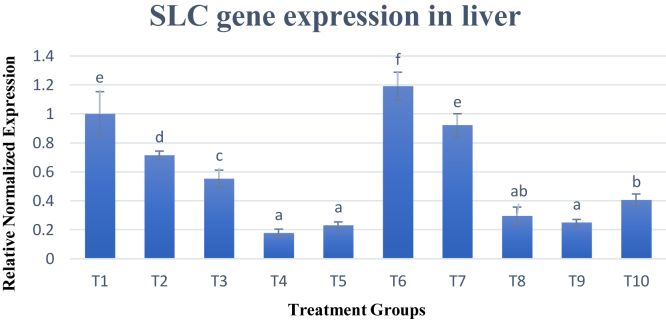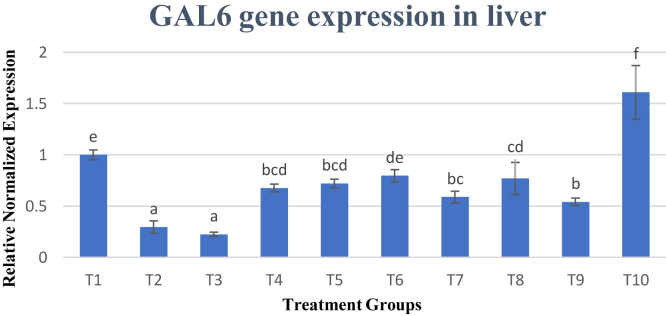Abstract
Globally, the poultry industry is 1 of the most advanced livestock industries. Feed contributes to the biggest proportion (65–70%) of the production cost. Most feed ingredients in Malaysia are imported, which contributes to the high food bill annually, and alternative feed formulation may help decrease the cost of poultry feed. Feed formulation are improved to efficiently meet the dietary requirements of the broilers and 1 of the ways is by reducing the level of crude protein in the diet while supplementing essential amino acids. In this study, the effects of methionine and lysine, which are the 2 most limiting amino acids in the chicken diet, were supplemented in a low crude protein diet, and its effects on the growth and expression of immunity genes such as MUC2, SLC, GAL6, and LEAP-2 were studied. A total of 300 Cobb500 broilers were tested with 10 different dietary treatments. Experimental treatment diets consist of high, standard, and low levels of methionine and lysine in the diet with reduced crude protein. The control group consists of diet with standard levels of lysine, methionine, and crude protein as recommended for Cobb500 broilers. Ribonucleic acid was extracted from the jejunum, spleen, and liver for gene expression analysis which was performed with real-time polymerase chain reaction using SYBR Green chemistry. Results of the growth performance at 6 wk showed improved feed conversion ratio when lysine was increased by 0.2% in a low crude protein diet at 1.96 ± 0.11. Gene expression of MUC2 gene in the jejunum showed a significant increase across all experimental diets with the treatment with higher lysine in low crude protein diet with the highest increase of 3.8 times as compared with the control diet. The other genes expressed in the spleen and liver were mostly downregulated. It was concluded that supplementation of high lysine with standard methionine in a low crude protein diet performed better in terms of lowest feed conversion ratio and high upregulation of MUC2 gene.
Key words: broiler, lysine, methionine, immunity gene, gene expression
Introduction
The poultry industry is the most advanced livestock industry in the world. Poultry includes birds such as duck, turkey, ostrich, quail, and chicken that provide both egg and meat (Al-Nasser et al., 2007). In Malaysia, owing to the rapid growth, poultry represents 75% of the total output and accounts for 70% of the total meat consumed whereby consumption of chicken and duck meat is about 38 kg per capita (Ariffin et al., 2014). Chicken meat is the most popular and cheapest choice because it does not have dietary or religious restriction such as beef and pork meat (Mohamed et al., 2013). However, raw ingredients for poultry feed such as cereal grains, animal proteins, and microingredients (vitamins and minerals) are almost entirely imported resulting in the high cost of total food bill each year (Loh, 2002). The high cost of poultry feed may be decreased with alternative feed formulations to efficiently meet the dietary requirements of the chickens.
For more than 50 yr, it has been known that adding purified amino acids allowed levels of crude protein (CP) to be reduced in the chicken diet (Pesti, 2009). Availability of commercial feed-grade amino acids that are affordable to supplement lowered CP content in the diet is more economical as compared with feeding chickens with higher-CP diets. The limiting order of essential amino acids in chickens varies in accordance with age, type of diet fed, and the experimental assay used to assess the amino acid limitation. It was established that methionine, lysine, and threonine are the 3 most limiting essential amino acid in corn–soybean meal chicken diet (Fernandez et al., 1994). Methionine, the most limiting amino acid in broiler diets, has several functions such as a precursor for cystine, source of dietary sulfur, and serves as an integral portion of body protein (Ojano-Dirain and Waldroup, 2002). Meanwhile, lysine is particularly important for muscle development especially the breast muscle of the chicken (Tesseraud et al., 1996). The link between lysine and methionine was confirmed by Hickling et al. (1990) who confirmed that response of lysine is influenced by methionine levels in the diet.
Regulation of gene expression by amino acids may occur in any step during transcription, translation, or post-translational modifications (Wu, 2009). Excess or deficiency of certain amino acids such as leucine, valine, lysine, and methionine cause changes in immune responses in chickens (Konashi et al., 2000). It was found that prolonged durations of a low-protein diet will cause amino acid levels in the plasma to decrease and is associated with high levels of infection (Fafournoux et al., 2000). There are two types of immunity in the chicken, which are innate immunity and adaptive immunity (humoral and cell-mediated) (Erf, 2004). The immunity genes chosen for this study are involved in the innate immunity of chickens to provide natural genetic resistance against pathogens.
The main component of the mucus layer lining the gastrointestinal tract is mucins (Montagne et al., 2004). Its main function is to protect the gastrointestinal tract from aggressors (chemical, physical, or bacterial) as a form of innate immunity in poultry (Erf, 2004). Besides preventing infection from pathogens, the mucus layer also acts as a diffusion barrier to uptake nutrients such as amino acids into the lumen of the intestine and slowly discharges the amino acid mixture into the peripheral blood (Baracos, 2004). Among the mucin genes, mucin 2 (MUC2) is a secreted mucin and a major structural component of the mucus layer (van der Sluis et al., 2009). MUC2 is essential for the maintenance of the mucus layer on the surface of the intestinal lumen, and any changes in the diet may affect the expression and integrityof the mucus layer.
The solute carrier family 11 member 1 (SLC11A1) gene (previously known as natural resistance–associated macrophage protein 1) is part of an ancient family of a divalent cation antiporter protein (Doiphode et al., 2009). SLC11A1 transport ions such as manganese (Mn2+), which is essential for the survival of pathogens as it is the cofactor for superoxide dismutase, and inactivation of this enzyme eliminates the pathogens (Jabado et al., 2000). Expression of SLC11A1 gene has been known to be influenced by pH and metal ions in the cells. However, there have not been any studies regarding the effect of amino acids on the gene.
Gallinacins are β-defensins found in poultry and are part of the family of antimicrobial peptides. Antimicrobial peptides are polypeptides of fewer than 100 amino acids that display antimicrobial activity (Ganz, 2003). Gallinacins is comprised of a family of 13 genes known as gallinacins 1-13 (GAL1-13) that are expressed in various tissues with variable expressions in the body. GAL6 in particular are strongly expressed in the liver, kidneys, and gall bladder (Lynn et al., 2004). Antimicrobial activity of GAL6 indicated that the cell wall of pathogens is permeabilized, thus affecting the protein synthesis and DNA/RNA replication of pathogens, which causes cell lysis (Van Dijk et al., 2007). The novel cationic liver-expressed antimicrobial peptide 2 (LEAP-2) was first discovered in chickens by Lynn et al. (2003) using expressed sequence tags. It is part of the defense system in avian and inducible when infected with Salmonella in chicks (Townes et al., 2004) and in reproductive organs such as testis and ovary of sexually matured chickens (Michailidis, 2010). However, studies revealed that supplementation of Lactobacillus probiotics and enroflaxin did not induce LEAP-2 gene expression in healthy broilers (Pavlova et al., 2016).
Therefore, this study aims to see the effects of adding variable amount of lysine and methionine in a low crude protein diet on the chicken's growth performance. The gene expression of immunity genes such as MUC2, SLC, GAL6, and LEAP-2 were also analyzed to examine the effects of different concentrations of lysine and methionine supplemented in a low crude protein diet. This ensures that the best and most suitable feed formulation are chosen for the broilers in terms of growth and immunity.
Materials and methods
Birds and Housing
A total of 300, 1-day-old chicks (Cobb500) were obtained from a local hatchery. The chicks were raised in open house battery cages, wing banded, weighed, and randomly assigned into ten treatment groups. Each dietary treatment group consisted of 5 replicate groups, and each group had 6 birds. On day 4 and 14, chickens were vaccinated against Newcastle disease and infectious bronchitis. Vaccination against infectious bursal disease was administered on day 21. Vaccinations were performed via eye drops. Water and experimental feed were provided ad libitum. Starter diets were fed to the chicks for the first 21 D followed by the finisher diet from day 22 until 42. The feeding experiment was conducted for 6 wk. The experimental animals received humane care as outlined and approved by Institutional Animal Care and Use Committee for the Care and Use of Animals for Scientific Purposes (Research Policy, Universiti Putra Malaysia).
Experimental Diet
The control diet (treatment 1 [T1]) consisted of a diet formulated to contain the standard required levels of lysine, methionine, and crude protein, which is 1.2% lysine, 0.46% methionine, and 21% crude protein for the starter diet and 1.05% lysine, 0.43% methionine, and 18% crude protein for the finisher diet as recommended nutrient requirement for Cobb500 broilers (Cobb-Vantress, 2013). Other experimental treatments had lower crude protein content of 19% for a starter diet and 16% for a finisher diet. Diets were formulated by Shazali (2015), using FeedLIVE (Thailand) software. Amino acids levels were adjusted to 3 different levels which were high, standard, and low. Methionine (99% feed grade; Evonik Industries, Belgium) and lysine (98.5% feed grade; ADM, USA) were mixed into the diet fed to the broilers. For a starter diet, it consisted of 1.4, 1.2, and 1.0% lysine and 0.51, 0.46, and 0.41% methionine in 19% low crude protein diet, whereas for a finisher diet, it consisted of 1.25, 1.05, and 0.85% lysine and 0.48, 0.43, and 0.38% methionine in 16% low crude protein diet. The formulated diet treatments are shown in Tables 1 and 2.
Table 1.
Starter diet experimental feed formulation with different levels of lysine and methionine supplementation in low crude protein diet.
| Ingredients (kg) | Dietary treatments |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | |
| Corn | 18.63 | 19.57 | 19.59 | 19.62 | 19.60 | 19.61 | 19.63 | 19.67 | 19.62 | 19.62 |
| Soybean meal | 13.77 | 10.35 | 10.35 | 10.35 | 10.44 | 10.44 | 10.46 | 10.55 | 10.62 | 10.62 |
| Wheat pollard | 6.56 | 8.66 | 8.66 | 8.66 | 8.66 | 8.66 | 8.66 | 8.57 | 8.56 | 8.57 |
| Crude palm oil | 2.03 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 1.99 | 2.00 | 2.01 |
| Fish meal | 2.12 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.27 | 2.27 | 2.27 |
| Dicalcium phosphate | 0.275 | 0.234 | 0.234 | 0.234 | 0.234 | 0.234 | 0.234 | 0.234 | 0.234 | 0.234 |
| Calcium carbonate | 0.495 | 0.522 | 0.522 | 0.522 | 0.522 | 0.522 | 0.522 | 0.522 | 0.522 | 0.522 |
| Choline chloride | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 |
| Salt | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 |
| Mineral premix | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 |
| Vitamin premix | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 | 0.450 |
| Antioxidant | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 |
| Toxin binder | 0.046 | 0.046 | 0.046 | 0.046 | 0.046 | 0.046 | 0.046 | 0.046 | 0.046 | 0.046 |
| L-Lysine | 0.000 | 0.207 | 0.207 | 0.207 | 0.090 | 0.090 | 0.090 | 0.000 | 0.000 | 0.000 |
| DL-Methionine | 0.045 | 0.081 | 0.059 | 0.036 | 0.081 | 0.058 | 0.035 | 0.080 | 0.057 | 0.034 |
| L-Threonine | 0.009 | 0.050 | 0.050 | 0.050 | 0.048 | 0.062 | 0.048 | 0.046 | 0.045 | 0.045 |
| Total | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 |
| Parameter | ||||||||||
| Lysine (%) | 1.2 | 1.4 | 1.4 | 1.4 | 1.2 | 1.2 | 1.2 | 1.0 | 1.0 | 1.0 |
| Methionine (%) | 0.46 | 0.51 | 0.46 | 0.41 | 0.51 | 0.46 | 0.41 | 0.51 | 0.46 | 0.41 |
| Crude protein (%) | 21 | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 |
Table 2.
Finisher diet experimental feed formulation with different levels of lysine and methionine supplementation in low crude protein diet.
| Ingredients (kg) | Dietary treatments |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | |
| Corn | 44.55 | 47.03 | 47.03 | 47.03 | 46.98 | 47.03 | 46.98 | 47.02 | 47.02 | 47.03 |
| Soybean meal | 19.80 | 15.35 | 15.38 | 15.44 | 15.20 | 16.20 | 16.20 | 16.87 | 16.88 | 16.92 |
| Wheat pollard | 13.42 | 16.01 | 16.02 | 16.02 | 15.48 | 15.48 | 15.48 | 14.94 | 14.99 | 14.99 |
| Crude palm oil | 4.46 | 4.50 | 4.50 | 4.50 | 4.50 | 4.50 | 4.59 | 4.59 | 4.59 | 4.59 |
| Fish meal | 4.05 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 |
| Dicalcium phosphate | 0.297 | 0.747 | 0.747 | 0.747 | 0.747 | 0.747 | 0.747 | 0.747 | 0.747 | 0.747 |
| Calcium carbonate | 1.035 | 1.062 | 1.062 | 1.062 | 1.062 | 1.062 | 1.062 | 1.062 | 1.062 | 1.062 |
| Choline chloride | 0.029 | 0.029 | 0.029 | 0.029 | 0.029 | 0.029 | 0.029 | 0.029 | 0.029 | 0.029 |
| Salt | 0.207 | 0.225 | 0.225 | 0.225 | 0.225 | 0.225 | 0.225 | 0.225 | 0.225 | 0.225 |
| Mineral premix | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 |
| Vitamin premix | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 |
| Antioxidant | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 |
| Toxin binder | 0.093 | 0.093 | 0.093 | 0.093 | 0.093 | 0.093 | 0.093 | 0.093 | 0.093 | 0.093 |
| L-Lysine | 0.078 | 0.518 | 0.518 | 0.514 | 0.264 | 0.264 | 0.264 | 0.015 | 0.015 | 0.014 |
| DL-Methionine | 0.100 | 0.189 | 0.144 | 0.097 | 0.185 | 0.140 | 0.094 | 0.183 | 0.136 | 0.090 |
| L-Threonine | 0.075 | 0.189 | 0.189 | 0.187 | 0.174 | 0.174 | 0.174 | 0.163 | 0.162 | 0.162 |
| Total | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| Parameter | ||||||||||
| Lysine (%) | 1.05 | 1.25 | 1.25 | 1.25 | 1.05 | 1.05 | 1.05 | 0.85 | 0.85 | 0.85 |
| Methionine (%) | 0.43 | 0.48 | 0.43 | 0.38 | 0.48 | 0.43 | 0.38 | 0.48 | 0.43 | 0.38 |
| Crude protein (%) | 18 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
Data Collection and Analysis
On a weekly basis, individual BW and feed intake (FI) were recorded. Mortality was checked daily, and weights of dead birds were used to adjust feed conversion ratio (FCR). Data collected for 6 wk were recorded, and calculations for body weight gain (BWG) and FI of the birds were performed. The FCR was calculated as follows: total feed consumed by birds/total weight gain, and the results were analyzed using Statistical Analysis Software (SAS, 2013).
Sample collection
At the end of the experiment, 3 birds per cage (15 birds per treatment) were randomly selected and weighed before being slaughtered. The slaughter process was conducted at the Department of Animal Science abattoir, Faculty of Agriculture, Universiti Putra Malaysia. The birds were slaughtered in accordance with the halal slaughter procedure as outlined in the Standards Malaysia 1,500: (MS1500 2,009). The organs such as the liver, spleen, and small intestine were collected and frozen in liquid nitrogen before being kept in −80°C until further analysis.
RNA Extraction
For intestine samples, the small intestine was separated into 3 regions: the duodenum (from the gizzard outlet to the end of the duodenal loop), jejunum (from the duodenal loop to Meckel's diverticulum), and ileum (from Meckel's diverticulum to the beginning of the caeca). The jejunum portion used for sampling is approximately 5 cm distal from the duodenal loop and 5 cm before Meckel's diverticulum. Immediately after separation, the jejunum contents were removed and flushed with distilled water. The lumen of the intestine was exposed by cutting longitudinally, and the mucosal content was scrapped using a metal spatula and kept in a microcentrifuge tube for RNA extraction. The jejunum section was chosen particularly because it is a major site of nutrient absorption in poultry (Horn et al., 2009). Organs such as the spleen and liver were sliced into small pieces to be used for RNA extraction.
RNA extraction was performed using RNeasy Mini Kit (Qiagen, Germany) as per the manufacturer's protocol with some minor modifications. The organs (spleen and liver) were grinded in liquid nitrogen using mortar and pestle until it became fine powder, and the powder was transferred to a microcentrifuge tube for RNA extraction with DNase treatment.
Extracted RNA was assessed for its quantity, quality, and integrity via absorbance reading using a NanoPhotometer Classic (Implen, Germany), agarose gel electrophoresis, and an RNA 6000 bioanalyzer chip (Agilent, USA). RNA samples with RNA integrity number reading of 6.5 and higher were used in the next procedure.
Gene Expression Studies
Reverse transcription was performed using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA) in accordance with the manufacturer's protocol. The cDNA obtained was directly used as a template in the next reaction.
The gene expression of MUC2, SLC, GAL6, and LEAP-2 genes was studied using quantitative real-time PCR (qPCR). Two reference genes were used for normalization, which were beta-actin and glyceraldehyde-3-phosphate dehydrogenase genes. The primer sequences for all the genes used for qPCR are shown in Table 3.
Table 3.
Primer sequences and accession number for primers used in qPCR.
| Gene | Primer sequence | Accession No. |
|---|---|---|
| ß-actin | Forward: GCTCTGACTGACCGCGTTAC Reverse: GCTCTGACTGACCGCGTTAC |
L08165 |
| GAPDH | Forward: AAGGCGAGATGGTGAAAGTC Reverse: TTGATGGCCACCACTTGGAC |
NM_204305.1 |
| MUC2 | Forward: TCACCCTGCATGGATACTTGCTCA Reverse: TGTCCATCTGCCTGAATCACAGGT (Horn et al., 2009) |
JX284122.1 |
| SLC | Forward: GCCCTGCTATGGCATCATTG Reverse: ACATTGCTGGCGTCAGTTTG |
U40598 |
| GAL6 | Forward: CTCTTCCAGGCTGCTCCAGCTTAC Reverse: TTAGGAGCTAGGTGCCCATTTG |
AY534894 |
| LEAP-2 | Forward: CTTCTGAGACTGAAGCGGATGAC Reverse: TCACTCGGAGGCCGTTCTAAG |
AY534899 |
The qPCR reaction was performed using a Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies, USA) in accordance with the manufacturer's protocol. Efficiency curves were performed for each primer set, and amplification efficiency between 90 and 110% was considered good with correlation coefficient R2 values of >0.99 (Rebrikov and Trofimov, 2006). All reactions were analyzed in triplicates, and no-template controls were included. Concentrations of primers used were 300 nmol as the final concentration for each reaction and a cDNA template of 100 ng was used in each reaction. Gene expression studies were carried out consisting of triplicates for every treatment and each set of primers. The qPCR amplification was performed using a Bio-Rad CFX96 machine with the amplification steps as follow: 3 min at 95°C followed by 40 cycles of 5 s at 95°C and 10 s at 60°C. After the qPCR cycle, melt curve peak was generated by increasing the temperature by 0.5°C every 10 s from 65°C to 95°C. The same PCR cycles were used for all sets of gene primers including the annealing temperature.
Statistical analysis
The quantification cycle values obtained from the amplification were analyzed using the Bio-Rad CFX Manager 3.1. The levels of transcripts were normalized to beta-actin and glyceraldehyde-3-phosphate dehydrogenase genes. The quantification cycle values were converted to linear units called relative normalized expression, and standard deviation values were calculated by the software. Mean ± SD values for each group were calculated, and differences between groups were analyzed using SPSS statistics software (SPSS 23 for Windows, Chicago, IL). One-way analysis of variance of the data was calculated, and Duncan's multiple range test was performed as a post hoc test to measure specific differences between the groups. Statistical significance was declared at P ≤ 0.05.
Results
Growth Performance
The experimental diet treatment effects on the growth of the chickens were calculated and shown in Table 4. Parameters such as BWG, FI, FCR, and growth rate (GR) were calculated. For the first 3 wk, in terms of BWG and FCR, there was no significant (P > 0.05) difference between the treatment groups and control (T1). After 6 wk, there were significant differences in terms of BWG, FI, and FCR. Among the treatment groups, reduction of lysine in the low crude protein diet gave the least BWG, FI, and GR. However, FCR were higher for the groups with less lysine supplied in the diet, which are T8–T10. Overall comparison between the groups indicated that only treatment 3 (T3) showed an improved FCR of 1.96 ± 0.11 as compared with T1 which gave a ratio of 2.17 ± 0.08.
Table 4.
Growth performance of broilers at week 3 and week 6 with different dietary treatments.
| Parameter | Dietary treatments |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | |
| Week 3 | ||||||||||
| Body weight gain, (g) | 541.94 ± 531.42a,b | 526.67 ± 38.00a,b | 586.67 ± 30.86a | 512.39 ± 82.22a,b | 541.67 ± 64.93a,b | 525.17 ± 62.10a,b | 531.39 ± 92.37a,b | 495.4 ± 68.55a,b | 463.22 ± 21.86b | 513.89 ± 35.51a,b |
| Feed intake, (g) | 1,238.89 ± 97.66a | 1,205.56 ± 34.69a,b | 1,127.78 ± 75.15b | 1,155.56 ± 63.10b | 1,255.56 ± 75.15a | 1,200 ± 44.10a,b | 1,155.56 ± 34.69b | 1,094.44 ± 9.62b | 1,172.22 ± 118.24a,b | 1,166.67 ± 16.67a,b |
| Feed conversion ratio | 2.29 ± 0.11a,b | 2.30 ± 0.23a,b | 1.92 ± 0.15b | 2.29 ± 0.33a,b | 2.33 ± 0.18a,b | 2.31 ± 0.33a,b | 2.22 ± 0.36a,b | 2.24 ± 0.35a,b | 2.53 ± 0.24a | 2.28 ± 0.19a,b |
| Week 6 | ||||||||||
| Body weight gain, (g) | 2,125.36 ± 21.27a | 2,056.95 ± 127.76a,b | 2,093.00 ± 128.03a,b | 2,008.99 ± 160.06a,b,c | 2,120.07 ± 24.92a | 1,861.37 ± 98.16c,d | 1,942.39 ± 41.89b,c | 1,698.11 ± 110.44d | 1,522.22 ± 70.40e | 1,698.62 ± 16.38d |
| Feed intake, (g) | 4,612.22 ± 114.23a | 4,455.56 ± 198.34a,b | 4,087.78 ± 197.27c,d | 4,222.22 ± 145.30b,c | 4,440.56 ± 74.65a,b | 4,093.33 ± 112.60c,d | 4,155.56 ± 250.47b,c | 3,781.11 ± 203.32d | 4,025.56 ± 267.51c,d | 3,986.67 ± 88.38c,d |
| Feed conversion ratio | 2.17 ± 0.08b,c | 2.17 ± 0.07b,c | 1.96 ± 0.11d | 2.11 ± 0.20c,d | 2.09 ± 0.05c,d | 2.20 ± 0.08b,c | 2.14 ± 0.09b,c,d | 2.23 ± 0.10b,c | 2.65 ± 0.17a | 2.35 ± 0.03b |
| Growth rate | 2,065.92 ± 58.60a,b | 2,024.39 ± 130a,b,c | 2,060.04 ± 90.07a,b,c | 1,894.61 ± 245.68b,c | 2,123.69 ± 29.64a | 1,850.27 ± 119.24c,d | 1,923.68 ± 39.19a,b,c | 1,686.27 ± 108.69d,e | 1,502.73 ± 78.29e | 1,688.88 ± 25.17d,e |
a-eRow with no common letter differs significantly (P < 0.05).
Data shown are average of 5 groups per treatment with 6 birds per group.
Gene Expression
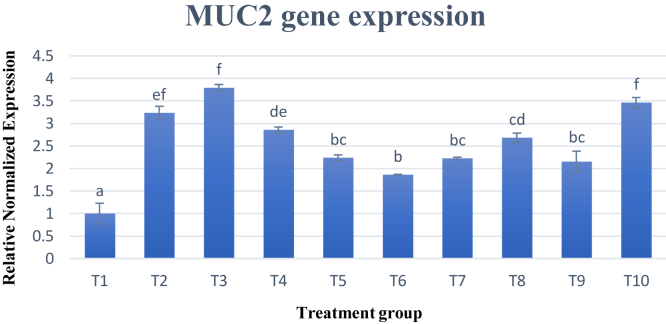
Gene expression of MUC2 gene was analyzed in the jejunum of the small intestine. Results obtained were analyzed and presented in a bar graph as shown in Figure 1. Overall, MUC2 gene expression was significantly (P < 0.05) increased across all the experimental diets when compared with the control diet (T1). Treatment 3 showed the most significant (P < 0.05) increase in gene expression at 3.8 times more than T1.
Figure 1.
MUC2 gene expression in the jejunum. Changes in MUC2 gene expression were normalized to ß-actin and GAPDH reference genes and expressed relative to the control (T1). Values are mean ± SD for an average of 15 biological replicates and 3 technical replicates. a–fBars with no common letter differs significantly (P < 0.05). Abbreviations: ß-actin, beta-actin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MUC2, mucin 2.
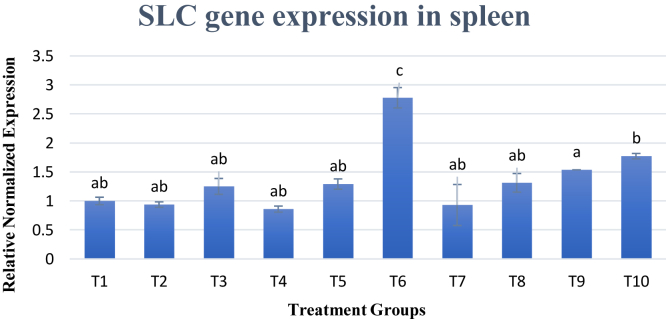
Meanwhile, SLC gene expression was analyzed in both the spleen and liver. Results were shown in Figures 2 and 3 for the spleen and liver, respectively. In the spleen, SLC gene expression across all experimental diets had no difference except treatment 6 (T6) that had significantly (P < 0.05) increased upregulation of SLC gene expression at 2.8 times more than T1. Meanwhile, in the liver, SLC gene expression was significantly (P < 0.05) decreased across most of the diets as compared with that of T1. Only T6 showed a significant (P < 0.05) increase in gene expression at 1.19-fold.
Figure 2.
Gene expression of SLC gene in spleen. Changes in SLC gene expression in spleen are normalized to ß-actin and GAPDH reference genes and expressed relative to the control (T1). Values are means ± SD for an average of 15 biological replicates and 3 technical replicates. a–cBars with no common letter differs significantly (P < 0.05). Abbreviations: ß-actin, beta-actin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SLC, solute carrier family.
Figure 3.
Gene expression of SLC gene. Changes in SLC gene expression in liver are normalized to ß-actin and GAPDH reference genes and expressed relative to the control (T1). Values are means ± SD for an average of 15 biological replicates and 3 technical replicates. a–fBars with no common letter differs significantly (P < 0.05). Abbreviations: ß-actin, beta-actin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SLC, solute carrier family.
GAL6 gene expression was tested in the liver, and results in Figure 4 showed that dietary treatment does significantly (P < 0.05) affect the gene expression of GAL gene. The most noticeable difference is in treatment 10 (T10) being the only treatment that showed up-regulation of GAL6 gene as compared with T1. In contrast, T2 and T3 had the lowest expression of only 0.30 and 0.22 times, respectively. which was significantly (P < 0.05) lower than T1. Other treatment groups, T4–T9, also showed similar trend of down regulation of the GAL gene expression with only T6 showing no significant (P < 0.05) decrease as compared with T1.
Figure 4.
Gene expression of GAL6 gene. Changes in GAL6 gene expression are normalized to ß-actin and GAPDH reference genes and expressed relative to the control (T1). Values are means ± SD for an average of 15 biological replicates and 3 technical replicates. a–fBars with no common letter differs significantly (P < 0.05). Abbreviations: ß-actin, beta-actin; GAL6, gallinacin 6; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
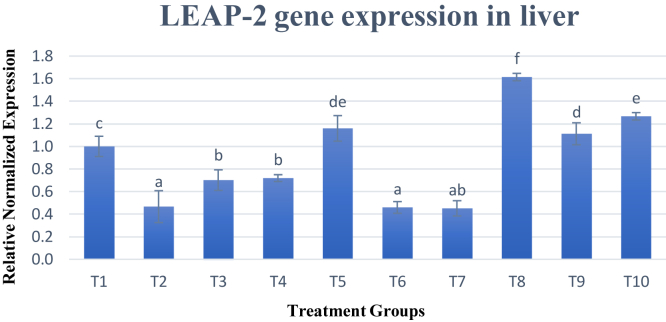
Chicken liver-expressed antimicrobial peptide 2 (LEAP-2) gene expression was tested in the liver, and results varied between different treatment groups as shown in Figure 5. Half the treatment groups showed gene expression that was significantly (P < 0.05) lower than the control group (T1), whereas the other half of the treatment groups showed significant (P < 0.05) increase in gene expression as compared with T1. Treatment 8 showed the most increase in gene expression which is 1.61 times followed by T10, T5, and T9 which had an expression of 1.27, 1.16, and 1.11 times, respectively.
Figure 5.
Gene expression of LEAP gene. Changes in LEAP-2 gene expression are normalized to ß-actin and GAPDH reference genes and expressed relative to the control (T1). Values are means ± SD for an average of 15 biological replicates and 3 technical replicates. a–fBars with no common letter differs significantly (P < 0.05). Abbreviations: ß-actin, beta-actin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LEAP-2, liver-expressed antimicrobial peptide 2.
Discussion
The growth performance in Table 4 indicated that increasing the lysine content by 0.2% with the standard requirement of methionine (T3) in a low crude protein diet significantly reduced the FCR, while GR of the chickens was not affected. This is in agreement with the study by Kamran et al. (2004) who suggested the dietary crude protein level for broilers could be reduced provided that essential amino acid levels are maintained as per the dietary recommendations. The feed formulation chosen for this study was based on a study whereby reducing the dietary CP by 2% with amino acids supplementation was shown to improve growth performance (Shazali et al., 2019). From the results obtained, treatments with lower levels of lysine consistently gave depressed results in terms of BWG and FI. Lysine deficiency could reduce the body weight up to 40 to 45% because of lysine being used mainly for muscle protein production in broilers (Tesseraud et al., 1996). The breast meat contains high levels of lysine, and reduction of lysine in the diet has been reported to affect the breast meat accretion (Rezaei et al., 2004). This is in line with our findings where the weight of the broilers was significantly reduced in diets with reduced levels of lysine. The effect of methionine is less evident on the growth performance of the broilers.
Overall, T3 supplemented with additional lysine of 0.2% with the standard methionine level of 0.43% in a reduced CP diet gave the best results in terms of FCR. This is in agreement with past studies by Rezaei et al. (2004) that reducing dietary protein with addition of lysine was able to improve feed efficiency and breast meat yield in broiler chickens. The chickens would be able to perform as those fed with higher-CP diets provided that optimal essential amino acids are provided in the feed (Kamran et al., 2004). Despite reduced FI in the dietary treatments, the BWG of broilers did not decrease but were similar to the control group, which means feed was more efficiently converted into live weight gain. This is because crystalline amino acids added into the feed are more digestible as compared with protein-bound amino acids (Park, 2006). The economic evaluation of the reduction of 2% CP which is compensated by only an increase of 0.2% lysine while maintaining the standard methionine levels were calculated for starter and finisher diets. For a starter diet, for a treatment group that consists of 30 broilers, it would save RM13.97 (US$ 3.34) based on cost calculated from the present study, whereas for a finisher diet, a saving of RM13.75 (US$ 3.29) was computed. Moreover, reduction in CP is advantageous for the environment as well. It is known that reduction of CP by 9% would be able to reduce ammonia gas excretion by 31% and nitrogen in the litter by 16.5% (Ferguson et al., 1998).
From the gene expression pattern observed, increasing lysine by 0.2% in the feed significantly (P < 0.05) increased the expression of MUC2 gene the most. This correlates with the growth performance study (Table 4) that indicated T3 performed the best in terms of FCR. The lowest FCR indicates that feed was efficiently converted into live weight of broilers despite the lower FI. Other studies have shown that excess or deficiency of threonine can greatly affect the immune system because it is a major component of intestinal mucin by functioning as cellular signaling mechanism, antibody production, and affecting the jejunal mucosal concentration (Li et al., 2007). However, there have been a lack of studies studying the effect of methionine and lysine, the two most limiting amino acids in the chicken diet on the gene expression of mucins in the jejunum of broilers. From this study, it was observed that a reduction in CP increased MUC2 gene expression significantly regardless if lysine and methionine was increased, decreased, or maintained compared with the control, T1. Previous study has shown that reduction of CP decreased goblet cell numbers in the jejunum; however, supplementation of threonine was able to compensate for these changes (Abbasi et al., 2014). From this study, it was observed that similar to threonine, lysine and methionine supplementation were able to increase mucin expression in the jejunum despite reduction of CP. As T3 showed the most increase in gene expression, we can correlate this with the growth performance, which also indicated that T3 were able to outperform the rest of the treatment groups in terms of FCR. Increasing the dietary supplementation of lysine in the diet may improve not only the growth but also the health of the chickens by strengthening the immunity of the chickens as proven with the upregulation of the MUC2 gene. An explanation for this is that the intestine has been found to be of critical importance in actively regulating essential amino acid flow to the body as a whole (van Goudoever et al., 2000). Crystalline amino acids increase the availability of amino acids for absorption when compared with amino acids in intact proteins, which is the reason reducing the CP with supplementation of amino acids would not be detrimental to the broiler performance (Abbasi et al., 2014). This indicates that small fluctuations of amino acids in the diet can affect gene expression in the small intestine as mucins play an important part in the intestine of the chickens not only as a defense mechanism but also displays capabilities of digesting and absorbing nutrients for the body (Horn et al., 2009). Dietary protein was able to alter the recovery of mucins in the endogenous protein of the small intestine (Montagne et al., 2004). This is particularly important as mucins are constantly being synthesized and secreted and results in changes in the charge and viscosity of mucin (Horn et al., 2009). The importance of MUC2 especially in providing innate immunity was studied intensively as it is the major mucin in the intestines. As the gut is considered as the first line of defense when infected with pathogens, a mucus layer with reduced function would leave the host immunocompromised against enteral bacteria (Law et al., 2007). It was established that MUC2 consists of two layers, an inner adherent layer and an outer loosely adherent layer forming the mucus, and it was revealed that the inner layer is free from bacteria, which suggests that it has properties such as small pore size that physically block bacteria from entering (Johansson and Hansson, 2010).
Overall results for SLC gene expression in both the spleen and liver were similar, whereby T6 was the best dietary treatment for SLC gene expression. Treatment group 6 consisted of methionine and lysine of standard levels in a diet of low crude protein. This dietary profile was able to slightly improve the gene expression of SLC in both the spleen and liver. It is apparent that maintaining lysine and methionine to the standard requirement when the crude protein is lowered is best for increasing SLC gene expression. Changes in levels of essential amino acids such as lysine and methionine could instead reduce the gene expression of this gene. Possible reason for this is that SLC gene functions to modify the intraphagosomal environment to hinder microbial replication by working as metal ion transporters. Therefore, amino acid levels in the diet were not able to increase the expression of this gene as it may be more sensitive toward the changes of metals such as Fe2+, Mn2+, and Zn2+ in the body. For example, in yeast, the SLC1 ortholog has been shown to have a high affinity for Mn2+ but is inhibited by Zn2+. In flies, on the other hand, the SLC1 ortholog was shown to be inhibited by Mn2+ and Fe2+ ions (Goswami et al., 2001). The SLC gene is expressed solely in professional phagocytes and may be involved in modifying the intracytoplasmic milieu to suppress replication of unrelated pathogens (Cellier et al., 1995). Despite numerous studies on the effects of divalent cations on the expression of SLC gene, there have been no previously reported studies on the effects of amino acids on this gene. Based on this study, it is revealed that lowering the crude protein in the diet significantly reduced (P < 0.05) expression of SLC gene. However, when limiting essential amino acids such as lysine and methionine were supplemented at the standard required amount, the SLC gene expression was increased.
The GAL6 gene in the liver organ showed downregulation of expression in most of the dietary treatments except T10 where decreased supplementation of lysine and methionine increased the gene expression GAL6 gene. It is well defined that microbicidal activity of cationic peptides such as GAL6 is affected by different environmental elements such as pH, temperature, ionic strength, and microbial growth phase (Van Dijk et al., 2007). In chickens, gallinacins display antimicrobial activity across a vast spectrum in both gram-negative and gram-positive bacteria by penetrating the membrane of the bacteria to inhibit the synthesis of protein, RNA, and DNA (Hasenstein and Lamont, 2007). There have been no reported studies on the effect of CP on GAL6 expression before this. The effect of reduced CP in the diet of broilers was significant (P < 0.05) reduction of GAL6 gene expression in the liver. Diets with increased lysine content (T2–T4) had the lowest expression of GAL6. Possible reason for this was an observation that GAL6 has aspartic acid (Asp) at the N-terminal and replacement of Asp with glycine and serine caused it to have less antimicrobial activity and structure (Van Dijk et al., 2007). Lysine which is a positively charged amino acid can interact with Asp which is negatively charged neutralizing the charge causing them to form a salt bridge when in close proximity (King et al., 1991). This may reduce the expression of GAL6 when there is a higher content of lysine in the diet especially when CP was reduced. Interestingly, T10 which contained low lysine and methionine was the only treatment which had upregulation of the GAL6 gene.
The gene expression pattern of LEAP-2 gene varies as per the lysine supplementation in the chicken diet. Treatment 8–T10 which had lower amount of lysine by 0.2% improved the LEAP gene expression in the liver, while increased supplementation of lysine in the diet decreased the expression as observed in T2–T4. Meanwhile, T5 which had the standard required amount of lysine with a higher methionine level by 0.05% showed positive results. From the outcome, lysine supplementation played a bigger role in regulating the LEAP-2 gene expression in the liver. High amounts of lysine reduced the expression of LEAP-2 as observed in T2–T4, whereas lower amounts of lysine increased the expression as seen in T8–T10. It was also noted that dietary treatments with increased methionine with standard or lower lysine levels performed better. Comparison between treatment groups T8–T10, which had lower levels of lysine indicated that the group with high levels of methionine, T8, performed the best among these 3 groups. This pattern is similar in the groups fed with standard levels of lysine (T5–T7) whereby T5 outperformed the other two groups significantly. The reasoning behind this may be because of the role played by methionine in the secretion of chicken LEAP-2. LEAP-2 is secreted as a propeptide with the length of 53 amino acids which will be proteolytically cleaved to release a 40-amino acid-long mature peptide characterized by the formation of two disulphide bonds made up by 4 cysteines (Townes et al., 2004). Cysteine is catabolized via transsulfuration from homocysteine that was transmethylated intracellularly from methionine (Riedijk et al., 2007). Methionine is a precursor for cysteine production in the body system and plays a crucial role in cellular protein function. Treatments supplemented with higher amount of methionine such as T5 and T8 increased the expression of LEAP-2 gene as excess methionine will be used for metabolism of cysteine to form the LEAP-2 peptide. Chicken LEAP-2 is part of the epithelial innate defense system, which hinders the interaction of potentially pathogenic microbes with the epithelial surfaces and prevents invasion of the tissues in the animal (Townes et al., 2004). However, although T8–T10 seem best for the upregulation of LEAP-2 gene which may correlate with increased immunity for broilers, T8–T10 gave the worst GR performance for the broilers.
From the results obtained, we can conclude that altering the diet of the chicken will significantly alter the gene expression of immunity genes in the chickens. However, different amino acid treatments affected different immunity genes differently and there was no treatment where all immunity genes were upregulated together. Because there was no significant pattern correlating immunity gene expression with chicken growth performance, it is recommended to do further analysis in the future to strengthen the hypothesis on the correlation of diet with the immune system of broilers. For example, challenge studies with specific pathogens can be performed on the treated groups, and the amino acid and cytokine profiling can be determined from the sera of the broiler to further correlate amino acid supplementation with immunity. In addition, microscopic evaluation of the structures of the treated broilers such as the intestine and liver may provide further information on the effect of the diet treatment.
Conclusion
In conclusion, this study revealed that lowering the CP with increased supplementation of lysine (T3) could lower the FCR while maintaining the broiler's GR. In addition, T3 also upregulates MUC2 gene expression the most, although MUC2 gene was upregulated in all treatments, indicating that lowering CP was the determinant for upregulation of MUC2 rather than lysine and methionine supplementation. The reduction in CP would reduce nitrogen emission and help reduce feed cost. Treatment 6 was the best treatment for upregulation of SLC, whereas T10 upregulated both GAL6 and LEAP-2 significantly. However, it should be noted that the chicken GR for T6 and T10 was significantly lower than that for the control. Therefore, these treatments are not recommended. In future, challenge studies with pathogens should be conducted to study the effect of an infection on the expression of these genes.
Acknowledgments
The authors would also like to thank Nurhazirah (Universiti Putra Malaysia, Serdang, Selangor) for assistance in the animal trials. The experimental animals received humane care as outlined and approved by Institutional Animal Care and Use Committee for the Care and Use of Animals for Scientific Purposes (Research Policy, Universiti Putra Malaysia. Reference number: UPM/IACUC/AUP-R081/2016). This project was fully sponsored by the Long-Term Research Grant Scheme (LRGS) under the Ministry of Education, Malaysia (LRGS, MOE) under research programme of “Enhancing the competitiveness and sustainability of the poultry industry for food security” (Grant No: UPM/700-1/3/LRGS).
Conflict of Interest Statement: The authors declare that they have no competing interests.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.03.013.
Supplementary data
References
- Abbasi M.A., Mahdavi A.H., Samie A.H., Jahanian R. Effects of different levels of dietary crude protein and threonine on performance, humoral immune responses and intestinal morphology of broiler chicks. Braz. J. Poult. Sci. 2014;16:35–44. [Google Scholar]
- Al-Nasser A., Al-Khalifa H., Al-Saffar A., Khalil F., Al-Bahouh M., Ragheb G., Al-Haddad A., Mashaly M. Overview of chicken taxonomy and domestication. Worlds. Poult. Sci. J. 2007;63:285–300. [Google Scholar]
- Ariffin A.D., Mohtar S., Baluch N. 4th International Conference on Technology and Operations Management (ICTOM04); Kuala Lumpur, Malaysia: 2014. Broiler industry on short supply chain in Malaysia. Proc; pp. 34–46. [Google Scholar]
- Baracos V.E. Animal Models of amino acid metabolism: a Focus on the intestine. J. Nutr. 2004;134:1656S–1659S. doi: 10.1093/jn/134.6.1656S. [DOI] [PubMed] [Google Scholar]
- Cellier M., Prive G., Belouchi A., Kwan T., Rodrigues V., Chia W., Gros P. Nramp defines a family of membrane proteins. Proc. Natl. Acad. Sci. 1995;92:10089–10093. doi: 10.1073/pnas.92.22.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb-Vantress . 2013. Management guides.http://www.cobb-vantress.com/academy/managementguides Accessed Oct. 2013. [Google Scholar]
- Doiphode A., Rajaravindra K.S., Das D., Mitra A. Molecular cloning and characterization of SLC11A1 cDNA in Japanese Quail (Coturnix Coturnix Japonica) Vet. Immunol. Immunopathol. 2009;129:143–146. doi: 10.1016/j.vetimm.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Erf G.F. Cell-mediated immunity in poultry. Poult. Sci. 2004;83:580–590. doi: 10.1093/ps/83.4.580. [DOI] [PubMed] [Google Scholar]
- Fafournoux P., Bruhat A., Jousse C. Amino acid regulation of gene expression. Biochem. J. 2000;351:1–12. doi: 10.1042/0264-6021:3510001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N.S., Gates R.S., Taraba J.L., Cantor A.H., Pescatore A.J., Straw M.L., Ford M.J., Burnham D.J. The effect of dietary crude protein on growth, ammonia concentration, and litter composition in broilers. Poult. Sci. 1998;77:1481–1487. doi: 10.1093/ps/77.10.1481. [DOI] [PubMed] [Google Scholar]
- Fernandez S.R., Aoyagi S., Han Y., Parsons C.M., Baker D.H. Limiting order of amino acids in corn and soybean meal for growth of the chick. Poult. Sci. 1994;73:1887–1896. doi: 10.3382/ps.0731887. [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Goswami T., Bhattacharjee A., Babal P., Searle S., Moore E., Li M., Blackwell J.M. Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochem. J. 2001;519:511–519. doi: 10.1042/0264-6021:3540511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstein J.R., Lamont S.J. Chicken gallinacin gene Cluster Associated with Salmonella response in advanced Intercross line. Avian Dis. Dig. 2007;51:561–567. doi: 10.1637/0005-2086(2007)51[561:CGGCAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hickling D., Guenter W., Jackson M.E. The effects of dietary methionine and lysine on broiler chicken performance and breast meat yield. Can. J. Anim. Sci. 1990;70:673–678. [Google Scholar]
- Horn N.L., Donkin S.S., Applegate T.J., Adeola O. Intestinal mucin dynamics: response of broiler chicks and White Pekin ducklings to dietary threonine. Poult. Sci. 2009;88:1906–1914. doi: 10.3382/ps.2009-00009. [DOI] [PubMed] [Google Scholar]
- Jabado N., Jankowski A., Dougaparsad S., Picard V., Grinstein S., Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 2000;192:1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E.V., Hansson G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes. 2010;1:51–54. doi: 10.4161/gmic.1.1.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran Z., Mirza M.A., Alsan-ul-Haq, Mahmood S. Effect of decreasing dietary protein levels with optimal amino acids profile on the performance of broilers. Pak. Vet. J. 2004;24:165–168. [Google Scholar]
- King S.C., Hansen C.L., Wilson T.H. The interaction between aspartic acid 237 and lysine 358 in the lactose carrier of Escherichia coli. BBA - Biomembr. 1991;1062:177–186. doi: 10.1016/0005-2736(91)90390-t. [DOI] [PubMed] [Google Scholar]
- Konashi S., Takahashi K., Akiba Y. Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Br. J. Nutr. 2000;83:449–456. [PubMed] [Google Scholar]
- Law G.K., Bertolo R.F., Adjiri-Awere A., Pencharz P.B., Ball R.O. Adequate oral threonine is critical for mucin production and gut function in neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1293–G1301. doi: 10.1152/ajpgi.00221.2006. [DOI] [PubMed] [Google Scholar]
- Li P., Yin Y.-L., Li D., Woo Kim S., Wu G. Amino acids and immune function. Br. J. Nutr. 2007;98:237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- Loh T.C. Livestock production and the feed industry in Malaysia. Anim. Sci. 2002;18:329–339. [Google Scholar]
- Lynn D.J., Higgs R., Gaines S., Tierney J., James T., Lloyd A.T., a Fares M., Mulcahy G., O’Farrelly C. Bioinformatic discovery and initial characterisation of nine novel antimicrobial peptide genes in the chicken. Immunogenetics. 2004;56:170–177. doi: 10.1007/s00251-004-0675-0. [DOI] [PubMed] [Google Scholar]
- Lynn D.J., Lloyd A.T., O’Farrelly C. In silico identification of components of the Toll-like receptor (TLR) signaling pathway in clustered chicken expressed sequence tags (ESTs) Vet. Immunol. Immunopathol. 2003;93:177–184. doi: 10.1016/s0165-2427(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Michailidis G. Expression of chicken LEAP-2 in the reproductive organs and embryos and in response to Salmonella enterica infection. Vet. Res. Commun. 2010;34:459–471. doi: 10.1007/s11259-010-9420-3. [DOI] [PubMed] [Google Scholar]
- Mohamed Z., Shamsudin M.N., Latif I.A., Mu′azu A.U. Proc. of the International Conference on Social Science Research (ICSSR 2013); Penang, Malaysia: 2013. Measuring competition along the supply chain of the Malaysian poultry industry; pp. 1454–1466. [Google Scholar]
- Montagne L., Piel C., Lallels J.P. Effect of diet on mucin kinetics and composition: nutrition and health implications. Nutr. Rev. 2004;62:105–114. doi: 10.1111/j.1753-4887.2004.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Ojano-Dirain C., Waldroup P. Evaluation of lysine, methionine and threonine needs of broilers three to six week of age under moderate temperature stress. Int. J. Poult. Sci. 2002;1:16–21. [Google Scholar]
- Park B.C. Amino acid imbalance-biochemical mechanism and nutritional aspects. Asian-Australasian J. Anim. Sci. 2006;19:1361–1368. [Google Scholar]
- Pavlova I., Milanova A., Danova S., Fink-Gremmels J. Enrofloxacin and probiotic Lactobacilli influence PepT1 and LEAP-2 mRNA expression in poultry. Probio. Antimicrob. Proteins. 2016;8:215–220. doi: 10.1007/s12602-016-9225-y. [DOI] [PubMed] [Google Scholar]
- Pesti G.M. Impact of dietary amino acid and crude protein levels in broiler feeds on biological performance. J. Appl. Poult. Res. 2009;18:477–486. [Google Scholar]
- Rebrikov D.V., Trofimov D.Y. Real-time PCR: a review of approaches to data analysis. Appl. Biochem. Microbiol. 2006;42:455–463. [PubMed] [Google Scholar]
- Rezaei M., Nassiri Moghaddam H., Pour Reza J., Kermanshahi H. The effects of dietary protein and lysine levels on broiler performance, carcass characteristics and N excretion. Int. J. Poult. Sci. 2004;3:148–152. [Google Scholar]
- Riedijk M.A., Stoll B., Chacko S., Schierbeek H., Sunehag A.L., van Goudoever J.B., Burrin D.G. Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc. Natl. Acad. Sci. 2007;104:3408–3413. doi: 10.1073/pnas.0607965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistic SAS Institute Inc; Cary, NC: 2013. SAS User Guide. [Google Scholar]
- Shazali N. Universiti Putra Malaysia; Selangor, Malaysia: 2015. Growth performance, gut morphology and immune response of broiler chickens fed low protein diets supplemented with lysine and methionine. Master Thesis. [Google Scholar]
- Shazali N., Loh T.C., Foo H.L., Samsudin A.A. Gut microflora and intestinal morphology changes of broiler chickens fed reducing dietary protein supplemented with lysine, methionine, and threonine in tropical environment. Brazillian J. Anim. Sci. 2019;48 [Google Scholar]
- Van Dijk A., Veldhuizen E.J.A., Kalkhove S.I.C., Tjeerdsma-Van Bokhoven J.L.M., Romijn R.A., Haagsman H.P. The β-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against food-borne pathogens. Antimicrob. Agents Chemother. 2007;51:912–922. doi: 10.1128/AAC.00568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis M., Schaart M.W., de Koning B. a E., Schierbeek H., Velcich A., Renes I.B., van Goudoever J.B. Threonine metabolism in the intestine of mice: loss of mucin 2 induces the threonine catabolic pathway. J. Pediatr. Gastroenterol. Nutr. 2009;49:99–107. doi: 10.1097/MPG.0b013e3181a23dbe. [DOI] [PubMed] [Google Scholar]
- van Goudoever J.B., Stoll B., Henry J.F., Burrin D.G., Reeds P.J. Adaptive regulation of intestinal lysine metabolism. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11620–11625. doi: 10.1073/pnas.200371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseraud S., Maaa N., Peresson R., Chagneau A.M. Relative responses of protein turnover in three different skeletal muscles to dietary lysine deficiency in chicks. Br. Poult. Sci. 1996;37:641–650. doi: 10.1080/00071669608417893. [DOI] [PubMed] [Google Scholar]
- Townes C.L., Michailidis G., Nile C.J., Hall J. Induction of cationic chicken liver-expressed antimicrobial peptide 2 in response to Salmonella enterica infection. Infect. Immun. 2004;72:6987–6993. doi: 10.1128/IAI.72.12.6987-6993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.