Key Points
Question
Does treatment with vocimagene amiretrorepvec (Toca 511) and flucytosine (Toca FC), compared with standard of care (SOC), improve survival among patients with recurrent glioblastoma and anaplastic astrocytoma?
Findings
In this randomized open-label clinical trial of 403 patients assigned to receive Toca 511/FC or SOC, the primary end point of overall survival was not met (11.1 months for the Toca 511/FC group and 12.22 months for the SOC group). Secondary end points did not demonstrate statistically significant differences and rates of adverse events were similar in the 2 groups.
Meaning
Toca 511 and Toca FC treatment did not improve survival for patients with recurrent glioblastoma and anaplastic astrocytoma.
Abstract
Importance
New treatments are needed to improve the prognosis of patients with recurrent high-grade glioma.
Objective
To compare overall survival for patients receiving tumor resection followed by vocimagene amiretrorepvec (Toca 511) with flucytosine (Toca FC) vs standard of care (SOC).
Design, Setting, and Participants
A randomized, open-label phase 2/3 trial (TOCA 5) in 58 centers in the US, Canada, Israel, and South Korea, comparing posttumor resection treatment with Toca 511 followed by Toca FC vs a defined single choice of approved (SOC) therapies was conducted from November 30, 2015, to December 20, 2019. Patients received tumor resection for first or second recurrence of glioblastoma or anaplastic astrocytoma.
Interventions
Patients were randomized 1:1 to receive Toca 511/FC (n = 201) or SOC control (n = 202). For the Toca 511/FC group, patients received Toca 511 injected into the resection cavity wall at the time of surgery, followed by cycles of oral Toca FC 6 weeks after surgery. For the SOC control group, patients received investigators’ choice of single therapy: lomustine, temozolomide, or bevacizumab.
Main Outcomes and Measures
The primary outcome was overall survival (OS) in time from randomization date to death due to any cause. Secondary outcomes reported in this study included safety, durable response rate (DRR), duration of DRR, durable clinical benefit rate, OS and DRR by IDH1 variant status, and 12-month OS.
Results
All 403 randomized patients (median [SD] age: 56 [11.46] years; 62.5% [252] men) were included in the efficacy analysis, and 400 patients were included in the safety analysis (3 patients on the SOC group did not receive resection). Final analysis included 271 deaths (141 deaths in the Toca 511/FC group and 130 deaths in the SOC control group). The median follow-up was 22.8 months. The median OS was 11.10 months for the Toca 511/FC group and 12.22 months for the control group (hazard ratio, 1.06; 95% CI 0.83, 1.35; P = .62). The secondary end points did not demonstrate statistically significant differences. The rates of adverse events were similar in the Toca 511/FC group and the SOC control group.
Conclusions and Relevance
Among patients who underwent tumor resection for first or second recurrence of glioblastoma or anaplastic astrocytoma, administration of Toca 511 and Toca FC, compared with SOC, did not improve overall survival or other efficacy end points.
Trial Registration
ClinicalTrials.gov Identifier: NCT02414165
This randomized clinical trial compares overall survival for patients receiving tumor resection followed by vocimagene amiretrorepvec (Toca 511) with flucytosine (Toca FC) vs standard of care.
Introduction
High-grade gliomas (HGGs), including grade III anaplastic astrocytoma (AA) and grade IV glioblastoma, are the most aggressive malignant primary brain tumors.1 Patients with glioblastoma and AA receive maximal safe resection plus radiotherapy with concurrent chemotherapy temozolomide, followed by maintenance temozolomide as standard of care (SOC) treatment. This results in a median overall survival (mOS) ranging from 12.7 months to 3.9 years depending on the tumor grade and the molecular and genetic profile.2,3,4 Despite aggressive treatment, nearly all HGGs eventually recur, and there are no effective treatments for this population. For recurrent glioblastoma, the median progression-free survival is as short as 1.8 months5 and mOS ranges from 7.1 to 9.8 months.6,7,8 Chemotherapies such as lomustine and temozolomide have been approved by the US Food and Drug Administration for treating glioblastoma and AA, but the OS improvement in the recurrent setting is minimal.9 New treatments are needed to improve the prognosis of this patient population.
Vocimagene amiretrorepvec (Toca 511) is an investigational γ retroviral replicating vector encoding a transgene for an optimized yeast cytosine deaminase, an enzyme that converts 5-fluorocytosine (5-FC) into 5-fluorouracil in the tumor microenvironment without systemic 5-fluorouracil adverse effects.10 Preclinical data indicated that Toca 511 and 5-FC treatment kills tumor cells and nearby immunosuppressive cells such as myeloid-derived suppressor cells and tumor-associated macrophages, leading to T-cell priming and durable systemic antitumor immune activity.11 Multiyear durable complete responses have been observed in recurrent HGG patients in a phase 1 resection-injection trial (NCT01470794) with Toca 511/FC.12 Integrated Toca 511 was commonly detected in tumor and transiently detected in blood. No evidence for clonal expansion of cells with integrated Toca 511 DNA, or preferential retrieval of integration sites near oncogenes was observed.13
A phase 2/3 randomized clinical trial of Toca 511 and Toca FC vs SOC for treatment of patients with recurrent glioblastoma and AA has been completed. Efficacy, safety, and baseline molecular and immunological results are reported here.
Methods
Study Design and Oversight
This was a multicenter, randomized, open-label study of Toca 511 and Toca FC vs SOC that was investigator’s choice of single-agent chemotherapy (lomustine or temozolomide) or bevacizumab for patients with first or second recurrence of glioblastoma or AA. Patients were recruited from 67 centers in the US, Canada, Israel, and South Korea, with 58 sites enrolling patients. The trial was conducted from November 30, 2015, to December 20, 2019. This study was approved by the institutional review board and institutional biosafety committee for each site, and all patients provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials. The trial protocol is available in Supplement 1.
Patient Selection and Follow-up
Patients aged 18 to 75 years with first or second recurrence of histologically proven glioblastoma or AA were eligible. Additional key inclusion criteria were prior first-line multimodal therapy; tumor size between 1 and 5 cm; adequate laboratory values for surgery; and Karnofsky Performance Status score of at least 70. Key exclusion criteria were multifocal tumor; any active infection requiring systemic antibiotic, antifungal, or antiviral therapy within the previous 4 weeks; or received bevacizumab in the recurrent setting.
Randomization and Masking
Patients were randomized at the time of resection in a 1:1 ratio to either Toca 511 and Toca FC (Toca 511/FC), or SOC group. Stratification was by isocitrate dehydrogenase 1 (IDH1) variant status (variation vs wild type), Karnofsky Performance Status score (70-80 vs 90-100) and geographical region (United States vs Canada vs outside North America). Further methodologic details on randomization and masking are provided in the eMaterials and eMethods in Supplement 2.
Study Procedure
Patients in the Toca 511/FC group received 1 dose of approximately 4 mL of Toca 511 (108 TU/mL) injected into the resection cavity wall at the time of resection. Video training was provided for virus delivery as well as optional mentorship from experienced investigators from the similar phase 1 trial (NCT01470794). Approximately 6 weeks after resection, patients began the first cycle with a 7-day course of oral Toca FC dosed at 220 mg/kg/d, and repeated every 6 weeks. Toca FC was taken by patients as long as the drug was tolerated and the investigator believed patients were obtaining benefit. For the SOC group, patients began the first cycle approximately 6 weeks after resection, and the schedule was as follows: lomustine at 110 mg/m2 repeated every 6 weeks; temozolomide either at 50 mg/m2 once daily continuously or at 150 mg/m2 once daily for 5 consecutive days per 28-day cycle that could be increased to 200 mg/m2 once daily for 5 consecutive days in the following 28-day cycles; or bevacizumab at 10 mg/kg by intravenous infusion every 2 weeks. The SOC group treatment was continued until confirmed progression or end of treatment.
Study End Points
The primary end point was overall survival (OS), and the secondary end points were safety, durable response rate (DRR), duration of durable response, durable clinical benefit rate, OS and DRR by IDH1 variant status, and 12-month OS using modified Response Assessment in Neuro-Oncology criteria by independent radiologic review. Two additional secondary end points are not reported herein: patient reported outcome and quality of life and progression-free survival (see the trial protocol in Supplement 1). Further methodologic details on study end points are provided in eMaterials and eMethods in Supplement 2.
Exploratory Patient Molecular Profiling
Methodologic details of viral safety testing, peripheral blood monitoring, and tumor profiling are provided in eMaterials and eMethods in Supplement 2.
Statistical Analysis
The primary end point was OS, measured from randomization date to death due to any cause; the secondary end points were DRR, durable clinical benefit rate, duration of durable response, OS and DRR by IDH1 variant status, and 12-month OS. The 257 events were needed to detect a hazard ratio (HR) of 0.685 at a 2-sided α of .05 and a power of 85%. The OS end point incorporated group sequential design with the O’Brien-Fleming boundaries as implemented by Lan-DeMets α spending method to avoid inflation of the type I error rate. For the secondary end points, the Holm procedure was planned to adjust for multiplicity. All efficacy analyses included the intent-to-treat population (ITT), and all safety analyses included all randomized patients who underwent resection. Further details on statistical analysis are provided in the eMaterials and eMethods in Supplement 2.
Results
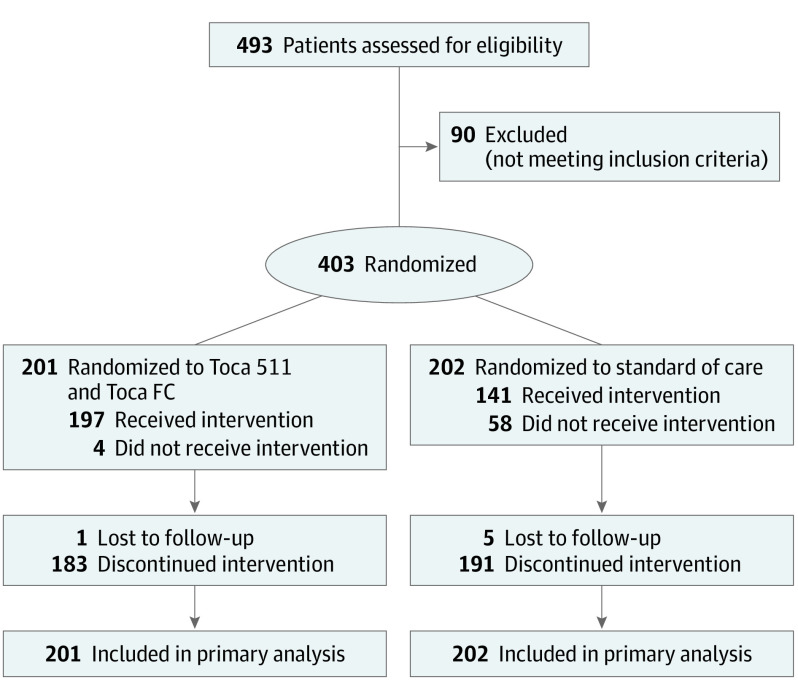
A total of 403 patients (median [SD] age: 56 [11.46] years; 62.5% [252] men) underwent randomization from November 2015 to November 2018 (187 patients in the phase 2 and 216 patients in the phase 3) at 58 institutions in 4 countries, with an enrollment pause from February 2017 to October 2017 between the phase 2 and the phase 3 portion (Figure 1). The last-known follow-up date was May 2019. Patient demographic characteristics and neuro-oncology history were balanced between the two groups (Table 1), and were also balanced between the patients in phase 2 and the patients in phase 3 (eTable 1 in Supplement 2).
Figure 1. Patient Flow in the TOCA 5 Randomized Clinical Trial.
Toca 511 indicates vocimagene amiretrorepvec; Toca FC, flucytosine.
Table 1. Baseline Demographics and Neuro-Oncology History for Intent-to-Treat Population.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Toca 511 and Toca FC (n = 201) | Standard of care (n = 202) | Total (N = 403) | |
| Age, y | |||
| Median | 57.0 | 56.0 | 56.0 |
| <65 | 155 (77.1) | 164 (81.2) | 319 (79.2) |
| ≥65 | 46 (22.9) | 38 (18.8) | 84 (20.8) |
| Sex | |||
| Male | 125 (62.2) | 127 (62.9) | 252 (62.5) |
| Female | 76 (37.8) | 75 (37.1) | 151 (37.5) |
| Race | |||
| White | 174 (86.6) | 173 (85.6) | 347 (86.1) |
| Karnofsky Performance Status score category | |||
| 70-80 | 64 (31.8) | 66 (32.7) | 130 (32.3) |
| 90-100 | 137 (68.2) | 136 (67.3) | 273 (67.7) |
| Region | |||
| US | 156 (77.6) | 158 (78.2) | 314 (77.9) |
| Non US | 45 (22.4) | 44 (21.8) | 89 (22.1) |
| Tumor histology at study entry from investigator | |||
| Glioblastoma | 171 (85.1) | 183 (90.6) | 354 (87.8) |
| Anaplastic Astrocytoma | 30 (14.9) | 19 (9.4) | 49 (12.2) |
| IDH1 variant status | |||
| Present (variant) | 27 (13.4) | 31 (15.3) | 58 (14.4) |
| Absent (wild type) | 174 (86.6) | 171 (84.7) | 345 (85.6) |
| No. of recurrences | |||
| 1 | 173 (86.1) | 170 (84.2) | 343 (85.1) |
| 2 | 28 (13.9) | 32 (15.8) | 60 (14.9) |
Abbreviations: HGGs, high-grade gliomas; Toca 511, vocimagene amiretrorepvec; Toca FC, flucytosine.
For the Toca 511/FC group, 199 patients (99%) received Toca 511 at the time of surgery, and 197 patients (98%) received both Toca 511 and at least one cycle of Toca FC. For the SOC group, 141 patients (70%) received at least one cycle of drug (Figure 1). At data cutoff, the median number of cycles of Toca FC taken by patients on the Toca 511/FC arm was 2 cycles. Maximal survival was achieved in patients who received up to 4 cycles of Toca FC (eFigure 1 in Supplement 2). For the SOC group, the median cycle of metronomic temozolomide was 3, of temozolomide was 3, of lomustine was 2, and of bevacizumab was 4 (eTable 2 in Supplement 2).
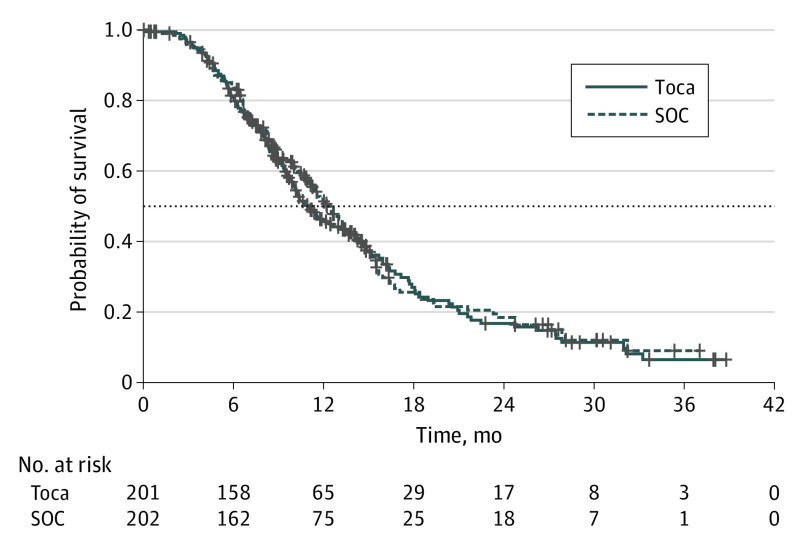
At final analysis, 271 deaths were observed, with 141 deaths in the Toca 511/FC group and 130 deaths in the SOC group. The median follow-up for all randomized patients was 22.8 months. A total of 29 patients (9.0% [18 of 201] on the Toca 511/FC arm and 5.4% [11 of 202] on the SOC arm) were continuing treatment at data cutoff. The mOS in the ITT population was 11.1 months for the Toca 511/FC arm and 12.22 months for the SOC arm (HR, 1.06; 95% CI, 0.83-1.35; P = .62) (Figure 2), which did not meet the statistical significance for the study’s primary end point. The preplanned secondary end points also did not demonstrate statistically significant differences in outcomes (eTable 3 in Supplement 2).
Figure 2. Kaplan-Meier Overall Survival Curves for Intent-to-Treat Population.
Toca indicates vocimagene amiretrorepvec (Toca 511) followed by flucytosine (Toca FC), SOC, standard of care.
In preplanned subgroup analyses, there was no statistically significant effect on mOS in the Toca 511/FC group among patients with IDH1-variant tumors and AA (HR, 0.64; 95% CI:, 0.33-1.24; P = .19; HR, 0.57; 95% CI, 0.28-1.14; P = .11, respectively) (Figures 3; eFigures 2a and 2b in Supplement 2). In the preplanned subgroup analysis of patients at second recurrence (n = 60), improvement in OS was observed, with an mOS of 21.8 months for the Toca 511/FC group (n = 28) and 11.1 months for the SOC group (n = 32) (HR, 0.43; 95% CI, 0.21-0.87; P = .02) (Figure 3; eFigure 2c in Supplement 2) although these numbers would not be statistically significant when adjusted for multiplicity. Demographic characteristics and neuro-oncology history in this subgroup were balanced between the 2 groups (eTable 4 in Supplement 2). In further exploratory post hoc subset analyses in the second recurrence subpopulation, while patient numbers were very small, there was an improvement in survival for patients with IDH1-variant tumors, with mOS not reached for the Toca 511/FC group (n = 8), and 10.9 months for the SOC group (n = 10) (HR, 0.14, 95% CI, 0.03-0.69; P < .001). Also with very small numbers, there was an improvement in survival for patients with AA, with mOS not reached for the Toca 511/FC group (n = 7) and 9.07 months for the SOC group (n = 6) (HRs were not estimated) (eFigure 3 in Supplement 2).
Figure 3. Forest Plot of Preplanned Subgroup Analyses.
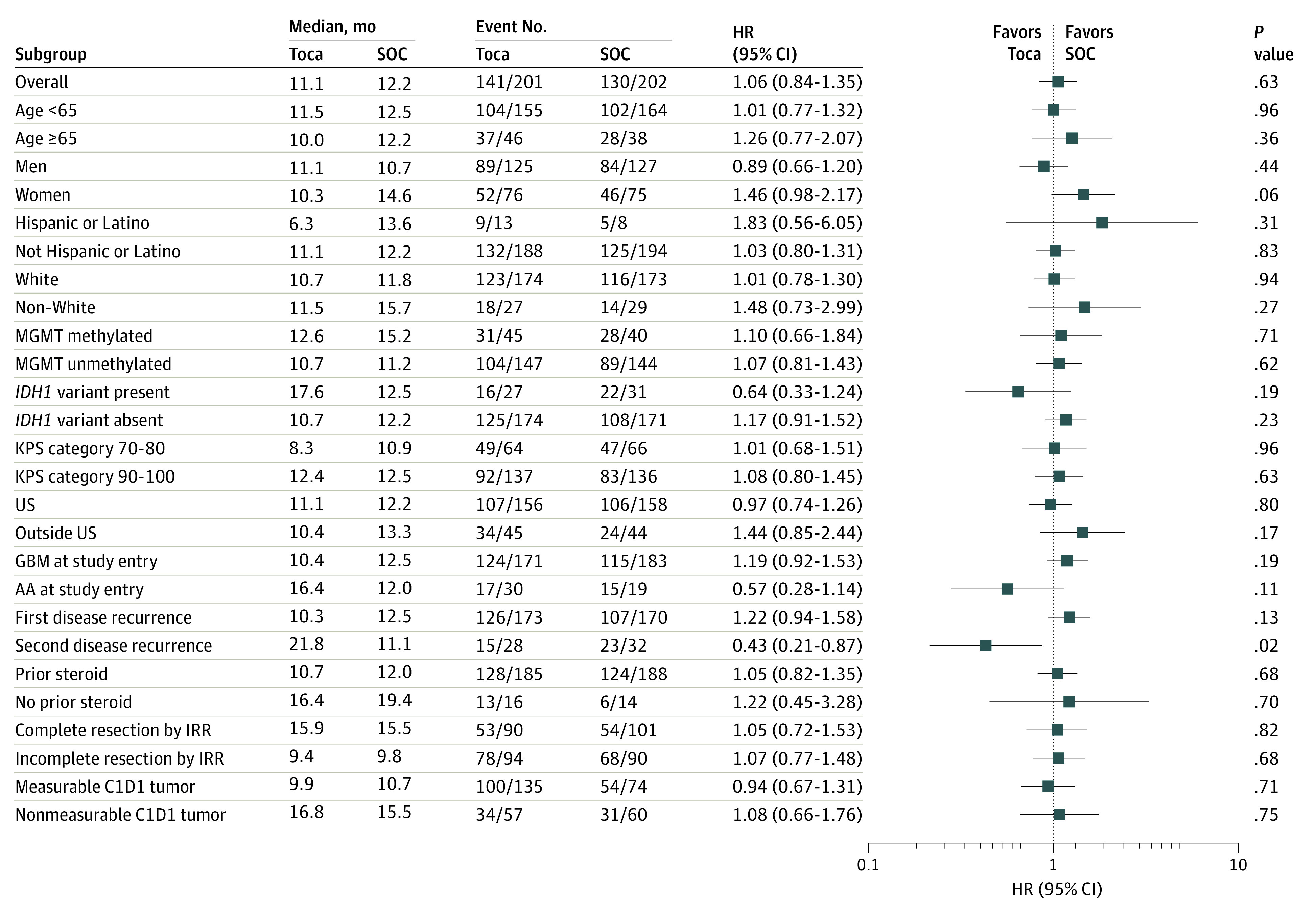
AA indicates anaplastic astrocytoma; C1D1, cycle 1 day 1; GBM, glioblastoma multiforme; HR, hazard ratio; IDH1, isocitrate dehydrogenase 1; KPS, Karnofsky performance status; MGMT, O6-methylgaunine-DNA-methyltransferase; SOC, standard of care; and Toca, vocimagene amiretrorepvec (Toca 511) followed by flucytosine (Toca FC).
The safety population included 100% (201 of 201) of patients in the Toca 511/FC group and 99% (199 of 202) of patients in the SOC group. No treatment-associated adverse events resulted in deaths in either group, and the most common adverse events for both groups are reported in Table 2.
Table 2. Most Common (≥5%) Treatment-Emergent Adverse Events.
| Grade 3-4 TEAEs | No. (%) | |
|---|---|---|
| Toca 511 and Toca FC (n = 201) | Standard of care (n = 199) | |
| Aphasia | 16 (8.0) | 7 (3.5) |
| Hemiparesis | 15 (7.5) | 5 (2.5) |
| Headache | 13 (6.5) | 10 (5.0) |
| Seizure | 8 (4.0) | 11 (5.5) |
| White blood cell count decreased | 0 (0) | 10 (5.0) |
| TEAEs leading to treatment discontinuation | 2 (1.0) | 5 (2.5) |
| TEAEs leading to death | 6 (3.0) | 3 (1.5) |
Abbreviations: TEAEs, treatment-emergent adverse events; Toca 511, vocimagene amiretrorepvec; Toca FC, flucytosine.
The presence of Toca 511 DNA and RNA was sampled longitudinally throughout the course of treatment.14 Quantitative Toca 511 DNA was observed in 4.5% of patients at cycle 1 day 1 and no viral DNA signal was observed at cycle 4-day 1 or beyond in any patients out to 1.5 years (eTable 5 in Supplement 2). Most patients showed quantitative viral RNA signal postsurgery (70.9%) but had cleared signal before cycle 1 day 1 of Toca FC (eTable 5 in Supplement 2). Once a patient’s viral RNA signal dropped below quantitative levels, no additional quantitative signal was detected thereafter. These results are consistent with prior experience under similar trial conditions (NCT01470794).14
Exploratory baseline molecular and immune profiling were performed to better understand the patient population and balance in this study (eFigures 4-32 in Supplement 2). Analyses of tumor obtained pretreatment during resection indicated that IDH1-variant tumors had a favorable immune cell composition for an immuno-oncology therapy compared with IDH1-wild type tumors (eFigure 26 in Supplement 2), including lower levels of M0 macrophages, higher levels of CD4 memory cells, resting NK cells, and resting dendritic cells than IDH1-wild type tumors. Immune cell composition was similar between the Toca 511/FC and SOC groups, including by ITT population, by subgroups such as histology, number of recurrences, and IDH1 status (eFigure 22 in Supplement 2). Wild-type IDH1 tumors preferentially expressed mRNAs encoding proteins involved in innate immune responses (eFigure 23 in Supplement 2), which could inhibit Toca 511 infection. Variant IDH1 tumors exhibited higher lymphoid compartment cells in baseline peripheral blood mononuclear cells compared with wild-type IDH1 tumors, suggesting that patients with a variant IDH1 tumor have a more robust peripheral immune cell population (eFigure 29 in the Supplement 2).
Discussion
In a disease with an extremely high unmet need, recent trials such as CheckMate-143 with nivolumab,15 INTELLANCE 2 with Depatux-M, GLOBE with VB-111 in combination with bevacizumab, have not shown efficacy benefits in recurrent HGG setting.16,17 Similarly, TOCA 5 did not meet primary objective of improved OS or the secondary end points. Overall, there were no observed significant biases in demographic and specified stratification markers (Table 1), or known molecular prognostic markers (eFigure 4 in Supplement 2) between the groups. Toca 511 and Toca FC were well tolerated with safety comparable to the SOC group, similar to previously reported and as expected in this setting. All patients in the Toca 511/FC group either showed no detectable peripheral virus or had transient signal that was cleared before cycle 4. Baseline peripheral blood immune cell health was also comparable between groups. However, subgroups that appeared to do better on treatment group showed more robust baseline immune cells and reduced immune suppressive cells (eFigures 30 and 31 in Supplement 2). The median numbers of cycle for Toca FC and SOC were low and likely incompatible with the amount given to long-term survivors or to the proposed mechanism of action of Toca 511/FC reported previously11,12; more cycles might be required to see a therapeutic effect. Suboptimal virus delivery was not practical to assess in this brain cancer setting and whether enough virus was delivered to patients remains unknown.
A preplanned subgroup analysis identified a subgroup with better outcomes. Patients with second recurrence appear to have better outcomes with Toca 511/FC treatment compared with SOC. These differences seem to be independent of other prognostic factors as the tumor molecular profiles between patients at first recurrence or second recurrence are generally similar and lack significant group imbalances between the two groups (eFigures 13, 14, and 28 in Supplement 2). Within the second recurrence population, while patient numbers were small, there was an improvement in survival for patients with IDH1-variant tumors and patients with AA. Patients with IDH1 variants or AA histology have a notably better prognosis than those with IDH1-wildtype and GBM histology as evidenced by longer OS and higher rates of 5-year survival.18,19,20 These slower growing, clinically less aggressive (indolent) HGG tumors may have more potential to respond to therapy that may stimulate a local immune response, such as Toca 511 and Toca FC, given the delayed nature of response to immune therapy noted by others in the field.21
Although the numbers analyzed were small, a consistent pattern of baseline immune potential was observed in treatment subgroups that demonstrated improvements in survival to Toca 511/FC treatment compared with SOC (eFigures 24, 26-31 in Supplement 2). The uniqueness of the potential mechanisms of action of Toca 511 and Toca FC may contribute to the efficacy advantage observed in these patients. In the phase 1 resection-injection trial (NCT01470794), a durable response rate of 21.7% was observed in patients who met the phase 2/3 trial entry criteria with a median duration of response follow-up of at least 35.7 months as of August 2017.12,14 In these patients, responses were observed at approximately 6 to 19 months after Toca 511 administration, consistent with an immunologic-based response.
Limitations
This study has limitations. Study limitations include the small number of cycles of patients receiving treatment, the very small number of patients included in the subgroup analysis, insufficient number of blood and tissue samples collected for biomarker analysis, and variations of Toca 511 distribution in the resection cavity wall.
Conclusions
In this multicenter randomized clinical trial of patients who underwent tumor resection for first or second recurrence of glioblastoma or anaplastic astrocytoma, administration of Toca 511 and Toca FC, compared with SOC, did not improve overall survival or the secondary efficacy end points. In a small subgroup analysis, a treatment effect was seen in a few patients at second recurrence. The results of this study may help to inform future study designs including population selection and minimum number of Toca FC treatment cycles.
Trial Protocol
eMaterials and eMethods
eReferences
eFigure 1. Kaplan-Meier overall survival curve with Toca FC cycle numbers.
eFigure 2. Kaplan-Meier overall survival curve for pre-planned subpopulations.
eFigure 3. Kaplan-Meier overall survival curve for subpopulations within the second recurrence.
eFigure 4. Workflow for whole exome sequencing and RNA sequencing data analyses.
eFigure 5. Quality assessment of phase 2 and phase 3 portions of Toca 5 tumor RNA sequencing.
eFigure 6. Assessment of Toca 5 RNA sequencing data quality.
eFigure 7. Boxplot comparing intra-tumor Pearson correlations in mRNA expression vs inter-tumor Pearson correlations in mRNA expression.
eFigure 8. mRNA expression profiles of Toca 5 tumors.
eFigure 9. Molecular classification of Toca 5 tumor samples.
eFigure 10. Relationships between patients’ tumor molecular classification and survival.
eFigure 11. Sequencing coverage metrics across targeted regions for normal and tumor samples.
eFigure 12. Summary of DNA sequencing analyses of Toca 5 tumors.
eFigure 13. Comparison of genetic profiles from 1st and 2nd recurrence tumors.
eFigure 14. Comparison of genetic profiles from 2nd recurrence patients in the two treatment arms.
eFigure 15. Relationships between tumor DNA alterations and survival.
eFigure 16. Tumor copy number variants inferred from exome sequencing data.
eFigure 17. CNV subtypes for IDH1-wildtype GBM patients.
eFigure 18. Mutational signatures in hypermutated tumors.
eFigure 19. Number of predicted tumor neoantigens correlates with tumor mutational burden.
eFigure 20. Relationships between tumor purity and molecular subtype.
eFigure 21. Pairwise correlations among genetic alterations, RNA subtypes and histology.
eFigure 22. Relative and absolute abundance of leukocyte populations in Toca 5 tumors.
eFigure 23. mRNA expression differences between IDH1 mutant tumors and IDH1 wild-type tumors.
eFigure 24. Comparison between treatment-arms of immune cell population levels in tumors at time of surgery as measured by iSort.
eFigure 25. Stratification of patients based on tumor immune cell levels inferred by iSort.
eFigure 26. Differences in immune cell composition between IDH1 mutant tumors and IDH1 wildtype tumors.
eFigure 27. Differences in immune cell and neoantigen composition between AA tumors and GBM tumors.
eFigure 28. Differences in immune cell composition between first and second recurrence tumors.
eFigure 29. Differences in peripheral immune cell composition between IDH1 wildtype and IDH1 mutant patients.
eFigure 30. Differences in peripheral immune cell composition between AA and GBM patients.
eFigure 31. Differences in peripheral immune cell composition between patients at first and second recurrence.
eFigure 32. Baseline peripheral immune balance between the control arm and the Toca 511/FC arm
eTable 1. Baseline demographics and neuro-oncology history for Phase 2 and Phase 3 patients.
eTable 2. Toca FC and SOC cycle numbers and schedule for patients.
eTable 3. Secondary endpoints for Toca 5 randomized clinical trial.
eTable 4. Baseline patient characteristics for patients in second recurrence.
eTable 5. Toca 511 viral RNA and DNA signal by cycle.
eTable 6. Proposed etiology for SBS Mutational signatures, from COSMIC.
Data Sharing Statement
References
- 1.Chamberlain MC. Treatment options for glioblastoma. Neurosurg Focus. 2006;20(4):E19. doi: 10.3171/foc.2006.20.4.12 [DOI] [PubMed] [Google Scholar]
- 2.Clarke JL, Ennis MM, Yung WK, et al. ; North American Brain Tumor Consortium . Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro-oncol. 2011;13(10):1118-1124. doi: 10.1093/neuonc/nor110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang S, Zhang P, Cairncross JG, et al. Phase III randomized study of radiation and temozolomide versus radiation and nitrosourea therapy for anaplastic astrocytoma: results of NRG Oncology RTOG 9813. Neuro-oncol. 2017;19(2):252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups . National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 5.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro-oncol. 2007;9(1):29-38. doi: 10.1215/15228517-2006-025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212-3218. doi: 10.1200/JCO.2012.47.2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943-953. doi: 10.1016/S1470-2045(14)70314-6 [DOI] [PubMed] [Google Scholar]
- 8.Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168-1174. doi: 10.1200/JCO.2009.23.2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry JR, Bélanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28(12):2051-2057. doi: 10.1200/JCO.2009.26.5520 [DOI] [PubMed] [Google Scholar]
- 10.Ostertag D, Amundson KK, Lopez Espinoza F, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro-oncol. 2012;14(2):145-159. doi: 10.1093/neuonc/nor199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell LA, Lopez Espinoza F, Mendoza D, et al. Toca 511 gene transfer and treatment with the prodrug, 5-fluorocytosine, promotes durable antitumor immunity in a mouse glioma model. Neuro-oncol. 2017;19(7):930-939. doi: 10.1093/neuonc/nox037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cloughesy TF, Landolfi J, Vogelbaum MA, et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-oncol. 2018;20(10):1383-1392. doi: 10.1093/neuonc/noy075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan DJ, Zhu JJ, Diago OR, et al. Molecular analyses support the safety and activity of retroviral replicating vector Toca 511 in patients. Clin Cancer Res. 2018;24(19):4680-4693. doi: 10.1158/1078-0432.CCR-18-0619 [DOI] [PubMed] [Google Scholar]
- 14.Cloughesy TF, Landolfi J, Hogan DJ, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8(341):341ra75. doi: 10.1126/scitranslmed.aad9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6(7):1003-1010. doi: 10.1001/jamaoncol.2020.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cloughesy TF, Brenner A, de Groot JF, et al. ; GLOBE Study Investigators . A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro-oncol. 2020;22(5):705-717. doi: 10.1093/neuonc/noz232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Den Bent M, Eoli M, Sepulveda JM, et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro-oncol. 2020;22(5):684-693. doi: 10.1093/neuonc/noz222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm SA, Chamberlain MC. Anaplastic astrocytoma. CNS Oncol. 2016;5(3):145-157. doi: 10.2217/cns-2016-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SongTao Q Lei Y, Si G, et al. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. 2012;103(2):269-273. [DOI] [PubMed]
- 20.Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75(17):1560-1566. doi: 10.1212/WNL.0b013e3181f96282 [DOI] [PubMed] [Google Scholar]
- 21.Anagnostou V, Yarchoan M, Hansen AR, et al. Immuno-oncology trial endpoints: capturing clinically meaningful activity. Clin Cancer Res. 2017;23(17):4959-4969. doi: 10.1158/1078-0432.CCR-16-3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMaterials and eMethods
eReferences
eFigure 1. Kaplan-Meier overall survival curve with Toca FC cycle numbers.
eFigure 2. Kaplan-Meier overall survival curve for pre-planned subpopulations.
eFigure 3. Kaplan-Meier overall survival curve for subpopulations within the second recurrence.
eFigure 4. Workflow for whole exome sequencing and RNA sequencing data analyses.
eFigure 5. Quality assessment of phase 2 and phase 3 portions of Toca 5 tumor RNA sequencing.
eFigure 6. Assessment of Toca 5 RNA sequencing data quality.
eFigure 7. Boxplot comparing intra-tumor Pearson correlations in mRNA expression vs inter-tumor Pearson correlations in mRNA expression.
eFigure 8. mRNA expression profiles of Toca 5 tumors.
eFigure 9. Molecular classification of Toca 5 tumor samples.
eFigure 10. Relationships between patients’ tumor molecular classification and survival.
eFigure 11. Sequencing coverage metrics across targeted regions for normal and tumor samples.
eFigure 12. Summary of DNA sequencing analyses of Toca 5 tumors.
eFigure 13. Comparison of genetic profiles from 1st and 2nd recurrence tumors.
eFigure 14. Comparison of genetic profiles from 2nd recurrence patients in the two treatment arms.
eFigure 15. Relationships between tumor DNA alterations and survival.
eFigure 16. Tumor copy number variants inferred from exome sequencing data.
eFigure 17. CNV subtypes for IDH1-wildtype GBM patients.
eFigure 18. Mutational signatures in hypermutated tumors.
eFigure 19. Number of predicted tumor neoantigens correlates with tumor mutational burden.
eFigure 20. Relationships between tumor purity and molecular subtype.
eFigure 21. Pairwise correlations among genetic alterations, RNA subtypes and histology.
eFigure 22. Relative and absolute abundance of leukocyte populations in Toca 5 tumors.
eFigure 23. mRNA expression differences between IDH1 mutant tumors and IDH1 wild-type tumors.
eFigure 24. Comparison between treatment-arms of immune cell population levels in tumors at time of surgery as measured by iSort.
eFigure 25. Stratification of patients based on tumor immune cell levels inferred by iSort.
eFigure 26. Differences in immune cell composition between IDH1 mutant tumors and IDH1 wildtype tumors.
eFigure 27. Differences in immune cell and neoantigen composition between AA tumors and GBM tumors.
eFigure 28. Differences in immune cell composition between first and second recurrence tumors.
eFigure 29. Differences in peripheral immune cell composition between IDH1 wildtype and IDH1 mutant patients.
eFigure 30. Differences in peripheral immune cell composition between AA and GBM patients.
eFigure 31. Differences in peripheral immune cell composition between patients at first and second recurrence.
eFigure 32. Baseline peripheral immune balance between the control arm and the Toca 511/FC arm
eTable 1. Baseline demographics and neuro-oncology history for Phase 2 and Phase 3 patients.
eTable 2. Toca FC and SOC cycle numbers and schedule for patients.
eTable 3. Secondary endpoints for Toca 5 randomized clinical trial.
eTable 4. Baseline patient characteristics for patients in second recurrence.
eTable 5. Toca 511 viral RNA and DNA signal by cycle.
eTable 6. Proposed etiology for SBS Mutational signatures, from COSMIC.
Data Sharing Statement