Abstract
Hyperammonemia is the pathological accumulation of ammonia in the blood, which can occur in many different clinical settings. Most commonly in adults, hyperammonemia occurs secondary to hepatic dysfunction; however, it is also known to be associated with other pathologies, surgeries, and medications. Although less common, hyperammonemia has been described as a rare, but consistent complication of solid organ transplantation. Lung transplantation is increasingly recognized as a unique risk factor for the development of this condition, which can pose grave health risks—including long-term neurological sequelae and even death. Recent clinical findings have suggested that patients receiving lung transplantations may experience postoperative hyperammonemia at rates as high as 4.1%. A wide array of etiologies has been attributed to this condition. A growing number of case studies and investigations suggest disseminated opportunistic infection with Ureaplasma or Mycoplasma species may drive this metabolic disturbance in lung transplant recipients. Regardless of the etiology, hyperammonemia presents a severe clinical problem with reported mortality rates as high as 75%. Typical treatment regimens are multimodal and focus on 3 main avenues of management: (1) the reduction of impact on the brain through the use of neuroprotective medications and decreasing cerebral edema, (2) augmentation of mechanisms for the elimination of ammonia from the blood via hemodialysis, and (3) the diminishment of processes producing predominantly using antibiotics. The aim of this review is to detail the pathophysiology of hyperammonemia in the setting of orthotopic lung transplantation and discuss methods of identifying and managing patients with this condition.
Keywords: Hyperammonemia, ammonia, lung transplantation, Ureaplasma, Mycoplasma hominis, dialysis, critical care
Introduction
Ammonia (NH3) is an ordinary metabolite in the human body, however, supraphysiologic levels in the systemic circulation can result in profound neurological damage and even death. This hyperammonemic state has been observed in response to a variety of clinical conditions, including post lung transplantation. Though rare, hyperammonemia can be a grave condition in these patients, with estimates of its all-cause mortality being 50% to 75%.1-5 Surviving patients are often burdened with significant long-term neurological sequelae, such as cognitive impairment.1,6 The most common cause of the disorder in adults is hepatic dysfunction—particularly cirrhosis.7-9 In children, it is classically associated with inborn-errors-of-metabolism concerning urea cycle enzymes and transporters,1,10,11 collectively called urea cycle disorders (UCDs). More recently, however, hyperammonemia has been increasingly linked to organ transplantation—particularly in lung transplant recipients.
In the United States, 36 528 solid organ transplants were performed in 2018—more than 2500 of which were lung transplants—the sixth consecutive year in which a new record high of organ transplantation was reached.12 Data show the median survival after lung transplant between 2009 and 2016 is 6.5 years, up from 6.1 years between 1999 and 2008 and 4.3 years between 1990 and 1998.13 Survival curves show mortality is highest within the first few months post-transplant and the slope declines in subsequent years.14 The leading causes of death post lung transplantation continue to be infection and graft failure within the first year.14 Still, other early causes of morbidity and mortality remain a serious concern, including hyperammonemia.
Current estimates report the incidence of hyperammonemia subsequent to lung transplantation as 1% to 4.1%,4,5,15-17 and this condition carries a 67% 30-day mortality. In contrast, lung transplant patients with physiologic NH3 levels have a 17% post-transplant mortality rate for the same time period.18,19 Onset of symptoms following lung transplant is typically observed within 2 weeks of surgery.17 Unfortunately, the early identification of hyperammonemia in the postoperative period, while the patient is often still in the intensive care unit, can be confounded by other factors. For example, the altered mental state associated with high ammonia levels could be attributed to postoperative delirium, sedation due to analgesia, or immune suppression.3 Delayed diagnosis in the recovery period likely contributes to the overall poor outcomes. This review will focus on the pathophysiology, clinical presentation, diagnosis, and treatment of hyperammonemia with regards to orthotopic lung transplantation patients.
Pathophysiology
The pathophysiology of hyperammonemia is multifactorial. The production of NH3 in the human body—as shown in Figure 1—occurs primarily within the gut via the breakdown of proteins and through the natural metabolic processes of the gut microbiome.2,7-9,20-22 NH3 is then transported through the portal circulation to hepatocytes where 90% is converted to urea through the urea cycle (UC) and the remainder is converted to glutamine (Gln) via glutamine synthetase (GS).1,7,9,10,16,20,21,23-25 This enzyme serves an identical function in non-hepatic tissues such as the brain and skeletal muscle.2,7-9,22 Gln can then be used for energy production in the gut or be excreted in the urine.24 Secondarily, NH3 is produced by deamination in skeletal muscle2,7-9,22 and the kidneys.2,7-9,22,26 The kidneys are able to regulate NH3 levels by shifting their production to excretion ratio to favor excretion in times of elevated NH3.7,8 A myriad of different pathological derangements in these pathways can lead to hyperammonemic states in conditions such as cirrhosis, UCD’s, chronic kidney disease, hemorrhagic shock, and others—all of which are largely well understood and reported in the literature. The etiology of the hyperammonemia syndrome in a lung transplant recipient, however, is somewhat less well known.
Figure 1.
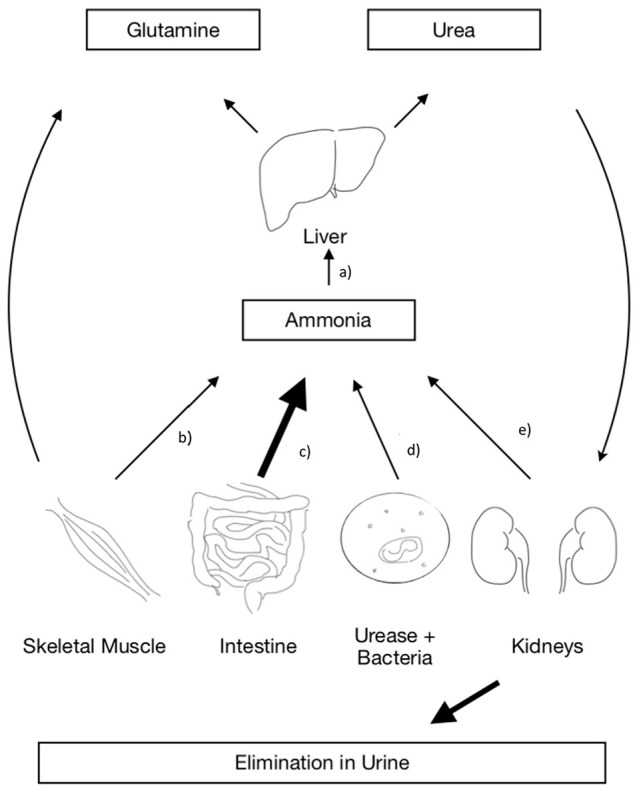
The urea cycle. The figure depicts the key organs responsible for producing, metabolizing, and eliminating ammonia. (a) NH3 can accumulate in the liver either when the production in the body exceeds its ability to metabolize it or when the liver’s metabolic functions are deranged such as in the case of cirrhosis. (b) Skeletal muscle functions to both produce and eliminate ammonia, it can contribute to hyperammonemia when there is severe sarcopenia such as in the setting of calcineurin inhibitors and corticosteroids. (c) The gastrointestinal system is the primary producer of ammonia under physiologic conditions. Its rate of consumption can be elevated in the setting of high dietary protein. (d) Urease positive bacteria are only responsible for ammonia production in pathological states and play no role in the normal urea cycle. (e) The kidneys are able to produce and eliminate NH3 in the form of urea, they can shift their production to elimination ratio in times of excess.
Due to the rarity of the condition, there is a paucity of research dedicated to hyperammonemia following lung transplantation. It is well documented, however, that compared to other solid organ transplants, excluding the liver, idiopathic hyperammonemia most commonly affects lung transplant recipients.27 One of the more classical hypotheses suggests that physiological stress from orthotopic lung transplantation induces catabolism great enough to uncover an occult deficit in GS and the UC enzymes.5 Case studies of patients having other procedures have been published where the stress of surgery has been shown to uncover an enzyme deficiency and ultimately result in hyperammonemia.28 In support of this hypothesis, Tuchman et al reported a markedly reduced level of hepatic GS in 2 patients who developed hyperammonemia secondary to lung transplantation.29 More recent studies have shown similar findings of hepatic GS deficiencies in some, but not all, hyperammonemic patients observed post-orthotopic lung transplantation.16 An additional hypothesis that has been presented implicates calcineurin inhibitors and corticosteroids, due to their potential for inducing sarcopenia and limiting the ability of skeletal muscle to contribute toward fixing NH3 into glutamine.30 These putative mechanisms are consistent with findings that hyperammonemia following lung transplant is independent from liver disease.19
More recent investigations have focused on an extrahepatic cause for elevated NH3 in lung transplant recipients. Mycoplasma and Ureaplasma are organisms that fall under the class of Mollicutes and have been associated with cases of hyperammonemia. These species are difficult to isolate with standard hospital laboratory conditions and equipment,31 because they lack a cell wall for staining.
In 2015, Bharat et al identified systemic infections with either Ureaplasma parvum or Ureaplasma urealyticum in all 6 of their transplant patients who developed hyperammonemia and in none of their 20 control subjects.15 It has been hypothesized that these urease-producing bacteria that commonly inhabit the genitourinary tract may disseminate in immunosuppressed individuals and be responsible for development of hyperammonemia in lung transplant recipients.15 By converting urea into NH3 and CO2 these organisms are able to produce energy as well as generate a substrate for further ureagenesis by the host organism.32 This creates a dangerous cycle, where Ureaplasma are able to proliferate while progressively producing greater and greater amounts of NH3. Additional investigations have confirmed the presence of Ureaplasma in hyperammonemic lung recipients.3,33,34 Ureaplasma has also been detected in patients with hyperammonemia and other immunocompromising conditions. The first case of Ureaplasma-induced hyperammonemia in an immunosuppressed pediatric patient with acute myelogenous leukemia was recently published.35 Subsequent case reports have evinced further incidences of hyperammonemia secondary to disseminated Ureaplasma in other children with hematologic malignancies and impaired immunologic function,36 which notably resolved with antibiotic treatment of the infection. Systemic Ureaplasma infections have similarly been observed in patients experiencing hyperammonemia following hematopoietic cell transplantation37 and undergoing immunosuppression for cancer treatment.18 Furthermore, hyperammonemia has been described in a case report of a lung transplant recipient whose donor lung tested positive for Ureaplasma parvum on bronchioalveolar lavage prior to surgery.33 A murine model for these transplant patients has shown a causal relationship between systemic infection with Ureaplasma and hyperammonemia in immunosuppressed mice.17,38
Identifying this bacterial etiology of hyperammonemia is complicated by the difficulty of pre-transplant screening for Ureaplasma, as it does not culture on standard media or stain.15,33 Accordingly, prescreening for Ureaplasma is not performed and the true prevalence of Ureaplasma-derived hyperammonemia is difficult to quantify.
One similar etiology that has been recently described, although with some controversy, is systemic infection by Mycoplasma hominis. An organism more known for its ability to cause opportunistic infection in the renal transplant population, disseminated M. hominis infection has been isolated in patients presenting with hyperammonemia.18 Much like the Ureaplasma species, M. hominis is difficult to culture or stain, and therefore limits timely identification and diagnosis.34,39,40 M. hominis, however, raises NH3 levels through an alternative mechanism than Ureaplasma species. This organism depletes arginine, a necessary component of the UC,40 resulting in a pathological inability to clear NH3 from the circulation. M. hominis has recently been cited as a disseminated organism in patients who developed hyperammonemia following autologous skin transplant,41 as well as a lung transplant recipient.38 However, multiple case reports of confirmed M. hominis infection had retrospective PCR confirmed co-infection with Ureaplasma species, which may indicate that M. hominis may not be a lone cause of hyperammonemia.27 Future case studies of PCR confirmed infection are needed to further support or reject the hypothesis that M. hominis plays a causal role in the development of hyperammonemia in immunocompromised patients.
In all cases of hyperammonemia—regardless of the etiology—the mechanism of ammonia’s effect on the central nervous system is the same. Once in the systemic circulation, NH3 is able to cross the blood brain barrier through multiple mechanisms including gaseous diffusion, passive diffusion in its soluble form through membrane channels, and competitively through potassium channels.8 In the brain, NH3 is taken up by astrocytes and converted to glutamine through the action of GS.7,21 This results in a series of adverse events. The markedly elevated Gln increases the osmotic pressure, causing a disruption of aquaporins, ultimately leading to the cerebral edema and hypertension characteristic of hyperammonemia.7,21 Concomitantly, astrocytes release various pro-inflammatory cytokines, such as tissue necrosis factor alpha (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), and interferon (IFN).7,8 Astrocyte impairment and subsequent downregulation of their glutamate receptors can trigger excessive glutamatergic activity in adjacent synapses, leading to excitotoxicity that results in the encephalopathy and seizures commonly seen in hyperammonemia.22 Similar physiologic changes of increased GABAergic tone occur in the Purkinje cells of the cerebellum, which is likely responsible for the ataxia and myoclonus also seen with this metabolic disturbance.42 Delay in recognition and proper management can lead to significant long-term morbidity, including refractory status epilepticus, motor and cognitive impairment, cerebral palsy, and death.24
Clinical Presentation
The usual clinical course of hyperammonemia if left untreated has several defining signs and symptoms which can vary depending on the severity of the patient’s condition and with the patient’s age. In adults, the condition is typified by several classic symptoms, shown in Table 1, such as altered mental state, lethargy, disturbances in mood and personality, ataxia, vomiting, seizures, unconsciousness, and potentially death.2,6,8,11 In a recent study of adults in the intensive care unit (ICU) with elevated NH3 in the absence of acute or chronic liver disease, the most common symptom of hyperammonemia was encephalopathy with a prevalence of 71%.43 When NH3 accumulates chronically—such as in cirrhosis—the severity of the symptoms is attenuated somewhat by the compensatory mechanisms of the muscle and liver to clear NH3 from the blood.7 In the acute form, this protective mechanism has not had a chance to take effect.
Table 1.
Acute signs and symptoms of hyperammonemia.
| Altered consciousness |
| Seizures |
| Changes in mood |
| Changes in personality |
| Nausea and vomiting |
| Ataxia |
| Lethargy |
| Poor feedinga |
This item is for consideration in pediatric patients.
In acute hyperammonemia—which occurs in patients receiving organ transplantations—the lack of compensation can lead to potentially catastrophic complications not seen in the chronic form. Acute plasma concentrations of NH3 ⩾ 200 µM have been associated with cerebral edema and cerebral herniation.2,7,11 This is in addition to the other hallmark signs of altered mentation, ataxia, vomiting, and seizures.2,6,8,11 Survival in these acute cases has been linked to early treatment of cerebral hypertension and measures that slow down metabolism.2 Initial symptoms of hyperammonemia in lung recipients may be masked by concurrent administration of sedatives, calcineurin-inhibitors, and/or narcotic analgesia3 in the immediate postoperative period.
Diagnostics
Given that early signs and symptoms of hyperammonemia post lung transplant are easily attributed to more common causes such postoperative delirium and sedation due to analgesia, more aggressive laboratory monitoring is likely appropriate. In a 2018 study by Larangeira et al, the authors suggest using a NH3 cut-off of 92 µmol/L as the value to begin treatment of intracranial hypertension.44 Use of this laboratory value may possess increased significance since radiographic findings associated with acute hyperammonemia are not well recognized in the adult literature.6 With the recent identification of a possible association between disseminated infection and hyperammonemia in lung transplant recipients, screening of the donor lung prior to or post-transplant for the culprit organisms might be of value. However, as previously mentioned, both Mycoplasma and Ureaplasma species are difficult to isolate with standard hospital laboratory conditions and equipment.31 While culture is the gold standard of detection, these species require specialized media and conditions, and up to 5 days of growth to identify.31 PCR assays have been developed and used to identify infection in some patients. A rapid real-time PCR assay described by Cunningham et al for detection of these Mollicutes lowers detection time to 3 hours with high sensitivity and specificity, but its use was limited to isolates originating solely from urogenital infections.31 PCR was also successfully used to identify Ureaplasma parvum by Smith et al in a pediatric, hyperammonemic patient, whose infection had become disseminated.36 Somerville et al similarly implemented a real-time PCR screening program to test fluid from bronchioalveolar lavage of recently transplanted lungs. Their results support the association between Mollicute infection and hyperammonemia syndrome. These authors recommend early surveillance of donors and/or recipients with PCR testing as a method to prevent the rare but fatal consequence of hyperammonemia.45
Treatment
The majority of prior investigations on the management of hyperammonemia have focused predominantly on cases secondary to liver failure or UCD’s. As such, many of the treatment modalities discussed here rely on data from investigations of those populations, with specific research into treatment of hyperammonemia post lung transplant lacking in the scientific literature. As these interventions are frequently used in case studies of transplant recipients and primarily utilize ways to reduce NH3 and provide neuroprotection, they have been included in this review along with recommendations targeted more directly at microbial causes. Traditionally because of the condition’s complexity, an approach that includes neuroprotective and anti-inflammatory medications, directly removing NH3 from the blood, and decreasing NH3 production from its source should be considered in any hyperammonemic patient.46 Neuroprotective and anti-inflammatory medications such as N-acetylcysteine, indomethacin, propofol, minocycline, and mannitol are used to reduce cerebral edema and blood flow.2,7 The use of indomethacin has proven controversial, due to its potential for causing renal failure.7 Induced hypothermia has also been indicated as a potential therapy for reducing the inflammation and cerebral edema that occurs during hyperammonemia.7,47
Hemofiltration and dialysis have been successfully employed to directly reduce NH3 levels in patients’ blood.2,47 While many reviewers recommend aggressive early implementation of dialysis,7,8,10,21,48 the specific method of dialysis that is most beneficial is dependent on the individual patient, available equipment, and dialysis expertise.49 Although case reports have demonstrated successful lowering of blood NH3 levels with peritoneal dialysis,48 popular opinion is that this method is ineffective and not recommended.49 Multiple case reports demonstrate the effectiveness of continuous renal replacement therapy (CRRT) at lowering toxic ammonia levels in patients with severe hyperammonemia (>1000 mg/dL),50 including cases where NH3 levels were refractory to a trial of peritoneal dialysis.51 A recent retrospective study of critically ill children with metabolic diseases has demonstrated the efficacy of CRRT at quickly reducing NH3 levels. Case reports have also shown a benefit to early initiation of high dose intermittent hemodialysis (IHD) in adult lung transplant recipients.47 Studies comparing these methods have shown mixed results for treating acute hyperammonemia. CRRT prevents continued build-up of NH3 levels that may occur between dialysis sessions in the intermittent form,2,47 but some clinicians argue for the efficacy of aggressive intermittent hemodialysis with flow titration to the climbing NH3 levels between sessions.47 A 2017 retrospective institutional and systematic review of hyperammonemic lung transplant recipients found mortality of 40% with intermittent hemodialysis, 75% with continuous veno-venous hemodialysis, and 100% in patients that did not receive renal replacement therapy to remove NH3,52 making it clear just how crucial a role dialysis plays for these patients. As the literature continues to grow, further studies are needed to determine the best method to reduce mortality in individual patient populations.
There are also many treatment options for hyperammonemia that target upregulating the elimination of NH3 or downregulating its production. These interventions—shown in Table 2—have a number of different modes of action and have an established benefit in the treatment of chronic hyperammonemia. Lactulose administration, currently considered the foundation of therapeutics in general cases of hyperammonemia,53 has the ability to limit NH3 absorption from the gut. By acidification of the colon, lactulose indirectly ionizes NH3 into non-absorbable and excretable ammonium (NH4+).8,21 A meta-analysis published in 2016 comparing non-absorbable disaccharides to placebo showed lactulose has both a therapeutic and preventative effect on patients with hepatic encephalopathy.54 While a mainstay in the treatment for chronic hyperammonemia, lactulose is not effective in the treatment of acute hyperammonemia such as that seen in lung transplant patients, having been shown to have no effect on improving patient mortality.2,7 This would likely be especially true in cases of hyperammonemia secondary to systemic Ureaplasma infection, where the source of excessive NH3 production is not located in the gut.
Table 2.
Pharmacologic interventions.
| Intervention | Purpose |
|---|---|
| N-acetylcysteine, indomethacin, propofol, and mannitol | To reduce cerebral edema |
| Lactulose | To acidify the colon to decrease NH3 absorption |
| Sodium benzoate | To decrease glycine metabolism |
| Sodium phenylacetate, sodium phenylbutyrate, glycerol phenylbutyrate, branched chain amino acids | To decrease glutamine metabolism |
| L-arginine/L-citrulline, carglumic acid, L-ornithine/L-aspartate | To promote urea production |
| Antibiotics | To eliminate NH3 producing microbes |
Other treatments aim to increase the body’s natural pathways to eliminate NH3 and to reduce its production. Agents including L-arginine,2,7 L-citrulline, L-ornithine, L-aspartate, and carglumic acid, all of which promote the UC and thus the fixation of NH3 from the blood, are recognized interventions in hyperammonemic patients.8 Patients with hepatitis C at the pre-cirrhotic stage were shown to have significantly decreased blood concentrations of NH4+ and improvement in psychometric testing after a trial of L-ornithine-L-aspartate (LOLA).55 Additionally, LOLA has been shown to restore muscle protein synthesis in sarcopenic patients, thus enhancing skeletal muscle’s natural ability to metabolize NH3 from the blood.56 Conversely, reducing the production of NH3by preventing the degradation of Gln and glycine—2 amino acids responsible for much of its physiologic production—has been suggested. Sodium benzoate,2,7,8 sodium phenylacetate,2,7,8 sodium phenylbuterate,8,46 glycerol phenylbuterate,8 and branch chain amino acids (BCAAs)2,8 have all been used for this purpose. Additionally, the institution of set protocols for treating these patients may prove beneficial, as one study investigating a protocol for determining when to administer combined intravenous sodium benzoate and sodium phenylacetate was shown to significantly reduce the length of time to administration of these scavengers.57 Unfortunately, this study did not show a benefit to patient morbidity and mortality.57
Non-pharmacological recommendations—detailed in Table 3—in addition to hemodialysis, include adjustments to diet. Decreasing meat protein from the diet, whether by restriction of protein or replacement with dairy-based or vegetable protein, has been suggested as a method to limit substrates for ammoniagenesis.22,47 Although the high dietary protein requirements of patients with cirrhosis has made this challenging in traditional hyperammonemic patients, this poses less of an obstacle in the setting of lung transplantation. Restriction or modification of total parenteral nutrition in the ICU setting may additionally be advisable, as this mode of feeding has been independently associated with hyperammonemia.58
Table 3.
Non-pharmacologic interventions.
| Intervention | Purpose |
|---|---|
| Intermittent hemodialysis | To directly dialyze NH3 from the blood |
| Continuous renal replacement therapy | To dialyze NH3 and perhaps prevent toxic build-up between dialysis sessions |
| Peritoneal dialysis | To directly remove NH3a |
| Restriction/elimination of total parenteral nutrition | To reduce substrates for aminogenesis |
| Dietary protein restriction | To reduce substrates for aminogenesis in the chronic setting |
| Hypothermia | To reduce inflammation |
The effectiveness of peritoneal dialysis is contested. Although case studies have shown it being used successfully, choosing peritoneal dialysis would not be recommended in the setting of available alternatives.
Although antibiotic administration is a mainstay of treatment for hyperammonemic patients, it is usually targeted at reducing the gut microbiome’s NH3 production via agents such as rifaximin.21 In the case of disseminated Ureaplasma and Mycoplasma species, this is unlikely to be effective as the source of NH3 is elsewhere. The effective selection of antibiotics for patients with disseminated Ureaplasma or Mycoplasma infections requires special consideration. These Mollicutes, like all Mycoplasmas, display innate resistance to β-lactams, sulfonamides, trimethoprim, and rifampicin.59,60 M. hominis is uniformly resistant to macrolides,18 resistance to macrolides, quinolones, lincosamides, and tetracycline have also been observed in Ureaplasma.60 Dhawan et al has, however, shown that Ureaplasma isolates were susceptible specifically to doxycycline in 91% of cases, josamycin in 86%, ofloxacin in 77%, and azithromycin in 71%, whereas isolates of M. hominis were uniformly susceptible to doxycycline, josamycin, and ofloxacin.61 These data are summarized in Table 4. In a case study of a patient who developed hyperammonemia resulting from U. parvum infection transmitted by a donor lung, Fernandez et al demonstrated that targeted antibiotic therapy with azithromycin and doxycycline resolved the hyperammonemia.32 Further studies continue to support Mycoplasma and Ureaplasma’s overwhelming susceptibility to doxycycline.62
Table 4.
General mollicute antibiotic information.
| Ureaplasma species | Mycoplasma species | |
|---|---|---|
| Specific susceptibilities | Doxycycline (91%), josamycin (86%), ofloxacin (77%), azithromycin (71%) | Doxycycline (100%), josamycin (100%), ofloxacin (100%) |
| General resistances | β-lactams, sulfonamides, trimethoprim, and rifampicin, macrolides, quinolones, lincosamides, tetracycline | β-lactams, sulfonamides, trimethoprim, and rifampicin, macrolides (100%) |
Information provided in parentheses indicates the percent of isolates in which the organisms were susceptible or resistant to the specific antibiotic.
Due to the difficulty in identifying these Mollicutes with standard laboratory practices, empiric antibiotics for suspected disseminated urease-producing infections should be considered in any acutely hyperammonemic immunocompromised patient, as early treatment could be life-saving.34 Case reports of successful treatment of hyperammonemia syndrome in immunocompromised individuals often involve multimodal approaches to treatment, combining antimicrobial agents with ammonia-lowering medications and CRRT, as well as airway protection via mechanical ventilation.5,27,37,41,47 Unfortunately, despite advances in earlier identification of the potential causes of hyperammonemia and treatment modalities, mortality from hyperammonemia following orthotopic lung transplantation remains high.
Hyperammonemia secondary to organ transplantation remains an understudied medical condition, with most of the extant literature being limited to case studies. Further investigations into this postoperative complication are required to establish a best treatment plan for caring for these critically ill patients.
Conclusion
Hyperammonemia is a rare but potentially catastrophic complication of organ transplantation. The etiology of this metabolic disturbance is likely rooted in opportunistic bacterial infections by Ureaplasma and Mycoplasma species. However, the extent to which catabolic stress and otherwise occult deficits within the patients’ metabolic pathways may contribute to the incidence of this disease is still unknown. In any patient with new onset altered mental status—especially in the lung transplant recipients in the critical care setting and in the initial 30-day post-operative period—checking an ammonia level even when the cause seems clear could help steer management. Early initiation of treatment is crucial for improving survival and outcomes. Currently, targeted therapies against systemic bacterial infections appear to be one of the best methods of treatment for these postoperative transplant recipients. Despite not addressing the cause of the toxicity, early and aggressive hemodialysis has been shown to be an effective method at lowering blood ammonia levels in patients with severe acute hyperammonemia. While no universal standardized treatment plan has been established, a case-dependent multimodal approach appears to be key to improving mortality. In cases with high suspicion for hyperammonemia early hemodialysis, antibiotics that cover U. parvum and M. hominis, and implementation of neuroprotective measures are likely to best address and treat this metabolic derangement. Antibiotic prophylaxis in donors may also merit consideration. Further research into the treatment of hyperammonemia and in determining which patients are at greatest risk is desperately needed to remedy the unacceptably high morbidity and mortality that is threatening the lives of some of the most vulnerable patients.
Footnotes
Declaration of conflicting interests:The author (s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author (s) received no financial support for the research, authorship, and/or publication of this article.
Author’s Note: All work was performed at Temple University: Lewis Katz School of Medicine, Philadelphia, PA, USA.
Authors’ Contributions: RFL: literature review, detailed composition and editing of manuscript, original graphics. Matthew S Silverman: literature review and editing of manuscript. ESH: resource management and editing of manuscript. KDG: literature review, editing of manuscript and resource management.
ORCID iDs: Robert F Leger  https://orcid.org/0000-0002-2013-4685
https://orcid.org/0000-0002-2013-4685
Ksenia D Guvakova  https://orcid.org/0000-0001-8553-9686
https://orcid.org/0000-0001-8553-9686
References
- 1. Haberle J, Boddaert N, Burlina A, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pallavi R, Matejak-Popis B. Hyperammonemia: a silent killer. Am J Ther. 2016;23:E591-E593. [DOI] [PubMed] [Google Scholar]
- 3. McLaughlin DC, Mallea JM, Ng LK. Hyperammonemia presenting as refractory status epilepticus after lung transplant in a patient positive for Ureaplasma parvum. Indian J Crit Care Med. 2018;22:463-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen C, Bain KB, Iuppa JA, et al. Hyperammonemia syndrome after lung transplantation: a single center experience. Transplantation. 2016;100:678-684. [DOI] [PubMed] [Google Scholar]
- 5. Moffatt-Bruce SD, Pesavento T, Von Viger J, et al. Successful management of immunosuppression in a patient with severe hyperammonemia after lung transplantation. J Heart Lung Transplant. 2008;27:801-803. [DOI] [PubMed] [Google Scholar]
- 6. U-King-Im J, Yu E, Bartlett E, Soobrah R, Kucharczyk W. Acute hyperammonemic encephalopathy in adults: imaging findings. Am J Neuroradiol. 2011;32:413-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest. 2007;132:1368-1378. [DOI] [PubMed] [Google Scholar]
- 8. Liu J, Lkhagva E, Chung HJ, Kim HJ, Hong ST. The pharmabiotic approach to treat hyperammonemia. Nutrients. 2018;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clericetti CM, Milani GP, Lava SAG, Bianchetti MG, Simonetti GD, Giannini O. Hyperammonemia associated with distal renal tubular acidosis or urinary tract infection: a systematic review. Pediatr Nephrol. 2018;33:485-491. [DOI] [PubMed] [Google Scholar]
- 10. Machado MC, da Silva FP. Hyperammonemia due to urea cycle disorders: a potentially fatal condition in the intensive care setting. J Intensive Care. 2014;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Batshaw ML. Hyperammonemia. Curr Probl Pediatr. 1984;14:6-69. [DOI] [PubMed] [Google Scholar]
- 12. Administration HRS. Organ donation statistics. June 16, 2019, https://www.organdonor.gov/statistics-stories/statistics.html#transplants.
- 13. Chambers DC, Cherikh WS, Goldfarb SB, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-fifth adult lung and heart-lung transplant report—2018; focus theme: multiorgan transplantation. J Heart Lung Transplant. 2018;37:1169-1183. [DOI] [PubMed] [Google Scholar]
- 14. Thabut G, Mal H. Outcomes after lung transplantation. J Thorac Dis. 2017;9:2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bharat A, Cunningham SA, Budinger GS, et al. Disseminated Ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci Transl Med. 2015;7:284re283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lichtenstein GR, Yang YX, Nunes FA, et al. Fatal hyperammonemia after orthotopic lung transplantation. Ann Intern Med. 2000;132:283-287. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Greenwood-Quaintance KE, Karau MJ, et al. Ureaplasma parvum causes hyperammonemia in a pharmacologically immunocompromised murine model. Eur J Clin Microbiol Infect Dis. 2017;36:517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nowbakht C, Edwards AR, Rodriguez-Buritica DF, et al. Two cases of fatal hyperammonemia syndrome due to Mycoplasma hominis and Ureaplasma urealyticum in immunocompromised patients outside lung transplant recipients. Paper presented at: Open Forum Infectious Diseases; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lichtenstein GR, Kaiser LR, Tuchman M, et al. Fatal hyperammonemia following orthotopic lung transplantation. Gastroenterology. 1997;112:236-240. [DOI] [PubMed] [Google Scholar]
- 20. Lama M, Shannon S, Davin Q. Methamphetamine intoxication encephalopathy associated with hyperammonemia. Psychosomatics. 2016;57:325-329. [DOI] [PubMed] [Google Scholar]
- 21. Jover-Cobos M, Khetan V, Jalan R. Treatment of hyperammonemia in liver failure. Curr Opin Clin Nutr Metab Care. 2014;17:105-110. [DOI] [PubMed] [Google Scholar]
- 22. Auron A, Brophy PD. Hyperammonemia in review: pathophysiology, diagnosis, and treatment. Pediatr Nephrol. 2012;27:207-222. [DOI] [PubMed] [Google Scholar]
- 23. Soria LR, Nitzahn M, De Angelis A, et al. Hepatic glutamine synthetase augmentation enhances ammonia detoxification. J Inherit Metab Dis. 2019;42:1128-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Savy N, Brossier D, Brunel-Guitton C, et al. Acute pediatric hyperammonemia: current diagnosis and management strategies. Hepat Med. 2018;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Häberle J. Clinical and biochemical aspects of primary and secondary hyperammonemic disorders. Arch Biochem Biophys. 2013;536:101-108. [DOI] [PubMed] [Google Scholar]
- 26. Ratner S, Nocito V, Green DE. Glycine oxidase. J Biol Chem. 1944;152:119-133. [Google Scholar]
- 27. Matson KM, Sonetti DA. Successful treatment of Ureaplasma-induced hyperammonemia syndrome post-lung transplant. Transpl Infect Dis. 2019;21:e13022. [DOI] [PubMed] [Google Scholar]
- 28. Castineira J, Goltser Y, Vila M, et al. Postbariatric surgery hyperammonemia: a rare cause of encephalopathy. ACG Case Rep J. 2019;6(7):e00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tuchman M, Lichtenstein GR, Rajagopal B, et al. Hepatic glutamine synthetase deficiency in fatal hyperammonemia after lung transplantation. Ann Intern Med. 1997;127:446-449. [DOI] [PubMed] [Google Scholar]
- 30. Seethapathy H, Fenves AZ. Pathophysiology and management of hyperammonemia in organ transplant patients. Am J Kidney Dis Off J Natl Kidney Found. 2019;74:390-398. [DOI] [PubMed] [Google Scholar]
- 31. Cunningham SA, Mandrekar JN, Rosenblatt JE, Patel R. Rapid PCR detection of Mycoplasma hominis, Ureaplasma urealyticum, and Ureaplasma parvum. Int J Bacteriol. 2013;2013:168742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith D, Russell W, Ingledew W, Thirkell D. Hydrolysis of urea by Ureaplasma urealyticum generates a transmembrane potential with resultant ATP synthesis. J Bacteriol. 1993;175:3253-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fernandez R, Ratliff A, Crabb D, Waites KB, Bharat A. Ureaplasma transmitted from donor lungs is pathogenic after lung transplantation. Ann Thorac Surg. 2017;103:670-671. [DOI] [PubMed] [Google Scholar]
- 34. Aguilar C, Gohir W, Tikkanen J, et al. Ureaplasma spp. and Mycoplasma hominis PCR in respiratory samples from lung transplant recipients with hyperammonemia syndrome and cerebral edema. J Heart Lung Transplant. 2018;37:S360-S360. [Google Scholar]
- 35. Placone N, Kao RL, Kempert P, et al. Hyperammonemia from Ureaplasma infection in an immunocompromised child. J Pediatr Hematol Oncol. 2019;42(2):e114-e116. [DOI] [PubMed] [Google Scholar]
- 36. Smith M, Crews JD, Cheek N, Srivastava R, Appachi E. Hyperammonemic encephalopathy due to Ureaplasma parvum infection in an immunocompromised child. Pediatrics. 2019;144:e20190601. [DOI] [PubMed] [Google Scholar]
- 37. Graetz R, Meyer R, Shehab K, Katsanis E. Successful resolution of hyperammonemia following hematopoietic cell transplantation with directed treatment of Ureaplasma parvum infection. Transpl Infect Dis. 2018;20:4. [DOI] [PubMed] [Google Scholar]
- 38. Wang XH, Karau MJ, Greenwood-Quaintance KE, et al. Ureaplasma urealyticum causes hyperammonemia in an experimental immunocompromised murine model. PLoS One. 2016;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanghavi D, Pannu J, Peters S, Scott J, Kennedy C, Wylam M. 1153: Mycoplasma hominis infection post heart-lung transplant patients- a case series. Crit Care Med. 2014;42:A1630. [Google Scholar]
- 40. Wylam ME, Kennedy CC, Hernandez NM, et al. Fatal hyperammonaemia caused by Mycoplasma hominis. Lancet. 2013;382:1956-1956. [DOI] [PubMed] [Google Scholar]
- 41. Wang W, Gao S, Kang Y, Yu L, Liu Y, Shen Z. Successful management of hyperammonemia syndrome in a patient after skin transplantation: a case report and a literature review on 41 patients. Zhonghua wei zhong bing ji jiu yi xue. 2019;31:367-370. [DOI] [PubMed] [Google Scholar]
- 42. Hassan SS, Baumgarten TJ, Ali AM, et al. Cerebellar inhibition in hepatic encephalopathy. Clin Neurophysiol. 2019;130:886-892. [DOI] [PubMed] [Google Scholar]
- 43. Sakusic A, Sabov M, McCambridge AJ, et al. Features of adult hyperammonemia not due to liver failure in the ICU. Crit Care Med. 2018;46:e897-e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Larangeira AS, Tanita MT, Dias MA, et al. Analysis of cerebral blood flow and intracranial hypertension in critical patients with non-hepatic hyperammonemia. Metab Brain Dis. 2018;33:1335-1342. [DOI] [PubMed] [Google Scholar]
- 45. Somerville L, Sligl W, Zelyas N, Lien D, Preiksaitis J. Surveillance for Mycoplasma/Ureaplasma infection in lung transplant recipients (LTRs) [abstract]. Am J Transplant. 2017;17(suppl 3):C257. [Google Scholar]
- 46. Said VJ, Garcia-Trujillo E. Beyond lactulose: treatment options for hepatic encephalopathy. Gastroenterol Nurs. 2019;42:277-285. [DOI] [PubMed] [Google Scholar]
- 47. Anwar S, Gupta D, Ashraf MA, et al. Symptomatic hyperammonemia after lung transplantation: Lessons learnt. Hemodial Int. 2014;18:185-191. [DOI] [PubMed] [Google Scholar]
- 48. Mehta K. Dialysis therapy in children. J Indian Med Assoc. 2001;99:368-373. [PubMed] [Google Scholar]
- 49. Matsumoto S, Häberle J, Kido J, Mitsubuchi H, Endo F, Nakamura K. Urea cycle disorders—update. J Hum Genet. 2019;64:833-847. [DOI] [PubMed] [Google Scholar]
- 50. Santa Maríaa FC, Espinosab JR, Hernándezc PG. Continuous venovenous hemofiltration in neonates with hyperammonemia. A case series. Rev Child Pediatr. 2018;89:74-78. [DOI] [PubMed] [Google Scholar]
- 51. Falk MC, Knight JF, Roy LP, et al. Continuous venovenous haemofiltration in the acute treatment of inborn errors of metabolism. Pediatr Nephrol. 1994;8:330-333. [DOI] [PubMed] [Google Scholar]
- 52. Krutsinger D, Pezzulo A, Blevins AE, et al. Idiopathic hyperammonemia after solid organ transplantation: primarily a lung problem? A single-center experience and systematic review. Clin Transplant. 2017;31:e12957. [DOI] [PubMed] [Google Scholar]
- 53. Hadjihambi A, Khetan V, Jalan R. Pharmacotherapy for hyperammonemia. Expert Opin Pharmacother. 2014;15:1685-1695. [DOI] [PubMed] [Google Scholar]
- 54. Gluud LL, Vilstrup H, Morgan MY. Nonabsorbable disaccharides for hepatic encephalopathy: a systematic review and meta-analysis. Hepatology. 2016;64:908-922. [DOI] [PubMed] [Google Scholar]
- 55. Buyeverov A, Bogomolov P, Mayev I, Matsievich M, Uvarova O. Possibilities of therapeutic correction of hyperammonemia and minimal hepatic encephalopathy in patients with chronic hepatitis C at the pre-cirrhotic stage. Tei Arkh. 2019;91:52-58. [DOI] [PubMed] [Google Scholar]
- 56. Butterworth RF. L-Ornithine L-Aspartate for the treatment of sarcopenia in chronic liver disease: the taming of a vicious cycle. Can J Gastroenterol Hepatol. 2019;2019:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brossier D, Goyer I, Ziani L, et al. Influence of implementing a protocol for an intravenously administered ammonia scavenger on the management of acute hyperammonemia in a pediatric intensive care unit. J Inherit Metab Dis. 2019;42:77-85. [DOI] [PubMed] [Google Scholar]
- 58. Devasahayam JVM, John SG, Assar S, et al. Hyperammonemia as an adverse effect in parenteral nutrition. In: Rajendram R, Preedy VR, Patel VB, eds. Diet and Nutrition in Critical Care. New York, NY: Springer; 2015:2065-2078. [Google Scholar]
- 59. Waites KB, Schelonka RL, Xiao L, Grigsby PL, Novy MJ. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin Fetal Neonatal Med. 2009;14:190-199. [DOI] [PubMed] [Google Scholar]
- 60. Kokkayil P, Dhawan B. Ureaplasma: current perspectives. Indian J Med Microbiol. 2015;33:205-214. [DOI] [PubMed] [Google Scholar]
- 61. Dhawan B, Malhotra N, Sreenivas V, et al. Ureaplasma serovars & their antimicrobial susceptibility in patients of infertility & genital tract infections. Indian J Med Res. 2012;136:991. [PMC free article] [PubMed] [Google Scholar]
- 62. Jang YS, Min JW, Kim YS. Positive culture rate and antimicrobial susceptibilities of Mycoplasma hominis and Ureaplasma urealyticum. Obstet Gynecol Sci. 2019;62:127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]


