Abstract
Elevated levels of arsenic and uranium have been detected in water sources near abandoned uranium mines in the Southwest. Evidence suggests uranium exposure increases the likelihood of immune dysfunction and this study investigates the impact of arsenic and uranium on human immune cell lines. Concentration-dependent cytotoxicity occurred following exposure to arsenite, whereas cells remained viable after 48-hour treatment with up to 100 μM uranyl acetate despite uptake of uranium into cells. Arsenite stimulated an oxidative stress response as detected by Nrf-2 nuclear accumulation and induction of HMOX-1 and NQO1, which was not detected with up to 30 μM uranyl acetate. Cellular oxidative stress can promote DNA damage and arsenite, but not uranium, stimulated DNA damage as measured by pH2AX. Arsenic enhanced the cytotoxic response to etoposide suggesting an inhibition of DNA repair, unlike uranium. Similarly, uranium did not inhibit PARP-1 activity. Because uranium reportedly stimulates oxidative stress, DNA damage and cytotoxicity in adherent epithelial cells, the current study suggests distinct cell type differences in response to uranium that may relate to generation of oxidative stress and associated downstream consequences. Delineating the actions of uranium across different cell targets will be important for understanding the potential health effects of uranium exposures.
Keywords: uranium, arsenic, oxidative stress, DNA damage, immunotoxicity, T-lymphocyte
1. INTRODUCTION
Arsenic and uranium are naturally occurring elements found in soil, rocks and ores [1–3] and often co-occur in water and soils near abandoned uranium mines in the Western United States [4–7]. Evidence of elevated arsenic and uranium was found in half of unregulated water sources on the Navajo Nation; arsenic and uranium levels were above the Environmental Protection Agency (EPA) set maximum contamination level (MCL) in 15% and 12.5% of those sources, respectively [6,8]. Furthermore, biomonitoring confirms elevated levels of urinary uranium in residents of the Navajo Nation, an area with numerous abandoned mine sites [9]. The potential for chronic exposure to arsenic and uranium for people residing near uranium mine sites has raised questions about the risk of adverse health outcomes [8,10].
The human health consequences of environmental exposure to arsenic include increased risk of cancer, cardiovascular disease, immune dysfunction and other health effects [1,3,11–13]. Although less is known about the health effects of uranium, research suggests uranium exposure is associated with cancer, diabetes and an increased likelihood for developing multiple chronic diseases including hypertension and kidney disease [2,8,10]. Natural uranium is a heavy metal comprised of three isotopes: 238U, 235U and 234U; uranium remaining in mine waste after extraction of enriched uranium is > 99.9% 238U [2,10]. There is increased interest in understanding the effects of uranium as a metal in biological systems.
Generation of oxidative stress and DNA damage are common cellular responses to metal exposure and may reflect shared mechanisms of toxicity for arsenic and uranium [14]. DNA damage can be caused directly by oxidative stress or by inhibition of DNA repair [14–16]. Arsenic induces oxidative stress that may cause DNA damage via mitochondrial dysfunction, depletion of antioxidants, and by increasing the activity of NADPH oxidase [17–19]. In addition, arsenic inhibits the DNA repair protein Poly(ADP-Ribose) Polymerase 1 (PARP-1) leading to DNA repair deficiencies [16,20]. Less is known about oxidative stress and DNA damage response to uranium and the effects of uranium exposure appears to vary by cell type. However, uranium-induced increase in ROS and/or DNA damage has been reported in a number of experimental cell models [21–27].
Much of the experimental research on depleted uranium has focused on effects in cells representing tissues along possible exposure, accumulation or excretion routes [21,25,28,29], but there is evidence that uranium alters the balance of the immune system leading to reduced immune surveillance or increased immune activity [30–33]. Both arsenic and uranium are proposed immunotoxic agents [11,12,30,32,34,35]. Arsenic exposure is linked to altered immune cell populations both in animals and humans [35–38]. In utero arsenic exposure was associated with altered T-cells populations [39] suggesting T-cells are sensitive to the immunotoxic effects of arsenic. Similarly, a decrease in hematologic markers, including lymphocytes, was associated with increased uranium exposure in humans [32].
Given the imbalance of evidence regarding the immunotoxicity of arsenic and uranium, the goal of this study was to compare the impact of arsenic and uranium on human immune cell lines. Human T-cells (Jurkat) were chosen as a model for this study based on evidence for metal effects in this immune cell population [34,38,40,41] and importance of well-functioning, properly differentiated, populations of T-cells for mounting an appropriate immune response. A human monocyte line (THP-1) was included for select studies to determine whether any responses were T-cell lineage specific. Distinct differences in cell response to metal with regard to cytotoxicity, induction of oxidative stress response and DNA damage were observed in both cell lines with the findings supporting greater toxicity for arsenic when compared to uranium.
2. MATERIALS AND METHODS
2.1. Chemicals
Uranium, as uranyl acetate (UA) (99.6% purity) (Electron Microscopy Science, PA, USA) was comprised of 99.9% 238U and 0.1% 235U according to the product’s technical bulletin. Uranyl acetate was chosen because the uranyl ion has a +VI valence in aqueous medium, mimicking an environmental exposure. UA had a radioactive activity of 0.51 μCi g−1 and was handled according to the regulations set forth by the Radiation Safety office at the University of New Mexico. Arsenic in the form of sodium arsenite (AS) (99% purity) (Fluka Chemie, Buchs, Germany) was used for experimental treatments. Ten millimolar stock solutions of UA and one hundred millimolar stock solutions of AS were prepared in MilliQ water and sterilized using a 0.22-μm syringe filter. Working solutions were prepared by diluting the stock with complete cell growth medium. Etoposide (ETOP) (Millipore Sigma, MA, USA) was suspended in dimethylsulfoxide (DMSO) (Sigma-Aldrich St. Louis, MO) at a concentration of 100 mM and stored at −20°C protected from light. Catalase (Cat) (Sigma-Aldrich, MO, USA), lyophilized from bovine liver, was made fresh before use by combining 0.5 mg of Cat with 5 mL of RPMI medium. The solution was filter sterilized using 0.2 μm membrane filter.
2.2. Cell culture
Jurkat human T-lymphocytic clone E6.1 cells were provided by Trevigen® (Gaithersburg, MN, USA) and THP-1 human monocytic cells (TIB-202) were purchased from ATCC® (Rockville, MD, USA). Both cell lines were maintained in RPMI 1640 medium (Gibco Life Technologies, New York, NY, USA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA, USA) and 2mM L-glutamine per ATCC® culture methods. β-mercaptoethanol was not included in THP-1 culture medium because of its antioxidant properties. Cells were maintained at a density between 0.1 × 106 and 1.0 × 106 per ml of media. Cells were passaged when cell density reached ~0.8 × 105 by diluting cells to 0.1 × 105 cells per ml in growth medium. Control of THP-1 cell density was carefully maintained because alterations of cytokine profiles and cellular morphology have been observed in cultures at 2.0 × 106 cells per ml [42]. Cell passage did not exceed twenty-five from vendor provided stocks. Cell cultures were maintained at 37 °C in a 95% air/5% CO2 humidified incubator.
2.3. Cell Viability Assay
Jurkat cells (2.0 × 105 cells per ml) were treated with AS (0–100 μM) or UA (0–100 μM) and incubated in a 96-well plate for 6, 24 and 48 hours. PrestoBlue® (Life Technologies, CA, USA) was added to each well at a final volume of 10% according to manufacturers’ instructions, then plates were returned to the cell culture incubator for an additional 2 hours. Fluorescence was measured using a microplate reader (SpectraMAx M2e, Molecular Devices) with excitation 555 nm and emission 585 nm. Values were normalized to untreated controls. Additionally, Jurkat cells were treated with sodium acetate to verify that the acetate component of UA was not having an impact on cytotoxicity. To test whether metal treatment affected cell viability in the presence of a DNA damaging agent, Jurkat cells (2.0 × 105 cells per ml) were pre-treated for 2 hours with 5 μM ETOP solubilized in DMSO. The concentration of DMSO did not exceed 0.05%. Etoposide was removed by pelleting the cells and washing with 1X phosphate buffered saline (PBS) (Sigma-Aldrich, MO, USA). Cells were then resuspended in growth medium with AS (10 μM) or UA (30 μM) and incubated in a 96-well plate for 24 hours. Cell viability was measured as described above. Values were normalized to no treatment (no etoposide, no metal) controls. Viability in THP-1 cells was performed as described above except these cells were seeded at 2.5 × 105 cells per ml before treatment.
2.4. Western Blot
Jurkat cells were treated with AS or UA for times indicated in the Figure Legends. After exposures, cells were washed three times in 1X PBS, and protein was collected in lysis buffer (20 mM Tris, pH 7.5; 150 mM NaCl; 1 mM Ethylenediaminetetraacetic acid (EDTA); 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA); 1% Triton X-100; 2.5 mM sodium pyrophosphate; 1 mM β-glycerophosphate; 1 mM sodium vanadate) plus 1% Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific, MA, USA). Nuclear protein was collected using the EpiQuik Nuclear Extraction Kit (EpiGentek, NY, USA) according to the manufacturer’s instructions. Protein content was quantified using a BCA protein assay kit (Thermo Fisher Scientific, MA, USA) and samples were stored at −80°C until use.
Western blot analysis was performed using 15 or 20 μg protein (total protein) or 10 μg (nuclear protein) per sample, and all samples were resolved on a 10% polyacrylamide Tris-HCl gel. An overnight transfer was run at 25V, 4°C. Each membrane was stained with Ponceau for 5 minutes and imaged on a ProteinSimple FluorChem™ R imager (ProteinSimple, CA, USA), then cut so that multiple targets could be probed without stripping. The following primary antibodies were used according to manufacturers’ recommendation for protein detection: BLM RecQ Like Helicase (BLM) (Cell Signaling, cat# 2742); β-tubulin (Santa Cruz, cat# sc-9104), Heme Oxygenase 1 (HO-1) (Abcam, cat# ab13243); MutL Homolog (MLH1) (Cell Signaling, cat# 3515), Nuclear factor erythoid 2 (NFE2)-related factor 2 (NRF-2) (Cell Signaling, cat# 12721); 8-Oxoguanine DNA Glycosylase (OGG1) (Abcam, cat# ab124741), PARP-1 (Cell Signaling, cat# 9542), Proliferating Cell Nuclear Antigen (PCNA) (Cell Signaling, 2586); Xeroderma Pigmentosum, Complementation Group A (XPA) (Abcam, cat# ab2352); Xeroderma Pigmentosum, Complementation Group C (XPC) (Abcam, cat# 21078), Xeroderma Pigmentosum, Complementation Group F (XPF) (Abcam, cat# ab76948); and X-Ray Repair Cross Complementing 1 (XRCC1) (Abcam, cat# ab1838). The membranes were then rinsed two times in 0.05% Tris buffered saline with Tween 20 (TBST).
For each blot, the same solution was used as block and as a diluent for both the primary and secondary antibodies. This diluent was either 5% dry milk (w/v) in 0.05% TBST or 5% bovine serum albumin (BSA) (w/v) in 0.05% TBST according to the manufacturer’s recommendation for each antibody. Each membrane was blocked on a shaker for 2–4 hours at room temperature, then the primary antibody was added, and the blots were incubated on a rocker at 4°C overnight. The blots were rinsed three times in 0.05% TBST then incubated in secondary antibody on a shaker at room temperature for 1 hour. The blots were rinsed three times in 0.05% TBST then imaged on the ProteinSimple FluorChem R (Protein Simple, CA, USA) using SuperSignal™ West Pico or Femto Chemiluminescent Substrate (Thermo Fisher Scientific, MA, USA). Densitometry was performed using AlphaView SA Version 3.4.0.0 (Protein Simple, CA, USA).
2.5. Gene expression analysis of oxidative stress
RNA expression changes of genes associated with oxidative stress response (HMOX1 and NQO1) were quantified using RT-qPCR. Total RNA was isolated using TRIzol (Invitrogen, CA, USA) per manufacturer instructions, and quantified using a Nanodrop ONE spectrophotometer (Thermo Fisher Scientific, MA, USA). cDNA was generated from 1 μg of RNA using the SuperScript IV VILO Master Mix with ezDNase (Thermo Fisher Scientific, MA, USA). The following SYBR Green PCR primers (Bio-Rad, CA, USA) were used to investigate gene expression changes: HMOX1 (F-TCCTGGCTCAGCCTCAAATG; R – CGTTAAACACCTCCCTCCCC), NQO1 (F – CGCAGACCTTGTGATATTCCAG; R – CGTTTCTTCCATCCTTCCAGG) and 18S (F – CGGAGGTTCGAAGACGATCAGATA; R – TTGGTTTCCCGGAAGCTGCC). For each independent experiment triplicate quantitative RT-PCR (QPCR) reactions using iTaq universal SYBR green Supermix (Bio-Rad, CA, USA) were amplified using a ViiA 7 Real-Time PCR System (Thermo Fisher Scientific, MA, USA). The following cycling parameters were used: 95 °C for 10 min, 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Gene expression changes were calculated using the double delta Ct analysis method with 18S as a housekeeping gene.
2.6. PAR Elisa
Jurkat and THP-1 cells were assayed for Poly (ADP-ribose) (PAR) as a reflection of PARP-1 activity in response to metal exposure. Two wells of each cell type were treated with the following: no treatment; 3 μM AS; 1, 3, or 10 μM UA. After 24 hours, 25 μM ETOP was added to one well per treatment and allowed to incubate for a further 4 hours.
The cells were washed three times in 1X PBS, and protein was collected in the lysis buffer provided with the PARP in vivo Pharmacodynamic Assay II Kit (Trevigen, MD, USA) plus 1% Halt Protease Inhibitor Cocktail (Thermo Scientific, MA, USA). Protein content was quantified using a BCA protein assay kit (Thermo Scientific, MA, USA) and samples were stored at −80°C until use. Samples were analyzed for PAR using the PARP ELISA kit according to the manufacturer’s instructions. Samples were run in duplicate. For THP-1 cells, 10 μg protein were loaded per well and for Jurkat cells, 2.5 μg protein were loaded per well. Samples were read on a Molecular Devices SpectraMax i3x microplate reader.
2.7. Inductively coupled plasma mass spectrometry (ICP-MS)
Cell pellets were collected after metal exposure and washed three times with 1X PBS. Cell counts were obtained during the final PBS wash. Cell pellets contained 4.7 × 106 mean number of cells prior to digestion. Pellets were digested in full strength OmniTrace Nitric Acid (Millipore Sigma, MA, USA), overnight at room temperature and diluted with Chelex-treated MilliQ water to 5% nitric acid. Samples were stored at −20 °C. ICP-MS analysis for arsenic and uranium was performed by the Arizona Laboratory for Emerging Contaminants (ALEC) at the University of Arizona, Tucson, AZ. Samples were run the Agilent 8900 ICP-QQQ with a uranium limit of detection at 0.0001 μg/L.
2.8. Immunocytochemistry
Jurkat cells were plated at a density of 7.0 × 105 cells per ml in RPMI or pretreated with catalase (Cat) (from bovine liver, cat# C1345; 3422U/mg powder) in RPMI for 30 min. Cells were then treated with AS or UA as indicated for 6 hours. Additional cells were treated with 0.25 mM tert-Butyl hydroperoxide (TBHP) to serve as a control. Cells were washed with 1X PBS and fixed in 4% formaldehyde at 37°C for 10 minutes, then chilled on ice for 1 minute. The formaldehyde was removed, and 90% cold methanol was added then incubated on ice for 30 minutes. Two washes in incubation buffer (0.5% BSA in PBS) were performed, and then blocked for 30 minutes at room temperature in incubation buffer + 5% goat serum. Cells were washed three times, and pH2AX antibody (Cell Signaling, cat# 2577) was added at a 1:100 dilution in incubation buffer, then incubated at 4 °C overnight. Secondary antibody conjugated to AlexaFluor 647 (Cell Signaling, cat# 9720S) was added at a 1:1000 dilution in incubation buffer and incubated at room temperature in the dark for 1 hour. Cells were washed one time in incubation buffer, one time in 1X PBS, and resuspended in 100μl 1X PBS. 50μl of cells were added and air dried on slide and SlowFade™ Diamond Antifade Mountant (Invitrogen, CA, USA) was added and sealed. Images were collected with an Olympus IX83 fluorescence microscope equipped with a DP80 digital camera and cellSens Dimension 1.18 (Olympus American, PA, USA) software. Processing of images was completed using cellSens Dimension 1.16.
2.9. Statistical Analysis
At least three independent experiments were performed for each assay and averages ± standard error of the mean (SEM) are reported. Statistical analysis for the viability, Nuclear factor erythoid 2 (NFE2)-related factor 2 (Nrf-2) and oxidative stress markers at the 6-hour time point were performed using one-way ANOVAs with post hoc analysis of treatment vs control using Dunnett’s Multiple Comparison Test. Oxidative stress marker time-course and PARP activity was analyzed using two-way ANOVA with post hoc analysis of treatment vs control using Dunnett’s Multiple Comparison Test. pH2Ax staining was analyzed using one-way ANOVAs with Tukey’s post hoc comparison.
3. RESULTS
3.1. Arsenic, but not uranium, causes a dose-dependent decrease in viability despite accumulation of uranium in cells
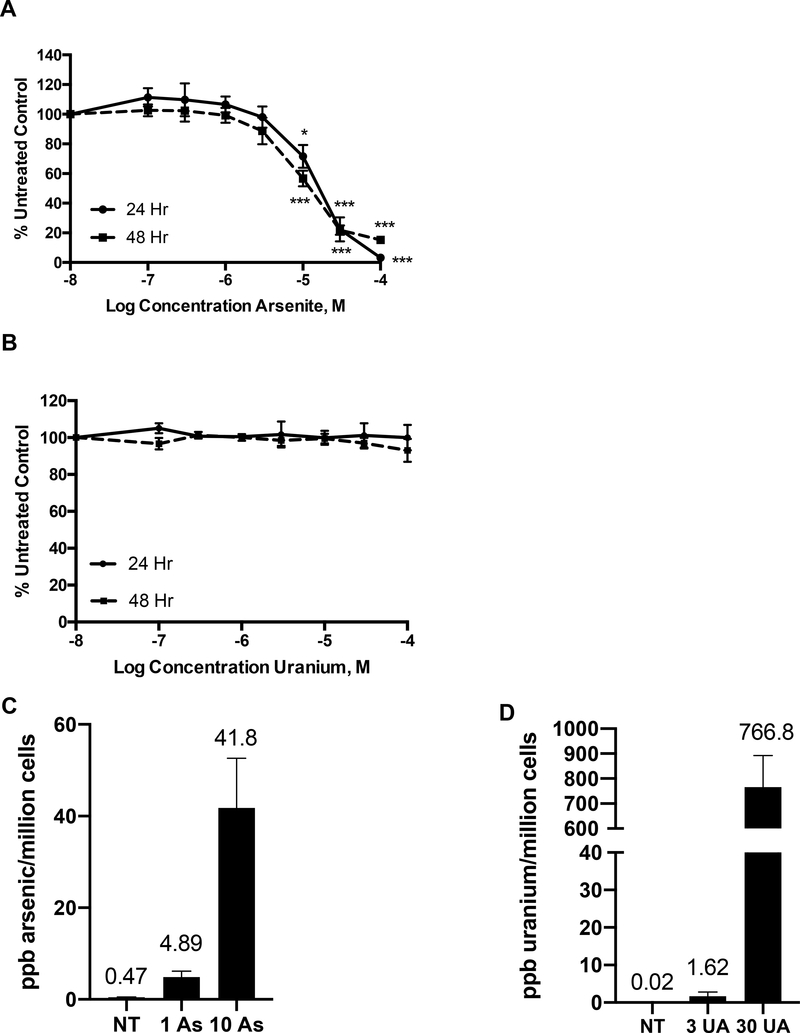
To study the cytotoxicity of arsenic and uranium in Jurkat cells, the cells were exposed to increasing concentrations (0–100 μM) of AS or UA and cellular viability was assessed after 6, 24 and 48 hours. No significant changes in viability were evident for AS concentrations up to 10 μM AS at 6 hours and decreased viability was observed at and above 10 μM AS after 24 hours of exposure (Fig 1A, Supplemental Fig. 2A). A similar concentration dependence was reported for THP-1 cells at 24 and 48 hours [43]. In contrast, no reduction of viability was observed in Jurkat cells (Fig. 1B, Supplemental Fig. 2A) or THP-1 cells (Supplemental Fig. 1A) treated with UA up to 100 μM at any time point. UA toxicity was also assessed in normal peripheral blood mononuclear cells and there was no reduction of viability up to 30 μM UA exposure (data not shown). The lack of UA cytotoxicity was not due to the inability of UA to enter the cells. Jurkat cells were exposed to AS (1 μM and 10 μM) or UA (3 μM and 30 μM) for 24 hours and intracellular arsenic and uranium levels were measured by ICP-MS. Arsenic levels measured 4.89 and 41.8 ppb/million cells at 1 μM and 10 μM AS treatment, respectively (Fig. 1C) and uranium accumulation was 1.62 and 766.8 ppb/million cells at 3 μM and 30 μM UA treatment, respectively (Fig 1D). In THP-1 cells intracellular arsenic levels were 6.67 and 57.1 ppb/million cells at 1 μM and 10 μM AS, respectively (Supplemental Fig. 1B) and uranium levels were 0.70 and 13.1 ppb/million cells at 3 μM and 30 μM UA treatment, respectively (Supplemental Fig. 1C). These findings confirm uranium uptake into both cell lines. Even though uranium uptake is approximately 18-fold greater for 30 μM uranium than 10 μM As, this data demonstrates the lack of uranium response isn’t a function of less metal in the cells.
Figure 1. Viability is reduced by AS but not UA despite accumulation of UA in Jurkat cells.
Jurkat cells were exposed to AS or UA for 24 and 48 hr and viability was measured as described in Materials and Methods. (A) A dose dependent decrease in viability was observed with arsenic exposure at 24 hr (solid line) or 48h (dashed lines). (B) No decrease in viability was observed in cells exposed to UA up to 100 μM for 24 or 48 hr. Jurkat cells were exposed to AS (1 μM or 10 μM) or UA (3 μM or 30 μM) for 24 hr and intra-cellular accumulation of arsenic (C) or uranium (D) was measured by ICP-MS. NT=no treatment control. Graphs represent mean ± SEM of at least 3 independent experiments. *p≤0.05, ***p≤0.001
3.2. Arsenic, but not uranium, stimulates an oxidative stress signaling
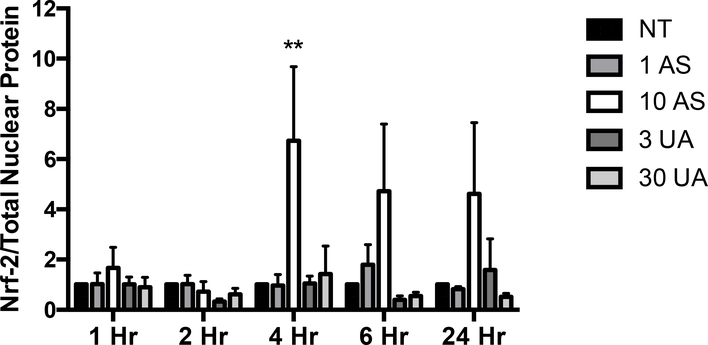
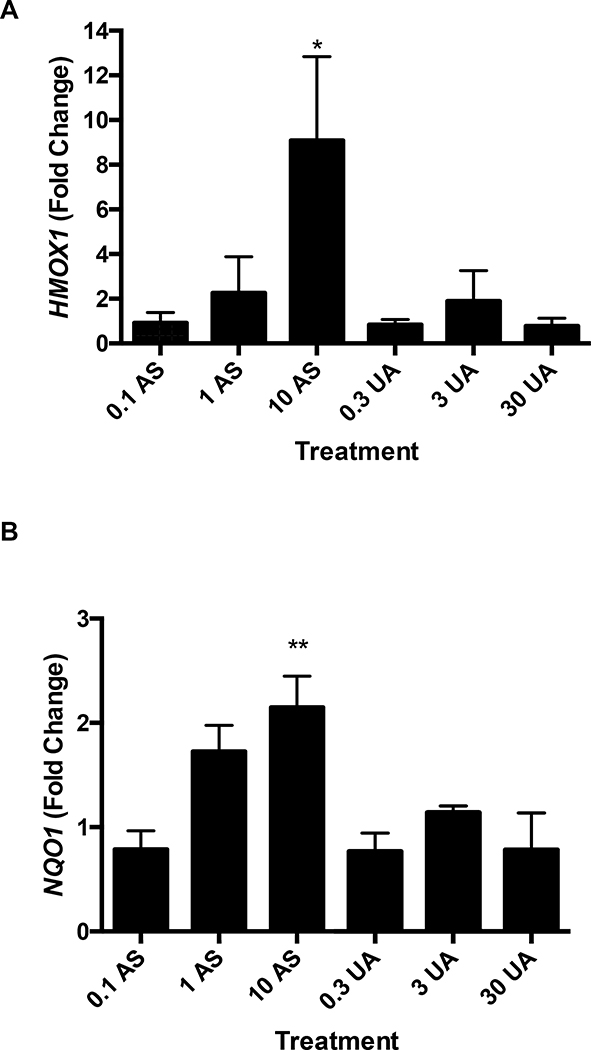
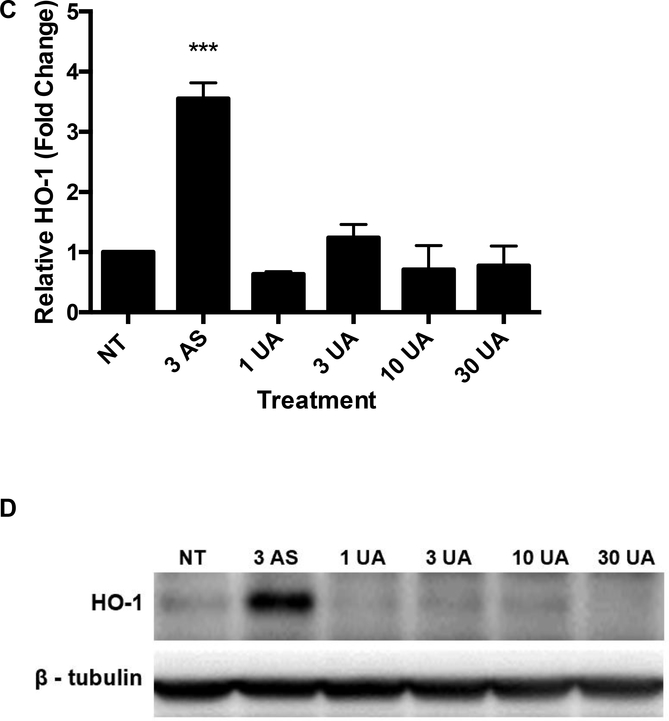
An oxidative stress signaling response was detected for AS. Nuclear translocation of Nrf-2 was observed in response to 10 μM AS exposure after 4 and 6 hours (Fig. 2). Induction of oxidative stress and nuclear translocation of Nrf-2, initiates a cascade of responses including changes in expression of ROS responsive genes such as HMOX1 and NQO1. Exposure to 10 μM AS increased HMOX1 expression 9.08-fold and NQO1 expression 2.14-fold (Fig. 3A and B). The increase in transcription of HMOX1 is confirmed by a 3.55-fold increase in heme oxygenase-1 (HO-1) protein after treatment with 3 μM AS for 6 hours (Fig. 3C and D). No increase in expression of NQO1 or HMOX1 was detected after 6-hour UA exposure up to 30 μM (Fig. 3A and B). To ensure UA did not produce a delayed response, expression of HMOX1 and NQO1 were measured after 24 hours of treatment with no changes evident in the UA exposed cells (Supplemental Fig. 3A and B). UA exposure did not increase HO-1 protein production after 6 hours (Fig. 3C and D). AS treatment induced an oxidative stress response in THP-1 cells as measured by HMOX1 and NQO1 gene expression; however, UA treatment did not significantly impact HMOX1 or NQO1 expression in these cells (Supplemental Fig. 3C and D).
Figure 2. AS, but not UA, stimulated Nrf-2 nuclear localization.
Nuclear translocation of Nrf-2 was measured by western blot analysis. Results are expressed as a ratio of nuclear Nrf-2 to total protein and values shown are normalized to the untreated control (NT). The graph represents mean ± SEM of 3 independent experiments. **p≤0.01
Figure 3. AS, but not UA, exposure increases HMOX1 and NQO1 expression and subsequent HO-1 protein production.
Jurkat cells were treated with the indicated concentrations of AS or UA for 6 hr and RNA was harvested for qRT-qPCR. (A) HMOX1 expression increased significantly upon exposure to 10 μM AS, but not UA. (B) NQO1 expression increased significantly in cells exposed to 10 μM AS, but not UA. (C) HO-1 protein production increased significantly after 6 hours in response to 10 μM AS. HO-1 levels were normalized to β-tubulin and reported values are relative to the untreated control (NT). No response was observed with UA. (D) Representative western blot of HO-1 protein. Graphs represent mean ± SEM of at least 3 independent experiments. *p≤0.05, **p≤0.01, ***p≤0.001
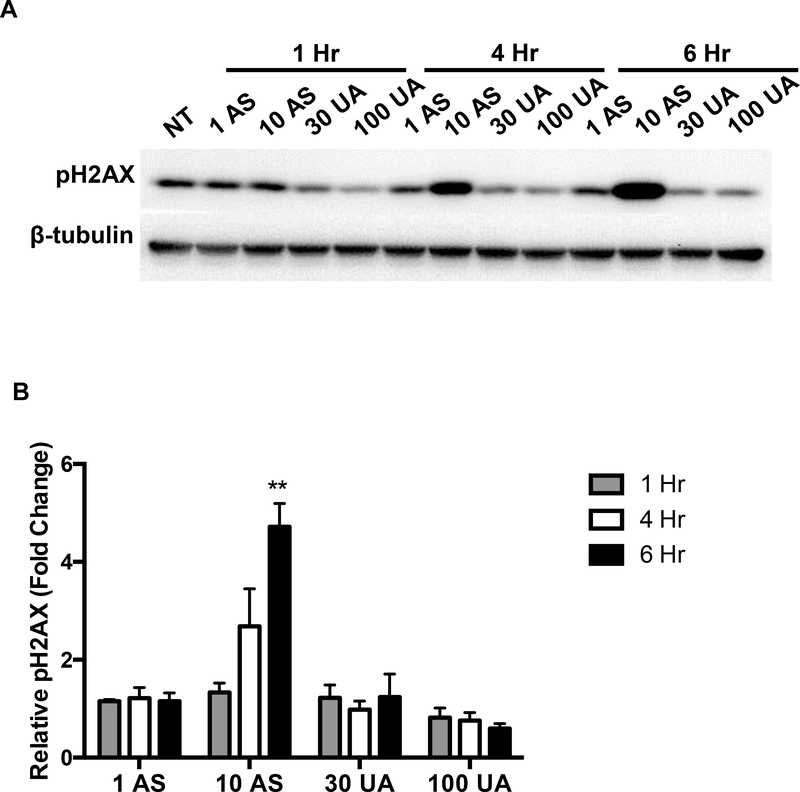
3.3. Arsenic, but not uranium, induces DNA damage as detected by pH2AX
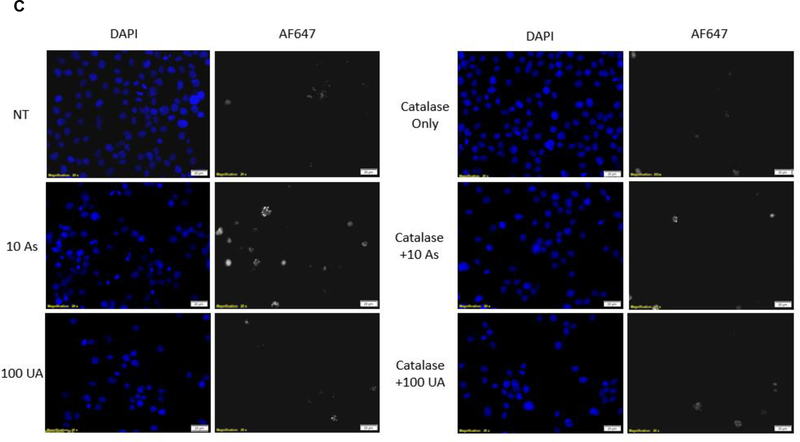
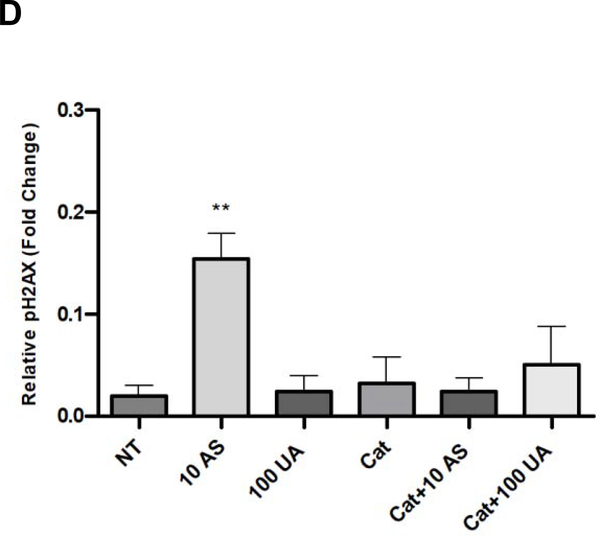
Metal-induced oxidative stress can lead to strand breaks in DNA [14]. H2AX proteins located near the sites of DNA strand breaks are readily phosphorylated at Ser139, and passively recruit DNA repair enzymes [44]. pH2AX is commonly used as a marker for DNA damage. An increase in pH2AX was detected in Jurkat cells treated with 10 μM AS for 6 hours whereas no detectable increase was observed in cells treated up to 100 μM UA (Fig. 4A & B). Fluorescent images and corresponding quantification of Jurkat cells stained for pH2AX support the immunoblot results confirming that AS causes DNA damage which can be suppressed by pre-treatment with the ROS scavenger catalase while UA at the concentrations tested does not (Fig 4C & D).
Figure 4. DNA damage in response to metal treatment.
Jurkat cells were treated with metals at the indicated times and concentrations (μM). (A) Representative western blot showing pH2AX in Jurkat cells in response to metal treatment. (B) Bar graph depicting quantification of western blots by densitometry. pH2AX levels were normalized to β-tubulin and reported values are relative to the untreated control (NT). Graph represents mean ± SEM of 3 independent experiments. (C) Catalase mitigates Arsenic induced DNA damage. Jurkat cells were pretreated with catalase (Cat) for 30 min when indicated and treated with metal (10μM AS, 100μM UA) for 6h. Cells were fixed post-metal exposure and stained with pH2AX. Representative images for each treatment group are shown. Scale bar is 25 μm. (D) Bar graph depicting quantification of pH2AX intensity. Bars represent mean ± SEM of the intensity per nuclei of at least 3 images from each treatment group from 3 independent experiments, normalized to TBHP control. **p≤0.01.
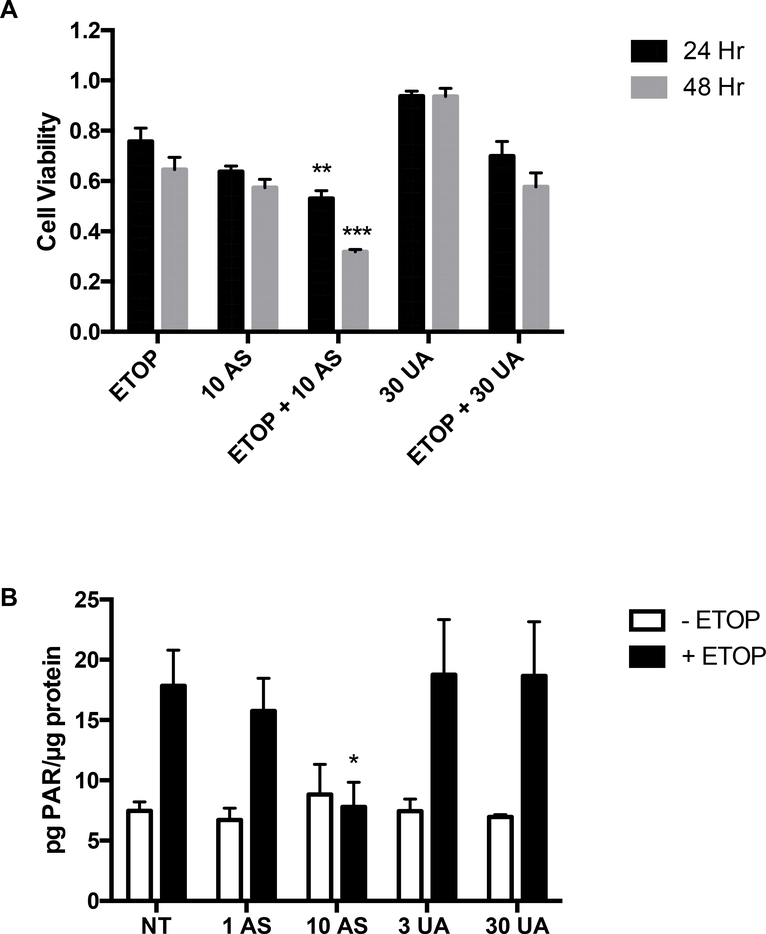
3.4. Arsenic, but not uranium, enhanced the cytotoxicity of cells with induced DNA damage
Certain metals such as arsenic interfere with DNA repair at concentrations lower than those required to elicit detectable DNA damage and can sensitize cells to a separate DNA damaging insult [16]. Jurkat cells were treated for 2 hours with the DNA damaging agent etoposide at 5 μM, a concentration that caused less than 50% loss of cell viability (Fig. 5A). Cells were rinsed and then placed in medium containing 10 μM AS or 30 μM UA and incubated for an additional 24 or 48 hours. The combination of AS plus etoposide was more cytotoxic than etoposide alone, but UA treatment had no significant impact on etoposide-mediated cytotoxicity in Jurkat cells (Fig. 5A) and THP-1 cells (Supplemental Fig. 4A).
Figure 5. AS, but not UA, sensitizes and reduces PARP-1 activity in Jurkat cells when combined with the DNA damaging agent etoposide.
Jurkat cells were treated for 2 hr with 5 μM ETOP and then exposed to 10 μM AS or 30 μM UA for 24 and 48 hr and viability measured as described in Materials and Methods. (A) AS but not UA treatment sensitized Jurkat cells to DNA damage induced by ETOP. (B) Jurkat cells were treated with AS (1 or 10 μM), or UA (3 or 10 μM) for 24 hr. One well of each concentration was then treated with 25 μM ETOP for 4 hr before protein was collected and samples were analyzed for PAR using the PARP ELISA kit according to the manufacturer’s instructions. AS, but not UA treatment decreased the activity of PARP-1 in response to ETOP induced DNA damage. Graphs represent mean ± SEM of at least 3 independent experiments. *p≤0.05, ** p≤0.01, ***p≤0.001
Inhibition of DNA repair by metals has been investigated as a mechanism for increased DNA damage when metals are combined with another DNA damaging insult [16,20] and PARP-1 has been reported to be a target for arsenic and uranium [16,20,22]. PARP-1 activity is stimulated by etoposide-induced DNA damage and this response is significantly reduced in AS treated Jurkat cells (Fig. 5B) and THP-1 cells (Supplemental Fig. 4B), but UA did not inhibit PARP-1 activity in either cell line. To further test the potential for UA to modulate DNA repair process, we compared the effects of AS and UA in Jurkat cells on expression of certain DNA repair proteins reported in the literature to be modulated by metals [45,46]. We did not detect significant changes in protein levels of nine DNA repair proteins by either AS or UA treatment (Supplemental Table 1).
4. DISCUSSION
Metals such as arsenic and uranium have numerous biological actions and research findings support the likelihood for cumulative impacts on human immune function, inflammation and autoimmunity [12,32,34,35,37–39]. Imbalances in immune cell numbers or function can lead to autoimmunity or decreased defense against pathogens and tumors. In vivo studies show that exposure to environmentally relevant arsenic levels induce significant changes in T and B cell function [35] and other researchers have reported decreases in T-regulatory (Treg) cell populations with arsenic exposure [47,48]. Relatively little is known about the effects of uranium on immune function in cell culture or in vivo models; however, uranium ingestion has been reported to induce inflammatory cytokine production in mice [49,50], affect macrophage viability [51], and induce TNF-α secretion [33,52]. This study is novel as it seeks to delineate the actions of uranium (compared to arsenic) at environmentally relevant concentrations in order to better understand the mechanisms of uranium toxicity on the immune system and elucidate the cause of immune dysfunction seen in population exposure studies.
Mechanisms commonly attributed to metal toxicity include increased production of ROS contributing to DNA damage, reduced DNA repair through interactions with zinc binding proteins and genotoxicity [14–16,20,21]. Cell-based and in vivo studies support the relationship between arsenic and oxidative stress, DNA damage and inhibition of DNA repair in immune cells and other cell types [14,16,19,20,35]. In the present study, we found that arsenic stimulated oxidative stress signaling, DNA damage and cytotoxicity in human T-lymphocyte and monocyte cell lines further confirming the current evidence that immune cells are sensitive to arsenic exposure. The consistency in which arsenic exerts its actions through oxidative stress, Nrf2 induction and inhibition of DNA damage repair in multiple cell types, and the fact that it co-localizes with uranium in areas of mixed metal waste is why we used arsenic as a mechanistic control in contrast to uranium whose actions are not well understood in immune/non-adherent cells.
In contrast to arsenic, there is limited information on the effects of uranium on oxidative stress and DNA damage in immune cells. At high concentrations at or in excess of 100 μM, uranium exposure stimulates oxidative stress in lung, kidney, osteoblast cells and zebrafish [24,25,30,46,53,53,54]. We did not detect evidence of oxidative stress signaling in human lymphocytes or monocytes despite uranium uptake into these cells. These findings are consistent with the lack of evidence for detectable oxidative stress biomarkers in human populations exposed to uranium [9] and limited accumulation of uranium in immune organs of mice [55]. We did not detect DNA damage in response to uranium although uranium has been implicated in decreased DNA damage repair in PBMCs from a population exposed to uranium mine waste [56]. Uranium enhances DNA damage in multiple cell types [21,24,27,57] typically at exposures greater than 50 μM in contrast to 30 μM used in this study. We selected concentrations based on levels in surface water samples adjacent to an abandoned uranium mine in New Mexico with a range of 35.3 to 772 μg L-1 (ppb) [4]. The lowest concentration tested in our studies (3 μM) falls within these measured environmental levels.
There appear to be notable cell type differences in response to uranium. Many cells, including Jurkat and THP-1 cells, remain viable at uranium concentrations greater than or equal to 100 μM [26,27,33] whereas keratinocytes and bronchial epithelial cells displayed cytotoxicity at lower exposures [22,46]. Although uranium cytotoxic response has been shown to be enhanced in the context of DNA repair deficiency [27] or co-exposure to an additional DNA damaging insult [22,58], no such interaction was evident in Jurkat cells treated with uranium and etoposide suggesting that uranium was not interfering with DNA repair processes in the human T-lymphocyte line. Decreased DNA repair efficiency and reduced expression of DNA repair proteins was reported in bronchial epithelial cells [46], but we did not detect changes in DNA repair protein expression or decreased PARP-1 activity in the Jurkat cells. In human keratinocytes, uranium disrupted the zinc finger function of PARP-1 and decreased PARP-1 activity as we previously observed in response to arsenic [16,22]. A possible explanation for the differences between Jurkat cells and keratinocytes may be the ability of uranium to stimulate an oxidative stress response. In keratinocytes and bronchial epithelial cells uranium increased production of ROS [22,46]. We reported previously that inhibition of PARP activity by arsenic required both As binding and As-dependent stimulation of NADPH-oxidase leading to generation of oxidative stress [59]. The response to arsenic in both of these immune cell lines was similar to that reported for keratinocytes, so the absense of oxidative stress signaling by uranium in Jurkat or THP-1 cells could account for the lack of inhibition of PARP activity by uranium.
There is ample evidence that arsenic and uranium both have genotoxic potential [15,24,26] that may have an adverse effect on immune cell function. Depending on the cell type there may be shared mechanisms for arsenic and uranium such as generation of ROS, DNA damage and inhibition of DNA repair. In other cases, deficits in an induced oxidative stress response may render cells resistant to uranium as we report for Jurkat and THP-1 cells. Although oxidative stress is a common mechanism in metal toxicity, it is possible that the adverse effects of uranium on the immune system are independent of oxidative stress or are related to other mechanisms such as formation of uranium DNA adducts [26], disruption of DNA binding proteins [60] or changes in gene expression related to immune cell function [33,50]. Furthering our knowledge of the underlying mechanisms by which uranium affects immune function will be essential to understanding the potential health risks in exposed populations.
Supplementary Material
Supplemental Figure 1. Viability is not reduced by UA despite accumulation of UA in THP-1 cells. THP-1 cells were exposed to UA for 24 and 48 hr and viability was measured as described in Materials and Methods. (A) No decrease in viability was observed with UA exposure at 24 hr (solid line) or 48h (dashed lines). THP-1 cells were exposed to AS (1 μM or 10 μM) or UA (3 μM or 30 μM) for 24 hr and intra-cellular accumulation of arsenic (B) or uranium (C) was measured by ICP-MS. NT=no treatment control. Graphs represent mean ± SEM of at least 3 independent experiments.
Supplemental Figure 2. No significant alterations to cell viability observed with acute AS and UA treatments and sodium acetate exposure compared to untreated controls. (A) Jurkat cells were exposed to AS or UA for 6 hr and viability was measured as described in Materials and Methods. AS (up to 10 μM) and UA (up to 100 μM) for 6 hr did not alter cellular viability. (B) Jurkat cells were treated with sodium acetate (NaAc) to verify that the acetate component of UA was not having an impact on cytotoxicity. Graphs represent mean ± SEM of at least 3 independent experiments.
Supplemental Figure 3. Time course of HMOX1 and NQO1 expression in Jurkat and THP-1 cells. Jurkat (A, B) and THP-1 (C, D) cells were treated as indicated in the figure and HMOX-1 (A, C) or NQO1 (B, D) expression was measured by RT-qPCR. Significant increases in expression of HMOX1 and NQO1 were detected with AS treatment in both cell lines. No significant increases were detected for UA. HMOX1 and NQO1 levels were normalized to 18S and reported values are relative to the untreated control (NT). Graphs represent mean ± SEM of at least 3 independent experiments. *p≤0.05, **p≤0.01, #p≤0.0001
Supplemental Figure 4. Arsenic but not uranium reduces PARP-1 activity in THP-1 cells when combined with the DNA damaging agent etoposide. THP-1 cells were treated for 2 hr with 5 μM ETOP and then exposed to 10 μM AS or 30 μM UA for 24 and 48 hr and viability measured as described in Materials and Methods. (A) AS, but not UA treatment sensitized THP-1 cells to DNA damage induced by ETOP. (B) THP-1 cells were treated with AS (3 μM), or UA (3 or 10 μM) for 24 hr. One well of each concentration was then treated with 25 μM ETOP for 4 hr before protein was collected and samples were analyzed for PAR using the PARP ELISA kit according to the manufacturer’s instructions. AS but not UA treatment decreased the activity of PARP-1 in response to ETOP induced DNA damage. Graphs represent mean ± SEM of at least 3 independent experiments. *p≤0.05
Highlights.
Uranium is not cytotoxic to Jurkat cells, despite intracellular accumulation
Uranium is not able to generate oxidative stress induced DNA damage
Uranium does not inhibit DNA repair
Uranium produces distinct responses compared to arsenic in Jurkat cells
Acknowledgements
We would like to extend our gratitude to Mary Kay Amistadi at the University of Arizona Laboratory for Emerging Contaminants for her help with the ICP-MS analysis of Jurkat and THP-1 cell pellets. This work was supported by the National Institutes of Health [Competing Revision to 1R01ES021100 NIH Revision Awards for Creating Virtual Consortium for Translational/ Transdisciplinary Environmental Research (ViCTER)(R01); 1P50ES026102 UNM Center for Native Environmental Health Equity NIEHS/NIMHD P50ES026102 & USEPA (#83615701) and METALS Superfund Research Center 5P42ES025589–02. Additional support for Shared Resources was provided by 2P30 CA118100 UNM Comprehensive Cancer Center NCI and training support for Drs. Dashner-Titus and Duncan by 5K12GM088021–08 Academic Science Education and Research Training (ASERT) Program. S. Alvarez was supported by a PREP/Fly Base fellowship from the National Institutes of Health 5R25HG007630.
Disclaimers
This material was developed in part under Assistance Agreement No. 83615701 awarded by the U.S. Environmental Protection Agency to the University of New Mexico Health Sciences Center. It has not been formally reviewed by EPA. The views expressed are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
A portion of the research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Numbers P50ES026102 and 5P42ES025589–02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AS
Sodium Arsenite
- Cat
Catalase
- DMSO
Dimethyl Sulfoxide
- ETOP
etoposide
- pH2AX
phosphorylated H2AX, HMOX1, Heme Oxygenase 1
- H2DCFDA
2’,7’-Dichlorodihydrofluorescein Diacetate
- ICP-MS
Inductively coupled plasma mass spectrometry
- NQO1
NAD(P)H Quinone Dehydrogenase 1
- Nrf-2
Nuclear factor erythoid 2 (NFE2)-related factor 2
- PARP-1
Poly(ADP-Ribose) Polymerase 1
- PBS
Phosphate buffered saline
- ROS
reactive oxygen species
- SEM
Standard error of the mean
- TBHP
tert-Butyl hydroperoxide
- TBST
Tris Buffered Saline with Tween 20
- UA
Uranyl Acetate
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Agency for Toxic Substances & Disease Registry, Toxicological Profile: Arsenic, (2007). http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=22&tid=3#bookmark07 (accessed July 21, 2011). [PubMed]
- [2].Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological profile for Uranium., (2013). https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=440&tid=77 (accessed December 5, 2018). [PubMed]
- [3].Agency for Toxic Substances and Disease Registry (ATSDR), Addendum to the Toxicological Profile for Arsenic, (2016). https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&cad=rja&uact=8&ved=2ahUKEwi78---vYDhAhXBc98KHQTsB90QFjABegQIBBAC&url=https%3A%2F%2Fwww.atsdr.cdc.gov%2Ftoxprofiles%2FArsenic_addendum.pdf&usg=AOvVaw25jKa698QD825_ND10__WB (accessed March 13, 2019).
- [4].Blake JM, De Vore CL, Avasarala S, Ali A-M, Roldan C, Bowers F, Spilde MN, Artyushkova K, Kirk MF, Peterson E, Rodriguez-Freire L, Cerrato JM, Uranium mobility and accumulation along the Rio Paguate, Jackpile Mine in Laguna Pueblo, NM, Environmental Science: Processes & Impacts. 19 (2017) 605–621. 10.1039/C6EM00612D. [DOI] [PubMed] [Google Scholar]
- [5].Eggers M, Doyle J, Lefthand M, Young S, Moore-Nall A, Kindness L, Other Medicine R, Ford T, Dietrich E, Parker A, Hoover J, Camper A, Community Engaged Cumulative Risk Assessment of Exposure to Inorganic Well Water Contaminants, Crow Reservation, Montana, International Journal of Environmental Research and Public Health. 15 (2018) 76 10.3390/ijerph15010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hoover J, Gonzales M, Shuey C, Barney Y, Lewis J, Elevated Arsenic and Uranium Concentrations in Unregulated Water Sources on the Navajo Nation, USA, Expo Health. 9 (2017) 113–124. 10.1007/s12403-016-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hoover JH, Coker E, Barney Y, Shuey C, Lewis J, Spatial clustering of metal and metalloid mixtures in unregulated water sources on the Navajo Nation – Arizona, New Mexico, and Utah, USA, Science of The Total Environment. 633 (2018) 1667–1678. 10.1016/j.scitotenv.2018.02.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lewis J, Hoover J, MacKenzie D, Mining and Environmental Health Disparities in Native American Communities, Current Environmental Health Reports. 4 (2017) 130–141. 10.1007/s40572-017-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dashner-Titus EJ, Hoover Joseph, Li L, Lee J-H, Du R, Liu KJ, Traber MG, Ho E, Lewis J, Hudson LG, Metal exposure and oxidative stress markers in pregnant Navajo Birth Cohort Study participants, Free Radical Biology and Medicine. 124 (2018) 484–492. 10.1016/j.freeradbiomed.2018.04.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brugge D, Buchner V, Health effects of uranium: new research findings, Rev Environ Health. 26 (2011) 231–249. [DOI] [PubMed] [Google Scholar]
- [11].Dangleben NL, Skibola CF, Smith MT, Arsenic immunotoxicity: a review, Environmental Health. 12 (2013). 10.1186/1476-069X-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ferrario D, Gribaldo L, Hartung T, Arsenic Exposure and Immunotoxicity: a Review Including the Possible Influence of Age and Sex, Curr Environ Health Rep. 3 (2016) 1–12. 10.1007/s40572-0160082-3. [DOI] [PubMed] [Google Scholar]
- [13].Erdei E, Shuey C, Pacheco B, Cajero M, Lewis J, Rubin RL, Elevated autoimmunity in residents living near abandoned uranium mine sites on the Navajo Nation, J. Autoimmun. (2019). 10.1016/j.jaut.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K, Redox- and non-redox-metal-induced formation of free radicals and their role in human disease, Archives of Toxicology. 90 (2016) 1–37. 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- [15].Bhattacharjee P, Banerjee M, Giri AK, Role of genomic instability in arsenic-induced carcinogenicity. A review, Environ Int. 53 (2013) 29–40. 10.1016/j.envint.2012.12.004. [DOI] [PubMed] [Google Scholar]
- [16].Hudson LG, Cooper KL, Atlas SR, King BS, Lui KJ, Arsenic: Exposure Sources, Health Risks and Mechanisms of Toxicity, in: Arsenic: Exposure Sources, Health Risks, and Mechanisms of Toxicity, Wiley, Hoboken, New Jersey, 2016: pp. 291–315. [Google Scholar]
- [17].Cooper KL, Liu KJ, Hudson LG, Enhanced ROS production and redox signaling with combined arsenite and UVA exposure: Contribution of NADPH oxidase, Free Radical Biology and Medicine. 47 (2009) 381–388. 10.1016/j.freeradbiomed.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Flora SJS, Arsenic-induced oxidative stress and its reversibility, Free Radical Biology and Medicine. 51 (2011) 257–281. 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- [19].Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M, Arsenic: toxicity, oxidative stress and human disease: Toxicity of arsenic, Journal of Applied Toxicology. (2011) n/a-n/a. 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- [20].Hartwig A, Metal interaction with redox regulation: an integrating concept in metal carcinogenesis?, Free Radic. Biol. Med. 55 (2013) 63–72. 10.1016/j.freeradbiomed.2012.11.009. [DOI] [PubMed] [Google Scholar]
- [21].Asic A, Kurtovic-Kozaric A, Besic L, Mehinovic L, Hasic A, Kozaric M, Hukic M, Marjanovic D, Chemical toxicity and radioactivity of depleted uranium: The evidence from in vivo and in vitro studies, Environmental Research. 156 (2017) 665–673. 10.1016/j.envres.2017.04.032. [DOI] [PubMed] [Google Scholar]
- [22].Cooper KL, Dashner EJ, Tsosie R, Cho YM, Lewis J, Hudson LG, Inhibition of poly(ADP-ribose)polymerase-1 and DNA repair by uranium, Toxicology and Applied Pharmacology. 291 (2016) 13–20. 10.1016/j.taap.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Daraie B, Pourahmad J, Hamidi-Pour N, Hosseini M-J, Shaki F, Soleimani M, Uranyl acetate induces oxidative stress and mitochondrial membrane potential collapse in the human dermal fibroblast primary cells, Iran J Pharm Res. 11 (2012) 495–501. [PMC free article] [PubMed] [Google Scholar]
- [24].Garmash SA, Smirnova VS, Karp OE, Usacheva AM, Berezhnov AV, Ivanov VE, Chernikov AV, Bruskov VI, Gudkov SV, Pro-oxidative, genotoxic and cytotoxic properties of uranyl ions, Journal of Environmental Radioactivity. 127 (2014) 163–170. 10.1016/j.jenvrad.2012.12.009. [DOI] [PubMed] [Google Scholar]
- [25].Periyakaruppan A, Kumar F, Sarkar S, Sharma CS, Ramesh GT, Uranium induces oxidative stress in lung epithelial cells, Arch Toxicol. 81 (2007) 389–395. 10.1007/s00204-006-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stearns DM, Yazzie M, Bradley AS, Coryell VH, Shelley JT, Ashby A, Asplund CS, Lantz RC, Uranyl acetate induces hprt mutations and uranium–DNA adducts in Chinese hamster ovary EM9 cells, Mutagenesis. 20 (2005) 417–423. 10.1093/mutage/gei056. [DOI] [PubMed] [Google Scholar]
- [27].Yellowhair M, Romanotto MR, Stearns DM, Clark Lantz R, Uranyl acetate induced DNA single strand breaks and AP sites in Chinese hamster ovary cells, Toxicology and Applied Pharmacology. 349 (2018) 29–38. 10.1016/j.taap.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bao Y, Wang D, Li Z, Hu Y, Xu A, Wang Q, Shao C, Chen H, Efficacy of a novel chelator BPCBG for removing uranium and protecting against uranium-induced renal cell damage in rats and HK-2 cells, Toxicology and Applied Pharmacology. 269 (2013) 17–24. 10.1016/j.taap.2013.02.010. [DOI] [PubMed] [Google Scholar]
- [29].Thiebault C, Carriere M, Milgram S, Simon A, Avoscan L, Gouget B, Uranium Induces Apoptosis and Is Genotoxic to Normal Rat Kidney (NRK-52E) Proximal Cells, Toxicological Sciences. 98 (2007) 479–487. 10.1093/toxsci/kfm130. [DOI] [PubMed] [Google Scholar]
- [30].Gagnaire B, Bado-Nilles A, Sanchez W, Depleted Uranium Disturbs Immune Parameters in Zebrafish, Danio rerio: An Ex Vivo/In Vivo Experiment, Archives of Environmental Contamination and Toxicology. 67 (2014) 426–435. 10.1007/s00244-014-0022-x. [DOI] [PubMed] [Google Scholar]
- [31].Hao Y, Ren J, Liu J, Yang Z, Liu C, Li R, Su Y, Immunological changes of chronic oral exposure to depleted uranium in mice, Toxicology. 309 (2013) 81–90. 10.1016/j.tox.2013.04.013. [DOI] [PubMed] [Google Scholar]
- [32].Wagner SE, Burch JB, Bottai M, Pinney SM, Puett R, Porter D, Vena JE, Hébert JR, Hypertension and hematologic parameters in a community near a uranium processing facility, Environmental Research. 110 (2010) 786–797. 10.1016/j.envres.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wan B, Fleming JT, Schultz TW, Sayler GS, In vitro immune toxicity of depleted uranium: effects on murine macrophages, CD4+ T cells, and gene expression profiles, Environ. Health Perspect. 114 (2006) 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lourenço J, Pereira R, Pinto F, Caetano T, Silva A, Carvalheiro T, Guimarães A, Gonçalves F, Paiva A, Mendo S, Biomonitoring a human population inhabiting nearby a deactivated uranium mine, Toxicology. 305 (2013) 89–98. 10.1016/j.tox.2013.01.011. [DOI] [PubMed] [Google Scholar]
- [35].Xu H, Wang X, Burchiel SW, Toxicity of environmentally-relevant concentrations of arsenic on developing T lymphocyte, Environmental Toxicology and Pharmacology. 62 (2018) 107–113. 10.1016/j.etap.2018.07.003. [DOI] [PubMed] [Google Scholar]
- [36].Attreed SE, Navas-Acien A, Heaney CD, Arsenic and Immune Response to Infection During Pregnancy and Early Life, Curr Environ Health Rep. 4 (2017) 229–243. 10.1007/s40572-017-0141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Burchiel SW, Lauer FT, Beswick EJ, Gandolfi AJ, Parvez F, Liu KJ, Hudson LG, Differential Susceptibility of Human Peripheral Blood T Cells to Suppression by Environmental Levels of Sodium Arsenite and Monomethylarsonous Acid, PLoS ONE. 9 (2014) e109192 10.1371/journal.pone.0109192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Martin-Chouly C, Morzadec C, Bonvalet M, Galibert M-D, Fardel O, Vernhet L, Inorganic arsenic alters expression of immune and stress response genes in activated primary human T lymphocytes, Molecular Immunology. 48 (2011) 956–965. 10.1016/j.molimm.2011.01.005. [DOI] [PubMed] [Google Scholar]
- [39].Nygaard UC, Li Z, Palys T, Jackson B, Subbiah M, Malipatlolla M, Sampath V, Maecker H, Karagas MR, Nadeau KC, Cord blood T cell subpopulations and associations with maternal cadmium and arsenic exposures, PLOS ONE. 12 (2017) e0179606 10.1371/journal.pone.0179606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Conde P, Acosta-Saavedra LC, Goytia-Acevedo RC, Calderon-Aranda ES, Sodium arsenite-induced inhibition of cell proliferation is related to inhibition of IL-2 mRNA expression in mouse activated T cells, Archives of Toxicology. 81 (2007) 251–259. 10.1007/s00204-006-0152-7. [DOI] [PubMed] [Google Scholar]
- [41].Tenorio EP, Saavedra R, Differential effect of sodium arsenite during the activation of human CD4+ and CD8+ T lymphocytes, International Immunopharmacology. 5 (2005) 1853–1869. 10.1016/j.intimp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- [42].Aldo PB, Craveiro V, Guller S, Mor G, Effect of culture conditions on the phenotype of THP-1 monocyte cell line, Am J Reprod Immunol. 70 (2013) 80–86. 10.1111/aji.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wong CP, Dashner-Titus EJ, Alvarez SC, Chase TT, Hudson LG, Ho E, Zinc Deficiency and Arsenic Exposure Can Act Both Independently or Cooperatively to Affect Zinc Status, Oxidative Stress, and Inflammatory Response, Biological Trace Element Research. (2019). 10.1007/s12011-019-1631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kuo LJ, Yang L-X, Gamma-H2AX - a novel biomarker for DNA double-strand breaks, In Vivo. 22 (2008) 305–309. [PubMed] [Google Scholar]
- [45].Andrew AS, Warren AJ, Barchowsky A, Temple KA, Klei L, Soucy NV, O’Hara KA, Hamilton JW, Genomic and Proteomic Profiling of Responses to Toxic Metals in Human Lung Cells, Environmental Health Perspectives. (2008). 10.1289/txg.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jin F, Ma T, Guan H, Yang Z-H, Liu X-D, Wang Y, Jiang Y-G, Zhou P-K, Inhibitory effect of uranyl nitrate on DNA double-strand break repair by depression of a set of proteins in the homologous recombination pathway, Toxicology Research. 6 (2017) 711–718. 10.1039/C7TX00125H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gera R, Singh V, Mitra S, Sharma AK, Singh A, Dasgupta A, Singh D, Kumar M, Jagdale P, Patnaik S, Ghosh D, Arsenic exposure impels CD4 commitment in thymus and suppress T cell cytokine secretion by increasing regulatory T cells, Sci Rep. 7 (2017) 7140 10.1038/s41598-017-07271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hernández-Castro B, Doníz-Padilla LM, Salgado-Bustamante M, Rocha D, Ortiz-Pérez MD, Jiménez-Capdeville ME, Portales-Pérez DP, Quintanar-Stephano A, González-Amaro R, Effect of arsenic on regulatory T cells, J. Clin. Immunol. 29 (2009) 461–469. 10.1007/s10875-009-9280-1. [DOI] [PubMed] [Google Scholar]
- [49].Dublineau I, Grison S, Linard C, Baudelin C, Dudoignon N, Souidi M, Marquette C, Paquet F, Aigueperse J, Gourmelon P, Short-term Effects of Depleted Uranium on Immune Status in Rat Intestine, Journal of Toxicology and Environmental Health, Part A. 69 (2006) 1613–1628. 10.1080/15287390600629825. [DOI] [PubMed] [Google Scholar]
- [50].Dublineau I, Grandcolas L, Grison S, Baudelin C, Paquet F, Voisin P, Aigueperse J, Gourmelon P, Modifications of inflammatory pathways in rat intestine following chronic ingestion of depleted uranium, Toxicol. Sci. 98 (2007) 458–468. 10.1093/toxsci/kfm132. [DOI] [PubMed] [Google Scholar]
- [51].Kalinich JF, Ramakrishnan N, Villa V, McClain DE, Depleted uranium-uranyl chloride induces apoptosis in mouse J774 macrophages, Toxicology. 179 (2002) 105–114. [DOI] [PubMed] [Google Scholar]
- [52].Gazin V, Kerdine S, Grillon G, Pallardy M, Raoul H, Uranium induces TNF alpha secretion and MAPK activation in a rat alveolar macrophage cell line, Toxicol. Appl. Pharmacol. 194 (2004) 49–59. [DOI] [PubMed] [Google Scholar]
- [53].Hao Y, Liu C, Huang J, Gu Y, Li H, Yang Z, Liu J, Wang W, Li R, Ghrelin protects against depleted uranium-induced apoptosis of MC3T3-E1 cells through oxidative stress-mediated p38-mitogen-activated protein kinase pathway, Toxicology and Applied Pharmacology. 290 (2016) 116–125. 10.1016/j.taap.2015.10.022. [DOI] [PubMed] [Google Scholar]
- [54].Zheng J, Zhao T, Yuan Y, Hu N, Tang X, Hydrogen sulfide (H2S) attenuates uranium-induced acute nephrotoxicity through oxidative stress and inflammatory response via Nrf2-NF-κB pathways, Chemico-Biological Interactions. 242 (2015) 353–362. 10.1016/j.cbi.2015.10.021. [DOI] [PubMed] [Google Scholar]
- [55].Bolt AM, Medina S, Lauer FT, Xu H, Ali A-M, Liu KJ, Burchiel SW, Minimal uranium accumulation in lymphoid tissues following an oral 60-day uranyl acetate exposure in male and female C57BL/6J mice, PLoS ONE. 13 (2018) e0205211 10.1371/journal.pone.0205211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Au WW, McConnell MA, Wilkinson GS, Ramanujam VM, Alcock N, Population monitoring: experience with residents exposed to uranium mining/milling waste, Mutat. Res. 405 (1998) 237–245. [DOI] [PubMed] [Google Scholar]
- [57].Miller AC, Stewart M, Rivas R, Preconceptional paternal exposure to depleted uranium: transmission of genetic damage to offspring, Health Phys. 99 (2010) 371–379. 10.1097/HP.0b013e3181cfe0dd. [DOI] [PubMed] [Google Scholar]
- [58].Wilson J, Zuniga MC, Yazzie F, Stearns DM, Synergistic cytotoxicity and DNA strand breaks in cells and plasmid DNA exposed to uranyl acetate and ultraviolet radiation, J Appl Toxicol. 35 (2015) 338–349. 10.1002/jat.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhou X, Cooper KL, Sun X, Liu KJ, Hudson LG, Selective Sensitization of Zinc Finger Protein Oxidation by Reactive Oxygen Species through Arsenic Binding, J. Biol. Chem. 290 (2015) 18361–18369. 10.1074/jbc.M115.663906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hartsock WJ, Cohen JD, Segal DJ, Uranyl Acetate as a Direct Inhibitor of DNA-Binding Proteins, Chem. Res. Toxicol. 20 (2007) 784–789. 10.1021/tx600347k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Viability is not reduced by UA despite accumulation of UA in THP-1 cells. THP-1 cells were exposed to UA for 24 and 48 hr and viability was measured as described in Materials and Methods. (A) No decrease in viability was observed with UA exposure at 24 hr (solid line) or 48h (dashed lines). THP-1 cells were exposed to AS (1 μM or 10 μM) or UA (3 μM or 30 μM) for 24 hr and intra-cellular accumulation of arsenic (B) or uranium (C) was measured by ICP-MS. NT=no treatment control. Graphs represent mean ± SEM of at least 3 independent experiments.
Supplemental Figure 2. No significant alterations to cell viability observed with acute AS and UA treatments and sodium acetate exposure compared to untreated controls. (A) Jurkat cells were exposed to AS or UA for 6 hr and viability was measured as described in Materials and Methods. AS (up to 10 μM) and UA (up to 100 μM) for 6 hr did not alter cellular viability. (B) Jurkat cells were treated with sodium acetate (NaAc) to verify that the acetate component of UA was not having an impact on cytotoxicity. Graphs represent mean ± SEM of at least 3 independent experiments.
Supplemental Figure 3. Time course of HMOX1 and NQO1 expression in Jurkat and THP-1 cells. Jurkat (A, B) and THP-1 (C, D) cells were treated as indicated in the figure and HMOX-1 (A, C) or NQO1 (B, D) expression was measured by RT-qPCR. Significant increases in expression of HMOX1 and NQO1 were detected with AS treatment in both cell lines. No significant increases were detected for UA. HMOX1 and NQO1 levels were normalized to 18S and reported values are relative to the untreated control (NT). Graphs represent mean ± SEM of at least 3 independent experiments. *p≤0.05, **p≤0.01, #p≤0.0001
Supplemental Figure 4. Arsenic but not uranium reduces PARP-1 activity in THP-1 cells when combined with the DNA damaging agent etoposide. THP-1 cells were treated for 2 hr with 5 μM ETOP and then exposed to 10 μM AS or 30 μM UA for 24 and 48 hr and viability measured as described in Materials and Methods. (A) AS, but not UA treatment sensitized THP-1 cells to DNA damage induced by ETOP. (B) THP-1 cells were treated with AS (3 μM), or UA (3 or 10 μM) for 24 hr. One well of each concentration was then treated with 25 μM ETOP for 4 hr before protein was collected and samples were analyzed for PAR using the PARP ELISA kit according to the manufacturer’s instructions. AS but not UA treatment decreased the activity of PARP-1 in response to ETOP induced DNA damage. Graphs represent mean ± SEM of at least 3 independent experiments. *p≤0.05