Abstract
Introduction:
Stroke is a sexually dimorphic disease. While women account for more stroke deaths, recent data show that after adjusting for age and pre-stroke functional status, mortality is higher in men. Immune responses are key determinants of stroke outcome and may differ by sex. This study examined sex differences in central and peripheral T cell immune responses, systemic effects on gut permeability and microbiota diversity and behavioral outcomes after stroke in aged mice. We hypothesized that there are sex differences in the immune response to stroke in aged animals.
Methods:
C57BL/6N mice (20-22 months) were subjected to 60 min middle cerebral artery occlusion, or sham surgery. T cells were quantified in brain and blood at 3, 7 and 15 days (d) post-stroke by flow cytometry. Peripheral effects on gut permeability and microbiota diversity, as well as neurological function were assessed up to 14d, and at 21d (cognitive function) post-stroke. Brain glial fibrillary acidic protein (GFAP) expression was evaluated at 42d post-stroke.
Results and Discussion:
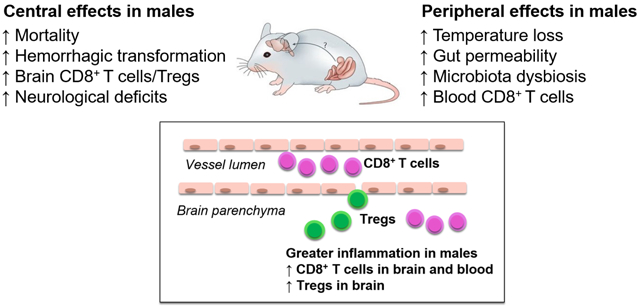
Mortality (50% vs 14%, p<0.05) and hemorrhagic transformation (44% vs 0%) were significantly higher in males than in females. No difference in infarct size at 3d were observed. Peripherally, stroke induced greater gut permeability of FITC-dextran in males at d3 (p<0.05), and non-reversible alterations in microbiota diversity in males. Following the sub-acute phase, both sexes demonstrated a time-dependent increase of CD4+ and CD8+ T cells in the brain, with significantly higher levels of CD8+ T cells and Regulatory T cells in males at d15 (p<0.01). Aged males demonstrated greater neurological deficits up to d5 and impaired sensorimotor function up to d15 when assessed by the corner asymmetry test (p<0.001 and p<0.01, respectively). A trend in greater cognitive decline was observed at d21 in males. Increased GFAP expression in the ischemic hemisphere, indicating astroglial activation and gliosis, was demonstrated in both males and females 42d post-stroke. Our findings indicate that despite a similar initial ischemic brain injury, aged male mice experience greater peripheral effects on the gut and ongoing central neuroinflammation past the sub-acute phase after stroke.
Keywords: Ischemic stroke, T cells, neuroinflammation, sex differences, middle cerebral artery occlusion, CD8+ T cells, Tregs, flow cytometry
Graphical Abstract
1. Introduction
Stroke is a cerebrovascular disease that presents with sex differences in risk, prevalence and outcome (Benjamin et al., 2018). While the onset of stroke is earlier in men, the lifetime risk is higher in women due to the larger population of aged women (Ahnstedt et al., 2016; Towfighi et al., 2007). Women suffer from poor functional outcomes, are less likely to get discharged to home and account for higher number of stroke deaths (Ahnstedt et al., 2016; Glader et al., 2003; Go et al., 2014; Reeves et al., 2009). However, age, pre-stroke disability and stroke severity are major drivers of outcome and mortality after stroke in clinical populations (Lisabeth et al., 2015). This is evident in a recent meta-analysis study where sex differences in the mortality rate ratio were fully reversed after adjusting for age, pre-stroke function, stroke severity, as well as a history of atrial fibrillation (Phan et al., 2017). In unadjusted analysis, women were 35% more likely than men to be dead 1 year after the stroke, while in adjusted analysis mortality was higher in men (Phan et al., 2017). Sex differences in human stroke is complex; as the response to injury and stroke etiology vary with age. Differences cannot be fully explained by sex disparities in demographics, pre-stroke function and clinical factors (Lisabeth et al., 2015; Phan et al., 2019).
Sex differences in ischemic stroke are not limited to humans but also apparent in the experimental setting. Numerous studies have shown that young female animals are protected from ischemic stroke and demonstrate smaller brain injury than age-matched males (Alkayed et al., 1998; Hall et al., 1991; Manwani et al., 2013). These sex differences have mostly been attributed to sex hormones, as the protection is lost and brain injury is aggravated in ovariectomized and reproductively senescent middle-aged females (Alkayed et al., 2000; Dubal et al., 1998; Manwani et al., 2013; Simpkins et al., 1997). Aged animals of both sexes sustain smaller ischemic injury after stroke (young females still exhibit the smallest injury) yet aged animals have higher mortality and morbidity, a paradox that is also evident in humans (Agarwal et al., 2013; Liu et al., 2012; Manwani et al., 2013; Roussel et al., 2009). In prior studies, we found no significant differences in infarct volumes or mortality in aged males versus aged females (20-22 months), but this study was limited, as only acute endpoints were examined (24 hours) (Manwani et al., 2013). Although stroke is a disease of the elderly, and despite these apparent sex differences, a limited number of studies on ischemic stroke have included aged animals of both sexes. The present study is the first that not only studies sex differences in aged animals, but incorporates chronic time-points of 15, 21 and 42 days after ischemic stroke, in addition to subacute time-points of 3 and 7 days.
Ischemic stroke induces a profound inflammatory response that is considered as an important pathological mechanism and contributor to stroke outcome (Chamorro et al., 2012). Neutrophils have been implicated in many detrimental processes in the acute phase of stroke, including capillary plugging, the no-reflow phenomenon, oxidative stress and hemorrhagic transformation (del Zoppo et al., 1991; Garcia et al., 1994; Jickling et al., 2015). Despite promising experimental data, early clinical studies failed to show benefit from neutrophil targeted therapies (Jickling et al., 2015). More recently, examination and targeting of other peripheral immune cells in ischemic stroke have been investigated (Jickling et al., 2015; Kleinschnitz et al., 2011; Liesz et al., 2011; Yilmaz et al., 2006). Yilmaz et al. demonstrated that CD4+ and CD8+ T cells contribute to the inflammatory response and brain injury associated with experimental stroke (Yilmaz et al., 2006). This was later supported by Liesz et al. showing infiltrating T cells were the principal mediators of delayed ischemic injury (Liesz et al., 2011). The delayed and prolonged dynamics of T cell brain infiltration provides a promising target for stroke therapy. It was recently demonstrated that aging is associated with an accumulation of resident effector memory CD8+ T cells in brain (Ritzel et al., 2016). However, T cell responses after stroke in aged animals have been less studied, especially in aged females (Ahnstedt and McCullough, 2019). Biological sex is an important factor to include in studies of immune responses as significant male-female differences exist in healthy and disease states throughout the lifespan (reviewed in (Ahnstedt and McCullough, 2019)). Therefore, the current study was aimed to investigate temporal and sex-specific T cell immune responses after ischemic stroke in mice of advanced age (>20 months) to more closely mimic an aging human population. In addition, neurological deficits, sensorimotor and cognitive function were evaluated in these animals. Ischemic stroke is increasingly recognized as a systemic disease with reported effects on spleen, heart and gut. As the gut hosts a large immune cell pool, in particular T cells, we determined peripheral effects on gut permeability, fecal microbiota diversity and short-chain fatty acid levels.
2. Methods
2.1. Animals
Aged (20-22 months) C57BL/6N mice were purchased from the National Institute of Aging (NIA) and acclimatized in house for two months before use. All animals had access to chow and water ad libitum. Animal procedures were performed in accordance with National Institutes of Health Guidelines for the care and use of laboratory animals and approved by the Animal Welfare Committee at the University of Texas Health Science Center at Houston, Texas (AWC-15-0140).
2.2. Middle cerebral artery occlusion model of ischemic stroke
Cerebral ischemia was induced by the intraluminal filament model of middle cerebral artery occlusion for 60 min, as described previously (Koizumi et al., 1986; Longa et al., 1989). Animals were kept under general isoflurane anesthesia on a temperature-controlled heating pad to maintain rectal temperatures of ~37°C during the initial filament placement. Bupivacaine (0.25%) was administered subcutaneously at the skin incision site (ventral midline neck incision). Right middle cerebral artery occlusion was performed by the insertion and advancement of a 0.23 mm silicone-coated monofilament (6.0) (Doccol, Redlands, CA) into the right internal carotid artery. Animals were allowed to awaken after occlusion to confirm intra-ischemic neurological deficits, and then re-anesthetized for suture removal/reperfusion. Postoperative care included 1cc 0.9% NaCl subcutaneously two times a day and soft chow for the first 7 days. Mice were only excluded on surgical grounds e.g. confirmed bleed around the right external carotid artery stump or at base of the skull (6 females and 2 males).
2.3. Placement of wireless temperature telemeter
In some mice, core body temperature was monitored by a miniature wireless Implantable Programmable Temperature Transponder (IPTT-300, BioMedic DataSystems) placed under the skin on the back of the animal. A previous study showed similar measurements in core temperature between this method and when an abdominal thermocouple was placed (Feketa et al., 2014).
2.4. Gut permeability assay in vivo
Intestinal permeability in vivo was assessed by oral gavage of 4000-Da fluorescent dextran (Sigma-Aldrich) to mice that had been fasted for 5 h (600 mg/kg, 100 mg/ml). Blood was collected one hour after gavage and plasma was prepared by centrifugation at 3000xg for 6 min. Undiluted plasma was analyzed for FITC-dextran concentration using a fluorescence spectrophotometer (Infinite 200, Tecan, Männedorf, Switzerland) at the excitation wavelength of 485 nm and the emission wavelength of 535 nm. Standard curves for calculating the FITC-dextran concentration in the samples was prepared by diluting FITC-dextran in non-treated plasma.
2.5. Terminal histopathology
Three and 42 days post stroke, animals were euthanized by intra-peritoneal avertin overdose followed by transcardial perfusion. Brain tissues were cleared and fixed prior to removal by transcardial perfusion with heparinized PBS followed by perfusion with 4% paraformaldehyde (PFA). Subsequently, brains were placed in 4% PFA for 24 hours, and then transferred to a dehydrating solution (30% w/v sucrose in PBS). Brains were cut into 30-μm sections using a sliding microtome (HM 450, Thermo Fisher, Houston, Texas), and stored in anti-freeze solution (30% glycerol, 30% ethylene glycol in PBS) at −20°C. Every eight section were mounted onto glass slides, and each slide was simultaneously stained with cresyl violet (5 w/v%, pH 3.9) to visualize the infarct area via cell death. Analysis of the infarct size was quantified and analyzed using the Sigma Scan Pro5™ computer software as described previously (Liu et al., 2009; Ritzel et al., 2017; Swanson et al., 1990).
For glial fibrillary protein (GFAP) analysis, brain sections at +0.74 mm and −1.28 mm from bregma were mounted onto glass slides and placed in 10 mM sodium citrate buffer pH 6 containing 0.05% Tween-20 followed by 30 minutes of incubation in a rice cooker. Following, 1 h blocking with PBS/5%BSA/0.01% Tween-20, sections were incubated with mouse anti-GFAP direct-conjugated to Cy3 overnight at 4°C. Sections were microscoped with a Leica DMi8 microscope at 10X. Analysis was performed by an investigator blinded to the study details by measuring the mean gray value in 5 pre-selected regions of interests (ROIs, see Figure 5C).
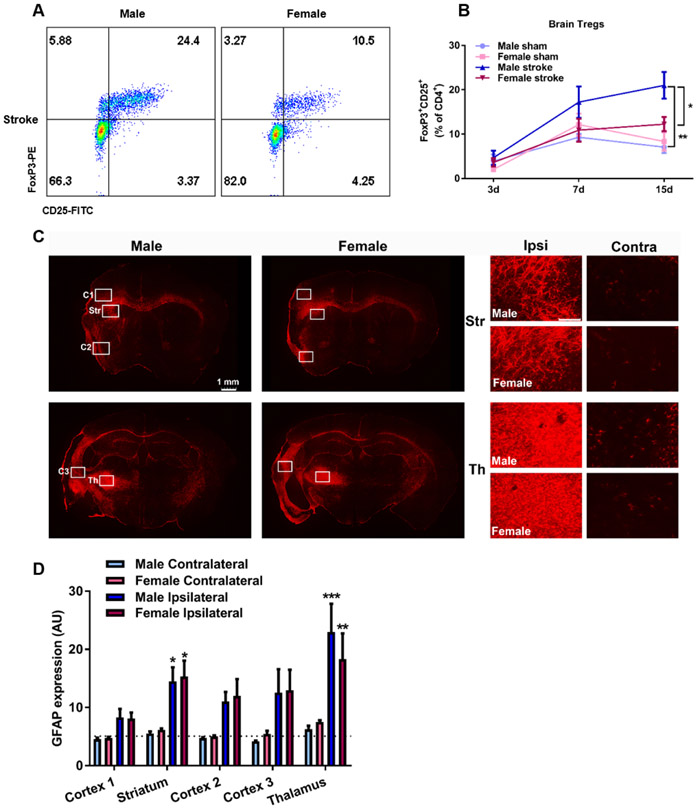
Fig. 5.
Infiltration of Regulatory T cells (Tregs) and increased glial fibrillary acidic protein (GFAP) expression in the brain after ischemic stroke. A) Representative flow cytometry dot plots of brain FoxP3+CD25+CD4+ T cells in male and female 15 days post-stroke. B) Quantitative analysis of Tregs at 3d, 7d and 15d post-stroke. Two-way ANOVA, *p<0.05 male stroke vs female stroke, ***p<0.001 male stroke vs male sham, sham: n=5-6, stroke: n=7-12. C) Representative images of GFAP expression in the brain 42d post-stroke, regions of interests are depicted. C1-3= Cortex area 1-3, Str = Striatum, Th = Thalamus, Right panel scale bar: 500 μm D) Quantitative analysis of GFAP in five different regions were performed in a blinded manner. GFAP expression was increased in striatum and thalamus ROIs, Two-way ANOVA, *p<0.05, **p<0.01, ***p<0.001 Ipsilateral vs Contralateral within respective sex, n=5-6. Average sham expression is depicted by the dotted line.
2.6. Flow cytometry
Mice were euthanized with avertin and blood was drawn by cardiac puncture using heparin-coated needles. Subsequently, the mice were transcardially perfused with 60 mL of cold, sterile PBS, and the brains were collected. The brainstem, olfactory bulbs and cerebellum were removed before the brain was divided into ipsilateral and contralateral hemispheres. The ipsilateral hemisphere was placed in red phenol-red free RPMI 1640 medium (Thermo-Fischer Scientific Waltham, MA) supplemented with 5% charcoal-stripped fetal bovine serum (Sigma-Aldrich, St Louis, MO), 25 mM HEPES and Pen-strep. The brain tissue was mechanically and enzymatically digested in collagenase/dispase (0.03 mg/ml, Roche Diagnostics, Risch, Schweiz) and DNASE I (0.6 mg/ml, Roche Diagnostics) at 37°C and 45 rpm for 45 min. The cell suspension was filtered through a 70 μm filter. Leukocytes were collected from the interphase of a 70/30% Percoll gradient followed by blocking of Fc receptors with CD16/32 (BioLegend, San Diego, CA) prior to staining of surface markers. Cells were stained for viability (Fixable Live/Dead Aqua Stain, Thermo Fisher Scientific, Waltham, MA) for 30 minutes, followed by incubation with primary antibodies (CD45-BV605, CD8-BV421 and CD69-PE-Cy7 from Biolegend, and CD11b-PerCP-Cy5.5, CD4-APC, CD25-FITC and CD3-APC-Cy7 from TONBO Biosciences, San Diego, CA) for 30 min at room temperature. Subsequently, leukocytes were fixed and permeabilized with FoxP3 staining buffer set (eBioscience, Thermo Fisher Scientific) and stained with FoxP3-PE (eBioscience, Thermo Fisher Scientific) for 45 min at room temperature. Leukocytes were re-suspended in FACS buffer and count bright counting beads (Thermo Fischer Scientific) were added prior to reading in a Cytoflex S flow cytometer (Beckman-Coulter, Brea, CA).
2.6. Fecal collection and 16S sequencing
Fecal samples containing microbiota representative of the distal colon were collected from mice at baseline, and 3, 7 and 14d post-stroke, and stored in sterile tubes at −80°C until processing. Bacteria taxa in each sample were analyzed by amplifying the V4 to V5 hypervariable regions of 16S ribosomal RNA using high-throughput sequence analysis (Illumina MisSeq platform, Illumina, San Diego, CA) (Nelson et al., 2014). 16S rRNA sequences were clustered into operational taxonomic units (OTUs), with 97% similarity, by closed reference OUT-picking using the UCLUST algorithm and GreenGenes reference database (v13.5) (Caporaso et al., 2010; DeSantis et al., 2006; McDonald et al., 2012). Sequences were controlled for chimeras using ChimeraSlayer with standard options as implemented in Quantitative Insights Into Microbial Ecology (QIIME versions 1.6 and 1.7) (Caporaso et al., 2010). Sequences that were not clustered were identified using the Ribosomal Database Project to the lowest taxonomic level possible. Before any downstream analysis, the data were randomly rarified to 10,000 sequences per sample.
2.8. Short chain fatty acids analysis
Short chain fatty acids (SCFAs) were analyzed in fecal samples as previously described (Han et al., 2015). Briefly, fecal samples were diluted 1:10 (w/v) in 50% aqueous acetonitrile and homogenized. SCFAs in the supernatant were derivatized using 12C6-3-nitrophenlhydrazine (200mM) and (N-ethyl-N’-(dimethylaminopropyl) carbodiimide hydrolyzed urea derivative (120mM). Thereafter, samples were spiked with derivatized 13C6-3NPH-HCl. Aliquots (5 μl) were analyzed by liquid chromatography/mass spectrometry (electrospray ionization negative mode) using an Acquity UPLC HSS T3 1.8 mm, 2.13100mm high-performance liquid chromatography column.
2.9. Behavioral testing
Behavioral testing was performed at the same time of the day for each testing time point. All animals were screened prior to use, and animals with any pre-stroke paw bias were excluded. Animals were then randomly assigned into stroke/sham groups. All testing was performed by a blinded investigator. Before testing, the animals were allowed to acclimatize for 1 hour in the testing room in their home cages. The equipment was cleaned with 70% ethanol between trials. A camera was placed above the testing arena of NORT and Barnes maze to monitor performance through a video tracking system (EthoVision XT, Noldus, Wageningen, The Netherlands).
2.9.1. Neurological deficit scoring:
Neurological deficits were assessed using the neurological deficit scoring (NDS) system as described previously (Bederson et al., 1986; Li et al., 2004). NDS was scored at reperfusion and then daily up to 14d post-stroke using a 5-point scale: 0 = no deficit: 1 = forelimb weakness and torso turning to the ipsilateral side when held by tail; 2 = circling to affected side; 3 = unable to bear weight on the affected side; and 4 = no spontaneous locomotor activity, or barrel rolling.
2.9.2. Corner test:
Sensorimotor asymmetry was assessed at 3, 7 and 14d post-stroke using the corner test as described previously with minor modifications (Zhang et al., 2002). The mouse was placed between two cardboard pieces (30 × 20 × 1 cm3). Thereafter the two plexiglas boards were slowly moved to form a corner of 30° with a small opening along the joint between the two boards. When facing the corner both sides of the vibrissae were stimulated by the two boards.
The mouse then rears forward and upward before turning around to face the open end. Mice subjected to ischemic stroke will preferentially turn toward the non-impaired, ipsilateral (right) side while mice subjected to sham surgery will turn either left or right with no preference. Twenty trials were performed for each mouse and a turning score of right turns over total number of turns was calculated. This test detects integrated sensorimotor function as it involves both stimulation of vibrissae (sensory/neglect) and rearing (motor response).
2.9.3. Adhesive removal test:
This test evaluates both somatosensory and motor function and was adapted from Bouet et al. (Bouet et al., 2009). Briefly, an adhesive tape circle (Ø 12 mm) was placed on the impaired, contralateral (left) forelimb paw. Initially, we performed pilot experiments with an additional adhesive placed on the non-impaired, ipsilateral (right) forelimb paw, but after ischemic stroke in mice of this advanced age (>20 months) and early time-point after stroke (3 and 7d) none of the mice succeeded in removing the adhesive before the time cap of 3 minutes. Time to removal of the adhesive (in seconds) was recorded for each mouse.
2.9.4. Hang wire test:
Deficits in motor strength were tested using the hang wire test (Spychala et al., 2018) at 3 and 7d post-stroke. Mice were placed in the center of a wire-cage top (18 × 9 inches) that was slowly inverted and placed 36 inches above a cage containing regular bedding. Latency to fall (in seconds) was recorded and 300 seconds was set as the time out period.
2.9.5. Novel object recognition test:
In this test performed at d14, the mouse was first presented with two similar objects (familiarization session), and then one of the objects was replaced by a novel object during a second session (test session) to provide an index of recognition memory. The current protocol entailed no habituation phase and both the familiarization and test session was limited to 10 min with 5 min in between trials (Leger et al., 2013). The two objects used were bottle caps of different size, texture and brightness with the goal to maximize the difference between the objects without unintended induced preference for one of the objects.
2.9.6. Barnes maze:
Spatial learning memory was evaluated by Barnes maze, a test performed on an elevated circular platform (Ø: 36 inches) with 20 evenly spaced holes (Ø: 2 inches) (Sunyer et al., 2007). A randomly chosen hole was designated as the escape hole allowing animals to escape the platform into a dark rectangular box below the platform. By using spatial clues positioned around the platform, mice learn the location of the escape hole during training trials. All training trials and the test trial were performed in a dark room, and the entire platform was lit with bright white light. At d21 following stroke, animals received 3 training trials followed by a test trial 4 hours later. During the first training trial, the mouse was placed in the center of the arena and then guided to the escape hole in a clear cylinder. The mouse was allowed to explore the escape hole for 1 minute. During the second and third trials the animal was again placed in the center of the platform and allowed to explore the arena freely for 5 minutes. If the animal did not find the escape hole by the end of the trial it was guided to it using the same clear cylinder. The arena was cleaned carefully between trials. The test trial was terminated when the animal entered the escape hole, or at the end of the test period which was set to 3 minutes. Any animals that did not manage to find the escape hole at least once during the training trials was excluded.
2.9. Statistical Methods
Descriptive graphs were provided for outcomes (Figure 1-6). For data analysis, one way or two-way ANOVA were used to analyze the sex effect and/or effect of the other factor (Figure 1A, 2B, 3A-D, 4B-E, 5B, 5D, 6C-6E), and Sidak’s method was used for multiple comparison adjustment. Note that in Figure 4B-C, and 5B, the mice on different days are not the same, so two-way ANOVA was used. For outcomes repeatedly measured on the same mice (Figure 2A, 2C, 6A, 6B), linear mixed models or generalized estimating equation (GEE) models were used to account for the within-subject correlation, with multiple testing adjusted for group comparisons on different time points. Finally, Kaplan-Meier estimator (Figure 1B) and cumulative incidence function (Figure 6F) were provided for survival analysis and log rank test was used for group comparison. All analyses were performed in SAS 9.4 (Cary, NC) or GraphPad Prism 7.
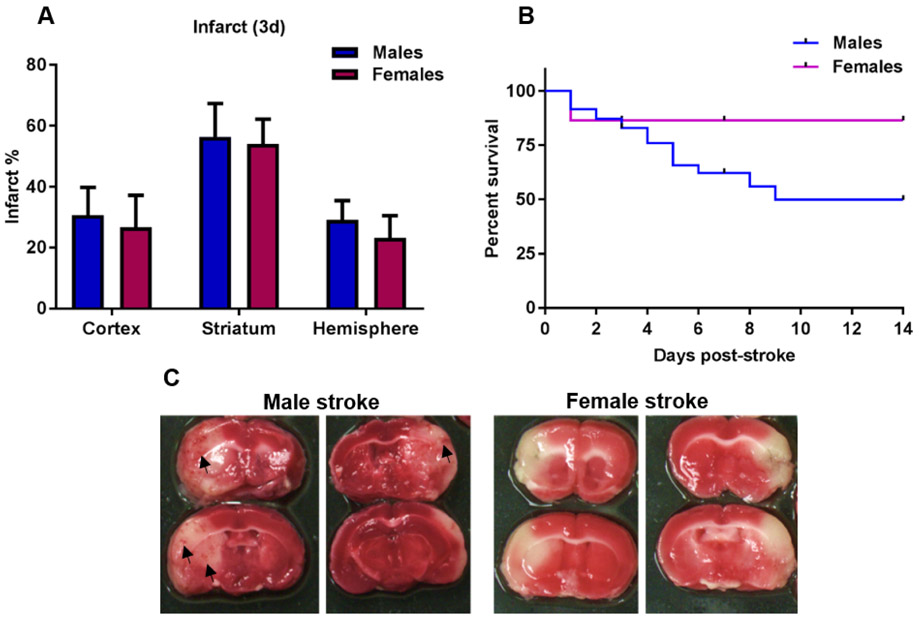
Fig. 1.
Aged males experience higher mortality and hemorrhagic transformation rates than females despite no difference in infarct size. A) Percent infarcted brain area in cortex, striatum and hemisphere 3 days post-stroke measured on 2,3,5-triphenyltetrazolium chloride (TTC) stained brain sections, n=5-6. B) Kaplan Meier survival analysis demonstrate significantly higher mortality in males vs females, Log-rank (Mantel-Cox) test p<0.05, n=44-47. C) Representative TTC images of male (4 days post-stroke) and female (1 day post-stroke) mortality cases. Hemorrhagic transformation (depicted with arrow heads) were found in 44% of the males and in 0% of the females that died.
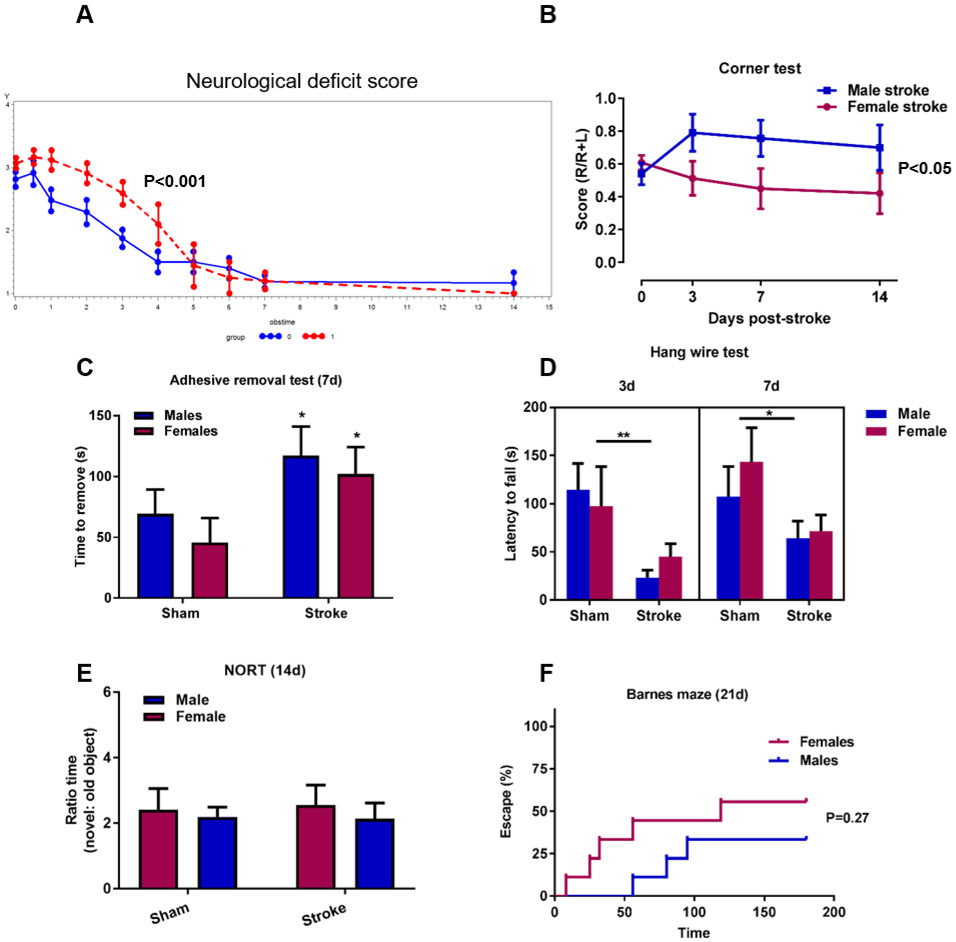
Fig. 6.
Greater early neurological deficits, impaired recovery and cognitive deficits in males. A) Neurological deficit scores (NDS) were greater in males (red dotted line) the first days after stroke (p<0.0001) despite a similar initial injury on day 0. The decreasing rate of the score were significantly slower in males indicating impaired recovery compared to females (blue solid line) (P=0.015), R package for joint modelling of longitudinal NDS and time-to-event data. 0: females, 1: males, obstime = days post-stroke. B) Corner test to evaluate sensorimotor deficits show significantly greater right turn preference in aged males compared to females. *p<0.05, linear mixed model with robust estimate male stroke vs female stroke, n=8-12. C) Adhesive removal taste demonstrate longer elapsed time to removal in males and females 7d post-stroke compared to sham groups, Two-way ANOVA, effect of stroke, *p<0.05, sham: n=6, stroke: n=8-9. D) Motor strength evaluated with the hang-wire test demonstrate stroke-induced deficits in both sexes 3d and 7d after stroke, Two-way ANOVA, effect of stroke *p<0.05, **p<0.01, sham: n=6, stroke: n=9-10. E) Novel object recognition test at 14d demonstrated no discrimination for the new object over the old object. G) Barnes maze was used to evaluate cognitive deficits in a 21d chronic cohort. Time to find the escape hole and escape success rate (%) is graphed demonstrating a tendency towards longer time to escape in males, Log-rank (Mantel-Cox), P=0.27, n=9.
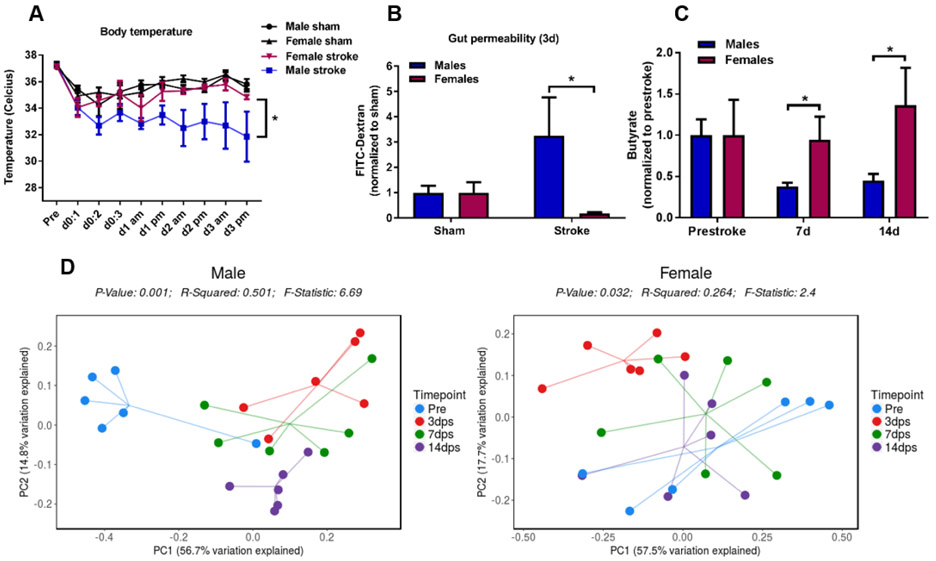
Fig. 2.
Stroke induces greater systemic effects on temperature regulation and gut characteristics in aged males than in females. A) Core body temperature was significantly lower in male stroke mice compared to females, linear mixed model with robust estimate, Tukey-Kramer multiple comparison adjustment, * p<0.05, sham: n=6, stroke: n=9-10. B) Gut permeability i.e. gut leakiness of 4 kDa FITC-dextran was significantly greater in males 3 days after stroke than in females, Two-way ANOVA with Sidak’s multiple comparison test, * p<0.05, n=6-7. C) Short-chain fatty acid (SCFA) analysis demonstrate greater loss of fecal butyrate in males than in females 7 days post-stroke, generalized estimating equation (GEE) model, **p<0.01, n=6. D) 16s sequencing of fecal samples collected pre- and post-stroke revealed long-lasting changes in β-diversity (“richness”) in aged males depicted as a Principal Coordinates Analysis (PCoA) plot, whereas in aged females the β-diversity was restored by day 7 post-stroke.
Fig. 3.
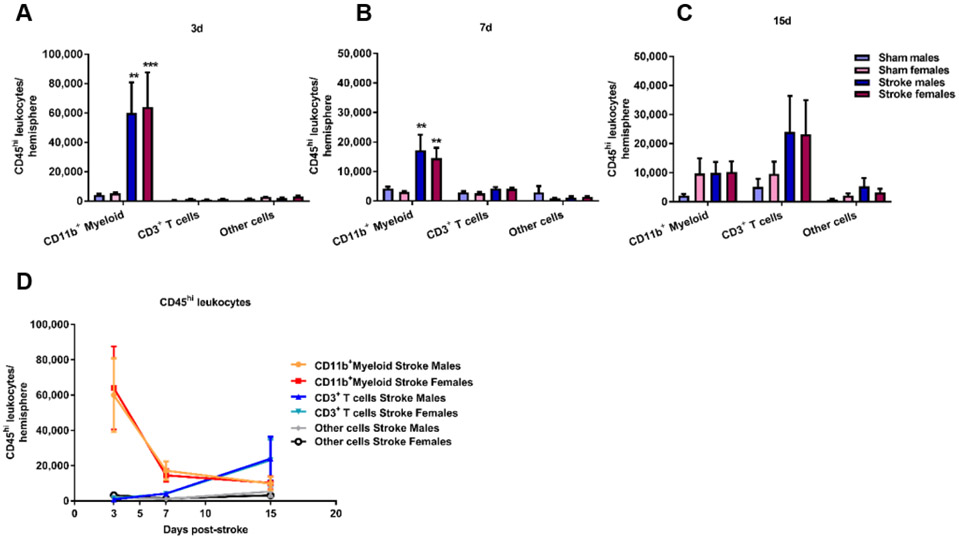
Temporal changes of brain-infiltrating myeloid and lymphoid cells after ischemic stroke in aged mice. Flow cytometry analysis on immune cells derived from the ipsilateral hemisphere 3 days (A), 7 days (B) and 15 days (C) post-stroke demonstrate an early increase of myeloid cells (CD45hiCD11b+) and a delayed increase in CD3+ T cells (D). Two-way ANOVA with Sidak’s multiple comparison test, **p<0.01, ***p<0.001 vs sham.
Fig. 4.
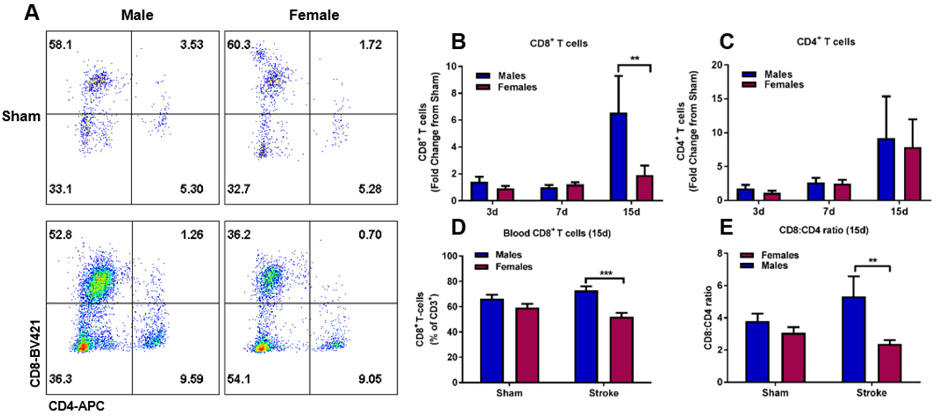
Stroke induces a time-dependent and sex-specific increase in brain CD8+ T cells after ischemic stroke. A) Representative flow cytometry dot plot of CD4+ and CD8+ T cells at 15 days post-stroke. Quantitative analysis of CD8+ (B) and CD4+ (C) T cells demonstrate significantly elevated levels of CD8+ T cells at 15 days in males compared to females, Two-way ANOVA with Sidak’s multiple comparison test, p<0.01, n=7-12. Quantitative analysis of blood CD8+ and CD4+ T cells demonstrate significantly higher CD8+ T cell levels in male stroke mice compared to females (D) and a higher CD8:CD4 ratio (E), Two-way ANOVA with Sidak’s multiple comparisons test, ***p<0.001, *p<0.05, sham: n=6, stroke: n=8-12.
3. Results
3.1. Higher mortality and hemorrhagic transformation in aged male mice after ischemic stroke
Despite similar brain injury at day 3, a time-point when the infarct has fully evolved, (Figure 1A), a significant sex-specific effect on survival probability was observed. Aged males had a significantly higher mortality compared to aged females up to 15 days post-stroke (Figure 1B, Log-Rank Mantel Cox test p<0.05). In 16 of the male mortality cases, 7 (44%) had hemorrhagic transformation with petechial bleedings (Figure 1C), while none of the females that died had hemorrhagic transformation (n=6). It should be noted that the female mortalities all occurred on day 1 as can be seen in Figure 1B, while male mortalities occurred from day 1 to day 9 post-stroke.
3.2. Aged male mice experience greater detrimental systemic effects on body temperature regulation, gut permeability and gut microbiota diversity
As seen in the Kaplan Meier curves depicted in Figure 1A, the survival probability in males and females start to deviate at day 3 post stroke suggesting other factors than the size of the ischemic core are important drivers of mortality. At this time-point we observed more pronounced sickness behavior in the aged male mice such as lack of grooming and a significant loss of body temperature (Figure 2A, male stroke vs female stroke: p<0.05, linear mixed model with robust estimate, Tukey-Kramer multiple comparison adjustment). As ischemic stroke has been shown to induce profound systemic effects on spleen, heart and gut, we examined gut permeability i.e. gut leakiness to further evaluate the peripheral effects of stroke. 4 kDa FITC-dextran was orally gavaged followed by measurement in plasma collected 1 h later. As seen in Figure 2B, aged males had 3.25 times higher permeability of FITC-dextran (leaky gut) compared to sham males, and significantly higher FITC-dextran concentration in the circulation when compared to stroke females (Figure 2B, p<0.05). In line with this, we found that levels of fecal butyrate, a short-chain fatty acid known to promote a healthy gut barrier was significantly reduced after stroke in males (Figure 2C, male stroke vs female stroke: p<0.05, generalized estimating equation (GEE) model). Other studies have demonstrated stroke-induced alterations in the gut microbiome (Singh et al., 2016; Spychala et al., 2018), but until now sex differences in the microbiota diversity after stroke in aged mice has not been evaluated. To this end, we performed 16S rRNA sequencing in fecal samples collected pre-stroke, and 3, 7 and 14 days post-stroke. As can be seen in Figure 2D, β-diversity was altered by the experimental stroke in both males and females at 3 days post-stroke (red) compared to pre-stroke (blue, p<0.01 and p<0.05, respectively). Thereafter, microbiota diversity in females is restored (similar to the pre-stroke), whereas in males, diversity at 7 and 14 days post-stroke remains altered compared to pre-stroke (p<0.01). Taken together, these results demonstrate that ischemic stroke induces more long-lasting changes in microbiota diversity in aged males compared to aged females.
3.3. Sex-specific and temporal effects of ischemic stroke on CD4+ and CD8+ T cell responses
After determining peripheral and systemic effects of ischemic stroke in aged mice, we next determined the central effects on brain T cell immune responses. We performed a temporal and quantitative analysis of post-ischemic inflammation by flow cytometry of the ischemic hemispheres at 3, 7 and 15 days post-stroke. Similar to previous observations in young male mice (Gelderblom et al., 2009), we demonstrate a peak brain infiltration of myeloid cells (CD45hi, CD11b+) at day 3 post-stroke (Sham males vs Stroke males: p<0.01, Sham females vs Stroke females: p<0.001, Figure 3A), that over time decreases significantly while the lymphocyte proportion (CD45hi, CD11b−) increases (Figure 3B-D). Overall, there were no sex differences in total myeloid cell numbers and total lymphocyte cell numbers over time (Figure 3D).
The importance of T cell immune responses in aging (Ritzel et al., 2016) and in the response to stroke has been shown in prior literature (Liesz et al., 2011; Yilmaz et al., 2006). However, less is known about the T cell immune responses in aged animals over 20 months, specifically the temporal responses and whether there are sex-specific effects. Therefore, we studied brain CD4+ and CD8+ T cell numbers at 3, 7 and 15 days following MCAO (representative dot plot at 15d, Figure 4A). As depicted in Figure 4B, we observed a delayed increase in CD8+ T cells at 15 days after stroke relative to sham (Two-way ANOVA, effect of time p<0.05). The infiltration of peripheral CD8+ T cells was significantly greater in aged males (stroke to sham fold change, males: 6.6x vs 1.9x in females, Figure 4B, p<0.01). This effect was specific to CD8+ T cells as no sex difference was observed in CD4+ T cells at 15d (Figure 4C). Similar to the observation seen in brain at 15d, aged males had higher levels of CD8+ T cells in blood than females (Figure 4D, p<0.001), with no significant changes in CD4+ T cells (Supplementary Figure S1). As a result, the ratio of CD8+ to CD4+ T cells were significantly higher in males than in females at this time-point (5.3 vs 2.4, Figure 4E, p<0.01). It is important to highlight that the observed sex differences in brain and blood CD8+ T cell levels do not reflect a sex difference in infarct size as aged male and female mice exhibited similar extent of brain injury after ischemic stroke (Figure 1A).
3.4. Regulatory T cell infiltration to the brain is pronounced in aged males after ischemic stroke
In the immune system there is a drive for balance between inflammatory and anti-inflammatory mechanisms. Tregs are an anti-inflammatory T cell subtype with the important tasks of preserving immune homeostasis. These cells limit extensive inflammation upon injury (Liesz et al., 2015). Our flow cytometry data showed a significant increase in pro-inflammatory cytotoxic CD8+ T cells at 15d post-stroke in aged males. We next measured the infiltration of anti-inflammatory Tregs, defined as CD4+CD25+FoxP3+ in the post-ischemic brain (representative dot plot at 15d, Figure 5A). In support of previous studies (Ito et al., 2019; Stubbe et al., 2013), we observed a delayed accumulation of Tregs in the brain after ischemic stroke (Figure 5B, Two-way ANOVA, effect of time p<0.001). This increase in Tregs was significantly higher in males than in females at 15d post-stroke (21.0% of CD4+ T cells vs 12.2%, Figure 5B, p<0.05, absolute counts at 15d, male stroke: 1222±757 vs female stroke: 637±330). The higher level of Tregs in the brain at d15 in parallel to the observations of elevated pro-inflammatory CD8+ T cells may be indicative of ongoing inflammation necessitating continued Treg infiltration to maintain homeostasis.
Recent data suggest that Tregs can promote neurological recovery by suppressing astrogliosis during the chronic phase of ischemic stroke (Ito et al., 2019). Therefore, we investigated whether there are sex differences in the expression of glial fibrillary acidic protein (GFAP) in brain sections from an additional 42d cohort as a marker of astroglial activation and gliosis (representative brain section and regions of interest, Figure 5C). While we did not observe a sex difference in GFAP expression, we did observe elevated levels of GFAP in the ipsilateral hemispheres of both males and females at 42d as an effect of ischemic stroke (Figure 5D, male ipsilateral vs male contralateral: p<0.001, female ipsilateral vs female contralateral: p<0.001, Two-way ANOVA with Tukey’s multiple comparisons test). Further, GFAP expression in thalamus was statistically higher compared to the three different cortical regions but not to striatum (p<0.05, Two-way ANOVA with Tukey’s multiple comparisons test). No statistical difference in GFAP expression was observed between sham and the contralateral groups (the average sham expression is depicted by the dotted line). Our data indicate that neurodegenerative processes are ongoing in multiple brain regions at a chronic time-point of 42d after ischemic stroke in both aged male and female mice.
3.5. Ischemic stroke in aged mice induced greater neurological deficits and impaired recovery in males.
It is well-known that immune responses are key contributors to outcome and recovery after ischemic stroke (Chamorro et al., 2012). Furthermore, T cells have been implicated in neuronal plasticity and cognitive function in health and disease (Kipnis et al., 2012; Selvaraj and Stowe, 2017; Zarif et al., 2018). We therefore performed a battery of behavior tests to assess neurological deficits, motor function, and sensorimotor function at 3, 7 and 14 days after stroke. An additional chronic cohort was evaluated for cognitive function at 21 days post-stroke. To avoid habituation and stress due to multiple behavioral testing, cohorts were tested using different tests and also selected based on the time-point, e.g. the 3d time-point is not suitable for cognitive tests as the mice are still recovering. Neurological deficits are reported as neurological deficit scores (Bederson et al., 1986) and are depicted in Figure 6A. As seen, although males and females experienced similar deficits acutely after ischemic stroke, recovery was impaired in aged males demonstrated by the slower declining slope in male animals (Figure 6A, p<0.05). Sensorimotor function assessed by the corner test showed greater asymmetric preference in aged males than in females from day 3 to day 14 (Figure 6B, male stroke vs females stroke, p<0.05, linear mixed model with robust estimate). This was not due to baseline differences in asymmetric preference, as the turning score (number of right turns divided by the total number of turns) was similar in males and females pre-stroke (0.54 and 0.61 respectively, Unpaired t-test, not significant, n=19). Also assessing sensorimotor function, the adhesive removal test showed stroke-induced deficits in both males and females (Figure 6C, p<0.05), with no sex-specific effects. Forelimb strength evaluated by the hang wire test demonstrated impaired grip force in both sexes at day 3 and 7 post stroke (Figure 6D, p<0.01 and p<0.05).
Cognitive decline after ischemic stroke is well recognized in patients (Kuźma et al., 2018) and in mice, and the role of T cells in cognitive function has been an area of substantial interest over the past decade (Filiano et al., 2017; Kipnis et al., 2012). Therefore we assessed cognitive function at d14 using the novel object recognition test (NORT). At this time-point both male and female mice regardless of the type of surgery, were able to discriminate the novel object (Figure 6E). Acknowledging that 14 days after the stroke surgery may be too early to assess cognitive decline, we assessed short-term spatial memory retention in an additional cohort at day 21 using Barnes maze test. We observed a trend in sex differences in cognitive function at day 21. A smaller proportion of male mice succeeded to find the escape hole compared to females (6/9 vs 4/9, Figure 6F, P=0.27) and the time to escape was longer (P=0.22, Supplementary Figure S2), suggesting that sex differences in cognitive decline after ischemic stroke may emerge over time but require further evaluation at more chronic time-points.
4. Discussion
In this study, we report several novel findings after ischemic stroke in aged mice. These include; (1) A higher mortality and significantly increased rates of hemorrhagic transformation in aged males compared to females despite equivalent infarct size at day 3 post-stroke; (2) Systemic effects of stroke were more pronounced in males, including enhanced gut permeability and long-lasting changes in fecal microbiota diversity; (3) Brain T cell immune responses were temporally distinct and sex-specific as demonstrated by a greater infiltration of CD8+ T cell and Tregs at 15 days post-stroke in males; (4) Ischemic stroke induced greater sensorimotor deficits in aged males compared to age-matched females. These data suggest that despite a similar initial ischemic brain injury, aged male mice experience greater peripheral effects and central immune changes over time.
T cells are increasingly recognized as important players in the detrimental inflammatory cascade after ischemic stroke (Selvaraj and Stowe, 2017). T cells accumulate in the brain over the first 3-5 days following ischemic stroke and can persist as late as 7 weeks post-stroke but the specific dynamics and numbers depend on the experimental stroke model used (permanent versus transient, and occlusion times) (Doyle et al., 2015; Feng et al., 2017; Gelderblom et al., 2009; Liesz et al., 2011; Selvaraj and Stowe, 2017; Stubbe et al., 2013; Vindegaard et al., 2017; Xie et al., 2018). They contribute substantially to secondary brain injury and their delayed and prolonged temporal pattern make them excellent candidate targets for therapeutic intervention. Indeed, Rag−/− mice, which lack T and B cells, showed decreased lesion volumes and neurological deficits compared to wild-type mice when subjected to ischemia and reperfusion (Yilmaz et al., 2006). Further, the findings indicate that CD4+ and CD8+ T cells contribute to inflammatory responses, brain injury and neurological deficits after ischemic stroke (Yilmaz et al., 2006).
Our data are similar to a previous study that characterized peripheral leukocyte infiltration to the brain after ischemic stroke in young mice (Gelderblom et al., 2009); an early spike in myeloid cells followed by a delayed increase in overall lymphocytes and CD3+ T cells, but the dynamics are different. While T cell infiltration in young mice peaked at day 3 post-stroke, aged mice showed only slightly increased levels of brain infiltrating T cells from day 3 to 7 but these continued to significantly increase from day 7 to 15 until reaching approximately 24,000 cells/hemisphere at day 15. The delayed infiltration of T cells might be due to antigen-dependent mechanisms as clonal expansion of T cells has been detected first at day 7 and 14 after stroke (Liesz et al., 2013). After an ischemic stroke, autoimmune responses can develop when T cells react to brain antigens from injured neurons and glia, either within the brain itself or systemically, as the blood brain barrier becomes compromised after ischemic stroke which allows for lymphocytes to enter and brain antigens to leak into the periphery (Gill and Veltkamp, 2016; Jin et al., 2018; Selvaraj and Stowe, 2017).
Age-related changes in immunity are well-known (Bupp et al., 2018) and are likely contribute to the differences seen in T cell dynamics after a severe insult such as ischemic stroke. A previous study from our group found that aging itself is associated with increased recruitment of T cells, in particular CD8+ T cells, to the brain (Ritzel et al., 2016). These CD8+ T cells were primed to potentiate neuroinflammation after ischemic brain injury. Our current findings emphasize the importance of utilizing aged stroke models when studying immune responses to potentially identify novel therapeutic targets.
While we did not observe sex-differences in overall T cell levels, there was a sex-specific and time-dependent infiltration of specific subpopulations, i.e. CD8+ T cells and Tregs. Aged male brains had approximately 6-fold higher levels of CD8+ T cells at day 15 compared to brains from sham animals, while in females the respective number was 2-fold. This was specific as the overall numbers of CD4+ T cell numbers were not different in males and females. CD8+ T cells are potent inflammatory T cells with strong cytolytic actions via release of perforin, granzymes, and interferon gamma (IFN-γ) (Janeway et al., 2001). Through the use of transgenic animals including lymphocyte deficient Rag1−/− or Rag2−/− mice, perforin-1−/− and IFN-γ−/− and specific depletion of T cell subsets such as CD8+ T cells, CD4+ T cells and natural killer T cells, emerging evidence point to a key role for cytotoxic CD8+ T cells in mediating brain injury and neurological deficits after ischemic stroke (Li et al., 2017; Liesz et al., 2011; Mracsko et al., 2014; Yilmaz et al., 2006).
Interestingly, some of these studies demonstrate that depletion of CD8+ T cells had a delayed protective effect on infarct size at day 7 and day 14, although the neutralizing antibody was given before induction of permanent MCAO (Liesz et al., 2011; Mracsko et al., 2014). We observed a delayed and prominent increase of CD8+ T cells in brain and blood at day 15 in aged male mice indicating ongoing neuroinflammation at this time-point. In parallel, sensorimotor deficits in the corner test were significantly greater in males compared to females up to day 14. Further, cognitive testing at day 14 and day 21 indicate a greater cognitive decline in aged males. Interestingly, CD8+ T cells can inhibit neurite outgrowth (Pool et al., 2012) which may impair the recovery process after ischemic stroke. Related to cognitive function, CD8+ T cell infiltration has been observed in the hippocampus of tau transgenic mice and cortex of patients with frontotemporal dementia (Laurent et al., 2017). Chronic depletion of T cells using neutralizing CD3-antibody prevented hippocampal T cell infiltration and reversed spatial memory deficits (Laurent et al., 2017). Interestingly, vascular dementia patients have elevated levels of circulating CD8+ T cells (Guoping et al., 2015). In contrast, overwhelming evidence by Kipnis and others describe how T cells function to support learning and memory under healthy conditions and that T cell deficiency leads to cognitive dysfunction (Filiano et al., 2017; Kipnis et al., 2012, 2004). In the uninjured state, T cells do not enter the brain parenchyma, but a considerable number are found in the meninges that surround the brain. Further studies are needed to pinpoint the specific mechanisms of delayed CD8+ T cell infiltration after ischemic stroke in aged mice and their effect on long-term cognitive function.
Another T cell subtype demonstrated to have a delayed and prolonged temporal profile after ischemic stroke are Tregs (Ito et al., 2019; Stubbe et al., 2013). Tregs belong to a subpopulation of T cells that have the important task of maintaining immune tolerance and promoting tissue homeostasis. This is the first study to investigate the temporal pattern of Treg infiltration to the brain after ischemic stroke in aged animals. Under normal conditions the number of Tregs in the brain is low, however as reported in the current study and by others (Ito et al., 2019; Stubbe et al., 2013), there is a delayed accumulation of Tregs at 14-15 days after ischemic stroke. Our results in aged animals indicate that approximately 20-30% of the CD4+ T cells were composed of FoxP3+ Tregs, slightly lower than the previously reported 30-40% in young male mice (Ito et al., 2019; Stubbe et al., 2013). Interestingly, we did not observe this delayed increase in FoxP3+ Tregs after ischemic stroke in females. To our knowledge, no prior experimental stroke studies have investigated brain Treg responses in females, let alone in aged females. However, two studies of spleen Tregs after stroke reported no sex differences in cell numbers during the acute phase (96 h) (Dotson et al., 2016, 2015). One clinical study reported significantly elevated levels of circulating Tregs in male patients up to three weeks after the stroke compared to controls, while no significant changes were observed in females (Yan et al., 2012). As the primary function of Tregs is to suppress the function and proliferation of cytotoxic T cells, the accumulation of brain Tregs observed in male mice that was not seen in females is likely a consequence of the significantly elevated levels of CD8+ T cells in the circulation and brains of aged males at this time-point.
Ischemic stroke not only exhibits central effects on the brain, but is recognized as a systemic disease with dramatic effects on peripheral organs such as gut and spleen (Benakis et al., 2016; Chauhan et al., 2018; Crapser et al., 2016; Dotson et al., 2015; Spychala et al., 2018). Previous work from our lab has demonstrated that ischemic stroke in aged male mice is associated with increased gut permeability (leaky gut) (Crapser et al., 2016). However, up until now, these systemic effects have never been investigated in aged females or in aged male mice. In support of the prior literature (Crapser et al., 2016), we report an approximately 3 times greater permeability of FITC-dextran in male stroke animals relative to male sham animals. Surprisingly, evidence of stroke-induced gut permeability was not seen in the aged female mice. In line with the results of increased gut permeability in males but not in females, we further demonstrated that aged males experienced a greater stroke-induced loss of fecal butyrate, a short-chain fatty acid known to promote intestinal barrier function (Kelly et al., 2015; Monolayers et al., 2009). Additionally, aged males had long-lasting changes in fecal microbiota diversity after ischemic stroke at day 14, while in females, stroke-induced changes normalized by day 7. Overall, these results suggest that both central T cell immune responses and peripheral effects on gut permeability and microbiota diversity are greater in aged males than in females. In parallel to the proposed breach in the gut barrier, we observed a higher incidence of hemorrhagic transformation in brains from aged males, suggesting that not only the gut barrier is compromised but also the blood brain barrier (BBB). The concept of a leaky gut, leaky brain and the gut-brain axis has been emerging in diseases such as celiac disease, depression, Alzheimer’s as well as in ischemic stroke (Benakis et al., 2016; Cryan et al., 2019; Durgan et al., 2019; Obrenovich, 2018). Our current data showing stroke-induced gut permeability and dysbiosis in aged males support our previous studies that have demonstrated an increased gut permeability in aged male mice after ischemic stroke and the gut microbiota as a modifier of stroke outcome (Crapser et al., 2016; Spychala et al., 2018).
It is well-known that sex differences are seen in the clinical stroke population. Many studies have shown that women have worse stroke outcomes, and that women have higher mortality after stroke compared to men (Ahnstedt et al., 2016; Reeves et al., 2008). In the current study we report sex differences in the mortality after ischemic stroke, with significantly higher mortality in aged males compared to females. By using an aged stroke model we mimic the aged stroke population, however the aged mice were of exactly the same age with the same genetic background, and these mice have no sex differences in comorbidities and pre-stroke disability that have been seen in human stroke patients. In the clinical stroke population, women are on average 5 years older when they have their first stroke, an important aspect to consider. In fact, a recent large meta-analysis found that including pre-stroke disability, comorbidities and other factors in the analysis, mortality shifted from women being 35% more likely than men to be dead 1 year after the stroke in unadjusted analysis, to women being 19% less likely to die in adjusted analysis (Phan et al., 2017). Similar, Renoux et al. found no evidence of worse outcome in women when age and pre-stroke disability were adjusted for (Renoux et al., 2017). Hence, the current study closely resembles the mortality data from these clinical studies. However, a new study shows that women have more severe ischemic strokes than men (Phan et al., 2019). While adjusting for pre-stroke factors including an older age in women, greater pre-stroke dependency and higher prevalence of atrial fibrillation significantly attenuated the observed sex differences in severity, it did not fully explain them (female:male RR 1.35 vs 1.20 in adjusted analysis) (Phan et al., 2019). This highlights the complexity of sex differences in ischemic stroke and emphasizes the need for further studies.
Limits:
This is the first study to investigate T cell responses in the acute, as well as the chronic phase after ischemic stroke in aged animals of both sexes. We evaluated the temporal and sex-dependent dynamics of peripheral leukocyte infiltration into the ischemic brain, in particular T cell responses, further studies are needed to fully understand the mechanisms underlying T cell dynamics in stroke outcome and the specific T cell phenotypes. Further, aged stroke models come with increased mortality that was especially notable in the aged male mice which raises a potential for survival bias. Nevertheless, in the animals that did survive we found that neuroinflammation, sensorimotor deficits and cognitive decline were more prominent in aged males compared to females.
5. Conclusions
The present study reveals significant sex differences in central T cell responses as well as greater peripheral effects after ischemic stroke in aged mice. Our findings show that males have a more chronic neuroinflammatory response compared to females during the chronic phase of stroke, which may be associated with greater cognitive decline long-term after stroke.
Supplementary Material
Highlights.
Aged males had greater mortality than females despite equivalent stroke injury
Systemic effects on gut permeability and microbiota diversity were larger in males
Brain T cell immune responses were temporally distinct and more pronounced in males
Sensorimotor deficits were greater in aged males than in females
Acknowledgments
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) grant R01 NS108779, NS096493 (United States, to LDM), and the American Heart Association (AHA) Postdoctoral Fellowship #17POST33660010 (to HA).
Footnotes
Declaration of Competing Interest
None.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Scoffings DJ, Simon Jones P, et al. , 2013. Interaction of age with the ischaemic penumbra, leptomeningeal collateral circulation and haemodynamic variables in acute stroke: A pilot study. J. Neurol. Neurosurg. Psychiatry 84, 271–276. 10.1136/jnnp-2012-303258 [DOI] [PubMed] [Google Scholar]
- Ahnstedt H, McCullough LD, 2019. The impact of sex and age on T cell immunity and ischemic stroke outcomes. Cell. Immunol In Press. 10.1016/j.cellimm.2019.103960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnstedt H, McCullough LD, Cipolla MJ, 2016. The Importance of Considering Sex Differences in Translational Stroke Research. Transl Stroke Res. 10.1007/s12975-016-0450-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, et al. , 1998. Gender-linked brain injury in experimental stroke. Stroke 29, 159–65; discussion 166. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Murphy SJ, Traystman RJ, et al. , 2000. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke 31, 161–168. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, et al. , 1986. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17, 472–476. [DOI] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, et al. , 2016. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med 22, 516–523. 10.1038/nm.4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, et al. , 2018. Heart Disease and Stroke Statistics— 2018 Update: A Report From the American Heart Association, Circulation. 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- Bouet V, Boulouard M, Toutain J, et al. , 2009. The adhesive removal test: A sensitive method to assess sensorimotor deficits in mice. Nat. Protoc 4, 1560–1564. 10.1038/nprot.2009.125 [DOI] [PubMed] [Google Scholar]
- Bupp MRG, Potluri T, Fink AL, et al. , 2018. The confluence of sex hormones and aging on immunity. Front. Immunol 9 10.3389/fimmu.2018.01269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, et al. , 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro Á, Meisel A, Planas AM, et al. , 2012. The immunology of acute stroke. Nat. Rev. Neurol 8, 401–410. 10.1038/nrneurol.2012.98 [DOI] [PubMed] [Google Scholar]
- Chauhan A, Al Mamun A, Spiegel G, et al. , 2018. Splenectomy protects aged mice from injury after experimental stroke. Neurobiol. Aging 61, 102–111. 10.1016/j.neurobiolaging.2017.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapser J, Ritzel R, Verma R, et al. , 2016. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging (Albany. NY). 8, 1049–1063. 10.18632/aging.100952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, O’Riordan KJ, Cowan CSM, et al. , 2019. The Microbiota-Gut-Brain Axis. Physiol. Rev 99, 1877–2013. 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Schmid-Schönbein GW, Mori E, et al. , 1991. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 22, 1276–1283. 10.1161/01.STR.22.10.1276 [DOI] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, et al. , 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol 72, 5069–5072. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson AL, Wang J, Chen Y, et al. , 2016. Sex differences and the role of PPAR alpha in experimental stroke. Metab. Brain Dis 31, 539–547. 10.1007/s11011-015-9766-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson AL, Wang J, Saugstad J, et al. , 2015. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J. Neuroimmunol 278, 289–298. 10.1016/j.jneuroim.2014.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Quach LN, Sol+R M, et al. , 2015. B-Lymphocyte-Mediated Delayed Cognitive Impairment following Stroke. J. Neurosci 35, 2133–2145. 10.1523/JNEUROSCI.4098-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, et al. , 1998. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab 18, 1253–1258. 10.1097/00004647-199811000-00012 [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Lee J, McCullough LD, et al. , 2019. Examining the Role of the Microbiota-Gut-Brain Axis in Stroke. Stroke 50, 2270–2277. 10.1161/strokeaha.119.025140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feketa VV, Zhang Y, Cao Z, et al. , 2014. Transient receptor potential melastatin 8 channel inhibition potentiates the hypothermic response to transient receptor potential vanilloid 1 activation in the conscious mouse. Crit. Care Med 42, e355–e363. 10.1097/CCM.0000000000000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Liao S, Wei C, et al. , 2017. Infiltration and persistence of lymphocytes during late-stage cerebral ischemia in middle cerebral artery occlusion and photothrombotic stroke models. J. Neuroinflammation 14, 1–12. 10.1186/s12974-017-1017-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AJ, Gadani SP, Kipnis J, 2017. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat. Rev. Neurosci 18, 375–384. 10.1038/nrn.2017.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Yoshida Y, et al. , 1994. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat). Am J Pathol 144, 188–199. [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, et al. , 2009. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40, 1849–1857. 10.1161/STROKEAHA.108.534503 [DOI] [PubMed] [Google Scholar]
- Gill D, Veltkamp R, 2016. Dynamics of T cell responses after stroke. Curr. Opin. Pharmacol 26, 26–32. 10.1016/j.coph.2015.09.009 [DOI] [PubMed] [Google Scholar]
- Glader EL, Stegmayr B, Norrving B, et al. , 2003. Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke 34, 1970–1975. 10.1161/01.STR.0000083534.81284.C5 [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, et al. , 2014. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 129, e28–e292. 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guoping P, Wei W, Xiaoyan L, et al. , 2015. Characteristics of the peripheral T cell immune response of patients at different stages of vascular cognitive impairment. Immunol. Lett 168, 120–125. 10.1016/j.imlet.2015.09.015 [DOI] [PubMed] [Google Scholar]
- Hall ED, Pazara KE, Linseman KL, 1991. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab 11, 292–298. 10.1038/jcbfm.1991.61 [DOI] [PubMed] [Google Scholar]
- Han J, Lin K, Sequeira C, et al. , 2015. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 854, 86–94. 10.1016/j.aca.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Ito M, Komai K, Mise-Omata S, et al. , 2019. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 10.1038/s41586-018-0824-5 [DOI] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, et al. , 2001. Immunobiology: The Immune System In Health And Disease, Immuno Biology 5 Garland Science, New York: 10.1111/j.1467-2494.1995.tb00120.x [DOI] [Google Scholar]
- Jickling GC, Liu D, Ander BP, et al. , 2015. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 35, 888–901. 10.1038/jcbfm.2015.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W-N, Gonzales R, Feng Y, et al. , 2018. Brain Ischemia Induces Diversified Neuroantigen-Specific T-Cell Responses That Exacerbate Brain Injury. Stroke STROKEAHA.118.020203. 10.1161/STROKEAHA.118.020203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CJ, Zheng L, Campbell EL, et al. , 2015. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671. 10.1016/j.chom.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, et al. , 2004. T cell deficiency leads to cognitive dysfunction: Implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc. Natl. Acad. Sci 101, 8180–8185. 10.1073/pnas.0402268101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC, 2012. Pro-cognitive properties of T cells. Nat. Rev. Immunol 12, 663–9. 10.1038/nri3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Schwab N, Kraft P, et al. , 2011. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 115, 3835–3842. 10.1182/blood-2009-10-249078 [DOI] [PubMed] [Google Scholar]
- Koizumi J, Yoshida Y, Nakasawa T, et al. , 1986. Experimental studies of ischemic brain edema, I: A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke 8, 1–8. [Google Scholar]
- Kuźma E, Lourida I, Moore SF, et al. , 2018. Stroke and dementia risk: A systematic review and meta-analysis. Alzheimers. Dement 14, 1416–1426. 10.1016/j.jalz.2018.06.3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent C, Dorothée G, Hunot S, et al. , 2017. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain 140, 184–200. 10.1093/brain/aww270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger M, Quiedeville A, Bouet V, et al. , 2013. Object recognition test in mice. Nat. Protoc 8, 2531–2537. 10.1038/nprot.2013.155 [DOI] [PubMed] [Google Scholar]
- Li M, Li Z, Yao Y, et al. , 2017. Astrocyte-derived interleukin-15 exacerbates ischemic brain injury via propagation of cellular immunity. Proc. Natl. Acad. Sci 114, E396–E405. 10.1073/pnas.1612930114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, et al. , 2004. Chronic behavioral testing after focal ischemia in the mouse: Functional recovery and the effects of gender. Exp. Neurol 187, 94–104. 10.1016/j.expneurol.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Liesz A, Hu X, Kleinschnitz C, et al. , 2015. Functional role of regulatory lymphocytes in stroke: facts and controversies. Stroke. 46, 1422–30. 10.1161/STROKEAHA.114.008608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Karcher S, Veltkamp R, 2013. Spectratype analysis of clonal T cell expansion in murine experimental stroke. J. Neuroimmunol 257, 46–52. 10.1016/j.jneuroim.2013.01.013 [DOI] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Mracskó É, et al. , 2011. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain 134, 704–720. 10.1093/brain/awr008 [DOI] [PubMed] [Google Scholar]
- Lisabeth LD, Reeves MJ, Baek J, et al. , 2015. Factors influencing sex differences in poststroke functional outcome. Stroke 46, 860–863. 10.1161/STROKEAHA.114.007985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Benashski SE, Persky R, et al. , 2012. Age-related changes in AMP-activated protein kinase after stroke. Age (Omaha). 34, 157–168. 10.1007/s11357-011-9214-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Schafer DP, Mccullough LD, 2009. TTC , Fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion 179, 1–8. 10.1016/j.jneumeth.2008.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa E, Weinstein P, Carlson S, et al. , 1989. Reversible Middle Cerebral Artery Occlusion Without Craniectomy in Rats. Stroke 20, 84–91. [DOI] [PubMed] [Google Scholar]
- Manwani B, Liu F, Scranton V, et al. , 2013. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol 249, 120–131. 10.1016/j.expneurol.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Price MN, Goodrich J, et al. , 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6, 610–618. 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monolayers C-C, Peng L, Li Z, et al. , 2009. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase. J Nutr 139, 1619–1625. 10.3945/jn.109.104638.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mracsko E, Liesz A, Stojanovic A, et al. , 2014. Antigen Dependently Activated Cluster of Differentiation 8-Positive T Cells Cause Perforin-Mediated Neurotoxicity in Experimental Stroke. J. Neurosci 34, 16784–16795. 10.1523/JNEUROSCI.1867-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MC, Morrison HG, Benjamino J, et al. , 2014. Analysis, optimization and verification of illumina-generated 16s rRNA gene amplicon surveys. PLoS One 9 10.1371/journal.pone.0094249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrenovich M, 2018. Leaky Gut, Leaky Brain? Microorganisms 6, 1–13. 10.3390/microorganisms6040107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan HT, Blizzard CL, Reeves MJ, et al. , 2017. Sex Differences in Long-Term Mortality After Stroke in the INSTRUCT (INternational STRoke oUtComes sTudy). Circ. Cardiovasc. Qual. Outcomes 10, e003436 10.1161/CIRCOUTCOMES.116.003436 [DOI] [PubMed] [Google Scholar]
- Phan HT, Reeves MJ, Blizzard CL, et al. , 2019. Sex differences in severity of stroke in the INSTRUCT study: A meta analysis of individual participant data. J. Am. Heart Assoc 8, 1–12. 10.1161/JAHA.118.010235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool M, Rambaldi I, Darlington PJ, et al. , 2012. Neurite outgrowth is differentially impacted by distinct immune cell subsets. Mol. Cell. Neurosci 49, 68–76. 10.1016/j.mcn.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, et al. , 2008. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 7, 915–926. 10.1016/S1474-4422(08)70193-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MJ, Fonarow GC, Zhao X, et al. , 2009. Quality of care in women with ischemic stroke in the GWTG program. Stroke 40, 1127–1133. 10.1161/STROKEAHA.108.543157 [DOI] [PubMed] [Google Scholar]
- Renoux C, Coulombe J, Li L, et al. , 2017. Confounding by Pre-Morbid Functional Status in Studies of Apparent Sex Differences in Severity and Outcome of Stroke. Stroke 48, 2731–2738. 10.1161/STROKEAHA.117.018187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Crapser J, Patel AR, et al. , 2016. Age-Associated Resident Memory CD8 T Cells in the Central Nervous System Are Primed To Potentiate Inflammation after Ischemic Brain Injury. J. Immunol 10.4049/jimmunol.1502021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Patel AR, Spychala M, et al. , 2017. Multiparity improves outcomes after cerebral ischemia in female mice despite features of increased metabovascular risk. PNAS 5673–5682. 10.1073/pnas.1607002114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel BD, MacRez R, Jullienne A, et al. , 2009. Age and albumin D site-binding protein control tissue plasminogen activator levels: Neurotoxic impact. Brain 132, 2219–2230. 10.1093/brain/awp162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj UM, Stowe AM, 2017. Long-term T cell responses in the brain after an ischemic stroke. Discov. Med 24, 323–333. [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, et al. , 1997. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg 87, 724–730. 10.3171/jns.1997.87.5.0724 [DOI] [PubMed] [Google Scholar]
- Singh V, Roth S, Llovera G, et al. , 2016. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J. Neurosci 36, 7428–7440. 10.1109/MSSC.2013.2278093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spychala MS, Venna VR, Jandzinski M, et al. , 2018. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol 84, 23–36. 10.1002/ana.25250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe T, Ebner F, Richter D, et al. , 2013. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J. Cereb. Blood Flow Metab 33, 37–47. 10.1038/jcbfm.2012.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer B, Patil S, Höger H, et al. , 2007. Barnes maze, a useful task to assess spatial reference memory in the mice. Protoc. Exch 198, 58–68. 10.1038/nprot.2007.390 [DOI] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, et al. , 1990. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab 10, 290–293. 10.1038/jcbfm.1990.47 [DOI] [PubMed] [Google Scholar]
- Towfighi A, Saver JL, Engelhardt R, et al. , 2007. A midlife stroke surge among women in the United States. Neurology 69, 1898–1904. 10.1212/01.wnl.0000268491.89956.c2 [DOI] [PubMed] [Google Scholar]
- Vindegaard N, Muñoz-Briones C, El Ali HH, et al. , 2017. T-cells and macrophages peak weeks after experimental stroke: Spatial and temporal characteristics. Neuropathology 37, 407–414. 10.1111/neup.12387 [DOI] [PubMed] [Google Scholar]
- Xie L, Li W, Hersh J, et al. , 2018. Experimental ischemic stroke induces long-term T cell activation in the brain. 10.1177/0271678X18792372 [DOI] [PMC free article] [PubMed]
- Yan J, Read SJ, Henderson RD, et al. , 2012. Frequency and function of regulatory T cells after ischaemic stroke in humans. J. Neuroimmunol 243, 89–94. 10.1016/j.jneuroim.2011.12.019 [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, et al. , 2006. Role of T lymphocytes and interferon-γ in ischemic stroke. Circulation 113, 2105–2112. 10.1161/CIRCULATIONAHA.105.593046 [DOI] [PubMed] [Google Scholar]
- Zarif H, Nicolas S, Guyot M, et al. , 2018. Brain , Behavior , and Immunity CD8 + T cells are essential for the effects of enriched environment on hippocampus-dependent behavior , hippocampal neurogenesis and synaptic plasticity. Brain Behav. Immun 69, 235–254. 10.1016/j.bbi.2017.11.016 [DOI] [PubMed] [Google Scholar]
- Zhang L, Schallert T, Zhang Z, et al. , 2002. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J. Neurosci. Methods 117, 207–214. 10.1016/S0165-0270(02)00114-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.