Key Points
Question
What is the prevalence of high tumor mutational burden (TMB-H), defined as 10 or more mutations per megabase, and what is its association with overall survival among patients with 10 rare solid tumor types not treated with immunotherapy?
Findings
In this cohort study among 2589 patients, nearly 13% had TMB-H tumors. Overall survival among patients with TMB-H tumors did not differ from that observed among patients with non–TMB-H tumors when receiving nonimmunotherapy treatments.
Meaning
These findings suggest that TMB-H was not associated with overall survival in patients with these cancer types in the absence of immunotherapy.
This cohort study examines tumor mutational burden in patients with uncommon cancers not receiving immunotherapy as a biomarker associated with overall survival.
Abstract
Importance
Tumor mutational burden (TMB) is a potential biomarker associated with response to immune checkpoint inhibitor therapies. The prognostic value associated with TMB in the absence of immunotherapy is uncertain.
Objective
To assess the prevalence of high TMB (TMB-H) and its association with overall survival (OS) among patients not treated with immunotherapy with the same 10 tumor types from the KEYNOTE-158 study.
Design, Setting, and Participants
This retrospective cohort study evaluated the prognostic value of TMB-H, assessed by Foundation Medicine (FMI) and defined as at least 10 mutations/megabase (mut/Mb) in the absence of immunotherapy. Data were sourced from the deidentified Flatiron Health–FMI clinicogenomic database collected up to July 31, 2018. Eligible patients were aged 18 years or older with any of the following solid cancer types: anal, biliary, endometrial, cervical, vulvar, small cell lung, thyroid, salivary gland, mesothelioma, or neuroendocrine tumor. Patients with microsatellite instability–high tumors were excluded from primary analysis. For OS analysis, patients were excluded if immunotherapy started on the FMI report date or earlier or if patients died before January 1, 2012, and patients were censored if immunotherapy was started later than the FMI report date. Data were analyzed from November 2018 to February 2019.
Main Outcomes and Measures
Overall survival was analyzed using the Kaplan-Meier method and Cox proportional hazards model, adjusting for age, sex, cancer types, practice type, and albumin level.
Results
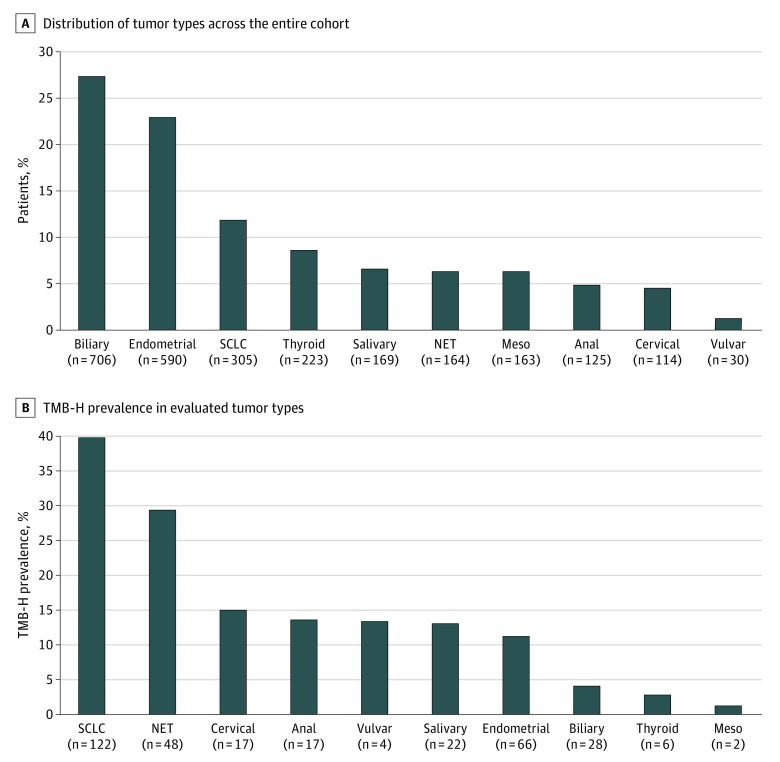
Of 2589 eligible patients, 1671 (64.5%) were women, and the mean (SD) age was 63.7 (11.7) years. Median (interquartile range) TMB was 2.6 (1.7-6.1) mut/Mb, and 332 patients (12.8%) had TMB-H (≥10 mut/Mb). Prevalence of TMB-H was highest among patients with small cell lung cancer (40.0%; 95% CI, 34.7%-45.6%) and neuroendocrine tumor (29.3%; 95% CI, 22.8%-36.6%) and lowest was among patients with mesothelioma (1.2%; 95% CI, 0.3%-4.4%) and thyroid cancer (2.7%; 95% CI, 1.2%-5.7%). Adjusted hazard ratio for OS of patients not treated with immunotherapy with TMB-H vs those without TMB-H was 0.94 (95% CI, 0.77-1.13). Comparable results were observed when including patients with high microsatellite instability tumors and calculating OS from first observed antineoplastic treatment date.
Conclusions and Relevance
These findings suggest that prevalence of TMB-H varies widely depending on tumor type and TMB-H does not appear to be a factor associated with OS among patients across these cancer types treated in the absence of immunotherapy.
Introduction
Tumor mutational burden (TMB), defined as the total number of somatic mutations per coding area of a tumor genome, is an emerging clinical biomarker associated with response to immune checkpoint inhibitor (ICI) therapy.1,2 TMB has been shown to vary markedly among tumor types as well as among patients within tumor types.3,4 Higher TMB is commonly observed in cancers associated with mutagens, such as ultraviolet light exposure in melanoma and smoking in non–small-cell lung cancer (NSCLC),4 with prevalence of high TMB (TMB-H; defined here as ≥10 mutations/megabase [mut/Mb]) in approximately 52% of patients with melanoma and 38% to 42% of patients with NSCLC.3 By comparison, less than 5% prevalence of TMB-H has been reported in other tumor types, including mesothelioma, sarcomas, and prostate cancers.3
High TMB has been associated with increased expression of tumor-specific neoantigens,5 a subset of which can be recognized by the immune system. Higher numbers of somatic mutations in tumor DNA have been hypothesized to increase the likelihood of the immune system recognizing and eliminating tumor cells during treatment with ICI therapy.3 Evidence supporting this hypothesis from randomized clinical trials has yet to emerge, to our knowledge. However, in a 2018 meta-analysis,6 significant associations between TMB and objective response rate (ORR) were reported across multiple tumor types for patients receiving anti–programmed cell death 1 or anti–programmed cell death ligand 1 therapy. Additionally, higher TMB (albeit assessed with differing analytic methods and defined with differing thresholds) has been associated with response to ICI therapy in several tumor types, including NSCLC,7,8,9,10,11 small cell lung cancer (SCLC),12 melanoma,13 and colorectal cancer.14 Evidence has been equivocal as to whether higher TMB is associated with outcomes independent of therapy. In the Lung Adjuvant Cisplatin Evaluation-Bio-II study, nonsynonymous TMB of more than 8 mut/Mb was associated with favorable outcomes, such as overall survival (OS), progression-free survival, and lung cancer–specific survival, in patients with NSCLC,15 whereas other studies have indicated significantly longer disease-free survival in patients with lung adenocarcinomas with low TMB16 or no association between TMB and OS in patients with SCLC.17 Little is known about the association of TMB-H with outcomes among patients with less common types of cancer not treated with immunotherapy.
The KEYNOTE-158 study18 is an ongoing phase 2 multicohort study prospectively evaluating multiple biomarkers, including TMB, for associations with response to monotherapy with the anti–programmed cell death 1 monoclonal antibody pembrolizumab in 10 less common types of advanced cancer. Preliminary findings from the KEYNOTE-158 study indicated that patients with TMB-H, defined as 10 or more mut/Mb (assessed in formalin-fixed and paraffin-embedded [FFPE] tissue using the FoundationOne [F1] clinical trial assay based on the F1 Companion Diagnostic [CDx] assay platform; Foundation Medicine), had an ORR of 29% (95% CI, 21%-39%) compared with 6% (95% CI, 5%-8%) for patients with non–TMB-H tumors.19 Because KEYNOTE-158 is a single-group nonrandomized study, it is uncertain whether associations between TMB-H and outcomes represent evidence for TMB-H as a biomarker associated with response to pembrolizumab monotherapy or evidence of an association between TMB-H and prognosis. Currently, to our knowledge, there are no real-world data to describe the prevalence of TMB-H or its association with outcomes in the 10 tumor types evaluated in KEYNOTE-158 when such patients received standard-of-care nonimmunotherapy treatment. Using the deidentified Flatiron Health (FH)–Foundation Medicine (FMI) clinicogenomic database (CGDB), we therefore investigated prevalence of TMB-H and its potential association with OS in a real-world data set of patients not receiving immunotherapy for the 10 tumor types evaluated in KEYNOTE-158.
Methods
Institutional review board approval of the study protocol was obtained prior to study conduct and included a waiver of informed consent because there was no more than minimal risk, the study could not be practicably conducted without waiver or alteration, and the study could not be practicably conducted without use of protected health information. This study is reported following Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Design and Patients
This was a retrospective, observational study across 10 tumor types evaluated in the KEYNOTE-158 study18 using data from the CGDB collected up to July 31, 2018, the data cutoff date for this analysis. The CGDB links deidentified electronic health record (EHR)–derived patient-level data from the nationwide FH network (comprised of >265 oncology practices across the US, representing more than 2 million patients with cancer) with targeted comprehensive genomic profiling data from FMI, when such testing was performed as part of routine clinical care. Clinical data from FH included structured clinical and demographic data, such as laboratory values and drug orders and administrations; stage at diagnosis and date of diagnosis were not available. Genomic data from FMI included specimen features (eg, pathologic tumor purity) and alteration-level details (eg, TMB, genomic position, reference and alternate alleles, mutant allele count, and minor allele frequency). Tumor types were as reported by the ordering physician when submitting a patient’s tumor sample to FMI, with confirmation by a pathologist at FMI.
For this analysis, eligible patients were aged 18 years or older as of FMI report date (representing the earliest date on which FMI communicated test results to the ordering physician with respect to a single test requisition); had a valid measurement of TMB (per comprehensive genomic profiling by FMI); had at least 1 documented clinic visit observed in the CGDB after January 1, 2011; had a pathologist-confirmed solid tumor of 1 of the following types: anal carcinoma, biliary adenocarcinoma, cervical carcinoma, endometrial carcinoma, mesothelioma, neuroendocrine tumor (NET), salivary gland carcinoma, SCLC, thyroid carcinoma, or vulvar carcinoma. Patients were excluded if they had confirmed cancer other than the prespecified tumor types or if their cancer type was not otherwise specified. Patients were excluded from OS analysis if they had received immunotherapy on or before their FMI report date or were censored at the time of immunotherapy receipt if they began immunotherapy after their FMI report date.
Assessment of TMB
For assessment of TMB in the primary analysis, FFPE tumor tissue specimens were analyzed using either the most recent bait sets of FMI’s solid tissue assay, corresponding to the F1 assay or its successor, F1CDx; comparability of TMB values between F1 and F1CDx have been described previously.20 TMB was defined as the number of somatic base substitutions and short insertions and deletions identified from coding regions within the FMI test, filtering out known or likely oncogenic driver mutations to reduce bias. The resultant count was divided by the size of the genomic region interrogated to yield a resultant number of mutations per megabase.21 This method for TMB assessment has been analytically validated20 and demonstrates strong agreement with whole exome sequencing. For the primary analysis, TMB-H had the same cutoff used for the KEYNOTE-158 analysis18 (ie, ≥10 mut/Mb), but for an additional exploratory analysis, the cutoff was 13 or more mut/Mb, a threshold that has been evaluated in other clinical trials.11
Statistical Analysis
The primary objectives were to evaluate the association between TMB and OS from time of FMI report date among patients receiving routine clinical care for any of the 10 tumor types included in KEYNOTE-15818 and to evaluate prevalence of TMB-H in these tumor types. All statistical analyses were prespecified. The primary analysis was descriptive and combined all tumor types included in the analysis. Patients were included in the primary analysis if they were tested using either of the most recent bait sets of FMI’s solid tissue assay. Patients with confirmed microsatellite instability–high (MSI-H) cancers were excluded from the primary analysis of this study. Prevalence of TMB-H (ie, TMB ≥10 mut/Mb) was reported with corresponding 95% CIs calculated using Wilson score interval. OS from FMI report date (the primary index date) to the date of death of any cause or censor date (the date of a patient’s first use of immunotherapy, if any, otherwise the date of a patient’s last visit in the FH network) was analyzed with the Kaplan-Meier method, stratified by TMB status (TMB-H vs non–TMB-H) with corresponding 95% CIs. Cox proportional hazards modeling was performed to compare OS by TMB status (TMB-H vs non–TMB-H) and was adjusted for age at the FMI report date, sex, type of cancer, type of practice (ie, community vs academic), and last serum albumin concentration (≥3 g/dL vs <3 g/dL [to convert to grams per liter, multiply by 10]) within 90 days before FMI report date.
Sensitivity analyses were performed for patients with the following different scenarios: histological characteristics (all vs tumor-specific), MSI-H cancers (included vs excluded), bait sets (all vs F1/F1CDx only), index date (first antineoplastic treatment date vs FMI report date), and differing TMB cutoffs (≥10 mut/Mb vs ≥13 mut/Mb).
Data were analyzed using R statistical software version 3.6.1 (R Project for Statistical Computing). A 2-sided 95% CI (97.5%, 2.5% quantiles) was used. Data were analyzed from November 2018 to February 2019.
Results
Baseline Characteristics
A total of 2992 patients with any of the 10 tumor types and who were not treated with immunotherapy prior to FMI report were included. For primary analysis, 109 patients (3.6%) with confirmed MSI-H cancer were excluded per the prespecified analysis plan; of these, 101 patients (92.7%) had endometrial cancer. An additional 294 patients (9.8%) were excluded because they were tested using an older version of the F1 assay. Overall, 2589 patients were included in the primary analysis (eFigure in the Supplement); the mean (SD) age of these patients was 63.7 (11.7) years, and most patients were women (1671 patients [64.5%]), and White individuals (1803 patients [69.6%]). The 3 most common tumor types in the overall cohort were biliary (706 patients [27.3%]), endometrial (590 patients [22.8%]), and SCLC (305 patients [11.8%]) (Figure 1A and Table 1). The median (interquartile range) duration of follow-up was 5.4 (1.5-11.3) months from the date of FMI report and 13.0 (6.0-24.6) months from the date of the first antineoplastic treatment, with 1877 patients (72.5%) recorded as having at least 1 order or administration for antineoplastic therapy as of the FMI report date (Table 1). Neither median follow-up from FMI report date nor median follow-up from first antineoplastic date differed significantly between patients with TMB-H and those without TMB-H.
Figure 1. Distribution of Tumor Types and High Tumor Mutational Burden (TMB-H) Across the Entire Cohort .
Analysis included all tumor types. Evaluation of TMB-H was performed using the FoundationOne assay or FoundationOne Companion Diagnostic platforms. TMB-H was defined as 10 or more mutations per megabase. All patients had at least 1 documented clinical visit observed in the clinicogenetic database after January 1, 2011. Meso indicates mesothelioma; NET, neuroendocrine tumor; SCLC, small cell lung cancer.
Table 1. Baseline Characteristics of Study Population for Primary Analysis.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Overall (N = 2589) | Non–TMB-H (n = 2257) | TMB-H (n = 332) | |
| Sexa | |||
| Women | 1671 (64.5) | 1460 (64.7) | 211 (63.6) |
| Men | 917 (35.4) | 796 (35.3) | 121 (36.4) |
| Age, y | |||
| Mean (SD) | 63.7 (11.7) | 63.6 (11.8) | 64.1 (11.1) |
| <65 | 1262 (48.7) | 1092 (48.4) | 170 (51.2) |
| ≥65 | 1327 (51.3) | 1165 (51.6) | 162 (48.8) |
| Raceb | |||
| White | 1803 (69.6) | 1572 (69.6) | 231 (69.6) |
| Black | 146 (5.6) | 129 (5.7) | 17 (5.1) |
| Asian | 60 (2.3) | 55 (2.4) | 5 (1.5) |
| Other or missing | 580 (22.4) | 501 (22.2) | 79 (23.8) |
| Practice type | |||
| Academic | 451 (17.4) | 402 (17.8) | 49 (14.8) |
| Community | 2138 (82.6) | 1855 (82.2) | 283 (85.2) |
| Last serum albumin concentration within 90 d prior to FMI report date | |||
| ≥3 g/dL | 1289 (49.8) | 1106 (49.0) | 183 (55.1) |
| <3 g/dL | 88 (3.4) | 79 (3.5) | 9 (2.7) |
| Missing | 1212 (46.8) | 1072 (47.5) | 140 (42.2) |
| FMI assays | |||
| F1 | 2490 (96.2) | 2173 (96.3) | 317 (95.5) |
| F1CDx | 99 (3.8) | 84 (3.7) | 15 (4.5) |
| Documented antineoplastic drug use as of index date | |||
| No | 712 (27.5) | 641 (28.4) | 71 (21.4) |
| Yes | 1877 (72.5) | 1616 (71.6) | 261 (78.6) |
| MSI status | |||
| Non–MSI-Hc | 1710 (66.0) | 1492 (66.1) | 218 (65.7) |
| MSI unknown | 879 (34.0) | 765 (33.9) | 114 (34.3) |
| Tumor type | |||
| Biliary | 706 (27.3) | 678 (30.0) | 28 (8.4) |
| Endometrial | 590 (22.8) | 524 (23.2) | 66 (19.9) |
| SCLC | 305 (11.8) | 183 (8.1) | 122 (36.7) |
| Thyroid | 223 (8.6) | 217 (9.6) | 6 (1.8) |
| Salivary | 169 (6.5) | 147 (6.5) | 22 (6.6) |
| Mesothelioma | 163 (6.3) | 161 (7.1) | 2 (0.6) |
| NET | 164 (6.3) | 116 (5.1) | 48 (14.5) |
| Anal | 125 (4.8) | 108 (4.8) | 17 (5.1) |
| Cervical | 114 (4.4) | 97 (4.3) | 17 (5.1) |
| Vulvar | 30 (1.2) | 26 (1.2) | 4 (1.2) |
| Follow-up from FMI report date, mo | |||
| Mean (SD) | 8.1 (8.8) | 8.1 (8.8) | 7.8 (8.7) |
| Median (IQR) | 5.4 (1.5-11.3) | 5.5 (1.5-11.5) | 4.8 (1.9-10.2) |
| Follow-up from first antineoplastic dated | |||
| Mean (SD) | 18.2 (18.0) | 18.2 (18.2) | 17.8 (16.1) |
| Median (IQR) | 13.0 (6.0-24.6) | 13.1 (5.9-24.7) | 12.2 (6.6-23.8) |
Abbreviatons: F1, FoundationOne; F1CDx, F1 Companion Diagnostic; FMI, Foundation Medicine; IQR, interquartile range; MSI, microsatellite instability; MSI-H, high MSI; NET, neuroendocrine tumor; SCLC, small cell lung cancer; TMB-H, high tumor mutational burden.
SI conversion factor: To convert albumin to grams per liter, multiply by 10.
Missing for 1 patient.
Race was documented in the electronic health record as reported by the patient or oncology clinic at the point of care and mapped to standardized categories.
Non–MSI-H includes patients with microsatellite stable status and microsatellite stability indeterminate status. A total of 109 patients with MSI-H status were excluded from the primary analysis.
Limited to patients with a record of antineoplastic administration in the Flatiron Health electronic health record–derived deidentified database.
Prevalence of TMB-H
Of 2589 patients included for primary analysis, 332 (12.8%) had TMB-H (≥10 mut/Mb). TMB status varied by tumor type, with the median (interquartile range) TMB of 2.6 (1.7-6.1) mut/Mb in the entire cohort and with a range from 1.7 mut/Mb among patients with mesothelioma, salivary, or thyroid cancer to 8.7 mut/Mb among patients with SCLC. Prevalence of TMB-H was highest in patients with SCLC (40.0%; 95% CI, 34.7%-45.6%), NET (29.3%; 95% CI, 22.8%-36.6%), and cervical cancer (14.9%; 95% CI, 9.5%-22.6%) whereas prevalence of TMB-H was lowest in patients with mesothelioma (1.2%; 95% CI, 0.3%-4.4%), thyroid cancer (2.7%; 95% CI, 1.2%-5.7%), and biliary cancer (4.0%; 95% CI, 2.8%-5.7%) (Figure 1B). Among 164 patients with NETs, there were 90 patients with lung large cell NET (41 patients [45.6%] with TMB-H), 51 patients with colon NET (6 patients [11.8%] with TMB-H), 17 patients with small intestine NET (1 patient [5.9%] with TMB-H), and 6 patients with rectum NET (0 patients with TMB-H). Prevalence of TMB-H in the cohort was 9.2% (95% CI, 8.1%-10.3%) using the alternative TMB-H cutoff of 13 or more mut/Mb.
Association of TMB-H With OS
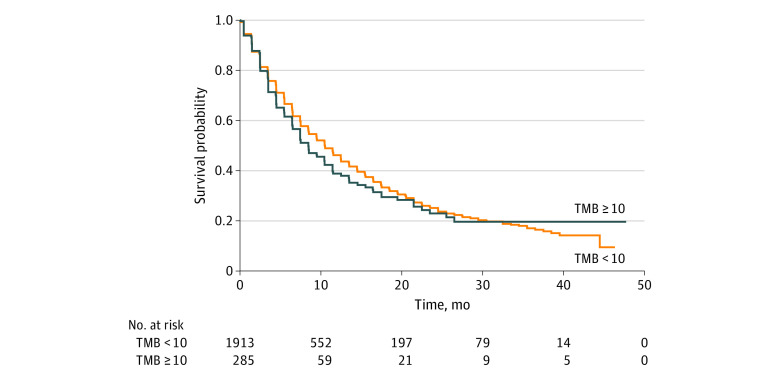
A total of 2517 patients were included in the primary analysis of OS after excluding 69 patients (2.7%) who received immunotherapy before the index date and 3 patients (0.1%) who died before January 1, 2012. Of 320 patients in the TMB-H group, 140 (43.8%) had an OS event, and of 2197 patients in the non–TMB-H group, 956 (43.5%) had an OS event. In the primary analysis population, OS across all cancer types was similar among patients with TMB-H and without TMB-H, with median OS of 8.4 (95% CI, 7.4-11.4) months in the TMB-H group and 10.5 (95% CI, 9.5-11.5) months in the non–TMB-H group (Figure 2 and Table 2). The hazard ratio (HR) for OS for TMB-H vs non–TMB-H groups was 1.11 (95% CI, 0.93-1.33) in unadjusted analyses and 0.94 (95% CI, 0.77-1.13) in adjusted analyses (Table 2). Consistent with the overall cohort, across each individual tumor type, the unadjusted and adjusted HRs for OS contained 1 (Table 2).
Figure 2. Kaplan-Meier Estimate of Overall Survival Among Patients With High Tumor Mutational Burden (TMB) and Without High TMB.
All patients were included for primary analysis. Survival was defined as the time from Foundation Medicine report date to the date of death due to any cause or censor date. High TMB was defined as 10 or more mutations per megabase.
Table 2. TMB-H Prevalence and OS Analysis by TMB Status.
| Cancer | No. | TMB-H | TMB, median (IQR) | OS (Mo, 95% CI) | HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % (95% CI) | No.a | TMB-H | Non-TMB-H | Unadjusted | Adjusted | |||
| Total | 2589 | 332 | 12.8 (11.6-14.2) | 2.6 (1.7-6.1) | 2517 | 8.4 (7.4-11.4) | 10.5 (9.5-11.5) | 1.11 (0.93-1.33) | 0.94 (0.77-1.13) |
| SCLC | 305 | 122 | 40.0 (34.7-45.6) | 8.7 (5.2-13.1) | 281 | 6.4 (5.4-7.5) | 7.4 (5.5-10.5) | 1.06 (0.76-1.48) | 1.03 (0.74-1.44) |
| NET | 164 | 48 | 29.3 (22.8-36.6) | 5.2 (2.6-10.4) | 159 | 10.4 (6.4-NA) | 6.4 (4.5-10.5) | 0.81 (0.48-1.39) | 0.83 (0.48-1.44) |
| Cervical | 114 | 17 | 14.9 (9.5-22.6) | 4.4 (2.6-7.0) | 110 | NA (6.4-NA) | 7.4 (4.4-11.5) | 0.31 (0.07-1.27) | 0.32 (0.08-1.31) |
| Anal | 125 | 17 | 13.6 (8.7-20.7) | 4.4 (2.6-7.8) | 119 | 7.4 (2.5-NA) | 7.5 (5.5-15.4) | 0.86 (0.41-1.79) | 0.84 (0.40-1.79) |
| Vulvar | 30 | 4 | 13.3 (5.3-29.7) | 4.1 (2.6-8.1) | 29 | 8.5 (0.5-NA) | 6.5 (2.5-NA) | 1.21 (0.26-5.70) | 1.18 (0.22-6.29) |
| Salivary | 169 | 22 | 13.0 (8.8-18.9) | 1.7 (0.9-4.4) | 164 | 4.5 (3.5-NA) | 15.5 (10.5-21.5) | 1.27 (0.60-2.68) | 1.20 (0.48-2.99) |
| Endometrial | 590 | 66 | 11.2 (8.9-14.0) | 3.5 (1.7-5.2) | 587 | 11.4 (8.5-26.5) | 13.5 (11.5-15.4) | 1.14 (0.75-1.72) | 1.15 (0.75-1.75) |
| Biliary | 706 | 28 | 4.0 (2.8-5.7) | 2.6 (0.9-3.5) | 697 | 11.5 (7.4-NA) | 8.4 (7.4-10.4) | 0.71 (0.39-1.29) | 0.65 (0.35-1.19) |
| Thyroid | 223 | 6 | 2.7 (1.2-5.7) | 1.7 (0.9-3.5) | 220 | 10.2 (1.5-NA) | 27.5 (21.5-NA) | 1.70 (0.41-7.02) | 1.64 (0.39-6.96) |
| Mesothelioma | 163 | 2 | 1.2 (0.3-4.4) | 1.7 (0.9-2.6) | 151 | NA | 12.5 (8.5-15.5) | NA | NA |
Abbreviations: HR, hazard ratio; IQR, interquartile range; NA, not available/applicable; NET, neuroendocrine tumor; OS, overall survival; SCLC, small cell lung cancer; TMB, tumor mutational burden; TMB-H, high TMB.
Patients were excluded from OS analysis if the start of immunotherapy was earlier than or equal to FMI report date (69 patients) or had death date prior to January 1, 2012 (3 patients).
Sensitivity analyses were conducted to evaluate whether the outcome was affected by the criteria used for analysis. These sensitivity analyses were conducted at both TMB-H cutoffs (ie, ≥10 mut/Mb and ≥13 mut/Mb), using the F1 or F1CDx tested population vs patients tested using any FMI bait set, including and excluding patients with MSI-H cancers, and alternative index dates for calculation of OS (using the first observed antineoplastic treatment date or FMI report date). At the alternative TMB-H cutoff of ≥13 mut/Mb, the adjusted HR for OS was 0.84 (95% CI, 0.67-1.05). The 95% CIs of the HRs for sensitivity analyses all contained 1, suggesting there was no association in OS between patients with different TMB status across these tumor types in either unadjusted or unadjusted analyses (Figure 3).
Figure 3. Sensitivity Analyses Evaluating Association Between Tumor Mutational Burden (TMB) and OS Among Patients With All 10 Tumor Types.
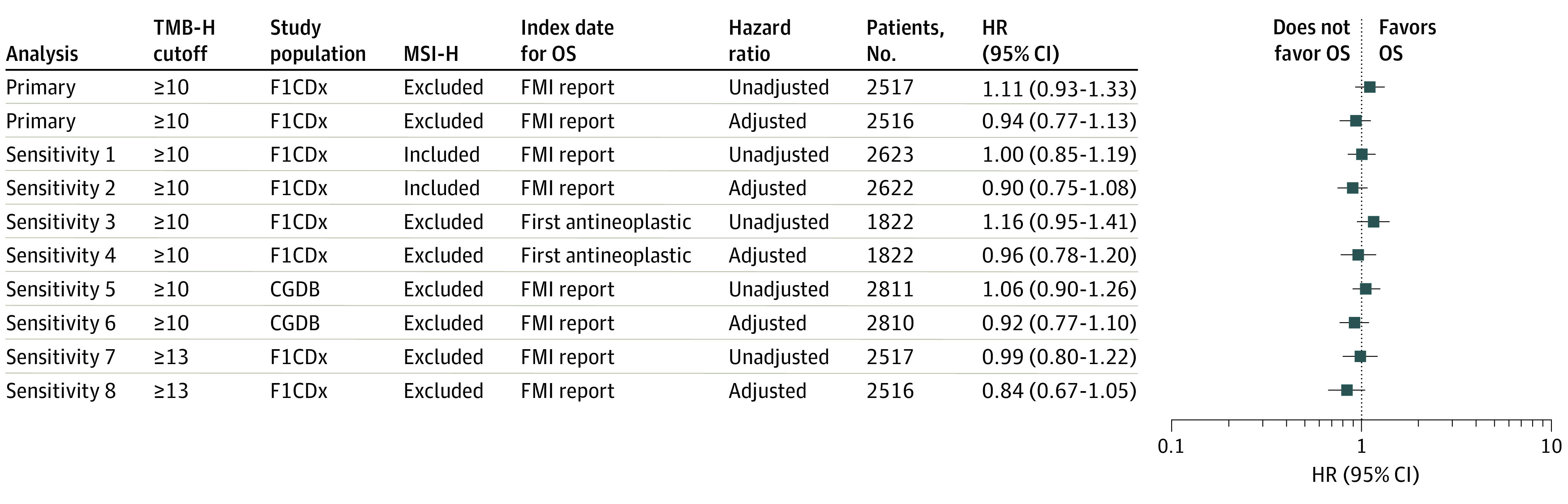
The study population was derived from patients tested by FoundationOne (F1) assay or F1 Companion Diagnostic (CDx) assay vs any FMI assay. OS was calculated from Foundation Medicine (FMI) report date vs date of first antineoplastic treatment. CGDB indicates clinicogenomic database; HR, hazard ratio; MSI-H, high microsatellite instability; TMB, tumor mutational burden; TMB-H, high TMB (≥10 or ≥13 mutations per megabase).
Discussion
This retrospective cohort study using a real-world data set evaluated prevalence of TMB-H (defined as ≥10 mut/Mb) and its association with OS (ie, its prognostic association) among patients with 1 of 10 rare solid tumor types who were not treated with immunotherapy. Median TMB ranged from 1.7 mut/Mb among patients with thyroid cancer, salivary cancer, and mesothelioma to 8.7 mut/Mb among patients with SCLC. Prevalence of TMB-H varied widely across tumor types, ranging from 1.2% in patients with mesothelioma to 40.0% in patients with SCLC. Prevalence of TMB-H exceeded 10% in 7 of 10 tumor types evaluated. These results indicate marked differences in TMB-H prevalence rates across a wide range of cancers.
We found no difference when comparing HRs for OS across all patients and within each tumor group. Sensitivity analyses, which evaluated the effect of using a different cutoff for TMB-H (≥13 mut/Mb), including patients with MSI-H tumors, including patients tested with any FMI bait set, and calculating OS from the first observed antineoplastic treatment date, similarly found no difference in OS for patients with TMB-H tumors vs those with non–TMB-H tumors. Based on these findings, TMB-H was not associated with overall survival among patients with these cancer types when not treated with immunotherapy.
Evidence from other studies regarding associations between OS and TMB-H in patients not treated with immunotherapy has been equivocal.22,23,24,25,26,27,28 For example, higher TMB (ie, >100 somatic nonsynonymous mutations) was associated with more favorable OS vs lower TMB in patients with ovarian cancer who had not received immunotherapy.25,27 In contrast, a 2018 study22 reported that a very high TMB (ie, >62 mut/Mb) was associated with shorter OS in patients with resected NSCLC who had not received chemotherapy or immunotherapy before surgery. Consistent with our finding for SCLC, in which no association was found between TMB-H and OS based on HR estimates for OS comparing patients with TMB-H vs without TMB-H, previous studies have found no association between TMB-H and improved OS in SCLC,17 gastroesophageal adenocarcinoma,29 and across a selection of 10 tumor types not included in our study: bladder, breast, colorectal, esophagogastric, head and neck, renal cell carcinomas, glioma, melanoma, and NSCLC.1 These differences could result from different study populations, treatment settings, TMB testing assays, or TMB-H definition.
An association between TMB-H and improved efficacy of immunotherapies has been reported in several tumor types, suggesting TMB-H may be a potential biomarker associated with response to these treatments.7,13,30 An analysis of the KEYNOTE-158 study using the F1CDx assay evaluated associations between TMB and outcomes with pembrolizumab monotherapy. Findings from the KEYNOTE-158 study18 indicated an improved ORR among patients with TMB-H (≥10 mut/Mb) after pembrolizumab monotherapy in the same tumor types as those included in the current study, reporting an ORR of 29% among patients with TMB-H (28% when MSI-H patients were excluded) compared with 6% for patients without TMB-H. Median OS for TMB-H was 11.7 (95% CI, 9.1-19.1) months compared with 12.8 (95% CI, 11.1-14.1) months for patients without TMB-H,19 although the many potential confounding factors associated with assessments in such pooled estimates of OS must be noted. In the context of a single-group study, such as KEYNOTE-158,18 it can be difficult to evaluate whether differences in outcomes (or lack thereof) are representative of a predictive or prognostic association. Our results suggest that assessment of any difference in outcomes by TMB status in KEYNOTE-158 was not confounded by a prognostic association of TMB-H.
The association between TMB-H and clinical outcomes may further be strengthened by co-occurrence of another marker of genomic instability, such as MSI-H.21,31 Chalmers et al21 reported that 97% of specimens with MSI-H also had TMB-H and that such co-occurrences are observed more frequently in certain cancer types, especially in gastrointestinal cancers, but rarely in melanoma and lung carcinoma. As such, comparative analysis of biomarkers associated with treatment response and their association with rare solid tumor types may help provide accurate and improved usage of immunotherapies.
Consistent with our findings, evidence from previous studies suggests that prevalence of TMB-H varies widely among tumor types,3,4 with cancers related to chronic mutagenic exposures, such as lung (tobacco) and malignant melanoma (ultraviolet light), exhibiting the highest prevalence of TMB-H.3,4 Yarchoan et al3 recently reported frequencies of TMB-H in 9887 FFPE clinical cancer specimens in 35 tumor types, including most tumor types evaluated in this study, using the F1 assay. Their studies showed the highest prevalence of TMB-H was reported in 41% of SCLCs, followed by 17% of cervical cancers, 15% of anal cancers, 9% of NET, 8% of endometrial cancers, and 1% of mesotheliomas.3 In this study, prevalence of TMB-H observed in the NET cohort (29.3%) was higher possibly owing to the anatomic site distribution of the NETs: of 164 patients with NETs, 90 had lung large cell NET, of whom approximately half had TMB-H.
Strengths of this investigation include use of patient-level data from routine clinical practice. Additionally, this study used analytically and clinically validated assays, which are highly concordant with respect to assessment of TMB.32
Limitations
This study has some limitations, including the small sample size of patients with TMB-H-for some tumor types and potential differences in immunotherapy usage across tumor types. Further research would be needed to explore whether the prognostic association of TMB varies by tumor type or TMB-H cutoff. Because TMB is a continuous variable, dichotomization of TMB in this study has inherent limitations,33 and future analyses will need to consider whether there are dose-dependent associations of TMB across different tumor types. The population, clinical characteristics, and outcomes in this study were captured from EHRs of a nationwide network of community and academic oncology practices, and thus treatments or outcomes that occurred outside the FH network and were not documented in the patient’s record may be missing. Furthermore, because this study relied on structured EHR data for cohort selection, there may be unmeasured confounders in the comparison, including certain data elements not ascertainable without EHR review, such as date of diagnosis, stage, additional therapies (eg, radiation therapy), or comorbidities. Because of the lack of availability of the diagnosis date, a novel index date (FMI report date) was used for primary analysis. Sensitivity analyses using an alternative date of first documented antineoplastic therapy showed similar results. Prior to conducting this analysis, we compared HRs and 95% CIs for OS using structured index dates (ie, date of first antineoplastic, FMI report date) vs abstractor confirmed index dates (ie, advanced diagnosis date, first-line start date) across several other FH-FMI clinicogenomic data sets with abstractor confirmed data available and found that the structured and abstractor-confirmed index dates yielded similar results. As a result, we used the structured index date of FMI report date for this analysis. Other limitations include the potential for differences in characteristics for patients included in this study (ie, those for whom TMB testing was performed) and the broader population of patients with cancer and that MSI-H status was not ascertained in one-third of patients. Additionally, this study was not designed to evaluate whether specific mutations or types of mutations (eg, frameshift vs nonframeshift) were associated with prognosis.
Conclusions
This diagnostic/prognostic study found that prevalence of TMB-H varied widely across a range of solid tumors, and there was no association between TMB-H status and OS across the evaluated tumor types. These findings indicate that TMB-H does not have a prognostic association among patients with these tumor types in the absence of immunotherapy.
eFigure. Patient Selection for Primary Analysis Population
References
- 1.Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202-206. doi: 10.1038/s41588-018-0312-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singal G, Miller PG, Agarwala V, et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non–small cell lung cancer using a clinicogenomic database. JAMA. 2019;321(14):1391-1399. doi: 10.1001/jama.2019.3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarchoan M, Albacker LA, Hopkins AC, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight. 2019;4(6):e126908. doi: 10.1172/jci.insight.126908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. ; Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain . Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415-421. doi: 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544-548. doi: 10.1038/nature25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizvi NA, Cho BC, Reinmuth N, et al. LBA6—durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line treatment for metastatic non-small cell lung cancer: MYSTIC. Ann Oncol. 2018;29(suppl 10):x40-x41. doi: 10.1093/annonc/mdy511.005 [DOI] [Google Scholar]
- 7.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ready N, Hellmann MD, Awad MM, et al. First-line nivolumab plus ipilimumab in advanced non–small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019;37(12):992-1000. doi: 10.1200/JCO.18.01042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reck M, Schenker M, Lee KH, et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non-small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer. 2019;116:137-147. doi: 10.1016/j.ejca.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 10.Herbst RS, Lopes G, Kowalski DM, et al. LBA79—association between tissue TMB (tTMB) and clinical outcomes with pembrolizumab monotherapy (pembro) in PD-L1–positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Ann Oncol. 2019;30(suppl 5):v916-v917. doi: 10.1093/annonc/mdz394.077 [DOI] [Google Scholar]
- 11.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi: 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2019;35(2):329. doi: 10.1016/j.ccell.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 13.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207-211. doi: 10.1126/science.aad0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabrizio DA, George TJ Jr, Dunne RF, et al. Beyond microsatellite testing: assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J Gastrointest Oncol. 2018;9(4):610-617. doi: 10.21037/jgo.2018.05.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devarakonda S, Rotolo F, Tsao MS, et al. Tumor mutation burden as a biomarker in resected non-small-cell lung cancer. J Clin Oncol. 2018;36(30):2995-3006. doi: 10.1200/JCO.2018.78.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Liang H, Lin C, et al. Molecular subtyping and prognostic assessment based on tumor mutation burden in patients with lung adenocarcinomas. Int J Mol Sci. 2019;20(17):4251. doi: 10.3390/ijms20174251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47-53. doi: 10.1038/nature14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov . Study of pembrolizumab (MK-3475) in participants with advanced solid tumors (MK-3475-158/KEYNOTE-158). Accessed October 5, 2020. https://clinicaltrials.gov/ct2/show/NCT02628067
- 19.Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353-1365. doi: 10.1016/S1470-2045(20)30445-9 [DOI] [PubMed] [Google Scholar]
- 20.Food and Drug Administration . FoundationOne CDx: summary of safety and effectiveness data. Accessed December 19, 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p170019
- 21.Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi: 10.1186/s13073-017-0424-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owada-Ozaki Y, Muto S, Takagi H, et al. Prognostic impact of tumor mutation burden in patients with completely resected non–small cell lung cancer: brief report. J Thorac Oncol. 2018;13(8):1217-1221. doi: 10.1016/j.jtho.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 23.Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587-13598. doi: 10.18632/oncotarget.7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz S, Tian Y, Cecchi F, et al. The prognostic role of microsatellite status, tumor mutational burden, and protein expression in CRC. J Clin Oncol. 2018;36(4)(suppl):572. doi: 10.1200/JCO.2018.36.4_suppl.57229272162 [DOI] [Google Scholar]
- 25.Simpson D, Ferguson R, Marinez CN, et al. Mutation burden as a potential prognostic marker of melanoma progression and survival. J Clin Oncol. 2017;35(15)(suppl):9567. doi: 10.1200/JCO.2017.35.15_suppl.9567 [DOI] [Google Scholar]
- 26.Birkbak NJ, Kochupurakkal B, Izarzugaza JM, et al. Tumor mutation burden forecasts outcome in ovarian cancer with BRCA1 or BRCA2 mutations. PLoS One. 2013;8(11):e80023. doi: 10.1371/journal.pone.0080023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SE, Park K, Lee E, et al. Clinical implication of tumor mutational burden in patients with HER2-positive refractory metastatic breast cancer. Oncoimmunology. 2018;7(8):e1466768. doi: 10.1080/2162402X.2018.1466768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown SD, Warren RL, Gibb EA, et al. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014;24(5):743-750. doi: 10.1101/gr.165985.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou KI, Peterson BF, Serritella A, et al. Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin Cancer Res. 2020;clincanres.2085.2020. doi: 10.1158/1078-0432.CCR-20-2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellmann MD, Nathanson T, Rizvi H, et al. Genomic features of response to combination immunotherapy in patients with advanced non–small-cell lung cancer. Cancer Cell. 2018;33(5):843-852.e4. doi: 10.1016/j.ccell.2018.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12(1):54. doi: 10.1186/s13045-019-0738-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FoundationOne CDx . FoundationOne CDx technical information. Accessed December 19, 2019. https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019C.pdf
- 33.Naggara O, Raymond J, Guilbert F, Roy D, Weill A, Altman DG. Analysis by categorizing or dichotomizing continuous variables is inadvisable: an example from the natural history of unruptured aneurysms. AJNR Am J Neuroradiol. 2011;32(3):437-440. doi: 10.3174/ajnr.A2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Patient Selection for Primary Analysis Population