Abstract
Polymorphisms within the prion protein gene (Prnp) are an intrinsic factor that can modulate chronic wasting disease (CWD) pathogenesis in cervids. Although wild European reindeer (Rangifer tarandus tarandus) were infected with CWD, as yet there have been no reports of the disease in North American caribou (R. tarandus spp.). Previous Prnp genotyping studies on approximately 200 caribou revealed single nucleotide polymorphisms (SNPs) at codons 2 (V/M), 129 (G/S), 138 (S/N), 146 (N/n) and 169 (V/M). The impact of these polymorphisms on CWD transmission is mostly unknown, except for codon 138. Reindeer carrying at least one allele encoding for asparagine (138NN or 138SN) are less susceptible to clinical CWD upon infection by natural routes, with the majority of prions limited to extraneural tissues. We sequenced the Prnp coding region of two caribou subspecies (n = 986) from British Columbia, Saskatchewan, Yukon, Nunavut and the Northwest Territories, to identify SNPs and their frequencies. Genotype frequencies at codon 138 differed significantly between barren‐ground (R. t. groenlandicus) and woodland (R. t. caribou) caribou when we excluded the Chinchaga herd (p < .05). We also found new variants at codons 153 (Y/F) and 242 (P/L). Our findings show that the 138N allele is rare among caribou in areas with higher risk of contact with CWD‐infected species. As both subspecies are classified as Threatened and play significant roles in North American Indigenous culture, history, food security and the economy, determining frequencies of Prnp genotypes associated with susceptibility to CWD is important for future wildlife management measures.
Keywords: caribou, caribou conservation, chronic wasting disease, genotyping, prion protein
1. INTRODUCTION
Chronic wasting disease (CWD) was first discovered in 1967 in captive mule deer in Colorado (Williams & Young, 1980). Since then, the disease has spread to wild and farmed cervids in 26 US states and three Canadian provinces (https://www.usgs.gov/media/images/distribution‐chronic‐wasting‐disease‐north‐america‐0; Hannaoui, Schatzl, & Gilch, 2017). Recently, CWD has also been reported in wild cervids in Europe (Benestad, Mitchell, Simmons, Ytrehus, & Vikøren, 2016). CWD is a prion disease, also known as a transmissible spongiform encephalopathy (TSE), of cervid species. Prion diseases are caused by conversion of the endogenous cellular prion protein (PrPC) into its infectious isoform, PrPSc, which is the major component of prions (Prusiner, 1998). This conformational transition is catalysed by PrPSc coming into contact with PrPC (Prusiner, 1998), and PrPC is most abundantly expressed in the central nervous system (CNS) (Linden et al., 2008). The CNS is a major site of PrPSc conversion and accumulation, leading to neurodegeneration and ultimately death (Prusiner, 1998). In naturally occurring CWD, prions are acquired through peripheral routes, such as oral uptake from environmental reservoirs (Gilch et al., 2011). Prions are highly resistant to many major forms of inactivation (Giles, Woerman, Berry, & Prusiner, 2017). It is exceptionally challenging and not possible to contain and eradicate CWD to date, as prions are shed in excrement and bodily fluids of infected free‐ranging cervids (Cheng, Hannaoui, et al., 2017; Haley et al., 2011; Henderson et al., 2015; Safar et al., 2008). This results in contamination of the environment, particularly soil, with prions that remain infectious for years (Bartelt‐Hunt & Bartz, 2013; Kuznetsova, McKenzie, Cullingham, & Aiken, 2020; Pritzkow et al., 2018; Somerville et al., 2019). So far, wild and farmed cervid species naturally infected with CWD include white‐tailed deer (Odocoileus virginianus), mule deer (O. hemionus), elk (Cervus canadensis), red deer (C. elaphus) and moose (Alces alces sp.) in North America (Baeten, Powers, Jewell, Spraker, & Miller, 2007; Hannaoui, Amidian, et al., 2017; Kurt & Sigurdson, 2016; Pirisinu et al., 2018; Williams & Young, 1980, 1982, 1993), as well as reindeer (Rangifer tarandus tarandus), red deer (C. elaphus) and moose (A. a. alces) in Europe (Benestad et al., 2016; Pirisinu et al., 2018; Vikøren et al., 2019).
There are currently four caribou subspecies residing in North America: the Peary (R. t. pearyi), Grant's (R. t. granti), barren‐ground (R. t. groenlandicus), and woodland (R. t. caribou) caribou (National Museum of Canada, 1962). Peary caribou populations are located in the Canadian Arctic, on islands north of the Northwest Territories (NT) and Nunavut (Mallory & Boyce, 2019). Barren‐ground caribou herds migrate throughout vast areas of NT, Nunavut and Yukon, with historical ranges in Northern Alberta and Saskatchewan (COSEWIC, 2016). Grant's caribou, consisting mainly of the Porcupine herd, are found in Yukon and Alaska, and are also often classified as barren‐ground caribou (Festa‐Bianchet, Ray, Boutin, Côté, & Gunn, 2011). Woodland caribou are divided into two major ecotypes comprising boreal and mountain populations (Festa‐Bianchet et al., 2011). Boreal woodland caribou populations span from British Columbia (BC) to Québec, while mountain woodland caribou mainly reside in BC, Yukon and NT (Festa‐Bianchet et al., 2011). Many caribou populations in Canada are listed as either Threatened or Endangered (COSEWIC, 2016; Thomas & Gray, 2002). Recent observations of cervid populations in Saskatchewan show an overlap in habitat between white‐tailed deer, including CWD‐infected deer, and boreal caribou herds (Hebblewhite & Fortin, 2017; Hervieux et al., 2013; McLoughlin et al., 2019; Saskatchewan Ministry of Environment, 2013). Because CWD is becoming increasingly prevalent in free‐ranging cervids in Saskatchewan (CWD map of positive tests, 2020; Kahn, Dubé, Bates, & Balachandran, 2004), this brings into light the possibility of CWD invading the R. tarandus populations in this province. All cervid species tested so far have been shown to be susceptible to CWD infection, albeit some only in experimental settings (Balachandran et al., 2010; Hamir et al., 2011; Mitchell et al., 2012; Moore et al., 2016; Nalls et al., 2013; Sigurdson et al., 1999). Furthermore, wild reindeer in Europe acquired CWD (Benestad et al., 2016). While as yet wild caribou in Canada are reportedly free from CWD, transmission is likely to happen naturally under current conditions.
The prion protein gene (Prnp) sequence is largely identical among cervid species, with only a small number of single nucleotide polymorphisms (SNPs) present in the gene pool (Robinson, Samuel, O’Rourke, & Johnson, 2012). Nevertheless, Prnp polymorphisms can modulate susceptibility to CWD (Angers et al., 2014; Green et al., 2008; Hannaoui, Amidian, et al., 2017; Hannaoui, Schatzl, et al., 2017; Johnson et al., 2011). Previous studies reported a Prnp polymorphism resulting in a substitution from serine to asparagine at codon 138 (S138N) unique to fallow deer (Dama dama) and reindeer/caribou (Hamir et al., 2011; Mitchell et al., 2012; Moore et al., 2016; Rhyan et al., 2011; Robinson et al., 2019). This polymorphism has been associated with lower susceptibility to CWD in both species under experimental conditions, depending on the route of prion infection (Mitchell et al., 2012; Moore et al., 2016; Rhyan et al., 2011). Fallow deer, naturally homozygous for asparagine at codon 138 (138NN), did not develop clinical disease nor accumulate detectable amounts of PrPSc in their CNS or lymphatic organs, after 6 years of exposure to CWD‐infected mule deer (Rhyan et al., 2011). Nevertheless, fallow deer were susceptible to intracerebral CWD infection, where PrPSc and spongiform degeneration was detected in the CNS after a prolonged incubation period of 51 months post‐infection, whereas deer usually succumb to the disease between 12–34 months (Hamir et al., 2011; Kahn et al., 2004). In two other studies, no PrPSc was found in the CNS of reindeer homozygous or heterozygous for asparagine at codon 138 (138SN or 138NN) infected peripherally with prions, with the exception of one animal (Mitchell et al., 2012; Moore et al., 2016). A prolonged CWD incubation period with the absence of typical clinical CWD symptoms was also seen in a 138SN reindeer infected orally (Mitchell et al., 2012). The S138N polymorphism is present in caribou herds in Alaska and Alberta, but not reported in wild reindeer in Norway so far (Cheng, Musiani, Cavedon, & Gilch, 2017; Güere et al., 2020; Happ, Huson, Beckmen, & Kennedy, 2007). Other Prnp polymorphisms in the Rangifer species include codons V2M, G129S, N146n (synonymous substitution), V169M, N176D, S225Y, and a 24 base pair deletion in the octapeptide repeat region (Cheng, et al., 2017; Robinson et al., 2012; Wik et al., 2012). Recent studies reported that the prevalence of the 225S allele and a combination of the 2M‐129S‐169M variant was higher in CWD‐positive wild reindeer from the Nordfjella region in Norway (Güere et al., 2020).
Here, we report the prevalence and distribution of R. tarandus prion protein polymorphisms obtained from 986 caribou samples from BC, Yukon, NT, Nunavut and Saskatchewan. We identified two novel prion protein polymorphisms, one in barren‐ground (Y153F) and one in woodland (P242L) caribou. The frequencies of previously reported polymorphisms found in this study were not significantly different across herds and subspecies. At the herd level, the 138N allele was exceptionally prevalent in the Chinchaga boreal woodland caribou population in BC, in agreement with previous studies in Alberta (Cheng, Musiani, et al., 2017). When we excluded this woodland herd from the analyses, we found that codon 138 was significantly different between the two subspecies, with 138NN and 138SN genotypes being more frequent in barren‐ground than woodland caribou. Notably, spatial differences were found among different herds, with the lowest prevalence of the 138N allele in woodland caribou of the mountain ecotype in BC, and the boreal woodland caribou herds in SK.
2. MATERIALS AND METHODS
2.1. Samples
We obtained a total of 986 frozen caribou blood samples or tissue from herds in BC (n = 397), NT/Nunavut (n = 462), Saskatchewan (n = 101) and Yukon (n = 26). These samples were collected between 2004–2018 and represent woodland and barren‐ground caribou populations in western Canada (Figure 1). The NT/Nunavut samples consist of the barren‐ground herds Bathurst (n = 87), Beverly and Ahiak (n = 107), Bluenose East (n = 90), Bluenose West (n = 50), Cape Bathurst (n = 27), Tuktoyaktuk Peninsula (n = 15) and woodland boreal herds Hay and Cameron (n = 54) and those from the North Slave region (n = 19). Yukon samples comprise of the Porcupine herd (R. t. granti) (n = 19), which will be included as barren‐ground from here onwards, and woodland caribou of the mountain ecotype including Horseranch (n = 2), Little Rancheria (n = 2) and Carcross (n = 2) herds. BC woodland caribou herds included in this study were Calendar (n = 35), Chinchaga (n = 59), Parker (n = 3), Prophet (n = 9), Maxhamish (n = 37), Snake‐Sahtaneh (n = 70), and Fort Nelson (n = 4) of the boreal ecotype and Muskwa (n = 6), Frog (n = 6), Itcha‐Ilgachuz (n = 59), Wolverine (n = 10), Atlin (n = 26), Finlay (n = 8), Gataga (n = 8), Graham (n = 11), Pink Mountain (n = 17), and Hart South (n = 29) of the mountain ecotype. Out of the 986 samples, 14 animals did not have herd information.
FIGURE 1.
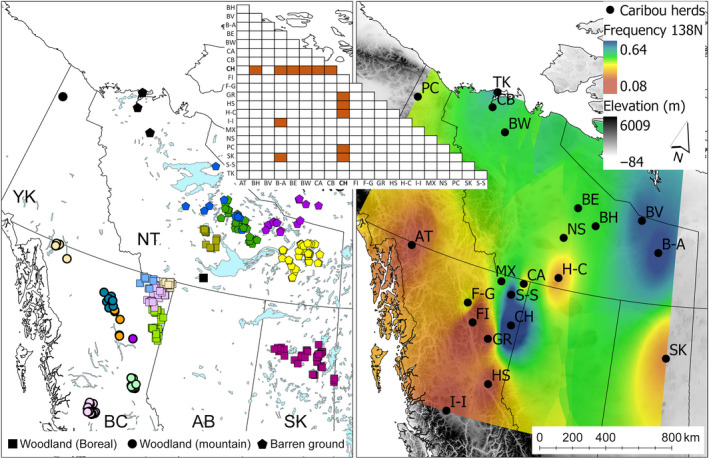
Left panel shows the distribution of caribou samples used in this study with colours representing the herds, and symbol shapes representing the subspecies/ecotype. Black symbols represent herds without individual coordinates. Top panel indicates significant pairwise genotypic comparisons among herds following p‐value correction for multiple tests. Right panel is an interpolation of the 138N allele frequency across herds, individual coordinates were averaged for centroids. Herds are labelled using the following abbreviations: AT, Atlin; B‐A, Beverly/Ahiak; BE, Bluenose east; BH, Bathurst; BV, Beverly; BW, Bluenose west; CA, Calendar; CB, Cape Bathurst; CH, Chinchaga; F‐G, Frog/Gataga; FI, Finlay; GR, Graham; H‐C, Hay/Cameron; HS, Hart south; I‐I, Itcha‐Ilgachuz; MX, Maxhamish; NS, North Slave; PC, Porcupine; SK, SK1; S‐S, Snake‐Sahtaneh; TK, Tuktoyaktuk
2.2. DNA extraction, polymerase chain reaction (PCR) and sequencing
We extracted DNA from frozen clotted blood using the MagMAX‐96 DNA Multi‐Sample Kit (ThermoFisher Scientific, cat #4413022) in a 96‐well format following the manufacturer's protocol. The Prnp open reading frame in exon 3 was amplified using a protocol from Cheng, Musiani, et al. (2017). PCR reaction conditions were as follows: 10 µl of genomic DNA (equivalent to 100 ng of genomic DNA), 2 µl of 10 µM forward primer (5′‐ CCT AGT TCT CTT TGT GGC CAT GTG ‐3′ or 5′‐ GGG CAT ATG ATG CTG ACA CCC TCT TT ‐3′), 2 µl of 10 µM reverse primer (5′‐ TGA GGA AAG AGA TGA GGA GGA TCA C 3′ or 5′‐ GAG AAA AAT GAG GAA AGA GAT GAG GAG G ‐3′), 10 µl of 10x Pfu buffer (Agilent), 1 µl of 10 mM dNTP (Invitrogen), 0.5 µl of Pfu polymerase (Agilent) and nuclease‐free water in a total reaction volume of 50 µl. Conditions for the PCR were 4 min of initial denaturation at 94°C, followed by 39 cycles of denaturation at 94°C for 30 s, annealing at 63°C for 30 s and extension at 72°C for 1 min, with a final extension at 72°C for 10 min. The runs were performed in 96‐well plates using the GeneAmp PCR System 9700 (Applied Biosystems) or the T100 (Bio‐Rad). No‐template controls were randomly included in every run to ensure no cross‐contamination of samples. Amplified samples were run on a 2% agarose gel at 95V for 1 hr. Bands were visualized under UV light, excised using a clean blade, and amplified DNA was retrieved and purified using a commercial gel extraction kit (Qiagen, cat #28706). Sequencing of products were performed at Eton Bioscience (San Diego) with the first primer set used in the PCR reaction above.
2.3. TOPO cloning
Gel‐extracted PCR products from samples with novel SNPs detected in the chromatograms were cloned using the TOPO Zero Blunt cloning kit (ThermoFisher Scientific, cat #450245) and transformed into TOP10 cells (ThermoFisher Scientific, cat #C404010) according to the manufacturer's protocol. Ten colonies from each sample were screened for the Prnp insert using colony PCR with Taq DNA Polymerase (GenScript, cat #E00043) and the first primer set mentioned above. Plasmid DNA was extracted from positive colonies using a commercial kit (Omega Bio‐tek, cat #D6945‐2) and sent for sequencing at the University of Calgary core sequencing facility or Eton Bioscience (San Diego) using the common M13F and M13R primers present in the vector sequence.
2.4. SNPs and statistical analyses
To find SNPs, both known and novel, caribou Prnp sequences were analysed using the sangeranalyseR package (Aneichyk et al., 2018; https://github.com/roblanf/sangeranalyseR) in R Studio and Geneious v.10.2.6 (Kearse et al., 2012; http://www.geneious.com). Statistical significance of genotype and allele frequencies between herds and subspecies were determined using using an exact G‐test, chi‐squared or Fisher's exact test in Genepop and/or the Genetics package (https://CRAN.R‐project.org/package=genetics) in R Studio. p‐values were adjusted using the fdr correction with the p.adjust function in R Studio. Prnp genotypes for each SNP were also analysed for deviations from Hardy‐Weinberg Equilibrium, and for linkage disequilibrium using the Genetics package. To visualize the spatial variation in the frequency of 138N we generated a heatmap using the interpolation tool within ArcGIS Pro (ESRI Software). Centroid locations were estimated for herds without geographic coordinates, and we did not include herds with fewer than six individuals. Frog and Gataga herds were merged as they had small sample sizes, were spatially adjacent, and their genotype frequencies did not differ.
3. RESULTS
Seven Prnp polymorphisms and a deletion from nucleotide (nt) 249 to 272 have been reported in caribou/reindeer (R. tarandus spp.). The polymorphisms are V→M at codon 2, G→S at codon 129, S→N at codon 138, N→n (synonymous substitution) at codon 146, V→M at codon 169, N→D at codon 176 and S→Y at codon 225 and (41,48,49,51). The 24 base pair (bp) deletion, the 176 N→D and the 225 S→Y polymorphisms have only been reported in European reindeer. All sequences from our North American caribou samples were homozygous for serine at codon 225 and none had the 24 bp deletion. The polymorphism at codon 2 is located in the signal peptide sequence of PrPC, which is cotranslationally cleaved off, thus we excluded this codon from our analyses. Out of 986 samples, 708 successful reads were obtained for Prnp codon 129 (71.81%), 726 for codon 138 (73.63%), 697 for codon 146 (70.69%), 724 for codon 169 (73.43%) and 708 for codon 225 (71.81%). Only individuals with genotype data at codons 129, 138, 146 and/or 169 and herd data, and only herds with greater than five individuals, were included in the herd level analyses (n = 756). Statistical tests show that all alleles in the Prnp codons analysed here were in Hardy‐Weinberg equilibrium (chi‐squared and Fisher's exact tests), suggesting that none of these Prnp polymorphisms were under selection pressure. Pairwise linkage disequilibrium analyses also showed us that the majority of SNP haplotype pairs, with the exception of codon 146, were genetically linked (D′ = 0.86–0.99; p < .05). This is expected as these SNPs are located physically close to each other within the Prnp open reading frame (ORF) on exon 3.
We found two barren‐ground caribou that were heterozygous for N and D at codon 176 (Figure S1). European reindeer are known to carry the D variant at codon 176, but this is the first report of its presence in caribou in North America. Furthermore, we found two novel Prnp SNPs that have not been previously reported in any species; a Y→F substitution at codon 153 in three NT barren‐ground caribou (all heterozygous Y/F) and a P→L substitution at codon 242 in 12 BC woodland caribou (nine heterozygous P/L and three homozygous L/L). We performed TOPO cloning on all samples with possible SNPs to exclude any sequencing artifacts.
We assessed the genotypic differentiation between all caribou herds (n = 21 herds, with individuals >5 per herd, Frog‐Gataga combined) at codons 129, 138, 146 and 169 in Genepop. Only codon 138 had significantly different pairwise comparisons following p‐value correction; the majority of the significant comparisons were between Chinchaga and the other herds (Figure 1 and Table 1). We also performed Grubbs’ test for outliers, and based on the N allele frequency at codon 138, the Chinchaga herd was determined an outlier amongst all caribou herds (Z = 2.27, p < .05). Mean allele frequency of 138N was 0.32 across all herds, while the frequency in the Chinchaga herd was 0.64. There was no significant difference between the barren‐ground and woodland caribou subspecies at all codons (Table S1), but when we omitted the Chinchaga herd, the genotype frequencies at codon 138 between barren‐ground and woodland populations differed significantly (p < .01) (Figure 2). In addition, codon 138 also significantly differed between barren‐ground and woodland caribou of the mountain ecotype (p < .05) (Figure 2). These differences are highlighted in the heatmap for the 138N allele (Figure 1). We also show that individual animals can carry more than one polymorphic allele (Table 2). We report that the Prnp codon associated with reduced CWD susceptibility is found in higher frequencies in the Chinchaga woodland population, and higher in migratory barren‐ground than woodland caribou populations when this herd is excluded from analyses.
Table 1.
Herd allele frequencies at Prnp codon 138
| Herd | Subspecies | Ecotype | Abbrev. | Sample size | Codon 138 | |
|---|---|---|---|---|---|---|
| N‐freq | S‐freq | |||||
| Atlin | Woodland | Mountain | AT | 9 | 0.222 | 0.778 |
| Bathurst | Barren‐ground | NA | BH | 79 | 0.342 | 0.658 |
| Beverly | Barren‐ground | NA | BV | 18 | 0.417 | 0.583 |
| Beverly/Ahiak | Barren‐ground | NA | B‐A | 77 | 0.435 | 0.565 |
| Bluenose E | Barren‐ground | NA | BE | 83 | 0.343 | 0.657 |
| Bluenose W | Barren‐ground | NA | BW | 46 | 0.348 | 0.652 |
| Calendar | Woodland | Boreal | CA | 34 | 0.294 | 0.706 |
| Cape Bathurst | Barren‐ground | NA | CB | 26 | 0.346 | 0.654 |
| Chinchaga | Woodland | Boreal | CH | 51 | 0.637 a | 0.363 |
| Finlay | Woodland | Mountain | FI | 6 | 0.083 | 0.917 |
| Frog/Gataga | Woodland | Mountain | F‐G | 11 | 0.318 | 0.682 |
| Graham | Woodland | Mountain | GR | 7 | 0.214 | 0.786 |
| Hart South | Woodland | Mountain | HS | 17 | 0.206 | 0.794 |
| Hay/Cameron | Woodland | Boreal | H‐C | 45 | 0.256 | 0.744 |
| Itcha‐Ilgachuz | Woodland | Mountain | I‐I | 30 | 0.200 | 0.800 |
| Maxhamish | Woodland | Boreal | MX | 12 | 0.333 | 0.667 |
| North Slave | Woodland | Boreal | NS | 14 | 0.321 | 0.679 |
| Porcupine | Barren‐ground | NA | PC | 19 | 0.316 | 0.684 |
| SK woodland | Woodland | Boreal | SK | 82 | 0.250 | 0.750 |
| Snake‐Sahtaneh | Woodland | Boreal | S‐S | 33 | 0.439 | 0.561 |
| Tuktoyaktuk | Barren‐ground | NA | TK | 15 | 0.400 | 0.600 |
Significantly different from most other herds.
FIGURE 2.
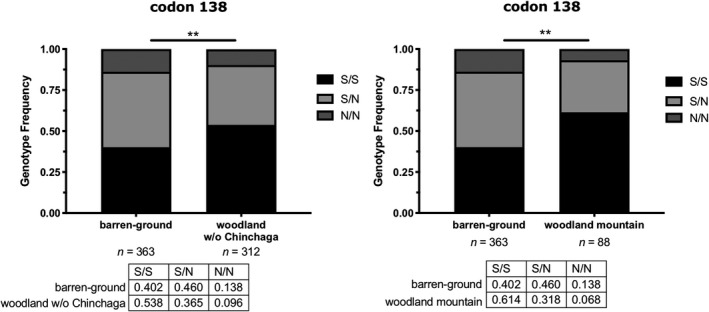
When the boreal woodland Chinchaga herd was omitted from statistical analyses, the difference in codon 138 between barren‐ground and woodland caribou (left) and between barren‐ground and woodland caribou of the mountatin ecotype (right) was statistically significant at a 5% significance level (p = .002 and .001, respectively). Tests were performed using a chi‐squared test in the Genetics package in R Studio and graphs were generated in Graphpad Prism 8
Table 2.
Number of individuals per haplotype group present in caribou herds in western Canada (codons 129/138/169; 146 is excluded as it is a synonymous substitution)
| Caribou subspecies | GSV | GNV | GSM | GNM | SSV | SNV | SSM | SNM |
|---|---|---|---|---|---|---|---|---|
| Barren‐ground (R. t. groenlandicus) | 112 | 178 | 1 | 0 | 19 | 9 | 6 | 4 |
| Woodland (R. t. caribou) | 132 | 152 | 0 | 0 | 12 | 7 | 1 | 0 |
4. DISCUSSION
Currently, there have been no reports of CWD in wild caribou in North America. However, CWD has been identified in their wild relatives in Europe (reindeer) (Benestad et al., 2016). They are members of the same species, R. tarandus; hence, there is concern that CWD will expand, or has already transmitted, to caribou in the wild. The S138N Prnp polymorphism has been described in caribou and fallow deer (Cheng, Musiani, et al., 2017; Hamir et al., 2011; Happ et al., 2007; Wik et al., 2012) and the pseudogene of mule deer and white‐tailed deer (Brayton, O’Rourke, Lyda, Miller, & Knowles, 2004; O’Rourke, 2004). Here, we report the prevalence of the S138N polymorphism in Prnp sequences of 726 caribou samples from two provinces (BC, Saskatchewan) and three territories (Yukon, NT, and Nunvaut) in western Canada. Our results give an overall picture of the 138N allele frequency being higher in the northern migratory barren‐ground herds when compared to both boreal and mountain woodland caribou populations, with the exception of the boreal woodland Chinchaga herd. We also report the presence of the N176D polymorphism in caribou for the first time as well as two novel Prnp polymorphisms, Y153F and P242L. As the genotype frequencies in the other three polymorphisms (G129S, N146n and V169M) do not differ significantly between subspecies and herds, we will not discuss them further.
This S138N polymorphism is linked with strongly reduced susceptibility to clinical CWD upon natural routes of prion infection (Hamir et al., 2011; Mitchell et al., 2012; Moore et al., 2016; Rhyan et al., 2011). In fallow deer, where both alleles at codon 138 encode asparagine (138NN), peripheral exposure to CWD‐infected deer did not result in disease (Rhyan et al., 2011). However, they are susceptible to intracerebral infection with white‐tailed deer and elk CWD prions, with prolonged survival times ranging between 24 to 59 months post‐infection (Hamir et al., 2011), indicating that 138N PrPC per se can be converted into PrPSc and does not confer absolute resistance to prion infection. In reindeer, the 138N allele has been associated with reduced susceptibility to natural CWD infection routes in experimental settings. Only one animal had detectable prions in the obex, while in the rest prions were limited to peripheral organs (Mitchell et al., 2012; Moore et al., 2016). All reindeer that tested positive for CWD in the Nordfjella region in Norway are of the 138SS genotype, and the 138N allele has not been reported in that population of reindeer (Güere et al., 2020). In addition, the substitution from serine to asparagine at position 138 impacted in vitro prion conversion in a previous study (Raymond et al., 2000). Together, these data suggest this may be an important site for disease modulation. Structurally, serine is a small polar (hydrophilic) noncharged residue. An exchange to asparagine, which is a larger polar noncharged residue, can alter packing of side chains as serine may fit into pockets of bigger residues.
While the newly identified polymorphisms have no known exposure to CWD, we provide some insight into their potential role in disease susceptibility based on their locations and amino acid changes. The polymorphism at codon 242 is located in the C‐terminal of the PrPC sequence that is cotranslationally cleaved off when the GPI‐anchor is added. Thus, it is unlikely that the P242L polymorphism plays a role in determining PrPC properties that influence the pathogenesis of CWD. The Y153F polymorphism is located within the first alpha‐helix of PrPC (Wopfner et al., 1999). Tyrosine (Y) is identical to phenylalanine (F) and only differ in a hydroxyl group present in tyrosine. A previous study reported that the YYR (Tyrosine‐Tyrosine‐Arginine) motif at position 149–151 in human PrP, corresponding to 152–154 in cervids, is exposed in the PrPSc conformation and constitutes a PrPSc‐specific epitope (Paramithiotis et al., 2003). The Y153F substitution might impact recognition by PrPSc‐specific antibodies developed based on the YYR motif, but whether it actually plays a role in PrPC to PrPSc conversion is not known. The N→D polymorphism at codon 176, previously reported only in the Eurasian reindeer, was found in two NT barren‐ground animals in this study. R. tarandus spp. is divided into two lineages based on microsatellite loci and mitochondrial DNA sequences (Yannic et al., 2014). The barren‐ground populations belong to the Euro‐Beringia clade that inhabit western Canada and Eurasia (Yannic et al., 2014). This shows the possibility that the Prnp gene variants are preserved within the lineage. Another explanation is the introduction of Eurasian reindeer into Alaska which were later herded into the Inuvik region in the 1930s, highlighting possibilities of introgression between caribou and reindeer in NT (Klein, 1980). Polymorphisms and their locations with respect to PrPC are visualized in Figure 3.
FIGURE 3.
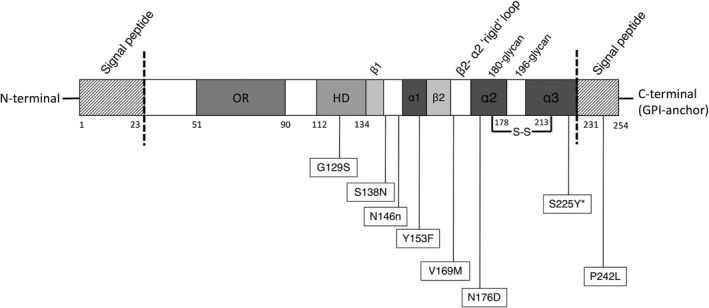
Positions of caribou polymorphisms reported in this study within the prion protein sequence. GPI, glycosylphosphatidylinositol; HD, hydrophobic domain; OR, octapeptide repeat. *All animals were homozygous serine (S) at codon 225
Landscape features, such as major valleys and separation by rivers, have shown to be more accurate in describing caribou genetic structure than classification by subspecies and ecotype (Serrouya et al., 2012). We therefore examined genotypic differentiation at the herd level, finding Chinchaga was significantly different from most of the other herds at the 138 locus. Boreal woodland populations in the Chinchaga range, both in BC and Alberta, inhabit only the peatlands where lichen is abundant (Leech, Whittaker, & the Doig River First Nation, 2016; O’Leary, Saxena, & DeCoursey, 2002). Furthermore, the overlay with landscape motifs in Figure 1 indicates a possibility that the Chinchaga population in BC is located in a secluded habitat surrounded by higher elevation ground, which may result in reduced gene flow. These provide possible reasons for the high 138N allele prevalence in the Chinchaga caribou herd, where geographic isolation limits genetic flow to and from other adjacent herds. A similarly high 138N prevalence has also been previously reported in the Chinchaga caribou population in Alberta (Cheng, Musiani, et al., 2017). Although there are likely many factors impacting free‐ranging caribou populations, we propose that the high 138N allele prevalence is advantageous should CWD expand to the Chinchaga population, as caribou in this area are at high risk of extinction (Leech et al., 2016; O’Leary et al., 2002).
Woodland boreal caribou in Saskatchewan are divided into two major populations, the northern SK1 range that inhabits the Boreal Shield and the southern SK2 range, further categorized into west, central and east, populating the Saskatchewan Boreal Plains (Saskatchewan Ministry of Environment, 2013). The SK2 range habitat overlaps with white‐tailed deer, which are natural hosts of CWD (McLoughlin et al., 2019). Genetic information on these populations show that caribou in the SK2 range often migrate to the SK1 range but not vice versa, excepting high gene flow in the central range (Figure S2) (Priadka et al., 2018). Our results show that the majority of the SK1 range caribou harbor the wild‐type 138SS Prnp genotype (97.5%), which is associated with susceptibility to natural CWD infection routes (Güere et al., 2020; Mitchell et al., 2012; Moore et al., 2016). Should these herds contract CWD, they will increase transmission probability to woodland and barren‐ground caribou populations in NT. In this study, barren‐ground caribou have significantly higher 138N Prnp allele frequencies (36.8%) than woodland caribou (27.9%), in particular of the mountain ecotype (22.7%), when the outlier Chinchaga herd was removed from the analyses. As barren‐ground caribou migrate across vast areas of land (COSEWIC, 2016), they will pose a risk of widespread prion contamination if infected, eventually reaching the east and west coasts, and even Alaska.
Climate change and industrial development have reduced the size of remaining caribou habitat leading to range retraction and increased contact between caribou and other cervids (especially white‐tailed deer) and predator species, either directly or indirectly (Bauduin, McIntire, St‐Laurent, & Cumming, 2018; Dawe & Boutin, 2016; Hebblewhite & Fortin, 2017; Hervieux et al., 2013; Leech et al., 2016; Tennant et al., 2020). Predators have been shown to pass prions in their faeces after consuming CWD‐infected cervids (Krumm, Conner, Hobbs, Hunter, & Miller, 2010; Nichols, Fischer, Spraker, Kong, & VerCauteren, 2015), and mountain lions preferentially prey on CWD‐infected deer (Krumm et al., 2010), which further increases the probability of prion dissemination. Should wild caribou come into contact with these prions, an additional risk factor is imposed to their already Threatened status, as the disease has been shown to drive the decline of white‐tailed and mule deer populations (DeVivo et al., 2017; Edmunds et al., 2016). Although CWD has not yet been detected in free‐ranging caribou in Canada, transmission to these animals from infected captive or free‐ranging cervids remains a concern for wildlife scientists and managers. Thus, CWD control measures and strategies for caribou are already part of current wildlife disease management guidelines (CWD map of positive tests, 2020; Gagnier, Laurion, & DeNicola, 2020; Williams, Miller, Kreeger, Kahn, & Thorne, 2002; Zimmer, Boxall, & Adamowicz, 2011). Our findings highlight herds with the highest susceptibility to CWD, assuming the 138N allele reduces susceptibility. Of the herds we examined, mountain caribou herds and those in the SK1 range in Saskatchewan are at the highest risk (Figure 1). We hope these findings help in mitigating CWD transmission to caribou by contributing to future decisions and planning of CWD surveillance and preventive measures in Canada.
In conclusion, the 138N Prnp allele, associated with less susceptibility to CWD, is found in caribou populations in Alaska (Happ et al., 2007), Alberta (Cheng, Musiani, et al., 2017), British Columbia, the Northwest Territories, Nunavut, Saskatchewan, and Yukon. It is more prevalent in barren‐ground than woodland caribou populations, with statistical significance when the woodland Chinchaga herd was excluded from the analyses. This high 138N allele frequency in the Chinchaga range is probably due to landscape and geographic isolation. In the SK1 range in Saskatchewan, which overlaps with barren‐ground caribou range, the 138N Prnp allele is present in much lower frequencies, thus increasing caribou susceptibility to contracting clinical CWD. This caribou population can serve as a gateway to possible CWD transmission from infected cervids, thus bringing into light the risk of CWD expansion into woodland and barren‐ground caribou in western North America.
AUTHOR CONTRIBUTIONS
M.I.A. wrote the paper. S.G. and M.I.A. designed the research. M.I.A., A.S., S.Y.S., and Y.H. performed the research. H.F., and P.D.M. provided samples. M.I.A., A.S., C.C., G.M., and S.G. analysed data. A.S., S.Y.S., H.F., P.D.M., C.C., G.M., and S.G. provided comments and edits to the manuscript.
Supporting information
Fig S1
Fig S2
Table S1
Table S2
Legends
ACKNOWLEDGEMENTS
We would like to acknowledge our collaborators Bryan Macbeth, Maeve Winchester and Helen Schwantje at the British Columbia Ministry of Forests, Lands, Natural Resource Operations, and Rural Development and Jane Harms at the Department of Environment, Yukon for providing caribou blood or tissue samples, as well as the Veterinary Medicine Faculty Teaching Facility at the University of Calgary. We acknowledge funding for this research from Genome Canada, Alberta Prion Research Institute and Alberta Agriculture and Forestry through Genome Alberta, and the University of Calgary. SG is supported by the Canada Research Chair program. PDM was supported by a Natural Sciences and Engineering Research Council (NSERC) Collaborative Research and Development grant.
Arifin MI, Staskevicius A, Shim SY, et al. Large‐scale prion protein genotyping in Canadian caribou populations and potential impact on chronic wasting disease susceptibility. Mol Ecol. 2020;29:3830–3840. 10.1111/mec.15602
Contributor Information
Catherine I Cullingham, Email: catherinecullingham@cunet.carleton.ca.
Sabine Gilch, Email: sgilch@ucalgary.ca.
DATA ACCESSIBILITY
Caribou ID, genotype, herd and capture coordinates are listed in Table S2. Novel Prnp polymorphisms are submitted to Genbank with accession numbers MT361766 and MT361767.
REFERENCES
- Aneichyk, T. , Hendriks, W. T. , Yadav, R. , Shin, D. , Gao, D. , Vaine, C. A. , … Talkowski, M. E. (2018). Dissecting the causal mechanism of X‐linked dystonia‐parkinsonism by integrating genome and transcriptome assembly. Cell, 172(5), 897–909.e21. 10.1016/j.cell.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers, R. , Christiansen, J. , Nalls, A. V. , Kang, H.‐E. , Hunter, N. , Hoover, E. , … Telling, G. C. (2014). Structural effects of PrP polymorphisms on intra‐ and interspecies prion transmission. Proceedings of the National Academy of Sciences of the United States of America, 111(30), 11169–11174. 10.1073/pnas.1404739111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten, L. A. , Powers, B. E. , Jewell, J. E. , Spraker, T. R. , & Miller, M. W. (2007). A Natural case of chronic wasting disease in a free‐ranging moose (Alces alces shirasi). The Journal of Wildlife Diseases, 43(2), 309–314. 10.7589/0090-3558-43.2.309 [DOI] [PubMed] [Google Scholar]
- Balachandran, A. , Harrington, N. P. , Algire, J. , Soutyrine, A. , Spraker, T. R. , Jeffrey, M. , … O‐Rourke, K. I. (2010). Experimental oral transmission of chronic wasting disease to red deer (Cervus elaphus elaphus): Early detection and late stage distribution of protease‐resistant prion protein. Canadian Veterinary Journal, 51(2), 169–178. [PMC free article] [PubMed] [Google Scholar]
- Bartelt‐Hunt, S. L. , & Bartz, J. C. (2013). Behavior of prions in the environment: Implications for prion biology. PLoS Pathogens, 9(2), e1003113 10.1371/journal.ppat.1003113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauduin, S. , McIntire, E. , St‐Laurent, M.‐H. , & Cumming, S. G. (2018). Compensatory conservation measures for an endangered caribou population under climate change. Scientific Reports, 8, 16438 10.1038/s41598-018-34822-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benestad, S. L. , Mitchell, G. , Simmons, M. , Ytrehus, B. , & Vikøren, T. (2016). First case of chronic wasting disease in Europe in a Norwegian free‐ranging reindeer. Veterinary Research, 47(1), 88 10.1186/s13567-016-0375-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton, K. A. , O’Rourke, K. I. , Lyda, A. K. , Miller, M. W. , & Knowles, D. P. (2004). A processed pseudogene contributes to apparent mule deer prion gene heterogeneity. Gene, 326, 167–173. 10.1016/j.gene.2003.10.022 [DOI] [PubMed] [Google Scholar]
- Cheng, Y. C. , Hannaoui, S. , John, T. R. , Dudas, S. , Czub, S. , & Gilch, S. (2017). Real‐time quaking‐induced conversion assay for detection of CWD prions in fecal material. Journal of Visualized Experiments, 127, e56373 10.3791/56373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y. C. , Musiani, M. , Cavedon, M. , & Gilch, S. (2017). High prevalence of prion protein genotype associated with resistance to chronic wasting disease in one Alberta woodland caribou population. Prion, 11(2), 136–142. 10.1080/19336896.2017.1300741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSEWIC (2016). COSEWIC assessment and status report on the caribou, Rangifer tarandus, Barren‐ground population, in Canada (p. 123). Ottawa, ON: Committee on the Status of Endangered Wildlife in Canada; Retrieved from http://publications.gc.ca/collections/collection_2017/eccc/CW69‐14‐746‐2017‐eng.pdf [Google Scholar]
- CWD map of positive tests|Chronic Wasting Disease (2020). Government of Saskatchewan. Retrieved from https://www.saskatchewan.ca/residents/environment‐public‐health‐and‐safety/wildlife‐issues/fish‐and‐wildlife‐diseases/chronic‐wasting‐disease/cwd‐map [Google Scholar]
- Dawe, K. L. , & Boutin, S. (2016). Climate change is the primary driver of white‐tailed deer (Odocoileus virginianus) range expansion at the northern extent of its range; land use is secondary. Ecology and Evolution, 6(18), 6435–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVivo, M. T. , Edmunds, D. R. , Kauffman, M. J. , Schumaker, B. A. , Binfet, J. , Kreeger, T. J. , … Cornish, T. E. (2017). Endemic chronic wasting disease causes mule deer population decline in Wyoming. PLoS One, 12(10), e0186512 10.1371/journal.pone.0186512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds, D. R. , Kauffman, M. J. , Schumaker, B. A. , Lindzey, F. G. , Cook, W. E. , Kreeger, T. J. , … Cornish, T. E. (2016). Chronic wasting disease drives population decline of White‐Tailed deer. PLoS One, 11(8), e0161127 10.1371/journal.pone.0161127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa‐Bianchet, M. , Ray, J. C. , Boutin, S. , Côté, S. D. , & Gunn, A. (2011). Conservation of caribou (Rangifer tarandus) in Canada: An uncertain future. Canadian Journal of Zoology, 89(5), 419–434. [Google Scholar]
- Gagnier, M. , Laurion, I. , & DeNicola, A. J. (2020). Control and surveillance operations to prevent chronic wasting disease establishment in free‐ranging white‐tailed deer in Québec, Canada. Animals (Basel), 10(2), 238 10.3390/ani10020283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilch, S. , Chitoor, N. , Taguchi, Y. , Stuart, M. , Jewell, J. E. , & Schätzl, H. M. (2011). Chronic wasting disease. Topics in Current Chemistry, 305, 51–77. [DOI] [PubMed] [Google Scholar]
- Giles, K. , Woerman, A. L. , Berry, D. B. , & Prusiner, S. B. (2017). Bioassays and inactivation of prions. Cold Spring Harbor Perspectives in Biology, 9(8), a023499 10.1101/cshperspect.a023499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, K. M. , Browning, S. R. , Seward, T. S. , Jewell, J. E. , Ross, D. L. , Green, M. A. , … Telling, G. C. (2008). The elk PRNP codon 132 polymorphism controls cervid and scrapie prion propagation. Journal of General Virology, 89(2), 598–608. 10.1099/vir.0.83168-0 [DOI] [PubMed] [Google Scholar]
- Güere, M. E. , Våge, J. , Tharaldsen, H. , Benestad, S. L. , Vikøren, T. , Madslien, K. , … Tranulis, M. A. (2020). Chronic wasting disease associated with prion protein gene (PRNP) variation in Norwegian wild reindeer (Rangifer tarandus). Prion, 14(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley, N. J. , Mathiason, C. K. , Carver, S. , Zabel, M. , Telling, G. C. , & Hoover, E. A. (2011). Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: Potential mechanisms of prion shedding and transmission. Journal of Virology, 85(13), 6309–6318. 10.1128/JVI.00425-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamir, A. N. , Greenlee, J. J. , Nicholson, E. M. , Kunkle, R. A. , Richt, J. A. , Miller, J. M. , & Hall, M. (2011). Experimental transmission of chronic wasting disease (CWD) from elk and white‐tailed deer to fallow deer by intracerebral route: Final report. Canadian Journal of Veterinary Research, 75(2), 152–156. [PMC free article] [PubMed] [Google Scholar]
- Hannaoui, S. , Amidian, S. , Cheng, Y. C. , Duque Velásquez, C. , Dorosh, L. , Law, S. , … Gilch, S. (2017). Destabilizing polymorphism in cervid prion protein hydrophobic core determines prion conformation and conversion efficiency. PLoS Pathogens, 13(8), e1006553 10.1371/journal.ppat.1006553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannaoui, S. , Schatzl, H. M. , & Gilch, S. (2017). Chronic wasting disease: Emerging prions and their potential risk. PLoS Pathogens, 13(11), e1006619 10.1371/journal.ppat.1006619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happ, G. M. , Huson, H. J. , Beckmen, K. B. , & Kennedy, L. J. (2007). Prion protein genes in caribou from Alaska. Journal of Wildlife Diseases, 43(2), 224–228. 10.7589/0090-3558-43.2.224 [DOI] [PubMed] [Google Scholar]
- Hebblewhite, M. , & Fortin, D. (2017). Canada fails to protect its caribou. Science, 358(6364), 730–731. [DOI] [PubMed] [Google Scholar]
- Henderson, D. M. , Denkers, N. D. , Hoover, C. E. , Garbino, N. , Mathiason, C. K. , & Hoover, E. A. (2015). Longitudinal detection of prion shedding in saliva and urine by chronic wasting disease‐infected deer by real‐time quaking‐induced conversion. Journal of Virology, 89(18), 9338–9347. 10.1128/JVI.01118-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieux, D. , Hebblewhite, M. , DeCesare, N. J. , Russell, M. , Smith, K. , Robertson, S. , & Boutin, S. (2013). Widespread declines in woodland caribou (Rangifer tarandus caribou) continue in Alberta. Canadian Journal of Zoology, 91(12), 872–882. [Google Scholar]
- Johnson, C. J. , Herbst, A. , Duque‐Velasquez, C. , Vanderloo, J. P. , Bochsler, P. , Chappell, R. , & McKenzie, D. (2011). Prion protein polymorphisms affect chronic wasting disease progression. PLoS One, 6(3), e17450 10.1371/journal.pone.0017450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn, S. , Dubé, C. , Bates, L. , & Balachandran, A. (2004). Chronic wasting disease in Canada: Part 1. Canadian Veterinary Journal, 45(5), 397–404. [PMC free article] [PubMed] [Google Scholar]
- Kearse, M. , Moir, R. , Wilson, A. , Stones‐Havas, S. , Cheung, M. , Sturrock, S. , … Drummond, A. (2012). Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28(12), 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, D. R. (1980). Conflicts between domestic reindeer and their wild counterparts: A review of Eurasian and North American experience. Arctic, 33(3), 739–756. [Google Scholar]
- Krumm, C. E. , Conner, M. M. , Hobbs, N. T. , Hunter, D. O. , & Miller, M. W. (2010). Mountain lions prey selectively on prion‐infected mule deer. Biology Letters, 6(2), 209–211. 10.1098/rsbl.2009.0742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt, T. D. , & Sigurdson, C. J. (2016). Cross‐species transmission of CWD prions. Prion, 10(1), 83–91. 10.1080/19336896.2015.1118603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova, A. , McKenzie, D. , Cullingham, C. , & Aiken, J. M. (2020). Long‐term incubation PrPCWD with soils affects prion recovery but not infectivity. Pathogens (Basel Switzerland), 9(4). 10.3390/pathogens9040311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech, S. M. , Whittaker, C. & the Doig River First Nation (2016). Madziih (caribou) Tsáá? ché ne dane traditional knowledge and restoration study. Report prepared for DFN and the David Suzuki Foundation by the Firelight Group December 2016. p. 60. [Google Scholar]
- Linden, R. , Martins, V. R. , Prado, M. A. M. , Cammarota, M. , Izquierdo, I. , & Brentani, R. R. (2008). Physiology of the prion protein. Physiologial Reviews, 88(2), 673–728. 10.1152/physrev.00007.2007 [DOI] [PubMed] [Google Scholar]
- Mallory, C. D. , & Boyce, M. S. (2019). Prioritization of landscape connectivity for the conservation of Peary caribou. Ecology and Evolution, 9(4), 2189–2205. 10.1002/ece3.4915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin, P. D. , Superbie, C. , Stewart, K. , Tomchuk, P. , Neufeld, B. , Barks, D. , … Johnstone, J. F. (2019). Population and habitat ecology of boreal caribou and their predators in the Saskatchewan boreal shield. Final report. Department of Biology, University of Saskatchewan, Saskatoon. p. 238. 2013–2018. [Google Scholar]
- Mitchell, G. B. , Sigurdson, C. J. , O’Rourke, K. I. , Algire, J. , Harrington, N. P. , Walther, I. , … Balachandran, A. (2012). Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PLoS One, 7(6), e39055 10.1371/journal.pone.0039055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, S. J. , Kunkle, R. , Greenlee, M. H. W. , Nicholson, E. , Richt, J. , Hamir, A. , … Greenlee, J. (2016). Horizontal transmission of chronic wasting disease in reindeer. Emerging Infectious Diseases, 22(12), 2142–2145. 10.3201/eid2212.160635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls, A. V. , McNulty, E. , Powers, J. , Seelig, D. M. , Hoover, C. , Haley, N. J. , … Mathiason, C. K. (2013). Mother to offspring transmission of chronic wasting disease in reeves’ muntjac deer. PLoS One, 8(8), e71844 10.1371/journal.pone.0071844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Museum of Canada (1962). Revision of the reindeer and caribou, genus Rangifer. (Bulletin National Museum of Canada; No. 177). [Google Scholar]
- Nichols, T. A. , Fischer, J. W. , Spraker, T. R. , Kong, Q. , & VerCauteren, K. C. (2015). CWD prions remain infectious after passage through the digestive system of coyotes (Canis latrans). Prion, 9(5), 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary, D. , Saxena, A. , & DeCoursey, C. (2002). Biophysical inventory of chinchaga wildland park (p. 72). Valleyview, AB: Alberta Community Development Parks and Portected Areas. [Google Scholar]
- O'Rourke, K. I. , Spraker, T. R. , Hamburg, L. K. , Besser, T. E. , Brayton, K. A. , & Knowles, D. P. (2004). Polymorphisms in the prion precursor functional gene but not the pseudogene are associated with susceptibility to chronic wasting disease in white‐tailed deer. Journal of General Virology, 85(5), 1339–1346. 10.1099/vir.0.79785-0 [DOI] [PubMed] [Google Scholar]
- Paramithiotis, E. , Pinard, M. , Lawton, T. , LaBoissiere, S. , Leathers, V. L. , Zou, W.‐Q. , … Cashman, N. R. (2003). A prion protein epitope selective for the pathologically misfolded conformation. Nature Medicine, 9(7), 893–899. 10.1038/nm883 [DOI] [PubMed] [Google Scholar]
- Pirisinu, L. , Tran, L. , Chiappini, B. , Vanni, I. , Di Bari, M. , Vaccari, G. , … Benestad, S. L. (2018). Novel Type of Chronic Wasting Disease Detected in Moose (Alces alces), Norway. Emerging Infectious Diseases, 24(12), 2210–2218. 10.3201/eid2412.180702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priadka, P. , Manseau, M. , Trottier, T. , Hervieux, D. , Galpern, P. , McLoughlin, P. D. , & Wilson, P. J. (2018). Partitioning drivers of spatial genetic variation for a continuously distributed population of boreal caribou: Implications for management unit delineation. Ecology and Evolution, 14, e4682 10.1002/ece3.4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritzkow, S. , Morales, R. , Lyon, A. , Concha‐Marambio, L. , Urayama, A. , & Soto, C. (2018). Efficient prion disease transmission through common environmental materials. Journal of Biological Chemistry, 293(9), 3363–3373. 10.1074/jbc.M117.810747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner, S. B. (1998). Prions. Proceedings of the National Academy of Sciences of the United States of America, 95(23), 13363–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, G. J. , Bossers, A. , Raymond, L. D. , O’Rourke, K. I. , McHolland, L. E. , Bryant, P. K. III , … Caughey, B. (2000). Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. The EMBO Journal, 19(17), 4425–4430. 10.1093/emboj/19.17.4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyan, J. C. , Miller, M. W. , Spraker, T. R. , McCollum, M. , Nol, P. , Wolfe, L. L. , … O'Rourke, K. I. (2011). Failure of fallow deer (Dama dama) to develop chronic wasting disease when exposed to a contaminated environment and infected mule deer (Odocoileus hemionus). Journal of Wildlife Diseases, 47(3), 739–744. 10.7589/0090-3558-47.3.739 [DOI] [PubMed] [Google Scholar]
- Robinson, A. L. , Williamson, H. , Güere, M. E. , Tharaldsen, H. , Baker, K. , Smith, S. L. , … Houston, F. (2019). Variation in the prion protein gene (PRNP) sequence of wild deer in Great Britain and mainland Europe. Veterinary Research, 50(1), 59 10.1186/s13567-019-0675-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, S. J. , Samuel, M. D. , O’Rourke, K. I. , & Johnson, C. J. (2012). The role of genetics in chronic wasting disease of North American cervids. Prion, 6(2), 153–162. 10.4161/pri.19640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar, J. G. , Lessard, P. , Tamgüney, G. , Freyman, Y. , Deering, C. , Letessier, F. , … Prusiner, S. B. (2008). Transmission and detection of prions in feces. Journal of Infectious Diseases, 198(1), 81–89. 10.1086/588193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saskatchewan Ministry of Environment (2013). Conservation strategy for boreal woodland caribou (Rangifer tarandus caribou) in Saskatchewan. (Fish and Wildlife Technical Report 2014). Retrieved from http://publications.gov.sk.ca/documents/66/89807‐English.pdf [Google Scholar]
- Serrouya, R. , Paetkau, D. , McLellan, B. N. , Boutin, S. , Campbell, M. , & Jenkins, D. A. (2012). Population size and major valleys explain microsatellite variation better than taxonomic units for caribou in western Canada. Molecular Ecology, 21(11), 2588–2601. 10.1111/j.1365-294X.2012.05570.x [DOI] [PubMed] [Google Scholar]
- Sigurdson, C. J. , Williams, E. S. , Miller, M. W. , Spraker, T. R. , O’Rourke, K. I. , & Hoover, E. A. (1999). Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). Journal of General Virology, 80(10), 2757–2764. 10.1099/0022-1317-80-10-2757 [DOI] [PubMed] [Google Scholar]
- Somerville, R. A. , Fernie, K. , Smith, A. , Bishop, K. , Maddison, B. C. , Gough, K. C. , & Hunter, N. (2019). BSE infectivity survives burial for five years with only limited spread. Archives of Virology, 164(4), 1135–1145. 10.1007/s00705-019-04154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant, J. M. , Li, M. , Henderson, D. M. , Tyer, M. L. , Denkers, N. D. , Haley, N. J. , … Hoover, E. A. (2020). Shedding and stability of CWD prion seeding activity in cervid feces. PLoS One, 15(3), e0227094 10.1371/journal.pone.0227094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, D. C. , & Gray, D. R. (2002). Update COSEWIC status report on the woodland caribou rangifer tarandus caribou in Canada. In COSEWIC assessment and update status report on the the Woodland Caribou Rangifer tarandus caribou in Canada. Ottawa, ON: Committee on the Status of Endangered Wildlife in Canada; pp. 1–98. Retrieved from http://www.sararegistry.gc.ca/virtual_sara/files/cosewic/sr_woodland_caribou_e.pdf. [Google Scholar]
- Vikøren, T. , Våge, J. , Madslien, K. I. , Røed, K. H. , Rolandsen, C. M. , Tran, L. , … Benestad, S. L. (2019). First detection of chronic wasting disease in a wild red deer (Cervus elaphus) in Europe. Journal of Wildlife Diseases, 55(4), 970 10.7589/2018-10-262 [DOI] [PubMed] [Google Scholar]
- Wik, L. , Mikko, S. , Klingeborn, M. , Stéen, M. , Simonsson, M. , & Linné, T. (2012). Polymorphisms and variants in the prion protein sequence of European moose (Alces alces), reindeer (Rangifer tarandus), roe deer (Capreolus capreolus) and fallow deer (Dama dama) in Scandinavia. Prion, 6(3), 256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, E. S. , Miller, M. W. , Kreeger, T. J. , Kahn, R. H. , & Thorne, E. T. (2002). Chronic wasting disease of deer and elk: A review with recommendations for management. The Journal of Wildlife Management, 66(3), 551 10.2307/3803123 [DOI] [Google Scholar]
- Williams, E. S. , & Young, S. (1980). Chronic wasting disease of captive mule deer: A spongiform encephalopathy. Journal of Wildlife Diseases, 16(1), 89–98. 10.7589/0090-3558-16.1.89 [DOI] [PubMed] [Google Scholar]
- Williams, E. S. , & Young, S. (1982). Spongiform encephalopathy of rocky mountain elk. Journal of Wildlife Diseases, 18(4), 465–471. 10.7589/0090-3558-18.4.465 [DOI] [PubMed] [Google Scholar]
- Williams, E. S. , & Young, S. (1993). Neuropathology of chronic wasting disease of mule deer (Odocoileus hemionus) and elk (Cervus elaphus nelsoni). Veterinary Pathology, 30(1), 36–45. [DOI] [PubMed] [Google Scholar]
- Wopfner, F. , Weidenhöfer, G. , Schneider, R. , von Brunn, A. , Gilch, S. , Schwarz, T. F. , … Schatzl, H. M. (1999). Analysis of 27 mammalian and 9 avian PrPs reveals high conservation of flexible regions of the prion protein. Journal of Molecular Biology, 289(5), 1163–1178. [DOI] [PubMed] [Google Scholar]
- Yannic, G. , Pellissier, L. , Ortego, J. , Lecomte, N. , Couturier, S. , Cuyler, C. , … Côté, S. D. (2014). Genetic diversity in caribou linked to past and future climate change. Nature Climate Change, 4(2), 132–137. 10.1038/nclimate2074 [DOI] [Google Scholar]
- Zimmer, N. , Boxall, P. C. , Adamowicz, W. L. (Vic) . (2011). The impact of chronic wasting disease and its management on hunter perceptions, opinions, and behaviors in Alberta, Canada. Journal of Toxicology and Environmental Health, Part A, 74(22‐24), 1621–1635. 10.1080/15287394.2011.618988 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Table S2
Legends
Data Availability Statement
Caribou ID, genotype, herd and capture coordinates are listed in Table S2. Novel Prnp polymorphisms are submitted to Genbank with accession numbers MT361766 and MT361767.


