Abstract
FOXM1, a member of the Forkhead transcriptional family, plays an important role in the EMT process, and transforming growth factor-β1 (TGF-β1) has been identified as the most potent factor that can independently induce EMT in various types of cancer cells. Here we examine the important role of FOXM1 in TGF-β1-induced EMT and investigate the mechanism underlying the relationship between TGF-β1 and FOXM1. Lentivirus-mediated transfection was used to stably upregulate the expression of FOXM1, and a small interfering RNA (siRNA) was introduced to silence the expression of FOXM1. Transwell and wound-healing assays were then performed to assess the invasion and motility potential of non-small cell lung cancer (NSCLC) cells. The NSCLC cell lines exhibited EMT characteristics, including an elongated fibroblastoid shape, induced expression of EMT marker proteins, and increased migratory and invasive potential after induction with TGF-β1. The overexpression of FOXM1 enhanced TGF-β1-induced EMT in NSCLC cells. Knockdown of FOXM1 reversed TGF-β1-induced EMT in NSCLC cell lines but had no effect on the phosphorylation level of ERK. Additionally, U0126, an ERK signaling inhibitor, exerted a reversible effect on TGF-β1-induced EMT and inhibited FOXM1 expression. FOXM1 regulated by the ERK pathway can mediate TGF-β1-induced EMT in NSCLC and is a potential target for the treatment of NSCLC.
Key words: FOXM1, Epithelial–mesenchymal transition (EMT), TGF-β1, ERK, Non-small cell lung cancer
INTRODUCTION
Non-small cell lung cancer (NSCLC) accounts for 80% of all lung cancers, is one of the most common malignant tumors worldwide, and the leading death cause of lung cancer is metastasis. Tumor metastasis involves several steps, including separation, migration, invasion, and the formation of a new tumor nodule.
The epithelial–mesenchymal transition (EMT) is a cellular mechanism recognized as a central feature of normal tissue development (1). Recent studies have revealed that EMT is a key process contributing to cancer metastasis that is characterized by the loss of the epithelial marker E-cadherin, an increase in the mesenchymal markers vimentin and N-cadherin, and an increase in migratory and invasive behavior (2). Although several cytokines are involved in EMT, transforming growth factor-β1 (TGF-β1) has been identified as the most potent factor that can independently induce EMT in various types of cancer cells (3). The role of TGF-β1 in cancer metastasis is also confirmed by the fact that neutralizing antibodies for TGF-β1 can suppress cancer metastasis (4).
Forkhead box M1 (FOXM1) is a member of the Forkhead family of transcription factors that share homology within the Winged Helix/Forkhead box DNA-binding domain. In proliferating cells, FOXM1 regulates the G2/M transition of the cell cycle through the transcriptional activation of Cdc25B phosphatase, Plk1, AuroraB kinase, and cyclin B1 (5). Consistent with its role in cell cycle progression, increased FOXM1 expression is found in a variety of human cancers (6–10). Recent studies have demonstrated that FOXM1 plays an important role in the EMT process. FOXM1 can induce EMT by directly activating the promoter of the EMT-associated transcriptional factors Snail (11) and Slug (12). Furthermore, FOXM1 was found to be involved in EMT of A549 cells induced by TGF-β1 (13,14). However, the underlying mechanism linking TGF-β1 and FOXM1 remains unclear.
In the present study, we investigated whether FOXM1 regulated by the ERK pathway can mediate TGF-β1-induced EMT in NSCLC.
MATERIALS AND METHODS
Cell Culture, Reagents, and Treatments
Lung cancer cell lines A549 and H1299 were cultured in Dulbecco’s modified Eagle’s minimum (Gibco BRL), and H1650 and H1975 were cultured in RPMI-1640 medium (Gibco BRL); both media were supplemented with 10% fetal bovine serum (Hyclone, Inc., USA). The cell lines were incubated under standard conditions at 37°C in a humidified atmosphere containing 5% CO2. TGF-β1 was purchased from PeproTech, Inc. (Rocky Hill, NJ, USA), and U0126, a small molecular inhibitor of the ERK pathway, was purchased from Sigma Aldrich, Inc. (St. Louis, MO, USA).
Western Blot and Antibodies
Equal amounts of protein (50–200 μg/lane) were extracted from whole-cell lysates and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to polyvinylidene difluoride (PVDF) membranes (Millipore, USA), the proteins were incubated with primary antibodies (FOXM1 rabbit mAb, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA; E-cadherin, vimentin, ERK, p-ERK rabbit mAb, 1:1,000, Cell Signaling Technology, Beverly, MA, USA), followed by the appropriate HRP-conjugated secondary antibodies. Immunoreactive proteins were detected using an enhanced chemiluminescence detection kit (Thermo Scientific, Rockford, IL, USA).
RT-PCR
Total RNA from cultured cells was isolated with the Trizol reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA) and then resuspended in diethyl pyrocarbonate (DEPC)-treated water. Reverse transcription was performed according to the manufacturer’s instructions (TaKaRa, Dalian, China). The primer sequences utilized were as follows: FOXM1 sense, 5′-AGCGACAGGTTAAGGTTGAG-3′, antisense, 5′-GTGCTGTTGATGGCGAATTG-3′; E-cadherin sense, 5′-TGCCCAGAAAATGAAAAAGG-3′, antisense, 5′-GTGTATGTGGCAATGCGTTC-3′; vimentin sense, 5′-GAGAACTTTGCCGTTGAAGC-3′, antisense, 5′-CTCAATGTCAAGGGCCATCT-3′; GAPDH sense, 5′-ACGGATTTGGTCGTATTGGGCG-3′, antisense, 5′-CTCCTGGAAGATGGTGATGG-3′. The PCR products were separated by 1.5% agarose gel electrophoresis.
Transwell Migration and Invasion Assay
In vitro migration assays were performed in a Transwell chamber (Corning Costar, USA) containing a membrane filter (8-μm pore size). To measure cell invasion overnight, Transwell filters were coated on the lower chamber with 5 μg/ml of Bio-Coat Matrigel (BD Biosciences, USA) to reconstitute the matrix of the basement membrane. Cells were seeded at a density of 2.0 × 104 per insert in 200 μl of serum-free medium and transferred to wells filled with 600 μl culture medium containing 10% fetal bovine serum (FBS) as a chemoattractant. After 24 h of incubation, the noninvading cells on the top of the membrane were removed by scraping. The invading cells on the bottom of the membrane were fixed in 4% paraformaldehyde and stained with 0.05% crystal violet. The number of invading cells was then counted in nine random high-power fields per well at 200× magnification under a light microscope.
Transient Transfection of FOXM1 siRNA
FOXM1-specific small interfering RNA (siRNA) oligonucleotides were purchased from GenePharma (Shanghai GenePharma Co., Shanghai). The sequences of the double-stranded siRNA oligonucleotides utilized were 5′-GGACCACUUUCCCUACUUUTT-3′ (sense), 5′-AAAGUAGGGAAAGUGGUCCTT-3′ (antisense). The negative control siRNA sequences were 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense), 5′-ACGUGACACGUUCGGAGAATT-3′ (antisense). Human lung carcinoma H1650 and H1299 cells after treatment with TGF-β1 (5 ng/ml) were transfected with siRNA (100 nM) using the DharmaFECT4 siRNA transfection reagent (Dharmacon, Chicago, IL, USA) according to the manufacturer’s protocol.
Stable Transfection of FOXM1
The plasmid EX-Z5438-LV135 and Lenti-Pac HIV Expression Packaging Kit were purchased from GeneCopoeia, Inc. (Guangzhou, China). Human lung carcinoma H1650 cells, which were tested to confirm low FOXM1 expression, were stably transfected according to the manufacturer’s instructions.
Wound-Healing Assay
Cells were seeded into six-well plates and cultured overnight to form a confluent monolayer. After scratching with a sterile pipette tip, the cells were rinsed gently with phosphate-buffered saline to remove the detached cells and incubated with a medium containing 0.5% FBS. Imaging of the wounded areas was performed at the indicated time points. The distances between the two edges of the scratched cells were measured, and the healing rate was calculated using the following formula: healing rate = (the distance before healing − the distance after healing)/the distance before healing × 100%.
Statistical Analyses
Student’s t test and an ANOVA were used to compare the results, as appropriate. All statistical analyses were performed using the software of SPSS 17.0. A value of p < 0.05 was considered to be statistically significant.
RESULTS
TGF-β1-Induced EMT in NSCLC
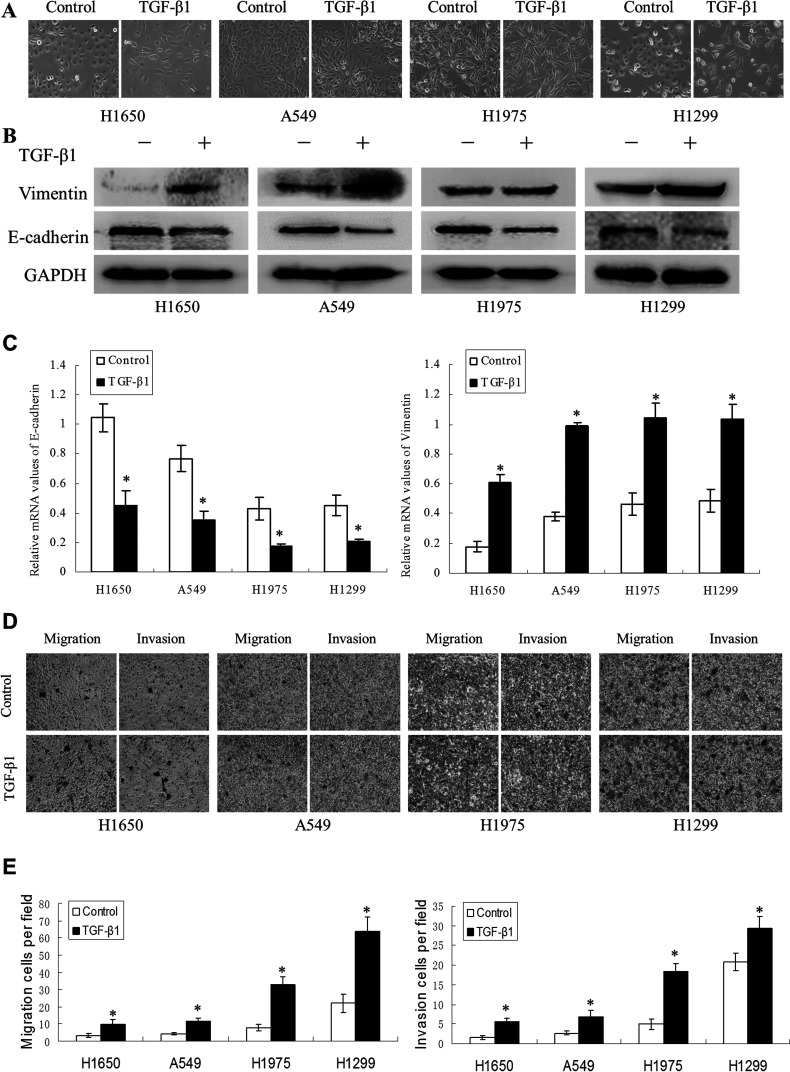
To address the effect of TGF-β1 on EMT, we first detected morphological changes in four NSCLC cell lines at 72 h after TGF-β1 treatment. As shown in Figure 1A, TGF-β1 (5 ng/ml) treatment promoted a change in the cell phenotype established by a loss of cell–cell contact and an elongated, fibroblastoid morphology, whereas the untreated cells maintained a uniform cobblestone morphology, with adherence and tight junctions. The morphological changes in the cell lines undergoing EMT were accompanied by a shift in specific molecular changes from an epithelial to a mesenchymal repertoire. As shown in Figure 1B and C, compared with untreated cells, the TGF-β1-induced cell lines exhibited decreased E-cadherin and increased vimentin expression at both the protein and mRNA levels. To further determine the effects of TGF-β1 on the migratory and invasive ability of the cell lines, we evaluated changes in migratory ability using Transwell assays. TGF-β1 could enhance the ability of NSCLC cell lines to invade through the Transwell membrane (Fig. 1D and E). Taken together, these data demonstrated that NSCLC cell lines exhibit phenotypes consistent with EMT after TGF-β1 induction.
Figure 1.
Transforming growth factor (TGF)-β1-induced EMT and increased migration ability in non-small lung cancer cell lines. (A) The H1650, A549, H1975, and H1299 cell lines treated with TGF-β1 (5 ng/ml) for 72 h displayed EMT phenotypic changes, including a loss of cell–cell contact and an elongated, fibroblastoid morphology. Magnification: 200×. The expression of epithelial and mesenchymal markers was determined by Western blotting (B) and RT-PCR (C) in the four types of NSCLC cell lines induced with TGF-β1. (D, E) Transwell assays were performed in the TGF-β1-treated cell lines (*p < 0.01) compared with the control.
FOXM1 Enhanced TGF-β1-Induced EMT in H1650 Cells
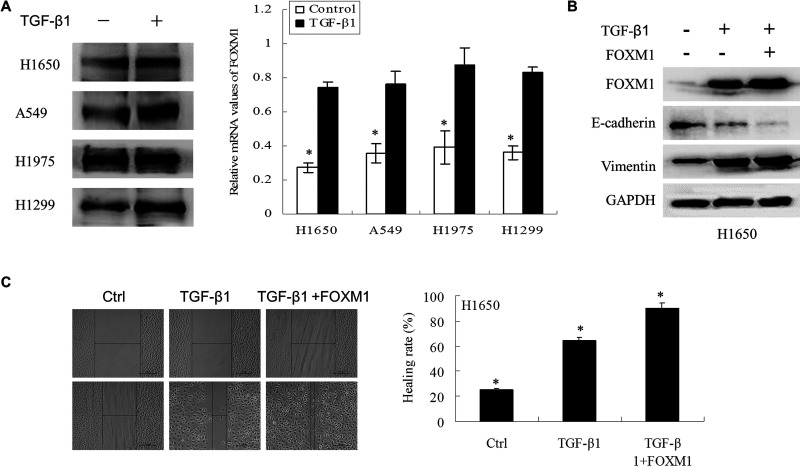
Emerging evidence has shown that FOXM1 plays an important role in EMT induction. As shown in Figure 2A, TGF-β1 increased the expression of FOXM1 both at the protein and mRNA levels. These data suggest that TGF-β1 might induce EMT through the increased expression of FOXM1.
Figure 2.
FOXM1 enhanced TGF-β1-induced EMT in NSCLC cells. (A) NSCLC cells treated with TGF-β1 (5 ng/ml) for 2 days were analyzed by Western blotting and RT-PCR to detect protein and mRNA changes in FOXM1. (B) Vector and FOXM1 stably overexpressing H1650 cells were treated with TGF-β1 (5 ng/ml) for 2 days. The proteins were then extracted, and Western blotting was performed to detect FOXM1, E-cadherin, and vimentin. (C) Wound-healing assays were used to detect the migration capacity of H1650 cells after treatment with TGF-β1 (5 ng/ml) for 3 days in the vector and FOXM1 groups.
To investigate the role of FOXM1 in TGF-β1-induced EMT, the expression of FOXM1, E-cadherin, and vimentin was detected in vector-transfected and FOXM1-overexpressing H1650 cells treated with TGF-β1. In the current study, H1650 cells underwent a very dramatic EMT when the cells were stimulated by a combination of TGF-β1 and exogenous FOXM1. Western blotting showed that TGF-β1 induced the upregulation of FOXM1 and vimentin, which was enhanced by exogenous FOXM1 in H1650 cells (Fig. 2B and C). However, the expression of E-cadherin was more decreased in the FOXM1-overexpressing H1650 cells than in the cells transfected with the empty vector. Additionally, the results of a wound-healing assay revealed a significant increase in cell migration in cells with high FOXM1 expression prior to treatment with TGF-β1 compared with cells treated with TGF-β1 alone at 24 h. These results suggested that FOXM1 enhanced TGF-β1-induced EMT in NSCLC cells.
FOXM1 Is Necessary for TGF-β1-Induced EMT
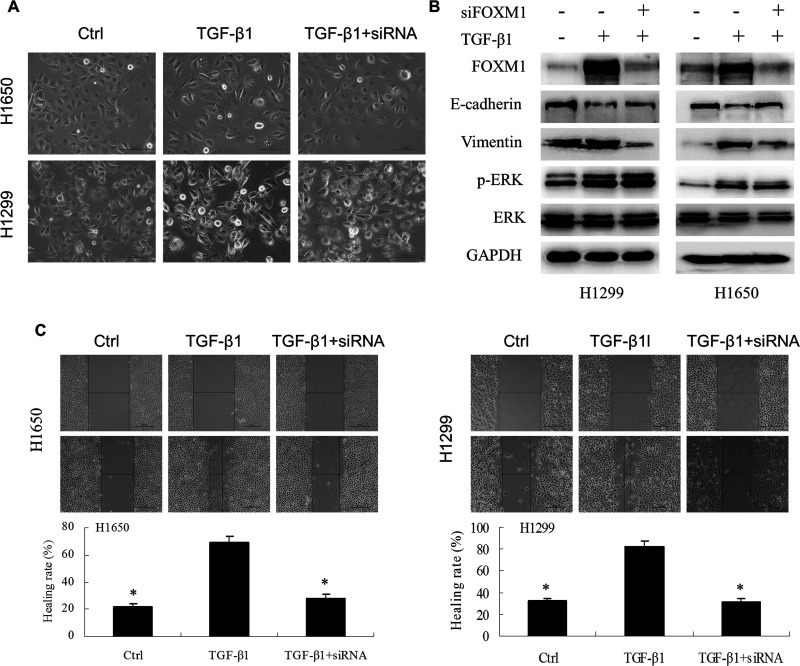
Given that FOXM1 was increased during TGF-β1-induced EMT, we addressed whether a siRNA targeting FOXM1 could reverse the EMT phenotype by knocking down the expression of FOXM1 in NSCLC cell lines. As shown in Figure 3A, the knockdown of FOXM1 in NSCLC cell lines reversed the change in the cell phenotype established by a loss of cell–cell contact and an elongated, fibroblastoid morphology; the control cells maintained a uniform cobblestone morphology, with adherence and tight junctions. Furthermore, in both H1650 and H1299 cells, the siRNA targeting FOXM1 also inhibited the expression of vimentin and upregulated the expression of E-cadherin, though the phosphorylation level of ERK was not changed (Fig. 3B). In addition, the wound-healing assay indicated a significant decrease in the migration ability of cells transfected with the FOXM1 siRNA after treatment with TGF-β1 compared with cells treated with TGF-β1 alone at 24 h (p < 0.01, Fig. 3C). The above results suggested that FOXM1 played an important role in TGF-β1-induced EMT in NSCLC cell lines and that knockdown of FOXM1 could inhibit TGF-β1-induced EMT.
Figure 3.
Knockdown of FOXM1 in NSCLC reversed the phenotype of TGF-β1-induced EMT. (A) H1650 and H1299 cells were incubated with TGF-β1 (5 ng/ml) for 72 h and then transfected with siRNA FOXM1 for 48 h. (A) Morphological changes were examined. (B) Western blot analysis of epithelial marker E-cadherin and mesenchymal marker vimentin. The effect of siRNA targeting FOXM1 on ERK and p-ERK was also detected. (C) The migration capacity of H1650 and H1299 cells treated with TGF-β1 and siRNA FOXM1 was determined by wound-healing assays (*p < 0.01).
The ERK Signaling Pathway Mediates TGF-β1-Induced EMT in NSCLC Through the Activation of FOXM1
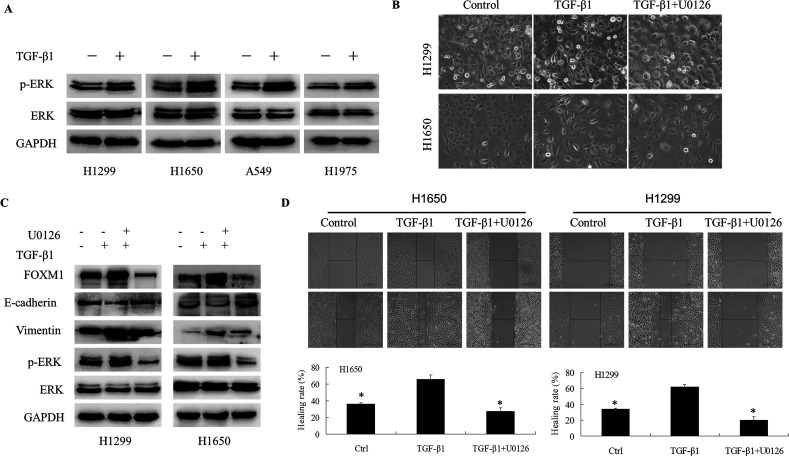
As shown in Figure 4A, the phosphorylation level of ERK was upregulated after treatment with TGF-β1 in four different types of NSCLC cell lines. To further investigate the role of the ERK pathway in TGF-β1-induced EMT, U0126, a reversible ERK pathway inhibitor, was used. As expected, U0126 could inhibit the expression of p-ERK (Fig. 4B). Next, we tested whether U0126 could affect EMT markers in TGF-β1-treated H1650 and H1299 cell lines. As shown in Figure 4B, the protein level of E-cadherin was upregulated after treatment with TGF-β1 and U0126, whereas the levels of vimentin were significantly downregulated compared with the TGF-β1-treated cell lines. Interestingly, the expression of FOXM1 was notably decreased after treatment with U0126. Furthermore, U0126 reversed the change in the cell phenotype established by a loss of cell–cell contact and an elongated, fibroblastoid morphology (Fig. 4C). Moreover, the migratory potential of the A549 cell line was determined using a wounding-healing assay, and the migratory ability of the cell lines treated with TGF-β1 and U0126 was significantly reduced compared with cells treated with TGF-β1 alone (Fig. 4D). These results demonstrated that U0126 was able to inhibit TGF-β1-induced EMT and FOXM1 expression in NSCLC cell lines, suggesting that the ERK signaling pathway mediates TGF-β1-induced EMT in NSCLC through the upregulation of FOXM1.
Figure 4.
U0126 reversed the phenotype of TGF-β1-induced EMT in NSCLC cell lines. H1650 and H1299 cells were incubated with TGF-β1 (5 ng/ml) for 72 h, and then U0126 (10 µM) was added for another 48 h. (A) Morphological changes were examined in control cells (RP1640) and cells treated with TGF-β1 (5 ng/ml) and TGF-β1 and U0126. (B) The expression of ERK, p-ERK, FOXM1, E-cadherin, and vimentin was determined by Western blotting. (D) The migration capacity of H1650 and H1299 cells treated with TGF-β1 and U0126 was determined by wound-healing assays (**p < 0.01).
DISCUSSION
In this study, we confirmed that the NSCLC cell lines exhibited EMT characteristics, including an elongated fibroblastoid shape, a switching of EMT marker proteins, and increased migratory and invasive potential after induction with TGF-β1. We also demonstrated that FOXM1 is necessary for TGF-β1-induced EMT in NSCLC cell lines. Additionally, U0126, an ERK signaling inhibitor, exerted a reversible effect on TGF-β1-induced EMT and inhibited FOXM1 expression, which indicated the critical role of ERK signaling in the process of EMT and that FOXM1 is a potential target for the treatment of NSCLC.
The process of EMT is involved in tumor migration, invasion, and dissemination, thus facilitating tumor progression (15). TGF-β1 has been recognized as a regulator of EMT in advanced-stage human cancers and is the most widely used inducer of EMT for in vitro studies (16). In this study, we confirmed that TGF-β1 could induce EMT characteristics in NSCLC cell lines, which was consistent with previous studies (17,18).
FOXM1 is a proliferation-specific transcription factor that is critical for the orderly progression of the cell cycle. Recent studies suggest that FOXM1 is a potential therapeutic target for the development of anticancer treatments (19–21). In pancreatic cancer (22), prostate cancer (23), and clear cell renal cell carcinoma (7), the downregulation of FOXM1 leads to the inhibition of invasion of these cancer cells. Additionally, there have been implications with regard to FOXM1’s role in regulating the EMT process of several human cancers. Hyun Jung Park et al. (24) suggested that, in addition to its well-characterized function in proliferation, the deregulation of FOXM1b is a major driving force for multiple steps in tumor metastasis. FOXM1 can promote the migration and invasion capabilities of various cancers by regulating the EMT process in these cells, which is one of the common mechanisms for cancer metastasis. Accumulating evidence has indicated that FOXM1 promotes metastasis by regulating EMT in cancer progression (9). In breast cancer, FOXM1 promotes EMT by stimulating the transcription of EMT-related genes, such as Slug (12). In lung cancer, FOXM1 has also been shown to increase EMT during radiation-induced pulmonary fibrosis by enhancing the expression of Snail (11). Additionally, the overexpression of FOXM1 can lead to indirect upregulation of ZEB1 and ZEB2, inducing the EMT process through microRNA-200b (miR-200b) downregulation (25). Previous studies have suggested that FOXM1 can promote the EMT process in cancer cells by regulating EMT-associated transcriptional factors, such as Slug, Snail, and ZEB1/2. In accordance with the above results, our data showed that FOXM1 not only enhances TGF-β1-induced EMT but also plays an important role in this process.
However, the interaction between TGF-β1, as a cytokine, and transcription factor FOXM1 is not clear. TGF-β1 was found to induce EMT via Smad-dependent and Smad-independent pathways (26). Through the Smad-mediated pathway, TGF-β1 signals are transduced by transmembrane serine/threonine kinase type II and type I receptors (TbR II and TbR I) and intracellular mediator Smads (27). In the non-Smad signaling pathway, TGF-β1 receptors interact with the MAPK pathway (28). Raf/MEK/MAPK signaling could stimulate FOXM1 nuclear translocation, and transactivating activity offers an explanation for this mitogenic dependency (29). Accordingly, we hypothesized that the ERK pathway is involved in the interaction between TGF-β1-induced EMT and FOXM1. To test the deep relationship between FOXM1 and the ERK pathway after treatment with TGF-β1, we used a FOXM1-specific siRNA and U0126, an ERK small molecular inhibitor, to downregulate FOXM1 expression and block the ERK pathway, respectively. Our results showed that U0126 could significantly decrease FOXM1 expression, whereas the FOXM1 siRNA did not alter the p-ERK levels. FOXM1 might mediate TGF-β1-induced EMT, downstream of the ERK pathway. This is the first report regarding how TGF-β1 interacts with FOXM1 in NSCLC. However, whether TGF-β1 affects the expression of other signaling pathways, such as PI3K/AKT, needs to be further investigated.
In summary, our results demonstrated that FOXM1 was upregulated in TGF-β1-induced EMT and that the inhibition of FOXM1 resulted in the antitumor activity associated with EMT reversal. These findings may provide new insight into understanding the mechanisms of lung cancer progression, offering a new biomarker for lung cancer treatment.
ACKNOWLEDGMENTS
This work was supported by Shanghai Jiaotong University School of Medicine Science Fund Project (11XJ22014), Hospital Foundation of No. 3 People’s Hospital, which is affiliated with the Shanghai Jiao-Tong University School of Medicine (syz2011-05), Education Fund for outstanding young teachers of Shanghai (zzjdyx12111), and Science and Technology Commission of Shanghai Municipality (10JC1409200).
REFERENCES
- 1. Thiery J. P; Acloque H.; Huang R. Y.; Nieto M. A. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–890. [DOI] [PubMed] [Google Scholar]
- 2. Kraljevic Pavelic S.; Sedic M.; Bosnjak H.; Spaventi S.; Pavelic K. Metastasis: New perspectives on an old problem. Mol. Cancer 2011; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ikushima H.; Miyazono K. TGFbeta signalling: A complex web in cancer progression. Nat. Rev. Cancer 2010; 10: 415–424. [DOI] [PubMed] [Google Scholar]
- 4. Biswas S.; Guix M.; Rinehart C.; Dugger T. C.; Chytil A.; Moses H. L.; Freeman M. L.; Arteaga C. L. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J. Clin. Invest. 2007; 117: 1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Costa R. H.; Kalinichenko V. V.; Major M. L.; Raychaudhuri P. New and unexpected: Forkhead meets ARF. Curr. Opin. Genet. Dev. 2005; 15: 42–48. [DOI] [PubMed] [Google Scholar]
- 6. Sun H. C.; Li M.; Lu J. L.; Yan D. W.; Zhou C. Z.; Fan J. W.; Qin X. B.; Tang H. M.; Peng Z. H. Overexpression of Forkhead box M1 protein associates with aggressive tumor features and poor prognosis of hepatocellular carcinoma. Oncol. Rep. 2011; 25: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 7. Xue Y. J.; Xiao R. H.; Long D. Z.; Zou X. F.; Wang X. N.; Zhang G. X.; Yuan Y. H.; Wu G. Q.; Yang J.; Wu Y. T.; Xu H.; Liu F. L.; Liu M. Overexpression of FoxM1 is associated with tumor progression in patients with clear cell renal cell carcinoma. J. Transl. Med. 2012; 10: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu D.; Zhang Z.; Kong C. Z. High FOXM1 expression was associated with bladder carcinogenesis. Tumour Biol. 2013; 34: 1131–1138. [DOI] [PubMed] [Google Scholar]
- 9. Xu N.; Jia D.; Chen W.; Wang H.; Liu F.; Ge H.; Zhu X.; Song Y.; Zhang X.; Zhang D.; Ge D.; Bai C. FoxM1 is associated with poor prognosis of non-small cell lung cancer patients through promoting tumor metastasis. PloS One 2013; 8: e59412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu X. R.; Chen Y. H.; Liu D. M.; Sha J. J.; Xuan H. Q.; Bo J. J.; Huang Y. R. Increased expression of forkhead box M1 protein is associated with poor prognosis in clear cell renal cell carcinoma. Med. Oncol. 2013; 30: 346. [DOI] [PubMed] [Google Scholar]
- 11. Balli D.; Ustiyan V.; Zhang Y.; Wang I. C.; Masino A. J.; Ren X.; Whitsett J. A.; Kalinichenko W.; Kalin T. V. Foxm1 transcription factor is required for lung fibrosis and epithelial-to-mesenchymal transition. EMBO J. 2013; 32: 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang C.; Chen H.; Tan G.; Gao W.; Cheng L.; Jiang X.; Yu L.; Tan Y. FOXM1 promotes the epithelial to mesenchymal transition by stimulating the transcription of slug in human breast cancer. Cancer Lett. 2013; 340: 104–112. [DOI] [PubMed] [Google Scholar]
- 13. Li J.; Wang Y.; Luo J.; Fu Z.; Ying J.; Yu Y.; Yu W. miR-134 inhibits epithelial to mesenchymal transition by targeting FOXM1 in non-small cell lung cancer cells. FEBS Lett. 2012; 586: 3761–3765. [DOI] [PubMed] [Google Scholar]
- 14. Ke Y.; Zhao W.; Xiong J.; Cao R. miR-149 inhibits non-small-cell lung cancer cells EMT by targeting FOXM1. Biochem. Res. Int. 2013; 2013: 506731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huber M. A.; Kraut N.; Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 2005; 17: 548–558. [DOI] [PubMed] [Google Scholar]
- 16. Muraoka-Cook R. S.; Shin I.; Yi J. Y.; Easterly E.; Barcellos-Hoff M. H.; Yingling J. M.; Zent R.; Arteaga C. L. Activated type I TGFbeta receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene 2006; 25: 3408–3423. [DOI] [PubMed] [Google Scholar]
- 17. Masaki S.; Masutani H.; Yoshihara E.; Yodoi J. Deficiency of thioredoxin binding protein-2 (TBP-2) enhances TGF-β signaling and promotes epithelial to mesenchymal transition. PLoS One 2012; 7: e39900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ha B.; Kim E. K.; Kim J. H.; Lee H. N.; Lee K. O.; Lee S. Y.; Jang H. H. Human peroxiredoxin 1 modulates TGF-beta1-induced epithelial-mesenchymal transition through its peroxidase activity. Biochem. Biophys. Res. Commun. 2012; 421: 33–37. [DOI] [PubMed] [Google Scholar]
- 19. Wang Z.; Ahmad A.; Li Y.; Benerjee S.; Kong D.; Sarkar F. H. Forkhead box M1 transcription factor: A novel target for cancer therapy. Cancer Treat. Rev. 2010; 36: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen H.; Yang C.; Yu L.; Xie L.; Hu J.; Zeng L; Tan Y. Adenovirus-mediated RNA interference targeting FOXM1 transcription factor suppresses cell proliferation and tumor growth of nasopharyngeal carcinoma. J. Gene Med. 2012; 14: 231–240. [DOI] [PubMed] [Google Scholar]
- 21. Yang C.; Chen H.; Yu L.; Shan L.; Xie L.; Hu J.; Chen T.; Tan Y. Inhibition of FOXM1 transcription factor suppresses cell proliferation and tumor growth of breast cancer. Cancer Gene Ther. 2013; 20: 117–124. [DOI] [PubMed] [Google Scholar]
- 22. Wang Z.; Banerjee S.; Kong D.; Li Y.; Sarkar F. H. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007; 67: 8293–8300. [DOI] [PubMed] [Google Scholar]
- 23. Lynch T. P.; Ferrer C. M.; Jackson S. R.; Shahriari K. S.; Vosseller K.; Reginato M. J. Critical role of O-Linked beta-N-acetylglucosamine transferase in prostate cancer invasion; angiogenesis; and metastasis. J. Biol. Chem. 2012; 287: 11070–11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park H. J.; Gusarova G.; Wang Z.; Carr J. R.; Li J.; Kim K. H.; Qiu J.; Park Y. D.; Williamson P. R.; Hay N.; Tyner A. L.; Lau L. F.; Costa R. H.; Raychaudhuri P. Deregulation of FoxM1b leads to tumour metastasis. EMBO Mol. Med. 2011; 3: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bao B.; Wang Z.; Ali S.; Kong D.; Banerjee S.; Ahmad A.; Li Y.; Azmi A. S.; Miele L.; Sarkar F. H. Overexpression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J. Cell. Biochem. 2011; 112: 2296–2306. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Derynck R.; Zhang Y. E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003; 425: 577–584. [DOI] [PubMed] [Google Scholar]
- 27. Miyazono K.; ten Dijke P.; Heldin C. H. TGF-beta signaling by Smad proteins. Adv. Immunol. 2000; 75: 115–157. [DOI] [PubMed] [Google Scholar]
- 28. Moustakas A.; Heldin C. H. Non-Smad TGF-β signals. J. Cell Sci. 2005; 118:3573–3584. [DOI] [PubMed] [Google Scholar]
- 29. Ma R. Y.; Tong T. H.; Cheung A. M.; Tsang A. C.; Leung W. Y.; Yao K. M. Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J. Cell Sci. 2005; 118: 795–806. [DOI] [PubMed] [Google Scholar]