Key Points
Question
What are the trends in age-standardized incidence rates of cutaneous squamous cell carcinoma (cSCC) from 1989 to 2017 and the estimated trends up to 2027 in the Netherlands and how are these rates associated with the inclusion of multiple cSCCs per patient?
Findings
This cohort study found that, between 1989 and 2017, age-standardized incidence rates of a first cSCC almost tripled for male patients and increased almost 5-fold for female patients; a further 10-year increase of 23% among male patients and 29% among female patients was estimated. Counting multiple cSCCs per patient additionally increased the cSCC burden by approximately 58% for men and 35% for women.
Meaning
These findings call for revision of skin cancer health policies to cope with and subsequently halt the high increase in cSCC incidence.
Abstract
Importance
Until now, most studies on cutaneous squamous cell carcinoma (cSCC) incidence rates concerned only the first cSCC per patient. Given the increase in incidence rates and the frequent occurrence of subsequent cSCCs per patient, population-based data on the incidence rates of both first and multiple cSCCs are needed.
Objectives
To calculate annual age-standardized incidence rates for histopathologically confirmed first and multiple cSCCs per patient and to estimate future cSCC incidence rates up to 2027.
Design, Setting, and Participants
A nationwide population-based epidemiologic cohort study used cancer registry data on 145 618 patients with a first histopathologically confirmed cSCC diagnosed between January 1, 1989, and December 31, 2017, from the Netherlands Cancer Registry and all patients with multiple cSCCs diagnosed in 2017.
Main Outcomes and Measures
Age-standardized incidence rates for cSCC—standardized to the European Standard Population 2013 and United States Standard Population 2000—were calculated per sex, age group, body site, and disease stage. A regression model with positive slope was fitted to estimate cSCC incidence rates up to 2027.
Results
A total of 145 618 patients in the Dutch population (84 572 male patients [58.1%]; mean [SD] age, 74.5 [11.5] years) received a diagnosis of a first cSCC between 1989 and 2017. Based on incident data, European Standardized Rates (ESRs) increased substantially, with the highest increase found among female patients from 2002 to 2017, at 8.2% (95% CI, 7.6%-8.8%) per year. The ESRs for first cSCC per patient in 2017 were 107.6 per 100 000 person-years (PY) for male patients, an increase from 40.0 per 100 000 PY in 1989, and 68.7 per 100 000 PY for female patients, an increase from 13.9 per 100 000 PY in 1989, which corresponds with a US Standardized Rate of 71.4 per 100 000 PY in 2017 for men and 46.4 per 100 000 PY in 2017 for women. Considering multiple cSCCs per patient, ESRs increased by 58.4% for men (from 107.6 per 100 000 PY to 170.4 per 100 000 PY) and 34.8% for women (from 68.7 per 100 000 PY to 92.6 per 100 000 PY). Estimation of ESRs for the next decade show a further increase of 23.0% for male patients (ESR up to 132.4 per 100 000 PY [95% prediction interval, 125.8-139.0 per 100 000 PY]) and 29.4% for female patients (ESR up to 88.9 per 100 000 PY [95% prediction interval, 84.3-93.5 per 100 000 PY]).
Conclusions and Relevance
This nationwide epidemiologic cohort study suggests that incidence rates of cSCC keep increasing, especially among female patients, and that the occurrence of multiple cSCCs per patient significantly adds to the current and future burden on dermatologic health care. Revision of skin cancer policies are needed to halt this increasing trend.
This cohort study calculates annual age-standardized incidence rates for histopathologically confirmed first and multiple cutaneous squamous cell carcinoma per patient and estimates future cutaneous squamous cell carcinoma incidence rates up to 2027.
Introduction
Cutaneous squamous cell carcinoma (cSCC) is the second most common skin cancer among white-skinned individuals and its incidence is still increasing.1,2 Several population-based studies across the world have illustrated this increasing trend, with the highest numbers reported in Australia3,4 and certain regions of the United States5 and slightly lower numbers in European countries.6,7,8 Given the high incidence rates of cSCC along with the associated costs and possibility of fatal progression, reliable and up-to-date data on cSCC epidemiologic characteristics are important to optimize patient care and to provide essential information for health care policy makers.9 Nonetheless, national cancer registries in many countries do not routinely record cSCC cases owing to its high incidence, consequently relying on incomplete data sources such as surveys, claims, or treatment data.1 Also, if cSCCs are registered, only the first tumor per patient is usually registered. However, because the occurrence of multiple cSCCs in a single patient is common, it is important to also evaluate the burden of multiple cSCCs per patient.10 Since 1989, the Netherlands Cancer Registry (NCR) records all histopathologically confirmed first cSCCs per patient in the Netherlands. In September 2016, the NCR began recording multiple cSCCs per patient, further approaching the true disease burden. The aims of this study were to investigate annual age-standardized incidence rates of the first primary cSCC in the Netherlands during a period of nearly 30 years (1989-2017), provide recent nationwide incidence data on multiple cSCCs per patient, and estimate future incidence rates of cSCC up to 2027.
Methods
Study Design, Setting, and Population
Data on all patients with histopathologically confirmed cSCC between January 1, 1989, and December 31, 2017, were retrieved from the nationwide NCR.11 The NCR is based on notification of all histopathologically confirmed malignant neoplasms by the nationwide network and registry of histopathology and cytopathology (PALGA).12 Up to September 1, 2016, patients’ demographic characteristics and tumor characteristics were obtained manually from their medical files by trained NCR personnel. Cases of cSCC with only a general practitioner or pathologist as the treating medical specialist were excluded to warrant reliable trend analyses, owing to variation in registration rules over the years and between regional registries. As a result of the high incidence rates of skin cancer, the NCR replaced manual registration with an automatic linkage to PALGA since September 1, 2016. This change in registration method enabled reliable estimations by including cSCCs that were diagnosed by general practitioners, pathologists, or private clinics without further hospital referral. Moreover, multiple cSCCs per patient were registered since September 1, 2016. This study followed the code of conduct of the Foundation Federation of Dutch Medical Scientific Societies,13 which states that informed consent is not needed for studies in which all data are anonymized and not reducible to the patient level.
All pathology reports with topography “skin” (C44) from the International Classification of Diseases for Oncology, third edition (ICD-O-3)14 with the following morphology codes were considered invasive cSCC: 8010/3, 8051/3, 8070/3-8076/3, 8078/3, 8081/3, 8083/3, and 8084/3. Anatomical subsite of the ICD-O-3 was categorized as follows: lips (code C44.0), eyelid (code C44.1), ear (code C44.2), face (code C44.3), scalp and neck (code C44.4), trunk (code C44.5), arms (code C44.6), legs (code C44.7), and unknown or overlapping (codes C44.8 and C44.9).
Starting September 1, 2016, pathology reports with the following characteristics were considered a report of a new cSCC per patient: a different ICD-O-3 anatomical subsite than in the previous report, the same ICD-O-3 anatomical subsite as in the previous report but another lateralization than in the previous report, the same ICD-O-3 anatomical subsite and lateralization as in the previous report at least 3 months after the previous report. Pathology reports with the same ICD-O-3 anatomical subsite and lateralization as in the previous report within 3 months were counted as 1 cSCC.
To study trends, the study period was divided into 6 periods (1989-1993, 1994-1998, 1999-2003, 2004-2008, 2009-2013, and 2014-2015). Given the change in registration method, the years 2016 and 2017 were evaluated separately. Age at cSCC diagnosis was divided into 3 categories (<70, 70-79, and ≥80 years) and stage (the TNM staging system) was evaluated postoperatively according to the then-current edition.15 When postoperative stage was unknown or surgery was not performed, the clinical TNM stage was used. Information on the historical Dutch population size was retrieved from Statistics Netherlands.16
Statistical Analysis
Annual crude and age-standardized incidence rates per 100 000 person-years (PY) were calculated using the population size (determined on January 1 each year) obtained from Statistics Netherlands. To calculate age-standardized incidence rates, the European Standard Population (ESP) 201317 and United States Standard Population (USSP) 200018 were used. The ESP and USSP are theoretical population structures resembling the European and US population structure, totaling 100 000, which is used in the weighting of incidence data to produce European Standardized Rates (ESRs) and US Standardized Rates (USSRs). Although crude rates could show biased values because of the population’s age distribution, the use of age-standardized incidence rates prevents this bias from occurring. For our main analyses, we used ESRs because the ESP resembles the Dutch population data best. European Standardized Rates were calculated according to sex, age group, body site, and disease stage. Age standardization to other standard populations than ESP and USSP was performed as well.
To evaluate trends in incidence rates for cSCC over time, we calculated the estimated annual percentage change (EAPC) with corresponding 95% CIs using joinpoint regression analyses that identify periods with a statistically significant change in incidence rates.19 To prevent a spurious increasing trend as a result of the automatic linkage to PALGA since mid-2016, the joinpoint regression analyses were restricted to the years up to 2015 in our main analysis. For the years 2013 to 2015, a flawed trend was expected because skin cancers were increasingly treated in private practices during this period but no NCR registration of these tumors took place.20 Therefore, in a revised analysis, we omitted the years 2013 to 2015 and included the years 2016 and 2017 to perform a trend analysis most likely representing the true incidence rates.
To estimate incidence rates of cSCC up to 2027, 2 models were fitted with a positive slope and 95% prediction intervals (PIs) were calculated.21,22 The fitted models were Ecit = nit(αi + βit) and Ecit = nitαi(1 + βt), where nit is the number of PY in age group i and year t; αi, βi, and β are the model parameters; and Ecit is the expected number of cases in the corresponding stratum. The first model assumes linear changes for the incidence cit/nit over time t. The second model assumes proportionality of the slope (αiβ) to the intercept (αi) in the linear trend, hereby preserving the age-dependent pattern of incidence rates within the period of estimation. Consequently, age-specific estimations can be made with greater precision. The second model showed the best fit and was used for our cSCC estimations.
All analyses were stratified by sex. All tests were 2-sided with P < .05 considered statistically significant. Statistical analyses were performed using SPSS, version 25.0 statistical software (SPSS Inc); STATA, version 14 with the predaaap macro (StataCorp); and Joinpoint Regression Program, version 4.7.0.0 (IMS Inc).19
Results
Incidence of First cSCC per Patient
From 1989 through 2017, 145 618 patients in the Dutch population received a diagnosis of a first cSCC. Of these, 84 572 were male (58.1%), with an overall mean (SD) age of 74.5 [11.5] years. The age-standardized incidence rates of male patients with a first cSCC almost tripled during the 29-year study period (ESR, 40.0 per 100 000 PY in 1989 and 107.6 per 100 000 PY in 2017; USSR, 26.5 per 100 000 PY in 1989 and 71.4 per 100 000 PY in 2017). For female patients, the age-standardized incidence rates increased almost 5-fold (ESR, 13.9 per 100 000 PY in 1989 and 68.7 per 100 000 PY in 2017; USSR, 9.3 per 100 000 PY in 1989 and 46.4 per 100 000 PY in 2017).
The ESRs and USSRs for cSCC per period of diagnosis and age group are shown in Table 1. The ESRs stratified per body site and stage are shown in eTable 1 in the Supplement and the age-standardized incidence rates for each year separately, including age standardizations to other standard populations, are included in eTable 2 in the Supplement.
Table 1. Numbers and Age-Standardized Incidence Rates of First Cutaneous Squamous Cell Carcinoma in the Netherlands (1989-2017).
| Characteristic | No. of cases | 1989-1993 | 1994-1998 | 1999-2003 | 2004-2008 | 2009-2013 | 2014-2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|---|---|---|
| European Standardized Rate per 100 000 person-years for male patients | |||||||||
| Overall | 84 572 | 40.1 | 43.7 | 45.8 | 58.0 | 75.1 | 79.6 | 97.1 | 107.6 |
| Age, y | |||||||||
| <70 | 25 676 | 11.8 | 12.4 | 12.0 | 14.4 | 17.0 | 16.3 | 20.8 | 24.7 |
| 70-79 | 31 039 | 151.9 | 173.0 | 175.1 | 221.8 | 293.5 | 316.4 | 391.2 | 418.8 |
| ≥80 | 27 857 | 325.4 | 348.7 | 394.7 | 514.0 | 682.1 | 742.6 | 879.8 | 974.9 |
| United States Standardized Rate per 100 000 person-years for male patients | |||||||||
| Overall | 84 572 | 26.5 | 29.0 | 30.3 | 38.4 | 49.6 | 52.6 | 64.2 | 71.4 |
| Age, y | |||||||||
| <70 | 25 676 | 8.0 | 8.3 | 8.0 | 9.5 | 11.3 | 10.7 | 13.8 | 16.4 |
| 70-79 | 31 039 | 152.8 | 174.2 | 176.4 | 223.3 | 295.5 | 318.8 | 394.1 | 422.1 |
| ≥80 | 27 857 | 310.5 | 338.3 | 381.5 | 500.4 | 660.1 | 722.9 | 854.0 | 951.6 |
| European Standardized Rate per 100 000 person-years for female patients | |||||||||
| Overall | 61 046 | 14.5 | 17.3 | 19.9 | 27.6 | 39.7 | 44.1 | 59.7 | 68.7 |
| Age, y | |||||||||
| <70 | 17 509 | 4.5 | 5.7 | 6.6 | 9.4 | 13.1 | 13.2 | 18.1 | 21.8 |
| 70-79 | 18 214 | 47.9 | 58.1 | 66.1 | 95.0 | 148.0 | 176.7 | 244.4 | 281.7 |
| ≥80 | 25 323 | 125.7 | 141.5 | 165.2 | 219.9 | 301.5 | 338.1 | 442.7 | 492.9 |
| United States Standardized Rate per 100 000 person-years for female patients | |||||||||
| Overall | 61 046 | 9.7 | 11.7 | 13.5 | 18.6 | 26.7 | 29.6 | 40.3 | 46.4 |
| Age, y | |||||||||
| <70 | 17 509 | 3.2 | 4.1 | 4.6 | 6.4 | 9.0 | 8.9 | 12.4 | 15.0 |
| 70-79 | 18 214 | 48.2 | 58.5 | 66.6 | 95.6 | 148.8 | 216.7 | 245.8 | 283.1 |
| ≥80 | 25 323 | 118.3 | 136.0 | 160.4 | 214.4 | 294.4 | 401.2 | 435.7 | 486.2 |
Stratified per age group, patients 80 years or older showed the highest age-standardized incidence rates for both sexes with an ESR as high as 974.9 per 100 000 PY for male patients (USSR, 951.6 per 100 000 PY) and 492.9 per 100 000 PY for female patients (USSR, 486.2 per 100 000 PY) in 2017 (Table 1). The face was the site with the most cSCCs in both male (30 473 of 84 572 [36.0%]) and female patients (27 357 of 61 046 [44.8%]), and two-thirds of all cSCCs were diagnosed in stage I (male patients, 56 450 of 84 572 [66.7%]; female patients, 40 821 of 61 046 [66.9%]) (eTable 1 in the Supplement).
Prominent sex-specific differences were found regarding body site of cSCCs. Although the face showed the highest ESRs for both male and female patients, the ears and scalp and neck were much less affected in female patients than in male patients (eTable 1 in the Supplement). The ESR of cSCCs located on the ears was the second-highest affected location up to 2006 for male patients; after 2006, the scalp and neck region was the second-highest affected location (eFigure 1 in the Supplement). For female patients, the arms showed the second-highest ESRs instead.
Time Trends for cSCC Incidence
Joinpoint regression analyses with EAPCs for the years preceding the change in registration method (1989-2015) are shown in Figure 1A. Significant increases in cSCC incidence were found in the first 2 time periods for both sexes: the ESR of cSCC for male patients increased by 1.3% (95% CI, 1.0%-1.7%) between 1989 and 2003 and increased by 6.5% (95% CI, 5.4%-7.6%) between 2003 and 2011. Incidence rates in males were higher than incidence rates in females, but the EAPCs in females showed slightly greater slopes: 2.9% (95% CI, 2.3%-3.5%) between 1989 and 2002 and 8.2% (95% CI, 7.0%-9.5%) between 2002 and 2011. Although the years 2013 to 2015 showed a flattening inclination in ESRs, this observed trend did not reach significance. Our revised analysis showed almost equal EAPC values as in our main analysis. However, the increasing trend now prolonged from 2002 to 2017, with an EAPC of 5.7% (95% CI, 5.2%-6.1%) for male patients and 8.2% (95% CI, 7.6%-8.8%) for female patients, was revealed (Figure 1B).
Figure 1. European Standardized Rate (ESR) and Joinpoint Analyses of Cutaneous Squamous Cell Carcinoma (cSCC) in the Netherlands for Male and Female Patients.
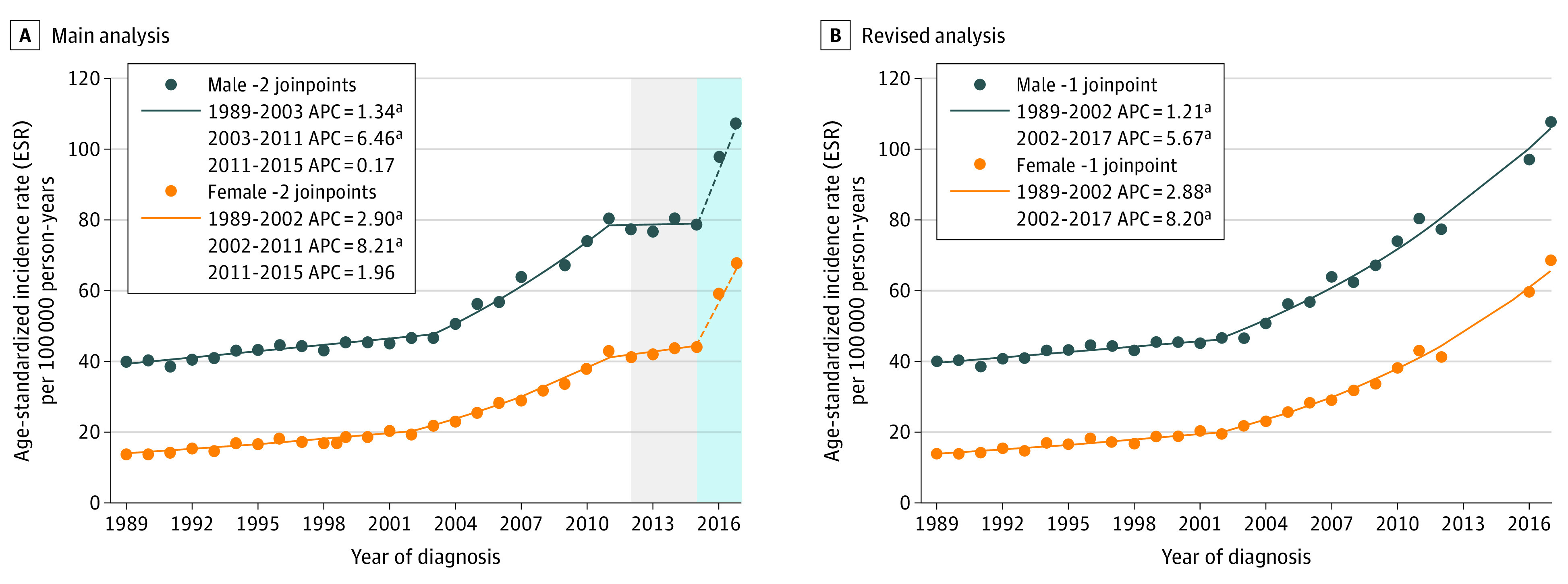
A, Main analysis: ESRs per 100 000 person-years for the period from 1989 to 2017 and joinpoint analyses with estimated annual percentage change (APC) for the period from 1989 to 2015, in dark blue for male patients and orange for female patients. The gray background for the period from 2012 to 2015 depicts a spurious flattening owing to nonregistration of patients with cSCC who were increasingly treated in private practices in this period. The light blue background for the years 2016 to 2017 emphasizes the optimized cSCC registration method. The dashed portions of the lines from 2015 to 2017 indicate that no trend analyses were conducted for this period. B, Revised analysis: ESRs per 100 000 person-years and estimated APCs for the period from 1989 to 2017, without the years 2013 to 2015, in dark blue for male patients and orange for female patients. This curve reflects the true burden of cSCC most accurately. aSignificant APC value.
Multiple cSCCs per Patient in 2017
Analysis of the total number of cSCCs in 2017 resulted in 19 359 cSCCs in 17 512 patients. Two-thirds of all patients had a single cSCC in 2017 (11 943 [68.2%]), and one-third of all patients (5569 [31.8%]) had multiple cSCCs in 2017. Most patients with multiple cSCCs (n = 5089) already had 1 or more cSCCs diagnosed before 2017, and 8.6% (n = 480) developed both their first and subsequent tumors in 2017 (Figure 2A). Figure 2B shows the distribution of the number of cSCCs (2, 3, and ≥4) among patients in 2017 with multiple cSCCs. This is the distribution of cSCCs in 1 calendar year in the NCR and not the total distribution of multiple cSCCs per patient. Location-specific distributions of cSCCs between the first and subsequent cSCCs were comparable (eFigure 2 in the Supplement).
Figure 2. Distribution of Number of Patients With Single and Multiple Cutaneous Squamous Cell Carcinomas (cSCCs) in 2017.
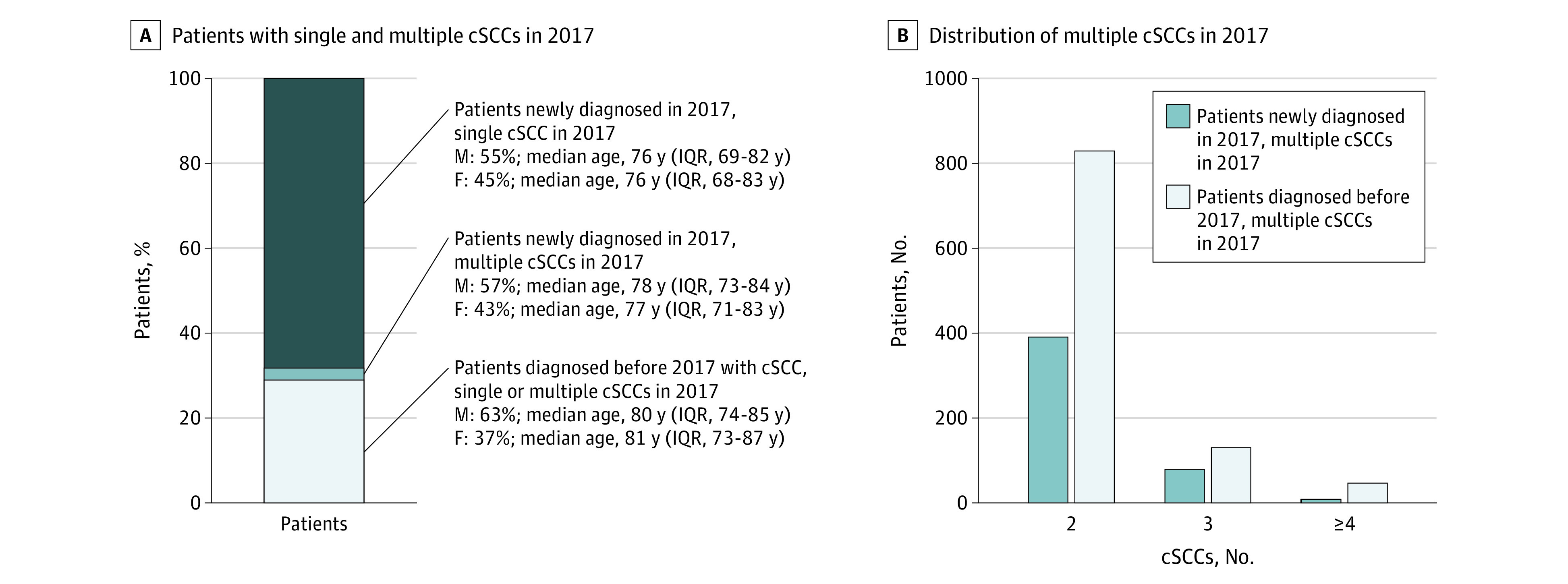
A, Patients with 1 or multiple cSCCs in 2017, including their sex and age distribution. B, Distribution of multiple cSCCs (2, 3, or ≥4) per patient in the same year. IQR indicates interquartile range.
Table 2 shows the outcome of including multiple cSCCs per patient in 2017 compared with including only the first cSCC. When only the first cSCC per patient is considered, 31.1% of all cSCCs in that year (6013 of 19 359) are being disregarded. This means that almost one-third of the overall burden of cSCCs in 2017 was caused by subsequent cSCCs per patient. In terms of ESRs, this corresponds with an increase of 58.4% for male patients (from 107.6 to 170.4 per 100 000 PY) and an increase of 34.8% for female patients (from 68.7 to 92.6 per 100 000 PY).
Table 2. Absolute Numbers, Crude Rates, and ESRs for First and Multiple cSCCs in 2017.
| Characteristic | Patients in 2017 | ||
|---|---|---|---|
| Male | Female | All | |
| cSCCs, No. | |||
| First | 7261 | 6085 | 13 346 |
| All | 11 146 | 8213 | 19 359 |
| Additional | 3885 | 2128 | 6013 |
| CR first cSCC per 100 000 person-years | 85.7 | 71.8 | 78.1 |
| CR multiple cSCCs per 100 000 person-years | 131.5 | 95.4 | 113.3 |
| ESR first cSCC per 100 000 person-years | 107.6 | 68.7 | 84.3 |
| ESR multiple cSCCs per 100 000 person-years | 170.4 | 92.6 | 123.3 |
Abbreviations: CR, crude rate; cSCC, cutaneous squamous cell carcinoma; ESR, European Standardized Rate.
Estimation of Future cSCC Incidence Rates
Based on the incidence rates of a first cSCC between 2008 and 2017, analyses resulted in an expected number of 12 273 newly diagnosed male patients with cSCC (95% PI, 11 682-12 864) and 9780 newly diagnosed female patients with cSCC (95% PI, 9269-10 289) in 2027. Compared with the incidence of a first cSCC in 2017 (male patients, 7261; and female patients, 6085 [Table 2]), this estimate corresponds with a 69.0% increase in the number of male patients and 60.7% increase in the number of female patients. The estimated ESRs for 2027 are 132.4 per 100 000 PY (95% PI, 125.8-139.0 per 100 000 PY) for male patients and 88.9 per 100 000 PY (95% PI, 84.3-93.5 per 100 000 PY) for female patients, equivalent to an increase of 23.0% in ESRs among male patients and a 29.4% increase in ESRs among female patients between 2017 and 2027.
Discussion
In this nationwide population-based study with nearly 150 000 patients with cSCC, the incidence rates of cSCC increased for both sexes and in all age groups, with the highest increase during the period from 2002 to 2017. Although male patients showed the highest overall incidence rates of cSCC, the mean annual increase in incidence was markedly higher for female patients of all age groups, irrespective of body site. Data on multiple cSCCs in 2017 showed that incidence rates increased by approximately 58% for men and 35% for women when subsequent tumors per patient were included, which has been an underexposed group until now in most cSCC studies worldwide. Given the even higher estimated incidence rates for 2027 and presence of multiple tumors per patient, a better understanding of epidemiologic characteristics of cSCC is important for all stakeholders (eg, physicians and health care policy makers) to cope with the high volume of patients with cSCC.
We found the highest ESRs for cSCCs located on the face and scalp and neck of male patients and on the face and arms of female patients, which is in line with findings of previous studies.7,8,23,24 The male-specific locations mainly reflect the UV light–damaged skin owing to a history of outdoor work and a short haircut or baldness. Patients with a cSCC on one of these most common locations were also the patients with more subsequent cSCCs, again suggesting the relevance of high cumulative UV light exposure. The fact that body sites that are generally covered, such as the trunk and legs, showed the highest location-specific increases in ESR over time in both sexes is most likely associated with changed tanning habits.7,25 Because male patients already had the highest cSCC incidence rates, the relative association of augmentened outdoor recreational activities with cSCC incidence might have been larger for female patients, explaining the steeper increase in cSCC incidence for female patients as found for the recent years.
The age-standardized incidence rates in our population are among the highest in Europe1,7,8 except for the United Kingdom.23 Higher age-standardized incidence rates of cSCC have been found in Australia,3,4,26 New Zealand,27 Scotland,28 and the United States.5 Besides variances in exposure to risk factors (eg, differences in latitude and climate, skin type and genetic predisposition, sunbed usage, outdoor activities, and clothing style), dissimilarities in keratinocyte carcinoma registration procedures, accustomed health-seeking behavior, and access to health care might explain the differences in cSCC incidence across countries. Regarding trends over time, the EAPCs found in the Netherlands were almost equal to the EAPCs in Belgium29 and higher compared with Denmark,30 Sweden,31 and Norway8 in the same study periods.
Our analyses showed that the age-standardized incidence rates of cSCC will continue to increase, which will have major implications on health care planning and cost management and hopefully stimulate further prevention strategies. We also showed that 30% of all cSCCs in a year are missed when only the first cSCC per patient is registered and all subsequent cSCCs are disregarded. This finding corresponds with the underrepresentation of 30% to 50% as found in studies from Australia26 and New Zealand,27 emphasizing that the true burden caused by cSCCs is much higher than shown in most studies considering only the incidence of first cSCC per patient.
Limitations
This study has some limitations. Despite the nationwide population-based setting of our study, as well as the highly advanced registration system of the NCR, we may still have underestimated the cSCC incidence rates, as patients treated by general practitioners or private practices without further hospital referral were not registered until 2016. Furthermore, cSCCs diagnosed on the same body site within 3 months’ time were considered recurrences and therefore were not counted as new tumors, which may have caused an underestimation of the true number of cSCCs per patient as shown for 2017. However, recurrences could have occurred after the 3-month window as well, meaning that the reported number of subsequent primary cSCCs for 2017 could have comprised recurrent cSCCs. Also, while recurrences were not counted as new tumors, in reality they add to the burden of skin cancer care, which is therefore even higher than we presented. Another downside of the automatic linkage system was that the recording of tumor stages decreased significantly because no manual check of medical records was being performed since September 2016, leading to high proportions of unknown tumor stages since 2016. Hence, we obtained more truthful incidence rates but could not assess an association with each tumor stage. Moreover, only histopathologically confirmed cSCCs were registered, missing a proportion of patients who were treated without histologic confirmation or who did not seek any medical care. However, because the cSCC guideline from the Dutch Society for Dermatology and Venereology states that all removed cSCCs should follow histopathologic examination, we expect the proportion of nonhistologically confirmed cSCCs to be rather small.32 Last, the underestimation of ESRs was mainly apparent from 2013 to 2015, when skin cancers were increasingly treated in private practices with no NCR registration thereof.20 To account for this spurious fluctuation, we performed a revised regression analysis that reflected the real incidence trend in the population as close to reality as possible.
Conclusions
Both the current and estimated incidence rates up to 2027 show a high and still increasing trend for cSCC incidence. Compared with all other cancers, the cSCC incidence rates are highest and keep increasing, while the incidence rates of almost all other malignant neoplasms in the Netherlands have flattened.33 We therefore emphasize the importance and value of complete nationwide cSCC registries with the same quality as for other invasive malignant neoplasms. Considering the even higher burden on dermatologic care when multiple cSCC data are included, our results call for revision of skin cancer health policies to be able to cope with the rising burden of keratinocyte carcinoma management. Full attention is needed to identify high-risk cSCCs or patients at risk for multiple cSCCs to establish efficient follow-up regimens.34 Ultimately, primary prevention will remain the key strategy to halt the increasing trend in cSCC incidence and the occurrence of multiple cSCCs per patient.
eTable 1. Numbers and European Standardized Rates of First cSCC Stratified by Body Site and Stage in Males and Females
eTable 2. Incidence Rates (per 100,000 Person-Years) of cSCC in the Netherlands in Males and Females From 1989 to 2017, Age-Standardized to the European Standard Population 2013 (European Standardized Rate [ESR 2013]), US Standard Population 2000 (United States Standardized Rate [USSR]), European Standard Population 1976 (European Standardized Rate [ESR 1976]), World Standard Population 1968 (World Standardized Rate [WSR]) and World (WHO 2000-2025) Standard Population (World Standardized Rate [WSR 2000-2025])
eFigure 1. Three Year Moving ESR Averages for cSCC per Body Site in Males (A) and Females (B)
eFigure 2. Body Site Specific Distribution of First cSCCs (A) and Subsequent cSCCs (B)
References
- 1.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069-1080. doi: 10.1111/j.1365-2133.2012.10830.x [DOI] [PubMed] [Google Scholar]
- 2.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237-247. doi: 10.1016/j.jaad.2017.08.059 [DOI] [PubMed] [Google Scholar]
- 3.Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985. Med J Aust. 2006;184(1):6-10. doi: 10.5694/j.1326-5377.2006.tb00086.x [DOI] [PubMed] [Google Scholar]
- 4.Staples M, Marks R, Giles G. Trends in the incidence of non-melanocytic skin cancer (NMSC) treated in Australia 1985-1995: are primary prevention programs starting to have an effect? Int J Cancer. 1998;78(2):144-148. doi: [DOI] [PubMed] [Google Scholar]
- 5.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151(10):1081-1086. doi: 10.1001/jamadermatol.2015.1187 [DOI] [PubMed] [Google Scholar]
- 6.Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48(13):2046-2053. doi: 10.1016/j.ejca.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 7.Korhonen N, Ylitalo L, Luukkaala T, et al. . Characteristics and trends of cutaneous squamous cell carcinoma in a patient cohort in Finland 2006-2015. Acta Derm Venereol. 2019;99(4):412-416. doi: 10.2340/00015555-3110 [DOI] [PubMed] [Google Scholar]
- 8.Robsahm TE, Helsing P, Veierød MB. Cutaneous squamous cell carcinoma in Norway 1963-2011: increasing incidence and stable mortality. Cancer Med. 2015;4(3):472-480. doi: 10.1002/cam4.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trakatelli M, Ulrich C, del Marmol V, Euvrard S, Stockfleth E, Abeni D. Epidemiology of nonmelanoma skin cancer (NMSC) in Europe: accurate and comparable data are needed for effective public health monitoring and interventions. Br J Dermatol. 2007;156(suppl 3):1-7. Published correction appears in Br J Dermatol. 2007;157(3):634. doi: 10.1111/j.1365-2133.2007.07861.x [DOI] [PubMed] [Google Scholar]
- 10.Flohil SC, van der Leest RJ, Arends LR, de Vries E, Nijsten T. Risk of subsequent cutaneous malignancy in patients with prior keratinocyte carcinoma: a systematic review and meta-analysis. Eur J Cancer. 2013;49(10):2365-2375. doi: 10.1016/j.ejca.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 11.Integraal Kankercentrum Nederland. Over werkgroepen. Accessed November 25, 2019. https://www.werkgroepeniknl.nl/
- 12.Casparie M, Tiebosch AT, Burger G, et al. . Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Association of Universities in the Netherlands Netherlands code of conduct for research integrity. Published 2018. Accessed September 22, 2020. http://www.vsnu.nl/files/documents/Netherlands Code of Conduct for Research Integrity 2018.pdf
- 14.Fritz A, Percy C, Jack A, eds. International Classification of Diseases for Oncology (ICD-O). 3rd ed World Health Organization; 2002. [Google Scholar]
- 15.Brierley JD, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours. 8th ed Wiley-Blackwell; 2017. [Google Scholar]
- 16.Central Bureau of Statistics website. Accessed April 15, 2019. https://www.cbs.nl/
- 17.Eurostat European Comission Revision of the European Standard Population. Accessed December 19, 2019. https://ec.europa.eu/eurostat/documents/3859598/5926869/KS-RA-13-028-EN.PDF/e713fa79-1add-44e8-b23d-5e8fa09b3f8f
- 18.Surveillance, Epidemiology, and End Results Program Standard populations (millions) for age-adjustment. Accessed December 19, 2019. https://seer.cancer.gov/stdpopulations/
- 19.Division of Cancer Control & Population Sciences, National Cancer Institute. Joinpoint trend analysis software. Accessed March 29, 2020. https://surveillance.cancer.gov/joinpoint
- 20.Vektis Share of independent treatment centers continues to increase. Accessed December 12, 2019. https://www.vektis.nl/nieuws/aandeel-zelfstandige-behandelcentra-blijft-toenemen
- 21.Dyba T, Hakulinen T. Do cancer predictions work? Eur J Cancer. 2008;44(3):448-453. doi: 10.1016/j.ejca.2007.11.014 [DOI] [PubMed] [Google Scholar]
- 22.Dyba T, Hakulinen T, Päivärinta L. A simple non-linear model in incidence prediction. Stat Med. 1997;16(20):2297-2309. doi: [DOI] [PubMed] [Google Scholar]
- 23.Venables ZC, Nijsten T, Wong KF, et al. . Epidemiology of basal and cutaneous squamous cell carcinoma in the U.K. 2013-15: a cohort study. Br J Dermatol. 2019;181(3):474-482. doi: 10.1111/bjd.17873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youl PH, Janda M, Aitken JF, Del Mar CB, Whiteman DC, Baade PD. Body-site distribution of skin cancer, pre-malignant and common benign pigmented lesions excised in general practice. Br J Dermatol. 2011;165(1):35-43. [DOI] [PubMed] [Google Scholar]
- 25.Bränström R, Kristjansson S, Ullén H. Risk perception, optimistic bias, and readiness to change sun related behaviour. Eur J Public Health. 2006;16(5):492-497. [DOI] [PubMed] [Google Scholar]
- 26.Pandeya N, Olsen CM, Whiteman DC. The incidence and multiplicity rates of keratinocyte cancers in Australia. Med J Aust. 2017;207(8):339-343. [DOI] [PubMed] [Google Scholar]
- 27.Elliott BM, Douglass BR, McConnell D, Johnson B, Harmston C. Incidence, demographics and surgical outcomes of cutaneous squamous cell carcinoma diagnosed in Northland, New Zealand. N Z Med J. 2018;131(1475):61-68. [PubMed] [Google Scholar]
- 28.Brewster DH, Bhatti LA, Inglis JH, Nairn ER, Doherty VR. Recent trends in incidence of nonmelanoma skin cancers in the east of Scotland, 1992-2003. Br J Dermatol. 2007;156(6):1295-1300. [DOI] [PubMed] [Google Scholar]
- 29.Callens J, Van Eycken L, Henau K, Garmyn M. Epidemiology of basal and squamous cell carcinoma in Belgium: the need for a uniform and compulsory registration. J Eur Acad Dermatol Venereol. 2016;30(11):1912-1918. [DOI] [PubMed] [Google Scholar]
- 30.Birch-Johansen F, Jensen A, Mortensen L, Olesen AB, Kjær SK. Trends in the incidence of nonmelanoma skin cancer in Denmark 1978-2007: rapid incidence increase among young Danish women. Int J Cancer. 2010;127(9):2190-2198. [DOI] [PubMed] [Google Scholar]
- 31.Hussain SK, Sundquist J, Hemminki K. Incidence trends of squamous cell and rare skin cancers in the Swedish national cancer registry point to calendar year and age-dependent increases. J Invest Dermatol. 2010;130(5):1323-1328. [DOI] [PubMed] [Google Scholar]
- 32.Oncoline. cSCC guideline from the Dutch Society for Dermatology and Venereology. Accessed March 29, 2020. https://www.oncoline.nl/plaveiselcelcarcinoom-van-de-huid
- 33.IKNL. Incidence rates of all cancers in the Netherlands (1989-2018). Accessed July 8, 2020. https://iknlsawebprod.blob.core.windows.net/mediacontainer/iknl/media/pdfs/kankersoorten/iknl_huidkanker-in-nl_rapport_nkr.pdf
- 34.Pelster MW, Wayne JD, Yoo S. Outcomes of patients with multiple cutaneous squamous cell carcinomas. JAMA Oncol. 2016;2(1):130-131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Numbers and European Standardized Rates of First cSCC Stratified by Body Site and Stage in Males and Females
eTable 2. Incidence Rates (per 100,000 Person-Years) of cSCC in the Netherlands in Males and Females From 1989 to 2017, Age-Standardized to the European Standard Population 2013 (European Standardized Rate [ESR 2013]), US Standard Population 2000 (United States Standardized Rate [USSR]), European Standard Population 1976 (European Standardized Rate [ESR 1976]), World Standard Population 1968 (World Standardized Rate [WSR]) and World (WHO 2000-2025) Standard Population (World Standardized Rate [WSR 2000-2025])
eFigure 1. Three Year Moving ESR Averages for cSCC per Body Site in Males (A) and Females (B)
eFigure 2. Body Site Specific Distribution of First cSCCs (A) and Subsequent cSCCs (B)


