Abstract
The gut not only plays a key role in digestion and absorption of nutrients but also forms a physical barrier and first line of defense between the host and the luminal environment. A functional gut barrier (mucus and epithelial cells with tight junctions [TJ]) is essential for optimal health and efficient production in poultry. In current broiler system, chicks are deprived of food and water up to 72 h due to uneven hatching, hatchery procedures, and transportation. Post-hatch feed delay results in lower BW, higher FCR and mortality, and delayed post-hatch gut development. Little is known about the effects of early neonatal development and delayed feeding immediately post-hatch on gut barrier function in chickens. Therefore, the aim of the present study was to characterize the expression pattern of gut barrier–related and TJ-related genes in the small intestine of broiler chickens during early development and delay in access to feed. Newly hatched chicks received feed and water immediately after hatch or were subjected to 48 h delayed access to feed to mimic commercial hatchery setting and operations. Birds were sampled (n = 6) at −48, 0, 4, 24, 48, 72, 96, 144, 192, 240, 288, and 336 h post-hatch. Jejunum and ileum were collected, cleaned of digesta, and snap-frozen in liquid nitrogen or fixed in paraformaldehyde. The relative mRNA levels of gut barrier- and TJ-related protein genes were measured by quantitative PCR and analyzed by 2-way ANOVA. In both tissues, changes (P < 0.05) in gene expression pattern of gut barrier–related and TJ-related genes were detected due to delayed access to feed post-hatch and/or development. In general, expression of TJ-related genes was downregulated while mRNA levels of gut barrier-related genes were upregulated during development. Histological differences and changes in mucin staining due to age and treatment were observed. These results suggest that delayed access to feed post-hatch may affect TJ structure and/or function and therefore gut barrier function and overall health of the chicken small intestine.
Key words: tight junctions, gut barrier, permeability, gene and protein expression, chicken
Introduction
In the current commercial broiler production systems, chicks hatch over a 24 to 36 h period (Careghi et al., 2005) and are removed from the hatcher at once when most chickens have hatched (de Gouw et al., 2017). After removal from the hatcher, chicks undergo selection, vaccination, sexing, sorting, and are then transported to farms. During this period (up to 72 h) chicks have no access to feed and water (Careghi et al., 2005; van de Ven et al., 2009; Willemsen et al., 2010). It has been shown that prolonged lack of access to feed post-hatch results in lower body weight at placement, lower organ weight and post-hatch growth, higher feed conversion ratio (FCR) and mortality, and delay in gastrointestinal tract (GIT) development (Bigot et al., 2003; Careghi et al., 2005; van de Ven et al., 2011, 2013; de Jong et al., 2017). Moreover, delayed feeding affected yolk utilization, slaughter weight, breast meat yield, and depressed immunological development (Noy et al., 1996; Dibner et al., 1998; Halevy et al., 2000; Gonzales et al., 2003; Juul-Madsen et al., 2004; Shira et al., 2005). In the GIT, delayed feeding for up to 36 h has been shown to decrease crypt depth and percentage of proliferating cells in crypts, depress villi surface, and alter the morphology of the microvilli (Uni et al., 1998; Geyra et al., 2001a). We have shown previously, that delayed feeding post-hatch inhibited the upregulation of lipogenic genes and lipogenic transcription factor genes until feeding was initiated (Richards et al., 2010) and affected expression pattern of some Ca and P transporter genes (Proszkowiec-Weglarz et al., 2019).
The gut barrier is composed of an extrinsic mucus layer, intestinal epithelial cells, tight junctions (TJ), and lamina propria. The mucus layer is divided into an outer layer associated with bacteria and loosely attached to the epithelium while the inner layer is characterized by high concentration of IgA and mucin, and is adherent to the epithelium. The mucosa forms a first protective layer of the intestinal epithelium that prevents against damages and pathogens, serves as a substrate for commensal bacteria fixation (Linden et al., 2008), provides an appropriate environment for brush border enzymes, and facilitates nutrient absorption (Smirnov et al., 2006). Intestinal epithelial cells are responsible for digestion, absorption, and pathogen recognition through expression of innate immune system receptors, as well as antimicrobial peptide release, and secretion of hormones, neurotransmitters, cytokines and chemokines (Yen and Wright, 2006; Abreu, 2010; Chen et al., 2015). One of the major components of the intestinal barrier is the formation of TJ between epithelial cells (Anderson and Van Itallie, 1995; Mitic et al., 2000). Tight junctions are multi-protein complexes that seal the paracellular space between adjacent epithelial cells and regulate the permeability of the intestinal barrier (Chen et al., 2015; Awad et al., 2017). The functional state of TJ is immensely dynamic. They open and close when exposed to a range of stimuli, including nutrients, absorption processes, hormonal or neuronal signals, various cellular pathways, and inflammatory mediators (Barekatain et al., 2019b). A TJ consists of zona occludens-1 (ZO-1), occludins (OCLN), claudins (CLDN), and actin-myosin cytoskeletal proteins (Gil-Cardoso et al., 2016). Occludins and CLDN are the main transmembrane proteins that contribute to the paracellular seal while ZO-1 serves as cytoplasmic plaque proteins that interacts with both transmembrane and cytoskeletal proteins (Fanning et al., 1998; Yu and Turner, 2008).
Nutritional and health status in poultry is linked with gut health, understood as correlation between immune system, gut microbial balance, and macro and micro structural integrity of the gut (Yegani and Korver, 2008). Therefore, the aim of this study was to investigate the effect of delayed access to feed immediately post-hatch on gut barrier function and integrity in broiler chicken.
Materials and methods
Animals and Experimental Protocols
All animal care procedures were approved by the USDA-ARS Institutional Animal Care and Use Committee. The experimental design was described in detail by Proszkowiec-Weglarz et al. (2019). In brief, fertile Ross 708 broiler chicken eggs (250 eggs) were obtained from a local hatchery (Perdue Hatchery, Hurlock, MD) and incubated under standard conditions. Birds were removed from the hatcher in 3 batches (within 180–240 min after occlusion) and randomly distributed between treatment groups (14–15 hatchlings per battery pen total). Hatchlings were divided into 2 treatment groups randomly (n = 6 battery pens for each treatment). One group received feed (a commercial type corn-soybean meal–based starter diet, Proszkowiec-Weglarz et al. (2019)) and water immediately after placement (FD) while the second received water immediately but had delayed access to feed for 48 h (NFD) to mimic commercial hatchery setting and operation.
Birds were sampled at hatch (0 h, wet chicks, within 30 min from hatch), and 4, 24, 48, 72, 96, 144, 192, 240, 288, and 336 h after start of feeding. In addition, embryos were sampled at embryonic (e) day 19 (−48 h, n = 6). One chick per pen, selected at random, was sacrificed by cervical dislocation, and 1 cm of the upper jejunum and 1 cm in the middle of the distal part of the ileum were collected, cleaned of digesta, and snap-frozen in liquid nitrogen for RNA and protein isolation. In addition, jejunal and ileal tissues were collected into 4% paraformaldehyde for histological analysis.
RNA Isolation and Reverse Transcription–Quantitative PCR
Total RNA extraction and 2-step reverse transcription-quantitative PCR were performed as described previously (Proszkowiec-Weglarz et al., 2019). Primer sequences, designed using Primer3 software (Untergasser et al., 2007) for OCLN, ZO-1 and ZO-2, CLDN 1, CLDN 4, and CLDN 5, junctional adhesion molecule (JAM) 2 and JAM3, fatty acid binding protein 2 (FABP2), mucin 2 (MUC2), immunoglobulin A (IgA), and its polymeric immunoglobulin receptor (pIgR), are listed in Table 1. The obtained data were normalized to the geometric mean (Vandesompele et al., 2002) of 4 housekeeping genes (β-actin, GAPDH, ubiquitin, and β2-microglobulin), analyzed, and presented as described in Proszkowiec-Weglarz et al. (2019). Primer sequences for housekeeping genes were published previously (Proszkowiec-Weglarz et al., 2019).
Table 1.
Gene-specific primers used for the analysis of mRNA levels using quantitative real-time RT-PCR (Untergasser et al., 2007).1
| Gene2 | GenBank accession No.3 | Forward primer (5’→3′) | Reverse primer (5’→3′) | Amplicon size (bp) |
|---|---|---|---|---|
| CLDN 1 | NM_001013611 | GGTGAAGAAGATGCGGATGG | TCTGGTGTTAACGGGTGTGA | 139 |
| CLDN 4 | AY435420 | ATCGCCCTGTCCGTCATC | ACCACGCAGTTCATCCACAG | 137 |
| CLDN5 | NM_204201 | AGGTGTCAGCCTTCATCGAC | CCAGGATGGAATCGTACACC | 123 |
| FABP2 | NM_001007923 | AGGCTCTTGGAACCTGGAAG | CTTGGCTTCAACTCCTTCGT | 139 |
| IgA | S40610 | GAAGGTCTCCGTGGAGGATT | ACGTTGACGTGAGAGGCTTT | 129 |
| JAM 2 | XM_015299112 | CTGCTCCTCGGGTACTTGG | CCCTTTTGAAAATTTGTGCTTGC | 135 |
| JAM 3 | XM_417876 | CCAGAGTGTTGAGCTGTCCT | AGAATTTCTGCCCGAGTTGC | 147 |
| MUC2 | NM_001318434 | AAACAACGGCCATGTTTCAT | GTGTGACACTGGTGTGCTGA | 127 |
| OCLN | NM_205128 | GATGGACAGCATCAACGACC | CTTGCTTTGGTAGTCTGGGC | 142 |
| pIgR | ENSGALT000000013534 | CAAGGGAGTACGGAGCAAAC | CTTTGTCTCAGCGGTGCTTT | 116 |
| ZO-1 | XM_015278975 | GCCAACTGATGCTGAACCAA | GGGAGAGACAGGACAGGACT | 141 |
| ZO-2 | NM_204918 | TCAGCAACAGCAAGGTGAAG | GCACCCATGGCAGTAAGGTA | 102 |
All primers used for expression analysis were designed using primer3 program (http://bioinfo.ut.ee/primer3-0.4.0/primer3/); (Untergasser et al., 2007).
Abbreviations of the gene names are defined in text.
Reference chicken gene sequences that contain the corresponding PCR products list.
Transcript from Ensembl genome assembly (http://www.ensembl.org/Gallus_gallus/Info/Index), no prediction of this gene is available in GenBank.
Protein Extraction, Western Blot Analysis, and ELISA Assay
Tissues were homogenized using ice-cold T-Per lysis buffer (Thermo Fischer Scientific, Inc., Waltham, MA) containing 1 mmol/L phenylmethylsulfonyl fluoride (Thermo Fisher Scientific, Inc.) and Halt protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific, Inc.), and the homogenate was centrifuged at 10,000 × g for 10 min. Protein concentration in collected supernatant was quantified using the Coomassie Plus (Bradford) assay kit (Thermo Fisher Scientific, Inc.). For Western Blot analysis, 50 μg of total protein was boiled for 5 min in Laemmli sample buffer (Bio-Rad, Hercules, CA) and resolved under reducing conditions by SDS-PAGE on a 10% gel. After transferring to a polyvinylidene fluoride membrane (GE Healthcare Life Science, Amersham, Pittsburgh, PA) for 30 min at 25 V with a Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell (Bio-Rad) in Tris-Glycine buffer containing 15% methanol, membranes were blocked for 2 h at room temperature (RT) in Odyssey Blocking buffer (LI-COR Biosciences, Lincoln, NE) and incubated overnight at 4°C with mouse monoclonal antibody for CLDN 1 and CLDN 5 (Santa Cruz Biotechnology, Dallas, TX). Membranes were then incubated with IRDye donkey anti-mouse antibody (1:15,000; LI-COR) diluted in PBS containing 0.1% Tween-20 (Thermo Fisher Scientific, Inc.). Immunoreactive bands were detected using Odyssey Infrared Imaging System and software (version 3.0.30; LI-COR). For housekeeping protein (alpha-tubulin) detection, membranes were stripped with NewBlot IR Stripping buffer (LI-COR) according to manufacturer's recommendation, blocked, and reprobed with mouse monoclonal alpha-tubulin antibody (Santa Cruz Biotechnology) and IRDye donkey anti-mouse antibody (1:15,000; LI-COR) in PBS with 0.1% Tween-20. The band intensities for CLDN 1 and 5 were normalized to corresponding alpha-tubulin band intensities before statistical analysis. Jejunal and ileal samples between −48 h and 72 h post-hatch were analyzed via Western Blot.
ZO-1 protein expression level in the ileal tissue was determined using a commercially available chicken TJP/ZO-1 ELISA kit (LifeSpan BioSciences, Inc., Seattle, WA) according to the manufacturer's instructions. The optical density was determined using plate reader (SpectraMax M2, Molecular Devices, San Jose, CA) set to 450 nm. Results were calculated from standard curve using SpectraMax M2 software.
Histological Staining
Jejunal and ileal samples collected into freshly prepared 4% paraformaldehyde were incubated overnight, followed by 70% ethanol storage until processing. Paraffin-embedded sections of the samples were prepared for subsequent histological staining by a professional histology service (Histoserv, Inc., Germantown, MD). Mucosal staining was performed using Alcian blue-PAS staining (Histoserv, Inc.). Each section was photographed at full illumination using 20× or 40× objectives on an Olympus BX-40 microscope with an Olympus DP-70 digital camera (Olympus, America, Inc., Center Valley, PA).
Statistical Analysis
Normal distribution of data was verified by UNIVARIATE analysis (SAS© Institute, Cary, NC). Box Cox transformation (Box and Cox, 1964) was employed for non-normal variables using R package (R-Core-Team, 2020). Transformed data were analyzed by 2-way ANOVA using the general linear models (GLM, SAS Institute). Age (h post-hatch), treatment (FD vs. NFD), and their interaction were set as the fixed effects. Main effects were not analyzed separately if the interaction between them was significant. Significance was set at P < 0.05.
Results
Gene Expression
Only age × treatment interactions are depicted in Figure 1, Figure 2, Figure 3, Figure 4, while all fixed effects are presented in Table 2. Age effect on mRNA expression for genes with no significant interaction between fixed effects is presented in Supplementary Figure 1 (jejunum) and Supplementary Figure 2 (ileum).
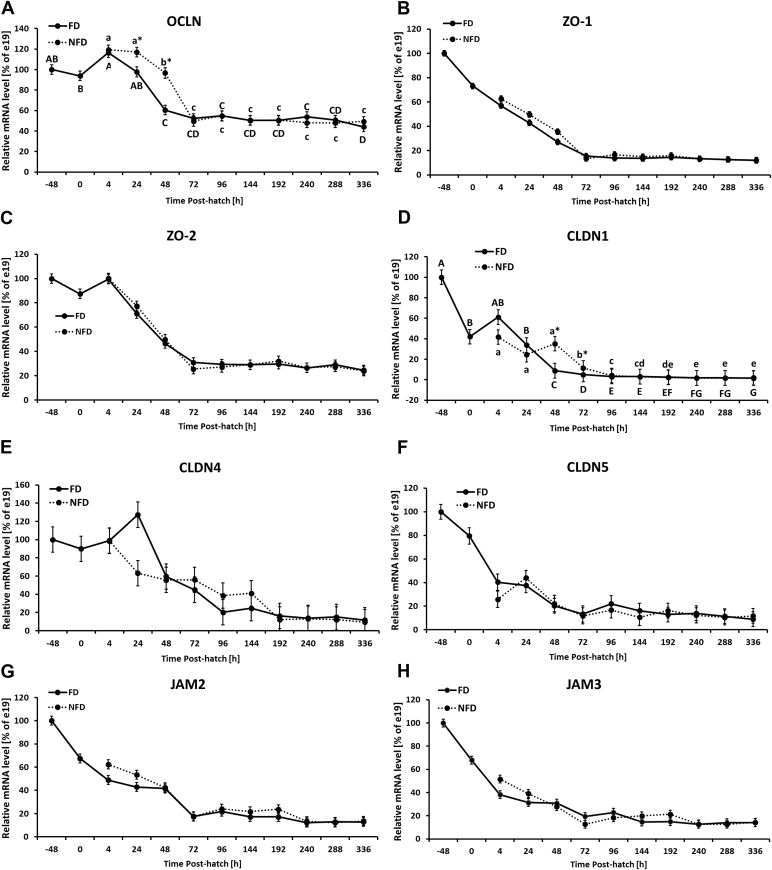
Figure 1.
Effect of delay in feed access for the first 48 h post-hatch on jejunal level of mRNA for tight junction–related genes: (A) occludin (OCLN), (B) zonula occluden-1 (ZO-1), (C) zonula occluden-2 (ZO-2), (D) claudin 1 (CLDN 1), (E) claudin 4 (CLDN 4), (F) claudin 5 (CLDN 5), (G) junctional adhesion molecule 2 (JAM2), and (H) junctional adhesion molecule 3 (JAM3). The expression level of e19 (−48 h) birds was set to 100% and the other values are presented as % of the −48 h data. Each value represents mean ± SE of 6 birds. Different letters (large characters for fed (FD) group and small characters for not fed (NFD) group) denote statistically significant (P < 0.05) differences between means within treatment. Stars denote statistically significant (P < 0.05) differences between treatment groups for each time point.
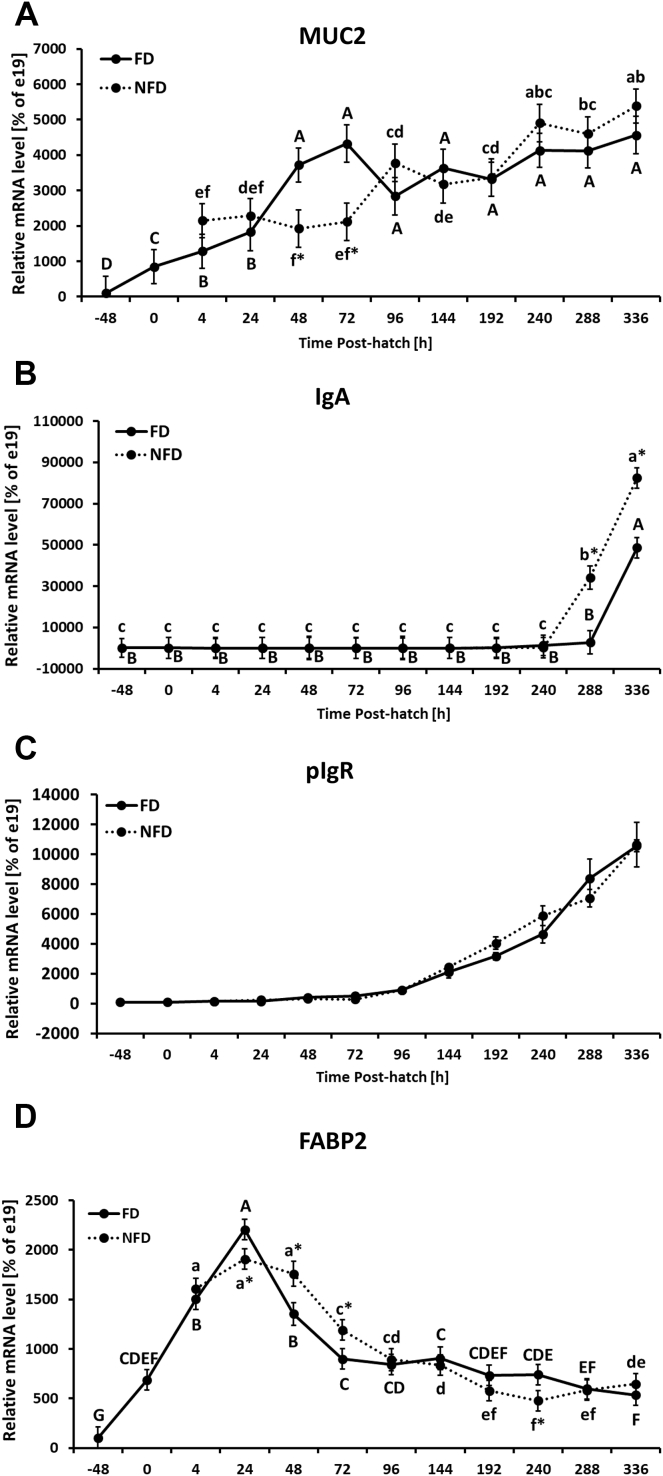
Figure 2.
Effect of delay in feed access for the first 48 h post-hatch on jejunal level of mRNA for gut barrier–related genes: (A) mucin 2 (MUC2), (B) immunoglobulin A (IgA), (C) polymeric immunoglobulin receptor (pIgR), and (D) fatty acid binding protein 2 (FABP2). The expression level of e19 (−48 h) birds was set to 100% and the other values are presented as % of the −48 h data. Each value represents mean ± SE of 6 birds. Different letters (large characters for fed [FD] group and small characters for not fed [NFD] group) denote statistically significant (P < 0.05) differences between means within treatment. Stars denote statistically significant (P < 0.05) differences between treatment groups for each time point.
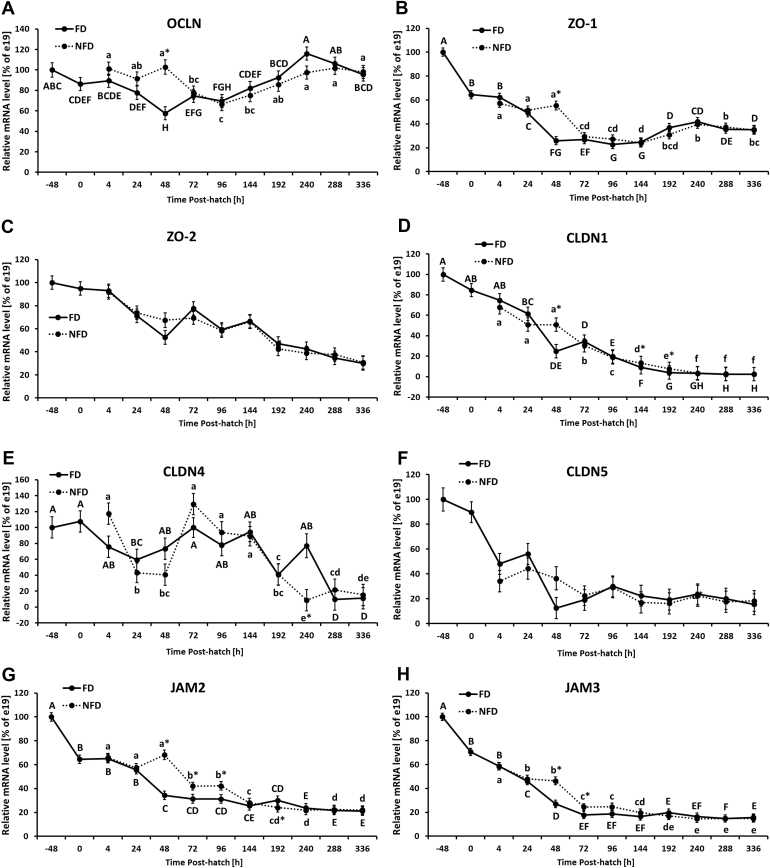
Figure 3.
Effect of delay in feed access for the first 48 h post-hatch on ileal level of mRNA for TJ genes: (A) occludin (OCLN), (B) zonula occluden-1 (ZO-1), (C) zonula occluden-2 (ZO-2), (D) claudin 1 (CLDN 1), (E) claudin 4 (CLDN 4), (F) claudin 5 (CLDN 5), (G) junctional adhesion molecule 2 (JAM2), and (H) junctional adhesion molecule 3 (JAM3). The expression level of e19 (−48 h) birds was set to 100% and the other values are presented as % of the −48 h data. Each value represents mean ± SE of 6 birds. Different letters (large characters for fed [FD] group and small characters for not fed [NFD] group) denote statistically significant (P < 0.05) differences between means within treatment. Stars denote statistically significant (P < 0.05) differences between treatment groups for each time point.
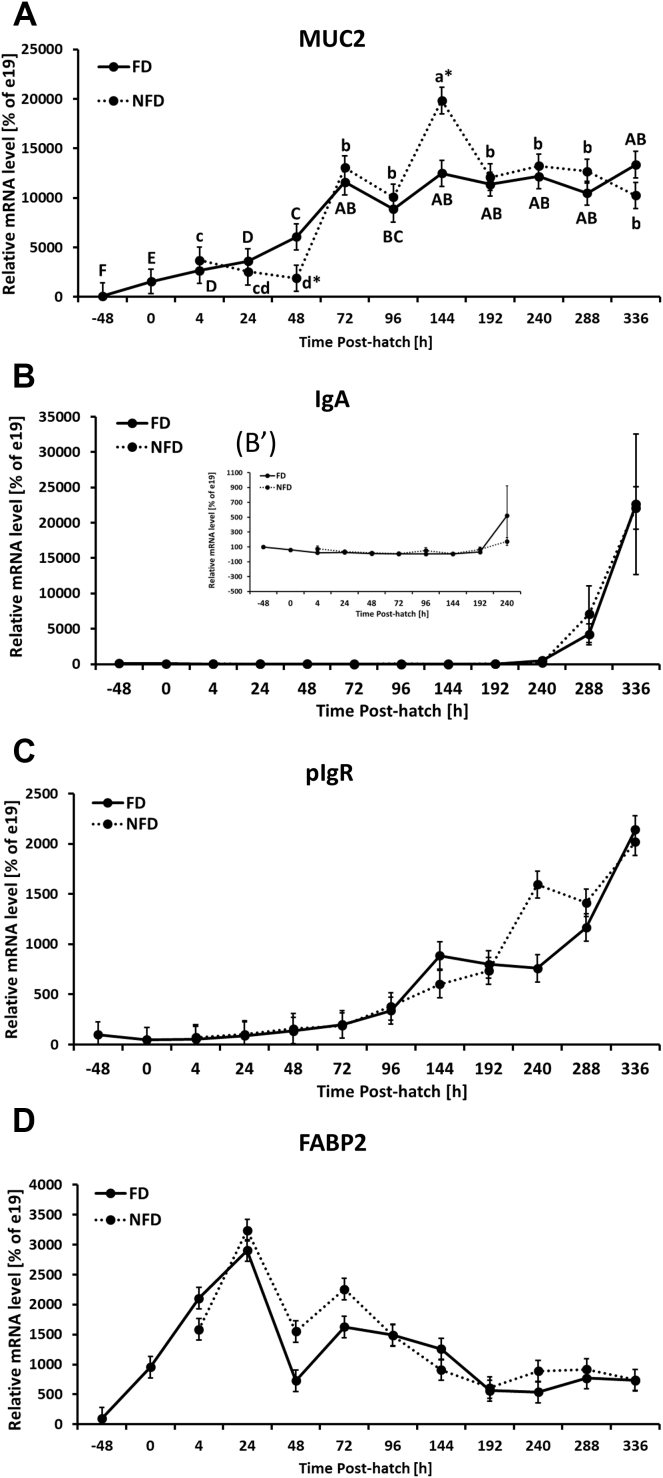
Figure 4.
Effect of delay in feed access for the first 48 h post-hatch on ileal level of mRNA for gut barrier–related genes: (A) mucin 2 (MUC2), (B) Immunoglobulin A (IgA), (C) polymeric immunoglobulin receptor (pIgR), and (D) fatty acid binding protein 2 (FABP2). Insert figure B′ represents IgA mRNA expression level from −48 to 240 h post-hatch. The expression level of e19 (−48 h) birds was set to 100% and the other values are presented as % of the −48 h data. Each value represents mean ± SE of 6 birds. Different letters (large characters for fed [FD] group and small characters for not fed [NFD] group) denote statistically significant (P < 0.05) differences between means within treatment. Stars denote statistically significant (P < 0.05) differences between treatment groups for each time point.
Table 2.
The effect of delay access to feed post-hatch (treatment) on tight junction and gut barrier–related gene expression during development (age).
| Gene1 | Pr > F |
||
|---|---|---|---|
| Treatment | Age | Treatment × age | |
| Jejunum | |||
| OCLN | 0.0375 | <0.0001 | 0.0020 |
| ZO-1 | 0.0079 | <0.0001 | 0.2744 |
| ZO-2 | 0.8346 | <0.0001 | 0.9338 |
| CLDN1 | 0.0630 | <0.0001 | 0.0008 |
| CLDN4 | 0.5392 | <0.0001 | 0.3583 |
| CLDN5 | 0.4597 | <0.0001 | 0.7074 |
| JAM2 | 0.0376 | <0.0001 | 0.8968 |
| JAM3 | 0.3077 | <0.0001 | 0.2315 |
| MUC2 | 0.8793 | <0.0001 | 0.0224 |
| IgA | 0.1637 | <0.0001 | <0.0001 |
| pIgR | 0.7063 | <0.0001 | 0.1923 |
| FABP2 | 0.8968 | <0.0001 | 0.0507 |
| Ileum | |||
| OCLN | 0.0175 | <0.0001 | 0.0022 |
| ZO-1 | 0.0497 | <0.0001 | <0.0001 |
| ZO-2 | 0.9476 | <0.0001 | 0.8658 |
| CLDN1 | 0.0396 | <0.0001 | 0.0129 |
| CLDN4 | 0.3269 | <0.0001 | <0.0001 |
| CLDN5 | 0.7965 | <0.0001 | 0.2260 |
| JAM2 | 0.0016 | <0.0001 | <0.0001 |
| JAM3 | 0.0159 | <0.0001 | 0.0022 |
| MUC2 | 0.5740 | <0.0001 | <0.0001 |
| IgA | 0.0049 | <0.0001 | 0.7042 |
| pIgR | 0.2687 | <0.0001 | 0.0349 |
| FABP2 | 0.7063 | <0.0001 | 0.4031 |
Gene abbreviations are deciphered in the text of the manuscript.
Jejunum
The expression pattern of TJ-related genes in the jejunum is presented in Figure 1. All 8 genes were significantly (P < 0.0001) affected by age with overall decreasing expression during post-hatch development (Figure 1). Significantly (P < 0.05) elevated OCLN mRNA expression was observed in NFD birds at 24 and 48 h post-hatch in comparison to FD chickens (Figure 1A). There was no age × treatment interaction for ZO-1 expression, but treatment effect was significant and NFD birds were characterized by a higher level of expression in comparison to the FD group with a more prominent effect between 4 and 24 h post-hatch (Figure 1B, Supplementary Figure 1A). The expression level of ZO-1 decreased (P < 0.05) over time with the lowest and constant level from 72 h post-hatch onward (Supplementary Figure 1A). ZO-2 mRNA expression was not affected by access to feed post-hatch (Figure 1C). In both groups, its expression was significantly downregulated at hatch and from 24 h post-hatch onward in comparison to −48 h post-hatch. From 72 h post-hatch until the end of the experiment, no changes in ZO-2 expression were observed (Supplementary Figure 1B, Table 2). CLDN1 mRNA expression was significantly upregulated in NFD birds at 48 and 72 h post-hatch in comparison to the FD group (Figure 1D). Overall, CLDN1 mRNA was downregulated during development, showing the lowest mRNA level during second week of development in both treatment groups (Figure 1D). No significant interaction between age and treatment as well as treatment effect were observed for CLDN 4 and 5 in the jejunum (Figures 1E, 1F). Expression of both CLDN genes was significantly downregulated during development (Supplementary Figures 1C, 1D, Table 2). In case of JAM2, NFD birds had higher expression (P = 0.0376) in comparison to FD birds (Supplementary Figure 1E), while no significant age × treatment interaction was observed (Figure 1G). Similar expression profile was observed for JAM3 (Figure 1H), but neither treatment effect nor interaction between main effects was significant (Table 2). For both genes, significant downregulation during development was observed (Supplementary Figures 1E, 1F). In case of gut barrier-related genes, MUC2 gene expression was significantly upregulated during development in both treatment groups, with higher expression level of MUC2 mRNA in FD birds between 48 and 72 h post-hatch in comparison to NFD group (Figure 2A, Table 2). IgA mRNA expression level was significantly upregulated at the end of the second week post-hatch with lower expression (P < 0.05) in FD birds at 288 and 336 h post-hatch in comparison to NFD group (Figure 2B, Table 2). Regardless of access to feed post-hatch, expression of pIgR was gradually upregulated during development starting at 96 h post-hatch and showing the highest mRNA level at 336 h post-hatch (Figure 2C, Supplementary Figure 1G, Table 2). Increase in FABP2 mRNA expression was observed from −48 h until 24 h post-hatch and was followed by gradual decrease during development in both treatment groups. FD birds had significantly higher FABP2 mRNA expression at 24 h post-hatch and lower expression at 48 and 72 h post-hatch in comparison to NFD birds (Figure 2D, Table 2).
Ileum
FD birds were characterized by decreasing OCLN mRNA expression from −48 h until 48 h post-hatch that was followed by gradual increase. Similar expression pattern was detected for NFD birds. The NFD group had significantly higher OCLN expression at 48 h post-hatch in comparison to the FD group (Figure 3A, Table 2). Ileal ZO-1 mRNA expression gradually decreased from −48 h post-hatch reaching the lowest expression between 48 and 144 h post-hatch and was followed by slight increase between 192 and 288 h post-hatch in FD birds. ZO-1 mRNA expression in NFD birds followed the same expression pattern except for 48 h post-hatch when the expression level in NFD was significantly higher in comparison to FD birds (Figure 3B, Table 2). In contrast to ZO-1, ZO-2 was not affected by access to feed post-hatch. Gradual downregulation of ZO-2 mRNA expression was observed during development (Figure 3C and Supplementary Figure 2A, Table 2). Both treatment groups were characterized by decreasing mRNA level of CLDN 1 from −48 h post-hatch until 144 h post-hatch with NFD birds characterized by significantly (P < 0.05) higher level at 48, 144, and 192 h post-hatch (Figure 3D, Table 2). In general, CLDN 4 expression level was downregulated during development in both treatment groups with NFD birds having significantly lower CLDN 4 mRNA expression 240 h post-hatch in comparison to FD group (Figure 3E, Table 2). CLDN 5 mRNA expression was characterized by a gradual decrease post-hatch in both treatment groups with no significant effect of delayed access to feed or interaction between age and treatment (Figure 3F and Supplementary Figure 2B, Table 2). In FD birds, JAM2 mRNA level was significantly downregulated during first 48 h post-hatch and stayed at similarly low level until the end of the experiment (336 h post-hatch), while NFD birds were characterized by significantly higher JAM 2 mRNA expression from 48 to 96 h and slightly (P < 0.05) lower expression at 192 h post-hatch in comparison to FD birds and (Figure 3G, Table 2). Similar expression pattern was observed for JAM 3 mRNA expression for both treatment groups with NFD birds being characterized by higher (P < 0.05) mRNA expression of JAM 3 at 48 and 72 h post-hatch as compared to FD birds (Figure 3H, Table 2). Significant increase in MUC2 mRNA expression during development was observed in both treatment groups, with lower (P < 0.05) mRNA level at 48 h and higher (P < 0.05) at 144 h post-hatch in NFD birds in comparison to the FD group (Figure 4A, Table 2). IgA mRNA level was significantly upregulated at the end of second week post-hatch with no significant difference between FD and NFD groups or interaction between age and treatment (Figure 4B and Supplementary Figure 2C, Table 2). Similarly to IgA, the mRNA expression of its receptor (pIgR) was not significantly affected by delayed access to feed post-hatch (Figure 4C, Table 2). pIgR expression level was gradually upregulated (P < 0.05) in both treatment groups, FD and NFD, starting at 24 h post-hatch, with the highest level at 336 h post-hatch (Figure 4D, Table 2). No significant interaction between age and treatment as well as lack of effect of access to feed was observed for FABP2 mRNA expression in the ileum (Figure 4D, Table 2). However, analysis of expression data only between 24 and 96 h post-hatch for both treatment groups revealed significant (P = 0.0126) upregulation of FABP2 expression in NFD birds (data not shown). FABP2 mRNA expression was also affected by development (age). Increase in FABP2 mRNA expression from −48 to 24 h post-hatch was followed by decrease in both treatment groups (Supplementary Figure 2E, Table 2). Significantly lower FABP2 mRNA level was observed at 192 h post-hatch in comparison to earlier time points (Supplementary Figure 2E, Table 2).
Protein Expression
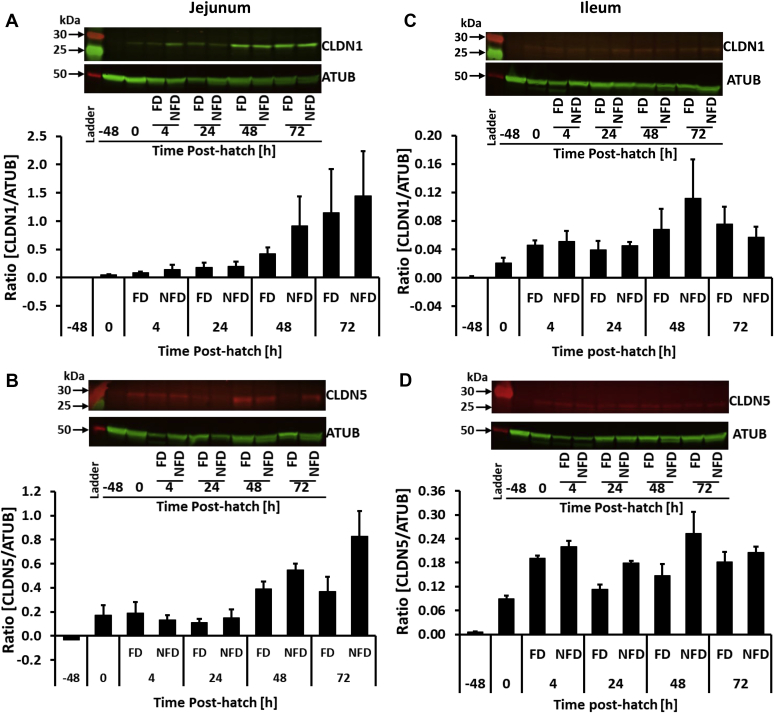
Owing to limited availability of commercial antibody for chicken TJ-related proteins, we were only able to determine CLDN 1 and 5 protein expression in the jejunum and ileum using Western blot (Figure 5). In addition, we determined ZO-1 protein level using ELISA (Figure 6). The ileum was selected for the ELISA assay because only in this tissue ZO-1 mRNA expression was affected by delayed access to feed. Overall, relatively low expression levels of both proteins, CLDN 1 and 5, were observed during Western blot analysis, especially in comparison to loading control alpha-tubulin (Figure 5).
Figure 5.
Effect of delay in feed access for the first 48 h post-hatch on jejunal (A) claudin 1 (CLDN 1) and (B) claudin 5 (CLDN 5), and ileal (C) CLDN 1 and (D) CLDN 5 protein expression analyses by Western Blot and quantified by Odyssey Imaging System. A representative Western blot depicting detection of CLDN 1 and 5, and alpha-tubulin (ATUB) are presented above each quantification figure. Each value represents mean ± SE of 4 birds.
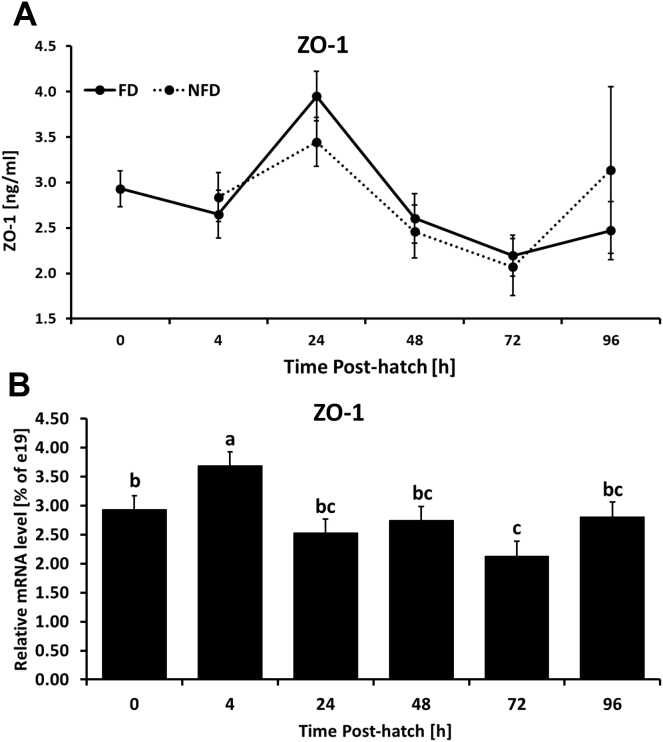
Figure 6.
(A) Effect of delay in feed access for the first 48 h post-hatch on ileal protein level for zonula occluden (ZO-1) determined by ELISA assay. (B) Effect of age on ileal protein level for ZO-1. Each value represents mean ± SE of 6 birds. Different letters denote statistically significant (P < 0.05) differences between means within treatment.
Jejunum
No significant effect of treatment or interaction of main effects was detected for CLDN 1 protein expression. However, CLDN 1 protein level was upregulated (P = 0.0055) during first 72 h post-hatch in both treatment groups (Figure 5A). A similar pattern was observed for CLDN 5 in jejunum (Figure 5B). However, a trend in delayed access to feed (P = 0.0637) and interaction between treatment and age (P = 0.0702) effects were observed, with NFD having higher CLDN 5 protein expression in comparison to FD birds (Figure 5B).
Ileum
CLDN 1 and CLDN 5 protein expression in the ileum was not affected by access to feed early post-hatch. Levels of both proteins were affected by age (P = 0.0022 and P < 0.0001, respectively). No significant interaction between treatment and age was observed in case of CLDN 1 and 5 (Figures 5C, 5D). ELISA revealed no changes in ZO-1 protein due to delayed access to feed post-hatch during first 96 h post-hatch (Figure 6A). ZO-1 protein level was downregulated over time in comparison to 4 h post-hatch regardless of treatment (Figure 6B, P = 0.0022). No interaction between age and treatment was observed for ZO-1 protein.
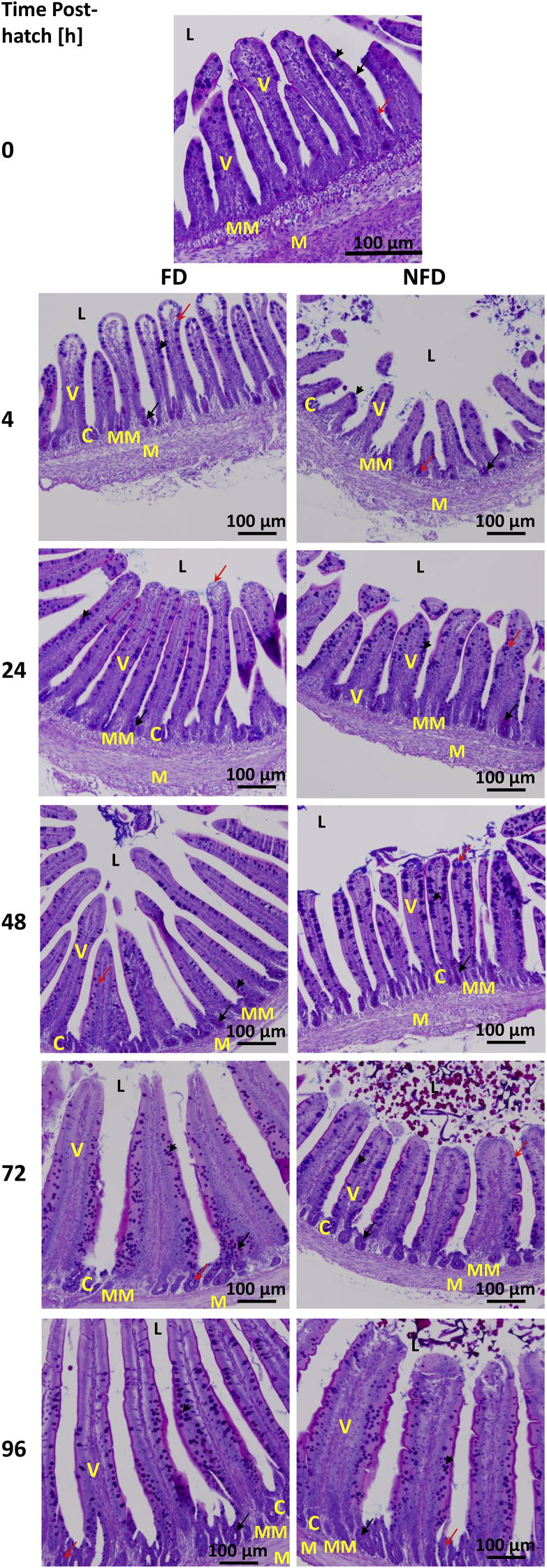
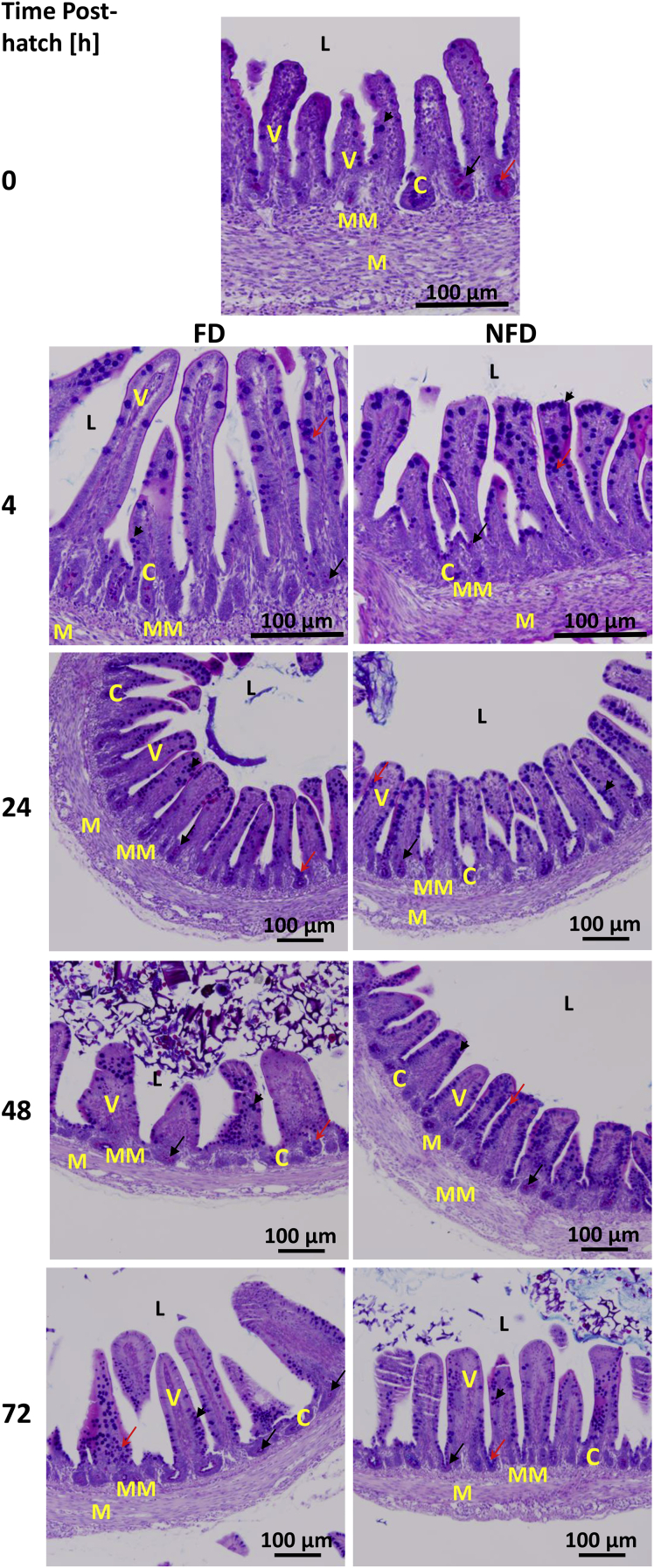
Histological Analysis
Figures 7 and 8 depict the histological changes and patterns of mucin staining during post-hatch development (from hatch to 96 h post-hatch and from hatch to 72 h, respectively) in both treatment groups (FD and NFD) in jejunum and ileum, respectively. These time points were selected based on changes in MUC2 mRNA expression and the effect of delayed access to feed on MUC2 gene expression in both tissues. Summary of the visual assessment of the proportion of cells stained pink (neutral mucin), blue (acidic mucin), and purple (mixed mucin) in the jejunum (Figure 7) and ileum (Figure 8) is presented in Table 3.
Figure 7.
Effect of delay in feed access for the first 48 h post-hatch on jejunal mucin staining. Paraffin sections of jejunum collected from 0 to 96 h post-hatch were stained with Alcian blue-PAS. The black arrow indicates neutral mucin staining; red arrow indicates acidic mucin and arrow head indicates mixed mucin staining. Abbreviations: C, crypt; L, intestinal lumen; M, smooth muscle; MM, muscularis mucosa; V, villi.
Figure 8.
Effect of delay in feed access for the first 48 h post-hatch on ileal mucin staining. Paraffin sections of the ileum collected from 0 to 72 h post-hatch were stained with Alcian blue-PAS. Black arrow indicates neutral mucin staining; red arrow indicates acidic mucin and arrow head indicates mixed mucin staining. Abbreviations: C, crypt; L, intestinal lumen; M, smooth muscle; MM, muscularis mucosa; V-villi.
Table 3.
Variation in mucin composition in the jejunum and ileum of birds with full access to feed (FD) post-hatch or subjected to delay in feeding (NFD) early post-hatch.
| Time [h] | Site | Mucin | Jejunum |
Ileum |
||
|---|---|---|---|---|---|---|
| FD | NFD | FD | NFD | |||
| 01 | Villi | Neutral Acidic Mixed |
++ + +++ |
+ + ++ |
||
| Crypt2 | Neutral Acidic Mixed |
-------- | ++ ++ + |
|||
| 4 | Villi | Neutral Acidic Mixed |
+++ ++ +++ |
++ ++ ++ |
+ ++ ++ |
+ + +++ |
| Crypt | Neutral Acidic Mixed |
++++ − + |
+ ++ + |
++ − ++ |
+ + + |
|
| 24 | Villi | Neutral Acidic Mixed |
+ ++ ++ |
+ + +++ |
++ + +++ |
+ +++ ++ |
| Crypt | Neutral Acidic Mixed |
+ +++ ++ |
+++ + ++ |
+++ + ++ |
++ + ++ |
|
| 48 | Villi | Neutral Acidic Mixed |
+ + +++ |
+ ++ +++ |
++ − +++ |
+ +++ ++ |
| Crypt | Neutral Acidic Mixed |
++ + ++ |
+++ + + |
+++ ++ ++ |
+++ + ++ |
|
| 72 | Villi | Neutral Acidic Mixed |
+++ − +++ |
++ ++ ++ |
++ ++ +++ |
+ +++ ++ |
| Crypt | Neutral Acidic Mixed |
++ ++ + |
++ + + |
+++ − ++ |
++ +++ + |
|
| 963 | Villi | Neutral Acidic Mixed |
++ − ++ |
++ −/+ +++ |
||
| Crypt | Neutral Acidic Mixed |
++ + ++ |
++ + ++ |
|||
−/+++ Visual assessment of the proportion of cells stained pink (neutral mucin), blue (acidic mucin), and purple (mixed mucin) in the jejunum (Figure 7) and ileum (Figure 8).
At hatch, there was only one group of birds, the birds were split into FD and NFD after hatch.
There were no distinguishable crypts visible at hatch in the jejunum.
Selection of samples for mucin staining was based on MUC2 mRNA expression changes during development.
Jejunum
At hatch (0 h), jejunum was characterized by small villi, almost non-distinguishable crypts, and high level of mixed (neutral and acidic, violet) and neutral (pink) mucin staining with less visible staining for acidic mucin (light blue). Four hour post-hatch, jejunal sections of FD birds were characterized by elongated villi and defined crypts. Mucin stained predominantly as mixed mucin with neutral mucin present mostly in crypts. In comparison to FD birds, NFD jejunal sections were characterized by shorter villi, smaller crypts and less mucin staining (Figure 7). Changes in length (further elongation) of the villi, presence of acidic mucin in crypts, mixed mucin along the villi, and less neutral mucin in crypts in comparison to 4 h post-hatch were characteristic for jejunal section 24 h post-hatch in FD birds. NFD birds were characterized by smaller and thicker villi with mixed mucin staining along the villi and neutral mucin presence in crypts at that post-hatch time. In comparison to 24 h post-hatch and NFD at 48 h post-hatch, FD group was characterized by longer villi 48 h post-hatch. Overall, more neutral and mixed mucin staining, and more neutral mucin in crypts were present in FD birds 48 h post-hatch while NFD birds were characterized by more elongated villi in comparison to 24 h post-hatch, more mucin staining in comparison to FD birds with neutral mucin present in crypts, and mixed mucin staining along the villi (Figure 7). At 72 h post-hatch, FD birds had thicker villi with a lot of mucin along the villi, presence of neutral mucin at the base of villi, acidic in crypts, and mixed mucin along the villi. NFD birds were characterized by neutral mucin staining in crypts and mixed and acidic mucin along the villi (Figure 7). At 96 h post-hatch, FD birds had much longer villi than NFD birds and were characterized by neutral and mixed mucin staining along the villi. NFD birds were characterized by shorter and thicker villi and more neutral mucin present along the villi (Figure 7 and Table 3).
Ileum
Similarly to jejunum, ileal sections at hatch (0 h) were characterized by small villi but had visible crypts. Crypts at this age were characterized by neutral and acidic mucin staining while only some staining was visible along the villi (Figure 8). At 4 h post-hatch, FD birds had longer villi, bigger crypts, and neutral and mixed mucin staining in crypts, while NFD had smaller villi and crypts, lower staining for mucin in crypts, and a lot of mixed mucin along the villi. At 24 h post-hatch, both treatment groups seemed to have similar villi length with a lot of mixed mucin staining along the villi, and neutral and mixed staining in crypts in FD birds, and a lot of acidic mucin along the villi and neutral mucin in crypts in NFD birds. Acidic and neutral mucin in crypts while neutral and mixed along villi were present 48 h post-hatch in ileum of FD birds. NFD birds were characterized by acidic and mixed mucin staining along the villi, and neutral mucin staining in crypts 48 h post-hatch. At 72 h post-hatch, comparable staining for mucin was observed in the ileum in FD and NFD birds with neutral mucin in crypts and neutral and mixed along villi in FD birds, and acidic and neutral mucin staining in NFD birds crypts, and mixed and neutral mucin along the villi in the NFD group (Figure 8 and Table 3).
Discussion
Intestinal permeability can be regulated by several factors including diet, intestinal microflora, stress, diseases, infections, toxins, antibiotics, and withholding feed (Kansagra et al., 2003; Schumann et al., 2005; Bischoff et al., 2014; Gilani et al., 2018a, b). Increased intestinal permeability in chickens is often associated with compromised health and performance, bacterial translocation, coccidiosis, immune activation, and lameness (Wideman et al., 2012; Chapman, 2014; Chen et al., 2015; Gilani et al., 2016). The aim of our study was to investigate changes in gut barrier function and integrity by determining mRNA and protein expression of gut barrier function and TJ-related genes in broiler chickens that were subjected to delayed access to feed immediately post-hatch.
Mucin, IgA, and pIgR represent first line of immune defense in the gastrointestinal tract while TJ proteins have been found to be fundamentally important in controlling intestinal permeability (Groschwitz and Hogan, 2009). It has been reported that barrier function can be assessed indirectly in vivo by characterization of TJ proteins through quantification of mRNA expression, determination of phosphorylation, protein folding, and through localization of TJ protein (Awad et al., 2017). In our study, greater number of TJ-related genes in the ileum were affected by lack of feed for the first 48 h in comparison to the jejunum. It has been previously shown that expression of genes associated with gut barrier and health is primarily modulated in the ileum (Palamidi and Mountzouris, 2018; Barekatain et al., 2019b). Moreover, in the chicken, mRNA expression patterns of nearly all TJ-related genes showed downregulation during the first 2 wk post-hatch, while during the same period, mRNA level of gut barrier-related genes was upregulated. The only exceptions were FABP2 and ileal OCLN. OCLN shows a trend of relatively stable expression over time in both treated group, FD and NFD. It is unknown why this gene did not follow the pattern of other TJ-related genes, especially in the ileum. Our data suggest that in the ileum, OCLN gene could be constitutively expressed. FABP2 is not considered to be a part of the gut barrier but rather a marker related to changes in gut permeability. It has been shown that reduction of FABP2 mRNA expression in germ-free birds was associated with loss of epithelial cell content and intestinal barrier failure (Chen et al., 2015). In broiler chickens, FABP2 has been shown to play a role in lipogenesis, fatty acid transport, and abdominal fat content (Hu et al., 2010; Hughes and Piontkivska, 2011; Guo et al., 2014). Therefore, it is possible that the changes of FABP2 mRNA that we observed are related more to the other role of FABP2 than as a marker of gut barrier failure. In chickens, the immediate post-hatch period is characterized by dramatic changes in lipid metabolism due to changes in feed type. During embryonic development, chicks rely on lipid-rich yolk, while after hatch, they are exposed to feed that is relatively high in carbohydrates and low in fat (Richards et al., 2010).
In contrast to gene expression analysis, protein expression pattern of TJ-related proteins remains largely unknown. In this experiment, we were able to determine protein expression of CLDN 1 and 5 in the jejunum and ileum of FD and NFD birds during first 72 h post-hatch using Western blot and commercially available antibodies. In contrast to mRNA expression, protein expression was upregulated during post-hatch time with a trend (P < 0.1) for NFD birds having higher protein expression. Moreover, we showed upregulation of ZO-1 protein using ELISA assay in ileum 4 h post-hatch that was followed by a decrease. Similarly to CLDN 1 and 5 protein expression, no significant differences between FD and NFD groups were detected. Claudins serve as backbones of TJ complexes and their role is regarded as one of the most relevant immunohistochemical markers for TJ (Barekatain et al., 2019b). Lack of corresponding changes in CLDNs and ZO-1 expression profile between mRNA and protein suggests that post-translational modification could be involved in the regulation of protein activity. In mammals, phosphorylation of claudins has been shown to affect their localization and interaction with other proteins, therefore changing intestinal permeability (Findley and Koval, 2009). Other TJ-related proteins have also been shown to undergo posttranslational modifications.
In contrast to our results, study in humans showed high intestinal permeability in neonates that undergo downregulation during development (Kerr et al., 2015). Rouwet et al. (2002) suggested that the intestinal barrier is not completely developed at birth and its postnatal maturation occurs coincidentally along with increasing bacterial colonization (Udall et al., 1981a, b). The overexpression of mRNA encoding TJ-related genes in birds not fed immediately after hatch could be a result of a compensatory mechanism protecting the gastrointestinal tract from pathogenic bacteria and restoring intestinal permeability. A similar mechanism was observed by Barekatain et al. (2019a) in chicks fed dexamethasone. Our results also suggest that mRNA expression of TJ-related genes comes as a first line of defense and is highly activated before hatch (at the end of the embryonic development) and at the hatch, to prepare the sterile gastrointestinal tract to exposure to feed and bacteria. Similar expression pattern was observed in birds for defensin genes, which are highly expressed during embryonic development and at hatch and are then downregulated during first weeks post-hatch (Bar-Shira and Friedman, 2006; Zhang and Sunkara, 2014).
In contrast to TJ-related genes, mRNA expression of gut barrier function genes such as MUC2 or IgA seems to require relatively more time for upregulation of expression to occur. In our experiment, gradual increase of MUC2 mRNA was observed after hatch in both tissues with significant delay in MUC2 mRNA increase in NFD birds. After hatch, birds are exposed to a variety of antigens including commensal and pathogenic bacteria, therefore mucosal development is critically important (Zhang et al., 2015) considering that immune system of birds and their intestinal barrier are not fully developed at that time (Rouwet et al., 2002; Huang et al., 2019). It is possible that the maturation of goblet cells upon exposure to antigens from food or bacteria is required for upregulation of MUC2 gene expression. It has been previously shown that mucus secretion from goblet cells is regulated by the host sensing the presence of gut microbes or their metabolites (Kim and Ho, 2010). Similar increase in MUC2 mRNA expression during development and after hatch in GIT has been previously observed by others (Zhang et al., 2015; Woodfint et al., 2017). Delay in MUC2 mRNA expression after hatch in NFD birds as a result of lack of feed for the first 48 h post-hatch could result in deterioration of intestinal barrier, lower protection against pathogens, and overall decrease in gut health. It has been suggested that a decrease in MUC2 expression may be associated with deterioration of intestinal mucosa (Forder et al., 2012) and can lead to spontaneous inflammation of the small intestine (Van der Sluis et al., 2006). Therefore, MUC2 has been used as a marker for gut health in poultry and in other species (Forder et al., 2012; Chen et al., 2015). Moreover, it has been shown that dietary restriction can lead to regression in mucosal development (Uni et al., 1998) while MUC2 mRNA expression is reduced in intestine of germ-free birds (Cheled-Shoval et al., 2014) suggesting a role of the bacterial population in mucus development. Because of the undeveloped gut barrier and immune system immediately after hatch, presence of mucus is important for gut protection during the first hours post-hatch (Moreira Filho et al., 2019) and it has been suggested that enhancement of mucin secretion in young chickens during the development of the gut mucosal barrier could be beneficial in preventing the invasion of pathogenic bacteria and toxins (Murai et al., 2018). Lack of feed for the first 48 h seems to have a deteriorating effect on the chicks' ability to protect itself from pathogenic bacteria and toxins.
In this study, the upregulation of MUC2 between 48 and 72 h post-hatch was corresponded with MUC2 histological staining. Lack of feed immediately after hatch not only led to visible morphological changes in the small intestine of chickens but was also shown to affect mucin dynamics (Uni et al., 2003). It has also been shown that presence of intestinal microflora in the GIT influences mucin dynamics and mucin development (Cheled-Shoval et al., 2014). In this experiment, we observed changes in mucin composition (based on histological staining) during the jejunum and ileum development post-hatch but also due to lack of feed immediately post-hatch in both tissues. Mucin itself can be classified into neutral mucin, sialic acid containing mucin, and ester sulphate containing mucin (Deplancke and Gaskins, 2001). It has been shown that before hatch, an acidic type of mucin prevails, while after hatch, neutral mucin is coproduced in goblet cells (Cheled-Shoval et al., 2014). The physiological relevance of distinct mucin types is not well understood but it has been suggested that it can influence their protective properties (Deplancke and Gaskins, 2001). It has been also suggested that acidic mucin protects against bacterial translocation because it appears to be less prone to degradation by bacterial enzymes and proteases (Deplancke and Gaskins, 2001). Therefore, presence of acidic mucin in early stages of development could be important due to an immune defense system that is not fully functional (Deplancke and Gaskins, 2001). In agreement with that data, we have shown increased staining for acidic and mixed mucin in NFD birds compared to FD birds between 24 and 72 h post-hatch.
Mucins provide adhesive sites for IgA and make up the first line of intestinal defense (Everett et al., 2004). IgA is the most abundant immunoglobulin isotype at mucosal sites (Brandtzaeg, 2010) and is an important factor in preventing infection and regulating the composition of microbiota (Den Hartog et al., 2016). In agreement with previous report (Zhang et al., 2015), IgA was poorly expressed during the first week of life and increased during second week of life in both the jejunum and ileum. Lack of IgA expression during the first week post-hatch in birds has been explained by the presence of maternal IgA from the egg and their role in passive immune mechanism (Bar-Shira et al., 2014). Similarly to other gut barrier-related genes, IgA mRNA showed an opposite expression pattern to TJ-related genes. In the jejunum, increase in IgA expression was initiated 48 h earlier when compared to the FD group, while no effect of lack of feed on IgA mRNA was observed in the ileum. Increase in IgA mRNA in the jejunum in NFD birds could be explained by the presence of compensatory mechanism due to lower MUC2 expression and/or faster depletion of maternal IgA. The difference in reaction of jejunum and ileum to lack of feed immediately after hatch could also be explained by presence of different bacterial populations occupying these 2 parts of the gastrointestinal tract (Choi et al., 2014). Besides the differences in upregulation of IgA expression in the jejunum of FD and NFD groups, it is worth to mention the huge difference in their relative expression. At 288 h and 336 h post-hatch, the IgA mRNA expression in the NFD chicks was, respectively, 3 × 102 and 1.6 times higher than in FD birds. This could suggest that NFD birds were experiencing possible low level of inflammation (“chronic type”) due to delay in access to feed post-hatch. Changes in the proinflammatory and anti-inflammatory interleukins such as Il-1β, Il-6, Il-8, and TGFβ as well as in TLR2, 4 and INFγ were observed during the first 2 wk post-hatch in NFD birds, supporting the hypothesis of presence of “chronic type” inflammation in the gastrointestinal tract of these chickens (authors' unpublished data).
Function of IgA depends on its transport from the lamina propria, where it is secreted, via pIgR located on the basolateral side of mucosal epithelial cells (Johansen et al., 1999; Kaetzel, 2005). Expression profile of pIgR mRNA was very similar to that of IgA in the jejunum and ileum with the exception of time of increased expression and reaction to lack of feed immediately post-hatch. Expression level of pIgR was upregulated in both tissues at the end of first week post-hatch, and in contrast to IgA, lack of feed had impact on pIgR mRNA level in the ileum but not in the jejunum. It seems that pIgR mRNA expression compensated with a lack of reaction to absence of feed immediately post-hatch. It is interesting, that pIgR expression was upregulated during development much earlier than IgA mRNA level. In contrast to our results, Zhang et al. (2015) observed similar expression profile of IgA and pIgR in the chicken ileum (increased during second week post-hatch). Moreover, the relative expression of the gut barrier–related genes such as IgA or pIgR over time was an order of magnitude higher than those at embryonic stage while changes in TJ-related genes over time were only 5-10 times lower over time in comparison to embryonic stage.
Small intestine of hatched chicks is immature and undergoes dramatic changes during the first days post-hatch (Uni et al., 2000). Immediately after hatch, functional maturation of GIT is triggered by microbiota and dietary antigens (Den Hartog et al., 2016). Similarly to our observation, rudimentary crypts have been found immediately post-hatch in small intestine and only one crypt per villi has been observed (Uni et al., 2000). Defined and developed crypts have been observed in small intestine of chickens within 2 to 3 D post-hatch (Uni et al., 2000; Geyra et al., 2001b). Development of crypts was accompanied by elongation and development of villi in both the jejunum and ileum. It has been previously shown that narrow villi have greater nutrient absorption area while widening of villi results in less area for nutrient absorption (Chen et al., 2015). Moreover, crypt depth and the ratio of crypt depth to villi height has been associated with measures of efficiency because increase of crypt depth or ratio indicates greater need for cell proliferation to maintain gut barrier integrity (Uni et al., 1998; Awad et al., 2009, 2011). Although in this manuscript we do not report measurements associated with GIT morphology, the presented microscope pictures clearly indicate changes in morphology of the small intestine not only during GIT maturation and development, but also due to delayed access to feed immediately post-hatch. Most available literature indicates that feeding immediately post-hatch leads to accelerated development of gut morphology (Noy and Sklan, 1998), while lack of feed for 24 to 36 h post-hatch leads to retarded growth in GIT segments of the intestine, alteration in crypt depth and microvilli (Uni et al., 1998), and morphology of the small intestine (Geyra et al., 2001b). However, changes in intestinal morphology due to lack of feed for the first 48 h post-hatch were not observed by Potturi et al. (2005).
In our previous study (Proszkowiec-Weglarz et al., 2019), we have shown that NFD birds were characterized by significantly lower body weight beginning at day 6 post-hatch and until the end of the experiment (day 14). However, at the end of the experiment, the feed intake was not significantly different between treatment groups. It is possible that the lower body weight that was observed in NFD birds is a result of changes in gut barrier function and overcompensation of these functions due to delay in access to feed early post-hatch. Increase in these processes may have come at the expense of growth parameters in NFD birds. On the other hand, FD birds showed relatively enhanced TJ integrity and gut barrier function at later stages of growth without compromised growth.
The impact of changes in gut barrier and gut integrity genes due to delay in access to feed early post-hatch, on responses to stress later in life, is largely unknown. More data are required to determine if changes observed early in life during gastrointestinal and immune system development will affect the response of broilers to challenges such heat stress, bacterial, or parasites infections. However, it has been shown that expression of TJ-related and gut barrier–related genes is modulated by different stresses encountered later in life (Shao et al., 2013; Song et al., 2014; Li et al., 2015; Yang et al., 2015; Chen et al., 2018; Liu et al., 2018; Goo et al., 2019).
In conclusion, we have shown that lack of access to feed for the first 48 h post-hatch is associated with changes in mRNA expression of genes related to TJ and gut barrier function. The data suggest that delayed access to feed post-hatch may affect TJ structure and/or function, gut barrier function, and overall health of the small intestine in the chickens. Moreover, our data suggest the presence of compensatory mechanisms in the expression of TJ-related genes to possibly counterbalance negative changes in gut barrier-related genes, mainly MUC2. Finally, our data strongly support the fact that immediate access to feed post-hatch is important not only for GIT development, but also for gastrointestinal barrier and overall animal health.
Acknowledgments
The work was funded by in house USDA-ARS CRIS # 8042-31000-108-00D. The authors would like to thank Dr. Matt Kramer (USDA, ARS, NEA Bioinformatics) for his help with statistical analysis.
Conflict of Interest Statement: The authors declare no conflict of interest.
Footnotes
Mention of trade name, proprietary product, or specific equipment does not constitute guarantee or warranty by USDA and does not imply its approval to the exclusion of other suitable products.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.06.023.
Supplementary data
References
- Abreu M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- Anderson J.M., Van Itallie C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Ghareeb K., Abdel-Raheem S., Bohm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Ghareeb K., Bohm J. Evaluation of the chicory inulin efficacy on ameliorating the intestinal morphology and modulating the intestinal electrophysiological properties in broiler chickens. J. Anim. Physiol. Anim. Nutr. (Berl) 2011;95:65–72. doi: 10.1111/j.1439-0396.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Hess C., Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins (Basel) 2017;9 doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shira E., Friedman A. Development and adaptations of innate immunity in the gastrointestinal tract of the newly hatched chick. Dev. Comp. Immunol. 2006;30:930–941. doi: 10.1016/j.dci.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bar-Shira E., Cohen I., Elad O., Friedman A. Role of goblet cells and mucin layer in protecting maternal IgA in precocious birds. Dev. Comp. Immunol. 2014;44:186–194. doi: 10.1016/j.dci.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Barekatain R., Chrystal P.V., Howarth G.S., McLaughlan C.J., Gilani S., Nattrass G.S. Performance, intestinal permeability, and gene expression of selected tight junction proteins in broiler chickens fed reduced protein diets supplemented with arginine, glutamine, and glycine subjected to a leaky gut model. Poult. Sci. 2019;98:6761–6771. doi: 10.3382/ps/pez393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barekatain R., Nattrass G., Tilbrook A.J., Chousalkar K., Gilani S. Reduced protein diet and amino acid concentration alter intestinal barrier function and performance of broiler chickens with or without synthetic glucocorticoid. Poult. Sci. 2019;98:3662–3675. doi: 10.3382/ps/pey563. [DOI] [PubMed] [Google Scholar]
- Bigot K., Mignon-Grasteau S., Picard M., Tesseraud S. Effects of delayed feed intake on body, intestine, and muscle development in neonate broilers. Poult. Sci. 2003;82:781–788. doi: 10.1093/ps/82.5.781. [DOI] [PubMed] [Google Scholar]
- Bischoff S.C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.D., Serino M., Tilg H., Watson A., Wells J.M. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box G.E.P., Cox D.R. An analysis of transformations. J. R. Stat. Soc. 1964;26B:211–252. [Google Scholar]
- Brandtzaeg P. Homeostatic impact of indigenous microbiota and secretory immunity. Benef. Microbes. 2010;1:211–227. doi: 10.3920/BM2010.0009. [DOI] [PubMed] [Google Scholar]
- Careghi C., Tona K., Onagbesan O., Buyse J., Decuypere E., Bruggeman V. The effects of the spread of hatch and interaction with delayed feed access after hatch on broiler performance until seven days of age. Poult. Sci. 2005;84:1314–1320. doi: 10.1093/ps/84.8.1314. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Milestones in avian coccidiosis research: a review. Poult. Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- Cheled-Shoval S.L., Gamage N.S., Amit-Romach E., Forder R., Marshal J., Van Kessel A., Uni Z. Differences in intestinal mucin dynamics between germ-free and conventionally reared chickens after mannan-oligosaccharide supplementation. Poult. Sci. 2014;93:636–644. doi: 10.3382/ps.2013-03362. [DOI] [PubMed] [Google Scholar]
- Chen J., Tellez G., Richards J.D., Escobar J. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front Vet. Sci. 2015;2:14. doi: 10.3389/fvets.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Cheng Y., Li Y., Wen C., Zhou Y. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 2018;119:1254–1262. doi: 10.1017/S0007114518000740. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Kim G.B., Cha C.J. Spatial heterogeneity and stability of bacterial community in the gastrointestinal tracts of broiler chickens. Poult. Sci. 2014;93:1942–1950. doi: 10.3382/ps.2014-03974. [DOI] [PubMed] [Google Scholar]
- de Gouw P., van de Ven L.J.F., Lourens S., Kemp B., van den Brand H. Effects of dust, formaldehyde and delayed feeding on early postnatal development of broiler chickens. Res. Vet. Sci. 2017;112:201–207. doi: 10.1016/j.rvsc.2017.04.021. [DOI] [PubMed] [Google Scholar]
- de Jong I.C., van Riel J., Bracke M.B.M., van den Brand H. A 'meta-analysis' of effects of post-hatch food and water deprivation on development, performance and welfare of chickens. PLoS One. 2017;12:e0189350. doi: 10.1371/journal.pone.0189350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hartog G., De Vries-Reilingh G., Wehrmaker A.M., Savelkoul H.F., Parmentier H.K., Lammers A. Intestinal immune maturation is accompanied by temporal changes in the composition of the microbiota. Benef Microbes. 2016;7:677–685. doi: 10.3920/BM2016.0047. [DOI] [PubMed] [Google Scholar]
- Deplancke B., Gaskins H.R. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001;73:1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Knight C.D., Kitchel M.L., Atwell C.A., Downs A.C., Ivey F.J. Early feeding and development of the immune system in neonatal poultry. J. Appl. Poult. Res. 1998;7:425–436. [Google Scholar]
- Everett M.L., Palestrant D., Miller S.E., Bollinger R.R., Parker W. Immune exclusion and immune inclusion: a new model of host-bacterial interactions in the gut. Clin. Appl. Immunol. Rev. 2004;4:321–332. [Google Scholar]
- Fanning A.S., Jameson B.J., Jesaitis L.A., Anderson J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Findley M.K., Koval M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life. 2009;61:431–437. doi: 10.1002/iub.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forder R.E., Nattrass G.S., Geier M.S., Hughes R.J., Hynd P.I. Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult. Sci. 2012;91:1335–1341. doi: 10.3382/ps.2011-02062. [DOI] [PubMed] [Google Scholar]
- Geyra A., Uni Z., Sklan D. The effect of fasting at different ages on growth and tissue dynamics in the small intestine of the young chick. Br. J. Nutr. 2001;86:53–61. doi: 10.1079/bjn2001368. [DOI] [PubMed] [Google Scholar]
- Geyra A., Uni Z., Sklan D. Enterocyte dynamics and mucosal development in the posthatch chick. Poult. Sci. 2001;80:776–782. doi: 10.1093/ps/80.6.776. [DOI] [PubMed] [Google Scholar]
- Gil-Cardoso K., Gines I., Pinent M., Ardevol A., Blay M., Terra X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016;29:234–248. doi: 10.1017/S0954422416000159. [DOI] [PubMed] [Google Scholar]
- Gilani S., Howarth G., Kitessa S., Forder R., Tran C., Hughes B. New biomarkers for intestinal permeability induced by lipopolysaccharide in chickens. Anim. Prod. Sci. 2016;56:1984–1997. [Google Scholar]
- Gilani S., Howarth G.S., Nattrass G., Kitessa S.M., Barekatain R., Forder R.E.A., Tran C.D., Hughes R.J. Gene expression and morphological changes in the intestinal mucosa associated with increased permeability induced by short-term fasting in chickens. J. Anim. Physiol. Anim. Nutr. (Berl.) 2018;102:e653–e661. doi: 10.1111/jpn.12808. [DOI] [PubMed] [Google Scholar]
- Gilani S., Howarth G.S., Tran C.D., Barekatain R., Kitessa S.M., Forder R.E.A., Hughes R.J. Reduced fasting periods increase intestinal permeability in chickens. J. Anim. Physiol. Anim. Nutr. (Berl) 2018;102:e486–e492. doi: 10.1111/jpn.12712. [DOI] [PubMed] [Google Scholar]
- Gonzales E., Kondo N., Saldanha E.S., Loddy M.M., Careghi C., Decuypere E. Performance and physiological parameters of broiler chickens subjected to fasting on the neonatal period. Poult. Sci. 2003;82:1250–1256. doi: 10.1093/ps/82.8.1250. [DOI] [PubMed] [Google Scholar]
- Goo D., Kim J.H., Choi H.S., Park G.H., Han G.P., Kil D.Y. Effect of stocking density and sex on growth performance, meat quality, and intestinal barrier function in broiler chickens. Poult. Sci. 2019;98:1153–1160. doi: 10.3382/ps/pey491. [DOI] [PubMed] [Google Scholar]
- Groschwitz K.R., Hogan S.P. Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Liu D., Zhao X., Li C., Guo Y. Xylanase supplementation of a wheat-based diet improved nutrient digestion and mRNA expression of intestinal nutrient transporters in broiler chickens infected with Clostridium perfringens. Poult. Sci. 2014;93:94–103. doi: 10.3382/ps.2013-03188. [DOI] [PubMed] [Google Scholar]
- Halevy O., Geyra A., Barak M., Uni Z., Sklan D. Early posthatch starvation decreases satellite cell proliferation and skeletal muscle growth in chicks. J. Nutr. 2000;130:858–864. doi: 10.1093/jn/130.4.858. [DOI] [PubMed] [Google Scholar]
- Hu G., Wang S., Tian J., Chu L., Li H. Epistatic effect between ACACA and FABP2 gene on abdominal fat traits in broilers. J. Genet. Genomics. 2010;37:505–512. doi: 10.1016/S1673-8527(09)60070-9. [DOI] [PubMed] [Google Scholar]
- Huang L., Luo L., Zhang Y., Wang Z., Xia Z. Effects of the dietary probiotic, Enterococcus faecium NCIMB11181, on the intestinal barrier and system immune status in Escherichia coli O78-challenged broiler chickens. Probiotics Antimicrob. Proteins. 2019;11:946–956. doi: 10.1007/s12602-018-9434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A.L., Piontkivska H. Evolutionary diversification of the avian fatty acid-binding proteins. Gene. 2011;490:1–5. doi: 10.1016/j.gene.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen F.E., Pekna M., Norderhaug I.N., Haneberg B., Hietala M.A., Krajci P., Betsholtz C., Brandtzaeg P. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 1999;190:915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul-Madsen H.R., Su G., Sorensen P. Influence of early or late start of first feeding on growth and immune phenotype of broilers. Br. Poult. Sci. 2004;45:210–222. doi: 10.1080/00071660410001715812. [DOI] [PubMed] [Google Scholar]
- Kaetzel C.S. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Kansagra K., Stoll B., Rognerud C., Niinikoski H., Ou C.N., Harvey R., Burrin D. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G1162–G1170. doi: 10.1152/ajpgi.00243.2003. [DOI] [PubMed] [Google Scholar]
- Kerr C.A., Grice D.M., Tran C.D., Bauer D.C., Li D., Hendry P., Hannan G.N. Early life events influence whole-of-life metabolic health via gut microflora and gut permeability. Crit. Rev. Microbiol. 2015;41:326–340. doi: 10.3109/1040841X.2013.837863. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang H., Chen Y.P., Yang M.X., Zhang L.L., Lu Z.X., Zhou Y.M., Wang T. Bacillus amyloliquefaciens supplementation alleviates immunological stress and intestinal damage in lipopolysaccharide-challenged broilers. Anim. Feed Sci. Technol. 2015;208:119–131. doi: 10.3382/ps/pev124. [DOI] [PubMed] [Google Scholar]
- Linden S.K., Sutton P., Karlsson N.G., Korolik V., McGuckin M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Lin L., Wang J., Zhang F., Wang J.P. Dietary cysteamine hydrochloride protects against oxidation, inflammation, and mucosal barrier disruption of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. 2018;96:4339–4347. doi: 10.1093/jas/sky292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitic L.L., Van Itallie C.M., Anderson J.M. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- Moreira Filho A.L.B., Ferket P.R., Malheiros R.D., Oliveira C.J.B., Aristimunha P.C., Wilsmann D.E., Givisiez P.E.N. Enrichment of the amnion with threonine in chicken embryos affects the small intestine development, ileal gene expression and performance of broilers between 1 and 21 days of age. Poult. Sci. 2019;98:1363–1370. doi: 10.3382/ps/pey461. [DOI] [PubMed] [Google Scholar]
- Murai A., Kitahara K., Terada H., Ueno A., Ohmori Y., Kobayashi M., Horio F. Ingestion of paddy rice increases intestinal mucin secretion and goblet cell number and prevents dextran sodium sulfate-induced intestinal barrier defect in chickens. Poult. Sci. 2018;97:3577–3586. doi: 10.3382/ps/pey202. [DOI] [PubMed] [Google Scholar]
- Noy Y., Uni Z., Sklan D. Routes of yolk utilisation in the newly-hatched chick. Br. Poult. Sci. 1996;37:987–995. doi: 10.1080/00071669608417929. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Yolk utilisation in the newly hatched poult. Br. Poult. Sci. 1998;39:446–451. doi: 10.1080/00071669889042. [DOI] [PubMed] [Google Scholar]
- Palamidi I., Mountzouris K.C. Diet supplementation with an organic acids-based formulation affects gut microbiota and expression of gut barrier genes in broilers. Anim. Nutr. 2018;4:367–377. doi: 10.1016/j.aninu.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potturi P.V., Patterson J.A., Applegate T.J. Effects of delayed placement on intestinal characteristics in turkey poults. Poult. Sci. 2005;84:816–824. doi: 10.1093/ps/84.5.816. [DOI] [PubMed] [Google Scholar]
- Proszkowiec-Weglarz M., Schreier L.L., Miska K.B., Angel R., Kahl S., Russell B. Effect of early neonatal development and delayed feeding post-hatch on jejunal and ileal calcium and phosphorus transporter genes expression in broiler chickens1. Poult. Sci. 2019;98:1861–1871. doi: 10.3382/ps/pey546. [DOI] [PubMed] [Google Scholar]
- R-Core-Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Richards M.P., Proszkowiec-Weglarz M., Rosebrough R.W., McMurtry J.P., Angel R. Effects of early neonatal development and delayed feeding immediately post-hatch on the hepatic lipogenic program in broiler chicks. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010;157:374–388. doi: 10.1016/j.cbpb.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Rouwet E.V., Heineman E., Buurman W.A., ter Riet G., Ramsay G., Blanco C.E. Intestinal permeability and carrier-mediated monosaccharide absorption in preterm neonates during the early postnatal period. Pediatr. Res. 2002;51:64–70. doi: 10.1203/00006450-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Schumann A., Nutten S., Donnicola D., Comelli E.M., Mansourian R., Cherbut C., Corthesy-Theulaz I., Garcia-Rodenas C. Neonatal antibiotic treatment alters gastrointestinal tract developmental gene expression and intestinal barrier transcriptome. Physiol. Genomics. 2005;23:235–245. doi: 10.1152/physiolgenomics.00057.2005. [DOI] [PubMed] [Google Scholar]
- Shao Y., Guo Y., Wang Z. Beta-1,3/1,6-glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult. Sci. 2013;92:1764–1773. doi: 10.3382/ps.2013-03029. [DOI] [PubMed] [Google Scholar]
- Shira E.B., Sklan D., Friedman A. Impaired immune responses in broiler hatchling hindgut following delayed access to feed. Vet. Immunol. Immunopathol. 2005;105:33–45. doi: 10.1016/j.vetimm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Smirnov A., Tako E., Ferket P.R., Uni Z. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci. 2006;85:669–673. doi: 10.1093/ps/85.4.669. [DOI] [PubMed] [Google Scholar]
- Song J., Xiao K., Ke Y.L., Jiao L.F., Hu C.H., Diao Q.Y., Shi B., Zou X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014;93:581–588. doi: 10.3382/ps.2013-03455. [DOI] [PubMed] [Google Scholar]
- Udall J.N., Colony P., Fritze L., Pang K., Trier J.S., Walker W.A. Development of gastrointestinal mucosal barrier. II. The effect of natural versus artificial feeding on intestinal permeability to macromolecules. Pediatr. Res. 1981;15:245–249. doi: 10.1203/00006450-198103000-00009. [DOI] [PubMed] [Google Scholar]
- Udall J.N., Pang K., Fritze L., Kleinman R., Walker W.A. Development of gastrointestinal mucosal barrier. I. The effect of age on intestinal permeability to macromolecules. Pediatr. Res. 1981;15:241–244. doi: 10.1203/00006450-198103000-00008. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ganot S., Sklan D. Posthatch development of mucosal function in the broiler small intestine. Poult. Sci. 1998;77:75–82. doi: 10.1093/ps/77.1.75. [DOI] [PubMed] [Google Scholar]
- Uni Z., Geyra A., Ben-Hur H., Sklan D. Small intestinal development in the young chick: crypt formation and enterocyte proliferation and migration. Br. Poult. Sci. 2000;41:544–551. doi: 10.1080/00071660020009054. [DOI] [PubMed] [Google Scholar]
- Uni Z., Smirnov A., Sklan D. Pre- and posthatch development of goblet cells in the broiler small intestine: effect of delayed access to feed. Poult. Sci. 2003;82:320–327. doi: 10.1093/ps/82.2.320. [DOI] [PubMed] [Google Scholar]
- Untergasser A., Nijveen H., Rao X., Bisseling T., Geurts R., Leunissen J.A. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007;35:W71–W74. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven L.J., van Wagenberg A.V., Groot Koerkamp P.W., Kemp B., van den Brand H. Effects of a combined hatching and brooding system on hatchability, chick weight, and mortality in broilers. Poult. Sci. 2009;88:2273–2279. doi: 10.3382/ps.2009-00112. [DOI] [PubMed] [Google Scholar]
- van de Ven L.J., van Wagenberg A.V., Debonne M., Decuypere E., Kemp B., van den Brand H. Hatching system and time effects on broiler physiology and posthatch growth. Poult. Sci. 2011;90:1267–1275. doi: 10.3382/ps.2010-00876. [DOI] [PubMed] [Google Scholar]
- van de Ven L.J., van Wagenberg A.V., Decuypere E., Kemp B., van den Brand H. Perinatal broiler physiology between hatching and chick collection in 2 hatching systems. Poult. Sci. 2013;92:1050–1061. doi: 10.3382/ps.2012-02534. [DOI] [PubMed] [Google Scholar]
- Van der Sluis M., De Koning B.A., De Bruijn A.C., Velcich A., Meijerink J.P., Van Goudoever J.B., Buller H.A., Dekker J., Van Seuningen I., Renes I.B., Einerhand A.W. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman R.F., Jr., Hamal K.R., Stark J.M., Blankenship J., Lester H., Mitchell K.N., Lorenzoni G., Pevzner I. A wire-flooring model for inducing lameness in broilers: evaluation of probiotics as a prophylactic treatment. Poult. Sci. 2012;91:870–883. doi: 10.3382/ps.2011-01907. [DOI] [PubMed] [Google Scholar]
- Willemsen H., Debonne M., Swennen Q., Everaert N., Careghi C., Han H., Bruggeman K., Tona K., Decuypere E. Delay in feed access and spread of hatch: importance of early nutrition. Worlds Poult. Sci. J. 2010;66:177–188. [Google Scholar]
- Woodfint R.M., Chen P.R., Ahn J., Suh Y., Hwang S., Lee S.S., Lee K. Identification of the MUC2 promoter as a strong promoter for intestinal gene expression through generation of transgenic quail expressing GFP in gut epithelial cells. Int. J. Mol. Sci. 2017;18:196. doi: 10.3390/ijms18010196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Wang A., Zeng X., Hou C., Liu H., Qiao S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015;15:32. doi: 10.1186/s12866-015-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegani M., Korver D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T.H., Wright N.A. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- Yu D., Turner J.R. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim. Biophys. Acta. 2008;1778:709–716. doi: 10.1016/j.bbamem.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Sunkara L.T. Avian antimicrobial host defense peptides: from biology to therapeutic applications. Pharmaceuticals (Basel) 2014;7:220–247. doi: 10.3390/ph7030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Eicher S.D., Applegate T.J. Development of intestinal mucin 2, IgA, and polymeric Ig receptor expressions in broiler chickens and Pekin ducks. Poult. Sci. 2015;94:172–180. doi: 10.3382/ps/peu064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.