Abstract
An imbalance of angiogenesis contributes to many pathologies such as cancer, arthritis and retinopathy, hence molecules that can modulate angiogenesis are of considerable therapeutic importance. Despite many reports on the promising antiangiogenic properties of naturally occurring flavonoids, no flavonoids have progressed to the clinic for this application. This systematic review and meta-analysis therefore evaluates the antiangiogenic activities of a wide range of flavonoids and is presented in two sections. The first part of the study (Systematic overview) included 402 articles identified by searching articles published before May 2020 using ScienceDirect, PubMed and Web of Science databases. From this initial search, different classes of flavonoids with antiangiogenic activities, related pathologies and use of in vitro and/or in/ex vivo angiogenesis assays were identified. In the second part (Meta-analysis), 25 studies concerning the antiangiogenic evaluation of flavonoids using the in vivo chick chorioallantoic membrane (CAM) assay were included, following a targeted search on articles published prior to June 2020. Meta-analysis of 15 out of the 25 eligible studies showed concentration dependent antiangiogenic activity of six compared subclasses of flavonoids with isoflavones, flavonols and flavones being the most active (64 to 80% reduction of blood vessels at 100 µM). Furthermore, the key structural features required for the antiangiogenic activity of flavonoids were derived from the pooled data in a structure activity relationship (SAR) study. All in all, flavonoids are promising candidates for the development of antiangiogenic agents, however further investigations are needed to determine the key structural features responsible for their activity.
Keywords: flavonoids, angiogenesis, inflammation, cancer, in-vivo angiogenesis, CAM assay, SAR
1. Introduction
Angiogenesis is the process of forming new blood vessels. Physiologically, angiogenesis is pivotal for tissue growth and regeneration [1] which is beneficial for many processes including embryogenesis and wound healing. Regulation of angiogenesis is complex and is maintained by the balance between endogenous stimulators (e.g., vascular endothelial growth factor (VEGF), platelet derived growth factors (PDGFs) and hypoxia-inducible factors (HIFs)), and inhibitors (e.g., angiostatin and endostatin). Other body conditions also contribute to the regulation of angiogenesis under physiological conditions. For example, certain metabolic demands such as the need for more oxygen can induce VEGF secretion and angiogenesis in heart and brain tissues [2,3]. Since angiogenesis affects many organs and tissues in the body, an imbalance in its regulation has been associated with different pathologies [4]. For instance, cancer, rheumatoid arthritis and diabetic retinopathy feature an upregulation of proangiogenic factors [5]. Conversely, if antiangiogenic factors were upregulated, several cardiovascular diseases are more likely to happen [6]. The use of drugs like Bevacizumab (Avastin®, Genentech) and Aflibercept (Eylea®, Regeneron) for the treatment of cancer and ocular diseases, emphasizes the imperative medicinal applications of antiangiogenic agents [7,8].
Flavonoids are widely distributed in fruits, vegetables and nuts. They are one of the most important chemical classes of natural compounds showing various pharmacological profiles that include anticancer [9,10,11], anti-inflammatory [12], cardioprotective [13] and neuroprotective activities [14].
The antiangiogenic activity of flavonoids has been extensively studied over the last two decades. Several studies document the ability of flavonoids to inhibit the proliferation and migration of endothelial cells by interfering with key angiogenesis signaling cascades such as the mitogen activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) pathways. Nevertheless, they can inhibit the expression of major proangiogenic factors such as VEGF and matrix metalloproteinases (MMPs) [2,7,15].
Researchers rely on different in vitro and in/ex vivo assays to quantitatively assess the effects of chemical compounds on angiogenesis [16,17]. Each of these assays can probe one or more of the different steps involved in the angiogenesis process such as cell proliferation, migration and tubulogenesis.
Despite considerable research concerning the antiangiogenic activities of flavonoids, to date they have neither progressed to the market nor clinical trials for that purpose. Therefore, the aim of this review is to systematically assess the antiangiogenic activities of flavonoids to provide greater insight into their potential as therapeutic agents. This study is comprised of two parts: Section 1 provides a systematic overview of the classes of flavonoids that have been investigated for their antiangiogenic activities, along with a summary of the different in vitro and/or in/ex vivo angiogenesis assays that have been used; Section 2 is a meta-analysis study of a quantitatively comparative subset of data, based on the in vivo chick chorioallantoic membrane (CAM) assay, to statistically evaluate the antiangiogenic effects of flavonoids.
2. Results
2.1. Section 1: Systematic Overview
2.1.1. Search Results
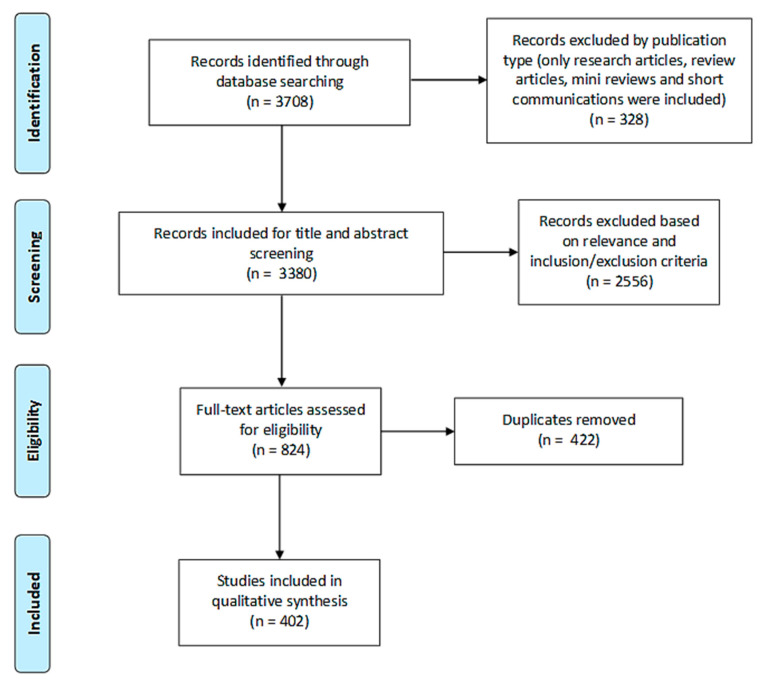
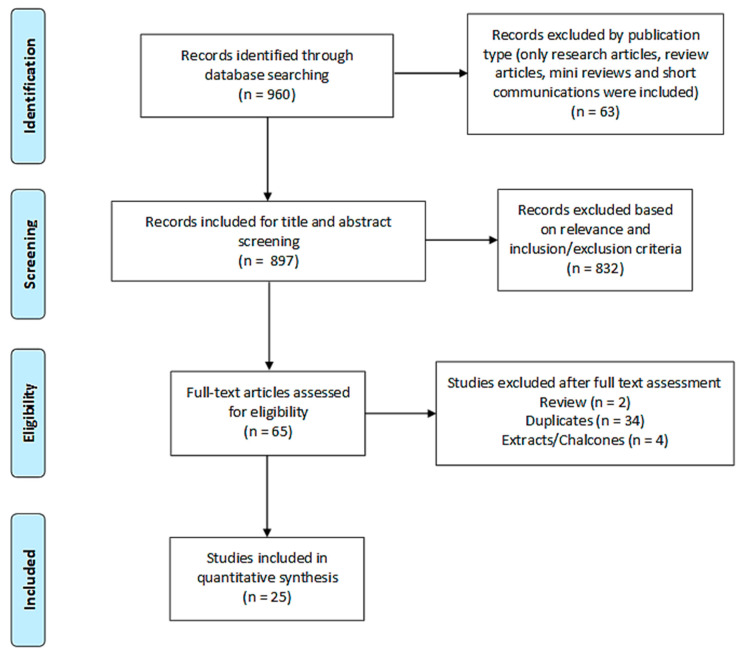
For Section 1 of the study, 3708 records were initially identified in three electronic databases (1555 from ScienceDirect, 1984 from PubMed and 169 from Web of Science). Search results were then limited to research articles, review articles, short communications and systematic reviews and the remaining 3380 articles were subjected to title and abstract screening. 2556 records were found to be irrelevant of the subject in focus or did not fulfill the inclusion criteria. After the removal of duplicates (422), 402 articles were finally included in the qualitative analysis for Section 1 of this study (Figure 1).
Figure 1.
PRISMA flow diagram of study search and selection process of Section 1.
2.1.2. Study Characteristics
The pool of studies included was classified with respect to: (a) flavonoid class (Figure 2), (b) flavonoid name, (c) disease, (d) in vitro test and (e) in/ex vivo tests. Characteristics of the included studies are summarized in Table 1 (flavonols are used as a representative example in Table 1 and Table S1 contains similar data for all classes of flavonoids). A total of 402 research and review articles were considered. All of the included articles reported angiogenesis related in vitro and/or in/ex vivo assays for different classes of flavonoids.
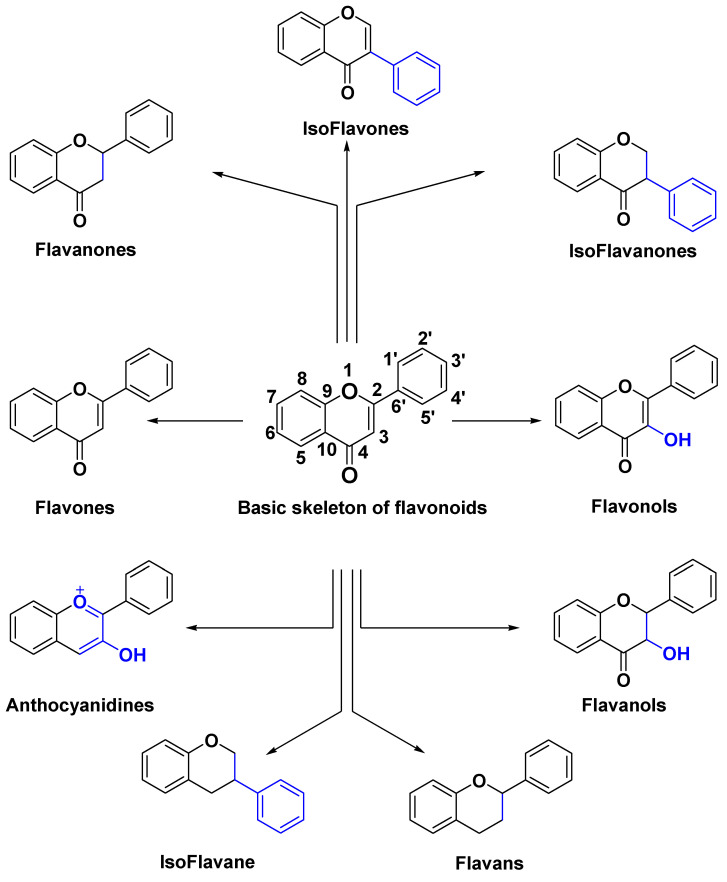
Figure 2.
Chemical structures of classes of flavonoids.
Table 1.
Characteristics of the studies included in Section 1 for flavonols subclass (see Table S1 for all 9 subclasses).
| Flavonol | Disease | In Vitro Tests | In/Ex Vivo Tests | Author, Year |
|---|---|---|---|---|
| Beturetol | Angiogenesis | CAM | Hisanori Hattori, 2011 [18] | |
| Casticin | Cancer * | Shanaya Ramchandani, 2020 [19] | ||
| Denticulatain | Lung Cancer | ZFM | Da Song Yang, 2015 [20] | |
| Dihydrokaempferide | Angiogenesis | CAM | Hisanori Hattori, 2011 [18] | |
| Fisetin | Cancer * | Dharambir Kashyap, 2018 [21] | ||
| Cancer * | Thamaraiselvan Rengarajan, 2016 [22] | |||
| Cancer * | Deeba N.Syed, 2016 [23] | |||
| Cancer * | Lall K. Rahul, 2016 [24] | |||
| Breast Cancer | In | Cheng Fang Tsai, 2018 [25] | ||
| Breast Cancer | WH, In | Xu Sun, 2018 [26] | ||
| Breast Cancer | WH, In | Mets in mice | Jie Li, 2018 [27] | |
| Cervical Cancer | In | Ruey Hwang Chou, 2013 [28] | ||
| Glioma | In | Chien Min Chen, 2015 [29] | ||
| Hepatic Cancer | In | Xiang Feng Liu, 2017 [30] | ||
| Leukemia | In | Anna Klimaszewska-Wiśniewska, 2019 [31] | ||
| Lung Cancer | WH, In | Saba Tabasum, 2019 [32] | ||
| Lung Cancer | WH, In, Ad | Junjian Wang, 2018 [33] | ||
| Prostate Cancer | WH, In, Ad | Chi Sheng Chien, 2010 [34] | ||
| Renal Cancer | In | Yih Shou Hsieh, 2019 [35] | ||
| Retinopathy | RbCN | A M Joussen, 2000 [36] | ||
| Galangin | Hepatic Cancer * | Dengyang Fang, 2019 [37] | ||
| Angiogenesis | TF, Ad | Jong Deog Kim, 2006 [38] | ||
| Glioma | TF, In | CAM, MD in mice | Daliang Chen, 2019 [39] | |
| Glioma | In | Deqiang Lei, 2018 [40] | ||
| Hepatic Cancer | WH, In, Ad | Shang Tao Chien, 2015 [41] | ||
| Ovarian Cancer | TF | CAM | Haizhi Huang, 2015 [42] | |
| Renal Cancer | WH, In | Jingyi Cao, 2016 [43] | ||
| Renal Cancer | In | Yun Zhu, 2018 [44] | ||
| Gossypin | Gastric Cancer | In | Wang Li, 2019 [45] | |
| Herbacetin | Melanoma | In | Lei Li, 2019 [46] | |
| Hyperoside | Arthritis | WH, In | CIAM in mice | Xiang Nan Jin, 2016 [47] |
| Icariin | Bone disease * | Xin Zhang, 2014 [48] | ||
| Cancer * | Meixia Chen, 2016 [49] | |||
| Angiogenesis | TF, In | RAR | Byung Hee Chung, 2008 [50] | |
| Esophageal Cancer | In | Zhen Fang Gu, 2017 [51] | ||
| Ovarian Cancer | WH | Pengzhen Wang, 2019 [52] | ||
| Wound healing | EWM in rats | Wangkheirakpam Ramdas Singh, 2019 [53] | ||
| Icariside | Cancer * | Meixia Chen, 2016 [49] | ||
| Glioma | WH, In | Kai Quan, 2017 [54] | ||
| Isoviolanthin | Hepatic Cancer | WH, In | Shangping Xing, 2018 [55] | |
| Isosakuranetin | Angiogenesis | CAM | Hisanori Hattori, 2011 [18] | |
| Kaempferol | Cancer * | Allen Y. Chen, 2013 [56] | ||
| Cancer * | Dharambir Kashyap, 2017 [57] | |||
| Angiogenesis | WH, TB, In | Hsien Kuo Chin, 2018 [58] | ||
| Angiogenesis | WH, TB | ZFM | Fang Liang, 2015 [59] | |
| Angiogenesis | CAM | Shigenori Kumazawa, 2013 [60] | ||
| Angiogenesis | TF, Ad | Jong Deog Kim, 2006 [38] | ||
| Diabetes | EWM in rats | Yusuf Özay, 2019 [61] | ||
| Glioma | WH | Vivek Sharma, 2007 [62] | ||
| Glioma | In | Mets in mice | S.C. Shen, 2006 [63] | |
| Hepatic Cancer | WH, In | Mets in mice | Youyou Qin, 2015 [64] | |
| Hepatic Cancer | In | Genglong Zhu, 2018 [65] | ||
| Lung Cancer | WH, In | Eunji Jo, 2015 [66] | ||
| Medulloblastoma | Ad | David Labbé, 2009 [67] | ||
| Oral Cancer | In | Chiao Wen Lin, 2013 [68] | ||
| Osteosarcoma | WH, In, Ad | Hui Jye Chen, 2013 [69] | ||
| Ovarian Cancer | CAM | Haitao Luo, 2009 [70] | ||
| Pancreatic Cancer | In | Jungwhoi Lee, 2016 [71] | ||
| Renal Cancer | WH, In | Mets in mice | Tung Wei Hung, 2017 [72] | |
| Retinal Vascularization | WH, In | Hsiang Wen Chien, 2019 [73] | ||
| Kaempferol-3-O-[(6-caffeoyl)-β- glucopyranosyl (1→3) α-rhamnopyranoside]-7-O-α-rhamnopyranoside | Angiogenesis | WH | Marco Clericuzio, 2012 [74] | |
| Kaempferide | Angiogenesis | CAM | Hisanori Hattori, 2011 [18] | |
| Morin | Arthritis | WH, TB | CIAM in rats | Ni Zeng, 2015 [75] |
| Arthritis | WH, TB | CIAM in rats | Mengfan Yue, 2018 [76] | |
| Leukemia | Ad | Nagaja Capitani, 2019 [77] | ||
| Melanoma | WH | Hua Wen Li, 2016 [78] | ||
| Myricetin | Melanoma * | Nam Joo Kang, 2011 [79] | ||
| Angiogenesis | TF, Ad | Jong Deog Kim, 2006 [38] | ||
| Breast Cancer | In | CAM, MD in mice, RAR | Zhiqing Zhou, 2019 [80] | |
| Breast Cancer | WH, In, Ad | Mets in mice | Yingqian Ci, 2018 [81] | |
| Glioma | WH, In | Wen Ta Chiu, 2010 [82] | ||
| Hepatic Cancer | In | Noriko Yamada, 2020 [83] | ||
| Hepatic Cancer | WH, In | Hongxin Ma, 2019 [84] | ||
| Lung Cancer | WH, In, Ad | Yuan Wei Shih, 2009 [85] | ||
| Medullobalstoma | In, Ad | David Labbé, 2009 [67] | ||
| Ovarian Cancer | TF | CAM | Haizhi Huang, 2015 [42] | |
| Quercetin | Breast Cancer * | Maryam Ezzati, 2020 [86] | ||
| Cancer * | Si-min Tang, 2020 [87] | |||
| Cancer * | Dharambir Kashyap, 2016 [88] | |||
| Colorectal Cancer * | Saber G. Darband, 2018 [89] | |||
| Angiogenesis | WH, In | Nu Ry Song, 2014 [90] | ||
| Angiogenesis | WH, TB | ZFM | Chen Lin, 2012 [91] | |
| Angiogenesis | TF, Ad | Jong Deog Kim, 2006 [38] | ||
| Bladder Cancer | WH, In | Yu Hsiang Lee, 2019 [92] | ||
| Breast Cancer | WH | Divyashree Ravishankar, 2015 [93] | ||
| Breast Cancer | MD in mice | Xin Zhao, 2016 [94] | ||
| Breast Cancer | CAM | Soo Jin Oh, 2010 [95] | ||
| Breast Cancer | WH, In | Asha Srinivasan, 2016 [96] | ||
| Breast Cancer | WH, In | Cheng Wei Lin, 2008 [97] | ||
| Breast Cancer | In | Amilcar Rivera Rivera, 2016 [98] | ||
| Cancer | TF | ZFM | Daxian Zhao, 2014 [99] | |
| Cancer | TF, In | CAM | Wen Fu Tan, 2003 [100] | |
| Cancer | MD in mice | Xiangpei Zhao, 2012 [101] | ||
| Cancer | WH, In | Lung Ta Lee, 2004 [102] | ||
| Cancer | WH | Dong Eun Lee, 2013 [103] | ||
| Colorectal Cancer | WH, In | Mets in mice | Ji Ye Kee, 2016 [104] | |
| Glioma | WH | Hong Chao Pan, 2015 [105] | ||
| Glioma | WH, In | Wen Ta Chiu, 2010 [82] | ||
| Glioma | WH, In | Yue Liu, 2017 [106] | ||
| Glioma | In | Jonathan Michaud-Levesque, 2012 [107] | ||
| Glioma | WH | Alessandra Bispo da Silva, 2020 [108] | ||
| Glioma | WH, TB, In | Yue Liu, 2017 [109] | ||
| Hepatic Cancer | In | Noriko Yamada, 2020 [83] | ||
| Hepatic Cancer | WH, In | Jun Lu, 2018 [110] | ||
| Lung Cancer | WH | Anna Klimaszewska-Wiśniewska, 2017 [111] | ||
| Lung Cancer | In | Tzu Chin Wu, 2018 [112] | ||
| Lung Cancer | In | Yo Chuen Lin, 2013 [113] | ||
| Medulloblastoma | In, Ad | David Labbé, 2009 [67] | ||
| Melanoma | In | Mun Kyung Hwang, 2009 [114] | ||
| Melanoma | In | Hui Hui Cao, 2015 [115] | ||
| Melanoma | WH, In | Mets in mice | Hui Hui Cao, 2014 [116] | |
| Oral Cancer | In | Junfang Zhao, 2019 [117] | ||
| Osteoblasts | In | Tae Wook Nam, 2008 [118] | ||
| Osteosarcoma | WH, In, Ad | Shenglong Li, 2019 [119] | ||
| Osteosarcoma | WH, In | Mets in mice | Haifeng Lan, 2017 [120] | |
| Osteosarcoma | WH, Ad | Kersten Berndt, 2013 [121] | ||
| Pancreatic Cancer | WH, In | Ying Tang Huang, 2005 [122] | ||
| Pancreatic Cancer | WH, In | Yu Dinglai 2017 [123] | ||
| Prostate Cancer | WH, In | Firdous Ahmad Bhat, 2014 [124] | ||
| Prostate Cancer | TF, In | MD in mice | Feiya Yang, 2016 [125] | |
| Retinoblastoma | In | Wei Song, 2017 [126] | ||
| Quercetin-3-O-[(6-caffeoyl)-β-glucopyranosyl(1→3) α-rhamnopyranoside]-7-O-α-rhamnopyranoside | Angiogenesis | WH | Marco Clericuzio, 2012 [74] | |
| Rutin | Angiogenesis | CAM | César Muñoz Camero, 2018 [127] | |
| Angiogenesis | CAM | Shigenori Kumazawa, 2013 [60] | ||
| Cancer | WH, In, Ad | Mohamed ben Sghaier, 2016 [128] | ||
| Glioma | WH | Alessandra Bispo da Silva, 2020 [108] | ||
| Neuroblastoma | WH, In | Hongyan Chen, 2013 [129] | ||
* Review article; TB, Tube Formation; WH, Wound Healing; In, Invasion; Ad, Adhesion; Mets, Metastasis; CAM, Chick Chorioallantoic Membrane; MPA, Matrigel Plug Assay; RAR, Rat Aortic Ring; EWM, Excision Wound Model; SF, Skin Flap; RRN, Rat Retinal Neovascularization; MAR, Mice Aortic Ring; MD, Microvessel Density; MRN, Mice Retinal Neovascularization; MCN, Mice Corneal Neovascularization; RbCN, Rabbit Corneal Neovascularization; ZFM, Zebra Fish Model; RCN, Rat Corneal Neovascularization; CIAM, Collagen Induced Arthritis Model; DASM, Dorsal air Sac Model; IWM, Incision Wound Model.
2.1.3. Data Analysis
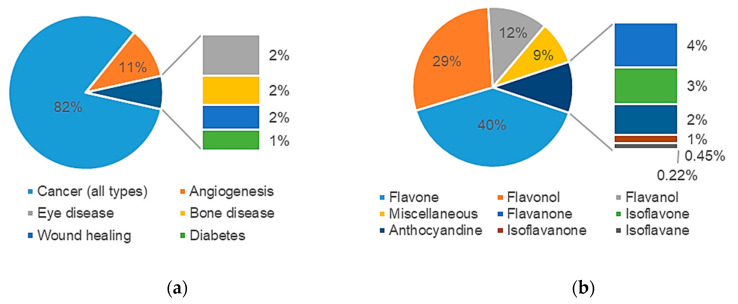
The majority of articles (332, 82%) focused on the implications of angiogenesis on cancer growth and metastasis. 7% of the articles studied antiangiogenic effects of flavonoids on other diseases such as diabetes, bone and eye diseases, whilst 11% focused on the antiangiogenic activity of flavonoids without application to a specific pathology (Figure 3a). A profiling of the studies retrieved with respect to chemical class of flavonoids is shown in Figure 3b.
Figure 3.
Profiling of papers retrieved in Section 1 with respect to: (a) pathology type; (b) chemical class of flavonoid.
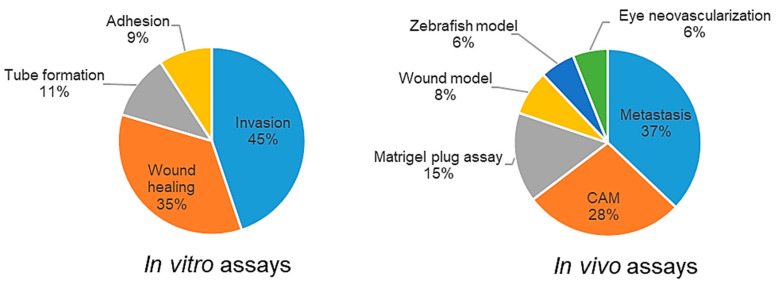
Figure 4 summarizes the types of in vitro and in vivo assays that were utilized in the studies. From a pool of 342 research articles included in this study, 152 articles (44%) reported a combination of in vitro and in/ex vivo assays in their studies. The percentage of research articles that depended only on in/ex vivo tests to evaluate antiangiogenic activity of flavonoids were comparatively low compared to those conducting only in vitro assays (3% vs. 53%, respectively).
Figure 4.
Types of assays used for in vitro and in vivo antiangiogenic evaluation of flavonoids.
2.2. Section 2: Meta-Analysis
2.2.1. Search Results
The second subset search, which is the basis of the meta-analysis forming Section 2 of this study, followed the same general methodology as detailed in the initial overview. 960 records were identified from four electronic databases (381 from ScienceDirect, 496 from PubMed, 65 from Web of Science and 18 from Google Scholar). 25 research articles were finally included in the quantitative analysis after the sequential steps of screening and sifting, as shown in Figure 5.
Figure 5.
PRISMA flow diagram of study search and selection process of Section 2.
2.2.2. Study Characteristics
The main study characteristics of the research articles included in Section 2 for the meta-analysis are summarized in Table 2 by study name.
Table 2.
Characteristics of the studies included in Section 2.
| Author, Year | Flavonoid | Angiogenesis Promoter | Cell Line | Concentration | Time, Duration of Treatment | Results Representation | n |
|---|---|---|---|---|---|---|---|
| Soo Jin Oh, 2010 [95] | Quercetin | NA | TAMR-MCF-7 | 3, 10, 30 µM | NA | Number of branches | 5 to 7 |
| Chiu-Mei Lin, 2006 [130] | Wogonin | LPS (1µg/mL) | NA | 10−5, 10−6, 10−7, 10−8 M | 10th day, 48 h | Percentage of vascular counts (%) | 3 |
| Ling-Zhi Liu, 2011 [131] | Acacetin | NA | OVCAR-3 | 10 µM | 9th day, 4 days | Relative angiogenesis | 5 |
| Kai Zhao, 2018 [132] | Wogonin, LW-215 | NA | NA | Wogonin: 80 ng/CAM, LW-215: 2, 4, 8 ng/CAM | 10th day, 48 h | The number of new vessels (% of control) | 3 |
| Haizhi Huang, 2015 [42] | Galangin, myricetin | NA | OVCAR-3 | G: 40 µM, M: 20 µM | 9th day, 5 days | Blood vessels (%) | 6 |
| Olga Viegas, 2019 [133] | Cyanidin, C-3-O-glucoside, delphinidin, D-3-O-glucoside | NA | NA | 20, 40, 80, 100, 200 µM | 11th day, 48 h | % of control | 5 |
| Wen-fu Tan, 2003 [100] | Quercetin | NA | NA | 25, 50, 100 nmol/10 µL/CAM | 10th day, 48 h | Microscopic pictures | 10 |
| Rajesh Gacche, 2010 [134] | Flavone, 3/5/6/7/-Hydroxy flavone | NA | NA | 10, 50, 100 µM | 10th day, 48 h | Antiangiogenic activity (%) of selected flavonoids | 8 |
| R.N. Gacche, 2011 [135] | 3, 6-DHF, 3, 7-DHF, 5, 7-DHF, apigenin, genistein, kaempferol, luteolin, fisetin, rutin, quercetin | NA | NA | 10, 50, 100 µM in 0.05% DMSO/20 µL/CAM | 10th day, 48 h | Antiangiogenic activity (%) of selected flavonoids | 8 |
| R.N. Gacche, 2015 [136] | 4′-Methoxy flavone, 3-Hydroxy-7-methoxy flavone, Formononetin, Biochanin-A, Diosmin, Hesperitin, Hesperidin, 2′-Hydroxy flavanone, 4′-Hydroxy flavanone, 7-Hydroxy flavanone, Myricetin, Taxifolin, Silibinin, Silymarin, Naringenin, Naringin, Catechin | NA | NA | 10, 50, 100 µM in 0.05% DMSO/20 µL/CAM | 10th day, 48 h | Antiangiogenic activity (%) of selected flavonoids | 8 |
| Yan Chen, 2010 [137] | LYG-202 | NA | NA | 2.4, 12, 60 ng/CAM | 10th day, 48 h | Percentage of vascular counts (% of control) | 10 |
| Hisanori Hattori, 2011 [18] | Beturetol, isosakuranetin | NA | NA | 300 ng/CAM | 5th day, 7 days | Inhibition % of angiogenesis at 300 ng/CAM. | 10 |
| Yujie Huang, 2019 [138] | Wogonoside | NA | MDA-MB-231, MDA-MB-468 | 50, 100, 200 ng/CAM | 10th day, 48 h | Number of new vessels (% cells) | 3 |
| Yan Chen, 2009 [139] | Wogonoside | LPS (1µg/mL) | NA | 1.5, 15, 150 ng/CAM | 10th day, 48 h | Number of vessels (% of LPS) | 10 |
| Xiaobo Li, 2017 [140] | Luteolin | Gas6 (300 ng/mL) | NA | 10, 20 µM | 6th day, 48 h | Relative vascular density (% of control) | 3 |
| Siva Prasad Panda, 2019 [141] | TMF | NA | EAT | 10, 17, 25 µg/mL | 5th day, 11 days | Microscopic pictures | 5 |
| Yujie Huang, 2016 [142] | Wogonoside | NA | MCF-7 | 50, 100, 200 ng/CAM | 10th day, 48 h | Number of new vessels (% MCF-7) | 3 |
| Tariq A. Bhat, 2013 [143] | Acacetin | NA | NA | 50 µM | 6th day, (every 48 h for 8 days) | % capillary formation | 5 independent areas on CAMs for each treatment |
| Jing Fang, 2007 [144] | Apigenin | NA | OVCAR-3, PC-3 | OVCAR-3: 7.5, 15 µM, PC-3: 10, 20 µM |
9th day, 4 days | Quantification of blood vessels on the CAM | 8 |
| Jianchu Chen, 2015 [145] | Nobiletin | NA | A2780 | 20 µM | 9th day, 5 days | Blood vessel count | 10 |
| Poyil Pratheeshkumar, 2012 [146] | Luteolin | NA | NA | 20, 40 µM | 8th day, 48 h | Relative vascular density | 3 |
| Chiu-Mei Lin, 2006 [147] | Wogonin | IL-6 (10 ng/mL) | NA | 10−5, 10−6, 10−7, 10−8 M | 10th day, 48 h | Percentage of vascular count (%) | 3 |
| Dongqing Zhu, 2016 [148] | Baicalin, baicalein | NA | NA | 0.5, 2, 10, 50 µg/mL and 0.2, 1, 5 mg/mL | 7.5th day, 48 h | Number of new blood vessels | 30 |
| Haitao Luo, 2009 [70] | Kaempferol | NA | OVCAR-3 | 20 µM | 9th day, 5 days | Blood vessel count | 5 |
| Laure Favot, 2003 [149] | Delphinidin | NA | NA | 2, 10, 25, 50 µg | 8th day, 48 h | Microscopic pictures | 5 |
n = number of CAMs used in each experiment; NA, Not available; DHF, Dihydroxyflavone; TMF, Trimethoxyflavonoid; TMAR, Tamoxifen breast cancer resistant cell line; MCF-7, Breast cancer cell line; LPS, Lipopolysaccharide; OVCAR-3, Ovarian cancer cell line; MDA-MB-231, MDA-MB-468, Triple negative breast cancer cell lines; Gas6, Growth arrest specific 6; EAT, Mouse breast carcinoma (Ehrlich-Lettre Ascites); PC-3, Prostate cancer cell line; A2780, ovarian cancer cell line; IL-6, Interleukin 6.
2.2.3. Meta-Analysis (Antiangiogenic Effect of Flavonoids on CAMs)
25 studies reporting the CAM assay for the in vivo evaluation of flavonoids were eligible for the meta-analysis. The number of blood vessels relative to the control was used as the outcome measure, the lower the ratio the higher the antiangiogenic activity. The studies were grouped into 3 sub-sets based on the controls used. In the first set (12 studies), the normal vasculature of the CAM was used as a control without any interventions that would induce angiogenesis. The second and third sets, however, tested the antiangiogenic activity of flavonoids on CAMs with abnormal angiogenesis using either proangiogenic factors for set 2 (4 studies) or cancer cell lines for set 3 (9 studies). 10 studies [18,95,100,132,137,139,140,141,145,149] out of the 25 eligible studies were not included in any of the conducted meta-analyses as they failed to report the required data outcomes or did not fit under any particular subgroup.
Set 1: Antiangiogenic effect of flavonoids under normal conditions
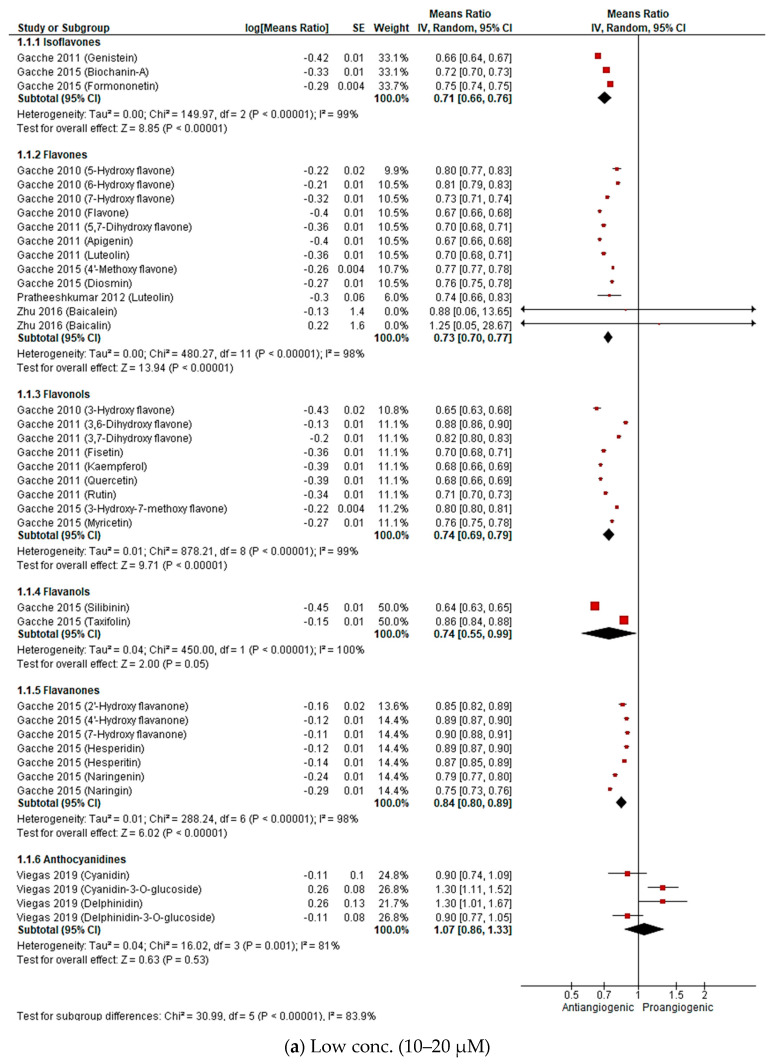
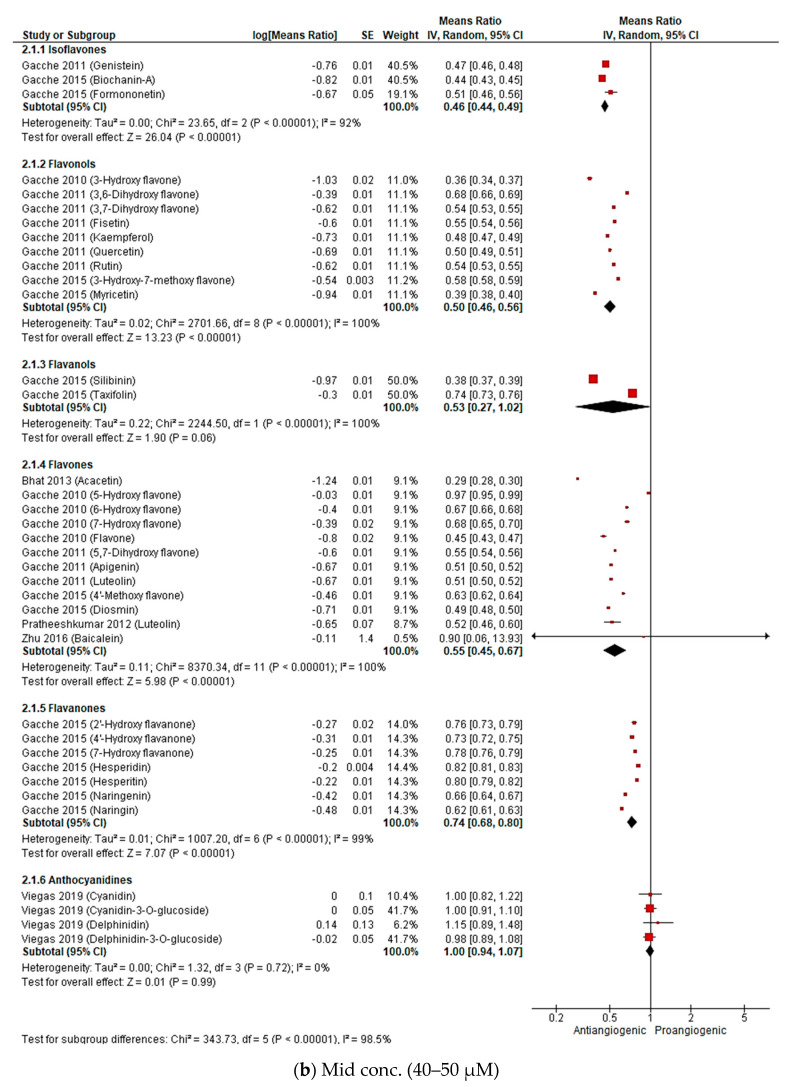
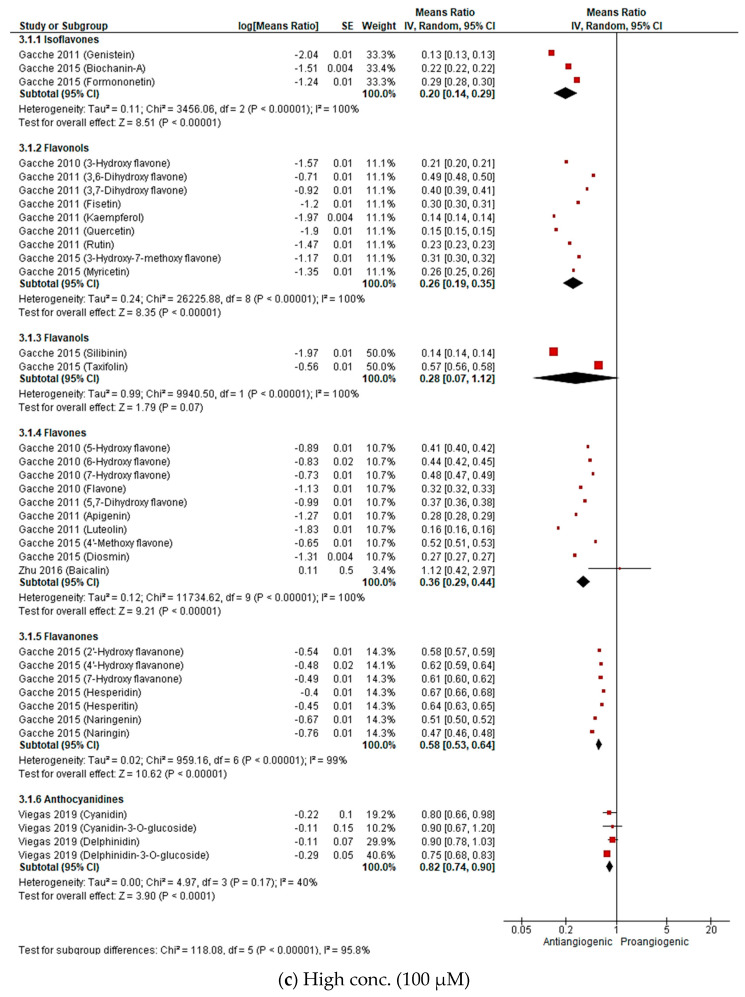
To ensure consistency in our comparison, for the meta-analysis of set 1, the concentrations were grouped into three ranges i.e., low (10–20 µM), medium (40–50 µM) and high (100 µM). Flavonoids were sub grouped based on their chemical class as shown in Figure 6. Pooled results indicate that all subclasses, except for anthocyanidines, demonstrate concentration dependent antiangiogenic activity expressed as a reduction in the number of blood vessels in a CAM. For the flavonols subgroup, for instance, the overall means ratios (summary estimates of antiangiogenic activity of a subgroup of flavonoids relative to control) were 0.74 (95%CI: 0.69, 0.79; p-value < 0.00001), 0.50 (95%CI: 0.46, 0.56; p-value < 0.00001) and 0.26 (95%CI: 0.19, 0.35; p-value < 0.00001) for the low, mid and high concentrations, respectively. On the other hand, the anthocyanidines subgroup exhibited only a minor overall reduction of 18% at the highest concentration and a slightly proangiogenic effect (overall means ratio: 1.07; 95%CI: 0.86, 1.33; p-value: 0.53) at 20 µM.
Figure 6.
Forest plots of means ratio and 95% confidence interval (CI) of number of blood vessels relative to control at 3 concentration ranges as calculated by inverse variance (IV) method: (a) low (10–20 µM); (b) medium (40–50 µM); (c) high (100 µM).
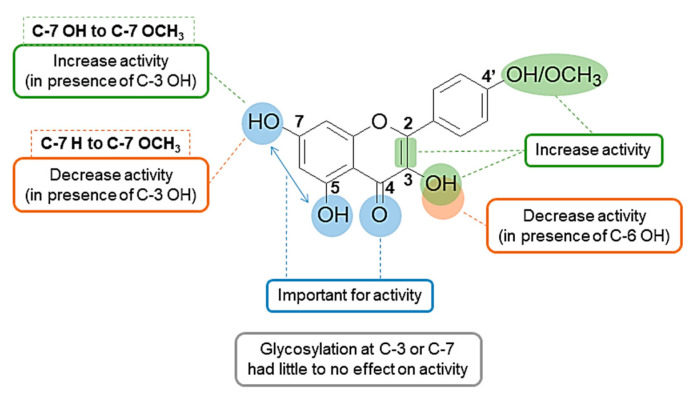
In addition to the forest plot analysis that gives a general idea about the overall in vivo antiangiogenic activity of flavonoids and identifies trends of activity among the different subclasses, some structure activity relationship (SAR) conclusions were drawn from the pooled results (Figure 7).
Figure 7.
Summary of antiangiogenic SAR of flavonoids.
First, there was no correlation between the number of hydroxyl groups and antiangiogenic activity. However, the position of the hydroxyl groups appeared to be of importance as most of the highly active flavonoids had hydroxyl groups at positions 3, 5 and 7 and/or 4′ (e.g., as demonstrated for 3-OH flavone, acacetin, biochanin A, apigenin, silibinin and kaempferol). The 7-OH group can be considered to be of greatest importance for activity since 7-OH flavone showed higher activity in the low and medium concentrations compared to the 5-OH analogue. Absence of the 3-OH group caused up to a 14% decrease in activity at the 50 and 100 µM concentrations, as demonstrated, for example, for 3-OH flavone vs. flavone, kaempferol vs. apigenin and 3,7-diOH flavone vs. 7-OH flavone. This was also true for quercetin vs. luteolin but with only a trivial drop of activity of 1 to 2%. However, this was not the case for 3,6-diOH flavone vs. 6-OH flavone where removal of the 3-OH group slightly increased the activity by 1 to 5% at the mid and high concentrations.
Secondly, unsaturation of the C2 and C3 bond is a common feature of most of the highly active flavonoids and is important for activity. 7-OH flavone and 7-OH flavanone are two good examples that exemplify this, as demonstrated by a reduction of the number of vessels: 27%, 32% and 52% for 7-OH flavone and 10%, 22% and 39% for 7-OH flavanone at 10 µM, 50 µM, and 100 µM, respectively.
Third, there are examples of where the presence of a methoxy group at position 4′ increases activity (e.g., biochanin A, diosmin and formononetin). However, the presence of a methoxy group at C7 caused a decrease in the activity when compared to the unsubstituted analogue (ie for the 3-OH flavone vs. 3-OH-7-OCH3 flavone, reduction of number of vessels: 35% and 20% at 10 µM, 64% and 42% at 50 µM, 79% and 69% at 100 µM, respectively). On the other hand, conversion of the 7-OH group in 3,7-diOH flavone to a 7-OCH3 group in 3-OH-7-OCH3 flavone slightly improved the activity (reduction of number of vessels) from 18% to 20% at 10 µM and from 63% to 69% at 100 µM, respectively. Finally, glycosylation at positions 3 or 7 showed neither a pronounced nor a consistent effect on the antiangiogenic activity of flavonoids. While a decrease in activity was observed with quercetin vs. rutin, hesperitin vs. hesperidin and cyanidin vs. cyanidin-3-O-glucoside, an increase was observed in the cases of naringin vs. naringenin and delphinidin vs. delphinidin-3-O-glucoside.
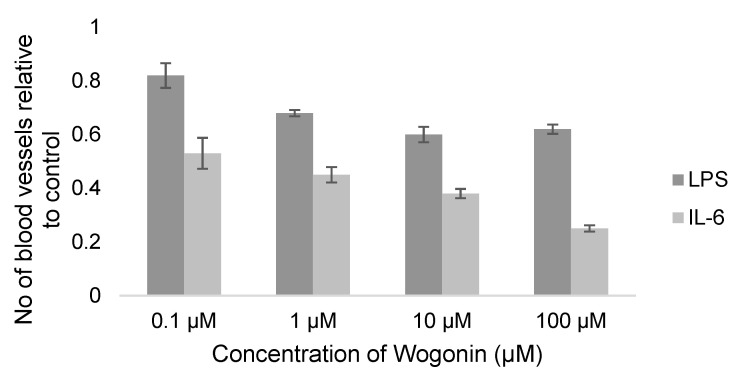
Set 2: Antiangiogenic effect of flavonoids under inflammatory conditions
Lin et al. evaluated the antiangiogenic activity of the flavone wogonin on LPS (the main component of gram negative bacterial membrane) and IL-6 induced angiogenesis in two reports [130,147]. The documented reduction in the number of CAM blood vessels by wogonin was shown to be dose dependent in both cases but more prominent in the case of IL-6 induced angiogenesis (75% as opposed to 38% in the case of LPS induced angiogenesis at 100 µM) (Figure 8). The authors also probed the possible mechanisms of wogonin’s inhibition of this inflammation-induced angiogenesis through different in vitro techniques such as western blotting and polymerase chain reaction (PCR) in which both LPS and IL-6 resulted in an upregulation of the IL-6/IL-6R pathway [130,147]. Although wogonin attenuated the IL-6/IL-6R pathway and levels of VEGF in both cases, it exhibited different expression of downstream vascular endothelial growth factor receptors (VEGFRs). Only VEGFR2 expression was downregulated with wogonin LPS-induced angiogenesis inhibition as opposed to VEGFR1 downregulation with IL-6 induced angiogenesis inhibition. This data needs further investigation in order to understand why these two similar mechanisms lead to the downregulation of two different downstream receptors (VGFR2 and VEGFR1) and to address the impact of this on the antiangiogenic potency. Inhibition of LPS-induced angiogenesis was also reported for wogonoside, which is the 7-glucuronic acid of wogonin, by Chen et al. [139] 150 ng/CAM of wogonoside reduced neo-vascularization of CAMs by 43%. Additionally, wogonoside downregulated mammalian toll-like receptor (TLR4), extracellular signal-regulated kinase (ERK1/2) and p38MAPK in a western blotting assay [139].
Figure 8.
Reported antiangiogenic effect of wogonin on LPS and IL-6 induced angiogenesis ± SEM.
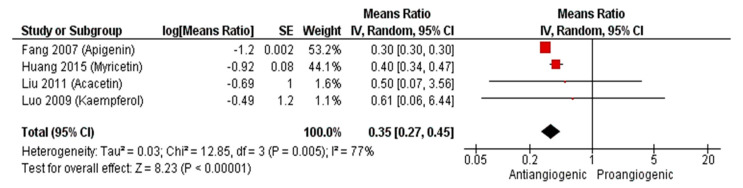
Set 3: Antiangiogenic effect of flavonoids under tumor conditions.
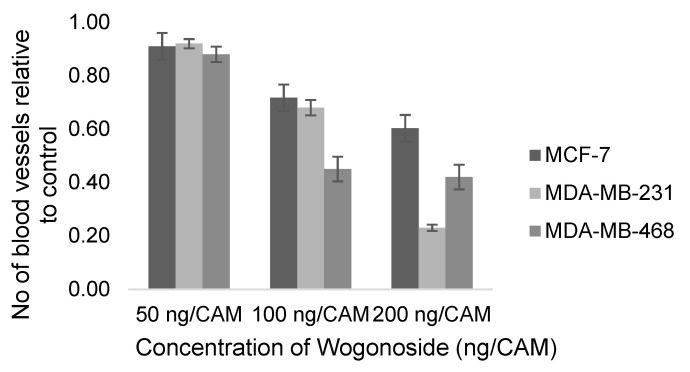
Since angiogenesis plays a vital role in tumor growth and metastasis, several studies have focused on the antiangiogenic evaluation of promising cytotoxic agents. Figure 9 shows the estimated antiangiogenic effect of the 4 flavonoids apigenin, myricetin, acacetin and keampferol on the ovarian cancer cell line (OVCAR-3) at 10–20 µM. The reduction in the number of CAM blood vessels ranged from 30 to 60% with an overall summary outcome of 0.35 (95%CI: 0.27, 0.45; p-value < 0.00001). HIFα and VEGF were significantly downregulated, as evidenced by immunoblotting analysis of CAM OVCAR-3 tissues that were treated with apigenin or acacetin [131,144]. The antiangiogenic activity of the flavone wogonoside was evaluated on the estrogen receptor positive (MCF-7) and two triple negative breast (MDA-MB-231 and MDA-MB-468) cancer cell lines by Huang et al. [138,142]. At 50 ng/CAM, wogonoside’s effect on the 3 cell lines was not prominent (Figure 10). However, a 55% reduction of the number of blood vessels was observed at 100 ng/CAM for the MDA-MB-468 cell line. A two-fold increase in the concentration of wogonoside to 200 ng/CAM did not, however, result in an increased antiangiogenic effect on the same cell line. On the other hand, reduction of the neo-vascularization for the MDA-MB-231 cell line increased from 32% to 77% upon increasing the concentration from 100 to 200 ng/CAM. Huang et al. demonstrated the ability of wogonoside to target the Hedgehog signaling pathway, which is upregulated in triple negative breast cancer, in MDA-MB-231 and MDA-MB-468 cell lines [138]. Expression of the Hedgehog downstream transmembrane protein smoothened (SMO) and glioma-associated oncogene homolog protein (Gli), is significantly increased in triple negative breast cancer [150] leading to an elevation in VEGF levels [151]. According to Huang and his colleagues, wogonoside promoted SMO degradation and inhibited Gli1 activity as well as expression of VEGF [138].
Figure 9.
Forest plot of means ratio and 95% confidence interval (CI) of number of blood vessels relative to control of flavonoids on OVCAR-3 cell lines.
Figure 10.
Reported antiangiogenic effect of wogonoside on breast cancer cell lines; MCF-7, MDA-MB-231 and MDA-MB-468 ± SEM.
2.2.4. Sensitivity Analysis
The high heterogeneity (I2 > 80%) observed for all subgroups in the generated forest plots, except for the anthocyanidines subgroup at the mid and high concentrations analyses (I2 = 0% and 40%, respectively), was expected given that each class included different flavonoid molecules. In that context, a sensitivity analysis was conducted by a leave-one-out strategy to assess the robustness of the results and determine the contribution of each flavonoid to heterogeneity. Overall, the results showed good robustness and the overall summary estimates did not show significant changes upon the systematic removal of individual studies (Tables S2–S4). This was the case in all subgroups with the exception of the flavanol subgroup which showed some difference in the overall summary at all concentrations. At the 40–50 µM range for instance, the overall pooled means ratio changed from 0.53 (95%CI: 0.27, 1.02, I2 = 100%) to 0.74 (95%CI: 0.73, 0.76, NA) and 0.38 (95%CI: 0.37, 0.39, NA) upon removal of the Gacche 2015 (Silibinin) and Gacche 2015 (Taxifolin) flavonoids, respectively (Table S3). This indicates that data provided on the flavanols subgroup is not sufficient to draw meaningful conclusions. Likewise, heterogeneity (I2) of the subgroups totals did not show significant change, with very few exceptions, upon implementation of the leave-one-out strategy (Tables S2–S4). This might be due to the fact that most of the flavonoids in a single subgroup belong to the same study, consequently, there are no differences in their experimental designs. In that case heterogeneity is believed to be either of clinical or statistical origin.
3. Discussion
Flavonoids have been reported to modulate several angiogenic factors and cascades in either a proangiogenic or an antiangiogenic manner which is postulated to be dose dependent [2,148]. A good illustration of this dual effect is demonstrated by the flavone baicalin; low doses were reported to stimulate angiogenesis [152] whilst high doses showed an inhibitory effect [153]. Due to the emerging importance of the use of angiogenesis modulators in the treatment of various pathological conditions including cancer, diabetes, bone, eye, cardiovascular and neurological disorders, the identification of flavonoids altering angiogenesis has gained new significance [2,154]. To the best of our knowledge, no systematic reviews have been conducted to quantitatively assess the antiangiogenic effects of flavonoids, despite the potential of such a study to have a positive impact on the treatment of serious health issues like cancer and rheumatoid arthritis. Given the breadth of the literature related to the antiangiogenic effects of flavonoids, a systematic search of the literature was initially conducted in this research program to identify (a) the extent to which angiogenesis modulation effects had been proposed for flavonoids and (b) the most widely used in vitro and in/ex vivo assays to determine the antiangiogenic activities of flavonoids.
Various study designs have been used in the literature to report on the antiangiogenic activity of chemical compounds. There are a number of comprehensive reviews in the literature comparing the different available angiogenesis assay models [16,17,155,156]. Although in vitro studies are less expensive and quicker to perform than in vivo studies, the results do not always convert into the same effect, in vivo. In vitro assays usually focus on monitoring the individual steps of angiogenesis such as migration or proliferation of endothelial cells rather than the collective formation of new tube-like structures [16]. In vivo assays offer the considerable advantage of mimicking more closely the body’s physiological conditions which is particularly important in angiogenic studies due to the complex nature of the process. While in vivo angiogenesis assays can be more informative, they present some cost, time and experimental design limitations. Inflammation resulting from the trauma that is caused by some assays, for instance, can stimulate several proangiogenic factors which compromise the sensitivity and specificity of the results [17]. Hence, it is recommended that a combination of in vitro and in vivo assays is used to provide consistent and complementary results. In relation to this, 44% of the research articles included in the conducted preliminary search reported a combination of in vitro and in/ex vivo assays.
Herein, a meta-analysis study was carried out in order to quantitatively evaluate the antiangiogenic effects of flavonoids. Only articles implementing the CAM assay in their study design were included. This is because the CAM assay is currently the most widely used in vivo angiogenic assay and, as such, it allows a comparison across different flavonoid types and offers many advantages over other angiogenic assays [157,158,159]. For instance, it is fairly simple, inexpensive, suitable for large scale screening and also offers the important advantage of expressing almost all of the known angiogenic factors [17,156]. Set-up of the assay is briefly as follows: fertilized chicken eggs are incubated at 37 °C for 3 days, a small hole is made in the egg shell to remove some of the albumin in order to facilitate detachment of the CAM from the shell. Compounds under investigation are added to approximately 5 to 10 day old chicks on specific carriers, such as matrigel or sterile filter/plastic discs, through a small window cut in the egg shell. After 48 to 72 h, existing blood vessels or tubules can be visualized and evaluated by light or electron microscopy [17,156]. Nevertheless, the CAM test comes with certain limitations such as sensitivity to oxygen tension and difficulty of visualization of newly formed vessels due to the presence of pre-existing ones [157].
Meta-analysis of results of the antiangiogenic evaluation of flavonoids via the in vivo CAM assay showed increasing activities with increasing concentrations. The evaluated flavonoids also demonstrated antiangiogenic activities of varying potencies. In light of this, results were inspected to gain some insights on the SAR of antiangiogenic activity of flavonoids (Figure 7). Although SARs of chemical compounds change based on the sought pharmacological activity, there are some common structural features of flavonoids that are recognized as important for activity [160]. Combination of the C2=C3 double bond and a 4-C=O is favorable for the antiviral/bacterial [161], anticancer [162,163], cardioprotective [164], anti-inflammatory [165], and antioxidant [164] activities of flavonoids. This conjugation maintains the planarity of the molecule and helps with the electron delocalization between rings A and C which is important for interaction with several targets [160]. Similarly, the 5, 7 di-OH is important for many of the biological activities of flavonoids [164,166,167,168]. This can be explained by the fact that flavonoids exert different pharmacological activities that have mutual and/or overlapping mechanisms. For example, the antioxidant activity of flavonoids contributes to their anti-inflammatory activity and both contribute to their anticancer activity. Moreover, several targets in the body have structurally similar binding sites and this is a phenomenon that is partially responsible for drug promiscuity or polypharmacology (binding of a drug to multiple targets). This was, in fact, observed for binding of the flavonoid quercetin with phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3KCG) and the serine/threonine proto oncogene, PIM1 kinase [169].
With respect to the antiangiogenic activity of flavonoids, limited SAR studies have been reported. Lam et al. tested the antiangiogenic activity of a number of polymethoxylated flavonoids in vitro and in vivo [170]. The authors concluded that methylation of C5, C6, C7 and/or C4′ OH groups increased the activity which is in agreement with Ravishankar et al. [93] who reported the in vitro antiangiogenic activity of a number of quercetin and luteolin derivatives. Our results also suggest that the presence of a 4′-OCH3 increases the antiangiogenic activity. Despite this, there were some discrepancies between the aforementioned SAR conclusions. In this SAR analysis we showed that the presence of a 3-OH group enhanced the antiangiogenic activity, which is in contrast to the report from Ravishankar et al. that noted that the same 3-OH caused a drop in the activity yet methylation of that OH increased the activity [93]. A study by Lam et al. reported that glycosylation at C7 dramatically decreased the activity [170] while our study showed such modification to cause a minor or no decrease in the activity and even a slight increase in some cases. These inconsistencies are likely to be a result of the different experimental methodologies and flavonoid concentrations used in each study. Additionally, the different evaluated flavonoids might exert their antiangiogenic activities by binding to different targets that require different structural features. This highlights the need for larger scale studies to more fully probe the antiangiogenic SAR of flavonoids taking in consideration the employed mechanisms of action.
Since the relation between inflammation and angiogenesis is well established and many flavonoids possess anti-inflammatory activities, several studies assessed the antiangiogenic effects of flavonoids on inflammation-induced angiogenesis. Inflammatory cells like T-lymphocytes and macrophages secrete cytokines that can control the survival, proliferation, activation and migration of endothelial cells [171,172]. Endothelial cells can additionally produce several cytokines and chemokines themselves [173]. Flavonoids such as baicalin, quercetin and kaempferol caused a reduction in both inflammatory and angiogenic markers in cultured macrophages and human umbilical vein endothelial cells (HUVECs) [174,175].
Bacterial infections also trigger angiogenesis through inflammatory pathways. In that context, binding of LPS to the TLR4 receptor located on the surface of endothelial cells leads to upregulation of ERK1/2 and p38MAPK pathways and increases production of pro-inflammatory cytokines like IL-6 [176,177]. Pro-inflammatory cytokines like IL-6 and tumor necrosis factor α (TNFα) can interact with VEGF expression and promote angiogenesis [178,179]. The flavone, wogonin, and its glucoside, wogonoside, showed promising antiangiogenic activity against LPS induced angiogenesis [130]. Wogonin also inhibited IL-6 induced angiogenesis in a concentration dependent manner where it was reported to downregulate VEGFR1 not VEGFR2 genetic expression [147]. While VEGR2 is the main receptor for VEGF and is downregulated by many flavonoids [91,180], VEGFR1‘s role in angiogenesis is still not fully understood and needs further investigation.
As mentioned earlier, cancer is one of the most serious pathologies related to angiogenesis. When cells grow malignantly beyond a certain size, they need more vascularization to receive oxygen and nutrients i.e., tumors depend on angiogenesis to grow above a certain limit, and to metastasize [181]. The tumor vasculature is characterized by an imbalance between pro and anti-angiogenic factors where several angiogenic stimulators like VEGF and HIF are overexpressed. The HIFs are major regulators of angiogenesis and orchestrate many of the steps involved [182]. Under physiological conditions, HIFs are released in response to low oxygen levels in the blood (hypoxia) and stimulate angiogenesis at various levels from endothelial cell proliferation to activating the transcription of angiogenic genes like VEGF and platelet derived growth factor (PDGF). During malignancy, HIF dependent angiogenesis is activated either in response to the predominant hypoxic environment or by the genetic transformations caused by cancer. Flavonoids can downregulate HIFα and VEGF in different cancer cell lines such as OVCAR-3, A2780, MCF-7 and PC-3 [42,70,95,131,144,145]. Many studies have also reported the ability of the flavonoids 3-hydroxy flavone, hesperidin, apigenin, fisetin and many others to reduce tumor size, capillary density and metastasis of different cancers, such as osteosarcoma, melanoma, lung and breast cancers, in xenograft mice [26,183,184,185,186,187].
Although this meta-analysis demonstrated the overall promising in vivo antiangiogenic activity of flavonoids whether in normal, inflammatory or tumor conditions, there were some limitations to the study. First, the standard forms and guidelines used in a systematic analysis are only applicable for clinical or animal trials. Consequently, the quality of the retrieved studies and publication bias were not taken into account here, as this would be methodologically inappropriate. As such, large scale animal studies and meta-analyses evaluating the antiangiogenic activity of flavonoids are much needed in the future to provide more definitive conclusions about the role of flavonoids in angiogenesis.
Second, despite subgrouping flavonoids based on their chemical class and using the random effects model, heterogeneity remained high in this study. There are three types of heterogeneity as defined by the Cochrane handbook for systematic reviews, (i) clinical: differences in participants, interventions or outcomes, (ii) methodological: differences in study design, risk of bias and (iii) statistical: variation in intervention effects or results [188]. Looking deeper into the generated forest plots we concluded the cause of heterogeneity to be clinical and/or statistical. This is mainly because most of the flavonoids in a single subgroup are from the same study hence methodological heterogeneity was excluded. This was further supported by the fact that no single flavonoid was found to solely contribute to the heterogeneity when applying the leave-one-out strategy in the sensitivity analysis. In that case, heterogeneity is mainly due to the different flavonoids used in the study (variation in interventions) in addition to other factors like variable outcomes (number of blood vessels). This clinical heterogeneity can lead to a statistical heterogeneity manifested as a variation among the effects or results (ratio of means of number of blood vessels).
4. Materials and Methods
This review and meta-analysis were conducted according to Preferred Reporting Items for systematic reviews and Meta Analyses (PRISMA) guidelines [189].
4.1. Search Strategy
For Section 1, a literature search was conducted using ScienceDirect, PubMed and Web of Science databases between 3 April 2020 and 23 April 2020 with no time limits. The first set of keywords, (flavonoid, flavone, flavonol, flavanol, anthocyanidin, polyphenol) was combined systematically using the Boolean operator AND with the second set, (angiogenesis, antiangiogenic, proangiogenic, “cell migration”, “wound healing”) in all databases (Table S5).
With regards to the detailed meta-analysis for Section 2, the literature search was carried out using ScienceDirect, PubMed, Web of Science and Google Scholar databases between 8 June 2020 and 10 June 2020 with no time limits. The first set of keywords, (flavonoid, flavone, flavonol, flavanol, anthocyanidin, polyphenol) was combined systematically using the Boolean operator AND with the second set, (angiogenesis, “chick chorioallantoic membrane”, “in vivo angiogenesis”) in all databases (Table S6).
4.2. Inclusion and Exclusion Criteria
Studies were included in the Section 1 overview search if they met the following eligibility criteria: (i) natural or synthetic flavonoids (ii) in vitro, in vivo and/or ex vivo angiogenesis assays (iii) focus on cancer, diabetes, bone regeneration or eye diseases. For the meta-analysis Section 2, the inclusion criteria were: (i) natural or synthetic flavonoids (ii) in vivo CAM angiogenesis assays. Articles not written in English and/or focusing on chalcones, plant extracts/total flavonoids content, combination of compounds, nanoformulations, prodrugs, neurological disorders or cardiovascular diseases were excluded from both searches. This systematic review and meta-analysis followed PRISMA guidelines (Table S7).
4.3. Data Extraction
Initially, articles’ titles and abstracts were screened based on relevance and inclusion/exclusion criteria. Full texts were checked in some cases when abstracts failed to provide a detailed description. Eligible articles were retrieved and data extracted into a specially designed form. The first set of extracted data for Section 1 included title, publication type, year of publication, flavonoid, disease of focus and conducted in vitro and/or in/ex vivo angiogenesis assays. The second set of data were extracted for the meta-analysis Section 2 study and included title, year of publication, flavonoid, angiogenesis promotor, cancer cell line, concentration, time and duration of flavonoid treatment, results representation and number of CAMs used for each test concentration (n).
4.4. Data Analysis
Means of the number of blood vessels in a CAM relative to control were used as the outcome measure. Concentrations were reported in µM in all analyses except for analysis of wogonoside’s antiangiogenic effect on breast cancer cell lines in which ng/CAM was used. Values are represented as means ratio ± standard error of means (SEM). For studies reporting standard deviation (SD), the SEM was calculated by dividing SD by square root of the corresponding study sample size. Pool effect size was expressed as means ratio and 95% CI and was calculated using the inverse variance (IV) method. The random effects model was used because it accounts for between study variability. Heterogeneity was assessed using Higgins’ I2 measure where I2 ≥ 50% indicates substantial heterogeneity [190]. Sensitivity analysis was applied to evaluate the effect of each flavonoid on summary effect size and on heterogeneity. It is based on the sequential removal of one study at a time. Statistical analysis was performed using Review Manager Version 5.1 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and Microsoft Excel 2016.
5. Conclusions
Despite the promising antiangiogenic activity of flavonoids presented in many literature studies, no flavonoids have reached clinical trials for this application. This systematic review and meta-analysis therefore aimed to provide further insight into this area by evaluating the in vivo antiangiogenic activity of flavonoids as determined by the widely reported, clinically relevant CAM assay. A comprehensive overview of the antiangiogenic activities of flavonoids with regards to the class of flavonoids, pathology and assays used was presented. Results have shown that the biggest fraction of studies focused on the flavone subclass, cancer related angiogenesis, and in vitro assays. Furthermore, an overall evaluation of the in vivo antiangiogenic activity of flavonoids was offered focusing on SAR and mechanistic considerations. Isoflavones, flavonols and flavones were found to be the most active classes of flavonoids where antiangiogenic activity was dose dependent. Several structural features were considered, from which it was concluded that the position of the hydroxyl substituents and the degree of unsaturation are key for high activity. Even though there were some limitations such as the miscellany of the studied flavonoids and the high heterogeneity, this study provided substantial information that will underpin further investigations by addressing current gaps in the literature regarding the antiangiogenic activity of flavonoids, and highlighting their future prospective as potentially clinically active antiangiogenic agents.
Acknowledgments
We are grateful to the Newton-Mosharafa Fund for a scholarship that has funded MK’s PhD studies.
Supplementary Materials
The following are available online, Table S1: Study characteristics of Section 1, Tables S2–S4: Sensitivity analysis, Tables S5 and S6: Database search results, Table S7: PRISMA checklist.
Author Contributions
M.K., F.G. and H.M.I.O. designed the study. The literature search, documentation, data extraction and analysis were carried out by M.K. and supervised by F.G. and H.M.I.O. M.K. wrote the first draft of the manuscript. M.K., F.G. and H.M.I.O. edited and revised the manuscript and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Newton-Mosharafa Fund, through a scholarship to MK.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability: Samples of the compounds are not available.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carmeliet P., Jain R.K. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature. 2011;473:289–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diniz C., Suliburska J., Ferreira I.M. New Insights into the Antiangiogenic and Proangiogenic Properties of Dietary Polyphenols. Mol. Nutr. Food Res. 2017;61:1600912. doi: 10.1002/mnfr.201600912. [DOI] [PubMed] [Google Scholar]
- 3.Yancopoulos G.D., Davis S., Gale N.W., Rudge J.S., Wiegand S.J., Holash J. Vascular-Specific Growth Factors and Blood Vessel Formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 4.Shibuya M. VEGF-VEGFR System as a Target for Suppressing Inflammation and Other Diseases. Endocr. Metab. Immune Disord. Targets. 2015;15:135–144. doi: 10.2174/1871530315666150316121956. [DOI] [PubMed] [Google Scholar]
- 5.Gacche R.N., Meshram R.J. Angiogenic Factors as Potential Drug Target: Efficacy and Limitations of Anti-Angiogenic Therapy. Biochim. Biophys. Acta Rev. Cancer. 2014;1846:161–179. doi: 10.1016/j.bbcan.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Majewska I., Gendaszewska-Darmach E. Proangiogenic Activity of Plant Extracts in Accelerating Wound Healing a New Face of Old Phytomedicines. Acta Biochim. Polonica. 2011;58:449–460. doi: 10.18388/abp.2011_2210. [DOI] [PubMed] [Google Scholar]
- 7.Mirossay L., Varinská L., Mojžiš J. Antiangiogenic Effect of Flavonoids and Chalcones: An Update. Int. J. Mol. Sci. 2018;19:27. doi: 10.3390/ijms19010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sulaiman R.S., Basavarajappa H.D., Corson T.W. Natural Product Inhibitors of Ocular Angiogenesis. Exp. Eye Res. 2014;129:161–171. doi: 10.1016/j.exer.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raffa D., Maggio B., Raimondi M.V., Plescia F., Daidone G. Recent Discoveries of Anticancer Flavonoids. Eur. J. Med. Chem. 2017;142:213–228. doi: 10.1016/j.ejmech.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Ravishankar D., Rajora A.K., Greco F., Osborn H.M.I. Flavonoids as Prospective Compounds for Anti-Cancer Therapy. Int. J. Biochem. Cell Biol. 2013;45:2821–2831. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Abotaleb M., Samuel S., Varghese E., Varghese S., Kubatka P., Liskova A., Büsselberg D. Flavonoids in Cancer and Apoptosis. Cancers. 2018;11:28. doi: 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peluso I., Raguzzini A., Serafini M. Effect of Flavonoids on Circulating Levels of TNF-α and IL-6 in Humans: A Systematic Review and Meta-Analysis. Mol. Nutr. Food Res. 2013;57:784–801. doi: 10.1002/mnfr.201200721. [DOI] [PubMed] [Google Scholar]
- 13.Wang X., Ouyang Y.Y., Liu J., Zhao G. Flavonoid Intake and Risk of CVD: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Br. J. Nutr. 2014;111:1–11. doi: 10.1017/S000711451300278X. [DOI] [PubMed] [Google Scholar]
- 14.Beking K., Vieira A. Flavonoid Intake and Disability-Adjusted Life Years Due to Alzheimers and Related Dementias: A Population-Based Study Involving Twenty-Three Developed Countries. Public Health Nutr. 2010;13:1403–1409. doi: 10.1017/S1368980009992990. [DOI] [PubMed] [Google Scholar]
- 15.Mojzis J., Varinska L., Mojzisova G., Kostova I., Mirossay L. Antiangiogenic Effects of Flavonoids and Chalcones. Pharmacol. Res. 2008;57:259–265. doi: 10.1016/j.phrs.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Jain R.K., Schlenger K., Höckel M., Yuan F. Quantitative Angiogenesis Assays: Progress and Problems. Nat. Med. 1997;3:1203–1208. doi: 10.1038/nm1197-1203. [DOI] [PubMed] [Google Scholar]
- 17.Norrby K. In Vivo Models of Angiogenesis. J. Cell. Mol. Med. 2006;10:588–612. doi: 10.1111/j.1582-4934.2006.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hattori H., Okuda K., Murase T., Shigetsura Y., Narise K., Semenza G.L., Nagasawa H. Isolation, Identification, and Biological Evaluation of HIF-1-Modulating Compounds from Brazilian Green Propolis. Bioorg. Med. Chem. 2011;19:5392–5401. doi: 10.1016/j.bmc.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 19.Ramchandani S., Naz I., Lee J.H., Khan M.R., Ahn K.S. An Overview of the Potential Antineoplastic Effects of Casticin. Molecules. 2020;25:1287. doi: 10.3390/molecules25061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang D.S., Li Z.L., Peng W.B., Yang Y.P., Wang X., Liu K.C., Li X.L., Xiao W.L. Three New Prenylated Flavonoids from Macaranga Denticulata and Their Anticancer Effects. Fitoterapia. 2015;103:165–170. doi: 10.1016/j.fitote.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Kashyap D., Sharma A., Sak K., Tuli H.S., Buttar H.S., Bishayee A. Fisetin: A Bioactive Phytochemical with Potential for Cancer Prevention and Pharmacotherapy. Life Sci. 2018;194:75–87. doi: 10.1016/j.lfs.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Rengarajan T., Yaacob N.S. The Flavonoid Fisetin as an Anticancer Agent Targeting the Growth Signaling Pathways. Eur. J. Pharmacol. 2016;789:8–16. doi: 10.1016/j.ejphar.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Syed D.N., Adhami V.M., Khan N., Khan M.I., Mukhtar H. Exploring the Molecular Targets of Dietary Flavonoid Fisetin in Cancer. Semin. Cancer Biol. 2016;40:130–140. doi: 10.1016/j.semcancer.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lall R.K., Adhami V.M., Mukhtar H. Dietary Flavonoid Fisetin for Cancer Prevention and Treatment. Mol. Nutr. Food Res. 2016;60:1396–1405. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai C.F., Chen J.H., Chang C.N., Lu D.Y., Chang P.C., Wang S.L., Yeh W.L. Fisetin Inhibits Cell Migration via Inducing HO-1 and Reducing MMPs Expression in Breast Cancer Cell Lines. Food Chem. Toxicol. 2018;120:528–535. doi: 10.1016/j.fct.2018.07.059. [DOI] [PubMed] [Google Scholar]
- 26.Sun X., Ma X., Li Q., Yang Y., Xu X., Sun J., Yu M., Cao K., Yang L., Yang G., et al. Anti-cancer Effects of Fisetin on Mammary Carcinoma Cells via Regulation of the PI3K/Akt/MTOR Pathway: In Vitro and in Vivo Studies. Int. J. Mol. Med. 2018;42:811–820. doi: 10.3892/ijmm.2018.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Gong X., Jiang R., Lin D., Zhou T., Zhang A., Li H., Zhang X., Wan J., Kuang G., et al. Fisetin Inhibited Growth and Metastasis of Triple-Negative Breast Cancer by Reversing Epithelial-to-Mesenchymal Transition via PTEN/Akt/GSK3β Signal Pathway. Front. Pharmacol. 2018;9:772. doi: 10.3389/fphar.2018.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou R.H., Hsieh S.C., Yu Y.L., Huang M.H., Huang Y.C., Hsieh Y.H. Fisetin Inhibits Migration and Invasion of Human Cervical Cancer Cells by Down-Regulating Urokinase Plasminogen Activator Expression through Suppressing the P38 MAPK-Dependent NF-ΚB Signaling Pathway. PLoS ONE. 2013;8:e71983. doi: 10.1371/journal.pone.0071983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C.M., Hsieh Y.H., Hwang J.M., Jan H.J., Hsieh S.C., Lin S.H., Lai C.Y. Fisetin Suppresses ADAM9 Expression and Inhibits Invasion of Glioma Cancer Cells through Increased Phosphorylation of ERK1/2. Tumor Biol. 2015;36:3407–3415. doi: 10.1007/s13277-014-2975-9. [DOI] [PubMed] [Google Scholar]
- 30.Liu X.F., Long H.J., Miao X.Y., Liu G.L., Yao H.L. Fisetin Inhibits Liver Cancer Growth in a Mouse Model: Relation to Dopamine Receptor. Oncol. Rep. 2017;38:53–62. doi: 10.3892/or.2017.5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klimaszewska-Wiśniewska A., Grzanka D., Czajkowska P., Hałas-Wiśniewska M., Durślewicz J., Antosik P., Grzanka A., Gagat M. Cellular and Molecular Alterations Induced by Low-dose Fisetin in Human Chronic Myeloid Leukemia Cells. Int. J. Oncol. 2019;55:1261–1274. doi: 10.3892/ijo.2019.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabasum S., Singh R.P. Fisetin Suppresses Migration, Invasion and Stem-Cell-like Phenotype of Human Non-Small Cell Lung Carcinoma Cells via Attenuation of Epithelial to Mesenchymal Transition. Chem. Biol. Interact. 2019;303:14–21. doi: 10.1016/j.cbi.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Huang S. Fisetin Inhibits the Growth and Migration in the A549 Human Lung Cancer Cell Line via the ERK1/2 Pathway. Exp. Ther. Med. 2018;15:2667–2673. doi: 10.3892/etm.2017.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chien C.S., Shen K.H., Huang J.S., Ko S.C., Shih Y.W. Antimetastatic Potential of Fisetin Involves Inactivation of the PI3K/Akt and JNK Signaling Pathways with Downregulation of MMP-2/9 Expressions in Prostate Cancer PC-3 Cells. Mol. Cell. Biochem. 2010;333:169–180. doi: 10.1007/s11010-009-0217-z. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh M.-H., Tsai J.-P., Yang S.-F., Chiou H.-L., Lin C.-L., Hsieh Y.-H., Chang H.-R. Fisetin Suppresses the Proliferation and Metastasis of Renal Cell Carcinoma through Upregulation of MEK/ERK-Targeting CTSS and ADAM9. Cells. 2019;8:948. doi: 10.3390/cells8090948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joussen A.M. Treatment of Corneal Neovascularization with Dietary Isoflavonoids and Flavonoids. Exp. Eye Res. 2000;71:483–487. doi: 10.1006/exer.2000.0900. [DOI] [PubMed] [Google Scholar]
- 37.Fang D., Xiong Z., Xu J., Yin J., Luo R. Chemopreventive Mechanisms of Galangin against Hepatocellular Carcinoma: A Review. Biomed. Pharmacother. 2019;109:2054–2061. doi: 10.1016/j.biopha.2018.09.154. [DOI] [PubMed] [Google Scholar]
- 38.Kim J.D., Liu L., Guo W., Meydani M. Chemical Structure of Flavonols in Relation to Modulation of Angiogenesis and Immune-Endothelial Cell Adhesion. J. Nutr. Biochem. 2006;17:165–176. doi: 10.1016/j.jnutbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Chen D., Li D., Xu X., Qiu S., Luo S., Qiu E., Rong Z., Zhang J., Zheng D. Galangin Inhibits Epithelial-Mesenchymal Transition and Angiogenesis by Downregulating CD44 in Glioma. J. Cancer. 2019;10:4499–4508. doi: 10.7150/jca.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei D., Zhang F., Yao D., Xiong N., Jiang X., Zhao H. Galangin Increases ERK1/2 Phosphorylation to Decrease ADAM9 Expression and Prevents Invasion in A172 Glioma Cells. Mol. Med. Rep. 2018;17:667–673. doi: 10.3892/mmr.2017.7920. [DOI] [PubMed] [Google Scholar]
- 41.Chien S.-T., Shi M.-D., Lee Y.-C., Te C.-C., Shih Y.-W. Galangin, a Novel Dietary Flavonoid, Attenuates Metastatic Feature via PKC/ERK Signaling Pathway in TPA-Treated Liver Cancer HepG2 Cells. Cancer Cell Int. 2015;15:15. doi: 10.1186/s12935-015-0168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang H., Chen A.Y., Rojanasakul Y., Ye X., Rankin G.O., Chen Y.C. Dietary Compounds Galangin and Myricetin Suppress Ovarian Cancer Cell Angiogenesis. J. Funct. Foods. 2015;15:464–475. doi: 10.1016/j.jff.2015.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao J., Wang H., Chen F., Fang J., Xu A., Xi W., Zhang S., Wu G., Wang Z. Galangin Inhibits Cell Invasion by Suppressing the Epithelial-Mesenchymal Transition and Inducing Apoptosis in Renal Cell Carcinoma. Mol. Med. Rep. 2016;13:4238–4244. doi: 10.3892/mmr.2016.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y., Rao Q., Zhang X., Zhou X. Galangin Induced Antitumor Effects in Human Kidney Tumor Cells Mediated via Mitochondrial Mediated Apoptosis, Inhibition of Cell Migration and Invasion and Targeting PI3K/AKT/MTOR Signalling Pathway. J. BUON. 2018;23:795–799. [PubMed] [Google Scholar]
- 45.Wang L., Wang X., Chen H., Zu X., Ma F., Liu K., Bode A.M., Dong Z., Kim D.J. Gossypin Inhibits Gastric Cancer Growth by Direct Targeting AURKA and RSK2. Phyther. Res. 2019;33:640–650. doi: 10.1002/ptr.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L., Fan P., Chou H., Li J., Wang K., Li H. Herbacetin Suppressed MMP9 Mediated Angiogenesis of Malignant Melanoma through Blocking EGFR-ERK/AKT Signaling Pathway. Biochimie. 2019;162:198–207. doi: 10.1016/j.biochi.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Jin X.N., Yan E.Z., Wang H.M., Sui H.J., Liu Z., Gao W., Jin Y. Hyperoside Exerts Anti-Inflammatory and Anti-Arthritic Effects in LPS-Stimulated Human Fibroblast-like Synoviocytes in Vitro and in Mice with Collagen-Induced Arthritis. Acta Pharmacol. Sin. 2016;37:674–686. doi: 10.1038/aps.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X., Liu T., Huang Y., Wismeijer D., Liu Y. Icariin: Does It Have an Osteoinductive Potential for Bone Tissue Engineering? Phyther. Res. 2014;28:498–509. doi: 10.1002/ptr.5027. [DOI] [PubMed] [Google Scholar]
- 49.Chen M., Wu J., Luo Q., Mo S., Lyu Y., Wei Y., Dong J. The Anticancer Properties of Herba Epimedii and Its Main Bioactive Componentsicariin and Icariside II. Nutrients. 2016;8:563. doi: 10.3390/nu8090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung B.H., Kim J.D., Kim C.K., Kim J.W., Won M.H., Lee H.S., Dong M.S., Ha K.S., Kwon Y.G., Kim Y.M. Icariin Stimulates Angiogenesis by Activating the MEK/ERK- and PI3K/Akt/ENOS-Dependent Signal Pathways in Human Endothelial Cells. Biochem. Biophys. Res. Commun. 2008;376:404–408. doi: 10.1016/j.bbrc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Gu Z.F., Zhang Z.T., Wang J.Y., Xu B.B. Icariin Exerts Inhibitory Effects on the Growth and Metastasis of KYSE70 Human Esophageal Carcinoma Cells via PI3K/AKT and STAT3 Pathways. Environ. Toxicol. Pharmacol. 2017;54:7–13. doi: 10.1016/j.etap.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Wang P., Zhang J., Xiong X., Yuan W., Qin S., Cao W., Dai L., Xie F., Li A., Liu Z. Icariin Suppresses Cell Cycle Transition and Cell Migration in Ovarian Cancer Cells. Oncol. Rep. 2019;41:2321–2328. doi: 10.3892/or.2019.6986. [DOI] [PubMed] [Google Scholar]
- 53.Singh W.R., Devi H.S., Kumawat S., Sadam A., Appukuttan A.V., Patel M.R., Lingaraju M.C., Singh T.U., Kumar D. Angiogenic and MMPs Modulatory Effects of Icariin Improved Cutaneous Wound Healing in Rats. Eur. J. Pharmacol. 2019;858:172466. doi: 10.1016/j.ejphar.2019.172466. [DOI] [PubMed] [Google Scholar]
- 54.Quan K., Zhang X., Fan K., Liu P., Yue Q., Li B., Wu J., Liu B., Xu Y., Hua W., et al. Icariside II Induces Cell Cycle Arrest and Apoptosis in Human Glioblastoma Cells through Suppressing Akt Activation and Potentiating FOXO3A Activity. Am. J. Transl. Res. 2017;9:2508–2519. [PMC free article] [PubMed] [Google Scholar]
- 55.Xing S., Yu W., Zhang X., Luo Y., Lei Z., Huang D., Lin J., Huang Y., Huang S., Nong F., et al. Isoviolanthin Extracted from Dendrobium Officinale Reverses TGF-Β1-Mediated Epithelial–Mesenchymal Transition in Hepatocellular Carcinoma Cells via Deactivating the TGF-β/Smad and PI3K/Akt/MTOR Signaling Pathways. Int. J. Mol. Sci. 2018;19:1556. doi: 10.3390/ijms19061556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen A.Y., Chen Y.C. A Review of the Dietary Flavonoid, Kaempferol on Human Health and Cancer Chemoprevention. Food Chem. 2013;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kashyap D., Sharma A., Tuli H.S., Sak K., Punia S., Mukherjee T.K. Kaempferol—A Dietary Anticancer Molecule with Multiple Mechanisms of Action: Recent Trends and Advancements. J. Funct. Foods. 2017;30:203–219. doi: 10.1016/j.jff.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chin H.K., Horng C.T., Liu Y.S., Lu C.C., Su C.Y., Chen P.S., Chiu H.Y., Tsai F.J., Shieh P.C., Yang J.S. Kaempferol Inhibits Angiogenic Ability by Targeting VEGF Receptor-2 and Downregulating the PI3K/AKT, MEK and ERK Pathways in VEGF-Stimulated Human Umbilical Vein Endothelial Cells. Oncol. Rep. 2018;39:2351–2357. doi: 10.3892/or.2018.6312. [DOI] [PubMed] [Google Scholar]
- 59.Liang F., Han Y., Gao H., Xin S., Chen S., Wang N., Qin W., Zhong H., Lin S., Yao X., et al. Kaempferol Identified by Zebrafish Assay and Fine Fractionations Strategy from Dysosma versipellis Inhibits Angiogenesis through VEGF and FGF Pathways. Sci. Rep. 2015;5:14468. doi: 10.1038/srep14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumazawa S., Kubota S., Yamamoto H., Okamura N., Sugiyama Y., Kobayashi H., Nakanishi M., Ohta T. Antiangiogenic Activity of Flavonoids from Melia Azedarach. Nat. Prod. Commun. 2013;8:1719–1720. doi: 10.1177/1934578X1300801215. [DOI] [PubMed] [Google Scholar]
- 61.Özay Y., Güzel S., Yumrutaş Ö., Pehlivanoğlu B., Erdoğdu İ.H., Yildirim Z., Türk B.A., Darcan S. Wound Healing Effect of Kaempferol in Diabetic and Nondiabetic Rats. J. Surg. Res. 2019;233:284–296. doi: 10.1016/j.jss.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Sharma V., Joseph C., Ghosh S., Agarwal A., Mishra M.K., Sen E. Kaempferol Induces Apoptosis in Glioblastoma Cells through Oxidative Stress. Mol. Cancer Ther. 2007;6:2544–2553. doi: 10.1158/1535-7163.MCT-06-0788. [DOI] [PubMed] [Google Scholar]
- 63.Shen S.C., Lin C.W., Lee H.M., Chien L.L., Chen Y.C. Lipopolysaccharide plus 12-o-Tetradecanoylphorbol 13-Acetate Induction of Migration and Invasion of Glioma Cells in Vitro and in Vivo: Differential Inhibitory Effects of Flavonoids. Neuroscience. 2006;140:477–489. doi: 10.1016/j.neuroscience.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 64.Qin Y., Cui W., Yang X., Tong B. Kaempferol Inhibits the Growth and Metastasis of Cholangiocarcinoma in Vitro and in Vivo. Acta Biochim. Biophys. Sin. (Shanghai). 2015;48:238–245. doi: 10.1093/abbs/gmv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu G., Liu X., Li H., Yan Y., Hong X., Lin Z. Kaempferol Inhibits Proliferation, Migration, and Invasion of Liver Cancer HepG2 Cells by down-Regulation of MicroRNA-21. Int. J. Immunopathol. Pharmacol. 2018;32:2058738418814341. doi: 10.1177/2058738418814341. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Jo E., Park S.J., Choi Y.S., Jeon W.K., Kim B.C. Kaempferol Suppresses Transforming Growth Factor-Β1-Induced Epithelial-to-Mesenchymal Transition and Migration of A549 Lung Cancer Cells by Inhibiting Akt1-Mediated Phosphorylation of Smad3 at Threonine-179. Neoplasia. 2015;17:525–537. doi: 10.1016/j.neo.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Labbé D., Provençal M., Lamy S., Boivin D., Gingras D., Béliveau R. The Flavonols Quercetin, Kaempferol, and Myricetin Inhibit Hepatocyte Growth. J. Nutr. Biochem. Mol. Genet. Mech. 2009;139:646–652. doi: 10.3945/jn.108.102616.kinase. [DOI] [PubMed] [Google Scholar]
- 68.Lin C.W., Chen P.N., Chen M.K., Yang W.E., Tang C.H., Yang S.F., Hsieh Y.S. Kaempferol Reduces Matrix Metalloproteinase-2 Expression by down-Regulating ERK1/2 and the Activator Protein-1 Signaling Pathways in Oral Cancer Cells. PLoS ONE. 2013;8:e80883. doi: 10.1371/journal.pone.0080883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H.J., Lin C.M., Lee C.Y., Shih N.C., Peng S.F., Tsuzuki M., Amagaya S., Huang W.W., Yang J.S. Kaempferol Suppresses Cell Metastasis via Inhibition of the ERK-P38-JNK and AP-1 Signaling Pathways in U-2 OS Human Osteosarcoma Cells. Oncol. Rep. 2013;30:925–932. doi: 10.3892/or.2013.2490. [DOI] [PubMed] [Google Scholar]
- 70.Luo H., Rankin G.O., Liu L., Daddysman M.K., Jiang B.H., Chen Y.C. Kaempferol Inhibits Angiogenesis and VEGF Expression through Both HIF Dependent and Independent Pathways in Human Ovarian Cancer Cells. Nutr. Cancer. 2009;61:554–563. doi: 10.1080/01635580802666281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J., Kim J.H. Kaempferol Inhibits Pancreatic Cancer Cell Growth and Migration through the Blockade of EGFR-Related Pathway in Vitro. PLoS ONE. 2016;11:e0155264. doi: 10.1371/journal.pone.0155264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hung T.W., Chen P.N., Wu H.C., Wu S.W., Tsai P.Y., Hsieh Y.S., Chang H.R. Kaempferol Inhibits the Invasion and Migration of Renal Cancer Cells through the Downregulation of AKT and FAK Pathways. Int. J. Med. Sci. 2017;14:984–993. doi: 10.7150/ijms.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chien H.W., Wang K., Chang Y.Y., Hsieh Y.H., Yu N.Y., Yang S.F., Lin H.W. Kaempferol Suppresses Cell Migration through the Activation of the ERK Signaling Pathways in ARPE-19 Cells. Environ. Toxicol. 2019;34:312–318. doi: 10.1002/tox.22686. [DOI] [PubMed] [Google Scholar]
- 74.Clericuzio M., Tinello S., Burlando B., Ranzato E., Martinotti S., Cornara L., La Rocca A. Flavonoid Oligoglycosides from Ophioglossum vulgatum L. Having Wound Healing Properties. Planta Med. 2012;78:1639–1644. doi: 10.1055/s-0032-1315149. [DOI] [PubMed] [Google Scholar]
- 75.Zeng N., Tong B., Zhang X., Dou Y., Wu X., Xia Y., Dai Y., Wei Z. Antiarthritis Effect of Morin Is Associated with Inhibition of Synovial Angiogensis. Drug Dev. Res. 2015;76:463–473. doi: 10.1002/ddr.21282. [DOI] [PubMed] [Google Scholar]
- 76.Yue M., Zeng N., Xia Y., Wei Z., Dai Y. Morin Exerts Anti-Arthritic Effects by Attenuating Synovial Angiogenesis via Activation of Peroxisome Proliferator Activated Receptor-γ. Mol. Nutr. Food Res. 2018;62:1800202. doi: 10.1002/mnfr.201800202. [DOI] [PubMed] [Google Scholar]
- 77.Capitani N., Lori G., Paoli P., Patrussi L., Troilo A., Baldari C.T., Raugei G., D’Elios M.M. LMW-PTP Targeting Potentiates the Effects of Drugs Used in Chronic Lymphocytic Leukemia Therapy. Cancer Cell Int. 2019;19:67. doi: 10.1186/s12935-019-0786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li H.W., Zou T.-B., Jia Q., Xia E.Q., Cao W.J., Liu W., He T.P., Wang Q. Anticancer Effects of Morin-7-Sulphate Sodium, a Flavonoid Derivative, in Mouse Melanoma Cells. Biomed. Pharmacother. 2016;84:909–916. doi: 10.1016/j.biopha.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Kang N.J., Jung S.K., Lee K.W., Lee H.J. Myricetin Is a Potent Chemopreventive Phytochemical in Skin Carcinogenesis. Ann. N. Y. Acad. Sci. 2011;1229:124–132. doi: 10.1111/j.1749-6632.2011.06122.x. [DOI] [PubMed] [Google Scholar]
- 80.Zhou Z., Mao W., Li Y., Qi C., He Y. Myricetin Inhibits Breast Tumor Growth and Angiogenesis by Regulating VEGF/VEGFR2 and P38MAPK Signaling Pathways. Anat. Rec. 2019;302:2186–2192. doi: 10.1002/ar.24222. [DOI] [PubMed] [Google Scholar]
- 81.Ci Y., Zhang Y., Liu Y., Lu S., Cao J., Li H., Zhang J., Huang Z., Zhu X., Gao J., et al. Myricetin Suppresses Breast Cancer Metastasis through Down-Regulating the Activity of Matrix Metalloproteinase (MMP)-2/9. Phyther. Res. 2018;32:1373–1381. doi: 10.1002/ptr.6071. [DOI] [PubMed] [Google Scholar]
- 82.Chiu W.T., Shen S.C., Chow J.M., Lin C.W., Shia L.T., Chen Y.C. Contribution of Reactive Oxygen Species to Migration/Invasion of Human Glioblastoma Cells U87 via ERK-Dependent COX-2/PGE2 Activation. Neurobiol. Dis. 2010;37:118–129. doi: 10.1016/j.nbd.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Yamada N., Matsushima-Nishiwaki R., Kozawa O. Quercetin Suppresses the Migration of Hepatocellular Carcinoma Cells Stimulated by Hepatocyte Growth Factor or Transforming Growth Factor-α: Attenuation of AKT Signaling Pathway. Arch. Biochem. Biophys. 2020;682:108296. doi: 10.1016/j.abb.2020.108296. [DOI] [PubMed] [Google Scholar]
- 84.Ma H., Zhu L., Ren J., Rao B., Sha M., Kuang Y., Shen W., Xu Z. Myricetin Inhibits Migration and Invasion of Hepatocellular Carcinoma MHCC97H Cell Line by Inhibiting the EMT Process. Oncol. Lett. 2019;18:6614–6620. doi: 10.3892/ol.2019.10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shih Y.W., Wu P.F., Lee Y.C., Shi M.-D., Chiang T.A. Myricetin Suppresses Invasion and Migration of Human Lung Adenocarcinoma A549 Cells: Possible Mediation by Blocking the Erk Signaling Pathway. J. Agric. Food Chem. 2009;57:3490–3499. doi: 10.1021/jf900124r. [DOI] [PubMed] [Google Scholar]
- 86.Ezzati M., Yousefi B., Velaei K., Safa A. A Review on Anti-Cancer Properties of Quercetin in Breast Cancer. Life Sci. 2020;248:117463. doi: 10.1016/j.lfs.2020.117463. [DOI] [PubMed] [Google Scholar]
- 87.Tang S., Deng X., Zhou J., Li Q., Ge X., Miao L. Pharmacological Basis and New Insights of Quercetin Action in Respect to Its Anti-Cancer Effects. Biomed. Pharmacother. 2020;121:109604. doi: 10.1016/j.biopha.2019.109604. [DOI] [PubMed] [Google Scholar]
- 88.Kashyap D., Mittal S., Sak K., Singhal P., Tuli H.S. Molecular Mechanisms of Action of Quercetin in Cancer: Recent Advances. Tumor Biol. 2016;37:12927–12939. doi: 10.1007/s13277-016-5184-x. [DOI] [PubMed] [Google Scholar]
- 89.Darband S.G., Kaviani M., Yousefi B., Sadighparvar S., Pakdel F.G., Attari J.A., Mohebbi I., Naderi S., Majidinia M. Quercetin: A Functional Dietary Flavonoid with Potential Chemo-Preventive Properties in Colorectal Cancer. J. Cell. Physiol. 2018;233:6544–6560. doi: 10.1002/jcp.26595. [DOI] [PubMed] [Google Scholar]
- 90.Song N.R., Chung M.Y., Kang N.J., Seo S.G., Jang T.S., Lee H.J., Lee K.W. Quercetin Suppresses Invasion and Migration of H-Ras-Transformed MCF10A Human Epithelial Cells by Inhibiting Phosphatidylinositol 3-Kinase. Food Chem. 2014;142:66–71. doi: 10.1016/j.foodchem.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 91.Lin C., Wu M., Dong J. Quercetin-4′-o-β-d-Glucopyranoside (QODG) Inhibits Angiogenesis by Suppressing VEGFR2-Mediated Signaling in Zebrafish and Endothelial Cells. PLoS ONE. 2012;7:e31708. doi: 10.1371/journal.pone.0031708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee Y.H., Tuyet P.T. Synthesis and Biological Evaluation of Quercetin–Zinc (II) Complex for Anti-Cancer and Anti-Metastasis of Human Bladder Cancer Cells. Vitr. Cell. Dev. Biol. Anim. 2019;55:395–404. doi: 10.1007/s11626-019-00363-2. [DOI] [PubMed] [Google Scholar]
- 93.Ravishankar D., Watson K.A., Boateng S.Y., Green R.J., Greco F., Osborn H.M.I. Exploring Quercetin and Luteolin Derivatives as Antiangiogenic Agents. Eur. J. Med. Chem. 2015;97:259–274. doi: 10.1016/j.ejmech.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 94.Zhao X., Wang Q., Yang S., Chen C., Li X., Liu J., Zou Z., Cai D. Quercetin Inhibits Angiogenesis by Targeting Calcineurin in the Xenograft Model of Human Breast Cancer. Eur. J. Pharmacol. 2016;781:60–68. doi: 10.1016/j.ejphar.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 95.Oh S.J., Kim O., Lee J.S., Kim J.A., Kim M.R., Choi H.S., Shim J.H., Kang K.W., Kim Y.C. Inhibition of Angiogenesis by Quercetin in Tamoxifen-Resistant Breast Cancer Cells. Food Chem. Toxicol. 2010;48:3227–3234. doi: 10.1016/j.fct.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 96.Srinivasan A., Thangavel C., Liu Y., Shoyele S., Den R.B., Selvakumar P., Lakshmikuttyamma A. Quercetin Regulates β-Catenin Signaling and Reduces the Migration of Triple Negative Breast Cancer. Mol. Carcinog. 2016;55:743–756. doi: 10.1002/mc.22318. [DOI] [PubMed] [Google Scholar]
- 97.Lin C.W., Hou W.C., Shen S.C., Juan S.H., Ko C.H., Wang L.M., Chen Y.C. Quercetin Inhibition of Tumor Invasion via Suppressing PKCδ/ERK/AP-1-Dependent Matrix Metalloproteinase-9 Activation in Breast Carcinoma Cells. Carcinogenesis. 2008;29:1807–1815. doi: 10.1093/carcin/bgn162. [DOI] [PubMed] [Google Scholar]
- 98.Rivera Rivera A., Castillo-Pichardo L., Gerena Y., Dharmawardhane S. Anti-Breast Cancer Potential of Quercetin via the Akt/AMPK/Mammalian Target of Rapamycin (MTOR) Signaling Cascade. PLoS ONE. 2016;11:e0157251. doi: 10.1371/journal.pone.0157251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao D., Qin C., Fan X., Li Y., Gu B. Inhibitory Effects of Quercetin on Angiogenesis in Larval Zebra Fi Sh and Human Umbilical Vein Endothelial Cells. Eur. J. Pharmacol. 2014;723:360–367. doi: 10.1016/j.ejphar.2013.10.069. [DOI] [PubMed] [Google Scholar]
- 100.Tan W.F., Lin L.P., Li M.H., Zhang Y.X., Tong Y.G., Xiao D., Ding J. Quercetin, a Dietary-Derived Flavonoid, Possesses Antiangiogenic Potential. Eur. J. Pharmacol. 2003;459:255–262. doi: 10.1016/S0014-2999(02)02848-0. [DOI] [PubMed] [Google Scholar]
- 101.Zhao X., Shu G., Chen L., Mi X., Mei Z., Deng X. A Flavonoid Component from Docynia delavayi (Franch.) Schneid Represses Transplanted H22 Hepatoma Growth and Exhibits Low Toxic Effect on Tumor-Bearing Mice. Food Chem. Toxicol. 2012;50:3166–3168. doi: 10.1016/j.fct.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 102.Lee L.T., Huang Y.T., Hwang J.J., Lee A.Y.L., Ke F.C., Huang C.J., Kandaswami C., Lee P.P.H., Lee M.T. Transinactivation of the Epidermal Growth Factor Receptor Tyrosine Kinase and Focal Adhesion Kinase Phosphorylation by Dietary Flavonoids: Effect on Invasive Potential of Human Carcinoma Cells. Biochem. Pharmacol. 2004;67:2103–2114. doi: 10.1016/j.bcp.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 103.Lee D.E., Chung M.Y., Lim T.G., Huh W.B., Lee H.J., Lee K.W. Quercetin Suppresses Intracellular Ros Formation, MMP Activation, and Cell Motility in Human Fibrosarcoma Cells. J. Food Sci. 2013;78 doi: 10.1111/1750-3841.12223. [DOI] [PubMed] [Google Scholar]
- 104.Kee J.Y., Han Y.H., Kim D.S., Mun J.G., Park J., Jeong M.Y., Um J.Y., Hong S.H. Inhibitory Effect of Quercetin on Colorectal Lung Metastasis through Inducing Apoptosis, and Suppression of Metastatic Ability. Phytomedicine. 2016;23:1680–1690. doi: 10.1016/j.phymed.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 105.Pan H.C., Jiang Q., Yu Y., Mei J.P., Cui Y.K., Zhao W.J. Quercetin Promotes Cell Apoptosis and Inhibits the Expression of MMP-9 and Fibronectin via the AKT and ERK Signalling Pathways in Human Glioma Cells. Neurochem. Int. 2015;80:60–71. doi: 10.1016/j.neuint.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 106.Liu Y., Tang Z.G., Lin Y., Qu X.G., Lv W., Wang G.-B., Li C.L. Effects of Quercetin on Proliferation and Migration of Human Glioblastoma U251 Cells. Biomed. Pharmacother. 2017;92:33–38. doi: 10.1016/j.biopha.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 107.Michaud-Levesque J., Bousquet-Gagnon N., Béliveau R. Quercetin Abrogates IL-6/STAT3 Signaling and Inhibits Glioblastoma Cell Line Growth and Migration. Exp. Cell Res. 2012;318:925–935. doi: 10.1016/j.yexcr.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 108.da Silva A.B., Cerqueira Coelho P.L., das Neves Oliveira M., Oliveira J.L., Oliveira Amparo J.A., da Silva K.C., Soares J.R.P., Pitanga B.P.S., dos Santos Souza C., de Faria Lopes G.P., et al. The Flavonoid Rutin and Its Aglycone Quercetin Modulate the Microglia Inflammatory Profile Improving Antiglioma Activity. Brain. Behav. Immun. 2020;85:170–185. doi: 10.1016/j.bbi.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 109.Liu Y., Tang Z.G., Yang J.Q., Zhou Y., Meng L.H., Wang H., Li C.L. Low Concentration of Quercetin Antagonizes the Invasion and Angiogenesis of Human Glioblastoma U251 Cells. OncoTargets. Ther. 2017;10:4023–4028. doi: 10.2147/OTT.S136821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu J., Wang Z., Li S., Xin Q., Yuan M., Li H., Song X., Gao H., Pervaiz N., Sun X., et al. Quercetin Inhibits the Migration and Invasion of HCCLM3 Cells by Suppressing the Expression of P-Akt1, Matrix Metalloproteinase (MMP) MMP-2, and MMP-9. Med. Sci. Monit. 2018;24:2583–2589. doi: 10.12659/MSM.906172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klimaszewska-Wiśniewska A., Hałas-Wiśniewska M., Izdebska M., Gagat M., Grzanka A., Grzanka D. Antiproliferative and Antimetastatic Action of Quercetin on A549 Non-Small Cell Lung Cancer Cells through Its Effect on the Cytoskeleton. Acta Histochem. 2017;119:99–112. doi: 10.1016/j.acthis.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 112.Wu T.C., Chan S.T., Chang C.N., Yu P.S., Chuang C.H., Yeh S.L. Quercetin and Chrysin Inhibit Nickel-Induced Invasion and Migration by Downregulation of TLR4/NF-ΚB Signaling in A549 cells. Chem. Biol. Interact. 2018;292:101–109. doi: 10.1016/j.cbi.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 113.Lin Y.C., Tsai P.H., Lin C.Y., Cheng C.H., Lin T.H., Lee K.P.H., Huang K.Y., Chen S.H., Hwang J.J., Kandaswami C.C., et al. Impact of Flavonoids on Matrix Metalloproteinase Secretion and Invadopodia Formation in Highly Invasive A431-III Cancer Cells. PLoS ONE. 2013;8:e71903. doi: 10.1371/journal.pone.0071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hwang M.K., Song N.R., Kang N.J., Lee K.W., Lee H.J. Activation of Phosphatidylinositol 3-Kinase Is Required for Tumor Necrosis Factor-α-Induced Upregulation of Matrix Metalloproteinase-9: Its Direct Inhibition by Quercetin. Int. J. Biochem. Cell Biol. 2009;41:1592–1600. doi: 10.1016/j.biocel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 115.Cao H.H., Cheng C.Y., Su T., Fu X.Q., Guo H., Li T., Tse A.K.W., Kwan H.Y., Yu H., Yu Z.L. Quercetin Inhibits HGF/c-Met Signaling and HGFstimulated Melanoma Cell Migration and Invasion. Mol. Cancer. 2015;14:103. doi: 10.1186/s12943-015-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cao H.H., Tse A.K.W., Kwan H.Y., Yu H., Cheng C.Y., Su T., Fong W.F., Yu Z.L. Quercetin Exerts Anti-Melanoma Activities and Inhibits STAT3 Signaling. Biochem. Pharmacol. 2014;87:424–434. doi: 10.1016/j.bcp.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 117.Zhao J., Fang Z., Zha Z., Sun Q., Wang H., Sun M., Qiao B. Quercetin Inhibits Cell Viability, Migration and Invasion by Regulating MiR-16/HOXA10 Axis in Oral Cancer. Eur. J. Pharmacol. 2019;847:11–18. doi: 10.1016/j.ejphar.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 118.Nam T.W., Il Yoo C., Kim H.T., Kwon C.H., Park J.Y., Kim Y.K. The Flavonoid Quercetin Induces Apoptosis and Inhibits Migration through a MAPK-Dependent Mechanism in Osteoblasts. J. Bone Miner. Metab. 2008;26:551–560. doi: 10.1007/s00774-008-0864-2. [DOI] [PubMed] [Google Scholar]
- 119.Li S., Pei Y., Wang W., Liu F., Zheng K., Zhang X. Quercetin Suppresses the Proliferation and Metastasis of Metastatic Osteosarcoma Cells by Inhibiting Parathyroid Hormone Receptor 1. Biomed. Pharmacother. 2019;114:108839. doi: 10.1016/j.biopha.2019.108839. [DOI] [PubMed] [Google Scholar]
- 120.Lan H., Hong W., Fan P., Qian D., Zhu J., Bai B. Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma Cells. Cell. Physiol. Biochem. 2017;43:553–567. doi: 10.1159/000480528. [DOI] [PubMed] [Google Scholar]
- 121.Berndt K., Campanile C., Muff R., Strehler E., Born W., Fuchs B. Evaluation of Quercetin as a Potential Drug in Osteosarcoma Treatment. Anticancer Res. 2013;33:1297–1306. [PubMed] [Google Scholar]
- 122.Huang Y.T., Lee L.T., Lee P.P.H., Lin Y.S., Lee M.T. Targeting of Focal Adhesion Kinase by Flavonoids and Small-Interfering RNAs Reduces Tumor Cell Migration Ability. Anticancer Res. 2005;25:2017–2025. [PubMed] [Google Scholar]
- 123.Yu D., Ye T., Xiang Y., Shi Z., Zhang J., Lou B., Zhang F., Chen B., Zhou M. Quercetin Inhibits Epithelial–Mesenchymal Transition, Decreases Invasiveness and Metastasis, and Reverses IL-6 Induced Epithelial–Mesenchymal Transition, Expression of MMP by Inhibiting STAT3 Signaling in Pancreatic Cancer Cells. OncoTargets. Ther. 2017;10:4719–4729. doi: 10.2147/OTT.S136840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bhat F.A., Sharmila G., Balakrishnan S., Arunkumar R., Elumalai P., Suganya S., Raja Singh P., Srinivasan N., Arunakaran J. Quercetin Reverses EGF-Induced Epithelial to Mesenchymal Transition and Invasiveness in Prostate Cancer (PC-3) Cell Line via EGFR/PI3K/Akt Pathway. J. Nutr. Biochem. 2014;25:1132–1139. doi: 10.1016/j.jnutbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 125.Yang F., Jiang X., Song L., Wang H., Mei Z., Xu Z., Xing N. Quercetin Inhibits Angiogenesis through Thrombospondin-1 Upregulation to Antagonize Human Prostate Cancer PC-3 Cell Growth in Vitro and in Vivo. Oncol. Rep. 2016;35:1602–1610. doi: 10.3892/or.2015.4481. [DOI] [PubMed] [Google Scholar]
- 126.Song W., Zhao X., Xu J., Zhang H. Quercetin Inhibits Angiogenesis—Mediated Human Retinoblastoma Growth by Targeting Vascular Endothelial Growth Factor Receptor. Oncol. Lett. 2017;14:3343–3348. doi: 10.3892/ol.2017.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Camero C.M., Germanò M.P., Rapisarda A., D’Angelo V., Amira S., Benchikh F., Braca A., De Leo M. Anti-Angiogenic Activity of Iridoids from Galium Tunetanum. Braz. J. Pharmacogn. 2018;28:374–377. doi: 10.1016/j.bjp.2018.03.010. [DOI] [Google Scholar]
- 128.Ben Sghaier M., Pagano A., Mousslim M., Ammari Y., Kovacic H., Luis J. Rutin Inhibits Proliferation, Attenuates Superoxide Production and Decreases Adhesion and Migration of Human Cancerous Cells. Biomed. Pharmacother. 2016;84:1972–1978. doi: 10.1016/j.biopha.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 129.Chen H., Miao Q., Geng M., Liu J., Hu Y., Tian L., Pan J., Yang Y. Anti-Tumor Effect of Rutin on Human Neuroblastoma Cell Lines through Inducing G2/M Cell Cycle Arrest and Promoting Apoptosis. Sci. World J. 2013;2013 doi: 10.1155/2013/269165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin C.M., Chang H., Chen Y.H., Li S.Y., Wu I.H., Chiu J.H. Protective Role of Wogonin against Lipopolysaccharide-Induced Angiogenesis via VEGFR-2, Not VEGFR-1. Int. Immunopharmacol. 2006;6:1690–1698. doi: 10.1016/j.intimp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 131.Liu L.Z., Jing Y., Jiang L.L., Jiang X.E., Jiang Y., Rojanasakul Y., Jiang B.H. Acacetin Inhibits VEGF Expression, Tumor Angiogenesis and Growth through AKT/HIF-1α Pathway. Biochem. Biophys. Res. Commun. 2011;413:299–305. doi: 10.1016/j.bbrc.2011.08.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao K., Yuan Y., Lin B., Miao Z., Li Z., Guo Q., Lu N. LW-215, a Newly Synthesized Flavonoid, Exhibits Potent Anti-Angiogenic Activity in Vitro and in Vivo. Gene. 2018;642:533–541. doi: 10.1016/j.gene.2017.11.065. [DOI] [PubMed] [Google Scholar]
- 133.Viegas O., Faria M.A., Sousa J.B., Vojtek M., Gonçalves-Monteiro S., Suliburska J., Diniz C., Ferreira I.M.P.L.V.O. Delphinidin-3-O-Glucoside Inhibits Angiogenesis via VEGFR2 Downregulation and Migration through Actin Disruption. J. Funct. Foods. 2019;54:393–402. doi: 10.1016/j.jff.2019.01.039. [DOI] [Google Scholar]
- 134.Rajesh G., Harshala S., Dhananjay G., Jadhav A., Vikram G. Effect of Hydroxyl Substitution of Flavone on Angiogenesis and Free Radical Scavenging Activities: A Structure-Activity Relationship Studies Using Computational Tools. Eur. J. Pharm. Sci. 2010;39:37–44. doi: 10.1016/j.ejps.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 135.Gacche R.N., Shegokar H.D., Gond D.S., Yang Z., Jadhav A.D. Evaluation of Selected Flavonoids as Antiangiogenic, Anticancer, and Radical Scavenging Agents: An Experimental and in Silico Analysis. Cell Biochem. Biophys. 2011;61:651–663. doi: 10.1007/s12013-011-9251-z. [DOI] [PubMed] [Google Scholar]
- 136.Gacche R.N., Meshram R.J., Shegokar H.D., Gond D.S., Kamble S.S., Dhabadge V.N., Utage B.G., Patil K.K., More R.A. Flavonoids as a Scaffold for Development of Novel Anti-Angiogenic Agents: An Experimental and Computational Enquiry. Arch. Biochem. Biophys. 2015;577–578:35–48. doi: 10.1016/j.abb.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 137.Chen Y., Lu N., Ling Y., Wang L., You Q., Li Z., Guo Q. LYG-202, a Newly Synthesized Flavonoid, Exhibits Potent Anti-Angiogenic Activity in Vitro and in Vivo. J. Pharmacol. Sci. 2010;112:37–45. doi: 10.1254/jphs.09213FP. [DOI] [PubMed] [Google Scholar]
- 138.Huang Y., Fang J., Lu W., Wang Z., Wang Q., Hou Y., Jiang X., Reizes O., Lathia J., Nussinov R., et al. A Systems Pharmacology Approach Uncovers Wogonoside as an Angiogenesis Inhibitor of Triple-Negative Breast Cancer by Targeting Hedgehog Signaling. Cell Chem. Biol. 2019;26:1143–1158.e6. doi: 10.1016/j.chembiol.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen Y., Lu N., Ling Y., Gao Y., Wang L., Sun Y., Qi Q., Feng F., Liu W., Liu W., et al. Wogonoside Inhibits Lipopolysaccharide-Induced Angiogenesis in Vitro and in Vivo via Toll-like Receptor 4 Signal Transduction. Toxicology. 2009;259:10–17. doi: 10.1016/j.tox.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 140.Li X., Chen M., Lei X., Huang M., Ye W., Zhang R., Zhang D. Luteolin Inhibits Angiogenesis by Blocking Gas6/Axl Signaling Pathway. Int. J. Oncol. 2017;51:677–685. doi: 10.3892/ijo.2017.4041. [DOI] [PubMed] [Google Scholar]
- 141.Panda S.P., Panigrahy U.P., Prasanth D., Gorla U.S., Guntupalli C., Panda D.P., Jena B.R. A Trimethoxy Flavonoid Isolated from Stem Extract of Tabebuia chrysantha Suppresses Angiogenesis in Angiosarcoma. J. Pharm. Pharmacol. 2020:jphp.13272. doi: 10.1111/jphp.13272. [DOI] [PubMed] [Google Scholar]
- 142.Huang Y., Zhao K., Hu Y., Zhou Y., Luo X., Li X., Wei L., Li Z., You Q., Guo Q., et al. Wogonoside Inhibits Angiogenesis in Breast Cancer via Suppressing Wnt/β-Catenin Pathway. Mol. Carcinog. 2016;55:1598–1612. doi: 10.1002/mc.22412. [DOI] [PubMed] [Google Scholar]
- 143.Bhat T.A., Nambiar D., Tailor D., Pal A., Agarwal R., Singh R.P. Acacetin Inhibits in Vitro and in Vivo Angiogenesis and Downregulates Stat Signaling and VEGF Expression. Cancer Prev. Res. 2013;6:1128–1139. doi: 10.1158/1940-6207.CAPR-13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fang J., Zhou Q., Liu L.Z., Xia C., Hu X., Shi X., Jiang B.H. Apigenin Inhibits Tumor Angiogenesis through Decreasing HIF-1α and VEGF Expression. Carcinogenesis. 2007;28:858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 145.Chen J., Chen A.Y., Huang H., Ye X., Rollyson W.D., Perry H.E., Brown K.C., Rojanasakul Y., Rankin G.O., Dasgupta P., et al. The Flavonoid Nobiletin Inhibits Tumor Growth and Angiogenesis of Ovarian Cancers via the Akt Pathway. Int. J. Oncol. 2015;46:2629–2638. doi: 10.3892/ijo.2015.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pratheeshkumar P., Son Y.O., Budhraja A., Wang X., Ding S., Wang L., Hitron A., Lee J.C., Kim D., Divya S.P., et al. Luteolin Inhibits Human Prostate Tumor Growth by Suppressing Vascular Endothelial Growth Factor Receptor 2-Mediated Angiogenesis. PLoS ONE. 2012;7:e52279. doi: 10.1371/journal.pone.0052279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin C.M., Chang H., Chen Y.H., Wu I.H., Chiu J.H. Wogonin Inhibits IL-6-Induced Angiogenesis via down-Regulation of VEGF and VEGFR-1, Not VEGFR-2. Planta Med. 2006;72:1305–1310. doi: 10.1055/s-2006-951692. [DOI] [PubMed] [Google Scholar]
- 148.Zhu D., Wang S., Lawless J., He J., Zheng Z. Dose Dependent Dual Effect of Baicalin and Herb Huang Qin Extract on Angiogenesis. PLoS ONE. 2016;11:e0167125. doi: 10.1371/journal.pone.0167125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Favot L., Martin S., Keravis T., Andriantsitohaina R., Lugnier C. Involvement of Cyclin-Dependent Pathway in the Inhibitory Effect of Delphinidin on Angiogenesis. Cardiovasc. Res. 2003;59:479–487. doi: 10.1016/S0008-6363(03)00433-4. [DOI] [PubMed] [Google Scholar]
- 150.Hui M., Cazet A., Nair R., Watkins D.N., O’Toole S.A., Swarbrick A. The Hedgehog Signalling Pathway in Breast Development, Carcinogenesis and Cancer Therapy. Breast Cancer Res. 2013;15:1–14. doi: 10.1186/bcr3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cao X., Geradts J., Dewhirst M.W., Lo H.W. Upregulation of VEGF-A and CD24 Gene Expression by the TGLI1 Transcription Factor Contributes to the Aggressive Behavior of Breast Cancer Cells. Oncogene. 2012;31:104–115. doi: 10.1038/onc.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang K., Lu J., Mori T., Smith-Powell L., Synold T.W., Chen S., Wen W. Baicalin Increases VEGF Expression and Angiogenesis by Activating the ERR/PGC-1 Pathway. Cardiovasc. Res. 2011;89:426–435. doi: 10.1093/cvr/cvq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu J.-J., Huang T.-S., Cheng W.-F., Lu F.-J. Baicalein and Baicalin Are Potent Inhibitors of Angiogenesis: Inhibition of Endothelial Cell Proliferation, Migration and Differentiation. Int. J. Cancer. 2003;106:559–565. doi: 10.1002/ijc.11267. [DOI] [PubMed] [Google Scholar]
- 154.Morbidelli L. Polyphenol-Based Nutraceuticals for the Control of Angiogenesis: Analysis of the Critical Issues for Human Use. Pharmacol. Res. 2016;111:384–393. doi: 10.1016/j.phrs.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 155.Cockerill G.W., Gamble J.R., Vadas M.A. Angiogenesis: Models and Modulators. Int. Rev. Cytol. 1995;159:113–160. doi: 10.1016/S0074-7696(08)62106-3. [DOI] [PubMed] [Google Scholar]
- 156.Ribatti D., Vacca A. Models for Studying Angiogenesis in Vivo. Int. J. Biol. Markers. 1999;14:207–213. doi: 10.1177/172460089901400403. [DOI] [PubMed] [Google Scholar]
- 157.Staton C.A., Stribbling S.M., Tazzyman S., Hughes R., Brown N.J., Lewis C.E. Current Methods for Assaying Angiogenesis in Vitro and in Vivo. Int. J. Experimantal Biol. 2004;85:233–248. doi: 10.1111/j.0959-9673.2004.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nguyen M., Shing Y., Folkman J. Quantitation of Angiogenesis and Antiangiogenesis in the Chick Embryo Chorioallantoic Membrane. Microvasc. Res. 1994;47:31–40. doi: 10.1006/mvre.1994.1003. [DOI] [PubMed] [Google Scholar]
- 159.Ribatti D., Vacca A., Roncali L., Dammacco F. The Chick Embryo Chorioallantoic Membrane as a Model for in Vivo Research on Angiogenesis. Int. J. Dev. Biol. 1996;40:1189–1197. doi: 10.2174/1389201003379040. [DOI] [PubMed] [Google Scholar]
- 160.Wang T., Li Q., Bi K. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018;13:12–23. doi: 10.1016/j.ajps.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nguyen T.T.H., Moon Y.H., Ryu Y.B., Kim Y.M., Nam S.H., Kim M.S., Kimura A., Kim D. The Influence of Flavonoid Compounds on the in Vitro Inhibition Study of a Human Fibroblast Collagenase Catalytic Domain Expressed in E. coli. Enzyme Microb. Technol. 2013;52:26–31. doi: 10.1016/j.enzmictec.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 162.Krych J., Gebicka L. Catalase Is Inhibited by Flavonoids. Int. J. Biol. Macromol. 2013;58:148–153. doi: 10.1016/j.ijbiomac.2013.03.070. [DOI] [PubMed] [Google Scholar]
- 163.Huang Z., Fang F., Wang J., Wong C.W. Structural Activity Relationship of Flavonoids with Estrogen-Related Receptor Gamma. FEBS Lett. 2010;584:22–26. doi: 10.1016/j.febslet.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 164.Atrahimovich D., Vaya J., Khatib S. The Effects and Mechanism of Flavonoid-RePON1 Interactions. Structure-Activity Relationship Study. Bioorganic Med. Chem. 2013;21:3348–3355. doi: 10.1016/j.bmc.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 165.Çelik H., Koşar M. Inhibitory Effects of Dietary Flavonoids on Purified Hepatic NADH-Cytochrome B5 Reductase: Structure-Activity Relationships. Chem. Biol. Interact. 2012;197:103–109. doi: 10.1016/j.cbi.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 166.Sithisarn P., Michaelis M., Schubert-Zsilavecz M., Cinatl J. Differential Antiviral and Anti-Inflammatory Mechanisms of the Flavonoids Biochanin A and Baicalein in H5N1 Influenza A Virus-Infected Cells. Antiviral Res. 2013;97:41–48. doi: 10.1016/j.antiviral.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 167.Kothandan G., Gadhe C.G., Madhavan T., Choi C.H., Cho S.J. Docking and 3D-QSAR (Quantitative Structure Activity Relationship) Studies of Flavones, the Potent Inhibitors of p-Glycoprotein Targeting the Nucleotide Binding Domain. Eur. J. Med. Chem. 2011;46:4078–4088. doi: 10.1016/j.ejmech.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 168.Singh M., Kaur M., Silakari O. Flavones: An Important Scaffold for Medicinal Chemistry. Eur. J. Med. Chem. 2014;84:206–239. doi: 10.1016/j.ejmech.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 169.Haupt V.J., Daminelli S., Schroeder M. Drug Promiscuity in PDB: Protein Binding Site Similarity Is Key. PLoS ONE. 2013;8:e65894. doi: 10.1371/journal.pone.0065894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lam I.K., Alex D., Wang Y.H., Liu P., Liu A.L., Du G.H., Yuen Lee S.M. In Vitro and in Vivo Structure and Activity Relationship Analysis of Polymethoxylated Flavonoids: Identifying Sinensetin as a Novel Antiangiogenesis Agent. Mol. Nutr. Food Res. 2012;56:945–956. doi: 10.1002/mnfr.201100680. [DOI] [PubMed] [Google Scholar]
- 171.Lingen M.W. Historical Perspective Role of Leukocytes and Endothelial Cells in the Development of Angiogenesis in Inflammation and Wound Healing. Arch. Pathol. Lab. Med. 2001;125:67–71. doi: 10.1043/0003-9985(2001)1252.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 172.Naldini A., Carraro F. Role of Inflammatory Mediators in Angiogenesis. Curr. Drug Targets Inflamm. Allergy. 2005;4:3–8. doi: 10.2174/1568010053622830. [DOI] [PubMed] [Google Scholar]
- 173.Kofler S., Nickel T., Weis M. Role of Cytokines in Cardiovascular Diseases: A Focus on Endothelial Responses to Inflammation. Clin. Sci. 2005;108:205–213. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 174.Gong G., Wang H., Kong X., Duan R., Dong T.T.X., Tsim K.W.K. Flavonoids Are Identified from the Extract of Scutellariae Radix to Suppress Inflammatory-Induced Angiogenic Responses in Cultured RAW 264.7 Macrophages. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-35817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu W.-B., Hung D.K., Chang F.W., Ong E.T., Chen B.H. Anti-Inflammatory and Anti-Angiogenic Effects of Flavonoids Isolated from Lycium Barbarum Linnaeus on Human Umbilical Vein Endothelial Cells. Food Funct. 2012;3:1068–1081. doi: 10.1039/c2fo30051f. [DOI] [PubMed] [Google Scholar]
- 176.Zhang F.X., Kirschning C.J., Mancinelli R., Xu X.P., Jin Y., Faure E., Mantovani A., Rothe M., Muzio M., Arditi M. Bacterial Lipopolysaccharide Activates Nuclear Factor-ΚB through Interleukin-1 Signaling Mediators in Cultured Human Dermal Endothelial Cells and Mononuclear Phagocytes. J. Biol. Chem. 1999;274:7611–7614. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 177.Wu S.H., Liao P.Y., Dong L., Chen Z.Q. Signal Pathway Involved in Inhibition by Lipoxin A4 of Production of Interleukins Induced in Endothelial Cells by Lipopolysaccharide. Inflamm. Res. 2008;57:430–437. doi: 10.1007/s00011-008-7147-1. [DOI] [PubMed] [Google Scholar]
- 178.Bachelot T., Ray-Coquard I., Menetrier-Caux C., Rastkha M., Duc A., Blay J.Y. Prognostic Value of Serum Levels of Interleukin 6 and of Serum and Plasma Levels of Vascular Endothelial Growth Factor in Hormone-Refractory Metastatic Breast Cancer Patients. Br. J. Cancer. 2003;88:1721–1726. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Molostvov G., Morris A., Rose P., Basu S., Muller G. The Effects of Selective Cytokine Inhibitory Drugs (CC-10004 and CC-1088) on VEGF and IL-6 Expression and Apoptosis in Myeloma and Endothelial Cell Co-Cultures. Br. J. Haematol. 2004;124:366–375. doi: 10.1046/j.1365-2141.2003.04777.x. [DOI] [PubMed] [Google Scholar]
- 180.Fang J., Xia C., Cao Z., Zheng J.Z., Reed E., Jiang B.-H. Apigenin Inhibits VEGF and HIF-1 Expression via PI3K/AKT/P70S6K1 and HDM2/P53 Pathways. FASEB J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- 181.Carmeliet P., Jain R.K. Angiogenesis in Cancer and Other Diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 182.Zimna A., Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lu K.H., Chen P.N., Hsieh Y.H., Lin C.Y., Cheng F.Y., Chiu P.C., Chu S.C., Hsieh Y.S. 3-Hydroxyflavone Inhibits Human Osteosarcoma U2OS and 143B Cells Metastasis by Affecting EMT and Repressing u-PA/MMP-2 via FAK-Src to MEK/ERK and RhoA/MLC2 Pathways and Reduces 143B Tumor Growth in Vivo. Food Chem. Toxicol. 2016;97:177–186. doi: 10.1016/j.fct.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 184.Liu L.Z., Fang J., Zhou Q., Hu X., Shi X., Jiang B.H. Apigenin Inhibits Expression of Vascular Endothelial Growth Factor and Angiogenesis in Human Lung Cancer Cells: Implication of Chemoprevention of Lung Cancer. Mol. Pharmacol. 2005;68:635–643. doi: 10.1124/mol.105.011254. [DOI] [PubMed] [Google Scholar]
- 185.Byun E.B., Kim H.M., Song H.Y., Kim W.S. Hesperidin Structurally Modified by Gamma Irradiation Induces Apoptosis in Murine Melanoma B16BL6 Cells and Inhibits Both Subcutaneous Tumor Growth and Metastasis in C57BL/6 Mice. Food Chem. Toxicol. 2019;127:19–30. doi: 10.1016/j.fct.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 186.Deep G., Kumar R., Jain A.K., Agarwal C., Agarwal R. Silibinin Inhibits Fibronectin Induced Motility, Invasiveness and Survival in Human Prostate Carcinoma PC3 Cells via Targeting Integrin Signaling. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2014;768:35–46. doi: 10.1016/j.mrfmmm.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhao J., Xu J., Lv J. Identification of Profilin 1 as the Primary Target for the Anti-Cancer Activities of Furowanin A in Colorectal Cancer. Pharmacol. Rep. 2019;71:940–949. doi: 10.1016/j.pharep.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 188.Deeks J.J., Higgins J.P., Altman D.G. Cochrane Handbook for Systematic Reviews of Interventions. John and Wiley and Sons; Hoboken, NJ, USA: 2019. Analysing Data and Undertaking Meta-analyses; pp. 241–284. [DOI] [Google Scholar]
- 189.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., Estarli M., Barrera E.S.A., et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Rev. Esp. Nutr. Hum. Diet. 2016;20:148–160. doi: 10.1186/2046-4053-4-1. [DOI] [Google Scholar]
- 190.Huedo-Medina T.B., Sánchez-Meca J., Marín-Martínez F., Botella J. Assessing Heterogeneity in Meta-Analysis: Q Statistic or I 2 Index? Psychol. Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.