SUMMARY
Increasing evidence links cognitive-decline and Alzheimer’s disease (AD) to various sleep disorders, including obstructive sleep apnea (OSA). With increasing age, there are substantial differences in OSA’s prevalence, associated comorbidities and phenotypic presentation. An important question for sleep and AD researchers is whether OSA’s heterogeneity results in varying cognitive-outcomes in older-adults compared to middle-aged adults. In this review, we systematically integrated research examining OSA and cognition, mild cognitive-impairment (MCI) and AD/AD biomarkers; including the effects of continuous positive airway pressure (CPAP) treatment, particularly focusing on characterizing the heterogeneity of OSA and its cognitive-outcomes. Broadly, in middle-aged adults, OSA is often associated with mild impairment in attention, memory and executive function. In older-adults, OSA is not associated with any particular pattern of cognitive-impairment at cross-section; however, OSA is associated with the development of MCI or AD with symptomatic patients who have a higher likelihood of associated disturbed sleep/cognitive-impairment driving these findings. CPAP treatment may be effective in improving cognition in OSA patients with AD. Recent trends demonstrate links between OSA and AD-biomarkers of neurodegeneration across all age-groups. These distinct patterns provide the foundation for envisioning better characterization of OSA and the need for more sensitive/novel sleep-dependent cognitive assessments to assess OSA-related cognitive-impairment.
Keywords: Obstructive sleep apnea, Cognition, Mild cognitive impairment, Alzheimer’s disease, Biomarkers, Amyloid, Phosphorylated tau, Middle aged, Older adults
Introduction
Increasing evidence links cognitive decline and Alzheimer’s disease (AD) to various sleep disorders, including obstructive sleep apnea (OSA), insomnia, and circadian rhythm abnormalities [1–4]. OSA is the most common primary sleep disturbance in older adults and is characterized by intermittent hypoxia, sleep fragmentation and intrathoracic pressure swings. The overall estimated prevalence of OSA irrespective of daytime symptoms in the US is 10% for mild [5] and 4–6.5% for moderate-to-severe [6,7], but in older adults it is as high as 30–80% [8–10], depending on the population studied (e.g., community dwelling vs. nursing home) or how sleep respiratory indices (apnea hypopnea index {AHI3, AHI4 or AHI3a}) and their clinical cut-offs (AHI ≥5, ≥15 or ≥30) are defined.
OSA in young and middle-aged populations is associated with excessive daytime sleepiness (EDS) [8,9,11], hypertension [12,13], coronary heart disease [14–16], congestive heart failure [17], stroke [18], and multiple inflammatory and metabolic effects [19,20]. Further evidence in these populations supports a link between OSA and impaired cognitive function, including areas such as attention, memory and executive function [21–23]. However, some studies have shown that the incidence of cognitive impairment, EDS, hypertension and mortality associated with OSA decline with age [24]. While this may in some cases reflect a survivor bias, it also potentially suggests that older people with OSA may not suffer from the same OSA-related consequences seen in the young and middle-aged. OSA may present distinctly in older populations owing to several factors, including differences in the underlying risk factors for OSA (e.g., ventilatory control abnormalities vs. obesity) or to elements that are reduced in the older population, like the amount of expression of EDS or the cardiovascular response to arousals [25–27].
The great disparity in OSA’s prevalence, the possibility of varying comorbidities, and the distinct phenotypic presentation in young and middle-aged vs. older adults, poses an alluring question for sleep and aging researchers, which is whether OSA’s heterogeneity results in varying cognitive outcomes in older adults compared to middle-aged adults. If so, understanding the relationship between OSA and risk for AD, as well as appreciating the heterogeneity of OSA and its outcomes in young and middle-aged vs. older adults is crucial to better tailor preventive and treatment strategies for AD.
Recent narrative reviews on OSA, cognitive decline and AD described the cognitive profiles found in association with OSA in children and adults in general (young, middle-aged and older adults) [28,29]; explored shared pathophysiological mechanisms between OSA and AD [30], examined OSA-AD neurobiology and treatment for a Psychiatry audience; and discussed probable explanatory mechanisms linking OSA, depression and cognitive dysfunction [31–33]. Other narrative discussions focused on the probable explanatory mechanisms linking OSA to dementia as well as discussions focusing on biomarkers of dementia in OSA [34–36]. Previous systematic and meta-reviews focused on how OSA affects specific neurocognitive domains, producing inconsistent [37,38] and sometimes non-conclusive findings [39,40]. The only meta-review focusing on older adults and cognition reported a small association between OSA and cognitive dysfunction and suggested that some specific populations may be more at risk of adverse cognitive effects [41].
In this systematic review, we examine the link between OSA with cognitive performance/impairment, subsequent development of mild cognitive impairment (MCI) or dementia, and AD biomarkers including effects of continuous positive airway pressure (CPAP) with a particular focus in characterizing the heterogeneity of OSA and its cognitive outcomes in distinct clinical groups. We also explored: 1) possible mechanisms linking OSA as a precipitator of AD pathogenesis; as well as, 2) AD-type neurodegeneration as a contributing factor to the emergence of OSA. We systematically reviewed all clinical and epidemiological evidence. Where findings were discrepant, we focused on methodological differences among studies.
Methods
Search strategy
This review was conducted adhering to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement by Moher et al. [42]. A systematic literature search of bibliographic databases, including PubMed/Medline, Embase, Psych INFO and Cochrane library for clinical trials, identified all eligible studies (published prior to May 1st, 2019) that examined associations between: OSA and cognitive function, OSA and subsequent cognitive decline, and OSA and AD. Our search strategy utilized the combination of terms characterizing cognitive function, cognitive impairment or MCI, AD or AD pathology as the dependent variables; OSA as the independent variable; and a third set of terms specifying study types, including clinical and epidemiological studies. Furthermore, we performed a manual search of included articles to identify relevant references not identified by the automated search.
Selection criteria
Eligible studies had to meet the following selection criteria: 1) be original research investigations examining associations between OSA and cognition, OSA and cognitive decline, and/or OSA and AD, including studies examining the effect of CPAP on cognition; 2) be conducted in human adults; 3) include both healthy controls and OSA patients with between group comparisons (studies without controls that conducted within group comparisons based on OSA severity were also considered); 4) use objective neuropsychological cognitive tests (in studies examining cognition or cognitive decline as an outcome); 5) use objective measures of AD (in studies examining AD or AD pathology as an outcome); and, 6) use polysomnography or ‘clinical diagnosis’ for diagnosis of OSA. Seminal studies examining the effect of CPAP and other interventions on sleep parameters and cognition in AD patients with OSA were included. Studies conducted in OSA patients that did not include relevant cognitive parameters (i.e., executive, motor, verbal, attention, memory), and those that examined the effects of CPAP but did not include an examination of OSA vs. control at baseline, were excluded.
Reviewing procedure and data extraction
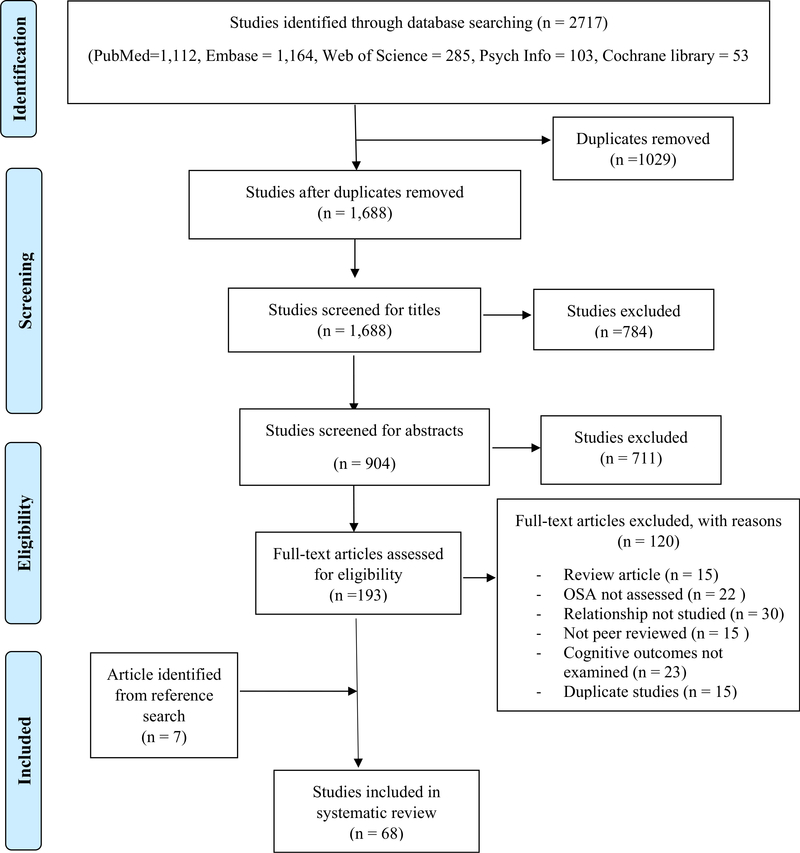
Independent examination of all titles and abstracts of identified eligible studies by the search strategy was performed by two authors (OB and RO) using EndNote X7. Where there were discordant decisions regarding inclusion, a resolution was reached by two other authors (AA and AV). Two authors (OB and MH) performed data extraction for each reference. Extracted fields included authors, year of publication, study design, study population, age, exposure and outcome assessment, statistical analytic methods used, covariates, and the main findings of the study. Two other authors (OU and AT) resolved discrepancy in the information extracted. Reviewers were not blinded to the authors or institutions. Fig. 1 shows a summary of the study selection and retrieval process.
Fig. 1.
Study retrieval and selection for obstructive sleep apnea, cognition and Alzheimer’s disease systematic review.
Assessment of study quality
We assessed the quality of included studies in this review, using an adaptation of the modified version of the Newcastle-Ottawa scale for quality assessment of observational studies [43], with addition of new items relevant to this review. Parameters used for the quality assessment included well-specified hypothesis, study design type, appropriately described sample, sample size, assessment and definition of OSA, cognitive impairment or AD, statistical analytic methods used, and approach used to adjust for potential confounders (see Table S2 in supplementary material). We utilized a star rating system with increasing number representing increasing quality, distinguishing low quality (<50% of the maximum number of stars), medium quality (≈55–70% of the maximum number of stars), and high quality (70% or more of the maximum number of stars). In general, majority (44 {65%}) of the studies were considered to be of high quality, 21 (31%) were of medium quality, and three (4%) were of low quality. Selection bias related to sampling, measurements of sleep and/or AD solely based on self-report and insufficient adjustment for core confounders were the main limitations (See Tables S3–S4 in supplementary material).
Age classifications
For the purpose of this study, included manuscripts were stratified by age (mean) of their study population. Young and middle-aged adults refers to ages 30–60 y; and older adults refers to ages >60 y.
Strength of association interpretation
Effect sizes from some of the reviewed studies included odds ratios (OR), hazard ratios (HR), Pearson’s correlation coefficient (r), beta estimates (β) and standardized mean differences (d). For purposes of interpretation of whether the associations observed were either weak to strong, we converted the different indices to a common index (see Table S5 in supplementary material for conversion formulae) [44]: d = 0.2 was considered a ‘weak’ effect size, 0.5 represents a ‘medium’ effect size and 0.8 a ‘strong’ effect size [45]. Where effect sizes were absent, an overall qualitative assessment incorporating parameters used for the quality assessment enabled result comparisons and interpretation between studies.
Results
OSA and cognition (cross-sectional studies)
Young and middle-aged adults
Table 1.1 contains the summary findings from studies that examined the association between OSA and cognition at a single time point in young and middle-aged adults. Altogether, there is substantial evidence suggesting weak to strong associations between OSA and cognitive performance on some measures of attention [46–49], memory [46,50–55], reaction time [55,56], psychomotor vigilance [55,57], information processing speed [49] and executive function [46,47,49,50,53–55]. Explanations and plausible mechanisms responsible for these findings in the middle-aged include daytime sleepiness or drowsiness from fragmented sleep because of frequent apneic episodes [58–60] and neurological damage due to intermittent hypoxia [61,62]. Specifically, deficits of attention and memory may be due to fragmented sleep and excessive daytime sleepiness [48,63], while motor function, executive function, reaction time and vigilance may be related to the severity of hypoxemia [64–66]. For example, in those studies where middle-aged adults with OSA who complained of EDS were compared to healthy controls, scores in memory and attention were consistently lower than normal [48,51,67]. Furthermore, correlation analysis revealed that EDs correlated with attention while nocturnal hypoxemia correlated with executive function and visual-constructive abilities [67]. However, a study that directly compared the effects of acute intermittent hypoxia (IH) versus sleep fragmentation (SF) 24 h following acquisition of the Morris water maze in rodents, demonstrated preservation of subsequent spatial memory following IH, but significantly worsened following SF [68].
Table 1.
Descriptive study characteristics and main findings: Obstructive sleep apnea and cognition.
| Authors, Year Published | Study Design, Setting (Study Quality) | Subjects |
Cognitive Domains | Adjusted Variables | Major Findings | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls |
OSA+ |
Gender | SDB Severity | |||||||
| N | Age | N | Age | |||||||
| OSA and Cognition (Cross-sectional Studies) Middle Aged (Mean Age: 30–59) | ||||||||||
| Alchantis et al., 2008 [56] | Cross-sectional, sleep clinic | 41 | 49 (33–63) | 58 | 49 (32–65) | N/A | AHI: range 31–137 OSAS; Range = 1–7 NC | Reaction time | Age, AHI, SaO2 min/mean, BMI | OSA patients, age ≥ 50 had decreased reaction times. Effect not seen in age <50. |
| Bawden et al., 2011 [46] | Cross-sectional | 20 | 27.6 (8.7) | 17 | 41.4 (13.0) | N/A | AHI: 5–15/>15–30/>30 | Global function, attention, memory, executive function | Age, education | OSA individuals had significantly impaired cognitive performance |
| Hrubos-Strom et al., 2012 [50] | Cross-sectional, community | N/A | N/A | 290 | 48.2 (11.2) | 162M 129F | AHI:<15/≥15 | Memory, Executive function | Age, sex, education | OSA severity significantly associated with cognitive function |
| Kloepfer et al., 2009 [51] | Cross-sectional | 20 | 47.4 (5.6) | 15 | 46.4 (5.9) | 22M | Clinical diagnosis: AHI >5 and questionnaire | Memory, global | Age, sex, IQ | Moderate OSA associated with impairment of procedural and verbal declarative memory |
| Mathieu et al., 2008 [47] | Cross-sectional, sleep clinic | Younger: 12 Older: 18 | Younger: 38.9 (2.2) Older: 62.5 (1.8) | Younger: 14 Older: 14 | Younger: 37.7 (2.0) Older: 62.3 (2.0) | 26M 2 F | AHI: 51 (4) OSAS younger; 43 (4) OSAS older | Attention, Executive Function, Memory | Age, education | ■No Group-by-Age interaction for cognitive performance. ■Main effects for both Group and Age |
| Naegele et al., 1995 [32] | Cross-sectional | 17 | 49 (3) | 17 | 49 (3) | All men | RDI > 10 | Attentional capacity, memory efficiency, long-term memory, frontal lobe function | Age, education, verbal IQ | Memory deficits were associated with the number of apneas and hypopneas per hour of sleep |
| Naegele et al., 2006 [52] | Cross-sectional | 95 | 47.4 | 95 | 48 | 144M 46F | RDI ≥ 10 | Memory | Age, education | OSA associated with impaired episodic memory, overall procedural memory performance, specific working memory capabilities |
| Nikodemova et al., 2013 [53] | Cross-sectional, community | n: 1148 | AHI 5–14 n: 298 AHI ≥ 15 n: 399 N: 1845 | 56.6 53.9 (10.1) age range: 30–81 | 1081M | AHI: <5/5–14/≥ 15 | Memory, Executive function | Age, sex, education, BMI | ■No significant association between SDB and cognitive performance in APOE4− ■AHI ≥15, APOE4+ associated with ↓cognitive function | |
| Salorio et al., 2002 [54] | Cross sectional | 24 | 44.2 (8.5) | 28 | 44.0 (7.9) | 34M 18F | Hypoxic episodes: 5–20/21–35/>35 episodes per hour | Long-term Learning and Memory, Executive control | Sex, education, IQ | OSA individuals exhibited poorer recall, less efficient semantic clustering, poorer use of semantic cues |
| Sharma et al., 2010 [55] | Cross-sectional | 25 | 45.6 (6.2) | 50 | 43 (7.5) | 63M 12F | AHI > 30 | Alertness, working memory, response inhibition, problem solving, executive function | Age, sex, education | ■OSA subjects had significantly impaired performance on tests on alertness, working memory, response inhibition, problem solving, executive function ■Significance disappeared after adjusting for delayed information processing |
| Quan et al., 2006 [49] | Cross-sectional, community | 74 | 57.4 (9.2) | 67 | 59.4 (9.2) | 84M 57F | AHI: ≤5 | Attention and vigilance, processing speed, executive function, motor learning, visuospatial ability, memory | Age, sex, education | No significant impact of OSAH and neuropsychological function ↑hypoxemia associated with ↓motor and processing speed |
| OSA and Cognition (Cross-sectional studies) Older Adults (Mean Age: 60 and older) | ||||||||||
| Berry et al., 1990 [69] | Cross-sectional, sleep clinic | 12 | 68.4 (2.2) | 8 | 68.6 (4.8) | Men only | AHI: 28 (12) OSAS; 3 (3)NC | Global, IQ, Memory | N/A | ■OSAS: ↓nonverbal IQ and nonverbal memory delayed recall |
| Boland et al., 2002 [74] | Cross-sectional, community | N/A | N/A | 1700 | 62 (range 52–75) | 837M | RDI median range: 0.4–23 | Attention, Executive Function, Memory | age, education, occupation, field center, diabetes, hypertension, body-mass index, CNS meds, alcohol use | ■No relationships |
| Blackwell et al., 2011 [70] | Cross-sectional, population-based | N/A | N/A | 2909 | 76 (6) | Only men | AHI: <5/5–14/≥ 30 | Global, Executive Function | Age, race, clinic, BMI, IADL, CVD comorbidities, antidepressant use, benzodiazepine use, depressive symptoms, education, alcohol use, smoking, physical activity, self-reported health | ■↓ time in REM, ↑ time in Stage 1 sleep, and ↑nocturnal hypoxemia are associated with poorer cognition |
| Foley et al., 2003 [75] | Cross-sectional, community | N/A | N/A | 718 | Range: 79–97 | Men only | 71% had AHI≥5; 19% had AHI ≥ 30 | Global, Attention, Executive Function, Construction, Memory, Language | Age, education, marital status | ■No relationships |
| Hayward et al., 1992 [76] | Cross-sectional, community | N/A | N/A | 96 | 78 (3.9) | 21M | RDI: 6 (6) | Attention, Executive Function, Memory, Language, Motor | Age, education | ■RDI not associated with memory, verbal, or motor factors |
| Ju et al., 2012 [71] | Case-control, sleep clinic | 21 | 68.7 (5.5) | 42 | 68 (4.4) | 37M 26F | AHI: Controls:< 15. OSA: >15 | General cognitive/intellectual ability, executive function | Age, sex, education, BMI | ■Significant findings with delayed recall and executive function |
| Kim et al., 2007 [57] | Cross-sectional, community | 395 | N/A | AHI: 5–15 (N = 127) AHI: >15 (N = 90) | 67.4 (3.8) | 346M 265W | AHI: Controls:< 5. OSA: 5–15 OSA: > 15 | psychomotor vigilance task (PVT) | sex, and BMI | ■↑AHI associated with PVT |
| Phillips et al., 1992 [77] | Cross-sectional, community | N/A | N/A | 92 | 64.2 (8.6) | 44M 48W | AHI: 3 (4) | Global, IQ, Attention, Executive Function, Memory, Language, Motor | None | ■No relationship between AHI and cognition |
| Sforza et al., 2010 [78] | Cross-sectional, community | 382 | Combined exposure and controls: 68 (1.8) | 445 | Combined exposure and controls: 68 (1.8) | 343M 484F | AHI: 20 (15); 53% had AHI≥15 | Global, Attention, Executive Function, Memory, Language | gender, BMI, diabetes, hypertension, education, anxiety, depression, self-reported sleep time | No significant associations |
| Spira et al., 2008 [72] | Cross-sectional, community | 391 | 82.7 (3.3) | 57 | 83.6 (4.3) | Women only | AHI: <30/≥ 30 | Global, Executive Function | Age, Education, SSRI (BMI and functional impairment were found to not have a significant impact on results) | ■↑AHI& ↑hypoxemia& ↑central apnea associated with cognition |
| Yesavage et al., 1985 [73] | Cross-sectional, sleep clinic | N/A | N/A | 41 | 69.5 (6.5) | Men only | RDI: 26 (30); 73% had RDI>5 | Attention, Executive Function, Memory | Age, depression, education, typical sleepiness/fatigue | ■RDI associated with attention and executive function |
| OSA and Cognition (Longitudinal studies) Older Adults (Mean Age: 60 and older) | ||||||||||
| Blackwell et al., 2015 [79] | Longitudinal, community based | 1504 | 75.8 (5.3) | 1132 | 76.4 (5.2) | Men only | ODI: <15, ≥15 AHI: <15, ≥15) | Global | Age, site, race, BMI, education, depressive symptoms, CVD comorbidities, Parkinson’s disease, IADL, benzodiazepine use, antidepressant use, self-reported health, physical activity, alcohol intake, smoking | ■No significant association between AHI and cognitive decline ■Modest association of nocturnal hypoxemia with global cognitive decline |
| Lutsey et al., 2016a [81] | Longitudinal | 521 | 60.7 (5.1) | 445 | 62 (4.9) | 435M 531F | AHI: <5/5–14.9/15–29.9/≥30 | Global | Age, sex, field center, education, alcohol intake, smoking, physical activity, APOE4, BMI, CRP, CVD comorbidities | No association between OSA and cognitive decline |
| Martin et al., 2015 [80] | Longitudinal, population based | N/A | N/A | 599 | 67.0 (1) | 224M 335F | AHI: <15/15–30/>30) | Global | Age, sex, education, follow-up length, BMI, ESS, CVD comorbidities, anxiety, depression | AHI indices associated slightly with decline in attention. No association with changes in executive and memory function |
| OSA and Cognition (RCT studies) Middle Aged (Mean Age: 30–59) | ||||||||||
| Canessa et al., 2011 [82] | Controlled clinical trial | 15 | 42.15 (6.64) | 17 | 44 (7.63) | All men | AH1: ≥ 30 | Memory, executive functions, attention, constructional abilities, abstract reasoning, vigilance, ESS | Age, education | OSA group had improved neurocognitive function after three months of CPAP |
| Castronovo et al., 2009 [83] | Controlled clinical trial | 14 | 42.15 (6.64) | 14 | 43.93 (7.78) | All men | AHI > 30 | Working memory, Brain activation (fMRI) | Age, education | OSA associated with decreases in activation after treatment |
| Castronovo et al., 2014 [84] | Controlled clinical trial | 15 | 42.15 (6.64) | 13 | 43.23 (7.63) | All men | AHI ≥ 30 | Global function, memory, attention, vigilance, abstract reasoning, visuospatial, verbal | Age, education | Almost complete reversal of white matter abnormalities after 12 mo of CPAP. Significant improvements in neuropsychological function |
| Ferini-Strambi et al., 2003 [67] | Controlled clinical trial | 23 | 55.8 (5.4) | 23 | 56.6 (6.1) | 40M 6F | AHI: Controls: <5, OSA: ≥5–40 | Processing speed, language, executive function, motor learning | Age, education | 15 d of CPAP treatment returned only visuospatial and motor skills to normal |
| Kushida et al., 2012 [85] | Controlled clinical trial | 542 | 50.8 (12.2) | 556 | 52.2 (12.2) | 719M 379F | AHI | Reaction time, Attention, Psychomotor vigilance, Memory | Age, Sex, Race, BMI & Sleep Study covariates | CPAP use resulted in mild, transient improvement in executive and frontal-lobe function in severe OSA |
| Saunamaki, Himanen, et al., 2009 [86] | Controlled clinical trial | 15 | 44 Range: 30–63 | 15 | 50 Range: 37–59 | All men | AHI:≤5/> 10 | WAIS-R | Age, education | OSAS associated with mild visually based cognitive dysfunction and reduced amount of sleep in the right hemisphere even after CPAP |
| Saunamaki et al., 2010 [87] | Controlled clinical trial | 17 | 44 Range: 30–63 | 20 | 50 Range: 37–65 | All men | AHI:≤5/> 10 | WAIS-R; Short term memory, working memory, verbal fluency, visuomotor tracking, visuospatial organization | Age, education, IQ | OSAS did not show any improvement on executive or visuospatial function even after long-term CPAP treatment |
| OSA and Cognition (RCT studies) Older Adults (Mean Age: 60 and older) | ||||||||||
| Aloia et al., 2003 [93] | Controlled clinical trial, clinic | Noncompliant | Compliant | N/A | RDI: 51 (20) OSAS compliant; 46 (22) OSAS noncompliant | Attention, Executive Function, Construction, Motor Speed, Memory, Language | Age, education, sleep apnea severity | ■↑RDI related to↓ verbal delayed recall memory ■↑sleep fragmentation and hypoxemia associated with↓ verbal delayed recall memory ■Cognitive benefits with CPAP compliance | ||
| 6 | 64.8 (2.6) | 6 | 64.8 (6.4) | |||||||
| Dalmases et al., 2015 [90] | Controlled clinical trial, community | 16 | 71.9 (6.0) | 17 | 70.8 (5.1) | 23M 10F | AHI: 55.49 (17.63) Control: 49.46 (15.75) CPAP: 61.16 (17.86) | Episodic, short-term memory, executive function, mental flexibility | Age, education | ■CPAP use associated with ↑ cognitive functioning ■CPAP use associated ↑ connectivity in the right middle front gyrus↑ and ↓cortical thinning |
| Martinez-Garcia et al., 2015 [91] | Controlled clinical trial, clinic | 109 | 75.6 (4.0) | 115 | 75.4 (3.8) | 153M 71F | AHI >30 | Executive function, visual attention, speed of processing, mental flexibility, and working memory | Age, BMI, sleep apnea severity | ■CPAP use associated with ↑quality of life, ↓ sleep-related symptoms, ↓ anxiety and depression, and ↑cognitive functioning in some areas |
| McMillan et al., 2014 [92] | Controlled clinical trial, clinic | 138 | 71.3 (4.6) | 140 | 70.9 (4.7) | 229M 49F | ODI: Control: 27.9 (18.5) CPAP: 29.4 (19.7) | Global cognition, TMT, digital symbol substitution test, reaction time | Age, sex, BMI, ODI | ■CPAP use improved daytime sleepiness ■No significant association between CPAP use and cognitive function, mood, functionality, nocturia, accidents, or cardiovascular events |
| OSA and Cognition (Quasi-experimental Study) Older Adults (Mean Age: 60 and older) | ||||||||||
| Richards et al, 2019 [94] | Clinical trial, community and clinic | 25 | 73.2 (8.6) | 29 | 67.4 (7.2) | 30M 24F | AHI ≥ 10 | Global cognition, attention, memory | Age, race, marital status | ■CPAP use was associated with ↑psychomotor/cognitive processing speed |
Abbreviations: Aβ40/42, amyloid beta-40/42; AD, Alzheimer’s disease; AHI ≥ 15, apnea hypopnea index of 15 or more events per hour of sleep; APOE, apolipoprotein epsilon4; BMI, body mass index, CDR, clinical dementia rating, CPAP, continuous pulmonary ambulatory pressure, CRP, c-reactive protein, CSF, cerebrospinal fluid; CVD, cardiovascular disease, DSM-IIIR/IV-TR, diagnostic and statistical manual of mental disorders; third edition/fourth edition, text revised; EDS, Excessive daytime sleepiness; ESS, Epworth sleepiness scale; F, female, GDS, global dementia scale, ICD-9/10, international classification of diseases ninth/tenth edition AD criteria; IADL, instrumental activities of daily living, IQ, intelligence quotient, M, male, MCI, mild cognitive impairment, MRI, magnetic resonance imaging; N, number of participants; NA, not applicable; N/A, not available; NC, normal cognition, NINCDS-ADRDA, national institute of neurological and communicative disorders and stroke and the Alzheimer’s disease and related disorders association; ODI: oxygen desaturation index; OSA, obstructive sleep apnea, OSAS, obstructive sleep apnea syndrome; RCT: randomized clinical trial; RDI, respiratory disturbance index, SaO2, saturated arterial oxygen, SCI, subjective cognitive decline, SDB, sleep disordered breathing; SSRI, selective serotonin reuptake inhibitor, TMT: trail making test; WAIS-R, Wechsler adult intelligence scale revised.
Older adults
Table 1.2 contains the summary findings from studies that examined the association between OSA and cognition at a single time point in late-life. Studies that restricted their populations to older adults (i.e., age 60 and older) generally show weaker, if any, links to impaired cognition [69–73]. Otherwise, most studies where potential confounders were accounted for showed null findings [74–78]. A seminal meta-analysis [41] of several of these studies that examined the association between OSA and cognition at a single time point in late-life including cognitive normal older adults mean age of 68.5 ± 3.9 y (range 55–82 y), showed a small negative association between OSA severity and combined measures of cognition as well as in processing speed and memory. However, this effect appeared to be driven by publication bias, with small case–control studies from sleep clinic populations observing the greatest associations [41], while larger cohort studies from community samples demonstrating no effects. OSA presenting with EDS could also drive this disparity, such that chronic or acute sleep loss could affect cognition both transiently and chronically, especially if the sleepiness is maintained through recurrent sleep restriction. An interpretation by the same authors is that the link between OSA severity and impaired cognition may be most pronounced in those seeking specialist assessments while absent in asymptomatic older adults or those with unrecognized symptoms.
OSA and cognition (longitudinal studies)
Older adults
Table 1.3 contains the summary findings from three studies that examined the association between OSA and cognition longitudinally in late-life. In the osteoporotic fractures in men (MrOS) study [79] a population-based prospective cohort that followed 2636 community-dwelling cognitively normal older men with a mean age of 76.0 ± 5.3 y for approximately 3-y, there was a modest association between nocturnal hypoxemia and subsequent decline in a global measure of cognition. In the prognostic indicator of cardiovascular and cerebrovascular events (PROOF) study [80], a population-based cohort that followed 559 community-dwelling cognitively normal older adults aged 67 at the study entry, after a follow-up period of approximately eight years; the AHI was associated with a slight decline in attention, which was more evident in subjects with severe OSA. In contrast, in the atherosclerosis risk in communities study (ARIC) study (81), which included a subset of 966 individuals who participated in the sleep heart and health cohort with a mean age of 61 at study entry, after a follow-up period of approximately 15 y, no evidence that OSA severity or nocturnal hypoxemia was associated with subsequent cognitive decline was found. The relationship between midlife OSA and later life cognition was also null. All three cohorts had several strengths including their large sample sizes, longitudinal study design, and use of extensive clinical and neuropsychological assessment. In addition, two studies used measurement standardization with z-scores [80,81], which has recently been deemed a more accurate method of minimizing measurement error. However, some methodological issues must be mentioned. First, the studies made use of healthy populations for which strict inclusion criteria were applied (e.g., excluding subjects with mild cognitive impairment, or including non-health-seeking asymptomatic community samples), which precluded the generalization of their data to clinical samples. Second, while cognitive assessments in these studies were performed at baseline and follow-up, neither a clinical evaluation nor ambulatory respiratory monitoring were available at the follow-up cycles. Third, some differences between the subjects examined at follow-up and those excluded after the first cognitive and/or at-home polygraphic studies were found [81] i.e., the AHI and indices of hypoxemia of the lost to follow-up/excluded subjects were more severe, and they differed in the rates of obesity and hypertension.
OSA and cognition (randomized controlled trials {RCTs})
Young and middle-aged adults
Table 1.4 contains the summary findings from studies examining the effect of CPAP treatment (short and long-term) on cognition in middle-aged OSA patients. The seven studies [67,82–87] analyzed showed that both short and long-term CPAP treatment improved some of the deficits associated with OSA in young and middle-aged adults, but there is substantial heterogeneity in the outcomes. It appears that CPAP is associated with improvements in attention and vigilance, but deficits in other domains tend to persist, despite treatment [88,89]. For example, after 3-months of CPAP treatment, Canessa et al. [82] observed significant improvements involving memory, attention, and executive functioning in OSA patients, while Saunamaki et al. [87] found no improvements in OSA patients’ visuospatial organizational skills or their mental set-shifting performance, after a 6-months treatment. Castronovo et al. [84] employed diffused tensor imaging (DTI) to examine changes in white matter (WM) integrity and cognition following CPAP treatment in severe OSA patients. Post-treatment, limited changes in white matter were seen after three months and an almost complete reversal of WM abnormalities was observed over the 12 mo. Additionally, significant improvements in memory, attention, and executive-function paralleled WM changes after treatment. These findings suggest that cognitive impairment seen in middle-aged patients with moderate to severe OSA may be due to damage of brain areas involved in those tasks, and highlight the potential prevention and/or therapeutic implications of CPAP, necessitating the need for providers to promptly diagnose and treat OSA patients at risk for cognitive impairment or AD. However, we should interpret the DTI-related WM changes over 3 and 12 mo of PAP treatment with caution. WM assessment in this study was exclusively done by DTI. Other WM estimates related to WM hyperintensities volume or vascular imaging were lacking.
Older adults
Table 1.5 contains the summary findings from studies examining the effect of CPAP treatment on cognition in older adult OSA patients. We identified four of such studies [37,9–92]. A similar picture emerges, with single-center small trials (n = 12 and n = 33) showing improvements in attention, psychomotor speed, memory and executive function [90,93], in clear contrast to the larger, multicenter clinical cohorts (n = 224 and n = 278) where CPAP shows significant improvement only in working memory or null effects [91,92] (although the low adherence and short CPAP usage {2 h} in the later negative trial might have diluted the treatment effect). In addition, participants were starting from a very high baseline. Therefore, the “null” effect may, in reality, be a ceiling effect [92]. It is also important to note that much of the literature employs a liberal definition of “high CPAP adherence”, therefore some of the limited benefits to CPAP treatment may be due to “high adherence” groups being too low in absolute adherence. In addition, baseline OSA severity may play a role as well, such that an individual with severe OSA and a high adherence to CPAP treatment may have greater benefits compared to another individual with mild to moderate OSA and a high adherence rate.
OSA and cognition (quasi-experimental study)
A quasi-experimental study with two comparison groups (pooled mean age of 70.1 ± 7.9 y): 1) an MCI, OSA, and CPAP-adherent group (MCI + CPAP, ≥4 h mean CPAP use per night for 1 y, n = 29); and 2) an MCI, OSA, CPAP-non-adherent group (MCI −CPAP, <4 h mean CPAP use per night for 1 y, n = 25), demonstrated significant improvements in psychomotor/cognitive processing speed in the MCI + CPAP group vs the MCI −CPAP group after adjustment for age, race, and marital status [94]. Moderate improvements were also observed for memory and everyday function at six months, and attention, daytime sleepiness, at one year in the MCI + CPAP group [94].
Summary on OSA and cognition
In young and middle-aged adults, cross-sectional studies have demonstrated that OSA is often associated with cognitive impairment. Longitudinal studies testing whether OSA in mid-life precedes cognitive decline are rare. Intermittent hypoxia and sleep fragmentation are the most likely cause of these cognitive and brain structural deficits in middle-aged OSA patients, with both short and long-term CPAP treatment improving certain cognitive domains. In contrast, cross-sectional and longitudinal studies in older adults show highly variable OSA-cognition associations, depending on the study type and setting, with small sleep clinic populations (i.e., more symptomatic patients) driving most of the positive findings. The characteristic lack of EDS in some older adults with OSA might decrease the sensitivity of standard cognitive tests as well as explain the negative findings. Other potential confounders’ specific to older adults are heterogeneity of OSA duration prior to evaluation, cognitive reserve, age-associated cognitive decline, survival bias, presence of prodromal AD, cerebrovascular disease or insulin resistance and diabetes, among others. Lastly, it is important to note that the majority of studies examining OSA’s role on cognitive memory have exclusively employed daytime tests, which do not provide much opportunity for sleep-dependent processing or consolidation to occur, in which opportunities for encoding and recall are separated by a period of sleep with or without OSA.
OSA and MCI/AD (cross-sectional studies)
Older adults
Table 2.1 contains the summary findings from two studies that examined the association between OSA and MCI at a single time-point in late-life. Dlugaj et al. [95] using a community-based sample, found no association between mild cognitive impairment (MCI) or any of its MCI sub-types and OSA-severity (the prevalence of OSA in patients with and without MCI was 27% and 26%, respectively) [95]. Kim et al. [96] using a clinic-based sample also found no association between MCI and the AHI indices (although the prevalence of OSA in patients with and without MCI was 77% and 73% in this case) [96]. Higher AHI however, was associated with lower language test performance among individuals with MCI but not among controls.
Table 2.
Descriptive Study Characteristics and Main Findings: Obstructive Sleep Apnea, and Mild Cognitive Impairment (MCI) and/or Alzheimer’s disease (AD).
| Authors, Year Published | Study Design | Subjects |
OSA assessment | Cognitive Domains | Adjusted Variables | Major findings | ||
|---|---|---|---|---|---|---|---|---|
| N | Age (Mean ± SD) | Gender | ||||||
| OSA and MCI/AD (Cross-sectional Studies) Older Adults (Mean Age: 60 and older) | ||||||||
| Dlugaj et al., 2014 [95] | Cross- sectional, population-based | 1793 | 63.8 (7.5) | 919M 874F | AHI | Memory, executive function | Age, sex, education | ■ SDB not associated with MCI or MCI subtypes (amnestic and non-amnestic) |
| Hoch et al., 1986 [97] | Cross-sectional | 80 | 71.5 (8.1) | 33M 57F | AHI | DSM-III | None | Significant association |
| Hoch et al., 1989 [99] | Cross-sectional | 27 | 74.5 (5.1) | 7M 20F | AHI | NICNDS-ADRDA, DSM-III | N/A | No association between OSA and dementia |
| Kim et al., 2011 [96] | Cross-sectional, clinic | 30 | 67.4 (3.8) | 42M 18W | AHI | Executive function, Language, Memory, Visuospatial construction | N/A | ■ ↑AHI associated with language with MCI |
| Reynolds et al., 1985 [98] | Cross-sectional | 61 | 69.7 (6.8) | 19M 42F | AI, AHI | DSM 3, Hamilton rating, Folstein score, and a modified Hachinski Ischemia score | Gender | Significant association between sleep apnea and dementia in women |
| Reynolds et al., 1987 [100] | Cross-sectional | 30 | 73.3 (9.1) | 3M 12F | 24 Channel polygraphs | DSM 3, Hamilton rating, Folstein score, and a modified Hachinski Ischemia score | N/A | No association between OSA and dementia |
| Smallwood et al., 1983 [101] | Cross-sectional | 55 | Range: 23–81 y | 45M 10F | AHI | DSM 3, neurological examination | Age, sex | No relationship between dementia and apnea severity |
| OSA and AD, All-cause MCI or Dementia (Longitudinal studies) | ||||||||
| Middle Aged (Mean Age: 30–59) | ||||||||
| Chang et al., 2013 [107] | Longitudinal, community based | Controls 7070 OSA 1414 | 55.5 (4.78) | M: 5034 F: 3450 | Clinical diagnosis (according to AASM guidelines) | ICD-9 CM Dementia diagnosis | Age, sex, CVD comorbidities, urbanization level, income | OSA was associated with increased Dementia risk than for the comparison group, and is an age, time, and gender dependent. |
| Older Adults (Mean Age: 60 and older) | ||||||||
| Lee et al., 2019 [122] | Longitudinal, Community | Controls: 3635 SDB: 727 | Range: 40–79 | M: 3332 F: 1030 | NHIS record of clinical diagnosis | ICD-10:G30 | Sex, age, CVD, hypertension, Type 2 DM, depression, BMI, smoking status, physical activity, and drinking | Those with SDB were 1.575 times more likely to develop AD |
| Lutsey et al., 2018 [105] | Longitudinal, Community | Controls: 849 OSA: 1100 | 63 (5.4) | M: 1073 F: 876 | Home PSG | TICSm, hospitalization codes). Neurocognitive exam | age, sex, field center, education, physical activity, ethanol intake, smoking status, leisure time physical activity, and APOE e4, BMI | Late-midlife OSA was associated with all-cause and Alzheimer’s disease dementia in later life. |
| Osorio et al., 2015 [106] | Prospective | 2285 | 74 (6.6) | 1101F | Self-reported | Self-report; diagnosis by clinician | APOE e4 status, sex, education, BMI, depression, cardiovascular disease, hypertension, diabetes, and age | Significant association between SDB and earlier age at cognitive decline |
| Yaffe et al., 2011 [103] | Longitudinal, Community | Controls: 193 SDB:105 | 82.3 (3.2) | Women only | AHI: ≥15 | Global, Attention, Executive Function, Memory | Age, race, BMI, education, smoking, diabetes, hypertension, antidepressant use, benzodiazepine use, non-diazepam anxiolytics use | ■ SDB: ↑hypoxemia had ↑risk of developing MCI or dementia over five year follow-up Sleep fragmentation and duration not associated with cognition |
| Yaffe et al., 2015 [104] | Longitudinal | AD: 4107 Dementia: 14380 | 67.7 (1.1) | Men Only | Not specified; clinical diagnosis | AD & Dementia (classified using ICD-9 codes) | Age, CVD comorbidities, obesity, depression, income, education | Those with a sleep apnea had a 20% and 27% increased risk for AD and dementia respectively |
| OSA and Cognition (RCT studies) | ||||||||
| Older Adults (Mean Age: 60 and older) | ||||||||
| Ancoli-Israel et al., 2008 [125] | RCT | 52 | 78.2 (7.2) | 39M 13F | Rechtschaffen and Kales criteria | Neuropsychological test battery | None | CPAP improved some cognitive functioning |
| Chong et al., 2006 [126] | RCT | 39 | 78.0 (7.04) | 29M 10F | RDI | NINCDS-ADRDA criteria | None | CPAP reduces sleepiness in those with AD and OSA |
| Cooke et al., 2009a [123] | RCT | 52 | 77.8 (7.3) | 39M 13F | Rechtschaffen and Kales criteria | NINCDS-ADRDA criteria, MMSE | None | After one night of CPAP use: deeper sleep, affects for three weeks |
| Cooke et al., 2009b [124] | RCT | 10 | 75.7 (5.9) | 7M 3F | AHI, PSQI, ESS, FOSQ | Neuropsychological test battery | None | Sustained CPAP use associated with less cognitive decline |
| Moraes et al., 2008 [127] | RCT | 23 | Control: 72.6 (11.0) Treatment: 76.9 (6.2) | 8M 15F | Rechtschaffen and Kales and AASM criteria | ADAS-cog | None | Donepezil treatment in AD individuals: improved AHI, oxygen saturation, and sleep duration |
Abbreviations: AASM: American academy of sleep medicine; Aβ40/42, amyloid beta-40/42; AD, Alzheimer’s disease; ADAS-cog: Alzheimer’s disease assessment scale-cognitive; AHI ≥ 15, apnea hypopnea index of 15 or more events per hour of sleep; APOE, apolipoprotein epsilon4; BMI, body mass index, CDR, clinical dementia rating, CPAP, continuous pulmonary ambulatory pressure, CRP, c-reactive protein, CSF, cerebrospinal fluid; CVD, cardiovascular disease, DSM-IIIR/IV-TR, diagnostic and statistical manual of mental disorders; third edition/fourth edition, text revised; EDS, Excessive daytime sleepiness; ESS, Epworth sleepiness scale; F, female, FOSQ: functional outcomes sleep questionnaire; GDS, global dementia scale, ICD-9/10, international classification of diseases ninth/tenth edition AD criteria; IADL, instrumental activities of daily living, IQ, intelligence quotient, M, male, MCI, mild cognitive impairment, MMSE: mini mental state examination; MRI, magnetic resonance imaging; N, number of participants; NA, not applicable; N/A, not available; NC, normal cognition, NINCDS-ADRDA, national institute of neurological and communicative disorders and stroke and the Alzheimer’s disease and related disorders association; ODI: oxygen desaturation index; OSA, obstructive sleep apnea, OSAS, obstructive sleep apnea syndrome; PSQI: Pittsburg sleep quality index; RCT: randomized clinical trial; RDI, respiratory disturbance index, SaO2, saturated arterial oxygen, SCI, subjective cognitive decline, SDB, sleep disordered breathing; SSRI, selective serotonin reuptake inhibitor, TICSm: Telephone interview for cognitive status TMT: trail making test; WAIS-R, Wechsler adult intelligence scale revised.
Studies examining associations between OSA and AD diagnosis are scarce and were conducted in community samples and nursing homes 2 to 3 decades ago. Findings are conflicting with two studies [97,98] demonstrating a significant association between AD and higher OSA prevalence, while three had null associations [99–101]. Nonetheless, a recent meta-analysis of these studies concluded that the aggregate odds ratio for OSA in AD vs. healthy control was 5.05 and homogeneous [102]. In addition, higher AHI was associated with worse cognitive and functional status, suggesting that severity of OSA worsened in the more advanced stages of AD. Given the cross-sectional nature of these analyses, the data cannot be interpreted for direction of causality or temporality. However, it does suggest the possibility of a reverse causation between these two disorders with higher incidence of OSA as cognitive decline progresses from MCI to AD as well as the re-emergence of associations between OSA severity and cognitive impairment, in this case likely related to neurodegeneration in addition to EDS and neurological damage due to OSA.
OSA and MCI/AD (longitudinal studies)
Middle-aged to older adults
Table 2.2 contains the summary findings from studies examining the association between OSA and dementia outcomes longitudinally in middle-aged to late-life. These studies tend to be more consistent in their findings. Yaffe et al. [103] in their seminal prospective study of OSA and cognition in older adult women without dementia at baseline (overall mean age of 82.3 ± 3.2 y), who were a sub-study of the study of osteoporotic fractures (SOF) cohort and were followed for approximately five years, found that older adult women with OSA had an 85% higher risk of developing MCI/Dementia at follow-up vs. those without OSA. In another study, Yaffe et al. [104] examined the relationship between a diagnosis of sleep disturbance and dementia in older adult male veterans with a mean age 67.7 ± 1.1 y. Sleep disturbance was significantly associated with higher risk of dementia and specifically, in a sub-analysis that included OSA patients, there were significant associations with higher risk of AD, vascular dementia and other dementias combined. Lutsey et al. [105] tested the hypotheses that late-midlife OSA and short and long sleep duration are associated with dementia over 15 y of follow-up in participants from the ARIC study; OSA and sleep duration were not associated with risk of incident dementia, however when using adjudicated outcomes (i.e., syndromic dementia and MCI as adjudicated by an expert panel), severe OSA (≥30 vs. <5 apnea-hypopnea events/hour) was associated with higher risk of all-cause dementia and AD dementia, however, associations were attenuated after controlling for cardiovascular risk factors. Osorio et al. [106] in a retrospective study using the Alzheimer’s disease neuroimaging initiative (ADNI) data determined that OSA patients had an earlier onset age to MCI or AD, and that CPAP use delayed the age of MCI onset. This study’s main limitation is the use of self-report for clinical diagnosis of OSA and CPAP use. Furthermore, Chang et al. [107] in a prospective matched-control cohort study utilizing data from Taiwan’s Health Insurance Database estimated dementia risk in OSA versus non-OSA patients in individuals 40 y and older, followed for five years. Results from the study showed a 70% higher risk of developing dementia among OSA compared to non-OSA individuals. This study also demonstrated sex-dependent, age-dependent and time-dependent associations of OSA and dementia. OSA females relative to males, OSA males aged 50–59 relative to females aged 50–59, and OSA females aged ≥70 y relative to males aged ≥70 y, were all at a higher risk of developing dementia in the first 2.5 y of follow-up. Notably, consistent evidence show that OSA is more common in men than women in the general population with a male-to-female ratio of approximately 1.5:1 [9,11,108,109]. Anatomical and physiological differences such as upper airway stability, ventilatory response to chemical stimuli or higher abdominal or neck fat, make men more susceptible to OSA [110–114]. The sex differences in prevalence also remains in older adults [9,115] though the prevalence of OSA seems to be higher in post-versus premenopausal women [116], somewhat suggesting that hormonal-related effects may be important in OSA pathogenesis in women. In addition, studies show women having lower AHI, more partial obstruction and shorter events, more respiratory effort related arousal (RERA) events and upper airway resistance syndrome (UARS), less severe OSA in non-rapid eye movement (NREM) sleep, and a higher prevalence of rapid eye movement (REM) related sleep apnea events compared to men independent of age, weight and influence of medications, such as anti-depressants [114,117–119]. With CPAP treatment, improvement in apnea symptoms, neurobehavioral performance, mood state and functional status does not vary by sex [120], though reversion of elevated markers of systemic inflammation occurred faster in men than women, possibly suggesting sex differences in CPAP effects on cardiovascular risk factors [121]. However, the clinical relevance of these results as it relates to cognitive outcomes are still unknown. Finally, Lee et al. [122] in a study utilizing data from the national health insurance service-health screening cohort (NHIS-HEALS) estimated AD risk in OSA versus propensity matched non-OSA patients, followed for 10–13 y. OSA patients showed a 58% higher risk of developing AD compared to non-OSA individuals.
We note here that in the previous section of cross-sectional studies, associations between OSA and cognition in later life are highly variable and vary based on the type and setting of study. Moreover, the risk of bias from the studies reviewed renders the evidence inconclusive. In contrast, longitudinal studies in older adults that examined the association between OSA and dementia outcomes show more consistently that OSA is associated with development of MCI or AD. However, several of these studies used self-reports, medical records or administrative claims connoting a clinical diagnosis of OSA, which incorporates abnormal sleep breathing events alongside associated symptoms (e.g., EDS) that prompted these subjects to seek a diagnosis. Thus, the link between OSA and cognitive decline to MCI or AD in these cases might also be driven by those seeking specialist assessments. To conclude, although the results from the SOF cohort provide the strongest evidence to date supporting the hypothesis that OSA precedes dementia, the high prevalence of AD in this age group (mean age 82 at inclusion) and absence of AD biomarker assessments do not preclude the possibility of reverse causation.
OSA and MCI/AD (RCTs)
Table 2.3 contains the summary findings from studies examining the effect of CPAP on sleep parameters and cognition in AD patients with OSA. All five RCTs identified in this review included older adult participants (mean age >70) and reported significant improvements in slow wave sleep (SWS) [123], mood [124], cognition [124,125], EDS [126], and AHI [127] in OSA patients with AD. More specifically, in a randomized placebo-controlled trial, Cooke et al. [123] compared the outcomes of 3-weeks of CPAP treatment with 3-weeks placebo CPAP in patients with AD and OSA. Results showed significant improvements in SWS after one night, with the improved effect extending for three weeks. Chong et al. [126] examined the effect of CPAP on EDS in mild-moderate AD/OSA patients, finding that sleepiness was significantly reduced after CPAP treatment. Furthermore, Ancoli-Israel et al. [125] compared CPAP–treatment vs. placebo for three weeks in AD patients, demonstrating significant cognitive improvements in the treatment arm. Post-hoc analyses showed particular improvements in episodic verbal learning and memory and executive functioning (cognitive flexibility and processing speed). In addition, a doubleblind, placebo-controlled study examining the effects of donepezil, a central acetylcholinesterase inhibitor, on OSA in AD patients, found that compared to baseline and placebo, 3-months donepezil treatment significantly improved AHI and oxygen saturation. Furthermore, REM sleep duration was significantly higher and Alzheimer’s disease assessment scale-cognitive (ADAS-cog) scores significantly improved [127].
Notably, there are currently no RCTs of CPAP in MCI patients with OSA. An important limitation in these RCTs on AD patients pertains to the power to detect meaningful changes across treatment arms, with some studies being underpowered to make definitive assumptions on the causality of the cognitive improvements. Other limitations include the examination of sleep parameters post-hoc while the study was powered for changes in cognition, inability to make causal inferences due to non-random group assignment (continued use vs discontinuation of CPAP), limited validation of sleepiness scales in older adult patients with AD, and generalizability issues. Despite these limitations, there is sufficient evidence to conclude that CPAP treatment may be effective in improving cognition in OSA patients with AD and that more better designed RCTs should follow.
Summary on OSA and MCI/AD
In young and middle-aged adults, longitudinal studies examining the association between OSA and dementia outcomes are extremely rare for obvious reasons. Given that dementia is an outcome related to cognitive aging, it is understandable why more studies are conducted in the elderly than in young and middle-aged adults. However, since AD is considered a life-course disease and presence of preclinical AD occurs prior to the onset of symptomatic AD, longitudinal epidemiological studies with longer follow-up periods starting from young and middle-aged adults are needed. Cross-sectional studies in older adults that examined the association between OSA and MCI show null findings. In contrast, cross-sectional studies that examined the association between OSA and AD show an aggregate odds ratio in older adults for OSA in AD vs. healthy controls of 5.05 (95% CI: 2.4–10.6) [102], however, reverse causation is a possibility in these cases. Longitudinal studies in older adults that examined the association between OSA and cognitive decline outcomes more consistently show that OSA is often associated with development of MCI or AD but positive findings might be driven by OSA patients seeking treatment in a similar way as those studies reviewed under “OSA and Cognition”. RCTs provide an insight into the causal associations between OSA and AD and are more compelling. All RCTs were conducted in older adults and showed that CPAP treatment not only improved sleep parameters (e.g., SWS, EDS) in AD patients with OSA, but it also increased cognitive function. These findings provide evidence that AD patients (particularly mild to moderate) with OSA may benefit from CPAP treatment.
OSA and AD pathology/biomarkers (cross-sectional studies)
Young and middle-aged adults
Table 3.1 contains the summary findings from studies assessing the association between OSA and specific AD neuropathology at a single time point. We identified five such studies conducted in middle-aged participants. For interpretation purposes, higher brain amyloid or tau burden, higher cerebrospinal fluid (CSF) tau burden, and lower CSF amyloid burden signify worse outcomes. Yun et al. [128] examined whether moderate to severe OSA increased brain amyloid burden relative to healthy controls in 38 participants (mean age 58.5 ± 4.2 y) from the population-based Korean genome and epidemiology study. After adjusting for potential confounders, OSA patients had higher amyloid in the right posterior cingulate gyrus and right temporal cortex, relative to controls. Results from this study however, should be interpreted with caution given the small sample size, unilaterality of findings, small cluster size and lack of difference between groups in unadjusted analyses. In addition, most OSA and control subjects were PiB negative, which was expected given the age of the sample. Bu et al. [129] examined whether hypoxia indices (AHI, oxygen desaturation index, as well as mean and lowest oxyhemoglobin saturations) were associated with higher serum levels of Aβ, total tau (T-tau) and phosphorylated tau 181 (P-tau181) using enzyme linked immunosorbent assay (ELISA) in a sample of 49 OSA patients (14 patients with mild, 13 with moderate and 18 with severe) relative to 44 simple snoring matched controls (pooled mean age of 43.5 ± 9.8) from a sleep clinic. They concluded that significantly higher levels of serum Aβ40, Aβ42, T-tau and P-tau181 were present in OSA patients compared to controls, suggesting a contribution of intermittent hypoxia to these novel markers of AD pathogenesis. Similarly, Motamedi et al. [130] examined whether T-tau and other biomarkers of inflammation were related to OSA severity. T-tau, Aβ40, Aβ42, c-reactive protein (CRP), TNF-α, interleukin (IL)-6, and IL-10 were measured in blood and compared between 28 participants with moderate-severe OSA, 22 subjects with mild OSA, and 24 healthy controls. The cohort included a sample of young middle-age active duty military personnel males (pooled mean age of 34.5 ± 7.9), and total biomarker concentrations were determined from plasma samples using an ultra-sensitive detection method, single molecule array (Simoa™), while CRP was assayed by ELISA. In this case, T-tau and IL-6 concentrations were elevated in participants with moderate-severe OSA, compared to those with mild OSA and healthy controls. It is worth pointing out that serum/plasma Aβ is non-specific and brain-derived amyloid constitutes only a tiny fraction of blood soluble Aβ and should be interpreted with caution when using current Simoa or ELISA methods. While current plasma/serum tau assays do not correlate significantly with CSF T-tau or CSF P-tau, plasma tau levels may nonetheless be useful in predicting AD risk [131]. Both Aβ and tau in plasma need to be assessed in cohorts with different sociodemographic characteristics, and in longitudinal studies of subjects stratified by amyloid or tau positron emission tomography (PET) imaging, or by CSF Aβ and tau profiles, as well as correlated with neuropathology findings.
Table 3.
Descriptive Study Characteristics and Main Findings: Obstructive Sleep Apnea, and Alzheimer’s disease Pathology.
| Authors, Year Published | Study Design | Subjects |
OSA assessment | Alzheimer’s disease assessment | Adjusted Variables | Major findings | ||
|---|---|---|---|---|---|---|---|---|
| N | Age (Mean ± SD) | Gender | ||||||
| Cross-sectional Studies | ||||||||
| OSA and AD pathology (Middle Aged (30–60)) | ||||||||
| Bu et al., 2015 [129] | Cross-sectional | 94 | 43.62 (9.78) | 67M 27F | AHI, ODI, MSaO2, LSaO2 | Amyloid beta levels | Age, sex | Significant association between hypoxia and amyloid levels |
| Ju et al., 2016 [132] | Cross-sectional | 41 | Control: 53.2 (5.7) OSA: 56.4 (4) | 23M 18F | AHI | CSF amyloid beta levels | None | CSF amyloid beta decreased in OSA group |
| Ju et al., 2018 [133] | Cross-sectional/Interventional | 18 | 56.9 (8.3) | 12M 6F | AHI | CSF amyloid beta levels | None | Treatment and improvement of OSA associated with ↑ slow wave activity and ↓ amyloid beta |
| Motamedi et al., 2018 [130] | Cross-sectional | 74 | 33.6 (7.87) | 71M 3F | AHI | Total tau and IL-6 | BMI, age, race, gender, total sleep time, hypertension | Moderate-severe OSA: ↑ tau concentrations |
| Yun et al., 2017 [128] | Cross-sectional | 38 | 56.7 (4.0) | M: 18 F: 20 | AHI | Neuropsychological test battery | Age, sex, education, APOE genotype, sleep duration, hypertension, diabetes, BMI, exercise, depressive mood, smoking, and alcohol drinking | Significant association between OSA and amyloid deposition |
| OSA and AD pathology Older Adults (Mean Age: 60 and older) | ||||||||
| Handa et al., 2019 [140] | Cross-sectional | 14 | 65 | 10M 4F | AHI, lowest SpO2, TDS | MMSE, HDS-R, C-PiB PET | None | No association between severity of OSA and amyloid beta deposition |
| Ligouri et al., 2017 [137] | Cross-sectional | 50 | 66.96 (7.98) | 33M 17F | AHI | SCI classified by cognitive test performance | Age, education | Significant association between OSA and CSF AD biomarkers |
| Mendes et al., 2018 [139] | Cross-sectional | 318 | 76.07 (3.5) | 114M 204F | Self-reported clinical diagnosis | Neuropsychological assessment, hippocampal volumetry, WMH volumetry, PET, | Age, sex, educational level, ApoE4 status, WMH volume | Obesity and excessive alcohol are associated with ↓ FDG-PET values OSA and mood disorders are related to↓ amyloid-PET SUV ratios |
| Osorio et al., 2014 [134] | Cross-sectional | 95 | 67.6 | 37M 58F | AHI | Neuropsychological test battery | Age, BMI, time interval between sleep study and lumbar puncture, ApoE4 status | Significant association between SDB and AD CSF biomarkers |
| Spira et al., 2014 [138] | Cross-sectional | 13 | 71.6 (7.8) | 7M 6F | AHI, ODI | Neuropsychological tests, GDS, CDR | None | SDB severity associated with amyloid deposition |
| Prospective Studies | ||||||||
| OSA and AD pathology Older Adults (Mean Age: 60 and older) | ||||||||
| Bubu et al., 2019 [142] | Prospective | 1639 | OSA+: 72.3 (7.1) OSA−: 73.9 (7.3) | 948M 691F | Self-reported clinical diagnosis | MMSE, CDR, florbetapir-PET, CSF biomarkers | Age, sex, BMI, education, CPAP-use, ApoE4 status, alcohol intake, baseline biomarker data, history of respiratory disease, hypertension, diabetes, history of cardiovascular disease, and history of TBI | In NL and MCI individuals, OSA was associated with increases in amyloid burden by both CSF and PET imaging measures, and CSF concentration of both T-tau & P-Tau tau over 2.5-years |
| Lutsey, Norby et al., 2016b [141] | Prospective | 312 | 61.7 (5.0) | 145M 167F | AHI, SHHS Sleep Habits Questionnaire | Neurocognitive exam, brain MRI | age, sex, field center, education, physical activity, ethanol intake, smoking status, leisure time physical activity, and APOE e4, BMI | No relationship between mid-life OSA and dementia over 15-years |
| Sharma et al., 2018 [136] | Prospective | 208 | 68.5 (7.4) | 129F | AHIall AHI4% | Amyloid beta levels | Age, sex, BMI and APOE4 | Significant association between OSA severity and increased amyloid burden over 2-years |
Abbreviations: AASM: American academy of sleep medicine; Aβ40/42, amyloid beta-40/42; AD, Alzheimer’s disease; ADAS-cog: Alzheimer’s disease assessment scale-cognitive; AHI ≥ 15, apnea hypopnea index of 15 or more events per hour of sleep; APOE, apolipoprotein epsilon4; BMI, body mass index, CDR, clinical dementia rating, CPAP, continuous pulmonary ambulatory pressure, CRP, c-reactive protein, CSF, cerebrospinal fluid; CVD, cardiovascular disease, DSM-IIIR/IV-TR, diagnostic and statistical manual of mental disorders; third edition/fourth edition, text revised; EDS, Excessive daytime sleepiness; ESS, Epworth sleepiness scale; F, female, FOSQ: functional outcomes sleep questionnaire; GDS, global dementia scale, ICD-9/10, international classification of diseases ninth/tenth edition AD criteria; IADL, instrumental activities of daily living, IQ, intelligence quotient, M, male, MCI, mild cognitive impairment, MMSE: mini mental state examination; MRI, magnetic resonance imaging; N, number of participants; NA, not applicable; N/A, not available; NC, normal cognition, NINCDS-ADRDA, national institute of neurological and communicative disorders and stroke and the Alzheimer’s disease and related disorders association; ODI: oxygen desaturation index; OSA, obstructive sleep apnea, OSAS, obstructive sleep apnea syndrome; PSQI: Pittsburg sleep quality index; RCT: randomized clinical trial; RDI, respiratory disturbance index, SaO2, saturated arterial oxygen, SCI, subjective cognitive decline, SDB, sleep disordered breathing; SSRI, selective serotonin reuptake inhibitor, TMT: trail making test; WAIS-R, Wechsler adult intelligence scale revised.
Finally, two studies by Ju et al. [132,133] (one cross-sectional and one interventional) have demonstrated associations between OSA and AD pathology in middle-aged participants that originated from both a community-based registry and a sleep clinic. In the cross sectional study, Ju et al. [132] examined CSF AD biomarkers and other neuronal derived protein in a group of 31 control (AHI<5) and 10 moderate to severe OSA patients (AHI>15) (pooled mean age of 54 ± 5.3 y). Aβ40 and Aβ42, as well as T-Tau, P-Tau181, neurogranin, SNAP-25, and VILIP-1 (all neuronally derived proteins) were all lower in OSA patients. Also relevant, there was a significant negative correlation between slow wave activity (SWA) (as measured by delta power), CSF Aβ40 and Aβ42 (i.e., lower SWA was associated with higher CSF Aβ levels) which was not found in OSA patients. In the interventional study [133] SWA and CSF Aβ were measured in participants with OSA before and 1–4 mo after treatment with CPAP. OSA treatment increased SWA and normalized the inverse association between SWA and CSF Aβ levels.
Older adults
In older adults, several cross-sectional studies have demonstrated associations between OSA and AD pathology. Osorio et al. [134] examined the association between OSA severity, cerebrospinal fluid (CSF) AD biomarkers, and apolipoprotein e (APOE) alleles in a sample of 95 cognitively normal older adults (pooled mean age 67.6 ± 7.7) recruited from the community in a memory clinic setting, demonstrating an association between OSA and CSF AD-biomarkers. Intermittent hypoxia was associated with increases in CSF T-Tau, P-Tau and Aβ42 in ApoE3+ and a trend towards decrease Aβ42 levels in ApoE4+, suggesting that hypoxia may be responsible for changes in CSF AD biomarkers but this could be dependent to the different stages of (pre)clinical disease, genotype and OSA severity (see also Discussion). Results from this study should be interpreted with caution as the cohort examined contains significant overlap with subjects in which we also found negative associations between SWA and CSF Aβ42 (i.e., lower SWA was associated with higher CSF Aβ42 levels) [135]. In addition, differences in OSA-AD biomarker relationships by APOE alleles were not replicated at cross-section in a follow-up study that included the same subjects but in a larger dataset (n = 179) [136]. Liguori et al. [137] compared CSF Aβ42, tau proteins, and lactate levels in OSA versus CPAP treated OSA and controls in subjective cognitive impairment (SCI) participants admitted to a sleep clinic (pooled mean age 67.2 ± 8.1). They concluded that OSA patients had lower CSF Aβ2, higher lactate levels, and higher T-tau/Aβ42 ratio compared to controls and CPAP treated OSA patients, with both these groups having similar AD-biomarker levels. These findings suggest that OSA may effect early AD biomarker changes that may be susceptible to CPAP treatment. In a small study (n = 13) with cognitively normal and older adult MCI patients from the community in a memory clinic setting, Spira et al. [138] showed that greater OSA severity was associated with higher brain amyloid burden globally and regionally in the precuneus in MCI but not in normal older adults (n = 8), although OSA severity in the latter group was either mild or normal (AHI4 = 7.6 ± 8.2). Although the sample size was small, this study was able to demonstrate effects using objective measures of OSA and AD pathology, suggesting that the sample was sufficient to demonstrate effects if one truly existed. This pattern, with observed associations between higher amyloid deposition measured by amyloid PET and higher AHI in a feedforward cycle [136,138] suggests an increase in AD progression risk by OSA, as Aβ accumulation and OSA severity become increasingly abnormal. Recently, Mendes et al. [139] documented an inverse association between self-reported OSA and brain amyloid-PET (i.e., OSA associated with less amyloid load compared to non-OSA subjects) in 20 older adult individuals from a sample of 318 older adults (mean age 76.1 ± 3.6 y) recruited from the community into a prospective monocentric cohort. Limitations of the study include the small sample size, OSA by self-report, and lack of data on OSA severity. Another study conducted in a cohort of 14 untreated cognitively normal OSA patients (pooled mean age of 65 ± 9.96), concluded that OSA severity (AHI) was not associated with Aβ burden measured by PiB-PET [140]. However, this study was limited by its small sample size and lack of controls without OSA.
OSA and AD pathology/biomarkers (longitudinal studies)
Middle-aged to older adults
Table 3.2 contains the summary findings from studies assessing the association between OSA and AD-specific neuropathology longitudinally. Though longitudinal studies in this area are sparse, Lutsey et al. [141] examined whether diagnosed OSA in the middle-aged was associated with adverse morphological brain changes 15 y later in participants from the ARIC study. After accounting for body mass index in a series of multivariate models, OSA at mid-life was not associated with indices/markers of brain health such as white matter lesion and local or global brain volume loss. A third of participants, however, did not attend follow-up neurocognitive assessments, introducing a potential selection bias. The study had also relatively few severe OSA patients, necessitating lumping of moderate and severe OSA patients together, which could have attenuated any association in severe OSA patients. CPAP use during the follow-up period was also not accounted for.
In contrast, in a follow-up study to our previously published analysis of OSA and AD biomarkers in community dwelling memory clinic setting, we failed to replicate our initial cross-sectional findings but documented that OSA severity was associated with higher amyloid burden (measured as longitudinal decreases in CSF Aβ42 and increases in PiB uptake) over a 2-y follow-up [136]. We then expanded the analysis of longitudinal examination from purely cognitively normal older individuals to those across the spectrum of dementia, from normal cognition, to MCI, to full AD, in a large population from the ADNI cohort. We found associations between self-reported clinical diagnosis of OSA with greater longitudinal increases in amyloid burden by both CSF and PET imaging measures, and CSF concentration of both total and phosphorylated tau over a 2.5-y period after adjusting for several pertinent cofactors, in the normal cognition and MCI groups [142]. No significant differences in the biomarker changes over time occurred in the AD group [142].
Summary on OSA and AD pathology/biomarkers
In middle-aged, and older adults, cross-sectional data suggest that there is an association between OSA and both established and novel biomarkers of AD pathology, although the results seem more conclusive in those studies that included clinical populations than those that were performed in community or memory clinic settings. Prospective studies examining whether OSA accelerates amyloid deposition and affects regional brain morphological changes that contribute to AD are sparse. The three prospective studies we examined showed contrasting associations between OSA and AD pathology. However, methodological issues related to selection and information biases may have been responsible.
Discussion
Altogether, over three decades of research has investigated OSA-cognition, OSA-MCI/AD diagnosis and OSA-AD pathology associations in the middle-aged and older adults. Studies examined in this review were conducted between 1983 and 2019. During the first decade, studies were fewer, of lower quality, mostly cross-sectional, small sample-sized, clinic based and in older adults. In the second decade, study population and setting cut across young and middle-aged to older adults, clinic based, and community based. Sample size were relatively larger and studies were of better quality. In the last decade and more recently, many studies have been larger, with samples drawn from the community. In addition, as the AD field moves towards a biological definition, more studies are now being conducted using neuroimaging and CSF measures of AD.
The data suggest the following: 1) in young and middle-aged adults, OSA is often associated with cognitive impairment. In older adults, cross-sectional and longitudinal associations between OSA and cognition are highly variable, depending on the study type and setting, with small sleep clinic populations (i.e., more symptomatic patients) driving most of the positive findings. 2) In young and middle-aged adults, cross-sectional and longitudinal studies examining the association between OSA and dementia outcomes in late life are extremely rare. Among older adults, cross-sectional studies have failed to demonstrate a higher prevalence of OSA among those with MCI compared to those with normal cognition; however, OSA is more prevalent among older individuals with AD and/or dementia than in those with normal cognitive function. OSA is also often associated with subsequent development of MCI or AD in older adults, but similar to the studies on cognitive outcomes, clinical patients who have a higher likelihood of associated disturbed sleep or cognitive consequences of OSA might drive these findings. 3) RCTs conducted both in the middle-aged and older adults show that CPAP treatment not only improved sleep parameters (SWS, EDS) in AD patients with OSA, but it also increased cognitive function. 4) Finally, there is a link between OSA and AD biomarkers of neurodegeneration (e.g., Aβ40, Aβ42, Total tau and P-tau), in the young and middle-aged using promising novel biomarkers for AD, as well as in several studies performed in older adults using more established AD biomarker outcomes.
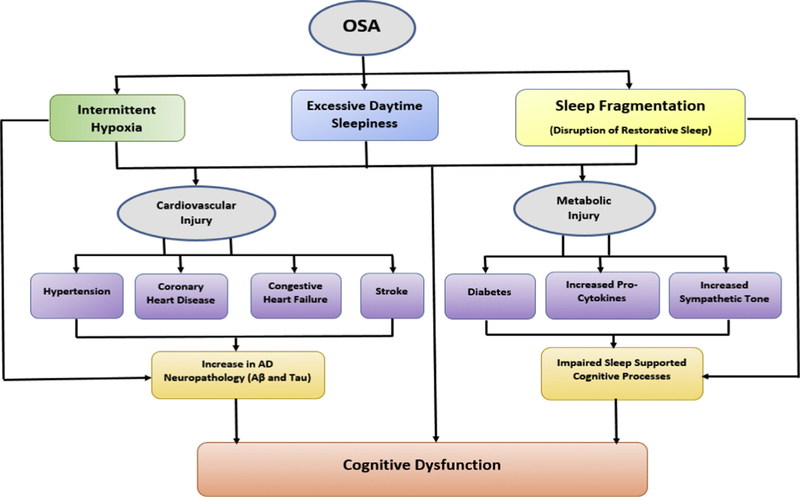
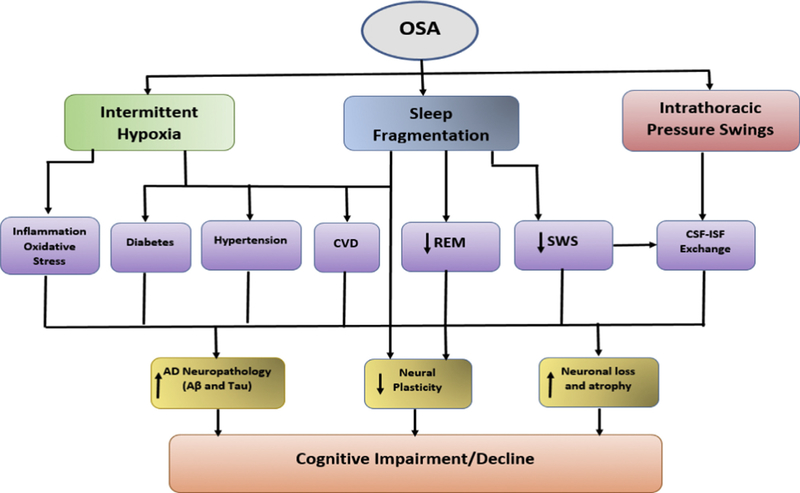
A pertinent question arises from the findings: Is there a physiologic explanation as to why OSA-cognitive associations are particularly pronounced in the middle-aged and variable in older adults? Studies suggest that the link between sleep and cognition weakens with increasing age because the aging brain is unable to adequately and efficiently facilitate specific sleep-supported cognitive processes [143,144]. If this is true, then it could have been responsible for the null or weaker results shown with cognition where associations were identified in older adults. It also implies that improving duration and quality of sleep in older adults may not significantly improve cognitive dysfunction because of diminished neural plasticity, increased neuronal loss and atrophy [145]. These neurobiological changes seen in older adults may also compromise memory consolidation processes, thus making elderly controls similar to OSA cases, and attenuating any difference that may exist when comparisons are done using standard neuropsychological testing. Scullin and Bliwise in their seminal review [145] make the case that a ‘functional weakening’ of the brain in their support of sleep-specific cognitive processes occurs as we age; in other words, that hippocampal-neocortical consolidation will not occur regardless of SWS quantity and spindle density, if the hippocampus, thalamus, neocortex, or hippocampal-neocortical connections are greatly disrupted by the aging process. However, while some studies in older adults show impaired sleep-dependent memory consolidation [146,147], others have reported no evidence of overnight sleep-dependent deficits [147,148], or shown that age differences manifest in sleep-based declarative memory but not in procedural memory consolidation [149]. It is also possible that cognitive impairment secondary to OSA is somewhat driven by impairment in attention and vigilance due to EDS. OSA patients have more lapses and/or longer reaction times in tasks requiring sustained attention, selective attention, or vigilance, and show an increase in reaction times in conditions requiring divided attention, when compared to healthy controls [62,64,150–153], and this could influence other aspects of cognitive deficits attributed to OSA [150,154,155]. In the young and middle-aged, symptomatic OSA with EDS may drive this lapse in attention. Older OSA patients are less likely to present with EDS [156], thus, elderly OSA patients may be able to perform as well as healthy controls in cognitive tasks that are generally impacted by attentional deficits. Notably, cardiovascular effects of OSA are also more pronounced in younger and middle-aged adults and include hypertension [12,13,84], coronary heart disease [14–16], congestive heart failure [17], and stroke [18]. Cardiovascular dysfunction in OSA together with chronic intermittent hypoxia, and hypercapnia, may induce axonal, glial or white matter damage, in multiple brain regions [157–159] The effect of intermittent hypoxia in precipitating hypertension [160,161], hypoperfusion [162,163], impaired glucose metabolism [164–166], adverse cardiovascular and metabolic consequences [167,168], beta amyloid deposition [169,170] and possibly tau hyperphosphorylation; ultimately may lead to particularly pronounced OSA-cognitive associations (Fig. 2) that over a long period of time progress to AD in late-life.
Fig. 2.
Possible intermediate mechanisms in the relationship between OSA and Cognitive Dysfunction in the middle-aged. Chronic exposure to intermittent hypoxia, excessive daytime sleepiness (EDS), disruption of restorative sleep from sleep fragmentation and increase in AD neuropathology may lead to increased cognitive dysfunction. EDS and disruption of restorative sleep are more pronounced in the middle-aged relative to the elderly. Cognitive dysfunction is mainly mediated via cardiovascular and metabolic injuries. Cardiovascular effects of OSA including hypertension, coronary heart disease, congestive heart failure and stroke can also lead to increase in AD neuropathology while metabolic injury effects including diabetes, increased pro-inflammatory cytokines and impaired sympathetic tone from disruption of restorative sleep can further impair sleep supported cognitive processes. OSA, obstructive sleep apnea; and AD, Alzheimer’s disease.
Another pertinent question from the findings is: What physiologic mechanisms underlie OSA’s association with development of MCI or AD in older adults? Disturbed sleep as seen in OSA possibly causes changes in sleep modulated cognitive functions across the lifespan such that there is weakening of and/or compensation attempts directed at sleep-cognition links [145]. Intermittent hypoxia, sleep fragmentation and intrathoracic pressure swings are the three main processes by which OSA is thought to induce neurodegenerative changes (Fig. 3). Studies of cerebral ischemia suggest that hypoxia promotes the accumulation of Aβ42 [170–173], while two mouse studies have shown that intermittent hypoxia is associated with increased Aβ production [174,175]. Concerning sleep fragmentation, actigraphy-assessed arousals and circadian rhythm disruption have also been shown to be associated with increased risk of MCI/dementia in older adults [176,177]. Chronic intermittent hypoxia, hypercapnia and hypertension in OSA can induce neuronal damage, including axons [158], white matter [157], and reduced DTI based mean diffusivity in multiple brain regions [178]. Studies also show grey matter loss in OSA patients compared to controls [159,179,180]. Because of the pathophysiological effect of hypoxia precipitating hypertension [160,161], hypoperfusion [162,163], impaired glucose metabolism [164–166], and adverse cardiovascular, and metabolic consequences [167,168]; ultimately these effects could lead to cognitive decline and progress to AD. These findings suggest that OSA elderly patients with cardiovascular consequences might be at higher risk of AD than those without OSA-related vascular symptoms. In addition, inflammation [181,182], and oxidative stress [183,184] upregulate neurocognitive impairment in OSA and sustained OSA-cognitive dysfunction overlay with that seen in AD-associated cognitive decline. Another indirect plausible mechanism by which OSA increases AD risk maybe through impairment in the CSF-ISF exchange promoted by the glymphatic system [132]. Mechanisms that could explain OSA inducing CSF-ISF exchange impairment include: 1) intrathoracic pressure swings from respiratory efforts against a closed airway that would impede the glymphatic flow of metabolites from ISF to CSF [185–187] (although the reverse could also be true, in other words an increase in flow secondary to the pressure increase); 2) a reduction in the clearance of subarachnoid CSF directly into dural lymphatic channels due to increased venous pressure that might be elevated in OSA; and 3) cerebral edema secondary to intermittent hypoxia. The latter mechanism has been proposed recently in a study of 71 subjects (age: 65.3 ± 5.6 y) in which severity of OSA correlated with higher cortical thickness of the prefrontal, parietal and posterior cingulate cortices [188], and could also explain the brain volume reductions observed in a study following OSA treatment with CPAP (i.e., pseudo-atrophy) which also suggests the existence of brain edema in severe OSA [189]. Intriguingly, mice exposed to intermittent hypoxia show reduced levels of AQP1 as well as areas of extensive gliosis compatible with cytotoxic edema [190].
Fig. 3.
Possible intermediate mechanisms in the relationship between OSA and Cognitive dysfunction in older adults. Chronic exposure to intermittent hypoxia may lead to increased inflammation and oxidative stress, diabetes, hypertension and CVD, all potentially contributing to AD pathology development. Sleep fragmentation, both by itself and by leading to decreased REM and SWS stages, can additionally promote AD pathogenesis. Intrathoracic pressure swings associated with OSA may disrupt CSF-ISF exchange integrity and lead to AD neuropathology accumulation. Furthermore, all the processes including hypoxia, fragmented sleep and intrathoracic pressure swings can cumulatively lead to decreased neural plasticity and neuronal loss and atrophy, thereby contributing to cognitive dysfunction. OSA, obstructive sleep apnea; CVD, cardiovascular disease; REM, rapid eye movement; SWS, slow wave sleep; CSF-ISF, cerebrospinal fluid-interstitial fluid; AD, Alzheimer’s disease.
Reduced SWS is another possible mechanism by which OSA precipitates AD pathogenesis and a possible explanation for some of the observed null findings, as matched control groups may have age-related impairment of SWS [191]. Apneas are more commonly observed in NREM1–2 and REM sleep and less commonly in SWS, which has been associated with a higher respiratory arousal threshold [192,193] as well as more stable breathing [194]. However, the temporal course of SWA has been shown to be slower in mild OSA [195], while severe OSA patients show up to a 40% rebound in SWS duration during OSA treatment with CPAP [196], which suggest that changes in SWS quality may also be involved in OSA pathology.
SWS has been suggested to promote opposite effects on Aβ dynamics, after CSF Aβ was found to fluctuate in a diurnal pattern in healthy adults, with lowest CSF Aβ levels around 10:00 h (which correspond to approximately 04:00 h sleep time, a point roughly 2/3 of the way through typical total sleep time), after which most SWS has occurred and when normal sleep is mostly cycling between stages NREM1–2 and REM [191]. This CSF Aβ decrease was later shown to be attenuated by prolonged wakefulness (i.e., higher CSF Aβ42 levels in the sleep deprived when compared to normal sleepers), while partial sleep deprivation with preserved SWS did not affect Aβ42 levels [191]. Further corroboration was made by two independent observational studies [132,135]; one that showed inverse associations between CSF Aβ peptides and SWA both in middle age (53.2 ± 5.7 y) [132], and another, in older adults (66.9 ± 8.3 y) [135], while a third study demonstrated increased CSF Aβ40 in middle age adults (54.1 ± 6.7 y) after selective SWS disruption using auditory tones delivered via headphones [197]. Recently, we showed that spindle density during NREM 2 sleep was negatively correlated with CSF Aβ42, P-tau and T-tau, with CSF T-tau being the most significantly associated with spindle density, after adjusting for age, sex and ApoE4. Spindle duration, count and fast spindle density were also negatively correlated with T-tau levels, suggesting that reduced spindles during N2 sleep may represent an early dysfunction related to tau, possibly reflecting axonal damage or altered neuronal tau secretion [198]. Lucey et al. [199] also recently demonstrated that frontal NREM SWA on the single-EEG lead Profiler was inversely associated with brain tau by PET in predominantly cognitively normal older adults, and suggesting that NREM SWA, changes may discriminate between tau pathology and cognitive impairment at the earliest stages of symptomatic AD. However, it is important to note that the single EEG is limited in capacity to assess SWA topographical differences in adults [199] and the antero-posterior shift that occurs in NREM power during consecutive NREM sleep periods [200].
Notably, while the effects of OSA on Aβ have been studied in both humans and animal models, much less is known about the effects of OSA on tau and its hyperphosphorylation, a crucial step in the formation of neurofibrillary tangles, a key feature of AD pathogenesis. Blood tau level has been investigated in OSA and is higher (see section on ‘OSA and AD Pathology’) [129,130]. However, current plasma/serum tau assays do not correlate significantly with CSF T-tau or CSF P-tau and positive findings are hard to interpret. For the first time, we demonstrated greater longitudinal increases in CSF concentration of both total and phosphorylated tau in OSA compared to controls [142]. Though not specifically investigating OSA’s effect, a recent study showed that the sleep–wake cycle regulates ISF tau, and that sleep deprivation increases ISF and CSF tau as well as tau pathology spreading [201]. The fundamental question though for researchers in the field, is whether OSA leads to pathophysiological processes involved in neurodegeneration pathogenesis of which tau plays a significant role and is not mediated by prior Aβ deposition. On the other hand, tau though higher in OSA may or may not be the more informative biomarker about the mechanisms underlying the link between OSA and dementia.
AD-type neurodegeneration as a contributing factor to the emergence of OSA
Little is known of how AD-type neurodegeneration may contribute to the emergence of OSA but there is evidence that the hippocampus might play a direct role in breathing or the response to abnormal breathing [202]. Hippocampal structures have been implicated in apneas, showing substantially increased activity accompanying inspiratory onset after apnea [202,203], and functional MRI studies show increased signal changes in many cortical regions including the hippocampal formation during the Valsalva maneuver [204]. In AD, neurofibrillary tangles (NFT), related to P-Tau protein pathology, consistently develop first in the lower brainstem and hippocampal formation during their earliest stages of the disease [205] and subsequently expand to the neocortical association areas [206,207], at which time, a diagnosis of AD is imminent especially with the presence of Aβ pathology. NFTs are closely correlated with hippocampal damage and the early symptoms of memory loss in AD [208]. Preexisting NFT pathology in AD (reflected in vivo by increases in P-Tau), and/or hippocampal atrophy, may therefore affect breathing and increase the risk of OSA. In addition, Alzheimer’s patients have been found to have a significantly higher proportion of NREM-related than REM-related apnea [97].
Recommendations for critical future directions
Methodological differences existed among studies reviewed and can be rightly viewed as a limitation in the field of OSA and AD research. Issues related to the single assessment of OSA in longitudinal studies, absent or incomplete CPAP intervention information during follow-up, and the possibility that the etiological timeframe relevant for the association between OSA and AD could be outside the examined period, variability in ways in which cognition was assessed, and issues relating to selection bias, are all opportunities for future improvements. Many studies utilized sleep clinic patients. It is clear that the likelihood of clinic attendance in such participants is associated with disturbed sleep, EDS, and possibly cognitive and cardiovascular consequences. Therefore, when analyses are conducted on only such participants, selection bias results are likely the outcome. In addition, not all studies accounted for the possible role of depression and its symptoms, which may be important mediator or confounder of the association between disturbed sleep and cognition [209,210]. Therefore, new research in the field should endeavor to separate causality relating to OSA and associated symptoms including the cardiovascular system, depression and cognition.
Despite methodological strengths such as use of PSG sleep measures, in-vivo measures of AD pathology and certain long follow-ups, these were not all present in all studies, therefore limiting the strength of causal inferences that can be made. Furthermore, future studies should examine whether these associations are causal, focusing on the mechanisms responsible for the somewhat different OSA effects seen at different ages or in different populations, including sex and race-specific OSA risk on cognitive decline and AD.
Future RCTs need to include dose–response studies stratified not only to the mild, moderate and severe categories of OSA, but also to include categories addressing duration of disease, extent of intermittent hypoxemia, fragmented sleep severity, and presence of comorbidities such as EDS and cardiovascular symptoms. Issues with design and sample size of double-blinded, placebo controlled clinical trials addressing the effect of CPAP exist but need to be improved. RCTs also need to consider the effect of EDS, which was recently shown to be longitudinally associated with amyloid deposition [211] and whether those randomized to “no treatment” or “sham treatment” should be given a drug like modafinil. Lastly, as Pan et al. [36] noted in their review, non-inferiority trials utilizing sleep apnea dental devices or other non-PAP therapeutics will also be beneficial.
Conclusion
OSA is often associated with cognitive impairment in young and middle-aged adults. In older adults, OSA is associated with the development of MCI or AD with clinic patients who have a higher likelihood of associated disturbed sleep and OSA-related consequences driving these findings. CPAP treatment may be effective in improving cognition in OSA patients with AD. Recent trends demonstrate links between OSA and AD biomarkers of neurodegeneration across all age groups. Intermittent hypoxia, sleep fragmentation, reduced SWS and intrathoracic pressure swings are possible mechanisms by which OSA induces neurodegenerative changes. This distinct pattern observed in OSA-cognition and OSA-AD associations in middle-aged, and older adults, provides the foundation for envisioning better characterization of OSA especially in late-life and the need for more sensitive/novel sleep-dependent cognitive assessments to assess OSA-related cognitive impairment. Future studies with improved designs addressing the longitudinal relationship between these two entities and the possible protective effect of CPAP treatment on AD biomarkers of neurodegeneration are required to better intervene in this pressing public health issue.
Supplementary Material
Practice points.
OSA is often associated with cognitive impairment inyoung and middle-aged adults. In older adults, OSA is associated with the development of MCI or AD with OSA patients seeking treatment driving these findings.
There is a link between OSA and AD biomarkers of neurodegeneration (e.g., Aβ40, Aβ42, total Aβ and P-tau 181) in cognitively normal individuals of all age groups.
OSA worsens metabolic injury that is particularly resplendent in middle-aged, and exacerbates neuronal injury and facilitates memory and cognitive impairment, that is particularly resplendent in older adults.
Intermittent hypoxia, sleep fragmentation, reduced SWSand intrathoracic pressure swings are possible mechanisms by which OSA induces neurodegenerative changes.
CPAP treatment may be effective in improving cognitionin OSA patients with AD.
Research agenda.
Future research with improved designs that address the temporal nature of the OSA-AD relationship and whether OSA leads to pathophysiological processes involved in AD neurodegeneration pathogenesis are needed.
Issues related to the single assessment of OSA in longitudinal studies, absent or incomplete CPAP intervention information during follow-up, possibility of etiological relevant timeframes being outside of the examined period, variability in cognitive assessments, and possible selection bias, are all opportunities for future improvements.
Future RCTs that include categories addressing duration of disease, intermittent hypoxemia extent, fragmented sleep severity, and presence of comorbidities; examining the possible protective effect of CPAP treatment on AD biomarkers of neurodegeneration in the preclinical stages of AD are required.
There is need for the development of more sensitive/novel sleep-dependent cognitive assessments to assess OSA-related impairment especially in older adults.
Acknowledgements
The authors would like to thank the librarians John Orriola and Allison Howard from the University of South Florida (USF) medical library for their assistance in developing the electronic search strategies. We also thank Drs. James Mortimer, Kevin Kip, and Alfred Mbah at USF Health, Dr. Dave Morgan at the Michigan State University and Dr. Amy Borenstein at University of California, San Diego for their expert guidance. This work was supported by grants from the NIH/NIA/NHLBI (CIRAD P30AG059303 Pilot, T32HL129953, R01HL118624, R21AG049348, R21AG055002, R01AG056031, R01AG022374, R21AG059179, R01AG056682, R01AG056531 and P30AG008051).
Abbreviations
- Aβ
amyloid beta
- AD
Alzheimer’s disease
- ADAS-cog
Alzheimer’s disease assessment scale-cognitive
- ADNI
Alzheimer’s disease neuroimaging initiative
- AHI
apnea hypopnea index
- APOE
apolipoprotein e
- ARIC
atherosclerosis risk in communities
- CPAP
continuous positive airway pressure
- CRP
C reactive protein
- CSF
cerebrospinal fluid
- DTI
diffused tensor imaging
- EDS
excessive daytime sleepiness
- ELISA
enzyme linked immunosorbent assay
- IL
interleukin
- MCI
mild cognitive impairment
- MrOS
the osteoporotic fractures in men
- NFT
neurofibrillary tangles
- NHIS-HEALS
national health insurance service-health screening
- NOS
Newcastle-Ottawa scale
- NREM
non-rapid eye move movement
- OSA
obstructive sleep apnea
- PET
positron emission tomography
- PSG
polysomnography
- PRISMA
preferred reporting items for systematic reviews and meta-analyses
- PROOF
prognostic indicator of cardiovascular and cerebrovascular events
- RCT
randomized controlled trials
- RDI
respiratory disturbance index
- REM
rapid eye movement
- SDB
sleep disordered breathing
- SHHS
sleep heart health study
- SIMOA
single molecule array
- SOF
study of osteoporotic fractures
- SWA
slow wave activity
- SWS
slow wave sleep
- TNF
tumor necrosis factor
- WM
white matter
Footnotes
Conflicts of interest
The authors do not have any conflicts of interest to disclose
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.smrv.2019.101250.
References
* The most important references are denoted by an asterisk.
- [1].Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastiao YV, Wen Y, et al. Sleep, cognitive impairment, and Alzheimer’s disease: a systematic review and meta-analysis. Sleep 2017;40(1). [DOI] [PubMed] [Google Scholar]
- [2].Naismith SL, Hickie IB, Terpening Z, Rajaratnam SM, Hodges JR, Bolitho S, et al. Circadian misalignment and sleep disruption in mild cognitive impairment. J Alzheimer’s Dis: JAD 2014;38(4):857–66. [DOI] [PubMed] [Google Scholar]
- [3].Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev 2018;40:4–16. [DOI] [PubMed] [Google Scholar]
- [4].Sindi S, Kareholt I, Johansson L, Skoog J, Sjoberg L, Wang HX, et al. Sleep disturbances and dementia risk: a multicenter study. Alzheimer’s Dementia: J Alzheimer’s Assoc 2018;14(10):1235–42. [DOI] [PubMed] [Google Scholar]
- [5].Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997;20(9):705–6. [DOI] [PubMed] [Google Scholar]
- [6].Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001;163(3 Pt 1):608–13. [DOI] [PubMed] [Google Scholar]
- [7].Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 1998;157(1):144–8. [DOI] [PubMed] [Google Scholar]
- [8].Heinzer R, Marti-Soler H, Haba-Rubio J. Prevalence of sleep apnoea syndrome in the middle to old age general population. The Lancet Respir Med 2016;4(2):e5–6. [DOI] [PubMed] [Google Scholar]
- [9].Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. The Lancet Respir Med 2015;3(4):310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zamarron C, Gude F, Otero Y, Alvarez JM, Golpe A, Rodriguez JR. Prevalence of sleep disordered breathing and sleep apnea in 50- to 70year-old individuals. A survey – Respiration – Int Rev Thorac Dis 1999;66(4):317–22. [DOI] [PubMed] [Google Scholar]
- [11].Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328(17):1230–5. [DOI] [PubMed] [Google Scholar]
- [12].Hla KM, Skatrud JB, Finn L, Palta M, Young T. The effect of correction of sleep-disordered breathing on BP in untreated hypertension. Chest 2002;122(4):1125–32. [DOI] [PubMed] [Google Scholar]
- [13].Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep 2008;31(6):795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Baniak LM, Chasens ER, Luyster FS, Strollo PJ Jr, Thunstrom E, Peker Y. Obstructive sleep apnea and self-reported functional impairment in revascularized patients with coronary artery disease in the RICCADSA trial. Sleep Breath = Schlaf & Atmung 2018;22(4):1169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Strausz S, Havulinna AS, Tuomi T, Bachour A, Groop L, Makitie A, et al. Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: a longitudinal population-based study in Finland. BMJ open 2018;8(10):e022752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang J, Song Y, Ji Y, Song Y, Cai S, Yu Y, et al. Correlation between coronary artery disease and obstructive sleep apnea syndrome and analysis of risk factors. Exp Ther Med 2018;15(6):4771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol 2013;169(3):207–14. [DOI] [PubMed] [Google Scholar]
- [18].Li M, Hou WS, Zhang XW, Tang ZY. Obstructive sleep apnea and risk of stroke: a meta-analysis of prospective studies. Int J Cardiol 2014;172(2): 466–9. [DOI] [PubMed] [Google Scholar]
- [19].Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metabol 2010;24(5):843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang X, Bi Y, Zhang Q, Pan F. Obstructive sleep apnoea and the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Respirology (Carlton, Vic) 2013;18(1):140–6. [DOI] [PubMed] [Google Scholar]
- [21].Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology (Carlton, Vic) 2013;18(1): 61–70. [DOI] [PubMed] [Google Scholar]
- [22].Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep 2013;36(9):1297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ahuja S, Chen RK, Kam K, Pettibone WD, Osorio RS, Varga AW. Role of normal sleep and sleep apnea in human memory processing. Nat Sci Sleep 2018;10:255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sanchez-de-la-Torre M, Campos-Rodriguez F, Barbe F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med 2013;1(1): 61–72. [DOI] [PubMed] [Google Scholar]
- [25].Dhar S, Shastri SR, Lenora RA. Aging and the respiratory system. Med Clin N Am 1976;60(6):1121–39. [DOI] [PubMed] [Google Scholar]
- [26].Malhotra A, Huang Y, Fogel R, Lazic S, Pillar G, Jakab M, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med 2006;119(1). 72.e9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miller MA, Wright H, Ji C, Cappuccio FP. Cross-sectional study of sleep quantity and quality and amnestic and non-amnestic cognitive function in an ageing population: the English Longitudinal Study of Ageing (ELSA). PLoS One 2014;9(6):e100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gagnon K, Baril AA, Gagnon JF, Fortin M, Decary A, Lafond C, et al. Cognitive impairment in obstructive sleep apnea. Pathol Biol 2014;62(5): 233–40. [DOI] [PubMed] [Google Scholar]
- [29].Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SC, Morrell MJ. Sleep apnoea and the brain: a complex relationship. The Lancet Respir Med 2015;3(5):404–14. [DOI] [PubMed] [Google Scholar]
- [30].Polsek D, Gildeh N, Cash D, Winsky-Sommerer R, Williams SCR, Turkheimer F, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev 2018;86: 142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Davies CR, Harrington JJ. Impact of obstructive sleep apnea on neurocognitive function and impact of continuous positive air pressure. Sleep Med Clin 2016;11(3):287–98. [DOI] [PubMed] [Google Scholar]
- [32].Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry: Off J Am Assoc Geriatr Psychiatr 2016;24(6):496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shastri A, Bangar S, Holmes J. Obstructive sleep apnoea and dementia: is there a link? Int J Geriatr Psychiatr 2016;31(4):400–5. [DOI] [PubMed] [Google Scholar]
- [34].Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res 2015;93(12):1778–94. [DOI] [PubMed] [Google Scholar]
- [35].Baril AA, Carrier J, Lafreniere A, Warby S, Poirier J, Osorio RS, et al. Biomarkers of dementia in obstructive sleep apnea. Sleep Med Rev 2018;42: 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pan W, Kastin AJ. Can sleep apnea cause Alzheimer’s disease? Neurosci Biobehav Rev 2014;47:656–69. [DOI] [PubMed] [Google Scholar]
- [37].Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc: JINS 2004;10(5):772–85. [DOI] [PubMed] [Google Scholar]
- [38].Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep 2003;26(3):298–307. [DOI] [PubMed] [Google Scholar]
- [39].Kilpinen R, Saunamaki T, Jehkonen M. Information processing speed in obstructive sleep apnea syndrome: a review. Acta Neurol Scand 2014;129(4):209–18. [DOI] [PubMed] [Google Scholar]
- [40].Vaessen TJ, Overeem S, Sitskoorn MM. Cognitive complaints in obstructive sleep apnea. Sleep Med Rev 2015;19:51–8. [DOI] [PubMed] [Google Scholar]
- [41].Cross N, Lampit A, Pye J, Grunstein RR, Marshall N, Naismith SL. Is obstructive sleep apnoea related to neuropsychological function in healthy older adults? A systematic review and meta-analysis. Neuropsychol Rev 2017;27(4):389–402. [DOI] [PubMed] [Google Scholar]
- [42].Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- [43].Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. 2011. [accessed 112514]. 2011, http://wwwohrica/programs/clinical_epidemiology/oxfordasp/.
- [44].Borenstein Michael HLVHJPT, Rothstein HR. Introduction to meta-analysis. John Wiley & Sons, Ltd.; 2009. [Google Scholar]
- [45].J C. Statistical power analysis for the behavioral sciences. New York, NY: Routledge Academic; 1998. [Google Scholar]
- [46].Bawden FC, Oliveira CA, Caramelli P. Impact of obstructive sleep apnea on cognitive performance. Arquivos de neuro-psiquiatria 2011;69(4):585–9. [DOI] [PubMed] [Google Scholar]
- [47].Mathieu A, Mazza S, Decary A, Massicotte-Marquez J, Petit D, Gosselin N, et al. Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patients. Sleep Med 2008;9(2): 112–20. [DOI] [PubMed] [Google Scholar]
- [48].Naegele B, Thouvard V, Pepin JL, Levy P, Bonnet C, Perret JE, et al. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep 1995;18(1):43–52. [PubMed] [Google Scholar]
- [49].Quan SF, Wright R, Baldwin CM, Kaemingk KL, Goodwin JL, Kuo TF, et al. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the sleep heart health study. Sleep Med 2006;7(6):498–507. [DOI] [PubMed] [Google Scholar]
- [50].Hrubos-Strom H, Nordhus IH, Einvik G, Randby A, Omland T, Sundet K, et al. Obstructive sleep apnea, verbal memory, and executive function in a community-based high-risk population identified by the Berlin Questionnaire Akershus Sleep Apnea Project. Sleep Breath = Schlaf & Atmung 2012;16(1):223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kloepfer C, Riemann D, Nofzinger EA, Feige B, Unterrainer J, O’Hara R, et al. Memory before and after sleep in patients with moderate obstructive sleep apnea. J Clin Sleep Med : JCSM : Off Publ Am Acad Sleep Med 2009;5(6):540–8. [PMC free article] [PubMed] [Google Scholar]
- [52].Naegele B, Launois SH, Mazza S, Feuerstein C, Pepin JL, Levy P. Which memory processes are affected in patients with obstructive sleep apnea? An evaluation of 3 types of memory. Sleep 2006;29(4):533–44. [DOI] [PubMed] [Google Scholar]
- [53].Nikodemova M, Finn L, Mignot E, Salzieder N, Peppard PE. Association of sleep disordered breathing and cognitive deficit in APOE epsilon4 carriers. Sleep 2013;36(6):873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Salorio CF, White DA, Piccirillo J, Duntley SP, Uhles ML. Learning, memory, and executive control in individuals with obstructive sleep apnea syndrome. J Clin Exp Neuropsychol 2002;24(1):93–100. [DOI] [PubMed] [Google Scholar]
- [55].Sharma H, Sharma SK, Kadhiravan T, Mehta M, Sreenivas V, Gulati V, et al. Pattern & correlates of neurocognitive dysfunction in Asian Indian adults with severe obstructive sleep apnoea. Indian J Med Res 2010;132: 409–14. [PubMed] [Google Scholar]
- [56].Alchanatis M, Zias N, Deligiorgis N, Liappas I, Chroneou A, Soldatos C, et al. Comparison of cognitive performance among different age groups in patients with obstructive sleep apnea. Sleep Breath = Schlaf & Atmung 2008;12(1):17–24. [DOI] [PubMed] [Google Scholar]
- [57].Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep 2007;30(10):1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cheshire K, Engleman H, Deary I, Shapiro C, Douglas NJ. Factors impairing daytime performance in patients with sleep apnea/hypopnea syndrome. Arch Intern Med 1992;152(3):538–41. [PubMed] [Google Scholar]
- [59].Telakivi T, Kajaste S, Partinen M, Koskenvuo M, Salmi T, Kaprio J. Cognitive function in middle-aged snorers and controls: role of excessive daytime somnolence and sleep-related hypoxic events. Sleep 1988;11(5):454–62. [PubMed] [Google Scholar]
- [60].Valencia-Flores M, Bliwise DL, Guilleminault C, Cilveti R, Clerk A. Cognitive function in patients with sleep apnea after acute nocturnal nasal continuous positive airway pressure (CPAP) treatment: sleepiness and hypoxemia effects. J Clin Exp Neuropsychol 1996;18(2):197–210. [DOI] [PubMed] [Google Scholar]
- [61].Findley LJ, Barth JT, Powers DC, Wilhoit SC, Boyd DG, Suratt PM. Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest 1986;90(5):686–90. [DOI] [PubMed] [Google Scholar]
- [62].Greenberg GD, Watson RK, Deptula D. Neuropsychological dysfunction in sleep apnea. Sleep 1987;10(3):254–62. [DOI] [PubMed] [Google Scholar]
- [63].Roehrs T, Merrion M, Pedrosi B, Stepanski E, Zorick F, Roth T. Neuropsychological function in obstructive sleep apnea syndrome (OSAS) compared to chronic obstructive pulmonary disease (COPD). Sleep 1995;18(5):382–8. [DOI] [PubMed] [Google Scholar]
- [64].Bedard MA, Montplaisir J, Richer F, Malo J. Nocturnal hypoxemia as a determinant of vigilance impairment in sleep apnea syndrome. Chest 1991;100(2):367–70. [DOI] [PubMed] [Google Scholar]
- [65].Bedard MA, Montplaisir J, Richer F, Rouleau I, Malo J. Obstructive sleep apnea syndrome: pathogenesis of neuropsychological deficits. J Clin Exp Neuropsychol 1991;13(6):950–64. [DOI] [PubMed] [Google Scholar]
- [66].Bliwise DL. Sleep apnea and cognitive function: where do we stand now? Sleep 1993;16(Suppl. 8):S72–3. [DOI] [PubMed] [Google Scholar]
- [67].Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP). Brain Res Bull 2003;61(1):87–92. [DOI] [PubMed] [Google Scholar]
- [68].Ward CP, McCoy JG, McKenna JT, Connolly NP, McCarley RW, Strecker RE. Spatial learning and memory deficits following exposure to 24 h of sleep fragmentation or intermittent hypoxia in a rat model of obstructive sleep apnea. Brain Res 2009;1294:128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Berry DT, Phillips BA, Cook YR, Schmitt FA, Honeycutt NA, Arita AA, et al. Geriatric sleep apnea syndrome: a preliminary description. J Gerontol 1990;45(5):M169–74. [DOI] [PubMed] [Google Scholar]
- [70].Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep 2011;34(10): 1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ju G, Yoon IY, Lee SD, Kim TH, Choe JY, Kim KW. Effects of sleep apnea syndrome on delayed memory and executive function in elderly adults. J Am Geriatr Soc 2012;60(6):1099–103. [DOI] [PubMed] [Google Scholar]
- [72].Spira AP, Blackwell T, Stone KL, Redline S, Cauley JA, Ancoli-Israel S, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc 2008;56(1):45–50. [DOI] [PubMed] [Google Scholar]
- [73].Yesavage J, Bliwise D, Guilleminault C, Carskadon M, Dement W. Preliminary communication: intellectual deficit and sleep-related respiratory disturbance in the elderly. Sleep 1985;8(1):30–3. [DOI] [PubMed] [Google Scholar]
- [74].Boland LL, Shahar E, Iber C, Knopman DS, Kuo TF, Nieto FJ. Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: the Sleep Heart Health Study. J Sleep Res 2002;11(3):265–72. [DOI] [PubMed] [Google Scholar]
- [75].Foley DJ, Masaki K, White L, Larkin EK, Monjan A, Redline S. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep 2003;26(5):596–9. [DOI] [PubMed] [Google Scholar]
- [76].Hayward L, Mant A, Eyland A, Hewitt H, Purcell C, Turner J, et al. Sleep disordered breathing and cognitive function in a retirement village population. Age Ageing 1992;21(2):121–8. [DOI] [PubMed] [Google Scholar]
- [77].Phillips BA, Berry DT, Schmitt FA, Magan LK, Gerhardstein DC, Cook YR. Sleep-disordered breathing in the healthy elderly. Clinically significant? Chest 1992;101(2):345–9. [DOI] [PubMed] [Google Scholar]
- *[78].Sforza E, Roche F, Thomas-Anterion C, Kerleroux J, Beauchet O, Celle S, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep 2010;33(4):515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Blackwell T, Yaffe K, Laffan A, Redline S, Ancoli-Israel S, Ensrud KE, et al. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2015;63(3): 453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[80].Martin MS, Sforza E, Roche F, Barthelemy JC, Thomas-Anterion C. Sleep breathing disorders and cognitive function in the elderly: an 8-year follow-up study. the proof-synapse cohort. Sleep 2015;38(2):179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lutsey PL, Bengtson LG, Punjabi NM, Shahar E, Mosley TH, Gottesman RF, et al. Obstructive sleep apnea and 15-year cognitive decline: the atherosclerosis risk in communities (ARIC) study. Sleep 2016;39(2):309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, Falini A, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med 2011;183(10):1419–26. [DOI] [PubMed] [Google Scholar]
- [83].Castronovo V, Canessa N, Strambi LF, Aloia MS, Consonni M, Marelli S, et al. Brain activation changes before and after PAP treatment in obstructive sleep apnea. Sleep 2009;32(9):1161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Castronovo V, Scifo P, Castellano A, Aloia MS, Iadanza A, Marelli S, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep 2014;37(9):1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[85].Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012;35(12):1593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Saunamaki T, Himanen SL, Polo O, Jehkonen M. Executive dysfunction in patients with obstructive sleep apnea syndrome. Eur Neurol 2009;62(4): 237–42. [DOI] [PubMed] [Google Scholar]
- [87].Saunamaki T, Himanen SL, Polo O, Jehkonen M. Executive dysfunction and learning effect after continuous positive airway pressure treatment in patients with obstructive sleep apnea syndrome. Eur Neurol 2010;63(4): 215–20. [DOI] [PubMed] [Google Scholar]
- [88].Kylstra WA, Aaronson JA, Hofman WF, Schmand BA. Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: a meta-analysis. Sleep Med Rev 2013;17(5):341–7. [DOI] [PubMed] [Google Scholar]
- [89].Pan YY, Deng Y, Xu X, Liu YP, Liu HG. Effects of continuous positive airway pressure on cognitive deficits in middle-aged patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Chin Med J 2015;128(17):2365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Dalmases M, Sole-Padulles C, Torres M, Embid C, Nunez MD, Martinez-Garcia MA, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized Pilot study. Chest 2015;148(5):1214–23. [DOI] [PubMed] [Google Scholar]
- [91].Martinez-Garcia MA, Chiner E, Hernandez L, Cortes JP, Catalan P, Ponce S, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J 2015;46(1):142–51. [DOI] [PubMed] [Google Scholar]
- *[92].McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, Davies RJ, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. The Lancet Respir Med 2014;2(10):804–12. [DOI] [PubMed] [Google Scholar]
- [93].Aloia MS, Ilniczky N, Di Dio P, Perlis ML, Greenblatt DW, Giles DE. Neuropsychological changes and treatment compliance in older adults with sleep apnea. J Psychosom Res 2003;54(1):71–6. [DOI] [PubMed] [Google Scholar]
- [94].Richards KC, Gooneratne N, Dicicco B, Hanlon A, Moelter S, Onen F, et al. CPAP adherence may slow 1-year cognitive decline in older adults with mild cognitive impairment and apnea. J Am Geriatr Soc 2019;67(3): 558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Dlugaj M, Weinreich G, Weimar C, Stang A, Dragano N, Wessendorf TE, et al. Sleep-disordered breathing, sleep quality, and mild cognitive impairment in the general population. J Alzheimer’s Dis: JAD. 2014;41(2): 479–97. [DOI] [PubMed] [Google Scholar]
- [96].Kim SJ, Lee JH, Lee DY, Jhoo JH, Woo JI. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am J Geriatr Psychiatry: Off J Am Assoc Geriatr Psychiatr 2011;19(4):374–81. [DOI] [PubMed] [Google Scholar]
- [97].Hoch CC, Reynolds CF 3rd, Kupfer DJ, Houck PR, Berman SR, Stack JA. Sleep-disordered breathing in normal and pathologic aging. J Clin Psychiatry 1986;47(10):499–503. [PubMed] [Google Scholar]
- [98].Reynolds CF 3rd, Kupfer DJ, Taska LS, Hoch CC, Sewitch DE, Restifo K, et al. Sleep apnea in Alzheimer’s dementia: correlation with mental deterioration. J Clin Psychiatry 1985;46(7):257–61. [PubMed] [Google Scholar]
- [99].Hoch CC, Reynolds CF 3rd, Nebes RD, Kupfer DJ, Berman SR, Campbell D. Clinical significance of sleep-disordered breathing in Alzheimer’s disease. Preliminary data. J Am Geriatr Soc 1989;37(2):138–44. [DOI] [PubMed] [Google Scholar]
- [100].Reynolds CF 3rd, Kupfer DJ, Hoch CC, Houck PR, Stack JA, Berman SR, et al. Sleep deprivation as a probe in the elderly. Arch Gen Psychiatr 1987;44(11):982–90. [DOI] [PubMed] [Google Scholar]
- [101].Smallwood RG, Vitiello MV, Giblin EC, Prinz PN. Sleep apnea: relationship to age, sex, and Alzheimer’s dementia. Sleep 1983;6(1):16–22. [DOI] [PubMed] [Google Scholar]
- [102].Emamian F, Khazaie H, Tahmasian M, Leschziner GD, Morrell MJ, Hsiung GY, et al. The association between obstructive sleep apnea and Alzheimer’s disease: a meta-analysis perspective. Front Aging Neurosci 2016;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[103].Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA : J Am Med Assoc 2011;306(6): 613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Yaffe K, Nettiksimmons J, Yesavage J, Byers A. Sleep quality and risk of dementia among older male veterans. Am J Geriatr Psychiatry: Off J Am Assoc Geriatr Psychiatr 2015;23(6):651–4. [DOI] [PubMed] [Google Scholar]
- [105].Lutsey PL, Misialek JR, Mosley TH, Gottesman RF, Punjabi NM, Shahar E, et al. Sleep characteristics and risk of dementia and Alzheimer’s disease: the Atherosclerosis Risk in Communities Study. Alzheimer’s Dementia: J Alzheimer’s Assoc 2018;14(2):157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015;84(19):1964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[107].Chang WP, Liu ME, Chang WC, Yang AC, Ku YC, Pai JT, et al. Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in taiwan. PLoS One 2013;8(10):e78655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B, Gislason T. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J 2016;47(1):194–202. [DOI] [PubMed] [Google Scholar]
- [109].Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis 2015;7(8):1311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jordan AS, Catcheside PG, O’Donoghue FJ, Saunders NA, McEvoy RD. Genioglossus muscle activity at rest and in response to brief hypoxia in healthy men and women. J Appl Physiol (Bethesda, Md: 1985) 2002;92(1): 410–7. [DOI] [PubMed] [Google Scholar]
- [111].Mohsenin V Effects of gender on upper airway collapsibility and severity of obstructive sleep apnea. Sleep Med 2003;4(6):523–9. [DOI] [PubMed] [Google Scholar]
- [112].O’Halloran KD, Lewis P, McDonald F. Sex, stress and sleep apnoea: decreased susceptibility to upper airway muscle dysfunction following intermittent hypoxia in females. Respir Physiol Neurobiol 2017;245: 76–82. [DOI] [PubMed] [Google Scholar]
- [113].Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications. Am J Respir Crit Care Med 1999;159(1):149–57. [DOI] [PubMed] [Google Scholar]
- [114].Ye L, Pien GW, Weaver TE. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med 2009;10(10):1075–84. [DOI] [PubMed] [Google Scholar]
- [115].Sforza E, Chouchou F, Collet P, Pichot V, Barthelemy JC, Roche F. Sex differences in obstructive sleep apnoea in an elderly French population. Eur Respir J 2011;37(5):1137–43. [DOI] [PubMed] [Google Scholar]
- [116].Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31(8):1071–8. [PMC free article] [PubMed] [Google Scholar]
- [117].Koo BB, Dostal J, Ioachimescu O, Budur K. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath = Schlaf & Atmung 2008;12(3):259–64. [DOI] [PubMed] [Google Scholar]
- [118].O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 2000;161(5):1465–72. [DOI] [PubMed] [Google Scholar]
- [119].Valipour A Gender-related differences in the obstructive sleep apnea syndrome. Pneumologie (Stuttgart, Germany) 2012;66(10):584–8. [DOI] [PubMed] [Google Scholar]
- [120].Ye L, Pien GW, Ratcliffe SJ, Weaver TE. Gender differences in obstructive sleep apnea and treatment response to continuous positive airway pressure. J Clin Sleep Med: JCSM: Off Publ Am Acad Sleep Med 2009;5(6): 512–8. [PMC free article] [PubMed] [Google Scholar]
- [121].Mermigkis C, Bouloukaki I, Mermigkis D, Kallergis E, Mavroudi E, Varouchakis G, et al. CRP evolution pattern in CPAP-treated obstructive sleep apnea patients. Does gender play a role? Sleep Breath = Schlaf & Atmung 2012;16(3):813–9. [DOI] [PubMed] [Google Scholar]
- [122].Lee JE, Yang SW, Ju YJ, Ki SK, Chun KH. Sleep-disordered breathing and Alzheimer’s disease: a nationwide cohort study. Psychiatry Res 2019;273: 624–30. [DOI] [PubMed] [Google Scholar]
- [123].Cooke JR, Ancoli-Israel S, Liu L, Loredo JS, Natarajan L, Palmer BS, et al. Continuous positive airway pressure deepens sleep in patients with Alzheimer’s disease and obstructive sleep apnea. Sleep Med 2009;10(10): 1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med: JCSM: Off Publ Am Acad Sleep Med 2009;5(4):305–9. [PMC free article] [PubMed] [Google Scholar]
- *[125].Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc 2008;56(11):2076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Chong MS, Ayalon L, Marler M, Loredo JS, Corey-Bloom J, Palmer BW, et al. Continuous positive airway pressure reduces subjective daytime sleepiness in patients with mild to moderate Alzheimer’s disease with sleep disordered breathing. J Am Geriatr Soc 2006;54(5):777–81. [DOI] [PubMed] [Google Scholar]
- [127].Moraes W, Poyares D, Sukys-Claudino L, Guilleminault C, Tufik S. Donepezil improves obstructive sleep apnea in Alzheimer disease: a doubleblind, placebo-controlled study. Chest 2008;133(3):677–83. [DOI] [PubMed] [Google Scholar]
- [128].Yun CH, Lee HY, Lee SK, Kim H, Seo HS, Bang SA, et al. Amyloid burden in obstructive sleep apnea. J Alzheimer’s Dis: JAD 2017;59(1):21–9. 10.3233/JAD-161047. [DOI] [PubMed] [Google Scholar]
- [129].Bu XL, Liu YH, Wang QH, Jiao SS, Zeng F, Yao XQ, et al. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep 2015;5:13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Motamedi V, Kanefsky R, Matsangas P, Mithani S, Jeromin A, Brock MS, et al. Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apnea. Sleep Med 2018;43:71–6. [DOI] [PubMed] [Google Scholar]
- [131].Pase MP, Beiser AS, Himali JJ, Satizabal CL, Aparicio HJ, DeCarli C, et al. Assessment of plasma total tau level as a predictive biomarker for dementia and related endophenotypes. JAMA Neurol 2019. May 1;76(5): 598–606. 10.1001/jamaneurol.2018.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[132].Ju YE, Finn MB, Sutphen CL, Herries EM, Jerome GM, Ladenson JH, et al. Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol 2016;80(1):154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ju YS, Zangrilli MA, Finn MB, Fagan AM, Holtzman DM. Obstructive sleep apnea treatment, slow wave activity, and amyloid-beta. Ann Neurol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Osorio RS, Ayappa I, Mantua J, Gumb T, Varga A, Mooney AM, et al. The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiol Aging 2014;35(6): 1318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Varga AW, Wohlleber ME, Gimenez S, Romero S, Alonso JF, Ducca EL, et al. Reduced slow-wave sleep is associated with high cerebrospinal fluid Abeta42 levels in cognitively normal elderly. Sleep 2016;39(11):2041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[136].Sharma RA, Varga AW, Bubu OM, Pirraglia E, Kam K, Parekh A, et al. Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly: a longitudinal study. Am J Respir Crit Care Med 2018. April 1;197(7):933–43. 10.1164/rccm.201704-0704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Liguori C, Mercuri NB, Izzi F, Romigi A, Cordella A, Sancesario G, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep 2017;40(5). [DOI] [PubMed] [Google Scholar]
- [138].Spira AP, Yager C, Brandt J, Smith GS, Zhou Y, Mathur A, et al. Objectively measured sleep and beta-amyloid burden in older adults: a Pilot study. SAGE Open Med 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Mendes A, Tezenas du Montcel S, Levy M, Bertrand A, Habert MO, Bertin H, et al. Multimorbidity is associated with preclinical Alzheimer’s disease neuroimaging biomarkers. Dement Geriatr Cognit Disord 2018;45(5–6):272–81. [DOI] [PubMed] [Google Scholar]
- [140].Handa SS, Baba S, Yamashita K, Nishizaka M, Ando S. The severity of obstructive sleep apnea syndrome cannot predict the accumulation of brain amyloid by imaging with [11C]-Pittsburgh compound B PET computed tomography in patients with a normal cognitive function. Ann Nucl Med 2019. July;33(7):541–4. 10.1007/s12149-019-01349-6 Epub 2019 Mar 18. [DOI] [PubMed] [Google Scholar]
- [141].Lutsey PL, Norby FL, Gottesman RF, Mosley T, MacLehose RF, Punjabi NM, et al. Sleep apnea, sleep duration and brain MRI markers of cerebral vascular disease and Alzheimer’s disease: the atherosclerosis risk in communities study (ARIC). PLoS One 2016;11(7):e0158758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[142].Bubu OM, Pirraglia E, Andrade AG, Sharma RA, Gimenez-Badia S, Umasabor-Bubu OQ, et al. Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep 2019. June 11;42(6). 10.1093/sleep/zsz048.pii:zsz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Kronholm E Sleep in cognitive life-time trajectory. Sleep Med 2012;13(7): 777–8. [DOI] [PubMed] [Google Scholar]
- [144].Scullin MK. Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging 2013;28(1):105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci: J Assoc Psychol Sci 2015;10(1):97–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Fogel SM, Albouy G, Vien C, Popovicci R, King BR, Hoge R, et al. fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum Brain Mapp 2014;35(8):3625–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Spencer RM, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learn Mem 2007;14(7):480–4. [DOI] [PubMed] [Google Scholar]
- [148].Tucker M, McKinley S, Stickgold R. Sleep optimizes motor skill in older adults. J Am Geriatr Soc 2011;59(4):603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Gui WJ, Li HJ, Guo YH, Peng P, Lei X, Yu J. Age-related differences in sleep-based memory consolidation: a meta-analysis. Neuropsychologia 2017;97: 46–55. [DOI] [PubMed] [Google Scholar]
- [150].Mazza S, Pepin JL, Naegele B, Plante J, Deschaux C, Levy P. Most obstructive sleep apnoea patients exhibit vigilance and attention deficits on an extended battery of tests. Eur Respir J 2005;25(1):75–80. [DOI] [PubMed] [Google Scholar]
- [151].Sforza E, Haba-Rubio J, De Bilbao F, Rochat T, Ibanez V. Performance vigilance task and sleepiness in patients with sleep-disordered breathing. Eur Respir J 2004;24(2):279–85. [DOI] [PubMed] [Google Scholar]
- [152].Shpirer I, Elizur A, Shorer R, Peretz RB, Rabey JM, Khaigrekht M. Hypoxemia correlates with attentional dysfunction in patients with obstructive sleep apnea. Sleep Breath = Schlaf & Atmung 2012;16(3):821–7. [DOI] [PubMed] [Google Scholar]
- [153].Tulek B, Atalay NB, Kanat F, Suerdem M. Attentional control is partially impaired in obstructive sleep apnea syndrome. J Sleep Res 2013;22(4): 422–9. [DOI] [PubMed] [Google Scholar]
- [154].Verstraeten E, Cluydts R. Executive control of attention in sleep apnea patients: theoretical concepts and methodological considerations. Sleep Med Rev 2004;8(4):257–67. [DOI] [PubMed] [Google Scholar]
- [155].Verstraeten E, Cluydts R, Pevernagie D, Hoffmann G. Executive function in sleep apnea: controlling for attentional capacity in assessing executive attention. Sleep 2004;27(4):685–93. [PubMed] [Google Scholar]
- [156].Dijk DJ, Groeger JA, Stanley N, Deacon S. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep 2010;33(2): 211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kiernan TE, Capampangan DJ, Hickey MG, Pearce LA, Aguilar MI. Sleep apnea and white matter disease in hypertensive patients: a case series. The Neurologist 2011;17(5):289–91. [DOI] [PubMed] [Google Scholar]
- [158].Ludemann P, Dziewas R, Soros P, Happe S, Frese A. Axonal polyneuropathy in obstructive sleep apnoea. J Neurol Neurosurg Psychiatr 2001;70(5): 685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep 2008;31(7): 967–77. [PMC free article] [PubMed] [Google Scholar]
- [160].Morrell MJ, Finn L, Kim H, Peppard PE, Badr MS, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Crit Care Med 2000;162(6):2091–6. [DOI] [PubMed] [Google Scholar]
- [161].Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342(19):1378–84. [DOI] [PubMed] [Google Scholar]
- [162].Corfield DR, Meadows GE. Control of cerebral blood flow during sleep and the effects of hypoxia. Adv Exp Med Biol 2006;588:65–73. [DOI] [PubMed] [Google Scholar]
- [163].Foster GE, Hanly PJ, Ostrowski M, Poulin MJ. Effects of continuous positive airway pressure on cerebral vascular response to hypoxia in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2007;175(7):720–5. [DOI] [PubMed] [Google Scholar]
- [164].Pallayova M, Steele KE, Magnuson TH, Schweitzer MA, Hill NR, Bevans-Fonti S, et al. Sleep apnea predicts distinct alterations in glucose homeostasis and biomarkers in obese adults with normal and impaired glucose metabolism. Cardiovasc Diabetol 2010;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol (Bethesda, Md: 1985) 2005;99(5):2008–19. [DOI] [PubMed] [Google Scholar]
- [166].Zizi F, Jean-Louis G, Brown CD, Ogedegbe G, Boutin-Foster C, McFarlane SI. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr Diabetes Rep 2010;10(1):43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Lurie A Metabolic disorders associated with obstructive sleep apnea in adults. Adv Cardiol 2011;46:67–138. [DOI] [PubMed] [Google Scholar]
- [168].Seetho IW, Wilding JP. Sleep-disordered breathing, type 2 diabetes and the metabolic syndrome. Chronic Respir Dis 2014;11(4):257–75. [DOI] [PubMed] [Google Scholar]
- [169].Guglielmotto M, Tamagno E, Danni O. Oxidative stress and hypoxia contribute to Alzheimer’s disease pathogenesis: two sides of the same coin. Sci World J 2009;9:781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF, et al. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J Biol Chem 2007;282(15):10873–80. [DOI] [PubMed] [Google Scholar]
- [171].Fisk L, Nalivaeva NN, Boyle JP, Peers CS, Turner AJ. Effects of hypoxia and oxidative stress on expression of neprilysin in human neuroblastoma cells and rat cortical neurones and astrocytes. Neurochem Res 2007;32(10): 1741–8. [DOI] [PubMed] [Google Scholar]
- [172].Nalivaevaa NN, Fisk L, Kochkina EG, Plesneva SA, Zhuravin IA, Babusikova E, et al. Effect of hypoxia/ischemia and hypoxic preconditioning/reperfusion on expression of some amyloid-degrading enzymes. Ann N Y Acad Sci 2004;1035:21–33. [DOI] [PubMed] [Google Scholar]
- [173].Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, et al. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci USA 2006;103(49):18727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Ng KM, Lau CF, Fung ML. Melatonin reduces hippocampal beta-amyloid generation in rats exposed to chronic intermittent hypoxia. Brain Res 2010;1354:163–71. [DOI] [PubMed] [Google Scholar]
- [175].Shiota S, Takekawa H, Matsumoto SE, Takeda K, Nurwidya F, Yoshioka Y, et al. Chronic intermittent hypoxia/reoxygenation facilitate amyloid-beta generation in mice. J Alzheimer’s Dis: JAD. 2013;37(2):325–33. [DOI] [PubMed] [Google Scholar]
- [176].Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep 2013;36(7):1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Schlosser Covell GE, Dhawan PS, Lee Iannotti JK, Hoffman-Snyder CR, Wellik KE, Caselli RJ, et al. Disrupted daytime activity and altered sleep-wake patterns may predict transition to mild cognitive impairment or dementia: a critically appraised topic. The Neurologist 2012;18(6):426–9. [DOI] [PubMed] [Google Scholar]
- [178].Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res 2012;90(10):2043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Torelli F, Moscufo N, Garreffa G, Placidi F, Romigi A, Zannino S, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage 2011;54(2):787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Morrell MJ, Jackson ML, Twigg GL, Ghiassi R, McRobbie DW, Quest RA, et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax 2010;65(10):908–14. [DOI] [PubMed] [Google Scholar]
- [181].Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 2015;14(4):388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Rosenzweig I, Williams SC, Morrell MJ. The impact of sleep and hypoxia on the brain: potential mechanisms for the effects of obstructive sleep apnea. Curr Opin Pulm Med 2014;20(6):565–71. [DOI] [PubMed] [Google Scholar]
- [183].Schild L, Reiser G. Oxidative stress is involved in the permeabilization of the inner membrane of brain mitochondria exposed to hypoxia/reoxygenation and low micromolar Ca2+. FEBS J 2005;272(14):3593–601. [DOI] [PubMed] [Google Scholar]
- [184].Zhu Y, Fenik P, Zhan G, Mazza E, Kelz M, Aston-Jones G, et al. Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model. J Neurosci: Off J Soc Neurosci 2007;27(37):10060–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci 2011;14(6):750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gartner J, Frahm J. Identification of the upward movement of human CSF in vivo and its relation to the brain venous system. J Neurosci: Off J Soc Neurosci 2017;37(9):2395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Kiviniemi V, Wang X, Korhonen V, Keinanen T, Tuovinen T, Autio J, et al. Ultra-fast magnetic resonance encephalography of physiological brain activity - glymphatic pulsation mechanisms? J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metabol 2016;36(6):1033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Baril AA, Gagnon K, Brayet P, Montplaisir J, De Beaumont L, Carrier J, et al. Gray matter hypertrophy and thickening with obstructive sleep apnea in middle-aged and older adults. Am J Respir Crit Care Med 2017;195(11): 1509–18. [DOI] [PubMed] [Google Scholar]
- [189].O’Donoghue FJ, Wellard RM, Rochford PD, Dawson A, Barnes M, Ruehland WR, et al. Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. Sleep 2012;35(1):41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Baronio D, Martinez D, Fiori CZ, Bambini-Junior V, Forgiarini LF, Pase da Rosa D, et al. Altered aquaporins in the brains of mice submitted to intermittent hypoxia model of sleep apnea. Respir Physiol Neurobiol 2013;185(2):217–21. [DOI] [PubMed] [Google Scholar]
- [191].Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol 2014;71(8):971–7. [DOI] [PubMed] [Google Scholar]
- [192].Ratnavadivel R, Stadler D, Windler S, Bradley J, Paul D, McEvoy RD, et al. Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax 2010;65(2): 107–12. [DOI] [PubMed] [Google Scholar]
- [193].Saboisky J, Eckert D, Malhotra A. Stable breathing through deeper sleeping. Thorax 2010;65(2):95–6. [DOI] [PubMed] [Google Scholar]
- [194].Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med 2004;170(11):1225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Ondze B, Espa F, Dauvilliers Y, Billiard M, Besset A. Sleep architecture, slow wave activity and sleep spindles in mild sleep disordered breathing. Clin Neurophysiol: Off J Int Fed Clin Neurophysiol 2003;114(5):867–74. [DOI] [PubMed] [Google Scholar]
- [196].Brillante R, Cossa G, Liu PY, Laks L. Rapid eye movement and slow-wave sleep rebound after one night of continuous positive airway pressure for obstructive sleep apnoea. Respirology (Carlton, Vic) 2012;17(3): 547–53. [DOI] [PubMed] [Google Scholar]
- [197].Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain: J Neurol 2017;140(8):2104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Kam K, Parekh A, Sharma RA, Andrade A, Lewin M, Castillo B, et al. Sleep oscillation-specific associations with Alzheimer’s disease CSF biomarkers: novel roles for sleep spindles and tau. Mol Neurodegener 2019;14(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Finelli LA, Achermann P, Borbely AA. Individual ‘fingerprints’ in human sleep EEG topography. Neuropsychopharmacology: Off Publ Am Coll Neuropsychopharmacol 2001;25(Suppl. 5):S57–62. [DOI] [PubMed] [Google Scholar]
- [200].Werth E, Achermann P, Borbely AA. Brain topography of the human sleep EEG: antero-posterior shifts of spectral power. Neuroreport 1996;8(1): 123–7. [DOI] [PubMed] [Google Scholar]
- [201].Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science (New York, NY) 2019;363(6429):880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Harper RM, Poe GR, Rector DM, Kristensen MP. Relationships between hippocampal activity and breathing patterns. Neurosci Biobehav Rev 1998;22(2):233–6. [DOI] [PubMed] [Google Scholar]
- [203].Poe GR, Rector DM, Harper RM. Hippocampal reflected optical patterns during sleep and waking states in the freely behaving cat. J Neurosci: Off J Soc Neurosci 1994;14(5 Pt 2):2933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Harper RM, Bandler R, Spriggs D, Alger JR. Lateralized and widespread brain activation during transient blood pressure elevation revealed by magnetic resonance imaging. J Comp Neurol 2000;417(2):195–204. [DOI] [PubMed] [Google Scholar]
- [205].Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011;70(11):960–9. [DOI] [PubMed] [Google Scholar]
- [206].Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl 1996;165:3–12. [DOI] [PubMed] [Google Scholar]
- [207].Braak H, Braak E. Staging of Alzheimer-related cortical destruction. Int Psychogeriatrics/IPA 1997;9(Suppl. 1):257–61. discussion 69–72. [PubMed] [Google Scholar]
- [208].Didic M, Barbeau EJ, Felician O, Tramoni E, Guedj E, Poncet M, et al. Which memory system is impaired first in Alzheimer’s disease? J Alzheimer’s Dis: JAD 2011;27(1):11–22. [DOI] [PubMed] [Google Scholar]
- [209].Hahn EA, Wang HX, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. Am J Geriatr Psychiatry: Off J Am Assoc Geriatr Psychiatr 2014;22(11):1262–71. [DOI] [PubMed] [Google Scholar]
- [210].Lichtenberg PA, Ross T, Millis SR, Manning CA. The relationship between depression and cognition in older adults: a cross-validation study. J Gerontol Ser B Psychol Sci Soc Sci 1995;50(1):P25–32. [DOI] [PubMed] [Google Scholar]
- [211].Carvalho DZ, St Louis EK, Knopman DS, Boeve BF, Lowe VJ, Roberts RO, et al. Association of excessive daytime sleepiness with longitudinal beta-amyloid accumulation in elderly persons without dementia. JAMA Neurol 2018;75(6):672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.