Abstract
Background and objective
Radiation pneumonitis (RP) could be a lethal complication of lung cancer treatment. No reliable predictors of RP severity have been recognized. This prospective pilot study was performed to identify early predictors of high grade lung toxicity and to evaluate clinical, biological or dosimetric features associated with different grades of toxicity.
Method
Sixteen patients with non-small cell lung cancer with indication of concurrent chemoradiotherapy using 60 Gy/2 Gy/fraction starting at cycle one of platinum based chemotherapy were included. Bronchoalveolar lavage (BAL), pulmonary function testing (PFT), and 18F-2-fluoro-2-deoxy-D-glucose positron-emission tomography was performed before radiotherapy (RT), after three weeks of treatment, and two months post-RT. For analysis, patients were grouped by grade (low [G1-G2] vs. high [G3-G5]). The two groups were compared to identify predictors of RP. Protein expression BAL and lung tissue metabolism was evaluated in two patients (RP-G1 vs. RP-G3). Categorical variables such as comorbidities, stages and locations were summarized as percentages. Radiation doses, pulmonary function values and time to RP were summarized by medians with ranges or as means with standard deviation. Longitudinal analysis PFT was performed by a T-test.
Results
All 16 patients developed RP, as follows: G1 (5 pts; 31.3%); G2 (5 pts; 31.3%); G3 (5 pts; 31.3%); and G5 (1 pts; 6.1%). Patients with high grade RP presented significant decrease (p = 0.02) in diffusing lung capacity for carbon monoxide (DLCO) after three weeks of RT. No correlation between dosimetric values and RP grades was observed. BAL analysis of the selected patients showed that CXCL-1, CD154, IL-1ra, IL-23, MIF, PAI-1 and IFN-γ were overexpressed in the lungs of the RP-G3 patient, even before treatment. The pre-RT SUVmax value in the RP-G3 patient was non-significantly higher than in the patient with RP-G1.
Conclusions
RT induces some degree of RP. Our data suggest that decrease in DLCO% is the most sensitive parameter for the early detection of RP. Moreover, we detect biological differences between the two grades of pneumonitis, highlighting the potential value of some cytokines as a prognostic marker for developing high grade lung toxicity. Further multicenter studies with larger sample size are essential to validate these findings.
Keywords: Non-small cell lung cancer, Radiation pneumonitis, Lung function, Wound healing
Background
Radiotherapy (RT) is a mainstay treatment for non-small cell lung cancer (NSCLC). Several studies have showed a benefit in local control and survival increasing biological equivalent doses [1]. However, its effectiveness is limited by the risk of radiation-induced lung injury (RILI). RILI is the result of an abnormal healing response to lung irradiation caused by damage to parenchymal cells, vasculature, and/or stroma followed by inflammatory cytokine release [2]. Diagnosis of RILI is based on nonspecific symptoms with or without abnormalities in pulmonary function tests (PFT). Radiographic changes usually reveal parenchymal abnormalities.
Radiation pneumonitis (RP), and pulmonary fibrosis (PF) represent, the acute and late phase of RILI. Symptomatic RILI has been described in 30% of cases, with mortality rates as high as 2% [3–6]. Distinctions between these phases is arbitrary because early and late effects of RT are a continuous spectrum of the same biological event. Early-RILI or RP is considered when symptoms appear within 12 week after lung RT and up to 6 months post-RT. X-rays is characterized by inhomogeneous opacity inside or outside the irradiation field and increased density of septal structures. Late-RILI or PF is a chronic lung damage that usually evolves over 6 to 24 months after RT. X-rays shows contracted, dense scar that occupies a much smaller volume than the originally irradiated volume. Also fibroelastosis pleuroparenchymal changes can be observed do to RT [7].
The relationship between the development of RILI and baseline patient characteristics, lung function parameters and radiation dose have been retrospectively investigated [3, 8, 9]. Some molecular biomarkers in blood and bronchoalveolar lavage (BAL) have been proposed [10–12]. In addition, imaging technologies such as 8F-2-fluoro-2-deoxy-D-glucose (18F-FDG) positron-emission computed tomography (PET/CT) are able to quantify the uptake of 18F-FDG in the lung as a marker of pulmonary inflammation [13–17]. Despite these advances, scarce data is available to show reliable predictive factors of RP, the effects of radiation on the lung, or the mechanisms leading to fibrosis and death in the context of RILI.
This prospective pilot study was conducted to identify early predictive factors of severity in RP and to evaluate the possible features associated with different grades of RP.
Methods and material
Patients
Patients diagnosed with NSCLC at our Institution with indication of concurrent chemoradiotherapy regimen using 60 Gy 2 Gy/fraction starting at cycle one of platinum based chemotherapy were prospectively included from January 2011 to March 2013. Inclusion criteria comprised: histologically confirmed NSCLC, inoperable locally advanced NSCLC, no previous thoracic RT. Exclusion criteria included: Karnofsky index < 70, interstitial lung disease (ILD), forced expiratory volume at first second (FEV1) < 30%, chronic respiratory failure, oral corticosteroid treatment, contraindication for bronchoscopy, or refusal to participate. The Ethics Committee of the University Hospital of Bellvitge and the Catalan Institute of Oncology approved the study protocol (PR206/08). Patients signed a written informed consent prior to inclusion.
Patients underwent BAL by fiberotpic-bronchoscopy, lung function testing, and 18FDG-PET/CT prior to initiation of RT, at the end of the third week of RT, and at two months post-RT. Patient consultations were once weekly from the time of study inclusion until RT completion. Thereafter, patients were evaluated every 15 days for 6 months and then monthly for one year. The follow-up visits included: medical history, physical examination and monthly chest X-rays. RP diagnosis was based on the appearance or worsening of dyspnea and cough, which may associate fever or chest pain, accompanied with changes of radiological images. RP diagnosis and imaging evaluation were made by the multidisciplinary clinical team (medical oncologist, radiation oncologist and thoracic radiologist). RP grade was scored according to the Common Terminology Criteria for Adverse Events version 4.0. (CTCAEv4.0) [18]. Patients were divided into 2 groups (low-grade RP [G1 and G2], and high-grade RP [G3-G5]), according to the CTCAEv4.0. The two groups were compared to identify early predictors for high-grade RP development.
Radiotherapy treatment
Treatment planning for the RT used a 3D technique. An specific CT scan over the thorax and upper abdomen with intravenous contrast was obtained [19]. Gross tumor volume (GTV) was contoured according to the PET/CT and diagnostic CT scan. No prophylactic nodal irradiation was performed. To cover subclinical disease, the GTV was expanded according to histological findings. The GTV was increased by 0.6 cm for squamous cell carcinomas and by 0.8 cm for adenocarcinomas to provide the clinical target volume (CTV) [20]. The planning target volume (PTV) was determined by adding 0.7 cm to the CTV in the lateral and anterior posterior direction and 1.5 cm in the cranio-caudal direction [21]. The mean dose to the PTV was 60 Gy according to standard protocols [22]. Organs at risk were contoured in accordance with treatment guidelines [23]. Dose constrains to the lungs were V20 < 35% (i.e., 35% of the healthy lung should receive ≤ 20 Gy) with a mean dose < 19 Gy. Radiotherapy and chemotherapy were started at the same time.
Pulmonary function testing
PFT parameters were measured according to European Respiratory Society guidelines [24] using computerized lung function testing equipment (Body Box 5500; Morgan Scientific). The parameters assessed included forced vital capacity (FVC), forced expiratory volume at first second (FEV1), and diffusing lung capacity for carbon monoxide (DLCO). The same technician performed the PFT at all follow-up consultations.
BAL sample collection
BAL was performed in both lungs (i.e., the irradiated and non-irradiated) by using four 40 mL aliquots of isotonic saline solution (0.45%) per wash through a fiberoptic-bronchoscope (Olympus BF-160) to facilitate the extraction of cytokines and chemokines from the alveolar space. The first sample was discarded; the second and the third samples were mixed and sent for cytological evaluation. The fourth aliquot was centrifuged (543.6 g × 5 min) into cellular fraction and supernatant, which was aliquoted for cytokine determination; both fractions were frozen at − 80 °C.
Protein array analysis of cytokine in BAL supernatant
To evaluate differences in individual predisposition to lung damage and tissue repair response, we evaluate BAL samples. In this preliminary report, we chose one representative patient from each study group: one from low grade RP (RP-G1) and another from high grade RP (RP-G3). Expression protein was assessed using the Human Cytokine Array Panel A (R&D Systems, Minneapolis, MN; USA). Protein concentration was measured in each sample [25]. Pixel density of the spots was analysed using the Multi Gauge V3.0 (FujiFilm, Palo Alto, CA; USA). Three independent readers compared the mean pixel density values in the spots.
Positron-emission computed tomography
Patients underwent PET/CT imaging with 18F-FDG according to standard practice [26]. They were asked to fast 6 h prior to the imaging session to ensure fasting blood glucose levels within the normal range (3.3–5.6 mmol/L). Patients received an intravenous administration of FDG per kg of body weight. The 18F-FDG-PET/CT scan was performed with a hybrid PET/CT scanner (General Electric Discovery ST). The whole-body acquisition protocol included a CT scan and a PET scan in a three-dimensional mode. No iodine intravenous contrast was administered. The CT data were used for attenuation correction and anatomic location of PET findings. The standardized uptake value (SUV) was used to measure uptake in the lungs.
Image analysis
Pre-treatment PET/CT image analyses of the two patients (i.e., RP-G1 and RP-G3) were performed to screen for possible differences in the SUV. This analysis was processed by three independent readers and evaluated using custom Matlab software (v2011a, Mathworks, Inc; Natick, MA; USA). The lung region of interest (ROI) was segmented semi-automatically. Overlap of central airway, liver, heart, diaphragm, and tumor in the lung ROI were manually removed. The resulting binary lung ROI was used for the analysis. The SUV was calculated from the PET attenuation corrected emission images [27]. SUV of voxels in the lung ROI were binned into histograms; the maximum SUV (SUVmax) was calculated according to the formula described by Petit el al. [15].
Statistical analysis
Since the prevalence of RP is variable due to differences in diagnostic scales [4], sample size was calculated using the “observed versus a reference mean” [28], which includes the reported prevalence of RP among the interstitial lung disease [29]. To detect a relevant clinical difference (alpha = 0.05 and beta = 0.1), 17 patients were required (assuming a follow-up loss rate of 20%).
Patients were divided into 2 groups (low-grade [G1 and G2], and high-grade [G3-G5]), according to the CTCAEv4.0. The two groups were compared to identify predictors for the early identification of RILI. Categorical variables were summarized as percentages. Ordinal categorical variables were summarized by medians with ranges or as means with standard deviation (SD). Longitudinal analysis of FEV1(%) and DLCO(%) was performed by a T-test. Differences were considered statistically significant for p < 0.05. All plots and analyses were performed using the statistical software R version 3.2.1 for Windows.
Results
Population
Seventeen patients were invited to participate and one refused. A total of 16 patients were included. Table 1 shows the characteristics of the cohort. All patients developed RP, with different grades of severity distributed as follows: 5 patients, G1 (31.3%); 5 patients, G2 (31.3%); 5 patients, G3 (31.3%); and 1 patient, G5 (6.1%) who died 3 weeks after beginning the treatment. The rest of them had one year of follow-up from the start of concurrent chemoradiotherapy. Patients were grouped by RP grade (low [G1-G2] vs. high [G3-G5]), with 10 and 6 patients in each group, respectively. Four patients from the high-grade group developed RILI in both lungs.
Table 1.
Clinical and treatment characteristics of the sample
| Characteristics | |
|---|---|
| No. of patients | 16 |
| Sex | |
| Male | 14 (87.5%) |
| Female | 2 (12.5%) |
| Age (range) | 63 (58.8–76) |
| Smoking history | |
| Current | 10 (62.5%) |
| Former | 5 (31.2%) |
| Never | 1 (6.2%) |
| Pulmonary function (range) | |
| FVC (%) | 101 (87–105.8) |
| FEV1 (%) | 85.5 (71.5–93.3) |
| FEV1/FVC | 67.9 (60.5–73) |
| DLCO (%) | 71 (57.2–87.5) |
| Comorbidities | |
| Hypertension | 5 (31.2%) |
| Diabetes | 3 (18.8%) |
| COPD | 11 (68.8%) |
| Heart disease | 2 (12.5%) |
| Vascular disease | 2 (12.5%) |
| Histologic type | |
| Adenocarcinoma | 4 (25%) |
| Squamous cell carcinoma | 9 (56.2%) |
| Large cell neuroendocrine carcinoma | 2 (12.5%) |
| NSCLC | 1 (6.2%) |
| Location | |
| Mediastinum | 1 (6.2%) |
| Hilar | 3 (18.8%) |
| Right upper lobe | 6 (37.5%) |
| Right inferior lobe | 2 (12.5%) |
| Left superior lobe | 3 (18.8%) |
| Left inferior lobe | 1 (6.2%) |
| Radiation doses (range) | |
| Mean dose (Gy) | 17.2 (12.7–22.9) |
| V20 (%) | 30 (20.3–35.8) |
| V5 (%) | 60 (47.5–65.8) |
| Pneumonitis grades | |
| 1 | 5 (31.3%) |
| 2 | 5 (31.3%) |
| 3 | 5 (31.3%) |
| 4 | 0 (0%) |
| 5 | 1 (6.1%) |
FVC: forced vital capacity, FEV1: forced expiratory volume in one second, DLCO: diffusing lung capacity for carbon monoxide, COPD: chronic obstructive pulmonary disease, NSCLC: non-small cell lung cancer
Table 2 shows the patient characteristics by RP group (low vs. high grade). There were no significance differences between the groups in terms of age, gender, comorbidities, PFT baseline values, cancer histology, stage and tumor localization. No differences were observed in the time of onset of RP and severity (p = 0,6642). The mean radiation dose was higher in the high-grade group [18 Gy (15.2 Gy-20.1 Gy) vs. 16.1 Gy (12 Gy-22.2 Gy)]; however, V20 was lower in the high-grade group [29.5 Gy (95% CI 23–30) vs. 32 Gy (95% CI 21–35)]. No significant correlation between dosimetric values and RP grades was observed.
Table 2.
Patient characteristics according to pneumonitis grade: low vs. high grade
| Characteristics | Low grade (G1–G2) | High grade (G3–G5) |
|---|---|---|
| No. of patients | 10 (62.5%) | 6 (37.5%) |
| Sex | ||
| Male | 9 (90%) | 5 (83.3%) |
| Female | 1 (10%) | 1 (16.7%) |
| Age (range) | 66.0 (59.2–67) | 59.5 (58.2–65.2) |
| Smoking history | ||
| Current | 8 (80%) | 2 (33.3%) |
| Former | 2 (20%) | 3 (50%) |
| Never | 0 (0%) | 1 (16.7%) |
| Pulmonary function (range) | ||
| FVC (%) | 100,5 (88.5- 106.5) | 102.5(82.5–104.8) |
| FEV1 (%) | 81.5 (59–93) | 89 (77–90.5) |
| FEV1/FVC | 64 (55–71.6) | 69.5 (67.9–80.3) |
| DLCO (%) | 71 (46.8–83.5) | 71.5 (63.5–92.2) |
| Comorbidities | ||
| Hypertension | 3 (30%) | 2 (33.3%) |
| Diabetes | 0 (0%) | 3 (50%) |
| COPD | 7 (70%) | 4 (66.7%) |
| Heart disease | 1 (10%) | 1 (16.7%) |
| Vascular disease | 2 (20%) | 0 (0%) |
| Histologic type | ||
| Adenocarcinoma | 3 (30%) | 1 (16.7%) |
| Squamous cell carcinoma | 5 (50%) | 4 (66.7%) |
| Large cell neuroendocrine carcinoma | 2 (20%) | 0 (0%) |
| NSCLC | 0 (0%) | 1 (16.7%) |
| Clinical stage | ||
| IIB | 3 (30%) | - |
| IIIA | 6 (60%) | 3 (50%) |
| IIIB | 1 (10%) | 5 (50%) |
| Location | ||
| Mediastinum | 1 (10%) | 0 (0%) |
| Hilar | 2 (20%) | 1 (16.7%) |
| Right upper lobe | 4 (40%) | 2 (33.3%) |
| Right inferior lobe | 1 (10%) | 1 (16.7%) |
| Left superior lobe | 1 (10%) | 2 (33.3%) |
| Left inferior lobe | 1 (10%) | 0 (0%) |
| Chemotherapy agents | ||
| Carboplatin-etoposide | 1 (10%) | 1 (16.7%) |
| Carboplatin-gemcitabina | 1 (10%) | 0 (0%) |
| Cisplatin-vinorelbine | 2 (20%) | 0 (0%) |
| Cisplatina-etoposide | 6 (60%) | 5 (83.3%) |
| Radiation doses (range) | ||
| Mean dose(Gy) | 16.1 (12–22.2) | 18 (15.2–20.1) |
| V20 (%) | 32 (21–35.8) | 29.5(23–30) |
| Onset of radiation pneumonitis (median) | 68,5 days | 111 days |
FVC: forced vital capacity, FEV1: forced expiratory volume in one second, DLCO: diffusing lung capacity for carbon monoxide, COPD: chronic obstructive pulmonary disease, NSCLC: non- small cell lung cancer
Pulmonary function testing
Baseline FEV1(%) and DLCO(%) at diagnosis was not associated with the different grade of RP development. No significant differences in mean values were found between the groups at the three time points (baseline, end of week three, and at two month post-RT) (Tables 3 and 4). However, by the end of the third week of RT, the DLCO(%) had decreased substantially in those cases that developed high-grade group (p = 0.0203) (Fig. 1-B). Furthermore, the DLCO(%) decline was even worse after 2 months post-RT in that same group (p = 0.0342) (Fig. 1-B). A FEV1(%) decline was observed after 2 months of RT but not earlier during the treatment (Fig. 1-A). Therefore, the DLCO(%) decrease during the RT allows to predict a high-grade RP even before starting respiratory symptoms.
Table 3.
Mean values in FEV1 between CTCAEv4.0 groups at baseline, end of third week, and two month post-RT
| Mean values | Low grade (G1–G2) | High grade (G3–G5) | p Value |
|---|---|---|---|
| Baseline | 78.2% (SD 21.2) | 88.5% (SD: 19.1) | 0.2803 |
| 3 weeks of RT | 93.4% (SD 17.5) | 86.2% (SD: 22.4) | 0.0668 |
| 2 months post-RT | 78.7% (SD: 12.0) | 76.4% (SD 11.8) | 0.3015 |
FEV1: forced expiratory volume in one second, CTCAEv4.0: common terminology criteria for Adverse Events version 4.0, RT: radiotherapy, SD: standard deviation
Table 4.
Mean values in DLCO between CTCAEv4.0 groups at baseline, end of third week, and two month post-RT
| Mean values | Low grade (G1–G2) | High grade (G3–G5) | p Value |
|---|---|---|---|
| Baseline | 67.7% (SD 22.4) | 83.3% (SD: 30.2) | 0.0719 |
| 3 weeks of RT | 62.8% (SD 11.5) | 65.6% (SD: 21.6) | 0.2136 |
| 2 months post- RT | 57% (SD: 13.6) | 55.2% (SD 16.7) | 0.0595 |
DLCO: diffusing lung capacity for carbon monoxide, CTCAEv4.0: common terminology criteria for adverse events version 4.0, RT: radiotherapy, SD: standard deviation
Fig. 1.
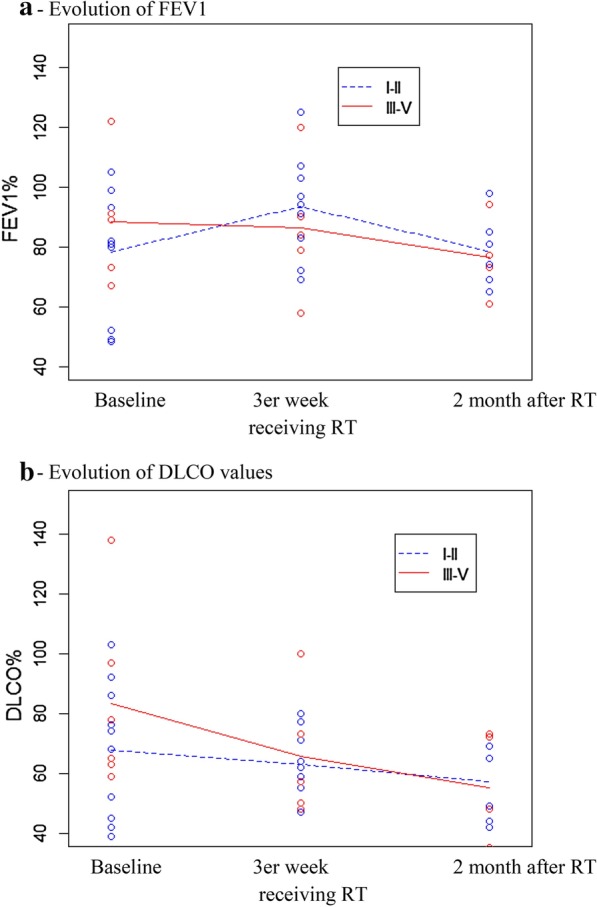
Pulmonary function test values at different time-points by CTCAEv4.0 groups (low-grade [GI and GII] and high-grade [GIII-GV]). a Evolution of FEV1: The points represent the FEV1 (%) value of each patient at the three different observation times. The straight, is the mean FEV1 (%) value at each observation time. CTCAEv4.0: Common Terminology Criteria for Adverse Events version 4.0 FEV1: forced expiratory volume in one second RT: radiotherapy. b Evolution of DLCO values: The points represent the DLCO (%) value of each patient at three different observation times. The straight line is the mean DLCO (%) value at each observation time. CTCAEv4.0: Common Terminology Criteria for Adverse Events version 4.0 DLCO: diffusing lung capacity for carbon monoxide RT: radiotherapy
Protein array analysis in the BAL supernatant
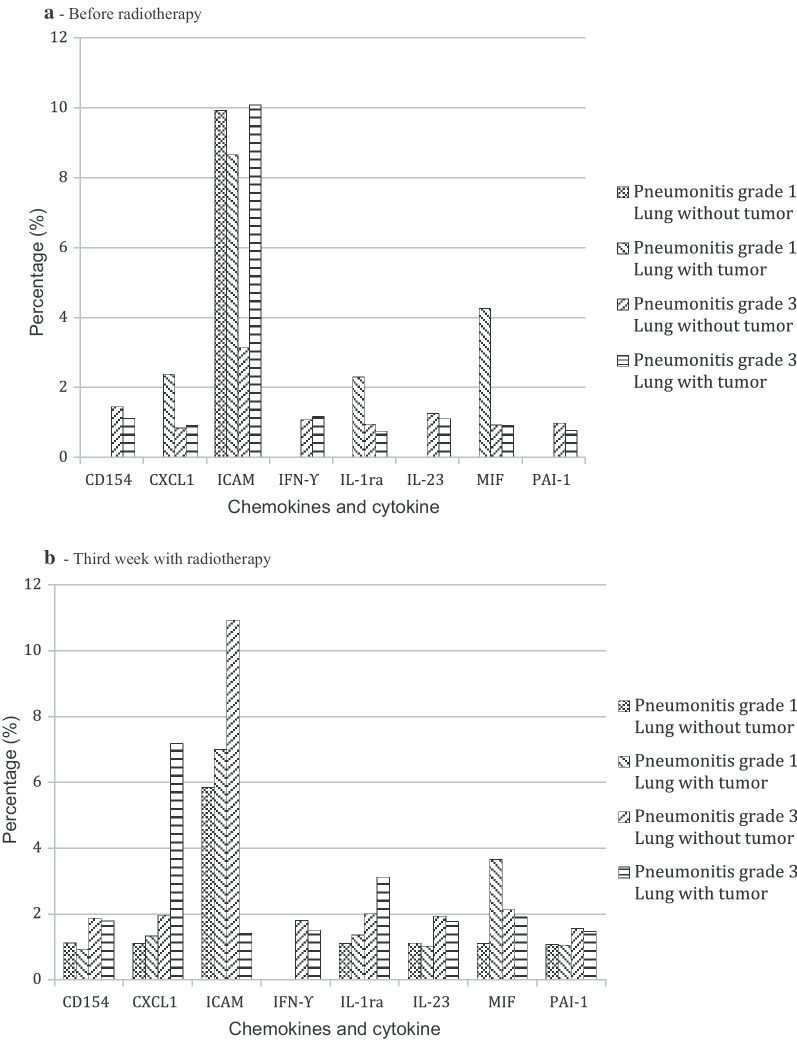
Different cytokine and chemokine expression profile (pre-RT and week 3) was found in a patient with RP-G1 compared to another with RP-G3 (Fig. 2). Before RT (Fig. 2-A), the only protein expressed in the tumor-free lung of the RP-G1 patient was ICAM while the tumor-free lung of the RP-G3 patient presented an overexpression of CD154, CXCL-1, ICAM, IFN-γ, IL-1ra, IL-23, MIF and PAI-1. In the lung with tumor (Fig. 2-A), the RP-G1 patient expressed CXCL-1, ICAM, IL-1ra and MIF while the RP-G3 patient showed those same cytokines but also expressed CD154, IL-23, IFN-γ and PAI-1. At the end of third week of RT (Fig. 2-B), a change in the cytokine and chemokine patterns was detected in both cases. RT induced expression of CD154, CXCL-1, IL-1ra, IL-23, MIF, and PAI-1 while reducing ICAM expression in both lungs of the RP-G1 patient. RT increased cytokine response of the most overexpressed proteins in the tumor and tumor-free lungs of the RP-G3 patient, with a higher expression of CD154, CXCL1, IL-1ra, IFN-γ, IL-23 and PAI-1 (Fig. 2-B).
Fig. 2.
Cytokine and chemokines in bronchoalveolar lavage in patients with grade 1 and grade 3 radiation pneumonitis. a Before radiotherapy. b Third week with radiotherapy
PET/CT image analysis
The pre-RT SUVmax value was calculated for the RP-G1 and RP-G3 patients. In both patients, the pre-treatment SUVmax was higher than normal (standardized lung SUVmax values, 0.05 ± 0.17) [30]. However, the RP-G3 patient had a non-significantly higher SUVmax (2.20 vs. 2, respectively).
Discussion
The present study demonstrates that RT induces RILI in all patients who undergo external RT with variable clinical manifestations and different degrees of lung damage. This variability in the degree of RILI suggests that severe RP may be associated with differences in individual predisposition. The early decrease of DLCO(%) was a predicting factor of severe RP development and thus could serve as a marker for early diagnosis and treatment modification.
The reported prevalence of RP in lung cancer patients ranges from 0 to 58% [4]. This variation is likely due to differences in diagnostic scales, non-specific symptoms, and the lack of standardized assessment protocols [4]. In the present study, standard follow-up protocols detected RILI in all of the patients but with different severity presentation. This finding suggests that all patients treated with RT are likely to develop RILI to a greater or lesser extent, which may depend on individual biological characteristics.
Previous studies have shown that subtle, local changes in PFT after RT can be used as indicators of acute and chronic lung damage, although published results are not always consistent [3, 9]. The largest and most consistent changes in PFT values after RT are observed in DLCO, which has been directly associated with respiratory morbidity [31]. In the present study, we evaluated the mean differences in FEV1(%) and DLCO(%) between low-grade RP patients [G1-G2] and high-grade RP patients [G3-G5]. We found no statistically significant differences between the two groups in FEV1(%) values at the different time points, thus leading us to conclude that FEV1(%) is not a predictor of RP, a finding that is consistent with other reports [9, 31]. By contrast, we found that a decrease in DLCO(%) 2 month post-RT was predictive of RP severity, in line with some previous reports [9, 31]. Importantly, the decline in DLCO(%) after three weeks of RT was an early predictor of severe RP. The explanatory power of this variable could be that histopathological alterations present during the latency phase (i.e., without clinical manifestations) for RILI may alter gas exchange [3]. Clearly, the ability to early predict severe RILI would be helpful to optimize therapeutic options.
Classically, RP grade has been associated with the radiation dose [3, 8]. In our study, the high-grade group received a higher mean radiation dose but lower V20 than the low-grade group. This finding is contrast with the meta-analysis published by Palma et al. [32] The absence of a significant association in this study between RP grade and radiation parameters could be due to the limited sample size, the small differences among patients with regards to the radiation dose and biological predisposition [9].
The 18FDG-PET/CT has been use to quantify increase cell glycolysis in the healthy lung tissue, excluding tumoral areas, like a marker of pulmonary inflammation through the uptake of 18FDG mesure by SUV [15-17]. It should be noted that both patients (RP-G1 and RP-G3) presented higher pre-RT SUVmax than normal values (0.05 ± 0.17) [30]. This finding could be explained by the role of chronic inflammation in lung cancer. Tumorgenesis includes a diverse leukocyte cells that has been considered key factors in tumor promotion since they release different variety of cytokines, chemokines, and cytotoxic. These mediators alter the adequate balance between pro-inflammatory and anti-inflammatory cytokines, favoring the increase of the first ones and producing a chronic inflammation state [33].
Accordingly, Castillo et al. [16, 17] demonstrated the predictive value of pre-treatment 18F-FDG lung uptake in the subsequent development of RP symptoms. In this study, RP-G3 patient had a higher SUVmax in healthy lung tissue respect RP-G1 patient.These findings support the experience already published by Catillo et al.
RILI produce an imbalance between type 1 and type 2 helper T-cells and abnormal fibroproliferative wound healing [34]. Variations among patients in terms of RP severity and lung repair capacity could be related to individual pre-treatment lung biomolecular conditions and genetic factors [35]. In the present study, increased expression of some mediators were obtained in BAL of tumor lungs in both patients (RP-G1 and RP-G3), although these proteins were also expressed in the tumor-free lung of the RP-G3 patient before RT. Previous studies indicate that IL-1ra is involved in acute inflammation [36]; MIF modulates RILI [37]; and CXCL1 promotes angiogenesis and thus contribute to the pathogenesis of PF. [34] Interestingly, CD154, IFN-γ, IL-23 and PAI-1 were expressed in both lungs (tumor and tumor-free lung) before RT only in the RP-G3 patient. These four cytokines have been described in animal models of lung fibrosis [38, 39]. Furthermore, a recent study reported that a truncated PAI-1 protein protects against RILI in a murine model [40]. Finally, Liu et al. found that rs7242 GT/GG genotypes located in the 3ÚTR of PAI-1 were associated with a significantly increased risk of RP [41]. Overall, our findings suggest a potential biological predisposition to lung damage and altered wound healing in RILI development, which would deserve a depth study to better understand pathogenesis.
Study strengths and limitations
We have to recognize some limitations: First, the small sample size which was calculated using “observed versus a reference mean” and even although we have enrolled sixteen patients, instead of including seventeen, we have not had any loss of follow up. Secondly, the low power of the biological lung and the PET/CT image analysis (only two patients). In this sense, this is a pilot study to identify if there are differences in biological features associated with different grades of RP. Our findings, warrant further investigation in a larger sample. The main strength of the study is that it is the first prospective study to evaluate patients with NSCLC through a longitudinal clinical and biological follow-up that demonstrate RT induces RILI in all cases but in some of them with a high-grade of lung injury and consequent altered wound repair.
Conclusion
RT treatment always induces some degree of lung injury and the extent of the damage is variable. Our data suggest that decrease in DLCO% is the most sensitive parameter for the early detection of severe RP. Moreover, we detect biological differences between the two grades of pneumonitis, highlighting the potential value of cytokines such as CXCL-1, CD154, IL-1ra, IL-23, MIF, PAI-1 and IFN-γ as a prognostic marker for developing high grade of lung toxicity. Further multicenter studies with larger sample size are essential to validate these preliminary findings.
Acknowledgements
Authors wish to thank Bradley Londres for editing the manuscript. Pilar Bayo and Gabriel Reynés (Department of Nuclear Medicine, Bellvitge Universitary Hospital and Physic Department, Catalan Institute of Oncology) for image analysis.
Abbreviations
- CTCAEv4.0
Common terminology criteria for adverse events version 4.0
- CXCL1
Chemokine (C-X-C motif) ligand 1
- CTV
Clinical target volume
- G1
Grade 1
- G2
Grade 2
- G3
Grade 3
- G5
Grade 5
- GTV
Gross tumour volume
- ICAM
Intercellular adhesion molecular
- IFN-γ
Interferon-gamma
- IL-23
Interleukin-23
- IL-1ra
Interleukin-1 receptor antagonist
- MIF
Macrophage migration inhibitory factor
- PTV
Planning target volume
- PAI-1
Plasminogen activator inhibitor type 1
- RT
Radiotherapy
- RP-G1
Radiation pneumonitis grade 1
- RP-G3
Radiation pneumonitis grade 3
- ROI
Region of interest
- TGF-β1
Transforming growth factor β-1
- V20
Healthy lung that should receive ≤ 20 Gy
Authors’ contributions
S.A., A.N and M.M. takes responsibility of the content and writing of manuscript. S.A., A.N., J.I.M., F.M, and M.M. designed the study. S.A., A.N, S.P., R.P, collected data. N.C and R.L performed the bronchoscopy S.A., L.R., R.C., E.C., and T.G., performed image analysis, S.A., A.M. and M.M., performed cytokine analysis A.N., R.C., S.P, E.C., A.M., J.I.M., N.C., R.L., L.R., R.P., F.M., J.D., and T.G., reviewed manuscript and approved final manuscript.
Funding
The Sociedad Española de Neumología y Cirugía Torácica (SEPAR) supported this project by a grant to finance the material expenses for the protein array analysis of cytokine, for statistical analysis and English corrector. The Insitut d’Investigació de Bellvitge (IDIBELL) supported this project by a grant to finance Samantha Aso PhD graduated student. These institutions haven’t participated in the design or development of the study or in the writing of the document.
Availability of data and materials
The datasets are available to all interested researchers on reasonable request from corresponding author.
Ethics approval and consent to participate
The Ethics Committee of the University Hospital of Bellvitge and the Catalan Institute of Oncology approved the study protocol (PR206/08). Patients signed a written informed consent prior to inclusion.
Consent for publication
Not applicable.
Competing interests
M.M: she’s consulting of Esteve-Teijin, Boehringer Ingelheim, Roche, Chiesi, GSK and Pfizer. Rest of authors: the authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arturo Navarro-Martin, Email: anavarro@iconcologia.net, Email: artnama79@gmail.com.
María Molina, Email: mariamolinamolina@hotmail.com.
References
- 1.Ma L, Men Y, Feng L, Kang J, Sun X, Yuan M, Jiang W, Hui Z. A current review of dose-escalated radiotherapy in locally advanced non-small cell lung cancer. Radiol Oncol. 2019;53(1):6–14. doi: 10.2478/raon-2019-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodemann HP. Molecular radiation biology: Perspectives for radiation oncology. Radiother Oncol. 2009;92:293–298. doi: 10.1016/j.radonc.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Madani I, De Ruyck K, Goeminne H, De Neve W, Thierens H, Van Meerbeeck J. Predicting risk of radiation-induced lung injury. J Thorac Oncol. 2007;2:864–874. doi: 10.1097/JTO.0b013e318145b2c6. [DOI] [PubMed] [Google Scholar]
- 4.Inoue A, Kunitoh H, Sekine I, Sumi M, Tokuuye K, Saijo N. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys. 2001;49:649–655. doi: 10.1016/s0360-3016(00)00783-5. [DOI] [PubMed] [Google Scholar]
- 5.Kong FM, Ten Haken R, Eisbruch A, Lawrence TS. Non-small cell lung cancer therapy-related pulmonary toxicity: An update on radiation pneumonitis and fibrosis. Semin Oncol. 2005;32:S42–S54. doi: 10.1053/j.seminoncol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Wang JY, Chen KY, Wang JT, Chen JH, Lin JW, Wang HC, Lee LN, Yang PC. Outcome and prognostic factors for patients with non-small-cell lung cancer and severe radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2002;54:735–741. doi: 10.1016/s0360-3016(02)02994-2. [DOI] [PubMed] [Google Scholar]
- 7.Portillo K, Arriaga I, Ruiz-Manzano J. Fibroelastosis pleuropulmonar: ¿es también una entidad idiopática? Arch Bronconeumol. 2015;51:509–514. doi: 10.1016/j.arbres.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XJ, Sun JG, Sun J, Ming H, Wang XX, Wu L, Chen ZT. Prediction of radiation pneumonitis in lung cancer patients: a systematic review. J Cancer Res Clin Oncol. 2012;138:2103–2116. doi: 10.1007/s00432-012-1284-1. [DOI] [PubMed] [Google Scholar]
- 9.Kong F-MS, Wang S. Nondosimetric risk factors for radiation-induced lung toxicity. Semin Radiat Oncol. 2015; 25:100–9. DOI: 10.1016/j.semradonc.2014.12.003 [DOI] [PMC free article] [PubMed]
- 10.Barthelemy-Brichant N, Bosquée L, Cataldo D, Corhay J-L, Gustin M, Seidel L, Thiry A, Ghaye B, Nizet M, Albert A, Deneufbourg L-M, Bartsch P, Nusgens B. Increased IL-6 and TGF-beta1 concentrations in bronchoalveolar lavage fluid associated with thoracic radiotherapy. Int J Radiat Oncol Biol Phys. 2004;58:758–767. doi: 10.1016/S0360-3016(03)01614-6. [DOI] [PubMed] [Google Scholar]
- 11.Rübe CE, Palm J, Erren M, Fleckenstein J, König J, Remberger K, Rübe C. Cytokine plasma levels: reliable predictors for radiation pneumonitis? PLoS ONE. 2008;3:e2898. doi: 10.1371/journal.pone.0002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong FM, Ao X, Wang L, Lawrence TS. The use of blood biomarkers to predict radiation lung toxicity: A potential strategy to individualize thoracic radiation therapy. Vol. 15, Cancer Control. 2008. p. 140–50. DOI: 10.1177/107327480801500206 [DOI] [PubMed]
- 13.Guerrero T, Johnson V, Hart J, Pan T, Khan M, Luo D, Liao Z, Ajani J, Stevens C, Komaki R. Radiation pneumonitis: local dose versus [18F] fluorodeoxyglucose uptake response in irradiated lung. Int J Radiat Oncol Biol Phys. 2007;68:1030–1035. doi: 10.1016/j.ijrobp.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 14.De RD, Houben A, Aerts HJWL, Dehing C, Wanders R, Öllers M, Dingemans A-MC, Hochstenbag M, Boersma L, Borger J, Dekker A, Lambin P. Increased 18F-deoxyglucose uptake in the lung during the first weeks of radiotherapy is correlated with subsequent Radiation-Induced Lung Toxicity (RILT): A prospective pilot study. Radiother Oncol. 2009;91:415–420. doi: 10.1016/j.radonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Petit SF, Van Elmpt WJC, Oberije CJG, Vegt E, Dingemans AMC, Lambin P, Dekker ALA, De Ruysscher D. [18F]fluorodeoxyglucose uptake patterns in lung before radiotherapy identify areas more susceptible to radiation-induced lung toxicity in non-small-cell lung cancer patients. Int J Radiat Oncol Biol Phys. 2011;81:698–705. doi: 10.1016/j.ijrobp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Castillo R, Pham N, Ansari S, Meshkov D, Castillo S, Li M, Olanrewaju A, Hobbs B, Castillo E, Guerrero TM. Pre-radiotherapy FDG PET predicts radiation pneumonitis in lung cancer. Radiat Oncol. 2014;9:74. doi: 10.1186/1748-717X-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo R, Pham N, Castillo E, Aso-Gonzales S, Ansari S, Hobbs B, Palacio D, Skinner H, Guerrero TM. Pre-radiation therapy fluorine helps identify patients with esophageal cancer at high risk for radiation pneumonitis. Radiology. 2015;275:822–831. doi: 10.1148/radiol.14140457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute of Cancer. Common Terminology Criteria for Adverse Events (CTCAE ), Version 4.0, DCTD, CTI, NIH, DHHS. NIH Publication. 2009. 0–71 p. DOI: 10.1186/s12955-016-0426-6
- 19.De Ruysscher D, Faivre-Finn C, Nestle U, Hurkmans CW, Le Péchoux C, Price A, Senan S. European organisation for research and treatment of cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. J Clin Oncol. 2010;28:5301–5310. doi: 10.1200/JCO.2010.30.3271. [DOI] [PubMed] [Google Scholar]
- 20.Giraud P, Antoine M, Larrouy A, Milleron B, Callard P, De Rycke Y, Carette MF, Rosenwald JC, Cosset JM, Housset M, Touboul E. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys. 2000;48:1015–1024. doi: 10.1016/s0360-3016(00)00750-1. [DOI] [PubMed] [Google Scholar]
- 21.Senan S, Chapet O, Lagerwaard FJ, Ten HRK. Defining target volumes for non-small cell lung carcinoma. Semin Radiat Oncol. 2004;14:308–314. doi: 10.1016/j.semradonc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, Diekemper R, Detterbeck FC, Arenberg DA. Treatment of stage III non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143:e314S–340S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 23.Kong FM, Ritter T, Quint DJ, Senan S, Gaspar LE, Komaki RU, Hurkmans CW, Timmerman R, Bezjak A, Bradley JD, Movsas B, Marsh L, Okunieff P, Choy H, Curran WJ, Jr, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: Atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys. 2011;81:1442–1457. doi: 10.1016/j.ijrobp.2010.07.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brusasco V, Crapo R, Viegi G. Coming together: The ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J. 2005;26:1–2. doi: 10.1183/09031936.05.00034205. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough FAL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 26.Boellaard R, Doherty MJO, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, Oyen WJ, Kotzerke J, Hoekstra OS, Pruim J, Marsden PK, Tatsch K, Hoekstra CJ, Visser EP, Arends B, Verzijlbergen FJ, Zijlstra JM, Comans EF, Lammertsma AA, Paans AM, Willemsen AT, Beyer T, Bockisch A, Schaefer-Prokpo C, Delbeke D, Baum RP, Chiti A and Krause BJ. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010; 37:181–200. DOI: 10.1007/s00259-009-1297-4 [DOI] [PMC free article] [PubMed]
- 27.Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991;32:623–648. [PubMed] [Google Scholar]
- 28.Marrugat J, Vila J, Pavesi M, Sanz F. Estimation of the sample size in clinical and epidemiological investigations. Med Clin (Barc). 1988;111(7):267–76. [PubMed] [Google Scholar]
- 29.Xaubet A, Ancochea J, Morell F, Rodríguez-Arias JM, Villena V, Blanquer R, Montero C, Sueiro A, Disdier C, Vendrell M, Spanish Group on Interstitial Lung Disease, SEPAR. Report on the Incidence of Interstitial Lung Diseases in Spain. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(1):64–70. [PubMed]
- 30.Ley JA, Borbón GA, Ochoa Carrillo FJ, Escobar RV, Rojas SH, Estrada G. Valor estandarizado de captación máximo, determinado con Tomografía Computarizada. “Primera experiencia en México” (Spanish). An Radiol Mex. 2007; 6:113–9.
- 31.Lopez Guerra JL, Gomez DR, Zhuang Y, Levy LB, Eapen G, Liu H, Mohan R, Komaki R, Cox JD, Liao Z. Changes in pulmonary function after three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, or proton beam therapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:E537–E543. doi: 10.1016/j.ijrobp.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB, De Petris L, Stitt L, Rodrigues G. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85(2):444–50. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomes M, Teixeira AL, Coelho A, Araújo A and Medeiros R. the role of inflammation in lung cancer. In: B. B. Aggarwal et al. (eds.), Inflammation and cancer, advances in experimental medicine and biology. Porto: Springer Basel; 2014. p. 1–23. DOI: 10.1007/978-3-0348-0837-8_1 [DOI] [PubMed]
- 34.Keane MP. The role of chemokines and cytokines in lung fibrosis. Vol. 17, European Respiratory Review. 2008. p. 151–6.
- 35.Tang W, Yang L, Qin W, Yi MX, Liu B, Yuan XL. Impact of genetic variant of HIPK2 on the risk of severe radiation pneumonitis in lung cancer patients treated with radiation therapy. Radiat Oncol. 2020;15(1):9. doi: 10.1186/s13014-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Scsnyder B, Akira S, Quesniaux V.F.J, Lagente V, Ryffel B and Couillin I. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007; 117:3786–99. DOI: 10.1172/JCI32285 [DOI] [PMC free article] [PubMed]
- 37.Mathew B, Jacobson JR, Siegler JH, Moitra J, Blasco M, Xie L, Unzueta C, Zhou T, Evenoski C, Al-Sakka M, Sharma R, Huey B, Bulent A, Smith B, Jayaraman S, Reddy NM, Reddy SP, Fingerle-Rowson G, Bucala R, Dudek SM, Natarajan V, Weichselbaum RR and Garcia J.G.N. Role of migratory inhibition factor in age-related susceptibility to radiation lung injury via NF-E2-related factor-2 and antioxidant regulation. Am J Respir Cell Mol Biol. 2013; 49:269–78. DOI: 10.1165/rcmb.2012-0291OC [DOI] [PMC free article] [PubMed]
- 38.Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, di Padova F, Fick L, Charron S, Lagente V, Eberl G, Le Bert M, Quesniaux VF, Huaux F, Leite-de-Moraes M, Ryffel B, Couillin I. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS ONE. 2011;6:e23185. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaufman J, Sime PJ, Phipps RP. Expression of CD154 (CD40 ligand) by human lung fibroblasts: differential regulation by IFN-gamma and IL-13, and implications for fibrosis. J Immunol. 2004;172:1862–1871. doi: 10.4049/jimmunol.172.3.1862. [DOI] [PubMed] [Google Scholar]
- 40.Senoo T, Hattori N, Tanimoto T, Furonaka M, Ishikawa N, Fujitaka K, Haruta Y, Murai H, Yokoyama A, Kohno N. Suppression of plasminogen activator inhibitor-1 by RNA interference attenuates pulmonary fibrosis. Thorax. 2010;65:334–340. doi: 10.1136/thx.2009.119974. [DOI] [PubMed] [Google Scholar]
- 41.Liu B, Tang Y, Yi M, Liu Q, Xiong H, Hu G, Yuan X. Genetic variants in the plasminogen activator inhibitor-1 gene are associated with an increased risk of radiation pneumonitis in lung cancer patients. Cancer Med. 2017;6:681–688. doi: 10.1002/cam4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available to all interested researchers on reasonable request from corresponding author.