Abstract
Information on sex differences in the association between chronotype and depression is scarce. We aimed to investigate these differences using data from the Korea National Health and Nutrition Examination Survey in 2016. Chronotypes were categorised based on mid-sleep time on free days corrected by sleep debt accumulated on workdays (MSFsc): early type, < mean MSFsc − 1 standard deviation (SD); intermediate type, between mean MSFsc − 1 SD and MSFsc + 1 SD; and late type, > mean MSFsc + 1 SD. A Patient Health Questionnaire-9 score of ≥ 10 indicated depression. Among 5550 non-shift working adults aged 19–80 years, the prevalence rates of depression in the early, intermediate, and late chronotype groups were 7.4%, 4.5%, and 9.3%, respectively. Women with late chronotype (odds ratio [OR] = 2.9, 95% confidence interval [CI] = 1.8–4.7) showed a higher risk of depression than women with intermediate chronotype after adjusting for covariates. Women with early chronotype did not show a significant difference in depression risk (OR = 1.3, 95% CI = 0.9–2.0). In conclusion, late chronotype is associated with an increased risk of depression in women but not in men. Early chronotype is not associated with depression in women or men.
Subject terms: Neuroscience, Diseases, Medical research, Neurology
Introduction
Depression is a prevalent disease that affects 4.4% of the global population1. Owing to associated symptoms and comorbid conditions, depression is a significant source of disability in affected individuals and imposes a huge social burden2. Depression was ranked as the single largest contributor to global disability in 2015 by the World Health Organization3.
Chronotype refers to an individual’s circadian propensity for when to wake and be active, and when to sleep. Early chronotype (morning type, advanced sleep phase) and late chronotype (evening type, delayed sleep phase) are two extreme types of chronotypes4. Previous studies have reported that individuals with late chronotype are more likely to have depression or depressive symptoms5,6.
Sex is closely related to both depression and chronotype. Women are twice as likely to be diagnosed with depression compared to men7. Women experience higher rates of comorbid anxiety, a higher rate of atypical features, more somatic and cognitive-affective symptoms, and respond more favourably to selective serotonin reuptake inhibitors than men8–10. Prefrontal-limbic abnormalities are more prominent in women11. In contrast, men respond more favourably to tricyclic antidepressants than women, and prefrontal-striatal abnormalities are more marked in men11,12. Sex differences in chronotype have also been reported, and most studies have shown that men are more prone to show the evening chronotype or late chronotype than women13.
To the best of our knowledge, only one study has reported a sex difference in the association between a delayed sleep–wake schedule and depression14. Unfortunately, the study was conducted in young adults aged 19–25 years and did not use validated instruments to determine a delayed sleep–wake schedule. Information on the influence of sex on the association between chronotype and depression in a general population-based setting using validated instruments is currently limited.
The seventh Korea National Health and Nutrition Examination Survey (KNHANES VII) is a nationwide representative cross-sectional survey conducted by the Korea Centers for Disease Control and Prevention. Data collected during this survey may provide an opportunity to evaluate sex differences in the association between chronotype and depression. KNHANES VII, conducted in 2016, included items for depression, average sleep duration, and bedtime and wakeup time on workdays and free days. The aim of the present study was to assess whether or not there was a sex difference in the association between chronotype and depression using data from KNHANES VII.
Results
Participants and chronotype
KNHANES VII, 2016, included data on 8,150 participants aged 1–80 years. We selected 6,382 adult participants aged 19–80 years. Among them, 201 participants were excluded because they worked in shifts, and 631 were excluded owing to incomplete data. Finally, data on 5,550 participants were used in the present study (Fig. 1). Demographic, socioeconomic, and lifestyle characteristics of the participants are summarised in Table 1.
Figure 1.
Flow chart depicting the participation of subjects in the seventh Korea National Health and Nutrition Examination Survey (KNHANES VII-1), 2016. Numbers in parentheses indicate representative numbers of the Korean population. *Shift workers were defined as those with regular day-night shift work, 24-h shift work, split-day work (≥ 2 work shifts in a day), or irregular shift work.
Table 1.
Demographic, socioeconomic, lifestyle, sleep, and chronotype characteristics of participants.
| All (n = 5,550) | Women (n = 3,222) | Men (n = 2,328) | P-value (women vs. men) | |
|---|---|---|---|---|
| Age, years (mean ± SE) | 46.7 ± 0.4 | 47.7 ± 0.5 | 45.7 ± 0.0 | < 0.001 |
| Chronotype, % (95% CI) | ||||
| Early type | 18.2 (16.7–19.9) | 17.9 (16.1–19.9) | 18.5 (16.8–20.4) | 0.534 |
| Intermediate type | 66.4 (64.5–68.2) | 68.1 (66.0–70.1) | 64.5 (61.8–67.2) | 0.018 |
| Late type | 15.4 (14.0–16.9) | 14.0 (12.3–15.9) | 16.9 (15.0–19.0) | 0.017 |
| Sleep duration, hours (mean ± SE) | ||||
| Workday | 7.0 ± 0.2 | 7.0 ± 0.3 | 7.0 ± 0.3 | 0.532 |
| Free day | 7.7 ± 0.3 | 7.7 ± 0.4 | 7.6 ± 0.3 | 0.090 |
| Average | 7.2 ± 0.2 | 7.2 ± 0.3 | 7.2 ± 0.3 | 0.302 |
| MSFsc (mean ± SE) | 3.5 ± 0.0 AM | 3.5 ± 0.0 AM | 3.6 ± 0.0 AM | 0.160 |
| Alcohol (≥ 2/week), % (95% CI) | 23.8 (22.5–25.1) | 12.2 (11.0–13.6) | 36.1 (33.9–38.3) | < 0.001 |
| Smoking (current), % (95% CI) | 21.8 (20.2–23.5) | 6.1 (5.1–7.4) | 38.5 (36.0–41.1) | < 0.001 |
| Job (yes), % (95% CI) | 61.8 (59.8–63.7) | 50.2 (47.8–52.7) | 74.1 (71.4–76.5) | < 0.001 |
| Education (≥ 12 yr), % (95% CI) | 75.7 (73.6–77.6) | 70.1 (67.6–72.5) | 81.6 (79.5–83.6) | < 0.001 |
| BMI, kg/m2 (mean ± SE) | 24.0 ± 0.7 | 23.4 ± 1.0 | 24.6 ± 0.9 | < 0.001 |
| Depression (PHQ score ≥ 10), % (95% CI) | 5.7 (4.9–6.6) | 7.2 (6.1–8.5) | 4.2 (3.2–5.4) | < 0.001 |
| PHQ-9 score (mean ± SE) | 2.7 ± 0.8 | 3.2 ± 0.1 | 2.1 ± 0.1 | < 0.001 |
BMI: body mass index, CI: confidence interval, MSFsc: mid-sleep time on free days corrected by sleep debt accumulated over the workdays, PHQ-9: Patients Health Questionnaire-9, SE: standard error.
The mean mid-sleep duration on workdays, mid-sleep duration on free days (MSF), and mid-sleep duration on free days corrected by sleep debt accumulated on workdays (MSFsc) were 3.2 ± 0.0, 3.8 ± 0.0, and 3.5 ± 0.0 h, respectively. The distribution of MSFsc showed an acceptable normality (Supplementary Figure S1, skewness: 0.822, kurtosis: 3.031)15.
Depression was more prevalent in women than in men
Among the 5,550 participants, 346 had Patient Health Questionnaire (PHQ)-9 scores of ≥ 10, and the prevalence of depression was calculated as 5.7% (95% confidence interval [CI] = 4.9–6.6%). The prevalence of depression in women was higher than that in men (4.2% [95% CI = 3.2–5.4%] vs. 7.2% [95% CI = 6.1–8.5%], p < 0.001). The mean PHQ-9 score in all participating women was higher than that in all participating men (3.2 ± 0.1 vs. 2.1 ± 0.1, p < 0.001). Among participants with depression, the PHQ-9 scores were not different between women and men (14.4 ± 0.3 vs. 13.9 ± 0.5 p = 0.224).
Late chronotype was more prevalent in men than in women
The prevalence of late chronotype was higher in men than women (16.9% vs. 14.0%, p = 0.017) whereas the prevalence of intermediate chronotype was lower in men than in women (64.5% vs. 68.1%, p = 0.018). The prevalence of early chronotype did not significantly differ between men and women (18.5% vs. 17.9%, p = 0.534).
Prevalence of depression was elevated in early and late chronotypes
The prevalence of depression was significantly different among those with early, intermediate, and late chronotypes (7.4% [95% CI = 5.9–9.3%] vs. 4.5% [95% CI = 3.7–5.4%] vs. 9.3% [95% CI = 7.0–12.1%], respectively, p < 0.001). Post hoc analyses revealed that there was a significant difference in the prevalence of depression between the early and intermediate chronotype groups (p = 0.001) and between the late and intermediate chronotype groups (p < 0.001). The prevalence of depression between the early and late chronotype groups was not significantly different (p = 0.212) (Fig. 2).
Figure 2.

Prevalence of depression in those with early, intermediate, and late chronotypes. Mean values are presented in boxes, and 95% confidence intervals are shown below and above the bars.
Risk of depression was elevated in late chronotype but not in early chronotype among all participants
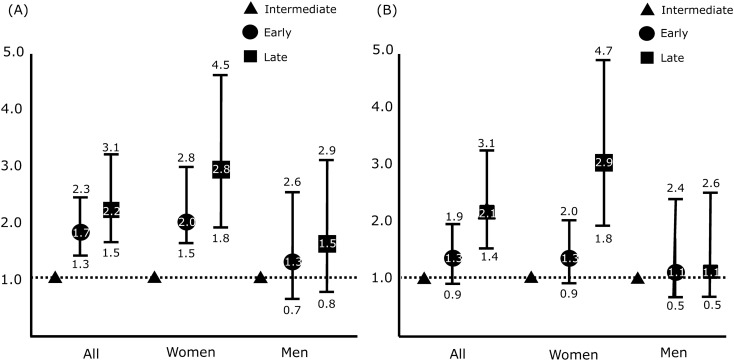
The risk of depression was significantly higher in participants with early (odds ratio [OR] = 1.7, 95% CI = 1.3–2.3) and late chronotypes (OR = 2.2, 95% CI = 1.5–3.1) than in those with intermediate chronotype, as shown by univariate analysis (Fig. 3A). After adjusting for covariates including age, body mass index (BMI), current job status, years of education, smoking status, alcohol intake, and average sleep duration, the association between depression risk and early chronotype was not significant (OR = 1.3, 95% CI = 0.9–2.0). In contrast, the association of the risk of depression with late chronotype remained significant after adjusting for covariates (OR = 2.9, 95% CI = 1.8–4.7) (Fig. 3B).
Figure 3.
Odds ratios with 95% CI for depression in those with early (round), late (square) and intermediate (triangle) chronotypes. (A) Univariate ORs and (B) Multivariate ORs adjusted for age, sex, alcohol intake status, smoking status, job, education level, BMI, and average sleep duration. BMI: body mass index, CI: confidence interval, OR: odds ratio.
Risk of depression was elevated in late chronotype but not in early chronotype in women
Among women, the risk of depression was higher in the early (OR = 2.0, 95% CI = 1.5–2.8) and late (OR = 2.8, 95% CI = 1.8–4.5) chronotype groups than in the intermediate chronotype group as shown by univariate analysis (Fig. 3A). After adjusting for covariates, the association of the risk of depression with late chronotype maintained its significance (OR = 2.9, 95% CI = 1.8–4.7). Nevertheless, its association with early chronotype lost significance (OR = 1.3, 95% CI = 0.9–1.9) (Fig. 3B).
Risk of depression was not associated with chronotype in men
Among men, the risk of depression was higher in the early (OR = 1.3, 95% CI = 0.7–2.6) and late (OR = 1.5, 95% CI = 0.8–2.9) chronotype groups than in the intermediate chronotype group, but this result was not significant as shown by univariate analyses (Fig. 3A). After adjusting for covariates, the non-significant association between the risk of depression and early (OR = 1.1, 95% CI = 0.5–2.4) and late (OR = 1.1, 95% CI = 0.5–2.6) chronotypes (Fig. 3 B) persisted.
Risk of depression according to chronotype in different age groups
We further assessed the risk of depression in participants with early and late chronotypes compared to that in those with intermediate chronotype by stratifying age into 20-year increments using multivariate logistic regression analyses adjusting for covariates (Table 2). The risk of depression was significantly elevated in all age groups among women with late chronotype. However, the risk of depression was not significantly different among age groups in women with early chronotype. In men with late chronotype, the risk of depression was not significantly different across age groups. In men with early chronotype, the risk of depression was only significantly elevated in the 40–60-year age group; no other age group showed significant differences in depression risk.
Table 2.
Risk of depression in participants with early and late chronotypes compared to that in those with intermediate chronotype according to age groups using multivariate analysis.
| Age groups, no. of corresponding participants | Intermediate chronotype, no. of depression cases/no. of corresponding participants | Early chronotype, OR and 95% CI, no. of depression cases/no. of corresponding participants | Late chronotype, OR and 95% CI, no. of depression cases/no. of corresponding participants |
|---|---|---|---|
| All participants | |||
| Aged 19–40 years, 1,642 | REF, 53/3579 | 1.8 (0.7–5.4), 5/52 | 1.9 (1.3–3.2), 42/472 |
| Aged 41–59 years, 2,039 | REF, 55/1613 | 2.2 (1.2–4.2), 22/276 | 3.1 (1.5–6.2), 12/150 |
| Aged 60–80 years, 1,869 | REF, 77/848 | 0.7 (0.5–1.1), 76/999 | 2.3 (0.8–6.7), 4/22 |
| Women | |||
| Aged 19–40 years, 953 | REF, 36/667 | 3.1 (0.9–10.5), 4/26 | 2.1 (1.1–4.2), 30/260 |
| Aged 41–59 years, 1,196 | REF, 36/967 | 1.9 (0.9–4.0), 14/146 | 4.6 (2.0–10.7), 8/83 |
| Aged 60–80 years, 1,073 | REF, 55/502 | 0.8 (0.5–1.3), 57/558 | 3.6 (1.1–11.2), 4/13 |
| Men | |||
| Aged 19–40 years, 689 | REF, 17/451 | 0.4 (0.1–4.1), 1/26 | 1.2 (0.5–3.2), 12/212 |
| Aged 41–59 years, 843 | REF, 19/646 | 3.0 (1.2–7.8), 8/130 | 1.5 (0.3–7.0), 4/67 |
| Aged 60–80 years, 796 | REF, 22/346 | 2.6 (0.9–7.7), 19/441 | –, 0/9* |
*No men aged 60–80 years with late chronotype had depression.
OR: odds ratio, CI: confidence internal, No.: number, REF: reference.
Discussion
The main findings of the present study were as follows: (1) the prevalence of depression was higher in participants with early and late chronotypes than in those with intermediate chronotype; (2) depression was more prevalent in women than in men, and late chronotype was more prevalent in men than in women, and (3) after adjusting for covariates, the risk of depression was significantly associated with late chronotype in women but not in men. The association of risk of depression with early chronotype did not significantly differ in both women and men.
Biological marker studies have shown that women had a phase advance in melatonin peak time and core body temperature relative to men16. A meta-analysis study including 164 studies that used questionnaires such as Morningness-Eveningness Questionnaire and Composite Scale of Morningness demonstrated that men were more evening-oriented than women13. An American nationwide study evaluating chronotype, based on sleep onset time, wakeup time, and the Munich Chronotype Questionnaire (MCTQ), showed that men were more prone to have late chronotype than women17. Our study also showed similar results. This proneness to late chronotype among men observed in the present study suggests that our study properly evaluated chronotypes in women and men.
What are the potential explanations for the sex difference in the association between depression and chronotype? First, there may be a sex difference in the role of melatonin. Melatonin has a sleep-promoting effect and is a key regulatory hormone for circadian regulation, including chronotype18,19. Circadian abnormalities are common findings in depression, and melatonin dysregulation; including lower nocturnal melatonin levels; phase advance of melatonin onset or offset; and a delay in the peak, onset, or offset of melatonin secretion; has been reported in depression20–23. Furthermore, melatonin levels may also be related to alterations in serotonin and norepinephrine levels, which are important neurotransmitters in depression24,25. Women showed earlier onset and a higher amplitude of melatonin secretion than men16. Nevertheless, sex differences in melatonin dysregulation in individuals with depression and late chronotype has not been reported.
Another potential explanation is the role of sex hormones. Sex hormones can independently affect both chronotype and depression. Women with morning chronotype show earlier increases in oestradiol levels during their menstrual cycles than women with intermediate chronotype26. Higher testosterone levels are related to higher eveningness in adolescents27. Oestrogen has antianxiety and antidepressant-like effects28. The present study found that women with late chronotype aged 60–80 years had an increased risk of depression due to reduced levels of sex hormones. This finding indicates that sex hormones are less likely to play a major role in the sex difference. Nevertheless, sex hormones may affect the sex difference through developmental hormone exposure (organisational effects) in addition to direct effects of hormones (activational effects).
Differences in behavioural and psychological factors could be additional potential explanations. A Dutch study including 859 adults revealed that the relationships between late chronotype and depressive symptoms were mediated via sleep quality, alcohol intake, and cognitive emotion regulation strategies29. Another Dutch cohort study found that late chronotype (evening type) was associated with higher cognitive reactivity (depressogenic cognition)30. This finding suggested that depressogenic cognitions mediated the association between chronotype and depression. Women have poorer sleep quality and consume less alcohol than men31,32. They also exhibit more depressogenic cognitive responses to life events than men33. Therefore, behavioural and psychological factors may contribute to the sex difference in the association between chronotype and depression.
Since populations differ with respect to the mean and width of chronotype distribution, how ‘early’ or ‘late’ someone can be considered changes with the reference population. Therefore, there is no definitive criteria or cut-off value of MSFsc for the classification of chronotype, and various cut-off values have been proposed for establishing chronotype with the MCTQ, using 2.5%, 15%, and 20% of extreme scores of the sample30,34,35. In the present study, we classified early and late chronotypes as mean < MSFsc + SD and > MSFsc − SD, which corresponded approximately to the upper and lower 16% of the distribution, respectively.
The prevalence of depression in participants with early chronotype was higher than that in participants with intermediate chronotype. Nevertheless, the risk of depression in participants with early chronotype did not significantly differ from that in those with intermediate chronotype. This discrepancy might be owing to the effects of covariates. We used age, sex, BMI, average sleep duration, job status, alcohol drinking status, smoking status, and education level as covariates. Age, sex, average sleep duration, BMI, alcohol intake status, and smoking status are significantly associated with both chronotype and depression17,36–38. The associations of covariates with early chronotype and depression might have mitigated the significance of the association between early chronotype and depression and resulted in a non-significant association between the two conditions in the multivariate analyses. Studies on the association between early chronotype and depression have reported conflicting results30,39–41. Application of different covariates might be a possible reason for these conflicting outcomes.
Some limitations of the present study should be mentioned. First, we did not use the exact version of the ultrashort MCTQ (μMCTQ) in calculating MSFsc. The µMCTQ is composed of six questions regarding shift working, number of workdays per week, and sleep onset time and wakeup time on workdays and free days42. We calculated MSFsc based on bedtime rather than sleep onset time, and we considered the number of workdays per week as five instead of evaluating each participant’s number of workdays per week. The difference between bedtime and sleep onset time is defined as sleep latency. Although the mean sleep latency in a polysomnographic study among individuals from the general population was 18.6 ± 18.3 min, and the difference between the MSFsc based on bedtime and the actual MSFsc was not expected to be large, there was a difference between the calculated MSFsc and the actual MSFsc determined based on sleep latency43. Although the μMCTQ did not include a question for alarm clock use, the original version of the MCTQ did. The use of an alarm clock on free days could affect the MSFsc44. Nevertheless, the μMCTQ showed a good correlation with dim-light melatonin onset, a biological marker of circadian rhythm42. In the present study, we could not investigate alarm clock use owing to the limit on the number of items. Second, we did not evaluate the light exposure of participants. Light exposure is a key factor for determining chronotype along with age and sex34. The timing of light exposure plays a differential role in the circadian phase. Early light exposure advances the cycle while late light exposure delays the circadian phase45. Time spent outside, light dose, day length, and daily radiance were significant factors related to light exposure that showed a close association with chronotype46. However, items of light exposure were not included in KNHANES 2016, and we could not include light exposure in the present analyses.
Nevertheless, the present study has several strengths. First, the present study used a dataset obtained from a large sample that represented the whole population of Korea. This enabled us to properly evaluate sex difference in the association between chronotype and depression after adjusting for potential covariates. Second, we included potential covariates in the analyses including job status, years of education, smoking status, and alcohol intake, which were reported to be significantly related with chronotype and/or depression. Our analyses will provide more accurate information on the sex difference in the association between chronotype and depression.
In conclusion, we evaluated the sex difference in the association between chronotype and depression using a general population-based sample representing the whole population of Korea. We found that late chronotype was associated with an increased risk of depression in women but not in men, compared to intermediate chronotype. The risk of depression was not significantly associated with early type in women or men. These findings suggest that the influence of chronotype differs according to sex. Future research is needed to elucidate the biological mechanisms underlying the mutual effects of chronotype and depression by sex.
Methods
Data and participants
We used data from the KNHANES VII, which was conducted in 2016. The KNHANES is a nationwide, cross-sectional survey, representative of the entire population of Korea. The Korea Centers for Disease Control and Prevention have been conducting this survey annually for assessing the health and nutritional status of Koreans since 1998. The KNHANES adopted a stratified multistage probability sampling design to obtain a nationally representative sample of non-institutionalised civilian Koreans for data collection each year. The KNHANES collects data on a wide range of characteristics including those on sociodemographics, health, and nutrition. A detailed description of the KNHANES has been published elsewhere47,48. We used the data on adult participants aged 19–80 years from the KNHANES VII.
Sleep duration
Participants’ average sleep duration was evaluated based on the response to the following two questions: ‘On average, at what time do you go to sleep and at what time do you wake up on workdays’? and ‘On average, at what time do you go to sleep and at what time do you wake up on free days’? Average sleep duration was calculated using the following formula: [(workdays sleep duration × 5) + (free days sleep duration × 2)]/7.
Chronotype
We classified chronotype based on the μMCTQ with some modification42. Data on the bedtime and wakeup time on workdays and free days were used for assessing the chronotype. Chronotype was determined based on the MSFsc. MSFsc was calculated as follows: MSFsc = MSF − 0.5 × [sleep duration on free days – (5 × sleep duration on workdays + 2 × sleep duration on free days)/7]49. The chronotype was classified into three groups based on MSFsc: early chronotype, < mean MSFsc − 1 standard deviation (SD); intermediate chronotype, between mean MSFsc − 1 SD and MSFsc + 1 SD; and late chronotype, > mean MSFsc + 1 SD. We assumed five workdays and two free days per week for all participants. The use of an alarm clock was not considered.
Depression
The PHQ-9 was used to assess the severity of depression50. A PHQ-9 score of ≥ 10 indicated depression. The Korean version of the PHQ-9 has been previously validated, and it showed 81.1% sensitivity and 89.9% specificity51.
Covariates
The role of covariates was investigated to further elucidate the relationship between chronotype and depression across sex-specific groups as well as the total study population. Socioeconomic and lifestyle characteristics including BMI, current job status, years of education, smoking status, and alcohol intake have been strongly correlated with chronotype and/or depression52–57.
The participants’ socioeconomic characteristics and lifestyle were assessed using health interviews and examinations. Alcohol intake was classified as follows: < 2 times/week vs. ≥ 2 times/week. Smoking status was classified as follows: current vs. never or former. Current job status was also documented (employed or non-employed). Furthermore, highest achieved education level was categorised into two groups: ≥ 12 years or < 12 years. We included sex, age (continuous), job status, alcohol intake status, smoking status, average sleep duration (continuous), and BMI (continuous) as covariates in multivariate logistic regression analyses.
Ethical approval
KNHANES VII, 2016, was a national study conducted for direct public benefit. In accordance with Article 2, Subparagraph 1 of the Bioethics and Safety Act, and Article 2, Paragraph 2, Subparagraph 1 of the Enforcement Rule of the same act, the present study did not require review by a research ethics board58. This study was also exempt for review by the Institutional Review Board of Severance Hospital, Yonsei University (No. 2020-2443-001). Written informed consent was obtained from all participants. This study was conducted in adherence with the KNHANES usage guidelines59 and the Declaration of Helsinki60.
Statistical analyses
We analysed the data of KNHANES VII, using sampling weights specified in the KNHANES, which account for the complex survey design, non-response, and post-stratification, to acquire nationally representative estimates. The data in the present study are presented as weighted means or weighted proportions for continuous or categorical variables, respectively. Categorical variables are represented as numbers and percentages, and continuous variables are represented as means ± standard errors.
OR was defined as the ratio of the odds of having depression in a selected group to the odds of having depression in an unselected group. These values were compared using the chi-squared test between the three independent chronotype groups. Multivariate logistic regression analysis was used to measure the adjusted ORs and 95% CIs for depression according to sex after controlling for covariates.
The complex sample analysis module of the Statistical Package for Social Sciences version 23.0 (SPSS 23.0; IBM, Armonk, NY, USA) was used for all statistical analyses. Statistical significance was set at p < 0.05 two-tailed.
Supplementary information
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2019R1F1A1053841).
Author contributions
K.M.K.: Conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing. S.M.H and K.H.: Data analysis and interpretation. W.J. K.: Collection and assembly of data. M.K.C.: Conception and design, data analysis and interpretation, and revision of the manuscript for intellectual content.
Data availability
The raw dataset used in this study is publicly available at https://knhanes.cdc.go.kr/knhanes/main.do.
Competing interests
M.K.C. was a site investigator for a multi-centre trial sponsored by Otsuka Korea, Novartis International AG, and Eli Lilly and Company. He worked as an advisory member for Teva and has received lecture honoraria from Allergan Korea, Handok-Teva, and Yuyu Pharmaceutical Company in the past 24 months. He received grants from Yonsei University College of Medicine (2018-32-0037) and National Research Foundation of Korea (2019R1F1A1053841). The other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75724-z.
References
- 1.World Health Organization Depression and Other Common Mental Disorders, Global Health Estimates https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf (2017).
- 2.Lepine JP, Briley M. The increasing burden of depression. Neuropsychiatr. Dis. Treat. 2011;7:3–7. doi: 10.2147/NDT.S19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet388, 1545–1602 (2016). [DOI] [PMC free article] [PubMed]
- 4.Adan A, et al. Circadian typology: A comprehensive review. Chronobiol. Int. 2012;29:1153–1175. doi: 10.3109/07420528.2012.719971. [DOI] [PubMed] [Google Scholar]
- 5.Muller MJ, Haag A. The concept of chronotypes and its clinical importance for depressive disorders. Int. J. Psychiatry Med. 2018;53:224–240. doi: 10.1177/0091217417749787. [DOI] [PubMed] [Google Scholar]
- 6.Au J, Reece J. The relationship between chronotype and depressive symptoms: A meta-analysis. J. Affect. Disord. 2017;218:93–104. doi: 10.1016/j.jad.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol. Bull. 2017;143:783–822. doi: 10.1037/bul0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus SM, et al. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the sequenced treatment alternatives to relieve depression study. Compr. Psychiatry. 2008;49:238–246. doi: 10.1016/j.comppsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuch JJ, Roest AM, Nolen WA, Penninx BW, de Jonge P. Gender differences in major depressive disorder: Results from the Netherlands study of depression and anxiety. J. Affect. Disord. 2014;156:156–163. doi: 10.1016/j.jad.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Young EA, et al. Sex differences in response to citalopram: a STAR*D report. J. Psychiatr. Res. 2009;43:503–511. doi: 10.1016/j.jpsychires.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong L, et al. Sex differences of gray matter morphology in cortico-limbic-striatal neural system in major depressive disorder. J. Psychiatr. Res. 2013;47:733–739. doi: 10.1016/j.jpsychires.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornstein SG, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am. J. Psychiatry. 2000;157:1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 13.Randler C, Engelke J. Gender differences in chronotype diminish with age: A meta-analysis based on morningness/chronotype questionnaires. Chronobiol. Int. 2019;36:888–905. doi: 10.1080/07420528.2019.1585867. [DOI] [PubMed] [Google Scholar]
- 14.Morita Y, Sasai-Sakuma T, Asaoka S, Inoue Y. The impact of a delayed sleep-wake schedule on depression is greater in women—A web-based cross-sectional study in Japanese young adults. Chronobiol. Int. 2015;32:952–958. doi: 10.3109/07420528.2015.1055756. [DOI] [PubMed] [Google Scholar]
- 15.Kim HY. Statistical notes for clinical researchers: Assessing normal distribution (2) using skewness and kurtosis. Restor. Dent. Endod. 2013;38:52–54. doi: 10.5395/rde.2013.38.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boivin DB, Shechter A, Boudreau P, Begum EA, Ng Ying-Kin NM. Diurnal and circadian variation of sleep and alertness in men vs naturally cycling women. Proc. Natl. Acad. Sci. USA. 2016;113:10980–10985. doi: 10.1073/pnas.1524484113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the US—Influence of age and sex. PLoS ONE. 2017;12:e0178782. doi: 10.1371/journal.pone.0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantermann T, Sung H, Burgess HJ. Comparing the Morningness-Eveningness Questionnaire and Munich ChronoType Questionnaire to the dim light melatonin onset. J. Biol. Rhythms. 2015;30:449–453. doi: 10.1177/0748730415597520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santhi N, et al. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc. Natl. Acad. Sci. USA. 2016;113:E2730–2739. doi: 10.1073/pnas.1521637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claustrat B, Chazot G, Brun J, Jordan D, Sassolas G. A chronobiological study of melatonin and cortisol secretion in depressed subjects: Plasma melatonin, a biochemical marker in major depression. Biol. Psychiatry. 1984;19:1215–1228. [PubMed] [Google Scholar]
- 21.Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168:259–261. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Mendlewicz J, et al. Abnormal 24 hour pattern of melatonin secretion in depression. Lancet. 1979;2:1362. doi: 10.1016/s0140-6736(79)92838-1. [DOI] [PubMed] [Google Scholar]
- 23.Rubin RT, Heist EK, McGeoy SS, Hanada K, Lesser IM. Neuroendocrine aspects of primary endogenous depression. XI. Serum melatonin measures in patients and matched control subjects. Arch. Gen. Psychiatry. 1992;49:558–567. doi: 10.1001/archpsyc.1992.01820070052008. [DOI] [PubMed] [Google Scholar]
- 24.Chuluyan HE, Rosenstein RE, Chang SM, Gálvez MM, Cardinali DP. Presynaptic effects of melatonin on norepinephrine release and uptake in rat pineal gland. J. Pineal. Res. 1991;10:165–173. doi: 10.1111/j.1600-079x.1991.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 25.Danilenko KV, Putilov AA, Russkikh GS, Duffy LK, Ebbesson SO. Diurnal and seasonal variations of melatonin and serotonin in women with seasonal affective disorder. Arctic Med. Res. 1994;53:137–145. [PubMed] [Google Scholar]
- 26.Michels KA, et al. The influences of sleep duration, chronotype, and nightwork on the ovarian cycle. Chronobiol. Int. 2020;37:260–271. doi: 10.1080/07420528.2019.1694938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagenauer MH, Lee TM. The neuroendocrine control of the circadian system: Adolescent chronotype. Front Neuroendocrinol. 2012;33:211–229. doi: 10.1016/j.yfrne.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van den Berg JF, Kivelä L, Antypa N. Chronotype and depressive symptoms in students: An investigation of possible mechanisms. Chronobiol. Int. 2018;35:1248–1261. doi: 10.1080/07420528.2018.1470531. [DOI] [PubMed] [Google Scholar]
- 30.Antypa N, et al. Associations between chronotypes and psychological vulnerability factors of depression. Chronobiol. Int. 2017;34:1125–1135. doi: 10.1080/07420528.2017.1345932. [DOI] [PubMed] [Google Scholar]
- 31.Wilsnack RW, Wilsnack SC, Kristjanson AF, Vogeltanz-Holm ND, Gmel G. Gender and alcohol consumption: Patterns from the multinational GENACIS project. Addiction. 2009;104:1487–1500. doi: 10.1111/j.1360-0443.2009.02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fatima Y, Doi SA, Najman JM, Mamun AA. Exploring gender difference in sleep quality of young adults: Findings from a large population study. Clin. Med. Res. 2016;14:138–144. doi: 10.3121/cmr.2016.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahles JJ, Harding KA, Mezulis AH, Hudson MR. Sex differences in domain-specific depressogenic cognitive responses to negative and positive life events. Personal. Individ. Differ. 2015;76:198–203. [Google Scholar]
- 34.Roenneberg T, et al. Human activity and rest in situ. Methods Enzymol. 2015;552:257–283. doi: 10.1016/bs.mie.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 35.KÅhnle, v. T. Quantitative analysis of human chronotypes https://edoc.ub.uni-muenchen.de/5168/1/Kuehnle_Tim.pdf (2006).
- 36.de Punder K, Heim C, Entringer S. Association between chronotype and body mass index: The role of C-reactive protein and the cortisol response to stress. Psychoneuroendocrinology. 2019;109:104388. doi: 10.1016/j.psyneuen.2019.104388. [DOI] [PubMed] [Google Scholar]
- 37.Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction. 1994;89:455–462. doi: 10.1111/j.1360-0443.1994.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 38.Wittmann M, Paulus M, Roenneberg T. Decreased psychological well-being in late 'chronotypes' is mediated by smoking and alcohol consumption. Subst. Use Misuse. 2010;45:15–30. doi: 10.3109/10826080903498952. [DOI] [PubMed] [Google Scholar]
- 39.Kim SJ, et al. Age as a moderator of the association between depressive symptoms and morningness-eveningness. J. Psychosom. Res. 2010;68:159–164. doi: 10.1016/j.jpsychores.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Merikanto I, et al. Circadian preference links to depression in general adult population. J. Affect. Disord. 2015;188:143–148. doi: 10.1016/j.jad.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 41.Furusawa M, et al. Relationship between morningness-eveningness typology and cumulative fatigue or depression among Japanese male workers. Ind. Health. 2015;53:361–367. doi: 10.2486/indhealth.2013-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghotbi N, et al. The µMCTQ: An ultra-short version of the Munich ChronoType Questionnaire. J. Biol. Rhythms. 2020;35:98–110. doi: 10.1177/0748730419886986. [DOI] [PubMed] [Google Scholar]
- 43.Zinkhan M, et al. Agreement of different methods for assessing sleep characteristics: A comparison of two actigraphs, wrist and hip placement, and self-report with polysomnography. Sleep Med. 2014;15:1107–1114. doi: 10.1016/j.sleep.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 44.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 45.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J. Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porcheret K, et al. Chronotype and environmental light exposure in a student population. Chronobiol. Int. 2018;35:1365–1374. doi: 10.1080/07420528.2018.1482556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SM, et al. Prevalence of diabetes and impaired fasting glucose in Korea: Korean National Health and Nutrition Survey 2001. Diabetes Care. 2006;29:226–231. doi: 10.2337/diacare.29.02.06.dc05-0481. [DOI] [PubMed] [Google Scholar]
- 48.Kim Y, Lee BK. Iron deficiency increases blood manganese level in the Korean general population according to KNHANES 2008. Neurotoxicology. 2011;32:247–254. doi: 10.1016/j.neuro.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Roenneberg T, et al. Epidemiology of the human circadian clock. Sleep Med. Rev. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Kroenke, K. & L Spitzer, R. The PHQ-9: A New Depression Diagnostic and Severity Measure 509-5212002).
- 51.Choi HS, et al. Standardization of the Korean Version of Patient Health Questionnaire-9 as a screening instrument for major depressive disorder. J. Korean Acad. Fam. Med. 2007;28:114–119. [Google Scholar]
- 52.Boden JM, Fergusson DM. Alcohol and depression. Addiction. 2011;106:906–914. doi: 10.1111/j.1360-0443.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- 53.Fleig D, Randler C. Association between chronotype and diet in adolescents based on food logs. Eat. Behav. 2009;10:115–118. doi: 10.1016/j.eatbeh.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. J. Addict. Dis. 1998;17:35–46. doi: 10.1300/J069v17n01_04. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz M, Hu Y, Martikainen P, Bobak M. Life course socioeconomic position and incidence of mid-late life depression in China and England: A comparative analysis of CHARLS and ELSA. J. Epidemiol. Commun. Health. 2019;73:817–824. doi: 10.1136/jech-2019-212216. [DOI] [PubMed] [Google Scholar]
- 56.Luppino FS, et al. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 57.Ross CE, Mirowsky J. Sex differences in the effect of education on depression: Resource multiplication or resource substitution? Soc. Sci. Med. 2006;63:1400–1413. doi: 10.1016/j.socscimed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 58.Lee J, Kim HR. The association between long working hours and high-sensitivity C-reactive protein in older aged individuals: the Korea National Health and Nutrition Examination Survey (KNHANES) 2015. J. Occup. Environ. Med. 2018;60:775–780. doi: 10.1097/JOM.0000000000001359. [DOI] [PubMed] [Google Scholar]
- 59.Korea Centers for Disease Control and Prevention Korea National Health and Nutrition Examination Survey https://knhanes.cdc.go.kr/knhanes/eng/index.do.
- 60.World Medical Association WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw dataset used in this study is publicly available at https://knhanes.cdc.go.kr/knhanes/main.do.