Abstract
The humanization of animals is a powerful tool for the exploration of human disease pathogenesis in biomedical research, as well as for the development of therapeutic interventions with enhanced translational potential. Humanized models enable us to overcome biologic differences that exist between humans and other species, while giving us a platform to study human processes in vivo. To become humanized, an immune-deficient recipient is engrafted with cells, tissues, or organoids. The mouse is the most well studied of these hosts, with a variety of immunodeficient strains available for various specific uses. More recently, efforts have turned to the humanization of other animal species such as the rat, which offers some technical and immunologic advantages over mice. These advances, together with ongoing developments in the incorporation of human transgenes and additional mutations in humanized mouse models, have expanded our opportunities to replicate aspects of human allotransplantation and to assist in the development of immunotherapies. In this review, the immune and tissue humanization of various species is presented with an emphasis on their potential for use as models for allotransplantation, graft versus host disease, and regenerative medicine.
INTRODUCTION
For decades, studies in immunology have benefited from the ability to experiment on small animal models. However, it is increasingly clear that the mechanistic gap between human and small animal immune responses is significant, leading to challenges in the translation of findings from animal to human that can, on occasion, have devastating results.1-3 Humanized laboratory animals have been developed to bridge this gap, and provide a powerful method for the preclinical assessment of human immune responses in the context of transplantation and regenerative medicine. The vast majority of these models are created using immunodeficient mice engrafted with human cells and tissues. Although humanized mice have been extremely useful in the study of many pathologies, the humanization of other species can also be advantageous. The size of other animals, such as rats, pigs, or nonhuman primates, facilitates more challenging surgical procedures, which would be difficult in mice. Additionally, the anatomy and physiology of larger species more closely resemble that of the human. Before the description of gene-specific nucleases (such as meganucleases, zinc finger nucleases [ZFNs], transcription activator-like effector nucleases [TALENs], and CRISPR/Cas9), targeted genome editing was largely restricted to mice, the only species in which robust embryonic stem (ES) cells were available. However, the recent evolution of gene-specific nucleases has allowed the generation of immunodeficient animals in all the above mentioned species. In this review, we aim to explore the qualities and benefits of the available immunodeficient animal models, their potential for reconstitution with human tissues, and how this is benefitting preclinical research in the field of transplantation.
MICE
Immunodeficient Mouse Models
Over the past 2 decades, mice have become the dominant rodent model in biomedical research. The prominence of mice owes largely to the development of methods for genetic manipulation, the subsequent establishment and characterization of murine strains—starting with the first knockout mouse in 19874—and the availability of a wide range of transgenic mice as well as antibodies targeting mouse antigens. Further technological advances targeting mouse gene expression have enabled the experimental reproduction of human allogeneic transplantation within in vivo models. For success, such models require: (1) host mice that are rendered genetically immunodeficient, (2) adoptive transfer and engraftment of human immune cells, and (3) transplantation of allogeneic human tissues.
The development of immunodeficient mouse models began following the description of the spontaneously arising severe combined immunodeficiency (scid) mutation in C.B-17 mice,5 which produced mice lacking effective adaptive immunity. The scid mutation affects the Prkdc gene for the DNA-dependent protein kinase catalytic subunit, which is critical for DNA repair during V(D)J recombination in T and B cell receptor generation. As a result, SCID mice are incapable of producing mature T and B cells. The experimental replication of this immunodeficiency was first achieved by knocking out the Rag2 gene, which similarly arrests V(D)J recombination and lymphocyte maturation.6 These immunodeficient models therefore permit hematopoietic reconstitution with adoptively transferred human peripheral mononuclear cells7 or stem cells,8 since effective adaptive antihuman responses can no longer be mounted. It later became clear that human cell reconstitution can be significantly enhanced by targeting mouse innate cells to further limit xenoreactivity. Crossing SCID mice with inbred nonobese diabetic (NOD) mice resulted in mice that not only had defective adaptive immunity but also absent complement C5 (rendering these mice deficient in hemolytic complement) and impaired macrophage and dendritic cell function.9-13 In these NOD-scid mice, human cell engraftment was up to 10-fold higher than in C.B-17-scid mice.14
Further improvement in human cell engraftment and reconstitution came with the null mutation of the interleukin (IL)-2 receptor common γ-chain,15 blocking the signaling pathways of IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 and, crucially, diminishing mouse natural killer (NK) cell function through impaired IL-15 signaling. Combining mutations of both adaptive (scid, Rag1/2) and innate (IL-2Rγc) immunity heralded a new wave of severely immunodeficient mice that include NOG (NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac), NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ), and BRG (BALB/c Rag2-/- Il2rgtm1Sug/JicTac) mice,16 expanding opportunities for successful engraftment of human hematopoietic cells. NOG mice and NSG mice differ in the nature of the IL-2 receptor common γ-chain mutation wherein NOG mice lack the cytoplasmic domain whereas NSG mice have a complete null allele. BRG mice differ from NOG/NSG mice being on the Balb/c rather than the NOD background but like NOG mice, they also lack the cytoplasmic tail of the IL-2 receptor common γ-chain. Current evidence suggests that human cells engraft better in NSG/NOG compared with BRG mice.17,18 This may be in part due to greater compatibility between human CD47 and NOD SIRPα (discussed below) but perhaps more importantly because the DNA repair defect conferred by the scid mutation severely impairs bone marrow stem cell repopulation potential, facilitating exogenous hematopoietic stem cell (HSC) engraftment in a manner not seen in Rag-mutant mice.19 In order to further improve human cell engraftment, later modifications aimed to prevent mouse macrophage-mediated phagocytosis of human hematopoietic cells, which results from impaired “don’t eat me” signaling via an incompatibility between human CD47 and murine SIRPα. Successful attempts to address this include the transfer of the NOD.Sirpa allele, a highly polymorphic variant of SIRPα with high affinity for human CD47,20,21 or the human Sirpa,22,23 to other genetic backgrounds. A summary of all immunodeficient mouse models used for humanized studies is shown in Table 1.
TABLE 1.
Immunodeficient mouse models
| Name | Strain | Phenotype | Reference |
|---|---|---|---|
| SCID | B6.CB17-Prkdcscid/SzJ | T and B cell deficiency | 5 |
| NOD-scid | NOD.CB17-Prkdcscid/J | T and B cell deficiency | 10 |
| Phagocytic tolerance | |||
| NSG | NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ | T, B, and NK cell deficiency | 15 |
| Phagocytic tolerance | |||
| NSG B2M, β2m KO NSG | NOD scid Il2rynull B2mnull | T, B, and NK cell deficiency | 24 |
| Xeno-GvHD-resistance | |||
| NSG HLA-A2/HDD | NOD.Cg-PrkdcscidIl2rgtm1WjlTg (HLA-A2/H2-D/B2M)1Dvs/Sz(NSG-HLA-A2/HHD) | T, B, and NK cell deficiency | 25 |
| Human HLA-A2 expression | |||
| NSG HLA-DR | NOG/HLA-DR4/I-Ab−/− | T, B, and NK cell deficiency | 26 |
| Human HLA-DR expression | |||
| NSG-SGM3 | NOD.Cg-PrkdcscidIl2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ | T, B, and NK cell deficiency | 27 |
| Human IL-3, GM-CSF and SCF expression | |||
| hIL-6 Tg NSG | NOD.Cg-PrkdcscidIl2rgtm1Wjl Tg(BAC1/2-IL6) | T, B, and NK cell deficiency | 28 |
| Human IL-6 expression | |||
| NSGW41 | NOD.Cg-KitW-41JPrkdcscid Il2rgtm1Wjl/WaskJ | T, B, and NK cell deficiency, Impaired HSC development | 29 |
| NSGWv/+ | NOD/SCIDIl2rg−/−KitWv/+ | T, B, and NK cell deficiency, Impaired HSC development | 30 |
| NSGWv | NOD/SCIDIl2rg−/− KitWv/Wv | T, B, and NK cell deficiency, Impaired HSC development | 30 |
| NBSGW | NOD,B6.SCID Il2rγ−/− KitW41/W41 | T, B, and NK cell deficiency, Impaired HSC development | 31 |
| NOG | NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac | T, B, and NK cell deficiency | 15 |
| Phagocytic tolerance | |||
| IL-15-NOG Tg | NOD.Cg-Prkdcscid IL-2Rrgcm1Sug Tg (CMV-IL2/IL15)1-1Jic/JicTac | T, B, and NK cell deficiency | 32 |
| Phagocytic tolerance, human IL-15 expression | |||
| NOG-EXL | NOD.Cg-Prkdcscid Il2rgtm1Sug Tg(SV40/HTLV-IL3,CSF2)10-7Jic/JicTac | T, B, and NK cell deficiency | 33 |
| Phagocytic tolerance, human GM-CSF & IL-3 expression | |||
| NOG-IL2 Tg | NOD.Cg-Prkdcscid IL-2rgcm1Sug Tg(CMV-IL2)4-2Jic/JicTac | T, B, and NK cell deficiency | 34 |
| Phagocytic tolerance, human IL-2 expression | |||
| DRAG | NOD.Cg-Rag1tm1Mom Il2rgtm1Wjl Tg(HLA-DRA,HLA-DRB1*0401)39-2Kito/ ScasJ | T, B, and NK cell deficiency | 35 |
| Phagocytic tolerance, human HLA class II expression | |||
| NRG | NOD.Cg-Rag1tm1Mom Il2rgtm1Wjl | T, B, and NK cell deficiency | 36 |
| Phagocytic tolerance | |||
| SRG | Tg(hSIRPA)Rag2−/−Il2rg−/− | T, B, and NK cell deficiency | 22 |
| Phagocytic tolerance | |||
| SRG-W41 | NOD, Rag2−/−Il2rg−/−KitWv/Wv | T, B, and NK cell deficiency | 37 |
| Phagocytic tolerance, Impaired HSC development | |||
| BALB scid | CBySmn.CB17-Prkdcscid | 38 | |
| BRG | BALB/c Rag2−/− Il2rgtm1Sug/JicTac | T, B, and NK cell deficiency | 20,39 |
| BRG hIL-3 hGM-CSF | BALB/c Rag2−/− IL-2Rgc−/− IL3h CSF2h | T, B, and NK cell deficiency | 40 |
| Human IL-3 & GM-CSF expression | |||
| BRGS | BALB/c Rag2−/− IL-2Rgc−/− NOD.sirpa | T, B, and NK cell deficiency | 20,21 |
| NOD SIRPα expression | |||
| BRGSF | BALB/c Rag2−/− IL-2Rgc−/−Flt3−/− | T, B, and NK cell deficiency, NOD SIRPα expression, impaired HSC development | 41 |
| BRgWv | BALB/cRag2−/−Il2rg−/−KitWv/Wv | T, B, and NK cell deficiency, impaired HSC development | 30 |
| MISTRG | M-CSF,IL-3,Sirpa, TPO, Rag2−/− IL-2Rgc−/− | T, B, and NK cell deficiency, human M-CSF, IL-3, GM-CSF, SIRPα, and TPO expression | 42 |
| HUMAMICE | C57BL/6 HLAA2+/+/DR1+/+/H2b2m−/−/IAb−/−/Rag2−/−/IL2Rg−/−/perf−/−- | T, B, and NK cell deficiency | 43 |
| No mouse MHC expression, human HLA expression | |||
| Nude mouse, athymic nude | Foxn1nu | T and NK cell deficiency | 44 |
GvHD, graft vs host disease; HSC, hematopoietic stem cell; MHC, major histocompatibility complex; NK, natural killer; NOD, nonobese diabetic; SCID, severe combined immunodeficiency.
Immune Humanization of Mice: Peripheral Blood Mononuclear Cell-based Models
Peripheral blood mononuclear cells (PBMCs) or leukocytes are the most commonly used cell product for humanization in transplant studies, providing a method that is both convenient and cost-effective. Mice humanized with human PBMCs robustly reconstitute T cells, which have well-established roles in rejection and tolerance. The early identification of donor-specific memory and the rapid rejection of second-set allografts45 made adaptive immune cells, especially T cells, the traditional focus of study into pathways of transplant rejection. Indeed, in experimental models, T cells are both necessary and sufficient for the rejection of most allografts.46,47 Whereas recreation of these features is important, a caveat of PBMC-humanization is that reconstitution is heavily lymphoid-biased: over 90% of human cells are T cells and the majority express an activated or memory phenotype.48 The disproportionately large fraction of the human leukocyte repertoire (usually ~5%–20%) may misrepresent the global immunologic reaction to transplantation, potentially limiting the relatability of experimental findings to the true human alloresponse.
T cell receptor (TCR) recognition of human major histocompatibility complex (MHC) mismatches drives allograft rejection and human graft versus host disease (GvHD). Curiously, MHC molecules display an inherent cross-species immunogenicity. This is beneficial to xenogenic models of GvHD where human CD8+ and CD4+ T lymphocytes recognize mouse MHC class I and II, respectively, allowing the transferred lymphocytes to simulate systemic proinflammatory responses.24 Moreover, the precursor frequency of human lymphocytes responsive to mouse MHC (0.5% in CD4+ cells and 3% in CD8+ cells24) is similar to the frequency of alloresponsive lymphocytes (3.9% of CD4+ cells and 2.5% of CD8+ T cells49). Yet, xenogeneic models of GvHD can also be confounded by the absence of indirect recognition, abundance of nonhuman antigens, and the differing distribution of mouse MHC. For example, mice do not constitutively express MHC class II in the vasculature, unlike humans; therefore, humanized GvHD models may underestimate CD4+ T cell allorecognition in vascular interactions. However, the development of anti-mouse responses is undesirable in many transplantation studies because it invariably leads to lethal xenogeneic GvHD,15,24 an expanding human leukocyte compartment that is not directed at the tissue in question, and a limited experimental window. One strategy to address this has been to produce NSG mice deficient in MHC class I and II expression.50 Intraperitoneal injection of human PBMC in such mice results in the long-term engraftment of functional CD4+ and CD8+ T cells, which retain the capacity to reject mismatched human islets.50
The additional incorporation of non-T cell components in experimental models is desirable for replicating a more complete immune system and human alloresponse. The importance of innate-mediated rejection is of increasing interest.51,52 In clinical studies, T cell depletion (eg, with alemtuzumab) has been shown to be insufficient to prevent renal or intestinal allograft rejection. In these studies, rejection was instead associated with monocytic and eosinophilic inflammation, respectively.53,54 Experimental cardiac allotransplantation experiments in alymphatic mice results in leukocyte infiltration and proinflammatory cytokine production, highlighting the presence of innate responses that can develop to allografts in the absence of T cells.55 Moreover, an array of innate immune cells, such as dendritic cells,56 NK,57 and mast cells,58 have been shown to display immunoregulatory properties important for tolerance induction, and their reconstitution in humanized animals is therefore of interest. Another important consideration is that, as humanized mouse models currently used in transplantation research do not support reconstitution of functional human antigen presenting cells in the recipient host, in vivo assessment of immune alloreactivity is limited to responses triggered by presentation of antigen via the direct pathway. Successful engraftment of functional professional human antigen presenting cells may therefore also ensure that all allorecognition pathways are integrated into the experimental model. To be able to incorporate the entire spectrum of innate and adaptive human immune responses into experimental models of transplantation, support for multilineage human hematopoietic cell reconstitution is key.
An important additional requirement of humanized animal models is engraftment or development of mature human B cells capable of effective antigen presentation and immunoglobulin production. We and others have demonstrated the ability of PBMC-humanized immunodeficient mice to engraft mature human B cells and produce human IgG and IgM (Figure 1A)7,59 however, the frequency of these antibody-producing cells is consistently low and highly variable. Our experience is that immunodeficient mice humanized with UCB HSCs generate significant numbers of human B cells60 (Figure 1B). Despite this, analyses of human B and T cells generated within different HIS mice models have revealed these B cells to be developmentally blocked,61 with defective peripheral maturation and humoral responses.16,62 UCB-humanized mice produce low levels of human IgM and IgG that increase with the development of T lymphocytes, an effect that is enhanced by the introduction of autologous (CD34− CD45+) leukocytes (Figure 1C–E). Initially, this was thought to result from an inability of mouse B lymphocyte stimulator (BAFF) to signal human B cells, suggested by the observation that administration of recombinant human BAFF to NOD-Rag1nullPrf1null mice humanized with PBMCs increased B cell engraftment and the ability to mount an antipneumococcal polysaccharide response.63 It has been reported that pre-B and immature B cells differ from mature B cells in not requiring BAFF for their survival.64-66 This is supported by a recent study in which expression of full-length human BAFF from cDNA in the endogenous mouse locus did not improve maturation of human B cells in HIS mice.67 An alternative strategy involves the induction of IL-6, which has previously been shown to increase IgG1 expression up to 400-fold.68 Knock-in of human IL-6 into HIS BRG mice increases levels of total and antigen-specific human IgG, with a concordant rise in memory and IgG+ human B cells, thymocytes, and peripheral T cells,69 the latter of which are essential for B cell maturation. Additional strategies being investigated include attempts to improve peripheral lymph node development in humanized mice, which among other benefits can increase CXCL-13 signaling.70 This has the potential to induce CD4+ cells to become follicular T helper cells during antigen stimulation,71,72 in turn providing stimulatory signals to B cells to mediate positive selection of high affinity B cells and differentiation of plasma cells in germinal centers.71,73 In transplantation research, as in cancer, infection, and autoimmunity, recapitulation of the human B cell response to disease and treatment in humanized animal models will be key in enhancing the accuracy of empirical research outputs that can safely be translated into clinical studies.
FIGURE 1.
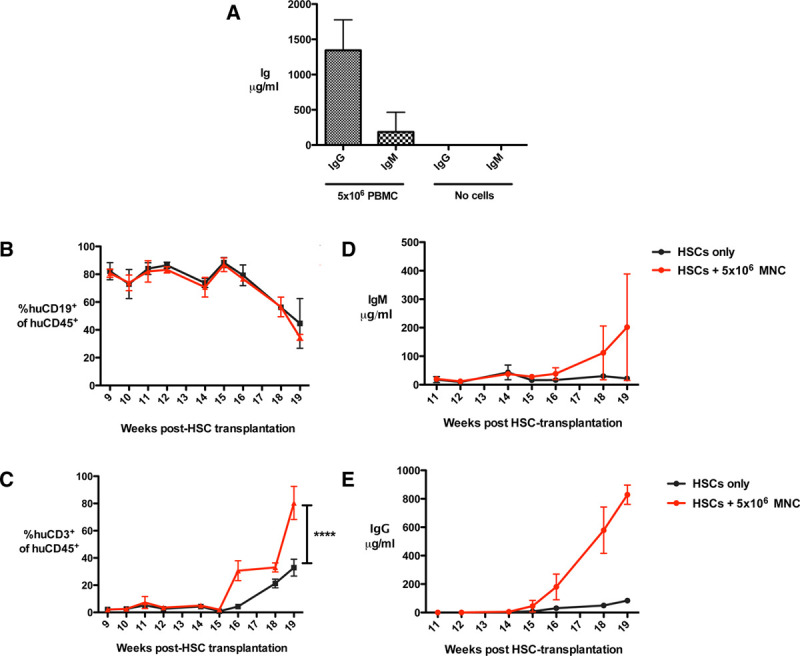
Antibody production following BALB/c-Rag2−/−cγ−/− mouse transplantation with human PBMC or HSC and a human skin graft. A, BALB/c-Rag2−/−cγ−/− mice received a human skin graft followed by 5 × 106 allogeneic PBMC (n = 9 mice) or no cells (n = 3 mice) 5 wk later. At the point of skin graft rejection (for those receiving PBMC) or long-term survival (>100 d, for those not receiving cells), mice were sacrificed and serum levels of human IgG and human IgM determined by ELISA. All mice receiving 5 × 106 PBMC achieved human leukocyte chimerism levels of >1% in the spleen. B–E, BALB/c-Rag2−/−cγ−/− mice received 4 Gy total body irradiation within 24 h of birth, followed shortly by an intrahepatic injection of 5 × 104 CD34+CD45−Lin−-enriched cells (derived from human UCB by magnetic bead isolation to a purity of over 92%). Six weeks later, mice received a human skin graft from a donor allogeneic to the HSC donor. One group received no further cells (n = 5 mice) and another group received an intraperitoneal injection of 5 × 106 mononuclear CD34−CD45+ cells (MNCs) from the same UCB donor 5 wk postskin transplantation (n = 4 mice). Peripheral blood was monitored from 9 wk onwards for the development of (B) live human 7AAD−CD45+CD19+ and (C) 7AAD−CD45+CD3+ leukocytes. Serum from peripheral blood was monitored from 9 wk onwards for the development of human (D) IgG and (E) IgM, measured by specific ELISA. Immunoglobulin was only detectable from wk 11 onwards. Data are represented as mean ± SD. HSC, hematopoietic stem cell; PBMC, peripheral blood mononuclear cell.
Immune Humanization of Mice: Stem Cell-based Models
PBMCs are characterized by a low frequency of self-renewing pluripotent hematopoietic cells and a high proportion of mature lineage-committed cells. Multilineage reconstitution must, therefore, be achieved with the use of HSC-based products rather than PBMCs. Sources of HSCs include human umbilical cord blood,30 adult bone marrow, fetal liver,74 and granulocyte colony-stimulating factor-mobilized adult PBMCs.75
NSG and BRG mice can support long-term multilineage hematopoietic reconstitution following human CD34+ HSC transplantation; however, studies have shown that the human T cells that develop are unable to recognize human HLA and mount human-restricted T cell responses because of selection on mouse MHC in the thymus.39,76 To overcome this obstacle, it is possible to implant human fetal liver and thymus tissue beneath the kidney capsules of SCID mice to produce “thymic organoids” capable of supporting human T cell development.77 To improve systemic reconstitution of T cells and other immune cell types while preserving this principle, the bone marrow-liver-thymus mouse model was created in the more supportive NSG strain, by transplanting human CD34+ cells intravenously and implanting human fetal thymus and liver tissue beneath the kidney capsule.78,79 Cells developing within this model show functional human-directed immune responses.80,81 To reduce the requirement for fetal tissue, a recent model instead utilized neonatal thymus, which has produced human cells capable of rejecting skin xenografts.82
Limitations in HSC humanization include (1) the requirement for myeloablative preconditioning of host mice to create a bone marrow niche in which human HSCs can engraft and (2) the challenges that remain in reconstituting entire the spectrum of immune cells.18,83 To overcome these, microenvironmental alterations to favor human hematopoiesis have been described (Figure 2). Methods include (1) mutation of critical murine growth factor receptors, such as the c-kit receptor (NSGW41, NSGWv/+, NSGWv, NBSGW, SRG-W41, and BRgWv mice),23,30 (2) inhibition of growth factor receptor function (eg, anti-c-kit receptor antibody),84 (3) exogenous human cytokine administration (eg, B lymphocyte stimulator for mature human B cell reconstitution63 and IL-7 analogues for T cell reconstitution75), and (4) knock-in of human cytokine genes, such as in SGM3,27 MISTRG,42 SRG-15,85 and hIL-6 Tg NSG28 strains, which include knock-ins of IL-3, IL-15, GM-CSF, M-CSF, or IL-6 to support engraftment of the wider human hematopoietic repertoire, including innate cells and regulatory T cells (Treg).27
FIGURE 2.
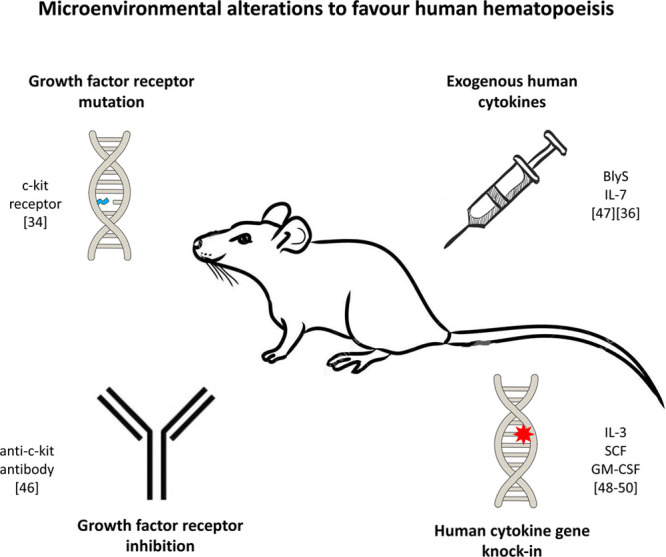
Microenvironment alterations to enhance human hematopoiesis. BlyS, B lymphocyte stimulator; GM-CSF, granulocyte macrophage colony-stimulating factor; IL, interleukin; SCF, stem cell factor.
Tissue Transplantation Into Humanized Mice
Once the challenge of human immune reconstitution is overcome, the interactions of these immune cells with specific tissues can be assessed. While responses are simulated by tumor engraftment, alloresponses are simulated by engraftment of human cells or tissues; the most common models being those that engraft human skin,86 islets of Langerhans,87 or blood vessels.88 The most widely used model is that of human skin allotransplantation.89-93 Skin grafts benefit from tissue accessibility permitting continuous visible monitoring and from an established progression of rejection in skin architecture and leukocyte infiltration. Moreover, skin is easily obtained as discarded tissue, with a single donor being able to provide sufficient tissue for multiple mice, providing a useful internal control. For example, our experience has shown that we are able to transplant skin to up to 50 mice from a single human donor.48 Acute and chronic rejection of human solid-organ transplants, such as kidney, heart, or lungs, is characterized by vascular injury.94 Rejection of the interposed artery segment in immune humanized mice is a highly relevant model, yet fails to represent the entire vascular tree. Capillaries of human origin are maintained in adult skin grafts on immunodeficient mice, while the graft is simultaneously permeated by mouse capillaries within the first 21 d posttransplantation (Figure 3A–C and Pober et al92). Immune humanization with PBMC selectively destroys human microvasculature, in a process that can be halted by the cotransfer of CD4+ Treg (Figure 3A–E). The humanization of mice with both PBMC and Treg can enable the long-term maintenance of human microvasculature in these skin grafts up to at least 100 d (Figure 3D and E). Some studies use alternate sources of capillaries, such as synthetic microvessels derived from endothelial colony-forming cells in cord blood.95-97
FIGURE 3.
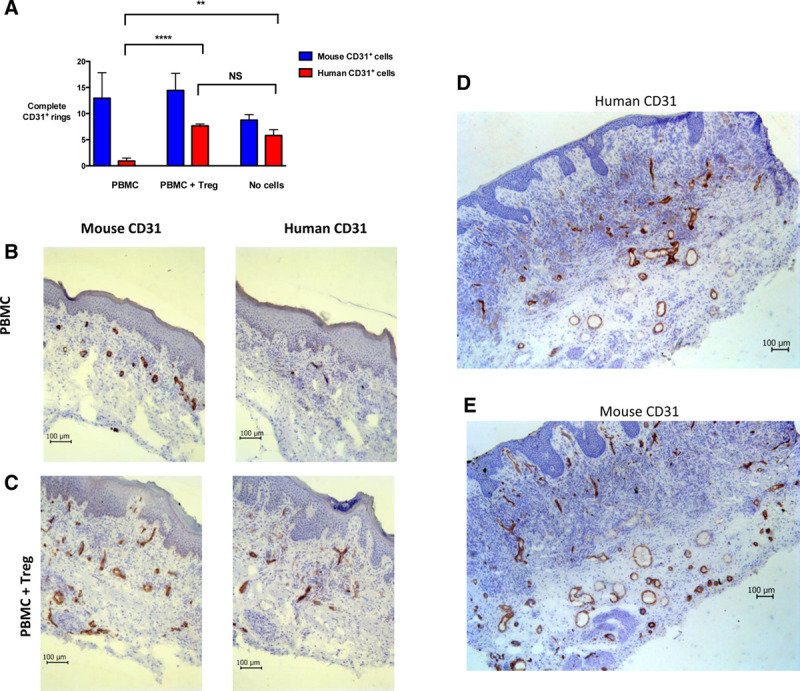
Human microvessels in human skin allografts are preserved in Treg-treated humanized BALB/c-Rag2−/−cγ−/− mice. BALB/c-Rag2−/−cγ−/− mice received a human skin graft and 5 wk later were injected with 5 × 106 allogeneic PBMCs (n = 3 mice) or 5 × 106 allogeneic PBMCs with 5 × 106 ex vivo-expanded CD127loCD25+CD4+ human Treg derived from the PBMC donor (ie, autologous Treg, n = 3 mice). A further group did not receive any cells (RPMI-1640 medium only, n = 2 mice). A, Twenty-one days postadoptive cellular transfer, skin grafts were procured and analyzed by immunohistochemistry for the number of complete cellular rings staining positive for either human CD31 or mouse CD31 within the dermis and epidermis. Complete rings were counted in the entirety of 3 separate sections (ie, in triplicate) per skin sample. Data are represented as mean values of the average of these triplicate values ± SD from a single in vivo assay using 1 PBMC donor and 1 skin donor, **P < 0.005, ****P < 0.0001, NS, not significant. Representative photomicrographs are shown of human skin sections procured 21 d postadoptive cellular transfer and stained by immunohistochemistry to visualize human CD31+ or mouse CD31+ cells in (B) mice receiving 5 × 106 PBMCs only and (C) mice receiving 5 × 106 PBMCs with 5 × 106 ex vivo-expanded Treg. Sections are counterstained with hematoxylin. The histology shown is representative of 6 stained sections for each type of stain for each of n = 3 mice for each group. Representative photomicrographs are shown of human skin sections from mice receiving PBMCs with Treg, procured >100 d postadoptive cellular transfer and stained by immunohistochemistry for (D) human CD31+ cells and (E) mouse CD31+ cells. Sections are counterstained with hematoxylin. Multiple complete human and mouse CD31+ rings are visible. Photomicrographs are representative of 6 sections from each of n = 4 mice. PBMC, peripheral blood mononuclear cell; Treg, regulatory T cell.
Human organs and tissues demonstrate unique immune functions and immune compartments that would ideally be modeled in homogenous tissues in vivo. This is clearly impractical in rodents, whose small size has traditionally limited the pool of suitable human tissues. Yet studies have ingenuously overcome this obstacle by generating or transferring human muscle, cartilage, bone, liver, kidneys, and intestines. Tissues such as cartilage,98 muscle,99 and ossicles100,101 may be of value in understanding the immunogenicity of vascularized composite allografts. Human satellite cells obtained from skeletal muscles can integrate with NSG mouse muscle to successfully produce muscle fibers and self-renew in situ.99 Allogeneic human articular chondrocytes in an agarose scaffold could successfully produce a cartilage matrix in NSG mice reconstituted with CD34+ HSC, without signs of antidonor responses.98 Recent advances in tissue engineering have generated protocols for creating 3-dimensional complex organoids from HSC or primary cells. The human bone niche can be reproduced by the subcutaneous differentiation of mesenchymal stromal cells that produce vascularized ossicles, to which human HSCs successfully home and reside.100,101 These ossicles have normal bone marrow architecture, a diverse cell repertoire including osteoclasts and osteoblasts, and organized hematopoietic clusters around sinusoids.
Human intestinal organoids have been produced from pluripotent stem cells; true to form, these organoids develop crypt-villus structures and are populated by Paneth and goblet cells.102 After implantation into the mesentery, organoids grow a vascular pedicle and can be joined to the murine intestine by anastomosis.102 Engraftment of fetal intestine into the mesentery of SCID mice is also a successful approach generating human intestinal architecture and supporting an enteric nervous system.103 Reconstruction of the mouse biliary tree with human extrahepatic cholangiocyte organoids has been demonstrated. Human cholangiocyte organoids grown on scaffolds could be surgically applied to repair and replace the gall bladder wall and common bile ducts.104 Engraftment of mice with human livers has been achieved by implantation of fetal liver105 or by the repopulation of immunodeficient mouse livers with human hepatocytes or pluripotent stem cells.106 Immunodeficient mice are genetically modified to impair hepatic homeostasis, creating space in the hepatic niche and a regenerative stimulus. Urokinase-type plasminogen activator transgenic mice or fumarylacetoacetate hydrolase (Fah) knockouts are common modifications for inducing toxicity in murine liver cells.107 The transfer of human hepatocytes has variable engraftment rates, but repopulation of up to 95% has been reported alongside a conserved liver microstructure.108 The Fah−/−Rag2−/−Il2rg−/− (FRG) mice combined with the NOD strain has been used to engraft both an immune system and repopulate the liver with human hepatocytes, creating dual chimerism and permitting the study of human hepatocyte-immune interactions.109 As an alternative to models requiring endogenous liver damage, induced pluripotent stem cells (iPS) can produce liver organoids comparable with adult human tissue in gene expression, protein secretion, and drug metabolism. After implantation into the livers of NSG mice, these organoids integrate with the native tissue and become vascularized within 4 wk.110
Of interest in modeling renal conditions, complex kidney organoids can be generated from pluripotent stem cells. Kidney organoids successfully contain organized compartments dedicated to nephrons, collecting ducts, stroma, and an endothelial network.111 Human kidney organoids can be transplanted into NSG mice, where they join onto the murine vasculature and undergo glomerular and tubular epithelium maturation.112
Preclinical Models for Immunotherapies
Control of immune responses is a requirement following allotransplantation to ensure graft function and survival. Faithful models of human biology lend themselves to the study of pathogenesis as well as the development of novel therapeutics. In the last 10 y, humanized mouse models have been broadly applied in preclinical studies for the development of the next generation of immunotherapies including costimulation and cytokine modulation, adoptive regulatory cell transfers, chimeric antigen receptor T cells, and nanotechnology for targeted drug delivery.
Immune humanization produces robust models of GvHD. This platform has provided the basis for the efficacy of adoptive CD4+ and CD8+ Treg therapy,90,113,114 mesenchymal stem cell transfers,115 costimulatory blockade such as anti-CD28 or anti-CD3 monoclonal antibodies,116,117 blockade of chemokine receptors,118 and small molecule drugs for the inhibition of JAK1/2 signaling.119
The advances in bioengineering human tissues and organs have the potential to enable studies of tissue-specific immune interactions with concurrent assessment of drug metabolism and off-target effects. However, the simultaneous engraftment of immune cells with adult allogeneic skin or vessels is currently the most prevalent model of tissue transplantation. Costimulatory and cytokine modulatory therapies have shown promise in these human tissue transplant models.89,120 Nanotechnology was recently applied to humanized models where arteries perfused with nanoparticles carrying silencing RNA could reduce endothelial MHC class II expression and could prevent alloimmune-mediated arteriopathy.121
Cell therapies rely on humanized models where differences in murine and human biology could have myriad effects on the fate of the transferred cell, different immune subsets, and the allogenic tissue. The adoptive transfer of polyclonal CD4+ and CD8+ Treg has been shown to prevent the rejection of human skin and vessel grafts48,86,88,90,122; these studies have inspired clinical trials into adoptive Treg transfer. Treg transfer therapies have been shown in humanized models to be modulated by their migratory potential,86 antigen-specificity,123 and number.86 To this end, the development of Treg bestowed with chimeric antigen receptors specific for alloantigens have been implemented in skin graft models to reduce alloimmune injury.93,124,125 Importantly, a number of national medical regulatory agencies have now started to acknowledge data from humanized models in submissions for clinical trial approval.
RATS
Immunodeficient Rat Models and Immune Humanization
Rats provide several advantages to mice in experimental studies in transplantation. First, rats are up to 10 times larger than mice, providing an advantage for technically demanding surgical studies. Their size enables the precise implantation of human tumors into relatively small anatomical locations, such as the prostate or specific areas of the brain, as well as enabling the implantation of organoids in orthotopic locations. Size of rats could benefit grafts that require immediate vascularization, such as human fetal vascularized organs, skin, and vessels. Second, there are no reports of shortened lifespan in rat strains used for generating immunodeficient models. This is unlike most NOD-derived immunodeficient mice, since they show reduced survival due to thymoma beginning at month 8,13 although survival may not be reduced for NSG mice.75 Third, rats share some immune characteristics with humans that mice do not.126 Although some of these are not relevant to immunodeficient models where rat T cells are absent, other characteristics remain pertinent such as macrophage expression of CD4 and/or CD8 or the expression of MHC class II on endothelial cells.127 A further advantage of immunodeficient rats is their potential to sustain a larger number of human cells, therefore allowing larger numbers of human immune cells to be procured from the spleen for functional or molecular studies (50–150 × 106 cells compared with 2–10 × 106 cells in mice128).
For many years, the only immunodeficient rats were nude rats established from a spontaneous mutation in the Foxn1 gene129 (Table 2). Similar to nude mice, nude rats are only deficient in T cells, while B and NK cell compartments are normal. Furthermore, they have a leaky phenotype whereby older rats produce T cells that develop from a spontaneous rearrangement of their TCRs.204,205 Novel immunodeficient rats have been generated with mutations in key genes, including Rag1,166-168 Rag2,169-171 and Il2rg.172 Rag1 and Rag2 KO rats have normal NK cell generation, and there are residual B and T cells in single Rag1, Rag2, or Il2rg mutants. The severity of immunodeficiency has been increased by combining mutations in the aforementioned genes and/or Prkdc.167,174,176,177
TABLE 2.
Transplantation and regenerative medicine models using immunodeficient species other than mice
| Species | Mutated genes | Immune phenotype | Transplantation model | References |
|---|---|---|---|---|
| Rat | ||||
| Foxn1 | T− (leaky) B+ NK+ | Human kidney stem cells, MSCs, neural stem cells, smooth muscle progenitors, retinal cells, pancreatic progenitors, hepatocytes, osteoblasts, astrocytes, oligodendrocytes, stromal stem cells in rotator cuff, bone regeneration, dental follicular cells, cardiomyocytes, intestinal cells | 130-165 | |
| Rag1 | T− (partially) B− (partially) NK+ | Human hepatocytes | 166-168 | |
| Rag2 | T− (partially) B− (partially) NK+ | Human fetal kidneys | 169,170,208 | |
| Il2rg | T− (partially) B− (partially) NK+ | Not described | 171,172,173 | |
| Prkdc | T− B− NK+ | Human iPS-derived neural precursors | 174,175,209 | |
| Il2rg and Prkdc | T− B− NK− | Human iPS, tumor cells and hepatocytes | 174 | |
| Rag1, Rag2 and Il2rg | T− B− NK− | Human cancer cells | 176 | |
| Rag1 and Il2rg | T− B− NK− | Human skin, tumor and hepatocytes | 177 | |
| IgM | T+ B− NK+ | Rat transplantation models | 178,179 | |
| IgM, Igκ, IgλhuIgs | Human antibodies | Not described | 180,181 | |
| Prkdc, Il2rg, hSIRPa | T− B− NK− Mo inhibition | Immune humanization, human cancer cells, iPS | 182 | |
| Rag1, Il2rg, hSIRPa | T− B− NK− Mo inhibition | Immune humanization, human GvHD | 128 | |
| Nonhuman primate | IL2RG | T− B− NK− | Not described | 183 |
| Pig | ||||
| IL2RG | T− B− NK− | Not described | 184-186 | |
| RAG1 and 2 | T− B− NK+ | Not described | 187 | |
| RAG1 | T− B− NK+ | Not described | 188 | |
| RAG2 | T− B− NK+ | Human iPS | 189-191 | |
| RAG2 and IL2RG | T− B− NK− | Not described | 192 | |
| ARTEMIS | T− B− NK+ | Human skin | 193,194 | |
| Zebra fish | ||||
| Rag1 | T− B− NK+ | Human tumors | 195 | |
| Rag2 | T− B− NK+ | Not described | 196 | |
| Prkdc and Il2rg | T− B− NK- | Not described | 197 | |
| Prkdc and Il2rg | T− B− NK− | Human tumors | 198 | |
| Rabbit | ||||
| RAG1 and RAG2 | T− B− NK+ | Human tumors | 199 | |
| RAG1, RAG2 and IL2RG | T− B− NK− | Not described | 200 | |
| FOXN1, RAG1, RAG2, IL2RG | T− B− NK− | Not described | 201 | |
| IgH | T+ B− NK+ | Not described | 202 | |
| Syrian hamster | ||||
| RAG1 | T− B− NK+ | Not described | 203 | |
GvHD, graft vs host disease; iPS, induced pluripotent stem cell; NK, natural killer.
Complement levels in most inbred mouse strains,9,11 including NOD-derived immunodeficient mice,12,13 are undetectable or very low. This led to the recent development of a complement-sufficient NSG strain.206 In contrast, rats have complement levels comparable with humans, as previously shown for several strains,11 including Sprague-Dawley (SD) rats deficient for Rag-1 and Il2rg (SD-RG) and expressing hSIRPα (RRGS).128
Rats selectively deficient for B cells and consequently immunoglobulin of all isotypes have been generated by deleting the Igh6 gene (orthologue of the IgM human gene) using ZFNs178 or CRISPR/Cas9179 technologies. Using ZFNs, rats have been generated that lack not only heavy chain immunoglobulins but also both kappa and lambda light chains. These animals were then subsequently humanized by transgenic expression of human immunoglobulin coding sequences allowing the production of fully human monoclonal antibodies of high affinity.180,181
A limitation of humanized allo-GvHD models in immunodeficient mice is the need for total body irradiation to observe clear clinical GvHD, which is increasingly disparate to clinical practice. This irradiation is not required in an immunodeficient rat model in which allo-GvHD was studied.128 Additionally, Prkdc mutations to obtain a SCID phenotype in many mouse strains (like all NOD-derived immunodeficient strains such as NSG and NOG) increase toxicity due to irradiation in certain models, such as in cancer treatments, since PRKDC is an enzyme essential in DNA repair and its absence generates uncontrolled toxicity in the host tissues. Some174,176,182 but not all177 immunodeficient rats also carry a mutation in the Prkdc gene.
As in mice, the molecular incompatibility between rat SIRPα and human CD47 could lead to the elimination of certain types of human cells by macrophages. A recent publication described a rat strain combining mutations in Prkdc, Il2rg, and the expression of human SIRPα, which allowed better immune humanization compared with animals without human SIRPα.182 Immune humanization in these animals was performed using CD34+ cells from fetal liver alone or in combination with autologous fetal thymus but not with human PBMCs. Despite immune humanization, this report did not describe their use to explore any immune response (such as skin rejection or antihuman tumor responses). Animals from the RRG line, with Rag1 and Il2rg mutations, indefinitely accepted human skin, tumors, and even hepatocytes, but did not accept human PBMCs.177 However, RRG animals crossed with rats expressing human SIRPα207 (RRGS animals) or with macrophage depletion allowed immune humanization using human PBMC.128 These rats humanized with PBMCs could develop GvHD and reject tumor cells. Furthermore, the presence of normal complement levels in RRGS animals allowed the successful prevention of acute GvHD by using a new depleting antihuman T cell polyclonal antibody. Immune humanization of RRGS animals with cord blood hCD34+ cells allowed immune humanization (unpublished). Altogether, these studies indicate that as for mice, immune humanization is more difficult than tissue or tumor humanization and that inhibition of macrophage-mediated phagocytosis of human immune cells by CD47−SIRPα interactions is necessary.
Tissue Humanization of Rats
Nude Foxn1RNU rats have been transplanted with a variety of human stem cells or organs in different models of transplantation and regenerative medicine, including models of intestinal transplantation,130 bronchus grafts,131 nephropathy,132 liver,133,134 cardiac infarct,135-137 bone and chondrocyte healing and regeneration,138-142 rotator cuff lesions,143,144 tendon lesions,145,146 brain or spinal trauma,147-155 urethral dysfunction,156,157 retinal degeneration,158-162 in vivo differentiation of ES cell-derived pancreatic beta cells,163 multiciliated airway cells for the generation of an artificial trachea,164 and periodontal tissues165 (Table 2).
The most recently developed models of immunodeficient rats engrafted with solid human tissues are summarized in Table 2. A rat strain, deficient in Fah and Il2rg, is suitable for the engraftment of human hepatocytes that demonstrate a competitive proliferative advantage in comparison to the resident rat hepatocytes.171 Rag1-deficient rats transplanted with human hepatocytes have been able to partially reconstitute the liver.167 Il2rg and Prkdc mutated rats have been shown to successfully engraft human iPS, tumors, and hepatocytes.174 Rag2-deficient rats have also been transplanted with human fetal kidneys (17–18 wk gestation) as a method to explore kidney organogenesis in vivo.208 Fetal kidneys increased their size through nephrogenesis and were functionally effective in prolonging the survival of nephrectomized recipients. This is a new animal model for the study of kidney ontogeny and preclinical toxicity of therapeutics agents. In a model of neonatal hypoxic brain injury, Prkdc-deficient rats transplanted with human neural precursor cells showed amelioration of lesions.209 It has also been demonstrated that rats from the RRG line bearing Rag1 and Il2rg mutations can indefinitely accept transplanted human skin and tumors.128
It is interesting to note that, in some models, immunodeficient rats seem to tolerate xenografts better than immunodeficient mice. This is the case for human pancreatic progenitors that matured faster in rats than mice.163 It is possible that accelerated maturation of beta cells was due to similar glucose levels in rats and humans during fasting or after glucose challenge, whereas mice showed high fasting glucose levels and dramatic glucose fluctuations peaking at higher levels after glucose challenge.163 Additionally, human tumors grew significantly faster and larger in SD-RG rats than in NSG mice and several fresh lung squamous tumors from patients (PDX model) were successfully implanted in this immunodeficient rat line.176 The use of immunodeficient rats for the generation of PDX tumors coupled with the humanization of immune responses will likely represent useful models for cancer research.
Rats do, however, have disadvantages when compared with mice. Their larger size implies higher breeding costs. There are also fewer established genetic modifications applicable in rats when compared with immunodeficient mice, such as expression of human cytokines and human MHC or elimination of dendritic cells. In the near future, the application of new genome editing nucleases, such as meganucleases,166 ZFNs,210 TALENs,211 and CRISPR/Cas9,212,213 will facilitate the development of rats with disease-specific mutations including with Cre-conditional mutations.214,215
IMMUNODEFICIENCY AND HUMANIZATION IN OTHER SPECIES
Pigs are an attractive species for the experimental implantation of human cells or tissues, because of their large size and physiological proximity to humans. Several lines of immunodeficient pigs carrying spontaneous or induced inactivation of RAG1, RAG2, ARTEMIS, or IL2RG alone or combined (RAG2 and IL2RG) have been described.216 However, in pigs that carry mutations of the IL2RG,184-186 RAG1,188 RAG2,191 RAG2, and IL2RG192 genes, there are no descriptions of transplantation of human cells or tissues. In T(−)B(−)NK(+) SCID pigs carrying spontaneous point mutations in the ARTEMIS gene,193 human cryopreserved deceased skin was accepted for at least 28 d.194 Since the successful in utero engraftment and differentiation of human CD34+ progenitors in immunocompetent pigs has been reported,217 it has been proposed that immunodeficient pigs could be used in this setting to generate large numbers of human cells.216 Human lymphocytes can recognize porcine MHC molecules and respond at similar levels to human allogenic MHC proteins.218,219 The homology between human and porcine MHC molecules means that antibodies against HLA class II antigens have a propensity to also bind swine leukocyte antigen class II antigens. In the context of future immune humanization of immunodeficient pigs, it is important to point out that porcine SIRPα binds to human CD47 and thus provides inhibition of pig macrophage phagocytosis of human leukocytes.220
Zebrafish offer the attractive characteristic of optical transparency and ease of breeding. Although immunodeficient zebrafish have been described, such as those deficient for Rag2,196 Rag1,195 or for both Prkdc and Il2rg,198 they have only been used for implanting human tumors and not for normal human tissue or cell transplantation. Human CD34+ HSCs have been transplanted into immunocompetent zebrafish where they are home to the caudal niche and engage endothelial cells and undergo cell division.221
Rabbits have excellent fecundity and a convenient size for many experimental procedures, while remaining small enough for maintenance as a laboratory animal. Immunocompromised rabbits have been produced with deficiencies in RAG1 and RAG2199; RAG1, RAG2, and IL2RG200 FOXN1, RAG1, RAG2, IL2RG,201 and IgH,202 but to date they have only been used for implantation of human tumor cells. Similarly, Syrian hamsters are small animal models used in several areas of research.222Syrian hamsters deficient for RAG1 have been described but have not yet been humanized.203Finally in nonhuman primates, immunocompromised marmosets mutated for IL2RG using ZFNs and TALENs have been described,183 but there have been no reports on immune humanization or transplantation of human tissues in these animals.
CONCLUSIONS
It is clear that the use of humanized animals is important in providing opportunities to understand the mechanisms of human immune responses to tissue transplants in vivo. These models provide an excellent path for the development and assessment of human-based immunotherapies in a human context. Regulatory agencies are starting to accept data from humanized models as indicative of therapeutic efficacy of a human-specific agent. A number of newer models have been developed to provide a more complete picture of human immune responses or to allow surgical procedures that would otherwise be impossible in mice. For most studies, this is not necessarily the case that the most advanced model should be used, rather that a specific model should be selected that answers the experimental question at hand. It is also important to note the significant limitations that exist. First, none of the models described fully reproduce all elements of a functional human immune system in the peripheral blood, and also in tissues as for macrophages and innate lymphoid cells.223 Second, human immune responses result from a complex interaction of cells between the peripheral, tissue, and lymphoid systems. Many of these elements do not exist even in the most advanced models. Third, these models do not yet provide a complete substitute for pharmacokinetic studies in larger animal models. However, it is arguable that some safety elements related to the effects on human leukocytes (eg, a cytokine storm) would be observed in a fully humanized animal. Nevertheless, agents that are found to be unsuccessful in a humanized animal are unlikely to be successful after translation, providing a method for filtering out therapies that would fail in expensive and risky early clinical trials.
PERSPECTIVES
In cancer studies, the outcomes of therapeutic adoptive T cell transfers could be modeled in immune-humanized mice engrafted with patient tumor (PDX models) and the therapeutic cell transfer.224 Patient material could also be used in generating relevant and insightful humanized models for transplantation. An example is a recent study that engrafted mice with pericardiophrenic artery segments obtained from the donor and PBMC from the recipient. Interestingly, histological changes in the artery were associated with development of chronic lung allograft dysfunction in the patient, indicating the presence of alloreactive T cells at time of transplant.225 Such models could provide a method for developing customized patient-specific therapies.
The importance of the microbiota in immune education is recognized, but the role of the microbiome in tolerance and rejection is an ongoing subject of research.226 Allotransplantation and immunosuppression are associated with changes in gut microbiota population frequency and diversity.227 Allogenic animal models have shown the ability of commensal bacteria in the gut or on the skin to influence allograft survival.228-230 In NSG mice reconstituted by neonatal administration of human CD34+ cells, antibiotics modified skin allograft tolerance and the success of the immunotherapy teplizumab.117 The impact of microbiota on allograft survival and sensitivity to immunointervention is a problem for the reproducibility of animal work, wherein animals housed in different facilities vary significantly in their microbiome composition.231,232 Indeed, mice from different animal houses have different capacities to tolerate or reject orthotopic lung allografts and the same principle applies to the tolerance of xenografts in immune humanized animals.231 Methods do exist to partially engraft human microbiota into mature murine intestinal environments, but the mammalian microbiome is species-specific and symbiotic.233-235 Not all human intestinal flora is maintained in a mouse intestine after transfer, resulting in bacterial diversity that is not representative of the original donor.
Rats have some characteristics that make them attractive for humanization. Their larger size makes them well suited for orthotopic implantation of human organoids of different tissues differentiated from human iPS or ES cells. New and exciting models are emerging using interspecies chimeras to generate organs from a different species in an animal knockout for a tissue master gene. For these models, the rat is often used.236,237 Nevertheless, species chimerism decreases with time during the embryo development and this could be due to in embryo immune responses, including SIRPα-CD47 incompatibilities that could benefit from the use of SIRPα humanized rats. Additionally, a number of rat knockout models that reproduce human genetic diseases better than mice, such as in dystrophin238 or Aire-deficient239 rats, could be used in an immunodeficient-humanized setting.
In nonmouse immunodeficient models, the rat is the only species that has been immune humanized. Yet very little has yet been performed in terms of tissue transplantation outside of current mouse models. It is likely that immune humanized pigs will be developed in the future. Given the recent advances in genetic modification, the tools now exist to produce immunodeficient animals in a range of species. The demand for immunodeficient recipients will likely increase in the future for the implantation of cells derived from human iPS and ES cells, such as hepatocytes, pancreatic beta cells, and retinal cells. Recent years have seen an explosion in the production of genetically humanized transgenic mice to sustain specific and functional components of human cells and tissues, which may move across species. Regenerative medicine is directly contributing to the pool of human tissues and organs that can be incorporated into other species, having already produced kidney organoids, muscle, cartilage, bone, intestines, bladder walls, and liver. The next step will be to apply these models to the study of cell, tissue, and solid organ transplantation and rejection. For example, antibody-mediated rejection could be modeled in transgenic mice with functional B cell antibody responses combined with a vascular transplant, or tissue-specific immunogenicity could be assessed using kidney organoid implantation together with T cell engraftment. The next generation of immunotherapies, such as cellular therapies and immunosuppressant-loaded nanoparticles, will require human immunity and tissues to thoroughly assess their functionality, immunogenicity, and target specificity. The development of advanced humanized animals with greater likeness to functional multilineage human immune systems will therefore permit the study and replication of more complex responses in allotransplantation, GvHD, and regenerative medicine.
Footnotes
This work was supported by generous funding from Biogenouest by Région Pays de la Loire, IBiSA program, TEFOR (Investissements d’Avenir French Government program, ANRII-INSB-0014), LabCom SOURIRAT project (ANR-14-LAB5-0008), Labex IGO project (Investissements d’Avenir French Government program, ANR-11-LABX-0016-01), IHU-Cesti project (Investissements d’Avenir French Government program, ANR-10-IBHU-005, Nantes Métropole and Région Pays de la Loire), Fondation Progreffe, the ReSHAPE 825392 EU Horizon 2020, Kidney Research UK, the Academy of Medical Sciences, the Wellcome Trust, and the Clarendon Fund and the Restore Research Trust. F.I. is a Wellcome Trust CRCD Fellow, J.H. is a KRUK Senior Fellow, and A.R.C. is an Oxford-Celgene Fellow.
The authors declare no conflicts of interest.
I.A. and F.I. planned and wrote the article. F.I. and J.H. performed the included experiments. G.A., S.M., A.R.C., and J.H. wrote and edited the article as well as performed bibliography searches. G.A., S.M., and A.R.C. contributed equally. F.I. and I.A.are both senior and corresponding authors.
REFERENCES
- 1.Kenter MJ, Cohen AF. Establishing risk of human experimentation with drugs: lessons from TGN1412. Lancet. 2006; 368:1387–1391. doi:10.1016/S0140-6736(06)69562-7 [DOI] [PubMed] [Google Scholar]
- 2.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006; 355:1018–1028. doi:10.1056/NEJMoa063842 [DOI] [PubMed] [Google Scholar]
- 3.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004; 172:2731–2738. doi:10.4049/jimmunol.172.5.2731 [DOI] [PubMed] [Google Scholar]
- 4.Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987; 51:503–512. doi:10.1016/0092-8674(87)90646-5 [DOI] [PubMed] [Google Scholar]
- 5.Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983; 301:527–530. doi:10.1038/301527a0 [DOI] [PubMed] [Google Scholar]
- 6.Shinkai Y, Rathbun G, Lam KP, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992; 68:855–867. doi:10.1016/0092-8674(92)90029-c [DOI] [PubMed] [Google Scholar]
- 7.Mosier DE, Gulizia RJ, Baird SM, et al. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988; 335:256–259. doi:10.1038/335256a0 [DOI] [PubMed] [Google Scholar]
- 8.Lapidot T, Pflumio F, Doedens M, et al. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992; 255:1137–1141. doi:10.1126/science.1372131 [DOI] [PubMed] [Google Scholar]
- 9.Wetsel RA, Fleischer DT, Haviland DL. Deficiency of the murine fifth complement component (C5). A 2-base pair gene deletion in a 5-exon. J Biol Chem. 1990; 265:2435–2440 [PubMed] [Google Scholar]
- 10.Serreze DV, Leiter EH. Defective activation of T suppressor cell function in nonobese diabetic mice. Potential relation to cytokine deficiencies. J Immunol. 1988; 140:3801–3807 [PubMed] [Google Scholar]
- 11.Ong GL, Mattes MJ. Mouse strains with typical mammalian levels of complement activity. J Immunol Methods. 1989; 125:147–158. doi:10.1016/0022-1759(89)90088-4 [DOI] [PubMed] [Google Scholar]
- 12.Baxter AG, Cooke A. Complement lytic activity has no role in the pathogenesis of autoimmune diabetes in NOD mice. Diabetes. 1993; 42:1574–1578. doi:10.2337/diab.42.11.1574 [DOI] [PubMed] [Google Scholar]
- 13.Shultz LD, Schweitzer PA, Christianson SW, et al. Multiple defects in innate and adaptive immunologic function in NOD/Ltsz-scid mice. J Immunol. 1995; 154:180–191 [PubMed] [Google Scholar]
- 14.Greiner DL, Shultz LD, Yates J, et al. Improved engraftment of human spleen cells in NOD/ltsz-scid/scid mice as compared with C.B-17-scid/scid mice. Am J Pathol. 1995; 146:888–902 [PMC free article] [PubMed] [Google Scholar]
- 15.Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma©(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002; 100:3175–3182. doi:10.1182/blood-2001-12-0207 [DOI] [PubMed] [Google Scholar]
- 16.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007; 7:118–130. doi:10.1038/nri2017 [DOI] [PubMed] [Google Scholar]
- 17.Pearson T, Shultz LD, Miller D, et al. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2R gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin Exp Immunol. 2008; 154:270–284. doi:10.1111/j.1365-2249.2008.03753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brehm MA, Cuthbert A, Yang C, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010; 135:84–98. doi:10.1016/j.clim.2009.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qing Y, Lin Y, Gerson SL. An intrinsic BM hematopoietic niche occupancy defect of HSC in scid mice facilitates exogenous HSC engraftment. Blood. 2012; 119:1768–1771. doi:10.1182/blood-2011-05-350611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legrand N, Huntington ND, Nagasawa M, et al. Functional CD47/signal regulatory protein alpha (SIRPα) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc Natl Acad Sci U S A. 2011; 108:13224–13229. doi:10.1073/pnas.1101398108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamauchi T, Takenaka K, Urata S, et al. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood. 2013; 121:1316–1325. doi:10.1182/blood-2012-06-440354 [DOI] [PubMed] [Google Scholar]
- 22.Strowig T, Rongvaux A, Rathinam C, et al. Transgenic expression of human signal regulatory protein alpha in rag2-/-gamma©-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A. 2011; 108:13218–13223. doi:10.1073/pnas.1109769108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labarthe L, Henriquez S, Lambotte O, et al. Frontline science: exhaustion and senescence marker profiles on human T cells in BRGSF-A2 humanized mice resemble those in human samples. J Leukoc Biol. 2020; 107:27–42. doi:10.1002/JLB.5HI1018-410RR [DOI] [PubMed] [Google Scholar]
- 24.King MA, Covassin L, Brehm MA, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009; 157:104–118. doi:10.1111/j.1365-2249.2009.03933.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shultz LD, Saito Y, Najima Y, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 2010; 107:13022–13027. doi:10.1073/pnas.1000475107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki M, Takahashi T, Katano I, et al. Induction of human humoral immune responses in a novel HLA-DR-expressing transgenic NOD/Shi-scid/γcnull mouse. Int Immunol. 2012; 24:243–252. doi:10.1093/intimm/dxs045 [DOI] [PubMed] [Google Scholar]
- 27.Billerbeck E, Barry WT, Mu K, et al. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rγ(null) humanized mice. Blood. 2011; 117:3076–3086. doi:10.1182/blood-2010-08-301507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ono R, Watanabe T, Kawakami E, et al. Co-activation of macrophages and T cells contribute to chronic GVHD in human IL-6 transgenic humanised mouse model. EBioMedicine. 2019; 41:584–596. doi:10.1016/j.ebiom.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahmig S, Kronstein-Wiedemann R, Fohgrub J, et al. Improved human erythropoiesis and platelet formation in humanized NSGW41 mice. Stem Cell Reports. 2016; 7:591–601. doi:10.1016/j.stemcr.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosgun KN, Rahmig S, Mende N, et al. Kit regulates HSC engraftment across the human-mouse species barrier. Cell Stem Cell. 2014; 15:227–238. doi:10.1016/j.stem.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 31.McIntosh BE, Brown ME, Duffin BM, et al. Nonirradiated NOD,B6.SCID IL2rγ-/- Kit(W41/W41) (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Reports. 2015; 4:171–180. doi:10.1016/j.stemcr.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katano I, Nishime C, Ito R, et al. Long-term maintenance of peripheral blood derived human NK cells in a novel human IL-15- transgenic NOG mouse. Sci Rep. 2017; 7:17230 doi:10.1038/s41598-017-17442-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito R, Takahashi T, Katano I, et al. Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. J Immunol. 2013; 191:2890–2899. doi:10.4049/jimmunol.1203543 [DOI] [PubMed] [Google Scholar]
- 34.Katano I, Takahashi T, Ito R, et al. Predominant development of mature and functional human NK cells in a novel human IL-2-producing transgenic NOG mouse. J Immunol. 2015; 194:3513–3525. doi:10.4049/jimmunol.1401323 [DOI] [PubMed] [Google Scholar]
- 35.Danner R, Chaudhari SN, Rosenberger J, et al. Expression of HLA class II molecules in humanized NOD.rag1ko.IL2RGCKO mice is critical for development and function of human T and B cells. PLoS One. 2011; 6:e19826 doi:10.1371/journal.pone.0019826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris DT, Badowski M, Balamurugan A, et al. Long-term human immune system reconstitution in non-obese diabetic (NOD)-Rag (-)-γ chain (-) (NRG) mice is similar but not identical to the original stem cell donor. Clin Exp Immunol. 2013; 174:402–413. doi:10.1111/cei.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller PH, Rabu G, MacAldaz M, et al. Analysis of parameters that affect human hematopoietic cell outputs in mutant c-kit-immunodeficient mice. Exp Hematol. 2017; 48:41–49. doi:10.1016/j.exphem.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rich BS, Honeyman JN, Darcy DG, et al. Endogenous antibodies for tumor detection. Sci Rep. 2014; 4:5088 doi:10.1038/srep05088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traggiai E, Chicha L, Mazzucchelli L, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004; 304:104–107. doi:10.1126/science.1093933 [DOI] [PubMed] [Google Scholar]
- 40.Willinger T, Rongvaux A, Takizawa H, et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci U S A. 2011; 108:2390–2395. doi:10.1073/pnas.1019682108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Mention JJ, Court N, et al. A novel Flt3-deficient HIS mouse model with selective enhancement of human DC development. Eur J Immunol. 2016; 46:1291–1299. doi:10.1002/eji.201546132 [DOI] [PubMed] [Google Scholar]
- 42.Rongvaux A, Willinger T, Martinek J, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014; 32:364–372. doi:10.1038/nbt.2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng Y, Liu B, Rubio MT, et al. Creation of an immunodeficient HLA-transgenic mouse (HUMAMICE) and functional validation of human immunity after transfer of HLA-matched human cells. PLoS One. 2017; 12:e0173754 doi:10.1371/journal.pone.0173754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pantelouris EM. Absence of thymus in a mouse mutant. Nature. 1968; 217:370–371. doi:10.1038/217370a0 [DOI] [PubMed] [Google Scholar]
- 45.Snell GD. Methods for the study of histocompatibility genes. J Genet. 1948; 49:87–108. doi:10.1007/bf02986826 [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg AS, Mizuochi T, Singer A. Analysis of T-cell subsets in rejection of Kb mutant skin allografts differing at class I MHC. Nature. 1986; 322:829–831. doi:10.1038/322829a0 [DOI] [PubMed] [Google Scholar]
- 47.Tilney NL, Strom TB, Kupiec-Weglinski JW. Humoral and cellular mechanisms in acute allograft injury. J Pediatr. 1987; 111Pt 21000–1003. doi:10.1016/s0022-3476(87)80044-6 [DOI] [PubMed] [Google Scholar]
- 48.Issa F, Hester J, Goto R, et al. Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation. 2010; 90:1321–1327. doi:10.1097/TP.0b013e3181ff8772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macedo C, Orkis EA, Popescu I, et al. Contribution of naïve and memory T-cell populations to the human alloimmune response. Am J Transplant. 2009; 9:2057–2066. doi:10.1111/j.1600-6143.2009.02742.x [DOI] [PubMed] [Google Scholar]
- 50.Brehm MA, Kenney LL, Wiles MV, et al. Lack of acute xenogeneic graft-versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. FASEB J. 2019; 33:3137–3151. doi:10.1096/fj.201800636R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu W, Li XC. An overview on non-T cell pathways in transplant rejection and tolerance. Curr Opin Organ Transplant. 2010; 15:422–426. doi:10.1097/MOT.0b013e32833b7903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaRosa DF, Rahman AH, Turka LA. The innate immune system in allograft rejection and tolerance. J Immunol. 2007; 178:7503–7509. doi:10.4049/jimmunol.178.12.7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H). Transplantation. 2003; 76:120–129. doi:10.1097/01.TP.0000071362.99021.D9 [DOI] [PubMed] [Google Scholar]
- 54.Wu T, Bond G, Martin D, et al. Histopathologic characteristics of human intestine allograft acute rejection in patients pretreated with thymoglobulin or alemtuzumab. Am J Gastroenterol. 2006; 101:1617–1624. doi:10.1111/j.1572-0241.2006.00611.x [DOI] [PubMed] [Google Scholar]
- 55.He H, Stone JR, Perkins DL. Analysis of robust innate immune response after transplantation in the absence of adaptive immunity. Transplantation. 2002; 73:853–861. doi:10.1097/00007890-200203270-00005 [DOI] [PubMed] [Google Scholar]
- 56.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007; 7:610–621. doi:10.1038/nri2132 [DOI] [PubMed] [Google Scholar]
- 57.Gill RG. NK cells: elusive participants in transplantation immunity and tolerance. Curr Opin Immunol. 2010; 22:649–654. doi:10.1016/j.coi.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Vries VC, Noelle RJ. Mast cell mediators in tolerance. Curr Opin Immunol. 2010; 22:643–648. doi:10.1016/j.coi.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosier DE, Gulizia RJ, Baird SM, et al. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991; 251:791–794. doi:10.1126/science.1990441 [DOI] [PubMed] [Google Scholar]
- 60.Brehm MA, Shultz LD, Greiner DL. Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes. 2010; 17:120–125. doi:10.1097/MED.0b013e328337282f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watanabe Y, Takahashi T, Okajima A, et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice). Int Immunol. 2009; 21:843–858. doi:10.1093/intimm/dxp050 [DOI] [PubMed] [Google Scholar]
- 62.Lang J, Ota T, Kelly M, et al. Receptor editing and genetic variability in human autoreactive B cells. J Exp Med. 2016; 213:93–108. doi:10.1084/jem.20151039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt MR, Appel MC, Giassi LJ, et al. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS One. 2008; 3:e3192 doi:10.1371/journal.pone.0003192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009; 9:491–502. doi:10.1038/nri2572 [DOI] [PubMed] [Google Scholar]
- 65.Treml JF, Hao Y, Stadanlick JE, et al. The BLyS family: toward a molecular understanding of B cell homeostasis. Cell Biochem Biophys. 2009; 53:1–16. doi:10.1007/s12013-008-9036-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mackay F, Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008; 19:263–276. doi:10.1016/j.cytogfr.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 67.Lang J, Zhang B, Kelly M, et al. Replacing mouse BAFF with human BAFF does not improve B-cell maturation in hematopoietic humanized mice. Blood Adv. 2017; 1:2729–2741. doi:10.1182/bloodadvances.2017010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suematsu S, Matsuda T, Aozasa K, et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1989; 86:7547–7551. doi:10.1073/pnas.86.19.7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu H, Borsotti C, Schickel JN, et al. A novel humanized mouse model with significant improvement of class-switched, antigen-specific antibody production. Blood. 2017; 129:959–969. doi:10.1182/blood-2016-04-709584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bar-Ephraïm YE, Mebius RE. Innate lymphoid cells in secondary lymphoid organs. Immunol Rev. 2016; 271:185–199. doi:10.1111/imr.12407 [DOI] [PubMed] [Google Scholar]
- 71.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014; 41:529–542. doi:10.1016/j.immuni.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015; 16:142–152. doi:10.1038/ni.3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sage PT, Sharpe AH. T follicular regulatory cells. Immunol Rev. 2016; 271:246–259. doi:10.1111/imr.12411 [DOI] [PubMed] [Google Scholar]
- 74.Holyoake TL, Nicolini FE, Eaves CJ. Functional differences between transplantable human hematopoietic stem cells from fetal liver, cord blood, and adult marrow. Exp Hematol. 1999; 27:1418–1427. doi:10.1016/s0301-472x(99)00078-8 [DOI] [PubMed] [Google Scholar]
- 75.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/ltsz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005; 174:6477–6489. doi:10.4049/jimmunol.174.10.6477 [DOI] [PubMed] [Google Scholar]
- 76.Chicha L, Tussiwand R, Traggiai E, et al. Human adaptive immune system rag2-/-gamma©-/- mice. Ann N Y Acad Sci. 2005; 1044:236–243. doi:10.1196/annals.1349.029 [DOI] [PubMed] [Google Scholar]
- 77.McCune JM, Namikawa R, Kaneshima H, et al. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988; 241:1632–1639. doi:10.1126/science.2971269 [DOI] [PubMed] [Google Scholar]
- 78.Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006; 12:1316–1322. doi:10.1038/nm1431 [DOI] [PubMed] [Google Scholar]
- 79.Lan P, Tonomura N, Shimizu A, et al. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006; 108:487–492. doi:10.1182/blood-2005-11-4388 [DOI] [PubMed] [Google Scholar]
- 80.Wege AK, Melkus MW, Denton PW, et al. Functional and phenotypic characterization of the humanized BLT mouse model. Curr Top Microbiol Immunol. 2008; 324:149–165. doi:10.1007/978-3-540-75647-7_10 [DOI] [PubMed] [Google Scholar]
- 81.Brainard DM, Seung E, Frahm N, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009; 83:7305–7321. doi:10.1128/JVI.02207-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown ME, Zhou Y, McIntosh BE, et al. A humanized mouse model generated using surplus neonatal tissue. Stem Cell Reports. 2018; 10:1175–1183. doi:10.1016/j.stemcr.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McDermott SP, Eppert K, Lechman ER, et al. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010; 116:193–200. doi:10.1182/blood-2010-02-271841 [DOI] [PubMed] [Google Scholar]
- 84.Gonzalez L, Strbo N, Podack ER. Humanized mice: novel model for studying mechanisms of human immune-based therapies. Immunol Res. 2013; 57:326–334. doi:10.1007/s12026-013-8471-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herndler-Brandstetter D, Shan L, Yao Y, et al. Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proc Natl Acad Sci U S A. 2017; 114:E9626–E9634. doi:10.1073/pnas.1705301114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Issa F, Hester J, Milward K, et al. Homing of regulatory T cells to human skin is important for the prevention of alloimmune-mediated pathology in an in vivo cellular therapy model. PLoS One. 2012; 7:e53331 doi:10.1371/journal.pone.0053331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu DC, Hester J, Nadig SN, et al. Ex vivo expanded human regulatory T cells can prolong survival of a human islet allograft in a humanized mouse model. Transplantation. 2013; 96:707–716. doi:10.1097/TP.0b013e31829fa271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nadig SN, Wieckiewicz J, Wu DC, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010; 16:809–813. doi:10.1038/nm.2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zaitsu M, Issa F, Hester J, et al. Selective blockade of CD28 on human T cells facilitates regulation of alloimmune responses. JCI Insight. 2017; 2:89381 doi:10.1172/jci.insight.89381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bézie S, Meistermann D, Boucault L, et al. Ex vivo expanded human non-cytotoxic CD8+CD45RCLOW/-Tregs efficiently delay skin graft rejection and GVHD in humanized mice. Front Immunol. 2017; 8:2014 doi:10.3389/fimmu.2017.02014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dawson NA, Lamarche C, Hoeppli RE, et al. Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight. 2019; 4:123672 doi:10.1172/jci.insight.123672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pober JS, Bothwell AL, Lorber MI, et al. Immunopathology of human T cell responses to skin, artery and endothelial cell grafts in the human peripheral blood lymphocyte/severe combined immunodeficient mouse. Springer Semin Immunopathol. 2003; 25:167–180. doi:10.1007/s00281-003-0135-1 [DOI] [PubMed] [Google Scholar]
- 93.Boardman DA, Philippeos C, Fruhwirth GO, et al. Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am J Transplant. 2017; 17:931–943. doi:10.1111/ajt.14185 [DOI] [PubMed] [Google Scholar]
- 94.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 kidney meeting report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018; 18:293–307. doi:10.1111/ajt.14625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shepherd BR, Enis DR, Wang F, et al. Vascularization and engraftment of a human skin substitute using circulating progenitor cell-derived endothelial cells. FASEB J. 2006; 20:1739–1741. doi:10.1096/fj.05-5682fje [DOI] [PubMed] [Google Scholar]
- 96.Abrahimi P, Qin L, Chang WG, et al. Blocking MHC class II on human endothelium mitigates acute rejection. JCI Insight. 2016; 1:e85293 doi:10.1172/jci.insight.85293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merola J, Reschke M, Pierce RW, et al. Progenitor-derived human endothelial cells evade alloimmunity by CRISPR/Cas9-mediated complete ablation of MHC expression. JCI Insight. 2019; 4:129739 doi:10.1172/jci.insight.129739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perrier-Groult E, Pérès E, Pasdeloup M, et al. Evaluation of the biocompatibility and stability of allogeneic tissue-engineered cartilage in humanized mice. PLoS One. 2019; 14:e0217183 doi:10.1371/journal.pone.0217183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garcia SM, Tamaki S, Lee S, et al. High-yield purification, preservation, and serial transplantation of human satellite cells. Stem Cell Reports. 2018; 10:1160–1174. doi:10.1016/j.stemcr.2018.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holzapfel BM, Hutmacher DW, Nowlan B, et al. Tissue engineered humanized bone supports human hematopoiesis in vivo. Biomaterials. 2015; 61:103–114. doi:10.1016/j.biomaterials.2015.04.057 [DOI] [PubMed] [Google Scholar]
- 101.Reinisch A, Hernandez DC, Schallmoser K, et al. Generation and use of a humanized bone-marrow-ossicle niche for hematopoietic xenotransplantation into mice. Nat Protoc. 2017; 12:2169–2188. doi:10.1038/nprot.2017.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cortez AR, Poling HM, Brown NE, et al. Transplantation of human intestinal organoids into the mouse mesentery: a more physiologic and anatomic engraftment site. Surgery. 2018; 164:643–650. doi:10.1016/j.surg.2018.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagy N, Marsiano N, Bruckner RS, et al. Xenotransplantation of human intestine into mouse abdomen or subcutaneous tissue: novel platforms for the study of the human enteric nervous system. Neurogastroenterol Motil. 2018; 30:e13212 doi:10.1111/nmo.13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sampaziotis F, Justin AW, Tysoe OC, et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017; 23:954–963. doi:10.1038/nm.4360 [DOI] [PubMed] [Google Scholar]
- 105.Irudayaswamy A, Muthiah M, Zhou L, et al. Long-term fate of human fetal liver progenitor cells transplanted in injured mouse livers. Stem Cells. 2018; 36:103–113. doi:10.1002/stem.2710 [DOI] [PubMed] [Google Scholar]
- 106.Yuan L, Liu X, Zhang L, et al. A chimeric humanized mouse model by engrafting the human induced pluripotent stem cell-derived hepatocyte-like cell for the chronic hepatitis B virus infection. Front Microbiol. 2018; 9:908 doi:10.3389/fmicb.2018.00908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Naritomi Y, Sanoh S, Ohta S. Utility of chimeric mice with humanized liver for predicting human pharmacokinetics in drug discovery: comparison with in vitro-in vivo extrapolation and allometric scaling. Biol Pharm Bull. 2019; 42:327–336. doi:10.1248/bpb.b18-00754 [DOI] [PubMed] [Google Scholar]
- 108.Azuma H, Paulk N, Ranade A, et al. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007; 25:903–910. doi:10.1038/nbt1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilson EM, Bial J, Tarlow B, et al. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res. 2014; 13Pt A404–412. doi:10.1016/j.scr.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ng SS, Saeb-Parsy K, Blackford SJI, et al. Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials. 2018; 182:299–311. doi:10.1016/j.biomaterials.2018.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Takasato M, Er PX, Chiu HS, et al. Generation of kidney organoids from human pluripotent stem cells. Nat Protoc. 2016; 11:1681–1692. doi:10.1038/nprot.2016.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van den Berg CW, Ritsma L, Avramut MC, et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports. 2018; 10:751–765. doi:10.1016/j.stemcr.2018.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hannon M, Lechanteur C, Lucas S, et al. Infusion of clinical-grade enriched regulatory T cells delays experimental xenogeneic graft-versus-host disease. Transfusion. 2014; 54:353–363. doi:10.1111/trf.12279 [DOI] [PubMed] [Google Scholar]
- 114.Zheng J, Liu Y, Liu Y, et al. Human CD8+ regulatory T cells inhibit GVHD and preserve general immunity in humanized mice. Sci Transl Med. 2013; 5:168ra9 doi:10.1126/scitranslmed.3004943 [DOI] [PubMed] [Google Scholar]
- 115.Tobin LM, Healy ME, English K, et al. Human mesenchymal stem cells suppress donor CD4(+) T cell proliferation and reduce pathology in a humanized mouse model of acute graft-versus-host disease. Clin Exp Immunol. 2013; 172:333–348. doi:10.1111/cei.12056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poirier N, Mary C, Dilek N, et al. Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab’ antibody. Am J Transplant. 2012; 12:2630–2640. doi:10.1111/j.1600-6143.2012.04164.x [DOI] [PubMed] [Google Scholar]
- 117.Gülden E, Vudattu NK, Deng S, et al. Microbiota control immune regulation in humanized mice. JCI Insight. 2017; 2:91709 doi:10.1172/jci.insight.91709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burger DR, Parker Y, Guinta K, et al. PRO 140 monoclonal antibody to CCR5 prevents acute xenogeneic graft-versus-host disease in NOD-scid IL-2Rynull mice. Biol Blood Marrow Transplant. 2018; 24:260–266. doi:10.1016/j.bbmt.2017.10.041 [DOI] [PubMed] [Google Scholar]
- 119.Betts BC, Bastian D, Iamsawat S, et al. Targeting JAK2 reduces GVHD and xenograft rejection through regulation of T cell differentiation. Proc Natl Acad Sci U S A. 2018; 115:1582–1587. doi:10.1073/pnas.1712452115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Leur K, Luk F, van den Bosch TPP, et al. The effects of an IL-21 receptor antagonist on the alloimmune response in a humanized mouse skin transplant model. Transplantation. 2019; 103:2065–2074. doi:10.1097/TP.0000000000002773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cui J, Qin L, Zhang J, et al. Ex vivo pretreatment of human vessels with siRNA nanoparticles provides protein silencing in endothelial cells. Nat Commun. 2017; 8:191 doi:10.1038/s41467-017-00297-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Issa F, Milward K, Goto R, et al. Transiently activated human regulatory T cells upregulate BCL-XL expression and acquire a functional advantage in vivo. Front Immunol. 2019; 10:889 doi:10.3389/fimmu.2019.00889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sagoo P, Ali N, Garg G, et al. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011; 3:83ra42 doi:10.1126/scitranslmed.3002076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.MacDonald KG, Hoeppli RE, Huang Q, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016; 126:1413–1424. doi:10.1172/JCI82771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boroughs AC, Larson RC, Choi BD, et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight. 2019; 5:126194 doi:10.1172/jci.insight.126194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wildner G. Are rats more human than mice? Immunobiology. 2019; 224:172–176. doi:10.1016/j.imbio.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 127.Ladhoff J, Fleischer B, Hara Y, et al. Immune privilege of endothelial cells differentiated from endothelial progenitor cells. Cardiovasc Res. 2010; 88:121–129. doi:10.1093/cvr/cvq109 [DOI] [PubMed] [Google Scholar]
- 128.Ménoret S, Ouisse LH, Tesson L, et al. In vivo analysis of human immune responses in immunodeficient rats. Transplantation. 2020. Apr; 104:715–723. doi:10.1097/TP.0000000000003047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Festing MF, May D, Connors TA, et al. An athymic nude mutation in the rat. Nature. 1978; 274:365–366. doi:10.1038/274365a0 [DOI] [PubMed] [Google Scholar]
- 130.Kitano K, Schwartz DM, Zhou H, et al. Bioengineering of functional human induced pluripotent stem cell-derived intestinal grafts. Nat Commun. 2017; 8:765 doi:10.1038/s41467-017-00779-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guihaire J, Itagaki R, Stubbendorff M, et al. Orthotopic tracheal transplantation using human bronchus: an original xenotransplant model of obliterative airway disorder. Transpl Int. 2016; 29:1337–1348. doi:10.1111/tri.12854 [DOI] [PubMed] [Google Scholar]
- 132.Santeramo I, Herrera Perez Z, Illera A, et al. Human kidney-derived cells ameliorate acute kidney injury without engrafting into renal tissue. Stem Cells Transl Med. 2017; 6:1373–1384. doi:10.1002/sctm.16-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Igarashi Y, Tateno C, Tanaka Y, et al. Engraftment of human hepatocytes in the livers of rats bearing bone marrow reconstructed with immunodeficient mouse bone marrow cells. Xenotransplantation. 2008; 15:235–245. doi:10.1111/j.1399-3089.2008.00483.x [DOI] [PubMed] [Google Scholar]
- 134.Pettinato G, Ramanathan R, Fisher RA, et al. Scalable differentiation of human ipscs in a multicellular spheroid-based 3D culture into hepatocyte-like cells through direct Wnt/β-catenin pathway inhibition. Sci Rep. 2016; 6:32888 doi:10.1038/srep32888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sougawa N, Miyagawa S, Fukushima S, et al. Laminin-511 supplementation enhances stem cell localization with suppression in the decline of cardiac function in acute infarct rats. Transplantation. 2019; 103:e119–e127. doi:10.1097/TP.0000000000002653 [DOI] [PubMed] [Google Scholar]
- 136.Saucourt C, Vogt S, Merlin A, et al. Design and validation of an automated process for the expansion of peripheral blood-derived CD34+ cells for clinical use after myocardial infarction. Stem Cells Transl Med. 2019; 8:822–832. doi:10.1002/sctm.17-0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao X, Chen H, Xiao D, et al. Comparison of non-human primate versus human induced pluripotent stem cell-derived cardiomyocytes for treatment of myocardial infarction. Stem Cell Reports. 2018; 10:422–435. doi:10.1016/j.stemcr.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pytlík R, Rentsch C, Soukup T, et al. Efficacy and safety of human mesenchymal stromal cells in healing of critical-size bone defects in immunodeficient rats. Physiol Res. 2017; 66:113–123. doi:10.33549/physiolres.933376 [DOI] [PubMed] [Google Scholar]
- 139.Saito A, Ooki A, Nakamura T, et al. Targeted reversion of induced pluripotent stem cells from patients with human cleidocranial dysplasia improves bone regeneration in a rat calvarial bone defect model. Stem Cell Res Ther. 2018; 9:12 doi:10.1186/s13287-017-0754-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mahmoud EE, Kamei N, Shimizu R, et al. Therapeutic potential of multilineage-differentiating stress-enduring cells for osteochondral repair in a rat model. Stem Cells Int. 2017; 2017:8154569 doi:10.1155/2017/8154569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang T, Nimkingratana P, Smith CA, et al. Enhanced chondrogenesis from human embryonic stem cells. Stem Cell Res. 2019; 39:101497 doi:10.1016/j.scr.2019.101497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Takahashi K, Ogura N, Tomoki R, et al. Applicability of human dental follicle cells to bone regeneration without dexamethasone: an in vivo pilot study. Int J Oral Maxillofac Surg. 2015; 44:664–669. doi:10.1016/j.ijom.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 143.Harada Y, Mifune Y, Inui A, et al. Rotator cuff repair using cell sheets derived from human rotator cuff in a rat model. J Orthop Res. 2017; 35:289–296. doi:10.1002/jor.23289 [DOI] [PubMed] [Google Scholar]
- 144.Gumucio JP, Flood MD, Roche SM, et al. Stromal vascular stem cell treatment decreases muscle fibrosis following chronic rotator cuff tear. Int Orthop. 2016; 40:759–764. doi:10.1007/s00264-015-2937-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kremen TJ, Bez M, Sheyn D, et al. In vivo imaging of exogenous progenitor cells in tendon regeneration via superparamagnetic iron oxide particles. Am J Sports Med. 2019; 47:2737–2744. doi:10.1177/0363546519861080 [DOI] [PubMed] [Google Scholar]
- 146.Nakano N, Matsumoto T, Takayama K, et al. Age-dependent healing potential of anterior cruciate ligament remnant-derived cells. Am J Sports Med. 2015; 43:700–708. doi:10.1177/0363546514561436 [DOI] [PubMed] [Google Scholar]
- 147.Lu P, Woodruff G, Wang Y, et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014; 83:789–796. doi:10.1016/j.neuron.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bohaciakova D, Hruska-Plochan M, Tsunemoto R, et al. A scalable solution for isolating human multipotent clinical-grade neural stem cells from ES precursors. Stem Cell Res Ther. 2019; 10:83 doi:10.1186/s13287-019-1163-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Beretta S, Cunningham KM, Haus DL, et al. Effects of human ES-derived neural stem cell transplantation and kindling in a rat model of traumatic brain injury. Cell Transplant. 2017; 26:1247–1261. doi:10.1177/0963689717714107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lien BV, Tuszynski MH, Lu P. Astrocytes migrate from human neural stem cell grafts and functionally integrate into the injured rat spinal cord. Exp Neurol. 2019; 314:46–57. doi:10.1016/j.expneurol.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 151.Nori S, Khazaei M, Ahuja CS, et al. Human oligodendrogenic neural progenitor cells delivered with chondroitinase ABC facilitate functional repair of chronic spinal cord injury. Stem Cell Reports. 2018; 11:1433–1448. doi:10.1016/j.stemcr.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nagoshi N, Khazaei M, Ahlfors JE, et al. Human spinal oligodendrogenic neural progenitor cells promote functional recovery after spinal cord injury by axonal remyelination and tissue sparing. Stem Cells Transl Med. 2018; 7:806–818. doi:10.1002/sctm.17-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Munter JP, Beugels J, Munter S, et al. Standardized human bone marrow-derived stem cells infusion improves survival and recovery in a rat model of spinal cord injury. J Neurol Sci. 2019; 402:16–29. doi:10.1016/j.jns.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 154.Haus DL, López-Velázquez L, Gold EM, et al. Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury. Exp Neurol. 2016; 281:1–16. doi:10.1016/j.expneurol.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 155.Yokobori S, Sasaki K, Kanaya T, et al. Feasibility of human neural stem cell transplantation for the treatment of acute subdural hematoma in a rat model: a pilot study. Front Neurol. 2019; 10:82 doi:10.3389/fneur.2019.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Y, Green M, Wen Y, et al. Efficacy and safety of immuno-magnetically sorted smooth muscle progenitor cells derived from human-induced pluripotent stem cells for restoring urethral sphincter function. Stem Cells Transl Med. 2017; 6:1158–1167. doi:10.1002/sctm.16-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakajima N, Tamaki T, Hirata M, et al. Purified human skeletal muscle-derived stem cells enhance the repair and regeneration in the damaged urethra. Transplantation. 2017; 101:2312–2320. doi:10.1097/TP.0000000000001613 [DOI] [PubMed] [Google Scholar]
- 158.McLelland BT, Lin B, Mathur A, et al. Transplanted hESC-derived retina organoid sheets differentiate, integrate, and improve visual function in retinal degenerate rats. Invest Ophthalmol Vis Sci. 2018; 59:2586–2603. doi:10.1167/iovs.17-23646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tu HY, Watanabe T, Shirai H, et al. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. Ebiomedicine. 2019; 39:562–574. doi:10.1016/j.ebiom.2018.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seiler MJ, Aramant RB, Jones MK, et al. A new immunodeficient pigmented retinal degenerate rat strain to study transplantation of human cells without immunosuppression. Graefes Arch Clin Exp Ophthalmol. 2014; 252:1079–1092. doi:10.1007/s00417-014-2638-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Seiler MJ, Lin RE, McLelland BT, et al. Vision recovery and connectivity by fetal retinal sheet transplantation in an immunodeficient retinal degenerate rat model. Invest Ophthalmol Vis Sci. 2017; 58:614–630. doi:10.1167/iovs.15-19028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thomas BB, Zhu D, Lin TC, et al. A new immunodeficient retinal dystrophic rat model for transplantation studies using human-derived cells. Graefes Arch Clin Exp Ophthalmol. 2018; 256:2113–2125. doi:10.1007/s00417-018-4134-2 [DOI] [PubMed] [Google Scholar]
- 163.Bruin JE, Asadi A, Fox JK, et al. Accelerated maturation of human stem cell-derived pancreatic progenitor cells into insulin-secreting cells in immunodeficient rats relative to mice. Stem Cell Reports. 2015; 5:1081–1096. doi:10.1016/j.stemcr.2015.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Okuyama H, Ohnishi H, Nakamura R, et al. Transplantation of multiciliated airway cells derived from human iPS cells using an artificial tracheal patch into rat trachea. J Tissue Eng Regen Med. 2019; 13:1019–1030. doi:10.1002/term.2849 [DOI] [PubMed] [Google Scholar]
- 165.Kaku M, Kitami M, Rosales Rocabado JM, et al. Recruitment of bone marrow-derived cells to the periodontal ligament via the stromal cell-derived factor-1/C-X-C chemokine receptor type 4 axis. J Periodontal Res. 2017; 52:686–694. doi:10.1111/jre.12433 [DOI] [PubMed] [Google Scholar]
- 166.Ménoret S, Fontanière S, Jantz D, et al. Generation of Rag1-knockout immunodeficient rats and mice using engineered meganucleases. FASEB J. 2013; 27:703–711. doi:10.1096/fj.12-219907 [DOI] [PubMed] [Google Scholar]
- 167.Tsuchida T, Zheng YW, Zhang RR, et al. The development of humanized liver with Rag1 knockout rats. Transplant Proc. 2014; 46:1191–1193. doi:10.1016/j.transproceed.2013.12.026 [DOI] [PubMed] [Google Scholar]
- 168.Zschemisch NH, Glage S, Wedekind D, et al. Zinc-finger nuclease mediated disruption of Rag1 in the LEW/Ztm rat. BMC Immunol. 2012; 13:60 doi:10.1186/1471-2172-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Noto FK, Adjan-Steffey V, Tong M, et al. Sprague dawley Rag2-null rats created from engineered spermatogonial stem cells are immunodeficient and permissive to human xenografts. Mol Cancer Ther. 2018; 17:2481–2489. doi:10.1158/1535-7163.MCT-18-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu Q, Zhou S, Fan C, et al. Biodistribution and residence time of adenovector serotype 5 in normal and immunodeficient mice and rats detected with bioluminescent imaging. Sci Rep. 2017; 7:3597 doi:10.1038/s41598-017-03852-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kuijk EW, Rasmussen S, Blokzijl F, et al. Generation and characterization of rat liver stem cell lines and their engraftment in a rat model of liver failure. Sci Rep. 2016; 6:22154 doi:10.1038/srep22154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mashimo T, Takizawa A, Voigt B, et al. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PLoS One. 2010; 5:e8870 doi:10.1371/journal.pone.0008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Samata B, Kikuchi T, Miyawaki Y, et al. X-linked severe combined immunodeficiency (X-SCID) rats for xeno-transplantation and behavioral evaluation. J Neurosci Methods. 2015; 243:68–77. doi:10.1016/j.jneumeth.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 174.Mashimo T, Takizawa A, Kobayashi J, et al. Generation and characterization of severe combined immunodeficiency rats. Cell Rep. 2012; 2:685–694. doi:10.1016/j.celrep.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 175.Ma Y, Shen B, Zhang X, et al. Heritable multiplex genetic engineering in rats using CRISPR/Cas9. PLoS One. 2014; 9:e89413 doi:10.1371/journal.pone.0089413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.He D, Zhang J, Wu W, et al. A novel immunodeficient rat model supports human lung cancer xenografts. Faseb J. 2019; 33:140–150. doi:10.1096/fj.201800102RR [DOI] [PubMed] [Google Scholar]
- 177.Ménoret S, Ouisse LH, Tesson L, et al. Generation of immunodeficient rats with Rag1 and Il2rg gene deletions and human tissue grafting models. Transplantation. 2018; 102:1271–1278. doi:10.1097/TP.0000000000002251 [DOI] [PubMed] [Google Scholar]
- 178.Ménoret S, Iscache AL, Tesson L, et al. Characterization of immunoglobulin heavy chain knockout rats. Eur J Immunol. 2010; 40:2932–2941. doi:10.1002/eji.201040939 [DOI] [PubMed] [Google Scholar]
- 179.Panzer SE, Wilson NA, Verhoven BM, et al. Complete B cell deficiency reduces allograft inflammation and intragraft macrophages in a rat kidney transplant model. Transplantation. 2018; 102:396–405. doi:10.1097/TP.0000000000002010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Osborn MJ, Ma B, Avis S, et al. High-affinity IgG antibodies develop naturally in Ig-knockout rats carrying germline human IgH/Igκ/Igλ loci bearing the rat CH region. J Immunol. 2013; 190:1481–1490. doi:10.4049/jimmunol.1203041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ouisse LH, Gautreau-Rolland L, Devilder MC, et al. Antigen-specific single B cell sorting and expression-cloning from immunoglobulin humanized rats: a rapid and versatile method for the generation of high affinity and discriminative human monoclonal antibodies. BMC Biotechnol. 2017; 17:3 doi:10.1186/s12896-016-0322-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang X, Zhou J, He J, et al. An immune system-modified rat model for human stem cell transplantation research. Stem Cell Reports. 2018; 11:514–521. doi:10.1016/j.stemcr.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sato K, Oiwa R, Kumita W, et al. Generation of a nonhuman primate model of severe combined immunodeficiency using highly efficient genome editing. Cell Stem Cell. 2016; 19:127–138. doi:10.1016/j.stem.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 184.Suzuki S, Iwamoto M, Saito Y, et al. Il2rg gene-targeted severe combined immunodeficiency pigs. Cell Stem Cell. 2012; 10:753–758. doi:10.1016/j.stem.2012.04.021 [DOI] [PubMed] [Google Scholar]
- 185.Watanabe M, Nakano K, Matsunari H, et al. Generation of interleukin-2 receptor gamma gene knockout pigs from somatic cells genetically modified by zinc finger nuclease-encoding mRNA. PLoS One. 2013; 8:e76478 doi:10.1371/journal.pone.0076478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kang JT, Cho B, Ryu J, et al. Biallelic modification of IL2RG leads to severe combined immunodeficiency in pigs. Reprod Biol Endocrinol. 2016; 14:74 doi:10.1186/s12958-016-0206-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huang J, Guo X, Fan N, et al. RAG1/2 knockout pigs with severe combined immunodeficiency. J Immunol. 2014; 193:1496–1503. doi:10.4049/jimmunol.1400915 [DOI] [PubMed] [Google Scholar]
- 188.Ito T, Sendai Y, Yamazaki S, et al. Generation of recombination activating gene-1-deficient neonatal piglets: a model of T and B cell deficient severe combined immune deficiency. PLoS One. 2014; 9:e113833 doi:10.1371/journal.pone.0113833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lee K, Kwon DN, Ezashi T, et al. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. Proc Natl Acad Sci U S A. 2014; 111:7260–7265. doi:10.1073/pnas.1406376111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Choi YJ, Kim E, Reza AMMT, et al. Recombination activating gene-2null severe combined immunodeficient pigs and mice engraft human induced pluripotent stem cells differently. Oncotarget. 2017; 8:69398–69407. doi:10.18632/oncotarget.20626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Suzuki S, Iwamoto M, Hashimoto M, et al. Generation and characterization of RAG2 knockout pigs as animal model for severe combined immunodeficiency. Vet Immunol Immunopathol. 2016; 178:37–49. doi:10.1016/j.vetimm.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 192.Lei S, Ryu J, Wen K, et al. Increased and prolonged human norovirus infection in RAG2/IL2RG deficient gnotobiotic pigs with severe combined immunodeficiency. Sci Rep. 2016; 6:25222 doi:10.1038/srep25222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Waide EH, Dekkers JC, Ross JW, et al. Not all SCID pigs are created equally: two independent mutations in the Artemis gene cause SCID in pigs. J Immunol. 2015; 195:3171–3179. doi:10.4049/jimmunol.1501132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Singer AJ, Tuggle C, Ahrens A, et al. Survival of human cadaver skin on severe combined immune deficiency pigs: proof of concept. Wound Repair Regen. 2019; 27:426–430. doi:10.1111/wrr.12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tokunaga Y, Shirouzu M, Sugahara R, et al. Comprehensive validation of T- and B-cell deficiency in rag1-null zebrafish: implication for the robust innate defense mechanisms of teleosts. Sci Rep. 2017; 7:7536 doi:10.1038/s41598-017-08000-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tang Q, Abdelfattah NS, Blackburn JS, et al. Optimized cell transplantation using adult rag2 mutant zebrafish. Nat Methods. 2014; 11:821–824. doi:10.1038/nmeth.3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tang Q, Iyer S, Lobbardi R, et al. Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing. J Exp Med. 2017; 214:2875–2887. doi:10.1084/jem.20170976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yan C, Brunson DC, Tang Q, et al. Visualizing engrafted human cancer and therapy responses in immunodeficient zebrafish. Cell. 2019; 177:1903–1914.e14. doi:10.1016/j.cell.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Song J, Zhong J, Guo X, et al. Generation of RAG 1- and 2-deficient rabbits by embryo microinjection of TALENs. Cell Res. 2013; 23:1059–1062. doi:10.1038/cr.2013.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang D, Xu J, Zhu T, et al. Effective gene targeting in rabbits using RNA-guided cas9 nucleases. J Mol Cell Biol. 2014; 6:97–99. doi:10.1093/jmcb/mjt047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Song J, Yang D, Ruan J, et al. Production of immunodeficient rabbits by multiplex embryo transfer and multiplex gene targeting. Sci Rep. 2017; 7:12202 doi:10.1038/s41598-017-12201-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Flisikowska T, Thorey IS, Offner S, et al. Efficient immunoglobulin gene disruption and targeted replacement in rabbit using zinc finger nucleases. PLoS One. 2011; 6:e21045 doi:10.1371/journal.pone.0021045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Miao J, Ying B, Li R, et al. Characterization of an N-terminal non-core domain of RAG1 gene disrupted Syrian hamster model generated by CRISPR Cas9. Viruses. 2018; 10:243 doi:10.3390/v10050243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Colston MJ, Fieldsteel AH, Dawson PJ. Growth and regression of human tumor cell lines in congenitally athymic (rnu/rnu) rats. J Natl Cancer Inst. 1981; 66:843–848 [PubMed] [Google Scholar]
- 205.Vaessen LM, Broekhuizen R, Rozing J, et al. T-cell development during ageing in congenitally athymic (nude) rats. Scand J Immunol. 1986; 24:223–235. doi:10.1111/j.1365-3083.1986.tb02089.x [DOI] [PubMed] [Google Scholar]
- 206.Verma MK, Clemens J, Burzenski L, et al. A novel hemolytic complement-sufficient NSG mouse model supports studies of complement-mediated antitumor activity in vivo. J Immunol Methods. 2017; 446:47–53. doi:10.1016/j.jim.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jung CJ, Ménoret S, Brusselle L, et al. Comparative analysis of piggyBac, CRISPR/Cas9 and TALEN mediated BAC transgenesis in the zygote for the generation of humanized SIRPA rats. Sci Rep. 2016; 6:31455 doi:10.1038/srep31455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chang NK, Gu J, Gu S, et al. Arterial flow regulator enables transplantation and growth of human fetal kidneys in rats. Am J Transplant. 2015; 15:1692–1700. doi:10.1111/ajt.13149 [DOI] [PubMed] [Google Scholar]
- 209.Beldick SR, Hong J, Altamentova S, et al. Severe-combined immunodeficient rats can be used to generate a model of perinatal hypoxic-ischemic brain injury to facilitate studies of engrafted human neural stem cells. PLoS One. 2018; 13:e0208105 doi:10.1371/journal.pone.0208105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Geurts AM, Cost GJ, Rémy S, et al. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol Biol. 2010; 597:211–225. doi:10.1007/978-1-60327-389-3_15 [DOI] [PubMed] [Google Scholar]
- 211.Tesson L, Usal C, Ménoret S, et al. Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol. 2011; 29:695–696. doi:10.1038/nbt.1940 [DOI] [PubMed] [Google Scholar]
- 212.Ménoret S, De Cian A, Tesson L, et al. Homology-directed repair in rodent zygotes using Cas9 and TALEN engineered proteins. Sci Rep. 2015; 5:14410 doi:10.1038/srep14410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li D, Qiu Z, Shao Y, et al. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat Biotechnol. 2013; 31:681–683. doi:10.1038/nbt.2661 [DOI] [PubMed] [Google Scholar]
- 214.Ma Y, Yu L, Pan S, et al. CRISPR/Cas9-mediated targeting of the Rosa26 locus produces Cre reporter rat strains for monitoring Cre-loxP-mediated lineage tracing. FEBS J. 2017; 284:3262–3277. doi:10.1111/febs.14188 [DOI] [PubMed] [Google Scholar]
- 215.Meek S, Mashimo T, Burdon T. From engineering to editing the rat genome. Mamm Genome. 2017; 28:302–314. doi:10.1007/s00335-017-9705-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Boettcher AN, Loving CL, Cunnick JE, et al. Development of severe combined immunodeficient (SCID) pig models for translational cancer modeling: future insights on how humanized SCID pigs can improve preclinical cancer research. Front Oncol. 2018; 8:559 doi:10.3389/fonc.2018.00559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fujiki Y, Fukawa K, Kameyama K, et al. Successful multilineage engraftment of human cord blood cells in pigs after in utero transplantation. Transplantation. 2003; 75:916–922. doi:10.1097/01.TP.0000057243.12110.7C [DOI] [PubMed] [Google Scholar]
- 218.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995; 155:5249–5256 [PubMed] [Google Scholar]
- 219.Hundrieser J, Hein R, Pokoyski C, et al. Role of human and porcine MHC DRB1 alleles in determining the intensity of individual human anti-pig T-cell responses. Xenotransplantation. 2019; 26:e12523 doi:10.1111/xen.12523 [DOI] [PubMed] [Google Scholar]
- 220.Boettcher AN, Cunnick JE, Powell EJ, et al. Porcine signal regulatory protein alpha binds to human CD47 to inhibit phagocytosis: implications for human hematopoietic stem cell transplantation into severe combined immunodeficient pigs. Xenotransplantation. 2019; 26:e12466 doi:10.1111/xen.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hamilton N, Sabroe I, Renshaw SA. A method for transplantation of human HSCs into zebrafish, to replace humanised murine transplantation models. F1000Res. 2018; 7:594 doi:10.12688/f1000research.14507.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Iwatsuki-Horimoto K, Nakajima N, Ichiko Y, et al. Syrian hamster as an animal model for the study of human influenza virus infection. J Virol. 2018; 92:e01693–e01700. doi:10.1128/JVI.01693-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Alisjahbana A, Mohammad I, Gao Y, et al. Human macrophages and innate lymphoid cells: tissue-resident innate immunity in humanized mice. Biochem Pharmacol. 2019; 113672 [Epub ahead of print. October 18, 2019]. doi:10.1016/j.bcp.2019.113672 [DOI] [PubMed] [Google Scholar]
- 224.Jespersen H, Lindberg MF, Donia M, et al. Clinical responses to adoptive T-cell transfer can be modeled in an autologous immune-humanized mouse model. Nat Commun. 2017; 8:707 doi:10.1038/s41467-017-00786-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Siemeni T, Knöfel AK, Ius F, et al. Transplant arteriosclerosis in humanized mice reflects chronic lung allograft dysfunction and is controlled by regulatory T cells. J Thorac Cardiovasc Surg. 2019; 157:2528–2537. doi:10.1016/j.jtcvs.2019.01.134 [DOI] [PubMed] [Google Scholar]
- 226.Sepulveda M, Pirozzolo I, Alegre ML. Impact of the microbiota on solid organ transplant rejection. Curr Opin Organ Transplant. 2019; 24:679–686. doi:10.1097/MOT.0000000000000702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Xiao J, Peng Z, Liao Y, et al. Organ transplantation and gut microbiota: current reviews and future challenges. Am J Transl Res. 2018; 10:3330–3344 [PMC free article] [PubMed] [Google Scholar]
- 228.Lei YM, Chen L, Wang Y, et al. The composition of the microbiota modulates allograft rejection. J Clin Invest. 2016; 126:2736–2744. doi:10.1172/JCI85295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lei YM, Sepulveda M, Chen L, et al. Skin-restricted commensal colonization accelerates skin graft rejection. JCI Insight. 2019; 4:e127569 doi:10.1172/jci.insight.127569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rey K, Manku S, Enns W, et al. Disruption of the gut microbiota with antibiotics exacerbates acute vascular rejection. Transplantation. 2018; 102:1085–1095. doi:10.1097/TP.0000000000002169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Guo Y, Wang Q, Li D, et al. Vendor-specific microbiome controls both acute and chronic murine lung allograft rejection by altering CD4+ Foxp3+ regulatory T cell levels. Am J Transplant. 2019; 19:2705–2718. doi:10.1111/ajt.15523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.McIntosh CM, Chen L, Shaiber A, et al. Gut microbes contribute to variation in solid organ transplant outcomes in mice. Microbiome. 2018; 6:96 doi:10.1186/s40168-018-0474-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Staley C, Kaiser T, Beura LK, et al. Stable engraftment of human microbiota into mice with a single oral gavage following antibiotic conditioning. Microbiome. 2017; 5:87 doi:10.1186/s40168-017-0306-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wrzosek L, Ciocan D, Borentain P, et al. Transplantation of human microbiota into conventional mice durably reshapes the gut microbiota. Sci Rep. 2018; 8:6854 doi:10.1038/s41598-018-25300-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Arrieta MC, Walter J, Finlay BB. Human microbiota-associated mice: a model with challenges. Cell Host Microbe. 2016; 19:575–578. doi:10.1016/j.chom.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 236.Hirabayashi M, Goto T, Hochi S. Pluripotent stem cell-derived organogenesis in the rat model system. Transgenic Res. 2019; 28:287–297. doi:10.1007/s11248-019-00161-2 [DOI] [PubMed] [Google Scholar]
- 237.Masaki H, Nakauchi H. Interspecies chimeras for human stem cell research. Development. 2017; 144:2544–2547. doi:10.1242/dev.151183 [DOI] [PubMed] [Google Scholar]
- 238.Larcher T, Lafoux A, Tesson L, et al. Characterization of dystrophin deficient rats: a new model for Duchenne muscular dystrophy. PLoS One. 2014; 9:e110371 doi:10.1371/journal.pone.0110371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ossart J, Moreau A, Autrusseau E, et al. Breakdown of immune tolerance in AIRE-deficient rats induces a severe autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy-like autoimmune disease. J Immunol. 2018; 201:874–887. doi:10.4049/jimmunol.1701318 [DOI] [PubMed] [Google Scholar]


