Abstract
The 70-kDa heat shock proteins (HSP70s) are a conserved class of chaperones that play critical roles during the normal life cycle of plants. HSP70s are particularly involved in the regulation of biotic and abiotic stress responses. In this paper, the potential roles of this protein were investigated. A reverse genetic approach was employed for transient silencing of hsp70 gene in tomato (Solanum lycopersicum L.) to evaluate different growth and physiological parameters under normal conditions and during the response to drought stress. A combined ANOVA (analysis of variance) and HCA (hierarchical clustering analysis) showed that hsp70 silencing led to severe growth retardation and mortality, significant membrane damage and leakage, decline in relative water content, low rate of pigment accumulation, and reduced antioxidant enzyme activity under normal and drought stress conditions. Among the different parameters, proline was the only trait that was unaffected by gene silencing and accumulated by similar amounts to that of nonsilent plants. In conclusion, HSP70 played critical roles in maintaining the cellular homeostasis of plants during adaptation to drought and under normal plant life conditions. It was speculated that proline was, to some extent, involved in improving the loss of protein folding or function resulting from HSP70 deficiency, and played a crucial role in the adaptation of plants on exposure to stress.
Keywords: Heat shock proteins (HSP), Hsp70s, Virus induced gene silencing (VIGS), Tomato, Drought stress
Introduction
The tomato plant (Solanum lycopersicum L.) is an important economic crop, and is widely cultivated throughout the world. The species has been considered as a model in studies to transfer agronomically important traits by introducing new genes (Paduchuri et al. 2010). One of the desired traits for crop plants is their adaptation to stress conditions, such as drought, especially for those in regions where there are frequent water shortages. Drought is one of the major causes of crop losses around the world (Ijaz et al. 2019). Drought stress on plants results in limited nutrient uptake and water retention, severe decline in germination rate and plant growth, decrease in leaf expansion, stem elongation, and root proliferation, as well as a reduction in the rate of photosynthesis (Kaya et al. 2006). Drought disrupts the normal transportation of solutes, causes electron leakage, and triggers the production of reactive oxygen species (ROS), which creates oxidative injury (Hussain et al. 2019). Plants adjust and adapt to drought stress to some degree by employing a wide range of defensive mechanisms at morphological, biochemical, physiological, cellular, or molecular levels (Osakabe et al. 2014; Farooq et al. 2009). Molecular responses occurring under drought stress are usually involved in the substantial changes in the expression of stress-related genes (Shinozaki and Yamaguchi-Shinozaki 2007; Singh et al. 2019). Among stress-related genes, heat shock proteins (HSPs) are the most common molecular components that are upregulated when plants are exposed to almost all types of stress (Wahid et al. 2007; Priya et al. 2019). These proteins are divided into five families according to their molecular weight: HSP100, HSP90, HSP70, HSP60, and small heat-shock proteins (sHSPs) (Al-Whaibi 2011; Usman et al. 2014). The 70-kDa heat shock proteins (HSP70s) are a conserved class of chaperones that play critical roles in cellular processes, such as folding of newly synthesized polypeptides, refolding of misfolded or aggregated proteins, translocation of proteins across organelles membranes, assembling or disassembling complex structure of a protein, controlling the activity of regulatory proteins, and assisting in the degradation of proteins (Bukau and Horwich 1998; Leborgne-Castel et al. 1999: Sable and Agarwal 2018). Apart from their roles in abiotic stresses, HSP70s also are known to be affiliated with viruses, which infect plants and animals. It was reported that following viral infection of a host cell HSP70s are recruited for regulation of different virus activities, including replication, synthesis, and folding of viral proteins (Aranda et al. 1996; Peremyslov et al. 1999). Moreover, HSP70s are shown to be involved in the regulation of host plant stress responses and could trigger the plant immune system against infection.
The deficiency in HSP70 members compared with other classes of HSPs in plant cells would lead to severe growth retardation, especially under stress conditions (Jungkunz et al. 2011). HSP70 in plants was speculated to play a dual role as a heat tolerance agent and a key factor for autoregulation of the heat shock response in plant cells (Lee and Schöffl 1996). It was shown that heat shock cognate Hsc70-1 was involved in improving growth, development, thermotolerance, and control of plant respose to heat shock (Sung and Guy 2003). Different roles of HSP70 under drought stress have been studied in various plants, such as sugarcane (Augustine et al. 2015), Arabidopsis thaliana, and wheat (Duan et al. 2011). It was suggested that among the three well-known classes of HSPs, HSP70 had a more prominent role under both salinity and stress-free life cycle of N. benthamiana (Anaraki et al. 2018).
The present work investigated the importance of HSP70 in tomato plant, as a crop model, during drought stress through a reverse genetic approach using a heterologous transient silencing method. The effect of gene silencing on the growth and physiology of tomato plants was evaluated to uncover the function of HSP70 protein in tomato under drought stress conditions. The endogenous hsp70 gene of Solanum lycopersicum L. was successfully silenced using a homolog fragment isolated from an evolutionary remote species, Capparis spinosa L. (with only 85% sequence similarity). This heterologous manner of silencing induced a mild negative effect on plant growth and survival, and might be ideal for analyzing the critical genes, such as hsps whose complete loss of function cause severe suppression of plant growth or result in plant death (Hosseini Tafreshi et al. 2012). The potential roles of HSP70 during drought stress conditions and the possible functional mechanisms also were discussed.
Materials and methods
Plant material
All experiments were conducted using Solanum lycopersicum cv. Punta Banda, as a tolerant tomato cultivar purchased from Native Seeds/SEARCH (USA).
Vectors preparation
The pTRV1 (YL192), pTRV2 (YL156), and pTRV2-NtPDS (YL124) vectors were obtained from the Arabidopsis Biological Resource Center, Ohio State University, USA. The construct pTRV2-GFP–containing TRV genome with a 296-bp fragment of GFP (Green Fluorescent Protein) was used as negative controls for the silencing results to distinguish between silencing and virus invasion. The silencing vector pTRV2-hsp70 contained a 474-bp fragment of hsp70 from a remote evolutionary species, Capparis spinosa L. According to our previous studies (Hosseini Tafreshi et al. 2012; Anaraki et al. 2018), this fragment size, which had about 85% sequence similarity with its corresponding part in the endogenous hsp70 gene of Solanum lycopersicum L. was experimentally efficient to successfully silence the collective expression of all tomato hsp70 genes at once.
The vectors were introduced into Agrobacterium tumefaciens strain LBA4404 using the heat shock method as previously described (Cui et al. 1994).
Virus-induced gene silencing
Tomato seeds were surface sterilized with 2.5% (v/v) sodium hypochlorite (NaOCl) solution for 15 min and rinsed 3–5 times with sterile distilled water. Then, sterile seeds cultivated in cellular seed trays containing peat moss were cultured in peat moss (Klasman-Deilmann GmbH, Germany) and grown in a growth chamber with 70% relative humidity under a photon flux density of 500–600 μmol m−2 s−1 in a 16 h light/8 h dark regime and with a temperature of 23 ± 2 °C. The 10-day-old seedlings were transplanted into 8-cm plastic pots containing peat moss, and irrigated with 0.1% Hoagland solution (Hoagland and Arnon 1950). The 3-week-old seedlings with the expanded cotyledons (2–4 true leaves) were used for plant infiltration. The pTRV1 and pTRV2- derivatives (containing hsp70, gfp, or pds inserts) were introduced into Agrobacterium tumefaciens strain LBA4404. The bacterial preparation and plant infiltration were done as previously described by Velásquez et al. (2009) with minor modification. The plants were infiltrated with four different mixtures of bacterial suspensions, including infiltration with buffer as the negative control, pTRV2-gfp as a negative control, pTRV2-Ntpds as a positive control of silencing, and pTRV2-hsp as the main heterologous silencing vector. All of the vectors were inoculated into the plants after mixing with pTRV1 at a ratio of 1 to 1. The plants were maintained at 20–22 °C for 9 days in a growth chamber for active viral infection and spread.
Drought treatment and experimental design
Nine days after the VIGS experiment, a group of silenced plants was treated with water deficiency stress with a 25% reduction in water capacity per week. The control plants were irrigated until the end of the period every 2 days with 100% capacity. The plants were monitored phenotypically during the 3 weeks of drought stress. The growth parameters and physiological indexes, including pigment content, relative water content, membrane leakage, lipid peroxidation, proline content, and activity of antioxidant enzymes, were recorded in tomatoes 25 days after drought stress started. Each treatment was repeated three times and arranged in a completely randomized design (CRD).
Real-time PCR
Total RNA was extracted using the Total RNA Extraction Mini Kit (RBC Bioscience, Taiwan) according to the manufacturer’s instructions, and then treated with RNase-free DNase I (Fermentas, Canada). It was reverse-transcribed into cDNA using the RevertAidTM First Strand cDNA Synthesis Kit (Fermentas, Canada) with 0.5 μg of total RNA and Oligo dT17 primer. The obtained cDNAs were used for gene expression analysis with real-time quantitative PCR. The RT-PCR was performed with StepOne Real-Time PCR System (Applied Biosystems, USA) using AccuPower ® 2X Greenstar qPCR Master Mix (Bioneer, Korea) following the program: 95 °C for 5 min, 94.5 °C for 10 s, 60 °C for 35 s, and 72 °C for 40 s for 42 cycles. To normalize the sample variance, the elongation factor 1 (EF1α) gene served as the internal control. This gene was previously indicated as one of the most stably expressed genes in Solanacean species under drought and osmotic stress conditions (Tang et al. 2017). The primers were designed according to the 5′ and 3′ UTR regions of the Slhsp70 gene to ensure gene-specificity of RT-PCR. The following primers were used for real-time RT-PCR:
F Slhsp70 5’-TGACAAGGGCAGACTCTCTAA-3′
R Slhsp70 5’-CCTTCTTCTTGTGCTCCTCATC-3’
F Slef1α 5’-ACCAGATTAACGAGCCCAAGAG-3′
R Slef1α 5’-CCAACAGGGACAGTACCAATAC-3’
The relative gene expression levels were calculated using the 2−△△Ct method.
Growth analysis
Growths parameters, such as shoot height and root length were determined using a graduated ruler. Fresh shoot and root weights were measured immediately after excision. The excised shoots and roots were dried in an oven at 65 °C until constant weight to record dry weights (Amjad et al. 2014).
Physiological and biochemical analysis
The Chl a, Chl b, and carotenoid contents were extracted in 80% (v/v) acetone and assayed as in the method of Lichtenthaler and Welburn (1983). The pigment values were expressed as mg g−1 fresh weight (FW). Relative water content (RWC) was measured on a newly expanded leaf detached from three plants per treatment, according to Ings et al. (2013). The proline content of leaves was extracted and measured using the method of Bates et al. (1973) based on the reaction of proline with ninhydrin. Proline concentration was determined using l-proline standard curve as μmol proline g−1 FW. Electrolyte leakage (EL) was determined to evaluate the stability of cells membrane by measuring the electrical conductivity of leaf extracts according to Liu et al. (2011). Lipid peroxidation was assayed as the indicator of the degree of damage to the membrane by measuring the amount of malondialdehyde (MDA) according to the method of Heath and Packer (1968). The results of the MDA values were expressed as nmol g−1F.W.
Enzyme extraction and assay
Fresh leaf samples (0.2 g) were homogenized in 1 ml of cold Na-phosphate buffer (50 mM, pH 7.0), containing 1% (w/v) polyvinyl pyrrolidone (PVP), 2 mM α-dithiothreitol and 2 mM EDTA in an ice bath. The homogenate was then centrifuged at 4 °C for 10 min at 3000g, and the supernatant was used as a source of enzyme extract to assay the activities of catalase (CAT) and ascorbate peroxidase (APX) (Aghaie et al. 2018).
The CAT activity was determined as previously described by (Beers and Sizer 1952; Zgallaï et al. 2006). The activity of this enzyme was measured by a decline in the rate of H2O2 in absorbance at 240 nm for 2 min. APX activity was measured based on Nakano and Asada (1981). The reaction was initiated by the addition of H2O2 and decrease absorbance rate at 290 nm for 2 min. Enzyme activity was determined by an absorbance coefficient of 2.8 mM−1 cm−1 for ascorbic acid. One unit of ascorbate peroxidase expressed as the oxidation of 1 μmol ascorbic acid in 1 min at 25 °C. Total superoxide dismutase (SOD) activity was measured by the amount of enzyme required to obtain a 50% inhibition of reduction of Nitro blue tetrazolium (NBT) to blue formazan at 560 nm using the method of Beauchamp and Fridovich (1971).
Statistical analysis
All experiments were replicated at least three times independently. SPSS package (Ver.19) was used to analyze the data for the analysis of variance (ANOVA). Duncan’s range test at P = 0.05 was used to compare the mean values. All graphs were generated using GraphPad Prism Software 6. HCA (hierarchical clustering analysis) was performed using all the measured parameters under drought and control conditions by ClustVis online tool (Metsalu and Vilo 2015). The heatmap function was used with row-wise scaling and Euclidean-based clustering.
Results
VIGS experiment in tomato
The Ntpds (phytoene desaturase) gene, as a positive control of VIGS, was silenced to confirm and evaluate VIGS experiment. The target gene in tomato under both drought-free control and drought stress conditions was also silenced. The results showed that after 3 weeks of silencing, the plants silenced for pds developed leaves with photobleached patches (Fig. 1). This phenotype, as an indication of pds silencing, showed that silencing efficiency was enough in plants under the control and stress conditions.
Fig. 1.
Phenotype of tomatoes in different silencing and stress conditions
Quantitative RT-PCR performed during the VIGS and drought treatments to investigate the changes in the mRNA level of hsp70 gene in tomatoes (Fig. 2). The results showed that the relative expression of Slhsp70 gene in the silenced plants was significantly reduced compared with those of the buffer-inoculated and pTRV2-gfp-infected plants (negative control), under drought-free control and drought stress conditions. The Slhsp70 mRNA level of silenced plants was reduced about 81%, and 91% compared with that of the buffered plants under the drought-free and drought stress conditions, respectively. However, no difference was observed between the Slhsp70 mRNA level of the buffered and gfp-infected plants under the control or stressed conditions.
Fig. 2.

The effect of VIGS on the relative expression of hsp70 gene in tomato under the control and drought stress conditions. Error bars indicate SD; similar letters are not significant (P < 0.05) based on Duncan test. Values are the mean of at least three independent experiments. Different letters indicate significant differences (P < 0.05) based on the Duncan’s test
It was also shown that drought stress significantly induced a high level of Slhsp70 expression in the buffered and pTRV2-gfp inoculated tomatoes, which was 285%, and 218% more than that of the control condition, respectively.
Silencing of Slhsp70 gene caused a significant decrease in plant growth under control and drought conditions
The ANOVA showed that the growth-related parameters, including shoot length, and shoot fresh and dry weights were significantly affected by the Slhsp70 silencing and drought stress, but not by the interaction of the two factors. Root length and dry weight were only affected by gene silencing but not by drought. However, root dry weight was significantly affected by independent drought and silencing treatments and also by the interaction of the two.
As shown in Fig. 1 and Table 1 the phenotype and growth parameters of tomato plants were significantly affected by Slhsp70 silencing. Generally, following silencing of Slhsp70, the growth parameters including shoot and root length and fresh and dry weight were decreased in well-watered (control) and drought-stressed plants. For example, the shoot length of silenced plants was reduced about 51%, and 61% compared with that of the buffered plants under drought-free and drought stress conditions, respectively,. There was also a significant difference between the growth parameters of pTRV2-gfp-inoculated (negative control) and buffered control plants under drought-free and stress conditions. For example, under drought-free and drought stress conditions, the shoot length of pTRV2-gfp-inoculated tomatoes was reduced about 24%, and 32%, respectively, compared with that of the buffered plants.
Table 1.
The effect of hsp70 silencing on growth-related parameters of tomato under drought-free and drought stress conditions. Values are the mean of at least three independent replicates. Different letters indicate significant differences (P < 0.05)
| Treatment | Shoot length (mm) | Root length (mm) | Shoot fresh Wt. (g) | Root fresh Wt. (g) | Shoot dry Wt. (g) | Root dry Wt. (g) | |
|---|---|---|---|---|---|---|---|
| Drought-free control | Buffer | 24.93 ± 1.70 a | 19.05 ± 0.99 a | 5.04 ± 0.53 a | 1.98 ± 0.21 a | 0.049 ± 0.006 a | 0.017 ± 0.001 a |
| pTRV2-gfp | 18.90 ± 1.67 c | 15.51 ± 2.2 b | 3.04 ± 0.26 c | 0.96 ± 0.12 c | 0.025 ± 0.005 c | 0.011 ± 0.005 c | |
| pTRV2-hsp70 | 11.96 ± 1.76 d | 8.12 ± 1.08 d | 1.40 ± 0.49 de | 0.38 ± 0.06 e | 0.015 ± 0.004 d | 0.005 ± 0.001 e | |
| Drought stress | Buffer | 22.16 ± 0.61 b | 20.81 ± 1.5 a | 3.87 ± 0.34 b | 1.29 ± 0.13 b | 0.035 ± 0.003 b | 0.014 ± 0.002 b |
| pTRV2-gfp | 15.06 ± 1.9 d | 12.50 ± 2.7 c | 2.03 ± 0.32 d | 0.62 ± 0.06 d | 0.017 ± 0.002 d | 0.009 ± 0.001 d | |
| pTRV2-hsp70 | 8.46 ± 0.95 e | 6.76 ± 2.2 e | 1.00 ± 0.33 e | 0.29 ± 0.11 e | 0.008 ± 0.003 e | 0.002 ± 0.005 f | |
On the other hand, drought intrinsically decreased all the growth parameters in tomatoes. For example, shoot length of drought-stressed plants was generally reduced about 18% compared with that of the drought-free control tomatoes.
The contents of pigments reduced following Slhsp70 silencing and drought stress
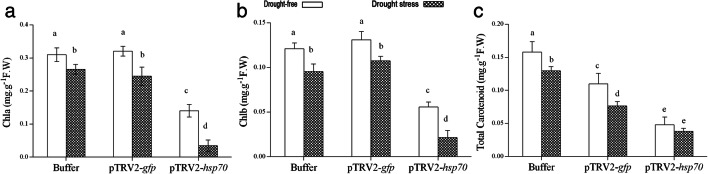
The content of pigments, including Chl a, Chl b, and total carotenoid were affected by both Slhsp70 silencing and drought stress but not by the interaction of the two factors. According to the results (Fig. 3b–d) the amounts of Chl a, Chl b, and total carotenoids were significantly decreased by about 56%, 56%, and 70% respectively in tomatoes silenced for Slhsp70 gene under drought-free conditions compared with buffered plants. A significant reduction in chlorophylls content took place when the plants coped with a combined effect of gene silencing and stress. Additionally, the viral infection (pTRV2-gpf inoculation) alone had no significant effect on chlorophyll content. However, it caused a 31% and 41% reduction in the amount of carotenoid under drought-free and drought-stressed conditions, respectively.
Fig. 3.
The effect of hsp70 silencing on a chlorophyll a, b chlorophyll b, and c total carotenoid in tomato plants under drought-free control or drought stress conditions. Error bars indicate SD; similar letters are not significant (P < 0.05) based on Duncan’s test. Values are the mean of at least three independent experiments. Different letters indicate significant differences (P < 0.05) based on Duncan’s test
Relative water content (RWC) decreased under both drought stress and Slhsp70 silencing
The results showed that RWC was significantly affected by both Slhsp70 silencing and drought stress, but not by the interaction of the two factors. As shown in Fig. 4a, the leaves of the plants infected with the pTRV2-gpf vector had similar RWC compared with those inoculated with buffer under drought-free control or drought stress conditions.
Fig. 4.
The effect of hsp70 silencing on a RWC, b electrolyte leakage, and c MDA content in tomato plants under drought-free control or drought stress conditions. Error bars indicate SD; similar letters are not significant (P < 0.05) based on Duncan’s test. Values are the mean of at least three independent experiments. Different letters indicate significant differences (P < 0.05) based on the Duncan’s test
Drought stress and Slhsp70 silencing increased electrolyte leakage
Our result revealed that the electrolyte leakage (EL) from the cells was significantly affected by both Slhsp70 silencing and drought stress but not by the interaction of the two factors. As shown in Fig. 4b, no significant difference in electrolyte leakage was observed between virus-infected (pTRV2-gpf-inoculated) and buffer-inoculated plants. In contrast, the plants silenced for Slhsp70 gene or those stressed with drought showed a higher rate of leakage. Compared with virus infection or buffer conditions, silencing of Slhsp70 gene induced an 85%, and 34% reduction in the electrolyte leakage of tomatoes under control and drought stress conditions, respectively.
Accumulation of MDA increased under drought stress and Slhsp70 silencing
Similar to RWC and El, MDA content of the cells was significantly affected by both Slhsp70 silencing and drought stress but not by the interaction of the two. The results showed (Fig. 4c) that virus infection (pTRV2-gpf inoculation) had no significant effect on the level of lipid peroxidation (MDA accumulation) in tomatoes when applied independently. On the other hand, a significant increase of MDA was observed in plants silenced for Slhsp70 or stressed by drought. The highest level of lipid peroxidation was observed in the Slhsp70-silenced tomatoes under drought stress, which was about 55% higher than that under drought-free conditions.
Proline accumulation was unaffected during gene silencing
Proline content of the cells was significantly affected by viral infection and drought stress and by the interaction of the two factors, as shown in Fig. 5a. The virus infection and drought stress significantly increased the amount of proline in tomatoes. The proline content of pTRV2-gpf inoculated plants increased by 263% when compared with that of the buffered plants under drought-free conditions. Higher levels of proline (350%) accumulated in the cells when biotic (viral infection) and abiotic (drought) stresses were simultaneously applied. More importantly, the results showed that the silencing of the Slhsp70 gene had no adverse effect on proline accumulation in tomatoes, so that the proline content of plants silenced for Slhsp70 was relatively similar to that of virus-infected plants under control and drought stress conditions.
Fig. 5.
The effect of hsp70 silencing on the activity of a CAT, b APX, and c SOD in tomato plants under drought-free control or drought stress conditions. Error bars indicate SD; similar letters are not significant (P < 0.05) based on Duncan’s test. Values are the mean of at least three independent experiments. Different letters indicate significant differences (P < 0.05) based on Duncan’s test
The effect of Slhsp70 silencing and drought stress on the antioxidant enzyme activity
The ANOVA showed that the activity of CAT and SOD enzymes was significantly affected by the type of inoculated vector, drought stress, and by the interaction of the two factors. However, the activity of another antioxidant enzyme, APX, was only affected by the type of vector and not affected by drought stress or the interaction of two factors. As shown in Fig. 4b–d, among the enzymes, the activity of two antioxidant enzymes, including CAT, and SOD increased as a result of drought stress by 27%, and 32% in buffered plants, respectively. The plants infected with pTRV2-gfp also increased their CAT, and SOD activity more than 28%, and 36% than those of the buffered plants. More importantly, a significant decrease in the activity of all three enzymes was observed after the silencing of Slhsp70 under both control and drought stress conditions. Compared with that of the buffered plants, a 410%, 191%, and 144% reduction in the activity of CAT, SOD, and APX were observed in tomatoes silenced for Slhsp70 gene, respectively.
HCA showed the district negative effect of Slhsp70 silencing on plants
The heatmap from hierarchical clustering analysis (HCA) grouped all the growth and physiological parameters into two main clusters (Fig. 6). Three physiological parameters were significantly increased under drought stress conditions, and were clustered in a distinct group I. Among these parameters, MDA and EL were significantly increased and proline was unaffected in plants silenced for Slhsp70 gene.
Fig. 6.
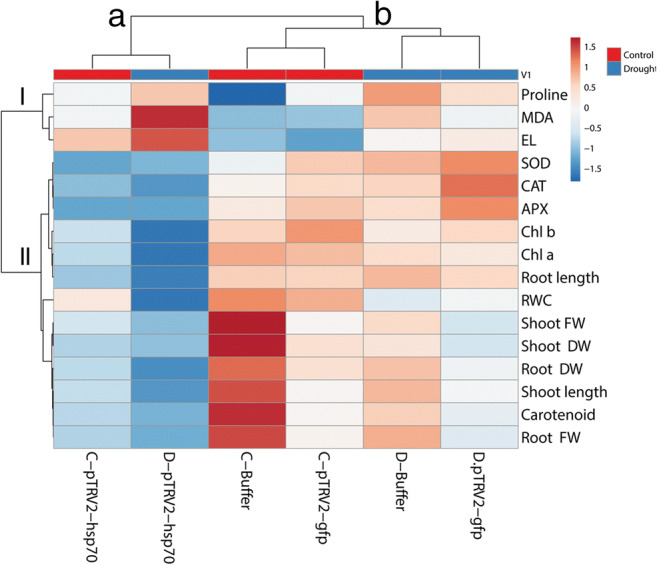
Hierarchical clustering analysis (HCA) of growth and physiological parameters of tomato plants inoculated with Buffer, pTRV2-gfp, or silenced for hsp70 gene (pTRV2-hsp70) under drought-free control (c) and drought stress (d) conditions
Another group of parameters (group II) was divided further into two subclusters; one including antioxidant enzymes (CAT, APX, and SOD), Chlorophyll a and b, and root length, which generally were reduced during Slhsp70 silencing in plants under both drought-free and drought stress conditions. Among these parameters, antioxidant enzymes were increased, and chlorophylls decreased by drought stress alone. Another subcluster of group II included growth-related traits (except root length), RWC, and carotenoid contents. These parameters were significantly influenced by Slhsp70 silencing and by drought stress and were the highest in buffered plants under drought-free control conditions.
The HCA was used to cluster different treatments. As indicated in the heatmap, all the treatments were divided into two main clusters (A and B). The Slhsp70 silencing was observed as a distinct and critical cluster A, including gene silencing under control (C-pTRV2-hsp70) and drought (D-pTRV2-hsp70) conditions. Generally, almost all of the parameters in cluster I and II increased and decreased under the treatments of cluster A, respectively.
The treatment in cluster B was subdivided in two clusters, one including the treatment related to drought-free control conditions (C-pTRV2-gfp and C. buffer) and another including the treatment related to drought conditions (D-pTRV2-gfp and D. buffer).
Discussion
Drought stress is one of the main limitations to plant growth and crop yield. Different methods are needed to develop genetically engineered drought-tolerant plants. These methods are mainly dependent on physiological, biochemical, and molecular studies about plant responses to drought (Bhargava and Sawant 2013). In this work, we provided an overview of the role of HSP70 in response to drought stress using a reverse genetic approach called VIGS in an important crop and model plant, tomato (Solanum lycopersicum). In recent years, VIGS has been identified as a useful tool for the functional characterization of genes associated with biotic and abiotic stress responses. The function of the genes is identified from transcript profiling of plants exposed to stressful conditions (Ramegowda et al. 2014). The TRV-based vectors have been successfully deployed in functional analysis of biotic and abiotic stress-responsive genes in model plants, such as Nicotiana benthamiana (Senthil-Kumar et al. 2007) and crop plants, such as tomato (Solanum lycopersicum and S. pimpinellifolium) (Virk et al. 2013; Senthil-Kumar and Udayakumar 2006). The silencing of hsp70 gene in tomato plants under both normal and drought stress conditions was analyzed. The results were supported with the examination of various growth and physiological parameters in S. lycopersicum. The silencing results were compared with those from negative controls that were inoculated with pTRV2-gfp, to compare the silencing results and symptoms of viral infection. Based on the results, the growth-related parameters of shoot and roots significantly declined in tomato plants that were separately exposed to drought (abiotic) or viral infection (biotic) stresses. Further reductions were observed when the plants were simultaneously exposed to both stresses. It was previously concluded that plants might show complex responses to the simultaneous occurrence of biotic and abiotic stresses (Suzuki et al. 2014; Xiong and Yang 2003). Similarly, several other studies also indicated that empty viral vectors (pTRV2) could decrease plant growth (Hartl et al. 2008; Wu et al. 2011). Meanwhile, the plants silenced for hsp70 gene showed a high mortality rate, and were significantly weaker than the negative controls. Consequently, the differences between the phenotypes and growth-related parameters resulted from silencing, or viral infection was clearly shown. This might indicate a general and critical role for hsp70 gene in maintaining the homeostasis and growth of plants under both normal and drought conditions. It was found that under normal (stress-free) conditions, HSP70 incorporation with its chaperone allies could help the newly produced proteins obtain their proper folding and also trigger the degradation of inactivated proteins (Sung et al. 2001). This critical role of HSP70 was highlighted among different classes of HSP proteins, in which the absence of AtHSP70 led to higher rate of retarded growth (Jungkunz et al. 2011). The higher degree of severe growth retardation in our study was observed in plants silenced for hsp70 gene and simultaneously exposed to drought. This could reveal another role for HSP70 in response to and/or adaptation to stress. This is in agreement with the fact that heat shock proteins play a significant role in stress tolerance mechanisms in plants (Divya et al. 2019). Anaraki et al. (2018) also found that the negative effect of heterologous silencing of hsp70 on the growth of Nicotiana benthamiana was significantly enhanced under salinity conditions.
In addition to growth, the hsp70 silencing significantly affected the majority of physiological parameters in tomato plants, which were higher than in those of drought treatment or viral infection. Among the parameters, the content of pigments, including chlorophylls and carotenoids showed a significant reduction after silencing of hsp70, especially under drought stress conditions. The results agreed with those presented by Anaraki et al. (2018), who found a remarked reduction of pigments in hsp70-silenced plants during salinity stress. It was previously emphasized that chaperone-like HSP70 helped the accumulation of chlorophyll precursors in the alga Chlamydomonas. Therefore, when the protein lost its function, the chlorophyll synthesis pathway could be significantly blocked.
A significant decline in chlorophyll content after hsp70 silencing may be due to lower light-harvesting capacity resulting in reduction in plant biomass and productivity. Carotenoids are central plant–based chemicals that have protective roles, especially in protecting of photosynthetic pigments (Egert and Tevini 2002). The presence of a positive correlation between carotenoid and chlorophyll contents has been reported (Javadi et al. 2017). Therefore, the decrease of chlorophylls in our study could be, at least in part, a result of pigment degradation triggered by carotenoid reduction. The carotenoids are also known to play a protective role in stabilizing the plasma membrane against membrane lipid peroxidation (Havaux 1998). This could explain the accumulation of high MDA content as a product of lipid peroxidation and the high percentage of leakage from the cells of hsp70-silenced tomatoes, especially under drought stress. It has been reported that silencing of other abiotic stress–related genes in some crops, such as wheat and carapa, reduced chlorophyll content and increased lipid peroxidation under drought and osmotic stresses (Kuzuoglu-Ozturk et al. 2012; Costa et al. 2010).
The activity of antioxidant enzymes (e.g., CAT, APX, and SOD) is among the most essential plant tools for scavenging stress-induced free radicals, such as dangerous ROS molecules in plant cells (Arora et al. 2002). Antioxidant enzymes activity declined significantly in tomato plants as a result of hsp70 silencing. The activity of these enzymes was relatively higher or unchanged in nonsilenced plants during drought stress. These results were in accordance with other studies that showed increased enzyme activity in different plant species under drought-stressed condition (Mirzaee et al. 2013; Klunklin and Savage 2017). However, plants that were silenced for hsp70 showed a low level of enzyme activity under both normal and stress conditions. It has been demonstrated that antioxidant enzymes could protect the plant cells from programmed cell death (PCD), especially under stress conditions (De Pinto et al. 2012). This might explain the occurrence of high mortality and necrosis in the leaves of hsp70-silenced plants in our study.
Proline as a main compatible solute is a typical metabolic response of plants during environmental stresses, such as drought and pathogen infection (Jangid and Dwivedi 2016; Fabro et al. 2004). Following drought stress or viral infection, reactive oxygen species (ROS) are produced in plants and induce programmed cell death in plant cells (Apel and Hirt 2004). In addition to carotenoids and antioxidant enzymes, proline is a potent scavenger of ROS and plays a vital role in ameliorating the destructive effects of ROS to cell membranes, enzymes activity, and DNA during stress, and prevents the induction of programmed cell death (Chen and Dickman 2005). According to our data, proline accumulation was significantly affected by drought stress. The results are supported by Jangid and Dwivedi (2016) and Pazarlar et al. (2013) who showed the accumulation of proline induced under both drought stress and TMV infection in tomato and pepper plants, respectively. Proline accumulation was not affected by hsp70 silencing any more than by a viral infection. This was partially in accordance with the results of Anaraki et al. (2018) who found a higher proline accumulation in the hsp70-silenced N. benthamiana plants under salinity and salt-free conditions. The higher proline content in their study compared with that of the present work could be related to the different plant species or different stress (i.e., salinity vs. drought). It was noteworthy, in the present study, that proline accumulation was the only critical physiological parameter whose reduction due to gene silencing was not more significant than those of the negative controls under control and stress conditions. It has been indicated that proline acted as a molecular chaperone and could mimic HSPs function by maintaining the protein integrity and stability or by improving their function when coping with various stress conditions (Sharma and Dubey 2005; Mishra and Dubey 2006).
Conclusion
The hsp70-silenced plants showed severe growth retardation and mortality, especially under drought stress conditions. Moreover, high cell membrane damage and leakage, reduced relative water content, lower accumulation of pigments, and lower antioxidant enzyme activity were induced in plants following hsp70 silencing under both normal and stress conditions. Proline was the only parameter that was unchanged after gene silencing. Therefore, it was speculated that this compatible solute might partially compensate for the loss of protein folding or function due to HSP70 deficiency, especially under stress conditions. It was also concluded that the hsp70 gene played a critical role under normal conditions and during plant response and adaptation to drought. The overexpression of this gene could provide a reliable approach in the plant genetic engineering discipline for the protection of crops against stress conditions.
Abbreviations
- ANOVA
Analysis of variance
- AsA
Ascorbic acid
- APX
Ascorbate peroxidase
- Car
Carotenoid
- CAT
Catalase
- Chl
Chlorophyll
- CRD
Completely randomized design
- DW
Dry weight
- EC
Electrical conductivity
- EL
Electrolyte leakage
- FW
Fresh weight
- GFP
Green fluorescent protein
- HCA
Hierarchical clustering analysis
- HSP
Heat shock proteins
- MDA
Malondialdehyde
- NaOCl
Sodium hypochlorite
- NBT
Nitro blue tetrazolium
- ROS
Reactive oxygen species
- RWC
Relative water content
- SOD
Superoxide dismutase
- TBA
Thiobarbituric acid
- TCA
Trichloroacetic acid
- TRV
Tobacco rattle virus
- TW
Turgid weight
Funding information
This work was financially supported by graduate study of University of Kashan under Grant No. 572212/15.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aghaie P, Hosseini Tafreshi SA, Ebrahimi MA, Haerinasab M. Tolerance evaluation and clustering of fourteen tomato cultivars grown under mild and severe drought conditions. Sci Hortic. 2018;232:1–12. [Google Scholar]
- Al-Whaibi MH. Plant heat-shock proteins: a mini review. King Saud Univ Sci. 2011;23:139–150. [Google Scholar]
- Amjad M, Akhtar J, Anwar-ul-Haq M, Ahmad R, Zaid M. Characterization of comparative response of fifteen tomato (Lycopersicon esculentum Mill.) genotypes to NaCl stress. J Agr Sci Tech. 2014;16(4):851–862. [Google Scholar]
- Anaraki ZE, Hosseini Tafreshi SA, Shariati M. Transient silencing of heat shock proteins showed remarkable roles for HSP70 during adaptation to stress in plants. Environ Exp Bot. 2018;155:142–157. [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Aranda MA, Escaler M, Wang D, Maule AJ. Induction of HSP70 and polyubiquitin expression associated with plant virus replication. Proc Natl Acad Sci. 1996;93:15289–15293. doi: 10.1073/pnas.93.26.15289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora A, Sairam R, Srivastava G. Oxidative stress and antioxidative system in plants. Curr Sci. 2002;82:1227–1238. [Google Scholar]
- Augustine SM, Narayan JA, Syamaladevi DP, Appunu C, Chakravarthi M, Ravichandran V, Subramonian N. Erianthus arundinaceus HSP70 (EaHSP70) overexpression increases drought and salinity tolerance in sugarcane (Saccharum spp. hybrid) Plant Sci. 2015;232:23–34. doi: 10.1016/j.plantsci.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Bates L, Waldren R, Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133–140. [PubMed] [Google Scholar]
- Bhargava S, Sawant K. Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breed. 2013;132:21–32. [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92(3):351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Chen C, Dickman MB. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc Natl Acad Sci U S A. 2005;102(9):3459–3464. doi: 10.1073/pnas.0407960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MA, Pinheiro HA, Shimizu ESC, Fonseca FT, dos Santos Filho BG, Moraes FKC, de Figueiredo DM. Lipid peroxidation, chloroplastic pigments and antioxidant strategies in Carapa guianensis (Aubl.) subjected to water-deficit and short-term rewetting. Trees. 2010;24(2):275–283. [Google Scholar]
- Cui W, Liu W, Wu G. A simple method for the transformation of Agrobacterium tumefaciens by foreign DNA. Chin J Biotechnol. 1994;11(4):267–274. [PubMed] [Google Scholar]
- De Pinto M, Locato V, De Gara L. Redox regulation in plant programmed cell death. Plant Cell Environ. 2012;35(2):234–244. doi: 10.1111/j.1365-3040.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- Divya K, Bhatnagar-Mathur P, Sharma KK, Reddy PS (2019) Heat shock proteins (Hsps) mediated signalling pathways during abiotic stress conditions. In: Plant Signaling Molecules. Elsevier, pp. 499–516
- Duan Y-H, Guo J, Ding K, Wang S-J, Zhang H, Dai X-W, Chen Y-Y, Govers F, Huang L-L, Kang Z-S. Characterization of a wheat HSP70 gene and its expression in response to stripe rust infection and abiotic stresses. Mol Biol Rep. 2011;38:301–307. doi: 10.1007/s11033-010-0108-0. [DOI] [PubMed] [Google Scholar]
- Egert M, Tevini M. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum) Environ Exp Bot. 2002;48(1):43–49. [Google Scholar]
- Fabro G, Kovács I, Pavet V, Szabados L, Alvarez ME. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol Plant-Microbe Interact. 2004;17:343–350. doi: 10.1094/MPMI.2004.17.4.343. [DOI] [PubMed] [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S (2009) Plant drought stress: effects, mechanisms and management. In: Sustainable Agriculture. Springer, pp. 153–188
- Hartl M, Merker H, Schmidt DD, Baldwin IT. Optimized virus-induced gene silencing in Solanum nigrum reveals the defensive function of leucine aminopeptidase against herbivores and the shortcomings of empty vector controls. New Phytol. 2008;179:356–365. doi: 10.1111/j.1469-8137.2008.02479.x. [DOI] [PubMed] [Google Scholar]
- Havaux M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998;3:147–151. [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular California agricultural experiment station 347 (2nd edit)
- Hosseini Tafreshi SA, Shariati M, Mofid MR, Nekui MK, Esmaeili A. Heterologous virus-inducedgene silencing as a promising approach in plant functional genomics. Mol Biol Rep. 2012;39(3):2169–2178. doi: 10.1007/s11033-011-0965-1. [DOI] [PubMed] [Google Scholar]
- Hussain S, Rao MJ, Anjum MA, Ejaz S, Zakir I, Ali MA, Ahmad N, Ahmad S (2019) Oxidative stress and antioxidant defense in plants under drought conditions. In: Plant Abiotic Stress Tolerance. Springer, pp. 207–219
- Ijaz M, Qamar S, Bukhari SA, Malik K (2019) Abiotic stress signaling in rice crop. In: Advances in Rice Research for Abiotic Stress Tolerance. Elsevier, pp. 551–569
- Ings J, Mur LA, Robson PR, Bosch M. Physiological and growth responses to water deficit in the bioenergy crop Miscanthus x giganteus. Front Plant Sci. 2013;4:468. doi: 10.3389/fpls.2013.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangid KK, Dwivedi P. Physiological responses of drought stress in tomato: a review. Int J Environ Agric Biotech. 2016;9:53. [Google Scholar]
- Javadi T, Rohollahi D, Ghaderi N, Nazari F. Mitigating the adverse effects of drought stress on the morpho-physiological traits and anti-oxidative enzyme activities of Prunus avium through β-amino butyric acid drenching. Sci Hortic. 2017;218:156–163. [Google Scholar]
- Jungkunz I, Link K, Vogel F, Voll LM, Sonnewald S, Sonnewald U. AtHsp70-15-deficient Arabidopsis plants are characterized by reduced growth, a constitutive cytosolic protein response and enhanced resistance to TuMV. Plant J. 2011;66:983–995. doi: 10.1111/j.1365-313X.2011.04558.x. [DOI] [PubMed] [Google Scholar]
- Kaya MD, Okçu G, Atak M, Çıkılı Y, Kolsarıcı Ö. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.) Eur J Agron. 2006;24:291–295. [Google Scholar]
- Klunklin W, Savage G. Effect on quality characteristics of tomatoes grown under well-watered and drought stress conditions. Foods. 2017;6:56. doi: 10.3390/foods6080056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuoglu-Ozturk D, Yalcinkaya OC, Akpinar BA, Mitou G, Korkmaz G, Gozuacik D, Budak H. Autophagy-related gene, TdAtg8, in wild emmer wheat plays a role in drought and osmotic stress response. Planta. 2012;236(4):1081–1092. doi: 10.1007/s00425-012-1657-3. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H, Welburn W. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans. 1983;11(5):591–592. [Google Scholar]
- Liu C, Liu Y, Guo K, Fan D, Li G, Zheng Y, Yu L, Yang R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ Exp Bot. 2011;71(2):174–183. [Google Scholar]
- Leborgne-Castel N, Jelitto-Van Dooren EP, Crofts AJ, Denecke J. Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell. 1999;11:459–470. doi: 10.1105/tpc.11.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Schöffl F. AnHsp70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF, and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol Gen Genet. 1996;252:11–19. doi: 10.1007/s004389670002. [DOI] [PubMed] [Google Scholar]
- Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43(W1):W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaee M, Moieni A, Ghanati F. Effects of drought stress on the lipid peroxidation and antioxidant enzyme activities in two canola (Brassica napus L.) cultivars. J Agric Sci Technol. 2013;15:593–602. [Google Scholar]
- Mishra S, Dubey RS. Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: role of proline as enzyme protectant. J Plant Physiol. 2006;163:927–936. doi: 10.1016/j.jplph.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Osakabe Y, Osakabe K, Shinozaki K, Tran L-SP. Response of plants to water stress. Front Plant Sci. 2014;5:86. doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paduchuri P, Gohokar S, Thamke B, Subhas M. Transgenic tomatoes-a review. Int J Adv Biotechnol Res. 2010;1:69–72. [Google Scholar]
- Pazarlar S, Gumus M, Öztekin GB. The effects of tobacco mosaic virus infection on growth and physiological parameters in some pepper varieties (Capsicum annuum L.) Not Bot Horti Agrobo. 2013;41:427–433. [Google Scholar]
- Peremyslov VV, Hagiwara Y, Dolja VV. HSP70 homolog functions in cell-to-cell movement of a plant virus. Proc Natl Acad Sci. 1999;96:14771–14776. doi: 10.1073/pnas.96.26.14771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya M, Dhanker OP, Siddique KH, HanumanthaRao B, Nair RM, Pandey S, Singh S, Varshney RK, Prasad PV, Nayyar H. Drought and heat stress-related proteins: an update about their functional relevance in imparting stress tolerance in agricultural crops. Theor Appl Genet. 2019;132:1607–1638. doi: 10.1007/s00122-019-03331-2. [DOI] [PubMed] [Google Scholar]
- Ramegowda V, Mysore K, Senthil-Kumar M. Virus-induced gene silencing is a versatile tool for unraveling the functional relevance of multiple abiotic-stress-responsive genes in crop plants. Front Plant Sci. 2014;5:323–323. doi: 10.3389/fpls.2014.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable A, Agarwal SK. Plant heat shock protein families: essential machinery for development and defense. Int J Biol Sci. 2018;4:51–64. [Google Scholar]
- Senthil-Kumar M, Govind G, Kang L, Mysore KS, Udayakumar M. Functional characterization of Nicotiana benthamiana homologs of peanut water deficit-induced genes by virus-induced gene silencing. Planta. 2007;225:523–539. doi: 10.1007/s00425-006-0367-0. [DOI] [PubMed] [Google Scholar]
- Senthil-Kumar M, Udayakumar M. High-throughput virus-induced gene-silencing approach to assess the functional relevance of a moisture stress-induced cDNA homologous to lea4. J Exp Bot. 2006;57:2291–2302. doi: 10.1093/jxb/erj200. [DOI] [PubMed] [Google Scholar]
- Sharma P, Dubey RS. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005;46:209–221. [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J Exp Bot. 2007;58:221–227. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- Singh PK, Srivastava D, Tiwari P, Tiwari M, Verma G, Chakrabarty D (2019) Drought tolerance in plants: molecular mechanism and regulation of signaling molecules. In: Plant Signaling Molecules. Elsevier, pp. 105–123
- Sung DY, Kaplan F, Guy CL. Plant Hsp70 molecular chaperones: protein structure, gene family, expression and function. Physiol Plant. 2001;113:443–451. [Google Scholar]
- Sung DY, Guy CL. Physiological and molecular assessment of altered expression of Hsc70-1 in Arabidopsis. Evidence for pleiotropic consequences. Plant Physiol. 2003;132(2):979–987. doi: 10.1104/pp.102.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R. Abiotic and biotic stress combinations. New Phytol. 2014;203:32–43. doi: 10.1111/nph.12797. [DOI] [PubMed] [Google Scholar]
- Tang X, Zhang N, Si H, Calderón-Urrea A. Selection and validation of reference genes for RT-qPCR analysis in potato under abiotic stress. Plant Methods. 2017;13:85. doi: 10.1186/s13007-017-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman MG, Rafii M, Ismail M, Malek M, Latif MA, Oladosu Y. Heat shock proteins: functions and response against heat stress in plants. Int J Sci Technol Res. 2014;3:204–218. [Google Scholar]
- Velásquez AC, Chakravarthy S, Martin GB (2009) Virus-induced gene silencing (VIGS) in Nicotiana benthamiana and tomato. J Vis Exp: JoVE (28) [DOI] [PMC free article] [PubMed]
- Virk N, Liu B, Zhang H, Li X, Zhang Y, Li D, Song F. Tomato SlMPK4 is required for resistance against Botrytis cinerea and tolerance to drought stress. Acta Physiol Plant. 2013;35:1211–1221. [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environ Exp Bot. 2007;61:199–223. [Google Scholar]
- Wu C, Jia L, Goggin F. The reliability of virus-induced gene silencing experiments using tobacco rattle virus in tomato is influenced by the size of the vector control. Mol Plant Pathol. 2011;12:299–305. doi: 10.1111/j.1364-3703.2010.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Yang Y. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid–inducible mitogen-activated protein kinase. Plant Cell. 2003;15:745–759. doi: 10.1105/tpc.008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgallaï H, Steppe K, Lemeur R. Effects of different levels of water stress on leaf water potential, stomatal resistance, protein and chlorophyll content and certain anti-oxidative enzymes in tomato plants. J Integr Plant Biol. 2006;48:679–685. [Google Scholar]