- González A.H., Rivadeneira P.S., Ferramosca A., Magdelaine N., Moog C.H. Impulsive zone mpc for type i diabetic patients based on a long-term model. IFAC-PapersOnLine. 2017;50:14729–14734. [Google Scholar]
- Hartemann A., Bensimon G., Payan C., Jacqueminet S., Bourron O., Nicolas N., Fonfrede M., Rosenzwajg M., Bernard C., Klatzmann D. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- He J., Zhang X., Wei Y., Sun X., Chen Y., Deng J., Jin Y., Gan Y., Hu X., Jia R. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat. Med. 2016;22:991–993. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- Kammula U., White D., Rosenberg S. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer. 1998;83:797–805. [PubMed] [Google Scholar]
- Khailaie S., Bahrami F., Janahmadi M., Milanez-Almeida P., Huehn J., Meyer-Hermann M. A mathematical model of immune activation with a unified self-nonself concept. Front. Immunol. 2013;4:474. doi: 10.3389/fimmu.2013.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreth J., Matsuoka K., Kim H.T., McDonough S.M., Bindra B., Alyea E.P.r., Armand P., Cutler C., Ho V.T., Treister N.S. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 2011;365:2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine F.M., Cherai M., Giverne C., Dimitri D., Rosenzwajg M., Trebeden-Negre H., Chaput N., Barrou B., Thioun N., Gattegnio B. Massive expansion of regulatory T-cells following interleukin 2 treatment during a phase I-II dendritic cell-based immunotherapy of metastatic renal cancer. Int. J. Oncol. 2009;35:569–581. doi: 10.3892/ijo_00000368. [DOI] [PubMed] [Google Scholar]
- Liao W., Lin J.X., Leonard W.J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni L., Raimondo D.M., Bossi L., Man C.D., Nicolao G.D., Kovatchev B., Cobelli C. Model predictive control of type 1 diabetes: an in silico trial. J. Diabetes Sci. Technol. 2007;1:804–812. doi: 10.1177/193229680700100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T.R. The main function of IL-2 is to promote the development of T regulatory cells. J. Leukoc. Biol. 2003;74:961–965. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- McDonald-Hyman C., Flynn R., Panoskaltsis-Mortari A., Peterson N., MacDonald K., Hill G., Luznik L., Serody J., Murphy W., Maillard I. Therapeutic regulatory T-cell adoptive transfer ameliorates established murine chronic GVHD in a CXCR5-dependent manner. Blood. 2016;128:1013–1017. doi: 10.1182/blood-2016-05-715896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaseri G., Alfonso J.C.L., Hatzikirou H., Meyer-Hermann M. A minimal modeling framework of radiation and immune system synergy to assist radiotherapy planning. J. Theor. Biol. 2020;486:110099. doi: 10.1016/j.jtbi.2019.110099. [DOI] [PubMed] [Google Scholar]
- Montaseri G., Boianelli A., Hernandez-Vargas E.A., Meyer-Hermann M. PK/PD-based adaptive tailoring of oseltamivir doses to treat within-host influenza viral infections. Prog. Biophys. Mol. Biol. 2018;139:31–42. doi: 10.1016/j.pbiomolbio.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Noack M., Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014;13:668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Rivadeneira P., Moog C. Impulsive control of single-input nonlinear systems with application to hiv dynamics. Appl. Math. Comput. 2012;218:8462–8474. [Google Scholar]
- Rivadeneira P.S., Caicedo M., Ferramosca A., González A.H. 2017 IEEE 56th Annual Conference on Decision and Control (CDC) IEEE; 2017. Impulsive zone model predictive control (iZMPC) for therapeutic treatments: application to HIV dynamics; pp. 4094–4099. [Google Scholar]
- Rivadeneira P.S., Ferramosca A., González A.H. MPC with state window target control in linear impulsive systems. IFAC-PapersOnLine. 2015;48:507–512. [Google Scholar]
- Rivadeneira P.S., Ferramosca A., González A.H. Impulsive zone model predictive control with application to type I diabetic patients. IEEE Conf. Cont. App. 2016;48:544–549. [Google Scholar]
- Rosenberg S.A. IL-2: The First Effective Immunotherapy for Human Cancer. J. Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun D., Rosenzwajg M., Joly F., Six A., Carrat F., Thibault V., Sene D., Cacoub P., Klatzmann D. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int. Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- Scheffold A., Huehn J., Hoefer T. Regulation of CD4+CD25+ regulatory T cell activity: it takes (IL-)two to tango. Eur. J. Immunol. 2005;35:1336–1341. doi: 10.1002/eji.200425887. [DOI] [PubMed] [Google Scholar]
- Sopasakis P., Patrinos p., Sarimveis H., Bemporad A. Model predictive control for linear impulsive systems. IEEE Tran. Autom. Cont. 2015;60:227–2282. [Google Scholar]
- von Spee-Mayer C., Siegert E., Abdirama D., Rose A., Klaus A., Alexander T., Enghard P., Sawitzki B., Hiepe F., Radbruch A. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2016;75:1407–1415. doi: 10.1136/annrheumdis-2015-207776. [DOI] [PubMed] [Google Scholar]
- Stone D., Celi L., Csete M. Engineering control into medicine. J. Crit. Care. 2015;30:652.e1-7. doi: 10.1016/j.jcrc.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Kuniyasu Y., Toda M., Sakaguchi N., Itoh M., Iwata M., Shimizu J., Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Thornton A., Shevach E. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Lu L., Jiang S. Regulatory T cells: customizing for the clinic. Sci. Transl. Med. 2011;3:83ps19. doi: 10.1126/scitranslmed.3001819. [DOI] [PubMed] [Google Scholar]
- Weiss L., Letimier F.A., Carriere M., Maiella S., Donkova-Petrini V., Targat B., Benecke A., Rogge L., Levy Y. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proc. Natl. Acad. Sci. U S A. 2010;107:10632–10637. doi: 10.1073/pnas.1000027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. Springer-Verlag; 2001. Impulsive Control Theory. [Google Scholar]
- Yee C., Thompson J., Byrd D., Riddell S., Roche P., Celis E., Greenberg P. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chua K.S., Guimond M., Kapoor V., Brown M.V., Fleisher T.A., Long L.M., Bernstein D., Hill B.J., Douek D.C. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat. Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Lu W., Liang C., Chen Y., Liu H., Qiu F., Dai Z. Chimeric antigen receptor (CAR) Treg: a promising approach to inducing immunological tolerance. Front. Immunol. 2018;9:2359. doi: 10.3389/fimmu.2018.02359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data produced or analyzed for this study are included in the published article.