Abstract
Alternative pre-mRNA splicing generates multiple mRNA isoforms of different structures and functions from a single gene. While the prevalence of alternative splicing control is widely recognized, and the underlying regulatory mechanisms have long been studied, the physiological relevance and biological necessity for alternative splicing are only slowly being revealed. Significant inroads have been made in the brain, where alternative splicing regulation is particularly pervasive and conserved. Various aspects of brain development and function (from neurogenesis, neuronal migration, synaptogenesis, to homeostasis of neuronal activity) involve alternative splicing regulation. Recent studies have begun to interrogate the possible role of alternative splicing in axon formation, a neuron-exclusive morphological and functional characteristic. We discuss how alternative splicing plays an instructive role in each step of axon formation. Converging genetic, molecular, and cellular evidence from studies of multiple alternative splicing regulators in different systems shows that a biological process as complicated and unique as axon formation requires highly coordinated and specific alternative splicing events.
Graphical Abstract
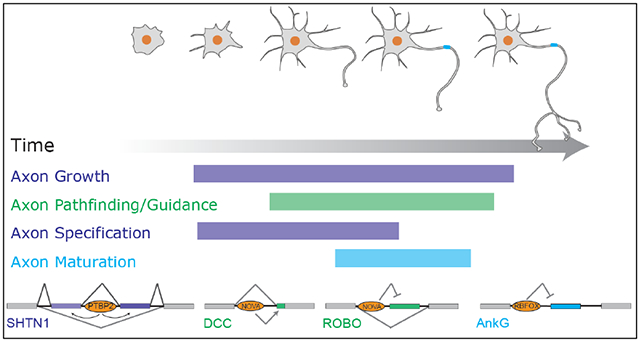
An instructive role of alternative splicing in each step of axon formation
Introduction
Over 90% of human multi-intron genes are subject to alternative splicing (Pan, Shai, Lee, Frey, & Blencowe, 2008; Wang et al., 2008). This prevalence is universal in all examined mammals, and likely all vertebrates (Barbosa-Morais et al., 2012; Merkin, Russell, Chen, & Burge, 2012). Moreover, alternative splicing is dynamic and context dependent. A single alternative splicing event can be influenced by intrinsic genetic programmes as well as extrinsic environmental factors (Fu & Ares, 2014). Significant progress has been made toward unveiling the regulation of alternative splicing (Black, 2003; Y. Lee & Rio, 2015). A general regulatory framework considers an array of trans factors interacting with cis elements on pre-mRNA to weigh on the choices of splice sites. Because almost all splicing regulators recognize degenerate primary RNA sequences (Dominguez et al., 2018), the exact biochemical mechanisms needed to achieve regulatory specificity are still enigmatic. However, the framework has been so far satisfactory to explain certain alternative splicing events. A complete understanding of alternative splicing regulation would be aided by identification of new splicing regulators, protein-RNA interaction rules, and regulatory checkpoints of spliceosome assembly.
The major challenge remaining is to understand the physiological and pathological functions of the vast and dynamic splicing landscape. Illuminating functional significance of alternative splicing for a specific biological context is helped by identifying the associated splicing regulators and alternative exons. Probably one of the most studied contexts is tissue-specific splicing and, among all tissues, brain-specific splicing stands out as particularly conserved in evolution (Barbosa-Morais et al., 2012; Merkin et al., 2012). Various studies support the involvement of alternative splicing in shaping the brain, including neurogenesis, synaptogenesis, and neuronal activity (Raj & Blencowe, 2015; C. K. Vuong, Black, & Zheng, 2016).
The brain is made of neuronal networks, which underlie an organism’s abilities in sensation, movement, learning, memory, emotion, behaviours, and cognition. This neural network is built upon neurons’ distinct axonal and dendritic appendages, which form synaptic connections. The compartmentalization of a single axon and multiple dendrites establishes axonal-dendritic polarity, which is essential for directional flow of network information, with the axon mainly responsible for transmitting and dendrites for receiving signals.
Recent studies have made new strides in unveiling the regulation and biological role of alternative splicing in axonogenesis. In this review, we discuss these new discoveries by focusing on RNA binding proteins, their genetic knockouts, and the downstream alternative splicing programs responsible for generating an axon. Due to space limitation we apologize for not able to include all published work.
[ALTERNATIVE SPLICING: REGULATION AND BIOLOGICAL FUNCTIONS]
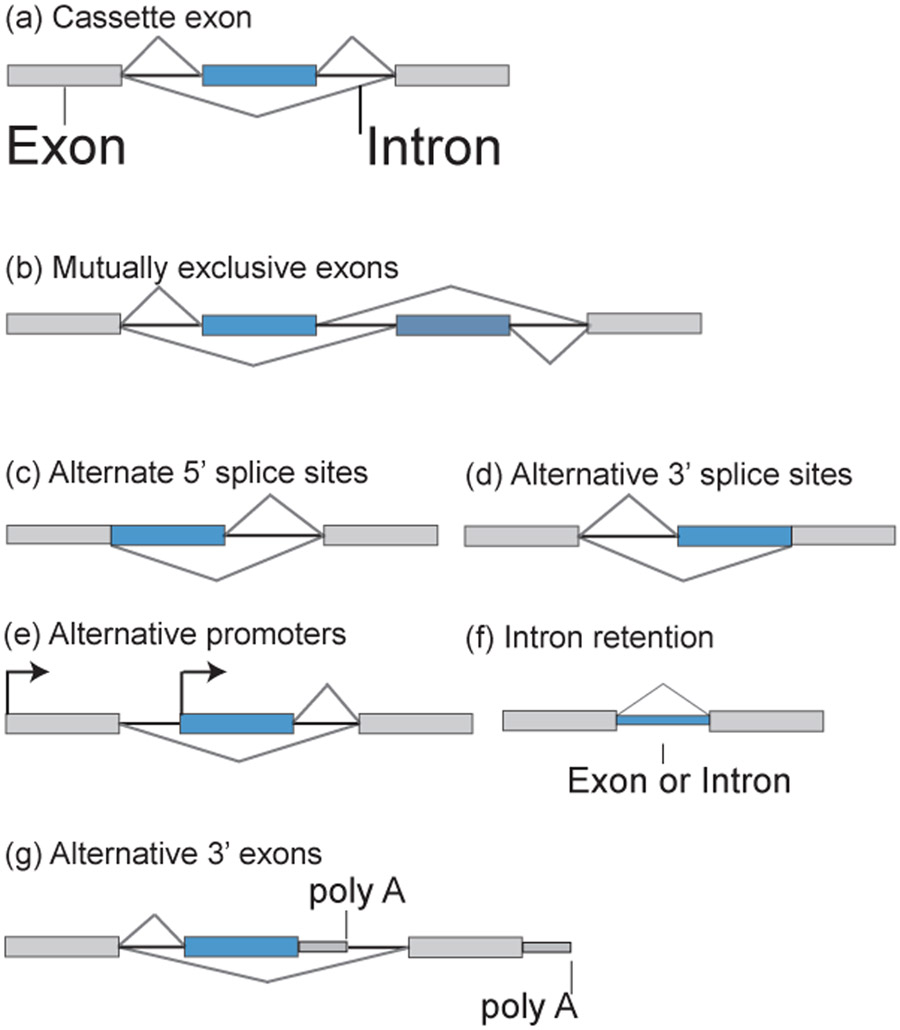
Splicing removes introns from the precursor mRNA and joins exons to generate the final mRNA product. Alternative splicing is a widespread gene regulatory mechanism that alters usages of splice sites to produce distinct RNA isoforms from a single gene locus (or, more precisely, precursor mRNA). Depending on the relative positions of the alternative splice sites, alternative splicing can be categorized by different patterns (Figure 1). The most common is alternative splicing of a cassette exon (skipped exon) that includes or excludes the whole exon from the mRNA. Mutually exclusive splicing refers to a pair of consecutive exons where only one of them is included. Alternative 5’ splicing is competition of (usually two) consecutive 5’ splice sites within a single exon. Conversely, alternative 3’ splicing is enacted by consecutive 3’ splice sites. Both patterns effectively change the length of an exon. Intron retention means a typically excised intron remains in the final mRNA. Alternative promoter refers to different first exons for the mRNA, while alternative 3’ exon means different last exons (and thus different 3’ UTR). These primitive alternative splicing patterns can co-exist in a single gene and can be combined to create more complicated splicing patterns.
Figure 1.
Patterns of alternative splicing
Alternative splicing is generally regulated by cis RNA elements in the exon and flanking introns that recruits cognate trans-acting pre-mRNA binding proteins (Fu & Ares, 2014; Y. Lee & Rio, 2015). The exact mechanisms of these splicing regulators affecting splice site choices remain an outstanding question. Only a limited number of RNA binding proteins are characterized as splicing regulators, but the final list is likely to expand significantly (Louie, Aigner, Bergalet, Zhou, & Su, 2018). Many such proteins appear to recognize short degenerate, and therefore overlapping, cis RNA elements at the level of primary sequences (Dominguez et al., 2018). The effect and activity of these splicing regulators can be influenced by their binding positions relative to the alternative exon, post-translational modifications, and protein-protein interactions. These complexities present challenges not only for mechanistic studies but also for phenotypic characterization.
In high eukaryotes almost all multi-exon genes produce distinct isoforms through alternative splicing (Pan et al., 2008; Wang et al., 2008). Global profiling of exon usage has become a common practice thanks to RNA-Seq. Although sequencing depth remains the critical determinant for the breadth of discoveries and the accuracy of splicing estimation, it is generally agreed that RNA-seq, in conjunction with state-of-the-art analytical methods, is a powerful tool to define the splicing landscape for samples with well-annotated genomes. Quantification of isoform expression is more challenging due to incomplete annotations and the shortfall of current technologies unable to simultaneously satisfy sequence depth and length (Bray, Pimentel, Melsted, & Pachter, 2016; Li & Dewey, 2011; Patro, Duggal, Love, Irizarry, & Kingsford, 2017; Trapnell et al., 2012; J. Zhang, Kuo, & Chen, 2015; Sika Zheng & Chen, 2009). A general assumption -- that various alternatively spliced RNA segments within a gene are independently regulated -- likely needs revision. Nevertheless, until long-read sequencing reaches the necessary depth and accuracy for whole transcriptome quantification, exon centric analysis will likely continue to dominate.
The degree to which alternative isoforms are biologically functional versus result from transcriptomic noises with little to no impact is of contentious debate (Blencowe, 2017; Sorek, Shamir, & Ast, 2004; Tress, Abascal, & Valencia, 2017; Sika Zheng & Black, 2013). Although some alternative splicing events are conserved, many others are not. Globally, the abundance of many isoforms can be predicted by a simple stochastic noise model (Melamud & Moult, 2009). Furthermore, in many cases the minor alternative isoform is expressed at a very low level, dampening the possibility of its biological relevance (Pickrell, Pai, Gilad, & Pritchard, 2010). Finally, in many experimental settings splicing regulation only shifts isoform ratios modestly, begetting scepticisms about their biological meanings.
Four criteria shall be met to fully establish biological functions of an alternative splicing event (Figure 2). First, the splicing change should be robust and consistent. Second, isoform changes at the mRNA level should lead to detectable changes in protein isoform composition or overall gene expression outputs. This requirement might seem over-cautious, but studies have suggested a large majority of isoforms may not even be translated (Tress et al., 2017). Third, the protein isoforms should have meaningful differences in molecular functions. This alone is challenging if a gene function has not been assigned. Studies of alternative splicing should also distinguish molecular functions from biological functions. The molecular function of alternative splicing, i.e., the impact of alternative splicing on the function of an encoded gene product, can be predicted when the gene function and its domain activity are characterized. Biochemical and molecular characterization of the two isoforms can be conducted without a biology context. By contrast, defining biological functions involves cell and animal studies. This brings up the fourth criterion: genetic necessity of the alternative exon. As definitive proof of functional relevance, genetic tests for necessity are largely missing for most alternative splicing events. Experimental testing to meet these four criteria is not trivial but may be necessary to firmly demonstrate the biological significance of a certain alternative splicing event.
Figure 2.
Different types of evidence to establish biological relevance of an alternative splicing event.
Tissue-specific splicing is one of the most robust outcomes of genetic regulation of alternative splicing. Indeed, many alternative splicing events reported predate the genomics era thanks to their tissue dependent splicing patterns. Among all major tissues, the brain shows the most alternative splicing events (Barbosa-Morais et al., 2012; Merkin et al., 2012). The root cause of this phenomenon is unknown but likely multi-faceted. The diversity of brain cell types may be a factor. Recent studies show most alternative splicing in the brain originates from neuronal cells rather than glia, epithelial cells, or other non-neuronal cells (Weyn-Vanhentenryck et al., 2018). Another contributor can be numerous neuronal subtypes that may exhibit distinct splicing patterns to meet their morphological and functional diversity.
Making sense of these neuron-specific splicing patterns remains an experimental and theoretical challenge, because investigating the biological function of each alternative splicing event can be a substantial project and how changes in splicing alter the protein function is usually unknown. Studies have focused on characterizing individual splicing regulators, which serve as anchors to link the specific neurobiological phenotypes their mutations reveal and the alternative splicing changes they regulate. Research during the past decade has shown several splicing factors and their regulatory network affect many aspects of neuronal development, including neurogenesis, synaptogenesis, and circuit function (C. K. Vuong et al., 2016). Recent studies have begun to elucidate the important roles of alternative splicing in axon formation (axonogenesis).
[AXON FORMATION]
Axon is a neuron’s cytoplasmic protrusion that typically conducts action potentials from the cell body and transmitting information to other cells. The other type of neuronal projection is a dendrite, which forms synapses with afferent axons to receive information from other neurons. Axon is not only the morphological hallmark of neurons, its formation establishes neuronal polarity and is prerequisite to synapse formation and circuit wiring, both of which underlie cognition and behaviours. Axon degeneration is one indicator for various neurodegenerative diseases and brain injury. Therefore, tremendous interest and effort have been placed on understanding and inducing axon regeneration. Herein, unless otherwise specified, “neuron” refers to hippocampal and cortical excitatory neurons, on which most axonongenesis studies are based.
Axonogenesis encompasses multiple integrated stages manifested through morphological and compositional changes of the axon (Figure 3). Neurons first protrude multiple, apparently indistinguishable, neurites. One of the neurites subsequently accelerates its growth to become the future axon (Cheng & Poo, 2012). In the mouse cortex, the initial stage of axon formation coincides with the migration of the neuronal cell body from the proliferation zone to the cortical plate (Hatanaka & Yamauchi, 2013). The leading process of the migrating neuron develops into a dendrite, and the trailing process into the axon. Meanwhile, the axon navigates through the complicated brain map and grows into its final targeting area. This path finding, or axon guidance, is an essential part of axonogenesis to assure correct circuit wiring of the nervous system.
Figure 3.
Stages of axon formation
While axonal growth elongates the process to be morphologically distinct from dendrites, axon specification refers to acquisition of the axonal fate through establishing molecular and structural hallmarks of axons (Cáceres, Ye, & Dotti, 2012). A classical molecular maker of axon specification is demarcation of microtubule-associated protein Tau1 at the distal part of the axon and simultaneous depletion of dendrite-associated microtubule-associated protein MAP2 (Kapitein & Hoogenraad, 2015). Following the initial specification, further specification includes establishment of microtubule polarity and acquisition of axon initial segment, which leads to functional maturation of axons (Szu-Yu Ho & Rasband, 2011). Axons generate branches, and sometimes form complex arborizations, at or before reaching their final destinations. Some of the excessive branches are subsequently pruned away by an activity-dependent matching process.
Axon growth, guidance, specification, maturation, branching, and pruning are terms describing different aspects of axonogenesis. While each aspect follows another, the exact boundaries of occurrences are not as clear cut. Pathfinding is essentially axon growth in the correct direction. Maturation may be regarded as functional specification of axons. Axon growth and axon specification are often used interchangeably in literature, as growth and specification seem to be concurrent. Despite the nuances between some of these macroscopic descriptions, the underlying molecular controls can be quite distinct, as exemplified by findings from mechanistic analysis of axon guidance (Chédotal, 2019). Molecular mechanisms of axon formation have been extensively reviewed (Barnes & Polleux, 2009; Cáceres et al., 2012; Cheng & Poo, 2012; Kevenaar & Hoogenraad, 2015; Namba et al., 2015; Zollinger, Baalman, & Rasband, 2015).
Even with substantial description of cell biology and molecular players, we still know little about genetic control of axonogenesis. For example, less work has been done to understand why only neurons and no other cell types have an axon. Studies of axon formation have substantially borrowed and benefited from findings of planar cell polarity, emphasizing the similarity of their mechanisms (Tissir & Goffinet, 2013). However, axonogenesis encompasses more complicated aspects than establishing cell polarity, and planar cells never grow an axon. To ensure axon production, one possibility is that neurons are uniquely equipped with a set of special regulatory rules.
[ALTERNATIVE SPLICING CONTROL OF EARLY AXONOGENESIS]
Two observations argue for robust intrinsic mechanisms controlling axon formation. First, primary cultured neurons extend one and only one axon in a symmetric in vitro environment without the in vivo microenvironment niche. Second, the success of differentiating embryonic and induced pluripotent stem cells into neurons in vitro further strengthens the idea of intrinsic regulation. As neuronal differentiation takes weeks in rodents and months in human to complete, transcriptome profiling of neuronal development typically has been performed with a relatively wide temporal resolution and provides limited information to interpret a specific developmental transition.
An unbiased transcriptome profiling of neurons before and after initial axon formation proved informative and unveiled the coordinated control of early axonogenesis (M. Zhang et al., 2019). Many genes change isoform expression during this period. Importantly, these alternative splicing events exhibit neural-specific splicing patterns, raising the possibility that neuron-specific splicing may be part of the cell type specific regulatory rules enabling axon formation exclusively in neurons.
This idea is further supported by the following. Over the years many genes have been documented to control axon formation. While these genes do not show changes in gene expression when a neuron grows out an axon, many change splicing (M. Zhang et al., 2019). Therefore, their contributions to axonongenesis are coordinated at the splicing level. A reasonable speculation is that these genes adopt new functions through alternative splicing that occurs only in neurons to take on the task of neuron-exclusive axon formation.
One example of such splicing change is in Shtn1, which switches expression from a long isoform (SHTN1L) to a short isoform (SHTN1S) during early axon formation. Excessive SHTN1S induces multiple (short) axons, so SHTN1S appears to regulate axon specification (Kubo et al., 2015). Initially proposed as a polarity gene, SHTN1S is induced and located at the axonal tip when neurons begin to protrude an axon (Toriyama et al., 2006). The induction of SHNT1S can be ascribed to the isoform switch from SHTN1L to SHTN1S. Gain and loss of function analyses show that SHNT1S does not promote axonal growth as SHTN1L does. This is because the SHNT1L-specific exon encodes a motif responsible for interacting with the cytoskeleton molecule actin, promoting actin polymerization, thus creating a driving force for the growth of axonal tips (M. Zhang et al., 2019).
SHTN1 regulation provides new insights to axon growth and specification. The gradual transition from SHTN1L to SHTN1S suggests axonal growth slightly precedes axonal specification (Figure 4a). This is consistent with a previous study that found axonal and dendritic cell fates can be switched even after initial axonal outgrowth (Goslin & Banker, 1989). Although disentangling axon growth from axon specification is difficult, the varying functions of SHTN1 isoforms show that growth and specification have distinct molecular drivers but are genetically coupled through alternative splicing.
Figure 4. Alternative splicing regulation of early axonogenesis.
(a) Early axonogenesis (axonal growth and specification) is coordinated by a neuron-specific alternative splicing program. A combinatorial and coordinated alternative splicing regulatory network encompassing many RBPs and alternative splicing events accompanies the morphological and molecular changes of axon formation. Alternative splicing of Shtn1 produces two isoforms. SHTN1L promotes axonal growth. SHNT1S promotes axon specification. F.S. indicates axon fate specification. (b) The axonongenesis-associated splicing program including Shtn1 is coordinated by PTBP2. PTBP2 loss decreases SHTN1L and reduce axonal elongation. Meanwhile, SHTN1S is increased and some Ptbp2−/− neurons can extend two TAU1+ axons.
De novo motif search points to PTBP2 as a master regulator of axonogenesis-associated splicing. PTBP2, also known as neuronal PTB or brain-specific PTB (nPTB or brPTB), is a member of PTBP family proteins best known to regulate alternative splicing (Markovtsov et al., 2000; Polydorides, Okano, Yang, Stefani, & Darnell, 2000). PTBP2 regulation of axonogenesis is corroborated by two transcriptome-wide analyses: 1) a significant proportion of axonogenesis-associated exons are affected by PTBP2 gain and loss; 2) CLIP-Seq confirms an overrepresented number of axonogenesis-associated alternative exons are targeted by PTBP2.
PTBP2 depletion impairs axon formation in vitro and in vivo, impeding axonal growth and simultaneously stimulating axon specification (Figure 4b). While Ptbp2−/− axons are generally shorter than wildtype axons, some Ptbp2 null neurons can extend two axons with molecular markers of developed axons: TAU1 and ANKG. These two distinct phenotypes further demonstrate the inequality between axon growth and specification. One possible explanation for the Ptbp2−/− phenotype is decreased expression of isoforms for axon growth and increased expression of isoforms for axon specification. In support of this hypothesis, Shtn1 splicing is regulated by PTBP2 such that SHNT1L is decreased and SHTN1S is increased in the knockout neurons. Compensation for the SHNT1L shortage partially rescues the axon length in Ptbp2−/− neurons. Therefore, alternative splicing regulation during early axon formation is partly to ensure balanced expression of the isoforms responsible for two integrated aspects of early axonongenesis.
PTBP2 expression is not only enriched in the brain versus other major tissues but also peaks during early axonogenesis in the brain (Sika Zheng, 2016; Sika Zheng et al., 2012). Its peak expression thus appears to serve a specific role for axon formation. Notably, PTBP2’s close paralog, PTBP1, is broadly expressed outside of the brain and is diminished when neural progenitors differentiate into neurons. PTBP2 and PTBP1 have overlapping RNA-binding activities but distinct splicing targets (J. K. Vuong et al., 2016). Our unpublished preliminary analysis of neural-specific Ptbp1 null mice have not identified obvious axonal defects, which might be due to PTBP2 compensation. PTBP1’s possible involvement was suggested by study of Cdc42, a Rho family GTPase (Yap, Xiao, Friedman, Je, & Makeyev, 2016). During early development, 3’ terminal exons of Cdc42 mRNA, change from exclusive exon 7 to a mix of either exon 6 or exon 7. Prevention of this alternative splicing event via knocking out exon 6 increased the axon number in some neurons. This splicing switch was regulated by PTBP1, at least in CAD neuroblastoma cells. Further comparisons of PTBP1 and PTBP2 and their exact roles in axon formation can shed light on why mammals evolve to have multiple PTBP family members.
[ALTERNATIVE SPLICING CONTROL OF AXON GUIDANCE]
The most studied axon guidance system is probably the commissural axons. Commissural axons projecting from the dorsal interneurons are attracted to the ventral midline, enter the midline, subsequently exit the midline, and never cross back (Figure 5a). Fundamental principles have been revealed regarding how extracellular guidance cues steer axonal growth paths via remodelling of the cytoskeleton in the growth cone (Chédotal, 2019). Attractive and repulsive signals (e.g., netrins, semaphorins, slits, ephrins, wnt, draxin, etc), their corresponding receptors (Dcc, Plexins, Neuropilins, Robos, Eph, Frz, etc), and downstream effectors have been identified. The precision of axon pathfinding requires dynamic and robust spatiotemporal regulation of guidance signals (Russell & Bashaw, 2018; Seiradake, Jones, & Klein, 2016). In the textbook version, netrin and DCC signalling are known to guide the axons toward the midline before crossing. Repulsion signalling mediated by slit and Robo1/2 is thought to be repressed to allow axons to enter and cross the midline. After crossing, though unknown mechanisms the Slit and ROBO signalling becomes activated to force the midline exit.
Figure 5. Alternative splicing regulation of axon guidance.
(a) In the spinal cord, growing commissural axons of dorsal interneurons are first attracted to the ventral midline. After crossing the midline, the axons are repulsed away and never cross back. (b) NOVA regulate the isoform expression of DCC and ROBO. (c) Proposed isoform expression for wildtype (WT) neurons before and after crossing the midline. (d) Proposed isoform changes in the Nova−/− before and after crossing the midline, and the associated phenotypes.
It is enigmatic how hundreds of millions (in rodents) and billions (in human) of neurons simultaneously navigate through the complex brain with resolute spatiotemporal precision given only a dozen guidance molecules are so far identified. Alternative splicing may be part of the solution, as many guidance molecules and their receptors express multiple isoforms (Cirulli & Yebra, 2007; Colak, Ji, Porse, & Jaffrey, 2013; Holmberg, Clarke, & Frisén, 2000; Keeling, Gad, & Cooper, 1997; Little, Rumballe, Georgas, Yamada, & Teasdale, 2002; Tamagnone et al., 1999; Yazdani & Terman, 2006; Ypsilanti, Zagar, & Chédotal, 2010). Probably the extreme case is Dscam1 in Drosophila, which can generate up to 38,000 isoforms (Schmucker et al., 2000). A different set of Dscam1 molecules are expressed in each neuron. Homophilic Dscam1 interactions induce self-avoidance to prevent clumping of the neurites of the same neuron during pathfinding (Hattori et al., 2009; Miura, Martins, Zhang, Graveley, & Zipursky, 2013; Wojtowicz, Flanagan, Millard, Zipursky, & Clemens, 2004). The expanded repertoire of regulatory molecules through alternative splicing theoretically allows more spatial and temporal control of axon guidance. However, in most cases the regulatory mechanism of isoform expression and functional diversity of isoforms are unknown.
Recent studies have begun to interrogate the biological meanings of alternative splicing for guidance molecules. New insights arrived from the study of the Nova knockouts. NOVA family proteins, NOVA1 and NOVA2, are neuronal splicing regulators. They contain three KH-type RNA-binding domains and bind stretches of YCAY sequences (Buckanovich & Darnell, 1997). NOVA knockout mice have deficits in cortical neuronal migration, neuromuscular junction formation, and slow inhibitory postsynaptic potential currents (Huang et al., 2005; Ruggiu et al., 2009; Yano, Hayakawa-Yano, Mele, & Darnell, 2010). Additional phenotypes were found in the younger Nova1/2 double knockout (dKO) embryonic brain: the agenesis of the corpus callosum in the cortex and the failure of the spinal cord interneuron axons to reach the midline, both of which are reminiscent of classic axon guidance defects (Johnson, Junge, & Chen, 2019; Leggere et al., 2016; Saito et al., 2016). The phenotypic defects in the spinal cord result from the synergistic actions of the misregulated Netrin/DCC and Slit/ROBO signalling (Figure 5b).
DCC is a netrin receptor and best known to mediate netrin-induced attraction of the growth cone. Similar to Nova1/2 dKO, Dcc knockout interfered with the guidance of the commissural axons (Fazeli et al., 1997). Alternative 3’ splice sites of exon 17 create two major Dcc isoforms, DCClong and DCCshort. NOVA binds a cluster of YCAY elements upstream of exon 17 and promotes the usage of the proximal splice site such that NOVA depletion increases the expression of DCCshort at the expense of DCClong (Figure 5c-d).
The Nova1/2 dKO phenotype is in part due to the splicing defects of Dcc. DCClong expression ameliorates the defects in NOVA dKO (Leggere et al., 2016). DCClong and DCCshort differ in the linker sequence between the fourth and fifth fibronectin repeats (Pierceall et al., 1994). Although the two isoforms bind netrin with similar affinities, they assemble differently with netrin resulting in different conformation (Kai Xu et al., 2014). The conformations may interpret the netrin signal differently and translate it into different cellular actions, although this hypothesis is yet to be tested. Alternatively, the two confirmations may induce antagonistic responses. It is also possible that DCCshort is inactive in the context of axon guidance, as DCCshort overexpression has no effect in rescuing Nova dKO (Leggere et al., 2016).
The defects of Nova dKO commissural axons are not fully rescued by DCClong overexpression. Matching endogenous DCClong expression level is difficult, but the incomplete rescue is likely due to additional NOVA targets that come into play. Furthermore, the Nova dKO phenotypes are more nuanced than failed axon growth into the midline. Although Nova dKO commissural axons are deficient in being attracted to the midline, about half of them do reach the midline. Those attracted to the midline either fail to cross the midline or cross the midline and grow in a wrong angle, indicative of enhanced repulsion activity at both sides of the midline (Figure 5d). In support of this idea, the midline crossing phenotype in Nova dKO is rescued when the repulsion signal is attenuated by Robo1 complete knockout (Johnson et al., 2019).
What could be the mechanisms of excessive repulsion? In the cortex, Nova dKO leads to misregulation of multiple axon guidance molecules in addition to Dcc. These include Robo2, Epha5, and Slit2, but their biological significance has not been tested (Saito et al., 2016). In the spinal cord, the splicing regulation of Robo1 and Robo2 was proven to be essential. ROBO1 and ROBO2 are receptors of the repulsive cue Slit. Alternative splicing of Robo1 and Robo2 exon 6b resulting in two distinct isoforms, ROBOlong and ROBOshort, that differ in a short linker between the third and fourth Ig domains. NOVA1/2 bind a cluster of YCAY motifs flanking exons 6b and repress their splicing (Figure 5d). Therefore, in the Nova dKO ROBOlong is increased and ROBOshort is decreased.
Chen’s group showed that mouse heterozygous deletions of exon 6b in Robo1/2, reducing ROBOlong, almost completely rescue the midline crossing defects in the Nova1/2 dKO (Johnson et al., 2019). This is because ROBOlong exhibits a stronger repulsive effect than ROBOshort before and after crossing. Under the condition of ROBOlong overexpression, fewer axons cross the midline and the postcrossing commissural axons are guided further away from the midline to a more lateral position, similar to what is observed in Nova dKO. The functional variation between the two ROBO isoforms were corroborated by their differential molecular effects. The two isoforms show similar binding affinities to SLIT but differ in the downstream canonical SLIT/ROBO signalling: ROBOlong has a stronger effect than ROBOshort in RAC42 activation and CDC42 inhibition.
These results not only describe the mechanistic actions of NOVA-mediated neuronal splicing but also provide new insights to the axon guidance field. For example, previous models assume silencing of ROBO signalling pre-crossing. However, before most axons cross the midline, ROBO1/2 almost exclusively express the long (and stronger) isoform at E10.5 (Johnson et al., 2019). Therefore, repulsive activities already exist pre-crossing and are strengthened by expressing the long isoforms to inhibit premature crossing of commissural axons. This is demonstrated by thicker ventral commissures in homozygous Robo1/2 exon6b knockout at E10.5.
A reasonable model for these new data follows (Figure 5c). The pre-crossing attraction signals (mediated by DCClong) are indispensable to counteract the repulsion signals (mediated by ROBOlong) for axons to reach the midline. After crossing, the attraction signals are attenuated and probably reduced to minimum, as DCClong is replaced by DCCshort. Meanwhile, Robo mRNA splices more to generate ROBOshort. ROBOlong is partially reduced so ROBO provides the right amount of repulsion to guide the axon to cross the midline. In essence, alternative splicing temporally controls the midline crossing.
NOVA regulation therefore acts as a temporal modifier of commissural axon crossing. Consistently, guidance defects in the Nova1/2 dKO are delayed rather than blocked and are somewhat alleviated by E12.5 (Johnson et al., 2019). Many important questions remain to be answered. Is NOVA expression controlled during axon pathfinding? Does NOVA depletion delay or block developmental regulation of ROBO and DCC? What is the biological consequence of modifying the timing of axon crossing? NOVA knockouts would not be a good model to directly answer this question because of their additional profound phenotypes in the later stage of development. The Robo exon 6b knockouts or cell type specific manipulation of NOVA may be a good model for behavioural tests.
The spatial specificity of these NOVA-mediated splicing control is also a puzzle. For example, the Robo exon6b knockout phenotype is found at the lumbar level but not at the branchial level (Johnson et al., 2019). In the cortex, corpus callosum is missing in the Nova2 KO brain; therefore axon crossing appears completely arrested. Axon guidance defects are also observed in Nova2 KO neurons innervating the ventral diaphragm and inner ear (Saito et al., 2016). To what degree these defects share molecular mechanisms with those in the spinal cord will be important in dissecting spatial regulation of axon guidance and the underlying biological logics of employing alternative splicing.
[ALTERNATIVE SPLICING CONTROL OF AXON MATURATION]
The axon initial segment (AIS) is a distinct microdomain of the proximal axon responsible for initiating action potentials (Nelson & Jenkins, 2017). AIS also serves as a structural barrier to separate the axonal compartment from the somatodendritic part of the neuron (Zollinger et al., 2015). Therefore, establishing AIS is one of critical step of axon maturation. A structural hallmark of AIS is a sub-membranous lattice containing periodic cytoskeleton rings composed of actin, βIV spectrin, and ankyrin G (ANKG) (Figure 6)(Ke Xu, Zhong, & Zhuang, 2013). ANKG is a scaffolding protein that enriches voltage gated sodium channels at AIS. Without ANKG, AIS cannot be maintained (Zollinger et al., 2015).
Figure 6. Alternative splicing regulation of axon maturation.
In wildtype (WT) neurons, ANKG expresses an isoform skipping exon 33 because of RBFOX repression. This isoform tethers ANKG to βIV spectrin and the periodic actin ring, thereby clustering sodium channels at the axon initial segment (AIS). In Rbfox knockout neurons, inclusion of exon 33 prevents ANKG from binding to βIV spectrin. As a result, AIS is not properly established and action potentials are impaired.
Insights into alternative splicing regulation of AIS formation were derived from studies of RBFOX proteins, a family of highly conserved RNA binding proteins. The RBFOX family contains RBFOX1, RBFOX2, and RBFOX3, and is preferentially expressed in neurons. Indeed, the widely used neuronal marker NeuN was identified as RBFOX3 (Kim, Adelstein, & Kawamoto, 2009). The best characterized function of RBFOX is to control alternative splicing. It is unclear how different RBFOX members differ in activity. However, they all contain the same single RRM domain recognizing (U)GCAUG sequence and appear to bind the same RNA targets in vivo, suggesting overlapping functions (Damianov et al., 2016; Weyn-Vanhentenryck et al., 2014). Different neuronal cell types can express a different combination of RBFOX proteins (Gehman et al., 2012; Hammock & Levitt, 2011). The functional redundancy of RBFOX proteins poses a challenge to understanding their physiological roles.
Zhang’s group interrogated RBFOX’s aggregative functions using a Rbfox triple knockout (tKO) mouse embryonic stem cell (ESC) line (Jacko et al., 2018). The major phenotype of the tKO neuron is in AIS formation. Assayed by various AIS markers, including ANKG, KCNQ3, and βIV spectrin, fewer tKO neurons contained AIS and in those that did the AIS length was shorter. Structurally, the periodicity of actin rings at the AIS is unaffected in tKO. However, the AnkG periodicity is disrupted, suggesting the defect can be as specific as impaired AnkG clustering at AIS.
RBFOX depletion leads to alternative usage of isoforms for multiple AIS components, including AnkG, Nfasc, and Nrcam. A noteworthy RBFOX-repressed cassette exon (exon 33) in AnkG encodes a peptide immediately upstream of and interfering with the function of its ZU5 domain for tethering ANKG to βIV spectrin and thus to the actin ring (Figure 6). As RBFOX is developmentally up-regulated, ANKG first expresses the inclusion isoform and later the exclusion isoform. Only the exclusion isoform interacts with βIV spectrin. Forced constitutive splicing and expression of the inclusion isoform leads to fewer neurons with ANKG at AIS and weaker ANKG clustering. Conversely, forced constitutive exclusion of this exon did not abolish ANKG localization at AIS; only some neurons appear to shorten the ANKG-marked AIS length. Therefore, recruitment of ANKG to the actin-βIV spectrin rings and normal maturation of AIS requires precise temporal splicing control of this alternative exon.
ANKG is unlikely to be the only causal target to explain the RBFOX tKO phenotype, because 15 more AIS constituents and over 500 additional genes change splicing in the absence of RBFOX (Jacko et al., 2018). Nevertheless, the involvement of alternative splicing regulation for axon maturation is clearly demonstrated by functional characterization of the two ANKG isoforms. The fact that overexpression of the ANKG exclusion isoform only marginally affects the RBFOX tKO phenotype suggests that the RBFOX dependent splicing program needs to be coordinated for axon maturation.
[ALTERNATIVE SPLICING CONTROL OF AXON REGENERATION]
Compared to the robust axon formation during embryonic development, adult axon regrowth (after injury) is rather limited. In mammals, only the peripheral nervous system, e.g., axons originating from the dorsal root ganglion, retains some regeneration capacity (Curcio & Bradke, 2018). In the central nervous system, most adult lesioned axons neither regenerate nor make functional connections. By contrast, invertebrate and non-mammalian vertebrate neurons generally exhibit a remarkable potential for regrowth in adults. There is substantial interest in understanding the mechanisms of regeneration and the lack thereof as well as translating that knowledge to enhance axon regeneration. Various cellular molecular mechanisms have been investigated, including intracellular signalling pathways, cytoskeletal dynamics and axonal transport, transcriptional and epigenetic regulation, etc. (Mahar & Cavalli, 2018). The roles of alternative splicing, or RNA binding proteins in general, remain to be determined.
A genetic screen for factors altering regeneration capacity in C. elegans identified UNC-75 (Lizhen Chen et al., 2016). Various Unc-75 loss-of-function mutants after injury exhibit normal growth cone formation but impaired axon extension for multiple neuron types. Therefore, UNC-75 is necessary for efficient axon regeneration in C. elegans.
UNC-75 is the invertebrate homolog of the mammalian CUGBP Elav-like family proteins, CELF. Nuclear CELF regulates alternative splicing, whereas cytoplasmic CELF controls mRNA stability and translation (Dasgupta & Ladd, 2012). There are six CELF member proteins and they are all expressed in the brain. Among them, CELF2 is specifically induced in adult dorsal root ganglion (DRG) neurons upon sciatic nerve crush, presumably as part of the intrinsic injury response (Lizhen Chen et al., 2016). DRG-specific Celf2 knockout exhibit reduced axon regeneration 3 days post sciatic nerve injury, confirming a conserved role of CELF2 for axon regeneration.
CELF2’s ability to program axon regeneration is unique among RNA binding proteins. Neither RBFOX (fox-1) or ELAVL (Exc-7) is required for axon regrowth in C. elegans, although both proteins genetically interact with CELF (Unc-75) in regulating some alternative splicing (Kuroyanagi, Watanabe, & Hagiwara, 2013; Norris et al., 2014). CELF2 expression in DRG neurons is down-regulated after birth, concurrent with DRG’s declined capability of regeneration over time (Mar, Bonni, & Sousa, 2014), further indicating that CELF2 level correlates with the ability of axon regeneration. Notably UNC-75 overexpression did not augment axon growth, suggesting a permissive role during regeneration.
Both UNC-75 and CELF2 are predominantly localized in the neuronal nucleus (Otsuka et al., 2009), suggesting that their mutant phenotypes are due to defects in alternative splicing regulation. One of the critical UNC-75 targets was found to be the alternative 3’ exon of Syntaxin (Unc-64). Similar to UNC-75, UNC64 appears dispensable for axon development but is required for axon regrowth. UNC-75 promotes the proximal 3’ exon to generate the neuronal isoform of Synatxin (UNC64A). Unc-75 mutants showed reduced expression of Unc64a and increased Unc64b (the non-neuronal isoform). Via overexpression analysis, Unc64a but not Unc64b is the functional isoform responsible for the Unc-75 phenotype (Lizhen Chen et al., 2016).
[FUTURE DIRECTIONS AND PERSPECTIVES]
Since neuron-specific alternative splicing may be one of the regulatory rules for axonogenesis, knowledge about neuronal splicing regulation during axon formation provides new perspectives for understanding the cell biology of axonogenesis as well as therapeutic strategies for boosting axon regeneration in neurodegenerative diseases and spinal cord injury. Some critical questions remain to be investigated.
Identifying functionally relevant alternative splicing events is essential. Classical gain and loss of function approaches on the isoform basis are still needed to discern the differences between isoforms. Perturbation of alternative splicing can also be achieved by CRISPR-Cas systems for either DNA targeting (Cas9, Cas12, etc) to completely remove the exon of interest or RNA targeting (Cas13, dCas9, etc) to interfere with the process of alternative splicing regulation (Shalem, Sanjana, & Zhang, 2015; Xiong, Chen, Lim, Zhao, & Qi, 2016). As these systems become scalable, together with high-speed and high-content imaging to streamline the process, morphology- or biomarker-based genetic screens will speed up the discovery. Of course, the success of screens hinges on the robustness and relevance of the readouts.
A biological process as complicated as axon formation almost certainly requires coordinated actions between many molecules. Traditional reductionist approaches may be at odds in discovering the underlying principles. Methods for interrogating multiple molecules at a time will be needed. These could include imaging of many differentially tagged molecules, structural analysis (e.g., cryoEM) of important complexes, large scale probing of protein-protein interactions at the isoform level, etc. The versatility of the CRISPR system to simultaneously hamper multiple splicing events will be also powerful, albeit limitations to its efficiency.
It is still essential to study alternative splicing regulators that coordinate multiple splicing events in axon formation. Detailed characterizations of RBP mutants have provided unprecedented insights to the cellular and molecular regulation of axon formation. Once important RBPs are identified, the regulatory relationships between RBPs and alternative splicing events can be assessed transcriptome-wide with various experimental and computational tools, including but not limited to CLIP-Seq, RNA-Seq, and functional screens of splicing reporters, etc (Liang Chen & Zheng, 2009; Feng et al., 2019; F. C. Y. Lee & Ule, 2018; Ule, Hwang, & Darnell, 2018; Wheeler, Van Nostrand, & Yeo, 2018; S. Zheng, 2016). Even though not every single target of an RBP is important, genetic interactions between its coordinated targets are presumably more possible than between two random alternative exons, which help prioritize exons of interest. The RBP mutants also provide indirect evidence their targets are linked to a specific process of axon formation.
This leads us to the following question: what is the direct target of a splicing regulator responsible for the observed phenotype? It is a legitimate question given the cross-regulation between splicing factors, a network of target exons by each splicing factor, and their pleiotropic cellular effects. The conventional test for a “direct” vs “indirect” target is the presence vs absence of CLIP-Seq signals . As robust as CLIP-Seq is for transcriptome-wide analysis, its limitation on specific genes of interest should also be recognized. Because of the low crosslinking efficiency of UV, CLIP-Seq has unestimatable but seemingly high false negatives, particularly for low abundant transcripts. Furthermore, nearly all CLIP-Seq show that most protein-RNA binding events may not be functional regarding their impacts on changing alternative splicing. Therefore, a holistic examination of evidence including but not limited to CLIP-Seq is needed.
Conclusion
Significant progress has been made to exemplify the functional significance of alternative splicing for axon formation. Alternative splicing changes the function of cytoskeleton associated proteins (e.g., SHTN1) to allow robust protrusion of a single axon, a structure no other cells types possess. Alternative splicing makes changes to intracellular signalling molecules (e.g., DCC, ROBO), and thereby finetunes the interpretation of extracellular signals to meet the needs of changing the orientation of axon pathfinding. Alternative splicing transforms scaffolding proteins (e.g., ANKG) to allow step-wise assembly and disassembly and stabilization of axon-specific structures like the axon initial segment. These events represent only a tip of the iceberg that encompasses a much larger splicing program associated with axon formation. The identification of RNA binding proteins controlling these splicing programs is a critical step and functional characterization of their mutants will propel new discoveries.
Temporal control is at the centre of these alternative splicing regulatory events. Either for a transient need of axon guidance or a permanent need of AIS formation, alternative splicing provides the solution. The timing of isoform changes coincides with the molecular demand for morphological changes, while the relevant genes are already expressed and have exerted their functions in the prior cellular state. Beyond temporal regulation, it is conceivable that alternative splicing is adopted as a regulatory strategy for the spatial control of neuronal morphology, e.g., to define axonal territories or to produce axons of differential properties for various neuronal subtypes.
Acknowledgments
I would like to thank Jo Gerrard for technical help in graphics. I apologize to those whose studies could not be cited due to space limitation.
Funding Information
S.Z. is supported by NIH grants 1R01NS104041 and 1R01MH116220.
Footnotes
No conflict of interest
References
- Barbosa-Morais NL, Irimia M, Pan Q, Xiong HY, Gueroussov S, Lee LJ, … Blencowe BJ (2012). The evolutionary landscape of alternative splicing in vertebrate species. Science, 338(6114), 1587–1593. [DOI] [PubMed] [Google Scholar]
- Barnes AP, & Polleux F (2009). Establishment of axon-dendrite polarity in developing neurons. Annual Review of Neuroscience, 32, 347–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DL (2003). Mechanisms of alternative pre-messenger RNA splicing. Annual Review of Biochemistry, 72, 291–336. [DOI] [PubMed] [Google Scholar]
- Blencowe BJ (2017). [Review of The Relationship between Alternative Splicing and Proteomic Complexity]. Trends in biochemical sciences, 42(6), 407–408. [DOI] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, & Pachter L (2016). Near-optimal probabilistic RNA-seq quantification. Nature Biotechnology, 34(5), 525–527. [DOI] [PubMed] [Google Scholar]
- Buckanovich RJ, & Darnell RB (1997). The neuronal RNA binding protein Nova-1 recognizes specific RNA targets in vitro and in vivo. Molecular and Cellular Biology, 17(6), 3194–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres A, Ye B, & Dotti CG (2012). Neuronal polarity: demarcation, growth and commitment. Current Opinion in Cell Biology, 24(4), 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chédotal A (2019). Roles of axon guidance molecules in neuronal wiring in the developing spinal cord. Nature Reviews. Neuroscience, 20(7), 380–396. [DOI] [PubMed] [Google Scholar]
- Cheng P-L, & Poo M-M (2012). Early events in axon/dendrite polarization. Annual Review of Neuroscience, 35, 181–201. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu Z, Zhou B, Wei C, Zhou Y, Rosenfeld MG, … Jin Y (2016). CELF RNA binding proteins promote axon regeneration in C. elegans and mammals through alternative splicing of Syntaxins. eLife, 5 10.7554/eLife.16072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, & Zheng S (2009). Studying alternative splicing regulatory networks through partial correlation analysis. Genome Biology, 10(1), R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli V, & Yebra M (2007). Netrins: beyond the brain. Nature Reviews. Molecular Cell Biology, 8(4), 296–306. [DOI] [PubMed] [Google Scholar]
- Colak D, Ji S-J, Porse BT, & Jaffrey SR (2013). Regulation of axon guidance by compartmentalized nonsense-mediated mRNA decay. Cell, 153(6), 1252–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio M, & Bradke F (2018). Axon Regeneration in the Central Nervous System: Facing the Challenges from the Inside. Annual Review of Cell and Developmental Biology, 34, 495–521. [DOI] [PubMed] [Google Scholar]
- Damianov A, Ying Y, Lin C-H, Lee J-A, Tran D, Vashisht AA, … Black DL (2016). Rbfox Proteins Regulate Splicing as Part of a Large Multiprotein Complex LASR. Cell, 165(3), 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta T, & Ladd AN (2012). The importance of CELF control: molecular and biological roles of the CUG-BP, Elav-like family of RNA-binding proteins. Wiley Interdisciplinary Reviews. RNA, 3(1), 104–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez D, Freese P, Alexis MS, Su A, Hochman M, Palden T, … Burge CB (2018). Sequence, Structure, and Context Preferences of Human RNA Binding Proteins. Molecular Cell, 70(5), 854–867.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, … Weinberg RA (1997). Phenotype of mice lacking functional Deleted in colorectal cancer (Dec) gene. Nature, 386(6627), 796–804. [DOI] [PubMed] [Google Scholar]
- Feng H, Bao S, Rahman MA, Weyn-Vanhentenryck SM, Khan A, Wong J, … Zhang C (2019). Modeling RNA-Binding Protein Specificity In Vivo by Precisely Registering Protein-RNA Crosslink Sites. Molecular Cell, 74(6), 1189–1204.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X-D, & Ares M Jr. (2014). Context-dependent control of alternative splicing by RNA-binding proteins. Nature Reviews. Genetics, 15(10), 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehman LT, Meera P, Stoilov P, Shiue L, O’Brien JE, Meisler MH, … Black DL (2012). The splicing regulator Rbfox2 is required for both cerebellar development and mature motor function. Genes & Development, 26(5), 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, & Banker G (1989). Experimental observations on the development of polarity by hippocampal neurons in culture. The Journal of Cell Biology, 108(4), 1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EAD, & Levitt P (2011). Developmental expression mapping of a gene implicated in multiple neurodevelopmental disorders, A2bp1 (Fox1). Developmental Neuroscience, 33(1), 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka Y, & Yamauchi K (2013). Excitatory cortical neurons with multipolar shape establish neuronal polarity by forming a tangentially oriented axon in the intermediate zone. Cerebral Cortex , 23(1), 105–113. [DOI] [PubMed] [Google Scholar]
- Hattori D, Chen Y, Matthews BJ, Salwinski L, Sabatti C, Grueber WB, & Zipursky SL (2009). Robust discrimination between self and non-self neurites requires thousands of Dscam1 isoforms. Nature, 461(7264), 644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg J, Clarke DL, & Frisén J (2000). Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature, 408(6809), 203–206. [DOI] [PubMed] [Google Scholar]
- Huang CS, Shi S-H, Ule J, Ruggiu M, Barker LA, Darnell RB, … Jan LY (2005). Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell, 123(1), 105–118. [DOI] [PubMed] [Google Scholar]
- Jacko M, Weyn-Vanhentenryck SM, Smerdon JW, Yan R, Feng H, Williams DJ, … Zhang C (2018). Rbfox Splicing Factors Promote Neuronal Maturation and Axon Initial Segment Assembly. Neuron, 97(4), 853–868.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V, Junge HJ, & Chen Z (2019). Temporal regulation of axonal repulsion by alternative splicing of a conserved microexon in mammalian Robo1 and Robo2. eLife, 8 10.7554/eLife.46042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, & Hoogenraad CC (2015). Building the Neuronal Microtubule Cytoskeleton. Neuron, 87(3), 492–506. [DOI] [PubMed] [Google Scholar]
- Keeling SL, Gad JM, & Cooper HM (1997). Mouse Neogenin, a DCC-like molecule, has four splice variants and is expressed widely in the adult mouse and during embryogenesis. Oncogene, Vol. 15, pp. 691–700. 10.1038/sj.onc.1201225 [DOI] [PubMed] [Google Scholar]
- Kevenaar JT, & Hoogenraad CC (2015). The axonal cytoskeleton: from organization to function. Frontiers in Molecular Neuroscience, 8, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, & Kawamoto S (2009). Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. The Journal of Biological Chemistry, 284(45), 31052–31061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y, Baba K, Toriyama M, Minegishi T, Sugiura T, Kozawa S, … Inagaki N (2015). Shootin1-cortactin interaction mediates signal-force transduction for axon outgrowth. The Journal of Cell Biology, 210(4), 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroyanagi H, Watanabe Y, & Hagiwara M (2013). CELF family RNA-binding protein UNC-75 regulates two sets of mutually exclusive exons of the unc-32 gene in neuron-specific manners in Caenorhabditis elegans. PLoS Genetics, 9(2), e1003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FCY, & Ule J (2018). Advances in CLIP Technologies for Studies of Protein-RNA Interactions. Molecular Cell, 69(3), 354–369. [DOI] [PubMed] [Google Scholar]
- Lee Y, & Rio DC (2015). Mechanisms and Regulation of Alternative Pre-mRNA Splicing. Annual Review of Biochemistry, 84, 291–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggere JC, Saito Y, Darnell RB, Tessier-Lavigne M, Junge HJ, & Chen Z (2016). NOVA regulates Dcc alternative splicing during neuronal migration and axon guidance in the spinal cord. eLife, 5 10.7554/eLife.14264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, & Dewey CN (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics, 12, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M, Rumballe B, Georgas K, Yamada T, & Teasdale RD (2002). Conserved modularity and potential for alternate splicing in mouse and human Slit genes. The International Journal of Developmental Biology, 46(4), 385–391. [PubMed] [Google Scholar]
- Louie AL, Aigner S, Bergalet J, Zhou B, & Su A (2018). A large-scale binding and functional map of human RNA binding proteins. bioRxiv. Retrieved from https://www.biorxiv.org/content/10.1101/179648v2.abstract [Google Scholar]
- Mahar M, & Cavalli V (2018). Intrinsic mechanisms of neuronal axon regeneration. Nature Reviews. Neuroscience, 19(6), 323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar FM, Bonni A, & Sousa MM (2014). Cell intrinsic control of axon regeneration. EMBO Reports, 15(3), 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovtsov V, Nikolic JM, Goldman JA, Turck CW, Chou MY, & Black DL (2000). Cooperative assembly of an hnRNP complex induced by a tissue-specific homolog of polypyrimidine tract binding protein. Molecular and Cellular Biology, 20(20), 7463–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamud E, & Moult J (2009). Stochastic noise in splicing machinery. Nucleic Acids Research, 37(14), 4873–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkin J, Russell C, Chen P, & Burge CB (2012). Evolutionary dynamics of gene and isoform regulation in Mammalian tissues. Science, 338(6114), 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura SK, Martins A, Zhang KX, Graveley BR, & Zipursky SL (2013). Probabilistic splicing of Dscam1 establishes identity at the level of single neurons. Cell, 155(5), 1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba T, Funahashi Y, Nakamuta S, Xu C, Takano T, & Kaibuchi K (2015). Extracellular and Intracellular Signaling for Neuronal Polarity. Physiological Reviews, 95(3), 995–1024. [DOI] [PubMed] [Google Scholar]
- Nelson AD, & Jenkins PM (2017). Axonal Membranes and Their Domains: Assembly and Function of the Axon Initial Segment and Node of Ranvier. Frontiers in Cellular Neuroscience, 11, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AD, Gao S, Norris ML, Ray D, Ramani AK, Fraser AG, … Calarco JA (2014). A pair of RNA-binding proteins controls networks of splicing events contributing to specialization of neural cell types. Molecular Cell, 54(6), 946–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka N, Tsuritani K, Sakurai T, Kato K, Matoba R, Itoh J, … Yoneda Y (2009). Transcriptional induction and translational inhibition of Arc and Cugbp2 in mice hippocampus after transient global ischemia under normothermic condition. Brain Research, 1287, 136–145. [DOI] [PubMed] [Google Scholar]
- Pan Q, Shai O, Lee LJ, Frey BJ, & Blencowe BJ (2008). Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nature Genetics, 40(12), 1413–1415. [DOI] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, & Kingsford C (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nature Methods, 14(4), 417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell JK, Pai AA, Gilad Y, & Pritchard JK (2010). Noisy splicing drives mRNA isoform diversity in human cells. PLoS Genetics, 6(12), e1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierceall WE, Reale MA, Candia AF, Wright CVE, Cho KR, & Fearon ER (1994). Expression of a Homologue of the Deleted in Colorectal Cancer (DCC) Gene in the Nervous System of Developing Xenopus Embryos. Developmental Biology, Vol. 166, pp. 654–665. 10.1006/dbio.1994.1345 [DOI] [PubMed] [Google Scholar]
- Polydorides AD, Okano HJ, Yang YY, Stefani G, & Darnell RB (2000). A brain-enriched polypyrimidine tract-binding protein antagonizes the ability of Nova to regulate neuron-specific alternative splicing. Proceedings of the National Academy of Sciences of the United States of America, 97(12), 6350–6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj B, & Blencowe BJ (2015). Alternative Splicing in the Mammalian Nervous System: Recent Insights into Mechanisms and Functional Roles. Neuron, 87(1), 14–27. [DOI] [PubMed] [Google Scholar]
- Ruggiu M, Herbst R, Kim N, Jevsek M, Fak JJ, Mann MA, … Darnell RB (2009). Rescuing Z agrin splicing in Nova null mice restores synapse formation and unmasks a physiologic defect in motor neuron firing. Proceedings of the National Academy of Sciences, Vol. 106, pp. 3513–3518. 10.1073/pnas.0813112106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SA, & Bashaw GJ (2018). Axon guidance pathways and the control of gene expression. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 247(4), 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Miranda-Rottmann S, Ruggiu M, Park CY, Fak JJ, Zhong R, … Darnell RB (2016). NOVA2-mediated RNA regulation is required for axonal pathfinding during development. eLife, 5 10.7554/eLife.14371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, … Zipursky SL (2000). Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell, 101(6), 671–684. [DOI] [PubMed] [Google Scholar]
- Seiradake E, Jones EY, & Klein R (2016). Structural Perspectives on Axon Guidance. Annual Review of Cell and Developmental Biology, 32, 577–608. [DOI] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, & Zhang F (2015). High-throughput functional genomics using CRISPR-Cas9. Nature Reviews. Genetics, 16(5), 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek R, Shamir R, & Ast G (2004). How prevalent is functional alternative splicing in the human genome? Trends in Genetics: TIG, 20(2), 68–71. [DOI] [PubMed] [Google Scholar]
- Szu-Yu Ho T, & Rasband MN (2011). Maintenance of neuronal polarity. Developmental Neurobiology, 71(6), 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, … Comoglio PM (1999). Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell, 99(1), 71–80. [DOI] [PubMed] [Google Scholar]
- Tissir F, & Goffinet AM (2013). Shaping the nervous system: role of the core planar cell polarity genes. Nature Reviews. Neuroscience, 14(8), 525–535. [DOI] [PubMed] [Google Scholar]
- Toriyama M, Shimada T, Kim KB, Mitsuba M, Nomura E, Katsuta K, … Inagaki N (2006). Shootin1: A protein involved in the organization of an asymmetric signal for neuronal polarization. The Journal of Cell Biology, 175(1), 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, … Pachter L (2012). Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature Protocols, 7(3), 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tress ML, Abascal F, & Valencia A (2017). Alternative Splicing May Not Be the Key to Proteome Complexity. Trends in Biochemical Sciences, 42(2), 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ule J, Hwang H-W, & Darnell RB (2018). The Future of Cross-Linking and Immunoprecipitation (CLIP). Cold Spring Harbor Perspectives in Biology, 10(8). 10.1101/cshperspect.a032243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong CK, Black DL, & Zheng S (2016). The neurogenetics of alternative splicing. Nature Reviews. Neuroscience, 17(5), 265–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong JK, Lin C-H, Zhang M, Chen L, Black DL, & Zheng S (2016). PTBP1 and PTBP2 Serve Both Specific and Redundant Functions in Neuronal Pre-mRNA Splicing. Cell Reports, 17(10), 2766–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, … Burge CB (2008). Alternative isoform regulation in human tissue transcriptomes. Nature, 456(7221), 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyn-Vanhentenryck SM, Feng H, Ustianenko D, Duffié R, Yan Q, Jacko M, … Zhang C (2018). Precise temporal regulation of alternative splicing during neural development. Nature Communications, 9(1), 2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyn-Vanhentenryck SM, Mele A, Yan Q, Sun S, Farny N, Zhang Z, … Zhang C (2014). HITS-CLIP and integrative modeling define the Rbfox splicing-regulatory network linked to brain development and autism. Cell Reports, 6(6), 1139–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler EC, Van Nostrand EL, & Yeo GW (2018). Advances and challenges in the detection of transcriptome-wide protein-RNA interactions. Wiley Interdisciplinary Reviews. RNA, 9(1). 10.1002/wrna.1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, & Clemens JC (2004). Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell, 118(5), 619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Chen M, Lim WA, Zhao D, & Qi LS (2016). CRISPR/Cas9 for Human Genome Engineering and Disease Research. Annual Review of Genomics and Human Genetics, 17, 131–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Wu Z, Renier N, Antipenko A, Tzvetkova-Robev D, Xu Y, … Nikolov DB (2014). Neural migration. Structures of netrin-1 bound to two receptors provide insight into its axon guidance mechanism. Science, 344(6189), 1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Zhong G, & Zhuang X (2013). Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science, 339(6118), 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Hayakawa-Yano Y, Mele A, & Darnell RB (2010). Nova2 regulates neuronal migration through an RNA switch in disabled-1 signaling. Neuron, 66(6), 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap K, Xiao Y, Friedman BA, Je HS, & Makeyev EV (2016). Polarizing the Neuron through Sustained Co-expression of Alternatively Spliced Isoforms. Cell Reports, 15(6), 1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani U, & Terman JR (2006). The semaphorins. Genome Biology, 7(3), 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypsilanti AR, Zagar Y, & Chédotal A (2010). Moving away from the midline: new developments for Slit and Robo. Development , 137(12), 1939–1952. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kuo C-CJ, & Chen L (2015). WemIQ: an accurate and robust isoform quantification method for RNA-seq data. Bioinformatics , 31(6), 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Ergin V, Lin L, Stork C, Chen L, & Zheng S (2019). Axonogenesis Is Coordinated by Neuron-Specific Alternative Splicing Programming and Splicing Regulator PTBP2. Neuron, 101(4), 690–706.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S (2016). Alternative splicing and nonsense-mediated mRNA decay enforce neural specific gene expression. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. 10.1016/j.ijdevneu.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S (2016). IRAS: High-Throughput Identification of Novel Alternative Splicing Regulators. Methods in Enzymology, 572, 269–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, & Black DL (2013). Alternative pre-mRNA splicing in neurons: growing up and extending its reach. Trends in Genetics: TIG, 29(8), 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, & Chen L (2009). A hierarchical Bayesian model for comparing transcriptomes at the individual transcript isoform level. Nucleic Acids Research, 37(10), e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Gray EE, Chawla G, Porse BT, O’Dell TJ, & Black DL (2012). PSD-95 is post-transcriptionally repressed during early neural development by PTBP1 and PTBP2. Nature Neuroscience, 15(3), 381–388, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger DR, Baalman KL, & Rasband MN (2015). The ins and outs of polarized axonal domains. Annual Review of Cell and Developmental Biology, 31, 647–667. [DOI] [PubMed] [Google Scholar]