Abstract
Main skin manifestations of COVID-19 have been recently classified. However, little is known about cutaneous histopathological patterns and the presence of SARS-CoV-2 in these skin lesions. We present a healthy 29-year-old man who developed a leucocytoclastic vasculitis for COVID-19 with positive SARS-CoV-2 PCR in skin biopsy.
Keywords: dermatology, vasculitis, infectious diseases
Background
In early 2020, a novel betacoronavirus named SARS-CoV-2 was identified in Wuhan City, Central China. SARS-CoV-2 spread rapidly to other regions, provoking a world pandemic. The virus affects mainly the respiratory system, causing bilateral pneumonia and, in some cases, acute respiratory distress syndrome. However, it can also affect other organs, including skin. Skin manifestations have been recently classified. Nevertheless, little is known about cutaneous histopathological patterns and the presence of SARS-CoV-2 in these skin lesions. In this study, we present a healthy 29-year-old man who developed a leucocytoclastic vasculitis in the context of advanced SARS-CoV-2 infection with positive SARS-CoV-2 PCR in skin biopsy.
Case presentation
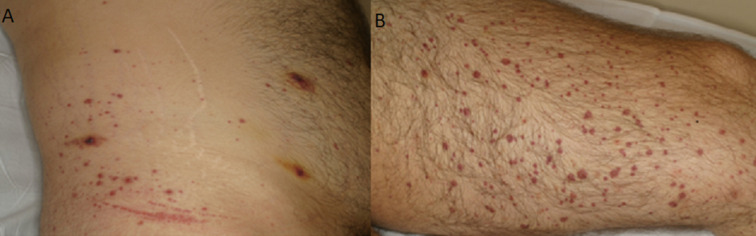
A previously healthy 29-year-old man was attended in our Sexual Health Clinic for evaluation of purple palpable papules, some of them with a necrotic centre, and serohaematic blisters on his abdomen, buttocks and both lower legs and feet that had appeared 2 days earlier (figure 1A, B). He also explained muscular pain in the lower legs, colic abdominal pain with diarrhoea and a single episode of hematochezia. He denied any new medication.
Figure 1.
Purple palpable papules, some of them with a necrotic centre and serohaematic blisters in the abdomen (A) and lower legs (B). Koebner phenomena can be observed due to pressure from tight-fitting undergarments.
He had been working during the SARS-CoV-2 pandemic as an orderly in a hospital. He had sex with men, with eventual no protective sex with different partners, and he is undergoing treatment with PrEP (pre-exposure prophylaxis) for 2 months.
One month before consultation, our patient was clinically diagnosed with COVID-19 due to fever, malaise and colitis. He received only symptomatic treatment, and these manifestations lasted for 10 days. While three different nasopharyngeal swabs for SARS-CoV-2 PCR done at this moment were negative, serological tests performed after 3 weeks from the onset of symptoms detected IgG and IgM antibodies against SARS-Cov-2. As a consequence of the onset of the skin lesions, the patient was admitted for further studies.
Investigations
The blood test showed no anaemia, normal white blood cell counts, elevated D-dimer of 1864 ng/ml (0–143 mg/dL) and erythrocyte sedimentation rate (ESR) of 31 mm/hour (0–15 mm/hour). The renal and hepatic functions were normal. Autoimmune tests for anti-nuclear antibodies (ANA 1/40), extractable nuclear antigen (ENA), antineutrophil cytoplasmic antibodies (ANCA), antiphospholipid antibodies and cryoglobulins were negative; proteinogram was normal; the analysis of gammaglobulins showed an IgA level of 260 mg/dL (70–400 mg/dL). In addition, 24-hour urinalysis was performed, showing no proteinuria nor haematuria. Sexually transmitted infection (STI) screening showed a primary syphilis (with negative rapid plasma reagin) that were treated with 2.4 MU intramuscular benzathine.
Lower extremity venous Doppler evaluation was performed due to lower limb pain and elevated D-dimer without detecting venous thrombosis. Cultures from stool and anal STI tests were negative. The colonoscopy showed minimal erythematous mucous areas in the rectum. The skin biopsy showed leucocytoclastic vasculitis, and direct immunofluorescence staining detected fibrinogen deposition. Finally, SARS-CoV-2 PCR from skin biopsy was positive (figure 2).
Figure 2.
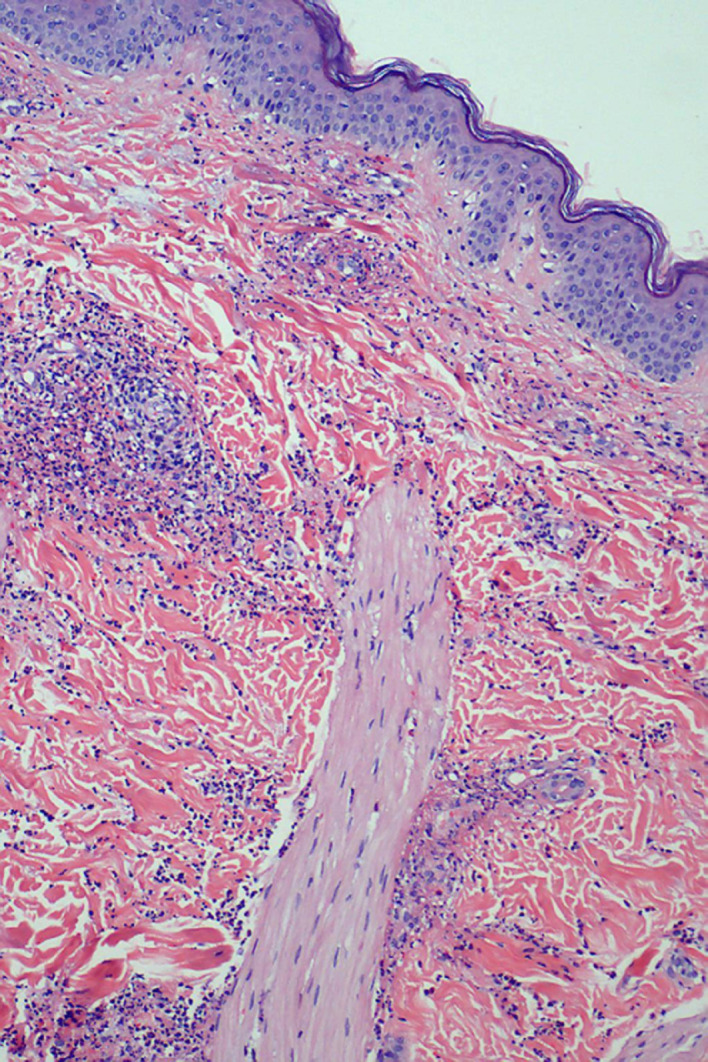
Histopathological examination. There is a heavy neutrophilic infiltrate in the walls of small vessels and their vicinity with leucocytoclasis. There is also fibrinoid necrosis and extravasation of red blood cells (H&E, ×10).
Differential diagnosis
Cutaneous leucocytoclastic vasculitis is a small vessel vasculitis that can be idiopathic or associated with a wide range of infections, drugs, connective tissue diseases or malignancies. Antibiotics, b-lactams in particular, are the most common drugs that can trigger this condition. In our case, the patient is undergoing treatment with PrEP (tenofovir and emtricitabine). However, there are very few reports suggesting that tenofovir can induce a leucocytoclastic vasculitis. He denied any new medication. He had no rashes, photosensitivity, oral ulcers, dry mouth, dry eyes or arthritis that made us suspect connective tissue diseases such as lupus erythematosus, Sjogren syndrome or rheumatoid arthritis. Furthermore, the autoimmune panel was negative. Another possibility was a systemic vasculitis, such as an ANCA-associated vasculitis (granulomatosis with poliangeitis, eosinophilic granulomatosis with poliangeitis), but the ANCA antibodies were negative; eosinophils were not elevated; and there were not any of the other characteristic systemic manifestations of those diseases (sinusitis, nephropathy and neuropathy). In fact, it was more likely to be Schonlein-Henoch IgA vasculitis, because it usually causes gastrointestinal bleeding and abdominal pain. However, it is a condition that affects younger people, and the laboratory analysis showed high levels of neither IgA nor haematuria. In addition, direct immunofluorescence on skin biopsy did not identify IgA deposition. Mixed cryoglobulinemic vasculitis may also manifest as a palpable purpura in the lower legs. Then again, however, it usually has other systemic manifestations, and there is a strong association with hepatitis C virus. Both hepatitis and HIV serological testing were negative in our patient.
Viral and bacterial infections could be another cause of leucocytoclastic vasculitis. We performed an STI screening (blood sample and anal swab) showing only a primary syphilis (with negative RPR) that was treated without any improvement in the skin lesions. Stool cultures were negative. In our case, it was remarkable that the patient had symptoms of COVID-19 infection 1 month before consultation with positive serological tests. Knowing that SARS-CoV-2 can cause different skin manifestations and after excluding common causes of leucocytoclastic vasculitis, we performed a SARS-CoV-2 PCR in skin biopsy with a positive result. This result suggests the presence of viral SARS-CoV-2 particles in the vessel endothelium. This result allowed the diagnosis of leucocytoclastic vasculitis with gastrointestinal involvement as a manifestation of COVID-19.
Treatment
Corticosteroids were started (0.5 mg/kg/day) with clinical improvement achieved after 5 days.
Outcome and follow-up
After the improvement of the lesions, the medication was progressively tapered. The skin lesions disappeared completely after 15 days and the patient remained asymptomatic after 3 weeks of follow-up.
Discussion
SARS-CoV-2 is a newly identified coronavirus that has spread rapidly from Wuhan to different countries around the world since December 2019.1 Spain is one of the most affected European countries, with 251 789 confirmed cases and 28 388 deaths at the time of this publication (6 July 2020).2
The most common symptoms at the onset of the illness are fever, dry cough, headache, fatigue and myalgia. More than half of the patients develop dyspnoea, with a median time from the onset of symptoms of 5 days. A high proportion of patients have bilateral pneumonia, and some of them progress rapidly to acute respiratory distress syndrome, which is highly related to mortality. The prognosis depends on age, comorbidities and immune status of the patients. An elevated D-dimer has been observed in among of patients with SARS-CoV-2 infections. In our patient, we can still detect an elevated D-dimer, but this probably is coming down from a higher value in the previous weeks.
Different reports have described increasing cases of COVID-19-related cutaneous manifestations, either in the early stage of the infection or as a late-onset manifestation.3 A recent series analysed the cutaneous involvement in 148 patients with COVID-19 hospitalised in Lecco Hospital, Lombardy, Italy.4 Skin lesions were described as rash, urticaria or chicken pox-like lesions. However, no clinical images were available in this study. Recently, a prospective study with 375 cases in Spain classified skin lesions in five different clinical patterns: pseudo-chilblains in acral areas in younger patients, vesicular eruptions, urticarial lesions, other maculopapular lesions that usually appear at the same time of other symptoms, and livedo or necrosis, with lesions suggesting occlusive vascular disease. The last one usually affects people with more severe conditions.5 Some patients presented purpuric lesions located in different areas. It has been also described a rash similar to dengue,6 acroischaemia in a critical patient,7 plaques in the heels8 and urticarial lesions.9 Unfortunately, most of these studies lack histopathological studies, which prevents obtainment of physiopathological conclusions.
Leucocytoclastic vasculitis is a small vessel vasculitis characterised by immune complex aggregates in the postcapillary venules, infiltration of polymorphonuclears cells, fibrinoid necrosis and leucocytoclasis.10 It can be idiopathic or related to autoimmune disease, malignancies, infection or medications. The most common manifestation consists of symmetrically distributed palpable purpura, most usually located in the lower legs, as seen in our patient. It may be accompanied by systemic symptoms such as fever, malaise or arthralgias. In less than half of the patients, vasculitis can affect other major organs, like kidneys or the gastrointestinal system. The latent period between inciting agent and leucocytoclastic vasculitis can be from 7 to 10 days to more than 2 weeks, which is the time required to produce a sufficient quantity of antibody to produce antigen–antibody complexes.
Mayor-Ibarguren et al described an 83-year-old woman who developed leucocytoclastic vasculitis with positive serum IgM and IgG against SARS-CoV-2 but negative oropharyngeal swab PCR, as seen in our patient.11 This could indicate that leucocytoclastic vasculitis develops as a late-onset manifestation of COVID-19 infection. Furthermore, it is believed that SARS-CoV-2 antigens may promote the development of antibodies, forming antigen–antibody complexes that target the vascular endothelium of the skin and provoking the appearance of the leucocytoclastic vasculitis. However, as many other skin lesions described in patients with COVID-19, there is no solid evidence about the role of SARS-CoV-2 in the ethiopathogenic mechanism of the skin lesions. A recent work from Colmenero et al has considered that the presence of viral particles in the endothelium from patients with chilblains and the histological evidence of vascular damage support a causal relation of the lesions with SARS‐CoV‐2.12
As far as we know, our work is the first to describe the presence of SARS-CoV-2 in a skin biopsy taken from leucocytoclastic vasculitis lesions. This finding supports a relationship between leucocytoclastic vasculitis and coronavirus instead of relating it with other infections or medications. Further works are needed to clarify if particles detected by PCR in skin tissue, specifically in endothelial cells of small dermal vessels, are viable virions or, more probably, just immune complex with non-viable viral particles. This second possibility may explain why our patient has a positive SARS-CoV-2 PCR in skin biopsy after 1 month of having COVID-19 infection. Finally, it should be studied if ulcerated skin lesions could be infective.
Learning points.
Cutaneous leucocytoclastic vasculitis is a small vessel vasculitis that could be associated with a wide spectrum of pathologies (infections, drugs, malignancies and autoimmune disorders)
SARS-CoV-2 can cause different types of skin lesions, with leucocytoclastic vasculitis being one of them.
The presence of positive PCR in skin biopsy is a reliable tool to ensure the association between SARS-CoV-2 infections and cutaneous manifestations.
Acknowledgments
We thank Dr B Almirante, Dr V Garcia-Patos and Dr A Anton for their intellectual contributions.
Footnotes
Twitter: @carlos_gocr
Contributors: All authors were involved in the care pathway of the patient and in the development of this article. MCG and CG-C were involved in the investigations, did the literature review and wrote the manuscript. BF was responsible for the acquisition of pathology results. MJB provided guidance in preparing the article and contributed substantially to revising the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Harapan H, Itoh N, Yufika A, et al. . Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health 2020;13:667–73. 10.1016/j.jiph.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centro de Coordinación de Alertas y Emergencias Sanitarias, Ministerio de Sanidad, Gobierno de España Actualización n°130. Enfermedad POR El coronavirus (COVID-19). Situación en España. Available: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCovChina/situacionActual.htm [Accessed 06 Jul 2020].
- 3.Skroza N, Bernardini N, Balduzzi V, et al. . A late‐onset widespread skin rash in a previous COVID‐19‐infected patient: viral or multidrug effect? J Eur Acad Dermatol Venereol 2020;34 10.1111/jdv.16633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol 2020;34:e212–3. 10.1111/jdv.16387 [DOI] [PubMed] [Google Scholar]
- 5.Galván Casas C, Català A, Carretero Hernández G, et al. . Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020;183:71–7. 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol 2020;82:e177. 10.1016/j.jaad.2020.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Cao W, Xiao M, et al. . [Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia]. Zhonghua Xue Ye Xue Za Zhi 2020;41:E006. 10.3760/cma.j.issn.0253-2727.2020.0006 [DOI] [PubMed] [Google Scholar]
- 8.Estébanez A, Pérez-Santiago L, Silva E, et al. . Cutaneous manifestations in COVID-19: a new contribution. J Eur Acad Dermatol Venereol 2020;34:e250–1. 10.1111/jdv.16474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J-J, Dong X, Cao Y-Y, et al. . Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730–41. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 10.Koutkia P, Mylonakis E, Rounds S, et al. . Leucocytoclastic vasculitis: an update for the clinician. Scand J Rheumatol 2001;30:315–22. 10.1080/030097401317148499 [DOI] [PubMed] [Google Scholar]
- 11.Mayor-Ibarguren A, Feito-Rodriguez M, Quintana Castanedo L, et al. . Cutaneous small vessel vasculitis secondary to COVID-19 infection: a case report. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16670. [Epub ahead of print: 22 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colmenero I, Santonja C, Alonso‐Riaño M, et al. . SARS‐CoV‐2 endothelial infection causes COVID‐19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol 2020. 10.1111/bjd.19327 [DOI] [PMC free article] [PubMed] [Google Scholar]