Abstract
Chemokines play important roles in homeostasis and inflammatory processes. While their roles in leukocyte recruitment are well-appreciated, chemokines play additional roles in the body, including mediating or regulating angiogenesis, tumor metastasis and wound healing. In this opinion article, we focus on the role of CXCR3 and its ligands in fibrotic processes. We emphasize differences of the effects of each ligand, CXCL9, CXCL10 and CXCL11, on fibroblasts in different tissues of the body. We include discussions of differences in signaling pathways that may account for protective or pro-fibrotic effects of each ligand in different experimental models and ex vivo analysis of human tissues. Our goal is to highlight potential reasons why there are disparate findings in different models, and to suggest ways in which this chemokine axis could be manipulated for the treatment of fibrosis.
Keywords: CXCR3, CXCL9, CXCL10, CXCL11, fibrosis, fibroblast, pericyte, endothelial cell
Introduction: understanding CXCR3’s typical and atypical functions
Chemokine receptors are a subgroup of class A G-protein coupled receptors (GPCRs) that are relatively conserved across eukaryotes 1. They bind to chemokine ligands, a special class of 8–10kDa chemotactic cytokines, which are classified based on their amino acid structure (e.g. CC, CXC, or CX3C) 2. With a few exceptions, most ligand-receptor relationships are promiscuous, meaning that a single chemokine receptor has multiple ligands and a single chemokine can bind to multiple receptors. As of now, there are 18 known chemokine receptors with Gαi-dependent chemotactic activity, and 5 atypical (non-chemotactic, recycling or scavenging) chemokine receptors in humans. Many chemokines are considered inflammatory, as they recruit leukocytes during inflammatory responses. However, there are also homeostatic chemokines that are important for immune cell maturation, tissue development, and angiogenesis. Homeostatic chemokines often exhibit tissue tropism, providing signals for recirculating immune cells, paracrine signals for cells that comprise tissues, and even tumor growth and metastasis 3.
CXCR3 is typically considered to be an inflammatory chemokine receptor because it is expressed by leukocytes that migrate towards interferon-induced ligands to sites of tissue inflammation 4. However, CXCR3 is also expressed on non-hematopoietic cells including endothelial cells, where it plays roles in promoting or inhibiting angiogenesis, and fibroblasts, in which it mediates wound healing responses.
There are several examples of diseases where inflammation precedes or is admixed with fibrosis, including infectious diseases (e.g. schistosomiasis, tuberculosis), cancers (e.g. pancreatic cancer, post-irradiation breast cancer) and autoimmune diseases (e.g. hepatitis, pulmonary fibrosis in scleroderma and skin fibrosis in morphea). Hallmarks of inflammatory fibrosis include infiltration of leukocytes; activation of endothelium; fibroblast activation, migration, proliferation and differentiation; production of collagen and other extracellular matrix proteins; and increased collagen bundle thickness and disorganization 5. Data from our lab and others have demonstrated that the CXCR3 chemokine axis can mediate protective or pro-fibrotic signals depending upon the context of the involved organs. In this opinion article, we will discuss potential reasons for disparate findings, and provide our opinions about how this system can be targeted therapeutically for the treatment of fibrosis.
CXCR3 signaling pathways in leukocytes
CXCR3 has four extracellular domains that bind its ligands (CXCL9, CXCL10, and CXCL11), and four intracellular domains that mediate the receptor’s different functions. The differential involvement of CXCR3 receptor domains in ligand binding and subsequent differences in downstream signaling contribute to the complex nature of this chemokine system, which has been mapped out in leukocytes using mutational constructs and competition binding assays. Like many other GPCRs, CXCR3-mediated chemotaxis is pertussis-toxin sensitive. However, CXCR3 activates several other pathways in addition to Gα subunit proteins, which we will review below.
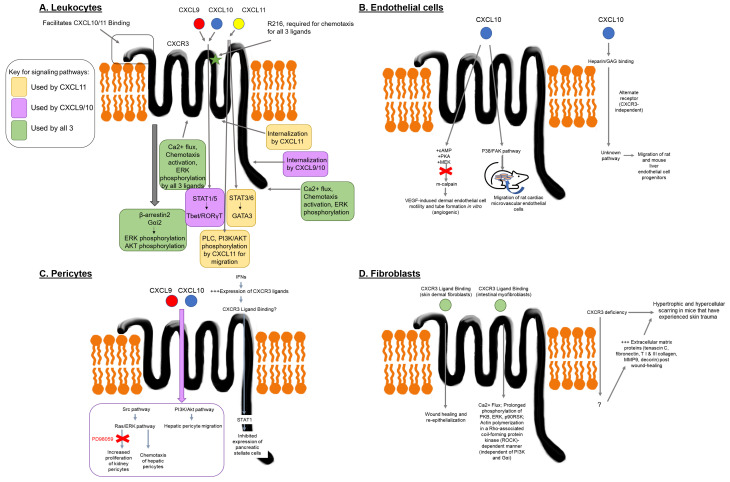
CXCR3 binding and activation requires ligand interactions with at least one sulfated tyrosine in the N terminus and an interaction with amino acid residue R216 in the second extracellular loop 6. The proximal 16 amino acid residues of the N terminus are required for CXCL10 and CXCL11 binding and activation, but not CXCL9 activation. R216 in the second extracellular domain plays no role in CXCL10 or CXCL11 binding or ligand-mediated internalization, but this residue is necessary to activate chemotaxis by all three CXCR3 ligands 7. Both the DRY site, which encompasses the R216, and the CXCR3 carboxyl terminus are essential for CXCL9-, CXCL10-, and CXCL11-induced chemotaxis, calcium mobilization, and Erk phosphorylation ( Figure 1A).
Figure 1. CXCR3 signaling pathways in different cell types.
( A) Major CXCR3 signaling pathways in leukocytes. CXCL9, CXCL10 or CXC11 bind to CXCR3 to mediate chemotaxis and T cell skewing. Different domains facilitate ligand binding, with the N terminus of CXCR3 facilitating binding of CXCL10 and CXC11. R216 in the second extracellular loop (green star) is required for chemotactic responses for all 3 ligands. All three ligands can induce calcium flux, pERK, and pAKT, though CXCL9/10 require Gαi2 for pERK and β-arrestin2 for pAkt. CXCL11 can activate PLC and PI3K/AKT to mediate migration independent of Gαi. Internalization of CXCR3 induced by CXCL9/10 requires the C terminus, whereas CXC11 requires the 3rd intracellular loop. CXCL9/10 can activate STAT1/5 to enforce Tbet/RORγT expression, whereas CXCL11 activates STAT3/6 to enforce GATA3 expression. ( B) CXCR3-dependent and independent signaling pathways in endothelial cells. CXCL10 activates cAMP, PKA and MEK in dermal endothelial cells to inhibit m-calpain and dampen angiogenesis. In cardiac microvascular endothelial cells, CXCL10 activates the p38/FAK pathway to induce migration, but not proliferation. CXCL10 also exerts effects on endothelial cells independently of CXCR3, but in a manner that requires GAG binding. ( C) CXCR3 signaling in pericytes. Pericytes activate Src, Ras/ERK and PI3K/AKT pathways downstream of CXCR3, which mediate chemotactic responses. Kidney pericytes exhibit increased proliferation downstream of CXCL9/10, which is ERK-dependent (inhibited by PD98059). Interferons inhibit proliferation of pancreatic stellate cells via STAT1, though it is unclear whether this response is via CXCR3 ligands. ( D) Fibroblast responses to CXCR3 ligands. Intestinal myofibroblasts exhibit calcium flux and phosphorylation of PKB, ERK, p90RSK induced by all three ligands. All three ligands induce actin polymerization in a Rho-associated coiled coil-forming protein kinase (ROCK)-dependent manner that is independent of PI3K and Gαi. Signaling in skin dermal fibroblasts has not been fully mapped, though CXCR3 deficiency leads to hypertrophic and hypercellular scarring in mice via increased extracellular matrix proteins, including tenascin C, fibronectin, type I & III collagen, MMP9 and decorin. Color key: CXCL9 = red; CXCL10 = blue; CXCL11 = yellow; CXCL9/10 = purple; CXCL9/10/11 = green.
CXCR3 ligands selectively activate different receptor internalization pathways via β-arrestin and Gαi family members. Differences in signaling pathway activation by each ligand is called biased agonism 8, 9. CXCL10-induced receptor internalization relies on the CXCR3 carboxyl terminus, dynamin and β-arrestin1 10. CXCL11 is the most potent inducer of CXCR3 internalization 11, and dominant negative dynamin and β arrestin 1 are unable to impede internalization 10. The third intracytoplasmic loop is required for maximal CXCL11-induced internalization ( Figure 1A).
In T cells, Gαi2 is required for mediating CXCR3 ligand signaling, whereas Gαi3 limits activation of this signaling pathway 12. Western blotting of peripheral blood leukocytes stimulated with CXCR3 ligands demonstrated that CXCL11 and, to a lesser extent, CXCL9/10 induce dose- and time-dependent phosphorylation of p44/42 MAPK (ERK) and Akt that is prevented by pertussis toxin treatment (inhibition of Gα subunit binding) 13. However, inhibition of MEK/ERK (U0126 or PD98059) does not prevent CXCL11-mediated chemotaxis, whereas PLC inhibition (U73122), PI3K (wortmannin) or, to a lesser extent, AKT inhibition (LY294002) does abrogate or reduce human T cell migration, respectively. Akt activation in human T cells was recently reported to be dependent upon β-arrestin2 14.
There is increasing evidence that chemokine receptors can mediate JAK/STAT signaling, which has typically been attributed to common gamma chain cytokine signaling 15. While JAK activation downstream of CXCR3 has not yet been studied, STAT activation in response to incubation with CXCL9/10/11 has been assessed in T cell cultures: addition of recombinant CXCL9/10 activates STAT1/STAT5 to promote Th1 and/or Th17 differentiation via Tbet/RORγT expression, whereas CXCL11 activates STAT3/STAT6 via GATA3 expression to augment regulatory function 16.
CXCR3 signaling pathways in vascular endothelial cells, smooth muscle cells, pericytes and fibroblasts
CXCR3 is also expressed by some non-hematopoietic cells, including endothelial cells, smooth muscle cells and fibroblast subsets. In endothelial cells, CXCR3 ligands mediate pro- or anti-angiogenic signals depending upon the model and tissue of origin. CXCL10 inhibits VEGF-induced dermal endothelial cell motility and tube formation in vitro via cAMP, PKA and MEK inhibition of m-calpain; this likely serves as a way to inhibit angiogenesis late in the wound healing process 17. CXCL10 exhibits angiostatic properties in non-small cell lung cancer and idiopathic pulmonary fibrosis 18, 19. CXCL10 is also able to induce angiostatic effects by binding glycosaminoglycans (GAGs) independently of CXCR3 20. However, CXCL10 induces migration, but not proliferation, of rat cardiac microvascular endothelial cells via the p38/FAK pathway 21 and rat and mouse liver endothelial cell progenitors through an unknown pathway 22 ( Figure 1B).
Pericytes are contractile cells on capillaries and post-capillary venules in tissues that play integral roles in tissue healing and remodeling 23. Examples include hepatic stellate cells in the liver, glomerular mesangial cells in the kidney and pancreatic stellate cells. Pericytes express CXCR3, and activate the Src, Ras/ERK, and PI3K/Akt pathways downstream of CXCR3 ligand binding 24. Inhibitor studies indicate that Src and subsequent Ras/ERK activation are required for chemotaxis of hepatic pericytes, which is in direct contrast to observations in T cells. PI3K/Akt also plays an important role in hepatic pericyte migration as evidenced by abrogation of CXCL10 migration in the presence of wortmannin or LY294002. In addition to chemotaxis, kidney pericytes exhibit increased proliferation downstream of CXCL9/10, which is ERK-dependent (inhibited by PD98059). Unlike hepatic pericytes, kidney pericytes exhibit a second wave of ERK phosphorylation following incubation with CXCL10. CXCR3 signaling in pancreatic stellate cells has not been as extensively mapped, but they respond to PDGF by activating Src-JAK2-STAT3 25. Interferons (IFNs), which drive expression of CXCR3 ligands, inhibit proliferation of pancreatic stellate cells via STAT1 26 ( Figure 1C).
CXCR3 plays a homeostatic role in wound healing and re-epithelialization responses by fibroblasts 27– 29. CXCR3 deficiency leads to hypertrophic and hypercellular scarring in mice that have experienced skin trauma 30, 31. While CXCR3-mediated signaling in skin fibroblasts has not been well-characterized, the consequence of loss of signaling during wound healing includes increases in extracellular matrix proteins including tenascin C, fibronectin, type I & III collagen, MMP9 and decorin 180 days post-wounding compared to WT controls 31.
Studies of CXCR3 signaling downstream of CXCL9 and CXCL10 in intestinal myofibroblasts have shown modest differences in signaling, including CXCL9/10-induced calcium flux at 10min versus 8min for CXCL11, and prolonged phosphorylation of PKB, ERK, p90RSK induced by all 3 ligands as compared to shorter phosphorylation time in peripheral blood leukocytes (e.g. 2-20min versus 1-2min) 32. All three ligands induce actin polymerization in a Rho-associated coiled coil-forming protein kinase (ROCK)-dependent manner that is independent of PI3K and Gαi ( Figure 1D). Further detailed signaling pathway analyses for CXCL9/10/11 signaling are warranted in non-hematopoietic cells from different organs.
Post-translational modifications, proteolytic processing and potential alternate receptors for the CXCR3 chemokine axis
Post-translational modifications of the CXCR3 chemokine axis modulates the function and signaling ability of the ligands and their receptor. CXCL10/11 have heparin binding sites that allow it to be presented on endothelium 33. CXCL10 presentation by the endothelium requires oligomerization 34. CXCL10/11 may be citrullinated by peptidylarginine deiminase, which inhibits their ability to induce chemotaxis and calcium flux and reduces their ability to bind heparin 35. CXCR3 itself requires tyrosine sulfation to bind to its ligands and mediate chemotaxis 6.
CXCL9/10/11 are cleaved/truncated by CD26, and CXCL11 is cleaved by CD13 36, 37. The CD26 truncations of CXCR3 ligands retain angiostatic activity while losing CXCR3-mediated signaling 38. The C’ terminus of CXCL9 can inhibit neutrophil migration via competition with CXCL8-mediated binding to heparin, heparan sulfate, and cellular GAGs, which normally facilitate adhesion to vessels and subsequent transmigration 39, 40. CD13 is expressed by endothelial and epithelial cells as well as fibroblasts in angiogenic tissue, but not normal tissue 41. Truncation of just the first two amino acids in CXCL11 by CD13 abrogates Akt and ERK phosphorylation and greatly reduces calcium flux to prevent migration of CXCR3-transfected CHO cells 36. Truncation of the first six amino acids still retains angiostatic activity as assessed by scratch assay of endothelial cell cultures.
There are two other isoforms of CXCR3: CXCR3-B which binds to CXCL4 and mediates angiostatic effects in cultured human endothelial cells 42; and CXCR3-alt which binds CXCL11 43. Of note, C57BL/6 (B6) mice do not express CXCR3-B 20. The roles of CXCR3-B and CXCR3-alt in fibrosis have not been studied. CXCR3 can also crosstalk with CXCR4 and CXCR7 via CXCL11 and CXCL12 44. CXCR4 mediates profibrotic effects in the liver, while CXCR7 mediates more homeostatic regenerative responses 45. CXCL9 can induce heterologous desensitization of CXCR4 to its ligand CXCL12 46. Notably, autoantibodies against CXCR3 and CXCR4 correlate with increased lung and skin disease severity in scleroderma patients, though it is unclear exactly how these impact signaling 47, 48. CXCL11 binds to CXCR7, which is expressed on activated endothelial cells, tumor cell lines and fetal liver cells 49. CXCL11 ligation by CXCR7, which has an affinity of 2-5nM, does not induce calcium flux or migration; rather it promotes survival and adhesion. CXCR7 has been proposed to be a scavenger receptor for CXCL11 50. CXCR7 can attenuate TGFβ signaling in the lung, though the role of CXCL11 in this process has not been studied 51, 52.
Profibrotic roles of CXCR3 ligands
CXCR3 and its ligands are reported to promote fibrosis in certain disease models and organs. An important caveat to bear in mind when assessing B6 mouse models is that CXCL11 is not expressed in this strain due to a null mutation. However, CXCL9 and CXCL10 knockout mice were generated using 129 oocytes and were backcrossed to B6. Therefore, WT B6 mice express CXCL9 and CXCL10, CXCL9-/- mice express CXCL10 and CXCL11, and CXCL10-/- mice express CXCL9 and CXCL11. This means that while CXCL11 cannot be directly assessed in B6 models, insights about its function can be gleaned by comparing CXCL9-/-, CXCL10-/- and WT B6 mice.
The nephrotoxic serum nephritis model of inflammatory kidney disease, which exhibits tubulointerstitial fibrosis, is dependent on CXCR3 and CXCL9, but not CXCL10, as determined by histopathology and loss of renal function 53. CXCR3-/- and CXCL9-/- mice had fewer intrarenal activated T cells and macrophages, as well as fewer IgG glomerular deposits and antigen-specific IgG in serum. These data suggest that CXCR3 and CXCL9 initiate nephritis through cell-mediated events, which ultimately promote tubulointerstitial fibrosis. CXCL10-/- animals developed kidney disease similar to WT controls, indicating that any potential antifibrotic role of CXCL11 in the kidney is potentially nullified by profibrotic effects of CXCL9. Similarly, any potential profibrotic role of CXCL11 in CXCL9-/- mice may be nullified by antifibrotic effects of CXCL10. However, a Balb/c mouse model of unilateral ureteral obstruction-induced renal tubulointerstitial fibrosis was exacerbated by JAK inhibition, and STAT3 played a protective role 54. Several factors may contribute to the disparate findings between these models, namely whether the process is immune-mediated or obstructive nephropathy, which other signals are being disrupted by JAK inhibition, and whether all three CXCR3 ligands are present to balance pro- versus anti-fibrotic signaling ( Table 1).
Table 1. Summary of the effects of each CXCR3 ligand in different organ systems and models of fibrosis.
| Ligand | Organ | Disease | Experimental
model |
Species | Effect on
fibroblasts/ fibrosis? |
Study outcomes | Reference(s) |
|---|---|---|---|---|---|---|---|
| CXCL9 | Heart | Myocardial
infarction |
Spontaneous;
isoproterenol- induced |
Human;
Rat |
Proliferation & migration | Increased fibrosis following MI; cytokines
released by myocardium induced expression of CXCL9 which promoted fibroblast proliferation & migration |
Lin et al., 2019 |
| Rheumatic fever | Spontaneous | Human | no direct effect
shown |
Increased migration of inflammatory
infiltrates specifically to valves and correlated with amount of cardiac fibrosis |
Faé et al., 2013 | ||
| Chagas
cardiomyopathy |
Spontaneous;
infduced |
Human;
Beagle dog |
no direct effect
shown |
Increased migration of inflammatory
infiltrates to the heart; polymorphism CXCL9 rs10336 CC was associated with protection from progression to severe CCC |
Nogueira et al., 2012 | ||
| Kidney | Inflammatory
Kidney Disease w tubulointerstitial fibrosis |
Nephrotoxic
serum nephritis |
Mouse | Pro-fibrotic | Pro-fibrotic: initiates nephritis through cell
mediated events |
Menke et al., 2008 | |
| Liver | Hepatic fibrosis | carbon
tetrachloride- induced |
Mouse | Anti-fibrotic | Angiostatic and antifibrotic via modulation
of stellate cell activation and endothelial cell inhibition. May or may not influence skewing of Th1-polarized, IFN-γ-positive cells in the liver. |
Sahin
et al., 2012,
Wasmuth et al., 2009 |
|
| Liver cirrhosis | Spontaneous | Human | no direct effect
shown |
Low levels correlated with better survival
following transjugular intrahepatic portosystemic shunt |
Berres et al., 2015 | ||
| Hepatitis C
Virus-associated fibrosis |
Spontaneous | Human | dependent upon
genotype |
Alleles/polymorphisms of CXCL9/10/11 are
associated with protection or promotion of fibrosis |
Jiménez-Sousa
et al., 2017,
Pineda-Tenor et al., 2015 |
||
| Lung | ? | not yet studied | |||||
| Pancreas | Chronic
pancreatitis |
Trinitrobenzene
sulfonic acid (TNBS) induced |
Rat | Anti-fibrotic | Attenuates fibrogenesis in vivo; has
antifibrotic effects in vitro |
Shen et al., 2013 | |
| Skin | Morphea | Spontaneous | Human | Pro-fibrotic | Serum levels are correlated with disease
activity |
O'Brien
et al., 2017;
Mertens et al., 2018 |
|
| Multiorgan | Systemic
scleroderma |
Spontaneous | Human | no direct effect
shown |
Increased levels are documented in disease |
Hasegawa
et al., 2011;
Liu et al., 2013; Rabquer et al., 2011 |
|
| CXCL10 | Heart | Chagas
cardiomyopathy |
Spontaneous;
induced |
Human;
Beagle dog |
no direct effect
shown |
Increased migration of inflammatory
infiltrates to the heart; polymorphism CXCL10 rs3921 GG was associated with protection from progression to severe CCC |
Nogueira et al., 2012 |
| Kidney | Inflammatory
Kidney Disease |
Nephrotoxic
serum nephritis |
Mouse | no direct effect
shown |
Not fully understood but seems to be
dispensable for pathology |
Menke et al., 2008 | |
| Liver | Hepatic fibrosis | carbon
tetrachloride- induced |
Mouse | Pro-fibrotic | CXCL10 prevents NK cells from inactivating
hepatic stellate cells |
Hintermann et al., 2010 | |
| Lung | Pulmonary
fibrosis |
Bleomycin
induced |
Mouse | Anti-fibrotic | Limits fibrosis by reducing fibroblast
migration to lung tissue |
Tager
et al., 2004;
Jiang et al., 2010 |
|
| Pancreas | ? | not yet studied | |||||
| Skin | Morphea | Spontaneous | Human | no direct effect
shown |
Serum levels are correlated with disease
activity |
Mertens et al., 2018 | |
| Multiorgan | Systemic
scleroderma |
Spontaneous | Human | no direct effect
shown |
Increased levels are documented in disease |
Hasegawa
et al., 2011;
Liu et al., 2013; Rabquer et al., 2011 |
|
| CXCL11 | Lung | Pulmonary
Fibrosis |
Bleomycin
induced |
Mouse | Anti-fibrotic | Systemic CXCL11 administration reduced
pulmonary collagen deposition, procollagen gene expression, and histopathologic fibroplasia and extracellular matrix deposition in the lung. CXCR3 is not expressed on fibroblasts; CXCL11 had no direct effect on pulmonary fibroblasts. |
Burdick et al., 2005 |
| Systemic
scleroderma |
Spontaneous | Human | no direct effect
shown |
High bronchoalveolar lavage fluid CXCL11
correlates with less risk of developing interstitial lung disease |
Cardarelli et al., 2012 | ||
|
CXCL4
(binds CXCR3-B) |
Liver | Hepatic fibrosis | carbon
tetrachloride- induced |
Mouse | no direct effect
shown |
Angiostatic: Directly interrupts VEGF signaling | Sulpice et al., 2004 |
Table 1 Complete References:
1. Lin C-F, Su C-J, Liu J-H, Chen S-T, Huang H-L, Pan S-L. Potential Effects of CXCL9 and CCL20 on Cardiac Fibrosis in Patients with Myocardial Infarction and Isoproterenol-Treated Rats. J Clin Med Res [Internet]. 2019 May 11;8(5). Available from: http://dx.doi.org/10.3390/jcm8050659
2. Faé KC, Palacios SA, Nogueira LG, Oshiro SE, Demarchi LMF, Bilate AMB, et al. CXCL9/Mig mediates T cells recruitment to valvular tissue lesions of chronic rheumatic heart disease patients. Inflammation [Internet]. 2013 Aug;36(4):800–11. Available from: http://dx.doi.org/10.1007/s10753-013-9606-2
3. Nogueira LG, Santos RHB, Ianni BM, Fiorelli AI, Mairena EC, Benvenuti LA, et al. Myocardial chemokine expression and intensity of myocarditis in Chagas cardiomyopathy are controlled by polymorphisms in CXCL9 and CXCL10. PLoS Negl Trop Dis [Internet]. 2012 Oct 25;6(10):e1867. Available from: http://dx.doi.org/10.1371/journal.pntd.0001867
4. Menke J, Zeller GC, Kikawada E, Means TK, Huang XR, Lan HY, et al. CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol [Internet]. 2008 Jun;19(6):1177–89. Available from: http://dx.doi.org/10.1681/ASN.2007111179
5. Sahin H, Borkham-Kamphorst E, Kuppe C, Zaldivar MM, Grouls C, Al-samman M, et al. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology [Internet]. 2012 May 19;55(5):1610–9. Available from: http://doi.wiley.com/10.1002/hep.25545
6. Pineda-Tenor D, Berenguer J, García-Álvarez M, Guzmán-Fulgencio M, Carrero A, Aldámiz-Echevarria T, et al. Single Nucleotide Polymorphisms of CXCL9-11 Chemokines Are Associated With Liver Fibrosis in HIV/HCV-Coinfected Patients. JAIDS Journal of Acquired Immune Deficiency Syndromes [Internet]. 2015 Apr 1 [cited 2020 Sep 23];68(4):386. Available from: https://journals.lww.com/jaids/fulltext/2015/04010/Single_Nucleotide_Polymorphisms_of_CXCL9_11.3.aspx?casa_token=2kMFCv_y5KcAAAAA:HJXjuc13C4IdQ0jXRz84X8bBYfKwrt3RWyPB1FpyLCOBTq2l4yTRsbYdS8OG9T0O0-hh-nBzVTb-_you33IXjJo
7. Jiménez-Sousa MÁ, Gómez-Moreno AZ, Pineda-Tenor D, Medrano LM, Sánchez-Ruano JJ, Fernández-Rodríguez A, et al. CXCL9-11 polymorphisms are associated with liver fibrosis in patients with chronic hepatitis C: a cross-sectional study. Clin Transl Med [Internet]. 2017 Jul 28;6(1):26. Available from: https://doi.org/10.1186/s40169-017-0156-3
8. Wasmuth HE, Lammert F, Zaldivar MM, Weiskirchen R, Hellerbrand C, Scholten D, et al. Antifibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology [Internet]. 2009 Jul;137(1):309–19, 319.e1–3. Available from: http://dx.doi.org/10.1053/j.gastro.2009.03.053
9. Berres M-L, Asmacher S, Lehmann J, Jansen C, Görtzen J, Klein S, et al. CXCL9 is a prognostic marker in patients with liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. J Hepatol [Internet]. 2015 Feb;62(2):332–9. Available from: http://dx.doi.org/10.1016/j.jhep.2014.09.032
10. Shen J, Gao J, Chen C, Lu H, Hu G, Shen J, et al. Antifibrotic role of chemokine CXCL9 in experimental chronic pancreatitis induced by trinitrobenzene sulfonic acid in rats. Cytokine [Internet]. 2013 Oct;64(1):382–94. Available from: http://dx.doi.org/10.1016/j.cyto.2013.05.012
11. O’Brien JC, Rainwater YB, Malviya N, Cyrus N, Auer-Hackenberg L, Hynan LS, et al. Transcriptional and Cytokine Profiles Identify CXCL9 as a Biomarker of Disease Activity in Morphea. J Invest Dermatol [Internet]. 2017 Aug;137(8):1663–70. Available from: http://dx.doi.org/10.1016/j.jid.2017.04.008
12. Mertens JS, de Jong EMGJ, Pandit A, Seyger MMB, Hoppenreijs EPAH, Thurlings RM, et al. Regarding “Transcriptional and Cytokine Profiles Identify CXCL9 as a Biomarker of Disease Activity in Morphea.” J Invest Dermatol [Internet]. ncbi.nlm.nih.gov; 2018 May;138(5):1212–5. Available from: http://dx.doi.org/10.1016/j.jid.2017.11.032
13. Hasegawa M, Fujimoto M, Matsushita T, Hamaguchi Y, Takehara K, Sato S. Serum chemokine and cytokine levels as indicators of disease activity in patients with systemic sclerosis. Clin Rheumatol [Internet]. 2011 Feb;30(2):231–7. Available from: http://dx.doi.org/10.1007/s10067-010-1610-4
14. Rabquer BJ, Tsou P-S, Hou Y, Thirunavukkarasu E, Haines GK 3rd, Impens AJ, et al. Dysregulated expression of MIG/CXCL9, IP-10/CXCL10 and CXCL16 and their receptors in systemic sclerosis. Arthritis Res Ther [Internet]. 2011 Feb 8;13(1):R18. Available from: http://dx.doi.org/10.1186/ar3242
15. Liu X, Mayes MD, Tan FK, Wu M, Reveille JD, Harper BE, et al. Correlation of interferon-inducible chemokine plasma levels with disease severity in systemic sclerosis. Arthritis & Rheumatism [Internet]. 2013;65(1):226–35. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/art.37742
16. Hintermann E, Bayer M, Pfeilschifter JM, Luster AD, Christen U. CXCL10 promotes liver fibrosis by prevention of NK cell mediated hepatic stellate cell inactivation. J Autoimmun [Internet]. 2010 Dec;35(4):424–35. Available from: http://dx.doi.org/10.1016/j.jaut.2010.09.003
17. Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GSV, Leary CP, et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol [Internet]. 2004 Oct;31(4):395–404. Available from: http://dx.doi.org/10.1165/rcmb.2004-0175OC
18. Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, et al. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest [Internet]. 2010 Jun;120(6):2049–57. Available from: http://dx.doi.org/10.1172/JCI38644
19. Cardarelli S, Facco M, Fittà C, Del Rosso A. CXCL11 in bronchoalveolar lavage fluid and pulmonary function decline in systemic sclerosis. Clinical and [Internet]. 2012; Available from: https://www.academia.edu/download/45798612/CXCL11_in_bronchoalveolar_lavage_fluid_a20160520-15769-1pwsbty.pdf
20. Burdick MD, Murray LA, Keane MP, Xue YY, Zisman DA, Belperio JA, et al. CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am J Respir Crit Care Med [Internet]. 2005 Feb 1;171(3):261–8. Available from: http://dx.doi.org/10.1164/rccm.200409-1164OC
21. Sulpice E, Contreres J-O, Lacour J, Bryckaert M, Tobelem G. Platelet factor 4 disrupts the intracellular signalling cascade induced by vascular endothelial growth factor by both KDR dependent and independent mechanisms. Eur J Biochem [Internet]. 2004 Aug;271(16):3310–8. Available from: http://dx.doi.org/10.1111/j.1432-1033.2004.04263.x
Morphea, or localized scleroderma, is an inflammatory fibrosing disease of the dermis and underlying tissue. Several studies have identified CXCR3 ligands as positively correlating with disease severity and activity in patients 55– 57. Systemic sclerosis, or scleroderma, also exhibits upregulation of CXCR3 ligands that correlates with disease severity 58. Preliminary studies from our laboratory support a pro-fibrotic role of CXCL9 in the skin: CXCL9-/- mice are protected from bleomycin-induced skin fibrosis, and in vitro treatment of mouse and human fibroblasts with CXCL9 induces transcription of collagen 1a1 (col1a1); these data are available on a preprint server and are currently undergoing peer review 59.
CXCL10 has pro-fibrotic effects in the liver, where it prevents NK cells from inactivating hepatic stellate cells 60. CXCL10-/- mice and WT mice treated with anti-CXCL10 antibody are protected from carbon tetrachloride-induced liver fibrosis. Hepatic stellate cells upregulate CXCR3 in response to carbon tetrachloride, and CXCL10 induces their migration but not proliferation. CXCL10 also mediates T and B cell aggregates in lymphoid tissue, which are essentially absent in CXCL10-/- mice ( Table 1).
Antifibrotic roles of CXCR3 ligands
While CXCL9 has pro-fibrotic effects in renal and skin tissue, CXCL9 has direct angiostatic and antifibrotic effects in experimental models of pancreas and liver fibrosis. In the trinitrobenzene sulfonic acid (TNBS) induced-pancreatitis rat model, administration of anti-CXCL9 antibody worsened fibrosis, whereas administration of recombinant CXCL9 improved fibrosis, as assessed by trichrome staining and hydroxyproline assay 61 ( Table 1). In vitro stimulation of pancreatic stellate cells with CXCL9 downregulated TGFβ1 and col1a1 production by confocal microscopy. Of note, antibody and recombinant CXCL9 were administered subcutaneously (s.c.) to rats in this model. We hypothesize that this route of administration may have pulled inflammatory infiltrates away from the gastrointestinal (GI) tract and towards the skin, considering there was 1.5ng/mL CXCL9 in serum and approximately 30µg was administered s.c. daily (assuming average weight of 300g/rat at a dose of 100 μg/kg body weight).
In the carbon tetrachloride-induced liver fibrosis model, CXCR3-/- mice exhibited augmented liver damage at 24h 62. Follow-up studies from the same laboratory used mice treated exogenously with CXCL9, which reduced the severity of liver fibrosis as assessed by Sirius red staining, hydroxyproline assay, and α-SMA expression 63 ( Table 1). In vivo CXCL9 treatment also inhibited angiogenesis as assessed by CD31 staining and ultrasound visualization of liver perfusion. However, CXCL9 treatment did not impact the number of Th1-polarized, IFN-γ-positive cells in the liver amongst treatment groups. Treatment of endothelial cells in vitro with CXCL9 was able to inhibit VEGF-mediated proliferation and migration via PLCγ, JNK and ERK. In vitro treatment of hepatic stellate cells reduced TGFβ and col1a1 by protein and RNA 64.
While CXCL10 has profibrotic effects in the liver, CXCL10 limits lung fibrosis in the murine model of bleomycin-induced pulmonary fibrosis ( Table 1). CXCR3-/- and CXCL10-/- mice display exaggerated pulmonary fibrosis after bleomycin administration, and transgenic mice overexpressing CXCL10 are protected from bleomycin-induced mortality 65, 66. Bleomycin did not alter the T cell cytokine milieu in CXCL10-/- mice, weakening the support for the idea that CXCL10 might limit fibrosis by skewing T cell polarization to the Th1 phenotype as demonstrated in hepatitis models. CXCL10 also did not decrease lung tissue-derived angiogenic activity and von Willebrand Factor expression after bleomycin delivery, despite that angiogenesis is considered a rate-limiting step in the development of pulmonary fibrosis. CXCR3 mRNA, but not protein, was detected in lung fibroblasts. Rather, direct interaction of the heparin-binding domain of CXCL10 and syndecan-4 on the lung interstitial compartment inhibits fibroblast recruitment, TGFβ signaling and subsequent fibrosis 67, 68. Similar findings were reported in myocardium, which required CXCL10 fibroblast responses through proteoglycans 69, and urethral fibrosis, in which CXCL10 signaling interfered with profibrotic TGFβ signaling 70.
Similar to CXCL10, CXCL11 attenuates lung fibrosis in the bleomycin mouse model and inhibits angiogenesis in the corneal micropocket assay 71 ( Table 1). A double-blind, placebo controlled study of 330 idiopathic pulmonary patients treated with subcutaneous IFN-γ 1b treatment exhibited increased CXCL11 in bronchoalveolar lavage fluid and plasma, with concomitant decreased elastin 72. The pro- and anti-fibrotic roles of the CXCR3 ligands in different organs are summarized in Table 2.
Table 2. Comparison of CXCR3 ligand actions in organ fibrosis.
| CXCL9 | CXCL10 | CXCL11 | |
|---|---|---|---|
| Profibrotic | Heart, Kidney, Skin | Heart, Liver | ? |
| Antifibrotic | Liver, Pancreas | Lung | Lung |
Potential therapeutic manipulations of CXCR3 for the treatment of fibrosis
To select how to manipulate CXCR3 and/or its ligands for the treatment of fibrosis, it is our opinion that the suspected cell-of-origin in the fibrotic response and the level of angiogenesis during fibrogenesis need to be assessed. Based on the evidence discussed above, we hypothesize that fibrosing disorders primarily mediated by pericyte-type cells that require ERK signaling and exhibit more angiogenesis as a disease feature would be more dependent upon CXCL10, and fibrosing disorders primarily mediated by fibroblast or myofibroblast-type cells that require AKT and JAK signaling and exhibit less vascular involvement would be more dependent upon CXCL9. For example, hepatic fibrosis has prominent vascular changes and is driven by hepatic stellate cells and CXCL10, whereas morphea has a low incidence of vascular changes and is driven by fibroblasts/myofibroblasts and CXCL9. GI organs, in which fibrosis is driven by pericytes, also generally seem to use CXCL9 for protective responses, whereas lung and skin, in which fibrosis is driven by fibroblast subsets, use CXCL10 for protective responses. Nuances in the signaling pathways, the relative chemokine responsiveness, as well as potential coreceptors, will need to be addressed in future studies. Technologies such as single cell RNA sequencing and proteomics may ultimately help resolve the heterogeneity of chemokine receptor and coreceptor expression, as well as preferential signaling pathway usage.
The first potential class of small molecules that could be used to disrupt CXCR3-mediated inflammatory fibrosis are JAK inhibitors. We and others have shown that JAK inhibitors prevent fibrosis in mice 73– 75, and demonstrated efficacy in our case studies of human morphea patients who were recalcitrant to standard therapies 73, 76. In our study of intradermal bleomycin injection in mice and human morphea tissue, we observed p-STAT1 and p-STAT3 activation in both immune infiltrates and cells with fibroblast morphology 73. Notably, STAT1, STAT3 and STAT5 have predicted binding sites in the collagen 1a1 (col1a1) promoter and enhancer regions (GeneCards), which may account for our observation that JAK inhibitors were able to suppress col1a1 transcription by human and mouse fibroblasts in vitro 73. We also noted that the JAK 1/2 inhibitor ruxolitinib yielded a slightly better p value than the JAK 3>>1>2 inhibitor tofacitinib for inhibition of dermal thickening in the intradermal bleomycin mouse model. These data are in agreement with previously published studies examining JAK2 as a driver of fibrosis in scleroderma fibroblasts and a bleomycin mouse model 74. Zhang et al demonstrated that following long-term selective inhibition of JAK2, JAK2 may be transphosphorylated by JAK1 to mediate fibrosis 77, supporting the use of a combination JAK1/2 inhibitor for treatment of fibrosis. It is interesting to note that ruxolitinib was originally FDA approved for myelofibrosis 78, and patients receiving ruxolitinib therapy often resolve fibrosis 79. While this is encouraging for potential repurposing of ruxolitinib for other fibrosing diseases, we would caution that careful tapering and monitoring is needed to prevent potential rebound effects 80, 81. Cessation of ruxolitinib can cause hyperphosphorylation of JAK2, increasing inflammation and subsequent fibrosis 82. Selecting a JAK1/2 inhibitor with a longer half-life, such as baricitinib 83, might be a safer option for patients who are tapering.
The second potential class of therapeutics would be agonist peptides to mimic the antifibrotic role of CXCL10 for lung fibrosis. As suggested by Tager and Jiang et al, maintaining heparin binding but excluding CXCR3 binding would mitigate potential toxicities related to T cell recruitment 65, 67. CXCL10-based therapeutics might also prove useful for improving lung fibrosis and function in patients recovering from infectious lung disease, particularly Sars-CoV2 infection/COVID-19 disease 84. Smith et al recently reported biased agonists of CXCR3 that can differentially mediate inflammation and migration of immune cells which they examined in the context of contact hypersensitivity in skin 14, providing a basis for the feasibility of this approach.
The third potential class of therapeutics would be agents that inhibit the pro-fibrotic signaling events mediated by CXCR3 ligands, such as CXCL9 in Th1/IFNγ-driven kidney disease or morphea. These approaches could include anti-CXCL9 blocking/neutralizing antibodies, CXCL9 siRNA, or antagonistic peptide ligands. Of note, antibody neutralization of CXCL10 for treatment of hepatic fibrosis may be challenging, as CXCL10 antibodies neutralize the free form and not endothelial-bound chemokine 85. Similar challenges may arise when attempting to neutralize CXCL9 with antibody, as would anti-drug antibody responses.
A fourth class of therapeutics could leverage the cell- or organ-specific context of chemokine expression. For example, stimulating γδ T-cells to produce CXCL10 in the lung could have therapeutic benefits in pulmonary fibrotic disease. Inhibiting macrophage production of CXCL9 in the skin could prevent collagen deposition in morphea. Drawing immune infiltrates away from the pancreas and towards the skin could reset the fibrotic process, as in the TNBS-induced rat model. Agents are in development to target specific cell types, such as antibody-drug conjugates 86 some with cleavable linkers 87, bispecific antibodies 88, 89 and nanoparticles 90, which can be preferentially phagocytosed by antigen presenting cells of the immune system. These could be leveraged to achieve the aforementioned goals of stimulating CXCL10 or inhibiting CXCL9 production by key cell types. Different drug delivery routes and systems may also help accomplish the goal of drawing immune cells away from the pancreas or other organs, with cutaneous administration via creams, injections or microneedle patches 91 helping to achieve a high local concentration.
A fifth class of therapeutics could leverage existing enzymatic cleavage of CXCR3 ligands. Recombinant peptides lacking amino-terminal amino acids can exert angiostatic effects, while inhibiting CXCR3-mediated migration. Administration of bioactive CD26 and/or CD13, or inhibitors of these enzymes, may modulate fibrotic processes in specific organs or diseases. CD26 inhibitors have been reported to reduce or prevent fibrosis in models of myocardial fibrosis, lung fibrosis and kidney fibrosis 92– 94, and a CD13 inhibitor improved fibrosis in a mouse model of silica-induced lung fibrosis 95.
Last, combination therapies targeting both prevention of inflammation and fibrosis in addition to promoting tissue remodeling will likely provide the best therapeutic outcome for fibrosis patients 96. Tissue remodeling will ultimately allow for breakdown of fibrotic plaques and better disease outcomes, which could be achieved through agonists or inducers of matrix metalloproteinases (MMPs) or antagonists of tissue inhibitors of MMPs (TIMPs).
Conclusion
The differential impact of CXCR3 and its ligands on tissues depends on disparate signaling pathways involving multiple cell types and potential coreceptors; the nuances of which should be addressed in future research involving single cell RNA sequencing and proteomics. As we have examined the known fibrotic and antifibrotic roles of CXCR3 and its ligands, we suggest that future therapeutic options should be centered around the suspected cell-of-origin in the fibrotic response, tissue-specific signaling factors and the degree of angiogenesis is during fibrosis. Likely, a combination of these therapies will have the best potential to ameliorate symptoms of patients with fibrosing diseases.
Data availability
No data are associated with this article.
Acknowledgements
We thank M. Tuzova and C. Garelli for insightful comments.
Funding Statement
JMR is supported by the National Institutes of Health [R56AR075761], a Career Development Award from the Dermatology Foundation, a Target Identification in Lupus Award from the Lupus Research Alliance and a Concept Award from the US Department of Defense Lupus Research Program [#LR190030].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Proudfoot AEI: Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2(2):106–15. 10.1038/nri722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Griffith JW, Sokol CL, Luster AD: Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. 10.1146/annurev-immunol-032713-120145 [DOI] [PubMed] [Google Scholar]
- 3. Zlotnik A, Burkhardt AM, Homey B: Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11(9):597–606. 10.1038/nri3049 [DOI] [PubMed] [Google Scholar]
- 4. Groom JR, Luster AD: CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89(2):207–15. 10.1038/icb.2010.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wynn TA: Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117(3):524–9. 10.1172/JCI31487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colvin RA, Campanella GSV, Manice LA, et al. : CXCR3 requires tyrosine sulfation for ligand binding and a second extracellular loop arginine residue for ligand-induced chemotaxis. Mol Cell Biol. 2006;26(15):5838–49. 10.1128/MCB.00556-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xanthou G, Williams TJ, Pease JE: Molecular characterization of the chemokine receptor CXCR3: evidence for the involvement of distinct extracellular domains in a multi-step model of ligand binding and receptor activation. Eur J Immunol. 2003;33(10):2927–36. 10.1002/eji.200324235 [DOI] [PubMed] [Google Scholar]
- 8. Zidar DA, Violin JD, Whalen EJ, et al. : Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci U S A. 2009;106(24):9649–54. 10.1073/pnas.0904361106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rajagopal S, Bassoni DL, Campbell JJ, et al. : Biased agonism as a mechanism for differential signaling by chemokine receptors. J Biol Chem. 2013;288(49):35039–48. 10.1074/jbc.M113.479113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colvin RA, Campanella GSV, Sun J, et al. : Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279(29):30219–27. 10.1074/jbc.M403595200 [DOI] [PubMed] [Google Scholar]
- 11. Sauty A, Colvin RA, Wagner L, et al. : CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell alpha chemoattractant (CXCL11). J Immunol. 2001;167(12):7084–93. 10.4049/jimmunol.167.12.7084 [DOI] [PubMed] [Google Scholar]
- 12. Thompson BD, Jin Y, Wu KH, et al. : Inhibition of G alpha i2 activation by G alpha i3 in CXCR3-mediated signaling. J Biol Chem. 2007;282(13):9547–55. 10.1074/jbc.M610931200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smit MJ, Verdijk P, van der Raaij-Helmer EMH: CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood. 2003;102(6):1959–65. 10.1182/blood-2002-12-3945 [DOI] [PubMed] [Google Scholar]
- 14. Smith JS, Nicholson LT, Suwanpradid J, et al. : Biased agonists of the chemokine receptor CXCR3 differentially control chemotaxis and inflammation. Sci Signal. 2018;11(555):eaaq1075. 10.1126/scisignal.aaq1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soriano SF, Serrano A, Hernanz-Falcó n P, et al. : Chemokines integrate JAK/STAT and G-protein pathways during chemotaxis and calcium flux responses. Eur J Immunol. 2003;33(5):1328–33. 10.1002/eji.200323897 [DOI] [PubMed] [Google Scholar]
- 16. Zohar Y, Wildbaum G, Novak R, et al. : CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Invest. 2014;124(5):2009–22. 10.1172/JCI71951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bodnar RJ, Yates CC, Wells A: IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. 2006;98(5):617–25. 10.1161/01.RES.0000209968.66606.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arenberg DA, Kunkel SL, Polverini PJ, et al. : Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med. 1996;184(3):981–92. 10.1084/jem.184.3.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keane MP, Arenberg DA, Lynch JP, 3rd, et al. : The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159(3):1437–43. [PubMed] [Google Scholar]
- 20. Campanella GSV, Colvin RA, Luster AD: CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS One. 2010;5(9):e12700. 10.1371/journal.pone.0012700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xia JB, Mao CZ, Chen ZY, et al. : The CXCL10/CXCR3 axis promotes cardiac microvascular endothelial cell migration via the p38/FAK pathway in a proliferation-independent manner. Exp Mol Pathol. 2016;100(2):257–65. 10.1016/j.yexmp.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 22. Ling CC, Ng KTP, Shao Y, et al. : Post-transplant endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling promotes liver tumor growth. J Hepatol. 2014;60(1):103–9. 10.1016/j.jhep.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 23. Attwell D, Mishra A, Hall CN, et al. : What is a pericyte? J Cereb Blood Flow Metab. 2016;36(2):451–5. 10.1177/0271678X15610340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonacchi A, Romagnani P, Romanelli RG: Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem. 2001;276(13):9945–54. 10.1074/jbc.M010303200 [DOI] [PubMed] [Google Scholar]
- 25. Masamune A, Satoh M, Kikuta K, et al. : Activation of JAK-STAT pathway is required for platelet-derived growth factor-induced proliferation of pancreatic stellate cells. World J Gastroenterol. 2005;11(22):3385–91. 10.3748/wjg.v11.i22.3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baumert JT, Sparmann G, Emmrich J, et al. : Inhibitory effects of interferons on pancreatic stellate cell activation. World J Gastroenterol. 2006;12(6):896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kroeze KL, Boink MA, Sampat-Sardjoepersad SC, et al. : Autocrine regulation of re-epithelialization after wounding by chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J Invest Dermatol. 2012;132(1):216–25. 10.1038/jid.2011.245 [DOI] [PubMed] [Google Scholar]
- 28. Yates CC, Whaley D, Hooda S, et al. : Delayed reepithelialization and basement membrane regeneration after wounding in mice lacking CXCR3. Wound Repair Regen. 2009;17(1):34–41. 10.1111/j.1524-475X.2008.00439.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huen AC, Wells A: The Beginning of the End: CXCR3 Signaling in Late-Stage Wound Healing. Adv Wound Care (New Rochelle). 2012;1(6):244–8. 10.1089/wound.2011.0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yates CC, Whaley D, Kulasekeran P, et al. : Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol. 2007;171(2):484–95. 10.2353/ajpath.2007.061092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yates CC, Krishna P, Whaley D, et al. : Lack of CXC chemokine receptor 3 signaling leads to hypertrophic and hypercellular scarring. Am J Pathol. 2010;176(4):1743–55. 10.2353/ajpath.2010.090564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kouroumalis A, Nibbs RJ, Aptel H, et al. : The chemokines CXCL9, CXCL10, and CXCL11 differentially stimulate G alpha i-independent signaling and actin responses in human intestinal myofibroblasts. J Immunol. 2005[cited 2019 Feb 15];175(8):5403–11. 10.4049/jimmunol.175.8.5403 [DOI] [PubMed] [Google Scholar]
- 33. Campanella GSV, Lee EMJ, Sun J, et al. : CXCR3 and heparin binding sites of the chemokine IP-10 (CXCL10). J Biol Chem. 2003;278(19):17066–74. 10.1074/jbc.M212077200 [DOI] [PubMed] [Google Scholar]
- 34. Campanella GSV, Grimm J, Manice LA, et al. : Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J Immunol. 2006;177(10):6991–8. 10.4049/jimmunol.177.10.6991 [DOI] [PubMed] [Google Scholar]
- 35. Loos T, Mortier A, Gouwy M, et al. : Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: a naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood. 2008;112(7):2648–56. 10.1182/blood-2008-04-149039 [DOI] [PubMed] [Google Scholar]
- 36. Proost P, Mortier A, Loos T: Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood. 2007;110(1):37–44. 10.1182/blood-2006-10-049072 [DOI] [PubMed] [Google Scholar]
- 37. Lambeir AM, Proost P, Durinx C, et al. : Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. 2001;276(32):29839–45. 10.1074/jbc.M103106200 [DOI] [PubMed] [Google Scholar]
- 38. Proost P, Schutyser E, Menten P, et al. : Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood. 2001;98(13):3554–61. 10.1182/blood.v98.13.3554 [DOI] [PubMed] [Google Scholar]
- 39. Vanheule V, Janssens R, Boff D, et al. : The Positively Charged COOH-terminal Glycosaminoglycan-binding CXCL9(74-103) Peptide Inhibits CXCL8-induced Neutrophil Extravasation and Monosodium Urate Crystal-induced Gout in Mice. J Biol Chem. 2015;290(35):21292–304. 10.1074/jbc.M115.649855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vanheule V, Boff D, Mortier A, et al. : CXCL9-Derived Peptides Differentially Inhibit Neutrophil Migration In Vivo through Interference with Glycosaminoglycan Interactions. Front Immunol. 2017;8:530. 10.3389/fimmu.2017.00530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pasqualini R, Koivunen E, Kain R, et al. : Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60(3):722–7. [PMC free article] [PubMed] [Google Scholar]
- 42. Lasagni L, Francalanci M, Annunziato F: An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197(11):1537–49. 10.1084/jem.20021897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ehlert JE, Addison CA, Burdick MD, et al. : Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J Immunol. 2004;173(10):6234–40. 10.4049/jimmunol.173.10.6234 [DOI] [PubMed] [Google Scholar]
- 44. Singh AK, Arya RK, Trivedi AK, et al. : Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev. 2013;24(1):41–9. 10.1016/j.cytogfr.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ding BS, Cao Z, Lis R, et al. : Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505(7481):97–102. 10.1038/nature12681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Giegold O, Ogrissek N, Richter C, et al. : CXCL9 causes heterologous desensitization of CXCL12-mediated memory T lymphocyte activation. J Immunol. 2013;190(7):3696–705. 10.4049/jimmunol.1101293 [DOI] [PubMed] [Google Scholar]
- 47. Weigold F, Günther J, Pfeiffenberger M, et al. : Antibodies against chemokine receptors CXCR3 and CXCR4 predict progressive deterioration of lung function in patients with systemic sclerosis. Arthritis Res Ther. 2018;20(1):52. 10.1186/s13075-018-1545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Recke A, Regensburger AK, Weigold F, et al. : Autoantibodies in Serum of Systemic Scleroderma Patients: Peptide-Based Epitope Mapping Indicates Increased Binding to Cytoplasmic Domains of CXCR3. Front Immunol. 2018;9:428. 10.3389/fimmu.2018.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burns JM, Summers BC, Wang Y, et al. : A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor developmen. J Exp Med. 2006;203(9):2201–13. 10.1084/jem.20052144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Naumann U, Cameroni E, Pruenster M, et al. : CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS One. 2010;5(2):e9175. 10.1371/journal.pone.0009175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guan S, Zhou J: CXCR7 attenuates the TGF-β-induced endothelial-to-mesenchymal transition and pulmonary fibrosis. Mol Biosyst. 2017;13(10):2116–24. 10.1039/c7mb00247e [DOI] [PubMed] [Google Scholar]
- 52. Cao Z, Lis R, Ginsberg M, et al. : Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med. 2016;22(2):154–62. 10.1038/nm.4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Menke J, Zeller GC, Kikawada E, et al. : CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol. 2008;19(6):1177–89. 10.1681/ASN.2007111179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koike K, Ueda S, Yamagishi SI, et al. : Protective role of JAK/STAT signaling against renal fibrosis in mice with unilateral ureteral obstruction. Clin Immunol. 2014;150(1):78–87. 10.1016/j.clim.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 55. O’Brien JC, Rainwater YB, Malviya N, et al. : Transcriptional and Cytokine Profiles Identify CXCL9 as a Biomarker of Disease Activity in Morphea. J Invest Dermatol. 2017;137(8):1663–70. 10.1016/j.jid.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Magee KE, Kelsey CE, Kurzinski KL, et al. : Interferon-gamma inducible protein-10 as a potential biomarker in localized scleroderma. Arthritis Res Ther. 2013;15(6):R188. 10.1186/ar4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mertens JS, de Jong A, Pandit A, et al. : Regarding "Transcriptional and Cytokine Profiles Identify CXCL9 as a Biomarker of Disease Activity in Morphea". J Invest Dermatol. 2018;138(5):1212–5. 10.1016/j.jid.2017.11.032 [DOI] [PubMed] [Google Scholar]
- 58. Rabquer BJ, Tsou PS, Hou Y, et al. : Dysregulated expression of MIG/CXCL9, IP-10/CXCL10 and CXCL16 and their receptors in systemic sclerosis. Arthritis Res Ther. 2011;13(1):R18. 10.1186/ar3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Richmond JM, Patel D, Watanabe T, et al. : An acute bleomycin inflammatory and fibrotic mouse model of morphea is dependent upon CXCL9 and CXCR3. medRxiv. 2019. 10.1101/19000844 [DOI] [Google Scholar]
- 60. Hintermann E, Bayer M, Pfeilschifter JM, et al. : CXCL10 promotes liver fibrosis by prevention of NK cell mediated hepatic stellate cell inactivation. J Autoimmun. 2010;35(4):424–35. 10.1016/j.jaut.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shen J, Gao J, Chen C, et al. : Antifibrotic role of chemokine CXCL9 in experimental chronic pancreatitis induced by trinitrobenzene sulfonic acid in rats. Cytokine. 2013;64(1):382–94. 10.1016/j.cyto.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 62. Zaldivar MM, Berres ML, Sahin H, et al. : The chemokine receptor CXCR3 limits injury after acute toxic liver damage. Lab Invest. 2012;92(5):724–34. 10.1038/labinvest.2012.48 [DOI] [PubMed] [Google Scholar]
- 63. Sahin H, Borkham-Kamphorst E, Kuppe C, et al. : Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology. 2012;55(5):1610–9. 10.1002/hep.25545 [DOI] [PubMed] [Google Scholar]
- 64. Wasmuth HE, Lammert F, Zaldivar MM, et al. : Antifibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology. 2009;137(1):309–19, 319.e1-3. 10.1053/j.gastro.2009.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tager AM, Kradin RL, LaCamera P, et al. : Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31(4):395–404. 10.1165/rcmb.2004-0175OC [DOI] [PubMed] [Google Scholar]
- 66. Jiang D, Liang J, Hodge J, et al. : Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114(2):291–9. 10.1172/JCI16861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jiang D, Liang J, Campanella GS, et al. : Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120(6):2049–57. 10.1172/JCI38644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tanino Y, Wang X, Nikaido T, et al. : Syndecan-4 Inhibits the Development of Pulmonary Fibrosis by Attenuating TGF-β Signaling. Int J Mol Sci. 2019;20(20):4989. 10.3390/ijms20204989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Saxena A, Bujak M, Frunza O, et al. : CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res. 2014;103(2):217–27. 10.1093/cvr/cvu138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xie H, Feng C, Fu Q, et al. : Crosstalk between TGF-β1 and CXCR3 signaling during urethral fibrosis. Mol Cell Biochem. 2014;394(1–2):283–90. 10.1007/s11010-014-2104-5 [DOI] [PubMed] [Google Scholar]
- 71. Burdick MD, Murray LA, Keane MP, et al. : CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am J Respir Crit Care Med. 2005;171(3):261–8. 10.1164/rccm.200409-1164OC [DOI] [PubMed] [Google Scholar]
- 72. Strieter RM, Starko KM, Enelow RI, et al. : Effects of interferon-gamma 1b on biomarker expression in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2004;170(2):133–40. 10.1164/rccm.200312-1670OC [DOI] [PubMed] [Google Scholar]
- 73. Damsky W, Patel D, Garelli CJ, et al. : Jak Inhibition Prevents Bleomycin-Induced Fibrosis in Mice and Is Effective in Patients with Morphea. J Invest Dermatol. 2020;140(7):1446–1449.e4. 10.1016/j.jid.2019.12.019 [DOI] [PubMed] [Google Scholar]
- 74. Dees C, Tomcik M, Palumbo-Zerr K, et al. : JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor β in systemic sclerosis. Arthritis Rheum. 2012;64(9):3006–15. 10.1002/art.34500 [DOI] [PubMed] [Google Scholar]
- 75. Wang W, Bhattacharyya S, Marangoni RG, et al. : The JAK/STAT pathway is activated in systemic sclerosis and is effectively targeted by tofacitinib. J Scleroderma Relat Disord. 2020;5(1):40–50. 10.1177/2397198319865367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim SR, Charos A, Damsky W, et al. : Treatment of generalized deep morphea and eosinophilic fasciitis with the Janus kinase inhibitor tofacitinib. JAAD Case Rep. 2018;4(5):443–5. 10.1016/j.jdcr.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang Y, Liang R, Chen CW, et al. : JAK1-dependent transphosphorylation of JAK2 limits the antifibrotic effects of selective JAK2 inhibitors on long-term treatment. Ann Rheum Dis. 2017;76(8):1467–75. 10.1136/annrheumdis-2016-210911 [DOI] [PubMed] [Google Scholar]
- 78. Deisseroth A, Kaminskas E, Grillo J, et al. : U.S. Food and Drug Administration approval: ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res. 2012;18(12):3212–7. 10.1158/1078-0432.CCR-12-0653 [DOI] [PubMed] [Google Scholar]
- 79. Molica M, Serrao A, Saracino R, et al. : Disappearance of fibrosis in secondary myelofibrosis after ruxolitinib treatment: new endpoint to achieve? Ann Hematol. 2014;93(11):1951–2. 10.1007/s00277-014-2096-y [DOI] [PubMed] [Google Scholar]
- 80. Tefferi A, Pardanani A: Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin Proc. 2011;86(12):1188–91. 10.4065/mcp.2011.0518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Coltro G, Mannelli F, Guglielmelli P, et al. : A life-threatening ruxolitinib discontinuation syndrome. Am J Hematol. 2017;92(8):833–8. 10.1002/ajh.24775 [DOI] [PubMed] [Google Scholar]
- 82. Tvorogov D, Thomas D, Liau NPD, et al. : Accumulation of JAK activation loop phosphorylation is linked to type I JAK inhibitor withdrawal syndrome in myelofibrosis. Sci Adv. 2018;4(11):eaat3834. 10.1126/sciadv.aat3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Markham A: Baricitinib: First Global Approval. Drugs. 2017;77(6):697–704. 10.1007/s40265-017-0723-3 [DOI] [PubMed] [Google Scholar]
- 84. Ackermann M, Verleden SE, Kuehnel M, et al. : Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–8. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bonvin P, Gueneau F, Buatois V, et al. : Antibody Neutralization of CXCL10 in Vivo Is Dependent on Binding to Free and Not Endothelial-bound Chemokine: IMPLICATIONS FOR THE DESIGN OF A NEW GENERATION OF ANTI-CHEMOKINE THERAPEUTIC ANTIBODIES. J Biol Chem. 2017;292(10):4185–97. 10.1074/jbc.M116.745877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zolot RS, Basu S, Million RP: Antibody-drug conjugates. Nat Rev Drug Discov. 2013;12(4):259–60. 10.1038/nrd3980 [DOI] [PubMed] [Google Scholar]
- 87. Bargh JD, Isidro-Llobet A, Parker JS, et al. : Cleavable linkers in antibody-drug conjugates. Chem Soc Rev. 2019;48(16):4361–74. 10.1039/c8cs00676h [DOI] [PubMed] [Google Scholar]
- 88. Wilhelm S, Tavares AJ, Dai Q, et al. : Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):16014 10.1038/natrevmats.2016.14 [DOI] [Google Scholar]
- 89. Labrijn AF, Janmaat ML, Reichert JM, et al. : Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–608. 10.1038/s41573-019-0028-1 [DOI] [PubMed] [Google Scholar]
- 90. Prow TW, Grice JE, Lin LL, et al. : Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev. 2011;63(6):470–91. 10.1016/j.addr.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 91. Prausnitz MR: Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56(5):581–7. 10.1016/j.addr.2003.10.023 [DOI] [PubMed] [Google Scholar]
- 92. Hirakawa H, Zempo H, Ogawa M, et al. : A DPP-4 inhibitor suppresses fibrosis and inflammation on experimental autoimmune myocarditis in mice. PLoS One. 2015;10(3):e0119360. 10.1371/journal.pone.0119360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kanasaki K, Shi S, Kanasaki M, et al. : Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014;63(6):2120–31. 10.2337/db13-1029 [DOI] [PubMed] [Google Scholar]
- 94. Liu Y, Qi Y: Vildagliptin, a CD26/DPP4 inhibitor, ameliorates bleomycin-induced pulmonary fibrosis via regulating the extracellular matrix. Int Immunopharmacol. 2020;87:106774. 10.1016/j.intimp.2020.106774 [DOI] [PubMed] [Google Scholar]
- 95. Kühlmann UC, Chwieralski CE, van den Brule S, et al. : Modulation of cytokine production and silica-induced lung fibrosis by inhibitors of aminopeptidase N and of dipeptidyl peptidase-IV-related proteases. Life Sci. 2009;84(1–2):1–11. 10.1016/j.lfs.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 96. Wynn TA, Ramalingam TR: Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–40. 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]