Abstract
The emergence of Zika virus (ZIKV) in Latin America brought to the fore longstanding concerns that forests bordering urban areas may provide a gateway for arbovirus spillback from humans to wildlife. To bridge urban and sylvatic transmission cycles, mosquitoes must co-occur with both humans and potential wildlife hosts, such as monkeys, in space and time. We deployed BG-Sentinel traps at heights of 0, 5, 10, and 15 m in trees in a rainforest reserve bordering Manaus, Brazil, to characterize the vertical stratification of mosquitoes and their associations with microclimate and to identify potential bridge vectors. Haemagogus janthinomys and Sabethes chloropterus, two known flavivirus vectors, showed significant stratification, occurring most frequently above the ground. Psorophora amazonica, a poorly studied anthropophilic species of unknown vector status, showed no stratification and was the most abundant species at all heights sampled. High temperatures and low humidity are common features of forest edges and microclimate analyses revealed negative associations between minimum relative humidity, which was inversely correlated with maximum temperature, and the occurrence of Haemagogus and Sabethes mosquitoes. In this reserve, human habitations border the forest while tamarin and capuchin monkeys are also common to edge habitats, creating opportunities for the spillback of mosquito-borne viruses.
Subject terms: Entomology, Alphaviruses, Dengue virus, Viral reservoirs, Viral vectors
Introduction
In Latin America, Zika (ZIKV, Flaviviridae: Flavivirus), dengue (DENV, Flaviviridae: Flavivirus), and chikungunya (CHIKV, Togaviridae: Alphavirus) viruses exist in human-endemic cycles of transmission involving Aedes aegypti and Ae. albopictus mosquitoes1,2. However, their ancestral lineages can be traced to the forests of Africa (ZIKV, CHIKV) and Southeast Asia (DENV), where each of these viruses is transmitted in sylvatic cycles among non-human primates by canopy-dwelling Aedes species1. There is significant concern that ZIKV could spill back into a New World sylvatic cycle, precluding regional elimination and creating an enduring threat to human health1. These concerns stem from similarities between the natural histories of yellow fever virus (YFV, Flaviviridae: Flavivirus) and ZIKV. In Africa, both viruses are maintained among many of the same monkey and mosquito species3–5. YFV was introduced to the Americas around 400 years ago6, after which it spilled back into a sylvatic cycle involving neotropical monkeys7 and Haemagogus and Sabethes species mosquitoes8,9. Recently, some of these same monkey and mosquito species have been shown to be susceptible to experimental infection with ZIKV10–13, and ZIKV has been isolated from monkeys in suburban neighborhoods in Brazil14,15. However, the virus has not been isolated from free-living forest mosquitoes, although at present, only low-numbers of the most likely sylvatic vector species have been screened16,17.
In light of these developments, there is a need to identify the species best poised to act as bridge vectors that could transfer ZIKV from humans to wildlife and back again. In this case, potential bridge vectors are those mosquitoes that are competent for ZIKV infection and bite both humans and potential wildlife reservoir species. The rate of contact between a vector and its host is a crucial component of vectorial capacity18, and a successful bridge vector must, therefore, co-occur in space and time with both humans and wildlife reservoirs. To elucidate potential bridge vectors, we previously investigated the distribution of mosquitoes in urban parks in Manaus, Brazil19. Manaus is a city of more than 2 million people surrounded by the Amazon rainforest, where abrupt urban-forest edges bring humans and animals into close contact and where DENV, CHIKV, and ZIKV circulate regularly. In these parks, Ae. albopictus and Ae. aegypti penetrated at least 100 m from the urban edge and may therefore serve as bridge vectors if they feed on both humans and monkeys. Haemagogus janthinomys, Sabethes glaucodaemon, and Sa. tridentatus were among multiple taxa of forest-dwelling mosquitoes present close to the urban-forest edge, but were rare19. However, these mosquitoes were sampled with BG-Sentinel traps placed at ground level, which likely favored the collection of ground-dwelling species and underrepresented the arboreal mosquitoes active at higher heights.
The main vectors of sylvatic YFV in the Americas, Hg. janthinomys, Hg. leucocelaenus, and Sa. chloropterus20,21, are arboreal species that all occur in the Amazon. All three are diurnally active mosquitoes inhabiting forest canopies20, a behavior termed “acrodendrophily”22, that lay eggs in natural containers such as tree holes or bamboo internodes23,24. Monkeys are thought to be an important bloodmeal source for these species25, although they will opportunistically feed on humans when in close contact20,26. There is also serological evidence that Hg. janthinomys and Hg. leucocelaenus feed on a range of other vertebrates including birds, rodents, and marsupials27,28.
Despite their acrodendrophilic habits, Hg. janthinomys, Hg. leucocelaenus, and Sa. chloropterus are not restricted to the forest canopy20. Both Hg. janthinomys and Sa. chloropterus are strongly acrodendrophilic25, 29–31, while Hg. leucocelaenus shows a more dispersed distribution between the canopy and ground32,33. However, there is some evidence that the stratification of Hg. janthinomys32 and Sa. chloropterus29 changes seasonally, and there are mainly anecdotal reports that acrodendrophilic species including Hg. janthinomys bite at ground level at forest edges20,34 and elsewhere where the canopy is disturbed30,32. There is also limited quantitative evidence that variation in microclimate may affect the vertical distribution of Haemagogus and Sabethes mosquitoes, with strongly acrodendrophilic species seeming to prefer hotter and less humid conditions associated with the forest canopy25,32. The physiological condition of mosquitoes in general may also influence their preferred activity height, as may external factors such as the distribution of their hosts35,36.
In this study, we investigated the composition and stratification of mosquito species at the Adolpho Ducke forest reserve which borders the city of Manaus, focusing on likely bridge vectors of mosquito-borne viruses between urban and sylvatic cycles. We predicted that known or probable bridge vectors would show distributions extending from the forest floor to the highest heights sampled, corresponding with strata most commonly occupied by humans and monkeys. In addition, we predicted that the most strongly acrodendrophilic species would be more abundant at relatively higher temperatures and lower humidity. Finally, we tested associations between 7-day cumulative rainfall in the weeks prior to collections and the occurrence of specific taxa, predicting that they would be consistent with the development times of particular species.
Methods
Study area
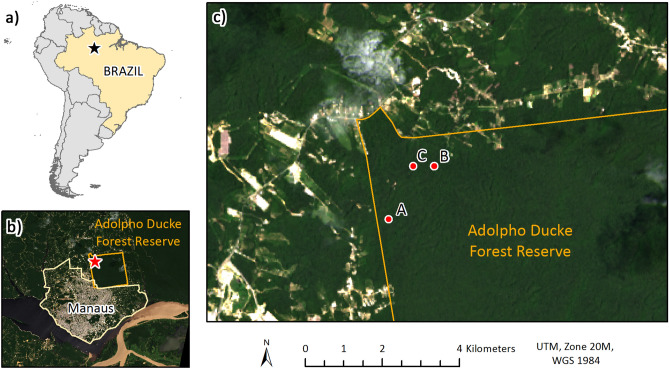
The Adolpho Ducke forest reserve is 100 km2 of primary rainforest37 situated north of Manaus in Amazonas State, Brazil (Fig. 1). The reserve borders Manaus in the northeast of the city where the two form an abrupt edge between urban and forest environments. The remaining boundaries border peri-urban or rural areas, which are contiguous with the Amazon rainforest. Canopy height in the region is 30–35 m with emergent trees reaching 50 m, while palms including Astrocaryum, Attalea, Bactris, and Geonoma species are abundant in the low-light understory. Ducke receives relatively light foot-traffic compared to other forest parks in Manaus, being mainly accessed by students and researchers. However, nearby communities, and densely populated urban areas in the southwest bring humans into close contact with the forest and its wildlife. In total, six monkey species have been recorded in the reserve38, namely the Guianan red howler monkey (Alouatta macconnelli), Guiana spider monkey (Ateles paniscus), tufted capuchin (Sapajus apella), bearded saki (Chiropotes chiropotes), golden-faced saki (Pithecia chrysocephala), and pied tamarin (Saguinus bicolor). Relative humidity exceeds 70% and annual precipitation ranges from 1750 to 2500 mm. The rainy season lasts from November to May while the dry season lasts from June to October37 (Fig. 2).
Figure 1.
Map showing location of (a) Manaus in Brazil, (b) the Adolpho Ducke forest reserve in Manaus and study area (star) in the northwest of the reserve, and (c) study sites A, B, and C, where collections were made. Orange outline shows the approximate extent of the Ducke reserve. Map created using ArcGIS Desktop 10.7.1 (ESRI, Redlands, California).
Figure 2.
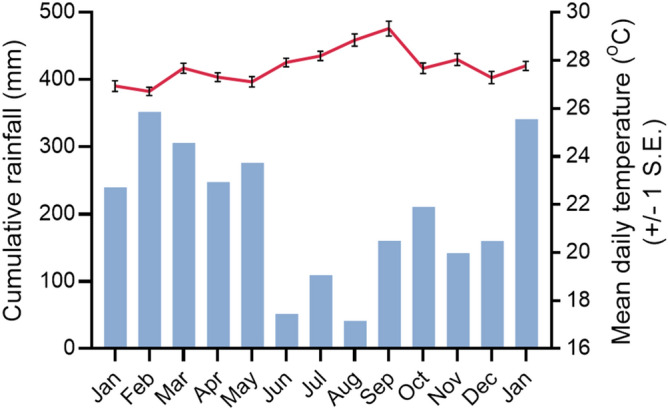
Weather during the study period from January 2019 until January 2020, recorded at the INMET meteorological station in Manaus39. Bars show cumulative monthly rainfall (mm) and line shows mean daily temperature per month ± 1 standard error (S.E.).
Study sites
Three study sites, A (central coordinates: 02.93776° S, 059.97646° W), B (central coordinates: 02.92537° S, 059.96582° W), and C (central coordinates: 02.92531° S, 059.97069° W), were established approximately 500–600 m from the forest edge in the north western corner of the reserve (Fig. 1). Site A was chosen based on prior knowledge of the mosquito fauna at this location, which included Haemagogus and Sabethes mosquitoes. Distance to forest edge was then controlled as a variable when choosing sites B and C, where these mosquitoes were also found to be present following pilot collections. Both Haemagogus and Sabethes species are thought to exhibit low dispersal when oviposition sites are abundant40 and distances of 500 m–2 km were, therefore, maintained between the sites to minimize spatial autocorrelation while ensuring that they were accessible. Each site was in a section of forest populated by large trees approximately 20–30 m in height and was close to an area of open canopy caused by at least one tree fall.
BG-Sentinel trap collections in trees
BG-Sentinel 2 traps (BioQuip, Rancho Dominguez, California) baited with CO2 (1 kg dry ice) and BG-Lure, a synthetic attractant containing ammonia, lactic acid, and caproic acid, were used to sample mosquitoes at heights of 0, 5, 10, and 15 m. Thirteen trees were sampled in total (Table 1); five at site A, and four at sites B and C. Trees were selected that (1) had appropriately high branches for suspending traps, (2) had either visible tree holes or were thought large enough to permit breeding by target species, (3) were situated within reasonable proximity to treefall gaps, and (4) were concentrated in areas where the mosquitoes of interest were present. For these reasons, the trees were clustered within 75 m at each site, but distances of > 10 m were maintained between them to minimize trap interference41. In each tree, a fishing line was placed over a branch using a Big Shot (SherrillTree, Greensboro, North Carolina) slingshot (Supplementary Fig. S1). The line was then substituted with a rope marked at 1 m intervals. A BG-Sentinel trap was tied to the rope and equipped with dry ice, which was wrapped in four sheets of newspaper and placed inside a sealable plastic bag with a 1 cm hole in the corner, before being raised to the desired height.
Table 1.
Identity and measurement of trees in which traps were placed at each site.
| Site | Family | Genus | Species | Local name | DBH (cm) |
|---|---|---|---|---|---|
| A | Elaeocarpaceae | Sloanea | guianensis | Urucurana | 30.3 |
| Lecythidaceae | Eschweilera | coriacea | Matamatá-branco | 99.4 | |
| Lecythidaceae | Eschweilera | pedicellata | Castanha Vermelha | 49.4 | |
| Melastomataceae | Mouriri | angulicosta | Muiraúba | 67.2 | |
| Sapotaceae | Pouteria | cladantha | Abiurana | 52.7 | |
| B | Apocynaceae | Geissospermum | argenteum | Acariquara-branca | 52.5 |
| Fabaceae | Dipteryx | polyphylla | Cumarurana | 68.5 | |
| Lecythidaceae | Eschweilera | pseudodecolorans | Ripeiro | 90.4 | |
| Lecythidaceae | Eschweilera | truncata | Matamatá | 75.2 | |
| C | Chrysobalanaceae | Licania | rodriguesii | Pajurazinho | 68.8 |
| Fabaceae | Pseudopiptadenia | psilostachya | Faveira-folha-fina | 35.0 | |
| Lecythidaceae | Corythophora | alta | Ripeiro Vermelho | 75.8 | |
| Sapotaceae | Micropholis | guyanensis | Risadinha | 46.5 |
DBH (cm) = diameter at breast height (1.3 m) in centimeters. See Supplementary file: Dataset for cross reference between tree species and mosquito collections.
When collecting at a site, one trap was placed in each tree at a randomly selected elevated height (5, 10, or 15 m) and one trap was placed at ground level (0 m) at the base of one or two of the trees. Traps were generally set before 11:00 and left to run for approximately 24 h before mosquitoes were collected. Different heights were then randomly selected, without replacement, for subsequent collections until all elevated and ground level heights had been sampled. The process was then repeated. The study design combined with occasional battery failure meant that there were days when no ground-level traps were running, or fewer traps were running at elevated heights, but overall, an equal number of collections were made at each of the four heights at each site. Collections started at site A on 24 January 2019 at the beginning of the rainy season (Fig. 2), followed by site B on 7 March and site C on 18 March. Once all sites had been established, collections were rotated between the three in a 3 × 3 Latin square design. From 17 June, all three sites were sampled simultaneously to increase the yield of target species before collections were suspended on 27 June 2019 at the beginning of the dry season when the abundance of target species diminished. Simultaneous collections were then restarted at sites B and C on 11 November 2019 following a period of substantial rainfall (Fig. 2), which was twice the monthly mean for September and October42, and collections continued until 9 January 2020.
Sample storage and permits
All mosquitoes were stored in the field on dry ice and were stored in the laboratory at the Fundação de Medicina Tropical Doutor Heitor Vieira Dourado at − 80 °C until identified. Permission to collect mosquitoes was provided by the Brazilian Ministry of the Environment (SISBIO 57003-6).
Environmental variables
In order to investigate variation in microclimate among heights and associations between microclimate and mosquito distributions, temperature (°C) and relative humidity (%) were recorded at 30-min intervals using Hygrochron iButton data loggers (Maxim Integrated, San Jose, California) placed in a catch bag (BioQuip, Rancho Dominguez, California) on the outside of each trap. These data were used to calculate the minimum, maximum, mean, daytime mean (06:00–18:00), and range of both variables. In addition, precipitation data were obtained from the automated INMET meteorological station (Code: Manaus-A101, OMM: 81730) located in the city of Manaus (3.103682° S, 60.015461° W) and were used to calculate several precipitation variables. These were defined as total rain (cumulative rainfall in mm during the ≈24 h sampling period), early rain (cumulative rainfall from 08:00 to 13:00 on the day traps were set), late rain (cumulative rainfall from 14:00 to 17:00 on the day traps were set), and daytime rain (cumulative rainfall from 08:00 to 17:00 on the day traps were set). Seven-day cumulative rainfall was also calculated at lags of 1, 2, 3, and 4 weeks prior to each collection to test associations between past rainfall and the occurrence of specific taxa.
Mosquito identifications
Individual mosquitoes were placed on a chill table (BioQuip, Rancho Dominguez, California) and were identified to genus and, where possible, species using a stereomicroscope and relevant taxonomic keys43–49. Genus and species names follow nomenclature in the Walter Reed Biosystematics Unit New Mosquito Classification50. All samples were stored at − 80 °C for future arbovirus screening.
Statistical analyses
Microclimate data were not normally distributed and variation in temperature and humidity parameters among heights was, therefore, analyzed using Kruskal–Wallis tests followed by post-hoc Wilcoxon Each Pair tests to compare differences between heights. Linear regression with site (A, B, or C) as the unit of replication was used to test the effect of height on the mean number of identified species and species diversity as measured by the Shannon–Wiener diversity index using the Vegan package in R v3.6.151. Two approaches were taken to compare mosquito communities at different heights: first, the Morisita overlap index was calculated to compare mosquito community overlap between collection sites and between sampled heights using PAST: Paleontological statistics software package52, second, a principal components analysis of relative frequencies of each species was conducted followed by hierarchical clustering of communities at each height. Due to the low abundance of Hg. janthinomys and Sa. chloropterus, Pearson’s chi-squared tests followed by post-hoc two-tailed Fisher’s exact tests were used to investigate the number of traps positive at each height for target species with large sample sizes. The latter test was also used to compare the number of traps positive at ground level with those above the ground when sample sizes were small. Pairwise Spearman’s rank analyses were used to investigate correlations between trap height, temperature, humidity, and rainfall variables except for rainfall lag, and nominal logistic regressions were used to investigate the impact of target variables on the occurrence of designated mosquito taxa at each height. Nominal logistic regressions were also used to test associations between rainfall lag and the occurrence of designated taxa. Statistical analyses were performed using JMP 1453 unless otherwise stated.
Results
Variation in microclimate among forest strata
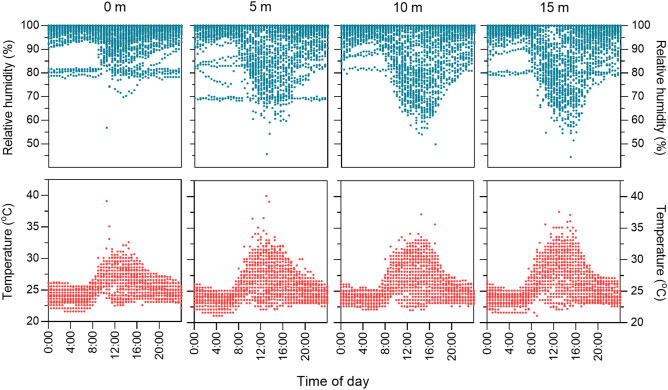
As expected, temperatures rose, and humidity fell with increasing height above the forest floor (Fig. 3). Spearman’s rank analyses showed that almost all temperature, humidity, and rainfall variables were significantly correlated (P < 0.05) with one another (Supplementary Table S1). Temperature and humidity variables, apart from maximum humidity (P = 0.57), were also significantly correlated with trap height, but rainfall and trap height were not correlated. There was a significant effect of height on minimum relative humidity (Kruskal–Wallis, DF = 3, χ2 = 75.6, P < 0.0001) and maximum temperature (DF = 3, χ2 = 55.8, P < 0.0001) (Fig. 4), which were chosen as two key variables that together captured variation in microclimate. Post-hoc Wilcoxon Each Pair analyses (exact P values listed in Supplementary Table S2) revealed that these differences were significant (P < 0.05) when comparing 0 m with all elevated heights for both variables; when comparing 5 m with 10 m for maximum temperature only; and when comparing 5 m with 15 m for both variables. However, differences were not significant for either variable when comparing 10 m with 15 m.
Figure 3.
Daily variation in microclimate among forest strata showing increasing temperature (°C) and decreasing relative humidity (%) with increasing height. Data recorded at 30-min intervals for duration of sampling.
Figure 4.
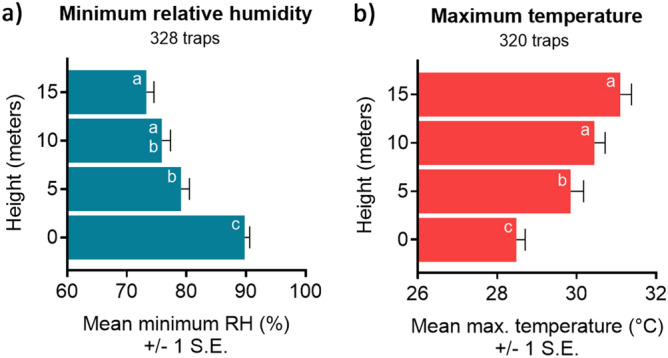
Variation in microclimate showing (a) mean minimum relative humidity (%) and (b) mean maximum temperature (°C) ± 1 standard error (S.E.) at each height sampled. Minimum relative humidity based on observations from 328 traps and maximum temperature based on observations from 320 trap collections. Different letters within bars indicate significant differences in occurrence between heights for each species; letters indicate within-panel comparisons only.
Relative abundance of mosquitoes
Each height was sampled 31 times at each site (N = 124 BG-Sentinel trap collections per site), equivalent to 372 collections across the three sites in total. These collections yielded 1834 mosquitoes representing 14 genera and 37 identified species (Fig. 5), of which 99.8% (1831/1834) were female (Supplementary file: Dataset). All specimens were identified to genus, but damage caused by trap fans, prolonged exposure to heavy rainfall, and high humidity often precluded species-level identifications, particularly of the more ornate species including those within the genus Sabethes. The number and percent of mosquitoes identified to species level within each genus were: Psorophora (1205/1407, 85.6%), Sabethes (75/133, 56.4%), Haemagogus (107/132, 81.1%), Culex (26/70, 37.1%), Orthopodoymia (20/22, 90.1%), Wyeomyia (6/21, 28.6%), Limatus (16/20, 80%), Trichoprosopon (12/15, 80%), Aedeomyia (4/4, 100%), Aedes (4/4, 100%), Mansonia (2/3, 66.7%), Anopheles (1/1, 100%), Coquillettidia (1/1, 100%), and Toxorhynchites (0/1, 0%). Overall, 1479 mosquitoes (80.6% of total collection) were identified to species, and these are summarized in Fig. 5.
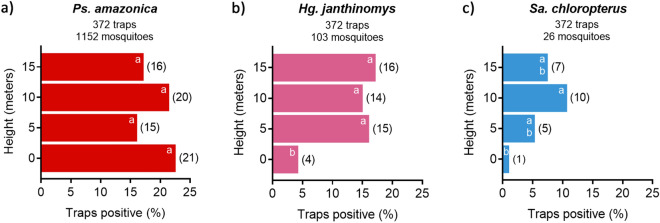
Figure 5.
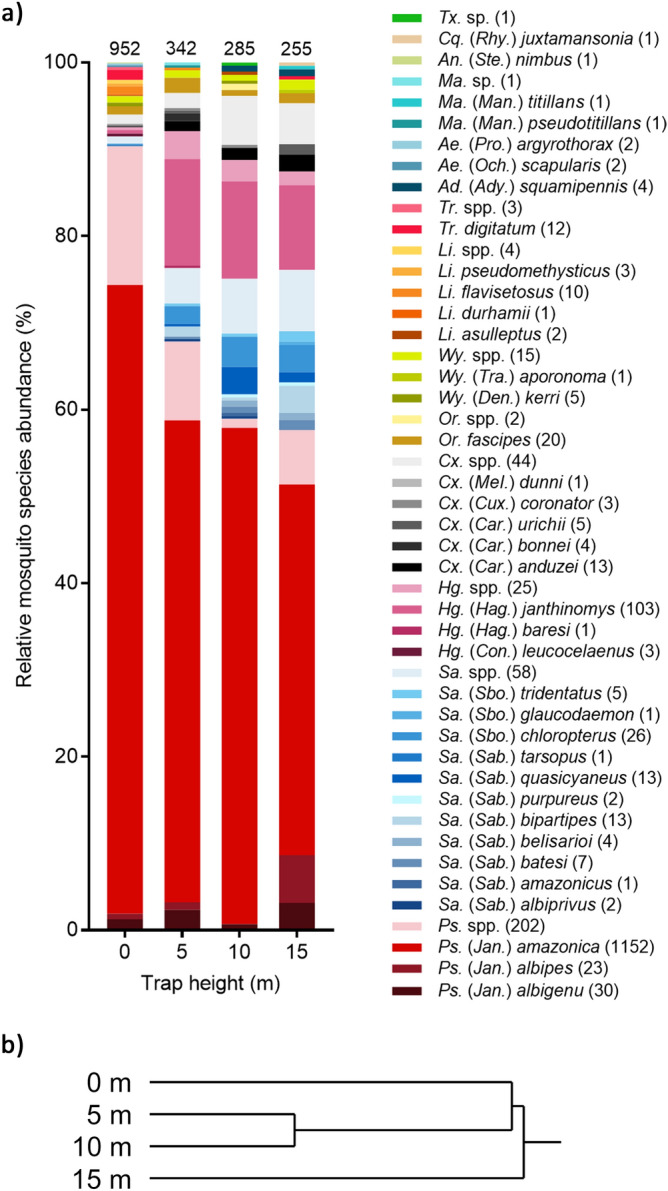
Mosquito species composition by height showing (a) the relative abundance of designated species collected using BG-Sentinel traps at each height (N = 93 collections) sampled across three sites combined (N = 372 collections). Number of mosquitoes collected at each height listed at top of bar; number of each species included in parentheses next to species name; genus and subgenus abbreviations follow Walter Reed Biosystematics Unit nomenclature50; sp. = single species, spp. = potentially multiple species. (b) shows hierarchical clustering of PC1 and PC2 from a principal components analysis of relative species frequency at each height sampled.
Psorophora amazonica dominated collections across all sites (77.9% of 1479 mosquitoes identified to species) and was the most abundant species at each height sampled, while Hg. janthinomys (7%) was the second most abundant of the identified species. Sabethes mosquitoes formed a similar proportion (7.3%) of the total catch and represented the most diverse genus with 11 identified species. However, while Sa. chloropterus was the most abundant species within its genus, only 26 specimens were collected, albeit in 23 separate trapping events. Due to the low abundance of target species, analyses focused on occurrence rather than abundance when investigating stratification.
Differences in mosquito community composition by collection site and height
Linear regression revealed no significant effect of trap height on the mean number of identified species (DF = 1,10, F = 0.47, P = 0.51) or on species diversity as measured by the Shannon–Wiener diversity index (F = 3.66, P = 0.085). Specimens that were not identified to species level were excluded from these analyses. The Morisita overlap index (Table 2) revealed substantial overlap in community composition at species level between the three sites and mosquito collections were therefore grouped for subsequent analysis of species-specific distributions. There was also substantial overlap among all heights sampled, contrary to expectations that mosquito communities would become more distinct moving from ground level to 15 m. In a principal components analysis of the relative frequency of each species at each height, the first two principal components (PC1 and PC2) explained 78.3% of the variation in the data. A hierarchical cluster analysis of PC1 and PC2 (Fig. 5) revealed that while mosquito communities at all heights overlapped substantially, the community at 0 m was distinct from the communities at 5 and 10 m, which were relatively similar to each other, and more distant from the community at 15 m.
Table 2.
Morisita overlap index by site and height based on species collected during the study period.
| Site | A | B | C | Height | 0 m | 5 m | 10 m | 15 m |
|---|---|---|---|---|---|---|---|---|
| A | 1 | 0 m | 1 | |||||
| B | 0.92 | 1 | 5 m | 0.95 | 1 | |||
| C | 0.88 | 0.99 | 1 | 10 m | 0.95 | 1 | 1 | |
| 15 m | 0.86 | 0.97 | 0.96 | 1 |
Specimens not identified to species level were excluded from analysis.
Species-specific distributions
Contingency table analyses revealed no significant effect of height on the occurrence, defined as the percent of traps positive, of Ps. amazonica (Pearson’s chi-squared, DF = 3, χ2 = 1.791, P = 0.62), but there was a significant effect on the occurrence of Hg. janthinomys (DF = 3, χ2 = 8.72, P = 0.033) and Sa. chloropterus (DF = 3, χ2 = 7.925, P = 0.048) (Fig. 6). Pairwise comparisons of height using two-tailed Fisher’s exact tests (exact P values listed in Supplementary Table S3) showed that Hg. janthinomys occurred less frequently at ground level than at all other heights (DF = 1, N = 186, P < 0.05 for all comparisons), but there was no detectable difference in occurrence when comparing heights above the ground. Sabethes chloropterus occurred less frequently at 0 m than at 10 m (DF = 1, N = 186, P < 0.01) and there was a similar trend when comparing 0 m with 15 m (DF = 1, N = 186, P = 0.06), but this was not significant and no other comparisons were significant for this species (DF = 1, N = 186, P > 0.05 for all comparisons). Two-tailed Fisher’s exact tests also revealed no significant difference in the occurrence of Sa. bipartipes (DF = 1, N = 372, P = 0.072) and Sa. quasicyaneus (DF = 1, N = 372, P = 0.072) when comparing traps positive at ground level (0 for both species) with those above the ground (11 and 10 traps positive for respective species), although there were only small numbers of traps positive for each species.
Figure 6.
Percent and number of traps positive at each height for (a) Ps. amazonica, (b) Hg. janthinomys, and (c) Sa. chloropterus using data pooled from across the three sites. Different letters within bars indicate significant differences in occurrence between heights for each species; letters indicate within-panel comparisons only. Number of traps positive at each height shown in brackets.
Associations between mosquito distributions and microclimate
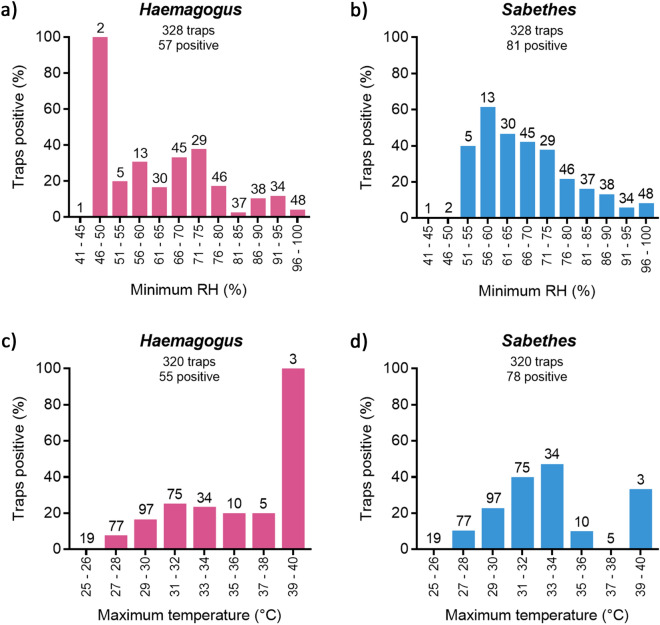
Nominal logistic regressions revealed negative associations between minimum relative humidity, which was negatively correlated with maximum temperature (Supplementary Table S1), and the occurrence of Haemagogus and Sabethes mosquitoes within each height sampled. For Haemagogus, this was significant at heights of 5 m (DF = 1, χ2 = 12.2, P = 0.0005) and 10 m (DF = 1, χ2 = 3.7, P = 0.05), but not at 0 m (DF = 1, χ2 = 2.4, P = 0.12) or 15 m (DF = 1, χ2 = 2.8, P = 0.09), although the trend was generally the same. For Sabethes, the association was significant at 0 m (DF = 1, χ2 = 5.4, P = 0.02), 5 m (DF = 1, χ2 = 6.4, P = 0.01), and 10 m (DF = 1, χ2 = 11.5, P = 0.0007), but not at 15 m (DF = 1, χ2 = 3.0, P = 0.08), although again, the trend was generally the same. The overall occurrence of both genera increased in frequency with increasing maximum temperature and decreasing relative humidity (Fig. 7). The negative association with minimum relative humidity within each height was not evident for Hg. janthinomys and Sa. chloropterus, the most abundant species in each genus, likely due to the small numbers of traps positive for each species. Furthermore, there was no significant relationship between minimum relative humidity and the occurrence of Psorophora mosquitoes, or specifically for Ps. amazonica, at any of the heights sampled.
Figure 7.
Associations between mosquitoes and microclimate showing percent of traps positive for (a) Haemagogus and (b) Sabethes mosquitoes in relation to minimum relative humidity (RH, shown at 5% intervals), and for (c) Haemagogus and (d) Sabethes mosquitoes in relation to maximum temperature (shown at 2 °C intervals). Number of traps in each humidity and temperature class shown above each bar.
Effects of 7-day cumulative rainfall on the occurrence of mosquitoes
Nominal logistic regressions revealed positive associations between rainfall in the weeks prior to collections and occurrence of key taxa. The occurrence of Haemagogus significantly increased with increasing 7-day cumulative rainfall at a lag of 1 week (DF = 1, χ2 = 3.9, P = 0.0495), while increasing rainfall at a lag of 3 weeks was associated with an increase in the occurrence of Sabethes (DF = 1, χ2 = 4.4, P = 0.0357). Significant increases in the occurrence of Psorophora were associated with increasing rainfall at lags of 1 week (DF = 1, χ2 = 16.5, P < 0.0001) and 4 weeks (DF = 1, χ2 = 15.3, P < 0.0001). These positive associations were replicated at species level for Sa. chloropterus (lag of 3 weeks, DF = 1, χ2 = 11.6, P = 0.0006) and Ps. amazonica (lag of 1 week, DF = 1, χ2 = 14.4, P = 0.0001; lag of 4 weeks, DF = 1, χ2 = 22, P < 0.0001), but not for Hg. janthinomys (lag of 1 week, DF = 1, χ2 = 2.8, P = 0.09), although the latter followed a similar trend.
Discussion
Our study investigated the composition and vertical stratification of mosquito species inside a large forest reserve bordering the city of Manaus, where abrupt edges between urban and forest environments bring humans, mosquitoes, and wildlife into close contact. Such areas increase permeability for the co-occurrence of hosts and bridge vectors, thereby elevating the risk of zoonotic arbovirus exchange54. This has the potential to cause outbreaks of novel diseases in urban populations, or, as is the concern for Zika virus in the Americas, lead to spillback and establishment of sylvatic cycles of transmission that could result in an enduring threat to human health1,55. Understanding the ecology and behavior of potential bridge vectors is, therefore, critical to understanding the risk of spillover and spillback.
We demonstrated that, as expected25,27,56, microclimate varied among forest strata, with the forest floor being cooler and more humid than at elevated heights, and with temperature increasing and humidity decreasing with increasing height. Despite this, we did not detect differences in the mean number (richness) of mosquito species, species diversity, or the overlap of mosquito communities based on the Morisita index. However, a principal components analysis did reveal a hierarchy in the structure of the mosquito community, which changed progressively with increasing height. Confalonieri and Costa Neto57 found that species diversity decreased with increasing height when sampling using handheld nets and CDC light traps at the Caxiuanã National Forest in Pará State, Brazil, but that differences were only significant between 0 and 8 m. In the same study, the composition of mosquito communities was found to be quite distinct between 0 and 30 m. Brant et al.58 also encountered significant differences in community composition between the ground and canopy (10–20 m) while investigating the stratification of mosquito vectors of zoonotic malaria in Borneo collected using human bait in evenings (18:00–22:00). When considering these findings, it is worth remembering that the use of human bait is inevitably biased towards the collection of human-biting species59,60, while BG-Sentinel and CDC light trap collections tend to detect a greater diversity of species60,61.
The high number of mosquitoes encountered in BG-Sentinel traps at ground level was driven by the high abundance of Ps. amazonica, which is an anthropophilic member of the Janthinosoma subgenus62 but not a known arbovirus vector. However, flaviviruses, including Ilhéus63 and St. Louis encephalitis64, have been isolated from Ps. ferox, another Janthinosoma species. Despite being the most abundant species at all heights sampled, there was no significant difference in the stratification of Ps. amazonica based on its occurrence in traps. Its vertical distribution from the forest floor to at least 15 m in height, along with previous observations that it occurs in relatively high abundance at forest edges in urban parks in Manaus62, should raise awareness of its potential to serve as a bridge vector, although more information about its feeding habits is needed. At present, little is known about the non-human blood hosts of Ps. amazonica, although monkey-baited CDC light traps have been successfully used to collect Ps. albigenu and Ps. ferox, both Janthinosoma species, on the ground and in forest canopy65.
In contrast, both Hg. janthinomys and Sa. chloropterus are known to transmit flaviviruses and may thus be capable of bridging human and sylvatic transmission cycles. YFV has repeatedly been detected in Hg. janthinomys, including recently in southeastern Brazil where infection rates of 3.4%66 (based on minimum infection rate) and 8.2%67 have been reported. Fewer YFV detections and lower infection rates have been reported in Sa. chloropterus, which is considered an important secondary vector66. The acrodendrophilic habits of these species are well documented and both showed significant differences in stratification in this study. Both were more likely to be present above the ground rather than at ground level, but of the two, Sa. chloropterus appeared to slightly favor heights above 5 m. Seventy-four percent of traps positive for this species were located at 10 m or higher, while for Hg. janthinomys, this value was 61%. The preference for Hg. janthinomys and Sa. chloropterus to occupy heights above the ground extends evidence that acrodendrophilic species are infrequently encountered at ground level in well-preserved tropical rainforest20,25, although several of our traps were situated near treefall gaps.
The significant breakpoint in stratification of Hg. janthinomys between 0 and 5 m corresponded with significant differences in minimum relative humidity and maximum temperature between ground level and all elevated heights. There was generally a less pronounced difference in microclimate among the 5, 10 and 15 m strata (Fig. 4), and there was no difference in stratification of this species among these heights. In one of the earliest field studies of Hg. janthinomys, Bates32 suspected that stratification was related to humidity and reported increases in abundance at ground level when humidity was lowest. This suspicion has often been echoed by others68–72, but there has been little empirical evidence to support it. Our results should be interpreted cautiously but suggest that a negative relationship exists between minimum relative humidity and the occurrence of Haemagogus and Sabethes mosquitoes and this was evident at several of the heights sampled. At heights where the relationship was not significant, results followed a similar trend. Collecting enough target mosquitoes in identifiable condition using BG-Sentinel traps was a challenge throughout the study, but since Hg. janthinomys formed 96.3% of the total identified Haemagogus catch, it seems reasonable to assume that these findings also apply to this species. Such a negative relationship with humidity would appear to agree with observations that Haemagogus and Sabethes mosquitoes increase in abundance at ground level in forest clearings and at forest edges20,26,31,32,34,68 where humidity is lower and temperature is higher than in undisturbed habitat19,73. As Bates32 suggested, occurrence at ground level may be further exacerbated during daytime hours when relative humidity is lowest (Fig. 3), increasing the risk of contact with humans and the bridge capacity of these mosquitoes.
Establishing links between rainfall and the appearance of vector species has potential use for modeling arbovirus disease dynamics74. We demonstrated that the appearance of Haemagogus, Sabethes, and Psorophora was related to seven-day cumulative rainfall in the weeks prior to collections. The occurrence of Haemagogus was associated with rainfall at a lag of 1 week, while Sabethes, including Sa. chloropterus, was associated with rainfall at a lag of 3 weeks. Some laboratory studies have reported slightly faster egg to adult development times for Haemagogus species75–77 than for Sa. chloropterus78, and our results appear to support this pattern under natural conditions. While little is known about the development times of Ps. amazonica, other Janthinosoma species undergo fairly rapid life cycles79–82. For Ps. varipes, this can be completed in around 16–19 days at 27 °C in the laboratory79, while successive broods of Ps. ferox have been observed to emerge about a month apart in Florida under natural conditions80. The association of Ps. amazonica with rainfall at lags of 1 and 4 weeks may, therefore, reflect the emergence of successive generations of this species resulting from a similar short life cycle. These findings indicate interesting variations in the life history of these taxa that should be examined more closely in the laboratory and in nature.
Our study had several limitations that may provide scope for further research. First, we terminated sampling at the end of the rainy season when mosquito abundance declines sharply. Extending our sampling window may have provided information about vector species persistence into the dry season, which is relevant to the maintenance cycles of sylvatic arboviruses. There is only limited evidence for transovarial transmission of YFV by Haemagogus species83,84, and while it has been shown that Hg. janthinomys7 and Sa. chloropterus29 may persist through the dry season, it is not known whether they remain sufficiently abundant to maintain transmission. Dry season sampling may also have provided further information about seasonal changes in stratification that have been observed for these species29,32. While BG-Sentinel trap collections should give reliable estimates of relative mosquito abundance, inferences about the absolute abundance of Hg. janthinomys, Sa. chloropterus, and Ps. amazonica are limited. It is not known how attractive BG-Sentinel traps are to these species, which were designed for the collection of Aedes species mosquitoes85. Similar collections using human bait, or a direct comparison of these methods would be worthwhile. Lastly, BG-Sentinel trap collections yielded insufficient numbers of identifiable Hg. janthinomys and Sa. chloropterus to perform microclimate analyses at species level, thus we were limited to analyzing associations of genera with microclimate.
The risk of arbovirus spillback will ultimately be influenced by the frequency of interactions between susceptible hosts and competent vectors in the presence of pathogens, and the stratification of mosquitoes relative to humans and monkeys may provide an indication of interactions most likely to take place. The higher occurrence and greater abundance of Hg. janthinomys relative to Sa. chloropterus suggests that the former may be the most likely of the known vectors to encounter humans at ground level, although neither was frequently encountered in BG-Sentinel traps on the forest floor. Both Hg. janthinomys and Sa. chloropterus have previously been collected at higher heights than sampled in this study25,31 and may, therefore, provide a route of arbovirus transmission between humans and monkeys active higher in the forest canopy. Of the monkeys present at the Ducke reserve, Ateles paniscus86,87 and Alouatta species86,88 are most frequently encountered above 20 m but seldom occur at forest edges. Both Chiropotes86 and Pithecia species86,89 often occur above 15 m, but the latter also occurs below 15 m and is more likely to enter edge habitats. However, Sapajus apella86,90,91 and Saguinus bicolor92,93 are the species most likely to be found below 15 m and at forest clearings and forest edges. It is in these edge habitats, where humans and these mosquito and monkey species coexist, that interactions between hosts and vectors are perhaps most likely to take place, creating opportunities for spillback of ZIKV and other mosquito-borne viruses.
Conclusions
The vertical stratification of Hg. janthinomys and Sa. chloropterus suggests that both are infrequently encountered at ground level in the Ducke reserve based on BG-Sentinel trap collections. However, the occurrence of both genera may increase at higher temperature and lower relative humidity, a characteristic feature of forest edges. These are habitats where monkeys such as Saguinus bicolor and Sapajus apella occupy the lower forest strata and are areas at risk of zoonotic arbovirus exchange when humans exist in close contact. Our previous work has shown that the spatial distribution of Ps. amazonica extends from the forest interior to its edge. The dominance of this species at all heights sampled during this study provides further evidence that its distribution overlaps with human and sylvatic arbovirus hosts in Manaus, where it should also be investigated as a potential bridge vector.
Supplementary information
Acknowledgements
The authors wish to acknowledge the contributions of Dr. Scott Weaver (UTMB) for advice on trapping methods; José Edmilson da Costa Souza for species identification of trees; Leandro Fernandes (UFAM) for assistance with mosquito collections; Izabele Guimarães, Italo Cotes, Ramon Linhares, Layne Borges, and Eloane Andrade (FMT-HVD) for assistance with sample processing and mosquito collections; Dr. Bárbara Chaves and Aleksey Lima (FMT-HVD) for assistance with administration and logistics; Raimundo Coelho (FMT-HVD) for driving and logistics; and, the Instituto Nacional de Pesquisas da Amazônia (INPA) for permission to use the Adolpho Ducke Reserve to collect samples for this study. The research was funded by an International Collaborations in Infectious Disease Research (ICIDR) grant U01 AI115577 awarded to NV by the National Institutes of Health (NIH), and a Consejo Nacional de Ciencia y Tecnología fellowship awarded to EHA by the Government of Mexico, 709139. MVGL is a CNPq fellow.
Author contributions
NV, KAH, and MVGL conceived and designed the work; NV, KAH, MVGL, and MG supervised; AH DV, CM, EHA ERC, JTAJ, FPA collected mosquitoes and field data; NFF identified mosquitoes; AH and DV curated data; KAH, AH, and EHA analyzed data; AH, KAH, EHA and MB prepared the manuscript; KAH, NV, MB, MG, VMS, and MVGL revised the manuscript. All authors reviewed and approved the manuscript.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kathryn A. Hanley, Email: khanley@nmsu.edu
Nikos Vasilakis, Email: nivasila@utmb.edu.
Supplementary information
is available for this paper at 10.1038/s41598-020-75178-3.
References
- 1.Althouse BM, et al. Potential for Zika virus to establish a sylvatic transmission cycle in the Americas. PLoS Negl. Trop. Dis. 2016;10:e0005055. doi: 10.1371/journal.pntd.0005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leta S, et al. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018;67:25–35. doi: 10.1016/j.ijid.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCrae AW, Kirya BG. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans. R. Soc. Trop. Med. Hyg. 1982;76:552–562. doi: 10.1016/0035-9203(82)90161-4. [DOI] [PubMed] [Google Scholar]
- 4.Hanley KA, et al. Fever versus fever: The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect. Genet. Evol. 2013;19:292–311. doi: 10.1016/j.meegid.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diallo D, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS ONE. 2014;9:e109442. doi: 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant JE, Holmes EC, Barrett AD. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007;3:e75. doi: 10.1371/journal.ppat.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugher JC, Boshell-Manrique J, Roca-García M, Osorno-Mesa E. Epidemiology of jungle yellow fever in eastern Colombia. Am. J. Epidemiol. 1944;39:16–51. doi: 10.1093/oxfordjournals.aje.a118895. [DOI] [Google Scholar]
- 8.Shannon RC, Whitman L, Franca M. Yellow fever virus in jungle mosquitoes. Science. 1938;88:110–111. doi: 10.1126/science.88.2274.110. [DOI] [PubMed] [Google Scholar]
- 9.Rodaniche ED, Galindo P. Isolation of yellow fever virus from Haemagogus mesodentatus, H. equinus and Sabethes chloropterus captured in Guatemala in 1956. Am. J. Trop. Med. Hyg. 1957;6:232–237. doi: 10.4269/ajtmh.1957.6.232. [DOI] [PubMed] [Google Scholar]
- 10.Chiu CY, et al. Experimental Zika virus inoculation in a New World monkey model reproduces key features of the human infection. Sci. Rep. 2017;7:17126. doi: 10.1038/s41598-017-17067-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karna AK, et al. Colonized Sabethes cyaneus, a sylvatic New World mosquito species, shows a low vector competence for Zika virus relative to Aedes aegypti. Viruses. 2018 doi: 10.3390/v10080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seferovic M, et al. Experimental Zika virus infection in the pregnant common marmoset induces spontaneous fetal loss and neurodevelopmental abnormalities. Sci. Rep. 2018;8:6851. doi: 10.1038/s41598-018-25205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanchiere JA, et al. Experimental Zika virus infection of Neotropical primates. Am. J. Trop. Med. Hyg. 2018;98:173–177. doi: 10.4269/ajtmh.17-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terzian ACB, et al. Evidence of natural Zika virus infection in Neotropical non-human primates in Brazil. Sci. Rep. 2018;8:16034. doi: 10.1038/s41598-018-34423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favoretto SR, et al. Zika virus in peridomestic Neotropical primates, northeast Brazil. EcoHealth. 2019;16:61–69. doi: 10.1007/s10393-019-01394-7. [DOI] [PubMed] [Google Scholar]
- 16.Pauvolid-Correa A, et al. Zika virus surveillance at the human–animal interface in west-central Brazil, 2017–2018. Viruses. 2019 doi: 10.3390/v11121164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abreu FVS, et al. Survey on non-human primates and mosquitoes does not provide evidences of spillover/spillback between the urban and sylvatic cycles of yellow fever and Zika viruses following severe outbreaks in southeast Brazil. Viruses. 2020 doi: 10.3390/v12040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith DL, et al. Ross, Macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 2012;8:e1002588. doi: 10.1371/journal.ppat.1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendy A, et al. Into the woods: Changes in mosquito community composition and presence of key vectors at increasing distances from the urban edge in urban forest parks in Manaus, Brazil. Acta Trop. 2020;206:105441. doi: 10.1016/j.actatropica.2020.105441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trapido H, Galindo P. Mosquitoes associated with sylvan yellow fever near Almirante, Panama. Am. J. Trop. Med. Hyg. 1957;6:114–144. doi: 10.4269/ajtmh.1957.6.114. [DOI] [PubMed] [Google Scholar]
- 21.Goenaga S, et al. Isolation of yellow fever virus from mosquitoes in Misiones province, Argentina. Vector Borne Zoonotic Dis. 2012;12:986–993. doi: 10.1089/vbz.2011.0730. [DOI] [PubMed] [Google Scholar]
- 22.Garnham PC, Harper JO, Highton RB. The mosquitos of the Kaimosi Forest, Kenya Colony, with special reference to yellow fever. Bull. Entomol. Res. 1946;36:473–496. doi: 10.1017/s000748530002410x. [DOI] [PubMed] [Google Scholar]
- 23.Galindo P, Carpenter SJ, Trapido H. Ecological observations on forest mosquitoes of an endemic yellow fever area in Panama. Am. J. Trop. Med. Hyg. 1951;31:98–137. doi: 10.4269/ajtmh.1951.s1-31.98. [DOI] [PubMed] [Google Scholar]
- 24.Galindo P, Carpenter SJ, Trapido H. A contribution to the ecology and biology of tree hole breeding mosquitoes of Panama. Ann. Entomol. Soc. Am. 1955;48:158–164. doi: 10.1093/aesa/48.3.158. [DOI] [Google Scholar]
- 25.Pinto CS, Confalonieri UEC, Mascarenhas BM. Ecology of Haemagogus sp. and Sabethes sp. (Diptera: Culicidae) in relation to the microclimates of the Caxiuanã National Forest, Pará, Brazil. Mem. Inst. Oswaldo Cruz. 2009;104:592–598. doi: 10.1590/S0074-02762009000400010. [DOI] [PubMed] [Google Scholar]
- 26.Downs WG, Aitken TH, Anderson CR. Activities of the Trinidad Regional Virus Laboratory in 1953 and 1954 with special reference to the yellow fever outbreak in Trinidad B.W.I. Am. J. Trop. Med. Hyg. 1955;4:837–843. doi: 10.4269/ajtmh.1955.4.837. [DOI] [PubMed] [Google Scholar]
- 27.Alencar J, et al. Feeding patterns of Haemagogus janthinomys (Diptera: Culicidae) in different regions of Brazil. J. Med. Entomol. 2005;42:981–985. doi: 10.1093/jmedent/42.6.981. [DOI] [PubMed] [Google Scholar]
- 28.Alencar J, et al. Feeding patterns of Haemagogus capricornii and Haemagogus leucocelaenus (Diptera: Culicidae) in two Brazilian states (Rio de Janeiro and Goias) J. Med. Entomol. 2008;45:873–876. doi: 10.1603/0022-2585(2008)45[873:fpohca]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Galindo P, Trapido H, Carpenter SJ, Blanton FS. The abundance cycles of arboreal mosquitoes during six years at a sylvan yellow fever locality in Panama. Ann. Entomol. Soc. Am. 1956;49:543–547. doi: 10.1093/aesa/49.6.543. [DOI] [Google Scholar]
- 30.Arnell JH. Mosquito studies (Diptera, Culicidae) XXXII. A revision of the genus Haemagogus. Contrib. Am. Entomol. Inst. 1973;10:1–174. [Google Scholar]
- 31.Pajot FX, Geoffroy B, Chippaux JP. Écologie d'Haemagogus janthinomys Dyar (Diptera, Culicidae) en Guyane française. Cah. ORSTOM ser. Entomol. Med. Parasitol. 1985;23:209–216. [Google Scholar]
- 32.Bates M. Observations on the distribution of diurnal mosquitoes in a tropical forest. Ecology. 1944;25:159–170. doi: 10.2307/1930689. [DOI] [Google Scholar]
- 33.Forattini OP, Lopes OS, Rabello EX. Investigações sôbre o comportamento de formas adultas de mosquitos silvestres no Estado de São Paulo. Brasil. Rev. Saude Publica. 1968;2:111–173. doi: 10.1590/S0034-89101968000200002. [DOI] [PubMed] [Google Scholar]
- 34.Downs, W. G. Natural history of yellow fever in the Americas. Report No. VIR/YF/71.14, p. 5 (World Health Organization, 1971).
- 35.Swanson DA, Adler PH. Vertical distribution of haematophagous Diptera in temperate forests of the southeastern U.S.A. Med. Vet. Entomol. 2010;24:182–188. doi: 10.1111/j.1365-2915.2010.00862.x. [DOI] [PubMed] [Google Scholar]
- 36.Swanson DA, Adler PH, Malmqvist B. Spatial stratification of host-seeking Diptera in boreal forests of northern Europe. Med. Vet. Entomol. 2012;26:56–62. doi: 10.1111/j.1365-2915.2011.00963.x. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira ML, Baccaro FB, Braga-Neto R, Magnusson WE, editors. Reserva Ducke: A bioversidade Amazônica através de uma grade. Manaus: Instituto Nacional de Pesquisas da Amazônia; 2011. p. 166. [Google Scholar]
- 38.Gordo M, Rodrigues LFR, Vidal MD, Spironelo WR, Ribeiro FRP, et al. Primatas. In: de Oliveira ML, Baccaro FB, Braga-Neto R, Magnusson WE, et al., editors. Reserva Ducke: A bioversidade Amazônica através de uma grade. Manaus: Instituto Nacional de Pesquisas da Amazônia; 2011. pp. 39–49. [Google Scholar]
- 39.Instituto Nacional de Meteorologia. Banco de dados meteorológicos para ensino e pesquisa, https://www.inmet.gov.br/portal/index.php?r=bdmep/bdmep (2020).
- 40.Dégallier N, et al. Release-recapture experiments with canopy mosquitoes in the genera Haemagogus and Sabethes (Diptera: Culicidae) in Brazilian Amazonia. J. Med. Entomol. 1998;35:931–936. doi: 10.1093/jmedent/35.6.931. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. Efficacy-testing of traps for control of Aedes spp. mosquito vectors. Report No. (WHO/CDS/NTD/VEM/2018.6), p. 42 (Geneva, 2018).
- 42.Diniz FA, Ramos AM, Rebello ERG. Brazilian climate normals for 1981–2010. Pesquisa Agropecuária Brasileira. 2018;53:131–143. doi: 10.1590/s0100-204x2018000200001. [DOI] [Google Scholar]
- 43.Lane, J. Neotropical Culicidae. Vol. 1 and 2, p. 1112 (University of São Paulo, São Paulo, Brazil, 1953).
- 44.Valencia JD. Mosquito studies (Diptera, Culicidae) XXXI. A revision of the subgenus Carrollia of Culex. Contrib. Am. Entomol. Inst. 1973;9:1–134. [Google Scholar]
- 45.Berlin OGW, Belkin JN. Mosquito studies (Diptera, Culicidae) XXXVI. Subgenera Aedinus Tinolestes and Anoedioporpa of Culex. Contrib. Am. Entomol. Inst. 1980;17:1–104. [Google Scholar]
- 46.Consoli R, Lourenço-de-Oliveira R. Principais mosquitos de importância sanitária no Brasil. Rio de Janeiro: Fundação Oswaldo Cruz (Fiocruz); 1994. p. 228. [Google Scholar]
- 47.Forattini OP. Culicidologia médica: Identificação, biologia, epidemiologia. São Paulo: University of São Paulo; 1996. p. 549. [Google Scholar]
- 48.Sallum MAM, Forattini OP. Revision of the Spissipes section of Culex (Melanoconion) (Diptera: Culicidae) J. Am. Mosq. Control Assoc. 1996;12:517–600. [PubMed] [Google Scholar]
- 49.Guimarães JH. Systematic database of Diptera of the Americas south of the United States: Family Culicidae. Plêiade: São Paulo; 1997. p. 286. [Google Scholar]
- 50.Walter Reed Biosystematics Unit. Systematic catalog of Culicidae, https://mosquitocatalog.org/default.aspx (2020).
- 51.Oksanen J, et al. The vegan package. Commun. Ecol. Pack. 2007;10:631–637. [Google Scholar]
- 52.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:9. [Google Scholar]
- 53.JMP v. 14 (SAS Institute Inc., Cary, NC, USA, 2018).
- 54.Borremans B, Faust C, Manlove KR, Sokolow SH, Loyd-Smith JO. Cross-species pathogen spillover across ecosystem boundaries: mechanisms and theory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019;374:18344. doi: 10.1098/rstb.2018.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lourenço-de-Oliveira R, Failloux AB. High risk for chikungunya virus to initiate an enzootic sylvatic cycle in the tropical Americas. PLoS Negl. Trop. Dis. 2017;11:e0005698. doi: 10.1371/journal.pntd.0005698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guimarães AE, Arlé M, Machado RNM. Mosquitos no Parque Nacional da Serra dos Orgãos, Estado do Rio de Janeiro, Brasil: II. Distribuição vertical. Mem. Inst. Oswaldo Cruz. 1985;80:171–185. doi: 10.1590/S0074-02761985000200008. [DOI] [Google Scholar]
- 57.Confalonieri UE, Costa Neto C. Diversity of mosquito vectors (Diptera: culicidae) in Caxiuanã, Pará, Brazil. Interdiscip. Perspect. Infect. Dis. 2012;2012:741273. doi: 10.1155/2012/741273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brant HL, et al. Vertical stratification of adult mosquitoes (Diptera: Culicidae) within a tropical rainforest in Sabah, Malaysia. Malar. J. 2016;15:370. doi: 10.1186/s12936-016-1416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silver JB, Service MW. Mosquito Ecology: Field Sampling Methods. 3. Dordrecht: Springer; 2008. p. 1494. [Google Scholar]
- 60.Obenauer PJ, Kaufman PE, Kline DL, Allan SA. Detection of and monitoring for Aedes albopictus (Diptera: Culicidae) in suburban and sylvatic habitats in north central Florida using four sampling techniques. Environ. Entomol. 2010;39:1608–1616. doi: 10.1603/en09322. [DOI] [PubMed] [Google Scholar]
- 61.Meeraus WH, Armistead JS, Arias JR. Field comparison of novel and gold standard traps for collecting Aedes albopictus in Northern Virginia. J. Am. Mosq. Control Assoc. 2008;24:244–248. doi: 10.2987/5676.1. [DOI] [PubMed] [Google Scholar]
- 62.Suárez-Mutis MC, Fé NF, Alecrim W, Coura JR. Night and crepuscular mosquitoes and risk of vector-borne diseases in areas of piassaba extraction in the middle Negro River basin, state of Amazonas, Brazil. Mem. Inst. Oswaldo Cruz. 2009;104:11–17. doi: 10.1590/S0074-02762009000100002. [DOI] [PubMed] [Google Scholar]
- 63.Causey OR, Causey CE, Maroja OM, Macedo DG. The isolation of arthropod-borne viruses, including members of two hitherto undescribed serological groups, in the Amazon region of Brazil. Am. J. Trop. Med. Hyg. 1961;10:227–249. doi: 10.4269/ajtmh.1961.10.227. [DOI] [PubMed] [Google Scholar]
- 64.Anderson CR, Aitken TH, Downs WG, Spence L. The isolation of St. Louis virus from Trinidad mosquitoes. Am. J. Trop. Med. Hyg. 1957;6:688–692. doi: 10.4269/ajtmh.1957.6.688. [DOI] [PubMed] [Google Scholar]
- 65.Andrews ES, et al. Species diversity, seasonal, and spatial distribution of mosquitoes (Diptera: Culicidae) captured in Aotus monkey-baited traps in a forested site near Iquitos, Peru. J. Med. Entomol. 2014;51:1127–1135. doi: 10.1603/me14058. [DOI] [PubMed] [Google Scholar]
- 66.Abreu FVS, et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019;8:218–231. doi: 10.1080/22221751.2019.1568180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinheiro GG, Rocha MN, de Oliveira MA, Moreira LA, Andrade Filho JD. Detection of yellow fever virus in sylvatic mosquitoes during disease outbreaks of 2017–2018 in Minas Gerais State, Brazil. Insects. 2019 doi: 10.3390/insects10050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boshell-Manrique J, Osorno-Mesa E. Observations on the epidemiology of jungle yellow fever in Santander and Boyaca, Colombia, September 1941, to April, 1942. Am. J. Epidemiol. 1944;40:170–181. doi: 10.1093/oxfordjournals.aje.a118984. [DOI] [Google Scholar]
- 69.Pinheiro FP, Travassos da Rosa AP, Moraes MA. An epidemic of yellow fever in Central Brazil, 1972–1973. II. Ecological studies. Am. J. Trop. Med. Hyg. 1981;30:204–211. doi: 10.4269/ajtmh.1981.30.204. [DOI] [PubMed] [Google Scholar]
- 70.Chadee DD, Tikasingh ES. Observations on the seasonal incidence and diel oviposition periodicity of Haemagogus mosquitoes (Diptera: Culicidae) in Trinidad, W.I.: Part I. Haemagogus janthinomys Dyar. Ann. Trop. Med. Parasitol. 1989;83:507–516. doi: 10.1080/00034983.1989.11812379. [DOI] [PubMed] [Google Scholar]
- 71.Chadee DD, Tikasingh ES. Observations on the seasonal incidence and diel oviposition periodicity of Haemagogus mosquitoes (Diptera: Culicidae) in Trinidad, W.I. II. Haemagogus equinus Theobald. Ann. Trop. Med. Parasitol. 1990;84:267–275. doi: 10.1080/00034983.1990.11812466. [DOI] [PubMed] [Google Scholar]
- 72.Chadee DD, Tikasingh ES, Ganesh R. Seasonality, biting cycle and parity of the yellow fever vector mosquito Haemagogus janthinomys in Trinidad. Med. Vet. Entomol. 1992;6:143–148. doi: 10.1111/j.1365-2915.1992.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 73.Laurance WF. Forest-climate interactions in fragmented tropical landscapes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:345–352. doi: 10.1098/rstb.2003.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian HY, et al. How environmental conditions impact mosquito ecology and Japanese encephalitis: an eco-epidemiological approach. Environ. Int. 2015;79:17–24. doi: 10.1016/j.envint.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Bates M. Observations on climate and seasonal distribution of mosquitoes in eastern Colombia. J. Anim. Ecol. 1945;14:17–25. doi: 10.2307/1396. [DOI] [Google Scholar]
- 76.Hovanitz W. Comparisons of mating behavior, growth rate, and factors influencing egg-hatching in South American Haemagogus mosquitoes. Physiol. Zool. 1946;19:35–53. doi: 10.1086/physzool.19.1.30151878. [DOI] [PubMed] [Google Scholar]
- 77.Bates M. The development and longevity of Haemagogus mosquitoes under laboratory conditions. Ann. Entomol. Soc. Am. 1947;40:1–12. doi: 10.1093/aesa/40.1.1. [DOI] [Google Scholar]
- 78.Galindo P. Bionomics of Sabethes chloropterus Humboldt, a vector of sylvan yellow fever in Middle America. Am. J. Trop. Med. Hyg. 1958;7:429–440. doi: 10.4269/ajtmh.1958.7.429. [DOI] [PubMed] [Google Scholar]
- 79.Aboualy A, Horsfall WR. Bionomics of Psorophora varipes, a model laboratory mosquito. J. Econ. Entomol. 1968;61:1657–1660. doi: 10.1093/jee/61.6.1657. [DOI] [PubMed] [Google Scholar]
- 80.Nielsen HT. Swarming and some other habits of Mansonia perturbans and Psorophora ferox (Diptera: Culicidae) Behaviour. 1964;24:67–89. doi: 10.1163/156853964x00229. [DOI] [PubMed] [Google Scholar]
- 81.Micieli VM, García JJ, Becnel JJ. Life cycle and epizootiology of Amblyospora ferocis (Microspora: Amblyosporidae) in the mosquito Psorophora ferox (Diptera: Culicidae) Folia Parasitol. (Praha) 2003;50:171–175. doi: 10.14411/fp.2003.031. [DOI] [PubMed] [Google Scholar]
- 82.Mello CF, Santos-Mallet JR, Tátila-Ferreira A, Alencar J. Comparing the egg ultrastructure of three Psorophora ferox (Diptera: Culicidae) populations. Braz. J. Biol. 2018;78:505–508. doi: 10.1590/1519-6984.171829. [DOI] [PubMed] [Google Scholar]
- 83.Dutary BE, Leduc JW. Transovarial transmission of yellow fever virus by a sylvatic vector, Haemagogus equinus. Trans. R. Soc. Trop. Med. Hyg. 1981;75:128. doi: 10.1016/0035-9203(81)90036-5. [DOI] [PubMed] [Google Scholar]
- 84.Mondet B, et al. Isolation of yellow fever virus from nulliparous Haemagogus (Haemagogus) janthinomys in eastern Amazonia. Vector Borne Zoonotic Dis. 2002;2:47–50. doi: 10.1089/153036602760260779. [DOI] [PubMed] [Google Scholar]
- 85.Biogents AG. The BG-Sentinel: Biogents' mosquito trap for researchers, https://www.bg-sentinel.com/ (2020).
- 86.Mittermeier RA, van Roosmalen MG. Preliminary observations on habitat utilization and diet in eight Surinam monkeys. Folia Primatol. (Basel) 1981;36:1–39. doi: 10.1159/000156007. [DOI] [PubMed] [Google Scholar]
- 87.van Roosmalen MGM. Habitat preferences, diet, feeding strategy and social organization of the black spider monkey [Ateles paniscus paniscus Linnaeus 1758] in Surinam. Acta Amaz. 1985;15:7–238. doi: 10.1590/1809-43921985155238. [DOI] [Google Scholar]
- 88.Youlatos D, Guillot D. Howler Monkey Positional Behavior in Howler Monkeys. Developments in Primatology: Progress and Prospects. New York: Springer; 2015. [Google Scholar]
- 89.Vié JC, Richard-Hansen C, Fournier-Chambrillon C. Abundance, use of space, and activity patterns of white-faced sakis (Pithecia pithecia) in French Guiana. Am. J. Primatol. 2001;55:203–221. doi: 10.1002/ajp.1055. [DOI] [PubMed] [Google Scholar]
- 90.Heymann EW, Encarnación C F, Canaquin Y JE. Primates of the Río Curaray, northern Peruvian Amazon. Int. J. Primatol. 2002;23:191–201. doi: 10.1023/A:1013262210863. [DOI] [Google Scholar]
- 91.Warner MD. Assessing habitat utilization by neotropical primates: a new approach. Primates. 2002;43:59–71. doi: 10.1007/BF02629577. [DOI] [PubMed] [Google Scholar]
- 92.Egler SG. Feeding ecology of Saguinus bicolor bicolor (Callitrichidae: Primates) in a relict forest in Manaus, Brazilian Amazonia. Folia Primatol. (Basel) 1992;59:61–76. doi: 10.1159/000156644. [DOI] [PubMed] [Google Scholar]
- 93.Gordo M, Calleia FO, Vasconcelos SA, Leite JJF, Ferrari SF. The Challenges of Survival in a Concrete Jungle: Conservation of the Pied Tamarin (Saguinus bicolor) in the Urban Landscape of Manaus, Brazil in Primates in Fragments. New York: Springer; 2013. pp. 357–370. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).