Abstract
Background
Recurrent pediatric medulloblastoma and ependymoma have a grim prognosis. We report a first-in-human, phase I study of intraventricular infusions of ex vivo expanded autologous natural killer (NK) cells in these tumors, with correlative studies.
Methods
Twelve patients were enrolled, 9 received protocol therapy up to 3 infusions weekly, in escalating doses from 3 × 106 to 3 × 108 NK cells/m2/infusion, for up to 3 cycles. Cerebrospinal fluid (CSF) was obtained for cellular profile, persistence, and phenotypic analysis of NK cells. Radiomic characterization on pretreatment MRI scans was performed in 7 patients, to develop a non-invasive imaging-based signature.
Results
Primary objectives of NK cell harvest, expansion, release, and safety of 112 intraventricular infusions of NK cells were achieved in all 9 patients. There were no dose-limiting toxicities. All patients showed progressive disease (PD), except 1 patient showed stable disease for one month at end of study follow-up. Another patient had transient radiographic response of the intraventricular tumor after 5 infusions of NK cell before progressing to PD. At higher dose levels, NK cells increased in the CSF during treatment with repetitive infusions (mean 11.6-fold). Frequent infusions of NK cells resulted in CSF pleocytosis. Radiomic signatures were profiled in 7 patients, evaluating ability to predict upfront radiographic changes, although they did not attain statistical significance.
Conclusions
This study demonstrated feasibility of production and safety of intraventricular infusions of autologous NK cells. These findings support further investigation of locoregional NK cell infusions in children with brain malignancies.
Keywords: immunotherapy, intraventricular infusions, natural killer cells, recurrent brain tumors
Key Points.
Intraventricular infusions of ex vivo autologous NK cells demonstrated safety.
Cryopreserved NK cells were safely delivered with evidence of persistence.
Importance of the Study.
Prognosis of children with recurrent medulloblastoma and ependymoma remains dismal. There is need for a cure and improvement in survival, in the absence of systemic toxicity. This study evaluated these unmet clinical needs through the conduct of the first-in-human phase I investigation of intraventricular infusions of autologous, ex vivo expanded NK cells, in these children. Leptomeningeal disease and dissemination through the CSF seen in these tumors also provided the rationale for the locoregional administration of NK cells. The study is the first to apply radiomic tools to mine data from clinical radiographic images of children undergoing immunotherapy. Our study suggests the need to reassess frequency of intraventricular infusions of NK cells, incorporate more extended follow-up periods, and evaluate the use of radiomics to facilitate clinical decisions in the next generation of trials with NK cells.
Medulloblastoma is the most common malignant pediatric brain cancer, having an incidence of approximately 0.74/100 000 person-year.1 Children older than 3 to 5 years are usually treated with maximal safe surgical resection of the tumor, focal or craniospinal irradiation (CSI), and chemotherapy based on the clinical criterion of standard risk (SR) or high-risk (HR) disease.2 Infants below 3 years of age are treated with irradiation avoiding strategies.3 Even though the survival rate has increased to 80–90% and 60–65% in SR and HR, respectively, morbidities and long-term side effects are concerning.4,5 Extensive molecular analysis and transcriptional profiling of large cohorts of medulloblastoma have now consistently identified 4 distinct molecular entities termed wingless (WNT), sonic hedgehog (SHH), Group 3, and Group 4, with distinct clinical and molecular features.6,7 Efforts to tailor therapy based on these subtypes are ongoing, with 25–40% of patients with medulloblastoma treated with radiation and chemotherapy relapse, depending on subgroup affiliation, specific cytogenetic alterations, and presence of metastasis at diagnosis.8 Recurrent medulloblastoma commonly seen in Groups 3 and 4 have poor survival rate of less than 10% even with various salvage therapies. An effective treatment has not been identified, likely due to the evolving and now well-known temporal and spatial pattern of these relapses, precluding tailored optimal treatment for recurrent tumors.9,10
Ependymomas are glial tumors arising from ependymal cells of the central nervous system (CNS), whose primary therapy consists of maximal surgical resection followed by radiation and some cases chemotherapy.11,12 Relapses are seen in about 30–50% of patients. Surgical resection, radiation, and targeted therapy are performed for these recurrences, yet overall survival (OS) remains around 50%.13 Recent advances in the molecular characterization of these neoplasms and efforts to profile targeted therapy have yet to yield an improvement of the long-term survival, more so for the younger children with posterior fossa type A tumors.14–16
Current treatment strategies cause significant morbidity, and the blood‒brain barrier (BBB) has precluded clinical translation of several new promising therapeutics for these patients. Thus, there is an unmet clinical need for not only new treatments, but also for locoregional techniques to circumvent problems posed by the BBB.
Though a clinical study has shown the safety of administering natural killer (NK) cells into brain tumors of adults, similar studies in children have not been reported.17 The dire need for a novel therapy, availability of preclinical data of efficacy and safety of NK cell infusions in animal models,18 and the reproducible ability to propagate large numbers of ex vivo expanded NK cells19 provided the impetus to conduct this first-in-human, phase I study in children with these recurrent tumors.
We conducted this study with autologous cells based on preclinical data of high functionality in expanded patient-derived NK cells and to avoid potential toxicity of allogeneic sources. We also restricted the trial to 3 cycles with 3 dose levels (2 dose escalation and one dose de-escalation), to obviate any concerning side effects associated with longer periods of multiple infusions, with a primary objective to demonstrate the feasibility of NK cell production, release, and safety of intraventricular infusions.
Our preclinical work demonstrated the cytolytic ability of ex vivo expanded NK cells against medulloblastoma cells in vitro.20,21 In a separate preclinical report, we also showed that NK cells labeled with a fluorine-19 (19F)–based perfluorocarbon emulsion could suppress medulloblastoma growth in mouse orthotopic models while enabling 19F MRI to provide feedback on the delivery of infused NK cells.18
Despite the rise in immunotherapeutic strategies, few patients have shown long-term improvement in survival. Developing tools are needed that not only can predict upfront which patients are likely to benefit from therapy, but also provide feedback on response during treatment.22 Radiomics is an emerging field that mines radiographic images to extract high-throughput data to provide a detailed characterization of the tumor.23,24 Radiomic-based biomarkers have been evaluated in immunotherapy for predicting clinical changes and tumor recurrence. We present data from a pilot radiomic study of the feasibility of applying this tool to assess changes in tumor features in response to immunotherapy. This will be the first study evaluating the role of radiomics in pediatric or NK cell immunotherapy.25
The primary objective of this dose-escalation study was to establish the feasibility of production, the safety of infusion, and the maximum tolerated dose (MTD) of autologous NK cells propagated ex vivo with feeder cells expressing interleukin (IL)-21 and administered directly into the ventricles. Secondary objectives included descriptive assessment of clinical and or radiological responses, CSF evaluation of cellular profile, persistence, and phenotype of infused NK cells. We also evaluated the feasibility of developing a reproducible radiomic signature to predict which patients would have radiographic changes and benefit from NK immunotherapy.
Materials and Methods
Ex Vivo Activation/Propagation of NK Cells
Previous studies have described improved ex vivo numeric expansion of NK cells with soluble cytokines, autologous feeder cells, and feeder cell lines engineered with co-stimulatory molecules such as membrane-bound IL-15.26 We previously demonstrated that NK cells can be robustly propagated to large numbers from peripheral blood mononuclear cells by coculturing with irradiated feeder cells derived from K562 cells and genetically modified to express co-stimulatory molecules, including membrane-bound IL-21.19 Here, we adapted this approach for reproducible manufacturing of patient-derived NK cells in compliance with current Good Manufacturing Practice (GMP). Three mL/kg of peripheral blood, up to a maximum of 150 mL, was obtained from patients for NK cell manufacture. Details of the NK cell harvest, depletion of T cells, and cryopreservation of NK cells are available in the Supplementary data. The data for viability of recovery after cryopreservation are shown in Supplementary Figure 1.
Eligibility
This study was open for patients less than 22 years of age with recurrent/refractory medulloblastoma, or ependymoma involving the brain and/or spine at original diagnosis or relapse. Adequate CSF flow was verified by MRI with cine sequence of the brain and spine, following catheter placement in the fourth ventricle as per Sandberg et al or lateral ventricle, before receiving NK cell infusion.27 A performance score of Lansky 50 or higher if ≤16 years of age or a Karnofsky score of 50 or higher if >16 years of age was required to be eligible on the trial.
Additional eligibility criteria include adequate bone marrow function defined by an absolute neutrophil count of ≥1000/μL, platelet count of ≥30 000, and hemoglobin of ≥9.0 g/dL. Signed informed consent and patient assent when appropriate was obtained.
Exclusion criteria included enrollment in another protocol, untreated infection, extracranial metastasis, and chronic corticosteroid dependence (except replacement therapy). Patients with extensive disease and/or comorbid conditions were also excluded.
Ethics and Study Oversight
Institutional review board approval was obtained for conducting the study. The Investigational New Drug office of the MD Anderson Cancer Center (MDACC) oversaw the adverse effects (AEs) and timely reporting updates of the clinical trial. Reporting to the FDA and other regulatory bodies was performed as required. General oversight of the trial was by the principal investigator of the study at MDACC.
Study Design and Treatment Plan
Enrolled patients were screened for autologous donation and then proceeded to peripheral blood collection to manufacture the autologous expanded NK cell product. Catheter placement was performed after all GMP release criteria of NK cells had been met and the product authorized for patient use. The criteria for collection of blood for NK cell expansion included that the patient be off systemic steroids for at least 3 days. Protocol also required that patients not have received any steroids within 72 hours of NK cell infusions (before and after infusions), as it could undermine therapeutic efficacy; no cytotoxic therapy or hematopoietic growth factor within 14 days before blood collection; and the absence of symptoms or signs of systemic infection that would preclude safe blood collection.
Criteria to start the first NK cell infusion included the following: NK cell products which have met all GMP release criteria, adequate CSF flow, and neurosurgical clearance to use the Ommaya connected to the intraventricular catheter. NK cell infusions were continued if patients tolerated prior infusions without dose-limiting toxicities (DLTs) and demonstrated no progressive disease (PD).
Infusion Procedure and Monitoring
Once patients were admitted to the infusion unit, vital signs and baseline physical evaluation including focused neurological exam were performed. Further details of infusion procedure, evaluations of blood counts, and MRI scans are shown in the Supplementary data.
Schedule of NK Cell Infusion
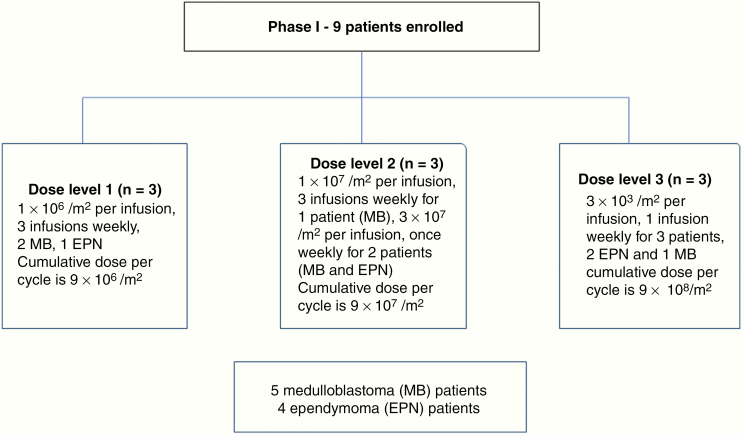
Each patient was scheduled to receive 3 cycles of NK cell infusion. Initially, each cycle was of 4 weeks duration with NK cell infusions thrice weekly for 3 weeks followed by a rest week. The dose of NK cells per infusion ranged from 106/m2 to 108/m2. The dose level and the number of NK cell infusion are shown in Fig. 1. Thrice-weekly infusions were administered to the first 4 patients (dose levels 1 and 2). Due to the logistics of frequent dosing, an amendment was submitted and approved for subsequent patients to triple the per-infusion dose (3 × 107/m2 to 3 × 108/m2) but administer only once weekly. The remaining 5 patients received the NK cells once weekly, with a minimum gap of 3 days between each infusion, and the fourth week continued a rest week. The weekly cumulative dose remained the same as initially planned. Patients were required to have a baseline MRI scan (prior to starting the NK cell infusion) and a scan after the first and end of the third cycle (end of therapy), and a follow-up MRI scan 30 days after the last NK cell infusion (end of follow-up study). Patients who progress clinically earlier will have MRI to confirm PD. Any additional MRI scans could be performed as indicated clinically. Later the protocol was amended and the requirement was a baseline MRI scan, a scan at the end of therapy after 3 cycles, and an end of study follow-up MRI scan 30 days after the last NK cell infusion. This was amended as MRI performed earlier showed evidence of increased fluid attenuated inversion recovery changes, while patient remained clinically stable. The Response Assessment in Neuro Oncology criteria were used to evaluate response to therapy.28
Fig. 1.
CONSORT diagram of patient enrollment, dose, and schedule of infusions.
AEs using the Common Terminology Criteria for Adverse Events (CTCAE) 4.0 were monitored during all visits till the end of study evaluation, which was defined as 30 days after the last NK cell infusion. Details of AE grading and evaluation are provided in the Supplementary data.
Feasibility Evaluation
Successful feasibility endpoint analysis required that >50% successful product generation of at least 6 patients and two-thirds of the planned infusion be achieved. This was a primary objective.
Statistical Considerations
Two dose escalation and one dose de-escalation were planned with a standard 3 + 3 design. The starting dose was level 1 as shown in Fig. 1. Before advancing/changing dose levels, a cohort summary was completed and submitted to the Investigational New Drug office for review. The MTD was the maximum dose at which less than one-third of patients experienced a DLT evaluated during cycle 1 of therapy.
For correlative studies, descriptive statistics and Student’s t-test are applied as indicated, using GraphPad Prism v8.0.2.
Cerebrospinal Fluid Evaluation Studies
Prior to each NK cell infusion during the first cycle and thereafter at least once during the remaining cycles, 3 mL of CSF was withdrawn for evaluation, using fluorescence-activated cell sorting or mass cytometry time-of-flight (CyTOF). Details of CSF evaluation are provided in the Supplementary data.
Radiomic Assessment
Of the 9 patients enrolled in this trial, 7 were evaluable for radiomic analysis. Additional details of methods of radiomic assessment are provided in the Supplementary data. Patients 1 and 5 were excluded from radiomic analysis, as 1 patient had only leptomeningeal disease (LMD) and the other presented at recurrence with a large hemorrhagic lesion. These phenotypes are known limitations for texture analysis and feature extraction. Of the 7 patients identified for radiomic analysis, 5 were evaluable for radiographic changes. Patients 3 and 7 were excluded, as their tumors were surgically removed before NK infusion, and had only LMD prior to initiating NK cell infusion, precluding evaluation of tumor size. We determined the feasibility of developing unique radiomic signatures to predict up front which patients would have radiological changes to NK cell immunotherapy. MR image segmentation and radiomic feature extraction were performed as previously described by our group.29 Radiomic features of progressive and stable lesions were compared with the generated radiomic signatures to identify differences. To reduce redundancy and identify a subset of useful and unique features, feature selection using LASSO (least absolute shrinkage and selection operator) regularization was performed. To assess the performance of the radiomic signature, we developed a multivariate logistic prediction model; we trained the model on all lesions (ie, stable and responding) to differentiate lesions that will not respond to immunotherapy.30 Unsupervised hierarchical clustering was further used to identify groups of patients and radiomic features.31
Results
Participants and Treatment
Nine participants were enrolled on this trial and received NK cell infusions, with 3 patients each on dose levels 1, 2, and 3, with doses ranging from 106/m2/dose to 3 × 108/m2/dose (Fig. 1).
Twelve patients were screened and only 9 were eligible. Three patients were deemed ineligible, due to failure in meeting GMP quality products for release, progressive clinical decline before NK cell infusion, and delay in postoperative recovery. Release specifications of expanded NK cells in all 9 eligible patients are shown in (Supplementary Figure 2).
Patient characteristics are shown in Table 1. The median age was 11 years 7 months (range, 8–18); 6 patients were female; 5 had medulloblastoma and 4 ependymoma.
Table 1.
Patient demographics and response
| Patient # | Dose Level / Number of Infusions Weekly | Age / Sex | PS | Pathology | Prior Treatments | Tumor Location Prior to NK Cell Infusion | Number of Infusions Received | Clinical /Radiological Response | Comments |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 / 3 | 18 /M | 80 | Medulloblastoma | 2 therapies | PF, LMD extending into foramen magnum | 21 / 27 | PD | Excluded from radiomic analysis, as had LMD only |
| 2 | 1 / 3 | 11 / M | 80 | Ependymoma | 3 surgeries/1 radiation therapy (chemo naïve) | Fourth ventricular tumor | 27 / 27 | PD | |
| 3 | 1 /3 | 15 / M | 80 | Medulloblastoma | 3 therapies | Fourth ventricular tumor, LMD across cerebellum | 9 / 27 | PD | |
| 4 | 2 / 3 | 16 / F | 60. | Medulloblastoma | 1 therapy | Tumor in cerebellar vermis, with LMD across PF | 24/27 | PD | Slurring of speech with dysphagia during second cycle, given steroids empirically for inflammation, symptoms improved, progressive gait and dysmetria at end of therapy for PD |
| 5 | 2 / 1 | 18 / F | 70 | Medulloblastoma | 3 therapies | Tumor recurrence at the left side of falx cerebi, hemorrhagic | 9/9 | PD | Excluded from radiomic analysis, as had hemorrhage in tumor |
| 6 | 2 /1 | 11 / M | 90 | Ependymoma | 3 surgeries and twice radiation therapy | Fourth ventricular mass extending into foramen of Luschka | 6//9 | PD | Increasing dysmetria after 6 infusions with evidence of PD |
| 7 | 3 / 1 | 9 / M | 70 | Ependymoma | 1 surgery and CSI | Fourth ventricular tumor extending into foramen of Luschka | 9/9 | PD, gait worsened | Dysarthria, gait abnormalities at beginning of treatment, these increased at end of therapy with PD, postoperative pseudomeningocele |
| 8 | 3 / 1 | 8 / M | 80 | Medulloblastoma | 1 surgery, 1 radiation therapy, and 2 lines of chemotherapy | Large right periventricular nodule with LMD in cerebellum | 5/9 | Radiographic response after 5 infusions (SD as decrease in size was <50%). No clinical improvement, PD after 3 weeks | 1 episode of seizure after 5th infusion, MRI showed decrease in size of lesion, received steroids for 2 days empirically for inflammation. No further infusions as patient progressed later to PD |
| 9 | 3 / 1 | 9 / F | 80 | Ependymoma | 3 surgeries, twice radiation therapy, vaccine therapy, and one targeted intrathecal chemotherapy | Multilocular mass along fourth ventricle, extending to foramen magnum | 2/9 | Tumor size slowly increased (SD) after 2 infusions, parents decided take off therapy. MRI remained same size (SD), noted 30 days after last NK cell infusion (end of study follow-up) | Patient taken off treatment per parental wish |
Abbreviations: CSI, craniospinal irradiation; LMD, leptomeningeal disease; PS, performance score; PF, posterior fossa; PD, progressive disease; SD, stable disease.
Lansky/Karnofsky performance score ranged 60–90. Three patients had LMD in addition to primary site of recurrence.
Safety Evaluation
This is the first study demonstrating the safety of 112 intraventricular NK cell infusions (98 through the fourth ventricle and 14 through the lateral ventricle), with no DLT. AEs are shown in Table 2. Most patients had grade 1 or 2 AEs, though 2 patients required hospitalization during therapy. The 2 patients who were admitted received 2–3 days of steroids; the neurological effects of transient dysarthria and speech improved and steroids were stopped. Further NK cell infusion proceeded after at least 72 hours off the short-term steroids, thus missing a dose of NK cells, yet following the needed time to restart NK cell infusion. AE was grade 1 fatigue. One patient who had multiple prior therapies had grade 1 thrombocytopenia at baseline, which increased to grade 2 during NK cell infusions and recovered to baseline grade 1 in 7 days. Bone marrow evaluation showed no evidence of unusual pathology. Primary objectives were achieved, which demonstrated the feasibility of NK cell production, release, and safety of intraventricular infusions.
Table 2.
Adverse events that were considered possibly/probably related to the NK cell infusion
| Clinical Events | # of Patients | Specific A/E | Dose Level 1 Grade (CTC) | Dose Level 2 Grade (CTC) | Dose Level 3 Grade (CTC) | Comment |
|---|---|---|---|---|---|---|
| Cardiac | 1 | Sinus tachycardia | 1 | Resolved spontaneously in 2 days | ||
| Nervous system | 2 | Dysmetria, dysarthria | 2 | 2 | 2 patient received steroids (one of them also had dysphagia), hospitalization, decreased symptoms within 2 days, then continued NK cell infusion | |
| 4 | Headache | 1 | 1 | 2 | ||
| 1 | Dysphagia | 2 | ||||
| 1 | Facial droop | 1 | ||||
| 1 | Seizure | 3 | (Patient 8) One episode of seizure, however MRI showed decrease in size of lesion, admitted for observation, received steroids for 2 days, given empirically for inflammation, no seizure recurrence during hospitalization (grade 3 AE for hospitalization). However seizure recurrence later with PD | |||
| 1 | Slurred speech | 3 | Intermittent, resolved with short course of steroids in 3 days (grade 3 for hospitalization) | |||
| General disorders | 2 | Fatigue | 1 | 1 | ||
| Nutritional disorder | 2 | Anorexia | 1 | |||
| Hematologic | 1 | Thrombocytopenia | Baseline grade 1, increased to grade 2 and resolved to grade 1 in 7 days | Bone marrow evaluation negative for infiltrative disease |
Radiographic Evaluation
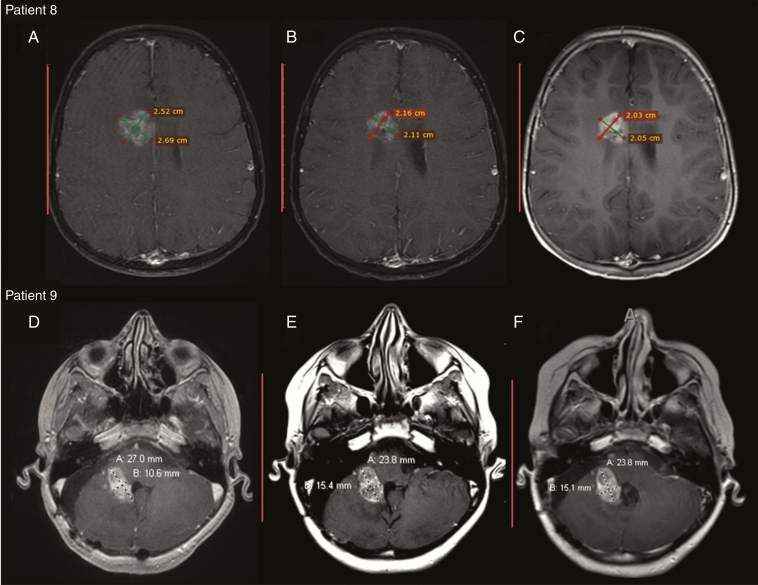
Patient 8 after 5 infusions developed a short spell of seizure, which spontaneously resolved. He had an MRI that showed response with nearly 30% reduction in size (so deemed a stable disease [SD] and not partial response). Patient was given a short course of 2 days of steroids and asked to return after 10 days (one dose of NK cell held as steroids were given). Ten days later he had headaches and MRI was performed locally, which showed further decrease in size of lesion (Fig. 2A–C). A few days later he started having seizures and later demonstrated PD. So this patient had a transient response before progressing.
Fig. 2.
(A–C) Patient 8: post-contrast T1 weighted MR images show irregular intra- and periventricular enhancing tumor in the right frontal lobe. (A) Pre-infusion tumor size and characteristics. (B) After the fifth infusion the tumor shows irregular ring enhancement with central necrosis. (C) Ten days following infusion the tumor continues to minimally decrease in size with increased enhancement. (D–F). Patient 9: post-contrast T1 weighted images through posterior fossa show enhancing medulloblastoma centered along the right foramen of Luschka to the pontomedullary cistern with invasion of adjacent pons and right middle cerebellar peduncle. (D) Post-contrast T1 image before NK cell infusion. (E) Post-contrast image after 2 NK cell infusions. There is increased size of the tumor by 9%. (F) Post-contrast image 30 days after the second NK cell infusion shows that the tumor remains stable in size.
Patient 9 had an MRI scan after second infusion which showed increase in tumor size to 9% from the baseline. The family decided to withdraw from therapy after 2 infusions. MRI scans performed 30 days later after the second infusion (end of therapy follow-up) showed SD with similar size of the lesion, with no signs of increased LMD or edema (Fig. 2D–F), even with no further infusions. All the patients showed PD (increased size of the lesion and or clinical progression) while on therapy or at follow-up at end of treatment, except patient 9.
CSF Evaluation and Biomarker Correlates
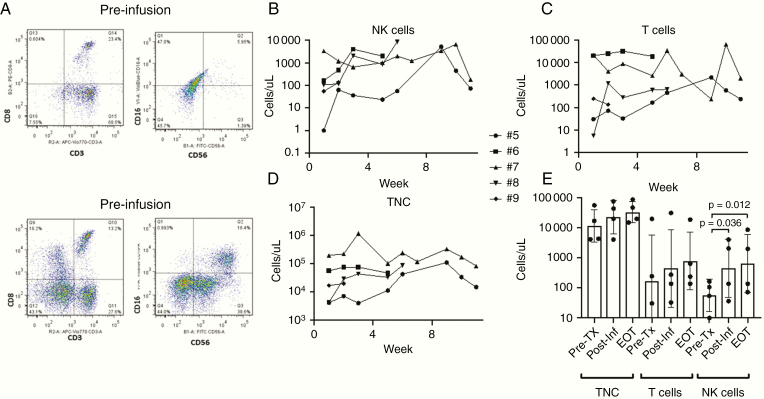
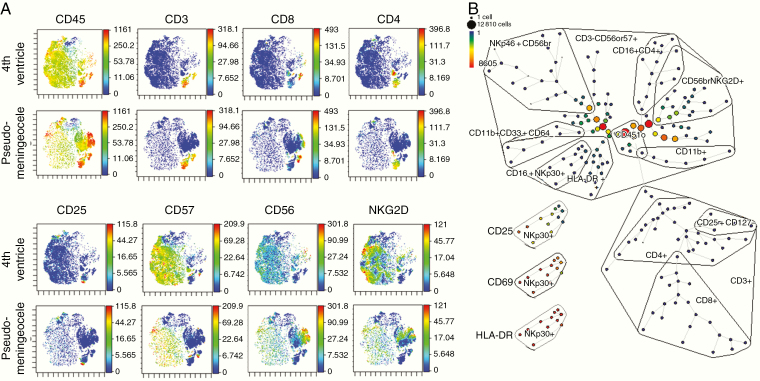
CSF samples obtained before each NK cell infusion showed pleocytosis with no evidence of infection. At low dose levels, NK cells were not consistently identified in the CSF by flow cytometry. Beginning with the change to once-weekly administration on dose level 2 (30-fold higher dose/infusion than dose level 1), NK cells were consistently present in all patient CSF samples (Fig. 3A; gating strategy shown in Supplementary Figure 3). Except for patient 7 (whose baseline cell counts were already very high), CSF NK cells increased significantly over time throughout the protocol therapy (Fig. 3B), whereas T cells (Fig. 3C) and total nucleated cells (TNCs) (Fig. 3D) did not. For these higher-dose patients, the mean change in absolute NK cells from pretreatment to 1 week after the first infusion or pretreatment to end of therapy was 8.2-fold (P= 0.03) or 11.6-fold (P= 0.01), respectively (Fig. 3E). For patient 7, who developed postoperative pseudomeningocele, CyTOF analysis of CSF from cycle 3 infusion 2 revealed a dominance of NK cells in the sample from the fourth ventricle with distinct phenotype from that in the pseudomeningocele, with dominant cluster of differentiation (CD)57 and NK group 2 member D (NKG2D) expression (Fig. 4A).
Fig. 3.
Flow cytometric assessment of CSF cell infiltrates shows total NK cells are increased acutely and sustained during the treatment period. CSF was stained for surface markers and analyzed as described (gating strategy shown in Supplementary Figure 3) and cell concentrations determined based on TNCs and percentage of subsets. Shown are results for all patients treated after dose-escalating to once-weekly infusions. (A) Representative flow plots from pretreatment (drawn prior to first infusion) and posttreatment (drawn prior to second treatment) samples. (B) NK-cell (CD3-CD16or56+) content, (C) T cell (CD3+) content, and (D) TNC content during the treatment period. (E) Comparison of each patient’s TNC, T-cell, and NK-cell content in CSF before any NK cell infusions (Pre-Tx), after the first infusion (Post-Inf), and at the end of protocol therapy (EOT). Shown are P-values that are <0.05 for ratio paired t-test.
Fig. 4.
Mass cytometry shows phenotypes of NK cells persistent locoregionally in the fourth ventricle and those migrating to a pseudomeningocele. Analysis of CSF samples after gating on nonviable, nondoublet, CD45+ cells. (A) ViSNE plots showing relative expression of common NK cell and T-cell markers. (B) SPADE analysis showing relative content of NK-cell and T-cell subpopulations, with high expression of activation markers CD25, CD69, and HLA-DR shown for the CD16+NKp30 subset.
Analysis by SPADE (Spanning-tree Progression Analysis of Density-normalized Events) revealed a broad range of NK cell subsets. Of note, a subset of CD16+NKp30+ NK cells showed a high expression of activation markers (human leukocyte antigen D related [HLA-DR+], CD69+, and CD25+) (Fig. 4B). The release specification of the NK cell products including sterility profile is shown in Supplementary Figure 2.
Radiomic Analysis
This first study evaluating the role of radiomics in pediatric adoptive immunotherapy identified radiomic signatures extracted from the NK cell treatment-naïve MRI scans in 7 patients. Radiographic response was evaluable in 5 out of the 7 patients; patients 1 and 5 were excluded, as mentioned earlier. A high radiomic performance was achieved; accuracy, sensitivity, and specificity were 100% but did not attain statistical significance, likely due to the low number of patients (Supplementary Figure 4A). The unsupervised hierarchical clustering method simultaneously clustered the patients and features into subclusters, yielding patient stratification labels and the low-dimension feature representation. As demonstrated in Supplementary Figure 4B, we identified 2 radiomic-based clusters that coincide with 2 patients (one with SD and the other with a radiological response) and those who had progressive disease, respectively.
Discussion
NK cells are known to play an important role in antitumor immunity, as shown in various human cancers.32–34 The potential of NK cells as effectors against brain tumors has been demonstrated in vitro and in vivo.35–37 A clinical study using NK cells has been conducted in brain tumors, but none to date have been explored in pediatric neuro-oncology.17 NK cells express a large number of receptors that trigger NK cell cytolytic activity on the recognition of specific ligands on target cells. Medulloblastoma upregulates ligands for NK cell activating receptors such as NKG2D and natural cytotoxicity receptors, which induces NK cell–mediated cytotoxicity and apoptosis of medulloblastoma cells.21 Increased NKG2D ligand expression in medulloblastoma and other pediatric brain tumors promotes reduced NK cell–mediated immune surveillance, and helps in creating a less immunosuppressive tumor microenvironment. This phenomenon likely improves NK cell trafficking and functions in pediatric brain tumors.38
As stated earlier, our preclinical work showed the cytotoxicity of NK cells in medulloblastoma and atypical teratoid rhabdoid tumor.18 Given the local route of delivery and the possibility of an adverse immunological response, we started at a lower dose with multiple infusions. The primary objective of feasibility/safety was achieved.
The study also evaluated antitumor activity based on imaging as a secondary objective. Patient 8 had a transient SD (with radiographic changes) before demonstrating PD. All patients showed increase in size of the lesion and or clinical decline during and/or at the end of therapy except patient 9, who had SD at end of the follow-up period.
CSF analysis using flow cytometry and CyTOF showed accumulation and the persistence of NK cells and the influx of CD3+ T cells, suggesting the cryopreserved cells retained viability and function. Here, in a few samples of sufficient size to allow in-depth CyTOF analysis, the T-cell component was observed to equally comprise CD4 and CD8 T cells with a very small component of CD25+CD127− regulatory T cells, suggesting that the infused NK cells promote effector T-cell migration into the CSF. Repeated infusions of cryopreserved NK cells were also associated with persistence and accumulation of NK cells (as seen in CSF biomarker evaluation).
One of the significant toxicities of immunotherapy is cytokine release, resulting in an influx of immune cells into the tumor and peritumoral area, seen after treatment with checkpoint inhibitors or adoptive immunotherapy.39–41 This often results in an increase in tumor size, with worsening of clinical features resulting in pseudoprogression disease (PsD). Differentiation between PsD and PD remains a therapeutic challenge. Longer periods of follow-up in immunotherapy-based clinical trials, showing decrease in tumor size and improved clinical features over time, have helped with resolution of this phenomenon.42 Multiple infusions of NK cells at short intervals, with a potentially amplified cytokine release and cellular infiltration (including NK and T cells), may have contributed to PsDs in our study; we were unable to conclusively rule out this possibility because of the short follow-up duration.
To our knowledge, this is the first report to include radiomic analyses to evaluate the feasibility of developing signature models as a component of pediatric immunotherapy study. The goal was to assess if radiomic signatures could predict upfront which patients will likely have radiographic responses when treated with NK cells. Although we were unable to achieve statistical significance, because of the small number of patients, a trend in the ability to predict radiographic changes could be discerned with increased accuracy and sensitivity, which was confirmed by hierarchal clustering (Supplementary Figure 4A, B). Larger studies using radiomics in immunotherapy showed predictive ability in other malignancies but attained lower predictive values of sensitivity and specificity, using different models and radiomic signatures.43 Few studies also showed radiomic data of texture features and could provide complementary diagnostic information for gliomas and predict molecular subtypes of medulloblastoma.44,45 The radiomic models used in our study to profile unique signatures need to be further evaluated for predictability in a larger cohort of patients.
We initiated this trial using autologous NK cells to demonstrate safety of expanded NK cells delivered by an intrathecal route. Despite being a phase I monotherapy safety/feasibility study with small patient numbers, multiple NK cell dose levels and intervals, and limited study duration of 3 cycles, which precluded differentiating PD from PsD, the current trial demonstrated safety of the approach. However, the cost, treatment delay, and limited doses inherent in generating patient-specific autologous products are significant. The potential problems of using autologous NK cells including freezing, thawing, and their diminished efficacy in contrast to allogenic cells are now known. In addition to the low function and exhaustion of autologous NK cells that are driven by exposure to transforming growth factor beta, tryptophan metabolites, adenosine, and chronic stimulation, allogeneic NK cells may be selected for additional anticancer function through killer immunoglobulin-like receptor (KIR) ligand mismatch and high activating KIR content.46–49 Thus, safety testing of NK cells from allogenic sources in this setting is warranted in future trials.
The current study was initiated with thrice-weekly infusions, which was logistically challenging. However, since adoptively transferred NK cells survived and persisted adequately at one-week infusion intervals, our next trials with allogenic cells are being designed with NK cell delivery at longer intervals, for more cycles, and with longer follow-up (at least 6 mo) to enable distinguishing PsD from PD. The role of radiomics will also be evaluated in a larger number of patients using the models and signatures described here. CSF immunological biomarkers including the cytokine profile will be interrogated. This trial has also revealed a pressing need for using non-invasive imaging modalities to track the delivery and fate of NK cells during treatment. Our preclinical study was successful in demonstrating that 19F-labeled NK cells can suppress medulloblastoma growth while enabling imaging feedback, which we expect will facilitate study and optimization of therapeutic paradigms in the future.18
Supplementary Material
Conflict of interest statement. DAL declares an equity interest, advisory role, and intellectual property licensing to CytoSen Therapeutics and Kiadis Pharma, and advisory role with Caribou BioSciences and Courier Biosciences. LJNC declares salary support from Ziopharma and MD Anderson Cancer Center, royalty from City of Hope, Immatics, intellectual property rights and patent holders from Sangamo BioSciences, MD Anderson Cancer Center, Ziopharm Oncology, contracted research with Ziopharm Oncology, ownership interests with Targazyme, Ziopharm Oncology, Immatics, Kiadis, Cell Chorus. KR declares conflict of interest with Takeda pharmaceuticals in licensing of CAR NK cells.
Authorship statement. SK, WZ, DIS, LK, JMJ, MER, DR, SG, HM, EJS, KR contributed to running of the clinical trial. DAL, LJNC, WZ, and VG helped in designing of the trial. EJS and KR provided guidance for the NK cell manufacture and oversight of the GMP. DAL, LJNC, VG, PT, RJN, AK, TI, RC, and GKB performed experiments and preclinical work and analyzed data. DDL provided statistical support.
Funding
This study was supported by Noah’s Light Foundation, Rally Foundation, Addi’s Faith Foundation, Jaxon’s FROG Foundation, Archer Foundation, Stripes Foundation, Texas4000, Meryl Charles Witmer Charitable Foundation and American Cancer Society.
References
- 1. Pui CH, Gajjar AJ, Kane JR, Qaddoumi IA, Pappo AS. Challenging issues in pediatric oncology. Nat Rev Clin Oncol. 2011;8(9):540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 3. Grundy RG, Wilne SH, Robinson KJ, et al. ; Children’s Cancer and Leukaemia Group (formerly UKCCSG) Brain Tumour Committee Primary postoperative chemotherapy without radiotherapy for treatment of brain tumours other than ependymoma in children under 3 years: results of the first UKCCSG/SIOP CNS 9204 trial. Eur J Cancer. 2010;46(1):120–133. [DOI] [PubMed] [Google Scholar]
- 4. Heitzer AM, Ashford JM, Harel BT, et al. Computerized assessment of cognitive impairment among children undergoing radiation therapy for medulloblastoma. J Neurooncol. 2019;141(2):403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eaton BR, Esiashvili N, Kim S, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol. 2016;18(6):881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho YJ, Tsherniak A, Tamayo P, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Northcott PA, Hielscher T, Dubuc A, et al. Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta Neuropathol. 2011;122(2):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shih DJ, Northcott PA, Remke M, et al. Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol. 2014;32(9):886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shih CS, Hale GA, Gronewold L, et al. High-dose chemotherapy with autologous stem cell rescue for children with recurrent malignant brain tumors. Cancer. 2008;112(6):1345–1353. [DOI] [PubMed] [Google Scholar]
- 10. Ramaswamy V, Taylor MD. Medulloblastoma: from myth to molecular. J Clin Oncol. 2017;35(21):2355–2363. [DOI] [PubMed] [Google Scholar]
- 11. Pejavar S, Polley MY, Rosenberg-Wohl S, et al. Pediatric intracranial ependymoma: the roles of surgery, radiation and chemotherapy. J Neurooncol. 2012;106(2):367–375. [DOI] [PubMed] [Google Scholar]
- 12. Bouffet E, Hawkins CE, Ballourah W, et al. Survival benefit for pediatric patients with recurrent ependymoma treated with reirradiation. Int J Radiat Oncol Biol Phys. 2012;83(5):1541–1548. [DOI] [PubMed] [Google Scholar]
- 13. Zacharoulis S, Ashley S, Moreno L, Gentet JC, Massimino M, Frappaz D. Treatment and outcome of children with relapsed ependymoma: a multi-institutional retrospective analysis. Childs Nerv Syst. 2010;26(7):905–911. [DOI] [PubMed] [Google Scholar]
- 14. Ramaswamy V, Taylor MD. Treatment implications of posterior fossa ependymoma subgroups. Chin J Cancer. 2016;35(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pajtler KW, Mack SC, Ramaswamy V, et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017;133(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benesch M, Mynarek M, Witt H, et al. Newly diagnosed metastatic intracranial ependymoma in children: frequency, molecular characteristics, treatment, and outcome in the prospective HIT series. Oncologist. 2019;24(9):e921–e929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishikawa E, Tsuboi K, Saijo K, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24(3b):1861–1871. [PubMed] [Google Scholar]
- 18. Kennis BA, Michel KA, Brugmann WB, et al. Monitoring of intracerebellarly-administered natural killer cells with fluorine-19 MRI. J Neurooncol. 2019;142(3):395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7(1):e30264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pérez-Martínez A, Fernández L, Díaz MA. The therapeutic potential of natural killer cells to target medulloblastoma. Expert Rev Anticancer Ther. 2016;16(6):573–576. [DOI] [PubMed] [Google Scholar]
- 21. Fernández L, Portugal R, Valentín J, et al. In vitro natural killer cell immunotherapy for medulloblastoma. Front Oncol. 2013;3:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li H, Zhu Y, Burnside ES, et al. MR imaging radiomics signatures for predicting the risk of breast cancer recurrence as given by research versions of MammaPrint, Oncotype DX, and PAM50 gene assays. Radiology. 2016;281(2):382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sandberg DI, Kerr ML. Ventricular access device placement in the fourth ventricle to treat malignant fourth ventricle brain tumors: technical note. Childs Nerv Syst. 2016;32(4):703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11): 1963–1972. [DOI] [PubMed] [Google Scholar]
- 29. Zinn PO, Singh SK, Kotrotsou A, et al. A coclinical radiogenomic validation study: conserved magnetic resonance radiomic appearance of periostin-expressing glioblastoma in patients and xenograft models. Clin Cancer Res. 2018;24(24):6288–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc Series B Stat Methodol. 1996;58(1):267–288. [Google Scholar]
- 31. Fred AL, Jain AK. Combining multiple clusterings using evidence accumulation. IEEE Trans Pattern Anal Mach Intell. 2005;27(6):835–850. [DOI] [PubMed] [Google Scholar]
- 32. Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2(11):850–861. [DOI] [PubMed] [Google Scholar]
- 33. Hsia JY, Chen JT, Chen CY, et al. Prognostic significance of intratumoral natural killer cells in primary resected esophageal squamous cell carcinoma. Chang Gung Med J. 2005;28(5):335–340. [PubMed] [Google Scholar]
- 34. Plonquet A, Haioun C, Jais JP, et al. ; Groupe d’étude des lymphomes de l’adulte Peripheral blood natural killer cell count is associated with clinical outcome in patients with aaIPI 2-3 diffuse large B-cell lymphoma. Ann Oncol. 2007;18(7):1209–1215. [DOI] [PubMed] [Google Scholar]
- 35. Castriconi R, Daga A, Dondero A, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182(6):3530–3539. [DOI] [PubMed] [Google Scholar]
- 36. Avril T, Vauleon E, Hamlat A, et al. Human glioblastoma stem-like cells are more sensitive to allogeneic NK and T cell-mediated killing compared with serum-cultured glioblastoma cells. Brain Pathol. 2012;22(2):159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poli A, Wang J, Domingues O, et al. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget. 2013;4(9):1527–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haberthur K, Brennan K, Hoglund V, et al. NKG2D ligand expression in pediatric brain tumors. Cancer Biol Ther. 2016;17(12):1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Plant AS, Koyama S, Sinai C, et al. Immunophenotyping of pediatric brain tumors: correlating immune infiltrate with histology, mutational load, and survival and assessing clonal T cell response. J Neurooncol. 2018;137(2):269–278. [DOI] [PubMed] [Google Scholar]
- 40. Oved JH, Barrett DM, Teachey DT. Cellular therapy: immune-related complications. Immunol Rev. 2019;290(1):114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cohen JV, Alomari AK, Vortmeyer AO, et al. Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res. 2016;4(3):179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Galldiks N, Kocher M, Langen KJ. Pseudoprogression after glioma therapy: an update. Expert Rev Neurother. 2017;17(11):1109–1115. [DOI] [PubMed] [Google Scholar]
- 43. Trebeschi S, Drago SG, Birkbak NJ, et al. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann Oncol. 2019;30(6):998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ditmer A, Zhang B, Shujaat T, et al. Diagnostic accuracy of MRI texture analysis for grading gliomas. J Neurooncol. 2018;140(3):583–589. [DOI] [PubMed] [Google Scholar]
- 45. Iv M, Zhou M, Shpanskaya K, et al. MR imaging-based radiomic signatures of distinct molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2019;40(1):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kannan GS, Aquino-Lopez A, Lee DA. Natural killer cells in malignant hematology: a primer for the non-immunologist. Blood Rev. 2017;31(2):1–10. [DOI] [PubMed] [Google Scholar]
- 47. Lee DA. Cellular therapy: Adoptive immunotherapy with expanded natural killer cells. Immunol Rev. 2019;290(1):85–99. [DOI] [PubMed] [Google Scholar]
- 48. Trikha P, Lee DA. The role of AhR in transcriptional regulation of immune cell development and function. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188335. [DOI] [PubMed] [Google Scholar]
- 49. Foltz JA, Moseman JE, Thakkar A, Chakravarti N, Lee DA. TGFbeta imprinting during activation promotes natural killer cell cytokine hypersecretion. Cancers (Basel) 2018;10(11) pii: E423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.