Abstract
We studied livestock abortion and various associated risk factors in the Ili region of northwest China. Livestock abortion prevalence was estimated and correlated with infections (Brucellosis, Salmonellosis, Mycoplasma and Chlamydia seropositivity) and management (farming type and contact with other herds/flocks) risk factors. A total of 2996 serum samples (1406 cow, 1590 sheep) were identified by RBPT (Rose Bengal Plate Test) and c-ELISA (competitive-enzyme linked immunosorbent assay), and they showed the overall seroprevalence of brucellosis in the study area was cow 6.76%, sheep 9.50%. The seroprevalence of brucellosis in X county was cow 7.06%, sheep 9.12%; in H county was cow 11.70%, sheep 10.80%; and in Q county was cow 4.22%, sheep 9.11%. The overall seroprevalence of Mycoplasma in the study area was cow 3.20%, sheep 6.42%. The seroprevalence of Mycoplasma in X county was cow 3.39%, sheep 7.98%; in H county was cow 5.26%, sheep 9.97%; and in Q county was cow 2.11%, sheep 4.33%. The Odds ratio of brucellosis for cow and sheep, respectively, were 45.909 [95% CI 26.912–78.317, P<0.001] and 70.507 [95% CI 43.783–113.544, P<0.001] times higher than other abortion-related factors including mixed farming, contact with other flocks and Mycoplasma infection. A total of 54 samples, including aborted cow (22), sheep (30) fetuses and milk samples (2), were identified as Brucella melitensis (B. melitensis) positive. A total of 38 Brucella were isolated from 16 aborted cow, 20 sheep fetuses and 2 milk samples. All of these isolates were identified, and confirmed, as B. melitensis. A phylogenetic tree showed that the Brucella isolates closely matched the B. melitensis biovar 3 isolated in Inner Mongolia, China, and B. melitensis isolated from Norway and India. These results suggest that B. melitensis biovar 3 is the main pathogen responsible for cow and sheep abortion and also pose a human health risk. Additionally, livestock reproduction can also be influenced by Mycoplasma infection and managerial factors (farming type and contact with other herds/flocks), especially in remote areas.
Introduction
Ruminants are a major source of meat production in China and are important for food security. Xinjiang Uygur Autonomous Region (XUAR) is located in northwest China and is a major ruminant production province. In 2017, beef production (0.43 million tons) and mutton production (0.58 million tons) in Xinjiang, respectively, accounted for the 6.8% and 12.4% in the total beef and mutton production in China. Ili is located in the western part of XUAR, where the economy is highly dependent on animal production [1, 2]. The combined number of cattle, sheep and goats is approximately 5.88 million in this region, in which the goats only accounted for the 2.1% because of economic value. The sheep, goats and cattle are reared under traditional systems, and confined sheep/goats or cattle ranches are the two main feeding systems.
Diseases and poor animal health are major risk factors for animal production in Ili. The viability of sheep and cattle production is largely determined by their reproductive ability, which is influenced by both genetic and environmental factors [3, 4]. Abortion is a serious threat to livestock, and it is also a public health issue as it is often induced by zoonotic microorganisms [5, 6].
Most ruminants are maintained by poor farmers as a way to increase family income. Abortion in sheep and dairy cows has a great impact on the animal production and the health of rural economies [7, 8]. The farming system and communal grazing are often involved in the spread of infectious organisms. There is a need for an improved diagnostics and specific control strategies for maintaining healthy livestock and public health safety [6]. Risk factors responsible for livestock abortion can be classified into infectious and non-infectious [9].
Infectious agents are the main causes of abortion in sheep and cattle as compared to non-infectious agents and are generally infectious to humans. The main etiological agents causing sheep and cattle abortion are Brucella, Salmonella, Mycoplasma, Chlamydia abortus and Toxoplasma gondii [9–12].
Ili is an endemic area for brucellosis with high incidences in sheep (4.21%) and cattle (6.91%) brucellosis in 2015 (Data from the Center for Animal Disease Control and Prevention of Ili). In this region, most farmers practice mixed farming (both sheep and dairy cows) and use a communal grazing system. Grazing in this environment can expose pregnant animals to pathogens [5, 13].
In recent years, the number of livestock abortions increased, according to the local Veterinary Department report. However, the causes of these abortions remained unknown. Hence, to protect and sustain the ruminant industry in the Ili region, we need to understand all of the reasons for animal abortion. Thus, the objectives of this study were to: 1) investigate the prevalence of abortion in Ili ruminant flocks and correlated its association with infectious agents (Brucella, Salmonella, Mycoplasma and Chlamydia abortus) and management (farming type and contact with other flocks) risk factors; 2) isolate and analyze genetic characteristics of the abortion-related pathogens.
Materials and methods
Ethics statement
All animals used in our experiment were treated humanely and in accordance with institutional animal care guidelines. Our study was approved by the Animal Care and Use Committee of Shihezi University.
Study design
A cross-sectional study was carried out in three counties of the Ili region (X county, H county and Q county) between March and July in both 2017 and 2018. Samples from cows and sheep were collected from smallholder farms. The selection strategies including regions, villages and farms were described in Arif et al [14]. These three counties were selected mostly based on operational convenience, but they also represent a range of agro-ecological zones. Villages within each county were selected randomly by an electronic calculation.
Herd selection
A total of 325 farms were selected from 25 villages in the three counties, and, given their availability, a maximum of five cows or sheep were randomly sampled in each farm. All of the livestock owners involved were informed about the purpose of this study and provided information about previous vaccinations. The study sampled non-vaccinated animals over two years of age. When there were more than five animals of the required age, five animals were selected randomly from the animals available.
Sample size
The study population included all the farms in selected villages, but the target population was all of the cows and sheep within the selected villages and all of the villages in selected counties. Several studies reported that Brucella is a main pathogen responsible for animal abortion in XUAR [15–17]. Therefore, the sample size was calculated according to the estimated prevalence of brucellosis in these three counties, and the assumed prevalence is listed in Table 1. The minimum sample number of sheep and cows required assumed a closed population, as described previously [18]. The sample size of cows and sheep was estimated to detect a reduction of at least 4% for cows and 6% for sheep brucellosis prevalence with a confidence of 95% and a power of 80% according the following equation [18]:
Table 1. The estimation of minimum required number in this study.
| County | Expect prevalence | Desire prevalence | Minimum require number | |||
|---|---|---|---|---|---|---|
| Cow | Sheep | Cow | Sheep | Cow | Sheep | |
| X | 9.5% | 8.3% | 2% | 3% | 150 | 296 |
| H | 8.1% | 6.7% | 2% | 3% | 201 | 316 |
| Q | 5.5% | 8.8% | 1.5% | 3% | 330 | 261 |
In this equation, n is the minimum number of samples required, Zα represents the value obtained from standard normal distribution for 95% confidence (1.96), Zβ represents the value obtained from standard normal distribution for a power of 80% (-0.84), p1 represents the estimated prevalence, an expected prevalence for cow and sheep brucellosis in these three counties (listed in Table 1), p2 represents the desired brucellosis prevalence for cows and sheep (listed in Table 1), q1 is (1-p1), q2 is (1-p2), p is (p1+p2)/2, and q is 1-p. The minimum number of cows and sheep required in these three counties is shown in Table 1.
Sample collection
A total of 2996 blood samples (1406 dairy cows and 1590 sheep) were collected from jugular veins using venoject needles (Venoject, China) and stored in 5 mL sterile vacutainer tubes. Additionally, 141 aborted fetuses (66 cow fetuses and 75 sheep fetuses) and 65 milk samples (42 cow milk and 23 ewe milk) were collected. Blood samples were centrifuged at 3000 rpm for 10 min, and the serum was separated into a new sterile tube and stored at -20°C until tested. The milk samples were transported to the laboratory and stored in 4°C. The aborted fetuses were stored at -20°C until processing.
Laboratory testing
All of the serum samples were screened for antibodies by RBPT and c-ELISA. Briefly, 30 μL of antigen was mixed with 30 μL of serum on a clean plate. After 3 min, any visible agglutination was considered as positive, and no agglutination was considered as negative. Positive or doubtful samples identified by RBPT were further tested with c-ELISA using the Svanovir Brucella-Ab-c-ELISA kits (Svanova Biotech, Uppsala, Sweden) according to the manufacturer’s instructions. The optical density (OD) of each samples were tested twice to obtain the average OD. The cutoff OD of 0.3 was used to identify positive reactions [19]. The sensitivity and specificity of these two methods have been validated as useful tools for brucellosis screening [20, 21]. Additionally, all of the serum sample were screened using the ELISA method to evaluate the changes of Chlamydia abortus-specific antibody titer. The mean value of OD was used to identify infected or non-infected livestock [22] and the Mycoplasma bovis-specific antibody concentration was determined by Mycoplasma bovis MilA IgG ELISA as described previously [23]. The antibodies against Salmonella spp were identified using an indirect ELISA kit as described previously [24].
Risk factors questionnaire
A questionnaire was filled out by participating farm owners. The questionnaire contained information about abortion history in the livestock during the previous two years, livestock management risk factors including history of contact with other animals (yes or no) and type of farming; sheep flocks (containing only sheep), cow herds (containing only cows) or mixed groups (containing both sheep and cows).
PCR examination
Samples of spleen, liver and stomach contents were collected aseptically from aborted fetuses of sheep or cows. The DNA extractions from tissue samples were performed using the TIANamp Genomic DNA Kit (TIANGEN BIOTECH CO., LTD, China) according to the manufacturer’s instructions. Nucleic acid extraction from raw milk was performed as previously described [25]. All of the samples were examined by PCR and the PCR primers used in this study are listed in S1 Table of the Supplementary Material.
Pathogen isolation
The Brucella was isolated from raw milk as previously described [26, 27]. Spleen, liver and stomach contents were crushed and cultured on Brucella serum dextrose agar composed of Brucella medium base (supplemented with Brucella selective antibiotic, OXOID, England) and 5%-10% heat-inactivated horse serum (GIBCO, New Zealand). Plates were incubated with, and without, 5%-10% carbon dioxide at 37°C after inoculation with sample materials. The plates were examined after 3–5 d for bacterial growth. A single clone was chosen for identification. The Salmonella spp., Mycoplasma bovis and Chlamydia abortus were isolated from aborted fetuses or milk samples as described previously [28–30].
Identification of isolates
The obtained single bacterial clones were identified using PCR targeting the 16S rRNA gene [31]. The PCR primers for examination of Salmonella spp., Mycoplasma bovis and Chlamydia abortus are listed in S1 Table of the Supplementary Material. The IS711 PCR primers were used to identify the species of Brucella. PCR products purification and sequencing was conducted as described above. Phylogenetic analysis of isolates was done according to the IS711 sequence. The sequence distance was determined by the neighbor-joining (NJ) method, and maximum-likelihood algorithms were analyzed using the Molecular Evolutionary Genetics Analysis (MEGA) 7 software [32]. The Brucella isolates were characterized by biochemical testing according to the standard strain identification method [33]. The carbon dioxide (CO2) requirement was tested on Brucella serum dextrose agar with and without CO2 during the first isolation. Agglutination by A, M and R monospecific antisera was detected by mixing the antisera with the isolate after dilution of the colony. This process was completed at the Center for Disease Prevention and control (CDC) of China in Beijing.
Estimation of true prevalence
An animal was considered seropositive if it was positive on both the RBPT and c-ELISA, and a herd was considered positive if it contained at least one seropositive animal. Data were saved in Microsoft Excel and used for risk factors analysis and prevalence calculations.
The true prevalence for the study area was estimated using the software Epitools according to the method described by Rogan and Gladen [34]. True prevalence was estimated using the common sensitivity of RBPT and c-ELISA tests [35] and the specificity of c-ELISA (0.996) test. The common sensitivity was estimated as 0.981, which was the outcome of RBPT (0.986) [36] and c-ELISA (0.995) (Svanova Biotech, Uppsala, Sweden).
Statistical analysis
To analyze the risk factors, a preliminary analysis of the data (univariate) was conducted to select the variables with P ≤ 0.05 by Chi-square test or Fisher’s exact test. Subsequently, the P ≤ 0.05 of variables was analyzed by multivariable logistic regression [37]. The collinearity was verified between each of the independent variables by correlation analysis, and a correlation coefficient >0.9 indicated the variables with strong collinearity. Because of the problem of multicollinearity, one or two variables were excluded from the multiple analysis based on the biological plausibility [38]. Confounding data were evaluated by adding new variables and then monitoring the changes in the model parameters. Large changes (>20%) in the regression coefficients were considered indicative of confounding. The calculations were made using SPSS software 17.0.
Results
Distribution of seroprevalence of four abortion-related pathogens in three counties
A total of 2,996 serum samples (1406 dairy cows and 1590 sheep) were collected from X county (cow 354, sheep 351), H county (cow 342, sheep 361) and Q county (cow 710, sheep 878) and then identified by RBPT, c-ELISA and ELISA. The overall brucellosis positivity for cows and sheep in the study area was cow 6.76%, sheep 9.50%, which was the highest rate among the four abortion-related diseases in this study (Table 2). The brucellosis positivity for cows and sheep in X county was cow 7.06%, sheep 9.12%; in H county was cow 11.70%, sheep 10.80%; and in Q county was cow 4.22%, sheep 9.11%, which is much higher than the seroprevalence of other pathogens in these three counties (Table 2). However, our results suggest that the Mycoplasma infection is an additional threat to livestock reproduction. The overall Mycoplasma positivity for cows and sheep in the study area was cow 3.20%, sheep 6.42% (Table 2). The Mycoplasma positivity for cows and sheep in X county was cow 3.39%, sheep 7.98%; in H county was cow 5.26%, sheep 9.97%; and in Q county was cow 2.11%, sheep 4.33% (Table 2), and its abortion rate for cows and sheep was 26.60% (12/45, P = 0.003) and 30.40% (31/102, P<0.001) (Table 3). The salmonellosis and Chlamydia abortus seroprevalence for cows and sheep in these three counties are shown in Table 2.
Table 2. Seroprevalence of brucellosis, salmonellosis, Mycoplasma and Chlamydia abortus in three counties.
| Variables | X County | H County | Q County | Overall |
|---|---|---|---|---|
| Brucellosis positivity | ||||
| Cow | 25/354 (7.06%) | 40/342 (11.70%) | 30/710 (4.22%) | 95/1406 (6.76%) |
| Sheep | 32/351 (9.12%) | 39/361 (10.80%) | 80/878 (9.11%) | 151/1590 (9.50%) |
| Salmonellosis positivity | ||||
| Cow | 3/354 (0.85%) | 4/342 (1.17%) | 3/710 (0.42%) | 10/1406 (0.71%) |
| Sheep | 6/351 (1.71%) | 6/361 (1.66%) | 4/878 (0.46%) | 16/1590 (1.01%) |
| Mycoplasma positivity | ||||
| Cow | 12/354 (3.39%) | 18/342 (5.26%) | 15/710 (2.11%) | 45/1406 (3.20%) |
| Sheep | 28/351 (7.98%) | 36/361 (9.97%) | 38/878 (4.33%) | 102/1590 (6.42%) |
| Chlamydia abortus positivity | ||||
| Cow | 1/354 (0.28%) | 1/342 (0.29%) | 3/710 (0.42%) | 5/1406 (0.36%) |
| Sheep | 2/351 (0.57%) | 1/361 (0.28%) | 4/878 (0.46%) | 7/1590 (0.44%) |
Table 3. Univariable analysis of abortion-related factors of livestock in the Ili region.
| Variables | No. of livestock sampled | No. of livestock with abortion | Rate of abortion | P-value |
|---|---|---|---|---|
| County | ||||
| X | 705 | 106 | 15.03% | 0.245 |
| H | 703 | 86 | 12.20% | |
| Q | 1588 | 204 | 12.80% | |
| Type of farming | ||||
| Cow | 160 | 8 | 5.00% | 0.016* |
| Mixed (Cow) | 203 | 25 | 12.30% | |
| Sheep | 141 | 11 | 7.80% | 0.005* |
| Mixed (Sheep) | 272 | 49 | 18.00% | |
| Contact with other flock | ||||
| No (Cow) | 76 | 6 | 7.90% | 0.008* |
| Yes (Cow) | 284 | 60 | 21.10% | |
| No (Sheep) | 114 | 11 | 9.60% | 0.002* |
| Yes (Sheep) | 331 | 75 | 22.70% | |
| Brucellosis positivity | ||||
| No (Cow) | 1311 | 99 | 7.55% | <0.0001* |
| Yes (Cow) | 95 | 75 | 78.90% | |
| No (Sheep) | 1439 | 96 | 6.67% | <0.0001* |
| Yes (Sheep) | 151 | 126 | 83.44% | |
| Salmonellosis positivity | ||||
| No (Cow) | 1396 | 174 | 12.46% | 0.233 |
| Yes (Cow) | 10 | 0 | 0% | |
| No (Sheep) | 1574 | 222 | 14.10% | 0.105 |
| Yes (Sheep) | 16 | 0 | 0% | |
| Mycoplasma positivity | ||||
| No (Cow) | 1361 | 162 | 11.90% | 0.003* |
| Yes (Cow) | 45 | 12 | 26.60% | |
| No (Sheep) | 1488 | 191 | 12.83% | <0.001* |
| Yes (Sheep) | 102 | 31 | 30.40% | |
| Chlamydia abortus positivity | ||||
| No (Cow) | 1401 | 174 | 12.41% | 0.400 |
| Yes (Cow) | 5 | 0 | 0% | |
| No (Sheep) | 1583 | 222 | 14.02% | 0.285 |
| Yes (Sheep) | 7 | 0 | 0% |
* Variables selected and subjected to the multiple analysis (P<0.05)
Other livestock management factors involved in abortion
Univariable analysis of abortion-related risk factors (Table 3) found no significant differences among the studied counties (P = 0.245); the abortion rate in the three regions ranged from 12.20% to 15.03%. However, the management factors were significantly correlated with sheep or cow abortion including the type of farming (cow P = 0.016, sheep P = 0.005) and contact with other herds or flocks (cow P = 0.008, sheep P = 0.002). Among the four pathogens, Brucella was the main reason for cow or sheep abortion, and the abortion rate of cow or sheep brucellosis was 78.90% and 83.44% (P<0.0001) (Table 3). Mycoplasma infection also posed a threat to cow and sheep reproduction, and the abortion rates were, respectively 26.60% (P = 0.003) and 30.40% (P<0.001) (Table 3).
Brucellosis is the main factor responsible for cow and sheep abortion
The abortion-related risk factors analyzed through multivariable logistic regression showed that brucellosis was the biggest risk factor for livestock abortion (Table 4). Our results also showed the brucellosis positivity was significantly associated with cow (P<0.001) and sheep (P<0.001) abortion in the Ili region, and its abortion rates for cow and sheep, respectively, were 78.9% (75/95) and 83.44% (126/151) (Table 3). The Exp (B) values of brucellosis for cow and sheep, respectively, were 45.909 [95% CI 26.912–78.317, P<0.001] and 70.507 [95% CI 43.783–113.544, P<0.001] times higher than other abortion-related factors including mixed farming, contact with other flocks and Mycoplasma infection (Table 4).
Table 4. Abortion-related risk factors of livestock in the Ili region.
| Risk factors | Logistic regression coefficient | Standard error | Wald | Exp(B) | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Mixed farming | ||||||
| Cow | 0.982 | 0.421 | 5.437 | 2.669 | 1.169–6.090 | 0.02 |
| Sheep | 0.954 | 0.351 | 7.374 | 2.597 | 1.304–5.171 | 0.007 |
| Contact with other flock | ||||||
| Cow | 1.139 | 0.450 | 6.425 | 3.125 | 1.295–7.542 | 0.011 |
| Sheep | 1.009 | 0.343 | 8.641 | 2.743 | 1.400–5.376 | 0.003 |
| Brucellosis positivity | ||||||
| Cow | 3.827 | 0.273 | 197.192 | 45.909 | 26.912–78.317 | <0.001 |
| Sheep | 4.256 | 0.243 | 306.461 | 70.507 | 43.783–113.544 | <0.001 |
| Mycoplasma positivity | ||||||
| Cow | 0.990 | 0.347 | 8.125 | 2.691 | 1.362–5.316 | 0.004 |
| Sheep | 1.087 | 0.229 | 22.564 | 2.965 | 1.893–4.643 | <0.001 |
Exp(B) represent the Odds ratio.
Molecular detection
In the present study, all of the 75 aborted sheep fetuses, 66 aborted cow fetuses, 42 milk and 23 ewe milk samples were screened by PCR targeting the 16s rRNA gene. A total of 54 samples (22 aborted cow fetuses, 30 aborted sheep fetuses, 1 milk and 1 ewe’s milk) were positive and were further identified as B. melitensis by targeting the IS711 gene (data not shown). However, all of these samples were negative for Salmonella spp., Mycoplasma bovis and Chlamydia abortus identified by PCR. The nucleotide sequences from this study have been deposited in the GeneBank database (IS711: MK913893-MK913898).
Identification of isolates
A total of 38 (70.37%) Brucella isolates were isolated from 54 positive samples, including 20 aborted sheep fetuses, 16 aborted cow fetuses and 1 milk sample and 1 ewe’s milk sample (Table 5). All of the isolates were positive for 16S rRNA. The Brucella differentiation was performed by PCR utilizing primers specific to the IS711 gene of B. melitensis. B. melitensis-specific DNA fragments with 731 bp were amplified from all isolates, and no DNA was observed in negative control samples. Only part of the results is presented in S1 Fig. Furthermore, all of the isolates were identified as B. melitensis biovar 3 by biochemical testing. The growth of all the 6 isolates on a medium with thionin at 40 μg/mL (1:25000) concentration and basic fuchsin at all concentrations suggested that these isolates were B. melitensis biovar 3. Only part of the results is presented in Table 6. No Salmonella spp., Mycoplasma bovis and Chlamydia abortus were isolated from aborted fetuses and milk samples.
Table 5. Comparison of PCR and culture results from aborted cow, sheep fetuses and milk samples.
| No. of samples | Host | PCR results | Culture results |
|---|---|---|---|
| 1–16 fetuses | cow | + | + |
| 17–22 fetuses | cow | + | - |
| 1 milk | cow | + | + |
| 1–20 fetuses | sheep | + | + |
| 21–30 fetuses | sheep | + | - |
| 1 milk | sheep | + | + |
Table 6. Species and biovar differentiation of the Brucella isolates.
| Brucella isolates | Source | Growth characteristics | Monospecific sera | Phage typing | Interpretation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urea | H2S | CO2 | BF | TH | A | M | Tb | Wb | BK2 | Fi | Iz | R/C | B. melitensis biovar 3 | ||
| DXY1 | Fetal spleen | ++ | - | - | + | + | + | + | NL | NL | CL | NL | PL | NL | B. melitensis biovar 3 |
| DXY3 | Fetal liver | ++ | - | - | + | + | + | + | NL | NL | CL | NL | PL | NL | B. melitensis biovar 3 |
| DXY6 | Milk | ++ | - | - | + | + | + | + | NL | NL | CL | NL | PL | NL | B. melitensis biovar 3 |
| DXY8 | Ewe’s milk | ++ | - | - | + | + | + | + | NL | NL | CL | NL | PL | NL | B. melitensis biovar 3 |
| DXY5794 | Stomach content | ++ | - | - | + | + | + | + | NL | NL | CL | NL | PL | NL | B. melitensis biovar 3 |
| DXY1954 | Stomach content | ++ | - | - | + | + | + | + | NL | NL | CL | NL | PL | NL | B. melitensis biovar 3 |
BF: basic fuchsin at 20 μL/mL (1/50,000 w/v), TH: thionin at 20 μL/mL (1/50,000 w/v), CL: confluent lysis, PL: partial lysis, NL: no lysis.
Phylogenetic analysis
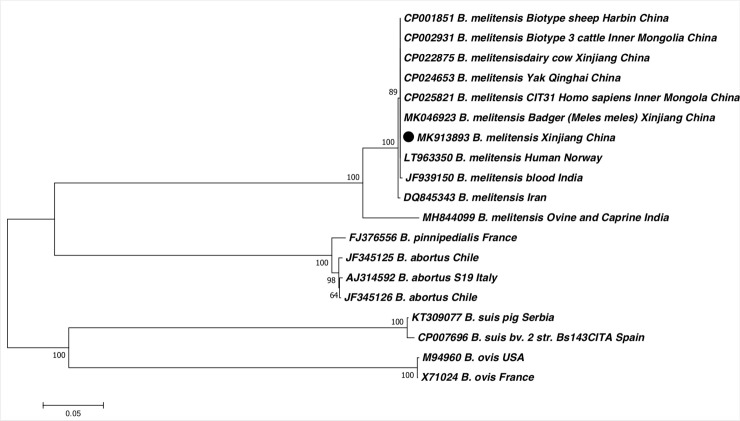
A phylogenetic tree was constructed based on the 731 bp sequence of the IS711 repetitive element for all isolates. After sequencing, we found that the IS711 gene sequences from all of these isolates showed 100% similarity (731/731bp). Phylogenetic analysis showed that the Brucella isolates closely matched those of B. melitensis biovar 3 isolated from cattle in Inner Mongolia, China. Isolates from Norway and India also showed 100% similarity to the isolates of the present study in clade 1 (Fig 1). The isolates of B. melitensis from other countries were placed into different clades based on low similarity to the Brucella isolates from this study (Fig 1).
Fig 1. Phylogenetic tree of the IS711 concatenated sequence of Brucella melitensis (⦁) isolated from aborted cow or sheep fetuses in this study and reference sequences from Brucella melitensis retrieved from the GenBank database.
The tree was constructed according to the neighbor-joining (NJ; 500 bootstrap replicates) and maximum–likelihood (ML, 1000 bootstrap replicates) analyses using MEGA7. The scale bar represents the inferred substitutions per nucleotide site.
Discussion
The livestock industry of the XUAR is a major source of its economic growth especially in some remote areas like Ili. However, there are few studies on the prevalence of brucellosis in this region. It has been reported that the brucellosis seroprevalence for cattle and sheep in Ili region was cattle 1.72%, sheep 1.95% in 2014 [39]. According to the data released from the Center for Animal Disease Control and Prevention of Ili in 2015, the brucellosis seroprevalence for cows and sheep were 6.91% and 4.21%. Although, the local control strategy was to vaccinate livestock for preventing brucellosis, animal brucellosis still occurred in an increasing number of cases in recent years. In the present study, we investigated the seroprevalence of abortion-related pathogens (Brucella, Salmonella, Mycoplasma and Chlamydia abortus) in three counties (X, H and Q). A total of 2996 cow and sheep serum samples were screened by RBPT, c-ELISA and ELISA. The resulting data showed that the brucellosis was widely prevalent in livestock in all of the studied counties. The seroprevalence for cows and sheep in X county was cow 7.06%, sheep 9.12%; in H county was cow 11.70%, sheep 10.80%; and in Q county was cow 4.22%, sheep 9.11% (Table 2). These data suggest that the disease is distributed within all of the Ili region and can potentially infect all of the susceptible livestock in this region. The results showed that Mycoplasma infection was also present and this can influence the livestock reproduction, although its seroprevalence was not as high as brucellosis. The seroprevalence for Mycoplasma infection in X county was cow 3.39%, sheep 7.98%; in H county was cow 5.26%, sheep 9.97%; and in Q county was cow 2.11%, sheep 4.33% (Table 2). The abortion rates of Mycoplasma positivity for cow and sheep were, respectively, 26.60% (12/45) and 30.40% (31/102) (Table 3). However, Wenhao Ni et al. [40], found that in 2018, the seroprevalence rates of Mycoplasma for Hazake sheep and Suffolk sheep were 22.2% and 8.3% in the Ili region. These data are similar to our results except that the higher seroprevalence in Hazake sheep may a breed-related difference.
Many reasons could induce abortion in pregnant animals include infectious factors and non-infectious factors, in which infectious factors include Brucella, Salmonella spp., Mycoplasma bovis, Chlamydia abortus, Listeria monocytogenes, bovine viral diarrhea virus (BVDV), Neospora enterica and T. gondii [10–12, 41, 42] and non-infectious factors involve heat stress, production stress, seasonal effect, chromosomal and single gene disorders [43–46]. We previously have found that the B. melitensis biovar 3 was the main cause of cow and sheep abortion in Nilka county (neighboring X county) in 2016 [47]. However, we could not rule out aborted fetuses caused by non-infectious factors, viral and parasitic agents, because we only examined bacterial agents in this study. In addition to the effects of pathogens on livestock abortion, we found that livestock abortion can also be influenced by livestock management systems including herd and flock size, mixed farming, grazing system and contact with other animals [48, 49]. We used univariable analysis to study management risk factors related to livestock abortion in the Ili region and found statistically significant links with the type of farming (cow P = 0.016, sheep P = 0.005) and contact with other herds/flocks (cow P = 0.008, sheep P = 0.002) (Table 3). This may be because these two management factors are easily overlooked by livestock owners.
Bacterial isolation is the gold standard for the diagnosis of brucellosis. We isolated a total of 38 B. melitensis biovar 3 isolates from 16 aborted cow fetuses, 20 aborted sheep fetuses and 1 milk and 1 ewe’s milk sample (Table 5). However, the 16 aborted fetuses that were positive for PCR but negative for culture probably occurred because contamination decreased the rate of Brucella isolation. Interestingly, all of the isolates from 16 aborted cow fetuses were identified as B. melitensis and 10 out of these 16 aborted cow fetuses were collected from mixed farming group. This finding is in agreement with previous report, 34 B. melitensis were isolated from cow aborted fetuses and milk in a farm in Ili region [47]. We also identified the Salmonella spp., Mycoplasma and Chlamydia abortus through PCR. But, no aborted fetuses were positive for these pathogens. These results show that B. melitensis biovar 3 is the dominant pathogen responsible for sheep and cow abortion.
RBPT and c-ELSA were combined to screen and diagnose brucellosis in China especially in some remote areas. The sensitivity and specificity of these two methods has been described previously [21, 50]. However, these two methods are not good tools for diagnosing brucellosis in the laboratory. We consider that the best way is bacterial isolation and identification. Molecular approaches appeared to be faster and more sensitive than traditional bacteriological tests [51, 52]. The 16S rRNA component of the 30S small subunit of prokaryotic ribosomes contains hyper-variable regions that provide species-specific signature sequences useful for bacterial identification. Therefore, the 16S rRNA gene can be used as the diagnostic target in the PCR for confirmatory identification of B. melitensis [53]. Several studies have demonstrated that the 16S rRNA can be used as a rapid tool for Brucella identification [53, 54]. In this study, we identified 38 Brucella isolates with PCR by targeting the 16S rRNA gene in the first round of screening and these were further identified as B. melitensis by the presence of the IS711 gene. The advantage of this method is that results can be obtained within one day as compared to seven days using traditional microbiological testing.
Brucellosis is principally an animal disease, but >500,000 human cases are reported each year globally [55]. Transmission to humans occurs primarily through contact with infected animals and consumption of contaminated food such as raw milk and its byproducts [56]. This study discovered B. melitensis biovar 3 isolates in raw milk and ewe’s milk. This result suggests that B. melitensis infection in cows and ewes is a public health issue in China. Infected cows and ewes, as disease reservoirs, can spread contaminated milk to the local human population. We recommend: i) increasing the regular quarantine of brucellosis and timely elimination of infected ewes or cows and their products and ii) implementing a vaccination program for livestock and iii) reducing mixed farming and avoiding contact with other herds/flocks and encouraging livestock owners to learn and adopt new management skills.
Conclusions
B. melitensis biovar 3 was identified as the main pathogen responsible for cow and sheep abortion. Mycoplasma infection, mixed farming and contact with other herds and flocks are strongly correlated with livestock abortion. An effective vaccination and control program is advocated for livestock owners in the Ili region to prevent the spread of brucellosis and Mycoplasma infection.
Supporting information
(XLSX)
(XLSX)
(TIF)
(XLSX)
(XLSX)
Acknowledgments
We would like to thank LetPub (www.letpub.com) for providing linguistic assistance during the preparation of this manuscript and Shengnan Song for helping construct the phylogenetic tree.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Granted No. U1803236, 31572491), National key research and development plan project in China (Granted No.2017YFD0500304).
References
- 1.Li Y-J, Li X-L, Liang S, Fang L-Q, Cao W-C. Epidemiological features and risk factors associated with the spatial and temporal distribution of human brucellosis in China. BMC infectious diseases. 2013;13(1):547 10.1186/1471-2334-13-547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Yin F, Zhang T, Yang C, Zhang X, Feng Z, et al. Spatial analysis on human brucellosis incidence in mainland China: 2004–2010. BMJ open. 2014;4(4):e004470 10.1136/bmjopen-2013-004470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flora HNB, Niba AT, Pougueu B, Dieudonné H, Nyuysemo IL, Joseph T. Factors affecting reproductive performance of crossbred dairy cattle at the Bambui regional centre of the institute of agricultural research for development Cameroon. 2019. [Google Scholar]
- 4.Mellado M, Valdéz R, García JE, López R, Rodríguez A. Factors affecting the reproductive performance of goats under intensive conditions in a hot arid environment. Small Ruminant Research. 63(1–2):110–118. [Google Scholar]
- 5.Benkirane A, Essamkaoui S, Idrissi AE, Lucchese L, Natale A. A sero-survey of major infectious causes of abortion in small ruminants in Morocco. Veterinaria Italiana. 2015;51(1):25–30. 10.12834/VetIt.389.1814.1 [DOI] [PubMed] [Google Scholar]
- 6.Ev Engelen, Luttikholt S, Peperkamp K, Vellema P, Brom RVd. Small ruminant abortions in The Netherlands during lambing season 2012–2013. Veterinary Record. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diallo A. Control of Peste des Petits Ruminants and Poverty Alleviation? J Vet Med B Infect Dis Vet Public Health. 2006;53(s1):11–13.16460350 [Google Scholar]
- 8.Kothalawala KACHA, Makita K, Kothalawalala H, Jiffry AM, Kono H. Association of farmers’ socio-economics with bovine brucellosis epidemiology in the dry zone of Sri Lanka. Preventive Veterinary Medicine. 2017;147:117 10.1016/j.prevetmed.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 9.Entrican G. Infectious Causes of Reproductive Failure in Livestock. 2009. [Google Scholar]
- 10.Dietz HH, Chriã©L M, Andersen TH, Jã¸Rgensen JC, Torpdahl M, Pedersen H, et al. Outbreak of Salmonella Dublin-associated abortion in Danish fur farms. Canadian Veterinary Journal-revue Veterinaire Canadienne. 2006;47(12):1201–1205. [PMC free article] [PubMed] [Google Scholar]
- 11.Hum S, Kessell A, Djordjevic S, Rheinberger R, Hornitzky M, Forbes W, et al. Mastitis, polyarthritis and abortion caused by Mycoplasma species bovine group 7 in dairy cattle. Australian Veterinary Journal. 2010;78(11):744–750. [DOI] [PubMed] [Google Scholar]
- 12.Navarro JA, Jn GDLF, Sánchez J, Martínez CM, Buendía AJ, Gutiérrez-Martín CB, et al. Kinetics of infection and effects on the placenta of Chlamydophila abortus in experimentally infected pregnant ewes. Veterinary Pathology. 2004;41(5):498 10.1354/vp.41-5-498 [DOI] [PubMed] [Google Scholar]
- 13.Holler LD. Ruminant Abortion Diagnostics. Veterinary Clinics of North America Food Animal Practice. 2012;28(3). 10.1016/j.cvfa.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 14.Arif S, Thomson PC, Hernandez-Jover M, Mcgill DM, Warriach HM, Heller J. Knowledge, attitudes and practices (KAP) relating to brucellosis in smallholder dairy farmers in two provinces in Pakistan. 2017;12(3):e0173365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CH, Xin-Cheng JI, Yang F, Wang ZB, Bayinchahan. Epidemiological Investigations of Infections with Neospora Caninum and Brucellosis in Abortion Dairy Cattle of Xinjiang Region. Xinjiang Agricultural Sciences. 2009:657–660. [Google Scholar]
- 16.Tian GZ, Cui BY, Piao DR, Zhao HY. Multi-locus variable-number tandem repeat analysis of ChineseBrucellastrains isolated from 1953 to 2013. Infectious Diseases of Poverty. 2017;6(1). 10.1186/s40249-017-0296-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jinping LI, Yan H, Yan J, Xiati D. Pathogenic investigation of brucellosis among farm animals in Aletai, Xinjiang Uyghur Autonomous Region, China. 2012. [Google Scholar]
- 18.Dohoo I, Martin S, Stryhn H. Veterinary Epidemiologic Research. Charlottetown: VER. Inc; 2009. [Google Scholar]
- 19.Matope G, Bhebhe E, Muma JB, Lund A, Skjerve E. Herd-level factors for Brucella seropositivity in cattle reared in smallholder dairy farms of Zimbabwe. Preventive Veterinary Medicine. 2010;94(3–4):213–221. 10.1016/j.prevetmed.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 20.Alton G, Maw J, Rogerson B, McPherson G. The serological diagnosis of bovine brucellosis: an evaluation of the complement fixation, serum agglutination and rose bengal tests. Australian Veterinary Journal. 1975;51(2):57–63. 10.1111/j.1751-0813.1975.tb09404.x [DOI] [PubMed] [Google Scholar]
- 21.Arif S, Heller J, Hernandez-Jover M, McGill DM, Thomson PC. Evaluation of three serological tests for diagnosis of bovine brucellosis in smallholder farms in Pakistan by estimating sensitivity and specificity using Bayesian latent class analysis. Preventive Veterinary Medicine. 2018;149:21–28. 10.1016/j.prevetmed.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 22.BuendíA AJ, Cuello F, Rio LD, Gallego MC, Caro MR, Salinas J. Field evaluation of a new commercially available ELISA based on a recombinant antigen for diagnosing Chlamydophila abortus (Chlamydia psittaci serotype 1) infection. Veterinary Microbiology. 2001;78(3):229–239. 10.1016/s0378-1135(00)00298-4 [DOI] [PubMed] [Google Scholar]
- 23.Wawegama NK, Browning GF, Kanci A, Marenda MS, Markham PF. Development of a recombinant protein-based enzyme-linked immunosorbent assay for diagnosis of Mycoplasma bovis infection in cattle. Clinical & Vaccine Immunology Cvi. 2014;21(2):196 10.1128/CVI.00670-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gharpure SJ, Prasath V. Development of an indirect ELISA for the detection of serum IgG antibodies against region IV of phase 1 flagellin of Salmonella enterica serovar Brandenburg in sheep. Journal of Medical Microbiology. 2009;58(12):1576–1581. [DOI] [PubMed] [Google Scholar]
- 25.Cremonesi P, Castiglioni B, Malferrari G, Biunno I, Vimercati C, Moroni P, et al. Improved method for rapid DNA extraction of mastitis pathogens directly from milk. Journal of Dairy Science. 2006;89(1):163–169. 10.3168/jds.S0022-0302(06)72080-X [DOI] [PubMed] [Google Scholar]
- 26.Langoni H, Chihara SM, Ilva AVD, Ardo RB, Onin FB, Endonça LJP, et al. Isolation of brucella spp from milk of brucellosis positive cows in São Paulo and Minas Gerais states. Brazilian Journal of Veterinary Research & Animal Science. 2000;37(6). [Google Scholar]
- 27.Naseri Z, Alikhani MY, Hashemi SH, Kamarehei F, Arabestani MR. Prevalence of the most common virulence-associated genes among Brucella Melitensis isolates from human blood cultures in Hamadan Province, West of Iran. Iranian journal of medical sciences. 2016;41(5):422 [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Cao X, Fu B, Chao Y, Cai J, Zhou J, editors. Identification and Characterization of\\r Chlamydia abortus\\r Isolates from Yaks in Qinghai, China 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sudda MM, Mtenga AB, Kusiluka LJ, Kassim N. Prevalence and Antibiotic Susceptibility of Escherichia coli and Salmonella spp. isolated from milk of zero grazed cows in Arusha City. African Journal of Microbiology Research. 2016;10(46):1944–1951. [Google Scholar]
- 30.Gioia G, Werner B, Nydam DV, Moroni P. Validation of a mycoplasma molecular diagnostic test and distribution of mycoplasma species in bovine milk among New York State dairy farms. Journal of Dairy Science. 2016;99(6):4668–4677. 10.3168/jds.2015-10724 [DOI] [PubMed] [Google Scholar]
- 31.Gee JE, De BK, Levett PN, Whitney AM, Novak RT, Popovic T. Use of 16S rRNA gene sequencing for rapid confirmatory identification of Brucella isolates. Journal of clinical microbiology. 2004;42(8):3649–3654. 10.1128/JCM.42.8.3649-3654.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology & Evolution. 2016;33(7):1870 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al SD, Tomaso H, Nöckler K, Neubauer H, Frangoulidis D. Laboratory-based diagnosis of brucellosis—a review of the literature. Part I: Techniques for direct detection and identification of Brucella spp. Clinical laboratory. 2003;49(9–10):487–505. [PubMed] [Google Scholar]
- 34.Rogan WJ, Gladen B. Estimating prevalence from the results of a screening test. American Journal of Epidemiology. 1978;107(1):71–6. 10.1093/oxfordjournals.aje.a112510 [DOI] [PubMed] [Google Scholar]
- 35.Matope G, Bhebhe E, Muma JB, Lund A, Skjerve E. Herd-level factors for Brucella seropositivity in cattle reared in smallholder dairy farms of Zimbabwe. Preventive Veterinary Medicine. 2010;94(3–4):213–221. 10.1016/j.prevetmed.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 36.Gorsich EE, Bengis RG, Ezenwa VO, Jolles AE. Evaluation of the sensitivity and specificity of an enzyme-linked immunosorbent assay for diagnosing brucellosis in african buffalo (Syncerus caffer). Journal of Wildlife Diseases. 2015;51(1):9–18. 10.7589/2013-12-334 [DOI] [PubMed] [Google Scholar]
- 37.Kardjadj M, Kouidri B, Metref D, Luka PD, Ben-Mahdi MH. Abortion and various associated risk factors in small ruminants in Algeria. Preventive Veterinary Medicine. 2016;123:97–101. 10.1016/j.prevetmed.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 38.Dohoo IR, Ducrot C, Fourichon C, Donald A, Hurnik D. An overview of techniques for dealing with large numbers of independent variables in epidemiologic studies. Preventive Veterinary Medicine. 1997;29(3):221–39. 10.1016/s0167-5877(96)01074-4 [DOI] [PubMed] [Google Scholar]
- 39.Ma X J JK, Shu Z, et al. Epidemiological investigation of animal Brucellosis in Xinjiang from 2012 to 2014. Animal Husbandry & Veterinary Medicine. 2016;48(5):111–114. [Google Scholar]
- 40.Wen-Hao NI, Ya-Nan Z, Yi-Yong L, Yan C, Lei C, Wen-Rong LI. Serological Investigation of Mycoplasma Pneumonia in Different Sheep Breeds in Xinjiang. Grass-Feeding Livestock. 2019. [Google Scholar]
- 41.Şevik M. The Role of Pestiviruses (BDV and BVDV) in Ruminant Abortion Cases in the Afyonkarahisar Province. 2018. [Google Scholar]
- 42.Masala G, Porcu R, Daga C, Denti S, Canu G, Patta C, et al. Detection of Pathogens in Ovine and Caprine Abortion Samples from Sardinia, Italy, by PCR. Journal of Veterinary Diagnostic Investigation Official Publication of the American Association of Veterinary Laboratory Diagnosticians Inc. 2007;19(1):96 10.1177/104063870701900116 [DOI] [PubMed] [Google Scholar]
- 43.Geoffrey HA, Noakes D.E., Pearson H., Parkinson T.J. Veterinary Reproduction & Obstetrics, sixth ed: Saunders Publishing; 1992. [Google Scholar]
- 44.Labèrnia J.,, and, López-Gatius F.,, and, et al. Influence of management factors on pregnancy attrition in dairy cattle. 1996. [DOI] [PubMed] [Google Scholar]
- 45.López-Gatius F, Santolaria P, Yániz J, Rutllant J, López-Béjar M. Factors affecting pregnancy loss from gestation Day 38 to 90 in lactating dairy cows from a single herd. Theriogenology. 2002;57(4):0–1261. 10.1016/s0093-691x(01)00715-4 [DOI] [PubMed] [Google Scholar]
- 46.Markusfeld-Nir O. Epidemiology of bovine abortions in Israeli dairy herds. Preventive Veterinary Medicine. 1997;31(3–4):245–255. 10.1016/s0167-5877(96)01142-7 [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Shengnan S, Benben W, Jiang Y, Wenxing W, Fei G, et al. Brucella melitensis Isolated from Aborted Cow and Sheep Fetuses in Northwest of China. Kafkas Üniversitesi Veteriner Fakültesi Dergisi. 2018;24(2). [Google Scholar]
- 48.Endrias Zewdu G, Abebe A, Tesfaye Sisay T, Getachew T, Girmay M, Maria V, et al. Some risk factors for reproductive failures and contribution of Toxoplasma gondii infection in sheep and goats of Central Ethiopia: A cross-sectional study. Research in Veterinary Science. 2013;95(3):894–900. 10.1016/j.rvsc.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 49.Mellado M, Valdéz R, García JE, López R, Rodríguez A. Factors affecting the reproductive performance of goats under intensive conditions in a hot arid environment. Small Ruminant Research. 2006;63(1–2):110–118. [Google Scholar]
- 50.Alton GG, Jones LM, Angus RD, Verger JM. Techniques for the brucellosis laboratory. 1988;13(6):420. [Google Scholar]
- 51.Casañas MC, Queipo-Ortuño MI, Rodriguez-Torres A, Orduña A, Colmenero JD, Morata P. Specificity of a Polymerase Chain Reaction Assay of a Target Sequence on the 31-Kilodalton Brucella Antigen DNA Used to Diagnose Human Brucellosis. European Journal of Clinical Microbiology & Infectious Diseases. 2001;20(2):127–131. 10.1007/pl00011242 [DOI] [PubMed] [Google Scholar]
- 52.Costa MD, Guillou JP, Garin‐Bastuji B, Thiébaud M, Dubray G. Specificity of six gene sequences for the detection of the genus Brucella by DNA amplification. Journal of Applied Bacteriology. 1996;81(3):267–275. 10.1111/j.1365-2672.1996.tb04328.x [DOI] [PubMed] [Google Scholar]
- 53.Singh A, Gupta VK, Kumar A, Singh VK, Nayakwadi S. 16S rRNA and Omp31 Gene Based Molecular Characterization of Field Strains of B. melitensis from Aborted Foetus of Goats in India. The Scientific World Journal. 2013;2013(1):1–7. 10.1155/2013/160376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barua A, Kumar A, Thavaselvam D, Mangalgi S, Prakash A, Tiwari S, et al. Isolation & characterization ofBrucella melitensisisolated from patients suspected for human brucellosis in India. Indian Journal of Medical Research. 2016;143(5):652–658. 10.4103/0971-5916.187115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: A re-emerging zoonosis. Veterinary Microbiology. 2010;140(3):392–398. 10.1016/j.vetmic.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention Section Editor: Rebecca Voelker. Brucella in Raw Milk Prompts Health Warning in Texas. Jama. 2017. 10.1001/jama.2017.15338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(TIF)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.