Abstract
INTRODUCTION:
The placenta employs an efficient and selective fatty acid transport system to supply lipids for fetal development. Disruptions in placental fatty acid transport lead to restricted fetal growth along with cardiovascular and neurologic deficits. Nevertheless, little is known about the molecular mechanisms involved in human placental fatty acid trafficking during the initial steps of uptake, or the importance of fatty acid chain length in determining uptake rates.
METHODS:
We employed BODIPY fluorophore conjugated fatty acid analogues of three chain lengths, medium (BODIPY-C5), long (BODIPY-C12), and very-long (BODIPY-C16), to study fatty acid uptake in isolated human trophoblast and explants using confocal microscopy. The three BODIPY-labeled fatty acids were added to freshly isolated explants and tracked for up to 30 minutes. Fatty acid uptake kinetics were quantified in trophoblast (cytotrophoblast and syncytiotrophoblast together) and the fetal capillary lumen.
RESULTS:
Long- (BODIPY-C12) and Very long-chain (BODIPY-C16) fatty acids accumulated more rapidly in the trophoblast layer than did medium-chain (BODIPY-C5) whereas BODIPY-C5 accumulated more rapidly in the fetal capillary than did the longer chain length fatty acids. The long-chain fatty acids, BODIPY-C12 and BODIPY-C16, are esterified and stored in lipid droplets in the cytotrophoblast layer, but medium-chain fatty acid, BODIPY-C5, is not.
DISCUSSION:
Fatty acids accumulate in trophoblast and fetal capillaries inversely according to their chain length. BODIPY-C5 accumulates in the fetal capillary in concentrations far greater than in the trophoblast, suggesting that medium-chain length BODIPY-labeled fatty acids are capable of being transported against a concentration gradient.
Introduction
The placenta delivers oxygen and nutrients to the fetus and produces pregnancy-sustaining hormones. Of the major nutrient classes transported by the placenta to support human development, the daily caloric requirement for fatty acids is the greatest[1]. Long-chain polyunsaturated fatty acids (LCPUFA) in particular, are crucial for developing embryonic and fetal organs including the lipid rich brain and cardiovascular systems[2]. Thus, their transport must increase with gestational age to match increasing fetal requirements[2–6]. The human placenta selectively takes up LCPUFA in their non-esterified form via several transport proteins and translocases[7,8] (FATP1, FATP4, FATP2, and FAT/CD36[9–12]) but when these systems are compromised, detrimental developmental consequences such as cognitive impairment and delayed brain maturation occur[13–15].
Placental transport of fatty acids is selective by fatty acid species. This selectivity in transport ensures the appropriate quantities of various fatty acid types are delivered to meet fetal lipid requirements for organ development. The mechanisms regulating the maternofetal transport of fatty acids, including sorting by chain length is less well understood compared than those that regulate glucose and amino acids transport[16]. Studies have proposed that subcellular processes such as fatty acid metabolism and esterification underlie the selective nature of fatty acid sorting and export of fatty acids from placenta[17–19], but these subcellular processes have yet to be discovered.
Unexpectedly, long-chain fatty acid BODIPY-C12 applied to the maternal side of the placenta demonstrates preferential shuttling into the cytotrophoblast where it is stored and metabolized, suggesting a preferential uptake and metabolism of fatty acids by one particular placental cell types[20,21]. While this process in the cytotrophoblast may underlie the intermediate esterification step involved in long-chain fatty acid transport proposed by others [18,19,22], it is unknown if the cytotrophoblast, or any placental cell type, exhibits specificity by fatty acid chain length.
The objective of this study was to quantify the uptake kinetics of different chain length fatty acids. We hypothesized that fatty acid uptake rates by trophoblast and transport into the fetal capillary would be dependent on chain length, with the shorter chain lengths being taken up much faster than the longer lengths. We employed BODIPY-labeled fatty acid analogues to track and quantify fatty acid uptake in human placental explants and isolated trophoblast.
Materials and Methods
Studies were approved by the Oregon Health & Science University (OHSU) Institutional Review Board (IRB# 5684), and all study subjects were provided written, informed consent.
Subject Details
Women (>37 wks gestation, Table 1) scheduled for cesarean section at OHSU were screened for enrollment. Exclusion criteria included multiple gestations, fetuses with chromosomal or structural anomalies including cardiac defects, preeclampsia, diabetes, maternal hypertension, and any other significant co-morbidity.
Table 1:
Maternal Characteristics
| Mean±S.D. (n=28) | 95% Confidence Interval | |
|---|---|---|
| Age (yr) | 31±5 | [ 29, 33 ] |
| Body Mass Index (BMI: kg/m2) | 27±8 | [ 24, 30 ] |
| Parity | 2±1 | [ 1 , 3 ] |
| Gestational Age (weeks) | 39±1 | [ 38, 39 ] |
| Birth Weight (g) | 3400±500 | [ 3200 , 3600 ] |
| Placenta Weight (g) | 546±120 | [ 500, 590 ] |
Tissue overview
We used twenty-eight placentas for different aspects of the study. In brief, placentas were collected at the time of delivery by cesarean section. The fetal membranes were removed prior to obtaining placental weight and dimensions. All placentas were processed within 30min of delivery. Placental tissue samples were used for (i) explants (<1mm3) isolated as previously described[10,20] for live imaging studies, and (ii) isolated cytotrophoblast cell studies for in vitro cell culture and Western Blotting.
Primary villous cytotrophoblast isolation and culture
Cytotrophoblast cells were isolated using trypsin-DNAse I digestion followed by Percoll enrichment as previously described[20,23]. Using Cytokeratin-7 and Vimentin immunohistochemical markers, the average CTB isolation purity used for these studies is 90.8% ± 0.3%. A representative image of the purity of our 8hr CTB is shown in Figure S1. Cytotrophoblasts were plated at a density of 3.0 × 105 cells cm−2 on tissue culture dishes or glass coverslips. Cells were cultured in Iscoves Modified Dulbecco’s Medium (IMDM, ATCC) supplemented with 10% Fetal Bovine Serum (FBS)(Gibco) and 100U/ml penicillin and 100μg/ml streptomycin and incubated in a 5% CO2/95% air incubator at 37°C. Cell viability was assessed by Trypan blue exclusion. After 8h in culture, nonadherent cells were removed by washing with pre-warmed culture media, and the media was subsequently replaced every 24h. Cells cultured on glass coverslips were used for fatty acid uptake studies, and Western Blot analyses were performed from plated tissue cultured cells. Cells were studied at 8h(cytotrophoblasts) or 72h(syncytiotrophoblast) when the majority of cytotrophoblasts have aggregated, fused and differentiated into multinucleated, syncytial giant cells[24].
Fluorescent fatty acid tracking and microscopy (explants, cells).
The BODIPY fluorophore is an intensely fluorescent, photostable, and intrinsically lipophilic molecule, unlike most other long-wavelength dyes. BODIPY conjugated fatty acids analogues undergo native-like cellular transport and metabolism, making them effective as a tracer for lipid trafficking[25]. The BODIPY-moiety effectively adds 4-6 carbons to the conjugated fatty acid (e.g. BODIPY-C12 is most similar to C-16 and C-18 natural fatty acids and BODIPY-C16 is similar to very long-chain fatty acids C-20 and C-22)[26]. BODIPY-C12, C16, C5 are well established as tools to study fatty acid uptake and esterification in multiple models[25].
We prepared 10μM solutions of BODIPY-C12 (Molecular Probes) by diluting a 2.5mM DMSO stock solution (1:250) in M199-HEPES containing 0.1% fatty acid-free bovine serum albumin (FAF-BSA) (Fisher Scientific)[20]. Explants and cells were exposed to 2μM BODIPY-fatty acids for 30min for most experiments because the uptake consistently reached a plateau by 15min.
Explants:
Explants were maintained in IMDM media. To assess explant viability, 2μL ethidium homodimer (Molecular Probes) was added to each well containing explants to distinguish dead regions of tissue. Imaging was restricted to regions with no evidence of ethidium homodimer uptake. For lipid droplet (LD) labeling, 1:500 of HCS LipidTOX Deep Red (Molecular Probes) was mixed into each well containing placental explants. After 30min, 2μM BODIPY-fatty acid solution was added to each well and mixed by trituration. Explants were immobilized for confocal microscopy using 8×8mm pieces of stainless steel mesh (TWP, Inc) and an incubation chamber (37°C; Okolab) was used.
Quantification of total BODIPY-C12 uptake in cells
Total BODIPY-C12 uptake was determined in cytotrophoblast cultures using a plate-reader assay as described[27]. Cytotrophoblast were plated at 3.0 × 105 cells cm−2 in 96-well plates (BD Falcon) and cultured as described. Cells were studied at 8h (cytotrophoblasts) and 72h of cell culture. Cells were pre-incubated in 80μL serum free IMDM for 1h with or without inhibitors. Inhibitors used included 10% FBS, Triacsin C to inhibit Long-chain Acyl-CoA Synthetase (Santa Cruz Biotech), and the FATP2 inhibitor, CB-2 (EMD Millipore). After 1h, 20μL of assay solution (25 μM BODIPY-fatty acid, 25 μM FAF-BSA, 10mg/ml Trypan Blue) was added to each well, mixed, and incubated for 20min at 37°C with 5% C02/95% air. Fluorescence (485nm excitation, 525nm emission) was measured using a BioTek Synergy H1 hybrid plate reader (BioTek). After measurement, the medium was removed, and plates were carefully washed with Hank’s Buffer for 10min at 37°C. Cells were then lysed using RIPA buffer (EMD Millipore) and protein was measured using a BCA method (Thermo Pierce).
Immunofluorescence
Cells cultured for 8h and 72h on glass coverslips were incubated with BODIPY-C12 for 30min as described above and fixed in 4% paraformaldehyde for 20min at room temperature. Samples were blocked and permeabilized in Block-aid (Life Technologies)/0.1% saponin (Sigma) for 1h before overnight incubation with primary antibody against desmoplakin (1:200, ab16434, Abcam) at 4°C. Following primary antibody incubation, samples were washed with PBS and labeled with secondary antibodies (donkey F(ab’)2 anti-mouse Alexa Fluor 647 (1:500, ab150103), for 1h at room temperature. The samples were washed and counterstained with 1 μg/ml Hoechst 33342 for 10min, mounted in Slowfade Diamond (Molecular Probes), and imaged using a Zeiss 880 LSM Confocal with Airyscan.
Image Analysis
For live-imaging of explants, 2 separate explants were imaged. All images were analyzed using Fiji software[28].The mean fluorescence intensity was measured in the trophoblast layer, representing a combination of the syncytiotrophoblast and cytotrophoblast, and the fetal capillary lumen using a consistently sized region of interest after automatic video stabilization. Because the BODIPY fluorophore is relatively insensitive to environmental conditions, quantification of fluorescence intensity is a reliable measure of tracer concentration[29].
Co-localization analyses were performed with Fiji using the automatic method of Costes et al.[30]. The Pearson co-localization coefficient indicates the degree to which BODIPY-C12 is being incorporated into the LD organelles identified by LipidTOX[31]. To segment and quantify particles, we used the analyze particles process on the thresholded images (Otsu method) in Fiji. This method distinguishes particles by their brightness relative to their surroundings.
Western Blotting
Protein was isolated after 8h or 72h of cell culture using RIPA lysis buffer (EMD Millipore) and concentrations were measured using a BCA protein assay kit (Thermo Pierce). SDS-PAGE, loaded with 15μg, and blotting was performed onto nitrocellulose using a Biorad Minicell (Biorad). Blots were probed with mouse anti-human FATP2 (1:500, ab175373, Abcam) and incubated overnight at 4°C. The blots were washed and probed with an anti-mouse HRP conjugated secondary antibody (1:1000, sc-2005, Santa Cruz Biotech) and developed for 5min using a chemilumiscent substrate (SuperSignal, Thermo Pierce). Gels were imaged using a UVP gel doc and all images were quantified using Fiji.
Statistical Methods
The linear portion(0-15min) of the uptake curve in explant studies was analyzed using linear regression. Two-way ANOVA with Sidak’s multiple comparisons test was used for all in vitro uptake plate reader experiments. Paired Student t-test was used for western blot quantification. P-values less than 0.05 were considered significant.
Results
To continuously quantify the uptake of various chain length fatty acids in live placenta tissue at two locations simultaneously, we used live-confocal microscopy and three BODIPY-labeled fatty acids differing only by fatty acid chain length incubated with human term placental explants. In all experiments, the BODIPY-fatty acids are too dilute to be detectable in the extra-villous space, but become visible as they accumulate in tissue. BODIPY-fatty acid uptake was first visible at the microvillous membrane and proceeded gradually inward toward the fetal capillaries. Within 15min of incubation, fatty acids of different chain lengths accumulated to differing degrees throughout the placental layers and in fetal capillaries(Figure 1A–C, Supplemental Movie S1,S2,S3).
Figure 1. Kinetic distribution of various fatty acid analogues during uptake in living human term placental explants imaged via confocal microscopy.
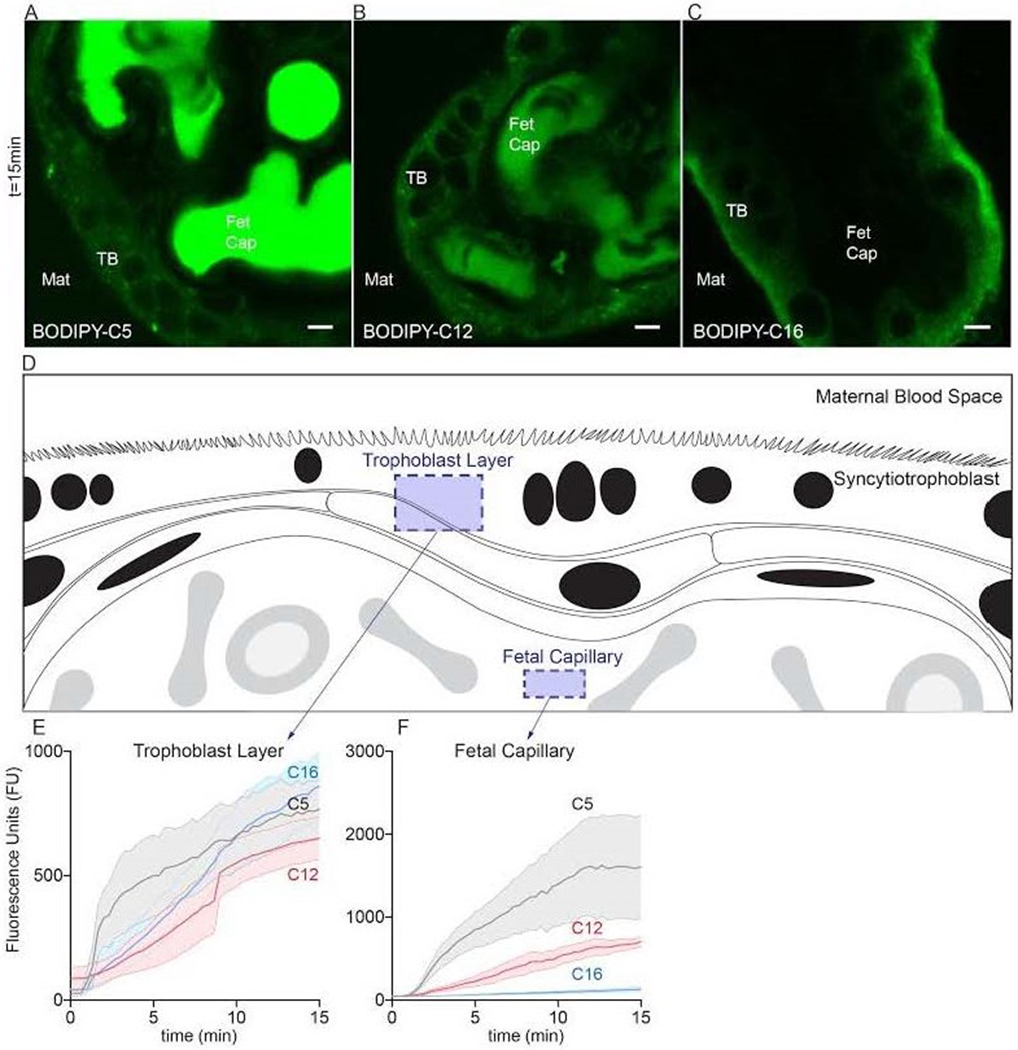
Explants isolated immediately after cesarean section (< 2h) were placed into HBSS onto a stage top incubator (37°C/ambient air) of a confocal microscope. 2μM fluorescent fatty acids were added to explant medium, representing the maternal blood space (Mat), after initialization the image acquisition. The tracer concentration in the medium was not sufficient to register a signal at these settings. Three fluorescent fatty acids were compared, BODIPY-C5 (medium-chain fatty acid), BODIPY-C12 (long-chain fatty acid), and BODIPY-C16 (very long-chain fatty acid). Uptake distributions varied according to fatty acid chain length over 20min of time. (A) BODIPY-C5 accumulated to the greatest degree within the fetal capillaries (Fet Cap) against an apparent concentration gradient; very little tracer was present in trophoblast (TB) or other placental cells at 20min. (B) BODIPY-C12 appeared to distribute equally between all layers and compartments of placental explants by 20min. (C) BODIPY-C16 largely incorporated into the trophoblast layer; little was present in capillaries at 20min. (D) Schematic representation of regions quantified in explants to produce panels E and F. (E,F) Quantification of total fluorescence, of BODIPY-C5, BODIPY-C12, and BODIPY-C16 at two different locations in explants over time. Data are Mean±SEM. Scale Bar: 5μm. n=5 placentas.
The uptake rates into trophoblast layers were different according to fatty acid chain length (BODIPY-C5: 42 ± 4.4 Fluorescence Units (FU) per min, FU•min−1, r2=0.29 , BODIPY-C12: 46 ± 2.9 FU•min−1, r2=0.54 and BODIPY-C16: 61 ± 3.3 FU•min−1, r2=0.59 p<0.001)(Figure 1E). By 15min, fluorescence in the trophoblast layer equilibrated and was not different between fatty acids, p>0.05(Figure 1E). BODIPY-C5, the shortest chain fatty acid, was rapidly transported into fetal capillaries where it accumulated(Figure 1A). For BODIPY-C5, the uptake rate was 110 ± 13 FU•min−1, r2=0.26 compared to 50 ± 2.5 %min−1, r2=0.64 for BODIPY-C12 and 6.1 ± 0.46 %min−1, r2=0.44 for BODIPY-C16 p<0.0001 (Figure 1F). Thus, as the fatty acid chain length increased, accumulation rates into fetal capillaries became slower. The mean fluorescence in the capillaries at 15min was greater for BODIPY-C5 (1610 ± 623 FU) than BODIPY-C12 (705 ± 57.4 FU p<0.05) or BODIPY-C16 131 ± 28.0 FU p<0.0001). B0DIPY-C12 and BODIPY-C16 mean fluorescence in capillaries at 15min was not different. Uptake of all fatty acid analogues plateaued by 30 minutes (not shown).
In other cell types, esterification of Long- (BODIPY-C12) and Very-long (BODIPY-C16), but not short-chain fatty acids mediates uptake and accumulation of fatty acids through incorporation into lipid droplets (LD)[32]. To determine whether esterification might be occurring in placental tissue and whether these processes are specific to long- and very long-chain fatty acids, we repeated the live explant studies using a LD counterstain, LipidTOX, to identify pre-existing LD and measure the extent of newly incorporated BODIPY-fatty acids. BODIPY-C5 highlighted punctate structures in the trophoblast layer (Figure 2A), but these punctate structures did not co-localize with LD (Figure 2B,C). In contrast, within min of addition of BODIPY-C12, numerous LD emerged and predominantly located in the cytotrophoblast(Figure 2D, Movie S4). BODIPY-C12 also incorporated into pre-existing LD (Figure 2E, F), signifying esterification of BODIPY-C12.
Figure 2. Comparison of short- and long-chain fatty acid uptake in human term placental explants after 15min of incubation with BODIPY-C5 or BODIPY- C12.
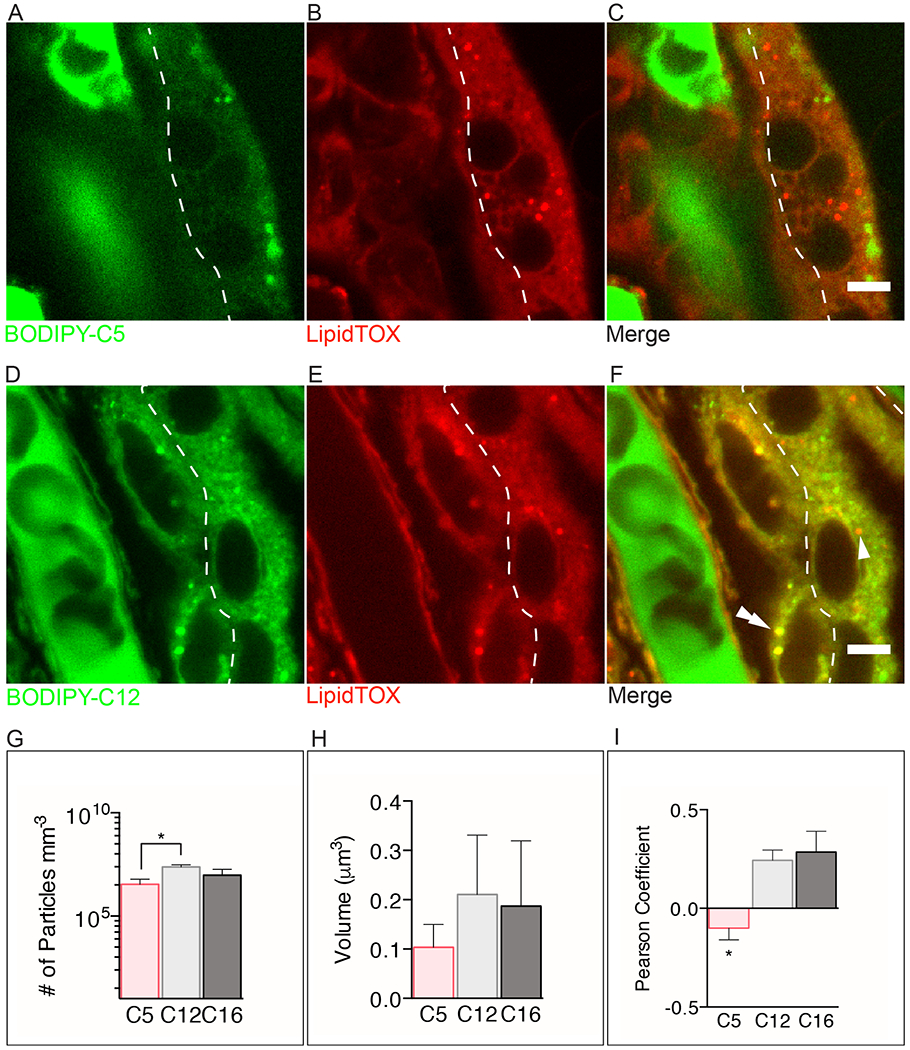
The dashed line separates the syncytial and cytotrophoblast layers. (A-C) BODIPY-C5 (green) (D-F) BODIPY-C12 (green) (A) The BODIPY-C5 fatty acid is found diffusely throughout all tissues and within immotile, unidentified punctate structures. (B) The green punctate structures shown in A are not stained with LipidTOX suggesting that they are not lipid droplets (LD). (C) Merge image shows that BODYPY-C5 is not esterified nor incorporated into LD. (D-F) In contrast, BODIPY-C12 is esterified and incorporated into LD (double arrowhead) in the cytotrophoblast but not the syncytiotrophoblast (arrowhead). (G) There are significantly more punctate structures in explants incubated with BODIPY-C12 than with BODIPY-C5 (log scale). (H) Volumes of BODIPY-C12, BODIPY-C16, and BODIPY-C5 particles are not different. (I) The long- and very long-chain fatty acids BODIPY-C12 and BODIPY-C16, respectively, co-localize with a greater frequency with LipidTOX stained droplets than does BODIPY-C5. This suggests that BODIPY-C12 and BODIPY-C16 are being incorporated into LD to a greater extent than BODIPY-C5. (A-F): Dashed line represents the syncytiotrophoblast-cytotrophoblast interface. Scale Bar: 5μm. (g-i): Data are mean ± SEM; n=5 BODIPY-C5, n=7 BODIPY-C12, n=5 BODIPY-C16. Unpaired t-test, *= p<0.05, **=p<0.01 vs BODIPY-C12.
We quantified the total number of BODIPY-containing particles, the bright punctate structures highlighted by BODIPY-fatty acids, as an estimate of LD accumulation after 15min. The number of particles (irrespective of cell layer) accumulated by 15min was higher with BODIPY-C12 (2×107 ± 7×106 particles mm−3) than BODIPY-C5 (4×106 ± 3×106 particles mm−3; p<0.05, Figure 2G), but was not different with BODIPY-C16 (1×107 ± 4×106 particles mm−3; p<0.05, Figure 2G). There was no difference between the volumes of the BODIPY-labeled particles incorporating BODIPY-C12 (0.21 ± 0.12 μm3) vs BODIPY-C5 (0.10 ± 0.046 μm3) vs BODIPY-C16 (0.19 ± 0.13 μm3) at 15min (Figure 2H). There was, however, greater co-localization (Pearson correlation) of LipidTOX and BODIPY-C12 (r= 0.24 ± 0.053), LipidTOX and BODIPY-C16 (r= 0.29 ± 0.11) than BODIPY-C5 and LipidTOX (r= −0.067 ± 0.063 p<0.05, Figure 2I).
Fatty acid esterification to Acyl-CoAs occurs concertedly with uptake [33]. We have previously shown that long-chain fatty acid esterification is significantly higher in the cytotrophoblast (CTB) compared to syncytiotrophoblast (SCT) [20]. To explore how different transport systems contribute to fatty acid uptake in each cell type, we tested a panel of inhibitors. We compared uptake of BODIPY-C5, BODIPY-C12, BODIPY-C16 in CTB at 8h compared to SCT at 72h to test in the presence of natural fatty acids present in fetal bovine serum, inhibition of FATP2 (CB-2), or ACSL (1,3,4,5) inhibition (Triacsin C).
Total uptake of BODIPY-fatty acid was quantified after 20min of incubation using a plate reader [27]. Consistent with explant experiments, minimal BODIPY-C5 accumulated within cells (Figure 3A). CTB uptake of BODIPY-C5 at 15min was not significantly different from the SCT (29±8.9 vs 15±7.4 Fluorescence Units (FU)×μg protein−1, p>0.05), and was not affected by any of the inhibitors (CTB: serum: 15±8.2 FU×μg protein−1, Triacsin C: 23±13 FU×μg protein−1, CB-2: 59±18 FU×μg protein−1, p>0.05 vs control; SCT: serum: 5.1±2.2 FU×μg protein−1, Triacsin C: 23±7.8 FU×μg protein−1, CB-2: 28±7.5 FU×μg protein−1, p>0.05 vs control) (Figure 3A).
Figure 3. Quantification of total BODIPY-fatty acid uptake in primary isolated human term trophoblast cells with and without inhibitors.
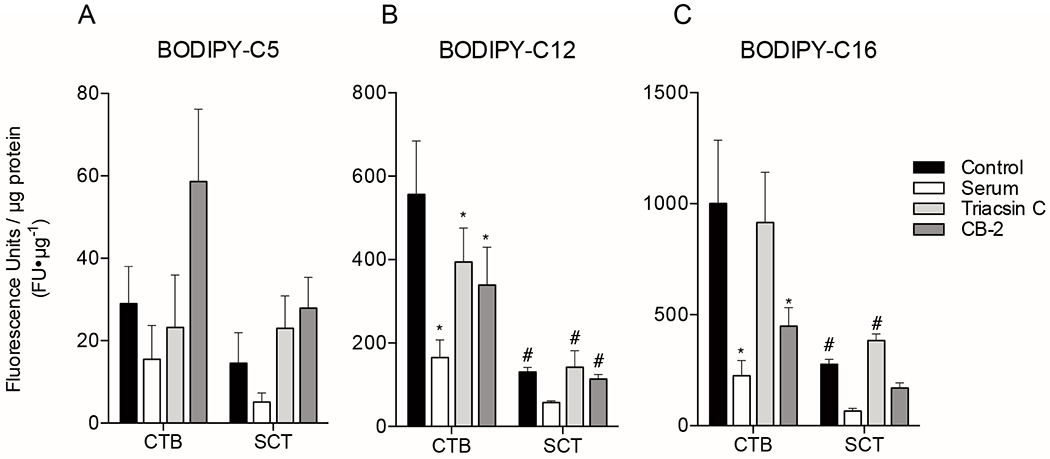
Primary human trophoblast were isolated and plated onto 96 well plates. Cells were pre-treated with inhibitors (fetal bovine serum [FBS], Triacsin C [Acyl-CoA inhibitor], CB-2 [FATP2 inhibitor]) 1h before addition of 2μM BODIPY-fatty acid, and total uptake was measured after 20min of incubation with BODIPY-fatty acid using plate reader fluorescence as a marker. Trophoblast tissues were assayed at two time points after initial plating, at 8h to measure cytotrophoblast uptake, or at 72h allow for differentiation to syncytiotrophoblast (SCT) before measuring uptake. (A) BODIPY-C5, (B) BODIPY-C12, and (C) BODIPY-C16 uptake was measured. These data indicate uptake of long and very long-chain fatty acids are transporter dependent. Data are Mean±SEM, n=5 placentas. *= p<0.05, **=p<0.01 vs corresponding cell type control. #=p<0.05 CTB vs SCT.
Total accumulation of BODIPY-C12 and BODIPY-C16 was substantially greater than BODIPY-C5. The accumulation of BODIPY-C12 by CTB was significantly higher than for SCT (556±128 vs. 131±10.6 FU×μg protein−1, p<0.0001). The uptake of BODIPY-C12 into CTB was inhibited by FBS (165±42.3 FU×μg protein−1, p<0.0001), Triacsin C (394±81.2 FU×μg protein−1, p<0.05), and CB-2 (339±91.2 FU×μg protein−1, p<0.01) (Figure 3B). In contrast, the uptake of BODIPY-C12 into SCT was not inhibited by FBS (57.5±3.62 FU×μg protein−1, p=n.s.), Triacsin C (142±39.5 FU×μg protein−1, p=n.s.), or CB-2 (114±10.8 FU×μg protein−1, p=n.s.)(Figure 3B).
Of the fatty acid analogues tested, BODIPY-C16 accumulated most in CTB. Uptake of BODIPY-C16 by CTB at 20min was greater than SCT (1000±285 vs. 277±23.4 FU×μg protein−1, p<0.001). The CTB uptake was decreased in the presence of FBS (225±68.8 FU×μg protein−1, p<0.0001) and CB-2 (445±84.0 FU×μg protein−1, p<0.01), but not triacsin C (915±227 FU×μg protein−1, p=n.s.)(Figure 3C). The SCT uptake was not different with FBS (66.7±11.6 FU×μg protein−1, p=n.s.), triacsin C (383±30.1 FU×μg protein−1, p=n.s.), or CB-2 (170±23.6 FU×μg protein−1, p=n.s.)(Figure 3C).
Differentiation of CTB to SCT is associated with suppression in long and very long-chain fatty acid uptake, but it is possible this effect is a byproduct of in vitro culture. Thus, we tested if blocking differentiation could preclude the suppression in long-chain and very long-chain fatty acid uptake. Similar to previous reports, we found CTB cultured for 72h predominantly fused and lost intercellular junctional divisions as evidenced by desmoplakin staining (Figure 4A), and SB203580 prevented fusion as discrete CTB demarcated by desmoplakin remained by 72h(Figure 4B). We measured total uptake of BODIPY-C12 in trophoblast cultured for 8h, 24h, and 72h with and without SB203580, p38 MAPK inhibitor to block differentiation. At 8h and 24h the uptake of BODIPY-C12 at 20min was not different between treated and untreated CTB: (8h: control 521±31.4 FU×μg protein−1 vs SB203580 495±42.8 FU×μg protein−1,p=n.s), (24h: control 316±35.7 FU×μg protein−1 vs SB203580 357±43.5 FU×μg protein−1, p=n.s)(Figure 4C). However, by 72h, when the majority of CTB have differentiated to SCT in the control group, uptake of BODIPY-C12 at 20min was greater in the SB203580 treated trophoblast (72h: control 248±41.8 FU×μg protein−1 vs SB203580 351±52.1 FU×μg protein−1, p<0.001)(Figure 4C).
Figure 4. The suppression in long-chain fatty acid uptake during differentiation of cytotrophoblasts to syncytiotrophoblast can be prevented with p38 MAPK inhibition.
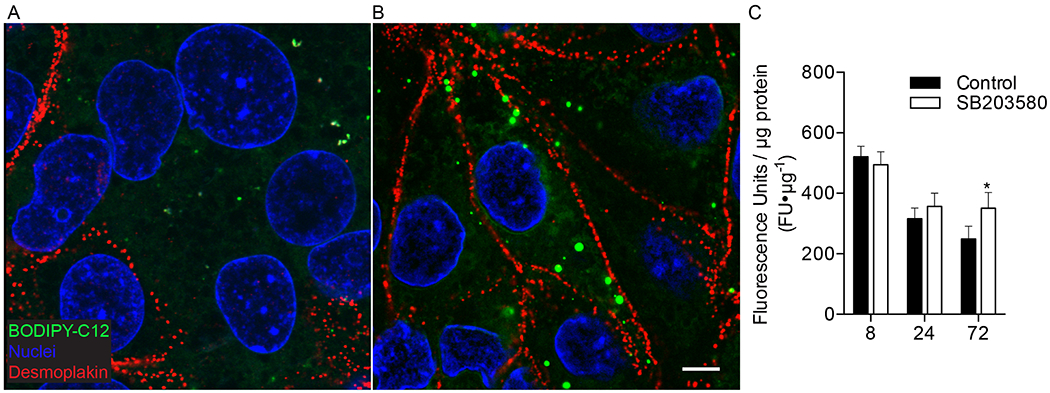
(A-B) Trophoblast were cultured for 72h with and without 10μM SB203580 (p38 MAPK inhibitor), incubated with 2μM BODIPY-C12 (green) for 20min, fixed and immunolabeled with desmoplakin (red) to visualize intercellular junctions and fatty acid uptake using confocal microscopy. Nuclei are labeled blue (Hoechst) (A) Desmoplakin shows large syncytial aggreggates in control 72h cultures, representing syncytiotrophoblast (SCT). (B) 72h cultures treated with SB203580 show cytotrophoblast (CTB) have aggregated, but remain as discrete cells, as evidenced by clearly demarcated desmoplakin labeled intercellular junctions, and suggests SB203580 maintains the CTB phenotype in vitro and prevents CTB differentiation to SCT (C) Total uptake of BODIPY-C12 long-chain fatty acid in isolated primary human term trophoblast was measured after 20min of incubation using a plate reader. Uptake was measured at 8, 24, or 72h after initial plating with and without a differentiation blocker SB203580. Treatment with SB203580 had no detectable effect on total uptake of BODIPY-C12 at 8 or 24h time points in culture. Trophoblast at 8 and 24h represent CTB because the majority of cells have not syncytialized at these time points. However, by 72h when CTB have largely fused to become SCT, uptake of BODIPY-C12 is greater in cultures treated with SB203580. These data indicate blocking differentiation of CTB to SCT is sufficient to preserve greater levels of BODIPY-C12 long-chain fatty acid uptake. Data are Mean±SEM, n=8 placentas.
We hypothesized that our observations of the greater CTB uptake of long and very long-chain fatty acids could be explained by higher levels of FATP2 protein levels, which is a LCPUFA transporter[34,35]. We tested this hypothesis by comparing CTB and SCT FATP2 levels via western blot. FATP2 protein levels were higher in CTB (2.3±0.25) than in SCT (1.5±0.083, p<0.01)(Figure 5).
Figure 5. FATP2 western blots of cytotrophoblast and syncytiotrophoblast in vitro.
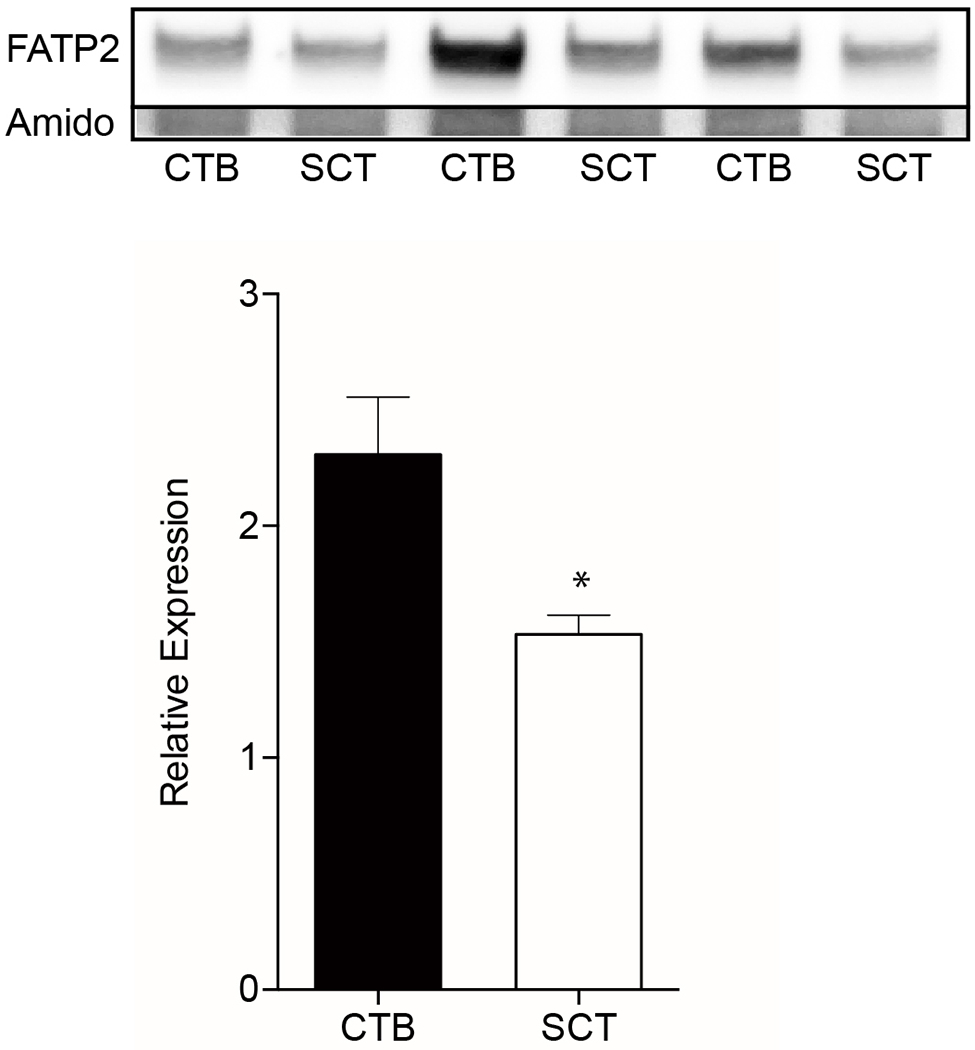
Primary human cytotrophoblast (CTB) were isolated and cultured for 8h and 72h to allow for differentiation to syncytiotrophoblast (SCT) before protein collection. Lysates were analyzed by western blots and probed for Fatty acid transporter type 2 (FATP2). These data suggest the decrease in BODIPY-C12 and/or BODIPY-C16 fatty acid uptake after syncytialization may be due to the reduction in FATP2 protein expression. FATP2 levels were normalized to total protein visualized by amido-black. Data are Mean±SEM, n=6 placentas
Discussion
We used live confocal microscopy to track fluorescently labeled fatty acid analogues in the human term placenta. The analogues represented medium- (BODIPY-C5), long- (BODIPY-C12) and very-long- (BODIPY-C16) fatty acids. We hypothesized that fatty acid uptake rate into trophoblast would be inversely proportional to chain length, consistent with passive diffusion. However, we were surprised to find such large differences in the uptake kinetics between the fatty acids tested. The rates of incorporation into the various cell layers of placenta were more different than expected by molecular weight (MW: BODIPY-C5: 320 Da; BODIPY-C12: 418 Da; BODIPY-C16: 474 Da)[36,37]. One possible explanation for our observations are differential transport rates by the action of selective transporters or co-transporters. The higher metabolic rate observed in CTB relative to SCT may support its role in transport and esterification, as these are metabolically demanding[21].
The shorter chain fatty acid, BODIPY-C5, accumulated to a lesser degree than the longer chain length fatty acids in the trophoblast layers and rapidly appeared within fetal capillaries. BODIPY-C5 appears to be highly permeable to trophoblast and accumulates in fetal capillaries, potentially reflecting a specific transport system. In contrast, BODIPY-C12 and BODIPY-C16, representing long- and very long-chain fatty acids, respectively, accumulated to high degrees in trophoblast. Within the 30min durations and concentrations tested, BODIPY-C12 accumulated in the capillaries, but little of BODIPY-C16 was found in the capillaries by 30min.
We have previously shown that BODIPY-C12 is stored in LD in the CTB and is FATP2 dependent[20]. Consistent with this, BODIPY-C12 and BODIPY-C16, known substrates of FATP2, were esterified and incorporated into LD in CTB but not the SCT. BODIPY-C5 esterification into LD was not detectable in explants or cells in culture. These results suggest chain length dependent uptake and metabolism of fatty acids in the human placenta.
Fatty acid esterification occurs concertedly during cellular uptake, but how esterification might be related placental fatty acid transport remains untested[19,22,38,39]. We investigated this question and others using a panel of transporter and esterification inhibitors in vitro. Trophoblast LD formation and BODIPY-C12 uptake were inhibited by phloretin, triacsin C, and CB-2. Phloretin is a non-specific inhibitor of protein-mediated transport, triacsin C inhibits Long-chain Acyl-CoA Synthetase (ACSL1,3,4 and 5)[40], and CB-2 inhibits FATP2[27].
Similar to our results observed in explants, minimal BODIPY-C5 accumulated within either CTB or SCT. Uptake of BODIPY-C5 appeared to be insensitive to competition with natural fatty acids in FBS. This may be because there is no natural lipid in FBS that competes with BODIPY-C5 transporters, or that its uptake is not transporter dependent. As expected, we observed no inhibition of uptake with Triacsin C, or CB-2, because these systems are specific for long- and very long-chain fatty acids[27,40]. BODIPY-C5 differs from the other two tracers we studied in that it is a medium-chain fatty acid with an odd number of carbons. The uptake and metabolism of odd chain and medium-chain fatty acids may be specialized to serve a crucial role in the placenta for the maintenance of mitochondrial Krebs cycle intermediates[41,42]. We found that the total uptake of BODIPY-C12 and BODIPY-C16 was greater than for BODIPY-C5, even by as much as an order of magnitude.
Similar to our previous report[20], we found CTB uptake of BODIPY-C12 was greater than SCT. Uptake of long- and very long-chain fatty acids into CTB were sensitive to competition from FBS fatty acids, ACSL inhibitor Triascin C (BODIPY-C12 only), and FATP2 inhibition with CB-2; but the SCT uptake was not affected by these inhibitors. The ACSL family effects long-chain fatty acids, but does not behave as a plasma membrane transporter. Because Triacsin C inhibits CTB BODIPY-C12 uptake, it appears that long- and very long-chain fatty acid uptake into CTB is facilitated by esterification.
FATP2 is especially relevant in the human placenta because it is the most highly expressed fatty acid transporter[43], and FATP2 is selective for the uptake and esterification of LCPUFA like Docosahexaenoate[34]. In the human placenta, FATP2 is appears to be localized to the CTB[11,20,43], but others have argued FATP2 is localized to the SCT as well. Consistent with the model that FATP2 is localized to the CTB, the greatest long- and very long-chain fatty acid uptake and esterification was observed in the CTB. Taken together, these data may suggest that CTB plays a unique role in the uptake of fatty acids similar to Docosahexaenoate. Other FATP isoforms (e.g. 1 and 4) expressed in placenta may be relevant to the trafficking phenomenon observed in this study, but these mechanisms were not specifically tested.
It is not immediately clear whether the lower uptake of BODIPY-C12 and BODIPY-C16 after trophoblast differentiation was associated with the reduction in the expression of FATP2[20]. We used a CTB differentiation inhibitor, SB203580, that directly blocks p38-MAPK activation, and CTB syncytialization in vitro[44,45]. With SB203580 treatment we observed a decrease in BODIPY-C12 uptake over the differentiation 72hr time course, but the decrease was not as large as found in normal differentiation. This effect was seen in spite of the fact that the syncytialization process appeared to be blocked by SB203580. Blocking differentiation of CTB was sufficient to preserve the ability of CTB to maintain higher levels of BODIPY-C12 uptake than would be expected if differentiation had occurred.
One unexpected result of our study was the degree of fatty acid tracer accumulation in fetal capillary spaces and the rapid rate at which the tracer intensity increased. Our experimental model differs from studies using the dually perfused placenta in that we allow tracer to accumulate in the capillaries in which there is no flow. It may possible that a portion of this fluorescence in the capillary represents the export of long-chain fatty acids incorporated into esterified lipids. While the concentration of unesterified fatty acids is less in umbilical than maternal blood, the absolute concentration of LCPUFA in esterified lipids is much higher in umbilical than maternal blood[46]. It is possible that the export of esterified BODIPY-fatty acids, presumably integrated within lipoproteins, is significant[47]. We cannot optically distinguish the form of lipid exported to fetal capillaries in our study, and future studies should address this. This study therefore highlights the significance of placental lipid processing in maternal-fetal lipid partitioning.
Other studies have suggested placental fatty acid transport is inadequately described by passive or facilitative mechanisms. La Fond et al. found arachidonic acid uptake, most similar in chain length to BODIPY-C16, is ATP and Na dependent in SCT basal plasma membrane[48]. BODIPY-C16 is similar in chain length to arachidonic acid, which accumulates in placental tissue greater than oleic acid[17]. Thomas et al. observed cord blood enrichment of medium- and long-chain fatty acids of partially oxidized long-chain fatty acids and suggested peroxisomal beta-oxidation may facilitate fatty acid transport[49]. These reports have proposed placental fatty acid transport appears to be regulated and is metabolically expensive.
All of the fatty acids we tested were taken up in trophoblast and fetal capillaries against a concentration gradient, which cannot be explained by diffusion alone. Similar to prior studies[10,50,51] the uptake of long and very long-chain fats are transporter dependent. The mechanism of fatty acid uptake by FATP and transporters complexed with ACSL involves an esterification step that utilizes 2-ATP equivalents per fatty acid molecule[52,53]. Unlike other placental nutrient transport systems, placental fatty acid transport appears to be regulated and metabolically demanding.
In conclusion, our experiments suggest fatty acid uptake in the human term placenta differs according to chain length. Selective esterification may account for differences in uptake in trophoblast cells. Differentiation of CTB to SCT results in a down regulation in long- and very long-chain fatty acid analogue uptake, suggesting these cells play differing roles in lipid transport and processing in the human term placenta. Our observations showing that BODIPY-fatty acids enter the SCT, the CTB and the fetal capillary against a concentration gradient are compatible only with the conclusion that these molecules are actively transported. Future investigations delving into the regulation of these kinetics and the pathological conditions caused by alterations in fatty acid transport and metabolism are needed.
Supplementary Material
Acknowledgements
This research was supported by the M. Lowell Edwards Endowment, NICHD P01HD034430 and R21 HD090529 (KT). Additional funding provided by Oregon Health and Science University School of Medicine Faculty Innovation Program (KT), the Tartar Trust Fellowship (KK). KK was funded by the Charles Patrick Memorial Fund and a Ruth L. Kirchstein National Research Service Award F30 HD084095-01. AV was funded by NIH WRHR 5 K12 HD085809. The authors thank Samantha Louey and Haeri Choi for assistance with experimental design.
Footnotes
Competing Interests
The authors have no competing interests to declare.
References:
- [1].Sparks JW, Girard JR, Battaglia FC, An estimate of the caloric requirements of the human fetus, Biol. Neonate 38 (1980) 113–119. [DOI] [PubMed] [Google Scholar]
- [2].Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW, Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements, Early Hum. Dev 4 (1980) 121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- [3].Weisinger HS, Armitage JA, Sinclair AJ, Vingrys AJ, Burns PL, Weisinger RS, Perinatal omega-3 fatty acid deficiency affects blood pressure later in life, Nat. Med 7 (2001) 258–259. doi: 10.1038/85354. [DOI] [PubMed] [Google Scholar]
- [4].Martinez M, Abnormal profiles of polyunsaturated fatty acids in the brain, liver, kidney and retina of patients with peroxisomal disorders, Brain Res. 583 (1992) 171–182. [DOI] [PubMed] [Google Scholar]
- [5].Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW, Infant cerebral cortex phospholipid fatty-acid composition and diet, Lancet. 340 (1992) 810–813. doi: 10.1016/0140-6736(92)92684-8. [DOI] [PubMed] [Google Scholar]
- [6].Innis SM, Essential fatty acid transfer and fetal development, Placenta. 26 Suppl A (2005) S70–5. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- [7].Haggarty P, Placental regulation of fatty acid delivery and its effect on fetal growth--a review, Placenta. 23 Suppl A (2002) S28–38. doi: 10.1053/plac.2002.0791. [DOI] [PubMed] [Google Scholar]
- [8].Haggarty P, Fatty acid supply to the human fetus, Annu. Rev. Nutr 30 (2010) 237–255. doi: 10.1146/annurev.nutr.012809.104742. [DOI] [PubMed] [Google Scholar]
- [9].Larqué E, Demmelmair H, Klingler M, De Jonge S, Bondy B, Koletzko B, Expression pattern of fatty acid transport protein-1 (FATP-1), FATP-4 and heart-fatty acid binding protein (H-FABP) genes in human term placenta, Early Hum. Dev 82 (2006) 697–701. doi: 10.1016/j.earlhumdev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- [10].Brass E, Hanson E, O’Tierney-Ginn PF, Placental oleic acid uptake is lower in male offspring of obese women, Placenta. 34 (2013) 503–509. doi: 10.1016/j.placenta.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lager S, Ramirez VI, Gaccioli F, Jang B, Jansson T, Powell TL, Protein expression of fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women, Placenta. 40 (2016) 60–66. doi: 10.1016/j.placenta.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Duttaroy A, Transport of fatty acids across the human placenta: A review, Progress in Lipid Research. 48 (2009) 52–61. doi: 10.1016/j.plipres.2008.11.001. [DOI] [PubMed] [Google Scholar]
- [13].Barker DJP, Thornburg KL, The obstetric origins of health for a lifetime, Clin Obstet Gynecol. 56 (2013) 511–519. doi: 10.1097/GRF.0b013e31829cb9ca. [DOI] [PubMed] [Google Scholar]
- [14].Rizzo T, Metzger BE, Burns WJ, Burns K, Correlations between antepartum maternal metabolism and child intelligence, N. Engl. J. Med 325 (1991) 911–916. doi: 10.1056/NEJM199109263251303. [DOI] [PubMed] [Google Scholar]
- [15].Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, et al. , Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study, Neurotoxicology. 29 (2008) 776–782. doi: 10.1016/j.neuro.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lager S, Powell TL, Regulation of Nutrient Transport across the Placenta, Journal of Pregnancy. 2012 (2012) 1–14. doi: 10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Haggarty P, Page K, Abramovich DR, Ashton J, Brown D, Long-chain polyunsaturated fatty acid transport across the perfused human placenta, Placenta. 18 (1997) 635–642. doi: 10.1016/S0143-4004(97)90004-7. [DOI] [PubMed] [Google Scholar]
- [18].Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG, The influence of placental metabolism on fatty acid transfer to the fetus, J. Lipid Res (2016) jlr.P072355. doi: 10.1194/jlr.P072355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gil-Sanchez A, Larque E, Demmelmair H, Acien MI, Faber FL, Parrilla JJ, et al. , Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake, Am. J. Clin. Nutr 92 (2010) 115–122. doi: 10.3945/ajcn.2010.29589. [DOI] [PubMed] [Google Scholar]
- [20].Kolahi K, Louey S, Varlamov O, Thornburg K, Real-Time Tracking of BODIPY-C12 Long-Chain Fatty Acid in Human Term Placenta Reveals Unique Lipid Dynamics in Cytotrophoblast Cells, PLoS ONE. 11 (2016) e0153522. doi: 10.1371/journal.pone.0153522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kolahi KS, Valent AM, Thornburg KL, Cytotrophoblast, Not Syncytiotrophoblast, Dominates Glycolysis and Oxidative Phosphorylation in Human Term Placenta, Sci Rep. 7 (2017) 42941. doi: 10.1038/srep42941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Coleman RA, Haynes EB, Synthesis and release of fatty acids by human trophoblast cells in culture, J. Lipid Res 28 (1987) 1335–1341. [PubMed] [Google Scholar]
- [23].Morrish DW, Dakour J, Li H, Xiao J, Miller R, Sherburne R, et al. , In vitro cultured human term cytotrophoblast: A model for normal primary epithelial cells demonstrating a spontaneous differentiation programme that requires EGF for extensive development of syncytium, Placenta. 18 (1997) 577–585. doi: 10.1016/0143-4004(77)90013-3. [DOI] [PubMed] [Google Scholar]
- [24].Douglas GC, King BF, Differentiation of human trophoblast cells in vitro as revealed by immunocytochemical staining of desmoplakin and nuclei, J Cell Sci. 96 ( Pt 1) (1990) 131–141. [DOI] [PubMed] [Google Scholar]
- [25].Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, et al. , Identification of the major intestinal fatty acid transport protein, Mol Cell. 4 (1999) 299–308. [DOI] [PubMed] [Google Scholar]
- [26].Rambold AS, Cohen S, Lippincott-Schwartz J, Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics, Dev Cell. 32 (2015) 678–692. doi: 10.1016/j.devcel.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sandoval A, Chokshi A, Jesch ED, Black PN, DiRusso CC, Identification and characterization of small compound inhibitors of human FATP2, Biochem. Pharmacol 79 (2010) 990–999. doi: 10.1016/j.bcp.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. , Fiji: an open-source platform for biological-image analysis, Nat. Methods 9 (2012) 676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hermanson GT, Bioconjugate Techniques, Academic Press, 2013. doi: 10.1016/B978-0-12\u003cbr\u003e\n-382239-0.00009-1. [DOI] [Google Scholar]
- [30].Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S, Automatic and quantitative measurement of protein-protein colocalization in live cells, Biophysj. 86 (2004) 3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kassan A, Herms A, Fernández-Vidal A, Bosch M, Schieber NL, Reddy BJN, et al. , Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains, J Cell Biol. 203 (2013) 985–1001. doi: 10.1083/jcb.201305142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang H, Starodub O, McIntosh A, Kier AB, Schroeder F, Liver fatty acidbinding protein targets fatty acids to the nucleus. Real time confocal and multiphoton fluorescence imaging in living cells, J Biol Chem. 277 (2002) 29139–29151. doi: 10.1074/jbc.M202923200. [DOI] [PubMed] [Google Scholar]
- [33].Richards MR, Harp JD, Ory DS, Schaffer JE, Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes, J. Lipid Res. 47 (2006) 665–672. doi: 10.1194/jlr.M500514-JLR200. [DOI] [PubMed] [Google Scholar]
- [34].Melton EM, Cerny RL, Watkins PA, DiRusso CC, Black PN, Human fatty acid transport protein 2a/very long chain acyl-CoA synthetase 1 (FATP2a/Acsvl1) has a preference in mediating the channeling of exogenous n-3 fatty acids into phosphatidylinositol, J Biol Chem. 286 (2011) 30670–30679. doi: 10.1074/jbc.M111.226316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Melton EM, Cerny RL, DiRusso CC, Black PN, Overexpression of human fatty acid transport protein 2/very long chain acyl-CoA synthetase 1 (FATP2/Acsvl1) reveals distinct patterns of trafficking of exogenous fatty acids, Biochemical and Biophysical Research Communications. 440 (2013) 743–748. doi: 10.1016/j.bbrc.2013.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thornburg KL, Binder ND, Faber JJ, Diffusion permeability and ultrafiltration-reflection-coefficients of Narand Cl- in the near-term placenta of the sheep, J. Dev. Physiol 1 (1979) 47–60. [PubMed] [Google Scholar]
- [37].Adams AK, Reid DL, Thornburg KL, Faber JJ, In vivo placental permeability to hydrophilic solutes as a function of fetal weight in the guinea pig, Placenta. 9 (1988) 409–416. [DOI] [PubMed] [Google Scholar]
- [38].Szabo AJ, Grimaldi RD, De Lellis R, Triglyceride synthesis by the human placenta. II. The effect of cyanide and fluoride on the incorporation of labeled palmitate into placental triglycerides, Am. J. Obstet. Gynecol 115 (1973) 263–266. [PubMed] [Google Scholar]
- [39].Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG, Computational modelling of fatty acid transport in the human placenta, Conf Proc IEEE Eng Med Biol Soc. 2015 (2015) 8054–8057. doi: 10.1109/EMBC.2015.7320262. [DOI] [PubMed] [Google Scholar]
- [40].Kaemmerer E, Peuscher A, Reinartz A, Liedtke C, Weiskirchen R, Kopitz J, et al. , Human intestinal acyl-CoA synthetase 5 is sensitive to the inhibitor triacsin C, World J. Gastroenterol 17 (2011) 4883–4889. doi: 10.3748/wjg.v17.i44.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gillingham MB, Connor WE, Matern D, Rinaldo P, Burlingame T, Meeuws K, et al. , Optimal dietary therapy of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency, Mol. Genet. Metab 79 (2003) 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Roe CR, Sweetman L, Roe DS, David F, Brunengraber H, Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride, J. Clin. Invest 110 (2002) 259–269. doi: 10.1172/JCI15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Weedon-Fekjaer MS, Dalen KT, Solaas K, Staff AC, Duttaroy AK, Nebb HI, Activation of LXR increases acyl-CoA synthetase activity through direct regulation of ACSL3 in human placental trophoblast cells, J. Lipid Res 51 (2010) 1886–1896. doi: 10.1194/jlr.M004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Johnstone ED, Sibley CP, Lowen B, Guilbert LJ, Epidermal growth factor stimulation of trophoblast differentiation requires MAPK11/14 (p38 MAP kinase) activation, Biology of Reproduction. 73 (2005) 1282–1288. doi: 10.1095/biolreprod.105.044206. [DOI] [PubMed] [Google Scholar]
- [45].Daoud G, Amyot M, Rassart E, Masse A, Simoneau L, Lafond J, ERK1/2 and p38 regulate trophoblasts differentiation in human term placenta, J. Physiol (Lond.). 566 (2005) 409–423. doi: 10.1113/jphysiol.2005.089326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Berghaus TM, Demmelmair H, Koletzko B, Fatty acid composition of lipid classes in maternal and cord plasma at birth, European Journal of Pediatrics. 157 (1998) 763–768. doi: 10.1007/s004310050931. [DOI] [PubMed] [Google Scholar]
- [47].Madsen EM, Lindegaard MLS, Andersen CB, Damm P, Nielsen LB, Human placenta secretes apolipoprotein B-100-containing lipoproteins, J Biol Chem 279 (2004) 55271–55276. doi: 10.1074/jbc.M411404200. [DOI] [PubMed] [Google Scholar]
- [48].Lafond J, Moukdar F, Rioux A, Ech-Chadli H, Brissette L, Robidoux J, et al. , Implication of ATP and sodium in arachidonic acid incorporation by placental syncytiotrophoblast brush border and basal plasma membranes in the human, Placenta. 21 (2000) 661–669. doi: 10.1053/plac.2000.0561. [DOI] [PubMed] [Google Scholar]
- [49].Thomas CR, Evans JL, Buttriss C, Lowy C, Lipid chain length alterations during placental transfer in the guinea pig, J. Dev. Physiol 7 (1985) 305–311. [PubMed] [Google Scholar]
- [50].Krammer J, Digel M, Ehehalt F, Stremmel W, Füllekrug J, Ehehalt R, Overexpression of CD36 and acyl-CoA synthetases FATP2, FATP4 and ACSL1 increases fatty acid uptake in human hepatoma cells, Int J Med Sci. 8 (2011) 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tobin KAR, Johnsen GM, Staff AC, Duttaroy AK, Long-chain polyunsaturated fatty acid transport across human placental choriocarcinoma (BeWo) cells, Placenta. 30 (2009) 41–47. doi: 10.1016/j.placenta.2008.10.007. [DOI] [PubMed] [Google Scholar]
- [52].Schwenk RW, Holloway GP, Luiken JJFP, Bonen A, Glatz JFC, Fatty acid transport across the cell membrane: regulation by fatty acid transporters, Prostaglandins Leukot. Essent. Fatty Acids 82 (2010) 149–154. doi: 10.1016/j.plefa.2010.02.029. [DOI] [PubMed] [Google Scholar]
- [53].Stahl A, Gimeno RE, Tartaglia LA, Lodish HF, Fatty acid transport proteins: a current view of a growing family, Trends Endocrinol. Metab 12 (2001) 266–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


