Abstract
The major Corona Virus Disease 2019 (COVID-19) outbreak caused tens of thousands of diagnosed patients quarantined and treated in designated hospitals in Wuhan, the epicenter of the disease in China. Evidence for the psychological problems of COVID-19 patients was limited. Here we report a cross-sectional study of the mental distress and sleep quality of patients in a single center in Wuhan. The study was based on a combined questionnaire of basic questions designed by the study group, Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), and Pittsburgh Sleep Quality Index (PSQI). On Feb 17th and Mar 14th, two groups of patients were recruited respectively in a designated hospital for COVID-19. Univariate analysis and regression models were used to identify predictors for patients' psychological distress and sleep quality. In total, there were 202 participants in our combined sample. The average SAS, SDS, and PSQI score of participants were 44.2, 51.7, and 9.3 respectively. Factors associated with SAS score include gender, subjective evaluation of disease symptoms, and evaluation of medical staffs' attitude. Gender, age, education level, frequency of contacting with family, subjective knowledge level of COVID 19, and evaluation of medical staffs’ attitude are associated with participants SDS score. Factors associated with PSQI score are age and subjective evaluation of disease symptoms.
Keywords: COVID-19, Mental health, Anxiety, Depression, Sleep condition
1. Introduction
In December 2019, local health facilities reported clusters of patients with pneumonia of unknown cause in Wuhan, China(Zhu et al., 2020). Later, the responsible pathogen was identified as a novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)(World Health Organization, 2020a; Wu et al., 2020; Zhou et al., 2020). Cases of pneumonia linked with SARS-CoV-2, named Corona Virus Disease 2019 (COVID-19) (World Health Organization, 2020a), have been reported worldwide. On Jan 30th, 2020, the World Health Organization (WHO) declared the outbreak a Public Health Emergency of International Concern (PHEIC)(World Health Organization, 2020b). Up to April 12th, 2020, there have been accumulatively 83,482 cases in China alone and 1,696,588 worldwide. The rapid outbreak of the disease caused over 100 thousand deaths totally(World Health Organization, 2020c). As a sudden pandemic sweeping over the world, COVID-19 brought problems to patients both physically and mentally. Previous studies of other similar epidemic diseases showed that patients might suffer from psychological problems include posttraumatic stress disorder, anxiety, depression, stress, psychological marginalization, etc.(Liu et al., 2012; Taylor et al., 2008; Wu et al., 2009)
Here we report a cross-sectional investigation carried out in a single-center in Wuhan, to find out factors associated with patients’ psychological distress and sleep quality.
2. Material and methods
2.1. Study design and participants
The study was carried out in accordance with the 2013 version of the Declaration of Helsinki. The Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (IORG No: IORG0003571) gave a final approval for this study. All subjects signed written informed consent for participation.
Two cross-sectional investigations were conducted on Feb 17th and Mar 14th, respectively. The two groups will be referred to as the Feb group and the Mar group in this article. All participants were recruited from the inpatients with COVID-19 in the authors’ institute. Diagnosis of COVID-19 was conducted according to the Diagnosis and Treatment Guideline for COVID-19 (fifth edition) issued by the National Health Commission of China(National Health Commission of China, 2020). More specifically, cases were confirmed using either chest CT scanning or nasopharyngeal swab testing with real-time reverse transcription-polymerase chain reaction (RT-PCR).
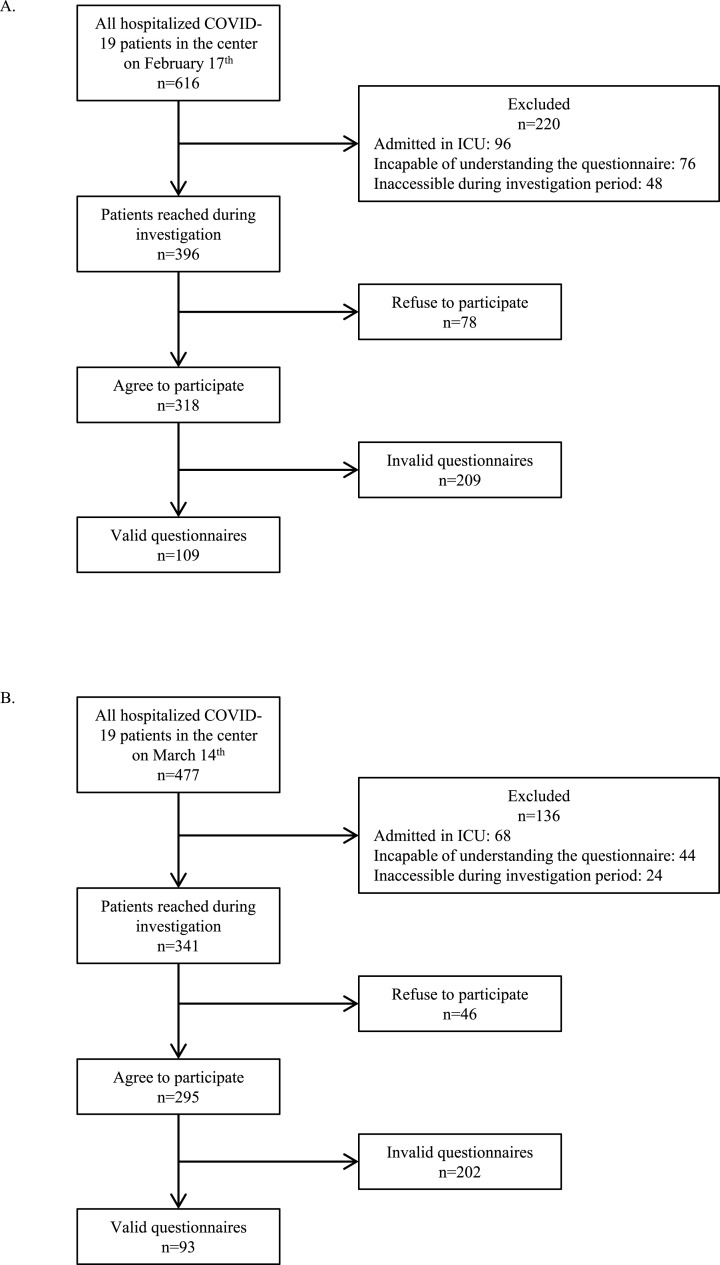
Inclusion criteria were 1) having stable consciousness states, 2) capable of understanding the questionnaire, and 3) accessible during the investigation period. On Feb 17th, there were 616 inpatients with COVID-19 in the institute. 396 (64.3%) of them were included according to the criteria described above. Among the included patients, 46 (11.6%) refused to participate in the survey. After excluding invalid ones, 109 (27.5%) questionnaires were included in statistical analysis. On Mar 14th, there were 477 inpatients, among whom 341 (71.5%) satisfied the inclusion criteria. 46 (13.5%) out of the included patients refused to participate. Finally, there were 93 (27.3%) valid questionnaires in the second round of the survey (Fig. 1 ).
Fig. 1.
Flow diagram of patient recruit. (A) Patient recruit process of the investigation conducted on February 17th. (B) Patient recruit process of the investigation conducted on March 14th.COVID-19 = corona virus disease 2019.
2.2. Data collection and evaluation
All recruited patients were given a questionnaire designed by the research group. Patients' basic demographic information was collected, including gender, age, marital status, and education level. Other questions included patients' chronic disease history, duration of hospitalization, conditions of family members (diagnosed with COVID-19 or not), frequency of contact with family members, subjective perception of their knowledge about the disease, subjective assessment of their disease severity, and evaluation of medical staffs’ attitudes. All this information was self-reported by the participants.
Anxiety was assessed by the Zung Self-Rating Anxiety Scale (SAS) developed by William W. K. Zung in 1971(Zung, 1971). SAS is a self-administered test to evaluate the levels of anxiety in patients with anxiety-related symptoms. It has 20 items with a four-point scale, from “a little of the time” to “most of the time”. 15 items are increasing anxiety level questions and five are decreasing anxiety level ones. The crude score is the sum of all the responses. The standard score is calculated by taking the integer of 1.25 times the crude score. The cut-off value of anxiety was defined as a standard score of at least 50. Patients with a standard score of 50–59, 60–69, and 70 or greater, were evaluated as having mild anxiety, moderate anxiety, and severe anxiety, respectively.
Depression level was assessed using the Zung Self-Rating Depression Scale (SDS)(William, 2014). Also developed by William Zung, SDS measures depression-associated psychological and somatic symptoms. It has 20 items concerning different manifestations of depressions. Ten questions are positively worded and ten are negatively worded, each scored on a four-point scale. The standard score is the integer of 1.25 times the crude score, which is defined as the sum of all responses. The normal range for the standard score is 25–49. The standard score ranges of 50–59, 60–69, and 70 and above are defined as mild depression, moderate depression, and severe depression, respectively.
Patients’ sleep quality was evaluated using the Pittsburgh Sleep Quality Index (PSQI), a self-report scale assessing the sleep quality of the respondents over a one-month interval(Buysse et al., 1989). PSQI contains 19 items, where four are open-ended questions and 15 require a rating from zero to three. The items are then grouped into seven components, addressing subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication, and daytime dysfunction. The global PSQI score is the addition of the seven component scores. Patients with global PSQI scores of four or less were defined as good sleepers. The cut-off value of poor sleepers was eight. Patients scoring five to seven were defined as average sleepers.
The participants completed all these three scales described above in a self-reported manner.
We confirmed the clinical type of patients by referring to the Electronic Medical Record (EMR) and discussing with relevant practitioners. Classification criteria follow the Diagnosis and Treatment Guideline for COVID-19 (fifth edition) issued by the National Health Commission of China(National Health Commission of China, 2020). Mild cases refer to patients with mild symptoms and no radiological findings indicating pneumonia. Ordinary cases are those who have symptoms including fever and respiratory manifestations, and radiological findings consistent with pneumonia. Criteria for severe cases include dyspnea (respiratory rate≥30 per minute), resting pulse oxygen saturation≤93%, and arterial partial pressure of oxygen (PaO2) over the fraction of inspired oxygen (FiO2) of ≤300 mmHg. Patients meeting any one of the criteria should be classified as severe cases. Critically ill patients are those who meet any one of the following criteria: 1) respiratory failure requiring mechanical ventilation, 2) shock, and 3) concurrent organ failure(s) requiring intensive care.
2.3. Statistical analyses
Data processing and statistical analyses were conducted using STATA, version 12.0 (copyright 1985–2011 StataCorp LP). Continuous variables were expressed as mean (SD) and compared using the two-sample t-test, Wilcoxon rank-sum test, one-way analysis of variance (ANOVA), or Kruskal-Wallis equality-of-populations rank test. If significant differences appeared in ANOVA or Kruskal-Wallis test, pairwise comparisons of means or rank means were carried out to further address the differences. Categorical variables were expressed as number (%) and compared using Pearson's chi-squared test or Fisher's exact test for nominal variables, or Wilcoxon rank-sum test for ordinal variables. Finally, two multiple regression models were developed for each scale. Model 1 was a stepwise regression model. The significance level for entering the model was 0.05. Model 2 included all factors investigated in the study, regardless of the confidence interval of the coefficients.
3. Results
3.1. Demographic characteristics
Overall, 202 participants were recruited in this study, among whom 109 were recruited on Feb 17th, and 93 were recruited on Mar 14th. Table 1 illustrates the general characteristics of the two groups and the combined population. 53% (107) of the patients in the combined population were male. The clinical type of COVID-19 were mild or ordinary in 124 patients (61.4%) and severe or critically ill in 78 patients (38.6%).
Table 1.
General characteristics of two groups of participants.
| Total (N = 202)a | February group (n = 109)a | March group (n = 93)a | p value | |
|---|---|---|---|---|
| Gender | 0.94b | |||
| Male | 107 (53) | 58 (53.2) | 49 (52.7) | |
| Female | 95 (47) | 51 (46.8) | 44 (47.3) | |
| Age, years | 0.43b | |||
| ≤41 | 59 (29.2) | 36 (33) | 23 (24.7) | |
| 42–51 | 66 (32.7) | 34 (31.2) | 32 (34.4) | |
| ≥52 | 77 (38.1) | 39 (35.8) | 38 (40.9) | |
| Marital status | 0.058b | |||
| Married | 186 (92.1) | 104 (95.4) | 82 (88.2) | |
| Single or divorced | 16 (7.9) | 5 (4.6) | 11 (11.8) | |
| Education level | 0.013b | |||
| Primary or secondary | 96 (47.5) | 43 (39.4) | 53 (57) | |
| Tertiary or higher | 106 (52.5) | 66 (60.6) | 40 (43) | |
| History of chronic disease | 0.99b | |||
| No | 115 (56.9) | 62 (56.9) | 53 (57) | |
| Yes | 87 (43.1) | 47 (43.1) | 40 (43) | |
| Hospitalization duration, days | <0.001b | |||
| ≤9 | 46 (22.8) | 36 (33) | 10 (10.8) | |
| 10–13 | 47 (23.3) | 38 (34.9) | 9 (9.7) | |
| ≥14 | 109 (54) | 35 (32.1) | 74 (79.6) | |
| Clinical type | 0.0098b | |||
| Mild or ordinary | 124 (61.4) | 58 (53.2) | 66 (71) | |
| Severe or critically ill | 78 (38.6) | 51 (46.8) | 27 (29) | |
| Family member diagnosed with COVID-19 | 0.35b | |||
| No | 108 (53.5) | 55 (50.5) | 53 (57) | |
| Yes | 94 (46.5) | 54 (49.5) | 40 (43) | |
| Frequency of contacting with family | 0.13b | |||
| 0–2/wk | 23 (11.4) | 9 (8.3) | 14 (15.1) | |
| ≥3/wk | 179 (88.6) | 100 (91.7) | 79 (84.9) | |
| Subjective knowledge level of COVID-19 | 0.75b | |||
| None | 23 (11.4) | 14 (12.8) | 9 (9.7) | |
| Common knowledge | 139 (68.8) | 73 (67) | 66 (71) | |
| Deep knowledge | 40 (19.8) | 22 (20.2) | 18 (19.4) | |
| Subjective evaluation of disease symptoms | 0.76b | |||
| Mild | 74 (36.6) | 37 (33.9) | 37 (39.8) | |
| Moderate | 86 (42.6) | 48 (44) | 38 (40.9) | |
| Severe | 28 (13.9) | 17 (15.6) | 11 (11.8) | |
| Not clear | 14 (6.9) | 7 (6.4) | 7 (7.5) | |
| Evaluation of medical staffs' attitude | 0.76c | |||
| Good | 192 (95) | 103 (94.5) | 89 (95.7) | |
| Normal or awful | 10 (5) | 6 (5.5) | 4 (4.3) |
aData are number (percentage over the total number of participants in corresponding group). COVID-19 = corona virus disease 2019.
bp values are generated from Pearson's chi-squared test.
cp values are generated from Fisher's exact test.
The two groups balanced on gender, age, marital status, and chronic disease history, while in the Mar group there were more participants whose education level was primary or secondary, compared with the Feb group (57.0% versus 39.4% respectively, p = 0.013). The average hospitalization time of the Mar group was significantly longer than that of the Feb group (23.6 days versus 12.1 days respectively, p < 0.001). Compared with patients recruited on Feb 17th, there were less severe or critically ill cases among patients recruited on Mar 14th (46.8% versus 29.0% respectively, p = 0.0098). There was no significant difference observed between the two groups when it came to their subjective perceptions of their knowledge about COVID-19, their symptoms, and medical staffs’ attitudes towards them.
3.2. Mental health status
Overall, patients got 44.2 (SD 8.4), 51.7 (SD 10.9), and 9.3 (SD 4.6) in SAS, SDS, and PSQI scales, as shown in Table 2 . In the Feb group, patients got 43.7 (SD 8.3) standard SAS scores on average, while those in the Mar group got 44.9 (SD 8.4) on average. Both scores fell into the normal range. The two patient groups showed no significant difference concerning standard SAS scores (p = 0.2). The average SDS scores of patients recruited in Mar were significantly higher than those of the Feb group (53.2 and 50.4 respectively, p = 0.047), while both scores fell into the score range of mild depression. When it came to sleep conditions, the average scores of participants recruited in Feb was 9.3, while that of those recruited in Mar was 9.5 (p = 0.77). These two scores fell in the PSQI score range for poor sleepers.
Table 2.
Scores of participants’ mental distress and sleep quality.
| Totala | February groupa | March groupa | p value | |
|---|---|---|---|---|
| SAS score | 44.2 (8.4) | 43.7 (8.3) | 44.9 (8.4) | 0.2b |
| SDS score | 51.7 (10.9) | 50.4 (10.9) | 53.2 (10.8) | 0.047b |
| PSQI score | 9.3 (4.6) | 9.3 (4.8) | 9.5 (4.4) | 0.77c |
aData are mean (standard deviation).
bp values comparing the February group and March group are generated from Wilcoxon rank-sum test.
cp values comparing the February group and March group are generated from two-sample t-test.
3.3. Factors associated with mental health status
The results of univariate analyses of the combined sample are shown in Table 3 . Notably, females got higher scores than males in both SAS assessment (average 45.8 versus 42.9 respectively, p = 0.013) and SDS assessment (average 54.6 versus 49.1 respectively, p < 0.001), while there was no significant difference in PSQI scores (average 9.1 versus 9.5 respectively, p = 0.55). Age was validated as a factor associated with patients' SDS scores (p = 0.025) and PSQI scores (p = 0.0019). Other predictors for lower SDS scores included education level of tertiary or higher (average 48.9 versus 54.8 for primary or secondary education level, p < 0.001), contact with family members for at least three times per week (average 50.9 versus 57.9 for less than three times per week, p = 0.0035), and subjective perceptions of having common or deep knowledge about COVID-19 (average 51.5 and 49.1 versus 57.4 for none knowledge, p = 0.045 and 0.010, respectively). Patients who perceived themselves as having moderate or severe symptoms got higher scores than those who thought they had just mild symptoms in PSQI (average 9.7 and 11.0 versus 8.0, p = 0.088 and 0.017, respectively) assessment. Thinking that medical staffs’ attitude were good predicted lower SAS scores (average 43.8 versus 51.6 for normal or awful attitudes, p = 0.012) and SDS scores (51.1 versus 62.8 for normal or awful attitudes, p < 0.001). There were no significant differences in the score of any of the three scales between patients of different hospitalization duration or different clinical types.
Table 3.
Univariate analyses of participants’ SAS, SDS, and PSQI scores.
| SAS scorea | p value | SDS scorea | p value | PSQI scorea | p value | |
|---|---|---|---|---|---|---|
| Gender | 0.013b | <0.001b | 0.55d | |||
| Male | 42.9 (7.9) | 49.1 (10.4) | 9.5 (4.8) | |||
| Female | 45.8 (8.7) | 54.6 (10.8) | 9.1 (4.5) | |||
| Age, years | 0.35c | 0.025c | 0.0019e | |||
| ≤41 | 42.9 (8.1) | 50 (12.1) | 7.6 (4.2) | |||
| 42–51 | 44.4 (9.2) | 50.1 (10.6) | 9.7 (4.7) | |||
| ≥52 | 45.1 (7.8) | 54.3 (9.8) | 10.4 (4.6) | |||
| Marital status | 0.56b | 0.16b | 0.76d | |||
| Married | 44.3 (8.3) | 52 (10.7) | 9.3 (4.6) | |||
| Single or divorced | 43.6 (9.8) | 48 (12.6) | 9.7 (5.5) | |||
| Education level | 0.18b | <0.001b | 0.38d | |||
| Primary or secondary | 44.8 (7.4) | 54.8 (9.8) | 9.6 (4.8) | |||
| Tertiary or higher | 43.7 (9.2) | 48.9 (11.2) | 9.1 (4.5) | |||
| History of chronic disease | 1b | 0.95b | 0.05d | |||
| No | 44 (8.3) | 51.6 (11.3) | 8.8 (4.6) | |||
| Yes | 44.5 (8.5) | 51.8 (10.5) | 10.1 (4.6) | |||
| Hospitalization duration, days | 0.39c | 0.19c | 0.27e | |||
| ≤9 | 45 (9.1) | 53.5 (10.5) | 9.8 (5.3) | |||
| 10–13 | 42.6 (7.5) | 49.4 (10.3) | 8.4 (4.5) | |||
| ≥14 | 44.6 (8.4) | 51.9 (11.3) | 9.6 (4.3) | |||
| Clinical type | 0.61b | 0.8b | 0.16d | |||
| Mild or ordinary | 43.8 (8.2) | 51.8 (11) | 9 (4.5) | |||
| Severe or critically ill | 44.9 (8.7) | 51.5 (10.8) | 9.9 (4.9) | |||
| Family member diagnosed with COVID-19 | 0.61b | 0.42b | 0.6d | |||
| No | 44.2 (7.3) | 52.2 (10.7) | 9.2 (4.6) | |||
| Yes | 44.2 (9.6) | 51.1 (11.2) | 9.5 (4.7) | |||
| Frequency of contacting with family | 0.1b | 0.0035b | 0.53d | |||
| 0–2/wk | 46.4 (6.8) | 57.9 (6.8) | 9.9 (5.2) | |||
| ≥3/wk | 43.9 (8.5) | 50.9 (11.1) | 9.3 (4.6) | |||
| Subjective knowledge level of COVID-19 | 0.094c | 0.0096c | 0.066e | |||
| None | 48.5 (10) | 57.4 (9.3) | 11.4 (5.8) | |||
| Common knowledge | 43.7 (7.8) | 51.5 (10.9) | 9 (4.3) | |||
| Deep knowledge | 43.7 (9) | 49.1 (10.8) | 9.3 (4.8) | |||
| Subjective evaluation of disease symptoms | 0.028c | 0.19c | 0.0064e | |||
| Mild | 41.8 (7.9) | 49.7 (12.4) | 8 (4.7) | |||
| Moderate | 45.1 (8.2) | 52.1 (9.4) | 9.7 (4.3) | |||
| Severe | 47.9 (9.6) | 53.6 (10.4) | 11 (4.6) | |||
| Not clear | 44.6 (6.6) | 55.9 (11.1) | 11 (4.8) | |||
| Evaluation of medical staffs' attitude | 0.012b | <0.001b | 0.46d | |||
| Good | 43.8 (8.1) | 51.1 (10.8) | 9.3 (4.7) | |||
| Normal or awful | 51.6 (10.3) | 62.8 (5.3) | 10.4 (4.4) |
aData are mean (standard deviation).
bp values comparing different groups are generated from Wilcoxon rank-sum test.
cp values comparing different groups are generated from Kruskal-Wallis equality-of-populations rank test.
dp values comparing different groups are generated from two-sample t-test.
ep values comparing different groups are generated from, one-way analysis of variance (ANOVA).
Table 4, Table 5, and Table 6 demonstrate the multivariate regression models developed for patients' SAS, SDS, and PSQI scores, respectively. All the three variables that showed association with patients' SAS scores in univariate analyses retained their significance in both regression models, including gender, subjective perception of disease severity, and personal evaluation of medical staffs' attitudes (Table 4). In models developed for SDS assessment, gender, education level, frequency of contact with family, and perception of medical staffs’ attitudes retained significant associations with the scores (Table 5). Age was not significantly associated with SDS scores anymore when other factors were adjusted. Notably, in both models, subjective cognition of the severity of their symptoms was associated with SDS scores with a positive coefficient. Age and subjective evaluation of their symptoms, which showed significant associations with PSQI scores in univariate analyses, retained their significance in both models (Table 6).
Table 4.
Multiple regression models for participants’ SAS scores.
| Modela |
Model 2b |
|||||
|---|---|---|---|---|---|---|
| Coefficientc | t value | P value | Coefficientc | t value | P value | |
| Gender | 3.09 (0.86–5.31) | 2.73 | 0.007 | 3.77 (1.28–6.27) | 2.98 | 0.003 |
| Age, years | 0.6 (−1.01–2.22) | 0.74 | 0.46 | |||
| Marital status | 0.02 (−4.31–4.35) | 0.01 | 0.99 | |||
| Education level | 0.04 (−2.42–2.49) | 0.03 | 0.98 | |||
| History of chronic disease | 0.96 (−1.59–3.51) | 0.74 | 0.46 | |||
| Hospitalization duration, days | 0.07 (−1.38–1.53) | 0.1 | 0.92 | |||
| Clinical type | 0.69 (−1.77–3.14) | 0.55 | 0.58 | |||
| Family member diagnosed with COVID-19 | −0.05 (−2.42–2.31) | −0.04 | 0.96 | |||
| Frequency of contacting with family | −2.34 (−5.98–1.3) | −1.27 | 0.21 | |||
| Subjective knowledge level of COVID-19 | −1.42 (−3.55–0.71) | −1.32 | 0.19 | |||
| Subjective evaluation of disease symptoms | 1.82 (0.55–3.09) | 2.83 | 0.005 | 1.58 (0.24–2.92) | 2.32 | 0.021 |
| Evaluation of medical staffs' attitude | 6.94 (1.8–12.08) | 2.66 | 0.008 | 6.34 (1.12–11.57) | 2.39 | 0.018 |
| Constant | 28.92 (22.25–35.6) | 8.54 | <0.001 | 32.09 (16.42–47.76) | 4.04 | <0.001 |
Model 1 is a stepwise linear regression model. Significance level for entering the model was 0.05. F = 7.85, adjusted R2 = 0.1063.
Model 2 is a linear regression model including all factors investigated in the study. F = 2.47, adjusted R2 = 0.1357.
Data are regression coefficient (95% confidence interval).
Table 5.
Multiple regression models for participants’ SDS scores.
| Model 1a |
Model 2b |
|||||
|---|---|---|---|---|---|---|
| Coefficientc | t value | p value | Coefficientc | t value | p value | |
| Gender | 5.4 (2.71–8.1) | 3.95 | <0.001 | 6.1 (3.1–9.1) | 4.01 | <0.001 |
| Age, years | 1.49 (−0.45–3.44) | 1.52 | 0.13 | |||
| Marital status | 1.26 (−3.95–6.46) | 0.48 | 0.63 | |||
| Education level | −5.16 (−7.88~-2.44) | −3.74 | <0.001 | −3.77 (−6.72~-0.82) | −2.52 | 0.012 |
| History of chronic disease | 0.79 (−2.28–3.85) | 0.51 | 0.61 | |||
| Hospitalization duration, days | −0.52 (−2.26–1.22) | −0.59 | 0.56 | |||
| Clinical type | −0.61 (−3.55–2.34) | −0.41 | 0.68 | |||
| Family member diagnosed with COVID-19 | −0.24 (−3.08–2.6) | −0.17 | 0.87 | |||
| Frequency of contacting with family | −5.75 (−10.03~-1.46) | −2.65 | 0.009 | −5.6 (−9.98~-1.23) | −2.53 | 0.012 |
| Subjective knowledge level of COVID-19 | −2.17 (−4.73–0.38) | −1.68 | 0.095 | |||
| Subjective evaluation of disease symptoms | 2.09 (0.55–3.62) | 2.68 | 0.008 | 1.75 (0.14–3.36) | 2.14 | 0.033 |
| Evaluation of medical staffs' attitude | 10.55 (4.33–16.77) | 3.35 | 0.001 | 9.7 (3.42–15.98) | 3.05 | 0.003 |
| Constant | 47.41 (35.7–59.13) | 7.98 | <0.001 | 44.16 (25.33–62.99) | 4.63 | <0.001 |
aModel 1 is a stepwise linear regression model. Significance level for entering the model was 0.05. F = 12.10, adjusted R2 = 0.2163.
bModel 2 is a linear regression model including all factors investigated in the study. F = 5.63, adjusted R2 = 0.2164.
cData are regression coefficient (95% confidence interval).
Table 6.
Multiple regression models for participants’ PSQI scores.
| Model 1a |
Model 2b |
|||||
|---|---|---|---|---|---|---|
| Coefficientc | t value | p value | Coefficientc | t value | p value | |
| Gender | 0.29 (−1.11–1.7) | 0.41 | 0.68 | |||
| Age, years | 1.08 (0.3–1.86) | 2.72 | 0.007 | 1.03 (0.13–1.94) | 2.25 | 0.026 |
| Marital status | −1.19 (−3.62–1.24) | −0.96 | 0.34 | |||
| Education level | 0.05 (−1.33–1.43) | 0.07 | 0.94 | |||
| History of chronic disease | 0.7 (−0.74–2.13) | 0.96 | 0.34 | |||
| Hospitalization duration, days | −0.35 (−1.17–0.46) | −0.85 | 0.4 | |||
| Clinical type | 0.1 (−1.28–1.47) | 0.14 | 0.89 | |||
| Family member diagnosed with COVID-19 | 0.16 (−1.17–1.48) | 0.23 | 0.82 | |||
| Frequency of contacting with family | −0.85 (−2.89–1.2) | −0.82 | 0.42 | |||
| Subjective knowledge level of COVID-19 | −0.52 (−1.72–0.67) | −0.86 | 0.39 | |||
| Subjective evaluation of disease symptoms | 0.98 (0.25–1.7) | 2.66 | 0.009 | 0.98 (0.23–1.73) | 2.56 | 0.011 |
| Evaluation of medical staffs' attitude | 0.06 (−2.87–3) | 0.04 | 0.97 | |||
| Constant | 5.23 (3.27–7.18) | 5.27 | <0.001 | 9.49 (0.69–18.29) | 2.13 | 0.035 |
aModel 1 is a stepwise linear regression model. Significance level for entering the model was 0.05. F = 9.67, adjusted R2 = 0.0794.
bModel 2 is a linear regression model including all factors investigated in the study. F = 1.88, adjusted R2 = 0.0501.
cData are regression coefficient (95% confidence interval).
4. Discussion
This is a descriptive study on the mental health status and sleep quality of COVID-19 patients who were hospitalized in different pandemic stages in a single center in Wuhan, the epicenter of the outbreak in China. Overall, 109 participants were recruited on Feb 17th and 93 participants on Mar 14th. On Feb 17th, the number of existing cases in Hubei province was 50,338 (Chinese Center for Disease Control and Prevention, 2020), a peak number of daily existing cases. By then, around 15.9% were severe or critically ill cases(Pan et al., 2020). To tackle the problem of the rising cases, China started to build several Fangcang shelter hospitals in February, for isolating and treating mild to moderate COVID-19 patients(Chen et al., 2020). These shelter hospitals accommodated around 12 thousand COVID-19 patients in total, and thus greatly lightened the burden of major hospitals in Wuhan. All the efforts paid off when a decline in the number of existing cases was observed. In mid-March, the number of existing cases in Hubei province had fallen to around 10 thousand(Chinese Center for Disease Control and Prevention, 2020). By then the overall proportion of severe or critically ill cases was around 10.3%(Pan et al., 2020). At the beginning of March, Wuhan shut down the Fangcang shelter hospitals one after another, transferring the remaining patients there to higher-level designated hospitals(China News, 2020). The change in patient population might make it improper to combine the two samples without addressing the effect of time. In this research, we found that the distribution of these two patient groups was different concerning education level, hospitalization duration, and clinical type. However, these variables were not associated with patients' mental health status, except that education level was associated with SDS scores. Therefore, we combined the two groups together, to address factors associated with patients’ mental health status in a greater sample.
Depression was a more common psychological problem seen in our participants as compared with anxiety. About half of the patients exhibited a mild level of depressive mood. Extra attention should be paid to female or elderly patients. Our study showed that gender might be an independent predictor for anxiety and depression status. Previous studies showed that females had a 1.95 odds ratio over males in terms of depression(Salk et al., 2017). This association of gender and psychological problems was also seen during emergent situations like disease outbreak and social adverse events(Brooks et al., 2020; Lin et al., 2007; Rubin et al., 2005; Taylor et al., 2008). Elderly people might already suffer from depression or other mental problems because of their vulnerability to mood disorders, comorbid diseases, early life adversities, etc.(Almeida, 2014; Thomas et al., 2017) In clinical practice, elderly patients require more attention since they always come with other factors that might affect their mental health. Understanding of the disease may also play a role in patients’ mental distress. Our results showed that those who had lower education levels or those who thought they did not have any knowledge about the disease were more likely to get higher scores in depression assessment. More contact with family members was associated with lower depression scores, as discovered in our study. Although visits to the wards were not permitted during the COVID-19 pandemic, contacts through social media might be comforting for the patients. Patients, who subjectively thought they had only mild symptoms or those who thought medical staffs were treating them well, generally got lower scores in mental distress assessments, which suggested better mental health status. However, these factors might either be the reasons for better mental health, or the results of it.
Previous studies showed that people in pandemic diseases might have sleep disorders(Ji et al., 2017; Lin et al., 2007; Mohammed et al., 2015). The environmental stressors, physical illness, separation from family and friends, and other comorbid psychological problems might contribute to this issue. Sleep problems were also notable in our participants. In our study, elderly people and those who had comorbid chronic disease(s) were more likely to have sleep problems. Patients who thought they had moderate or severe symptoms were more likely to suffer from sleep problems than those who thought they had mild ones.
It is worth noting that, it was patients' subjective perception of the severity of their disease, rather than the objective clinical classification, that was significantly associated with their mental distress and sleep quality. In clinical practice, patients' self-assessments of their symptoms and mental status might also provide information for health care workers. Those who assess themselves as being more severely sick might require more attention from the medical staffs to prevent mental illness. Patients' clinical type, as defined by the diagnosis guideline for COVID-19, showed no significant association with their SAS, SDS, or PSQI scores. This fact alerted that even patients classified as mild or ordinary type may suffer from similar mental distress and sleep problems as those classified as severe or critically ill type. Thus, maintaining patients' psychological well-being is equally important. Another finding of our study was that, although the proportion of severe or critically ill cases dropped greatly in the Mar group, there was no significant difference in the proportion of those who subjectively thought they had mild, moderate, or severe symptoms between two patient groups. This showed that there must be great mismatches between patients’ perception of their disease and their actual clinical type. Learning from this, it might be better if more attention could be attached to patient education to help them build positive perceptions of their illness.
We observed no significant differences in anxiety or sleep quality assessments between two patient groups. However, patients recruited on Mar 14th had higher average depression scores than those recruited on Feb 17th did. As stated before, the overall condition of the COVID-19 outbreak had changed greatly during this period. Late February and early March witnessed a drop in the number of newly confirmed cases and a rise in the recovery rate of the disease(Chinese Center for Disease Control and Prevention, 2020). Clinical trials of promising treatments and preventions had also been started. These were good news that may encourage COVID-19 patients. But in the meanwhile, patients recruited in March also experienced longer quarantine. It is likely that the negative effect of longer quarantine overweighs the positive effect of encouraging news on patients’ mental health. If the correlation between longer quarantine and higher depression level of the patients holds in larger descriptive studies or follow-up studies, governments might have to consider this adverse effect when prolonging quarantine.
A search on PubMed revealed thousands of studies with the keywords COVID-19 and mental health. However, most of the studies focus on the mental health status of either the general population (Qi et al., 2020; Skapinakis et al., 2020), certain population (Hoffman and Miller, 2020; Matsushima and Horiguchi, 2020), or the front-line workers (Nowicki et al., 2020; Sasaki et al., 2020). This study provides direct information of the mental health status of COVID-19 patients in a single center in Wuhan, and could serve as primary evidence for further studies addressing this issue.
There are some limitations in our study. First of all, the study was based on a self-reported questionnaire, which leads to information bias. The small sample size is another limitation of this research. Since we could only recruit patients who could cooperate with us and finish the questionnaire, there might also be selection bias during participant recruitment. The response rate in this study was relatively low, at around 30% in both groups. This was partly due to the difficulty of reaching the patients during the pandemic. Though widely accepted scales were used to assess patients' mental distress and sleep quality, these scales are all tools for screening rather than diagnosing mental diseases. Our study was a cross-sectional one. To find out more information about patients’ mental distress and sleep quality changes during and after the pandemic, future studies may look into the follow-up of patients.
To sum up, we reported a cross-sectional analysis of the mental distress and sleep quality of patients recruited in two different stages of the disease outbreak in Wuhan. Our findings provide clues for the psychological problems that COVID-19 patients might suffer from and identified some predictors that were associated with them.
SAS= Self-Rating Anxiety Scale. SDS= Self-Rating Depression Scale. PSQI= Pittsburgh Sleep Quality Index. COVID-19 = corona virus disease 2019.
CRediT authorship contribution statement
ZiYi Jiang: Formal analysis. PeiPei Zhu: Conceptualization. LiYuan Wang: Investigation, Resources. Ying Hu: Investigation, Resources. MingFan Pang: Formal analysis. ShunShing Ma: Conceptualization, Investigation, Resources, Writing - review & editing. Xin Tang: Writing - review & editing.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NNSFC grant 81470100 to X.T.) in data collection and patient recruitment.
References
- Almeida O.P. Prevention of depression in older age. Maturitas. 2014;79:136–141. doi: 10.1016/j.maturitas.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Brooks S.K., Webster R.K., Smith L.E., Woodland L., Wessely S., Neil Greenberg Fm, James Rubin FrcpG., Wessely FMedSci S., Greenberg FRCPsych N., James Rubin G. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;6736 doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatr. Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chen S., Zhang Z., Yang J., Wang J., Zhai X., Bärnighausen T., Wang C. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395 doi: 10.1016/s0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China News . 2020. All Fangcang Shelter Hospitals Are Suspended: Achieving Zero Cross-Infection and Zero Death; Bolstering Wuhan's Fight against COVID-19.http://www.chinanews.com/gn/2020/03-10/9120429.shtml (accessed 4.18.20) [Google Scholar]
- Chinese Center for Disease Control and Prevention . Chinese Cent. Dis. Control Prev. 2020. Coronavirus disease 2019 (COVID-19) situation of Hubei province [WWW document]http://2019ncov.chinacdc.cn/2019-nCoV/ (accessed 4.14.20) [Google Scholar]
- Hoffman J.A., Miller E.A. Addressing the consequences of school closure due to COVID‐19 on children's physical and mental well‐being. World Med. Heal. Policy wmh3. 2020;365 doi: 10.1002/wmh3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D., Ji Y.J., Duan X.Z., Li W.G., Sun Z.Q., Song X.A., Meng Y.H., Tang H.M., Chu F., Niu X.X., Chen G.F., Li J., Duan H.J. Prevalence of psychological symptoms among Ebola survivors and healthcare workers during the 2014-2015 Ebola outbreak in Sierra Leone: a cross-sectional study. Oncotarget. 2017;8:12784–12791. doi: 10.18632/oncotarget.14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-Y., Peng Y.-C., Wu Y.-H., Chang J., Chan C.-H., Yang D.-Y. The psychological effect of severe acute respiratory syndrome on emergency department staff. Emerg. Med. J. 2007;24:12–17. doi: 10.1136/emj.2006.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Kakade M., Fuller C.J., Fan B., Fang Y., Kong J., Guan Z., Wu P. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr. Psychiatr. 2012;53:15–23. doi: 10.1016/j.comppsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima M., Horiguchi H. The COVID-19 pandemic and mental well-being of pregnant women in Japan: need for Economic and Social Policy interventions. Disaster Med. Public Health Prep. 2020:1–11. doi: 10.1017/dmp.2020.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A., Sheikh T.L., Gidado S., Poggensee G., Nguku P., Olayinka A., Ohuabunwo C., Waziri N., Shuaib F., Adeyemi J., Uzoma O., Ahmed A., Doherty F., Nyanti S.B., Nzuki C.K., Nasidi A., Oyemakinde A., Oguntimehin O., Abdus-Salam I.A., Obiako R.O. An evaluation of psychological distress and social support of survivors and contacts of Ebola virus disease infection and their relatives in Lagos, Nigeria: a cross sectional study - 2014. BMC Publ. Health. 2015;15:1–8. doi: 10.1186/s12889-015-2167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of China . fifth ed. 2020. Diagnosis and Treatment Guideline for COVID-19.http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml (accessed 4.12.20) [Google Scholar]
- Nowicki G.J., Ślusarska B., Tucholska K., Naylor K., Chrzan-Rodak A., Niedorys B. The severity of traumatic stress associated with COVID-19 pandemic, perception of support, sense of security, and sense of meaning in life among nurses: research protocol and preliminary results from Poland. Int. J. Environ. Res. Publ. Health. 2020;17:6491. doi: 10.3390/ijerph17186491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q., Huang J., He N., Yu H., Lin X., Wei S., Wu T. Association of public health interventions with the epidemiology of the COVID-19 outbreak in wuhan, China. J. Am. Med. Assoc. 2020;2115 doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M., Li P., Moyle W., Weeks B., Jones C. Physical activity, health-related quality of life, and stress among the Chinese adult population during the COVID-19 pandemic. Int. J. Environ. Res. Publ. Health. 2020;17:6494. doi: 10.3390/ijerph17186494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G.J., Brewin C.R., Greenberg N., Simpson J., Wessely S. Psychological and behavioural reactions to the bombings in London on 7 July 2005: cross sectional survey of a representative sample of Londoners. Br. Med. J. 2005;331:606–611. doi: 10.1136/bmj.38583.728484.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk R.H., Hyde J.S., Abramson L.Y. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol. Bull. 2017;143:783–822. doi: 10.1037/bul0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N., Kuroda R., Tsuno K., Kawakami N. The deterioration of mental health among healthcare workers during the COVID-19 outbreak: a population-based cohort study of workers in Japan. Scand. J. Work. Environ. Health. 2020 doi: 10.5271/sjweh.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapinakis P., Bellos S., Oikonomou A., Dimitriadis G., Gkikas P., Perdikari E., Mavreas V. Depression and its relationship with coping strategies and illness perceptions during the COVID-19 lockdown in Greece: a cross-sectional survey of the population. Depress. Res. Treat. 2020:1–11. doi: 10.1155/2020/3158954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.R., Agho K.E., Stevens G.J., Raphael B. Factors influencing psychological distress during a disease epidemic: data from Australia's first outbreak of equine influenza. BMC Publ. Health. 2008;8 doi: 10.1186/1471-2458-8-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K.M., Redd L.A., Wright J.D., Hartos J.L. Sleep and mental health in the general population of elderly women. J. Prim. Prev. 2017;38:495–503. doi: 10.1007/s10935-017-0484-5. [DOI] [PubMed] [Google Scholar]
- William W.K. Encyclopedia of Quality of Life and Well-Being Research. Springer Netherlands; Dordrecht: 2014. Zung self-rating depression scale. [DOI] [Google Scholar]
- World Health Organization . 2020. Naming the Coronavirus Disease (COVID-19) and the Virus.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed 4.12.20) [Google Scholar]
- World Health Organization . 2020. Rolling Updates on Coronavirus Disease.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed 4.12.20) [Google Scholar]
- World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19)https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed 4.14.20) [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J., Pei Y., Yuan M., Zhang Y.-L., Dai F., Liu Y., Wang Q., Zheng J., Xu L., Holmes E.C., Zhang Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020 doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Fang Y., Guan Z., Fan B., Kong J., Yao Z., Liu X., Fuller C.J., Susser E., Lu J., Hoven C.W. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can. J. Psychiatr. 2009;54:302–311. doi: 10.1177/070674370905400504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung W.W.K. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]