Highlights
-
•
Tongue squamous cell carcinoma (TSCC) is most aggressive and highly prevalent subtype of head and neck cancer.
-
•
Majority of tobacco users are HPV−ve and have bad prognosis while HPV+ve TSCCs not using tobacco show better prognosis.
-
•
Tongue cancer stem cells (TCSCs) are resistant to chemo-radio therapy and responsible for cancer relapse.
-
•
Targeting TCSCs may provide efficient therapeutic strategy for relapse-free survival of TSCC patients.
Keywords: Tongue squamous cell carcinoma, HPV, Tongue cancer stem cells, Prognosis, Relapse-free survival
Abstract
The tongue squamous cell carcinoma (TSCC) is a highly prevalent head and neck cancer often associated with tobacco and/or alcohol abuse or high-risk human papillomavirus (HR-HPV) infection. HPV positive TSCCs present a unique mechanism of tumorigenesis as compared to tobacco and alcohol-induced TSCCs and show a better prognosis when treated. The poor prognosis and/or recurrence of TSCC is due to presence of a small subpopulation of tumor-initiating tongue cancer stem cells (TCSCs) that are intrinsically resistant to conventional chemoradio-therapies enabling cancer to relapse. Therefore, targeting TCSCs may provide efficient therapeutic strategy for relapse-free survival of TSCC patients. Indeed, the development of new TCSC targeting therapeutic approaches for the successful elimination of HPV+ve/−ve TCSCs could be achieved either by targeting the self-renewal pathways, epithelial mesenchymal transition, vascular niche, nanoparticles-based therapy, induction of differentiation, chemoradio-sensitization of TCSCs or TCSC-derived exosome-based drug delivery and inhibition of HPV oncogenes or by regulating epigenetic pathways. In this review, we have discussed all these potential approaches and highlighted several important signaling pathways/networks involved in the formation and maintenance of TCSCs, which are targetable as novel therapeutic targets to sensitize/eliminate TCSCs and to improve survival of TSCC patients.
Graphical abstract
Background
Head and neck cancer (HNC) is the 6th most predominant cancer globally and a major public health problem in India and South-East Asia [1,2]. In India, HNC is positioned first in males and third in females, accounting for over 205,325 new cancer cases and 122,834 deaths in 2018 [3]. HNCs are highly heterogeneous group of malignancies with variable rates of incidence, mortality and prognosis. These cancers originate mainly from the epithelium of the lip, cheek, floor of mouth, oropharynx, pharynx, or nasopharynx, larynx and tongue (the most common location) [4,5].
Among HNCs, human tongue squamous cell carcinoma (TSCC) has highest incidence and it is the most feared and predominant sub-type of HNCs, which accounts for ≥40% of all oral cancers. Majority of TSCCs (90%) are histologically originated from squamous cells and show different levels of cell differentiation. The most common anatomical sub-site of TSCC is the lateral border of the tongue and the left-side of the tongue is more frequently affected than the right-side because of majority of right-handed tobacco smokers inhaling smoke stream to their left-side [6]. It is often mis-interpreted as oropharyngeal carcinomas particularly those arise at the base of the tongue.
TSCCs are characterized by aggressive behavior, loco-regional recurrence and a higher rate of occult metastasis with poor prognosis [7], [8], [9]. Recently, number of studies have showed an increased incidence of TSCCs afflicting not only low-middle income countries (LMICs) but also in several high-income developed countries including US and UK [10], [11], [12], [13]. The incidence of oral-tongue cancers is highest in India compared to other nations of the world [14]. This is mainly because of constant use of a variety of tobacco products (Cigarette, Bidi, Khaini, Gutkha and Pan Masala) areca nut and various ingredients contained in betel leaf (Pan, a special chewable preparation most commonly used in India) etc. together with alcohol (including indigenous raw alcohol preparations) are the classical major etiological factors associated with the development of TSCCs [9,[15], [16], [17], [18]]. A concerted efforts and public awareness campaigns to educate the low or middle income group people regarding harmful health effects of tobacco and alcohol could lead to significant decrease in the incidence of HNC including TSCC. Furthermore, most alarming is the recent findings that incidence and proportion of TSCC specifically at the base of the tongue (BOT) is rising mainly in younger age population and none of the typical risk-factors (tobacco and alcohol) are found to be associated with the disease indicating other biological factors might be playing an important role in the development and progression of TSCCs [9,[19], [20], [21], [22]]. BOTSCCs seem to originate from lingual tonsillar tissue, since majority of BOTSCCs display tumor cell growth, proliferation and metastasis pattern as that of tonsil tumors, particularly in HPV+ve cases [23,24]. In recent decades, infection with specific types of high-risk human papillomaviruses (HR-HPVs) have been implicated as causative agents in the development and progression of a sub-set of TSCCs [9,20,[25], [26], [27]].
Thus, TSCCs are now divided into two distinct categories; i) HPV negative TSCCs and ii) oncogenic-HPV positive TSCCs. HR-HPV-positive TSCC patients show distinct clinical presentation, socioeconomic profiles as well as molecular and metabolic characteristics as compared to HPV negative TSCCs [9,25,28]. The prevalence of HR-HPV-induced TSCCs showed significant variability across populations and geographic regions including within the Indian subcontinent where the use of tobacco products is a part of the social-status and culture in certain sections of the society. It has been observed that HPV+ve TSCCs are mostly prevalent among non-tobacco users, who have distinct genetical, molecular and clinical characteristics and show better response to therapy [9,20,25,29]. It indicates that HPV associated TSCCs constitute an unique mechanism(s) of tumorigenesis, biological behavior and treatment outcomes [9,15,25,27,[30], [31], [32]].
Disappointingly enough, despite great advancement in diagnostic and surgical techniques, radiation therapy, targeted and combinatorial therapeutic approaches (chemo-radiotherapy and immuno-therapy), the overall 5-year survival rate of TSCC patients is only 50% and it has not increased significantly over the last several decades [11,29,33]. However, these traditional therapies are effective for few subsites of HNCs, hence a better understanding of the underlying mechanism for TSCC development will only help in identifying more reliable markers and therapeutic targets for early detection and effective treatment of this aggressive cancer [34,35]. The poor survival and the treatment failure of TSCC patients is due to high recurrence rate, aggressive metastasis, tumor heterogeneity, failure of immunological surveillance and resistant to traditional therapies. One of the main reasons for TSCC treatment failure is supposed to be related to the presence of a heterogeneous small subpopulation of cancer stem cells. The growing cancer research has revealed existence of this small subpopulation (∼0.05–2%) of “cancer initiating” or “cancer stem-like cells” (CSCs) in almost all cancers [36]. Similar to other cancers, TSCCs also have heterogeneous cancer stem-like cell population identified as tongue cancer stem cells (TCSCs) [37], [38], [39]. CSCs were first isolated and recognized in 1990 in leukemia using two stemness markers CD34+ and CD38 [40]. Subsequently, CSCs expressing various cell surface markers including CD133, CD44, ALDH, BMI-1, SOX2, OCT-4, nestin and NANOG, have been identified in several solid and non-solid tumors including TSCCs [39,[41], [42], [43], [44]]. These rare population (1–2%) of CSCs or progenitor cancer cells manifest specific stemness properties with a capability to form new tumor following self-renewal show multi-drug resistance and progressive metastasis and recurrence of tumor (Fig. 1). Therapeutic resistance of these cells has been attributed to their cytotoxic drug refluxing and self-renewal ability, stem cell plasticity, efficient DNA repair capacity, free-radical scavenging, alteration in cell cycle control mechanism and modulation of tumor microenvironment. These characteristics of CSCs play a critical role in provoking aggressive metastasis and poor prognosis of the disease which in turn is allusive of the fact that detection and targeted inhibition and/or sensitization of CSCs can be a new conceptual framework for TSCC treatment [45], [46], [47], [48].
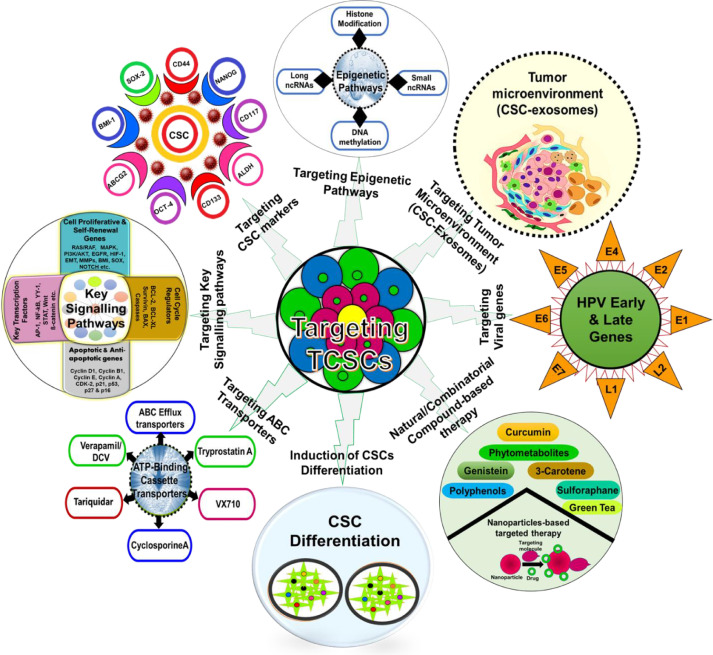
Fig. 1.
Different therapeutic strategies for tongue cancer stem cell targeting. Strategies for CSC targeting based on specific molecular characteristics including targeting the signaling network, targeting the stemness markers, inhibiting the tumor microenvironment, targeting with nanoparticle-based therapy, sensitizing cancer stem cells with natural and synthetic compounds or induction of CSC differentiation including targeting epigenomic pathways and ABC transporter cassette proteins.
Therefore, it has become imperative to understand the complex functional role of CSCs in the patho-physiology of the disease and deregulated self-renewal signaling pathways elucidating novel biomarkers for early diagnosis and designing novel treatment strategies for targeting CSCs.
CSCs have an active process of cytotoxic drug exclusion property because of expression of an ATP binding cassette (ABC) transporter transmembrane protein ABCG2 (a multidrug resistance protein) [49]. CSCs have been isolated from variety of tumours including TSCCs and explored to design novel therapeutic approaches that could target CSCs. However, despite concerted efforts, CSC-targeting strategies have not been efficiently translated to the clinic. This is partly due to partial understanding the molecular biology and signaling pathways underlying CSC treatment-resistance. The intra-tumoral heterogeneity and functional heterogeneity of CSCs depend on their nature of plasticity that modulates tumor invasiveness, metastasis and treatment response. The CSC population of various cancers including oral-tongue cancers are plastic in nature (bidirectional conversion of CSCs from non-CSC-state) that can gain stem cell features depending upon the tumor microenvironment, epigenetic and genomic modifications [50,51]. Further, the CSC plasticity can cause dynamic alterations in CSC markers expression and interactions with their niche [14, 52]. Certainly, CSC plasticity is an additional hurdle to overcome for an effective treatment of TCSC patients [52]. The CSC plasticity modulated by tumor microenvironment and cellular interactions that may participate in cellular transformation of cancer cells and provide protection to CSCs from chemo-radio therapeutic agents [52].
Since the survival of TSCC patients is poor as the current radio-chemo therapy treatments are not fully curative, isolation, identification and characterization of TCSCs and their efficient targeting must be taken into account when developing novel anti-cancer therapeutics. Deeper understanding of molecular mechanisms that contribute in tumor heterogeneity and CSC plasticity represent one of the greatest interests and challenges in translational cancer and cancer stem research.
Methods for identification, isolation and characterization of CSCs
The main purpose to study CSC biology is to design CSC-based remedies to treat chemo-radio resistant cancer cells which are presently untreatable by conventional therapies. But, the mechanistic understanding of CSCs to make them sensitive to chemo-radio therapy and identification of CSCs from the pool of cancer cells constitute a major experimental challenge in molecular oncology research. To date, there are at least four main methods are available for the isolation, identification and characterization of CSCs from cultured cancer cells or solid tumors based on the CSC properties [53,54]. These methods include the (i) dye efflux by multidrug resistant transporter proteins, (ii) tumorosphere-formation assays, (iii) the expression of CSC specific cell surface CD markers and (iv) single-cell omics technologies for analysis of individual CSCs from a mix population.
SP (Side population) analysis by dye efflux method
It is an important method for identification of SP cells from a subset of cancer cells population. SP analysis from cultured cancer cells using Hoechst 33,342 dye (a fluorescent DNA-binding dye that facilitates the efflux of this dye and other drugs by members of the ABC transporters) or dye cycle violet (DCV) or Rhodamine 123 staining and followed by fluorescence-activated cell sorting (FACS) analysis can provide a distinct cell population on the left quadrant of the dual-color emission spectra [55], [56], [57], [58]. These SP cells can be distinguished by their depletion using Hoechst transporter inhibitors Verapamil or FTC (Fumitremorgin C). SP cells have been identified and isolated from various cancers including TSCCs and this method does not need any cell specific stemness marker for isolation and sorting of CSCs from various tumor tissues and cells. Recently, several studies have identified SP cells in TSCC and characterized them as most aggressive and tumorigenic stem cell-like population [43,[59], [60], [61]]. However, SP analysis technique has some limitations such as low specificity, low purity of CSCs, dye concentration and toxicity etc. However, successful isolation or sorting of CSCs by FACS is mainly depend greatly on the flow cytometry and expertise in the technology.
Identification of CSCs by self-renewal and/or tonguosphere formation assays
The ability of CSCs to create spheres or tonguospheres after they were cultured under-low attachment surface and defined-conditioned medium (DCM) containing differential growth factors enhance CSC growth and maintenance [56,57,62]. Sphere-forming capacity is a “hallmark” and unique characteristic of CSCs and has been widely used to identify and characterize CSCs based on their self-renewal and differentiation ability at a single cell level more efficiently in-vitro non-adherent nutritionally deficient culture conditions [63]. Though, the identification and characterization of CSCs by functional sphere formation assays has been widely used in cancer stem cell research, numerous issues may limit the utility of this assay [64].
The disadvantages of this assay are to prove sphere clonality, inherently slower cell cycle kinetics, selection of single-cell derived spheres, differentiation potential of cultured cells may bias/affect due to use of high concentrations of exogenous growth factors and cell aggregation. [65]. Further, other challenges are that the assay may not be able to identify quiescent cells, a read-out of in-vivo stem cell frequency and efficient assessment of the cultured sphere's size and number. Recently, three-dimensional (3D) culturing technique has emerged as a very powerful tool that provides a proper microenvironment for development of clonal mini-organs, functional reporter of stem cells and progenitor cell activity. 3D cultures are ideal system and can be used as an alternative approach for the assessment of quiescent stem cells.
CSCs identification using potential stemness markers
This is the most common method for identification and characterization of CSCs based on the specific cell surface protein markers that nurture cells with CSC properties [66], [67], [68]. These markers are highly tumor specific and contain distinct cell-specific membrane proteins that distinguish them from non-stem cell population (NSP). Growing number of studies have identified the essential role of putative CSC markers in a variety of solid tumors but very few of them have been studied in TSCCs. ALDH, CD44 and CD133 cellular markers are very common surface markers used for the identification and sorting of CSCs in oral-tongue cancers.
i. ALDEFLUOR assay for ALDH activity: Recently, aldehyde dehydrogenase (ALDH), a cytosolic enzyme that is responsible for the oxidation of aldehydes to carboxylic acids. ALDH has been used for the identification and isolation of CSCs in variety of human tumors including TSCCs and it is now become an universal stemness marker for epithelial malignancies [69,70]. An elevated activity of detoxification enzyme ALDH in TCSCs have established and its overexpression is associated with multidrug resistance transport proteins [71]. The technique used to identify and sort high ALDH+ve (ALDH1A1) cell populations is called ALDEFLUOR assay [72]. With the emergence of this assay, identification and sorting of live cells from patient's samples have been possible [72,73] with high ALDH expression (ALDHhigh). It helps in functionally characterize the role of ALDHhigh cells in oral-tongue cancer progression. ALDEFLUOR is a non-immunological fluorescent reagent that measures the activity of ALDH enzyme through the cleavage of a BODIPY-Aminoacetaldehyde (BAAA) fluorescent substrate to its corresponding BODIPY-aminoacetate (BAA) carboxylic acid [74]. Diethylaminobenzaldehyde (DEAB), an ALDH inhibitor can be used as a control to measure the exact percentage of CSCs with ALDHhigh activity [72]. High ALDH+ve subpopulations in TSCCs cause more tumorigenic phenotype, aggressive metastasis and chemo-radiotherapy resistance [61,75]. Further, ALDH1+ve cells showed higher CSC-like characteristics than ALDH1−ve oral cancer cells [38,71]. Thus, specific targeting ALDH in TSCC significantly inhibits several CSC properties in cancer cells and may serve as a unique intracellular marker for identification of TCSCs.
ii. Identification of cellular marker CD44: CD44 is a well-known transmembrane glycoprotein marker that play a significant role in intercellular interactions, cell migration and cell adhesion [76]. It has been suggested that CD44 play a key role in cell growth, survival, differentiation, tumor progression and metastasis [77]. In a variety of cancers, interaction of CD44 with hyaluronic acid (HA), heparan sulphate and chondroitin sulphate leads to the binding of CD44 with growth factors and metalloproteinases and can activate tyrosine kinase receptors and promote cell growth, proliferation and survival [78]. Further, CD44 enable release of cancer cells to blood vessels during cancer metastasis. Prince et al. (2007) first identified CD44+ve subpopulation in HNC and showed higher tumor-initiating capability compared with CD44−ve subpopulation in xenograft mouse model suggesting the importance of CD44+ve CSCs in HNCs [47]. Following this, a large number of studies have shown that CD44+ve cell populations display higher potential for cell growth, proliferation, migration, differentiation, tumor sphere formation, and chemo-radio resistance in primary oral-tongue tissues and cell lines [47,[79], [80], [81]]. Thus, these studies indicate that CD44 is an important prognostic factor and can be used as a potential stemness marker to identify and isolate CSCs from TSCCs.
iii. Identification of CD133: Transmembrane glycoprotein CD133 (120 KDa in size), also known as prominin-1 or AC133 was one of the first stemness marker used for identification of neuroepithelial stem cells in 1997 and later defined in human hematopoietic stem cells. High CD133 cells display tumor initiation, progression, strong tumorigenicity, and CSCs maintenance. Highly strong expression of CD133 is associated with poorer survival and identified as a putative CSC marker in variety of cancers including TSCCs [82,83]. Several studies have revealed that CD133+ subpopulations increased proliferation, clonogenicity, tumor sphere formation, self-renewal and tumorigenicity in oral-tongue cancer cell lines [84,85]. The upregulation of CD133 is often identified in oral-tongue cancer stem cells, but its functional role as a CSC marker in TSCC is still not well-understood and requires further investigation.
Although, the accumulation of experimental data supporting the potential role of specific CSC markers in aggressive TSCCs [47,83] yet to date, no single full-proof TSCC specific stemness marker have been identified which might provide the best specificity for identifying/targeting TCSCs.
Single-cell omics technology for analysis of individual CSCs
Despite huge advancement in oral cancer treatment, the dynamics and complex tumor cell biology, extensive cell-to-cell variation and intra-tumoural heterogeneity are intrinsic and fundamental features of cancer and cancer stem cell populations which affect treatment efficacy and patient's survival, hence posing a significant challenge to developing targeted cancer therapies. However, these variations are masked when bulk of cells are employed for high-throughput single-cell omics technologies that provide precision to analyze cellular heterogeneity and detect distinct cell phenotypes within a ‘homogeneous’ stem cell population. Single cell omics are advanced technologies with high sensitivity and high-throughput with multiplexity that allow unbiased analysis of the individual cell type out of a complex subpopulation of cells at the level of genomics, transcriptomics, proteomics, epigenomics and metabolomics [86], [87], [88]. It provides a new opportunity to profile specific cells within heterogeneous tumours or CSCs and may help to discover new therapeutic targets for personalized medicine approaches for effective treatment [89,90]. The single cell isolation technique is useful for efficiency, purity and recovery of single cells and all these parameters provide huge advantages in the field of biomedical research [91].
TSCC being an extremely aggressive cancer, understanding potentials of TCSCs as prospective biomarkers and therapeutic targets, isolation and characterization of CSCs using a battery of techniques are of paramount importance to enrich effective diagnosis, prognosis and treatment of TSCC patients.
Various molecular approaches/signaling pathways for targeting TCSC
Targeting TCSCs by cell surface markers
In recent years, studies have been aimed at the application of stem cell biology in clinical medicine, particularly in regenerative medicine and its role in tumor recurrence and metastasis. Several CSC stemness markers have been identified mainly in the basal layers of oral-tongue mucosa which are essential for tissue homeostasis. The TCSCs overexpress specific surface markers as compared to NSP, therefore characterization of TCSC-specific stemness markers provide differential profiling of CSC populations which helps in the development of new drugs against those TSCC patients who develop resistance to conventional therapy.
Numerous studies suggest that TSCC harbours CSCs and specific stemness markers including BMP-4, CD44, OCT-4, NANOG, ALDH, CD133 and BMI-1 which have been identified and characterized in TSCC [47,[92], [93], [94], [95], [96]]. An earlier study by Kang et al. (2010) showed that CD133 may be used as a potential CSC marker for TSCC [93] while, Zou et al. (2011) revealed that TSCC cells (Tca8113) harbor 1.3% of ALDH1+ve cells, which show an increased proliferative, self-renewal capacity as compared to ALDH−ve cells. Therefore, ALDH has been considered as a potential therapeutic marker for TSCC [61]. Targeting ALDH with Aldi-6 and DEAB inhibited ALDH3A1 expression and blocked enzymatic activity of ALDH in TSCCs and TCSCs (see Table 2) [61,97].
Table 2.
Potential therapeutic agents that target TCSCs.
| S.N. | Inhibitors | Targeted gene/marker/pathway | Cancer cell type | References |
|---|---|---|---|---|
| 1. | Fumitremorgin C (FTC) & tariquidar (XR9576) | Inhibition of ABCG2 expression and function | TCSC | [123,212] |
| 2. | ABT-199 | Inhibit Bcl-2 and impairs mitochondrial functions in TSCC cells | TSCC | [210] |
| 3. | PTC-209, PTC596 | Reduced tumorsphere formation, the percentage of ALDH1+ subpopulation, BMI-1 expression and increased chemosensitivity against 5-FU and cisplatin in CAL27 TSCC cells | TSCC and TCSC | [51,112,114,116] |
| 4. | Gefitinib (EGFR tyrosine kinase inhibitor) | Inhibited EGFR signaling and reduced SOX2 expression in TSCC cells | TSCC | [213,214] |
| 5. | Combination therapy of SN-38 and gefitinib | inhibits the expression of CD44 stemness marker by promoting its lysosomal degradation in TSCC cells | TSCC | [110] |
| 6 | GSK1120212 | MEK1/2 inhibitor in TSCC | TSCC | [215] |
| 7 | Simvastatin & AR-42 (TAZ inhibitors) | TAZ (PDZ-binding motif) inhibited tumor growth in-vivo, reduced cell proliferation, migration and tumorsphere formation in TSCC cells | TSCC and TCSC | [216,217] |
| 8. | FLI-06 (NOTCH inhibitor) | Inhibited tumor growth and increased cell apoptosis, suppressed both the mRNA and protein expression of Notch receptor and block the proliferation and self-renewal of tongue cancer stem cells. | TSCC and TCSC | [143] |
| 9. | Aldi-6 and DEAB (ALDH inhibitor) | Aldi-6 inhibited ALDH3A1 and DEAB blocked enzymatic activity of ALDH in TSCC. | TSCC and TCSC | [61], [97] |
| TSCC and TCSC | [61,97] | |||
| 10. | T‐5224 (AP-1 inhibitor) | Inhibited the invasion, migration, MMP activity and prevented lymph node metastasis of TSCC cells | TSCC | [218] |
| 11. | NVP-AEE788 (tyrosine kinase inhibitor) | Inhibited EGF and VEGF signaling, cell growth and proliferation, induced apoptosis, and reduced the phosphorylation of EGFR/VEGFR-2/AKT in TSCC cells | TSCC | [130] |
Earlier findings suggest overexpression of ALDH1, CD44, OCT4 and SOX2 which can be used as independent prognostic stem cell markers for addiction habits, clinical staging and overall survival of TSCC [93]. Positive expression of OCT4 and SOX2 markers in TSCC cells [98] indicates that these markers help in reprogramming and development of tongue tumorigenesis [99]. Immunomagnetic microbead sorting method for isolation of high-purity CSCs revealed that CD133+ve and CD44+ve sorted subpopulation has capacity of elevated proliferation, invasion, self-renewal and tumor-formation which are indicative of their essential role in TSCC initiation, progression, metastasis and recurrence and hence they might serve as potential targets for TSCC treatment [59,61,93]. Recently, Baillie et al. (2016) identified subpopulation of CSCs along-with cytoplasmic localization of stemness markers (CD44, OCT4, SALL4, SOX2, NANOG and phosphorylated STAT3) in moderately differentiated TSCC (MDTSCC) which is indicative of a novel therapeutic target(s) in TSCC [59].
Numerous studies have revealed that altered overexpression of SOX2 is associated with cell proliferation, self-renewal, cancer cells plasticity, anti-apoptosis, tumorigenesis, tumor relapse and chemo-radio resistant leading to bad prognosis in TSCC patients [100], [101], [102], [103]. Interestingly, specific silencing of SOX2 in TSCC significantly inhibits self-renewal and invasion capacities, in-vivo tumorigenicity and increases chemo-radio sensitization [104]. Further, TSCC patients with SOX2high and MTA3Low (metastasis-associated protein 3) showed poorest prognosis suggesting SOX and MTA3 may serve as a potential prognostic markers for these patients [105].
Further, stem cell marker CD44 played significant role during aggressive metastasis of TSCC leading to increased cell proliferation, migration, invasion of TSCC cells [106,107]. The expression of CD44v6 and ABCG2 has also been correlated with lymph node metastasis, tumor progression, recurrence and poor survival of TSCC patients [108]. In addition, CD44+ TCSCs injection in nude mice formed larger tumor size and aggressive metastasis compared to CD44−TSCCs [107]. The expression of CD44 in TSCC and oropharynx is associated with a poor clinical outcome [109] and combination therapy with SN-38 and gefitinib inhibits the expression of CD44 by promoting its lysosomal degradation in TSCC cells (Table 2) [110].
Some studies have also reported altered regulation of BMI-1 and c-MYC which is correlated with progression, proliferation and invasion of tongue cancer and cancer stem cells [92,111].
BMI-1 (B lymphoma Mo-MLV insertion region 1 homolog) serves as an essential key factor of the polycomb repressive complex-1 (PRC1) and its overexpression often associates with advanced tumor stages, aggressive behavior, self-renewal, treatment resistance and poor prognosis in TSCC. Further, specific targeting and pharmacological inhibition of BMI-1 can inhibit tumor sphere formation and reduced tumorigenesis in TSCC [47,[112], [113], [114]]. Toshihiro Tanaka and co-workers (2016) used multicolor lineage tracing method and revealed that BMI1-positive tumorigenic cells display TCSC-like characteristics in a chemically induced tongue cancer mouse model [115]. Further, inhibition of BMI-1 through PTC-209, PTC596 inhibitors reduced BMI-1 expression, tumorsphere formation, the percentage of ALDH1+ve subpopulation and increased chemosensitivity against 5-FU and cisplatin in TSCC cells (Table 2) [51,112,114,116].
Certainly, these studies strongly suggest that all these stemness markers are indispensable and essential for identifying and characterizing CSCs including TCSCs for basic research and screening of anti-CSC drugs. However, these markers may not be sufficiently specific or may be poor therapeutic target(s). Therefore, it is reliable and most useful when the identified and isolated TCSCs using specific stemness marker(s) have the ability to develop tumorospheres in-vitro system and subsequently on the injection of these spheres can produce tumors in-vivo athymic mice.
Targeting ABC efflux transporters
ABC membrane efflux transporter superfamily proteins (ABCB1-MDR1, ABCC1-MRP1 and ABCG2-BCRP) play potential roles in drug metabolism and toxicity and its overexpression leads to higher efflux of cytotoxic drugs and hence chemo-radio resistance of cancer and CSCs (see Table 1). Side population fraction of cancer cells display increased ABCG2 expression, which facilitate drug resistance [60,117]. It has been shown that 90.9% of oral-tongue precancer lesions have higher ABCG2 expression which may help in early prediction of oral-tongue cancer progression [118]. Overexpression of efflux transporter proteins; ABCG2, ABCA3 and ABCG5 have been found in oral cancer SP cells [119], [120], [121]. Overexpression of solute carrier transporter SLC2A13 also correlated with tumorosphere formation ability and may serve as a putative molecular marker for TCSCs (Table 2) [122]. Recently, it has been shown that specific inhibition of ABCG2 via Fumitremorgin C (FTC) & tariquidar (XR9576) inhibitors (Table 2) reduced the expression and function of ABCG2 [17,123]. Therefore, targeting the two major superfamilies of efflux transporters; i) ATP-binding cassette transporters, and ii) the solute carrier (SLC) transporters will possibly significantly enhance the efficacy of cancer therapy.
Table 1.
Various targeted agents and therapeutic approaches against CSCs.
| I. Therapeutic strategies targeting cancer stem cell surface markers | ||
|---|---|---|
| Therapeutic approaches against Anti-CSC markers | Targeted CSC markers | References |
| H4C4, H90, P245, RO5429083 | Targeting CD44 | [202,203] |
| GV5 | Targeting CD44R1 | [202,203] |
| BsAb | Targeting CD3 or CD133 | [202,203] |
| OMP-21M18 | Targeting DLL4 (Delta-like ligand) | [202,203] |
| Vismodegib+ gemcitabine | Targeting CD44+/CD24+ | [202], [203], [204] |
| Catumaxomab | Targeting EpCAM | [202,203] |
| SL-401, SGN-123A, talacotuzumab, MGD006, KHK2823, CAR-T | Targeting CD123 | [202,203] |
| TTI-621 | Targeting CD47 | [202,203] |
| Amcasertib (BBI503), napabucasin (BBI608 | NANOG inhibitors | [203] |
| II. Therapeutic strategies targeting cancer signaling pathways | ||
| MK-0752 (ϒ-secretase inhibitors), RO4929097, Demcizumab (DLL inhibitors) | Targeting Notch pathway | [202,203] |
| GDC-0449 (Vismodegib), LDE225 (Sonidegib), Glasdegib, Cyclopamine, IPI26909 | Targeting Hedgehog pathways | [202,203,205] |
| OMP-54F28 (Ligand sequestration), PRI-724 (Inhibitors of β-catenin), CWP232291, Galunisertib (LY2157299), Fresolimumab (GC-1008), Trabedersen (AP 12009), DKK1 | Targeting Wnt/β-catenin pathway | [202,203,206] |
| III. Therapeutic strategies targeting CSC microenvironment | ||
| Plerixafor (CXCR4 inhibitor), Reparixin (CXCR1/2 inhibitor), Defactinib (FAK inhibitor), Sorafenib, Sunitinib, PX-478, Bevacizumab | Targeting CSC niches | [202,203] |
| IV. Therapeutic strategies targeting metabolic pathways | ||
| Venetoclax (Bcl2 inhibitors) | Targeting CSC metabolism | [203] |
| V. Therapeutic strategies targeting ATP binding cassette transporters/multiple drug resistance | ||
| ABC transporters, Verapamil, CDF (Difluorinated Curcumin), ALDH, Dofequidar (MS-209) | drug efflux transporters, MDR inhibitors | [202,203] |
| VI. Inducing CSC differentiation therapy | ||
| Smad inhibitors, retinoic acid (RA)-induced differentiation, BMPs, OSM (oncostatin) | Potential of differentiation therapy | [207,208] |
| VII. Targeting with anti-cancer and CSC-sensitizing phytocompounds | ||
| Curcumin | P53↑, PTEN↑, c‐Myc↓, k‐Ras↓, Bcl‐2↓, EGFR↓, SMO↓, GLI1↓, DNMT1↓, DNMT1, 3A and 3B↓pan‐HDAC inhibitor, Angiogenesis↓, Cancer‐associated fibroblasts↓, Cytotoxic effect of NK cells↑, CD8(+) T cells↑, Anti-CSC effect | [36,56,57,209] |
| Genistein | CD 163↓, p-STAT3↓, IL-10↓, IL-12↑, CD133↓, CD44↓, Twist1↓, N-cadherin↓, E-cadherin↑, CD133↓, CD44↓, ALDH1↓ | [209] |
| Berberine | P53↑, PTEN↑, c‐Myc↓, Bcl‐2↓, EGFR↓, DNMT1, DNMT3B↓ CREBBP, EP300, SIRT3, KDM6A, SETD7↑, HDAC8, H3K4me3, H3K27me3, H3K36me3↓, pan‐HDAC inhibitor, Angiogenesis↓, Anti-CSC effect | [74] |
| Resveratrol | p53 phosphorylation↑, mutant p53↓, PTEN↑; c‐Myc↓, Bcl‐2↓, k‐Ras↓, EGFR phosphorylation↓, β-catenin↓, Twist1↓, Snail↓, DNMT1, DNMT3A, DNMT3B↓, CD8(+) T cells↑, Angiogenesis↓, Cancer‐associated fibroblasts↓, HDAC1, HDAC2↓, CSC↓ | [209] |
| Dioscin | p53↑73; Bcl‐2↓73,74; VEGF‐A↓74, TET1↓, TET2↑, TET3↑, DNMT3A↑, Macrophage sensitivity↑ | [210] |
| Celastrol | p53 phosphorylation↑, c‐Myc↓, Bcl‐2↑, VEGF↑, VEGF↓, EGFR↓, Remodelling fibrotic and immunosuppressive tumor Microenvironment, Inflammatory microenvironment↓ | [210] |
| Silibinin | p53 acetylation↑, PTEN↑, c‐Myc↓, Bcl‐2↓, VEGF, EGFR↓DNMT1↓, H3K27me3↑, SIRT1↓, acH3, acH4↑, HDAC1‐3↓, Cancer‐associated fibroblasts↓, Tumor‐associated myeloid‐derived suppressor cells, Anti-CSC effect | [210] |
| Sulforaphane | SMO↓, GLI1↓, GLI2↓, Nanog↓, Oct-4↓, Bcl-2↑, Zeb-1↓, E-cadherin↓, VEGF↓, CR1↓, CR3↓, Nanog↓, ALDHH1A1↓, Wnt3↓, Notch4↓ | [209,211] |
Targeting CSC signaling pathways
TSCC including CSC generation involves various dysregulated cellular and molecular signaling pathways, some of them have been implicated in TCSC drug-resistance. Numerous altered signaling pathways such as PI3K/Akt, Notch, EMT, Wnt/b-catenin, STAT, AP-1 and NF-kB are associated with regulation of CSC properties and its self-renewal pathways involve in the development of various cancers including TSCCs [9,25,75,124,125]. Recently, studies have reported that dysregulation of PI3K/AKT and JAK/STAT pathways have been noted in the pathogenesis of head and neck cancers (HNCs) [126,127] and acts as potential therapeutic CSC-target(s). The altered activation of PI3K/AKT/mTOR signaling pathways play a critical role for cellular growth, transformation and in chemo-radio resistance in TSCC [128,129]. Further, NVP-AEE788, a tyrosine kinase inhibitor inhibited EGF and VEGF signaling, cell growth and proliferation, induced apoptosis, and reduced the phosphorylation of EGFR/VEGFR-2/AKT in TSCC cells (Table 2) [130]. It indicates that these signaling pathways have potential of therapeutic targets which may serve as promising network for the development of CSC-targeted chemo-radio sensitizing drugs for TSCC patients [129,131,132]. Abnormal/overactivation of Notch signaling pathway plays a key role in the CSC maintenance during TSCC and therapeutic targeting of this pathway prevents Notch receptor cleavage on the cell surface leading to inhibition of self-renewal and tumorigenicity of oral-tongue cancer stem-like cells thus acting as a potential chemotherapeutic target for TSCC patients [133], [134], [135], [136] (Table 1 & 2). In a recent study, aberrant expression of SOX8 correlated with activation of the Wnt/β-catenin and epithelial-mesenchymal transition (EMT) signaling leading to maintenance of stemness properties and treatment resistance in TSCC cells [137]. β-catenin also directly controls OCT4 expression and increases tumor growth in oral cancer stem-like cells [104]. Further, Liping Zhang et al. (2019) examined the expression of R-spondins proteins 2 (Rspo2) and leucine-rich repeat-containing G protein-coupled receptor 4 (LGR4) in TSCC cases and cell lines found that aberrant expression of Rspo2-LGR4 promotes cell proliferation, invasion, migration, self-renewal and EMT through the activation of Wnt/β-catenin signaling pathway [138].
Also, bone morphogenic protein 4 (BMP-4) expression promoted acquisition of TCSCs and EMT activation in TSCC cells [139]. Further, forced expression of EMT markers (Snail and Slug) enhanced cell invasion, migration and stemness features in TSCC cells. Altered expression of BMI-1 has been found to be correlated with self-renewal of CSC in HNC [47,140]. BMI-1+ve CSCs in TSCC cells have been associated with drug resistance in a mouse model [115,141]. Recently, Chen and co-workers (2017) investigated that BMI-1+ve CSCs can mediate invasive growth and lymph node metastasis in a mouse model and the primary oral tumors associated with high tumorigenicity, invasiveness and cisplatin-resistance leading to overexpression of transcription factor activator protein-1 (AP-1) [142]. Recently, it has been shown that AP-1 signaling involved in radio-sensitization of cervical and oral cancer stem cells and treatment of curcumin with conventional anti-cancer drugs significantly help for effective cancer treatment [9,56,134]. Further, we have shown that HR-HPV16 plays an essential role in maintaining stemness in cervical cancer [57] but in oral cancer [124] through functional interaction with NF-κB pathways, it lead to well differentiation of tumours and good prognosis.
Recently, Upadhyay et al. (2016), demonstrated that activation of NOTCH pathway levels is associated with spheroid formation, stemness markers expression and lymph node metastasis in TCSCs and may serve as therapeutic target for tongue cancer patients [135]. Further, upregulation of NOTCH1 enhances cells growth, proliferation and migration during tongue tumorigenesis [143] while inhibition of NOTCH1 with FLI-06 inhibitor could reduce tongue cells proliferation, migration, invasion and induce apoptosis both in-vivo and in-vitro [143] (Table 2). In addition, the treatment of ASCs (adipose-derived stem cells)-conditioned medium inhibited cisplatin-induced cell death and enhanced anti-apoptotic effects through activation of IGF-1R and ERK/AKT pathways in TSCC cells. Furthermore, p75 neurotrophin receptor (p75NTR) is a cell-surface receptor glycoprotein was significantly expressed in TSCC cells and showed CSC-like characteristics such as proliferation, self-renewal, multidirectional differentiation and tumorigenesis and may be considered as a potential stem cell marker for TSCC [144]. Moreover, upregulation of BCL-2 expression is associated with increased cell growth, proliferation and cisplatin treatment resistance in TSCC cell lines. Targeting Bcl-2 through ABT-199 inhibitor (Table 2) effectively suppresses BCL-2 expression and impairs mitochondrial functions in TSCC cells [145].
It is therefore suggested that current cancer research should focus towards novel treatment approaches either singly or combinatorial approaches against these signaling pathways that regulate CSC self-renewal, growth and differentiation. This will facilitate better understanding of TSCC pathobiology and provide cues to cure highly aggressive and metastatic tumors without relapse.
Targeting HPV-specific CSCs in TSCC
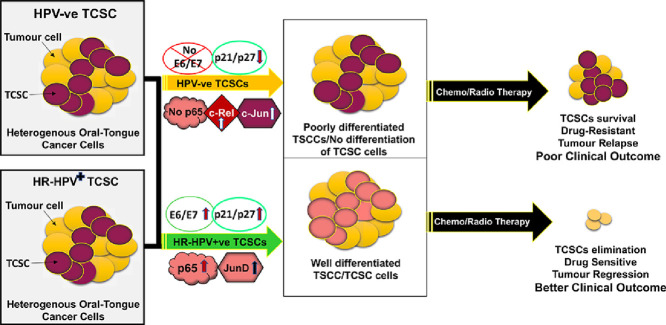
A large number of studies have now well established that infection of high-risk HPV (HR-HPV) specifically type 16 is an important carcinogenic agent responsible for a significant percentage of TSCCs. These HPV positive TSCCs possess a unique signaling mechanism for tumorigenesis as the patients show better prognosis with improved responsiveness to chemo-radio therapy leading to favorable survival rates as compared to tobacco or alcohol-induced HPV−ve TSCCs. Therefore, understanding the role played by HPV in TCSCs may improve treatment strategies as well as patient outcome [9,20,146,147]. Highly interesting is the findings that unlike cancer of cervix; in which HR-HPV is the major causative factor for tumorigenic transformation and invasive cancer, HPV infected TSCCs in contrast, show well differentiation of tumours with koilocytes (indicative of HPV) and better prognosis [20]. It indicates an inherent difference between HPV+ve and HPV−ve TCSCs [25], which also highlights the difference in tumor aggressiveness and response to treatment. CSCs share much of the genetic machinery with non-tumorigenic stem cells to maintain pluripotency and allow self-renewal and tumor formation. Therefore, it is tempting to speculate that HPV infection leads to establishment of the viral circular double-stranded DNA genome as a stable episome within the basal layer of epithelial cells and may alter pluripotency of several genes [148,149]. HR-HPVs that produce two principal transforming oncoproteins (E6/E7) play essential roles in viral integration, tumorigenic transformation and immune evasion by targeting cytokine expression including monocyte chemoattractant protein-1 (MCP-1) [150] to alter cell proliferation and interferon responses. HR-HPV E6 and E7 oncoproteins dysregulate two major tumor suppressor gene products p53 and pRb which also constitute important cellular regulatory pathways in maintaining stemness. Dysregulation of these genes leads to increased CSC production and aggressive carcinogenesis. It has been reported that HPV+ve oropharyngeal cancers show higher intrinsic CSC population than that in HPV−ve oral cancers and it is shown to be associated with HPVE6-mediated p53 inactivation [54]. p53 protein is deregulated by HPVE6 protein which is responsible for maintaining genomic integrity and stemness by altering expression of NANOG and NOTCH. Generally, p53 inhibits NOTCH expression but following HPV infection HPV-E6 binds to p53 and the inhibition to NOTCH is relieved leading to increased CSC production and tumorigenesis. HPV E7 protein on the other hand, binds to pRb which normally remains bound with E2F protein, the E2F becomes now free to bind to promoters of cell cycle regulatory genes allowing cells to produce cell cycle proteins and to progress from G1 to the S phase leading to increased cell division and oncogenesis. E2F is also bound to promoter of stemness factors such as NANOG and alters stem cell genes expression. Earlier, Lee et al. (2015) reported that four HPV+ve oral cancer cell lines show relatively a higher tumor growth and self-renewal capacity [151]. Interestingly, CSC marker CD44 expression is lower in patients with HPV-positive oral cancer as compared with HPV-negative oral cancer patients [152]. Further, Zhang et al. (2014) revealed that HPV+ve oropharyngeal cancers (OPSCCs) had a higher intrinsic CSC population as compared to HPV−ve tumors and elevated ALDH1 staining suggesting that CSC phenotype might be more important than CSC population density for disease aggressiveness and clinical outcome in OPSCCs [51]. Moreover, low percentage of CSCs in tumor bulk and high expression of CSC markers are associated with cisplatin drug resistant in HPV−ve OPSCCs implying CSC properties play a key role in treatment resistant cases [41]. Furthermore, in-vivo data suggests that CSCs from the bulk of HPV-positive oral cancer may respond better to platinum-based therapy [153,154]. On the other hand, it has also been demonstrated that HPV+ve oral cancer cell lines have a lower proportion of CSC than HPV−ve cells and radiation therapy stimulated tumor cell dedifferentiation proposing tumor CSC density in HPV+ve oral cancers have a better prognosis and treatment outcome [155]. These contradictory observations strongly suggest that additional biological or molecular features of CSC is responsible for the treatment response of oral-tongue cancer [51].
Recently, we have shown that a significantly higher subpopulation of CSCs in cervical cancer has been shown to overexpress HR-HPV E6 oncogene and found to regulate and maintain stemness properties via Hes1 signaling pathway [57]. We have further shown that HPV16 plays a crucial role through its functional interaction with NF-κB leading to well differentiation of tumours and better prognosis when treated [124]. Additionally, we have recently observed that higher expression and selective participation of NF-κB/c-Rel with p50 that in cross-talk with AP-1/Fra-2 in tongue cancer and cancer stem cells induced poor differentiation and aggressive tumorigenesis only in HPV−ve TSCC patients while HPV infection induced increased expression of p65 and p27 leading to well differentiation and better prognosis preferably in non-smoking TSCC patients [25,156]. These studies have established that CSCs with stemness markers along-with HPV infection could play an important role in the pathobiology and treatment outcome of oral-tongue cancer.
Targeting CSC-specific altered epigenetic pathways
Alterations in epigenetic pathways are commonly linked with tumor initiation, progression, differentiation and drug resistance. These alterations are also involved in cellular plasticity during cancer progression, and further contribute to the formation of CSCs. Increasing evidence suggests that CSCs expression can be influenced by epigenetic alterations in differential epigenetic signaling network such as histone modifications, involving DNA methylation, acetylation, post translational modifications and non-coding RNAs (ncRNAs) that dynamically control gene expression and are frequently occur in cancers including HNCs but the role of epigenetic alterations in TSCC certainly warrants further investigation [157], [158], [159], [160]. The epigenetic alterations are emerging as key markers for early diagnosis and prognosis of TSCCs. Altered DNA methylation patterns and histone modifications in cancer stem cells leading to aberrant expression of miRNAs, indicated distinct DNA methylation profiles in CSCs [161]. Histone protein modifications can also play an important epigenetic role in maintaining and controlling CSC properties in HNC [25,158,162,163]. Recently, several long non-coding RNAs (lncRNAs) are shown to play an important role in development, stemness, differentiation and cancer development. Recently, lncRNAs have been categorized on the basis of their association with various subtypes of HNCs and overexpression of metastasis-associated lung adenocarcinoma transcript1 (MALAT1) was found to be a prognostic biomarker for oral cancer patients. MALAT1 can also play a significant oncogenic marker for TSCC [164]. LncRNA HOTAIR was also found to be an essential marker for advanced grades of HNCs [165]. Emerging evidences demonstrate that significant role of P-element-induced wimpy testis (PIWI)-interacting RNAs (piRNAs) in cancer and other diseases, but it remains to be identified its role in CSCs formation. While, its role in germline stem cell maintenance and genome stability is well established and overexpression of PIWIL1 and PIWIL2 is associated with OCT4 and SOX2 overexpression in colon cancer, it would be interesting to look for the potential role of piRNAs in TSCC-specific CSCs.
Small ncRNAs the miRNAs, are also play key roles in tumorigenesis via increasing oncogene expression, inhibiting tumor suppressor gene function and contribute to drug resistance, initiate cell reprogramming, promote epithelial-mesenchymal transition (EMT) and stemness properties [158,166]. It has been demonstrated that in majority of oral cancer cases, miR-34b, miR-137, miR-193a, and miR-203 are located in close proximity to the CpG islands and are found silenced through hypermethylation [167]. Earlier, miR-145 has been found to be downregulated in ALDH+ and CD44+ oral cancer stem cells and modulation of miRNAs in CSCs will improve better understanding of the disease biology [168]. Reduced expression of miR-200b, miR-203 and let-7a and upregulation of miR-138, miR-183, miRNA-145 and miR-205 have been analysed in oral-tongue cancers, which suggest that altered expression of these miRNAs are associated with stem cell regulation in oral-tongue cancer [169]. Very recently, it has been reported that upregulation of miR-21 and miR-155 and reduced expression of miR-34a in HPV16-E6-positive CSCs is responsible for stemness maintenance in oral cancer cells [124]. These reports highlight the promising role of miRNAs in characterizing tongue cancer stem calls during oral-tongue carcinogenesis and targeting of these miRNAs may help in developing novel therapeutic strategies for effective management of TSCCs.
Targeting stem cell niches, tumor microenvironment and cancer stem cell-exosomes
The niche or heterogeneous tumor microenvironment plays a very significant role in the maintenance of CSCs which are responsible for chemo-radio resistance exist in a highly specialized niche. The main role of CSC niches to maintain and protect the CSCs, allowing them to resist standard anti-cancer therapies. The CSC niches allow the cell to remain dormant for a long time before initiating recurrent/distance metastasis [170]. Therefore, targeting whole tumor will not produce good results while targeting CSCs [171]. Furthermore, the cancer stem cells derived-exosomes are also crucial for tumor relapse, metastasis and maintenance. It can mediate cell to cell communication within the niches created in the tumor microenvironment to promote tumorigenesis. So it is possible that CSCs produce exosomes with unique characteristics and functions. Therefore, this is one of the best exosome cancer therapies to have chemo-radio therapeutic drug-loaded exosomes for targeting CSCs. There are also several studies now reported CSC derived exosomes containing specific type of miRNAs in renal, prostate, gastric and glioma cancers [172,173]. The exploitation of CSC-derived exosomes as drug delivery vehicles offers significant advantages as compared to other nanoparticulate drug delivery systems [174]. Exosomes are small extracellular membrane-based with different compositions that are involved in several biological and pathological processes of cancer and function as intercellular communicators by delivering their content to a target cell via membrane fusion or endocytosis [175,176]. Exosomes can be isolated from various body fluids including amniotic fluid, nasal lavage, saliva, serum, plasma, urine and CSCs etc. and used as micro-vesicles for therapeutic drug and gene delivery. As they are biocompatible, nontoxic, easy to produce, stable, easy to store, have a long life span and high cargo loading capacity [114,177,178], these characteristics making exosome a potential drug-delivery tool for the treatment of TSCCs [154,175,179]. Recent evidences have shown that exosome-based drug delivery has higher anti-tumor effects when compared to free drug delivery in mouse model [114]. Synthetic nanoparticles-based drug delivery have been employed to deliver drugs to bulk of the CSCs [180]. Further, it has been reported that exosomes derived from docetaxel- or adriamycin-resistant breast cancer cells contain distinct miRNAs which can decrease the chemo-sensitivity in targeted tumor cells [112]. Mutschelknaus and co-workers (2016) demonstrated a functional role of exosomes that transmitted pro-survival signals leading to tumorigenesis and radioresistant phenotype in TSCCs indicative of the fact that exosomes are potential targets to improved therapeutic strategies for better understanding of drug resistance TSCCs [175].
Chronic inflammation, secretion of inflammatory cytokines/chemokines and they have capacity to regulate proliferation, hypoxia and perivascular niches. Due to these characteristics, CSCs interact with heterogeneous tumor microenvironment leading to tumor progression and relapse [181], [182], [183]. Therefore, identification of CSC niches, CSC-derived exosomes and specific miRNAs there in and oral tumor microenvironment and understanding their interactions in TSCC will only allow us to gain the knowledge needed to develop targeted therapies against tumor progression and relapse (see Table 1 and Fig. 1).
Targeting CSCs by phytocompounds and nanoparticle-based therapy
CSCs are responsible for tumor heterogeneities and drug resistance to conventional therapies in many aggressive cancer types including TSCCs. These conventional treatment strategies such as surgery, radiotherapy, chemotherapy and immunotherapy are strategized depending on the tumor type, site, size, grade, stage and expression of specific biomarkers in TSCC patients. But these therapies are not much successful due to several limitations. These limitations are associated with systemic and local drug toxicity, cancer relapse, metastasis and self-renewal of CSCs or acquisition of CSC traits by non-CSCs. Hence, CSCs are becoming novel target for cancer therapy. Therefore, it is important to develop effective treatment/drugs that can target CSCs to prevent tumor relapse and reduce systemic toxicity. This will improve survival and quality of life of the TSCC patients. Emerging evidences suggest that herbal and nanoparticle-based therapy of CSCs can be achieved by several targets such as genetic or epigenetic signaling pathways, stemness markers, efflux transporters, HPV oncoproteins, CSC niches and CSC-derived exosomes containing specifc miRNAs which are critically responsible for self-renewal and maintenance of CSC phenotype and tumorigenicity. Use of nanoparticles with different sizes, configurations, chemical properties, and bio-functional compositions, may enhance both target selectivity and drug internalization in cancer cells [184,185]. Various nanomaterial including DNA (origami and tetrahedron), carbon (graphene oxide, carbon nanotubes and nano-diamond), noble metals (sphere AuNPs, gold nanorods and silver), organic nanoparticles (liposome and polymer) are emerging approaches in cancer stem cell therapy [186,187]. Nanoparticle-based delivery have high drug carrier capabilities, higher drug solubility and efficacy and site specific drug accumulation in cancer cells than conventional free drug delivery. These molecules also serve as suitable carriers of chemo-preventive herbal compounds for variety of cancer diagnosis and therapeutic outcome. NV-1 was a liposomal formulation having higher anticancer drug effect to breast cancer stem cells [180,186]. Recent studies also suggest that nano-formulation of herbal compound, curcumin (diferuloylmethane) can be used effectively for cancer and cancer stem therapy [180,188,189]. Nano-formulation of curcumin (NP-Cur) can induce cell necrosis and decrease the expression of several signaling pathways such as NF-κB, AP-1, VEGF, MMP-9, and TNF-α. It has been shown that delivery of Curcumin-difluorinated liposomes is useful for cisplatin resistant HNC therapy [190,191]. Cur-microemulsion formulation carry toxic effects and show damaged and ruptured tongue cancer cells after treatment than free Cur formulation [192]. Valproic acid, a histone deacetylase inhibitor (VPA) has been shown to downregulate the expression of stemness markers (OCT4, SOX2, and CD44) and can suppress self-renewal abilities of oral cancer stem cells [193]. Further, Nano-Cur can induce apoptotic death of TSCC and TCSCs with a low cytotoxic effect, curcumin being a strong antioxidant which is known to sensitize cancer and cancer stem cells [194]. These studies together indicate that development of anti-CSC drugs using nano-formulation may target growth and functionality of tongue cancer and cancer stem cells making them ideal approaches for effective TSCC/TCSC treatment outcome.
Inducing differentiation of CSC
Poor or undifferentiation is an essential hallmark of all cancer stem cells. While 99% of cells in a tumor are differentiated cells which are often killed by standard therapy, 1–2% cells are un-differentiated cancer stem-like cells which are responsible for tumor relapse and metastasis. Therefore, apart from targeting different genes or pathways, one of the best strategies for CSC-targeted therapy is though differentiation of CSCs that holds great promise for cancer treatment. Usually, differentiation therapy tend to have no toxic effect than conventional cancer treatment. In 1980, differentiation therapy had been adapted to leukemia CSCs to induce retinoic acid (RA)-induced differentiation in certain hematopoietic cells [195,196]. As CSCs have the ability to differentiate into several types of cells, including lineage-differentiated and non-cancerous-differentiated tumor cells after exposure to specific differentiation agents/signals, differentiation therapy might induce CSCs into terminally differentiated cancer cells, and then, these cells could be easily treated with alkylating agents or a combination of the two or more conventional therapies (Table 1). Treating CSCs through the induction of differentiation has been an attractive concept, but clinical development of differentiation-inducing agents to treat CSCs, especially CSC from solid tumours has been limited till date [196]. Few studies have shown that RA, a small lipophilic derivative of vitamin A, is a well-known inducer of differentiation and proliferation in normal stem cells [197]. All-trans retinoic acid (ATRA) is an inducer of differentiation, which can enhance sensitivity to anticancer therapies and decrease CSC motility, invasion and tumorigenicity in glioblastomas [198] and breast cancer [199]. BMP signaling is the most powerful inducer of differentiation and a variant type of BMP reduces tumorigenicity by inducing differentiation in glioblastomas [200,201]. Further, induction of mesenchymal-to-epithelial transition (MET) has been proposed as a new alternative as it has potential to reduce aggressive tumorigenicity of CSCs.
We have recently demonstrated that higher DNA binding and overexpression of AP-1/c-JUN with FRA-2 and NF-κB/c-REL with p50 promoted poor differentiation, aggressive tumorigenesis and worst prognosis only in HPV−ve TSCC patients, while HPV infection promoted selective activation of AP-1/JUND and NF-κB/p65 and upregulation of p27 leading to well differentiation and better prognosis mainly in non-smoking TSCC patients [9,25]. Thus, to eradicate TCSCs and prevent TSCC recurrence, induction of virus-based differentiation strategies may provide new insights for the treatment of solid tumors.
Concluding remarks
TSCCs are the most predominant and aggressive HNC subtype and despite numerous therapies have come into existence in the last few decades, late diagnosis, locoregional recurrence, aggressive metastasis and drug resistance have been the major confounders for the poor 5 years survival of TSCC patients. The treatment failures are mainly attributed to presence of a small subpopulation of ‘tongue cancer stem-like cells (TCSCs)’ within the tumor microenvironment. These TCSCs show hallmark abilities of self-renewal, cancer growth, metastasis, drug resistance, quiescence, apoptotic and immune evasion, DNA damage resistance and expression of drug efflux transporter proteins. Despite several attempts in studying the role of CSCs in tongue cancer and development of combinatorial therapeutic approaches that focus on targeting the stemness signaling pathways, stemness markers, blocking epithelial mesenchymal transition, CSC niche, stem cell plasticity, nano-particles based therapy, sensitizing of stem cells with pharmaceutical agents, modulation of epigenetic pathways and induction of differentiation may offer useful platform to eradicating high-risk TCSCs. Further understanding including single cell omics technology in CSC biology developing personalized and targeted therapies could provide recurrence-free treatment and improved quality of life for the TSCC patients. Targeting of TCSCs remains an attractive yet elusive therapeutic option, with the goal of increasing specificity and effectiveness in tumor eradication and prevention of recurrence as well as decreasing off-targeting and systemic toxicity. Further research for characterization and targeted delivery of therapies separately for HPV+ve/−ve TCSCs would be important because of their differential biology and prognostic characteristics.
Key Future directions
-
•
Therapy resistance and tumor a recurrence following standard treatment is quite common in TSCCs as TCSCs, a subpopulation of cancer cells are difficult to eliminate with conventional treatments. Therefore, strategic therapeutic interventions can be designed to target CSCs and/or CSC-exosomes to control aggressive metastasis and tumor relapse.
-
•
Identification of site-specific CSC markers for TSCC and development of alternative treatment strategies in combination with existing therapies are the critical steps in right direction for TCSC eradication with better understanding of disease pathogenesis and treatment outcome.
-
•
Furthermore, the use of emerging technologies including single cell omics, high-throughput DNA and RNRA sequencing and analysis of CSC-derived exosomal ncRNAs will help to discover new therapeutic avenues for personalized therapy and improved TSCC patient's survival outcome.
Declaration of Competing Interest
No conflicts of interest.
Acknowledgments
The authors gratefully acknowledge the financial support of J. C. Bose Fellowship from Department of Science & Technology (SR/S2/JCB-80/2007) and Indian Council of Medical Research (ICMR) (5/13/15/2015/NCD-III), Government of India to Bhudev C Das. This study was also supported by grants from the ICMR-Senior Research Fellowship to Prabhat Kumar (3/2/2/272/2014/NCD-III) and DST-SERB to Shilpi Gupta (PDF/2016/000017).
Footnotes
Rationale: Tongue squamous cell carcinoma (TSCC) is a predominant subtype of head and neck cancer with overall five year survival of only 50% and characterized by highly aggressive biological behavior, distant metastasis, tumor relapse and drug resistant. Majority of patients with tobacco habit are HPV−ve and have bad prognosis while HPV+ve TSCCs are mostly non-tobacco users who show well-differentiated tumors with much better treatment outcome. A small sub-population of tongue cancer stem cells (TCSCs) are resistant to chemo-radio therapy, escape immune surveillance and responsible for cancer relapse.
Selective targeting of TCSCs may provide efficient therapeutic strategy for relapse-free survival leading to improved quality of life and reduced mortality.
References
- 1.Siegel R., Ward E., Brawley O., Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Bosch F.X., Ritter D., Enders C., Flechtenmacher C., Abel U., Dietz A., Hergenhahn M., Weidauer H. Head and neck tumor sites differ in prevalence and spectrum of p53 alterations but these have limited prognostic value. Int. J. Cancer. 2004;111:530–538. doi: 10.1002/ijc.11698. [DOI] [PubMed] [Google Scholar]
- 5.Kamangar F., Dores G.M., Anderson W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 6.De Cicco C., Trifiro G., Calabrese L., Bruschini R., Ferrari M.E., Travaini L.L., Fiorenza M., Viale G., Chiesa F., Paganelli G. Lymphatic mapping to tailor selective lymphadenectomy in cN0 tongue carcinoma: beyond the sentinel node concept. Eur. J. Nucl. Med. Mol. Imaging. 2006;33:900–905. doi: 10.1007/s00259-006-0088-4. [DOI] [PubMed] [Google Scholar]
- 7.Bello I.O., Vered M., Dayan D., Dobriyan A., Yahalom R., Alanen K., Nieminen P., Kantola S., Laara E., Salo T. Cancer-associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011;47:33–38. doi: 10.1016/j.oraloncology.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Ganly I., Patel S., Shah J. Early stage squamous cell cancer of the oral tongue–clinicopathologic features affecting outcome. Cancer. 2012;118:101–111. doi: 10.1002/cncr.26229. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S., Kumar P., kour H., Sharma N., Saluja D., Bharti A., Das B.C. Selective participation of c-Jun with Fra-2/c-Fos promotes aggressive tumor phenotypes and poor prognosis in tongue cancer. Sci. Rep. 2015;5:16811. doi: 10.1038/srep16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byers R.M., Weber R.S., Andrews T., McGill D., Kare R., Wolf P. Frequency and therapeutic implications of "skip metastases" in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997;19:14–19. doi: 10.1002/(sici)1097-0347(199701)19:1<14::aid-hed3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi A.K., Engels E.A., Anderson W.F., Gillison M.L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 12.Davis S., Severson R.K. Increasing incidence of cancer of the tongue in the United States among young adults. Lancet. 1987;2:910–911. doi: 10.1016/s0140-6736(87)91393-6. [DOI] [PubMed] [Google Scholar]
- 13.Zelefsky M.J., Harrison L.B., Fass D.E., Armstrong J., Spiro R.H., Shah J.P., Strong E.W. Postoperative radiotherapy for oral cavity cancers: impact of anatomic subsite on treatment outcome. Head Neck. 1990;12:470–475. doi: 10.1002/hed.2880120604. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamurthy A., Ramshankar V. Early stage oral tongue cancer among non-tobacco users—an increasing trend observed in a South Indian patient population presenting at a single centre. Asian Pac. J. Cancer Prev. 2013;14:5061–5065. doi: 10.7314/apjcp.2013.14.9.5061. [DOI] [PubMed] [Google Scholar]
- 15.Llewellyn C.D., Johnson N.W., Warnakulasuriya K.A. Risk factors for squamous cell carcinoma of the oral cavity in young people—a comprehensive literature review. Oral Oncol. 2001;37:401–418. doi: 10.1016/s1368-8375(00)00135-4. [DOI] [PubMed] [Google Scholar]
- 16.Nair U., Bartsch H., Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis. 2004;19:251–262. doi: 10.1093/mutage/geh036. [DOI] [PubMed] [Google Scholar]
- 17.Ogden G.R. Alcohol and oral cancer. Alcohol. 2005;35:169–173. doi: 10.1016/j.alcohol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Warnakulasuriya S., Sutherland G., Scully C. Tobacco, oral cancer, and treatment of dependence. Oral Oncol. 2005;41:244–260. doi: 10.1016/j.oraloncology.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S., Kumar P., Das B.C. HPV: molecular pathways and targets. Curr. Probl. Cancer. 2018;42:161–174. doi: 10.1016/j.currproblcancer.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Mishra A., Bharti A.C., Varghese P., Saluja D., Das B.C. Differential expression and activation of NF-kappaB family proteins during oral carcinogenesis: role of high risk human papillomavirus infection. Int. J. Cancer. 2006;119:2840–2850. doi: 10.1002/ijc.22262. [DOI] [PubMed] [Google Scholar]
- 21.Bodner L., Manor E., Friger M.D., van der Waal I. Oral squamous cell carcinoma in patients twenty years of age or younger-review and analysis of 186 reported cases. Oral Oncol. 2013;50:84–89. doi: 10.1016/j.oraloncology.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Chitapanarux I., Lorvidhaya V., Sittitrai P., Pattarasakulchai T., Tharavichitkul E., Sriuthaisiriwong P., Kamnerdsupaphon P., Sukthomya V. Oral cavity cancers at a young age: analysis of patient, tumor and treatment characteristics in Chiang Mai university hospital. Oral Oncol. 2006;42:83–88. doi: 10.1016/j.oraloncology.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Kim K.Y., Lewis J.S., Jr., Chen Z. Current status of clinical testing for human papillomavirus in oropharyngeal squamous cell carcinoma. J. Pathol. Clin. Res. 2018;4:213–226. doi: 10.1002/cjp2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hafkamp H.C., Manni J.J., Haesevoets A., Voogd A.C., Schepers M., Bot F.J., Hopman A.H., Ramaekers F.C., Speel E.J. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int. J. Cancer. 2008;122:2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S., Kumar P., Kaur H., Sharma N., Saluja D., Bharti A.C., Das B. Constitutive activation and overexpression of NF-kappaB/c-Rel in conjunction with p50 contribute to aggressive tongue tumorigenesis. Oncotarget. 2018;9:33011–33029. doi: 10.18632/oncotarget.26041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tan P.F., Westra W.H., Chung C.H., Jordan R.C., Lu C., Kim H., Axelrod R., Silverman C.C., Redmond K.P., Gillison M.L. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Souza G., Kreimer A.R., Viscidi R., Pawlita M., Fakhry C., Koch W.M., Westra W.H., Gillison M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 28.Fakhry C., Westra W.H., Li S., Cmelak A., Ridge J.A., Pinto H., Forastiere A., Gillison M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 29.Gillison M.L., Koch W.M., Capone R.B., Spafford M., Westra W.H., Wu L., Zahurak M.L., Daniel R.W., Viglione M., Symer D.E., Shah K.V., Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 30.Facompre N., Nakagawa H., Herlyn M., Basu D. Stem-like cells and therapy resistance in squamous cell carcinomas. Adv. Pharmacol. 2012;65:235–265. doi: 10.1016/B978-0-12-397927-8.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castellsague X., Alemany L., Quer M., Halec G., Quiros B., Tous S., Clavero O., Alos L., Biegner T., Szafarowski T., Alejo M., Holzinger D., Cadena E., Claros E., Hall G., Laco J., Poljak M., Benevolo M., Kasamatsu E., Mehanna H., Ndiaye C., Guimera N., Lloveras B., Leon X., Ruiz-Cabezas J.C., Alvarado-Cabrero I., Kang C.S., Oh J.K., Garcia-Rojo M., Iljazovic E., Ajayi O.F., Duarte F., Nessa A., Tinoco L., Duran-Padilla M.A., Pirog E.C., Viarheichyk H., Morales H., Costes V., Felix A., Germar M.J., Mena M., Ruacan A., Jain A., Mehrotra R., Goodman M.T., Lombardi L.E., Ferrera A., Malami S., Albanesi E.I., Dabed P., Molina C., Lopez-Revilla R., Mandys V., Gonzalez M.E., Velasco J., Bravo I.G., Quint W., Pawlita M., Munoz N., de Sanjose S., Xavier Bosch F. HPV Involvement in Head and Neck Cancers: comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016;108:djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 32.Leemans C.R., Snijders P.J.F., Brakenhoff R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer. 2018;18:269–282. doi: 10.1038/nrc.2018.11. [DOI] [PubMed] [Google Scholar]
- 33.Nasman A., Du J., Dalianis T. A global epidemic increase of an HPV-induced tonsil and tongue base cancer - potential benefit from a pan-gender use of HPV vaccine. J. Intern. Med. 2019;287:134–152. doi: 10.1111/joim.13010. [DOI] [PubMed] [Google Scholar]
- 34.Marsh D., Suchak K., Moutasim K.A., Vallath S., Hopper C., Jerjes W., Upile T., Kalavrezos N., Violette S.M., Weinreb P.H., Chester K.A., Chana J.S., Marshall J.F., Hart I.R., Hackshaw A.K., Piper K., Thomas G.J. Stromal features are predictive of disease mortality in oral cancer patients. J. Pathol. 2011;223:470–481. doi: 10.1002/path.2830. [DOI] [PubMed] [Google Scholar]
- 35.Mroueh R., Haapaniemi A., Grenman R., Laranne J., Pukkila M., Almangush A., Salo T., Makitie A. Improved outcomes with oral tongue squamous cell carcinoma in Finland. Head Neck. 2017;39:1306–1312. doi: 10.1002/hed.24744. [DOI] [PubMed] [Google Scholar]
- 36.Bao B., Ahmad A., Azmi A.S., Ali S., Sarkar F.H. cancer stem cells (CSCs) and mechanisms of their regulation: implications for cancer therapy. Curr. Protoc. Pharmacol. 2013;14 doi: 10.1002/0471141755.ph1425s61. Unit 14 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weng J., Zhang H., Wang C., Liang J., Chen G., Li W., Tang H., Hou J. miR-373-3p targets DKK1 to promote EMT-induced metastasis via the Wnt/beta-catenin pathway in tongue squamous cell carcinoma. Biomed. Res. Int. 2017;2017 doi: 10.1155/2017/6010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prince M.E.P., Zhou L., Moyer J.S., Tao H., Lu L., Owen J., Etigen M., Zheng F., Chang A.E., Xia J., Wolf G., Wicha M.S., Huang S., Ren X., Li Q. Evaluation of the immunogenicity of ALDH(high) human head and neck squamous cell carcinoma cancer stem cells in vitro. Oral Oncol. 2016;59:30–42. doi: 10.1016/j.oraloncology.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reid P., Staudacher A.H., Marcu L.G., Olver I., Moghaddasi L., Brown M.P., Bezak E. Influence of the human papillomavirus on the radio-responsiveness of cancer stem cells in head and neck cancers. Sci. Rep. 2020;10:2716. doi: 10.1038/s41598-020-59654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lapidot T., Sirard C., Vormoor J., Murdoch B., Hoang T., Caceres-Cortes J., Minden M., Paterson B., Caligiuri M.A., Dick J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 41.Gunduz M., Gunduz E., Tamagawa S., Enomoto K., Hotomi M. Identification and chemoresistance of cancer stem cells in HPV-negative oropharyngeal cancer. Oncol. Lett. 2020;19:965–971. doi: 10.3892/ol.2019.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jakob M., Sharaf K., Schirmer M., Leu M., Kuffer S., Bertlich M., Ihler F., Haubner F., Canis M., Kitz J. Role of cancer stem cell markers ALDH1, BCL11B, BMI-1, and CD44 in the prognosis of advanced HNSCC. Strahlenther. Onkol. 2020 doi: 10.1007/s00066-020-01653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karatas H., Yemisci M., Eren-Kocak E., Dalkara T. Brain peptides for the treatment of neuropsychiatric disorders. Curr. Pharm. Des. 2018;24:3905–3917. doi: 10.2174/1381612824666181112112309. [DOI] [PubMed] [Google Scholar]
- 44.Shimokawa M., Ohta Y., Nishikori S., Matano M., Takano A., Fujii M., Date S., Sugimoto S., Kanai T., Sato T. Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature. 2017;545:187–192. doi: 10.1038/nature22081. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Moles M.A., Scully C., Ruiz-Avila I., Plaza-Campillo J.J. The cancer stem cell hypothesis applied to oral carcinoma. Oral Oncol. 2013;49:738–746. doi: 10.1016/j.oraloncology.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Kaveh K., Kohandel M., Sivaloganathan S. Replicator dynamics of cancer stem cell: selection in the presence of differentiation and plasticity. Math. Biosci. 2016;272:64–75. doi: 10.1016/j.mbs.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Prince M.E., Sivanandan R., Kaczorowski A., Wolf G.T., Kaplan M.J., Dalerba P., Weissman I.L., Clarke M.F., Ailles L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. U. S. A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satpute P.S., Hazarey V., Ahmed R., Yadav L. Cancer stem cells in head and neck squamous cell carcinoma: a review. Asian Pac. J. Cancer Prev. 2013;14:5579–5587. doi: 10.7314/apjcp.2013.14.10.5579. [DOI] [PubMed] [Google Scholar]
- 49.Shen B., Dong P., Li D., Gao S. Expression and function of ABCG2 in head and neck squamous cell carcinoma and cell lines. Exp. Ther. Med. 2012;2:1151–1157. doi: 10.3892/etm.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agliano A., Calvo A., Box C. The challenge of targeting cancer stem cells to halt metastasis. Semin. Cancer Biol. 2017;44:25–42. doi: 10.1016/j.semcancer.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Zhang M., Kumar B., Piao L., Xie X., Schmitt A., Arradaza N., Cippola M., Old M., Agrawal A., Ozer E., Schuller D.E., Teknos T.N., Pan Q. Elevated intrinsic cancer stem cell population in human papillomavirus-associated head and neck squamous cell carcinoma. Cancer. 2014;120:992–1001. doi: 10.1002/cncr.28538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biddle A., Gammon L., Liang X., Costea D.E., Mackenzie I.C. Phenotypic plasticity determines cancer stem cell therapeutic resistance in oral squamous cell carcinoma. EBioMedicine. 2016;4:138–145. doi: 10.1016/j.ebiom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 54.Swanson M.S., Kokot N., Sinha U.K. The role of HPV in head and neck cancer stem cell formation and tumorigenesis. Cancers (Basel) 2016;8:24. doi: 10.3390/cancers8020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiba T., Kita K., Zheng Y.W., Yokosuka O., Saisho H., Iwama A., Nakauchi H., Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 56.Tyagi A., Vishnoi K., Kaur H., Srivastava Y., Roy B.G., Das B.C., Bharti A.C. Cervical cancer stem cells manifest radioresistance: association with upregulated AP-1 activity. Sci. Rep. 2017;7:4781. doi: 10.1038/s41598-017-05162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tyagi A., Vishnoi K., Mahata S., Verma G., Srivastava Y., Masaldan S., Roy B.G., Bharti A.C., Das B.C. Cervical cancer stem cells selectively overexpress HPV oncoprotein E6 that controls stemness and self-renewal through upregulation of HES1. Clin. Cancer Res. 2016;22:4170–4184. doi: 10.1158/1078-0432.CCR-15-2574. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Byun Y., Ren Y.R., Liu J.O., Laterra J., Pomper M.G. Identification of inhibitors of ABCG2 by a bioluminescence imaging-based high-throughput assay. Cancer Res. 2009;69:5867–5875. doi: 10.1158/0008-5472.CAN-08-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baillie R., Itinteang T., Yu H.H., Brasch H.D., Davis P.F., Tan S.T. Cancer stem cells in moderately differentiated oral tongue squamous cell carcinoma. J. Clin. Pathol. 2016;69:742–744. doi: 10.1136/jclinpath-2015-203599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tabor M.H., Clay M.R., Owen J.H., Bradford C.R., Carey T.E., Wolf G.T., Prince M.E. Head and neck cancer stem cells: the side population. Laryngoscope. 2011;121:527–533. doi: 10.1002/lary.21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zou B., Sun S., Qi X., Ji P. Aldehyde dehydrogenase activity is a cancer stem cell marker of tongue squamous cell carcinoma. Mol. Med. Rep. 2012;5:1116–1120. doi: 10.3892/mmr.2012.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lobo N.A., Shimono Y., Qian D., Clarke M.F. The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds B.A., Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 64.Stingl J. Detection and analysis of mammary gland stem cells. J. Pathol. 2009;217:229–241. doi: 10.1002/path.2457. [DOI] [PubMed] [Google Scholar]
- 65.Pastrana E., Silva-Vargas V., Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Hajj M., Becker M.W., Wicha M., Weissman I., Clarke M.F. Therapeutic implications of cancer stem cells. Curr. Opin. Genet. Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Singh S.K., Clarke I.D., Hide T., Dirks P.B. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 68.Singh S.K., Clarke I.D., Terasaki M., Bonn V.E., Hawkins C., Squire J., Dirks P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 69.Marcato P., Dean C.A., Giacomantonio C.A., Lee P.W. Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011;10:1378–1384. doi: 10.4161/cc.10.9.15486. [DOI] [PubMed] [Google Scholar]
- 70.Shenoy A., Butterworth E., Huang E.H. ALDH as a marker for enriching tumorigenic human colonic stem cells. Methods Mol. Biol. 2012;916:373–385. doi: 10.1007/978-1-61779-980-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clay M.R., Tabor M., Owen J.H., Carey T.E., Bradford C.R., Wolf G.T., Wicha M.S., Prince M.E. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32:1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Storms R.W., Trujillo A.P., Springer J.B., Shah L., Colvin O.M., Ludeman S.M., Smith C. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc. Natl. Acad. Sci. U. S. A. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desiderio V., Papagerakis P., Tirino V., Zheng L., Matossian M., Prince M.E., Paino F., Mele L., Papaccio F., Montella R., Papaccio G., Papagerakis S. Increased fucosylation has a pivotal role in invasive and metastatic properties of head and neck cancer stem cells. Oncotarget. 2014;6:71–84. doi: 10.18632/oncotarget.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mahata S., Bharti A.C., Shukla S., Tyagi A., Husain S.A., Das B.C. Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol. Cancer. 2011;10:39. doi: 10.1186/1476-4598-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y., Li D., Li S. The Alox5 gene is a novel therapeutic target in cancer stem cells of chronic myeloid leukemia. Cell Cycle. 2009;8:3488–3492. doi: 10.4161/cc.8.21.9852. [DOI] [PubMed] [Google Scholar]
- 76.Vermeulen L., De Sousa E.M.F., van der Heijden M., Cameron K., de Jong J.H., Borovski T., Tuynman J.B., Todaro M., Merz C., Rodermond H., Sprick M.R., Kemper K., Richel D.J., Stassi G., Medema J.P. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 77.Chikamatsu K., Ishii H., Takahashi G., Okamoto A., Moriyama M., Sakakura K., Masuyama K. Resistance to apoptosis-inducing stimuli in CD44+ head and neck squamous cell carcinoma cells. Head Neck. 2011;34:336–343. doi: 10.1002/hed.21732. [DOI] [PubMed] [Google Scholar]
- 78.Ghatak S., Misra S., Toole B.P. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J. Biol. Chem. 2005;280:8875–8883. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]
- 79.Okamoto A., Chikamatsu K., Sakakura K., Hatsushika K., Takahashi G., Masuyama K. Expansion and characterization of cancer stem-like cells in squamous cell carcinoma of the head and neck. Oral Oncol. 2009;45:633–639. doi: 10.1016/j.oraloncology.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 80.Oliveira L.R., Oliveira-Costa J.P., Araujo I.M., Soave D.F., Zanetti J.S., Soares F.A., Zucoloto S., Ribeiro-Silva A. Cancer stem cell immunophenotypes in oral squamous cell carcinoma. J. Oral Pathol. Med. 2010;40:135–142. doi: 10.1111/j.1600-0714.2010.00967.x. [DOI] [PubMed] [Google Scholar]
- 81.Zhang P., Zhang Y., Mao L., Zhang Z., Chen W. Side population in oral squamous cell carcinoma possesses tumor stem cell phenotypes. Cancer Lett. 2009;277:227–234. doi: 10.1016/j.canlet.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 82.Hermann P.C., Huber S.L., Herrler T., Aicher A., Ellwart J.W., Guba M., Bruns C.J., Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Major A.G., Pitty L.P., Farah C.S. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013;2013 doi: 10.1155/2013/319489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei X.D., Zhou L., Cheng L., Tian J., Jiang J.J., Maccallum J. In vivo investigation of CD133 as a putative marker of cancer stem cells in Hep-2 cell line. Head Neck. 2009;31:94–101. doi: 10.1002/hed.20935. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Q., Shi S., Yen Y., Brown J., Ta J.Q., Le A.D. A subpopulation of CD133(+) cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett. 2009;289:151–160. doi: 10.1016/j.canlet.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 86.Gawad C., Koh W., Quake S.R. Single-cell genome sequencing: current state of the science. Nat. Rev. Genet. 2016;17:175–188. doi: 10.1038/nrg.2015.16. [DOI] [PubMed] [Google Scholar]
- 87.Mincarelli L., Lister A., Lipscombe J., Macaulay I.C. Defining cell identity with single-cell omics. Proteomics. 2018;18 doi: 10.1002/pmic.201700312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swanson M.S., Kokot N., Sinha U.K. The role of HPV in head and neck cancer stem cell formation and tumorigenesis. Cancers (Basel. 2016;8:24. doi: 10.3390/cancers8020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blainey P.C., Quake S.R. Dissecting genomic diversity, one cell at a time. Nat. Methods. 2014;11:19–21. doi: 10.1038/nmeth.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dwivedi S., Purohit P., Sharma P. Single cell omics approach: a paradigm shift in diagnosis and therapy of cancer. Indian J. Clin. Biochem. 2019;34:1–2. doi: 10.1007/s12291-019-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu F.W., Yu C.C., Hsieh P.L., Liao Y.W., Lu M.Y., Chu P.M. Targeting oral cancer stemness and chemoresistance by isoliquiritigenin-mediated GRP78 regulation. Oncotarget. 2017;8:93912–93923. doi: 10.18632/oncotarget.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayry V., Makinen L.K., Atula T., Sariola H., Makitie A., Leivo I., Keski-Santti H., Lundin J., Haglund C., Hagstrom J. Bmi-1 expression predicts prognosis in squamous cell carcinoma of the tongue. Br. J. Cancer. 2010;102:892–897. doi: 10.1038/sj.bjc.6605544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang C.F., Xu X.R., Wu T.F., Sun Z.J., Zhang W.F. Correlation of ALDH1, CD44, OCT4 and SOX2 in tongue squamous cell carcinoma and their association with disease progression and prognosis. J. Oral Pathol. Med. 2014;43:492–498. doi: 10.1111/jop.12159. [DOI] [PubMed] [Google Scholar]
- 94.Kang F.W., Wang K., Wu M., Wang Z.L., Zhu Y., Min R. [Biological characteristics of CD133+ subpopulation in tongue squamous cell carcinoma Tca8113 cell line] Hua Xi Kou Qiang Yi Xue Za Zhi. 2010;28:560–564. [PubMed] [Google Scholar]
- 95.Yu Y.H., Morales J., Feng L., Lee J.J., El-Naggar A.K., Vigneswaran N. CD147 and Ki-67 overexpression confers poor prognosis in squamous cell carcinoma of oral tongue: a tissue microarray study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015;119:553–565. doi: 10.1016/j.oooo.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zou J., Yu X.F., Bao Z.J., Dong J. Proteome of human colon cancer stem cells: a comparative analysis. World J. Gastroenterol. 2011;17:1276–1285. doi: 10.3748/wjg.v17.i10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim J., Shin J.H., Chen C.H., Cruz L., Farnebo L., Yang J., Borges P., Kang G., Mochly-Rosen D., Sunwoo J.B. Targeting aldehyde dehydrogenase activity in head and neck squamous cell carcinoma with a novel small molecule inhibitor. Oncotarget. 2017;8:52345–52356. doi: 10.18632/oncotarget.17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang X.D., Luo G., Wang X.H., Chen L.L., Ke X., Li Y. [Expression of Oct4 and Sox2 and their clinical significance in tongue squamous cell carcinoma] Zhonghua Kou Qiang Yi Xue Za Zhi. 2017;52:27–33. doi: 10.3760/cma.j.issn.1002-0098.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 99.Cai J., He B., Li X., Sun M., Lam A.K., Qiao B., Qiu W. Regulation of tumorigenesis in oral epithelial cells by defined reprogramming factors Oct4 and Sox2. Oncol. Rep. 2016;36:651–658. doi: 10.3892/or.2016.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bayo P., Jou A., Stenzinger A., Shao C., Gross M., Jensen A., Grabe N., Mende C.H., Rados P.V., Debus J., Weichert W., Plinkert P.K., Lichter P., Freier K., Hess J. Loss of SOX2 expression induces cell motility via vimentin up-regulation and is an unfavorable risk factor for survival of head and neck squamous cell carcinoma. Mol. Oncol. 2015;9:1704–1719. doi: 10.1016/j.molonc.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deng P., Wang J., Zhang X., Wu X., Ji N., Li J., Zhou M., Jiang L., Zeng X., Chen Q. AFF4 promotes tumorigenesis and tumor-initiation capacity of head and neck squamous cell carcinoma cells by regulating SOX2. Carcinogenesis. 2018;39:937–947. doi: 10.1093/carcin/bgy046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freier K., Knoepfle K., Flechtenmacher C., Pungs S., Devens F., Toedt G., Hofele C., Joos S., Lichter P., Radlwimmer B. Recurrent copy number gain of transcription factor SOX2 and corresponding high protein expression in oral squamous cell carcinoma. Genes Chromosomes Cancer. 2009;49:9–16. doi: 10.1002/gcc.20714. [DOI] [PubMed] [Google Scholar]
- 103.Schrock A., Bode M., Goke F.J., Bareiss P.M., Schairer R., Wang H., Weichert W., Franzen A., Kirsten R., van Bremen T., Queisser A., Kristiansen G., Heasley L., Bootz F., Lengerke C., Perner S. Expression and role of the embryonic protein SOX2 in head and neck squamous cell carcinoma. Carcinogenesis. 2014;35:1636–1642. doi: 10.1093/carcin/bgu094. [DOI] [PubMed] [Google Scholar]
- 104.Lee S.H., Oh S.Y., Do S.I., Lee H.J., Kang H.J., Rho Y.S., Bae W.J., Lim Y.C. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br. J. Cancer. 2014;111:2122–2130. doi: 10.1038/bjc.2014.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yao Z., Du L., Xu M., Li K., Guo H., Ye G., Zhang D., Coppes R.P., Zhang H. MTA3-SOX2 module regulates cancer stemness and contributes to clinical outcomes of tongue carcinoma. Front. Oncol. 2019;9:816. doi: 10.3389/fonc.2019.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saleem S., Jamshed A., Faisal S., Hussain R., Tahseen M., Loya A., Sutton C. Patterns of cancer cell sphere formation in primary cultures of human oral tongue squamous cell carcinoma and neck nodes. Cancer Cell Int. 2015;14:542. doi: 10.1186/s12935-014-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu T.F., Chen L., Bu L.L., Gao J., Zhang W.F., Jia J. CD44+ cancer cell-induced metastasis: a feasible neck metastasis model. Eur. J. Pharm. Sci. 2017;101:243–250. doi: 10.1016/j.ejps.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 108.Yanamoto S., Yamada S., Takahashi H., Kawasaki G., Ikeda H., Shiraishi T., Fujita S., Ikeda T., Asahina I., Umeda M. Predictors of locoregional recurrence in T1-2N0 tongue cancer patients. Pathol. Oncol. Res. 2013;19:795–803. doi: 10.1007/s12253-013-9646-9. [DOI] [PubMed] [Google Scholar]
- 109.de Moraes F.P., Lourenco S.V., Ianez R.C., de Sousa E.A., Silva M.M., Damascena A.S., Kowalski L.P., Soares F.A., Coutinho-Camillo C.M. Expression of stem cell markers in oral cavity and oropharynx squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017;123:113–122. doi: 10.1016/j.oooo.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 110.Nanbu T., Umemura N., Ohkoshi E., Nanbu K., Sakagami H., Shimada J. Combined SN-38 and gefitinib treatment promotes CD44 degradation in head and neck squamous cell carcinoma cells. Oncol. Rep. 2017;39:367–375. doi: 10.3892/or.2017.6105. [DOI] [PubMed] [Google Scholar]
- 111.Chou C.H., Yang N.K., Liu T.Y., Tai S.K., Hsu D.S., Chen Y.W., Chen Y.J., Chang C.C., Tzeng C.H., Yang M.H. Chromosome instability modulated by BMI1-AURKA signaling drives progression in head and neck cancer. Cancer Res. 2013;73:953–966. doi: 10.1158/0008-5472.CAN-12-2397. [DOI] [PubMed] [Google Scholar]
- 112.Chen D., Wu M., Li Y., Chang I., Yuan Q., Ekimyan-Salvo M., Deng P., Yu B., Yu Y., Dong J., Szymanski J.M., Ramadoss S., Li J., Wang C.Y. Targeting BMI1(+) cancer stem cells overcomes chemoresistance and inhibits metastases in squamous cell carcinoma. Cell Stem Cell. 2017;20:621–634. doi: 10.1016/j.stem.2017.02.003. e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He Q., Liu Z., Zhao T., Zhao L., Zhou X., Wang A. Bmi1 drives stem-like properties and is associated with migration, invasion, and poor prognosis in tongue squamous cell carcinoma. Int. J. Biol. Sci. 2015;11:1–10. doi: 10.7150/ijbs.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Q., Li Z., Wu Y., Huang R., Zhu Y., Zhang W., Wang Y., Cheng J. Pharmacological inhibition of Bmi1 by PTC-209 impaired tumor growth in head neck squamous cell carcinoma. Cancer Cell Int. 2017;17:107. doi: 10.1186/s12935-017-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tanaka T., Atsumi N., Nakamura N., Yanai H., Komai Y., Omachi T., Tanaka K., Ishigaki K., Saiga K., Ohsugi H., Tokuyama Y., Imahashi Y., Hisha H., Yoshida N., Kumano K., Okazaki K., Ueno H. Bmi1-positive cells in the lingual epithelium could serve as cancer stem cells in tongue cancer. Sci. Rep. 2016;6:39386. doi: 10.1038/srep39386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou Y., Zou C., Min L., Shi R., Zhang W., Luo Y., Peng J., Duan H., Tu C., Zhang H. [Regulation of human bone marrow mesenchymal stem cells osteogenic and adipogenic differentiations by Wnt10b adenoviral vector in vitro] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015;28:1284–1291. [PubMed] [Google Scholar]
- 117.Yajima T., Ochiai H., Uchiyama T., Takano N., Shibahara T., Azuma T. Resistance to cytotoxic chemotherapy-induced apoptosis in side population cells of human oral squamous cell carcinoma cell line Ho-1-N-1. Int. J. Oncol. 2009;35:273–280. [PubMed] [Google Scholar]
- 118.Feng J.Q., Mi J.G., Wu L., Ma L.W., Shi L.J., Yang X., Liu W., Zhang C.P., Zhou Z.T. Expression of podoplanin and ABCG2 in oral erythroplakia correlate with oral cancer development. Oral Oncol. 2012;48:848–852. doi: 10.1016/j.oraloncology.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 119.Grimm M., Krimmel M., Polligkeit J., Alexander D., Munz A., Kluba S., Keutel C., Hoffmann J., Reinert S., Hoefert S. ABCB5 expression and cancer stem cell hypothesis in oral squamous cell carcinoma. Eur. J. Cancer. 2012;48:3186–3197. doi: 10.1016/j.ejca.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 120.Hang D., Dong H.C., Ning T., Dong B., Hou D.L., Xu W.G. Prognostic value of the stem cell markers CD133 and ABCG2 expression in esophageal squamous cell carcinoma. Dis. Esophagus. 2012;25:638–644. doi: 10.1111/j.1442-2050.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- 121.Tsai L.L., Yu C.C., Chang Y.C., Yu C.H., Chou M.Y. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J. Oral Pathol. Med. 2011;40:621–628. doi: 10.1111/j.1600-0714.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- 122.Lee D.G., Lee J.H., Choi B.K., Kim M.J., Kim S.M., Kim K.S., Chang K., Park S.H., Bae Y.S., Kwon B.S. H(+)-myo-inositol transporter SLC2A13 as a potential marker for cancer stem cells in an oral squamous cell carcinoma. Curr. Cancer Drug Targets. 2011;11:966–975. doi: 10.2174/156800911797264752. [DOI] [PubMed] [Google Scholar]
- 123.Rabindran S.K., Ross D.D., Doyle L.A., Yang W., Greenberger L.M. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 124.Bano N., Yadav M., Das B.C. Differential inhibitory effects of curcumin between HPV+ve and HPV-ve oral cancer stem cells. Front. Oncol. 2018;8:412. doi: 10.3389/fonc.2018.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burger J.A., Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 126.Grandis J.R., Drenning S.D., Chakraborty A., Zhou M.Y., Zeng Q., Pitt A.S., Tweardy D.J. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J. Clin. Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Santuray R.T., Johnson D.E., Grandis J.R. New therapies in head and neck cancer. Trends Cancer. 2018;4:385–396. doi: 10.1016/j.trecan.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lui V.W., Peyser N.D., Ng P.K., Hritz J., Zeng Y., Lu Y., Li H., Wang L., Gilbert B.R., General I.J., Bahar I., Ju Z., Wang Z., Pendleton K.P., Xiao X., Du Y., Vries J.K., Hammerman P.S., Garraway L.A., Mills G.B., Johnson D.E., Grandis J.R. Frequent mutation of receptor protein tyrosine phosphatases provides a mechanism for STAT3 hyperactivation in head and neck cancer. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1114–1119. doi: 10.1073/pnas.1319551111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu C.C., Hung S.K., Lin H.Y., Chiou W.Y., Lee M.S., Liao H.F., Huang H.B., Ho H.C., Su Y.C. Targeting the PI3K/AKT/mTOR signaling pathway as an effectively radiosensitizing strategy for treating human oral squamous cell carcinoma in vitro and in vivo. Oncotarget. 2017;8:68641–68653. doi: 10.18632/oncotarget.19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yigitbasi O.G., Younes M.N., Doan D., Jasser S.A., Schiff B.A., Bucana C.D., Bekele B.N., Fidler I.J., Myers J.N. Tumor cell and endothelial cell therapy of oral cancer by dual tyrosine kinase receptor blockade. Cancer Res. 2004;64:7977–7984. doi: 10.1158/0008-5472.CAN-04-1477. [DOI] [PubMed] [Google Scholar]
- 131.Leiker A.J., DeGraff W., Choudhuri R., Sowers A.L., Thetford A., Cook J.A., Van Waes C., Mitchell J.B. Radiation enhancement of head and neck squamous cell carcinoma by the dual PI3K/mTOR inhibitor PF-05212384. Clin. Cancer Res. 2015;21:2792–2801. doi: 10.1158/1078-0432.CCR-14-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xia P., Xu X.Y. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am. J. Cancer Res. 2015;5:1602–1609. [PMC free article] [PubMed] [Google Scholar]
- 133.Lee S.H., Do S.I., Lee H.J., Kang H.J., Koo B.S., Lim Y.C. Notch1 signaling contributes to stemness in head and neck squamous cell carcinoma. Lab. Invest. 2016;96:508–516. doi: 10.1038/labinvest.2015.163. [DOI] [PubMed] [Google Scholar]
- 134.Shrivastava S., Steele R., Sowadski M., Crawford S.E., Varvares M., Ray R.B. Identification of molecular signature of head and neck cancer stem-like cells. Sci. Rep. 2015;5:7819. doi: 10.1038/srep07819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Upadhyay P., Nair S., Kaur E., Aich J., Dani P., Sethunath V., Gardi N., Chandrani P., Godbole M., Sonawane K., Prasad R., Kannan S., Agarwal B., Kane S., Gupta S., Dutt S., Dutt A. Notch pathway activation is essential for maintenance of stem-like cells in early tongue cancer. Oncotarget. 2016;7:50437–50449. doi: 10.18632/oncotarget.10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao Z.L., Zhang L., Huang C.F., Ma S.R., Bu L.L., Liu J.F., Yu G.T., Liu B., Gutkind J.S., Kulkarni A.B., Zhang W.F., Sun Z.J. NOTCH1 inhibition enhances the efficacy of conventional chemotherapeutic agents by targeting head neck cancer stem cell. Sci. Rep. 2016;6:24704. doi: 10.1038/srep24704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xie S.L., Fan S., Zhang S.Y., Chen W.X., Li Q.X., Pan G.K., Zhang H.Q., Wang W.W., Weng B., Zhang Z., Li J.S., Lin Z.Y. SOX8 regulates cancer stem-like properties and cisplatin-induced EMT in tongue squamous cell carcinoma by acting on the Wnt/beta-catenin pathway. Int. J. Cancer. 2017;142:1252–1265. doi: 10.1002/ijc.31134. [DOI] [PubMed] [Google Scholar]
- 138.Zhang L., Song Y., Ling Z., Li Y., Ren X., Yang J., Wang Z., Xia J., Zhang W., Cheng B. R-spondin 2-LGR4 system regulates growth, migration and invasion, epithelial-mesenchymal transition and stem-like properties of tongue squamous cell carcinoma via Wnt/beta-catenin signaling. EBioMedicine. 2019;44:275–288. doi: 10.1016/j.ebiom.2019.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139.Qiao B., Johnson N.W., Chen X., Li R., Tao Q., Gao J. Disclosure of a stem cell phenotype in an oral squamous cell carcinoma cell line induced by BMP-4 via an epithelial-mesenchymal transition. Oncol. Rep. 2011;26:455–461. doi: 10.3892/or.2011.1299. [DOI] [PubMed] [Google Scholar]
- 140.Siddique H.R., Saleem M. Role of BMI1, a stem cell factor, in cancer recurrence and chemoresistance: preclinical and clinical evidences. Stem Cells. 2012;30:372–378. doi: 10.1002/stem.1035. [DOI] [PubMed] [Google Scholar]
- 141.Squarize C.H., Castilho R.M., Abrahao A.C., Molinolo A., Lingen M.W., Gutkind J.S. PTEN deficiency contributes to the development and progression of head and neck cancer. Neoplasia. 2013;15:461–471. doi: 10.1593/neo.121024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen L., Li Y.C., Wu L., Yu G.T., Zhang W.F., Huang C.F., Sun Z.J. TRAF6 regulates tumour metastasis through EMT and CSC phenotypes in head and neck squamous cell carcinoma. J. Cell. Mol. Med. 2017;22:1337–1349. doi: 10.1111/jcmm.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gan R.H., Lin L.S., Xie J., Huang L., Ding L.C., Su B.H., Peng X.E., Zheng D.L., Lu Y.G. FLI-06 intercepts notch signaling and suppresses the proliferation and self-renewal of tongue cancer cells. Onco Targets Ther. 2019;12:7663–7674. doi: 10.2147/OTT.S221231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tong F., Geng J., Yan B., Lou H., Chen X., Duan C., He J., Zhang S., Xie H., Li H., Yuan D., Zhang F., Meng H., Wei L. Prevalence and prognostic significance of HPV in laryngeal squamous cell carcinoma in northeast China. Cell. Physiol. Biochem. 2018;49:206–216. doi: 10.1159/000492858. [DOI] [PubMed] [Google Scholar]
- 145.Xiong L., Tang Y., Liu Z., Dai J., Wang X. BCL-2 inhibition impairs mitochondrial function and targets oral tongue squamous cell carcinoma. Springerplus. 2016;5:1626. doi: 10.1186/s40064-016-3310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gillison M.L. Human papillomavirus and prognosis of oropharyngeal squamous cell carcinoma: implications for clinical research in head and neck cancers. J. Clin. Oncol. 2006;24:5623–5625. doi: 10.1200/JCO.2006.07.1829. [DOI] [PubMed] [Google Scholar]
- 147.Licitra L., Perrone F., Bossi P., Suardi S., Mariani L., Artusi R., Oggionni M., Rossini C., Cantu G., Squadrelli M., Quattrone P., Locati L.D., Bergamini C., Olmi P., Pierotti M.A., Pilotti S. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 148.Day P.M., Schelhaas M. Concepts of papillomavirus entry into host cells. Curr. Opin. Virol. 2014;4:24–31. doi: 10.1016/j.coviro.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gupta S., Kumar P., Tyagi A., Sharma K., Kaur H., Das B.C. Modern approaches to prevention and control of cancer of the uterine cervix in women. J. Indian Inst. Sci. 2012;92:353–564. [Google Scholar]
- 150.Kleine-Lowinski K., Rheinwald J.G., Fichorova R.N., Anderson D.J., Basile J., Munger K., Daly C.M., Rosl F., Rollins B.J. Selective suppression of monocyte chemoattractant protein-1 expression by human papillomavirus E6 and E7 oncoproteins in human cervical epithelial and epidermal cells. Int. J. Cancer. 2003;107:407–415. doi: 10.1002/ijc.11411. [DOI] [PubMed] [Google Scholar]
- 151.Lee S.H., Lee C.R., Rigas N.K., Kim R.H., Kang M.K., Park N.H., Shin K.H. Human papillomavirus 16 (HPV16) enhances tumor growth and cancer stemness of HPV-negative oral/oropharyngeal squamous cell carcinoma cells via miR-181 regulation. Papillomavirus Res. 2015;1:116–125. doi: 10.1016/j.pvr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rietbergen M.M., Martens-de Kemp S.R., Bloemena E., Witte B.I., Brink A., Baatenburg de Jong R.J., Leemans C.R., Braakhuis B.J., Brakenhoff R.H. Cancer stem cell enrichment marker CD98: a prognostic factor for survival in patients with human papillomavirus-positive oropharyngeal cancer. Eur. J. Cancer. 2014;50:765–773. doi: 10.1016/j.ejca.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 153.Spanos W.C., Nowicki P., Lee D.W., Hoover A., Hostager B., Gupta A., Anderson M.E., Lee J.H. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 154.Tang A.L., Owen J.H., Hauff S.J., Park J.J., Papagerakis S., Bradford C.R., Carey T.E., Prince M.E. Head and neck cancer stem cells: the effect of HPV–an in vitro and mouse study. Otolaryngol. Head Neck Surg. 2013;149:252–260. doi: 10.1177/0194599813486599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vlashi E., Chen A.M., Boyrie S., Yu G., Nguyen A., Brower P.A., Hess C.B., Pajonk F. Radiation-induced dedifferentiation of head and neck cancer cells into cancer stem cells depends on human papillomavirus status. Int. J. Radiat. Oncol. Biol. Phys. 2016;94:1198–1206. doi: 10.1016/j.ijrobp.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bano N., Yadav M., Mohania D., Das B.C. The role of NF-kappaB and miRNA in oral cancer and cancer stem cells with or without HPV16 infection. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205518. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 157.Demokan S., Dalay N. Role of DNA methylation in head and neck cancer. Clin. Epigenet. 2012;2:123–150. doi: 10.1007/s13148-011-0045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Giudice F.S., Pinto D.S., Jr., Nor J.E., Squarize C.H., Castilho R.M. Inhibition of histone deacetylase impacts cancer stem cells and induces epithelial-mesenchyme transition of head and neck cancer. PLoS One. 2013;8:e58672. doi: 10.1371/journal.pone.0058672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Righini C.A., de Fraipont F., Timsit J.F., Faure C., Brambilla E., Reyt E., Favrot M.C. Tumor-specific methylation in saliva: a promising biomarker for early detection of head and neck cancer recurrence. Clin. Cancer Res. 2007;13:1179–1185. doi: 10.1158/1078-0432.CCR-06-2027. [DOI] [PubMed] [Google Scholar]
- 160.Steinmann K., Sandner A., Schagdarsurengin U., Dammann R.H. Frequent promoter hypermethylation of tumor-related genes in head and neck squamous cell carcinoma. Oncol. Rep. 2009;22:1519–1526. doi: 10.3892/or_00000596. [DOI] [PubMed] [Google Scholar]
- 161.Wiklund E.D., Gao S., Hulf T., Sibbritt T., Nair S., Costea D.E., Villadsen S.B., Bakholdt V., Bramsen J.B., Sorensen J.A., Krogdahl A., Clark S.J., Kjems J. MicroRNA alterations and associated aberrant DNA methylation patterns across multiple sample types in oral squamous cell carcinoma. PLoS One. 2011;6:e27840. doi: 10.1371/journal.pone.0027840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chang H.H., Chiang C.P., Hung H.C., Lin C.Y., Deng Y.T., Kuo M.Y. Histone deacetylase 2 expression predicts poorer prognosis in oral cancer patients. Oral Oncol. 2009;45:610–614. doi: 10.1016/j.oraloncology.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 163.Chikamatsu K., Ishii H., Murata T., Sakakura K., Shino M., Toyoda M., Takahashi K., Masuyama K. Alteration of cancer stem cell-like phenotype by histone deacetylase inhibitors in squamous cell carcinoma of the head and neck. Cancer Sci. 2013;104:1468–1475. doi: 10.1111/cas.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou X., Liu S., Cai G., Kong L., Zhang T., Ren Y., Wu Y., Mei M., Zhang L., Wang X. Long non coding RNA MALAT1 promotes tumor growth and metastasis by inducing epithelial-mesenchymal transition in oral squamous cell carcinoma. Sci. Rep. 2015;5:15972. doi: 10.1038/srep15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Troiano G., Caponio V.C.A., Boldrup L., Gu X., Muzio L.L., Sgaramella N., Wang L., Nylander K. Expression of the long non-coding RNA HOTAIR as a prognostic factor in squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Oncotarget. 2017;8:73029–73036. doi: 10.18632/oncotarget.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sun X., Jiao X., Pestell T.G., Fan C., Qin S., Mirabelli E., Ren H., Pestell R.G. MicroRNAs and cancer stem cells: the sword and the shield. Oncogene. 2013;33:4967–4977. doi: 10.1038/onc.2013.492. [DOI] [PubMed] [Google Scholar]
- 167.Kozaki K., Imoto I., Mogi S., Omura K., Inazawa J. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancer. Cancer Res. 2008;68:2094–2105. doi: 10.1158/0008-5472.CAN-07-5194. [DOI] [PubMed] [Google Scholar]
- 168.Yu C.C., Tsai L.L., Wang M.L., Yu C.H., Lo W.L., Chang Y.C., Chiou G.Y., Chou M.Y., Chiou S.H. miR145 targets the SOX9/ADAM17 axis to inhibit tumor-initiating cells and IL-6-mediated paracrine effects in head and neck cancer. Cancer Res. 2013;73:3425–3440. doi: 10.1158/0008-5472.CAN-12-3840. [DOI] [PubMed] [Google Scholar]
- 169.Brito B.L., Lourenco S.V., Damascena A.S., Kowalski L.P., Soares F.A., Coutinho-Camillo C.M. Expression of stem cell-regulating miRNAs in oral cavity and oropharynx squamous cell carcinoma. J. Oral Pathol. Med. 2016;45:647–654. doi: 10.1111/jop.12424. [DOI] [PubMed] [Google Scholar]
- 170.Plaks V., Kong N., Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Garcia-Arranz M., Garcia-Olmo D., Herreros M.D., Gracia-Solana J., Guadalajara H., de la Portilla F., Baixauli J., Garcia-Garcia J., Ramirez J.M., Sanchez-Guijo F., Prosper F. Autologous adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistula: a randomized clinical trial with long-term follow-up. Stem Cells Transl. Med. 2019;9:295–301. doi: 10.1002/sctm.19-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Grange C., Tapparo M., Collino F., Vitillo L., Damasco C., Deregibus M.C., Tetta C., Bussolati B., Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 173.Sun Z.P., Li A.Q., Jia W.H., Ye S., Van Eps G., Yu J.M., Yang W.J. MicroRNA expression profiling in exosomes derived from gastric cancer stem-like cells. Oncotarget. 2017;8:93839–93855. doi: 10.18632/oncotarget.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Elena Varela J. [Burnout: prevent it is to advance] Rev. Enferm. 2015;38:40–44. [PubMed] [Google Scholar]
- 175.Mutschelknaus L., Peters C., Winkler K., Yentrapalli R., Heider T., Atkinson M.J., Moertl S. Exosomes derived from squamous head and neck cancer promote cell survival after ionizing radiation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Munagala R., Aqil F., Gupta R.C. Exosomal miRNAs as biomarkers of recurrent lung cancer. Tumour Biol. 2016;37:10703–10714. doi: 10.1007/s13277-016-4939-8. [DOI] [PubMed] [Google Scholar]
- 178.Srivastava A., Babu A., Filant J., Moxley K.M., Ruskin R., Dhanasekaran D., Sood A.K., McMeekin S., Ramesh R. Exploitation of exosomes as nanocarriers for gene-, chemo-, and immune-therapy of cancer. J. Biomed. Nanotechnol. 2016;12:1159–1173. doi: 10.1166/jbn.2016.2205. [DOI] [PubMed] [Google Scholar]
- 179.Pitt J.M., Andre F., Amigorena S., Soria J.C., Eggermont A., Kroemer G., Zitvogel L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Invest. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu B., Liu A., Wu H., Shen Q., Zhao T., Wang J. Hollow Au–Cu2O core-shell nanoparticles with geometry-dependent optical properties as efficient plasmonic photocatalysts under visible light. Langmuir. 2016;32:3085–3094. doi: 10.1021/acs.langmuir.6b00331. [DOI] [PubMed] [Google Scholar]
- 181.Birkeland A.C., Owen J.H., Prince M.E. Targeting head and neck cancer stem cells: current advances and future challenges. J. Dent. Res. 2015;94:1516–1523. doi: 10.1177/0022034515601960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim J.E., Kim S.K., Shin J., Se Y.B., Choi S.H., Park S.H., Choi S.A., Lee J.Y., Phi J.H., Wang K.C., Park C.K. A subpopulation of cancer stem cells identifies radiographic characteristics in glioblastoma. Oncol. Lett. 2017;13:1175–1182. doi: 10.3892/ol.2016.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ritchie K.E., Nor J.E. Perivascular stem cell niche in head and neck cancer. Cancer Lett. 2012;338:41–46. doi: 10.1016/j.canlet.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang X., El-Sayed I.H., Qian W., El-Sayed M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 185.Kong Y., Chen J., Gao F., Brydson R., Johnson B., Heath G., Zhang Y., Wu L., Zhou D. Near-infrared fluorescent ribonuclease-A-encapsulated gold nanoclusters: preparation, characterization, cancer targeting and imaging. Nanoscale. 2012;5:1009–1017. doi: 10.1039/c2nr32760k. [DOI] [PubMed] [Google Scholar]
- 186.Ahmad R., Fu J., He N., Li S. Advanced gold nanomaterials for photothermal therapy of cancer. J. Nanosci. Nanotechnol. 2016;16:67–80. doi: 10.1166/jnn.2016.10770. [DOI] [PubMed] [Google Scholar]
- 187.Tomuleasa C., Soritau O., Orza A., Dudea M., Petrushev B., Mosteanu O., Susman S., Florea A., Pall E., Aldea M., Kacso G., Cristea V., Berindan-Neagoe I., Irimie A. Gold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemoresistance of hepatocellular carcinoma-derived cancer cells. J. Gastrointestin. Liver Dis. 2012;21:187–196. [PubMed] [Google Scholar]
- 188.Marcazzan S., Varoni E.M., Blanco E., Lodi G., Ferrari M. Nanomedicine, an emerging therapeutic strategy for oral cancer therapy. Oral Oncol. 2018;76:1–7. doi: 10.1016/j.oraloncology.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 189.Yallapu M.M., Jaggi M., Chauhan S.C. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov. Today. 2011;17:71–80. doi: 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Basak S.K., Zinabadi A., Wu A.W., Venkatesan N., Duarte V.M., Kang J.J., Dalgard C.L., Srivastava M., Sarkar F.H., Wang M.B., Srivatsan E.S. Liposome encapsulated curcumin-difluorinated (CDF) inhibits the growth of cisplatin resistant head and neck cancer stem cells. Oncotarget. 2015;6:18504–18517. doi: 10.18632/oncotarget.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Singh S.P., Sharma M., Gupta P.K. Enhancement of phototoxicity of curcumin in human oral cancer cells using silica nanoparticles as delivery vehicle. Lasers Med. Sci. 2013;29:645–652. doi: 10.1007/s10103-013-1357-7. [DOI] [PubMed] [Google Scholar]
- 192.Lee K.H., Chow Y.L., Sharmili V., Abas F., Alitheen N.B., Shaari K., Israf D.A., Lajis N.H., Syahida A. BDMC33, a curcumin derivative suppresses inflammatory responses in macrophage-like cellular system: role of inhibition in NF-kappaB and MAPK signaling pathways. Int. J. Mol. Sci. 2012;13:2985–3008. doi: 10.3390/ijms13032985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee A.Y., Fan C.C., Chen Y.A., Cheng C.W., Sung Y.J., Hsu C.P., Kao T.Y. Curcumin inhibits invasiveness and epithelial-mesenchymal transition in oral squamous cell carcinoma through reducing matrix metalloproteinase 2, 9 and modulating p53-E-cadherin pathway. Integr. Cancer Ther. 2015;14:484–490. doi: 10.1177/1534735415588930. [DOI] [PubMed] [Google Scholar]
- 194.Mazzarino L., Loch-Neckel G., Bubniak Ldos S., Mazzucco S., Santos-Silva M.C., Borsali R., Lemos-Senna E. Curcumin-loaded chitosan-coated nanoparticles as a new approach for the local treatment of oral cavity cancer. J. Nanosci. Nanotechnol. 2015;15:781–791. doi: 10.1166/jnn.2015.9189. [DOI] [PubMed] [Google Scholar]
- 195.Breitman T.R., Selonick S.E., Collins S.J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc. Natl. Acad. Sci. U. S. A. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kawamata H., Tachibana M., Fujimori T., Imai Y. Differentiation-inducing therapy for solid tumors. Curr. Pharm. Des. 2006;12:379–385. doi: 10.2174/138161206775201947. [DOI] [PubMed] [Google Scholar]
- 197.Germain P., Iyer J., Zechel C., Gronemeyer H. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature. 2002;415:187–192. doi: 10.1038/415187a. [DOI] [PubMed] [Google Scholar]
- 198.Campos B., Wan F., Farhadi M., Ernst A., Zeppernick F., Tagscherer K.E., Ahmadi R., Lohr J., Dictus C., Gdynia G., Combs S.E., Goidts V., Helmke B.M., Eckstein V., Roth W., Beckhove P., Lichter P., Unterberg A., Radlwimmer B., Herold-Mende C. Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin. Cancer Res. 2010;16:2715–2728. doi: 10.1158/1078-0432.CCR-09-1800. [DOI] [PubMed] [Google Scholar]
- 199.Yan Y., Li Z., Xu X., Chen C., Wei W., Fan M., Chen X., Li J.J., Wang Y., Huang J. All-trans retinoic acids induce differentiation and sensitize a radioresistant breast cancer cells to chemotherapy. BMC Complement. Altern. Med. 2016;16:113. doi: 10.1186/s12906-016-1088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee J., Son M.J., Woolard K., Donin N.M., Li A., Cheng C.H., Kotliarova S., Kotliarov Y., Walling J., Ahn S., Kim M., Totonchy M., Cusack T., Ene C., Ma H., Su Q., Zenklusen J.C., Zhang W., Maric D., Fine H.A. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 2008;13:69–80. doi: 10.1016/j.ccr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tate C.M., Mc Entire J., Pallini R., Vakana E., Wyss L., Blosser W., Ricci-Vitiani L., D'Alessandris Q.G., Morgante L., Giannetti S., Larocca L.M., Todaro M., Benfante A., Colorito M.L., Stassi G., De Maria R., Rowlinson S., Stancato L. A BMP7 variant inhibits tumor angiogenesis in vitro and in vivo through direct modulation of endothelial cell biology. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pan Y., Ma S., Cao K., Zhou S., Zhao A., Li M., Qian F., Zhu C. Therapeutic approaches targeting cancer stem cells. J. Cancer Res. Ther. 2018;14:1469–1475. doi: 10.4103/jcrt.JCRT_976_17. [DOI] [PubMed] [Google Scholar]
- 203.Saygin C., Matei D., Majeti R., Reizes O., Lathia J.D. Targeting cancer stemness in the clinic: from hype to hope. Cell Stem Cell. 2019;24:25–40. doi: 10.1016/j.stem.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 204.Catenacci D.V., Junttila M.R., Karrison T., Bahary N., Horiba M.N., Nattam S.R., Marsh R., Wallace J., Kozloff M., Rajdev L., Cohen D., Wade J., Sleckman B., Lenz H.J., Stiff P., Kumar P., Xu P., Henderson L., Takebe N., Salgia R., Wang X., Stadler W.M., de Sauvage F.J., Kindler H.L. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2015;33:4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Brooks M.D., Burness M.L., Wicha M.S. Therapeutic implications of cellular heterogeneity and plasticity in breast cancer. Cell Stem Cell. 2015;17:260–271. doi: 10.1016/j.stem.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bogdahn U., Hau P., Stockhammer G., Venkataramana N.K., Mahapatra A.K., Suri A., Balasubramaniam A., Nair S., Oliushine V., Parfenov V., Poverennova I., Zaaroor M., Jachimczak P., Ludwig S., Schmaus S., Heinrichs H., Schlingensiepen K.H. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro. Oncol. 2010;13:132–142. doi: 10.1093/neuonc/noq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Murakami S., Ninomiya W., Sakamoto E., Shibata T., Akiyama H., Tashiro F. SRY and OCT4 are required for the acquisition of cancer stem cell-like properties and are potential differentiation therapy targets. Stem Cells. 2015;33:2652–2663. doi: 10.1002/stem.2059. [DOI] [PubMed] [Google Scholar]
- 208.Xia W., Lo C.M., Poon R.Y.C., Cheung T.T., Chan A.C.Y., Chen L., Yang S., Tsao G.S.W., Wang X.Q. Smad inhibitor induces CSC differentiation for effective chemosensitization in cyclin D1- and TGF-beta/Smad-regulated liver cancer stem cell-like cells. Oncotarget. 2017;8:38811–38824. doi: 10.18632/oncotarget.16402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Liskova A., Kubatka P., Samec M., Zubor P., Mlyncek M., Bielik T., Samuel S.M., Zulli A., Kwon T.K., Busselberg D. Dietary phytochemicals targeting cancer stem cells. Molecules. 2019;24 doi: 10.3390/molecules24050899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xiang Y., Guo Z., Zhu P., Chen J., Huang Y. Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med. 2019;8:1958–1975. doi: 10.1002/cam4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li S.H., Fu J., Watkins D.N., Srivastava R.K., Shankar S. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Mol. Cell. Biochem. 2012;373:217–227. doi: 10.1007/s11010-012-1493-6. [DOI] [PubMed] [Google Scholar]
- 212.Toyoda Y., Takada T., Suzuki H. Inhibitors of human ABCG2: from technical background to recent updates with clinical implications. Front. Pharmacol. 2019;10:208. doi: 10.3389/fphar.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Khalil A., Jameson M.J. The EGFR inhibitor gefitinib enhanced the response of human oral squamous cell carcinoma to cisplatin in vitro. Drugs R. D. 2017;17:545–555. doi: 10.1007/s40268-017-0204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lv X.X., Zheng X.Y., Yu J.J., Ma H.R., Hua C., Gao R.T. EGFR enhances the stemness and progression of oral cancer through inhibiting autophagic degradation of SOX2. Cancer Med. 2019;9:1131–1140. doi: 10.1002/cam4.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Desai A., Yan Y., Gerson S.L. Concise reviews: cancer stem cell targeted therapies: toward clinical success. Stem Cells Transl. Med. 2018;8:75–81. doi: 10.1002/sctm.18-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li Z., Wang Y., Zhu Y., Yuan C., Wang D., Zhang W., Qi B., Qiu J., Song X., Ye J., Wu H., Jiang H., Liu L., Zhang Y., Song L.N., Yang J., Cheng J. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol. Oncol. 2015;9:1091–1105. doi: 10.1016/j.molonc.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Su L., Wang S., Yuan T., Xie X., Fu X., Ji P., Zhong L., Liu W. Anti-oral squamous cell carcinoma effects of a potent TAZ inhibitor AR-42. J. Cancer. 2020;11:364–373. doi: 10.7150/jca.32436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kamide D., Yamashita T., Araki K., Tomifuji M., Tanaka Y., Tanaka S., Shiozawa S., Shiotani A. Selective activator protein-1 inhibitor T-5224 prevents lymph node metastasis in an oral cancer model. Cancer Sci. 2016;107:666–673. doi: 10.1111/cas.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]