Abstract
Background
There is a growing recognition of sex and gender influences in autism. Increasingly, studies include comparisons between sexes or genders, but few have focused on clarifying the characteristics of autistic girls’/women’s physical health.
Methods
A scoping review was conducted to determine what is currently known about the physical health of autistic girls/women. We screened 1112 unique articles, with 40 studies meeting the inclusion criteria. We used a convergent iterative process to synthesize this content into broad thematic areas.
Results
Autistic girls/women experience more overall physical health challenges compared to non-autistic girls/women and to autistic boys/men. Emerging evidence suggests increased prevalence of epilepsy in autistic girls/women compared to non-autistic girls/women and to autistic boys/men. The literature also suggests increased endocrine and reproductive health conditions in autistic girls/women compared to non-autistic girls/women. Findings regarding gastrointestinal, metabolic, nutritional, and immune-related conditions are preliminary and inconsistent.
Limitations
The literature has substantial heterogeneity in how physical health conditions were assessed and reported. Further, our explicit focus on physical health may have constrained the ability to examine interactions between mental and physical health. The widely differing research aims and methodologies make it difficult to reach definitive conclusions. Nevertheless, in keeping with the goals of a scoping review, we were able to identify key themes to guide future research.
Conclusions
The emerging literature suggests that autistic girls/women have heightened rates of physical health challenges compared to non-autistic girls/women and to autistic boys/men. Clinicians should seek to provide holistic care that includes a focus on physical health and develop a women’s health lens when providing clinical care to autistic girls/women.
Keywords: Autism, Physical health, Sex differences, Gender, Girls, Women, Scoping review
Background
Autism spectrum disorder (hereafter autism) is a neurodevelopmental condition characterized by early-onset social communication difficulties and repetitive, stereotyped behaviors. The estimated prevalence rate of autism is approximately 1% worldwide [1], with a higher prevalence among males than females [2, 3]. The widely reported male-to-female ratio for autism prevalence is 4–5:1 but large-scale, population-based epidemiological studies suggest that the ratio is in fact lower, at 3–4:1 [4]. The higher rates of autism among males reflect sex and gender differences in the likelihood of developing autism and potential gender biases in clinical assessment and diagnoses [5, 6].
Autism is highly associated with co-occurring health conditions [7]. It is hypothesized that this likely reflects complex epigenetic and pleiotropic gene–environment interactions and behavioral mechanisms [3, 8], which complicate the clinical presentation of autism. Co-occurring health conditions in autism are associated with varied developmental trajectories [9, 10] and unique social and psychological challenges experienced throughout the life course [3]. In order to improve our knowledge of sex-based and gender-based differences in autism [11], it is critical to better understand the prevalence and characteristics of co-occurring conditions in autistic girls and women.
Most research on co-occurring conditions in autistic girls/women relative to boys/men has focused on psychiatric conditions, suggesting increased internalizing psychopathology in autistic girls/women compared to in boys/men [12–14]. The latest meta-analysis on co-occurring psychiatric diagnoses in autistic people shows that studies with a higher proportion of girls/women tend to find higher rates of depression [15]. However, less attention has been paid to sex and gender differences in autism outside of the domain of mental health, especially regarding physical health. (Note that we define physical health in this context to encompass non-mental health conditions within the broad category of medical disorders or problems.) Accurate and in-depth information in this domain, especially concerning autistic girls/women, is essential to the provision of comprehensive and sex- and gender-sensitive health care and is important for elucidating clinically useful subgroups within the autism spectrum. In view of this, we conducted a scoping review of the literature, focused on the extent and range of research pertaining to the physical health of autistic girls/women. Two research questions guided our review: (1) What do we know about the physical health of autistic girls/women; and (2) How specific are these physical health concerns to autistic girls/women, as compared to autistic boys/men, and to non-autistic girls/women?
Methods
We conducted a scoping review of the literature following the methodological framework outlined by Arksey and O’Malley [16] and recent Preferred Reporting Items for Systematic Reviews and Meta-analyses standards for scoping reviews (PRISMA-ScR) [17]. Scoping reviews allow a broad survey of the literature in a particular area to determine existing themes and areas of inquiry that are under-researched. Scoping reviews typically do not conduct an assessment of bias in the research nor do they appraise or generate effect sizes [16]. We considered a scoping review to be the most appropriate approach for examining emerging evidence concerning the physical health of autistic girls/women since it was unclear what specific questions should be posed in this area given the limitations of current literature. Therefore, our purposes were to summarize the extent and range of research pertaining to physical health in autistic girls/women and to identify evidence gaps. In this way, we surveyed all of the literature with respect to physical health in autistic girls/women, without restrictions based on study design or comparison groups. Furthermore, as this literature often—unfortunately—conflates gender and sex, it is difficult to tease apart their respective effects. Hence, in interpreting the findings, references to “girls/women” were assumed to refer to biological, cis-gender females and references to “gender” were read very carefully to determine whether they referred to biological sex or gender identity.
We systematically searched the following databases according to PRISMA standards [18]: CINAHL, PubMed, EMBASE, PsycINFO, Scopus, and Web of Science (see Additional file 1: Appendix: Search Strategy). As this was a scoping review aimed at assessing general themes in the published literature rather than analyzing specific types of data, grey literature was not included in the searches. Autism and co-occurring physical health conditions were defined using a combination of keywords and controlled vocabulary applicable to each database (see Additional file 1: Appendix: Search Strategy). We purposely kept the definition of “physical health” as broad as possible, in order to gather a wide range of studies and gain a thorough coverage of the published literature with respect to non-mental health-related conditions. There was no publication type or date restriction at this stage, but the search results were limited to human studies and journal articles written in English. The final database search was performed on December 5, 2019, and references were managed using Mendeley (https://www.mendeley.com/).
A systematic selection process was used to determine the final articles included in this review. After duplicates were removed, two authors (CK and SB) screened titles and abstracts with support from senior authors (M-CL and GE), using broad criteria to allow for the inclusion of any potentially relevant study for further evaluation. Full-text articles were evaluated for inclusion by CK and SB. The pool of studies identified based on screening titles, abstracts, and consultations with senior authors determined the inclusion and exclusion criteria. At this stage, articles were included if they: (1) reported on co-occurring physical health conditions in people with a diagnosis of autism as defined by the DSM-IV, DSM-5, or ICD-10 criteria, or had direct relevance to the physical health of autistic girls/women; (2) included a clearly articulated sex-specific or gender-specific description or analysis of these conditions; (3) studied biological females only, or if the total female autism sample size was ≥ 15 and with at least one-eighth (12.5%) of the total autism sample being biologically female (to ensure that included studies had a sufficient number of girls/women to derive sex-specific or gender-specific information); (4) reported original, English-language research articles or reviews published in peer-reviewed scientific journals; and (5) in the case of review articles, used systematic search methods and included sex-specific or gender-specific analyses and interpretation. Articles were excluded if they were: (1) review articles using non-systematic search methodology; (2) opinion pieces; (3) editorials; (4) case reports; or (5) conference papers. Final decisions on which articles to include were made in discussion within the research team. Articles were grouped by main topic area and study design for organizational clarity. Data were extracted as shown in Tables 1 and 2, with relevant findings summarized in the Results section. We used a convergent iterative process involving multistage revisions among all authors to synthesize included studies into a series of themes that broadly summarize key findings in the literature.
Table 1.
Overview of included studies (n = 40)
| Years of publication | Country of origin | Study design |
|---|---|---|
| 2007–2020 |
North America = 17 Europe and UK = 12 Asia = 5 Middle East = 3 Australia = 3 Africa = 0 South America = 0 |
Systematic reviews and meta-analyses = 5 Reviews with systematic search methods = 2 Cross-sectional studies, with population/registry samples = 20 Cross-sectional studies, with clinic/community samples = 13 |
Table 2.
Summary of included studies (n = 40 unique studies, by themes and then by comparison groups)
| Author, country | Study design | Topic area | Sample size | Age (autism sample) | % Female of autism sample | % ID of autism sample | Comparison groups | Key findings |
|---|---|---|---|---|---|---|---|---|
| Theme 1: Overall Physical Health Status | ||||||||
| a. Autistic girls/women compared to autistic boys/men | ||||||||
|
Rydzewska et al. [19] UK |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions | N = 25,063 autism, N = 1,523,756 general population controls | 0 to 24 years | 20.7% (19,880 males, 5183 females) | 15.0% | Autistic boys/men | OR autistic girls/women compared to autistic boys/men (reference group): deafness 2.07 [95% CI 2.04–2.10], blindness 2.51 [2.12–2.97], physical disability 2.60 [2.50–2.71] |
|
Rydzewska et al. [20] UK |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions | N = 6649 autism, N = 3,739,935 general population controls | 25 + years | 30.7% (4610 males, 2039 females) | 29.4% | Autistic men | OR autistic women compared to autistic men (reference group): deafness 1.169 [95% CI 1.001–1.365], blindness 1.232 [1.051–1.443], physical disability 1.504 [1.333–1.697] |
|
Rydzewska et al. [21] UK |
Cross-sectional registry sample | Prevalence rates of general health status | N = 6649 autism, N = 3,739,935 general population controls | 25 + years | 30.7% (4610 males, 2039 females) | 29.4% | Autistic men | Among young adults (25–34 years), autistic women were more likely to have poorer general health compared to autistic men (43.9% autistic women vs. 35.7% autistic men reporting “poor general health”; χ2 = 13.2, df = 1, p < 0.001). No significant sex/gender differences in other age bands |
|
Supekar et al. [22] USA |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions | N = 4790 autism, N = 1,842,575 general population controls | All ages | Not reported for overall sample | Not reported | Autistic boys/men | Bowel disorders were overall more prevalent in autistic men, but there was a significant higher prevalence in autistic women > 35 years (23% autistic women vs. 10% autistic men, p < 0.05) |
|
Davignon et al. [23] USA |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions | N = 4123 autism, N = 20,615 ADHD, N = 2156 diabetes mellitus, N = 20,615 general population controls | 14 to 25 years | 19.3% (3326 males, 797 females) | 13% | Autistic boys/men | Rates of various health conditions mostly greater in autistic girls/women compared to autistic boys/men with the largest differences observed for allergy/immunologic conditions, infections, musculoskeletal conditions, neurologic conditions, and psychiatric conditions |
|
Jones et al. [24] USA |
Cross-sectional clinic/community sample | Prevalence rates for co-occurring health conditions | N = 92 autism, no controls | 23.5 to 50.5 years | 25% (69 males, 23 females) | N = 82 with data, 70% with ID | Autistic men | Autistic women had a median of 16 comorbid medical conditions, whereas autistic men had a median of 10 comorbid medical conditions, p = 0.01 |
|
Mason et al. [25] UK |
Cross-sectional registry sample | Physical quality of life | N = 370 autism, no controls | 17 to 80 years | 42.7% (199 males, 158 females, 13 not reported) | Not reported | Autistic boys/men | Autistic women reported poorer physical quality of life (mean = 45.98, SD = 19.57) than autistic men (mean = 52.98, SD = 17.32) |
|
Fortuna et al. [26] USA |
Cross-sectional registry sample | Overall health status | N = 255 autism, no controls | 18 to 71 years | 24.7% (192 males, 63 females) | N = 141 with data, 91% with ID | Autistic boys/men | Female sex/gender was associated with lower odds of good or excellent overall health: OR autistic women compared to autistic men (reference group) 0.5 [95% CI 0.2–1.0] |
|
Cashin et al. [27] Australia |
Review with systematic search methods | Physical health status | n = 6 studies, with samples ranging from N = 92 to N = 2075 autism | 18 + years | Not reported | Not reported | Autistic boys/men | 3 studies included sex-specific analyses, with inconsistent findings |
|
Rubenstein et al. [28] USA |
Review with systematic search methods | Sex differences in co-occurring conditions | n = 69 studies, with samples ranging from N = 28 to N = 337,000 | Not reported | Not reported | Not reported | Autistic boys/men | Insufficient research (hence evidence) to draw conclusions on sex differences in most co-occurring health conditions |
| b. Autistic girls/women compared to non-autistic girls/women | ||||||||
|
Cawthorpe et al. [29] Canada |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions | N = 2040 autism, N = 761,409 general population controls | All ages | 28.6% (1457 males, 583 females) | Not reported | Same-sex general population controls | Autistic girls/women had increased odds compared to non-autistic girls/women for most physical health conditions (encompassing almost all body systems), similar to that in autistic boys/men compared to non-autistic boys/men. Sex differential patterns were also found. (i) Conditions only elevated in autistic girls/women included: blood and blood-forming organ disorders (autistic female OR 1.35 [95% CI 1.11–1.65], autistic male 1.14 [0.96–1.35]), and endocrine, nutritional, metabolic diseases, and immunity disorders (autistic female 1.47 [1.25–1.73], autistic male 0.63 [0.56–0.71]). (ii) Conditions only elevated in autistic boys/men included: complications during mothers’ pregnancy/childbirth (autistic male 1.52 [1.07–2.15], autistic female 0.55 [0.44–0.68]), and genitourinary system diseases (autistic male 1.2 [1.08–1.33], autistic female 0.99 [0.81–1.20]) |
|
Croen et al. [30] USA |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions | N = 1507 autism, N = 15,070 general population matched controls | Adults (mean age 29.0 years, SD 12.2) | 26.9% (1102 males, 405 females) | 19.2% | Same-sex general population controls | Autistic women had increased odds compared to non-autistic women for most physical health conditions (encompassing almost all body systems), similar to that in autistic men compared to non-autistic men. Sex differential patterns were also found. (i) Conditions only elevated in autistic women included: stroke (autistic female OR 4.97 [99% CI 1.46–16.86], autistic male 1.48 [0.59–3.70]). (ii) Conditions only elevated in autistic men included: autoimmune diseases (autistic male 1.30 [1.01–1.68)], autistic female 1.12 [0.78–1.60]) and gastrointestinal disorders (autistic male 1.50 [1.25–1.79], autistic female 1.05 [0.80–1.39]) |
|
Hand et al. [31] USA |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions | N = 4685 autism, N = 46,850 matched controls | 65 years and older | 32.2% (3175 males, 1510 females) | 43.8% | Same-sex general population controls | Autistic women had increased odds compared to non-autistic women for most physical health conditions (encompassing almost all body systems), similar to that in autistic men compared to non-autistic men. No sex differential patterns were found. The three physical health conditions with the largest ORs in autistic women were epilepsy (OR 20.8 [95% CI 17.7–24.4]), Parkinson’s disease (8.2 [6.2–10.7]), and other gastrointestinal conditions (4.6 [4.1–5.1]) |
| Theme 2: Epilepsy and Related Neurological Conditions | ||||||||
| a. Autistic girls/women compared to autistic boys/men | ||||||||
|
Stacy et al. [32] USA |
Cross-sectional registry sample | Prevalence rates: including epilepsy | N = 913 autism | Weighted mean age 9.91 years (females 10.82 (SD 0.61) vs. males 9.71 (SD 0.27)) | 18.3% (746 males, 167 females) | Not reported | Autistic boys/men | No significant differences between autistic girls/women and autistic boys/men |
|
Amiet et al. [33] France |
Systematic review and meta-analysis | Prevalence rates: pooled risk ratio for epilepsy, and associations with ID | N = 1530 autism in sex/gender analyses from 14 studies | All ages | 22.2% in sex/gender analyses (1191 males, 339 females) | Not reported | Autistic boys/men | Lower risk for epilepsy in autistic boys/men compared to autistic girls/women (RR 0.55 [95% CI 0.45–0.66], p < 0.001; 34.5% in autistic girls/women vs. 18.5% in autistic boys/men) |
|
Ewen et al. [34] USA |
Cross-sectional registry sample | Prevalence rates: epilepsy | N = 6975 autism | 6 to 18 years | 18.7% (5671 males, 1304 females) | 20.8% | Autistic boys | Higher risk for epilepsy in autistic girls compared to autistic boys in a larger sub-cohort (RR 1.32 [95% CI 1.14–1.52], p < 0.05) but no significant findings in a smaller sub-cohort. Independent positive associations between epilepsy and intellectual disability, language impairment, core autism symptom, and motor dysfunction |
|
Viscidi et al. [35] USA |
Cross-sectional registry sample | Prevalence rates: epilepsy | N = 5815 autism | All ages, majority between 4 and 12 years (~ 75%) | 17.5% (4800 males, 1015 females) | N = 4894 with data, 15.5% | Autistic boys/men | Epilepsy was more prevalent in autistic girls/women (7.0%) than in autistic boys/men (3.9%, p < 0.001). Co-occurring epilepsy in autism was associated with older age, lower cognitive ability, poor adaptive language functioning, developmental regression, and more severe autism symptoms; most of the associations were driven by IQ |
|
Bowers et al. [36] USA |
Cross-sectional clinic/community sample | Prevalence rates: epilepsy, in preterm vs. term births | N = 883 autism | 0 to 18 years | 17.6% (728 males, 155 females) | N = 853 with data, 34.5% | Autistic boys | Seizure disorders were more frequent among autistic boys born preterm vs. those born term (17.0% vs. 8.5%, p = 0.01); no such preterm–term differences were found in autistic girls |
|
Ben-Itzchak et al. [37] Israel |
Cross-sectional clinic/community sample | Prevalence rates and sex differences in neurological phenotypes, including epilepsy | N = 663 autism | 1 to 15 years | 13.0% (577 males, 86 females) | 35.0%** | Autistic boys | Neurological anomalies were more prevalent in autistic girls than in autistic boys, including microcephaly (15.1% vs. 4.5%, χ2 = 15.0, df = 1, p < 0.001) and minor neurological–musculoskeletal deficits (73.8% vs. 57.1%, χ2 = 8.0, df = 1, p < 0.001), but no significant sex differences were found for seizures or macrocephaly |
|
*Supekar et al. [22] USA |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions, including epilepsy | N = 4790 autism, N = 1,842,575 general population controls | All ages | Not reported for overall sample | Not reported | Autistic boys/men | Overall higher prevalence of epilepsy in autistic girls/women (18.54%) than in autistic boys/men (15.14%, p < 0.05); this finding was modulated by age, that epilepsy was female-predominant in 0–18 years and 18–35 years, but male-predominant in > 35 years of age |
| b. Autistic girls/women compared to non-autistic girls/women | ||||||||
|
Ingudomnukul et al. [38] UK |
Cross-sectional clinic/community sample | Prevalence rates for co-occurring health conditions, including epilepsy | N = 54 autism, N = 74 mothers of autistic children, and N = 183 mothers of typically developing children (controls) | 19 to 63 years | 100% | Not reported | Same-sex general population controls | Epilepsy rates were higher in autistic women (7.4%) compared to control women (1.1%, p < 0.05) |
|
Pohl et al. [39] UK |
Cross-sectional clinic/community sample | Prevalence rates for co-occurring health conditions, including epilepsy | N = 415 autism, N = 415 age-matched controls | Mean age 36.39 years (SD 11.98) | 100% | Not reported | Same-sex general population controls | Epilepsy rates were higher in autistic women (4.1%) compared to non-autistic women (1.4%, p = 0.016) |
|
*Croen et al. [30] USA |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions, including epilepsy | N = 1507 autism, N = 15,070 general population matched controls | Adults (mean age 29.0 years, SD 12.2) | 26.9% (1102 males, 405 females) | 19.2% | Same-sex general population controls | Autistic women had increased odds compared to non-autistic women for epilepsy and recurrent seizures (OR 34.09 [99% CI 18.51–62.79]); a similar but smaller effect was found in autistic men compared to non-autistic men (11.53 [7.74–17.17]) |
|
*Hand et al. [31] USA |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions, including epilepsy | N = 4685 autism, N = 46,850 matched controls | 65 years and older | 32.2% (3175 males, 1510 females) | 43.8% | Same-sex general population controls | Autistic women had increased odds compared to non-autistic women for epilepsy (OR 20.8 [95% CI 17.7–24.4]) and Parkinson’s disease (8.2 [6.2–10.7]); a similar but smaller effect was found in autistic men compared to non-autistic men for epilepsy (18.0 [16.1–20.2]) |
| Theme 3: Endocrine and Reproductive Health Conditions | ||||||||
| a. Studies with no comparison group | ||||||||
|
Hamilton et al. [40] USA |
Cross-sectional clinic/community sample | Prevalence rates for menstruation complications | N = 124 autism | 10 to 25 years | 100% | Not reported | None | Autistic girls/women commonly experienced dysmenorrhea (91%), premenstrual syndrome (96%), and 33% endorsed autism-associated difficulties during the menstrual cycle (increased irritability/aggression before menses, worsening of autistic behaviors, and increased repetitive movements and obsessive behaviors) |
|
Bitsika and Sharpley [41] Australia |
Cross-sectional clinic/community sample | Effects of menarche on sensory features of autism | N = 53 autism | 6 to 17 years | 100% | Not reported | None | Autistic girls who had reached menarche had lower sensation seeking (less sensory interests) (F(25,27) = 2.113, p = 0.030) and multisensory processing (F(7,45) = 3.187, p = 0.008) compared to those who had not yet reached menarche |
| b. Autistic girls/women compared to non-autistic girls/women | ||||||||
|
*Croen et al. [30] USA |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions, including endocrine disorders | N = 1507 autism, N = 15,070 general population matched controls | Adults (mean age 29.0 years, SD 12.2) | 26.9% (1102 males, 405 females) | 19.2% | Same-sex general population controls | Autistic women had increased odds compared to non-autistic women for thyroid diseases (OR 1.85 [99% CI 1.20–2.85]); a similar but larger effect was found in autistic men compared to non-autistic men (3.34 [2.18–5.11]) |
|
*Hand et al. [31] USA |
Cross-sectional registry sample | Prevalence rates for co-occurring health conditions, including endocrine and menopausal disorders | N = 4685 autism, N = 46,850 matched controls | 65 years and older | 32.2% (3175 males, 1510 females) | 43.8% | Same-sex general population controls | Autistic women had increased odds compared to non-autistic women for thyroid disorders (OR 2.5 [95% CI 2.2–2.8]); a similar but larger effect was found in autistic men compared to non-autistic men (3.7 [3.3–4.0]). Autistic women did not differ from non-autistic women on rates of menopausal disorders (1.1 [0.9–1.5]) |
|
Steward et al. [42] UK |
Cross-sectional clinic/community sample | Autistic women’s experiences with menstruation | N = 123 autism, N = 114 controls | 16 to 60 + years | 100% | Not reported | Same-sex general population controls | Autistic women highlighted autism-specific issues during the menstrual cycle, including a cyclical amplification of autism-related challenges, sensory differences, and emotional regulation challenges, which had a significant negative impact on their lives |
|
*Ingudomnukul et al. [38] UK |
Cross-sectional clinic/community sample | Prevalence rates for co-occurring health conditions, including female-specific endocrine conditions | N = 54 autism, N = 74 mothers of autistic children, and N = 183 mothers of typically developing children (controls) | 19 to 63 years | 100% | Not reported | Same-sex general population controls | Autistic women, compared to control women, had higher rates of polycystic ovary syndrome (PCOS, 11.3% vs. 2.7%, p < 0.05), delayed puberty (7.4% vs. 0.5%, p < 0.01), hirsutism (29.6% vs. 4.4%, p < 0.001), irregular menstrual cycle (57.4% vs. 28.6%, p < 0.001), unusually painful periods (44.4% vs. 28.0%, p < 0.05), and history of severe acne (27.8% vs. 7.1%, p < 0.001) |
|
*Pohl et al. [39] UK |
Cross-sectional clinic/community sample | Prevalence rates for co-occurring health conditions, including female-specific endocrine conditions | N = 415 autism, N = 415 age-matched controls | Mean age 36.39 years (SD 11.98) | 100% | Not reported | Same-sex general population controls | Autistic women, compared to non-autistic women, had higher rates of irregular menstrual cycle (46.3% vs. 34.0%, p = 0.0002), unusually painful periods (39.3% vs. 26.3%, p = 0.00004), premenstrual syndrome in contraceptive pill users (24.0% vs. 13.8%, p = 0.001), severe acne in non-contraceptive pill users (21.3% vs. 5.9%, p = 0.002), precocious puberty (3.1% vs. 0.5%, p = 0.003), and early growth spurt (20.2% vs. 12.8%, p = 0.002) |
|
Cherskov et al. [43] UK |
Cross-sectional registry sample | Prevalence rates for polycystic ovary syndrome (PCOS) | N = 971 autism, N = 4855 general population controls | Mean age 30.3 years (SD 9.1) | 100% | Not reported | Same-sex general population controls | Prevalence of PCOS was higher in autistic women compared to non-autistic women (by Read code, 2.3% vs. 1.1%, p < 0.01, OR 2.01 [95% CI 1.22–3.30]; by NIH criteria, 7.4% vs. 3.1%, p < 0.001, 2.50 [1.87–3.35]; by Rotterdam criteria, 7.8% vs. 3.5%, p < 0.001, 2.33 [1.76–3.08]) |
|
Chiang et al. [44] Taiwan |
Cross-sectional registry sample | Prevalence rates for cancer, including ovarian cancer | N = 8438 autism, N = 76,332 general population controls | 0 to 25 + years | 17.9% (6931 males, 1507 females) | Not reported | Same-sex general population controls | Autistic girls/women had a higher standardized incidence ratio (SIR) for ovarian cancer compared to non-autistic girls/women (SIR 9.21 [95% CI 1.12–33.29]) |
|
Sundelin et al. [45] Sweden |
Cross-sectional registry sample | Pregnancy outcomes | N = 1382 autism (N = 2198 births), N = 503,846 general population controls (N = 877,742 births) | Adults of child-bearing age | 100% | Not reported | Same-sex general population controls | Autistic women, compared to non-autistic women, were at increased risks for preeclampsia (OR 1.34 [95% CI 1.08–1.66]), giving preterm birth (1.30 [1.10–1.54]), medically indicated preterm birth (1.41 [1.08–1.82]), and receiving elective cesarean delivery (1.44 [1.25–1.66]) |
| Miscellaneous Emerging Findings (all studies offered information regarding autistic girls/women in comparison with autistic boys/men) | ||||||||
| a. Additional neurological conditions | ||||||||
|
Memari et al. [46] Iran |
Cross-sectional clinic/community sample | Prevalence rates for co-occurring health conditions | N = 91 autism | 6 to 14 years | 25.3% (68 males, 23 females) | Not reported | Autistic boys | Autistic girls had higher prevalence of neurological conditions (~ 46%) than autistic boys (~ 19%, p = 0.02) |
|
Rubenstein et al. [47] USA |
Cross-sectional registry sample | Temporal trends in co-occurring health conditions | N = 6379 autism | 8-year-olds | 18.0% (5230 males, 1149 females) | 4.3% | Autistic boys | Rates of change of prevalence for neurological conditions over 2002–2010 were the same for autistic boys and autistic girls |
|
Mouridsen et al. [48] Denmark |
Cross-sectional registry sample | Prevalence rates for cerebral palsy | N = 4180 autism (ICD-10 Asperger’s syndrome) | 4 to 31 years | 17.9% (3431 males, 749 females) | 0% | Autistic boys/men | Increased rates for cerebral palsy in autistic people (0.65%) than in the general population (0.17%), but no difference between autistic girls/women (0.80%) and autistic boys/men (0.61%, p = 0.56) |
| b. Obesity and overweight | ||||||||
|
Broder-Fingert et al. [49] USA |
Cross-sectional registry sample | Prevalence rates for obesity and overweight | N = 2976 autism, N = 3696 general population controls | 2 to 20 years | 20.7% (2359 males, 617 females) | Not reported | Autistic boys/men | Autistic girls/women were less likely to be obese compared to autistic boys/men (OR 0.71 [95% CI 0.55–0.93]), but this did not hold for overweight (1.06 [0.81–1.39]) |
|
Garcia-Pastor et al. [50] USA |
Cross-sectional clinic/community sample | Prevalence rates for obesity and overweight | N = 78 autism | 7 to 48 years | 28.2% (56 males, 22 females) | Not reported | Autistic boys/men | Overweight and obesity were more prevalent in autistic men than in autistic women (p = 0.035); no differences were found between autistic girls and autistic boys |
| c. Gastrointestinal, metabolic, or nutritional problems | ||||||||
|
Yang et al. [51] China |
Cross-sectional clinic/community sample | Gastrointestinal symptoms | N = 169 autism, N = 172 controls | 3 to 12 years | 14.2% (145 males, 24 females) | Not reported | Autistic boys | Autistic girls had greater likelihood of gastrointestinal problems than autistic boys (OR 3.88 [95% CI 1.33–11.35], p = 0.013); more gastrointestinal symptoms were correlated with more severe core autistic symptoms |
|
Tseng et al. [52] Taiwan |
Systematic review and meta-analysis | Iron deficiency | n = 25 studies, with N = 1603 autism (across 3 analyses), N = 1507 controls | 0 to 18 years | 20.0% (no individual numbers) | Not reported | Autistic boys | No sex/gender differences in iron levels in autistic children |
|
Rossignol and Frye [53] USA |
Systematic review and meta-analysis | Mitochondrial disease (MD) | n = 65 studies, with N = 648 autism (among these N = 112 also with mitochondrial disease) | 0 to 20 years | 39% in autism plus MD, 19% in autism only (no individual numbers) | Not reported | Autistic boys/men | Autism–mitochondrial disease group had higher proportion of autistic girls/women (39%) than the autism-only group (19%, χ2 = 18.7, p < 0.0001); autistic boys/men are the majority in both groups |
|
Guo et al. [54] China |
Cross-sectional clinic/community sample | Vitamin A and vitamin D deficiencies | N = 332 autism, N = 197 controls | Mean age 4.87 years (SD 1.53) | 13.9% (286 males, 46 females) | Not reported | Autistic boys | Autistic girls had lower serum 25-OH vitamin D than autistic boys (p < 0.05) |
| d. Immune profile | ||||||||
|
Masi et al. [55] Australia |
Cross-sectional clinic/community sample | Cytokines | N = 144 autism | 2 to 18 years | 21.5% (113 males, 31 females) | Not reported | Autistic boys | In autistic girls, reduced levels of IL-1β, IL-8, MIP-1β, PDGF-BB and VEGF were associated with increased autism symptoms, while in autistic boys this was the case only for reduced PDGF-BB. Cytokine expression was moderated by sex/gender |
|
Hu et al. [56] China |
Cross-sectional registry sample | Cytokines | N = 87 autism, N = 41 controls | 1 to 6 years | 17.2% (72 males, 15 females) | Not reported | Autistic boys | Overall, autistic children had higher plasma levels of eotaxin, TGF-β1, and TNF-α than non-autistic children. In autistic girls, only the increase in eotaxin was statistically significant, whereas in autistic boys, the most consistent increase was in TGF-β1. Possible differential immune profiles in autistic girls vs. boys |
|
Saghazadeh et al. [57] Iran |
Systematic review and meta-analysis | Cytokines | n = 38 studies, with N = 1393 autism, N = 1094 controls | All ages | 36 studies with sex/gender info; 1582 males and 446 females in total sample; % of autistic females not reported | Not reported | Autistic boys/men | Overall, autistic individuals had higher concentrations of pro-inflammatory cytokines IFN-γ, IL-1β, IL-6, and TNF-α than controls; meta-regression revealed moderation effects of sex/gender (difference in the percentage of males between autism and control groups) for IL-1β and TNF-α |
| e. Autism symptoms associated with physical health | ||||||||
|
Moseley et al. [58] UK |
Systematic review and meta-analysis | Sex differences in autistic symptoms | N = 254 autism, N = 273 controls | Weighted mean age 36.4 years (females 37.5 (SD 14), males 35.3 (SD 13.4)) | 53.5% (118 males, 136 females) | 0% | Autistic boys/men | Autistic girls/women had more profound sensorimotor symptoms than autistic boys/men (t(252) = 4.346, p < 0.001) |
* Same study listed again but under different themes
** Calculated using mean and standard deviations from IQ scores, for males and females
CI confidence interval, OR odds ratio, RR risk ratio, SD standard deviation, SIR standardized incidence ratio
Results
Search results
We screened a total of 1112 unique citations and reviewed the full text of 201 articles, with 40 studies ultimately meeting the inclusion criteria (Fig. 1). The majority of the studies were from North America and Europe/UK, cross-sectional, and about general prevalence rates for health conditions in autistic individuals (Table 1). The articles included descriptive studies of autistic girls/women only, as well as studies of autistic individuals of all ages and functional levels that compared autistic girls/women to autistic boys/men and/or non-autistic girls/women (Table 2).
Fig. 1.
PRISMA flow diagram for study selection
Three major themes on the physical health status of autistic girls/women emerged from the existing literature: (1) Autistic girls/women experience more overall physical health conditions than autistic boys/men and non-autistic girls/women; (2) Epilepsy has been most commonly researched and seems more common in autistic girls/women compared to autistic boys/men and non-autistic girls/women; and (3) Autistic girls/women may experience more menstruation-related complications, endocrine and reproductive health conditions compared to non-autistic girls/women. Finally, studies that met the inclusion criteria but that did not align with the three major themes are described in a fourth section, Miscellaneous Emerging Findings. Details of sample characteristics, methodology, and statistics are provided in Table 2.
Summary of key themes
Theme 1: Overall Physical Health Status
Thirteen out of the 40 included studies focused on the overall physical health status of autistic girls/women [19–31], with respect to multiple conditions across body systems.
Autistic girls/women compared to autistic boys/men
Six studies explored the prevalence of various co-occurring physical health conditions in autistic girls/women and autistic boys/men [19–24]. Two of these six studies focused on population-based registry data: one in autistic children and youth (25,063 individuals with autism) [19] and the other in autistic adults (6649 with autism) [20]. Both reported higher odds for deafness, blindness, and physical disability in autistic girls/women compared to autistic boys/men [19, 20]. A third study [21], a follow-up on the same adult cohort in [20], found that autistic women in the 25–34 years of age range were more likely to report “poor general health” compared to autistic men [21]. Two additional studies reported on prevalence of physical health conditions as a percentage of their total registry-based autism sample [22, 23]. In 4123 autistic youth in a clinical registry, most physical health conditions were more common in autistic girls/women than in autistic boys/men (e.g., allergy/immunologic conditions, infections, musculoskeletal conditions, neurologic conditions) [23]. In 4790 autistic people of all ages from large medical registry cohorts, there was a higher prevalence of bowel disorders in autistic women compared to autistic men > 35 years of age [22]. The sixth study found a higher median number of medical comorbidities in autistic women compared to autistic men, in a community sample of 92 autistic adults [24].
Two studies further examined sex/gender as a predictor of physical quality of life and overall health status in 370 [25] and 255 [26] autistic adults. Female sex/gender was a significant predictor of lower physical health-related quality of life [25] and lower overall health [26]. Finally, two literature reviews on the physical health status of autistic adults regardless of sex/gender [27] and sex differences in developmental and medical endophenotypes in autism [28], respectively, reported there was insufficient research to reach consensus about sex/gender differences.
Autistic girls/women compared to non-autistic girls/women
Three studies with population-based registry samples examined the overall physical health status of autistic girls/women compared to non-autistic girls/women [29–31]. Two of them compared autistic adults to same-sex matched controls (2040 autistic individuals vs. 761,409 general population individuals [29] and 1507 autistic individuals vs. 15,070 general population controls [30]), and the other exclusively studied autistic seniors (≥ 65 years of age) who were enrolled in US-based Medicare (4685 with autism vs. 46,850 controls [31]). All three studies found elevated odds for most physical health conditions in autistic girls/women compared to non-autistic girls/women [29–31]. As a side note (also corresponding to the previous section), two of them also described odds ratios (ORs) of physical health conditions in autistic girls/women (reference to non-autistic girls/women) in contrast to autistic boys/men (reference to non-autistic boys/men) [29, 30]: Some conditions showed elevated ORs only in autistic girls/women, whereas others showed elevated ORs only in autistic boys/men (Table 2), alongside the majority of other conditions showing sex-similar patterns of elevated ORs across autistic individuals [29–31].
Theme 2: Epilepsy and Related Neurological Conditions
Eleven out of the 40 included studies specifically focused on, or included, epilepsy when examining sex/gender differences in neurological conditions in autistic individuals [22, 30–39].
Autistic girls/women compared to autistic boys/men
Five studies compared autistic girls/women to autistic boys/men with respect to epilepsy [22, 32–35]. While one study reported no sex differences in ORs for epilepsy [32], the other four studies found elevated rates of epilepsy in autistic girls/women compared to autistic boys/men. However, this finding was also associated with age, cognitive ability, adaptive functioning, language ability, and autism symptom severity [22, 33–35]. One meta-analysis with pooled sample sizes of 1530 autistic children and adults from 14 studies [33], and another study with 6975 autistic children from registry-based cohorts [34], found higher rates of epilepsy in autistic girls/women compared to autistic boys/men. They also found positive associations between rates of epilepsy and intellectual disability [33, 34], language impairment, core autism symptoms, and motor dysfunction [34]. Two other registry-based studies on prevalence rates for comorbid health conditions, including epilepsy, in 5815 [35] and 4790 [22] autistic individuals found higher prevalence of epilepsy in autistic girls/women compared to autistic boys/men. The sex difference patterns were modulated by age [22, 35], cognitive ability, adaptive language functioning, developmental regression, and autism symptom severity [35].
In addition, two studies specifically explored neurological profiles including seizure disorders in autistic children from community-based samples [36, 37]. One study of 883 autistic children found seizure disorders to be more frequent among autistic boys born preterm vs. those born term, while no preterm–term differences were found in autistic girls [36]. The other study on 663 autistic children found higher prevalence of neurological anomalies (e.g., microcephaly, minor neurological–musculoskeletal deficits) in autistic girls than in autistic boys, but no sex differences in seizures [37].
Autistic girls/women compared to non-autistic girls/women
Four studies reported risks of epilepsy in autistic girls/women against non-autistic girls/women [30, 31, 38, 39]. A population-based registry study of autistic adults found that autistic women were at significantly higher risks of having epilepsy than non-autistic women [30]. In another registry study on physical health in autistic seniors, epilepsy shows the largest OR among all conditions in autistic women compared to non-autistic women [31]. Similarly, two additional community-based studies reported elevated rates of epilepsy in autistic women compared to non-autistic women in a sample of 54 autistic women and 183 non-autistic women [38] and in another sample of 415 autistic women and 415 non-autistic women [39].
Theme 3: Endocrine and Reproductive Health Conditions
Ten out of the 40 included studies focused on endocrine and reproductive health conditions in autistic girls/women only [40, 41] or in comparison with non-autistic girls/women [30, 31, 38, 39, 42–45].
Studies with no comparison group
Two community-based studies reported menstruation-related health challenges in autistic girls/women [40, 41]. One online survey with 124 autistic girls/women found > 90% of them experienced dysmenorrhea and premenstrual syndrome (PMS), while one-third endorsed increased autism-associated difficulties during the menstrual cycle [40]. The other study on the effects of menarche on the sensory features of autism in 53 autistic girls reported that those who reached menarche had lower sensory interests and multisensory processing than those who had not yet reached menarche [41].
Autistic girls/women compared to non-autistic girls/women
Eight studies examined endocrine and reproductive health conditions in autistic girls/women compared to non-autistic girls/women [30, 31, 38, 39, 42–45]. A population-based registry study found that autistic women were at higher risks of having thyroid diseases than non-autistic women [30]. Another registry study on physical health in autistic seniors yielded similar findings regarding thyroid diseases, but autistic women had a comparable rate of menopausal disorders compared to non-autistic women [31]. A community-based qualitative study on 123 autistic and 114 non-autistic women’s experiences of menstruation found that, despite many overlapping challenges reported by both groups, autistic women highlighted amplification of autism-related (e.g., sensory and emotional regulation) challenges in sync with the menstrual cycle, which had significant negative impact on their lives [42]. Three studies focused on the prevalence of female-specific endocrine conditions: one from 54 autistic women and 183 non-autistic women [38] and the other from 415 autistic women and 415 non-autistic women [39] recruited from the community, as well as another registry-based study on nation-wide electronic health records of 971 autistic women and 4855 non-autistic women [43]. The findings demonstrated higher rates of irregular menstrual cycle [38, 39], unusually painful periods [38, 39], and polycystic ovary syndrome (PCOS) [38, 43] in autistic women compared to non-autistic women, along with a range of other endocrine-related conditions [38, 39]. Furthermore, a nation-wide registries study of cancer risks with 8438 autistic children, youth, and young adults compared to 76,332 general population controls found a higher incidence of ovarian cancer in autistic girls/women compared to non-autistic girls/women [44]. Finally, a registry-based study on pregnancy outcomes in 2198 births to 1382 autistic women of child-bearing age, compared to 877,742 births in 503,846 women from the general population, found increased risk of preeclampsia, giving preterm birth, and receiving elective cesarean delivery for autistic women compared to non-autistic women [45].
Miscellaneous emerging findings
Thirteen out of the 40 studies [46–58] were in emerging areas of research that did not align well with the three major themes; they also provided relatively inconsistent findings. All 13 studies offered information regarding autistic girls/women in comparison with autistic boys/men.
Additional neurological conditions
Three studies reported on neurological conditions beyond epilepsy in autism [46–48], with two examining neurological conditions as a broad category [46, 47] and the third, cerebral palsy [48]. One community-based study of 91 autistic children found that autistic girls had higher rates of neurological conditions than autistic boys [46]. A surveillance registry-based study found that in 6379 8-year-old autistic children across eight US sites, both autistic girls and boys showed stable rates of change in the prevalence of neurological conditions over 2002–2010 [47]. The final study, in a nation-wide cohort of 4180 autistic individuals (with ICD-10 Asperger’s syndrome), found increased prevalence of cerebral palsy in autistic individuals compared to the general population, without significant differences between autistic girls/women and boys/men [48].
Obesity and overweight
Two studies focused on obesity and overweight (via body mass index) [49, 50]. One registry-based study including 2976 autistic children and youth found that autistic girls were less likely to be obese compared to autistic boys, but they were equally likely to be overweight [49]. The other study, a community-based study of 78 autistic children, adolescents and adults, found that being overweight, as well as being obese, was more common in autistic men than in autistic women but with no differences found between autistic boys and autistic girls [50].
Gastrointestinal, metabolic, or nutritional problems
Four studies focused on gastrointestinal, metabolic, or nutritional conditions, comparing autistic girls/women and autistic boys/men [51–54]. One community-based study examined the prevalence of gastrointestinal problems in 169 autistic children, reporting that autistic girls had a greater likelihood of gastrointestinal problems than autistic boys and that more gastrointestinal symptoms were correlated with more severe core autistic symptoms [51]. Two were systematic reviews and meta-analyses: One, on peripheral iron levels and iron deficiency (1603 autistic children pooled from 25 studies across 3 analyses), found no sex/gender differences with respect to iron levels in autistic children [52]; the other, on mitochondrial dysfunction (648 autistic children and youth pooled from 65 studies), found a higher proportion of girls/women in the group of those with autism plus mitochondrial disease compared to those with autism only [53]. The final study, a community-based study on vitamins A and D deficiencies in 332 autistic children, reported that autistic girls had lower serum 25-OH vitamin D than autistic boys [54].
Immune profile
Three studies focused on sex-specific immunological features in autism [55–57]. Cytokine levels were measured in two studies of autistic children and adolescents from registry- and hospital-based cohorts: one with 144 (27 cytokines measured) [55] and the other, 87 (11 cytokines measured) [56] autistic individuals. Both studies found differences in the cytokine profiles of autistic girls compared to autistic boys but on different cytokines [55, 56]. A meta-analysis of 38 studies on 1393 autistic individuals regarding circulating pro-inflammatory cytokines also found sex/gender differences in cytokine patterns [57]. Altogether, these preliminary findings implicate potential sex/gender differences in immune profiles related to autism, though with inconsistent cytokine involvement.
Autism symptoms associated with physical health
Lastly, one meta-analysis examined sex/gender differences in 254 autistic adults regarding self-reported autistic characteristics, which included sensorimotor symptoms, some of which related to physical health, such as sensitivity to pain. Results revealed more severe sensorimotor symptoms in autistic women than in autistic men [58].
Discussion
The purpose of this scoping review was to explore what is known about co-occurring physical health conditions in autistic girls/women. Out of the 201 full-text articles we reviewed, only 40 met our inclusion criteria, mainly due to the paucity of reporting on sex or gender differences among populations with autism and the low percentages of autistic girls/women included in the current literature. There is a pressing need for more research that includes large numbers of autistic girls/women in order to better understand their physical health. This should be prioritized in order to advance the best clinical care for autistic individuals [11].
Emerging patterns of co-occurring physical health conditions are worth further examination and replication. With respect to Theme 1, the current literature suggests that autistic girls/women tend to have more overall physical health challenges and lower overall health and quality of life than do autistic boys/men [19–31]. However, apart from epilepsy, it is still unclear which specific conditions are consistently more prevalent in autistic girls/women compared to autistic boys/men or to non-autistic girls/women [29–31]. Such inconsistency could be related to the substantial heterogeneity in the autism population even within each sex [59] and could also be related to confounding factors (e.g., genetic etiological load or other neurodevelopmental disabilities). Based on Theme 2, epilepsy is the most studied neurological condition in autism and has been relatively consistently reported to be more prevalent in autistic girls/women than in autistic boys/men [22, 33–35]. Furthermore, these studies highlight heightened autism symptoms, language impairment, motor dysfunction, older age, and presence of intellectual disability as potential confounding factors to epilepsy prevalence in autism [22, 33–35].
Findings in Themes 1 and 2 regarding differences between autistic girls/women and autistic boys/men need to be interpreted in light of the general pattern of case ascertainment in autism research so far, and the complexity associated with sex/gender-related differences in comorbidity pattern (e.g., association with intellectual disability) and etiological load. With regard to ascertainment bias, some have suggested that the observed association between autism and epilepsy is largely driven by co-occurring intellectual disability [60], which is in turn confounded by the greater proportion of autistic girls/women with lower cognitive abilities [61] and shared neurobiological bases between autism and neurological anomalies [62]. Indeed, for a long time, girls/women who were clinically diagnosed with autism, and hence included in the registry and hospital-based datasets in the reviewed studies, tended to be those with lower IQ, early-recognized and more “classic” symptoms of autism [63]—those who tend to carry heightened etiological risk factors for neurodevelopmental and physical health issues. Meanwhile, sex- and gender-related barriers to girls/women receiving an autism diagnosis [2, 4, 11] may result in autistic girls/women without evident intellectual disability or “classic” symptoms of autism being under-represented in the current literature. Therefore, the observed differences between autistic boys/men and autistic girls/women in overall physical health status and epilepsy are likely confounded by sex- and gender-based ascertainment bias. It is also likely that the later recognition of autism in some girls/women has resulted in inadequate health care [64–66], further contributing to poorer overall health status. However, it remains unclear if the same pattern of male–female differences holds in autistic individuals who are so far under-recognized and undiagnosed.
At the same time, these observed sex/gender differences could be associated with variations in etiological load. At a group level, autistic girls/women tend to carry more de novo protein-truncating genetic variants likely causal to autism compared to autistic boys/men [67]. Given the pleiotropic effects of many autism-related genes, autistic individuals who carry these variants are more likely to experience other neurodevelopmental (e.g., intellectual disability) and physical health challenges (e.g., epilepsy, other neurological anomalies, cardiovascular defects, obesity) [8]. This means that the observed sex/gender differences may further reflect, and are confounded by, higher co-occurrence of neurodevelopmental disabilities and stronger de novo genetic etiological load in diagnosed autistic girls/women compared to autistic boys/men.
Another pattern that emerged (Theme 3) was the greater burden of co-occurring endocrine or reproductive health concerns in autistic girls/women (e.g., menstruation-related challenges, hormonal conditions, and ovarian cancer). Nevertheless, these findings should be viewed as preliminary, owing to the moderate sample sizes [40–42] and the reliance on self-report questionnaires—rather than direct clinical assessments—to characterize female-specific endocrine conditions [38–42]. Interestingly though, this theme can be hypothesis generating with implications for plausible biological mechanisms underlying autism, endocrine and other associated biological alternations. Some have speculated that endocrine dysregulation in autistic girls/women is partly indicative of altered prenatal sex steroid exposure [68, 69], which may contribute to both endocrine dysregulation and autism-related neurodevelopmental and behavioral characteristics [70–72]; see recent emerging empirical support [73–77]. How such prenatal endocrine factors contribute to the mechanisms leading to autism and sex differences in concurrent physical health disorders remains unclear and is an area requiring more in-depth mechanistic investigation. There is growing evidence supporting the role of multidirectional interactions between hormonal regulation, prenatal immune activation, epigenetic regulation in key brain regions, and postnatal environments in producing a range of distinct but related autistic phenotypes [78–80]. It is possible that there are shared mechanisms underlying autism and co-occurring endocrine and immune alterations, with sex differential mechanisms involved.
Finally, there is increasing evidence that gastrointestinal [81] and metabolic/nutritional conditions [82], including obesity and diabetes [83], have a high frequency of co-occurrence in autistic individuals. These conditions could involve shared etiological mechanisms with autism as well as with life experiences (e.g., lifestyle, health care support, medication use) of autistic people. The emerging but preliminary findings regarding sex/gender differences in these domains suggest that they may be especially important to the physical health of autistic girls/women. Elucidating associated sex/gender-related mechanisms requires more in-depth research.
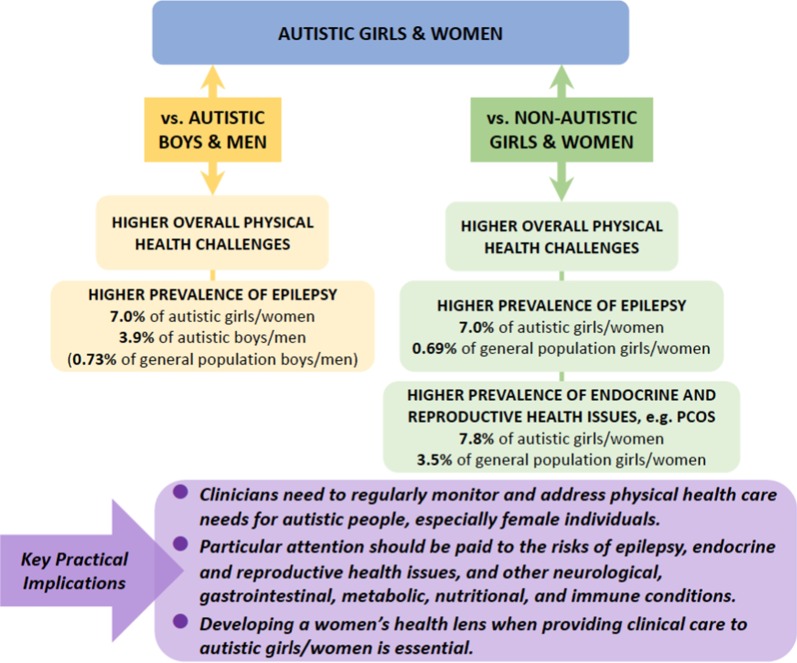
Clinical Implications (also see Fig. 2)
Fig. 2.
Exemplary findings and clinical implications. Prevalence rates of exemplary physical health conditions were
taken from the largest sample size studies included in this review (i.e., [43] for polycystic ovary syndrome [PCOS] and [35] for epilepsy) and a representative study in the general population (i.e., for epilepsy [93])
The finding that autistic girls/women experience more overall physical health challenges than non-autistic girls/women and autistic boys/men is of immediate clinical relevance. Improving physical health is integral to the care of all autistic individuals [84, 85]. Frontline and primary care clinicians should regularly monitor and address health care needs for autistic children, youth, and adults alike [86, 87], especially for female individuals [88]. For autistic girls/women, this scoping review provides indication for clinicians to particularly attend to the risks of epilepsy, endocrine and reproductive health issues, as well as other neurological, gastrointestinal, metabolic, nutritional, and immune conditions, among various physical health issues. To achieve holistic support, developing a women’s health lens when providing clinical care to autistic girls/women is essential and will significantly enrich sex- and gender-informed health care for all autistic people. Sex- and gender-informed health promotion strategies need to be applied across the life span. Conversely, improved attention to physical health in girls/women who also experience difficulties in social communication, restricted/stereotyped behaviors and sensory issues might also facilitate the identification of later recognized autism in girls/women [89].
Another key consideration is the interplay between physical and mental health. Autistic people are prone to experiencing mental health challenges (which we did not review here) [15]. However, many psychiatric medications for such challenges have side effects that are more commonly experienced in autistic compared to non-autistic individuals [84, 90, 91], contributing to heightened risk to physical health (e.g., weight gain and endocrine problems related to psychotropic medications). These findings have not yet been sufficiently studied in a sex-/gender-specific manner. Meanwhile, physical health challenges (e.g., epilepsy, hormonal dysregulation) can have detrimental impacts on mental health and affect mood and behavior. Such complexity and interplay may result in the high clinical needs and multiple service use that are common in the autism population, particularly among girls/women [64–66]. Many of these specific physical health challenges are treatable with the proviso that clinical trials need to disaggregate their data by sex and gender, which is unfortunately still insufficiently done for trials involving autistic people.
Timely diagnosis and treatment will enhance well-being associated with both physical and mental health of autistic individuals across the life span. This review has revealed that autistic girls/women are a population with unique health needs. Therefore, it requires us to develop comprehensive services that integrate developmental, mental, and physical health for autistic girls/women.
Limitations
There are several limitations to consider in interpreting our findings. First, it is possible that we were unable to identify all studies relevant to our guiding questions due to the heterogeneity in how physical health conditions were assessed and reported in the literature. Nevertheless, based on the principles of a scoping review, we have identified potential areas in the literature that warrant future investigation and areas with insufficient information as yet to make firm conclusions. Second, the decision to focus on physical health, thus excluding studies only focusing on psychiatric co-occurring conditions, meant that we could not explore how mental health and physical health are intertwined in autistic people, particularly in girls/women. Finally, our scoping review demonstrates that understandings of the physical health of autistic girls/women are still emerging. The limited number of studies in each theme, and their varying quality and research methodologies, makes it difficult to reach definitive conclusions.
However, several lessons about significant gaps in the clinical and research literature about autism can be learned from our review to inform future research directions. There is a lack of consistent, basic epidemiological information on the prevalence and incidence for co-occurring physical health conditions in the autism population by sex and, in particular, by gender. Reliable and valid measurement tools for physical health in autistic individuals need to be further developed. Additionally, there are insufficient longitudinal studies to chart the emergence of co-occurring conditions and randomized control trials to assess treatment for these conditions. There are also insufficient biological studies on the mechanisms of the development of physical health challenges in autism by sex and by gender. Further, there is significant underrepresentation of autistic girls/women in most studies, and only a small minority of studies are equipped to or have formally examined and reported sex/gender differences in their primary analyses. These highlight the ignorance about how sex and gender influence autism, perhaps due to a male-biased lens. The relative lack of awareness about women’s health and female experiences in the scientific and clinical knowledge about physical health and autism leaves girls/women diagnosed with autism at distinct disadvantages. Targeted research on autistic girls/women is clearly needed. Future studies on autism should be designed to achieve sex/gender equity by ensuring a male/female ratio closer to the general population rate (i.e., ~ 3:1) [4]. For research focusing on delineating sex-related or gender-related effects, balanced inclusion of participants with diverse sexes and genders should be targeted [2, 92]. Finally, it is extremely rare in the current empirical literature that sex (biological attributes) and gender (psychological, social, and cultural attributes) are defined and examined separately and in valid ways. These gaps need to be addressed in future research, alongside a clarification of sources of demographic, clinical, and etiological heterogeneity such as age, pubertal stage, developmental trajectories, intellectual functioning, and genetic background. Such clarification is fundamental for future studies to generate etiological and mechanistic insights by studying co-occurring physical health conditions in autism using sex- and gender-informed frameworks.
Conclusions
To our knowledge, this is the first scoping review on physical health in autistic girls/women. Emerging themes suggest that autistic girls/women tend to have heightened rates of a variety of co-occurring physical health challenges compared to autistic boys/men and non-autistic girls/women. Clinicians should provide holistic care that integrates not only developmental and mental health, but also physical health. Future studies need to include sufficient numbers of autistic girls/women to achieve adequate power, attend to physical health and the intertwined nature of developmental, mental, and physical health, and use sex- and gender-informed lenses.
Supplementary information
Additional file 1. Appendix: Search Strategy.
Acknowledgements
Not applicable.
Abbreviations
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- ICD
International Classification of Diseases
- OR
Odds ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- RR
Risk ratio
- SD
Standard deviation
- SIR
Standardized incidence ratio
Author contributions
M-CL, GE, and SB conceived and planned the study. CK and SB carried out the literature search, screening, extraction, and summary of data. M-CL and GE supervised the study and contributed to literature screening and summary of findings. CK, SB, M-CL, and GE drafted the manuscript. AT, YL, HKB, SHA, and PS contributed to the interpretation of findings and writing of the manuscript. M-CL and SHA obtained funding support for the study. CK and SB contributed equally as first authors. M-CL and GE contributed equally as senior authors. All authors read and approved the final manuscript.
Funding
M-CL is supported by a Canadian Institutes of Health Research (CIHR) Sex and Gender Science Chair (GSB 171373), the Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario (CAM-18-004), the Ontario Brain Institute via the Province of Ontario Neurodevelopmental Disorders (POND) Network (IDS-I l-02), and Women’s Xchange. M-CL and SHA are both supported by the O’Brien Scholars Program within the Child and Youth Mental Health Collaborative at the Centre for Addiction and Mental Health and The Hospital for Sick Children, Toronto, the Academic Scholars Award from the Department of Psychiatry, University of Toronto, and the Slaight Family Child and Youth Mental Health Innovation Fund via the Centre for Addiction and Mental Health Foundation. SHA is supported by funding from the US National Institutes of Mental Health, CIHR, and the Cundill Centre for Child and Youth Depression at the Centre for Addiction and Mental Health. AT is supported by the CIHR Post-doctoral Fellowship and the Azrieli Adult Neurodevelopmental Centre Fellowship. HKB is supported by a Tier 2 Canada Research Chair in Disability & Reproductive Health, CIHR, and the US National Institutes of Health. YL is supported by CIHR, the US National Institutes of Health, and the Azrieli Foundation. SB is supported by the Ontario Graduate Scholarship and the Department of Sociology, University of Toronto. GE is supported by the Wilfred and Joyce Posluns Chair in Women’s Brain Health and Aging, CIHR, Ontario Brain Institute, Alzheimer’s Society Canada, and Women’s Brain Health Initiative. The funders have no role in the design of the study, the collection, analysis, and interpretation of data, and writing of the manuscript.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Caroline Kassee and Stephanie Babinski are equal contribution joint first authors
Meng-Chuan Lai and Gillian Einstein are equal contribution joint senior authors
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13229-020-00380-z.
References
- 1.Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health. 2017;38:81–102. doi: 10.1146/annurev-publhealth-031816-044318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S. Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry. 2015;54:11–24. doi: 10.1016/j.jaac.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai MC, Lombardo MV, Baron-Cohen S. Autism. The Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 4.Loomes R, Hull L, Mandy WPL. What Is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56:466–474. doi: 10.1016/j.jaac.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Lai MC, Szatmari P. Sex and gender impacts on the behavioural presentation and recognition of autism. Curr Opin Psychiatry. 2020;33:117–123. doi: 10.1097/YCO.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 6.Whitlock A, Fulton K, Lai MC, Pellicano E, Mandy W. Recognition of girls on the autism spectrum by primary school educators: an experimental study. Autism Res. 2020;13:1358–1372. doi: 10.1002/aur.2316. [DOI] [PubMed] [Google Scholar]
- 7.Muskens JB, Velders FP, Staal WG. Medical comorbidities in children and adolescents with autism spectrum disorders and attention deficit hyperactivity disorders: a systematic review. Eur Child Adolesc Psychiatry. 2017;26:1093–1103. doi: 10.1007/s00787-017-1020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vorstman JAS, Parr JR, Moreno-De-Luca D, Anney RJL, Nurnberger JI, Hallmayer JF. Autism genetics: opportunities and challenges for clinical translation. Nat Rev Genet. 2017;18:362–378. doi: 10.1038/nrg.2017.4. [DOI] [PubMed] [Google Scholar]
- 9.Szatmari P, Georgiades S, Duku E, Bennett TA, Bryson S, Fombonne E, et al. Developmental trajectories of symptom severity and adaptive functioning in an inception cohort of preschool children with autism spectrum disorder. JAMA Psychiatry. 2015;72:276–283. doi: 10.1001/jamapsychiatry.2014.2463. [DOI] [PubMed] [Google Scholar]
- 10.Lord C, Bishop S, Anderson D. Developmental trajectories as autism phenotypes. Am J Med Genet. 2015;169:198–208. doi: 10.1002/ajmg.c.31440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandy W, Lai MC. Towards sex/gender informed autism research. Autism. 2017;21:643–645. doi: 10.1177/1362361317706904. [DOI] [PubMed] [Google Scholar]
- 12.Holtmann M, Bolte S, Poustka F. Autism spectrum disorders: sex differences in autistic behaviour domains and coexisting psychopathology. Dev Med Child Neurol. 2007;49:361–366. doi: 10.1111/j.1469-8749.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- 13.Tsakanikos E, Underwood L, Kravariti E, Bouras N, McCarthy J. Gender differences in co-morbid psychopathology and clinical management in adults with autism spectrum disorders. Res Autism Spectr Disord. 2011;5:803–808. doi: 10.1016/j.rasd.2010.09.009. [DOI] [Google Scholar]
- 14.Kreiser NL, White SW. ASD traits and co-occurring psychopathology: the moderating role of gender. J Autism Dev Disord. 2015;45:3932–3938. doi: 10.1007/s10803-015-2580-9. [DOI] [PubMed] [Google Scholar]
- 15.Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:819–829. doi: 10.1016/S2215-0366(19)30289-5. [DOI] [PubMed] [Google Scholar]
- 16.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 17.Tricco A, Lillie E, Zarin W, O’Brien K, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;167:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman D, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PMC free article] [PubMed]
- 19.Rydzewska E, Hughes-McCormack LA, Gillberg C, Henderson A, MacIntyre C, Rintoul J, et al. Prevalence of sensory impairments, physical and intellectual disabilities, and mental health in children and young people with self/proxy-reported autism: observational study of a whole country population. Autism. 2019;23:1201–1209. doi: 10.1177/1362361318791279. [DOI] [PubMed] [Google Scholar]
- 20.Rydzewska E, Hughes-Mccormack LA, Gillberg C, Henderson A, Macintyre C, Rintoul J, et al. Prevalence of long-term health conditions in adults with autism: observational study of a whole country population. BMJ Open. 2018;8:e023945–e023945. doi: 10.1136/bmjopen-2018-023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rydzewska E, Hughes-McCormack LA, Gillberg C, Henderson A, MacIntyre C, Rintoul J, et al. General health of adults with autism spectrum disorders – a whole country population cross-sectional study. Res Autism Spectr Disord. 2019;60:59–66. doi: 10.1016/j.rasd.2019.01.004. [DOI] [Google Scholar]
- 22.Supekar K, Iyer T, Menon V. The influence of sex and age on prevalence rates of comorbid conditions in autism. Autism Res. 2017;10:778–789. doi: 10.1002/aur.1741. [DOI] [PubMed] [Google Scholar]
- 23.Davignon MN, Qian Y, Massolo M, Croen LA. Psychiatric and medical conditions in transition-aged individuals with ASD. Pediatrics. 2018;141(Suppl 4):S335–S345. doi: 10.1542/peds.2016-4300K. [DOI] [PubMed] [Google Scholar]
- 24.Jones KB, Cottle K, Bakian A, Farley M, Bilder D, Coon H, et al. A description of medical conditions in adults with autism spectrum disorder: a follow-up of the 1980s Utah/UCLA autism epidemiologic study. Autism. 2016;20:551–561. doi: 10.1177/1362361315594798. [DOI] [PubMed] [Google Scholar]
- 25.Mason D, McConachie H, Garland D, Petrou A, Rodgers J, Parr JR. Predictors of quality of life for autistic adults. Autism Res. 2018;11:1138–1147. doi: 10.1002/aur.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortuna RJ, Robinson L, Smith TH, Meccarello J, Bullen B, Nobis K, et al. Health conditions and functional status in adults with autism: a cross-sectional evaluation. J Gen Intern Med. 2016;31:77–84. doi: 10.1007/s11606-015-3509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cashin A, Buckley T, Trollor JN, Lennox N. A scoping review of what is known of the physical health of adults with autism spectrum disorder. J Intellect Disabil. 2018;22:96–108. doi: 10.1177/1744629516665242. [DOI] [PubMed] [Google Scholar]
- 28.Rubenstein E, Wiggins LD, Lee L-C. A review of the differences in developmental, psychiatric, and medical endophenotypes between males and females with autism spectrum disorder. J Multihandicap Pers. 2015;27:119–139. doi: 10.1007/s10882-014-9397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cawthorpe D. Comprehensive description of comorbidity for autism spectrum disorder in a general population. Perm J. 2017;21:86–90. doi: 10.7812/TPP/16-088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, et al. The health status of adults on the autism spectrum. Autism. 2015;19:814–823. doi: 10.1177/1362361315577517. [DOI] [PubMed] [Google Scholar]
- 31.Hand BN, Angell AM, Harris L, Carpenter LA. Prevalence of physical and mental health conditions in Medicare-enrolled, autistic older adults. Autism. 2020;24:755–764. doi: 10.1177/1362361319890793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stacy ME, Zablotsky B, Yarger HA, Zimmerman A, Makia B, Lee L-C. Sex differences in co-occurring conditions of children with autism spectrum disorders. Autism. 2014;18:965–974. doi: 10.1177/1362361313505719. [DOI] [PubMed] [Google Scholar]
- 33.Amiet C, Gourfinkein I, Bouzamondo A, Tordjman S, Baulac M, Lechat P, et al. Epilepsy in autism is associated with intellectual disability and gender: evidence from a meta-analysis. Biol Psychiatry. 2008;64:577–582. doi: 10.1016/j.biopsych.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 34.Ewen JB, Marvin AR, Law K, Lipkin PH. Epilepsy and autism severity: a study of 6975 children. Autism Res. 2019;12:1251–1259. doi: 10.1002/aur.2132. [DOI] [PubMed] [Google Scholar]
- 35.Viscidi EW, Triche EW, Pescosolido MF, McLean RL, Joseph RM, Spence SJ, et al. Clinical characteristics of children with autism spectrum disorder and co-occurring epilepsy. PLoS ONE. 2013;8:e67797–e67797. doi: 10.1371/journal.pone.0067797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowers K, Wink LK, Pottenger A, McDougle CJ, Erickson C. Phenotypic differences in individuals with autism spectrum disorder born preterm and at term gestation. Autism. 2015;19:758–763. doi: 10.1177/1362361314547366. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Itzchak E, Ben-Shachar S, Zachor DA. Specific neurological phenotypes in autism spectrum disorders are associated with sex representation. Autism Res. 2013;6:594–604. doi: 10.1002/aur.1319. [DOI] [PubMed] [Google Scholar]
- 38.Ingudomnukul E, Baron-Cohen S, Wheelwright S, Knickmeyer R. Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm Behav. 2007;51:597–604. doi: 10.1016/j.yhbeh.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Pohl A, Cassidy S, Auyeung B, Baron-Cohen S. Uncovering steroidopathy in women with autism: a latent class analysis. Mol Autism. 2014;5:1–12. doi: 10.1186/2040-2392-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton A, Marshal MP, Murray PJ. Autism spectrum disorders and menstruation. J Adolesc Health. 2011;49:443–445. doi: 10.1016/j.jadohealth.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Bitsika V, Sharpley CF. The effects of menarche upon the sensory features of girls with autism spectrum disorder. J Dev Phys Disabil. 2018;30:755–759. doi: 10.1007/s10882-018-9617-x. [DOI] [Google Scholar]
- 42.Steward R, Crane L, Mairi Roy E, Remington A, Pellicano E. “Life is much more difficult to manage during periods”: autistic experiences of menstruation. J Autism Dev Disord. 2018;48:4287–4292. doi: 10.1007/s10803-018-3664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherskov A, Pohl A, Allison C, Zhang H, Payne RA, Baron-Cohen S. Polycystic ovary syndrome and autism: a test of the prenatal sex steroid theory. Transl Psychiatry. 2018;8:1–10. doi: 10.1038/s41398-018-0186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiang HL, Liu CJ, Hu YW, Chen SC, Hu LY, Shen CC, et al. Risk of cancer in children, adolescents, and young adults with autistic disorder. J Pediatr. 2015;166:418–423. doi: 10.1016/j.jpeds.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 45.Sundelin HEK, Stephansson O, Hultman CM, Ludvigsson JF. Pregnancy outcomes in women with autism: a nationwide population-based cohort study. Clin Epidemiol. 2018;10:1817–1826. doi: 10.2147/CLEP.S176910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Memari AH, Ziaee V, Mirfazeli FS, Kordi R. Investigation of autism comorbidities and associations in a school-based community sample. J Child Adolesc Psychiatr Nurs. 2012;25:84–90. doi: 10.1111/j.1744-6171.2012.00325.x. [DOI] [PubMed] [Google Scholar]
- 47.Rubenstein E, Schieve L, Wiggins L, Rice C, Van Naarden BK, Christensen D, et al. Trends in documented co-occurring conditions in children with autism spectrum disorder, 2002–2010. Res Dev Disabil. 2018;83:168–178. doi: 10.1016/j.ridd.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mouridsen SE, Rich B, Isager T. Cerebral palsy in individuals with a history of Asperger’s syndrome: a Danish nationwide register study based on hospital diagnoses. J Pediatr Neurol. 2013;11:29–34. [Google Scholar]
- 49.Broder-Fingert S, Brazauskas K, Lindgren K, Iannuzzi D, Van Cleave J. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408–414. doi: 10.1016/j.acap.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Pastor T, Salinero JJ, Theirs CI, Ruiz-Vicente D. Obesity status and physical activity level in children and adults with autism spectrum disorders: a pilot study. J Autism Dev Disord. 2019;49:165–172. doi: 10.1007/s10803-018-3692-9. [DOI] [PubMed] [Google Scholar]
- 51.Yang XL, Liang S, Zou MY, Sun CH, Han PP, Jiang XT, et al. Are gastrointestinal and sleep problems associated with behavioral symptoms of autism spectrum disorder? Psychiatry Res. 2018;259:229–235. doi: 10.1016/j.psychres.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 52.Tseng P, Cheng Y, Chen Y, Stubbs B, Whiteley P, Carvalho A, et al. Peripheral iron levels in children with autism spectrum disorders vs controls: a systematic review and meta-analysis. Nutr Res. 2018;50:44–52. doi: 10.1016/j.nutres.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2012;17:290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo M, Zhu J, Yang T, Lai X, Lei Y, Chen J, et al. Vitamin A and vitamin D deficiencies exacerbate symptoms in children with autism spectrum disorders. Nutr Neurosci. 2018;22:637–647. doi: 10.1080/1028415X.2017.1423268. [DOI] [PubMed] [Google Scholar]
- 55.Masi A, Breen EJ, Alvares GA, Glozier N, Hickie IB, Hunt A, et al. Cytokine levels and associations with symptom severity in male and female children with autism spectrum disorder. Mol Autism. 2017;8:1–11. doi: 10.1186/s13229-017-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu CC, Xu X, Xiong GL, Xu Q, Zhou BR, Li CY, et al. Alterations in plasma cytokine levels in chinese children with autism spectrum disorder. Autism Res. 2018;11:989–999. doi: 10.1002/aur.1940. [DOI] [PubMed] [Google Scholar]
- 57.Saghazadeh A, Ataeinia B, Keynejad K, Abdolalizadeh A, Hirbod-Mobarakeh A, Rezaei B. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: effects of age, gender, and latitude. J Psychiatr Res. 2019;115:90–102. doi: 10.1016/j.jpsychires.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Moseley RL, Hitchiner R, Kirkby JA. Self-reported sex differences in high-functioning adults with autism: a meta-analysis. Mol Autism. 2018;9:33–45. doi: 10.1186/s13229-018-0216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lombardo MV, Lai MC, Baron-Cohen S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol Psychiatry. 2019;24:1435–1450. doi: 10.1038/s41380-018-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berg AT, Plioplys S. Epilepsy and autism: is there a special relationship? Epilepsy Behav. 2012;23:193–198. doi: 10.1016/j.yebeh.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26:146–153. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veliskova J, Silverman JL, Benson M, Lenck-Santini PP. Autistic traits in epilepsy models: why, when and how? Epilepsy Res. 2018;144:62–70. [DOI] [PMC free article] [PubMed]
- 63.Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the simons simplex collection. J Am Acad Child Adolesc Psychiatry. 2014;53(329–340):e3. doi: 10.1016/j.jaac.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tint A, Weiss JA, Lunsky Y. Identifying the clinical needs and patterns of health service use of adolescent girls and women with autism spectrum disorder. Autism Res. 2017;10:1558–1566. doi: 10.1002/aur.1806. [DOI] [PubMed] [Google Scholar]
- 65.Tint A, Weiss JA. A qualitative study of the service experiences of women with autism spectrum disorder. Autism. 2018;22:928–937. doi: 10.1177/1362361317702561. [DOI] [PubMed] [Google Scholar]
- 66.Bargiela S, Steward R, Mandy W. The experiences of late-diagnosed women with autism spectrum conditions: an investigation of the female autism phenotype. J Autism Dev Disord. 2016;46:3281–3294. doi: 10.1007/s10803-016-2872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An J-Y, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180(568–584):e23. doi: 10.1016/j.cell.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467–520. doi: 10.1210/er.2015-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barrett ES, Swan SH. Stress and androgen activity during fetal development. Endocrinology. 2015;156:3435–3441. doi: 10.1210/en.2015-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gore AC, Martien KM, Gagnidze K, Pfaff D. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr Rev. 2014;35:961–991. doi: 10.1210/er.2013-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Auyeung B, Lombardo MV, Baron-Cohen S. Prenatal and postnatal hormone effects on the human brain and cognition. Pflugers Arch. 2013;465:557–571. doi: 10.1007/s00424-013-1268-2. [DOI] [PubMed] [Google Scholar]
- 72.Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011;9:e1001081–e1001081. doi: 10.1371/journal.pbio.1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baron-Cohen S, Tsompanidis A, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah M, et al. Foetal oestrogens and autism. Mol Psychiatry. 2019. [DOI] [PMC free article] [PubMed]
- 74.Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, et al. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. 2015;20:369–376. doi: 10.1038/mp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C, et al. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol Psychiatry. 2016;21:1441–1448. doi: 10.1038/mp.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee BK, Arver S, Widman L, Gardner RM, Magnusson C, Dalman C, et al. Maternal hirsutism and autism spectrum disorders in offspring. Autism Res. 2017;10:1544–1546. doi: 10.1002/aur.1797. [DOI] [PubMed] [Google Scholar]
- 77.Rotem RS, Chodick G, Davidovitch M, Hauser R, Coull BA, Weisskopf MG. Congenital abnormalities of the male reproductive system and risk of autism spectrum disorders. Am J Epidemiol. 2018;187:656–663. doi: 10.1093/aje/kwx367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meltzer A, Van De Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology. 2017;42:284–92. [DOI] [PMC free article] [PubMed]
- 79.Nardone S, Elliott E. The interaction between the immune system and epigenetics in the etiology of autism spectrum disorders. Front Neurosci. 2016;10:329–329. doi: 10.3389/fnins.2016.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCarthy MM, Wright CL. Convergence of sex differences and the neuroimmune system in autism spectrum disorder. Biol Psychiatry. 2017;81:402–410. doi: 10.1016/j.biopsych.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coury DL, Ashwood P, Fasano A, Fuchs G, Geraghty M, Kaul A, et al. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics. 2012;130(SUPPL. 2):S160–S168. doi: 10.1542/peds.2012-0900N. [DOI] [PubMed] [Google Scholar]
- 82.Ranjan S, Nasser JA. Nutritional status of individuals with autism spectrum disorders: do we know enough? Adv Nutr. 2015;6:397–407. doi: 10.3945/an.114.007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shedlock K, Susi A, Gorman GH, Hisle-Gorman E, Erdie-Lalena CR, Nylund CM. Autism spectrum disorders and metabolic complications of obesity. J Pediatr. 2016;178:183–187. doi: 10.1016/j.jpeds.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 84.Lai MC, Anagnostou E, Wiznitzer M, Allison C, Baron-Cohen S. Evidence-based support for autistic people across the lifespan: maximising potential, minimising barriers, and optimising the person–environment fit. Lancet Neurol. 2020;19:434–451. doi: 10.1016/S1474-4422(20)30034-X. [DOI] [PubMed] [Google Scholar]
- 85.Nicolaidis C, Raymaker D, McDonald K, Kapp S, Weiner M, Ashkenazy E, et al. The development and evaluation of an online healthcare toolkit for autistic adults and their primary care providers. J Gen Intern Med. 2016;31:1180–1189. doi: 10.1007/s11606-016-3763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karpur A, Lello A, Frazier T, Dixon PJ, Shih AJ. Health disparities among children with autism spectrum disorders: analysis of the national survey of children’s health 2016. J Autism Dev Disord. 2019;49:1652–1664. doi: 10.1007/s10803-018-3862-9. [DOI] [PubMed] [Google Scholar]
- 87.Mason D, Ingham B, Urbanowicz A, Michael C, Birtles H, Woodbury-Smith M, et al. A systematic review of what barriers and facilitators prevent and enable physical healthcare services access for autistic adults. J Autism Dev Disord. 2019;49:3387–3400. doi: 10.1007/s10803-019-04049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tint A, Hamdani Y, Sawyer A, Desarkar P, Ameis SH, Bardikoff N, et al. Wellness efforts for autistic women. Curr Dev Disord Rep. 2018;5:207–216. doi: 10.1007/s40474-018-0148-z. [DOI] [Google Scholar]
- 89.Lai MC, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2:1013–1027. doi: 10.1016/S2215-0366(15)00277-1. [DOI] [PubMed] [Google Scholar]
- 90.Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics. 2012;130:717–726. doi: 10.1542/peds.2012-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ameis SH, Kassee C, Corbett-Dick P, Cole L, Dadhwal S, Lai MC, et al. Systematic review and guide to management of core and psychiatric symptoms in youth with autism. Acta Psychiatr Scand. 2018;138:379–400. doi: 10.1111/acps.12918. [DOI] [PubMed] [Google Scholar]
- 92.Strang JF, van der Miesen AI, Caplan R, Hughes C, daVanport S, Lai MC. Both sex- and gender-related factors should be considered in autism research and clinical practice. Autism. 2020;24:539–543. doi: 10.1177/1362361320913192. [DOI] [PubMed] [Google Scholar]
- 93.Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon C-S, Dykeman J, et al. Prevalence and incidence of epilepsy. Neurology. 2017;88:296–303. doi: 10.1212/WNL.0000000000003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Appendix: Search Strategy.
Data Availability Statement
Not applicable.