Abstract
Chronic pain and inflammatory diseases can be regulated by complex mechanisms involving α7 nicotinic acetylcholine receptors (nAChRs), making this subtype a promising drug target for anti-inflammatory therapies. Recent evidence suggests that suchtreatment of inflammatory pain may rely on metabotropic-like rather than ionotropic activation of the α7 receptor subtype in non-neuronal cells. We previously identified para-trifluoromethyl (p-CF3) N,N-diethyl-N′-phenylpiperazinium (diEPP) iodide to be among the compounds classified as silent agonists, which are very weak α7 partial agonists that are able to induce positive allosteric modulator (PAM)–sensitive desensitization. Such drugs have been shown to selectively promote α7 ionotropic–independent functions. Therefore, we here further investigated the electrophysiological profile of p-CF3 diEPP and its in vivo antinociceptive activity using Xenopus oocytes expressing α7, α4β2, or α3β4 nAChRs. The evoked currents confirmed p-CF3 diEPP to be α7-selective with a maximal agonism 5% that of acetylcholine (ACh). Coapplication of p-CF3 diEPP with the type II PAM 4-naphthalene-1-yl-3a,4,5,9b-tetrahydro-3-H-cyclopenta[c]quinoline-8-sulfonic acid amide (TQS) produced desensitization that could be converted to PAM-potentiated currents, which at a negative holding potential were up to 13-fold greater than ACh controls. Voltage-dependence experiments indicated that channel block may limit both control ACh and TQS-potentiated responses. Although no p-CF3 diEPP agonist activity was detected for the heteromeric nAChRs, it was a noncompetitive antagonist of these receptors. The compound displayed remarkable antihyperalgesic and antiedema effects in in vivo assays. The antinociceptive activity was dose and time dependent. The anti-inflammatory components were sensitive to the α7-selective antagonist methyllycaconitine, which supports the idea that these effects are mediated by the α7 nAChR.
Introduction
Although the basis for nicotinic acetylcholine receptor (nAChR) signaling has been associated with ion channel activity and receptor permeability to cations (Na+, K+, and Ca2+) upon binding of specific ligands, the α7 nAChR subtype also displays an additional pharmacology related to a mode of signal transduction (Horenstein and Papke, 2017). Besides being widely distributed in the central and peripheral nervous systems, especially in the brain, the α7 nAChR has been found in non-neuronal cells and tissues not associated with ion channel transmission, such as lymphocytes, macrophages, and microglial cells (Zdanowski et al., 2015; Fujii et al., 2017). These non-neuronal cells are involved in the modulation of immune responses and neuropathic pain through the anti-inflammatory cholinergic pathway, which involves communication between the nervous and immune systems mediated by the vagal nerve (Tracey, 2007). Although the mechanisms regulating cytokine production and release are complex, the involvement of α7 nAChR has been extensively demonstrated (Tracey, 2007; Papke et al., 2015; Horenstein and Papke, 2017). In immune cells, the α7 nAChR appears to function as a metabotropic-like receptor (Papke, 2014; Kabbani and Nichols, 2018) by interacting with different pathways (i.e., JAK/STAT) (de Jonge et al., 2005), regulating tumor necrosis factor release from macrophages (Thomsen and Mikkelsen, 2012), and potentially coupling with G-proteins (Kabbani et al., 2013; King et al., 2015). Therefore, the α7 receptor subtype emerged as a promising target to treat disorders including inflammatory diseases and chronic neuropathic pain (Papke et al., 2015).
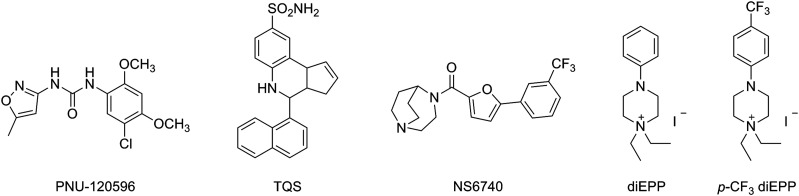
Modulation of the α7 nonionotropic effects may represent a better and alternative approach to classic ionotropic receptor activation to control and induce anti-inflammatory responses. Because of the diversity found in the distribution and functions of α7 nAChRs in the human body, molecules selectively promoting α7 nonionotropic signaling may reduce off-target effects compared with compounds inducing current-dependent effects, and therefore promote anti-inflammatory responses over cognitive effects. Some of the molecules selectively able to promote the α7 metabotropic-like function are known as silent agonists (Chojnacka et al., 2013; Papke et al., 2014; Quadri et al., 2016, 2017a,b). Silent agonists are very weak α7 partial agonists with the capability of selectively inducing and stabilizing receptor desensitization, which can be revealed by coapplication of a type II positive allosteric modulator (PAM) such as N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea (PNU-120596) (Hurst et al., 2005; Grønlien et al., 2007) or 4-naphthalene-1-yl-3a,4,5,9b-tetrahydro-3-H-cyclopenta[c]quinoline-8-sulfonic acid amide (TQS) (Grønlien et al., 2007) (Fig. 1). The α7 type II PAMs bind to sites distinct from the orthosteric agonist sites and do not induce significant receptor activation on their own but rather act on receptor agonist activation and/or desensitization by increasing α7’s intrinsically low open probability by means of at least two mechanisms. PAMs may reduce the steep energy barrier to enter the open state(s) and/or may decrease α7 desensitization by destabilizing the receptor’s desensitized state and inducing a PAM-sensitive, kinetically coupled conductive state. The combination of those two components results in activation of desensitized receptors (Williams et al., 2011b), allowing type II PAMs to reveal α7 desensitization induced by silent agonists. The α7-selective silent agonist 1,4-diazabicyclo[3.2.2]nonan-4-yl(5-(3-(trifluoromethyl)phenyl)furan-2-yl)methanone (NS6740) (Fig. 1), which induces strong desensitization but very little ion channel activation, proved to be efficacious in in vitro and in vivo inflammatory models (Thomsen and Mikkelsen, 2012; Papke et al., 2015). Indeed, in microglia cells NS6740 was more effective in reducing lipopolysaccharide-induced tumor necrosis factor-α release compared with efficacious α7 agonists such as choline (Thomsen and Mikkelsen, 2012). Moreover, different mouse models of chronic pain were investigated for testing the anti-inflammatory activity of NS6740, and the drug was efficacious for reducing inflammation associated with tonic inflammatory pain and peripheral neuropathy. In these models, the effects of NS6740 were consistent with nonionotropic signaling transduction (Papke et al., 2015).
Fig. 1.
Selected α7 nAChR ligand structures.
Here, we report our investigation of the anti-inflammatory profile of a weak α7 partial agonist with low channel activation efficacy, comparable to that of NS6740 but with a different molecular framework and more readily reversible desensitization. Structural modifications of the weak α7 silent agonist N,N-diethyl-N′-phenylpiperazinium (diEPP) (Papke et al., 2014) (Fig. 1) led to the identification of a much deeper desensitizer, related to a trifluoromethyl group in the para position of the aromatic ring, 1,1-diethyl-4-(4-(trifluoromethyl)phenyl)piperazin-1-ium iodide [para-trifluoromethyl (p-CF3) diEPP] (Quadri et al., 2016) (Fig. 1). Indeed, by introducing the p-CF3 group, we achieved a substantial increase in the PNU-120596-potentiated response compared with the parent compound diEPP while preserving the very weak partial agonist component. To better define the pharmacological profile of this promising α7 very weak partial agonist, we expanded the investigation of its effects on different nAChR subtypes and with the alternative type II PAM, TQS. Together with these extensive electrophysiological characterizations, we report the highlights of p-CF3 diEPP effects in in vivo models of hyperalgesia and edema.
Materials and Methods
In Vitro Methods
Heterologous Expression of nAChRs in Xenopus Laevis Oocytes.
Xenopus laevis oocytes were injected with human nAChR clones obtained from Dr. J. Lindstrom (University of Pennsylvania, Philadelphia, PA) to heterologously express nAChRs. To improve the level and speed of α7 subtype expression without affecting its pharmacological properties (Halevi et al., 2003) the human RIC3 clone was coinjected with α7. RIC3 was obtained from Dr. M. Treinin (Hebrew University, Jerusalem, Israel). Plasmid cDNAs were first linearized and purified, and then RNAs were prepared using the mMessage mMachine in vitro RNA transcription kit (Ambion, Austin, TX). Oocytes were surgically removed from mature Xenopus laevis frogs (Nasco, Ft. Atkinson, WI) and subsequently injected with appropriate nAChR subunit RNAs as described previously (Papke and Stokes, 2010). Frogs were maintained in the Animal Care Service facility at the University of Florida, and all procedures were approved by the University of Florida Institutional Animal Care and Use Committee. All studies were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf). Briefly, the frog was first anesthetized for 15–20 minutes in 1.5 l frog tank water containing 1 g of 3-aminobenzoate methanesulfonate buffered with sodium bicarbonate. The harvested oocytes were treated with 1.25 mg/ml collagenase (Worthington Biochemicals, Freehold, NJ) for 2 hours at room temperature in a calcium-free Barth’s solution (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgSO4, 15 mM HEPES, and 12 mg/l tetracycline, pH 7.6) to remove the follicular layer. Stage V oocytes were subsequently isolated and injected with 50 nl of 5–20 ng nAChR subunit RNA. Recordings were carried out 1–7 days after injection.
Chemicals.
Solvents and reagents were purchased from Sigma (St. Louis, MO). Cell culture supplies were purchased from Invitrogen (Carlsbad, CA). p-CF3 diEPP was synthesized as described previously (Quadri et al., 2016). TQS was synthesized as described previously by Dr. Ganesh A. Thakur (Kulkarni and Thakur, 2013). Fresh acetylcholine (ACh) stock solutions were made each day of experimentation. TQS and p-CF3 diEPP stock solutions were prepared in dimethylsulfoxide, stored at −20°C, and used for up to 1 month. TQS and p-CF3 diEPP solutions were prepared fresh each day at the desired concentration from the stored stock.
Two-Electrode Voltage-Clamp Electrophysiology.
Experiments were conducted using OpusXpress 6000A (Molecular Devices, Union City, CA). The OpusXpress recording system has previously been described in detail (Papke and Stokes, 2010). In brief, OpusXpress provides two-electrode voltage clamp of eight oocytes in parallel, including steady bath perfusion, drug delivery, and data acquisition. Both the voltage and current electrodes were filled with 3 M KCl. Oocytes were voltage clamped at −60 mV unless otherwise specified. For voltage-dependent experiments, oocytes were also voltage clamped at −90, −70, −50, and −30 mV throughout the whole experiment, including delivery of ACh controls, when p-CF3 diEPP was coapplied with ACh or also at +50 mV immediately prior to and during testing compound p-CF3 diEPP coapplied with TQS. The oocytes were bath perfused with Ringer’s solution (115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM HEPES, and 1 mM atropine, pH 7.2) at 2 ml/min for α7 receptors and at 4 ml/min for other subtypes. To evaluate the effects of experimental compounds compared with ACh-evoked responses of various nAChR subtypes expressed in oocytes, baseline control ACh responses were defined by two initial applications of ACh made before coapplications of experimental compounds with the control ACh. The agonist solutions were applied from a 96-well plate via disposable tips, and the test compounds were applied alone, coapplied with ACh, or coapplied with TQS. For the concentration-response study, drug applications alternated between ACh controls and experimental compounds to insure the stability of the baseline responses. Unless otherwise indicated, drug applications were 12 seconds in duration followed by a 181-second washout period for α7 receptors and 6 seconds with a 241-second washout for other subtypes. A typical recording for each oocyte constituted two initial control applications of ACh, an experimental compound application, and then a follow-up control application of ACh to determine the desensitization or possible rundown of the receptors. The control ACh concentrations were 60 µM for α7, 30 µM for α4β2, and 100 µM for α3β4. The responses of α4β2- and α3β4-expressing cells were measured as peak current amplitudes, and the α7 data were calculated as net charge, as previously described (Papke and Porter Papke, 2002). Data were collected at 50 Hz, filtered at 20 Hz, analyzed by Clampfit 9.2 (Molecular Devices) and Excel 2003 (Microsoft, Redmond, WA), and normalized to the averaged peak current or net charge response of the two initial ACh controls (Papke and Porter Papke, 2002). Data were expressed as mean ± S.E.M. from at least four oocytes for each experiment and plotted by KaleidaGraph 4.1.1 (Abelbeck Software, Reading, PA). Receptor-mediated activity has a limit of detection of approximately 0.05% of the ACh controls.
Multicell averages were calculated for display and comparisons of the raw data. Data from each cell were baseline corrected by subtracting the mean of the holding current for a 30-second period prior to the control ACh solution. The data were then normalized by dividing the control and experimental data by the peak current of the control response for each cell. Averages of the normalized data were calculated for each of the 10,322 points in each of the 206.44 second traces (acquired at 50 Hz), as well as for the S.E. for those averages.
In Vivo Methods
Animals.
Male adult (8–10 weeks of age) Institute of Cancer Research mice were obtained from Harlan Laboratories (Indianapolis, IN). Mice were housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care–approved animal care facility. They were housed in groups of four and had free access to food and water. The rooms were on a 12-hour light/dark cycle (lights on at 7:00 AM). All experiments were performed during the light cycle (between 7:00 AM and 7:00 PM), and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Animals were sacrificed via CO2 followed by cervical dislocation after the experiments were finished, unless noted otherwise. Any subjects that subsequently showed behavioral disturbances unrelated to the pain induction procedure were excluded from further behavioral testing.
Drugs.
Methyllycaconitine (MLA) citrate was purchased from RBI (Natick, MA). Complete Freund’s adjuvant (CFA) was purchased from Sigma-Aldrich (St. Louis, MO). p-CF3 diEPP was synthesized as previously described (Quadri et al., 2016) and dissolved in a mixture of 2:2:16 [2 volume ethanol/2 volume Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ)/16 volumes distilled water] and administered intraperitoneally for systemic injections. MLA was dissolved in physiologic saline (0.9% sodium chloride) and injected subcutaneously at a total volume of 1 ml/100 g body weight, unless noted otherwise. All doses are expressed as the free base of the drug.
Complete Freund’s Adjuvant–Induced Inflammatory Pain Model.
We explored the effects of p-CF3 diEPP in the CFA test, composed of inactivated and dried Mycobacterium tuberculosis and adjuvant, a widely used model of persistent inflammatory pain. The CFA model is based on hypersensitivity, paw swelling, and nuclear factor-κB–mediated transcription of tumor necrosis factor-α involved in the formation of the principal mediators of inflammation (Hartung et al., 2015). Mice were injected intraplantarly with 20 µl of CFA (50%, diluted in mineral oil). Mechanical sensitivity (see the measurement of the von Frey test) and paw diameter were measured before and 3 days after CFA injection. p-CF3 diEPP (1, 3.3, and 10 mg/kg) or vehicle was injected intraperitoneally on day 3 after CFA injection, and mice were tested for mechanical sensitivity at different time points (15, 30, 60, and 120 minutes) after drug injection.
We determined the α7 nAChR mediation in the effects of p-CF3 diEPP using subcutaneous injection of the α7 antagonist MLA in a separate group of mice. The α7 nicotinic antagonist MLA (10 mg/kg) or vehicle (saline) was injected subcutaneously 15 minutes before the p-CF3 diEPP (10 mg/kg, i.p.) or vehicle injection. Mechanical sensitivity was then tested 30 minutes after and paw diameter was measured 1 hour after p-CF3 diEPP injection.
Evaluation of Mechanical Sensitivity.
Mechanical sensitivity thresholds were determined according to the method of Chaplan et al. (1994) and as adapted in Bagdas et al. (2015). A series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) with logarithmically incremental stiffness ranging from 2.83 to 5.07 expressed as diameter sensitivity (ds) log 10 of 10 × force (in milligrams) was applied to the paw with a modified up-down method (Dixon, 1965). The mechanical threshold was expressed as log 10 of 10 × force (in milligrams), indicating the force of the von Frey hair to which the animal reacted (paw withdrawn, licking, or shaking). All behavioral testing on animals was performed in a blinded manner.
Measurement of Paw Edema.
The thickness of the CFA-treated paws was measured both before and after injections at the time points previously indicated using a digital caliper (Traceable Calipers, Friendswood, TX). Data were recorded to the nearest ±0.01 mm and expressed as change in paw thickness (the difference in the ipsilateral paw diameter before and after injection).
Locomotor Activity and Motor Coordination Measures.
We measured the impact of p-CF3 diEPP on mouse locomotor activity and coordination in two different tests. For the locomotor activity, mice were placed into individual Omnitech (Columbus, OH) photocell activity cages (28 × 16.5 cm). Interruptions of the photocell beams (two banks of eight cells each) were recorded for the next 30 minutes after injection of vehicle and p-CF3 diEPP (10 mg/kg, i.p.). Data were expressed as the number of photocell interruptions.
In a separate cohort of mice, we measured the impact of p-CF3 diEPP on motor coordination. For that, we used the rotarod test (IITC Inc. Life Science, Woodland Hills, CA). Mice were placed on textured drums (11/4 inch diameter) to avoid slipping. When an animal fell onto the individual sensing platforms, test results were recorded. Five mice were tested at a time using a rate of 4 rpm. Naive mice were trained until they remained on the rotarod for 3 minutes or 180 seconds. Animals that failed to meet this criterion within three trials were discarded. Thirty minutes after the intraperitoneal injection of vehicle or p-CF3 diEPP (10 mg/kg, i.p.), mice were placed on the rotarod for 3 minutes. If a mouse fell from the rotarod during this time period, it was scored as motor impaired. Percent impairment was calculated as follows: % impairment = (180 − test time)/(180 × 100).
Statistical Analysis.
The data were analyzed using GraphPad software, version 6.0 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M. Statistical analysis was done using the one- or two-way analysis of variance test, followed by the post hoc Tukey’s test. Unpaired Student’s t test was used for spontaneous activity. Values of P < 0.05 were considered significant.
Results
In Vitro Results
α7 nAChR.
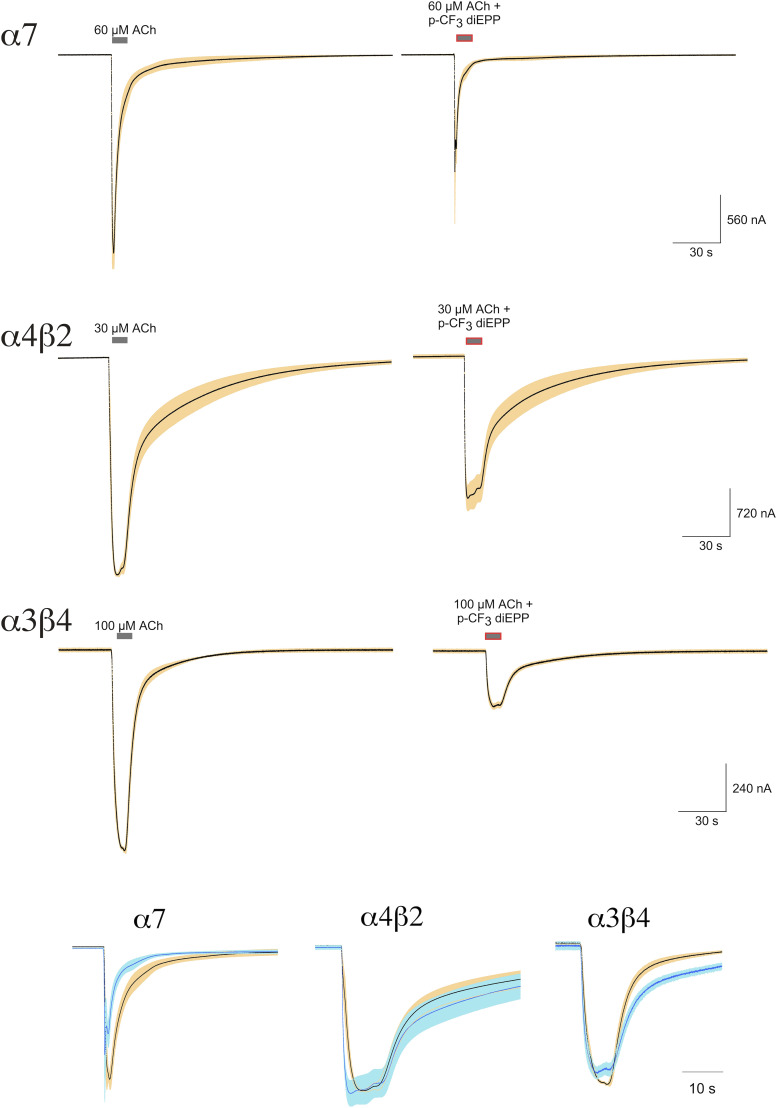
In previous experiments, p-CF3 diEPP was tested for its agonism at 30 μM on the α7 nAChR (Quadri et al., 2016). While it produced virtually no activation when applied alone, it generated large responses when coapplied with the type II PAM PNU-120596. We followed up those observations using the alternative type II PAM TQS. TQS has previously been reported to be a type II PAM (Grønlien et al., 2007) that produces no response when applied alone (data not shown). Multicell-averaged responses showing the activation of α7 nAChRs induced by p-CF3 diEPP applied alone or coapplied with TQS are shown in Fig. 2. It should be noted we use net charge as the primary measurement of α7 receptor function since concentration-dependent desensitization prevents the measurement of peak currents at high agonist concentrations (Papke and Porter Papke, 2002). To better evaluate its partial agonism activity at the α7 receptor, p-CF3 diEPP was tested at a broader range of concentrations (3, 10, 30, 100, and 300 μM), and it showed virtually no activation of the α7 receptor subtype when applied alone. The concentration-response curve (CRC) analysis evidenced an Imax (maximum current response of agonist) of 5% (absolute value 0.05 ± 0.002) of the 60 µM ACh response (or 4% of the ACh maximum response), with an EC50 (half-maximal effective concentration) of 26 ± 2.4 µM (Fig. 3A).
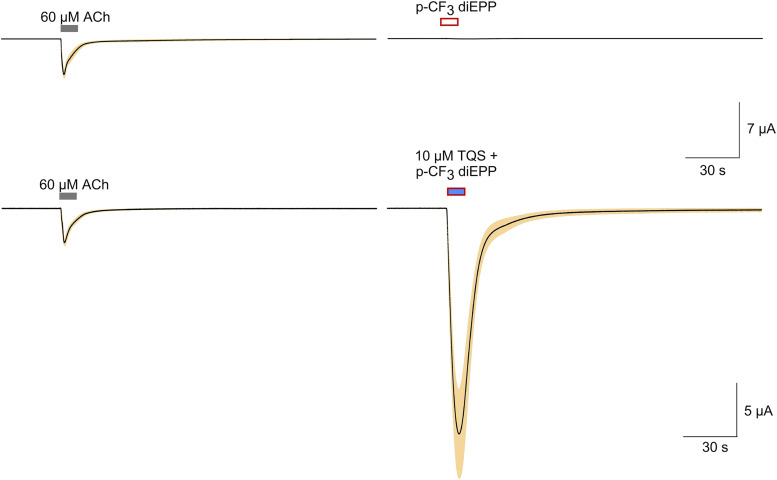
Fig. 2.
Multicell-averaged data traces for α7 responses induced by application of p-CF3 diEPP alone or coapplied with TQS. Averaged normalized responses to p-CF3 diEPP compared and scaled to ACh controls. The upper traces are the averaged responses of cells to 60 µM ACh (n = 5) obtained prior to the application of 30 µM p-CF3 diEPP. The average ACh control peak current amplitude was 5.05 ± 0.65 µA, as represented by the scale bar. The lower traces are the averaged responses of cells to 60 µM ACh (n = 8) compared with the averaged response to the coapplication of 30 µM p-CF3 diEPP and 10 µM TQS. The average ACh control peak current amplitude was 3.68 ± 0.76 µA, as represented by the scale bar. Each trace of 10,322 points is 206.44 seconds long. Responses of individual cells were each normalized to their responses to 60 µM ACh prior to the experimental applications; shown are the averaged normalized responses (solid lines) ± S.E.M. (tan area).
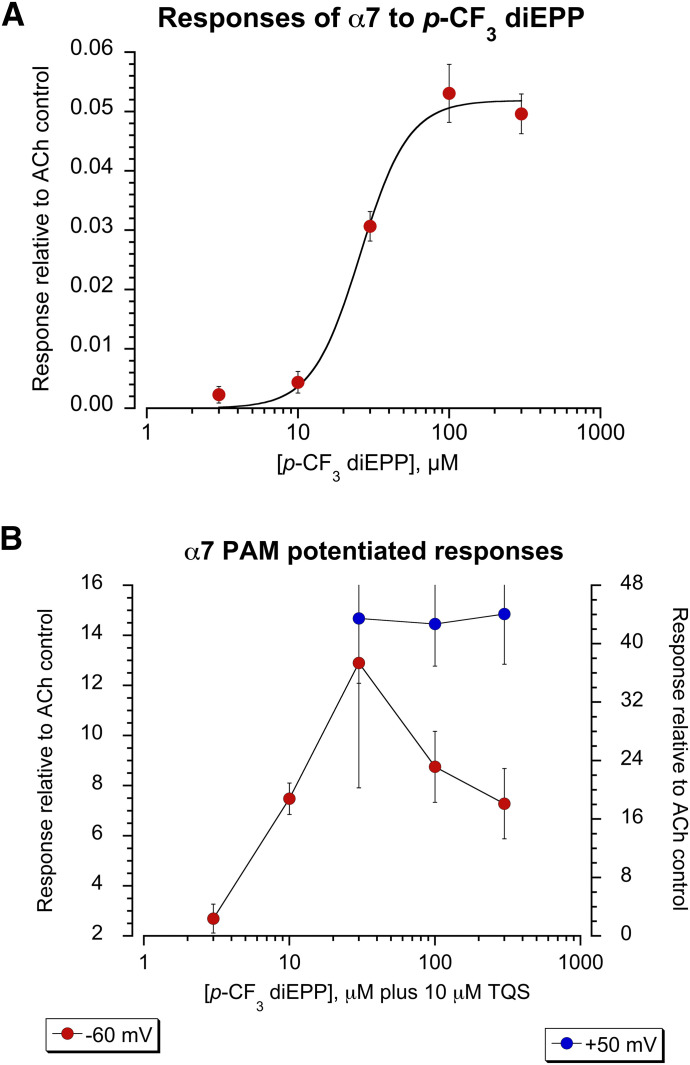
Fig. 3.
CRC data for the α7 nAChR responses evoked by p-CF3 diEPP applied alone or coapplied with TQS. (A) The α7 orthosteric activation evoked by p-CF3 diEPP applied alone at 3, 10, 30, 100, and 300 μM measured as net charge. Each point is the average (±S.E.M.) of at least five cells, normalized to 60 µM ACh control responses from the same cells. (B) The α7 activation evoked by p-CF3 diEPP (3, 10, 30, 100, and 300 µM) coapplied with 10 µM TQS at −60 mV (red, left-side scale) and at +50 mV (blue, right-side scale). The responses are reported as net charge and normalized to the average net charge value of two control applications of ACh (60 μM) at −60 mV. Each data point represents the average normalized response of at least four cells (±S.E.M.).
We then focused on investigating the desensitizing effects of p-CF3 diEPP on α7 according to the reported protocol (Chojnacka et al., 2013) and at a range of concentrations larger than previously reported (Quadri et al., 2016). Although both TQS and PNU-120596 are good type II PAMs, for the present study we chose TQS over PNU-120596 since we found the latter to suffer from greater experimental variability and less consistent results compared with TQS coapplied with p-CF3 diEPP. At the normal holding potential of −60 mV, the potentiated responses evoked by p-CF3 diEPP (3, 10, 30, 100, and 300 µM) coapplied with 10 µM TQS displayed an inverted-U-shaped curve with a maximum at 30 µM, corresponding to a response 13-fold greater than that induced by the 60 µM ACh control (net charge absolute value 12.9 ± 4.99 relative to 60 μM ACh control response) (Fig. 3B). These data confirmed the ability of the weak partial agonist p-CF3 diEPP to promote and stabilize α7 receptor desensitization. PAM-potentiated responses characterized by an inverted-U shape have been previously reported (Papke et al., 2015); therefore, we wanted to investigate the phenomenon as related to the p-CF3 diEPP responses. Specifically, we hypothesized that voltage-dependent channel block of α7 induced by p-CF3 diEPP may have been involved in the inverted-U shape of p-CF3 diEPP TQS-potentiated responses. Voltage-dependent channel block has been previously reported to affect other positively charged small molecules responses (Papke et al., 2014). To test this hypothesis, p-CF3 diEPP was coapplied with TQS at the positive holding potential of +50 mV and the responses were compared with those observed at the normal holding potential of −60 mV, according to a protocol previously used to determine channel-blocking activity (Papke et al., 2014) (Fig. 3B). It should be noted that while under normal conditions ACh-evoked α7 responses show strong inward rectification that would preclude conducting experiments at positive potentials; this rectification is relieved with the effect of a type II PAM (Peng et al., 2013). We tested p-CF3 diEPP at 30, 100, and 300 μM coapplied with 10 μM TQS to assess whether at +50 mV the PAM-potentiated responses would still invert at the higher concentrations of the drug. When coapplied with 10 μM TQS on α7 receptors at +50 mV, p-CF3 diEPP displayed comparable potentiated inward currents at the three different concentrations tested (net charge absolute values of 43.5 ± 8.87, 42.7 ± 5.78, and 44.1 ± 6.87 at 30, 100, and 300 μM, respectively) and did not display the invert-U phenomenon. According to the results, voltage-dependent channel block induced by coapplication of p-CF3 diEPP appears to be a limiting factor in the PAM-potentiated responses at the control negative holding potential.
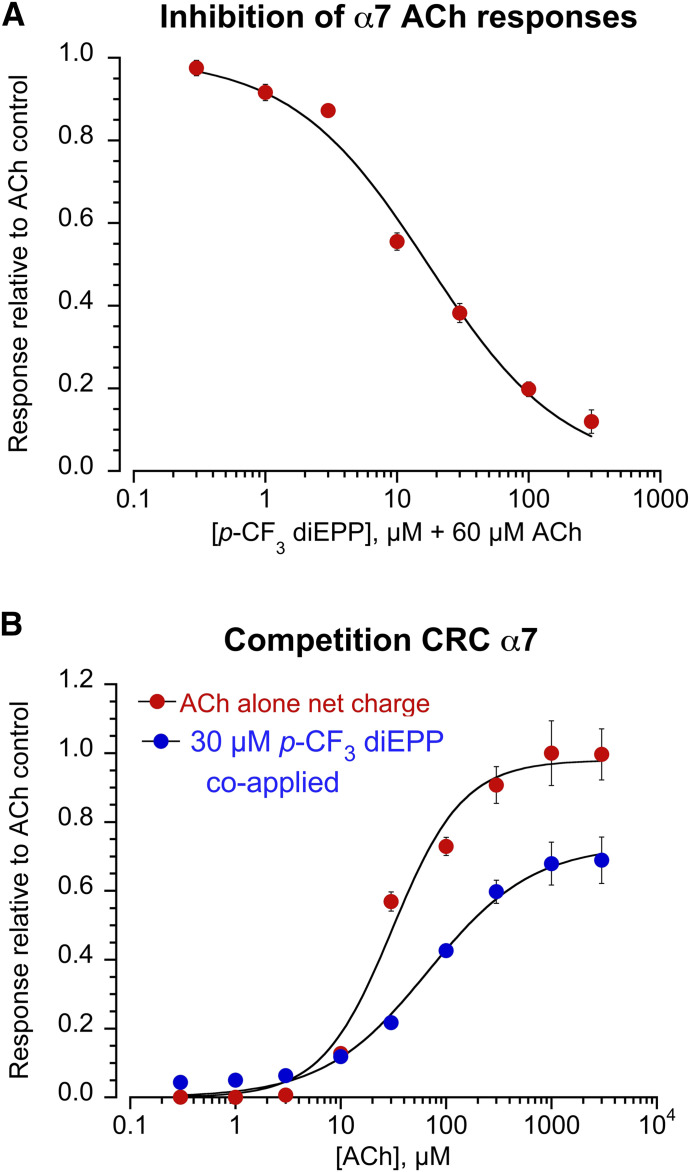
In the absence of the PAM, α7 silent agonists and very weak partial agonists are considered functional antagonists of the receptor and the drug potency can be evaluated in inhibition experiments. To investigate p-CF3 diEPP antagonism on the α7 receptor, 60 μM ACh was coapplied with increasing concentrations of the p-CF3 target compound (0.3, 1, 3, 10, 30, 100, and 300 μM). The corresponding inhibition CRC evidenced an IC50 (half-maximal inhibitory concentration) of 17 ± 2 µM, with almost complete receptor inhibition at the highest concentration tested (only 10% of the initial control ACh responses left) (Fig. 4A). We then investigated whether the observed α7 antagonism was competitive or not. To this end, 30 µM p-CF3 diEPP was coapplied with increasing concentrations of ACh (0.3, 1, 3, 10, 30, 100, and 300 μM, and 1 and 3 mM) (Fig. 4B). Compared with ACh applied alone, p-CF3 diEPP coapplication mostly affected ACh efficacy, with a 26% reduction in Imax (Imax = 98% ± 4% and 73% ± 3%, respectively, relative to 60 μM ACh control responses) while slightly affecting its potency, with a relatively small rightward shift of the EC50 (EC50 = 31 ± 5 and 67 ± 12 µM, respectively). These results suggested both noncompetitive and competitive antagonism of ACh responses by p-CF3 diEPP at the α7 nAChR.
Fig. 4.
CRC data for the α7 nAChR antagonism induced by p-CF3 diEPP. The responses are reported as net charge and normalized to the average net charge value of two control applications of ACh (60 μM). (A) Inhibition CRC for p-CF3 diEPP at 0.3, 1, 3, 10, 30, 100, and 300 μM coapplied with 60 μM ACh. Each data point represents the average normalized response of at least four cells (±S.E.M.). (B) Competition CRC data (blue) for 30 µM p-CF3 diEPP coapplied with different ACh concentrations (0.3, 1, 3, 10, 30, 100, and 300 µM, and 1 and 3 mM). For comparison, ACh CRC data alone (red) are shown at the same concentrations.
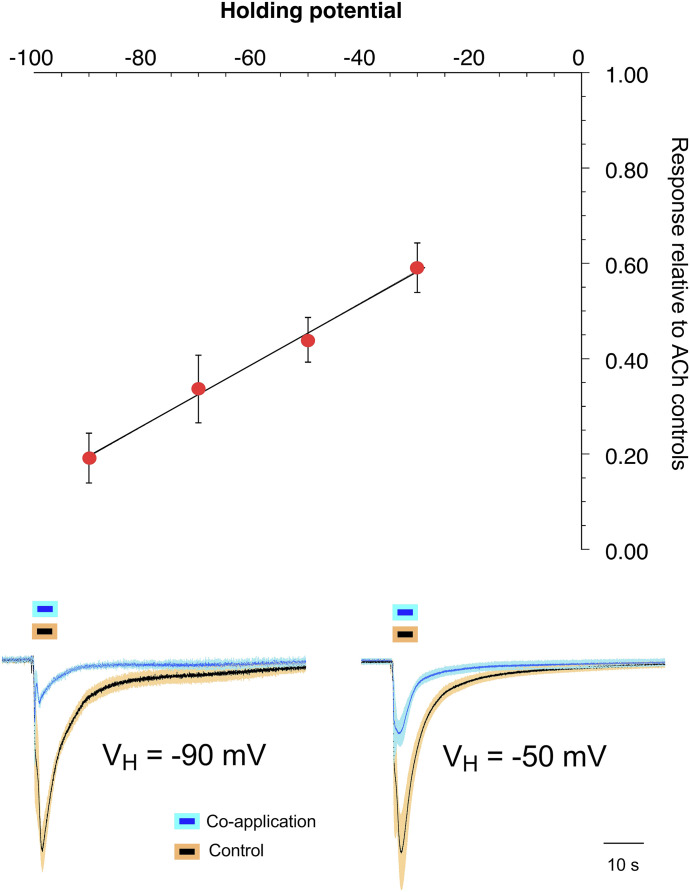
Considering the ACh response inhibition and the evidence that emerged for the inverted-U-shaped PAM-potentiated responses of the p-CF3 derivative, we hypothesized that α7 voltage-dependent channel block by p-CF3 diEPP was responsible for the reduced efficacy of ACh in the presence of p-CF3 diEPP. Therefore, we coapplied p-CF3 diEPP with ACh at different holding potentials (−90, −70, −50, and −30 mV) and compared the evoked responses to respective controls obtained at the same holding potentials to evaluate channel-blocking activity (Papke et al., 2014) (Fig. 5). As noted previously, data at positive holding potentials were not suitable for analysis due to inward rectification of α7 currents in the absence of a PAM.
Fig. 5.
Effects of voltage-dependent channel block induced by p-CF3 diEPP on ACh responses at the α7 nAChR. Responses evoked by coapplication of 30 μM p-CF3 diEPP with 60 μM ACh at different holding potentials (millivolts). The ACh residual responses measured in the presence of p-CF3 diEPP were 0.19 ± 0.05 at −90 mV (n = 5), 0.34 ± 0.07 at −70 mV (n = 5), 0.44 ± 0.05 at −50 mV (n = 7), and 0.59 ± 0.05 at −30 mV (n = 4) relative to 60 μM ACh control responses obtained at the same potentials. Averaged raw data traces showing the effects of holding potential on the inhibition of ACh currents in the presence of p-CF3 diEPP at −90 and −50 mV are shown below the graph. The control traces were scaled to have the same amplitude, and the coapplication responses are relative to the controls.
As a test concentration, we used 30 μM p-CF3 diEPP coapplied with 60 μM ACh, the same concentration used in the competition CRC test. The results at the different holding potentials suggest p-CF3 diEPP voltage-dependent channel block to be a factor limiting the ACh currents, consistent with the results obtained with the PAM-potentiated currents (Fig. 3). In the presence of p-CF3 diEPP, α7 residual ACh activation measured as net charge was 19% ± 5% at −90 mV, 34% ± 7% at −70 mV, 44% ± 5% at −50 mV, and 59% ± 5% at −30 mV. Results at the lowest (−90 mV) versus the two highest (−50 and −30 mV) holding potentials tested are statistically different, as well as data at −70 mV compared with −30 mV. These results collected at different holding potentials are, therefore, consistent with voltage-dependent channel block limiting the ACh responses that were coapplied with the p-CF3 compound, and possibly the efficacy of the drug when applied alone to α7 nAChRs is impacted by channel block.
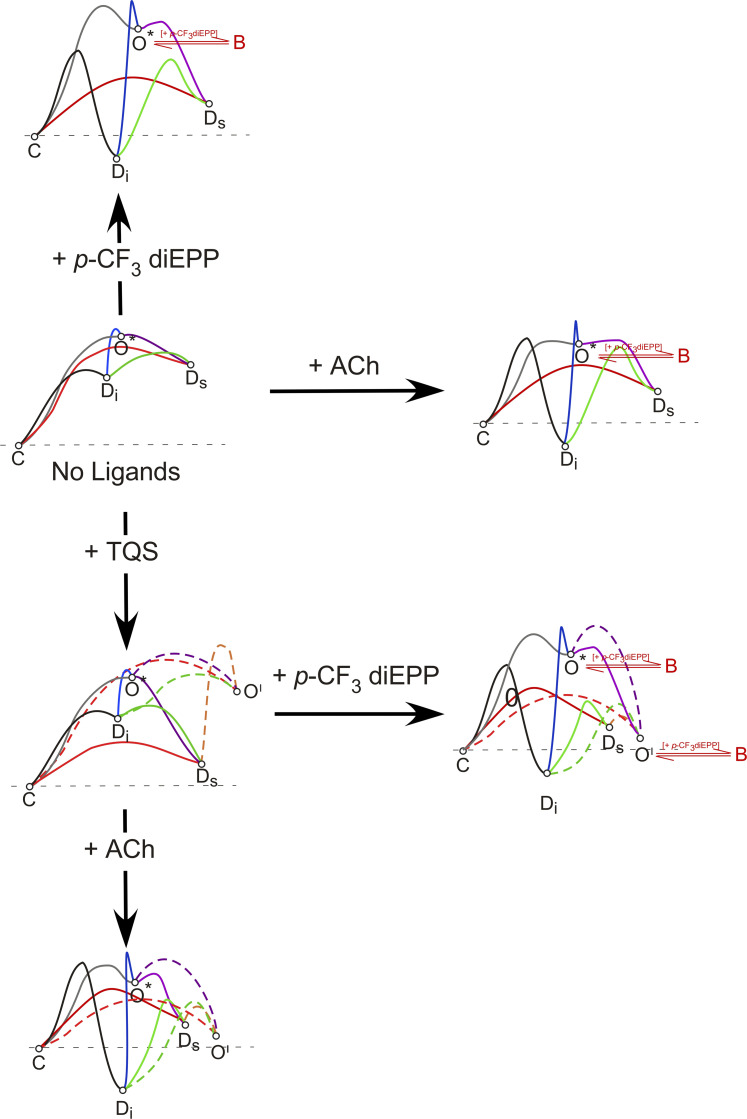
Based on the results collected on the α7 nAChR, we propose some hypothetical models representing the different conformational states distribution depending on receptor binding to the very weak partial agonist of interest, p-CF3 diEPP, in the presence or absence of ACh and/or TQS (Fig. 6). These models are based on those proposed for other silent agonists (Papke et al., 2014), which include high-energy barriers for entering the open state in the absence of the PAM, rapid activation and frequent reopenings in the presence of the PAM and p-CF3 diEPP, as well as concentration- and voltage-dependent block as additional limiting factors.
Fig. 6.
Hypothetical models for the energy landscape of the conformational states of α7 nAChR. The conformational changes and the corresponding hypothetical energy landscape variations for the α7 receptor as affected by the binding of the very weak partial agonist p-CF3 diEPP with or without the effects of coapplied TQS or ACh. Based on the α7 nAChR states identified in our previous studies, two forms of α7 desensitization [PAM-sensitive nonconducting desensitized state (Ds) and PAM-insensitive nonconducting desensitized state (Di)], a resting closed state (C), a low-probability open state (O*), and a PAM-dependent open state (O′) that can be coupled to the Ds state are shown here. The models refer to intermediate levels of agonist and allosteric modular binding at the orthosteric and allosteric sites, respectively. Such conditions were proven to be the most effective in promoting channel opening (Williams et al., 2011a). For each model, the vertical displacement of the states represents the absolute free energy of the various states, and the height of the energy barriers are inversely related to the rate constants for transitions between the states and are represented by the lines connecting the states. When no ligands are bound, the C state is most stable and transitions to other states are thermodynamically unlikely to happen. Once p-CF3 diEPP is applied, the thermodynamic landscapes of α7 receptors would rearrange in such a way that the probability of entering O* is somewhat increased but still low. Transition to the Ds or Di states becomes most likely. Upon application of TQS alone, the receptor is less likely to enter the desensitized state, and the additional O′ state enters the energy landscapes; however, channels are still unlikely to open. When p-CF3 diEPP is coapplied with TQS, the type II PAM favors the transition from the Ds to the O′ state, resulting in prolonged bursts of receptor openings. The channel blocking components of p-CF3 diEPP (B) affect both the low-probability open state (O*) and a PAM-dependent open state (O′).
Heteromeric nAChRs α4β2 and α3β4.
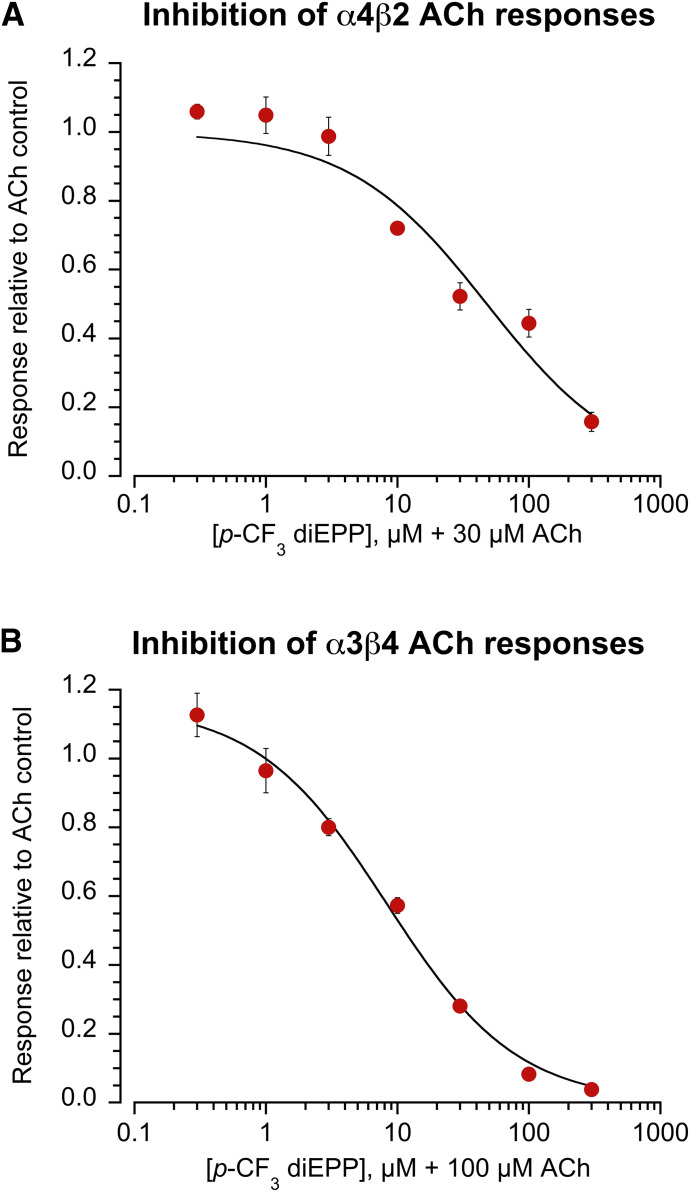
Nicotinic receptor subtype selectivity for activation and inhibition was investigated by testing the effects of p-CF3 diEPP on the heteromeric nAChRs α4β2 and α3β4 expressed in Xenopus laevis oocytes. When applied alone at 30 µM to cells expressing α4β2 or α3β4 nAChR, p-CF3 diEPP did not evoke responses above our reliable limit of detection, equal to ≈1% of our ACh controls (data not shown). To assess its antagonism, p-CF3 diEPP was tested at different concentrations (0.3, 1, 3, 10, 30, 100, and 300 µM) coapplied with ACh on α4β2 and α3β4 receptors (Fig. 7, A and B, respectively). On both receptor subtypes, p-CF3 diEPP produced inhibition of the ACh responses, with IC50 values of 48 ± 12 and 8.3 ± 1.5 on α4β2 and α3β4, respectively. Multicell averages of the raw data showing inhibition of ACh-evoked responses by coapplication with 30 µM p-CF3 diEPP at the heteromeric receptors α4β2 and α3β4 and at the homomeric α7 subtype are shown in Fig. 8. It is interesting to note from the scaled overlays at the bottom of Fig. 8 that only the p-CF3 diEPP–inhibited currents of α7 appear to have accelerated kinetics, often indicative of open channel block (Papke et al., 1994; Francis and Papke, 1996), suggesting that inhibition of heteromeric receptors may be mechanistically different.
Fig. 7.
Concentration-response studies for p-CF3 diEPP at the heteromeric nAChRs α4β2 and α3β4. The data represent the inhibition of receptor subtypes induced by coapplication of p-CF3 diEPP (0.3, 1, 3, 10, 30, 100, and 300 μM) with ACh control (100 µM for α3β4 and 30 µM for α4β2) at the α4β2 (A) and α3β4 (B) nAChRs. The responses are measured as the peak current and normalized to the ACh control applied alone. Each data point is the average normalized response of at least four cells (±S.E.M.).
Fig. 8.
Multicell-averaged raw data traces for inhibition of ACh-evoked responses by coapplications with 30 µM p-CF3 diEPP. The top traces are the averaged responses of cells (n = 5) expressing α7 nAChR to 60 µM ACh alone or coapplied with p-CF3 diEPP. The average ACh control peak current amplitude was 2.46 ± 0.38 µA. The middle traces are the averaged responses of cells (n = 5) expressing α4β2 nAChR to 30 µM ACh alone or coapplied with p-CF3 diEPP. The average ACh control peak current amplitude was 3.19 ± 0.62 µA. The bottom traces are the averaged responses of cells (n = 7) expressing α3β4 nAChR to 30 µM ACh alone or coapplied with p-CF3 diEPP. The average ACh control peak current amplitude was 1.05 ± 0.15 µA. Each trace of 10,322 points is 206.44 seconds long. Responses of individual cells were each normalized to their responses to ACh (60, 30, and 100 µM, respectively) prior to the experimental applications. Shown are the averaged normalized responses (solid lines) ± S.E.M. (tan area); shown at the bottom are kinetic comparisons of control and p-CF3 diEPP–inhibited currents scaled to the same amplitude.
In Vivo Results
p-CF3 diEPP Dose Dependently Attenuates CFA-Induced Inflammatory Pain in an α7-nAChR-Dependent Manner.
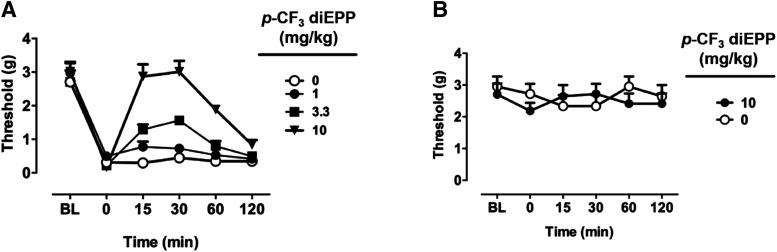
The tested compound p-CF3 diEPP dose dependently reduced CFA-induced mechanical hypersensitivity (Fdose × time(15,144) = 8.41, P < 0.0001, where Fdose denotes the bioavailability dose) (Fig. 9A). While being effective also at lower dosages of 1 and 3.3 mg/kg, post hoc analysis revealed that 10 mg/kg p-CF3 diEPP yielded maximal reversal of hypersensitivity similar to pretreatment baseline values at 15–30 minutes after injection. The effects of 10 mg/kg p-CF3 diEPP decreased to half-maximum at 1 hour after injection and became negligible at 2 hours. However, p-CF3 diEPP at 10 mg/kg did not alter von Frey responses in sham-treated mice (Fdose(5,72) = 0.744, P > 0.05) (Fig. 9B), since p-CF3 diEPP at the tested dose and vehicle provided comparable responses.
Fig. 9.
The effects of systemic p-CF3 diEPP in the CFA-induced chronic inflammatory pain model. (A) Antiallodynic effects after intraperitoneal administration of various doses of p-CF3 diEPP (1, 3.3, and 10 mg/kg). The mechanical paw withdrawal thresholds were determined 3 days after intraplantar injection of CFA (50%). (B) The effects of p-CF3 diEPP at 10 mg/kg on mechanical sensitivity after intraperitoneal injection in sham mice were determined using the von Frey test. BL, baseline; dose 0 = vehicle.
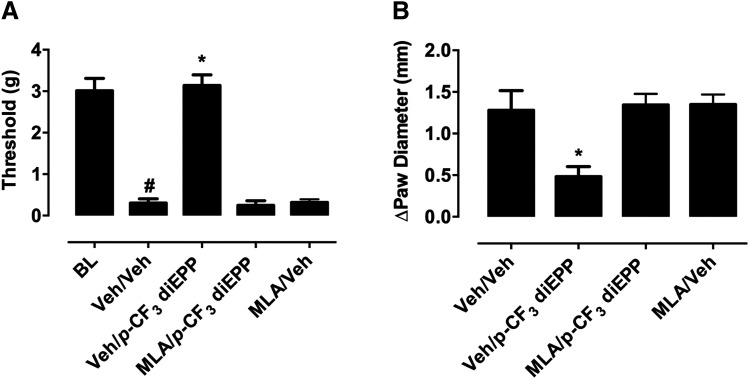
We next evaluated the possible role of α7 nAChRs in the antinociceptive and antiedema effects of p-CF3 diEPP in the CFA model and sought to confirm that the effects of p-CF3 diEPP were mediated by the α7 receptor subtype. To this end, we tested the effects of MLA on p-CF3 diEPP anti-inflammatory effects in two different experiments. Systemic (10 mg/kg) administration of the α7 nAChR antagonist MLA prevented the antiallodynic effects of p-CF3 diEPP (10 mg/kg, i.p.; F(6,24) = 66.44, P < 0.0001) (Fig. 10A). Indeed, administration of p-CF3 diEPP after MLA pretreatment resulted in suppression of p-CF3 diEPP anti-inflammatory effects by giving responses comparable to the control vehicle–treated mice. Similarly, MLA (10 mg/kg, s.c.) totally blocked the antiedema effect of 10 mg/kg p-CF3 diEPP (F(6,18) = 16.01, P < 0.0003) (Fig. 10B). Administration of 10 mg/kg, i.p, of p-CF3 diEPP significantly reduced the paw edema induced by CFA injection, and this effect was completely prevented when MLA was administered prior to p-CF3 diEPP.
Fig. 10.
The effects of systemic p-CF3 diEPP in the CFA-induced chronic inflammatory pain model. (A) To determine the blockade of the antiallodynic effect of p-CF3 diEPP by the α7 antagonist MLA, MLA was administered systemically (10 mg/kg, s.c.) 15 minutes before p-CF3 diEPP (10 mg/kg, i.p.) injection and mice were tested 30 minutes after administration. *P < 0.05, significantly different from vehicle-vehicle group; #P < 0.05, significantly different from the baseline (BL) group. (B) The anti-inflammatory effect of p-CF3 diEPP (10 mg/kg, i.p.) and its blockade by MLA. The effect was measured by the difference in the ipsilateral paw diameter (ΔPD) before and after CFA injection, and was assessed 1 hour after p-CF3 diEPP and/or MLA injection. *P < 0.05, significantly different from vehicle-vehicle group; Veh, Vehicle.
p-CF3 diEPP Does Not Alter Motor Activity or Motor Coordination.
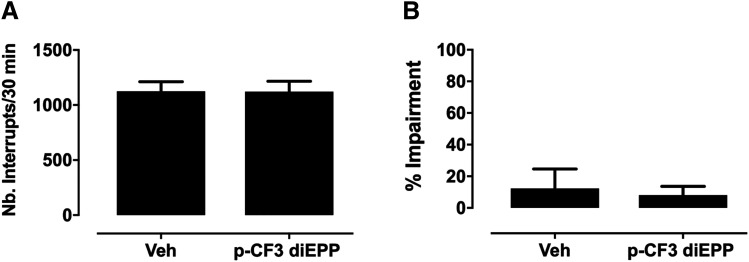
To establish that the observed effects of p-CF3 diEPP in CFA-induced mechanical hypersensitivity were not due to interference in locomotor activity and coordination during the experiments, we evaluated the effects of p-CF3 diEPP on mice locomotor activity and motor coordination at the highest effective dose in the antinociceptive tests. As seen in Fig. 11, 10 mg/kg, i.p., p-CF3 diEPP, a dose that totally reversed mechanical hypersensitivity, did not affect spontaneous locomotor activity (t = 0.02446, df = 14; P > 0.05) or performance in the rotarod test (t = 0.3132, df = 14; P > 0.05). Although these are not direct indicators of autonomic function, which might be compromised by ganglionic (α3β4 nAChR) blockade, they are consistent with animals that are not behaviorally impaired by autonomic dysfunction.
Fig. 11.
Effects of p-CF3 diEPP on motor activity and motor coordination in mice. (A) Mice were placed into photocell activity cages for 30 minutes after 30-minute intraperitoneal administration of vehicle or p-CF3 diEPP (10 mg/kg). Data are presented as the mean ± S.E.M. of the number of photocell interruptions. (B) Mice were placed on the rotarod for 3 minutes after 30-minute intraperitoneal administration of vehicle or p-CF3 diEPP (10 mg/kg). Data are presented as the mean ± S.E.M. of % impairment for each group. Data are given as the mean ± S.E.M. of six to eight animals for each group; Veh, Vehicle.
Discussion
α7 nAChR involvement in the regulation of inflammatory processes has drawn interest for targeting this receptor subtype for treatment of inflammation and pain-related diseases (de Jonge and Ulloa, 2007; Medhurst et al., 2008; Munro et al., 2012). In the present study, we investigated the in vitro and in vivo properties of p-CF3 diEPP, a very weak partial agonist that strongly desensitizes rather than activates α7 nAChR. Following up preliminary in vitro data on p-CF3 diEPP, we characterized p-CF3 diEPP over a full concentration-response range and confirmed that even at high concentrations it showed only very weak partial agonism (Imax 4% of ACh maximum).
To reveal the PAM-sensitive desensitized state of the α7 receptor induced by p-CF3 diEPP, we coapplied it with the type II PAM, TQS. Type II PAMs act on the α7 desensitized state by destabilizing it and inducing its kinetically coupled PAM-dependent conductive state (Williams et al., 2011b). This destabilization prompts prolonged receptor activation, which can be measured in two-electrode voltage-clamp experiments, and highlights the ability of some weak partial agonists that we characterize as silent agonists to induce high levels of PAM-sensitive α7 receptor desensitization. Our preliminary results on the PAM-potentiated net charge currents showed 30 µM p-CF3 diEPP coapplied with PNU-120596 evoked a response 62-fold greater than the one evoked by the 60 µM ACh control, indicating the induction of the PAM-sensitive nonconducting desensitized state by p-CF3 diEPP (Quadri et al., 2016). We further investigated the desensitizing effects of p-CF3 diEPP on α7 by coapplication with the alternative type II PAM TQS and at a broader range of concentrations. As noted previously, for the present studies TQS was selected over PNU-120596 given our empirical observation of lower experimental variability and higher reproducibility when coapplied with p-CF3 diEPP.
Similar to previous findings with the silent agonist NS6740 (Papke et al., 2015), the PAM-potentiated CRC of p-CF3 diEPP showed an inverted-U shape. Investigation of p-CF3 diEPP effects on ACh activation confirmed that it was a functional antagonist, consistent with the coapplication of a weak partial agonist with a full agonist. However, competition experiments suggested that the compound additionally acted as a noncompetitive antagonist of the α7 receptor, capable of reducing ACh efficacy while also decreasing its potency. Further investigating the nature of this noncompetitive component, we determined that the inhibition was voltage dependent, suggesting that similar noncompetitive activity might also limit the PAM-potentiated currents measured at the normal holding potential. The competition and voltage-dependence experiments were conducted with p-CF3 diEPP at 30 µM, the concentration associated with the peak of the inverted-U in the TQS-potentiated responses, and presumably concentrations greater than 30 µM of p-CF3 diEPP would show progressively higher degrees of α7 noncompetitive antagonism, resulting in the inverted-U. We confirmed that at a holding potential of +50 mV, there was little or no voltage-dependent channel block, and the p-CF3 diEPP PAM-potentiated responses did not show an inverted-U shape, with comparable responses at the three different concentrations tested. We typically observe that the reversal potential α7 currents in oocytes is approximately −10 mV, thus the driving force for outward currents at +50 mV would be only 20% greater than for inward currents at −60 mV. However, the 30 µM potentiated outward currents at +50 mV, compared with the −60 mV ACh controls, were 3-fold larger than the 30 µM potentiated outward currents at −60 mV, compared with the −60 mV ACh controls. These results suggest that, even at the normal holding potential, voltage-dependent channel block induced by p-CF3 diEPP limits both apparent efficacy as an agonist and the PAM-potentiated responses.
We further evaluated the effects of p-CF3 diEPP on heteromeric α4β2 and α3β4 nAChRs expressed in Xenopus laevis oocytes. The α4β2 subunits are a model for the primary high-affinity nicotine receptors of brain, while the α3β4 subunits are associated with autonomic ganglia and the adrenal gland (Papke, 2014). While no agonist activity was detected, p-CF3 diEPP displayed inhibition of both heteromeric receptor subtypes at potencies that were similar for α7. The mechanism of antagonism was not investigated for the heteromeric receptors, although it should be noted that it was readily reversible (data not shown). Additionally, as inspection of the raw data suggested, inhibition of heteromeric receptors may be qualitatively different from the inhibition of α7, since response kinetics of the heteromeric receptors were relatively unaffected (Fig. 8). The brain penetration of p-CF3 diEPP is unknown, thus it is unclear whether there would be in vivo effects associated with α4β2 inhibition. The closely related compound ASM024 is not considered to be a blood-brain barrier penetrant (Assayag et al., 2014). Furthermore, it seems unlikely that antagonism of either α3β4 or α4β2 would have many serious aversive effects given the historical use of the central nervous system–penetrant potent ganglionic antagonist mecamylamine as an antihypertensive therapy (Moyer et al., 1955; McQueen and Smirk, 1957).
Our in vivo data demonstrated that p-CF3 diEPP is effective in mouse models of hyperalgesia and edema and linked its activity to α7 nAChR mechanisms, based on the effects of preapplication of the α7-selective antagonist MLA. The analgesic-like properties of p-CF3 diEPP shown by our in vivo data could be ascribed to α7 signaling pathways independent of classic agonist ion channel activation, similar to NS6740 (Papke et al., 2015). As mentioned previously, the target compound lacked significant agonist activity, and indeed behaves as an antagonist of α7 ion channel currents. Nonetheless, it promotes desensitized states of the receptor, as revealed by potentiated responses evoked by PAM coapplication. Future studies could correlate the different nonconducting states wherein the receptor can exist with the anti-inflammatory effects reported here.
Because of its permanent positive charge, p-CF3 diEPP is most likely prevented from crossing the blood-brain barrier, and therefore its anti-inflammatory activity would be most likely localized and confined to peripheral immune cells. Such compartmentalization could possibly confer the compound of interest selectivity of action on peripheral over central systems. While standard α7 nAChR agonists have shown beneficial effects in chronic pain models in some studies (Gao et al., 2010; for a review, see Bagdas et al., 2017), this effect was not consistently seen in others. Interestingly, agents that have been shown to be effective in vivo have a remarkable range of pharmacological properties, ranging from the profoundly desensitizing agent NS6740 (Papke et al., 2015) to the potent allosteric ago-PAM GAT107 (Bagdas et al., 2016). What these agents have in common is their ability to induce nonconducting states that apparently mediate signal transduction (Papke et al., 2017). The results of this study and previous work identify multiple compounds, structurally unrelated, that have anti-inflammatory effects associated with the α7 nAChR. Are there unifying principles to develop a single structure-activity relationship, or are their multiple structure-activity relationships that have yet to be established? The answers to these questions represent the backdrop for additional design considerations. It is a concern that certain compounds may also have undesirable side effects. For example, NS6740 has been shown to antagonize the procognitive effects of other α7 channel activators (Briggs et al., 2009; Pieschl et al., 2017) and may directly reduce synaptic function in the hippocampus (Papke et al., 2018). Like GAT107, the type II PAM PNU-120596 is an extremely effective PAM that has been shown to be an active regulator of the cholinergic anti-inflammatory pathway (Freitas et al., 2013a,b). However, extreme levels of α7 channel activation by PNU-120596 have also been implicated to have cytotoxic effects due to calcium overload (Williams et al., 2012; Guerra-Álvarez et al., 2015). Due to these potential limitations, a compound like p-CF3 diEPP may represent an ideal middle road for future therapeutic development, inducing signal-transducing states without the likelihood of reducing cognitive function or cytotoxic effects.
In conclusion, these data shed light on the therapeutic potential of p-CF3 diEPP as well as other α7 very weak partial agonists for the treatment of inflammation and hyperalgesia by selectively targeting α7 nAChR desensitized conformations.
Acknowledgments
We acknowledge Alexander den Boef for conducting the OpusXpress experiments.
Abbreviations
- ACh
acetylcholine
- CFA
complete Freund’s adjuvant
- CRC
concentration-response curve
- diEPP
N,N-diethyl-N′-phenylpiperazinium
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
- NS6740
1,4-diazabicyclo[3.2.2]nonan-4-yl(5-(3-(trifluoromethyl)phenyl)furan-2-yl)methanon
- PAM
positive allosteric modulator
- p-CF3
para-trifluoromethyl
- p-CF3 diEPP
1,1-diethyl-4-(4-(trifluoromethyl)phenyl)piperazin-1-ium iodide
- PNU-120596
N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea
- TQS
4-naphthalene-1-yl-3a,4,5,9b-tetrahydro-3-H-cyclopenta[c]quinoline-8-sulfonic acid amide
Authorship Contributions
Participated in research design: Quadri, Papke, Horenstein, Damaj.
Conducted experiments: Bagdas, Toma, Stokes.
Contributed new reagents or analytic tools: Quadri, Horenstein.
Performed data analysis: Quadri, Bagdas, Toma, Papke, Damaj.
Wrote or contributed to the writing of the manuscript: Quadri, Papke, Horenstein, Damaj.
Footnotes
This research was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM57481] and a fellowship from the University of Milan to M.Q.
References
- Assayag EI, Beaulieu MJ, Cormier Y. (2014) Bronchodilatory and anti-inflammatory effects of ASM-024, a nicotinic receptor ligand, developed for the treatment of asthma. PLoS One 9:e86091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, AlSharari SD, Freitas K, Tracy M, Damaj MI. (2015) The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. Biochem Pharmacol 97:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Gurun MS, Flood P, Papke RL, Damaj MI. (2017) New insights on neuronal nicotinic acetylcholine receptors as targets for pain and inflammation: a focus on α7 nAChRs. Curr Neuropharmacol 16:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Wilkerson JL, Kulkarni A, Toma W, AlSharari S, Gul Z, Lichtman AH, Papke RL, Thakur GA, Damaj MI. (2016) The α7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain. Br J Pharmacol 173:2506–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs CA, Grønlien JH, Curzon P, Timmermann DB, Ween H, Thorin-Hagene K, Kerr P, Anderson DJ, Malysz J, Dyhring T, et al. (2009) Role of channel activation in cognitive enhancement mediated by α7 nicotinic acetylcholine receptors. Br J Pharmacol 158:1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. [DOI] [PubMed] [Google Scholar]
- Chojnacka K, Papke RL, Horenstein NA. (2013) Synthesis and evaluation of a conditionally-silent agonist for the α7 nicotinic acetylcholine receptor. Bioorg Med Chem Lett 23:4145–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge WJ, Ulloa L. (2007) The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 151:915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, et al. (2005) Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6:844–851. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. (1965) The up-and-down method for small samples. J Am Stat Assoc 60:967–978. [Google Scholar]
- Francis MM, Papke RL. (1996) Muscle-type nicotinic acetylcholine receptor delta subunit determines sensitivity to noncompetitive inhibitors, while gamma subunit regulates divalent permeability. Neuropharmacology 35:1547–1556. [DOI] [PubMed] [Google Scholar]
- Freitas K, Carroll FI, Damaj MI. (2013a) The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther 344:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Ghosh S, Ivy Carroll F, Lichtman AH, Imad Damaj M. (2013b) Effects of α7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology 65:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, Kawashima K. (2017) Expression and function of the cholinergic system in immune cells. Front Immunol 8:1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Hierl M, Clarkin K, Juan T, Nguyen H, Valk Mv, Deng H, Guo W, Lehto SG, Matson D, et al. (2010) Pharmacological effects of nonselective and subtype-selective nicotinic acetylcholine receptor agonists in animal models of persistent pain. Pain 149:33–49. [DOI] [PubMed] [Google Scholar]
- Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. (2007) Distinct profiles of α7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72:715–724. [DOI] [PubMed] [Google Scholar]
- Guerra-Álvarez M, Moreno-Ortega AJ, Navarro E, Fernández-Morales JC, Egea J, López MG, Cano-Abad MF. (2015) Positive allosteric modulation of alpha-7 nicotinic receptors promotes cell death by inducing Ca2+ release from the endoplasmic reticulum. J Neurochem 133:309–319. [DOI] [PubMed] [Google Scholar]
- Halevi S, Yassin L, Eshel M, Sala F, Sala S, Criado M, Treinin M. (2003) Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J Biol Chem 278:34411–34417. [DOI] [PubMed] [Google Scholar]
- Hartung JE, Eskew O, Wong T, Tchivileva IE, Oladosu FA, O’Buckley SC, Nackley AG. (2015) Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behav Immun 50:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein NA, Papke RL. (2017) Anti-inflammatory silent agonists. ACS Med Chem Lett 8:989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, et al. (2005) A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25:4396–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbani N, Nichols RA. (2018) Beyond the channel: metabotropic signaling by nicotinic receptors. Trends Pharmacol Sci 39:354–366. [DOI] [PubMed] [Google Scholar]
- Kabbani N, Nordman JC, Corgiat BA, Veltri DP, Shehu A, Seymour VA, Adams DJ. (2013) Are nicotinic acetylcholine receptors coupled to G proteins? BioEssays 35:1025–1034. [DOI] [PubMed] [Google Scholar]
- King JR, Nordman JC, Bridges SP, Lin MK, Kabbani N. (2015) Identification and characterization of a G protein-binding cluster in α7 nicotinic acetylcholine receptors. J Biol Chem 290:20060–20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AR, Thakur GA. (2013) Microwave-assisted expeditious and efficient synthesis of cyclopentene ring-fused tetrahydroquinoline derivatives using three-component povarov reaction. Tetrahedron Lett 54:6592–6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen EG, Smirk FH. (1957) Use of mecamylamine in the management of hypertension. BMJ 1:422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhurst SJ, Hatcher JP, Hille CJ, Bingham S, Clayton NM, Billinton A, Chessell IP. (2008) Activation of the α7-nicotinic acetylcholine receptor reverses complete Freund adjuvant-induced mechanical hyperalgesia in the rat via a central site of action. J Pain 9:580–587. [DOI] [PubMed] [Google Scholar]
- Moyer JH, Ford R, Dennis E, Handley CA. (1955) Laboratory and clinical observations on mecamylamine as a hypotensive agent. Proc Soc Exp Biol Med 90:402–408. [DOI] [PubMed] [Google Scholar]
- Munro G, Hansen R, Erichsen H, Timmermann D, Christensen J, Hansen H. (2012) The α7 nicotinic ACh receptor agonist compound B and positive allosteric modulator PNU-120596 both alleviate inflammatory hyperalgesia and cytokine release in the rat. Br J Pharmacol 167:421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL. (2014) Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol 89:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bagdas D, Kulkarni AR, Gould T, AlSharari SD, Thakur GA, Damaj MI. (2015) The analgesic-like properties of the α7 nAChR silent agonist NS6740 is associated with non-conducting conformations of the receptor. Neuropharmacology 91:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Chojnacka K, Horenstein NA. (2014) The minimal pharmacophore for silent agonism of the α7 nicotinic acetylcholine receptor. J Pharmacol Exp Ther 350:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Craig AG, Heinemann SF. (1994) Inhibition of nicotinic acetylcholine receptors by bis (2,2,6,6-tetramethyl- 4-piperidinyl) sebacate (Tinuvin 770), an additive to medical plastics. J Pharmacol Exp Ther 268:718–726. [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK. (2002) Comparative pharmacology of rat and human α7 nAChR conducted with net charge analysis. Br J Pharmacol 137:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Stokes C. (2010) Working with OpusXpress: methods for high volume oocyte experiments. Methods 51:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Stokes C, Damaj MI, Thakur GA, Manther K, Treinin M, Bagdas D, Kulkarni AR, Horenstein NA. (2017) Persistent activation of α7 nicotinic ACh receptors associated with stable induction of different desensitized states. Br J Pharmacol 175:1838–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Peng C, Kumar A, Stokes C. (2018) NS6740, an alpha7 nicotinic acetylcholine receptor silent agonist, disrupts hippocampal synaptic plasticity. Neurosci Lett 677:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Kimbrell MR, Tian C, Pack TF, Crooks PA, Fifer EK, Papke RL. (2013) Multiple modes of α7 nAChR noncompetitive antagonism of control agonist-evoked and allosterically enhanced currents. Mol Pharmacol 84:459–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieschl RL, Miller R, Jones KM, Post-Munson DJ, Chen P, Newberry K, Benitex Y, Molski T, Morgan D, McDonald IM, et al. (2017) Effects of BMS-902483, an α7 nicotinic acetylcholine receptor partial agonist, on cognition and sensory gating in relation to receptor occupancy in rodents. Eur J Pharmacol 807:1–11. [DOI] [PubMed] [Google Scholar]
- Quadri M, Matera C, Silnović A, Pismataro MC, Horenstein NA, Stokes C, Papke RL, Dallanoce C. (2017a) Identification of α7 nicotinic acetylcholine receptor silent agonists based on the spirocyclic quinuclidine-Δ2-isoxazoline scaffold: synthesis and electrophysiological evaluation. ChemMedChem 12:1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Papke RL, Horenstein NA. (2016) Dissection of N,N-diethyl-N′-phenylpiperazines as α7 nicotinic receptor silent agonists. Bioorg Med Chem 24:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Stokes C, Gulsevin A, Felts ACJ, Abboud KA, Papke RL, Horenstein NA. (2017b) Sulfonium as a surrogate for ammonium: a new α7 nicotinic acetylcholine receptor partial agonist with desensitizing activity. J Med Chem 60:7928–7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MS, Mikkelsen JD. (2012) The α7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-α release from microglia. J Neuroimmunol 251:65–72. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Peng C, Kimbrell MR, Papke RL. (2012) Intrinsically low open probability of α7 nicotinic acetylcholine receptors can be overcome by positive allosteric modulation and serum factors leading to the generation of excitotoxic currents at physiological temperatures. Mol Pharmacol 82:746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. (2011a) Investigation of the molecular mechanism of the α7 nicotinic acetylcholine receptor positive allosteric modulator PNU-120596 provides evidence for two distinct desensitized states. Mol Pharmacol 80:1013–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. (2011b) Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol 82:915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdanowski R, Krzyżowska M, Ujazdowska D, Lewicka A, Lewicki S. (2015) Role of α7 nicotinic receptor in the immune system and intracellular signaling pathways. Cent Eur J Immunol 40:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]