Abstract
In the dynamic era of climate change, agricultural farming systems are facing various unprecedented problems worldwide. Drought stress is one of the serious abiotic stresses that hinder the growth potential and crop productivity. Silicon (Si) can improve crop yield by enhancing the efficiency of inputs and reducing relevant losses. As a quasi-essential element and the 2nd most abundant element in the Earth’s crust, Si is utilized by plants and applied exogenously to combat drought stress and improve plant performance by increasing physiological, cellular and molecular responses. However, the physiological mechanisms that respond to water stress are still not well defined in Saccharum officinarum plants. To the best of our knowledge, the dynamics of photosynthesis responsive to different exogenous Si levels in Saccharum officinarum has not been reported to date. The current experiment was carried out to assess the protective role of Si in plant growth and photosynthetic responses in Saccharum officinarum under water stress conditions. Saccharum officinarum cv. ‘GT 42’ plants were subjected to drought stress conditions (80–75%, 55–50% and 35–30% of soil moisture) after ten weeks of normal growth, followed by the soil irrigation of Si (0, 100, 300 and 500 mg L−1) for 8 weeks. The results indicated that Si addition mitigated the inhibition in Saccharum officinarum growth and photosynthesis, and improved biomass accumulation during water stress. The photosynthetic responses (photosynthesis, transpiration and stomatal conductance) were found down-regulated under water stress, and it was significantly enhanced by Si application. No phytotoxic effects were monitored even at excess (500 mg L−1). Soil irrigation of 300 mg L−1 of Si was more effective as 100 and 500 mg L−1 under water stress condition. It is concluded that the stress in Saccharum officinarum plants applied with Si was alleviated by improving plant fitness, photosynthetic capacity and biomass accumulation as compared with the control. Thus, this study offers new information towards the assessment of growth, biomass accumulation and physiological changes related to water stress with Si application in plants.
Keywords: Biomass, Drought, Diurnal, Leaf gas exchange, Saccharum officinarum L., Silicon
Introduction
Water scarcity is one of the most crucial abiotic stresses for plants in the dynamic era of climate change. A number of studies have mentioned that water deficit is more harmful than other abiotic stresses (Chen et al., 2017; Verma et al., 2019a; Li et al., 2019; Raza et al., 2019; Verma et al., 2020). Under limited or highly variable water, plants have developed various mechanisms of resistance to water deficit by reduction of the plant life cycle (Mitra, 2001; Cia et al., 2012). Therefore, plants have evolved in various cellular and molecular strategies to cope with water deficit (Ijaz et al., 2017; Ali et al., 2018). Abiotic stresses are estimated to reduce about 51–82% agricultural crop production. The metabolic changes to water deficit in varieties with various responses to water stress have been well documented in different agricultural crops (Kang et al., 2011; Witt et al., 2012; Silvente, Sobolev & Lara, 2012; Zhao et al., 2014; Verma et al., 2019a, 2020).
Low soil water capacity in the dry season is one of the most important limitations to photosynthesis and consequently to Saccharum officinarum production (Chen et al., 2011; Verma et al., 2019a). Under limited water conditions, disturbances in photosynthetic apparatus at the molecular and cellular levels are associated with low electron transport through photosystem II (PS II) and/or with structural damages of PS II and the light harvesting complexes (Hura et al., 2007; Wu et al., 2008). Photosynthetic and growth responses are dependent on environmental variables and developmental phages. It is expected that changes in temperature, light intensity and available water content, and changes across the growth phases will influence the growth and physiological dynamics during diurnal cycle (De Souza et al., 2018; Verma et al., 2019a). Improving photosynthetic capacity is linked for the enhancement of biomass and crop productivity. Soil water content and nutrients can also play significant roles in sustaining the photosynthetic responses in agricultural crops (Shangguan, Shao & Dyckmans, 2000; Li et al., 2016, 2019; Khan et al., 2017; Verma et al., 2020). Under water deficiency, Si application can improve and/or enhance photosynthetic capacity, root growth-development, nutrient uptake and consequently increase crop productivity (Verma et al., 2019a, 2019b, 2019c; Li et al., 2019). Various agronomic strategies have been adopted for this purpose. One of the advanced strategies is the use of plant bio-stimulator to enhance the adaptability and protection of crop plants subjected to environmental stresses.
Silicon (Si) is the second most important element in the earth’s crust, which can improve crop resistance to reduce the negative impacts of biotic and abiotic stresses such as insufficient water, extreme air temperature, UV, cold, alkalinity, nutritional imbalance, heavy metal toxicity, plant pathogens and insect pests in various crop plants (Liang et al., 2007; Guo et al., 2016; Reynolds et al., 2016; Ju et al., 2017; Chen et al., 2018; Verma et al., 2019a). Plants generally take up Si in the form of silicic acid from soil and nutrient solutions and Si is the only nutrient element that is not detrimental when absorbing excess in the plant’s organ (Ma, Miyake & Takahashi, 2001; Ma & Yamaji, 2006; Chen et al., 2018). The maximum solubility of Si(OH)4 in solution is nearly 2 mM, and its concentration in soil solutions usually differ between 0.1 and 0.6 mM (Raven, 1983; Epstein, 1994). Moreover, orthosilicic acid (pKa1 = 9.84, pKa2 = 13.2, at 25 °C), the form of Si accessible to plants (Casey et al., 2004), is soluble in water only up to about 2 mM at 25 °C, above which polymerization into silica (SiO2) gels begins to occur (Ma, Miyake & Takahashi, 2001). Under similar situations, plant varieties have different abilities to accumulate Si, a reality that has been known, if poorly understood, for a long time.
The protective role of Si in metabolic, physiological and/or anatomical activities in crop plants against environmental stresses have been widely documented (Van Bockhaven, De Vleesschauwer & Höfte, 2013; Zhu & Gong, 2014; Shi et al., 2016; Verma et al., 2019a). The beneficial effects of Si against limited water supply/water deficiency have been extensively assessed in many crop plants, like Oryza sativa (Ming et al., 2012), Zea mays (Kaya, Tuna & Higgs, 2006; Amin et al., 2014), Triticum aestivum (Pei et al., 2010; Gong & Chen, 2012), Sorghum bicolor (Liu et al., 2014), Solanum lycopersicum (Shi et al., 2016), Saccharum spp. (Verma et al., 2019a, 2019b, 2019c, 2020), cucumber (Ma et al., 2004), Kentucky bluegrass (Saud et al., 2014), canola (Habibi, 2014) and alfalfa (Liu & Guo, 2013).
Sugarcane (Saccharum officinarum L.) is one of the most important cash crop in the globe due to its great demand for sugar and renewable energy sources to replace fossil fuels. Unlikely, in various regions, especially in the tropical and sub-tropical areas, the production of Saccharum officinarum is markedly decreased up to 60% due to availability of insufficient water for irrigation (Robertson et al., 1999; Verma et al., 2019a).
However, knowledge about how Si modulates the morphological, physiological and biomass accumulation in Saccharum officinarum “GT 42” during water stress remains elusive. Although the essentiality of this element to plants is still debated, there have been significant impacts in our understanding of the uptake of Si in plants. In addition, the present database regarding the precise amount of Si for its application method in Saccharum officinarum plants is limited. Therefore, the present study was conducted to investigate the possible impacts of exogenous application of Si on growth, biomass accumulation and photosynthetic responses in Saccharum officinarum plants subjected to water stress. Our work in Saccharum officinarum may help to better understand the mechanisms and functions for Si-mediated water stress tolerance in plants.
Materials and Methods
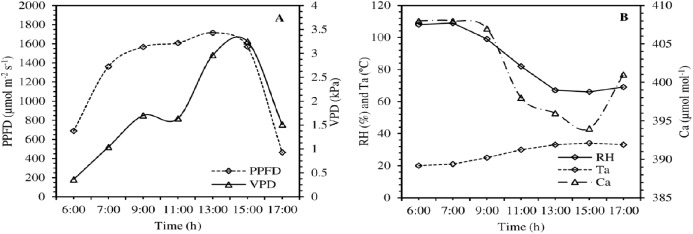
Saccharum officinarum “GT 42” single bud cane setts were planted in fertile farmland soil in greenhouse in March 2019 at Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China. After germination (8 weeks), the seedlings were shifted in pots (soil capacity 3.5 kg), and continued to receive full irrigation to keep moisture capacity at 100–95% for proper root development before treatment. For the stressed treatment, limited water was imposed by gradually withdrawing irrigation until 80–75%, 55–50% and 35–30% of soil moisture capacity, determined by Soil Moisture Meter (Top Instrument Co. Ltd., Zhejiang, China). At the end of July, silicon fertilizer was applied as 0, 100, 300 and 500 mg L−1, respectively, directly in the soil. Calcium metasilicate powder (Wollastonite, CaO.SiO2) was used as a source of Si. The irrigation water was applied to the plant roots to keep 100–95% (normal), 80–75% (mild), 55–50% (moderate) and 35–30% (severe) of soil moisture capacity, respectively. The total amount of Si solution applied to well water and stressed-plants was the same. The climatic variables (ambient air temperature (Ta), air relative humidity (RH), ambient CO2 concentration (Ca), photosynthetic photon flux density (PPFD) and vapor pressure deficit (VPD)) were recorded diurnally (Fig. 1). The experiment was designed as completely randomized with ten biological replicates.
Figure 1. Diurnal changes of climatic variables such as photosynthetically photon flux density (PPFD), vapor pressure deficit (VPD), air relative humidity (RH), air temperature (Ta) and ambient CO2 concentration (Ca) from 06:00 to 17:00 (A and B) on the measuring day.
Diurnal gas exchange
Photosynthesis (PN), stomatal conductance (gs) and transpiration rate (E) were observed at 60 days after treatment with limited water and exogenous use of Si, using portable photosynthesis system (LI-COR 6800; Biosciences, Lincoln, NE, USA). Diurnally leaf gas exchange was measured every 2 h from 07:00 to 17:00 in cloudless day on stressed and non-stressed plants (five replicates). The physiologically active leaves (middle part of leaf +1) were selected to place the leaf chamber for measuring all photosynthetic parameters at open environmental conditions without changing leaf angle/position.
Determination of morphological and biomass traits
The plant height and leaf area-expansion were observed by a measuring meter and Leaf Area Meter (CI-203 Area Meter; CID, Inc., Camas, WA, USA). At the end of experiment (60 days), the Saccharum officinarum plants were harvested, washed with running water and weighted. The plant organs were kept in paper bags, oven-dried at 65 ± 2 °C and dry mass was weighted until the weight was constant.
Model hypothesis
Physiological responses of plants are a result of complex chain reactions. Solar radiation is an essential input to start PN and other associated bio-enzymatic chemical reactions. E and gs are interrelated with PN process. Initially with increase in solar radiation PN increases and acquires the highest optimum PN. Soon after initiations of PN process inhibitive internal chain reactions also get started resulting in decline of PN and interrelated bio-enzymatic chemical chain reactions within the leaves even after steady increase in solar radiation. Higher temperature is an indicator of high kinetic energy which speeds up chemical reactions in addition to initiate the process. After an optimum temperature level the enzymes start becoming denatured retarding the PN process. For different plant species optimal temperature requirements differ (Herrmann, Haeder & Ghetti, 1997). Increase in solar radiation causes increase in temperature of the surrounding affecting the PN. Thus PN and related physiological responses are function of climatic parameters such as PPFD, RH, Ca and Ta.
Figures 1A and 1B shows the diurnal variations of PPFD, Ta, Ca, RH and VPD. The variation of PPFD is skewed. From an initial zero value (5:00) it reaches the peak between 9:00 and 13:00 and thereafter it starts declining continuously to a zero value (19:00) at sun set. It can be further seen from Fig. 1B that the temperature starts rising immediately after sunrise and reaches a peak value between 13:00 and 15:00 and starts declining thereafter. The sun set temperature is much higher than the sun rise temperature during the day of observation. The continuous reduction of relative air humidity with sun shine hour (Fig. 1B). Air humidity was observed to be 88% at 6:00 in the morning and declines to the tune of 36% at 17:00 in the evening. RH is minimum at 15:00 and starts increasing thereafter. The leaf temperature changes with time which follows the suit of Ta. Initially it remains higher but immediately after acquiring the peak it becomes lower than the ambient. The overall physiological response variation is determined by the pattern of solar radiation. It directly affects the rate and pattern of physiological responses. Before acquiring the photosynthesis peak rate, retarding and inhibitive processes also starts, which slow down the PN and other physiological responses. Biochemical changes taking place within the cell during the PN affect the physiological responses. Overall physiological responses are dependent on direct responsive factors such as solar radiation and inhibitive and retarding factors inside the plant cell and climatic conditions of the plant leaves such as decreasing solar radiation, increasing air and leaf temperature and depleting RH and their effect on physiological responses of plant cell. Change of physiological responses with respect to time can be hypothesized as below.
Changes of physiological parameters are directly proportional to summation of nth order responsive physiological response rate (p/tn). Mathematically it can be written as:
| (1) |
where,
p = physiological parameters
t = time
κ = order of the physiological parameter’s constant
n = order of the rate of physiological parameters
Eq. (1) can be expanded as below.
| (2) |
where,
α, β, γ … μ and ξ = individual order constants
Considering n = 2 and ignoring the higher terms on Eq. (1) reduces to the following form.
| (3) |
Equation (3) can be now rewritten as below:
| (4) |
where λ is proportionality constant.
Separating variables and integrating above equation one will obtain.
| (5) |
| (6) |
| (7) |
| (8) |
In Eq. (8) all the terms are constants which were replaced by another constant “ψ” and substituting the value of C = ψ into Eq. (6) one will get general solution of Eq. (4).
| (9) |
Taking antilog on the both side the solution can be rewritten as under:
| (10) |
Combining all the constant terms together the above equation will take the following forms:
| (11) |
where, ω = αλ and η = βλ.
The model cannot be defined at time t = 0, hence should be started with some opening value of physiological response against a given time.
Verification of the model
Physiological parameters such as PN, E and gs under drought stressed and normal conditions with Si application were fitted in the derived models (Eq. 10) and their parameters were worked out (Fig. S1; Table S1).
Cumulative photosynthetic responses
Cumulative responses are essential for assessing the performance of plants under limited water irrigation. Integration of Eq. (10) is difficult hence its numerical integration was obtained. Graphical integration is easier and can be used for field application. Cumulative photosynthetic responses were calculated numerically by integrating proposed model and presented in Tables S2–S4 and variations are shown in Figs. S2–S4.
| (12) |
where,
Pt = cumulative photosynthetic response from time t = 0 to time t = t.
Daily total photosynthetic CO2 assimilation (178.74–200.65 (control), 143.32–159.34 (mild), 111.25–120.21 (moderate) and 86.04–91.74 µmol CO2 m−1s−1 (severe stress)), transpiration rate (24.99–28.47 (control), 22.56–24.50 (mild) 19.41–20.89 (moderate) and 15.78–17.14 mmol CO2 m−1s−1 (severe stress)) and stomatal conductance (1,353.90–1,530.89 (control), 1,070.48–1,270.27 (mild), 746.78–867.65 (moderate) and 537.60–660.69 mmol H2O m−2 s−1 (severe stress)) were observed under limited water irrigation and Si application. There was almost 50% gain in photosynthetic parameters due to limited water and Si. It can be further seen from Tables S2 to S4 and Figs. S2 to S4 that by 12:00 at noon almost 60% photosynthetic responses are achieved and in the afternoon nearly 40% responses are achieved.
The experimental data were organized and processed between the limited water and Si application (± SD, n = 5). Data were analyzed by using GraphPad Prism 5.00 statistical software for windows (GraphPad Software, San Diego, CA, USA). One-way analysis of variance (ANOVA) was carried out to find out the significant differences among the treatment means at P < 0.05.
Results
Diurnal variation of environmental variables
On the measurement day, the PPFD enhanced steeply from 06:00 to 11:00, remained at highest levels up to 15:00, and then decline sharply. Under the impacts of PPFD diurnal changes, Ca and RH (Fig. 1) were at maximum in the early morning, followed by a sharp decline, remaining at relatively low levels during the midday period, and then began to enhance from 15:00. In contrast, Ta exhibited a diurnal trend similar to PPFD (Fig. 1).
Effect of silicon on growth and biomass traits
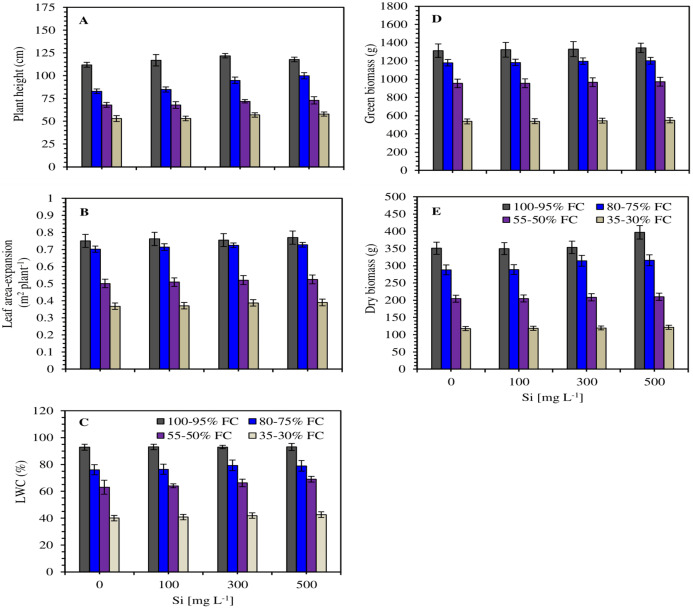
The Saccharum officinarum plants showed a drastic decline in plant height (PH), leaf area-expansion (LAE), leaf relative water content (LWC) and plant biomass (fresh and dry) under water stress, compared to well irrigation control (Figs. 2A–2E). The negative effects of low soil moisture capacity on growth and biomass were significantly (P < 0.05) mitigated and gradually enhanced with increasing Si levels (100–500 mg L−1). PH, LAE, RWC, fresh and dry mass of the plants in 100–95%, 80–75%, 55–50% and 35–30% of soil moisture capacity were found higher than the control without Si, with 2.41–20.48%, 0.65–6.12%, 0.17–9.54%, 0.14–2.42% and 0.30–13.12% increase, respectively (Figs. 2A–2E).
Figure 2. Effect of silicon (0, 100, 300 and 500 mg L−1) on plant height (A), leaf area-expansion (B), leaf water content (LWC) (C), fresh/green (D) and dry mass (E) in Saccharum officinarm plants against limited soil moisture capacity (100–95%, 80–75%, 55–50% and 35–30% of FC).
Data are means ± SD (n = 5). FC = field capacity.
Growth and biomass traits exhibited an initial increase with the application of Si (100–300 mg L−1) and then declined considerably at excess (500 mg L−1). Shoot fresh and dry biomass accumulation in Si-treated plants showed significant (P < 0.05) improvement compared to normal plants under stress condition. The 300 mg L−1 Si concentration exhibited the most significant effects on growth and biomass of Saccharum officinarum plants, followed by 500 mg L−1 Si (Fig. 2).
Water limitation and silicon effects on the diurnal changes of leaf gas exchange
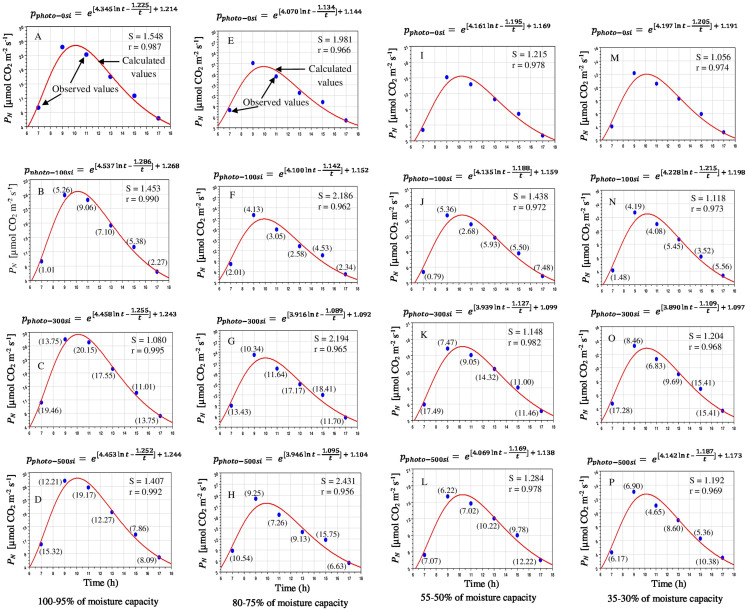
One of the prime impacts of water stress is on the physiological process of photosynthetic responses. Water stress caused a severe loss in photosynthesis. Diurnal changes of photosynthesis are shown in Fig. 3. Overall the pattern of PN mirrored that of gs. PN and gs maximum reached at 9:00 as PPFD and gs enhanced. Subsequently, PN steadily declined in all stressed plants and Si supplemented until a minimum value was reached in the early evening (17:00), similar to gs and E. Hence, gs was the main limiting factor for PN of mesophyll cells at this time of the day. At 17:00, photosynthetic values were found lowest due to low PPFD and gs. Water and fertilization did affect the trend in the diurnal variation of PN. Under different soil moisture levels such as 100–95, 80–75, 55–50 and 35–30%, the PN in the 300 mg L−1 treatment was higher than that of 500 mg L−1 and control (Figs. 3A–3P). The exogenous application of Si effects under non-limiting water and stressed conditions were significantly (P < 0.05) different at each measurement points (Table 1).
Figure 3. Diurnal effects of silicon on photosynthetic CO2 assimilation rate in Saccharum officinarm “GT 42” plants under limited water supply such as 100–95% (A–D), 80–75% (E–H), 55–50% (I–L) and 35–30% of soil moisture capacity (M–P) with four levels of Si concentrations, for example, 0, 100, 300 and 500 mg L−1.
Parenthesis values indicate percentage gain against control condition.
Table 1. Statistical analysis variance of four silicon concentrations on photosynthetic CO2 assimilation rate (PN), stomatal conductance (gs) and leaf transpirational rate (E) of Saccharum officinarum “GT 42” plants at diurnal and different soil water availabilities.
| Treatment (%FC) | Time (h) | |||||
|---|---|---|---|---|---|---|
| 07:00 | 09:00 | 11:00 | 13:00 | 15:00 | 17:00 | |
| Effect of Si on PN | ||||||
| 100–95 | NS | ** | ** | ** | NS | NS |
| 80–75 | ** | ** | ** | ** | ** | NS |
| 55–50 | ** | NS | NS | NS | NS | NS |
| 35–30 | NS | NS | NS | NS | NS | NS |
| Effect of Si on E | ||||||
| 100–95 | ** | ** | ** | ** | NS | ** |
| 80–75 | ** | ** | ** | ** | NS | NS |
| 55–50 | ** | ** | ** | ** | ** | NS |
| 35–30 | ** | ** | ** | NS | NS | ** |
| Effect of Si on gs | ||||||
| 100–95 | ** | ** | NS | ** | ** | ** |
| 80–75 | NS | ** | ** | ** | NS | NS |
| 55–50 | NS | NS | NS | NS | NS | ** |
| 35–30 | NS | NS | ** | NS | ** | NS |
Notes:
The Saccharum officinarum plants were exposed to four soil water conditioNS (control, mild, moderate and severe drought, corresponding to available soil water capacity between 100–95%, 80–75%, 55–50% and 35–30% of the field capacity) and four levels of silicon (0: no Si, 100, 300 and 500 mg L−1, n = 5 for each treatment).
Significant variatioNS between Si applicatioNS at specific time of the day for particular soil water field capacity (ANOVA, P < 0.05).
NS, no significant difference.
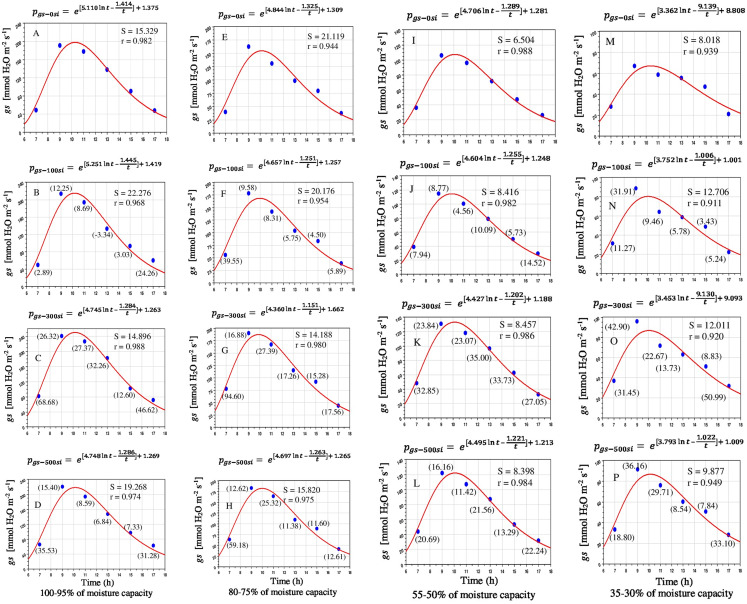
Stomata are the main limiting factor for carbon dioxide and vapor water exchange between plant leaves and the atmospheric conditions thus gs was directly controlled by PN and E. The diurnal changes of gs under all water limitations and Si treatments showed similar trends (Fig. 3). The relatively high gs levels were noted at 9:00–10:00, due to compensation for transpirational water loss before dark. Then, gs was significantly declined until evening (11:00–17:00). The gs was decreased with reducing soil moisture levels. The gs of plants receiving the 300 mg L−1 was consistently up-regulated compared to that in the 500 mg L−1 Si and that without Si application (Figs. 4A–4P). The impacts of Si application on gs under control and water stressed plants were statistically significant (P < 0.05) (Table 1).
Figure 4. Diurnal variation of silicon on stomatal conductance in Saccharum officinarm “GT 42” plants under limited water supply such as 100–95% (A–D), 80–75% (E–H), 55–50% (I–L) and 35–30% of soil moisture capacity (M–P) with four levels of Si concentrations, for example, 0, 100, 300 and 500 mg L−1.
Parenthesis values indicate percentage gain against control condition.
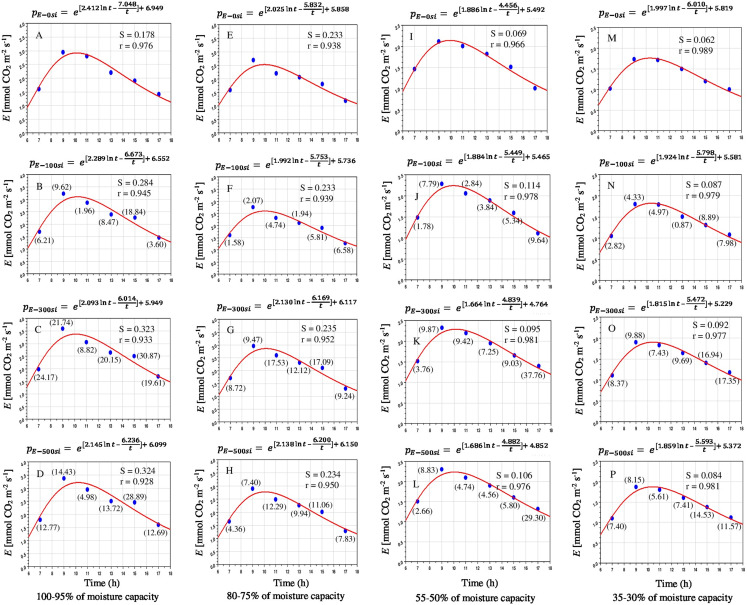
Leaf E water loss was compensated at dusk per day. Hence, based on the diurnal changes of the main environmental variables affecting leaf E under limited and sufficient soil moisture levels (Figs. 5A–5P). Under well and limited irrigation, leaf E was increased at a relatively higher from 7:00 to 9:00, followed by a significant and continuous decline from 11:00 to 17:00, reaching the minimum level at 17:00 (Fig. 5). Irrespective of Si application, E decreased with decreasing soil moisture capacity at each observation point during the day. Amended Si significantly affected leaf E at 100–95%, 80–75%, 55–50% and 35–30% of available soil moisture (Table 1). The exogenous application of Si enhanced the leaf E for most of the day time, especially in the plants grown under limited water irrigation.
Figure 5. Diurnal variation in transpiration rate (100–95%, A–D; 80–75%, E–H; 55–50%, I–L and 35–30% of FC, M–P) of Saccharum officinarm “GT 42” plants exposed to four irrigation levels and four silicon levels, n = 5. Si = 0, 100, 300 and 500 mg L−1.
Values in parentheses indicate percentage gain against control condition.
It may be seen from Figs. 3 to 5 that PN, gs and E started increasing after the sun rise and reaches maximum between 9:00 and 10:00 and started declining thereafter. The model values of PN, gs and E matches well with the observed values of diurnal variations of photosynthetic responses under water stress and Si application. The PN under mild, moderate and severe stress conditions gradually increased with increasing levels of Si. The correlation coefficients (r) between PN and water stress levels were found 0.987, 0.966, 0.978, 0.974 and 0.990–0.995, 0.956–0.966, 0.972–0.982, 0.968–0.973, respectively, in the treatments applied Si. Similarly diurnal variations of E were well explained by the model with value of r as 0.938–0.989 without Si and 0.928–0.981 with Si under limited water. Diurnal variation of gs was also well explained by the model with r as 0.939–0.988 without Si and 0.911–0.988 under limited water with Si. Limited water supply drastically suppressed the photosynthetic responses of Saccharum officinarum plant leaves (Table S1).
Discussion
Sufficient water is important for the appropriate growth and development of plants. Its effect on plants depends on their developmental stage. Water stress is one of the major environmental factors that affect the plant development, cellular, metabolism, productivity and quality of Saccharum officinarum (Verma et al., 2019a, 2019b, 2020). Higher plants have developed different types of mechanisms to tolerate stresses. The protective roles of Si in combating various environmental stresses have been widely reported (Van Bockhaven, De Vleesschauwer & Höfte, 2013; Zhu & Gong, 2014; Verma et al., 2019a, 2020). In this study, the protective role of Si was investigated in Saccharum officinarum plants during insufficient water supply.
In our study, the growth and biomass of Saccharum officinarum plants were markedly down-regulated after the plants were subjected to stress. However, the application of Si with soil irrigation decreased the severity of water stressed growth inhibition. It enhanced Saccharum officinarum tolerance to water stress in terms of promoting the plant’s growth and development (Fig. 2). Previous research has shown that Si application could increase water stress tolerance of plants. However, most of the studies have been conducted on Si-accumulating plants, whereas less information is available regarding the role of Si on water deficit tolerance of plants (Nikolic et al., 2007). The increase in growth and biomass accumulation is endorsed to the higher PN due to improved photosynthetic capacity of the stressed plants, which is in agreed with the previous demonstrations (Zhu & Gong, 2014; Liu et al., 2014; Shi et al., 2016; Verma et al., 2019a, 2020).
Our results further confirmed the findings of the previous reports that application of Si mitigated water stress and significantly affected plant growth and development in Oryza sativa (Ming et al., 2012), Zea mays (Kaya, Tuna & Higgs, 2006), Triticum aestivum (Pei et al., 2010; Gong & Chen, 2012), Sorghum bicolor (Liu et al., 2014), Solanum lycopersicum (Shi et al., 2016) and Saccharum officinarum (Verma et al., 2019a, 2020). Our results also imply a potential application of Si fertilizer in Saccharum officinarum crop production in tropical and sub-tropical regions.
In accordance with our experimental findings, leaf gas exchange significantly decreased during water stress with increasing stress levels (Figs. 3–5). Water deficit causes a major loss in LAE, LWC and photosynthetic pigments, which undeniably impairs and decreases PN, directly affecting plant performance (Shi et al., 2016; Verma et al., 2019a; Liang et al., 2019). Si-mediated up-regulation of leaf PN under drought could be attributed to improved or upgraded plant water status. In this experiment, the water status of Saccharum officinarum leaves were significantly improved by applied Si during drought. Our results showed that the applied Si mitigated the negative effects of water stress and enhanced the growth and biomass, improved the photosynthetic performance compared with the control plants during stress condition (Figs. 2–5). Our observations regarding enhancement in plant development and photosynthetic parameters due to exogenous use of Si during water stress condition are in accordance with other observations in various crops (Hattori et al., 2005; Pei et al., 2010; Ming et al., 2012; Shi et al., 2016; Chen et al., 2018; Ali et al., 2018; Verma et al., 2020). The improvement of growth by application of Si corresponded to the maintenance of higher PN (Fig. 3).
Drought stress can severely decrease the yield of Saccharum officinarum by inhibiting the photosynthetic responses (Passioura, 2007; Santos et al., 2009; Verma et al., 2019b, 2019c). At the initial sign of drought stress, plants close the stomata to avoid excess water loss by E and as a consequence, under moderate stress condition, PN is affected and is eventually inhibited by enhancing stress severity (Verma et al., 2019a, 2020). However, previous findings stated that an optimum level of Si fertilizer enhanced/improved the stomatal mechanisms/functions by enabling plants to reopen their stomata during water-deficit (Gong et al., 2005; Kaya, Tuna & Higgs, 2006; Chen et al., 2011; Liu et al., 2014; Verma et al., 2020). In this study, PN was significantly low in the water-stressed plants without Si application, undoubtedly due to stomatal limitations. Si treatments, however, were found to escalate PN and gs, and cause a simultaneous enhancement in LWC. The moderate to higher concentration of Si (300–500 mg L−1) as soil irrigation were more effective in enhancing growth and photosynthetic performance during water stress condition (Figs. 2–5).
In this study, however, the E of Saccharum officinarum leaves was enhanced by applied Si under drought stress (Fig. 5). The supplied Si treatments also resulted in an enhancement of E, possibly driven by the increased gs to maintain a steady state of PN against stress. Our results are in accordance with the previous studies like wheat, sorghum, rice, maize and tomato (Gong et al., 2005; Hattori et al., 2005; Chen et al., 2011; Shi et al., 2016) during abiotic stresses. Water deficiency and Si fertilization slightly affected the diurnal variations of Saccharum officinarum plants, which are positively correlated to the biological rhythm of the plants (Chen et al., 2016). However, compared with the plants cultivated under limited water without Si, Si generally enhanced plant growth and photosynthetic responses. An increase in soil moisture capacity is more effective than an increase in nutrient supply in improving the plant growth and development of Saccharum officinarum plants. Improvement of photosynthesis is the most important factor for overall plant performance. The change of PPFD during the natural diurnal cycle is the most important factor driving photosynthetic parameters (Paul & Pellny, 2003). Thus with maximum air RH and fully-irrigated plants, gas exchange changes in Saccharum officinarum plants is mainly driven by variations in sunlight intensity (Du et al., 2000), mainly resulting from the significantly direct or indirect dependance of C4 photosynthetic enzymes to light intensity (Leegood & Walker, 1999; De Souza et al., 2018; Verma et al., 2019a). Stomata-related increment of PN was found when Si improved gs and E (Chen et al., 2011; Savvas & Ntatsi, 2015; Verma et al., 2019b, 2019c).
The proposed model fitted quite well with the observed PN, gs and E. The PN fitted with “r” ranging from 0.956 to 0.995 and “S” 1.080 to 2.431; gs with “r” ranging from 0.911 to 0.988 and “S” from 6.540 to 22.276 and E with “r” from 0.928 to 0.989 and “S” from 0.062 to 0.324, respectively. The variations of model constants have a consistency in variations with changes in moisture status and applied Si levels (Figs. 3–5). The photosynthetic responses could be obtained for intermediate value of Si and moisture content of the soil by selecting the appropriate values of model constants. The model could be used to assess the photosynthetic advantages of Si application against water stress by integrating the model in terms of numerical values.
In summary, the results of this study revealed that application of Si might be an efficient approach for enhancing tolerance of Saccharum officinarum plants against water stress. It is also increased the growth, biomass accumulation and photosynthesis by protecting the leaf chlorophyl from degradation in Saccharum officinarum plants during stress. Further studies are needed to explore, how Si triggers the photosynthetic defense mechanism in Saccharum officinarum plants during drought stress. Thus, appropriate concentration is recommended for various crops to mitigate abiotic stresses.
Supplemental Information
Model constant and cumulative diurnal variation of photosynthetic parameters of limited water supply with different silicon levels.
Acknowledgments
We acknowledge all the members of the research team for their assistance in the field and laboratory work. We give our sincere thanks to the Guangxi Academy of Agricultural Sciences (GXAAS), Nanning, Guangxi, China for providing the necessary facilities for this experiment.
Funding Statement
The present study was supported by the grants from the Guangxi Special Fund for Scientific Base and Talent (GKAD17195100), the Fund for Guangxi Innovation Teams of Modern Agriculture Technology (gjnytxgxcxtd-03-01), the Fund of Guangxi Key Laboratory of Sugarcane Genetic Improvement (16-K-02-01) and the Fund of Guangxi Academy of Agricultural Sciences (2015YT02). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Xiu Peng Song, Email: xiupengsong@gxaas.net.
Yang Rui Li, Email: liyr@gxaas.net.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Krishan K. Verma conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Kai-Chao Wu analyzed the data, prepared figures and/or tables, and approved the final draft.
Chhedi Lal Verma conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Dong-Mei Li analyzed the data, prepared figures and/or tables, and approved the final draft.
Mukesh Kumar Malviya performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Rajesh Kumar Singh analyzed the data, prepared figures and/or tables, and approved the final draft.
Pratiksha Singh analyzed the data, prepared figures and/or tables, and approved the final draft.
Gan-Lin Chen conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Xiu Peng Song conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Yang Rui Li conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Raw measurements are available as a Supplemental File.
References
- Ali et al. (2018).Ali N, Schwarzenberg A, Yvin J-C, Hosseini SA. Regulatory role of silicon in mediating differential stress tolerance responses in two contrasting tomato genotypes under osmotic stress. Frontiers in Plant Science. 2018;9:1475. doi: 10.3389/fpls.2018.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin et al. (2014).Amin M, Ahmad R, Basra SMA, Murtaza G. Silicon induced improvement in morpho-physiological traits of maize (Zea Mays L.) under water deficit. Pakistan Journal of Agricultural Sciences. 2014;51:187–196. [Google Scholar]
- Casey et al. (2004).Casey WH, Kinrade SD, Knight CTG, Rains DW, Epstein E. Aqueous silicate complexes in wheat, Triticum aestivum L. Plant, Cell and Environment. 2004;27(1):51–54. doi: 10.1046/j.0016-8025.2003.01124.x. [DOI] [Google Scholar]
- Chen et al. (2018).Chen D, Wang S, Yin L, Deng X. How does silicon mediate plant water uptake and loss under water deficiency? Frontiers in Plant Science. 2018;9:281. doi: 10.3389/fpls.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2011).Chen W, Yao X, Cai K, Chen J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biological Trace Element Research. 2011;142(1):67–76. doi: 10.1007/s12011-010-8742-x. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2016).Chen Y, Liu L, Guo Q, Zhu Z, Zhang L. Effects of different water management options and fertilizer supply on photosynthesis, fluorescence parameters and water use efficiency of Prunella vulgaris seedlings. Biological Research. 2016;49(1):12. doi: 10.1186/s40659-016-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2017).Chen ZK, Niu YP, Ma H, Hafeez A, Luo HH, Zhang WF. Photosynthesis and biomass allocation of cotton as affected by deep-layer water and fertilizer application depth. Photosynthetica. 2017;55(4):638–647. doi: 10.1007/s11099-016-0675-y. [DOI] [Google Scholar]
- Cia et al. (2012).Cia MC, Guimaraes ACR, Medici LO, Chabregas SM, Azevedo RA. Antioxidant responses to water deficit by drought-tolerant and -sensitive sugarcane varieties. Annals of Applied Biology. 2012;161(3):313–324. doi: 10.1111/j.1744-7348.2012.00575.x. [DOI] [Google Scholar]
- De Souza et al. (2018).De Souza AP, Grandis A, ArenqueMusa BC, Buckeridge MS. Diurnal variation in gas exchange and nonstructural carbohydrates throughout sugarcane development. Functional Plant Biology. 2018;45(8):865–876. doi: 10.1071/FP17268. [DOI] [PubMed] [Google Scholar]
- Du et al. (2000).Du Y-C, Nose A, Kondo A, Wasano K. Diurnal changes in photosynthesis in sugarcane leaves: I. Carbon dioxide exchange rate, photosynthetic enzyme activities and metabolite levels relating to the C4 pathway and the Calvin cycle. Plant Production Science. 2000;3(1):3–8. doi: 10.1626/pps.3.3. [DOI] [Google Scholar]
- Epstein (1994).Epstein E. The anomaly of silicon in plant biology. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11–17. doi: 10.1073/pnas.91.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong & Chen (2012).Gong HJ, Chen KM. The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiologiae Plantarum. 2012;34(4):1589–1594. doi: 10.1007/s11738-012-0954-6. [DOI] [Google Scholar]
- Gong et al. (2005).Gong HJ, Zhu XY, Chen KM, Wang SM, Zhang CL. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Science. 2005;169(2):313–321. doi: 10.1016/j.plantsci.2005.02.023. [DOI] [Google Scholar]
- Guo et al. (2016).Guo B, Liu C, Ding N, Fu Q, Lin Y, Li H, Li N. Silicon alleviates cadmium toxicity in two cypress varieties by strengthening the exodermis tissues and stimulating phenolic exudation of roots. Journal of Plant Growth Regulation. 2016;35(2):420–429. doi: 10.1007/s00344-015-9549-y. [DOI] [Google Scholar]
- Habibi (2014).Habibi G. Silicon supplementation improves drought tolerance in canola plants. Russian Journal of Plant Physiology. 2014;61(6):784–791. doi: 10.1134/S1021443714060077. [DOI] [Google Scholar]
- Hattori et al. (2005).Hattori T, Inanaga S, Araki H, An P, Morita S, Luxova M, Lux A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiologia Plantarum. 2005;123(4):459–466. doi: 10.1111/j.1399-3054.2005.00481.x. [DOI] [Google Scholar]
- Herrmann, Haeder & Ghetti (1997).Herrmann H, Haeder DP, Ghetti F. Inhibition of photosynthesis by solar radiation in Dunalliella salina: relative efficiencies of UV-B, UV-A and PAR. Plant, Cell and Environment. 1997;20(3):359–365. doi: 10.1046/j.1365-3040.1997.d01-77.x. [DOI] [Google Scholar]
- Hura et al. (2007).Hura T, Hura K, Grzesiak M, Rzepka A. Effect of long-term drought stress on leaf gas exchange and fluorescence parameters in C3 and C4 plants. Acta Physiologiae Plantarum. 2007;29(2):103–113. doi: 10.1007/s11738-006-0013-2. [DOI] [Google Scholar]
- Ijaz et al. (2017).Ijaz R, Ejaz J, Gao S, Liu T, Imtiaz M, Ye Z, Wang T. Overexpression of annexin gene AnnSp2, enhances drought and salt tolerance through modulation of ABA synthesis and scavenging ROS in tomato. Scientific Report. 2017;7(1):12087. doi: 10.1038/s41598-017-11168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju et al. (2017).Ju S, Yin N, Wang L, Zhang C, Wang Y. Effects of silicon on Oryza sativa L. seedling roots under simulated acid rain stress. PLOS ONE. 2017;12(3):e0173378. doi: 10.1371/journal.pone.0173378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang et al. (2011).Kang Y, Han Y, Torres-Jerez I, Wang M, Tang Y, Monteros M, Udvardi M. System responses to long-term drought and re-watering of two contrasting alfalfa varieties. Plant Journal. 2011;68(5):871–889. doi: 10.1111/j.1365-313X.2011.04738.x. [DOI] [PubMed] [Google Scholar]
- Kaya, Tuna & Higgs (2006).Kaya C, Tuna L, Higgs D. Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. Journal of Plant Nutrition. 2006;29(8):1469–1480. doi: 10.1080/01904160600837238. [DOI] [Google Scholar]
- Khan et al. (2017).Khan A, Najeeb U, Wang L, Tan DKY, Yang G, Munsif F, Ali S, Hafeez A. Planting density and sowing date strongly influence growth and lint yield of cotton crops. Field Crops Research. 2017;209:129–135. doi: 10.1016/j.fcr.2017.04.019. [DOI] [Google Scholar]
- Leegood & Walker (1999).Leegood R, Walker R. Regulation of the C4 pathway. In: Sage RF, Monson RK, editors. ‘C4 Plant Biology. San Diego: Academic Press; 1999. pp. 89–131. [Google Scholar]
- Li et al. (2016).Li C, Nong Q, Solanki MK, Liang Q, Xie J, Liu X, Li Y, Wang W, Yang L, Li Y. Differential expression profiles and pathways of genes in sugarcane leaf at elongation stage in response to drought stress. Scientific Reports. 2016;6(1):25698. doi: 10.1038/srep25698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2019).Li JH, Wang YY, Li NN, Zhao RR, Khan A, Wang J, Luo HH. Cotton leaf photosynthetic characteristics, biomass production, and their correlation analysis under different irrigation and phosphorus application. Photosynthetica. 2019;57(4):1066–1075. doi: 10.32615/ps.2019.118. [DOI] [Google Scholar]
- Liang et al. (2019).Liang D, Ni Z, Xia H, Xie Y, Lv X, Wang J, Lin L, Deng Q, Luo X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Scientia Horticulturae. 2019;246:34–43. doi: 10.1016/j.scienta.2018.10.058. [DOI] [Google Scholar]
- Liang et al. (2007).Liang YC, Sun WC, Zhu YG, Christie P. Mechanisms of silicon mediated alleviation of abiotic stresses in higher plants: a review. Environmental Pollution. 2007;147(2):422–428. doi: 10.1016/j.envpol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Liu & Guo (2013).Liu HX, Guo ZG. Forage yield and water use efficiency of alfalfa applied with silicon under water deficit conditions. Philipp Agric Scientist. 2013;96:370–376. [Google Scholar]
- Liu et al. (2014).Liu P, Yin LN, Deng XP, Wang SW, Tanaka K, Zhang SQ. Aquaporin-mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. Journal of Experimental Botany. 2014;65(17):4747–4756. doi: 10.1093/jxb/eru220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2004).Ma CC, Li QF, Gao YB, Xin TR. Effects of silicon application on drought resistance of cucumber plants. Soil Science and Plant Nutrition. 2004;50(5):623–632. doi: 10.1080/00380768.2004.10408520. [DOI] [Google Scholar]
- Ma, Miyake & Takahashi (2001).Ma JF, Miyake Y, Takahashi E. Silicon as a beneficial element for crop plants. Studies in Plant Science. 2001;8:17–39. doi: 10.1016/S0928-3420(01)80006-9. [DOI] [Google Scholar]
- Ma & Yamaji (2006).Ma JF, Yamaji N. Silicon uptake and accumulation in higher plants. Trends in Plant Science. 2006;11(8):392–397. doi: 10.1016/j.tplants.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Ming et al. (2012).Ming DF, Pei ZF, Naeem MS, Gong HJ, Zhou WJ. Silicon alleviates PEG-Induced water-deficit stress in upland rice seedlings by enhancing osmotic adjustment. Journal of Agronomy and Crop Science. 2012;198(1):14–26. doi: 10.1111/j.1439-037X.2011.00486.x. [DOI] [Google Scholar]
- Mitra (2001).Mitra J. Genetics and genetic improvement of drought resistance in crop plants. Current Science. 2001;80:758–763. [Google Scholar]
- Nikolic et al. (2007).Nikolic M, Nikolic N, Liang YC, Kirkby EA, Romheld V. Germanium-68 as an adequate tracer for silicon transport in plants: characterization of silicon uptake in different crop species. Plant Physiology. 2007;143(1):495–503. doi: 10.1104/pp.106.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura (2007).Passioura J. The drought environment: physical, biological and agricultural perspectives. Journal of Experimental Botany. 2007;58(2):113–117. doi: 10.1093/jxb/erl212. [DOI] [PubMed] [Google Scholar]
- Paul & Pellny (2003).Paul M, Pellny T. Carbon metabolite feedback regulation of leaf photosynthesis and development. Journal of Experimental Botany. 2003;54(382):539–547. doi: 10.1093/jxb/erg052. [DOI] [PubMed] [Google Scholar]
- Pei et al. (2010).Pei ZF, Ming DF, Liu D, Wan GL, Geng XX, Gong HJ, Zhou WJ. Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. Journal of Plant Growth Regulation. 2010;29(1):106–115. doi: 10.1007/s00344-009-9120-9. [DOI] [Google Scholar]
- Raven (1983).Raven JA. The transport and function of silicon in plants. Biological Reviews of the Cambridge Philosophical Society. 1983;58(2):179–207. doi: 10.1111/j.1469-185X.1983.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Raza et al. (2019).Raza A, Razzaq A, Mehmood SS, Zou X, Zhang X, Lv Y, Xu J. Impact of climate change on crops adaptation and strategies to tackle its outcome: a review. Plants. 2019;8(2):34. doi: 10.3390/plants8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds et al. (2016).Reynolds OL, Padula MP, Zeng R, Gurr GM. Silicon: potential to promote direct and indirect effects on plant defense against arthropod pests in agriculture. Frontiers in Plant Science. 2016;7(73):744. doi: 10.3389/fpls.2016.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson et al. (1999).Robertson MJ, Inman-Bamber NG, Muchow RC, Wood AW. Physiology and productivity of sugarcane with early and mid-season water deficit. Field Crops Research. 1999;64(3):211–227. doi: 10.1016/S0378-4290(99)00042-8. [DOI] [Google Scholar]
- Santos et al. (2009).Santos MG, Ribeiro RV, Machado EC, Pimentel C. Photosynthetic parameters and leaf water potential of five common bean genotypes under mild water deficit. Biologia Plantarum. 2009;53(2):229–236. doi: 10.1007/s10535-009-0044-9. [DOI] [Google Scholar]
- Saud et al. (2014).Saud S, Li X, Chen Y, Zhang L, Fahad S, Hussain S, Sadiq A, Chen Y. Silicon application increases drought tolerance of Kentucky Bluegrass by improving plant water relations and morpho-physiological functions. Scientific World Journal. 2014;2014:368694. doi: 10.1155/2014/368694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvas & Ntatsi (2015).Savvas D, Ntatsi G. Biostimulant activity of silicon in horticulture. Scientia Horticulturae. 2015;196:66–81. doi: 10.1016/j.scienta.2015.09.010. [DOI] [Google Scholar]
- Shangguan, Shao & Dyckmans (2000).Shangguan ZP, Shao MG, Dyckmans J. Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. Journal of Plant Physiology. 2000;156(1):46–51. doi: 10.1016/S0176-1617(00)80271-0. [DOI] [Google Scholar]
- Shi et al. (2016).Shi Y, Zhang Y, Han W, Feng R, Hu Y, Guo J, Gong H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Frontiers in Plant Science. 2016;7:196. doi: 10.3389/fpls.2016.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvente, Sobolev & Lara (2012).Silvente S, Sobolev AP, Lara M. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLOS ONE. 2012;7(6):e38554. doi: 10.1371/journal.pone.0038554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockhaven, De Vleesschauwer & Höfte (2013).Van Bockhaven J, De Vleesschauwer D, Höfte M. Towards establishing broad-spectrum disease resistance in plants: silicon leads the way. Journal of Experimental Botany. 2013;64(5):1281–1293. doi: 10.1093/jxb/ers329. [DOI] [PubMed] [Google Scholar]
- Verma et al. (2019a).Verma KK, Liu XH, Wu KC, Singh RK, Song QQ, Malviya MK, Song XP, Singh P, Verma CL, Li YR. The impact of silicon on photosynthetic and biochemical responses of sugarcane under different soil moisture levels. Silicon. 2019a;12:1355–1367. [Google Scholar]
- Verma et al. (2020).Verma KK, Singh P, Song XP, Malviya MK, Singh RK, Chen GL, Solomon S, Li YR. Mitigating climate change for sugarcane improvement: role of silicon in alleviating abiotic stresses. Sugar Tech. 2020;22:741–749. doi: 10.1007/s12355-020-00831-0. [DOI] [Google Scholar]
- Verma et al. (2019b).Verma KK, Singh RK, Song QQ, Singh P, Zhang BQ, Song XP, Chen GL, Li YR. Silicon alleviates drought stress of sugarcane plants by improving antioxidant responses. Biomedical Journal of Scientific and Technical Research. 2019b;17(1):002957. doi: 10.26717/BJSTR.2019.17.002957. [DOI] [Google Scholar]
- Verma et al. (2019c).Verma KK, Wu K-C, Singh P, Malviya MK, Singh RK, Song X-P, Li YR. The protective role of silicon in sugarcane under water stress: photosynthesis and antioxidant enzymes. Biomedical Journal of Scientific and Technical Research. 2019c;15(2):002685. doi: 10.26717/BJSTR.2019.15.002685. [DOI] [Google Scholar]
- Witt et al. (2012).Witt S, Galicia L, Lisec J, Cairns J, Tiessen A, Araus JL, Palacios-Rojas N, Fernie AR. Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress. Molecular Plant. 2012;5(2):401–417. doi: 10.1093/mp/ssr102. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2008).Wu FZ, Bao WK, Li FL, Wu N. Effects of water stress and nitrogen supply on leaf gas exchange and fluorescence parameters of Sophora davidii seedlings. Photosynthetica. 2008;46(1):40–48. doi: 10.1007/s11099-008-0008-x. [DOI] [Google Scholar]
- Zhao et al. (2014).Zhao X, Wang W, Zhang F, Deng J, Li Z, Fu B. Comparative metabolite profiling of two rice genotypes with contrasting salt stress tolerance at the seedling stage. PLOS ONE. 2014;9(9):e108020. doi: 10.1371/journal.pone.0108020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu & Gong (2014).Zhu Y, Gong H. Beneficial effects of silicon on salt and drought tolerance in plants. Agronomy for Sustainable Development. 2014;34(2):455–472. doi: 10.1007/s13593-013-0194-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Model constant and cumulative diurnal variation of photosynthetic parameters of limited water supply with different silicon levels.
Data Availability Statement
The following information was supplied regarding data availability:
Raw measurements are available as a Supplemental File.