Abstract
Mitochondria are the first-line defense of the cell in the presence of stressing processes that can induce mitochondrial dysfunction. Under these conditions, the activation of two axes is accomplished, namely, (i) the mitochondrial unfolded protein response (UPRmt) to promote cell recovery and survival of the mitochondrial network; (ii) the mitophagy process to eliminate altered or dysfunctional mitochondria. For these purposes, the former response induces the expression of chaperones, proteases, antioxidant components and protein import and assembly factors, whereas the latter is signaled through the activation of the PINK1/Parkin and BNIP3/NIX pathways. These adaptive mechanisms may be compromised during aging, leading to the development of several pathologies including sarcopenia, defined as the loss of skeletal muscle mass and performance; and non-alcoholic fatty liver disease (NAFLD). These age-associated diseases are characterized by the progressive loss of organ function due to the accumulation of reactive oxygen species (ROS)-induced damage to biomolecules, since the ability to counteract the continuous and large generation of ROS becomes increasingly inefficient with aging, resulting in mitochondrial dysfunction as a central pathogenic mechanism. Nevertheless, the role of the integrated stress response (ISR) involving UPRmt and mitophagy in the development and progression of these illnesses is still a matter of debate, considering that some studies indicate that the prolonged exposure to low levels of stress may trigger these mechanisms to maintain mitohormesis, whereas others sustain that chronic activation of them could lead to cell death. In this review, we discuss the available research that contributes to unveil the role of the mitochondrial UPR in the development of sarcopenia, in an attempt to describe changes prior to the manifestation of severe symptoms; and in NAFLD, in order to prevent or reverse fat accumulation and its progression by means of suitable protocols to be addressed in future studies.
Keywords: mitochondrial homeostasis, aging, liver disease, skeletal muscle
1. Introduction
Highly metabolic tissues concentrate their energy demands on the participation of mitochondria, obtaining the majority of ATP through the electron transport chain coupled to ATP synthase, pointing to mitochondrial homeostasis as a key phenomenon to sustain organ functions. Imaging studies in living cells showed that mitochondria are in constant motion and undergo structural changes, depending on the cellular energy state [1]. The mitochondrial network moves to places with higher energy requirements along the cytoskeleton, allowing individual mitochondrion to merge with the mitochondrial network on both its external and internal membranes during this trafficking [2]. Evidence shows that the modification of the cellular metabolic state somehow determines the structure of the mitochondrial network, suggesting that each metabolic event could be associated with its own mitochondrial morphology [3]. This situation is clearly illustrated by the observation where the morphology of the mitochondrial network of various cell types rich in mitochondria depends on the energy demands to which these tissues are exposed. Cells in tissues with a high energy demand such as cardiomyocytes and myocytes exhibit a fused mitochondrial morphology, where there are a few very elongated tubules that group almost all of the mitochondria. Instead, cells in tissues that mainly metabolize digested nutrients from the diet, such as hepatocytes, have a fissioned mitochondrial network, where numerous and small isolated mitochondria coexist [4].
Mitochondrial biogenesis is the generation of new mitochondria. It is a critical process for development and differentiation, as well as being a response to eventual mitochondrial dysfunction as it restricts the transmission of harmful mtDNA mutations. Thus, an altered and dysfunctional mitochondrial biogenesis can cause severe disorders, including different pathologies, such as neuromuscular diseases [5]. Regulatory factors of mitochondrial biogenesis are the transcriptional family of the peroxisome proliferator-activated receptor γ (PPARγ) coactivator alpha (PGC-1α), which interacts with NRF-1 and NRF-2 to regulate protein expression. So, the generation of new mitochondria is a coordinated control of several pathways, where PGC-1α improves transcription by facilitating the interaction with the nuclear receptor family through specific LXXLL recognition domains [6]. Mitochondria form an independent compartment in the cell, providing its own translation and protein quality control machinery, including chaperones and proteases inside the mitochondrial matrix, which maintain mitochondrial homeostasis (proteostasis) [7]. In this respect, maintaining optimal protein structure for its adequate function in all cellular compartments including mitochondria is an absolute requisite for cell viability. Different types of chemical modifications occur in cellular proteins, including reversible ones, such as phosphorylation/dephosphorylation reactions involved in metabolic regulation. However, irreversible alterations are usually associated with loss of protein function and cell viability. One of them is the oxidation of target amino acids, which leads to partial protein unfolding or misfolding [8]. These alterations are due to the loss of the secondary and tertiary structures in the oxidative attack domains, which can be assessed by detecting individual modified amino acids [8] or by the spectrophotometric determination of protein carbonyls [9]. Enhancement in cellular protein oxidation in pro-oxidant conditions such as oxidative stress [10] implies proteome instability with loss of function, representing a stress condition at subcellular sites such as endoplasmic reticulum (ER) [11] and mitochondria [12]. Under these circumstances, cellular sensors are activated to trigger a transcriptional response known as unfolded protein response at ER (UPRer) and mitochondrial (UPRmt) sites, which promote cell survival through the recovery of organelle networks for optimal cellular function [11,12,13,14].
Proteostasis requires several molecular mechanisms to control protein folding, including the adaptation of protein synthesis and degradation, and processes facilitating the post-translational folding and maturation of proteins [7,12,13,14]. The UPRmt is rapidly activated in a time-course of hours to achieve proteostasis, thus representing an acute response that is executed through a multi-axes program [15], which induces pro-death signals under conditions of proteostasis failure or prolonged UPR activation [7,16]. Besides the excessive production of reactive oxygen species (ROS) leading to a proteotoxic stress, UPRmt can also be induced by depletion of oxidative phosphorylation (OXPHOS) components and loss of mitochondrial DNA (mtDNA). Recent evidence has shown that the decline in mitophagy because of the aging process can contribute as a cause of mitochondrial proteostasis [17]. In this regard, the accumulation of dysfunctional mitochondria could be due to impairment in mitophagy flux because of a decrease in mitophagy related proteins [17]. The precise mechanism through which mitophagy is linked to UPRmt is not yet unveiled. Some studies have shown that the inhibition of mitochondrial protease HSP90 induces PINK1 accumulation, ubiquitin phosphorylation at Ser65, Parkin activation and its recruitment to mitochondria, promoting mitophagy [18]. In this way, the inhibition of UPRmt and the subsequent loss of mitochondrial proteostasis, might produce dysfunctional mitochondria that can be a target for ubiquitination and mitophagy. Thus, if the mitochondrion is beyond repair, the entire organelle must be removed before it becomes cytotoxic and causes cellular damage [19]. In the aging process, mitophagy is significantly decreased, causing the accumulation of dysfunctional mitochondria which in turn has defective proteostasis and promotes the activation of the UPRmt. The excessive activation of this mechanism may also be detrimental to the cell [20]. In these scenarios, deleterious responses that underlie many pathological states, including sarcopenia and non-alcoholic fatty liver disease (NAFLD), can be activated (Table 1). These age-associated diseases are also characterized by the progressive loss of organ function due to the accumulation of ROS-induced damage to biomolecules, since the ability to counteract the continuous and large generation of ROS becomes increasingly inefficient with aging, positioning mitochondrial dysfunction as a central pathogenic mechanism for both pathological conditions.
Table 1.
UPRmt in the context of skeletal muscle and liver.
| Reference | Model | Main Findings |
|---|---|---|
| Cordeiro et al., 2020 [21] |
Young and old mice underwent 4 weeks of aerobic exercise training | Increased UPRmt markers in gastrocnemius muscle of aged mice |
| Tamura et al., 2017 [22] |
Young and aged mice received heat stress treatment | Remarkable improvements in age-related changes in soleus, but minor effects in gastrocnemius and plantaris |
| Memme et al., 2016 [23] |
Rats subjected to chronic contractile activity (CCA) for 1 to 7 days | UPRmt-specific markers were induced 10 to 80% between days 1 and 7 |
| Al-Furoukh et al., 2015 [24] |
ClpX overexpression in C2C12 mouse myoblasts and HEK293T cells | Upregulation of markers of the UPRmt |
| Gariani et al., 2016 [25] |
Mice fed with HFHS diet plus nicotinamide riboside | Induction of a SIRT1- and SIRT3-dependent UPRmt, preventing/reverting NAFLD |
| Quirós et al., 2017 [26] |
Multiomics approach in mammalian cells treated with 4 mitochondrial stressors | Identification of ATF4 as the main regulator of the stress response |
| Fiorese et al., 2016 [27] |
C. elegans lacking ATFS-1 and expressing a reporter of UPRmt | Rescue of UPRmt activation under stress conditions by ATF5 |
| Bennett et al., 2014 [28] |
Genome-wide RNAi screen for negative regulators of the UPRmt | UPRmt is neither necessary nor sufficient for lifespan extension |
| Michel et al., 2015 [29] |
Impairment of mtDNA expression | Triggering of ISR, but not of UPRmt |
| Zhao et al., 2002 [30] |
Mutated OTC to provoke mitochondrial protein accumulation | Induction of nuclear genes encoding for Hsp60, Hsp10 and ClpP |
1.1. Mitochondrial Proteostasis
Mitochondria have been extensively described as the powerhouse of the cell, and mitochondrial function has been placed as a reflection of the cell status [31]. In this regard, mitochondria are in charge of ATP production for muscle contraction, the maintenance of metabolic homeostasis, proper cellular functioning and viability [32]. Furthermore, mitochondria are the first-line responders to different stresses [33] and can adjust bioenergetics, thermogenesis, and oxidative stress responses in order to regulate perturbed homeostasis [34]. Thus, the mitochondrial network has developed mechanisms to combat impending mitochondrial dysfunction [35]. Since the mitochondrial genome encodes 13 proteins that are components of the OXPHOS respiratory complexes, the remaining mitochondrial proteome is encoded by nuclear genes, synthesized in the cytosol and imported into the different mitochondrial compartments through translocase of the outer membrane (TOM) and translocase of the inner membrane (TIM) translocators. Therefore, since most of the proteins in the mitochondria are synthesized by the cytosolic ribosomes, an adequate translocation of proteins to the mitochondria is essential. Mitochondrial biogenesis requires the import and trafficking of proteins synthesized in the cytosol into the intramitochondrial compartments. Proteins destined to the mitochondrial matrix are targeted to the inner membrane Tim17/23 translocon by their pre-sequences [36]. This is carried out using a mitochondrial targeting sequence (MTS), which is located at the N-terminal position of the protein to be imported. This positively charged 25–35 amphipathic amino acid sequence interacts with the TOM complex prior to import. Then, by going through the TOM complex in an unfolded state, the precursor proteins are translocated through the TIM23 complex, which requires the inner mitochondrial membrane (IMM) potential generated by the respiratory chain. The precursor proteins are then translocated to the mitochondrial matrix requiring a chaperone located in it, called mtHsp70, which interacts directly with the TIM23 complex [37,38]. Recent studies have also described the participation of TIM50 in the regulation of the translocation into mitochondria [39], details about translocation of proteins into mitochondria through TIM23 complex in detail can be found in reference [38]. Once in the matrix, the MTS is cleaved by the mitochondrial processing peptidase (MPP) [16]. Thus, chaperones and proteases play fundamental roles in protein folding after import. In this context, the import of most of the mitochondrial intermembrane space (IMS) proteins is mediated by the mitochondrial disulfide relay system linked to oxidative processes [40]. Moreover, mitochondria possess their own protein translation and quality control machinery depending on chaperones and proteases which degrade damaged or misfolded proteins, such as the AAA, LON and ClpXP proteases. These proteins located within the mitochondrial matrix are responsible for maintaining an adequate mitochondrial proteostasis [41].
1.2. Mitochondrial Unfolded Protein Response Signaling
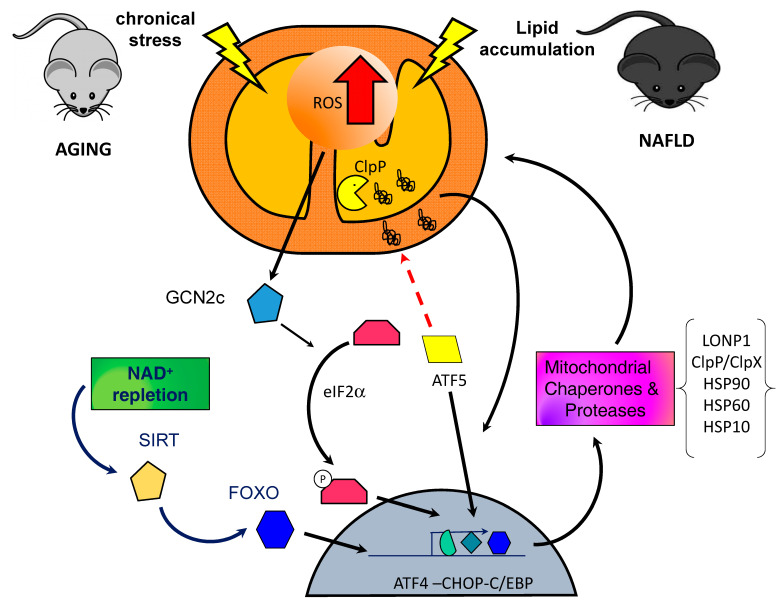
The transcriptional regulation of the UPRmt in mammalian cells is comparable to that of Caenorhabditis elegans and, likewise, is mediated by multiple bZIP transcription factors [27]. A functional ortholog of ATFS-1, activating transcription factor 5 (ATF-5), was discovered in mammals. It regulates mitochondrial import efficiency similar to what ATFS-1 does. Under non-stressful conditions, ATF-5 is actively imported into mitochondria for further degradation. However, in the presence of stress signals, it migrates to the nucleus triggering the transcription of various factors associated with mitochondrial proteostasis [27]. Among the transcription factors that have been linked to the UPRmt, ATF-5 stands out as the main signaling agent. However, also other participants can be listed as UPRmt responders: (i) CEBP homologous protein (CHOP), (ii) CCAAT/enhancer binding protein β (CEBPβ), (iii) and activating transcription factor 4 (ATF4) [30]. The expression of CHOP and ATF4 has also been linked to a process called integrated stress response (ISR), which is a mechanism dependent on phosphorylation of the eukaryotic translation initiation factor 2 alpha (eIF2α). In this regard, there is evidence that the ISR is necessary for the activation of UPRmt in response to mitochondrial dysfunction in mammals [42,43]. The activation of the ISR is mediated by four kinases that phosphorylate eIF2α in response to specific alterations. Particularly, the ISR kinase, protein kinase RNA-like ER kinase (PERK), responds to the accumulation of misfolded proteins in the ER; protein kinase RNA-activated (PKR) responds to cytosolic double stranded RNA; heme-regulated inhibitor kinase (HRI) is activated by depletion of the heme group [42], and general control non-derepressible 2 kinase (GCN2) is activated by mitochondrial stress, amino acid depletion, ROS and ribosome stalling [44,45]. Following eIF2α phosphorylation by one of these kinases, global protein synthesis is diminished, but the preferential translation of alternative reading frames coding for the transcription factors CHOP, ATF-4 and ATF-5 is preserved, which are associated with the induction of genes related to the UPRmt [26,43,46,47]. Furthermore, UPRmt produces a significant increase in various mitochondrial proteases including lon protease 1 (LONP1) [48] and ClpP/ClpXP; and chaperones heat shock protein 90 (Hsp90), mitochondrial heat shock protein 70 (mtHsp70), Hsp60 and Hsp10 [49] (Figure 1). The UPRmt has been described as a beneficial signaling pathway and it has also been linked to improvement in lifespan and in metabolic homeostasis. Nevertheless, a chronic increase in the UPRmt due to the constant presence of a stressor has been suggested to be detrimental to the cell [20,28].
Figure 1.
Proposed model of mitochondrial unfolded protein response (UPRmt) increased activation in non-alcoholic fatty liver disease (NAFLD) and Sarcopenia. The mechanisms that regulate the activation of UPRmt are still unveiled and are probably determined by the stress conditions present in the cell environment in a context-dependent way. Lipid accumulation in the liver and the presence of chronical stress during the aging process in skeletal muscle cells promote an increase in mitochondrial reactive oxygen species (ROS) production which triggers the phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2α) to trigger the integrated stress response (ISR). Moreover, the accumulation of misfolded and oxidized proteins which cannot be degraded inside the mitochondria by mitochondrial proteases promotes the translocation of activating transcription factor 5 (ATF-5) to the nucleus, increasing the transcription of mitochondrial chaperones and proteases. The prolonged exposure to the stress may continuously stimulate the response enhancing its effect until making it detrimental for the cell.
Mitochondria continually generate reactive oxygen species (ROS), which could eventually be detrimental for proteins, lipids, and DNA. Considering that damaged electron transport chain (ETC) proteins can produce even more ROS, mitochondria use quality control proteases to remove these potentially harmful proteins and respond to the protein stress deployed in the mitochondrial matrix [50]. Additionally, a mito-cellular response to cope with damaged outer mitochondrial membrane (OMM) proteins is carried out by the ubiquitin–proteasome quality control pathway [51], while some mitochondria also respond to genotoxic damage through DNA-repairing pathways found in the nucleus, recovering the function of the organelles [50]. Finally, autophagy is considered as the most radical quality control mechanism, signifying the total elimination of dysfunctional mitochondria (mitophagy).
1.3. Mitophagy
Mitophagy is the selective autophagic degradation of mitochondria and mediates the elimination of dysfunctional and/or superfluous mitochondria to maintain energy homeostasis [52]. Mitophagy pathways are stimulated by several cellular events involved in physiologic and pathophysiologic processes in different cell types, including cell differentiation, development, response to oxygen deprivation or mitochondrial damage among others [53]. In addition to the removal of damaged mitochondria, mammals can deploy specialized mitophagy pathways to remove mitochondria during different stages of development or basal conditions. Even though there are different effectors and receptors for mitophagy depending on the stimuli, a common feature is the microtubule-associated protein 1 light chain 3 (LC3) region of these proteins, which promotes mitochondrial sequestration [54]. Thus, mitophagy shares some common signaling pathways and key regulatory proteins with macroautophagy (LC3, p62 among others), there are also some specific mitochondrial proteins that either trigger or facilitate mitochondrial degradation [55].
1.3.1. The PINK1/Parkin Pathway
In healthy mitochondria, PTEN-induced putative kinase 1 (PINK1) acts as a stress sensor depending on mitochondrial potential. Under normal conditions, PINK1 is rapidly imported into mitochondria and undergoes cleavage and degradation. When mitochondrial potential is dissipated, PINK1 is stabilized on the outer mitochondrial membrane and recruits Parkin, which is a cytosolic E3 ubiquitin ligase that after being phosphorylated by PINK1, activates its E3 ligase activity. Parkin ubiquitinates various outer mitochondrial membrane proteins leading to subsequent autophagosomal engulfment and lysosomal degradation [54]. Recent studies have suggested that the PINK1/Parkin pathway is involved only in stress-activated mitophagy, in a mitochondrial potential-dependent way, while basal mitophagy is independent from these proteins’ activation and subsequent mitophagy [56]. Mitochondrial clearance balance is not only affected by the PINK1/Parkin pathway, but there is also a selective-removal mitophagy depending on the different mitochondrial stress stimuli [54].
1.3.2. BNIP3/NIX Pathway
BCL2 and adenovirus E1B 19-kDa-interacting protein 3 (BNIP3) and BNIP3-like (BNIP3L, also known as NIX) are mitochondria-localized proteins that are related to the BH3-only family. BNIP3 is primarily localized to the mitochondria and it is well documented that it induces loss of mitochondrial membrane potential, mitochondrial dysfunction and the activation of the mitochondrial pathways of apoptosis [57]. In addition to this role, it has been recently reported that BNIP3 induces mitochondrial autophagy in the absence of mitochondrial membrane permeabilization and Bax/Bak apoptosis proteins [57]. Moreover, Zhang et al. described an interaction between both the aforementioned mitophagy pathways, where BNIP3 interacts with PINK1 to inhibit its clearance, promoting Parkin recruitment and the initiation of PINK1/Parkin-mediated mitophagy [58].
2. Mitophagy and Associated Disorders in Skeletal Muscle
Skeletal muscle is characterized by its structural and functional plasticity, which provides an adaptation to variable physiological stimuli [59]. These physiological conditions are classified as acute and chronic changes related to physical activity and muscle workload. Skeletal muscle contracts due to changes in contractile machinery, Ca2+ concentration, and the metabolic capacity for energy production in the form of ATP that is carried out inside the mitochondria. Therefore, the mitochondrial network has evolved so that the mitochondrial production of ATP is adequate and can rapidly respond to the demands and requirements imposed by the activity in the muscle [60,61,62]. Thus, skeletal muscle mitochondria can be classified as subsarcolemal (SS) or intermyofibrillar (IMF) depending on the subcellular location. On the one hand, SS mitochondria are characterized by a large laminar shape located below the sarcolemma, while IMF mitochondria are smaller, more compact, and located among the contractile filaments [63,64,65].
Recently, three-dimensional electron microscopy studies revealed that skeletal muscle mitochondria could be classified into paravascular mitochondria (PVM), I-band mitochondria (IBM), fiber parallel mitochondria (FPM), and cross-fiber connection mitochondria (CFCM). These four types are interconnected to form a complex mitochondrial network and to be able to rapidly distribute energy through the myofibers [62]. Furthermore, skeletal muscle mitochondria serve as cellular sensors of metabolic demand and stress, where various forms of metabolic stress, including exercise, trigger responses in muscle mitochondria, generating signals corresponding to other compartments or cell tissues.
Loss or decrease in skeletal muscle function has serious consequences. In particular, the progressive decline in age-associated skeletal muscle function is related to metabolic dysfunction, susceptibility to chronic disease, loss of mobility, and an overall increase in mortality rate [66,67,68,69]. At the same time, this event is associated with the loss of mitochondrial protein homeostasis, which has been suggested to play a causal role in age-related skeletal muscle decline [70,71,72,73]. Sarcopenia is defined as a multifactorial syndrome that occurs with age and results in loss of skeletal muscle mass and its function [74]. This muscle mass loss begins to manifest approximately from 30 years of age. From then on, between 3 and 8% of muscle mass is lost, a phenomenon that accelerates significantly after the age of 60, with up to 40% of muscle mass losing after reaching 80 years of age [75,76]. This muscle mass decline has also been associated with loss in muscle function and strength, phenomena that are also related to mitochondrial dysfunction [77]. The causes of sarcopenia have been studied for many years and several hypotheses have been presented; however, the mechanisms that cause sarcopenia are still a matter of debate. Although physical changes begin to manifest at advanced ages, recent studies have shown that changes at the cellular and molecular levels precede the symptomatology of sarcopenia [78,79].
Mitochondrial biogenesis has been the focus of research during the last years and several research studies have linked this process with dysfunctional mitochondria in aging. Particularly in skeletal muscle, it has been reported that Nrf2 deficiency exacerbates frailty and sarcopenia during aging, at least partially by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner [80,81]. Moreover, deep sequencing analysis of the mtDNA mutator mouse, a mouse model with a proofreading-deficient mtDNA polymerase γ (POLG) and a valuable model system to study the contribution of mitochondrial dysfunction to aging, showed that the increase in mitochondrial biogenesis did not reduce the accumulation of mtDNA mutations but rather caused a small increase. These results indicate that increased muscle PGC-1α expression can improve some premature aging phenotypes in the mutator mice without reverting the accumulation of mtDNA mutations [82]. Studies in humans describe a significant decrease in PGC-1α in old individuals, together with a decrease in mitochondrial dynamics regulatory proteins. Conversely an increase in TOM22 and other protein import machinery proteins has been observed [71], suggesting a possible role of import machinery and mitochondrial proteostasis in aged muscle. In this regard, some studies indicate that skeletal muscle mitochondria in elderly humans and animals show accumulation of protein damage and release of ROS [70,83] that would contribute to global mitochondrial compromise and thus, to dysfunctional skeletal muscle tissue [83,84]. Studies in aged skeletal muscle in mice and humans have demonstrated altered expression of apoptotic and antiapoptotic proteins and enhanced susceptibility towards mitochondrial transition pore opening [85]. This recent evidence shows that the relationship between sarcopenia and mitochondria is still a matter of debate when defining the molecular mechanisms of muscle mass and function loss during aging.
Concerning mitophagy in skeletal muscle, current studies have described the decrease in this process with aging. In aged-mice skeletal muscle, PINK1 expression was reduced when compared to young mice muscle [86]. Interestingly, in a study with humans, despite no difference in Parkin expression between young and older muscles being observed, a lower ratio of Parkin relative to voltage-dependent anion channel (VDAC) in aged muscle was found, suggesting that the mitophagic potential may be impaired with aging (Figure 2) [84].
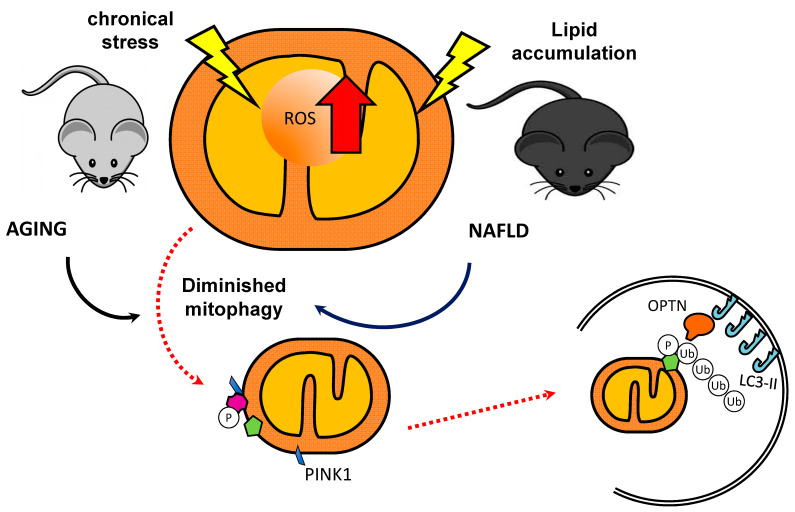
Figure 2.
Diminished mitophagy in NAFLD and Sarcopenia. Mitophagy-associated proteins are decreased during the aging process and have been linked with aging associated diseases. The accumulation of damaged mitochondria because of impaired mitophagy could be involved in the development of disease and organ malfunction.
Although there are few reports about muscle-specific silencing of autophagy/mitophagy genes, the most studied is the deletion of the autophagy related protein 7 (Atg7) gene, which encodes for a crucial component of both, Atg12 and LC3 ubiquitin-like conjugation systems involved in the expansion of the autophagosome membrane [87]. The deletion of the Atg7 gene, and subsequent defective mitophagy, causes an augmentation in protein carbonylation and accumulation of enlarged, swollen mitochondria in skeletal muscle and significantly shortens lifespan [88]. Thus, the decrease in mitophagy in skeletal muscle would be analogous to the characteristic mitochondrial phenotype traits related to aging [17]. Additionally, a higher accumulation of the autophagy markers p62 and LC3-II was observed in skeletal muscle of aged Fisher 344 BN rats [89], which may indicate diminished autophagic degradation in aging muscle, because LC3-II is catabolized during mitophagy. Furthermore, AMP-activated protein kinase (AMPK) plays a vital role in autophagy and mitophagy during aging and its activation in skeletal muscle seems to be diminished during senescence [90]. In response to muscle specific AMPK deletion, both SS and IMF mitochondrial sizes increased, and a significant reduction in mtDNA was observed [91]. Defects in mitochondrial metabolism provoke a decline in tissues and/or organs function. Likewise, malfunctioning of the mitochondrial proteostasis mechanisms owing to insufficient or impaired mitophagy may have an important effect upon homeostasis throughout the body. Mutations in Bcl2 phosphorylation sites inhibit the release of Beclin1 from the Bcl2-Beclin1 complex preventing the activation of autophagy, impairing exercise-induced increase in insulin sensitivity and failing to increase the translocation of GLUT-4 to the plasma membrane in skeletal muscle [92]. Moreover, it has been demonstrated that the inhibition of autophagy produces decreased endurance, altered glucose metabolism during exercise and impaired exercise-mediated protection against glucose intolerance induced by high fat diet [92].
Physical activity and muscle contraction have been established as powerful factors regulating mitophagy, which may potentially correct or attenuate dysfunctional mitophagy, during metabolic stress [93] and nutritional deficits such as cachexia [94]. In C26 tumor implanted mice, exercise attenuated cachexia-induced p62 and LC3 II/I accumulation indicating improved mitophagy. Additionally, AMPK activation via 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) administration suppressed p62 accumulation through promotion of mitophagy and accelerating the turnover of p62 accumulation in cachectic muscle [94]. Therefore, exercise and/or muscle contraction might be an effective therapy for the restoration of mitophagy balance, which would lead to proper mitochondrial and metabolic homeostasis of skeletal muscle. Thus, multiple studies point out the role of mitophagy over skeletal muscle under different metabolic conditions. However, additional investigations are required to establish a direct mechanism in the regulation of these processes. To deepen on the aforementioned view, several studies have attempted to understand the effects of resistance training on cellular systems over muscle aging. While some conflicting findings have been observed, it is almost undeniable that acute aerobic exercise is likely to increase autophagic responses in skeletal muscle [95]. For instance, it has been observed that aging muscles show higher expression of autophagic markers such as ATG7 and Beclin-1 when they undergo endurance training. Both markers are important in the autophagosome formation [96]. Furthermore, physically active elderly subjects have increased mRNA levels of autophagy markers such as Beclin-1, ATG7 and p62. Likewise, they also have increased mitophagy markers including BNIP3 and Parkin in skeletal muscle [97]. Therefore, it is possible that endurance training or chronic muscular activity might lead to a remodeling of the mitochondrial network in the skeletal muscle of elderly individuals, although, further studies are needed to fully understand these observations [96].
The decline of skeletal muscle oxidative capacity is a common feature of chronic diseases such as type 2 diabetes (T2D) [98], chronic obstructive pulmonary disease (COPD) [99] and congestive heart failure (CHF) [100]. Functional deficiencies, such as muscle dysfunction and decreased exercise capacity, are associated with lower muscle oxidative capacity that could eventually lead to disabilities or even handicaps. Additionally, impaired muscle oxidative capacity has been suggested to accelerate muscle wasting [101] and may increase the metabolic and cardiovascular risks [102]. Certainly, skeletal muscle mitochondrial quantity and its oxidative capacity are affected in chronic diseases. Although the number of studies on mitophagy in skeletal muscle of patients with the aforementioned chronic diseases is small, an increased presence of proteins related to mitophagy, such as BNIP3 and PARK2, has been observed together with a positive regulation of autophagy in vastus lateralis muscle of patients with COPD [103]. On the contrary, Kruse et al. found no differences in markers related to autophagy/mitophagy in vastus lateralis muscle of type 2 diabetes subjects [104]. However, none of these investigations evaluated mitochondrial content or oxidative capacity.
In skeletal muscle, Sakellariou et al. provided evidence that the regulation of mitochondrial ROS production could be affecting the PINK1/Parkin mitophagy pathway, without having any incidence in muscle atrophy [105]. In addition, recent evidence has shown that mitophagy mechanisms in high metabolic demand tissues, such as skeletal muscle, are independent of PINK1 activation in basal conditions [56]. On the other hand, Gouspillou et al. described that ablation of Parkin in skeletal muscle increases basal autophagic flux and BNIP3 levels, whereas skeletal muscle fibers from Park2 KO mice show increased mitochondrial dysfunction, together with a higher cross-sectional area of muscle fibers [106]. Following this research line, Peker et al. reported that Parkin KO muscles had dysfunctional mitochondria, but, in controversy with Gouspillou et al., Parkin KO muscles exhibited smaller myofiber area [107]. Other studies have reported that aging-induced Mitofusin-2 deficiency triggers a ROS-dependent adaptive signaling pathway through induction of HIF1α transcription factor and BNIP3 [108]. Studies in humans have described decreased mitophagy-related genes in skeletal muscle of physically inactive elderly women and a positive correlation between this decrease and the physical function or leg lean mass [95].
Since mitochondrial fission is usually linked to the mitophagy process, it could be considered as an indirect indicator of mitophagy. Thus, the decreased average mitochondrial size observed in the vastus lateralis muscle of patients with CHF [109] or T2D [110], suggests a mitochondrial fission/fusion balance inclined towards mitochondrial fission. Moreover, decreased expression of Mfn-2 is observed in skeletal muscle of type 2 diabetes patients [111], with no statistically significant difference being reported in skeletal muscle of CHF patients [112]. In spite of the unchanged gene expression, Molina et al. demonstrated that the protein expression of Mfn-2 decreased in skeletal muscle of CHF patients compared with sedentary subjects [113]. In relation to these mitochondrial variations in atrophy and aging, Romanello et al. described that the induction of mitochondrial fission by overexpressing proteins of the fission machinery produces a decrease in the area of muscle fibers and an increase in autophagy [114]. Along with these results, other studies have described a decrease in proteins linked to mitochondrial dynamics as age increases, thus relating mitochondrial morphology to aging [115]. The deficiency of mitochondrial fission processes during aging, could promote mitochondrial dysfunction due to the accumulation of damaged mitochondria, considering that mitochondrial fission processes precede mitophagy and maintain the homeostasis of these organelles [116]. In the context of studying mitochondrial morphology in aging, Leduc-Gaudet et al. reported highly fused mitochondrial structure in aged mouse muscle [117].
Nevertheless, as discussed above, the modifications in mitochondrial morphology during aging are still a matter of debate and none of these studies have shown how the proposed mechanisms are altered in middle age. Only a few recent studies have focused on describing changes prior to the state of manifesting severe sarcopenia symptoms. In this regard, del Campo et al. reported time-line changes in mitochondrial morphology and distribution in adult skeletal muscle fibers [79]. The results show that a mitochondrial network fused-like phenotype can be observed in 10–14-month-old mice. Moreover, muscle function and cross-sectional area begin to decline at the same age [79]. In addition to those findings, Sayed et al. showed that mice at an early stage of aging already display significant changes in muscle mitochondrial morphology and ultrastructure, which are suggestive of the onset of sarcopenia. Their results describe a significant decrease in the muscle-fiber cross-sectional area and an increase in intermyofibrillar mitochondria in skeletal muscle [78]. Altogether, these findings suggest that during middle age an impairment of mitochondrial homeostasis might occur previously to the development of sarcopenia symptoms and, consequently, this turnover point could be an interesting target to intervene. Likewise, it would be important to determine whether other mitochondrial mechanisms could be influencing the development of sarcopenia across the aging process, to shed light about imbalanced mitophagy in the course of chronic diseases and aging.
Given the aforementioned, the question is whether mitophagy in skeletal muscle would be a beneficial or harmful process. Mitophagy is vital for mitochondrial quality control and therefore a diminution or an alteration in this process could result in extensive mitochondrial damage, mitochondrial dysfunction, or cell death [118]. In this context, it would be more adequate considering mitophagy as a cellular process only, not intrinsically good or bad, but inclined toward one or the other outcomes according to the cellular needs at any given time.
In this respect, it seems logical relating mitophagy and UPRmt. The first process eliminates damaged components or eventually the whole organelle; the second one triggers the expression of proteases and chaperones to relieve mitochondrial stress provoked by misfolded proteins and promotes biogenesis to maintain proteostasis and mitochondrial function [119]. Therefore, both events could be connected through a common stressor mechanism that may benefit mitochondrial populations suitable for recovery in detriment of those tagged for mitophagy. While mitophagy tends to enclose the damage of dysfunctional mitochondria, stress responses such as UPRmt facilitate the recovery of mitochondria susceptible of being saved, resulting in a healthier mitochondrial network [35].
3. UPRmt in Sarcopenia
Age-associated atrophy contributes to decreased mobility and increased fragility, where many of the changes underlying the adaptive and plastic nature of the muscle converge into mitochondria. Thus, the maintenance of a functional mitochondrial network is critical for the preservation of skeletal muscle throughout life. During aging a significant increase in defective mitochondria is produced, which drives to a decline in the overall physiological functioning of the organism. This mitochondrial dysfunction is accompanied by reduced oxygen consumption with a corresponding diminution in respiratory complex activity [120]. Interestingly, this decline has been linked to the development of many age-related diseases such as Parkinson’s and coronary artery disease [121]. In a metabolic context, Nargund et al. found that the UPRmt activation was a positive regulator of glycolysis’ genes transcription suggesting that this compensatory mechanism promotes an alternative form of ATP production [122]. Concomitantly, OXPHOS transcript accumulation is diminished to match OXPHOS complex biogenesis rates to the protein folding and complex assembly capacity of the dysfunctional organelles, to finally restore mitochondrial respiration in an efficient manner [123].
Considering that skeletal muscle is one of the main metabolic regulators and the importance of mitochondrial homeostasis in its function, it is possible to establish a correlation between mitochondrial dysfunction and the decrease in muscle mass, where the UPRmt would have a fundamental role. In this regard, Chung et al. found that muscle-specific Crif1 (CR6-interacting factor 1) knock-out mice activated the UPRmt, stimulated the production of mitokines that regulate systemic energy homeostasis and induced progressive mitochondrial OXPHOS dysfunction, ultimately producing advanced muscular dystrophy and sarcopenia. In addition, it was observed that accumulation of abnormal, swollen mitochondria with disrupted cristae and some mice manifested traits of mitochondrial myopathy reduced muscle mass and lowered strength [124]. Tamura et al. stated that mitochondrial and endoplasmic reticulum stress and the activation of their compensatory mechanisms (UPRmt and UPRer) are involved in the pathogenesis of sarcopenia. Interestingly, heat shock treatment has been suggested as a strategy to counteract sarcopenia, improving age-induced mitochondrial and ER stress. In this regard, heat stress showed notable improvements in age-related changes in soleus muscle, but minor effects in gastrocnemius and plantaris muscles, suggesting that the outcomes vary qualitatively according to the skeletal muscle type [22]. Although these findings seem promising, more studies are needed to strengthen the concept of heat stress as a potential treatment for sarcopenia.
One of the first described physiological functions of UPRmt is associated with the lifespan extension observed after mild perturbations of OXPHOS in C. elegans [125]. The mitochondrial disturbances that lead to the lifespan extension also cause activation of the UPRmt [126] and it is important to note that the increased longevity of the C. elegans model requires multiple components of the UPRmt, such as histone lysine demethylases jmjd-3.1, matrix peptide exporter haf-1 and chaperone dve-1, which demonstrates that the pathway is protective during mitochondrial dysfunction [127]. However, how the UPRmt contributes to extending longevity remains unclear. In this context, the mitochondrial homeostasis and its variations are associated to lifespan and longevity, considering that mitochondrial dysfunction is frequently a characteristic of aging and different diseases. Many investigations have shown a positive correlation between UPRmt and longevity in C. elegans, Drosophila and mice [126]. However, this relationship is not straightforward because the UPRmt activation might lose its beneficial status and become detrimental, if the UPRmt activation is chronic [128], since the long-term implications of a constitutively active UPRmt promotes glycolytic and biosynthetic processes, which are characteristic traits of highly proliferative cells [35].
As organisms possess a high number of mtDNA in each cell, a small amount of mutated mtDNA is well tolerated possibly due to a much higher percentage of healthy mtDNA. However, deleterious mtDNA can accumulate as the organism grows old and disturb OXPHOS, impairing mitochondrial homeostasis and ultimately cellular function [129]. For instance, in a model of deleterious heteroplasmy in C. elegans, the UPRmt is activated, since the mtDNA lacks the genes necessary for the expression of various OXPHOS subunits [128]. The activation of UPRmt likely occurs in an attempt to maintain proteostasis and promote recovery of mitochondrial dysfunction [128]. Interestingly, the elimination of ATFS-1 resulted in a preferential reduction in mutated mtDNA together with an increase in wild-type mtDNA. In addition, the constitutive activation of the UPRmt in heteroplasmic worms was sufficient to cause an increase in mitochondrial biogenesis, which resulted in a preferential increase in deleterious mtDNA over the wild-type [128,130]. These data demonstrate that the activation of the UPRmt results in the spread of harmful mtDNA, although the mechanism is unclear. Similar results were observed in a mouse model of mitochondrial myopathy caused by an aberrant accumulation of mutated mtDNA, resulting in muscle OXPHOS impairment [131,132]. Interestingly, the inhibition of mTORC1, a factor that induces UPRmt, resulted in a reduction in the activation of UPRmt and limited the accumulation of deleterious mtDNA, which, in turn, reduced the progression of mitochondrial myopathy [131]. Furthermore, this inhibition limits mitochondrial biogenesis and induces an increase in mitophagy, suggesting a mechanism by which the UPRmt could antagonize mitophagy and promote the accumulation of harmful mtDNA [133].
In relation to exercise and stress response, a single bout of resistance exercise provokes the activation of the UPR, similar to what is observed after endurance training [134]. During exercise, both the UPRer and the UPRmt are upregulated during early stages of training. In addition, several investigations have studied the expression level of UPR genes and have shown that those gene variations occur according to the type of exercise training program [135,136,137]. The UPR activation precedes changes in mitochondrial biogenesis and autophagy, which together promote mitochondrial adaptations [23]. These data would suggest that the activation of the UPR might mediate some of the exercise-induced changes observed in skeletal muscle, but more evidence is required [23,134,138]. In this respect, it has been stated that physical activity improves metabolism and physical performance of old mice. Aerobic exercise (i) increases important proteins involved in mitochondrial functions and biogenesis, such as VDAC and SIRT1, along with mitochondrial encoded genes in skeletal muscle of aged mice; (ii) induces a mitonuclear imbalance, increasing the MTCO1/ATP5α ratio and UPRmt markers in skeletal muscle, including levels of protein Hsp60, LONP1 and Yme1L1 in gastrocnemius muscles of elderly mice [21]. Thus, these data suggest that the UPRmt activation could be triggered at least in part by exercise, improving mitochondrial metabolism and oxidative capacity in elderly individuals. Some studies support the idea that increasing the stress signaling via the UPRmt through the modulation of NAD+ levels may be a target to ameliorate mitochondrial function in aged tissues [139]. Supporting this idea, it has been observed that treatment with the NAD+ precursor nicotinamide riboside (NR) induces the UPRmt, which has an effect on mitochondrial activity modulating muscle stem cells senescence [140]. Considering the aforementioned, it would be reasonable to target mitochondria in search for treatment of sarcopenia. In this regard, resveratrol and polyphenols that enhance nuclear/mitochondrial protein interactions have not yet shown major effects in skeletal muscle [141,142]. Additionally, FGF21 has been shown to be a direct ATF4 target downstream of the UPRmt [143], while GDF15 secretion is induced in a CHOP-dependent manner during UPRmt. In several mouse models of defective mitochondria, skeletal muscle secretes FGF21 into circulation to exert an endocrine effect [143]. It has also been seen that GDF15 is induced and released from skeletal muscle upon multiple forms of mitochondrial stress [14]. The participation of these myokines in the signaling of UPRmt and mitochondrial maintenance may provide a partial scenario of how the organism communicates and manifests similar age-related mitochondrial adaptations in different organs (Figure 1), despite their differences in mitochondrial morphology due to cellular activity and energy needs [4].
4. Mitophagy and Non-Alcoholic Fatty Liver Disease (NAFLD)
As previously shown, mitophagy is imperative for the maintenance of the mitochondrial network, which is probably impaired in the process of aging, at least in skeletal muscle. Despite liver and skeletal muscle possess different energy demands and mitochondrial morphology, several studies have demonstrated that the efficiency of mitophagy is impaired during the aging process [144] and in the context of liver disease associated with aging or unhealthy eating habits (Figure 2) [145]. The evidence is clearer when it comes to possible therapeutic targets and pharmacological compounds that could address the pathophysiology of NAFLD through the modulation of mitophagy processes [146]. In this respect, an in vitro study proposes that liraglutide, a long-acting GLP-1 analog, attenuates mitochondrial dysfunction and ROS generation, with concomitant PINK1-mediated mitophagy enhancement and protection against lipid accumulation [147]. Besides, the anthocyanin cation cyanindin-3-O-glucoside improves NAFLD by promoting PINK1-mediated mitophagy in in vitro and in vivo models [148]. On the other hand, Li et al. demonstrated that high fat diet-induced liver damage is associated with Sirt3 downregulation linked to BNIP3-mediated inhibition of mitophagy, causing hepatocytes to undergo mitochondria-dependent death pathways [149]. These data point to the regulation of BNIP3 as a promising therapeutic target [150].
5. UPRmt and NAFLD
Development and progression of NAFLD are associated with a high energy intake and consumption of specific nutrients such as saturated fatty acids (FAs) (i.e., palmitate) [151], trans FAs [152] and carbohydrates (i.e., glucose, fructose) [153]. Besides, derangement in the intake of n-6 long-chain polyunsaturated FAs (n-6 LCPUFAs) and n-3 LCPUFAs enhancing the n-6/n-3 LCPUFA ratio [154] also occur, promoting the abnormal deposition of triglycerides (TGs) in the liver. The pathogenic mechanisms underlying NAFLD are beginning to be understood, placing the development of a drastic and progressive oxidative stress as a crucial molecular phenomenon triggering hepatocellular injury [155]. This redox imbalance is related to an enhanced and sustained ROS production from the start-point of overnutrition until NAFLD development, in which the initial excess FAs can be rapidly oxidized with consequent superoxide radical (O2•−) and hydrogen peroxide (H2O2) generation at complex I of the mitochondrial respiratory chain [156,157]. These processes are facilitated by the development of insulin resistance (IR) [158], which underlies the production of a pro-inflammatory state with upregulation of tumor necrosis factor-alpha (TNF-α) generation, due to activation of Kupffer cells [159], liver macrophages that contribute to ROS production through NADPH oxidase operation [160]. Under these conditions, the expression of liver microsomal cytochrome P450 2E1 is drastically enhanced [161,162], an enzyme characterized by its large capacity for ROS generation [163]. Consequently, liver oxidative stress in NAFLD leads to the oxidative deterioration of biomolecules, including PUFA peroxidation, DNA oxidation [164] and protein carbonylation. The latter alteration is observed in experimental (high-fat diet (HFD) with 60% of fat for 12 weeks) [165] and human [155] obesity, constituting a proteotoxic stimulus that may trigger UPRer, UPRmt or both.
Prolonged liver UPRer is associated with enhancements in the cellular levels of oxidized, unfolded proteins and saturated FAs such as palmitate, producing selective, structural effects in the ER. In the case of palmitate, this FA is incorporated into either TGs, forming the insoluble tripalmitin that is retained in the ER, or into phospholipids, disrupting the ER architecture [166]. Under these conditions, two branches of the UPRer are activated, namely, (i) the PERK-eIF2α-ATF4 pathway promoting de novo FA synthesis and TG formation via upregulation of the transcriptional regulator SREBP-1c; (ii) the IRE1α-XBP1 branch triggering TG synthesis via expression of PPARγ and the C/EBPs [167], thus inducing a steatotic response. Since NAFLD is a progressive disorder, chronic UPRer was proposed to favor the development of more drastic forms of the disease, including inflammatory and fibrotic responses ensuing liver failure [167,168].
Liver mitochondria are structurally and functionally deranged in NAFLD and its progressive form of injury non-alcoholic steatohepatitis (NASH) [169], features that can result from the interruption of the electron flow through the mitochondrial respiratory chain (MRC) that favors the direct transfer of electrons to O2 to produce O2•− and H2O2 [170]. Under conditions of ROS overproduction at the mitochondrial level, several alterations are induced, namely, (i) the oxidation of cardiolipin that destabilizes MRC complexes [171]; (ii) the stimulation of lipid peroxidation processes producing malondialdehyde and 4-hydroxynonenal, LCPUFA oxidation products that are inhibitors of cytochrome-c oxidase [170]; (iii) the direct oxidative deterioration of MRC complexes and mtDNA [170,171]. mtDNA oxidation is related to a lack of protective histones and limited repair mechanisms, thus making mtDNA highly susceptible to ROS, compromising the expression of components of the electron flow and ATP synthesis processes [170,172]. Consequently, mitochondrial dysfunction ensues in HFD-induced NAFLD, which is characterized by low activities of citrate synthase, MRC complex I and II [173], succinate dehydrogenase and cytochrome-c oxidase [174], concomitantly with a fall the respiratory control ratio [174], the NAD+/NADH ratio and the ATP content [173]. Importantly, age increases the susceptibility to HFD (48% of fat for 9 weeks)-induced hepatic steatosis, which is related to lower mitochondrial mass and FA oxidation and higher ROS generation [175]. Besides mitochondrial dysfunction in HFD-induced NAFLD [173,174], derangement of mitochondrial biogenesis is also encountered [169]. Mechanistically, this effect of HFD is associated with downregulation of hepatic PPAR-α-FGF21-AMPK-PGC-1α signaling cascade [173], in which loss in the activity of the transcriptional activator PGC-1α is related to (i) diminished PGC-1α mRNA expression; (ii) lower AMPK expression compromising PGC-1α activation by phosphorylation [176]; (iii) decreased NAD+-dependent Sirtuin 1 (SIRT1) activity by reduction in the NAD+/NADH ratio lowering PGC-1α activation by deacetylation [177]; (iv) failure in the physical interaction between PGC-1α and NRF-1 to promote mitochondrial biogenesis [178].
In general terms, the UPRmt is associated with several pathologies sharing a dysfunctional state of mitochondria induced by a proteotoxic stress [14], such as in NAFLD [173,174]. The response is understood in terms of a retrograde signaling pathway establishing a mitochondrion-to-nucleus communication to enhance the expression of chaperones and proteases as an adaptive mitohormetic mechanism [169,170]. Although UPRmt is considered to be useful to restore or prevent metabolic alterations leading to the development of NAFLD [161,169], the subject has received little attention at preclinical and clinical levels. In this context, studies using an alternate NAFLD model to the standard HFD (60% as fat for 12 weeks), namely, a high-fat (44.6%) high-sucrose (40.6%) (HFHS) diet for 18 weeks, revealed the development of steatosis and mitochondrial dysfunction with NAD+ and ATP depletion, concomitantly with enhanced mRNA expression of lipogenic, inflammatory and fibrotic markers [25]. Under these conditions, however, the HFHS diet did not alter the mRNA levels of the UPRmt markers ClpP, Hsp10 and Hsp60, despite the enhancement in those of UPRer [25]. Interestingly, HFHS diet-induced chronic hepatosteatosis was prevented or reversed by inducing UPRmt by means of supplementation with the NAD+ precursor nicotinamide riboside, an effect that was suggested to involve SIRT1, SIRT3 upregulation (Figure 1) [25]. The aforementioned allows one to establish the induction of the UPRmt by NAD+ replenishment as a potential common denominator treatment for diverse myopathies and NAFLD. However, it is yet to be determined if these improvements are owing to the UPRmt activation. These data point to abrogation of mitochondrial dysfunction as a crucial mechanism underlying NAFLD prevention, in agreement with a recent report using the co-administration of docosahexaenoic acid (DHA) and hydroxytyrosol (HT) along with HFD feeding [173], which warrants the assessment of UPRmt markers. Furthermore, the above strategy may also be important in the treatment of the more severe condition of NASH, since NASH patients subjected to a mixture of n-3 LCPUFAS (α-linolenic acid (ALA), EPA and DHA) for 6 months exhibit improvement of mitochondrial function with induction of the chaperone HSP60, in addition to attenuation of hepatic lipogenesis and ER stress [179].
In the scenario of obesity-induced NAFLD, the liver develops not only UPRer and mitochondrial dysfunction [170] but also physical communications between ER and mitochondria are established, defined as mitochondrial associated ER membranes (MAMs) [180], which are crucial in determining cell viability during nutrient overload. These MAMs are structural bridges that primarily exchange Ca2+, but also lipids and ROS, for which a structural and functional integrity is required to manifest normal ER-mitochondrial communications [181]. After disruption of MAM integrity, Ca2+ homeostasis is lost in association with UPRer, mitochondrial dysfunction, oxidative stress and IR, factors involved in NAFLD development and progression [181]. In this respect, genetic (ob/ob mice) and dietary (HFD) models of obesity showed reorganization of MAMs in the liver, with increased MAM formation resulting in higher Ca2+ flux from ER to mitochondria, enhanced oxidative stress and mitochondrial metabolic alterations [182]. Specifically, obesity upregulated the expression of proteins related to ER to mitochondria Ca2+ flux, namely, the Ca2+ channels IP3R1 and IP3R2, concomitantly with that of the ER-mitochondrial tethering protein PACS-2 [182]. Although normal links are necessary for adequate propagation of Ca2+ signals from ER to mitochondria, excessive MAM formation is deleterious to cells as in NAFLD, supporting the dependence of cell function and survival on the proper spacing between ER and mitochondria [183]. In this context, adenovirus-mediated knockdown of liver IP3R1 and PACS-2 improves mitochondrial function and metabolic performance pointing to MAM formation as a therapeutic target for NAFLD [182]. This could be approached with protocols abrogating mitochondrial dysfunction such as nicotinamide riboside [25] or DHA and HT co-administration [173] on future studies.
6. Conclusions
A thoughtful understanding of the cellular mechanisms underlying the physiological processes involved in sarcopenia and NAFLD is fundamental in order to develop therapeutic tools to attack the actual cause of the dysfunction. Despite this review being centered on the two aforementioned pathologies, the UPRmt seems to be related to many more, which is consistent with mitochondrial dysfunction as a common feature for diverse diseases. In fact, mitochondrial malfunctioning is present in various age-related pathologies. To unveil the precise mechanisms implicated would permit one to distinguish the failing step of the pathway, which would have profound clinical implications, because it might allow one to establish potential therapeutic targets. For example, increased levels of transcripts associated with UPRmt activation have been observed in patients with myopathy caused by mitochondrial disorders [184]. This observation is consistent with the activation of the UPRmt, but to be used as a therapeutic advantage is necessary to determine the mitochondrial-stress-induced transcription factors elicited in each pathological scenario, together with the effects of the transcriptional response over physiology.
Altogether, the above research suggests a major role of mitochondrial adaptations and removal in the pathogenesis of metabolic diseases (Table 1), nevertheless the results are still controversial, and the definitive mechanisms are far from being unveiled. In this context, sarcopenia and NAFLD exhibit pronounced mitochondrial dysfunction, thus compromising cell viability due to energy collapse. Mitochondrial dysfunction is associated with (i) mutations in genes encoding OXPHOS proteins and ATP synthase; (ii) oxidative stress due to excessive ROS generation that triggers the accumulation of damaged mtDNA, protein carbonylation and unfolding and phospholipid peroxidation particularly that of cardiolipin that destabilizes MRC complexes. Under these conditions, the canonical UPRmt axis is activated to promote cell survival and the recovery of the mitochondrial network to ensure optimal cellular functions. UPRmt activation underlying oxidative stress and ER stress conditions involves the phosphorylation of eIF2α by kinases GCN2 and PERK, phosphorylated eIF2α being able to attenuate global translation while favoring that of CHOP, ATF4 and ATF5. The latter transcription factors induce the expression of chaperones, proteases, antioxidant components limiting ROS toxicity, OXPHOS complex assembly factors and protein import components, which afford the recovery of mitochondrial functioning [5,12,20] (Figure 1). Besides UPRmt activation, mitophagy upregulation is also established to eliminate dysfunctional or altered mitochondria via PINK1/Parkin and BNIP3/NIX pathways. Although UPRmt activation and mitophagy provide protection under conditions of mild mitochondrial dysfunction underlying sarcopenia and NAFLD, future time-course studies are required to assess the effectiveness of strategies contributing to the recovery of proteostasis, since prolonged or dysregulated UPRmt activation is deleterious to the cell [5,12,23].
Abbreviations
| AICAR | 5-Aminoimidazole-4-carboxamide ribonucleotide |
| AMPK | AMP-activated protein kinase |
| Atg7 | Autophagy related protein 7 |
| Atg12 | Autophagy related protein 12 |
| ATP5α | ATP synthase subunit alpha |
| ATF-4 | Activating transcription factor 4 |
| ATF-5 | Activating transcription factor 5 |
| ATFS-1 | Activating transcription factor associated with stress |
| BAK | BCL2 homologous antagonist/killer |
| BAX | BCL2-like protein 4 |
| BNIP3 | BCL2 and adenovirus E1B 19-kDa-interacting protein 3 |
| BNIP3L | BNIP3-like |
| bZIP | Basic leucine zipper |
| C26 | Colon-26 carcinoma |
| CEBPβ | CCAAT/enhancer binding protein β |
| CFCM | Cross-fiber connection mitochondria |
| CHF | Congestive heart failure |
| CHOP | CEBP Homologous Protein |
| ClpP | Caseinolytic protease proteolytic subunit |
| ClpXP | ATP-dependent Clp protease ATP-binding subunit ClpX-like |
| COPD | Chronic obstructive pulmonary disease |
| Crif1 | CR6-interacting factor 1 |
| DHA | Docosahexaenoic acid |
| DNA | Deoxyribonucleic acid |
| Dve-1 | Homeobox domain-containing protein |
| eIF2α | Eukaryotic translation initiation factor 2 alpha |
| FA | Fatty acid |
| FGF21 | Fibroblast growth factor 21 |
| FPM | Fiber parallel mitochondria |
| GCN2 | General control non-derepressible 2 kinase |
| GDF15 | Growth differentiation factor 15 |
| Haf-1 | Matrix peptide exporter |
| HFD | High-fat diet |
| HFHS | High-fat and high-sucrose |
| HRI | Heme-regulated inhibitor kinase |
| Hsp10 | Heat shock protein 10 |
| Hsp60 | Heat shock protein 60 |
| Hsp90 | Heat shock protein 90 |
| HT | Hydroxytyrosol |
| IMM | Inner mitochondrial membrane |
| IMS | Intermembrane space |
| IP3R1 | Inositol 1,4,5-triphosphate receptor type 1 |
| IP3R2 | Inositol 1,4,5-triphosphate receptor type 2 |
| IRE1 | Inositol-requiring enzyme-1 |
| ISR | Integrated stress response |
| Jmjd-3.1 | Lysine-specific demethylase (JuMonJi Domain protein) |
| LC3 | Microtubule-associated protein 1 light chain 3 |
| LONP1 | Lon protease 1 |
| MAM | Mitochondrial associated ER membrane |
| Mfn-2 | Mitofusin 2 |
| MPP | Mitochondrial processing peptidase |
| MRC | Mitochondrial respiratory chain |
| MTCO1 | Mitochondrially encoded cytochrome c oxidase 1 |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| mtHsp70 | Mitochondrial heat shock protein 70 |
| MTS | Mitochondrial targeting sequence |
| LCPUFA | Long-chain polyunsaturated FA |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NRF-1 | Nuclear respiratory factor 1 |
| OMM | Outer mitochondrial membrane |
| OPTN | Optineurin protein |
| p62 | Classical receptor of autophagy |
| PACS-2 | Phosphofurin acidic cluster sorting protein 2 |
| PERK | Protein kinase RNA-like ER kinase |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PINK1 | PTEN-induced putative kinase 1 |
| PKR | Protein kinase RNA-activated |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PUFA | Polyunsaturated fatty acid |
| SIRT1 | Sirtuin 1 |
| SIRT3 | Sirtuin 3 |
| SREBP-1c | Sterol regulatory element-binding protein-1c |
| TIM | Translocase of the inner membrane |
| TNF-α | Tumor necrosis factor-alpha |
| TOM | Translocase of the outer membrane |
| VDAC | Voltage-dependent anion channel |
| XBP1 | X-box-binding protein-1 |
| Yme1L1 | Yme1-like 1 ATPase |
Author Contributions
N.C. searched for the articles; R.U.-V. wrote and reviewed some sections of the article and produced the table; L.A.V. and A.d.C. wrote, reviewed and corrected the article and produced the figures. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT In. 11190756 to AdC.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bereiter-Hahn J., Vöth M., Mai S., Jendrach M. Structural implications of mitochondrial dynamics. Biotechnol. J. 2008;3:765–780. doi: 10.1002/biot.200800024. [DOI] [PubMed] [Google Scholar]
- 2.Benard G., Rossignol R. Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid. Redox Signal. 2008;10:1313–1342. doi: 10.1089/ars.2007.2000. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi N., Ishihara N., Jofuku A., Oka T., Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 4.Duchen M.R. Mitochondria in health and disease: Perspectives on a new mitochondrial biology. Mol. Asp. Med. 2004;25:365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., Xu H. Translational regulation of mitochondrial biogenesis. Biochem. Soc. Trans. 2016;44:1717–1724. doi: 10.1042/BST20160071C. [DOI] [PubMed] [Google Scholar]
- 6.Dorn G.W., Vega R.B., Kelly D.P. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 2015;29:1981–1991. doi: 10.1101/gad.269894.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Münch C. The different axes of the mammalian mitochondrial unfolded protein response. BMC Biol. 2018;16:81. doi: 10.1186/s12915-018-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stadtman E.R. Metal ion-catalyzed oxidation of proteins: Biochemical mechanism and biological consequences. Free. Radic. Biol. Med. 1990;9:315–325. doi: 10.1016/0891-5849(90)90006-5. [DOI] [PubMed] [Google Scholar]
- 9.Reznick A.Z., Packer L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 10.Videla L.A., Fernández V., Cornejo P., Vargas R., Carrasco J., Fernández J., Varela N. Causal role of oxidative stress in unfolded protein response development in the hyperthyroid state. Free. Radic. Biol. Med. 2015;89:401–408. doi: 10.1016/j.freeradbiomed.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra J.D., Kaufman R.J. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi H.-S., Chang J.Y., Shong M. The mitochondrial unfolded protein response and mitohormesis: A perspective on metabolic diseases. J. Mol. Endocrinol. 2018;61:R91–R105. doi: 10.1530/JME-18-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malhi H., Kaufman R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shpilka T., Haynes C.M. The mitochondrial UPR: Mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 2018;19:109–120. doi: 10.1038/nrm.2017.110. [DOI] [PubMed] [Google Scholar]
- 15.Walter P., Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 16.Naresh N.U., Haynes C.M. Signaling and Regulation of the Mitochondrial Unfolded Protein Response. Cold Spring Harb. Perspect. Biol. 2019;11:a033944. doi: 10.1101/cshperspect.a033944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake J.C., Yan Z. Mitophagy in maintaining skeletal muscle mitochondrial proteostasis and metabolic health with ageing. J. Physiol. 2017;595:6391–6399. doi: 10.1113/JP274337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiesel F.C., James E.D., Hudec R., Springer W. Mitochondrial targeted HSP90 inhibitor Gamitrinib-TPP (G-TPP) induces PINK1/Parkin-dependent mitophagy. Oncotarget. 2017;8:106233–106248. doi: 10.18632/oncotarget.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quiles J.M., Gustafsson Å.B. Mitochondrial Quality Control and Cellular Proteostasis: Two Sides of the Same Coin. Front. Physiol. 2020;11:515. doi: 10.3389/fphys.2020.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngo J.K., Pomatto L.C.D., Davies K.J.A. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013;1:258–264. doi: 10.1016/j.redox.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordeiro A.V., Brícola R.S., Braga R.R., Lenhare L., Silva V.R.R., Anaruma C.P., Katashima C.K., Crisol B.M., Simabuco F.M., Silva A.S.R., et al. Aerobic Exercise Training Induces the Mitonuclear Imbalance and UPRmt in the Skeletal Muscle of Aged Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020 doi: 10.1093/gerona/glaa059. online ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Tamura Y., Matsunaga Y., Kitaoka Y., Hatta H. Effects of Heat Stress Treatment on Age-dependent Unfolded Protein Response in Different Types of Skeletal Muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72:299–308. doi: 10.1093/gerona/glw063. [DOI] [PubMed] [Google Scholar]
- 23.Memme J.M., Oliveira A.N., Hood D.A. Chronology of UPR activation in skeletal muscle adaptations to chronic contractile activity. Am. J. Physiol.-Cell Physiol. 2016;310:C1024–C1036. doi: 10.1152/ajpcell.00009.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Furoukh N., Ianni A., Nolte H., Hölper S., Krüger M., Wanrooij S., Braun T. ClpX stimulates the mitochondrial unfolded protein response (UPRmt) in mammalian cells. BBA — Mol. Cell Res. 2015;10:2580–2591. doi: 10.1016/j.bbamcr.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Gariani K., Menzies K.J., Ryu D., Wegner C.J., Wang X., Ropelle E.R., Moullan N., Zhang H., Perino A., Lemos V., et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatol. Baltim. Md. 2016;63:1190–1204. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quirós P.M., Prado M.A., Zamboni N., D’Amico D., Williams R.W., Finley D., Gygi S.P., Auwerx J. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J. Cell Biol. 2017;216:2027–2045. doi: 10.1083/jcb.201702058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiorese C.J., Schulz A.M., Lin Y.-F., Rosin N., Pellegrino M.W., Haynes C.M. The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr. Biol. CB. 2016;26:2037–2043. doi: 10.1016/j.cub.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett C.F., Vander Wende H., Simko M., Klum S., Barfield S., Choi H., Pineda V.V., Kaeberlein M. Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nat. Commun. 2014;5:3483. doi: 10.1038/ncomms4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel S., Canonne M., Arnould T., Renard P. Inhibition of mitochondrial genome expression triggers the activation of CHOP-10 by a cell signaling dependent on the integrated stress response but not the mitochondrial unfolded protein response. Mitochondrion. 2015;21:58–68. doi: 10.1016/j.mito.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Q., Wang J., Levichkin I.V., Stasinopoulos S., Ryan M.T., Hoogenraad N.J. A mitochondrial speci®c stress response in mammalian cells. EMBO J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen P.B., Yang J.S., Park Y. Adaptations of Skeletal Muscle Mitochondria to Obesity, Exercise, and Polyunsaturated Fatty Acids. Lipids. 2018;53:271–278. doi: 10.1002/lipd.12037. [DOI] [PubMed] [Google Scholar]
- 32.Chomentowski P., Coen P.M., Radiková Z., Goodpaster B.H., Toledo F.G.S. Skeletal muscle mitochondria in insulin resistance: Differences in intermyofibrillar versus subsarcolemmal subpopulations and relationship to metabolic flexibility. J. Clin. Endocrinol. Metab. 2011;96:494–503. doi: 10.1210/jc.2010-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan M.T., Hoogenraad N.J. Mitochondrial-nuclear communications. Annu. Rev. Biochem. 2007;76:701–722. doi: 10.1146/annurev.biochem.76.052305.091720. [DOI] [PubMed] [Google Scholar]
- 34.Manoli I., Alesci S., Blackman M.R., Su Y.A., Rennert O.M., Chrousos G.P. Mitochondria as key components of the stress response. Trends Endocrinol. Metab. TEM. 2007;18:190–198. doi: 10.1016/j.tem.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Melber A., Haynes C.M. UPRmt regulation and output: A stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018;28:281–295. doi: 10.1038/cr.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ting S.-Y., Yan N.L., Schilke B.A., Craig E.A. Dual interaction of scaffold protein Tim44 of mitochondrial import motor with channel-forming translocase subunit Tim23. eLife. 2017;6:e23609. doi: 10.7554/eLife.23609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mapa K., Sikor M., Kudryavtsev V., Waegemann K., Kalinin S., Seidel C.A.M., Neupert W., Lamb D.C., Mokranjac D. The conformational dynamics of the mitochondrial Hsp70 chaperone. Mol. Cell. 2010;38:89–100. doi: 10.1016/j.molcel.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Mokranjac D. How to get to the other side of the mitochondrial inner membrane—The protein import motor. Biol. Chem. 2020;401:723–736. doi: 10.1515/hsz-2020-0106. [DOI] [PubMed] [Google Scholar]
- 39.Caumont-Sarcos A., Moulin C., Poinot L., Guiard B., van der Laan M., Ieva R. Transmembrane Coordination of Preprotein Recognition and Motor Coupling by the Mitochondrial Presequence Receptor Tim50. Cell Rep. 2020;30:3092–3104. doi: 10.1016/j.celrep.2020.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Fischer M., Riemer J. The Mitochondrial Disulfide Relay System: Roles in Oxidative Protein Folding and Beyond. Int. J. Cell Biol. 2013;2013:1–12. doi: 10.1155/2013/742923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quirós P.M., Mottis A., Auwerx J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 2016;17:213–226. doi: 10.1038/nrm.2016.23. [DOI] [PubMed] [Google Scholar]
- 42.Pakos-Zebrucka K., Koryga I., Mnich K., Ljujic M., Samali A., Gorman A.M. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teske B.F., Fusakio M.E., Zhou D., Shan J., McClintick J.N., Kilberg M.S., Wek R.C. CHOP induces activating transcription factor 5 (ATF5) to trigger apoptosis in response to perturbations in protein homeostasis. Mol. Biol. Cell. 2013;24:2477–2490. doi: 10.1091/mbc.e13-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker B.M., Nargund A.M., Sun T., Haynes C.M. Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2. PLoS Genet. 2012;8:e1002760. doi: 10.1371/journal.pgen.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbosa C., Peixeiro I., Romão L. Gene expression regulation by upstream open reading frames and human disease. PLoS Genet. 2013;9:e1003529. doi: 10.1371/journal.pgen.1003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou D., Palam L.R., Jiang L., Narasimhan J., Staschke K.A., Wek R.C. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J. Biol. Chem. 2008;283:7064–7073. doi: 10.1074/jbc.M708530200. [DOI] [PubMed] [Google Scholar]
- 47.Vattem K.M., Wek R.C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bezawork-Geleta A., Brodie E.J., Dougan D.A., Truscott K.N. LON is the master protease that protects against protein aggregation in human mitochondria through direct degradation of misfolded proteins. Sci. Rep. 2015;5:17397. doi: 10.1038/srep17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Münch C., Harper J.W. Mitochondrial unfolded protein response controls matrix pre-RNA processing and translation. Nature. 2016;534:710–713. doi: 10.1038/nature18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker M.J., Tatsuta T., Langer T. Quality control of mitochondrial proteostasis. Cold Spring Harb. Perspect. Biol. 2011;3:a007559. doi: 10.1101/cshperspect.a007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka A., Cleland M.M., Xu S., Narendra D.P., Suen D.-F., Karbowski M., Youle R.J. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palikaras K., Lionaki E., Tavernarakis N. Coupling mitogenesis and mitophagy for longevity. Autophagy. 2015;11:1428–1430. doi: 10.1080/15548627.2015.1061172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fivenson E.M., Lautrup S., Sun N., Scheibye-Knudsen M., Stevnsner T., Nilsen H., Bohr V.A., Fang E.F. Mitophagy in neurodegeneration and aging. Neurochem. Int. 2017;109:202–209. doi: 10.1016/j.neuint.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei H., Liu L., Chen Q. Selective removal of mitochondria via mitophagy: Distinct pathways for different mitochondrial stresses. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2015;1853:2784–2790. doi: 10.1016/j.bbamcr.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 55.Lazarou M., Sliter D.A., Kane L.A., Sarraf S.A., Wang C., Burman J.L., Sideris D.P., Fogel A.I., Youle R.J. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McWilliams T.G., Prescott A.R., Montava-Garriga L., Ball G., Singh F., Barini E., Muqit M.M.K., Brooks S.P., Ganley I.G. Basal Mitophagy Occurs Independently of PINK1 in Mouse Tissues of High Metabolic Demand. Cell Metab. 2018;27:439–449. doi: 10.1016/j.cmet.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kubli D.A., Ycaza J.E., Gustafsson A.B. Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem. J. 2007;405:407–415. doi: 10.1042/BJ20070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang T., Xue L., Li L., Tang C., Wan Z., Wang R., Tan J., Tan Y., Han H., Tian R., et al. BNIP3 Protein Suppresses PINK1 Kinase Proteolytic Cleavage to Promote Mitophagy. J. Biol. Chem. 2016;291:21616–21629. doi: 10.1074/jbc.M116.733410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gan Z., Fu T., Kelly D.P., Vega R.B. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res. 2018;28:969–980. doi: 10.1038/s41422-018-0078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roger A.J., Muñoz-Gómez S.A., Kamikawa R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017;27:R1177–R1192. doi: 10.1016/j.cub.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Franzini-Armstrong C., Boncompagni S. The Evolution of the Mitochondria-to-Calcium Release Units Relationship in Vertebrate Skeletal Muscles. [(accessed on 14 August 2020)]; doi: 10.1155/2011/830573. Available online: https://www.hindawi.com/journals/bmri/2011/830573/ [DOI] [PMC free article] [PubMed]
- 62.Glancy B., Hartnell L.M., Malide D., Yu Z.-X., Combs C.A., Connelly P.S., Subramaniam S., Balaban R.S. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 2015;523:617–620. doi: 10.1038/nature14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riva A., Tandler B., Loffredo F., Vazquez E., Hoppel C. Structural differences in two biochemically defined populations of cardiac mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H868–H872. doi: 10.1152/ajpheart.00866.2004. [DOI] [PubMed] [Google Scholar]
- 64.Koves T.R., Noland R.C., Bates A.L., Henes S.T., Muoio D.M., Cortright R.N. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am. J. Physiol. Cell Physiol. 2005;288:C1074–C1082. doi: 10.1152/ajpcell.00391.2004. [DOI] [PubMed] [Google Scholar]
- 65.Ferreira R., Vitorino R., Alves R.M.P., Appell H.J., Powers S.K., Duarte J.A., Amado F. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics. 2010;10:3142–3154. doi: 10.1002/pmic.201000173. [DOI] [PubMed] [Google Scholar]
- 66.Rantanen T., Harris T., Leveille S.G., Visser M., Foley D., Masaki K., Guralnik J.M. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:M168–M173. doi: 10.1093/gerona/55.3.M168. [DOI] [PubMed] [Google Scholar]
- 67.Metter E.J., Talbot L.A., Schrager M., Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:B359–B365. doi: 10.1093/gerona/57.10.B359. [DOI] [PubMed] [Google Scholar]
- 68.Nair K.S. Aging muscle. Am. J. Clin. Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 69.Goodpaster B.H., Park S.W., Harris T.B., Kritchevsky S.B., Nevitt M., Schwartz A.V., Simonsick E.M., Tylavsky F.A., Visser M., Newman A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 70.Chabi B., Ljubicic V., Menzies K.J., Huang J.H., Saleem A., Hood D.A. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 71.Joseph A.-M., Adhihetty P.J., Buford T.W., Wohlgemuth S.E., Lees H.A., Nguyen L.M.-D., Aranda J.M., Sandesara B.D., Pahor M., Manini T.M., et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11:801–809. doi: 10.1111/j.1474-9726.2012.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kruse S.E., Karunadharma P.P., Basisty N., Johnson R., Beyer R.P., MacCoss M.J., Rabinovitch P.S., Marcinek D.J. Age modifies respiratory complex I and protein homeostasis in a muscle type-specific manner. Aging Cell. 2016;15:89–99. doi: 10.1111/acel.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marcinek D.J., Schenkman K.A., Ciesielski W.A., Lee D., Conley K.E. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J. Physiol. 2005;569:467–473. doi: 10.1113/jphysiol.2005.097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenberg I.H. Sarcopenia: Origins and clinical relevance. Clin. Geriatr. Med. 2011;27:337–339. doi: 10.1016/j.cger.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Short K.R., Bigelow M.L., Kahl J., Singh R., Coenen-Schimke J., Raghavakaimal S., Nair K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hughes V.A., Frontera W.R., Wood M., Evans W.J., Dallal G.E., Roubenoff R., Fiatarone Singh M.A. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:B209–B217. doi: 10.1093/gerona/56.5.B209. [DOI] [PubMed] [Google Scholar]
- 77.Ferri E., Marzetti E., Calvani R., Picca A., Cesari M., Arosio B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020;21:5236. doi: 10.3390/ijms21155236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sayed R.K.A., de Leonardis E.C., Guerrero-Martínez J.A., Rahim I., Mokhtar D.M., Saleh A.M., Abdalla K.E.H., Pozo M.J., Escames G., López L.C., et al. Identification of morphological markers of sarcopenia at early stage of aging in skeletal muscle of mice. Exp. Gerontol. 2016;83:22–30. doi: 10.1016/j.exger.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Del Campo A., Contreras-Hernández I., Castro-Sepúlveda M., Campos C.A., Figueroa R., Tevy M.F., Eisner V., Casas M., Jaimovich E. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging. 2018;10:34–55. doi: 10.18632/aging.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang D.-D., Fan S.-D., Chen X.-Y., Yan X.-L., Zhang X.-Z., Ma B.-W., Yu D.-Y., Xiao W.-Y., Zhuang C.-L., Yu Z. Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp. Gerontol. 2019;119:61–73. doi: 10.1016/j.exger.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 81.Kitaoka Y., Tamura Y., Takahashi K., Takeda K., Takemasa T., Hatta H. Effects of Nrf2 deficiency on mitochondrial oxidative stress in aged skeletal muscle. Physiol. Rep. 2019;7:e13998. doi: 10.14814/phy2.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dillon L.M., Williams S.L., Hida A., Peacock J.D., Prolla T.A., Lincoln J., Moraes C.T. Increased mitochondrial biogenesis in muscle improves aging phenotypes in the mtDNA mutator mouse. Hum. Mol. Genet. 2012;21:2288–2297. doi: 10.1093/hmg/dds049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Picard M., Ritchie D., Thomas M.M., Wright K.J., Hepple R.T. Alterations in intrinsic mitochondrial function with aging are fiber type-specific and do not explain differential atrophy between muscles. Aging Cell. 2011;10:1047–1055. doi: 10.1111/j.1474-9726.2011.00745.x. [DOI] [PubMed] [Google Scholar]
- 84.Gouspillou G., Sgarioto N., Kapchinsky S., Purves-Smith F., Norris B., Pion C.H., Barbat-Artigas S., Lemieux F., Taivassalo T., Morais J.A., et al. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J. 2014;28:1621–1633. doi: 10.1096/fj.13-242750. [DOI] [PubMed] [Google Scholar]
- 85.Tamilselvan J., Jayaraman G., Sivarajan K., Panneerselvam C. Age-dependent upregulation of p53 and cytochrome c release and susceptibility to apoptosis in skeletal muscle fiber of aged rats: Role of carnitine and lipoic acid. Free Radic. Biol. Med. 2007;43:1656–1669. doi: 10.1016/j.freeradbiomed.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 86.Zhou J., Yun Chong S., Lim A., Singh B.K., Sinha R.A., Salmon A.B., Yen P.M. Changes in macroautophagy, chaperone-mediated autophagy, and mitochondrial metabolism in murine skeletal and cardiac muscle during aging. Aging. 2017;9:583–596. doi: 10.18632/aging.101181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng Y., Yao Z., Klionsky D.J. How to control self-digestion: Transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 2015;25:354–363. doi: 10.1016/j.tcb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Masiero E., Agatea L., Mammucari C., Blaauw B., Loro E., Komatsu M., Metzger D., Reggiani C., Schiaffino S., Sandri M. Autophagy Is Required to Maintain Muscle Mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 89.Baehr L.M., West D.W.D., Marcotte G., Marshall A.G., De Sousa L.G., Baar K., Bodine S.C. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging. 2016;8:127–146. doi: 10.18632/aging.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Joseph A.-M., Adhihetty P.J., Wawrzyniak N.R., Wohlgemuth S.E., Picca A., Kujoth G.C., Prolla T.A., Leeuwenburgh C. Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PLoS ONE. 2013;8:e69327. doi: 10.1371/journal.pone.0069327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bujak A.L., Crane J.D., Lally J.S., Ford R.J., Kang S.J., Rebalka I.A., Green A.E., Kemp B.E., Hawke T.J., Schertzer J.D., et al. AMPK activation of muscle autophagy prevents fasting-induced hypoglycemia and myopathy during aging. Cell Metab. 2015;21:883–890. doi: 10.1016/j.cmet.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He C., Bassik M.C., Moresi V., Sun K., Wei Y., Zou Z., An Z., Loh J., Fisher J., Sun Q., et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vainshtein A., Grumati P., Sandri M., Bonaldo P. Skeletal muscle, autophagy, and physical activity: The ménage à trois of metabolic regulation in health and disease. J. Mol. Med. Berl. Ger. 2014;92:127–137. doi: 10.1007/s00109-013-1096-z. [DOI] [PubMed] [Google Scholar]
- 94.Pigna E., Berardi E., Aulino P., Rizzuto E., Zampieri S., Carraro U., Kern H., Merigliano S., Gruppo M., Mericskay M., et al. Aerobic Exercise and Pharmacological Treatments Counteract Cachexia by Modulating Autophagy in Colon Cancer. Sci. Rep. 2016;6:26991. doi: 10.1038/srep26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Drummond M.J., Addison O., Brunker L., Hopkins P.N., McClain D.A., LaStayo P.C., Marcus R.L. Downregulation of E3 Ubiquitin Ligases and Mitophagy-Related Genes in Skeletal Muscle of Physically Inactive, Frail Older Women: A Cross-Sectional Comparison. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:1040–1048. doi: 10.1093/gerona/glu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim Y., Triolo M., Hood D.A. Impact of Aging and Exercise on Mitochondrial Quality Control in Skeletal Muscle. Oxidative Med. Cell. Longev. 2017;2017:1–16. doi: 10.1155/2017/3165396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zampieri S., Pietrangelo L., Loefler S., Fruhmann H., Vogelauer M., Burggraf S., Pond A., Grim-Stieger M., Cvecka J., Sedliak M., et al. Lifelong physical exercise delays age-associated skeletal muscle decline. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:163–173. doi: 10.1093/gerona/glu006. [DOI] [PubMed] [Google Scholar]
- 98.Stanford K.I., Goodyear L.J. Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle. Adv. Physiol. Educ. 2014;38:308–314. doi: 10.1152/advan.00080.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maltais F., Decramer M., Casaburi R., Barreiro E., Burelle Y., Debigaré R., Dekhuijzen P.N.R., Franssen F., Gayan-Ramirez G., Gea J., et al. An Official American Thoracic Society/European Respiratory Society Statement: Update on Limb Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2014;189:e15–e62. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kinugawa S., Takada S., Matsushima S., Okita K., Tsutsui H. Skeletal Muscle Abnormalities in Heart Failure. Int. Heart. J. 2015;56:475–484. doi: 10.1536/ihj.15-108. [DOI] [PubMed] [Google Scholar]
- 101.van de Bool C., Gosker H.R., van den Borst B., Op den Kamp C.M., Slot I.G.M., Schols A.M.W.J. Muscle Quality is More Impaired in Sarcopenic Patients With Chronic Obstructive Pulmonary Disease. J. Am. Med. Dir. Assoc. 2016;17:415–420. doi: 10.1016/j.jamda.2015.12.094. [DOI] [PubMed] [Google Scholar]
- 102.Huffman K.M., Koves T.R., Hubal M.J., Abouassi H., Beri N., Bateman L.A., Stevens R.D., Ilkayeva O.R., Hoffman E.P., Muoio D.M., et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia. 2014;57:2282–2295. doi: 10.1007/s00125-014-3343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guo Y., Gosker H.R., Schols A.M.W.J., Kapchinsky S., Bourbeau J., Sandri M., Jagoe R.T., Debigaré R., Maltais F., Taivassalo T., et al. Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;188:1313–1320. doi: 10.1164/rccm.201304-0732OC. [DOI] [PubMed] [Google Scholar]
- 104.Kruse R., Vind B.F., Petersson S.J., Kristensen J.M., Højlund K. Markers of autophagy are adapted to hyperglycaemia in skeletal muscle in type 2 diabetes. Diabetologia. 2015;58:2087–2095. doi: 10.1007/s00125-015-3654-0. [DOI] [PubMed] [Google Scholar]
- 105.Sakellariou G.K., Pearson T., Lightfoot A.P., Nye G.A., Wells N., Giakoumaki I.I., Vasilaki A., Griffiths R.D., Jackson M.J., McArdle A. Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci. Rep. 2016;6:33944. doi: 10.1038/srep33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gouspillou G., Godin R., Piquereau J., Picard M., Mofarrahi M., Mathew J., Purves-Smith F.M., Sgarioto N., Hepple R.T., Burelle Y., et al. Protective role of Parkin in skeletal muscle contractile and mitochondrial function: Parkin is essential for optimal muscle and mitochondrial functions. J. Physiol. 2018;596:2565–2579. doi: 10.1113/JP275604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peker N., Donipadi V., Sharma M., McFarlane C., Kambadur R. Loss of Parkin impairs mitochondrial function and leads to muscle atrophy. Am. J. Physiol.-Cell Physiol. 2018;315:C164–C185. doi: 10.1152/ajpcell.00064.2017. [DOI] [PubMed] [Google Scholar]
- 108.Sebastián D., Sorianello E., Segalés J., Irazoki A., Ruiz-Bonilla V., Sala D., Planet E., Berenguer-Llergo A., Muñoz J.P., Sánchez-Feutrie M., et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016;35:1677–1693. doi: 10.15252/embj.201593084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guzmán Mentesana G., Báez A.L., Lo Presti M.S., Domínguez R., Córdoba R., Bazán C., Strauss M., Fretes R., Rivarola H.W., Paglini-Oliva P. Functional and Structural Alterations of Cardiac and Skeletal Muscle Mitochondria in Heart Failure Patients. Arch. Med. Res. 2014;45:237–246. doi: 10.1016/j.arcmed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 110.Kelley D.E., He J., Menshikova E.V., Ritov V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 111.Bach D., Naon D., Pich S., Soriano F.X., Vega N., Rieusset J., Laville M., Guillet C., Boirie Y., Wallberg-Henriksson H., et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: Effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–2693. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 112.Garnier A., Fortin D., Zoll J., N’Guessan B., Mettauer B., Lampert E., Veksler V., Ventura-Clapier R. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005;19:43–52. doi: 10.1096/fj.04-2173com. [DOI] [PubMed] [Google Scholar]
- 113.Molina A.J.A., Bharadwaj M.S., Van Horn C., Nicklas B.J., Lyles M.F., Eggebeen J., Haykowsky M.J., Brubaker P.H., Kitzman D.W. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients with Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail. 2016;4:636–645. doi: 10.1016/j.jchf.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Romanello V., Guadagnin E., Gomes L., Roder I., Sandri C., Petersen Y., Milan G., Masiero E., Del Piccolo P., Foretz M., et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010;29:1774–1785. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ibebunjo C., Chick J.M., Kendall T., Eash J.K., Li C., Zhang Y., Vickers C., Wu Z., Clarke B.A., Shi J., et al. Genomic and Proteomic Profiling Reveals Reduced Mitochondrial Function and Disruption of the Neuromuscular Junction Driving Rat Sarcopenia. Mol. Cell. Biol. 2013;33:194–212. doi: 10.1128/MCB.01036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Twig G., Elorza A., Molina A.J.A., Mohamed H., Wikstrom J.D., Walzer G., Stiles L., Haigh S.E., Katz S., Las G., et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leduc-Gaudet J.-P., Picard M., St-Jean Pelletier F., Sgarioto N., Auger M.-J., Vallée J., Robitaille R., St-Pierre D.H., Gouspillou G. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget. 2015;6:17923–17937. doi: 10.18632/oncotarget.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leermakers P.A., Gosker H.R. Skeletal muscle mitophagy in chronic disease: Implications for muscle oxidative capacity? Curr. Opin. Clin. Nutr. Metab. Care. 2016;19:427–433. doi: 10.1097/MCO.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 119.Schulz A.M., Haynes C.M. UPR(mt)-mediated cytoprotection and organismal aging. Biochim. Biophys. Acta. 2015;1847:1448–1456. doi: 10.1016/j.bbabio.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ben-Meir A., Yahalomi S., Moshe B., Shufaro Y., Reubinoff B., Saada A. Coenzyme Q-dependent mitochondrial respiratory chain activity in granulosa cells is reduced with aging. Fertil. Steril. 2015;104:724–727. doi: 10.1016/j.fertnstert.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 121.Duarte J.M.N., Schuck P.F., Wenk G.L., Ferreira G.C. Metabolic disturbances in diseases with neurological involvement. Aging Dis. 2014;5:238–255. doi: 10.14336/AD.2014.0500238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nargund A.M., Pellegrino M.W., Fiorese C.J., Baker B.M., Haynes C.M. Mitochondrial Import Efficiency of ATFS-1 Regulates Mitochondrial UPR Activation. Science. 2012;337:587–590. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nargund A.M., Fiorese C.J., Pellegrino M.W., Deng P., Haynes C.M. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt) Mol. Cell. 2015;58:123–133. doi: 10.1016/j.molcel.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chung H.K., Ryu D., Kim K.S., Chang J.Y., Kim Y.K., Yi H.-S., Kang S.G., Choi M.J., Lee S.E., Jung S.-B., et al. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J. Cell Biol. 2017;216:149–165. doi: 10.1083/jcb.201607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Copeland J.M., Cho J., Lo T., Hur J.H., Bahadorani S., Arabyan T., Rabie J., Soh J., Walker D.W. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr. Biol. CB. 2009;19:1591–1598. doi: 10.1016/j.cub.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 126.Durieux J., Wolff S., Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tian Y., Garcia G., Bian Q., Steffen K.K., Joe L., Wolff S., Meyer B.J., Dillin A. Mitochondrial Stress Induces Chromatin Reorganization to Promote Longevity and UPR(mt) Cell. 2016;165:1197–1208. doi: 10.1016/j.cell.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin Y.-F., Schulz A.M., Pellegrino M.W., Lu Y., Shaham S., Haynes C.M. Maintenance and propagation of a deleterious mitochondrial genome by the mitochondrial unfolded protein response. Nature. 2016;533:416–419. doi: 10.1038/nature17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wallace D.C., Chalkia D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb. Perspect. Biol. 2013;5:a021220. doi: 10.1101/cshperspect.a021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gitschlag B.L., Kirby C.S., Samuels D.C., Gangula R.D., Mallal S.A., Patel M.R. Homeostatic Responses Regulate Selfish Mitochondrial Genome Dynamics in C. elegans. Cell Metab. 2016;24:91–103. doi: 10.1016/j.cmet.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khan N.A., Nikkanen J., Yatsuga S., Jackson C., Wang L., Pradhan S., Kivelä R., Pessia A., Velagapudi V., Suomalainen A. mTORC1 Regulates Mitochondrial Integrated Stress Response and Mitochondrial Myopathy Progression. Cell Metab. 2017;26:419–428. doi: 10.1016/j.cmet.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 132.Tyynismaa H., Mjosund K.P., Wanrooij S., Lappalainen I., Ylikallio E., Jalanko A., Spelbrink J.N., Paetau A., Suomalainen A. Mutant mitochondrial helicase Twinkle causes multiple mtDNA deletions and a late-onset mitochondrial disease in mice. Proc. Natl. Acad. Sci. USA. 2005;102:17687–17692. doi: 10.1073/pnas.0505551102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gilkerson R.W., De Vries R.L.A., Lebot P., Wikstrom J.D., Torgyekes E., Shirihai O.S., Przedborski S., Schon E.A. Mitochondrial autophagy in cells with mtDNA mutations results from synergistic loss of transmembrane potential and mTORC1 inhibition. Hum. Mol. Genet. 2012;21:978–990. doi: 10.1093/hmg/ddr529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ogborn D.I., McKay B.R., Crane J.D., Parise G., Tarnopolsky M.A. The unfolded protein response is triggered following a single, unaccustomed resistance-exercise bout. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014;307:R664–R669. doi: 10.1152/ajpregu.00511.2013. [DOI] [PubMed] [Google Scholar]
- 135.Kim K., Kim Y.-H., Lee S.-H., Jeon M.-J., Park S.-Y., Doh K.-O. Effect of exercise intensity on unfolded protein response in skeletal muscle of rat. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014;18:211–216. doi: 10.4196/kjpp.2014.18.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Passos E., Ascensão A., Martins M.J., Magalhães J. Endoplasmic Reticulum Stress Response in Non-alcoholic Steatohepatitis: The Possible Role of Physical Exercise. Metabolism. 2015;64:780–792. doi: 10.1016/j.metabol.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 137.Pereira B.C., da Rocha A.L., Pinto A.P., Pauli J.R., de Souza C.T., Cintra D.E., Ropelle E.R., de Freitas E.C., Zagatto A.M., da Silva A.S.R. Excessive eccentric exercise-induced overtraining model leads to endoplasmic reticulum stress in mice skeletal muscles. Life Sci. 2016;145:144–151. doi: 10.1016/j.lfs.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 138.Wu J., Ruas J.L., Estall J.L., Rasbach K.A., Choi J.H., Ye L., Boström P., Tyra H.M., Crawford R.W., Campbell K.P., et al. The Unfolded Protein Response Mediates Adaptation to Exercise in Skeletal Muscle through a PGC-1α/ATF6α Complex. Cell Metab. 2011;13:160–169. doi: 10.1016/j.cmet.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mouchiroud L., Houtkooper R.H., Moullan N., Katsyuba E., Ryu D., Cantó C., Mottis A., Jo Y.-S., Viswanathan M., Schoonjans K., et al. The NAD+/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang H., Ryu D., Wu Y., Gariani K., Wang X., Luan P., DAmico D., Ropelle E.R., Lutolf M.P., Aebersold R., et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 141.Thoma A., Akter-Miah T., Reade R.L., Lightfoot A.P. Targeting reactive oxygen species (ROS) to combat the age-related loss of muscle mass and function. Biogerontology. 2020;21:475–484. doi: 10.1007/s10522-020-09883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morley J.E. Pharmacologic Options for the Treatment of Sarcopenia. Calcif. Tissue Int. 2016;98:319–333. doi: 10.1007/s00223-015-0022-5. [DOI] [PubMed] [Google Scholar]
- 143.Kim K.H., Jeong Y.T., Oh H., Kim S.H., Cho J.M., Kim Y.-N., Kim S.S., Kim D.H., Hur K.Y., Kim H.K., et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 144.Li Y., Ruan D.-Y., Jia C.-C., Zheng J., Wang G.-Y., Zhao H., Yang Q., Liu W., Yi S.-H., Li H., et al. Aging aggravates hepatic ischemia-reperfusion injury in mice by impairing mitophagy with the involvement of the EIF2α-parkin pathway. Aging. 2018;10:1902–1920. doi: 10.18632/aging.101511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou H., Du W., Li Y., Shi C., Hu N., Ma S., Wang W., Ren J. Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J. Pineal Res. 2018;64:e12450. doi: 10.1111/jpi.12450. [DOI] [PubMed] [Google Scholar]
- 146.Lin C.-W., Zhang H., Li M., Xiong X., Chen X., Chen X., Dong X.C., Yin X.-M. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J. Hepatol. 2013;58:993–999. doi: 10.1016/j.jhep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu X., Hao M., Liu Y., Ma X., Lin W., Xu Q., Zhou H., Shao N., Kuang H. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy. Eur. J. Pharmacol. 2019;864:172715. doi: 10.1016/j.ejphar.2019.172715. [DOI] [PubMed] [Google Scholar]
- 148.Li X., Shi Z., Zhu Y., Shen T., Wang H., Shui G., Loor J.J., Fang Z., Chen M., Wang X., et al. Cyanidin-3-O-glucoside improves non-alcoholic fatty liver disease by promoting PINK1-mediated mitophagy in mice. Br. J. Pharmacol. 2020;177:3591–3607. doi: 10.1111/bph.15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li R., Xin T., Li D., Wang C., Zhu H., Zhou H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018;18:229–243. doi: 10.1016/j.redox.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gao A., Jiang J., Xie F., Chen L. Bnip3 in mitophagy: Novel insights and potential therapeutic target for diseases of secondary mitochondrial dysfunction. Clin. Chim. Acta Int. J. Clin. Chem. 2020;506:72–83. doi: 10.1016/j.cca.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 151.Luukkonen P.K., Sädevirta S., Zhou Y., Kayser B., Ali A., Ahonen L., Lallukka S., Pelloux V., Gaggini M., Jian C., et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care. 2018;41:1732–1739. doi: 10.2337/dc18-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oteng A.-B., Loregger A., van Weeghel M., Zelcer N., Kersten S. Industrial Trans Fatty Acids Stimulate SREBP2-Mediated Cholesterogenesis and Promote Non-Alcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019;63:e1900385. doi: 10.1002/mnfr.201900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ouyang X., Cirillo P., Sautin Y., McCall S., Bruchette J.L., Diehl A.M., Johnson R.J., Abdelmalek M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Valenzuela R., Videla L.A. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011;2:644–648. doi: 10.1039/c1fo10133a. [DOI] [PubMed] [Google Scholar]
- 155.Videla L.A., Rodrigo R., Orellana M., Fernandez V., Tapia G., Quiñones L., Varela N., Contreras J., Lazarte R., Csendes A., et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin. Sci. Lond. Engl. 1979. 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 156.Yang S., Zhu H., Li Y., Lin H., Gabrielson K., Trush M.A., Diehl A.M. Mitochondrial adaptations to obesity-related oxidant stress. Arch. Biochem. Biophys. 2000;378:259–268. doi: 10.1006/abbi.2000.1829. [DOI] [PubMed] [Google Scholar]
- 157.Aronis A., Madar Z., Tirosh O. Mechanism underlying oxidative stress-mediated lipotoxicity: Exposure of J774.2 macrophages to triacylglycerols facilitates mitochondrial reactive oxygen species production and cellular necrosis. Free. Radic. Biol. Med. 2005;38:1221–1230. doi: 10.1016/j.freeradbiomed.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 158.Videla L.A., Rodrigo R., Araya J., Poniachik J. Insulin resistance and oxidative stress interdependency in non-alcoholic fatty liver disease. Trends Mol. Med. 2006;12:555–558. doi: 10.1016/j.molmed.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 159.Tsukamoto H. Redox regulation of cytokine expression in Kupffer cells. Antioxid. Redox Signal. 2002;4:741–748. doi: 10.1089/152308602760598882. [DOI] [PubMed] [Google Scholar]
- 160.Malaguarnera L., Rosa M.D., Zambito A.M., dell’Ombra N., Nicoletti F., Malaguarnera M. Chitotriosidase gene expression in Kupffer cells from patients with non-alcoholic fatty liver disease. Gut. 2006;55:1313–1320. doi: 10.1136/gut.2005.075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Orellana M., Rodrigo R., Varela N., Araya J., Poniachik J., Csendes A., Smok G., Videla L.A. Relationship between in vivo chlorzoxazone hydroxylation, hepatic cytochrome P450 2E1 content and liver injury in obese non-alcoholic fatty liver disease patients. Hepatol. Res. 2006;34:57–63. doi: 10.1016/j.hepres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 162.Weltman M.D., Farrell G.C., Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/S0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 163.Caro A.A., Cederbaum A.I. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu. Rev. Pharmacol. Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 164.Seki S., Kitada T., Yamada T., Sakaguchi H., Nakatani K., Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J. Hepatol. 2002;37:56–62. doi: 10.1016/S0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 165.Valenzuela R., Espinosa A., González-Mañán D., D’Espessailles A., Fernández V., Videla L.A., Tapia G. N-3 long-chain polyunsaturated fatty acid supplementation significantly reduces liver oxidative stress in high fat induced steatosis. PLoS ONE. 2012;7:e46400. doi: 10.1371/journal.pone.0046400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gentile C.L., Frye M.A., Pagliassotti M.J. Fatty acids and the endoplasmic reticulum in nonalcoholic fatty liver disease. BioFactors Oxf. Engl. 2011;37:8–16. doi: 10.1002/biof.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang X.-Q., Xu C.-F., Yu C.-H., Chen W.-X., Li Y.-M. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20:1768–1776. doi: 10.3748/wjg.v20.i7.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Leamy A.K., Egnatchik R.A., Young J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res. 2013;52:165–174. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yi H.S. Implications of Mitochondrial Unfolded Protein Response and Mitokines: A Perspective on Fatty Liver Diseases. Endocrinol. Metab. Seoul Korea. 2019;34:39–46. doi: 10.3803/EnM.2019.34.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wei Y., Rector R.S., Thyfault J.P., Ibdah J.A. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J. Gastroenterol. 2008;14:193–199. doi: 10.3748/wjg.14.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Paradies G., Paradies V., Ruggiero F.M., Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20:14205–14218. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Begriche K., Igoudjil A., Pessayre D., Fromenty B. Mitochondrial dysfunction in NASH: Causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 173.Ortiz M., Soto-Alarcón S.A., Orellana P., Espinosa A., Campos C., López-Arana S., Rincón M.A., Illesca P., Valenzuela R., Videla L.A. Suppression of high-fat diet-induced obesity-associated liver mitochondrial dysfunction by docosahexaenoic acid and hydroxytyrosol co-administration. Dig. Liver Dis. 2020;52:895–904. doi: 10.1016/j.dld.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 174.Teodoro J.S., Duarte F.V., Gomes A.P., Varela A.T., Peixoto F.M., Rolo A.P., Palmeira C.M. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: A possible role for SirT3 activation. Mitochondrion. 2013;13:637–646. doi: 10.1016/j.mito.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 175.Lohr K., Pachl F., Moghaddas Gholami A., Geillinger K.E., Daniel H., Kuster B., Klingenspor M. Reduced mitochondrial mass and function add to age-related susceptibility toward diet-induced fatty liver in C57BL/6J mice. Physiol. Rep. 2016;4:e12988. doi: 10.14814/phy2.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jäger S., Handschin C., St.-Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sugden M.C., Caton P.W., Holness M.J. PPAR control: It’s SIRTainly as easy as PGC. J. Endocrinol. 2010;204:93–104. doi: 10.1677/JOE-09-0359. [DOI] [PubMed] [Google Scholar]
- 178.Puigserver P., Spiegelman B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 179.Okada L.S.D.R.R., Oliveira C.P., Stefano J.T., Nogueira M.A., da Silva I.D.C.G., Cordeiro F.B., Alves V.A.F., Torrinhas R.S., Carrilho F.J., Puri P., et al. Omega-3 PUFA modulate lipogenesis, ER stress, and mitochondrial dysfunction markers in NASH—Proteomic and lipidomic insight. Clin. Nutr. 2018;37:1474–1484. doi: 10.1016/j.clnu.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 180.Rowland A.A., Voeltz G.K. Endoplasmic reticulum–mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012;13:607–615. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang J., He W., Tsai P.-J., Chen P.-H., Ye M., Guo J., Su Z. Mutual interaction between endoplasmic reticulum and mitochondria in nonalcoholic fatty liver disease. Lipids Health Dis. 2020;19:72. doi: 10.1186/s12944-020-01210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Arruda A.P., Pers B.M., Parlakgül G., Güney E., Inouye K., Hotamisligil G.S. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat. Med. 2014;20:1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Csordás G., Renken C., Várnai P., Walter L., Weaver D., Buttle K.F., Balla T., Mannella C.A., Hajnóczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tyynismaa H., Carroll C.J., Raimundo N., Ahola-Erkkilä S., Wenz T., Ruhanen H., Guse K., Hemminki A., Peltola-Mjøsund K.E., Tulkki V., et al. Mitochondrial myopathy induces a starvation-like response. Hum. Mol. Genet. 2010;19:3948–3958. doi: 10.1093/hmg/ddq310. [DOI] [PubMed] [Google Scholar]