Abstract
Background & Aims
Isolated autosomal-dominant polycystic liver disease (ADPLD) is generally considered a rare disease. However, the frequency of truncating mutations to ADPLD genes in large, population sequencing databases is 1:496. With the increasing use of abdominal imaging, incidental detection of hepatic cysts and ADPLD has become more frequent. The present study was performed to ascertain the incidence and point prevalence of ADPLD in Olmsted County, MN, USA, and how these are impacted by the increasing utilisation of abdominal imaging.
Methods
The Rochester Epidemiology Project and radiology databases of Mayo Clinic and Olmsted Medical Center were searched to identify all subjects meeting diagnostic criteria for definite, likely, or possible ADPLD. Annual incidence rates were calculated using incident cases during 1980–2016 as numerator, and age- and sex-specific estimates of the population of Olmsted County as denominator. Point prevalence was calculated using prevalence cases as numerator, and age- and sex-specific estimates of the population of Olmsted County on 1 January 2010 as denominator.
Results
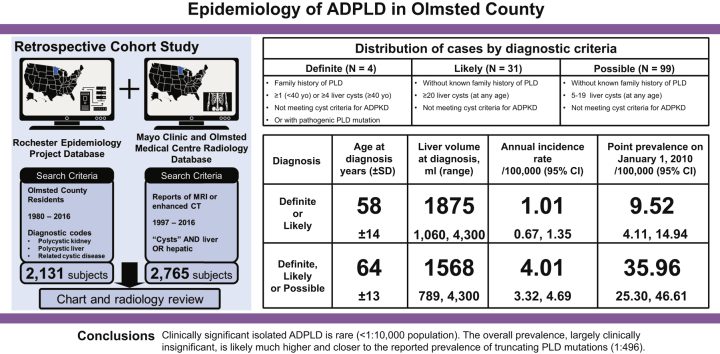
The incidence rate and point prevalence of combined definite and likely ADPLD were 1.01 per 100,000 person-years and 9.5 per 100,000 population, respectively. Only 15 of 35 definite and likely incident ADPLD cases had received a diagnostic code, and only 8 had clinically significant hepatomegaly. The incidence rates were much higher when adding possible cases, mainly identified through radiology databases, particularly in recent years and in older patients because of the increased utilisation of imaging studies.
Conclusions
Clinically significant isolated ADPLD is a rare disease with a prevalence <1:10,000 population. The overall prevalence of ADPLD, however, to a large extent not clinically significant, is likely much higher and closer to the reported genetic prevalence.
Lay summary
Isolated autosomal-dominant polycystic liver disease (ADPLD) is generally considered a rare disease. However, we demonstrate that it is a relatively common disease, which is rarely (<1:10,000 population) clinically significant.
Keywords: Autosomal-dominant polycystic liver disease, Polycystic liver disease, Hepatic cysts, Liver imaging, Epidemiology, Incidence, Prevalence
Abbreviations: ADPKD, autosomal-dominant polycystic kidney disease; ADPLD, autosomal-dominant polycystic liver disease; CT, computed tomography; HtLV, liver volume adjusted by height; IQR, inter-quartile range; MRI, magnetic resonance imaging; PLD, polycystic liver disease; US, ultrasound
Graphical abstract
Highlights
-
•
Isolated autosomal-dominant polycystic liver disease (ADPLD) is generally considered a rare disease.
-
•
Truncating mutations to ADPLD genes are fairly common (1:496) in large, population sequencing databases.
-
•
We identified 35 individuals meeting diagnostic criteria for definite or likely ADPLD and 99 additional patients with possible ADPLD.
-
•
The point prevalence of definite or likely ADPLD on 01/01/2010 was 9.5/100,000 or 36.0/100,000 population if adding possible cases.
-
•
Clinically significant isolated ADPLD is rare (<1:10,000 population), but the overall prevalence is likely much higher.
Introduction
Autosomal-dominant polycystic kidney disease (ADPKD), caused in most cases by mutations to PKD1 or PKD2, is characterised by progressive development and growth of cysts within the kidneys.1,2 These lead to the development of hypertension, pain, nephrolithiasis, haematuria, urinary tract infections, and eventually destruction of the renal parenchyma and kidney failure. It is a systemic disorder with cyst development in other organs and vascular and cardiac manifestations. Hepatic cysts or polycystic liver disease (PLD) constitute its most common extra-renal manifestation.[3], [4], [5]
For many years, it was generally believed that PLD was always associated with ADPKD.3 It is now recognised that PLD also occurs as a distinct genetic disorder with an autosomal-dominant pattern of inheritance (autosomal-dominant polycystic liver disease [ADPLD]).[6], [7], [8], [9] The first 2 genes associated with ADPLD, PRKCSH and SEC63, were identified in families characterised by relatively severe PLD in the absence of or with very few renal cysts.[10], [11], [12] Additional genes have more recently been identified to be variably associated with ADPLD,[13], [14], [15], [16], [17], [18], [19] but affected patients frequently exhibit renal cysts as well, pointing to a significant overlap between ADPLD and ADPKD.20
Whilst ADPKD is a relatively common disease with an estimated prevalence of 1:400–1,000 individuals,21 ADPLD has been considered to be much rarer with an estimated prevalence of 1:10,000.22 Recently, the combined frequency of truncating mutations to 1 of 6 ADPLD genes (PRKCSH, SEC63, GANAB, ALG8, SEC61B, and LRP5) in 2 large, population sequencing databases, gnomAD and BRAVO, was found to be 1:496.23 As PLD is usually asymptomatic, patients with PLD associated with ADPKD often come to medical attention because of the manifestations of the renal disease, whereas those with isolated ADPLD may remain undiagnosed. The mild and less symptomatic nature of isolated ADPLD compared with ADPKD may account for the discrepancy between the perceived clinical prevalence of ADPLD and the frequency of likely pathogenic ADPLD mutations.
With the increasing use of abdominal ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) for a multitude of purposes, incidental detection of hepatic cysts and ADPLD has become more frequent. The present study was performed to ascertain the incidence and point prevalence of ADPLD in Olmsted County, MN, USA, between 1980 and 2016, and how these estimates are impacted by the increasing utilisation of abdominal imaging.
Patients and methods
Research design
This is a retrospective cohort study conducted in Olmsted County, an area relatively isolated from other urban centres and with few healthcare providers, mainly Mayo Clinic, Olmsted Medical Center, and their affiliated facilities, delivering most healthcare to local residents. The study population was identified as part of a project to study the ‘Epidemiology of autosomal dominant polycystic kidney disease in Olmsted County’.21 The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Boards of Mayo Clinic and Olmsted Medical Center.
Data sources
We utilised the Rochester Epidemiology Project and the Mayo Clinic and Olmsted Medical Center radiology databases to identify subjects meeting our diagnostic criteria. The Rochester Epidemiology Project is a medical records linkage system incorporating data from all medical facilities in Olmsted County.[24], [25], [26] The reports of all imaging studies performed at a radiology department in Olmsted County have been stored in the Mayo Clinic or Olmsted Medical Center radiology databases. Abdominal CT and MRI scans have been electronically available since 1997, although before 2009, reports were only available from 1 site in the Rochester Epidemiology Project. We considered that the combination of these data sources provides the most powerful strategy to capture as many potential subjects with ADPLD as possible, and to accurately estimate the incidence or prevalence of ADPLD in this region.
Identification of potential subjects with ADPLD
We searched the Rochester Epidemiology Project for Olmsted County residents during the years 1980–2016 with diagnostic codes for polycystic liver, polycystic kidney, and related cystic disease diagnoses to capture the potential ADPLD subjects.21 We reviewed their medical records, abdominal imaging, and/or imaging reports, including US, CT, and MRI scans. Diagnostic criteria for definite, likely, and possible ADPLD are listed in Table 1.27 Patients not meeting these criteria were considered not to have ADPLD.
Table 1.
Diagnostic criteria for ADPLD.
| ADPLD | Criteria |
|---|---|
| Definite | |
| Likely | |
| Possible |
Cysts detected by ultrasound, computed tomography, or magnetic resonance imaging measuring ≥5 mm in diameter.
ADPKD, autosomal-dominant polycystic kidney disease; ADPLD, autosomal-dominant polycystic liver disease; PLD, polycystic liver disease.
The identification of undiagnosed ADPLD cases occurred through the retrieval of radiology images from Mayo Clinic and Olmsted Medical Center. The Mayo Clinic and Olmsted Medical Center radiology databases were searched for Olmsted County residents with reports of enhanced CT or (plain or enhanced) MRI scans between 1997 and 2016 containing the words ‘cysts’ and ‘liver’ or ‘hepatic’. We searched only subjects who underwent enhanced CT or (plain or enhanced) MRI scans because these imaging studies are more sensitive than non-enhanced CT or US scans to distinguish hepatic cysts from other lesions and to separate cystic from non-cystic tissue. The diagnostic criteria to classify patients as definite ADPLD, likely ADPLD, and possible ADPLD are the same as listed in Table 1.27,28 We reviewed the imaging studies in chronological order starting with the first study to get an accurate incidence date. If an older study had a poor imaging quality and we could not count the number of cysts, we reviewed subsequent studies to make the diagnosis. If subjects underwent multiple imaging studies, enhanced CT or plain or contrast MRI was given priority.
Actual abdominal images were not electronically available before 1997, and we evaluated such subjects by their medical charts and radiology reports. The number of liver cysts was recorded. If there was no information on the number of cysts in the reports, the cases were excluded. Liver volumes were measured when electronic images with full coverage of the livers were available. Hepatomegaly was defined as a liver volume adjusted by height (HtLV) exceeding 1,000 ml/m; HtLV between 1,000 and 1,800 ml/m was considered moderate, and HtLV exceeding 1,800 ml/m was considered severe hepatomegaly.29
Inclusion criteria for incident and prevalent cases
The incidence of ADPLD was defined as patients newly diagnosed in Olmsted County between January 1980 and December 2016. Olmsted County residents with liver cysts meeting our diagnostic criteria for ADPLD found incidentally on imaging studies were also identified. The incidence date was determined as the date when the diagnostic code was entered for the patients with diagnostic codes in the Rochester Epidemiology Project. The incidence date was determined as the date when the first imaging studies meeting our diagnostic criteria were performed for the patients from the radiology databases. ADPLD patients from all age ranges were included in this study. Only patients who had been Olmsted County residents for at least 1 yr before their diagnosis were considered incident cases. Anyone moving to Olmsted County to facilitate diagnosis or treatment for ADPLD was excluded. The aim of this study was to identify the incidence rate of ADPLD in Olmsted County, so all patients diagnosed as ADPLD in other facilities of this region as well as in Mayo Clinic were included in this study.
The point prevalence of ADPLD was defined as patients meeting our criteria for ADPLD who were Olmsted County residents on 1 January 2010. Patients who were diagnosed before 1980 and those who moved in Olmsted County after their diagnosis were also included for prevalence of ADPLD. The population of Olmsted County was 92,006 in 1980 and 144,248 in 2010.
Statistical analysis
Annual incidence rates of ADPLD per 100,000 person-years were calculated using incident cases of ADPLD as the numerator, and age- and sex-specific estimates of the population of Olmsted County as the denominator. The population at risk was estimated using US census data from 1980, 1990, 2000, and 2010, with linear interpolation for inter-census years. Overall incidence rates were age and sex adjusted, and standardised to The United States White Population: 2010. Incidence rates were also estimated separately for men and women and by age group. Estimates were provided for definite or likely, and for definite, likely, or possible ADPLD. In addition, incidence rates were estimated for patients with a diagnosis as well as all patients (patients with a diagnostic code and those with radiology only). Annual incidence rates were estimated averaging across 5-yr calendar periods. Finally, we created jitter plots to show the distribution of number of cysts by age group and sex amongst the ADPLD patients meeting ‘possible’ diagnostic criteria.
The point prevalence of ADPLD was calculated using prevalent cases of ADPLD on 1 January 2010 as the numerator, and age- and sex-specific estimates of the population of Olmsted County on 1 January 2010 as the denominator, as determined from the Rochester Epidemiology Project census.26 Prevalence rates were age- and sex adjusted, and standardised to The United States White Population: 2010. Prevalence rates were also estimated separately for men and women and by age group. Estimates were provided for likely alone and likely + possible diagnoses.
Results
We reviewed medical charts, abdominal images, or radiology reports for 2,131 subjects with diagnostic codes and 2,765 subjects from radiology (Fig. S1A and B). Between 1980 and 2016, 32 patients with incident ADPLD were identified by using diagnostic codes and 102 patients were identified from radiology. In total, 134 patients had incident ADPLD. Four patients had a definite diagnosis (3 with positive family history and 1 with a proven SEC63 mutation) and 31 patients had a likely diagnosis (Fig. 1). Because of the small number of definite diagnoses, the data for the patients with definite and likely diagnoses are presented together (Table S1). The remaining 99 patients had a possible diagnosis of ADPLD (Fig. 2).
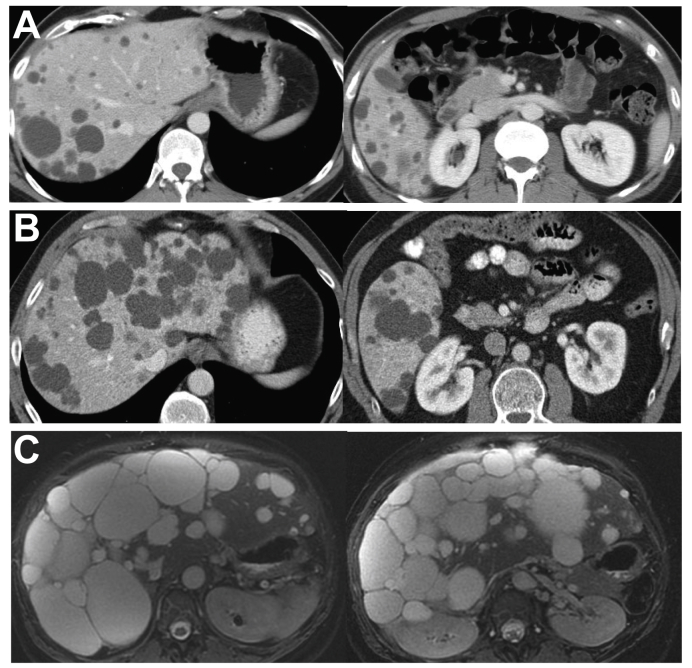
Fig. 1.
Representative abdominal MRI or CT images from patients with definite or likely ADPLD.
(A) A 34-yr-old man with a SEC63 mutation without known family history of PLD; liver volume 1,717 ml. (B) A 62-yr-old man without a family history of PLD; liver volume 3,365 ml. (C) A 45-yr-old woman with documented family history of PLD; liver volume 4,112 ml. ADPLD, autosomal-dominant polycystic liver disease; CT, computed tomography; MRI, magnetic resonance imaging; PLD, polycystic liver disease.
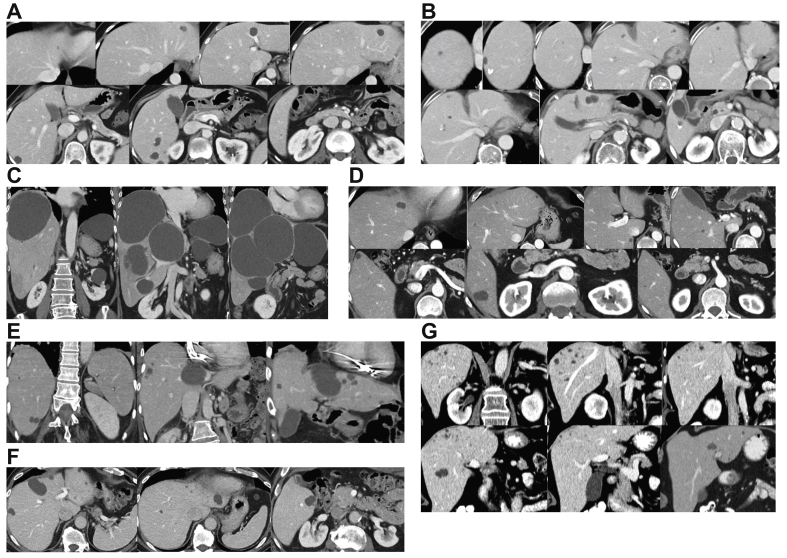
Fig. 2.
Representative abdominal or CT images from patients with possible ADPLD.
(A) A 49-yr-old woman; liver volume 1,287 ml. (B) A 64-yr-old woman; liver volume 1,642 ml. (C) A 77-yr-old woman; liver volume 3,502 ml. (D) A 51-yr-old man; liver volume 1,778 ml. (E) An 80-yr-old man; liver volume 1,385 ml. (F) An 81-yr-old man; liver volume 1,547 ml. (G) A 77-yr-old female; live volume 1,623 ml. ADPLD, autosomal-dominant polycystic liver disease; CT, computed tomography.
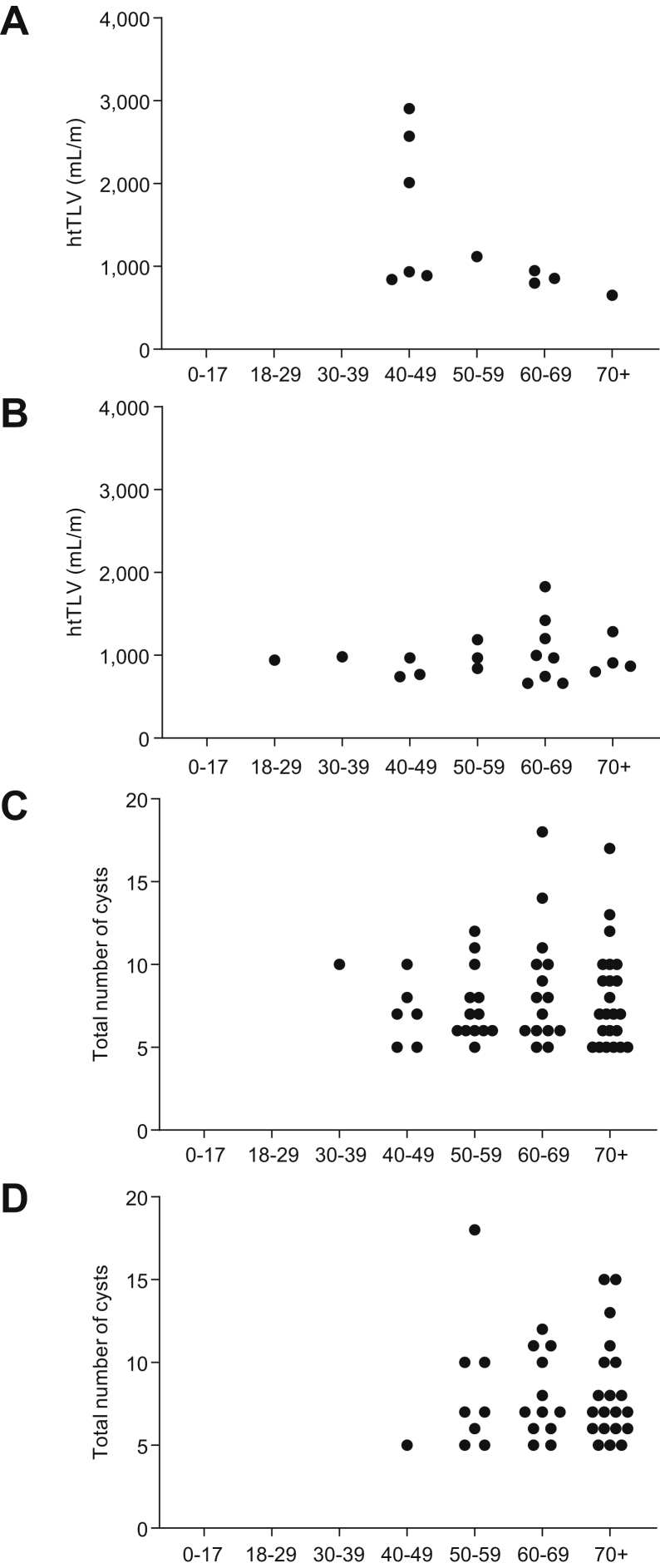
Patients with definite or likely ADPLD, compared with those with possible ADPLD, were younger and had larger liver volumes (median 1636; inter-quartile range [IQR] 1,375–2,054 ml vs. 1,381; 1,163–1,655 ml; p = 0.004) and height-adjusted liver volumes (median 940; IQR 800–1,187 ml/m vs. 824; 701–953 ml/m; p = 0.005) (Table 2). Five patients, 4 with a definite/likely diagnosis and 1 with a possible diagnosis, had severe hepatomegaly (HtLV >1800 ml/m) (Table 2). Four of the 5 patients with severe hepatomegaly were females and 1 of them required a partial liver resection. Nineteen patients, 5 with a definite/likely and 14 with a possible diagnosis, had moderate hepatomegaly (HtLV 1,000–1,800 ml/m). In both groups, with definite/likely or with possible diagnosis, there was a trend for larger height-adjusted liver volumes in women, but the difference was not statistically significant (Fig. 3A and B). The numbers of hepatic cysts in male and female patients with possible ADPLD were also similar (Fig. 3C and D). Forty-two per cent of definite or likely and 51% of possible ADPLD patients had a history of hypertension. There was a trend for more frequent history of nephrolithiasis in the patients with definite/likely ADPLD and of haematuria in the patients with possible ADPLD.
Table 2.
Characteristics of patients with ADPLD at diagnosis.
| All ADPLD patients | Definite or likely ADPLD | Possible ADPLD | |
|---|---|---|---|
| Age at diagnosis (yr) | (n = 134) | (n = 35) | (n = 99) |
| Mean (SD) | 64.4 (13.4) | 57.5 (13.7) | 66.9 (12.5) |
| Median (25th; 75th) | 65.5 (53; 74) | 59 (46; 66) | 67 (57; 76) |
| Range | 28–94 | 28–85 | 38–94 |
| Male, n (%) | 63 (47.0) | 22 (62.9) | 41 (41.4) |
| Caucasian, n (%) | 124/133 (93.2) | 31/35 (88.6) | 93/98 (94.9) |
| History of hypertension, n (%) | 63/129 (48.8) | 14/33 (42.4) | 49/96 (51.0) |
| History of haematuria, n (%) | 21/129 (16.3) | 3/33 (9.1) | 18/96 (18.8) |
| History of kidney stones, n (%) | 14/130 (10.8) | 7/34 (20.6) | 7/96 (7.3) |
| History of depression, n (%) | 29/129 (22.5) | 7/33 (21.2) | 22/96 (22.9) |
| Tobacco use ever, n (%) | 76/128 (59.4) | 20/32 (62.5) | 56/96 (58.3) |
| LV (ml) | (n = 107) | (n = 31) | (n = 76) |
| Mean (SD) | 1,568 (600) | 1,875 (837) | 1,442 (417) |
| Median (IQR) | 1,437 (1,220; 1,725) | 1,636 (1,375; 2,054) | 1,381 (1,163; 1,655) |
| Range | 789–4,300 | 1,060–4,300 | 789–3,502 |
| Height-adjusted LV (ml/m) | |||
| Mean (SD) | 930 (374) | 1,105 (534) | 858 (226) |
| Median (25th; 75th) | 845 (712; 981) | 940 (800; 1,187) | 824 (701; 953) |
| Range | 445–2,905 | 652–2,905 | 445–2,319 |
| <1,000, N (%) | 83 (77.6) | 22 (71.0) | 61 (80.3) |
| 1,000–1,800, N (%) | 19 (17.7) | 5 (16.1) | 14 (18.4) |
| H >1,800, N (%) | 5 (4.7) | 4 (12.9) | 1 (1.3) |
| Plasma creatinine (mg/dl) | (n = 126) | (n = 32) | (n = 94) |
| Mean (SD) | 0.91 (0.20) | 0.94 (0.20) | 0.90 (0.20) |
| Median (25th; 75th) | 0.9 (0.8; 1.0) | 0.9 (0.8; 1.0) | 0.9 (0.8; 1.0) |
| Range | 0.5–1.5 | 0.6–1.4 | 0.5–1.5 |
ADPLD, autosomal-dominant polycystic liver disease; IQR, inter-quartile range; LV, liver volume.
Fig. 3.
HtLV and cyst number by gender and age group.
(A) HtLV by age group in women with definite or likely ADPLD. (B) HtLV by age group in men with definite or likely ADPLD. (C) Number of cysts by age group in women with possible ADPLD. (D) Number of cysts by age group in men with possible ADPLD. ADPLD, autosomal-dominant polycystic liver disease; HtLV, height-adjusted liver volume.
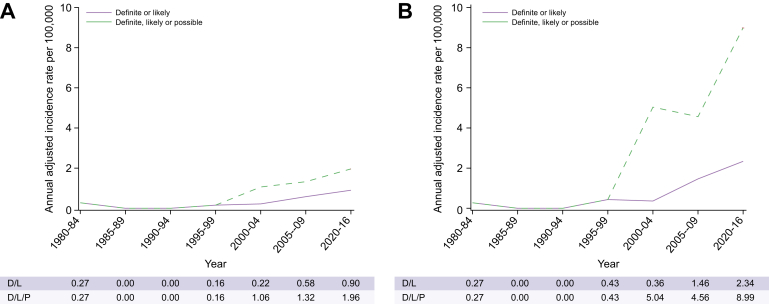
The overall age- and sex-adjusted annual incidence rate of definite or likely ADPLD was 1.01 (95% CI 0.67–1.35) and that of combined (definite, likely, or possible ADPLD) was 4.01 (95% CI 3.32–4.69) per 100,000 person-years (Table 3). The incidence rates were not different between males and females. They were very low before 1997 and increased progressively after 1997 as a result of image availability for our review and increasing utilisation of imaging studies (Fig. 4A and B). Incidence rates were greater in older groups (Table 3; Fig. S2A–D), reflecting the increasing utilisation of CT and MRI scans in older populations (Figs S3 and S4).
Table 3.
Annual incidence rates (1980–2016) of ADPLD per 100,000 person-years (95% CI) by age and sex in Olmsted County.
| Age group (yr) | Females |
Males |
Total |
|||
|---|---|---|---|---|---|---|
| n | Rate (95% CI)a | n | Rate (95% CI)a | n | Rate (95% CI)a | |
| Definite or likely ADPLD | ||||||
| 0–17 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| 18–29 | 0 | 0.00 | 1 | 0.27 (0.01; 1.48) | 1 | 0.13 (0.00; 0.71) |
| 30–39 | 0 | 0.00 | 1 | 0.28 (0.01; 1.57) | 1 | 0.14 (0.00; 0.79) |
| 40–49 | 6 | 1.90 (0.70; 4.13) | 3 | 0.97 (0.20; 2.83) | 9 | 1.44 (0.66; 2.73) |
| 50–59 | 3 | 1.17 (0.24; 3.42) | 4 | 1.63 (0.44; 4.17) | 7 | 1.40 (0.56; 2.87) |
| 60–69 | 3 | 1.76 (0.36; 5.15) | 9 | 5.91 (2.70; 11.22) | 12 | 3.72 (1.92; 6.50) |
| 70–110 | 1 | 0.46 (0.01; 2.56) | 4 | 2.90 (0.79; 7.43) | 5 | 1.41 (0.46; 3.29) |
| All ages | 13 | 0.68 (0.31; 1.06) | 22 | 1.40 (0.80; 1.99) | 35 | 1.01 (0.67; 1.35) |
| Definite, likely, or possible ADPLD | ||||||
| 0–17 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| 18–29 | 0 | 0.00 | 1 | 0.27 (0.01–1.48) | 1 | 0.13 (0.00–0.71) |
| 30–39 | 1 | 0.28 (0.01–1.57) | 1 | 0.28 (0.01–1.57) | 2 | 0.28 (0.03–1.02) |
| 40–49 | 11 | 3.48 (1.74–6.22) | 4 | 1.29 (0.35–3.31) | 15 | 2.40 (1.34–3.95) |
| 50–59 | 17 | 6.64 (3.87–10.62) | 12 | 4.89 (2.53–8.54) | 29 | 5.78 (3.87–8.30) |
| 60–69 | 18 | 10.58 (6.27–16.72) | 21 | 13.79 (8.54–21.09) | 39 | 12.10 (8.60–16.54) |
| 70–110 | 24 | 11.04 (7.07–16.43) | 24 | 17.42 (11.16–25.92) | 48 | 13.52 (9.97–17.92) |
| All ages | 71 | 3.82 (2.92–4.71) | 63 | 4.32 (3.24–5.39) | 134 | 4.1 (3.32–4.69) |
ADPLD, autosomal-dominant polycystic liver disease.
Rates for females and males are adjusted for age, whereas the total rate is adjusted for age and sex.
Fig. 4.
Trends in age- and sex-adjusted annual incidence of ADPLD per 100,000 over time, by diagnostic criteria.
Annual incidence of ADPLD identified from (A) diagnostic codes and (B) diagnostic codes and radiology. ADPLD, autosomal-dominant polycystic liver disease; D, definite; L, likely; P, possible.
Forty-four (41 with ADPLD previously identified in Olmsted County and 3 diagnosed elsewhere) lived in Olmsted County on 1 January 2010. The overall age- and sex-adjusted prevalence of definite or likely ADPLD was 9.5 (95% CI 4.1–14.9), and that of combined (definite, likely, or possible) ADPLD was 36.0 (95% CI 25.3–46.6) per 100,000 population on 1 January 2010 (Table 4).
Table 4.
Point prevalence (1 January 2010) of ADPLD per 100,000 population (95% CI) by age and sex in Olmsted County.
| Age group (yr) | Females |
Males |
Total |
|||
|---|---|---|---|---|---|---|
| N | Rate (95% CI)a | n | Rate (95% CI)a | n | Rate (95% CI)a | |
| Definite or likely ADPLD | ||||||
| 0–17 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| 18–29 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| 30–39 | 0 | 0.00 | 1 | 10.48 (0.27; 58.38) | 1 | 5.10 (0.13; 28.43) |
| 40–49 | 1 | 9.91 (0.25; 55.20) | 1 | 10.68 (0.27; 59.52) | 2 | 10.28 (1.24; 37.14) |
| 50–59 | 3 | 29.67 (6.12; 86.72) | 1 | 10.84 (0.27; 60.42) | 4 | 20.69 (5.64; 52.98) |
| 60–69 | 1 | 15.83 (0.40; 88.17) | 2 | 35.57 (4.31; 128.51) | 3 | 25.12 (5.18; 73.42) |
| 70–110 | 1 | 13.71 (0.35; 76.37) | 1 | 18.74 (0.47; 104.44) | 2 | 15.83 (1.92; 57.20) |
| All ages | 6 | 8.93 (1.77; 16.09) | 6 | 10.23 (41.96; 18.51) | 12 | 9.52 (4.11; 14.94) |
| Definite, likely or possible ADPLD | ||||||
| 0–17 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| 18–29 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| 30–39 | 0 | 0.00 | 1 | 10.48 (0.27; 58.38) | 1 | 5.10 (0.13; 28.43) |
| 40–49 | 1 | 9.91 (0.25; 55.20) | 2 | 21.37 (2.59; 77.18) | 3 | 15.42 (3.18; 45.06) |
| 50–59 | 9 | 89.02 (40.71; 168.99) | 4 | 43.37 (11.82; 111.06) | 13 | 67.25 (35.81; 114.99) |
| 60–69 | 6 | 94.95 (34.85; 206.67) | 4 | 71.15 (19.39; 182.17) | 10 | 83.75 (40.16; 154.01) |
| 70–110 | 10 | 137.06 (65.73; 252.06) | 7 | 131.21 (52.75; 270.34) | 17 | 134.59 (78.40; 215.49) |
| All ages | 26 | 39.26 (24.13; 54.39) | 18 | 32.33 (17.28; 47.39) | 44 | 35.96 (25.30; 46.61) |
ADPLD, autosomal-dominant polycystic liver disease.
Rates for females and males are adjusted for age, whereas the total rate is adjusted for age and sex.
Discussion
PLD is characterised by multiple cysts scattered throughout the liver parenchyma. It occurs in association with ADPKD or as a distinct genetic entity with autosomal-dominant inheritance (ADPLD). Nine genes have been associated with ADPLD (PRKCSH, SEC6, LRP5, GANAB, ALG8, ALG9, SEC61B, PKHD1, and DNAJB11).[10], [11], [12], [13], [14], [15], [16], [17], [18], [19] Together, they account for only 50–60% of the cases, suggesting that additional genes remain to be identified. Multiple hepatic cysts can also occur in association with other genetic disorders[30], [31], [32] and advanced chronic liver disease.33
The diagnosis of ADPLD relies on the demonstration of hepatic cysts and a pathogenic ADPLD mutation, the presence of hepatic cysts in the setting of a family history of ADPLD, or the presence of numerous hepatic cysts in the absence of or with only few renal cysts. As not all genetic causes of ADPLD have been identified, documentation of a family history of asymptomatic PLD is often lacking, and because simple hepatic cysts often develop in older individuals in the absence of a genetic cause, the diagnosis of ADPLD is often arbitrary. In a 2003 article describing the clinical profile of ADPLD, ≥20 hepatic cysts with a number of renal cysts below the diagnostic ADPKD criteria was required to diagnose ADPLD probands.27 A similar rule has been used in subsequent studies,[10], [11], [12] although some have used less stringent criteria (≥15 or ≥10 cysts).[34], [35], [36] For first-degree relatives of a proband, the presence of any hepatic cyst for individuals less than 40 yr of age and at least 4 hepatic cysts for those 40 yr old or older has been required for diagnosis.27,28
The criteria for the diagnosis of ADPLD have been based on the prevalence on simple hepatic cysts in the general population. It should be acknowledged that genotype–phenotype correlation studies to determine the number of cysts that warrants a diagnosis of ADPLD as opposed to multiple simple hepatic cysts have not been performed. Old studies using second-generation US machines found hepatic cysts in approximately 2% of individuals 40–70 yr in the general population and in up to 6% of those 70 yr of age or older.37,38 They are few in number, usually solitary, and only exceptionally more than 3. They are less common than renal cysts and develop at a later age. A more recent study using a third-generation US machine reported hepatic cysts in 11.3% of 1,541 patients.39 The frequency of hepatic cysts increased with age: 0%, 5.9%, 11.4%, 18.6%, 16.3%, and 23.4% of patients younger than 40, 40–49, 50–59, 60–69, 70–79, and 80–89 yr of age, respectively. Six patients had polycystic kidney disease and 8 had isolated PLD. The average number of cysts in the remaining 160 patients was 2 cysts per patient. Another study using contrast-enhanced spiral CT reported hepatic cysts in 17.8% of 617 patients.40 The frequency also increased with age: 2%, 14.4%, 20.6%, and 24.2% in patients younger than 40, 40–60, 60–80, and >80 yr of age. The number of cysts per patient was not provided.
In the present study, we have used conservative criteria for the diagnosis of ADPLD[10], [11], [12],27: definite, in the presence of multiple hepatic cysts and either a pathogenic ADPLD mutation or a family history of PLD; likely, with ≥20 cysts; and possible with 5–19 cysts. Using these criteria, only 4 Olmsted County patients met the criteria for definite ADPLD and only 35 met the criteria for definite or likely ADPLD during 1980–2016. The overall age- and sex-adjusted incidence rate of definite or likely ADPLD was 1.01 per 100,000 person-years compared with 3.06 per 100,000 person-years for definite or likely ADPKD.21 The overall age- and sex-adjusted 2010 point prevalence of definite or likely ADPLD was 9.5 per 100,000 compared with 68 per 100,000 population for definite or likely ADPKD.21 Furthermore, only 15 of 35 definite and likely incident ADPLD cases had received a diagnostic code, and only 8 (3 females and 5 males) had clinically significant hepatomegaly. The other 20 out of 35 likely ADPLD were identified from radiology databases, which means they had not been given any diagnostic codes related to liver cysts. These data confirm that ADPLD is diagnosed more rarely than ADPKD, and are consistent with statements in the literature that ADPLD, if restricted to clinically significant ADPLD, is a rare disease.
With the addition of possible ADPLD cases, incidence and prevalence were substantially higher, 4.01 per 100,000 person-years and 36.0 per 100,000 population, respectively. These rates, however, greatly underestimate the real prevalence of ADPLD, as possible cases were mainly identified by the review of the radiology databases and not all Olmsted County patients underwent abdominal imaging during the study period. Furthermore the radiology review was only possible starting in 1997. The degree to which these rates underestimate the real incidence and prevalence is illustrated by the increasing incidence rates between 1997 and 2016 and the higher incidence rates in older patients, both reflecting increasing utilisation of imaging studies. Therefore, it seems likely that the real prevalence of ADPLD, to a large extent not clinically significant, is likely much higher and closer to the reported genetic prevalence.23
Previous studies of ADPLD focused mainly on symptomatic disease and contained mainly female patients. In contrast, the present study is balanced with respect to sex, and shows that the incidence of ADPLD was similar in females and males, although the cystic disease appeared to be more severe and symptomatic in female patients. In its totality, the evidence suggests that the incidence of ADPLD is similar in males and females, but that the disease is more severe in females.
The main limitations of the current study because of its retrospective nature include the incomplete ascertainment of the cases (individuals without imaging studies or with only US or unenhanced CT scans), the possibility that some patients with multiple simple hepatic cysts might have been considered to have ADPLD, the paucity of genetic testing, and the low diversity of the Olmsted County population that limits the generalisability of the observations to more diverse populations.41
The incidence of likely or possible ADPLD has increased since 2000 (likely because of more frequent imaging), but the incidence of ADPLD with symptomatic hepatomegaly remains low. Most patients meeting our criteria for likely or possible ADPLD were found incidentally. Nevertheless, our results suggest that the incidence of ADPLD, including asymptomatic ADPLD, has been underestimated. The prevalence of likely ADPLD was also more than expected. Consistent with previous reports, the incidence of ADPLD was not different between females and males, but as reported in previous studies, PLD appeared to be more severe and symptomatic in females.[3], [4], [5] Genetic testing will be necessary to confirm a diagnosis of ADPLD in the patients classified as likely ADPLD, and to determine whether the patients with possible ADPLD have mutations in the already-known or in novel PLD genes.
Financial support
National Institute on Aging of the National Institutes of Health (R01AG034676); Mayo Clinic Robert M. and Billie Kelley Pirnie Translational Polycystic Kidney Disease Center; National Institute of Diabetes and Digestive and Kidney Diseases grant (DK090728).
Authors' contributions
T.S., A.M.C., B.F.K., F.T.C., M.C.H., P.S.K., P.C.H., and V.E.T. contributed to the conceptualisation of the paper. T.S., C.D.M., X.W., and V.E.T. contributed to data curation. T.S., A.M.C., J.M.K., and V.E.T. did the formal analysis. B.F.K., A.V.G., and T.L.K. contributed to the methodology. T.S. and V.E.T. wrote the original draft. T.S., A.M.C., P.S.K., P.C.H., and V.E.T. reviewed and edited the paper. V.E.T. contributed project administration, resources, and supervision. All authors reviewed and approved the final paper.
Conflicts of interest
The authors declare no conflicts of interest related to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
This project was performed along the Master's program in Center for Clinical and Translational Science in Mayo Clinic Graduate School. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under award number R01AG034676. This work was supported in part by the Mayo Clinic Robert M. and Billie Kelley Pirnie Translational Polycystic Kidney Disease Center and the National Institute of Diabetes and Digestive and Kidney Diseases grant DK090728. The authors thank Dr Ziad El-Zoghby (nephrology) and Ms Vicki C. Schmidt (radiology) for their assistance in the collection of clinical data, and Mr Scott M. Brue (programmer) and Mr Joseph J. Larson (statistician) for their help in analysing the data. The authors also thank Ms Marie E. Edwards and Mr Andrew J. Metzger (nephrology) for measuring the kidney or liver volumes of the enrolled patients, and Walter K. Kremers (statistician) for providing the power calculation for the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100166.
Supplementary data
References
- 1.Torres V.E., Harris P.C., Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 2.Cornec-Le Gall E., Alam A., Perrone R.D. Autosomal dominant polycystic kidney disease. Lancet. 2019;393:919–935. doi: 10.1016/S0140-6736(18)32782-X. [DOI] [PubMed] [Google Scholar]
- 3.Torres V. Polycystic liver disease. In: Watson M.T., editor. Polycystic Kidney Disease. Oxford Medical Publications; Oxford: 1996. pp. 500–529. [Google Scholar]
- 4.Chauveau D., Fakhouri F., Grunfeld J.P. Liver involvement in autosomal-dominant polycystic kidney disease: therapeutic dilemma. J Am Soc Nephrol. 2000;11:1767–1775. doi: 10.1681/ASN.V1191767. [DOI] [PubMed] [Google Scholar]
- 5.Everson G.T., Taylor M.R., Doctor R.B. Polycystic disease of the liver. Hepatology. 2004;40:774–782. doi: 10.1002/hep.20431. [DOI] [PubMed] [Google Scholar]
- 6.Berrebi G., Erickson R.P., Marks B.W. Autosomal dominant polycystic liver disease: a second family. Clin Genet. 1982;21:342–347. doi: 10.1111/j.1399-0004.1982.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 7.Karhunen P.J., Tenhu M. Adult polycystic liver and kidney diseases are separate entities. Clin Genet. 1986;30:29–37. doi: 10.1111/j.1399-0004.1986.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 8.Pirson Y., Lannoy N., Peters D., Geubel A., Gigot J.F., Breuning M. Isolated polycystic liver disease as a distinct genetic disease, unlinked to polycystic kidney disease 1 and polycystic kidney disease 2. Hepatology. 1996;23:249–252. doi: 10.1002/hep.510230208. [DOI] [PubMed] [Google Scholar]
- 9.Iglesias D.M., Palmitano J.A., Arrizurieta E., Kornblihtt A.R., Herrera M., Bernath V. Isolated polycystic liver disease not linked to polycystic kidney disease 1 and 2. Dig Dis Sci. 1999;44:385–388. doi: 10.1023/a:1026623005401. [DOI] [PubMed] [Google Scholar]
- 10.Li A., Davila S., Furu L., Qian Q., Tian X., Kamath P.S. Mutations in PRKCSH cause isolated sutosomal dominant polycystic liver disease. Am J Hum Genet. 2003;72:691–703. doi: 10.1086/368295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drenth J.P., te Morsche R.H., Smink R., Bonifacino J.S., Jansen J.B. Germline mutations in PRKCSH are associated with autosomal dominant polycystic liver disease. Nat Genet. 2003;10:10. doi: 10.1038/ng1104. [DOI] [PubMed] [Google Scholar]
- 12.Davila S., Furu L., Gharavi A.G., Tian X., Onoe T., Qian Q. Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat Genet. 2004;36:575–577. doi: 10.1038/ng1357. [DOI] [PubMed] [Google Scholar]
- 13.Cnossen W.R., te Morsche R.H., Hoischen A., Gilissen C., Chrispijn M., Venselaar H. Whole-exome sequencing reveals LRP5 mutations and canonical Wnt signaling associated with hepatic cystogenesis. Proc Natl Acad Sci U S A. 2014;111:5343–5348. doi: 10.1073/pnas.1309438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porath B., Gainullin V.G., Cornec-Le Gall E., Dillinger E.K., Heyer C.M., Hopp K. Mutations in GANAB, encoding the glucosidase IIα subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet. 2016;98:1193–1207. doi: 10.1016/j.ajhg.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besse W., Dong K., Choi J., Punia S., Fedeles S.V., Choi M. Isolated polycystic liver disease genes define effectors of polycystin-1 function. J Clin Invest. 2017;127:1772–1785. doi: 10.1172/JCI90129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Besse W., Choi J., Ahram D., Mane S., Sanna-Cherchi S., Torres V. A noncoding variant in GANAB explains isolated polycystic liver disease (PCLD) in a large family. Hum Mutat. 2018;39:378–382. doi: 10.1002/humu.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornec-Le Gall E., Olson R.J., Besse W., Heyer C.M., Gainullin V.G., Smith J.M. Monoallelic mutations to DNAJB11 cause atypical autosomal-dominant polycystic kidney disease. Am J Hum Genet. 2018;102:832–844. doi: 10.1016/j.ajhg.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Besse W., Chang A.R., Luo J.Z., Triffo W.J., Moore B.S., Gulati A. ALG9 mutation carriers develop kidney and liver cysts. J Am Soc Nephrol. 2019;30:2091–2102. doi: 10.1681/ASN.2019030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh V.T., Audrézet M.P., Sayer J.A., Ong A.C., Lefevre S., Le Brun V. Clinical spectrum, prognosis and estimated prevalence of DNAJB11-kidney disease. Clin Invest. 2020;98:476–487. doi: 10.1016/j.kint.2020.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornec-Le Gall E., Torres V.E., Harris P.C. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol. 2018;29:13–23. doi: 10.1681/ASN.2017050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suwabe T., Shukoor S., Chamberlain A.M., Killian J.M., King B.F., Edwards M. Epidemiology of autosomal dominant polycystic kidney disease in Olmsted County. Clin J Am Soc Nephrol. 2020;15:69–79. doi: 10.2215/CJN.05900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Aerts R.M.M., van de Laarschot L.F.M., Banales J.M., Drenth J.P.H. Clinical management of polycystic liver disease. J Hepatol. 2018;68:827–837. doi: 10.1016/j.jhep.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Lanktree M.B., Haghighi A., Guiard E., Iliuta I.A., Song X., Harris P.C. Prevalence estimates of polycystic kidney and liver disease by population sequencing. J Am Soc Nephrol. 2018;29:2593–2600. doi: 10.1681/ASN.2018050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocca W.A., Yawn B.P., St Sauver J.L., Grossardt B.R., Melton L.J., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Sauver J.L., Grossardt B.R., Yawn B.P., Melton L.J., 3rd, Pankratz J.J., Brue S.M. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St Sauver J.L., Grossardt B.R., Yawn B.P., Melton L.J., 3rd, Rocca W.A. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian Q., Li A., King B.F., Kamath P.S., Lager D.J., Huston J., 3rd Clinical profile of autosomal dominant polycystic liver disease. Hepatology. 2003;37:164–171. doi: 10.1053/jhep.2003.50006. [DOI] [PubMed] [Google Scholar]
- 28.Ravine D., Gibson R.N., Walker R.G., Sheffield L.J., Kincaid-Smith P., Danks D.M. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824–827. doi: 10.1016/s0140-6736(94)92026-5. [DOI] [PubMed] [Google Scholar]
- 29.Hogan M.C., Abebe K., Torres V.E., Chapman A.B., Bae K.T., Tao C. Liver involvement in early autosomal-dominant polycystic kidney disease. Clin Gastroenterol Hepatol. 2015;13:155–164.e6. doi: 10.1016/j.cgh.2014.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black M.E., Hedgire S.S., Camposano S., Paul E., Harisinghani M., Thiele E.A. Hepatic manifestations of tuberous sclerosis complex: a genotypic and phenotypic analysis. Clin Genet. 2012;82:552–557. doi: 10.1111/j.1399-0004.2012.01845.x. [DOI] [PubMed] [Google Scholar]
- 31.Friedrich C.A. Von Hippel-Lindau syndrome. A pleomorphic condition. Cancer. 1999;86:2478–2482. [PubMed] [Google Scholar]
- 32.Chetty-John S., Piwnica-Worms K., Bryant J., Bernardini I., Fischer R.E., Heller T. Fibrocystic disease of liver and pancreas; under-recognized features of the X-linked ciliopathy oral-facial-digital syndrome type 1 (OFD I) Am J Med Genet A. 2010;152A:2640–2645. doi: 10.1002/ajmg.a.33666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goossens N., Breguet R., De Vito C., Terraz S., Lin-Marq N., Giostra E. Peribiliary gland dilatation in cirrhosis: relationship with liver failure and stem cell/proliferation markers. Dig Dis Sci. 2017;62:699–707. doi: 10.1007/s10620-016-4421-x. [DOI] [PubMed] [Google Scholar]
- 34.Hoevenaren I.A., Wester R., Schrier R.W., McFann K., Doctor R.B., Drenth J.P. Polycystic liver: clinical characteristics of patients with isolated polycystic liver disease compared with patients with polycystic liver and autosomal dominant polycystic kidney disease. Liver Int. 2008;28:264–270. doi: 10.1111/j.1478-3231.2007.01595.x. [DOI] [PubMed] [Google Scholar]
- 35.van Keimpema L., de Man R.A., Drenth J.P. Somatostatin analogues reduce liver volume in polycystic liver disease. Gut. 2008;57:1338–1339. doi: 10.1136/gut.2008.155721. [DOI] [PubMed] [Google Scholar]
- 36.D'Agnolo H.M., Kievit W., Andrade R.J., Karlsen T.H., Wedemeyer H., Drenth J.P. Creating an effective clinical registry for rare diseases. United Eur Gastroenterol J. 2016;4:333–338. doi: 10.1177/2050640615618042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaines P.A., Sampson M.A. The prevalence and characterization of simple hepatic cysts by ultrasound examination. Br J Radiol. 1989;62:335–337. doi: 10.1259/0007-1285-62-736-335. [DOI] [PubMed] [Google Scholar]
- 38.Caremani M., Vincenti A., Benci A., Sassoli S., Tacconi D. Ecographic epidemiology of non-parasitic hepatic cysts. J Clin Ultrasound. 1993;21:115–118. doi: 10.1002/jcu.1870210207. [DOI] [PubMed] [Google Scholar]
- 39.Larssen T.B., Rorvik J., Hoff S.R., Horn A., Rosendahl K. The occurrence of asymptomatic and symptomatic simple hepatic cysts. A prospective, hospital-based study. Clin Radiol. 2005;60:1026–1029. doi: 10.1016/j.crad.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Carrim Z.I., Murchison J.T. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003;58:626–629. doi: 10.1016/s0009-9260(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 41.St Sauver J.L., Grossardt B.R., Leibson C.L., Yawn B.P., Melton L.J., 3rd, Rocca W.A. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.