Abstract
Immature Sertoli cell (SC) proliferation determines the final number of mature SCs and further regulates spermatogenesis. Accumulating evidence demonstrated that microRNAs (miRNAs) play an important role in SC proliferation, differentiation, and apoptosis. However, the effect and molecular mechanism of miRNA on bovine immature SC remain to be poorly understood. In this study, miRNA sequencing of testes collected in mature (24-mo old) and immature (neonatal) bulls was conducted to determine the miRNA expression profiles. MicroRNA-34b was one of the differentially expressed miRNAs and was selected for in-depth functional studies pertaining to SC growth. The results showed that miR-34b mimic transfection in primary Sertoli cells (PSC) inhibited cell proliferation and induced cell cycle arrested at G2 phase and decreased the expression of cell cycle-related genes such as CCNB1, CDK1, CDC25C, and C-MYC. MicroRNA-34b overexpression also leads to increased cell apoptosis, with proapoptotic genes P53 and BAX upregulated, while antiapoptotic gene BCL2 decreased. However, miR-34b knockdown had the opposite effects. Through a combination of transcriptome sequencing, bioinformatics analysis, dual-luciferase reporter assay, and Western blotting, mitogen-activated protein kinase kinase1 (MAP2K1), also known as MEK1, was identified as a target of miR-34b. In addition, PSC proliferation inhibition was mediated by cell cycle arrest and apoptosis with MAP2K1 interference. Overexpression of MAP2K1 effectively reversed the miR-34b-repressed PSC cell growth. Moreover, both miR-34b overexpression and MAP2K1 knockdown decreased the protein levels of P-ERK1/2, while MAP2K1 overexpression showed opposite effects. In summary, data suggest that miR-34b regulates PSC proliferation and apoptosis through the MEK/ERK signaling pathway. These data provide a theoretical and experimental framework for further clarifying the regulation of cell growth in PSC of bovine.
Keywords: apoptosis, cell cycle, mitogen-activated protein kinase kinase1, microRNA-34b, primary Sertoli cells
Introduction
Sertoli cells (SC), the only somatic cell that directly intersected with various stages of spermatozoa, play a central role in the spermatogenesis process. SC regulate spermatogenesis primarily by providing germ cells with physiological and nutritional support (Kao et al., 2012; Aitken and Baker, 2013), secreting proliferation- and differentiation-related factors, and forming blood–testis barrier (Heninger et al., 2006). Due to the fixed capacity for SC to support germ cells, the number of SC in the adult testis will determine the number of germ cells that can be supported during spermatogenesis and hence will determine the daily sperm production (Orth et al., 1988). Moreover, it is worth noting that only immature SC can undergo proliferation, which means the proliferative activity of immature SC determines the final number of mature SC and determines the extent of sperm production (Sinowatz and Amselgruber, 1986; Sharpe et al., 2003; Rebourcet et al., 2017). Thus, an understanding of the molecular mechanisms behind the process of SC proliferation and apoptosis is fundamental for further research regarding spermatogenesis and improving male fecundity and reproductive potential.
MicroRNAs (miRNAs) are small noncoding RNAs of 19 to 25 nucleotides that regulate gene expression posttranscriptionally by base-pairing to the 3′-untranslated region (3′UTR) of target mRNAs to induce mRNAs decay or inhibit translation (Valencia-Sanchez et al., 2006), which is crucial for development and specific biological processes, such as cellular proliferation, differentiation, mobility, and apoptosis (O’Rourke et al., 2006; Song and Tuan, 2006). Many miRNAs have been reported to regulate the SC proliferation and apoptosis in diverse species. For example, miR-196a, miR-449, miR-1285, miR-762, and miR-10b have been implicated to promote porcine immature SC proliferation (Jiao et al., 2015; Ma et al., 2016; Weng et al., 2019; Zhang et al., 2019; Gao et al., 2020), while miR-362, miR-26a, miR-34c, miR-375, and miR-638 show the opposite effects (Hu et al., 2017; Guo et al., 2018; Ran et al., 2018, 2019, 2020). These studies suggest that miRNA may play an important role in testicular development or SC proliferation. However, their role and molecular mechanisms in bovine testes have not been fully understood.
MicroRNA-34b, which has been reported to predominantly express in murine testes, plays an important role in the maintenance of testicular structure and functions (Oghbaei et al., 2018, 2020). It is well known that miR-34b belongs to the miR-34 family, which consists of three miRNAs (miR-34a, miR-34b, and miR-34c). It has been reported that miR-34 miRNAs are abundant in the testis of several species and appear important in spermatogenesis (Bouhallier et al., 2010; Tscherner et al., 2014; Wu et al., 2014). In somatic cells, known as tumor-suppressive miRNA, miR-34 is an integral component of the P53 network, and the ectopic expression of miR-34 induces cell cycle arrest and apoptosis in both primary and tumor-derived cell lines (Corney et al., 2007; He et al., 2007; Feng et al., 2019). Interestingly, another conserved miRNA family, miR-449, which consists of miR-449a, miR-449b, and miR-449c, has been demonstrated to structurally resemble those of the miR-34 members. Coincidentally, miR-449a/b has been reported to inhibit positive cell cycle regulators such as CDK6 and CDC25A expression, leading to cell cycle arrest at the G1 phase (Yang et al., 2009), and the upregulation of miR-449 was also reported during myoblast and epithelial cell differentiation, which suggested a role of miR-449 in directing cell cycle exit and differentiation (Greco et al., 2009; Marcet et al., 2011). Furthermore, double KO mice (lacking both miR-449 cluster and miR-34b/c cluster) showed partial perinatal lethality and infertility (Comazzetto et al., 2014; Wu et al., 2014). All these results suggested that miR-34 plays a vital role in the regulation of cell proliferation, differentiation, and apoptosis. To date, the miR-34 family has not been examined for its role in bovine SC functions, especially the mechanism of miR-34b action on cell proliferation and apoptosis remains largely unknown.
Mitogen-activated protein kinase kinase1 (MAP2K1), also known as MEK1, is a threonine/tyrosine kinase that activates ERK1 and ERK2, through phosphorylation, constituting the MEK/ERK cascade reaction (Tidyman and Rauen, 2009). It has been reported that the MEK/ERK signaling pathway plays an essential role in regulating tumor cell survival and proliferation (Ni et al., 2020; Zhou et al., 2020). In addition, the MAP2K1 has been reported to be a target gene of miR-34b/c in the hippocampus and insulin-producing cells (An et al., 2014; Bai et al., 2017). Therefore, in the present study, we hypothesized that miR-34b may be involved in the regulation of cell proliferation and apoptosis via target MAP2K1 in bovine SC. The specific objectives of our study sought to overexpress or inhibit miR-34b to establish functional relationships with MAP2K1 and clarify the specific mechanism of miR-34b regulating SC proliferation and apoptosis.
Materials and Methods
Ethics statement
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the College of Veterinary Medicine of Northwest A&F University, Shaanxi, China. All animal procedures were performed under the control of the Guidelines for Animal Experiments by the Committee for the Ethics on Animal Care and Experiments of Northwest A&F University.
Sample collection and RNA isolation
Qinchuan bulls used in this research were from the National Beef Cattle Improvement Centre (Yangling, China). Three Qinchuan bulls of similar body weight were biopsied in 24-mo old and neonatal, respectively. Surgical methods were used to collect the testes and washed with diethyl pyrocarbonate-treated water. Then, the samples were kept on ice and processed immediately for RNA extraction. Testicular tissue was suspended in RNA-later (Ambion Inc, Austin, TX), cut into small pieces, and immediately put in liquid nitrogen for the next extraction of RNA. RNA extraction is following the Trizol protocol (Invitrogen, Carlsbad, CA).
MicroRNA sequencing analysis
Small RNA library construction, sequencing, and miRNA prediction
Sequencing libraries were generated using NEBNext Multiplex Small RNA Library Prep Set for Illumina (NEB, USA) following the manufacturer’s recommendations. Six small RNA libraries, MB1, MB2, MB3, IB1, IB2, and IB3, were constructed and sequenced using Illumina Hiseq 2500 by Novogene Bioinformatics Technology Co. Ltd. (Beijing, China). After poor-quality reads were cleaned, clean reads were obtained from the total reads of MB1, MB2, MB3, IB1, IB2, and IB3, respectively (Table 1). After that, a certain range of length (18 to 40 nt) from clean reads was mapped to Bos taurus sequence by Bowtie without mismatch to analyze their expression (Langmead et al., 2009), and the length distribution analysis was also made, as shown in Supplementary Figure S1. Then, the mapped clean reads were used to search against miRbase 20.0 for miRNA prediction. The unannotated sequences were further analyzed through integrating the available software miREvo and mirdeep2 for novel miRNA prediction based on the characteristic of the hairpin structure of miRNA precursor (Friedlaender et al., 2012; Wen et al., 2012).
Table 1.
The summary information of sRNA-seq reads
| Sample | Raw reads | Bases | Error rate, % | Q20, % | Q30, % | Clean reads |
|---|---|---|---|---|---|---|
| MB1 | 12,035,650 | 0.602G | 0.01 | 98.21 | 96.50 | 11,808,021 (98.11%) |
| MB2 | 8,443,342 | 0.422G | 0.01 | 97.83 | 95.76 | 8,283,906 (98.11%) |
| MB3 | 9,640,521 | 0.482G | 0.01 | 97.93 | 95.95 | 9,481,798 (98.35%) |
| IB1 | 7,998,693 | 0.400G | 0.01 | 98.00 | 96.06 | 7,784,848 (97.33%) |
| IB2 | 9,839,059 | 0.492G | 0.01 | 98.17 | 96.39 | 9,683,438 (98.42%) |
| IB3 | 10,511,489 | 0.526G | 0.01 | 98.16 | 96.38 | 10,360,992 (98.57%) |
Differential analysis of miRNA
The differential expression analysis of mature bull (MB) and immature bull (IB) groups was performed using the DESeq R package (1.8.3). The P-values were adjusted using the Benjamini and Hochberg method. The corrected P-value of 0.05 was set as the threshold for significantly differential expression by default.
Primary SC isolation, culture, and identification
Primary Sertoli cells (PSC) were isolated from the testes of neonatal Qinchuan bulls (n = 3) and cultured as previously described (Yang et al., 2014; Tang et al., 2016). Briefly, the testes were decapsulated and digested in 1.0 mg/mL collagenase IV/DNase solution (Sigma, USA), and incubated at 37 °C in a humid environment containing 5% CO2 until the tubules were separated (about 45 min). After a washing step with PBS, the tubules were further digested with 2.5 mg/mL trypsin for 15 to 20 min at 37 °C. Then, the mixture was filtered using a 120-μm nylon cell strainer and washed with PBS. After decantation of the enzyme solution by centrifugation at 200 × g for 10 min, the cell pellet obtained was resuspended in DMEM/F12 (DF12, Gibco, Carlsbad, CA, USA) containing 15% fetal bovine serum (FBS, Gibco) and 1 × antibiotic-antimycotic (AA, Invitrogen, Carlsbad, CA, USA), and subsequently seeded onto plates pre-coated with 1% gelatin. Floating cells were slowly and gently aspirated after 2 to 4 h culture, and fresh medium was added for culture at 37 °C in a humid environment containing 5% CO2. The purity (>95%) of the PSC was identified through immunofluorescence by using the SC marker cytokeratin-18 (KRT-18) (Proteintech Group, USA, 1:300) (Supplementary Figure S2).
Cell transfection
For miRNA transfection, cells were treated with miR-34b mimic (50 nM), miR-34b inhibitor (200 nM), and the respective negative controls (50 and 200 nM) from RiboBio (RiboBio Co., Ltd., Guangzhou, China). The sequence for miR-34b mimic and inhibitor was as follows: miR-34b mimic, sense 5′-AGGCAGUGUAAUUAGCUGAUUG-3′ and antisense 5′-CAATCAGCTAATTACACTGCCT-3′; and miR-34b inhibitor, 5′-CAAUCAGC UAAUUACACUGCCU-3′. To clarify the specific mechanism of miR-34b regulating cell cycle and apoptosis in PSC, we confirmed MAP2K1 as the downstream target gene of miR-34b via TargetScan (http://www.targetscan.org) and then performed the second cell experiment in PSC transfected with si-MAP2K1 (50 nM) and its negative control siRNA (si-NC; 50 nM) from RiboBio (RiboBio Co., Ltd.) to verify the role of miR-34b-MAP2K1 axis that regulates cell cycle and apoptosis in PSC. The sequence with the maximum knockdown efficiency for si-MAP2K1 and si-NC was as follows: si-MAP2K1, sense 5′-GCCTAATCTCT TACTCTAA-3′ and antisense 5′-TTAGAGTAAGAGATTAGGC-3′; and control siRNA, sense 5′-GCATGTGGGTCTGGGTATT-3′ and antisense 5′-AATACCCAGACCCACATGC-3′.
Briefly, 4 × 105 PSC were plated in 35-mm dishes and cultured with 2 mL of DF12 supplemented with 10% FBS and 1 × AA for 24 h. Then, the medium was removed, and the small RNA and siRNA as well as their respective controls diluted in Opti-MEM were transfected into PSC using Lipofectamine RNAiMAX Reagent [Thermo Fisher Scientific (China) Co., Ltd., Shanghai, China] according to the manufacturer’s instructions. Cells were collected for analysis 48 h after transfection.
Construction of plasmid pcDNA3.1–MAP2K1 and MAP2K1 overexpression in PSC
The target CDS fragment of MAP2K1 was amplified by the PrimerSTAR HS DNA Polymerase (TaKaRa) using the cDNA generated from PSC with the following primers: forward primer 5′- CGCGGATCCatgcccaagaagaagccgac-3′ and reverse primer 5′-CCGCTCGAGttag acgccagccgcatggg-3′ (The Capital letters display BamH I and Hind III restriction enzyme sites, respectively). The PCR product was examined via electrophoresis using a 1% agarose gel. Then, the target fragment was purified using the Universal DNA Purification Kit (Tiangen Biotech, Beijing, China). Subsequently, the target fragment of MAP2K1 and pcDNA3.1(+) (Invitrogen, Carlsbad, CA, USA) was digested, respectively, by restriction enzymes of BamH I and Hind III. The enzyme-digested fragments were purified and ligated together using Solution I (TaKaRa). The ligation mixture was transferred into Escherichia coli DH5α. The constructed pcDNA3.1(+) plasmids containing the target fragment of MAP2K1 were extracted from positive clones and confirmed by sequencing and enzymatic digestion with the BamH I and Hind III restriction enzymes (Supplementary Figure S3).
For MAP2K1 overexpression, 2 µg of pcDNA3.1(+) empty vector or recombinant plasmid pcDNA3.1(+)–MAP2K1 combined with mimic negative control (NC; 50 nM) or miR-34b mimic (50 nM) was transfected into PSC using Lipofectamine 2000 reagent (Invitrogen, Waltham, MA). Cells were collected for analysis 48 h after transfection.
Cell proliferation assay
The PSC were plated in 96-well plates at a density of 1 × 104 per well and cultured for 24 h. Small RNA, siRNA, and constructed plasmid transfections were conducted as aforementioned. The effect of small RNA and siRNA on the proliferation of PSC was assessed every 12 h for 60 h using a Cell Counting Kit-8 (CCK-8; Beyotime, Shanghai, China). Specifically, 10 μL of CCK-8 was added to each well in each group at the designated times, and the wells were incubated for 1 h at 37 °C. The absorbance at 450 nm was measured using a microplate reader (Model 680, Bio-Rad, Hercules, CA, USA). All experiments were performed in triplicate.
Cell apoptosis assay
The PSC were plated into six-well plates at a density of 4 × 105 per well and incubated for 24 h. Before transfection with small RNA, siRNA, and constructed plasmid, all wells were washed to remove the unattached cells. Then, 48 h later, cells were gently digested with 0.5% trypsin (EDTA-free) and washed with PBS twice, and cells were centrifuged at 500 × g for 5 min after each wash. Apoptosis was assessed using an Annexin V-FITC/PI Apoptosis Detection Kit (Keygen Biotech, Nanjing, China) according to the manufacturer’s instructions. Briefly, the pelleted cells were resuspended in 500 µL of binding buffer, and 5 µL of Annexin V-FITC and Propidium Iodide was added, respectively, and mixed. Cells were then incubated at 25 °C for 10 min in the dark. Finally, the samples were subjected to flow cytometry (FACSAriaTM III Cell Sorter, BD, San Jose, CA USA). All data were analyzed using Flow.joX.10.0.7 software (Treestar, USA).
Cell cycle assay
The PSC were plated into six-well plates at a density of 4 ×105 per well and incubated for 24 h. Before transfection with small RNA, siRNA, and constructed plasmid, all wells were washed to remove the unattached cells. Then, 48 h later, cells were digested with 0.25% trypsin (Beyotime) and washed with PBS twice, and cells were centrifuged at 500 × g for 5 min after each wash. Following this, the pelleted cells were resuspended in 500 µL of DAPI (Beyotime). After incubating at 25 °C for 15 min in the dark, the samples were subjected to flow cytometry (FACSAriaTM III Cell Sorter, BD). All data were analyzed using modfit software.
RNA extraction and quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (TaKaRa, Dalian, China). The quality of RNA was verified using a NanoDrop 1000 [Thermo Scientific (China) Co., Ltd.]; the 260:280 nm optical density value was between 1.8 and 2.0. For mRNA expression analysis, the RNA samples were treated with RNase-free DNase (TianGen, Beijing, China), and cDNA was synthesized using the PrimeScript RT Reagent Kit (TaKaRa). For miRNA expression analysis, cDNA was synthesized using a miRNA 1st Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd., Nanjing, China). The gene GAPDH was used as an endogenous control for mRNA expression analysis, and the gene U6 was used as the endogenous control gene for miRNA expression analysis (Li et al., 2014). The primer sets used for amplification of mRNA and miRNA were listed in Table 2 and Table 3, respectively. qPCR reactions were performed using the THUNDERBIRD SYBR qPCR Mix (TOYOBO, Osaka, Japan) and a miRNA Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd.) on a CFX96 qPCR system (Bio-Rad, Hercules, CA, USA). The 2−∆∆Ct method was used for the quantitative analysis of relative gene expression levels.
Table 2.
Primers for miRNA qPCR
| Genes | Primer sequence |
|---|---|
| U6 | F: CGCTTCACGAATTTGCGTGTCAT |
| R:GCTTCGGCAGCACATATACTAAAAT | |
| miR-34b | F: GGGGAGGCAGTGTAATTAGC |
Table 3.
Primer sequences used for qPCR
| Genes | GeneBank accession no. | Sequence 5′–3′ | Amplicon, bp |
|---|---|---|---|
| CCNB1 | NM_001045872.1 | F: ACACCTACACCAAGTTTCAAATCA R: ATCGTAGTCCAGCATAGTTAGTTCC |
182 |
| CDK1 | NM_174016.2 | F: GGGTCAGCTGGCTACTCAAC R: AGTGCCCAAAGCTCTGAAAA |
138 |
| BCL2 | NM_001166486.1 | F: TGGAGGAGCTCTTCAGGGAC R: GTACTCGGTCATCCACAGGG |
140 |
| BAX | NM_011066.3 | F: GCGCATCGGAGATGAATTGG R: TAGAAAAGGGCGACAACCCG |
153 |
| P53 | NM_174201.2 | F: GAGCACTGCCTACCAACA R: CATCCAGAGCATCCTTCA |
150 |
| CDC25C | NM_001097996.2 | F: TCAGATGCTGGAGGAAGATTCA R: CCCTGGAACTCCCCTGATAGT |
150 |
| MAP2K1 | NM_001130752.1 | F: AGCCGTCCGGACTTGTTATG R: GCCCACGATATAAGGCGAGT |
122 |
| C-MYC | NM_001046273.2 | F: TCTCCTCTGCTGAGTCCTCC R: TTCTGACCTTTTGGCAGGGG |
164 |
| GAPDH | NM_007475.5 | F: GAGATCCTGCCAACATCAA R:CTTCTCCATGGTAGTGAAGAC | 83 |
Western blotting
After 48 h of transfection, cellular total protein was extracted with cell lysis buffer and then mixed with protein loading buffer and denatured at 100 °C for 10 min. Equal amounts of total cellular protein were resolved on 12% SDS-PAGE gels and then electrically transferred onto PVDF membranes (EMD Millipore, Billerica, MA, USA). After blocking with skim milk (BD, NJ, USA) for 1 h in Tris-buffered saline (TBST) containing 0.5% Tween 20 at 25 °C, the membranes were incubated with primary antibodies diluted in TBST overnight at 4 °C, and then with horseradish peroxidase (HRP)-conjugated secondary antibodies diluted in TBST at 25 °C for 1 h. Finally, the membranes were washed three times in TBST for 10 min. Peroxidase activity was detected using the ECL Super Sensitive Solution Kit (Advansta, Menlo Park, CA, USA). The immunoreactive bands were visualized using a Gel Image System (GBox-Chemi-XRQ, Syngene, Cambridge, UK) and digitized with Quantity One software (Bio-Rad Laboratories). The primary antibodies used were: rabbit anti-CCNB1 (Beyotime, 1:1,000), rabbit anti-CDK1 (Beyotime, 1:1,000), rabbit anti-MEK1 (Beyotime, 1:1,000), rabbit anti-BCL2 (Proteintech Group, 1:1,000), rabbit anti-BAX (BBI Life Science, Shanghai, China, 1:2,000), rabbit anti-P-ERK1/2 (Beyotime, 1:1,000), rabbit anti-ERK1/2 (Beyotime, 1:1,000), and mouse anti-β-actin (Sanjian Biotech, Tianjin, China; 1:2,000). The secondary antibodies used were: HRP-conjugated goat anti-rabbit (Zhongshan Golden Bridge Biotechnology, Nanjing, China; 1:2,000) and HRP-conjugated goat anti-mouse (Zhongshan Golden Bridge Biotechnology, Nanjing, China; 1:2,000). All immunogens used to generate primary antibodies were from human, mouse, and rat recombinant proteins, except anti-CCNB1 and anti-BAX, which were from human recombinant proteins.
Transcriptome sequencing data analysis
Raw reads were acquired from Illumina sequencing with the poor-quality reads cleaned. The clean reads were mapped to the reference genome of B. taurus (version UMD 3.1.1) via Tophat2 software (version 2.1.0; Kim et al., 2013). Only reads that matched perfectly or had one single mispairing were further analyzed and annotated on the basis of reference genome. Volcano plots comparing log10 (statistical relevance) to log2 (fold change) were generated using R (version 3.1.1, AT&T Bell Laboratories, New York, NY, USA), using the base plotting system and calibrate library. Kyoto encyclopedia of genes and genomes (KEGG) analyses were analyzed via Novomagic (Novogene Technology Co., LTD, Beijing, China), and pathway enrichment analyses were performed to categorize the considerably enriched functional classification or metabolic pathways in which differentially expressed genes (DEGs) operated.
MicroRNA target validation using luciferase reporter assay
The biological target of the miRNA was predicted by scanning the seed region between the bta-miR-34b seed region and the predicted gene on TargetScan (http://www.targetscan.org). The mature sequence of bta-miR-34b was obtained from the miRBase Sequence Database (http://www.mirbase.org). The sequence and the 3′UTR mRNA of MAP2K1 from B. taurus were aligned via the University of California-Santa Cruz Genome Bioinformatics program (http://genome.ucsc.edu). The MAP2K1-3′UTR wild-type (WT) and mutation type (MUT) were cloned into the psiCHECK-2 Vector (Promega, Madison, WI) using the Xhol I and Not I restriction enzyme sites. The 293T cells were cultured in 96-well plates and transfected when cells were grown to 70% confluence. Approximately, 0.16 μg of WT MAP2K1-3′UTR or MUT MAP2K1-3′UTR vector was cotransfected with 5 pmol miR-34b mimics or negative control into 293T cells (human embryonic kidney cell line) using Lipofectamine 2000 reagent. After 48 h of transfection, luciferase activity was measured using the luciferase reporter assay system (Promega) according to the manufacturer’s protocol. Renilla luciferase activity was normalized to the activity of firefly luciferase.
Data analysis and statistics
Data are expressed as the means ± S.E. Statistical significance was determined using Student’s t-test when two groups were compared. The ANOVA was performed using GraphPad Prism version 6.01 (GraphPad Software Inc., San Diego, CA). Differences were considered significant at P < 0.05. Each experiment was repeated at least three times.
Results
MicroRNA-34b differentially expressed during the development of testes
Identification of conserved miRNA
Using Bowtie, 6,277,457 (54.79%), 5,187,053 (63.43%), 5,246,050 (55.64%), 4,942,567 (63.96%), 5,378,027 (56.77%), and 6,562,773 (64.35%) clean reads after length selection (18 to 40 nt) were mapped to B. taurus genome. Then, the mapped reads from MB1, MB2, MB3, IB1, IB2, and IB3 were aligned with miRbase 20.0, and 488 conserved mature miRNAs were identified in B. taurus. Following the identification of conserved miRNA, with regard to the characteristics of the hairpin structure of miRNA precursor, software such as miREvo and mirdeep2 were integrated to predict novel miRNA (Friedlaender et al., 2012; Wen et al., 2012). Consequently, 26 putative novel miRNAs were identified.
MicroRNA differential expression profiles
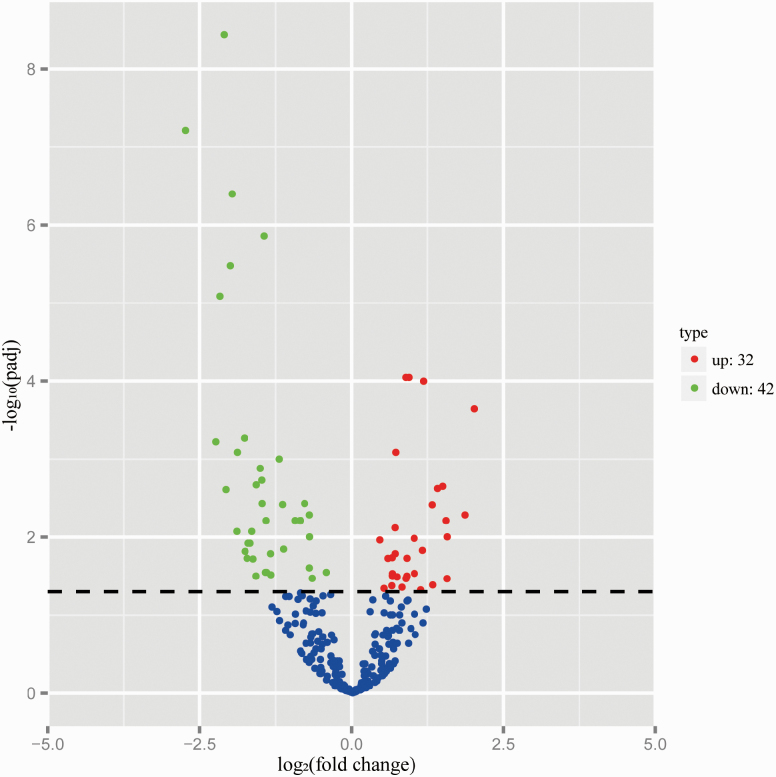
By combining the conserved and novel miRNA, a total of 514 miRNAs were identified. The subsequent differential expression revealed 74 significantly differentially expressed miRNAs in MB vs. IB group (P < 0.05), including 32 upregulated miRNAs and 42 downregulated miRNAs (Figure 1, Supplementary Table S1). Of which, miR-34b was highly expressed in MB groups (P < 0.01).
Figure 1.
Screening for miRNAs with differential expression in the MB and IB groups (n = 3 each). Among 514 miRNAs, 32 were upregulated, while 42 were downregulated. Red: upregulated; green: downregulated; blue: not significantly changed.
Transfection efficiency of miRNA and siRNA
Compared with mimic NC groups, the expression level of miR-34b was upregulated in miR-34b mimic-treated PSC (P < 0.01). In contrast, the expression of miR-34b was downregulated in miR-34b inhibitor-treated groups (P < 0.01). This indicated that miR-34b mimic and inhibitor had a high transfection efficiency and were suitable for further experiments (Figure 2A and B).
Figure 2.
Transfection efficiency of miR-34b mimic, miR-34b inhibitor, and si-MAP2K1. (A, B) PSC were transfected with miR-34b mimic (50 nM) or miR-34b inhibitor (200 nM) for 48 h; the miR-34b expression levels were quantified by qPCR, and the levels corrected relative to the levels of housekeeping gene U6. (C, D) PSC were transfected with NC (50 nM) or si-MAP2K1 (50 nM) for 48 h, and the MAP2K1 expression levels were quantified by qPCR and Western blot. Values are presented as means ± S.E. of three independent experiments; **P < 0.01.
After transfection of si-MAP2K1 for 48 h, total RNA and protein were extracted. qPCR results showed that the mRNA expression of MAP2K1 was downregulated by more than 70% in si-MAP2K1-transfected cells (P < 0.01). The MAP2K1 protein levels were also downregulated (P < 0.01). These data indicated that the designed siRNA could effectively inhibit the expression of MAP2K1 and could be used in subsequent experiments (Figure 2C and D).
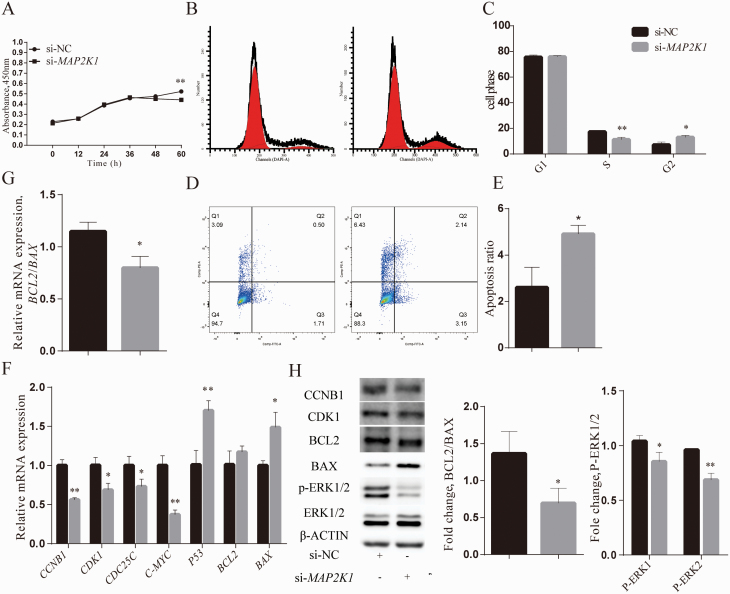
MicroRNA-34b suppresses proliferation and induces cell cycle arrest and apoptosis in PSC
Previous studies have shown that miRNAs play significant roles in cell proliferation, differentiation, and apoptosis. However, the physiological role of miR-34b in bovine PSC has been barely elucidated. To investigate the effect of miR-34b in PSC proliferation and apoptosis, miR-34b mimic and mimic NC were transfected into PSC. As shown in Figure 3A, the cell viability of PSC was attenuated after miR-34b overexpression compared with mimic NC groups (P < 0.01). Furthermore, flow cytometry analysis showed that the number of cells in the G2 phase was obviously increased with the transfection of miR-34b mimic (P < 0.01; Figure 3B and C). The apoptosis ratio of PSC was also elevated compared with the mimic NC group (P < 0.05) (Figure 3D and E). In accordance with these results, the mRNA expression levels of cell cycle-related genes, CCNB1 (P < 0.01), CDK1 (P < 0.01), CDC25C (P < 0.05), and C-MYC (P < 0.01), were markedly downregulated under miR-34b mimic transfection (Figure 3F), and the protein levels of CCNB1 and CDK1 were also decreased (Figure 3H). In contrast, the ratio between antiapoptotic gene BCL2 and proapoptotic gene BAX was decreased at both mRNA (P < 0.05) and protein (P < 0.01) levels in miR-34b mimic groups, and the mRNA level of P53 was also elevated compared with mimic NC group (P < 0.05; Figure 3F–H). Taken together, these results demonstrated that miR-34b inhibited the proliferation of PSC and promoted its apoptosis.
Figure 3.
miR-34b mimic transfection suppresses proliferation and induces cell cycle arrest and apoptosis in PSC. (A) CCK-8 assay detected the effect of miR-34b on PSC viability after PSC were transfected with mimic NC (50 nM) and miR-34b mimic (50 nM). (B, C) Cell cycle distribution was determined by flow cytometry assay after PSC were transfected with mimic NC and miR-34b mimic. (D, E) The percentage of apoptotic PSC was determined by flow cytometry. (F, G) The relative mRNA expression of the proliferation-related and apoptosis-related genes was quantified by qPCR, and the levels corrected relative to the levels of housekeeping gene GAPDH. (H) CCNB1, CDK1, BCL2, and BAX protein levels were analyzed by Western blot. Values are presented as means ± S.E. of three independent experiments; *P < 0.05, **P < 0.01.
Inhibition of miR-34b promotes proliferation and alleviates cell cycle arrest and apoptosis in PSC
Contrasted with the results observed in miR-34b mimic-transfected cells, transfection with miR-34b inhibitor promoted the proliferation of PSC compared with the inhibitor NC group (P < 0.01; Figure 4A). Flow cytometry analysis showed that the number of cells in the G2 phase was slightly but significantly decreased (P < 0.05; Figure 4B and C). Furthermore, the apoptosis ratio was modestly declined in miR-34b inhibitor-transfected cells (Figure 4D and E). Consistent with these results, the expression of cell cycle-related genes such as C-MYC (P < 0.05) and CDK1 (P < 0.01) was elevated at the mRNA level, and the expression ratio between antiapoptotic gene BCL2 and proapoptotic gene BAX was also upregulated at both mRNA (P < 0.01) and protein levels (Figure 4F–H).
Figure 4.
The inhibition of miR-34b promotes proliferation and alleviates cell cycle arrest and apoptosis in PSC. (A) CCK-8 assay detected the effect of miR-34b on PSC viability after PSC were transfected with inhibitor NC (200 nM) and miR-34b inhibitor (200 nM). (B, C) Cell cycle distribution was determined by flow cytometry assay after PSC were transfected with inhibitor NC and miR-34b inhibitor. (D, E) The percentage of apoptotic PSC was determined by flow cytometry. (F, G) The relative mRNA expression of the proliferation-related and apoptosis-related genes was quantified by qPCR, and the levels corrected relative to the levels of housekeeping gene GAPDH. (H) CCNB1, CDK1, BCL2, and BAX protein levels were analyzed by Western blot. Values are presented as means ± S.E. of three independent experiments; *P < 0.05, **P < 0.01.
Profile the miR-34b downstream genes by microarray and bioinformatics analysis
To screen the possible downstream genes regulated by miR-34b at mRNA levels in PSC, the genome-wide mRNA expression of PSC with or without miR-34b overexpression was performed by mRNA sequencing. A total of 318 genes were altered, and 193 genes were increased in miR-34b mimic-transfected cells, whereas 125 genes were decreased (P < 0.05; Figure 5A). Combined with the results predicted using TargetScan (http://www.targetscan.org), there were 22 downregulated genes and 4 upregulated genes intersected (P < 0.05; Table 4). Moreover, the pathway analysis based on the KEGG pathway database was manipulated. The KEGG enrichment analysis revealed that the VEGF signaling pathway, the MAPK signaling pathway, and the estrogen signaling pathway were all highly enriched (Figure 5B). Considering the gene function associated with cell fate control, MAP2K1 was selected for further analyses.
Figure 5.
DEGs between miR-34b mimic and mimic NC-transfected PSC. (A) Volcano plot of transcriptome in the PSC transfected with miR-34b mimic and mimic NC. (B) Top 20 signaling pathway items enriched in the significantly changed genes (n = 3). Size of the circle means the number of DEGs enriched.
Table 4.
The overlapped genes between bta-miR-34b predicted and differentially expressed in transcriptome sequencing
| Gene ID | Gene symbol | Mimic, read count | NC, read count | Log2, fold change | Corrected P-value |
|---|---|---|---|---|---|
| ENSBTAG00000018474 | IL6R | 67.97365 | 220.073 | −1.6946 | 2.98E-08 |
| ENSBTAG00000044048 | LMBR1L | 95.90157 | 243.6832 | −1.34653 | 0.000305 |
| ENSBTAG00000018898 | ARID4A | 258.2767 | 477.1153 | −0.88514 | 0.002151 |
| ENSBTAG00000019376 | FNDC3B | 2,155.856 | 3,401.804 | −0.65801 | 0.002431 |
| ENSBTAG00000021602 | CTTNBP2NL | 105.9182 | 224.1069 | −1.08126 | 0.003283 |
| ENSBTAG00000007335 | TPD52 | 372.1045 | 614.9744 | −0.72489 | 0.003572 |
| ENSBTAG00000021879 | VCL | 1,082.724 | 2,172.82 | −1.00481 | 0.007371 |
| ENSBTAG00000001754 | AHCYL2 | 280.7152 | 450.4876 | −0.68234 | 0.008565 |
| ENSBTAG00000033983 | MAP2K1 | 898.4603 | 1,542.265 | −0.77944 | 0.00948 |
| ENSBTAG00000032247 | PPP1R11 | 510.0173 | 848.2885 | −0.73428 | 0.01063 |
| ENSBTAG00000021245 | SPRY1 | 636.7399 | 1,122.49 | −0.81773 | 0.027961 |
| ENSBTAG00000018159 | CASP2 | 159.3814 | 284.9409 | −0.83827 | 0.027961 |
| ENSBTAG00000012789 | PRKD1 | 150.4904 | 253.3047 | −0.75128 | 0.030262 |
| ENSBTAG00000010843 | PGRMC2 | 1,872.271 | 2,628.574 | −0.4895 | 0.032101 |
| ENSBTAG00000048033 | SMIM15 | 26.98919 | 72.94197 | −1.43193 | 0.036746 |
| ENSBTAG00000021452 | TRANK1 | 790.7146 | 1,284.502 | −0.70003 | 0.037679 |
| ENSBTAG00000013678 | OSGIN2 | 185.4699 | 312.1997 | −0.75122 | 0.042925 |
| ENSBTAG00000002315 | RNF34 | 144.531 | 239.9375 | −0.73106 | 0.044131 |
| ENSBTAG00000018362 | TMEM109 | 1,231.691 | 2,265.342 | −0.87903 | 0.046013 |
| ENSBTAG00000010452 | PODXL | 85.80566 | 218.9088 | −1.35233 | 0.046272 |
| ENSBTAG00000013322 | POMGNT1 | 546.1977 | 807.947 | −0.56479 | 0.046578 |
| ENSBTAG00000005868 | HEBP1 | 1,134.375 | 1,890.091 | −0.73657 | 0.049218 |
| ENSBTAG00000020309 | G3BP1 | 3,548.796 | 1,803.971 | 0.976221 | 0.00011 |
| ENSBTAG00000005622 | LITAF | 1,337.329 | 694.1427 | 0.946233 | 0.00587 |
| ENSBTAG00000016664 | SLC2A4RG | 364.3316 | 194.8829 | 0.903131 | 0.007261 |
| ENSBTAG00000020035 | RCAN1 | 1,355.822 | 614.3679 | 1.141809 | 0.011416 |
Validation of MAP2K1 as a miR-34b target gene
To verify whether the MAP2K1 gene was downregulated by miR-34b in PSC, the expression level of MAP2K1 was detected. As shown in Figure 6, the mRNA and protein levels of MAP2K1 were significantly decreased by miR-34b mimic (P < 0.01; Figure 6A and B). To further confirm whether the MAP2K1 gene was directly targeted by miR-34b, the dual-luciferase reporter vectors of WT MAP2K1-3′ UTR or MUT MAP2K1-3′ UTR and miR-34b mimic were co-transfected into HEK 293T cells (Figure 6C). The Renilla/Firefly luciferase assay showed that the luciferase activity of WT MAP2K1-3′ UTR was downregulated (P < 0.01), whereas the luciferase activity of the MUT MAP2K1-3′ UTR did not change (Figure 6D). Moreover, it is worth noting that the protein levels of P-ERK1/2 were also downregulated by miR-34b overexpression (P < 0.05) and upregulated in miR-34b inhibitor-treated groups (P < 0.01; Figure 6E and F).
Figure 6.
MAP2K1 is the target gene of miR-34b in PSC. (A) Relative expression levels of MAP2K1 mRNA in PSC transfected with the miR-34b mimic (50 nM) and miR-34b inhibitor (200 nM) and their respective negative controls for 48 h. (B) Relative expression levels of MAP2K1, P-ERK1/2, and ERK1/2 protein in PSC transfected with the miR-34b mimic (50 nM) and miR-34b inhibitor (200 nM) and their respective negative controls for 48 h. (C) Sequence alignment of miR-34b and the 3′ UTR of MAP2K1 using TargetScan (http://www.targetscan.org) algorithms. (D) The 293T cells were cotransfected with the miR-34b mimic and luciferase reporter containing a fragment of the MAP2K1-3′ UTR harboring either the miR-34b-binding site (MAP2K1-3′ UTR WT) or a mutant (MAP2K1-3′ UTR MUT). (E, F) Relative expression levels of P-ERK1/2 and ERK1/2 protein in PSC transfected with the miR-34b mimic (50 nM) and miR-34b inhibitor (200 nM) and their respective negative controls for 48 h. Values are presented as means ± S.E.; *P < 0.05; **P < 0.01.
MAP2K1 knockdown suppresses proliferation and induces cell cycle arrest and apoptosis in PSC
To investigate the role of MAP2K1 in PSC proliferation and apoptosis, si-MAP2K1 and si-NC were transfected into PSC. As shown in Figure 7A, the cell viability of PSC was attenuated after MAP2K1 knockdown compared with the si-NC group (P < 0.01). Furthermore, flow cytometry analysis showed that the number of cells in the G2 phase was increased with the transfection of si-MAP2K1 (P < 0.05), while the S phase was decreased (P < 0.01; Figure 7B and C). The apoptosis ratio of PSC was also elevated compared with the si-NC group (P < 0.05; Figure 7D and E). In accordance with these results, the mRNA expression levels of cell cycle-related genes, CCNB1 (P < 0.01), CDK1 (P < 0.05), CDC25C (P < 0.05), and C-MYC (P < 0.01), were markedly downregulated under si-MAP2K1 transfection (Figure 7F), and the protein levels of CCNB1 and CDK1 were also decreased (Figure 7H). In contrast, the ratio between antiapoptotic gene BCL2 and proapoptotic gene BAX were decreased at both mRNA and protein levels (P < 0.05), and the mRNA level of P53 was also elevated compared with the si-NC group (P < 0.01; Figure 7F–H). In addition, the protein levels of P-ERK1 (P < 0.05) and P-ERK2 (P < 0.01) were markedly decreased after si-MAP2K1 transfection (Figure 7H). Taken together, these results demonstrated that MAP2K1 promoted the proliferation of PSC and inhibited its apoptosis.
Figure 7.
Knockdown of MAP2K1 suppresses proliferation and induces cell cycle arrest and apoptosis in PSC. (A) CCK-8 assay detected the effect of MAP2K1 knockdown on PSC viability after PSC were transfected with NC (50 nM) and siRNA-MAP2K1 (50 nM). (B, C) Cell cycle distribution was determined by flow cytometry assay after PSC were transfected with NC and siRNA-MAP2K1. (D, E) The percentage of apoptotic PSC was determined by flow cytometry. (F, G) The relative mRNA expression of the cell cycle-related and apoptosis-related genes was quantified by qPCR, and the levels corrected relative to the levels of housekeeping gene GAPDH. (H) CCNB1, CDK1, BCL2, BAX, P-ERK1/2, and ERK1/2 protein levels were analyzed by Western blot. Values are presented as means ± S.E. of three independent experiments.; *P < 0.05, **P < 0.01.
MAP2K1 overexpression attenuated the effects of miR-34b mimic in PSC
The results above demonstrated that knockdown of MAP2K1 had similar effects on cell proliferation and apoptosis as miR-34b overexpression. To further confirm that the effects of miR-34b were mediated by the downregulation of MAP2K1, three co-transfection treatments, including pcDNA3.1(+) + mimic NC, pcDNA3.1(+) + miR-34b mimic, and pcDNA3.1(+)–MAP2K1 + miR-34b mimic, were performed. As shown in Figure 8A, the cell viability of PSC was decreased in pcDNA3.1(+) + miR-34b mimic group, while this decrement was attenuated in the MAP2K1 overexpression group (P < 0.01). Furthermore, flow cytometry analysis showed that the number of cells in the G2 phase was increased with the transfection of pcDNA3.1(+) + miR-34b mimic (P < 0.05; Figure 8B and C), and the apoptosis ratio of PSC was also elevated compared with the pcDNA3.1(+) + mimic NC group (P < 0.01), while this effect was abolished by MAP2K1 overexpression (Figure 8D and E). In accordance with these results, the mRNA expression levels of cell cycle-related genes, CCNB1 (P < 0.01), CDK1 (P < 0.01), and CDC25C (P < 0.01), were markedly upregulated in pcDNA3.1(+)–MAP2K1 + miR-34b mimic group compared with pcDNA3.1(+) + miR-34b mimic group (Figure 8F), and the protein levels of CCNB1, CDK1, and P-ERK1/2 were also increased (P < 0.01; Figure 8H). In contrast, the ratio between antiapoptotic gene BCL2 and proapoptotic gene BAX was increased at both mRNA and protein levels, and the mRNA level of P53 was also downregulated compared with the pcDNA3.1(+) + miR-34b mimic group (Figure 8F and H). In addition, the protein levels of P-ERK1/2 were markedly decreased in the pcDNA3.1(+) + miR-34b mimic group (P < 0.05), while MAP2K1 overexpression showed the opposite effects (Figure 8H). Taken together, these results confirmed that the effects of miR-34b on PSC proliferation and apoptosis were mediated by MAP2K1 through the MEK/ERK pathway.
Figure 8.
MAP2K1 overexpression attenuates the effects of miR-34b on cell proliferation and apoptosis in PSC. Three co-transfection treatments including mimic NC + pcDNA3.1(+), miR-34b mimic + pcDNA3.1(+), and miR-34b mimic + pcDNA3.1(+)-MAP2K1 were performed. (A) The cell proliferation index was detected using the CCK-8 assay. (B, C) Cell cycle distribution was determined by flow cytometry assay. (D, E) The percentage of apoptotic PSC was determined by flow cytometry. (F, G) The relative mRNA expression of the cell cycle-related and apoptosis-related genes was quantified by qPCR, and the levels corrected relative to the levels of housekeeping gene GAPDH. (H) CCNB1, CDK1, BCL2, BAX, P-ERK1/2, and ERK1/2 protein levels were analyzed by Western blot. NC, mimic NC + pcDNA3.1(+); mimic, miR-34b mimic + pcDNA3.1(+); MEK1, miR-34b mimic + pcDNA3.1(+)-MAP2K1. Values are presented as means ± S.E. of three independent experiments.; asterisks indicate significant differences between the mimic NC + pcDNA3.1(+) and miR-34b mimic + pcDNA3.1(+) groups; *P < 0.05, **P < 0.01.
Discussion
It is well known that testes possess different structures and functions during different stages of development. At birth, the seminiferous tubules appear to be solid and are primarily composed of prepubertal spermatogonia and undifferentiated SC, which account for the increase in tubular size before the pubertal period (Sinowatz and Amselgruber, 1986; Li et al., 2016; Jiang et al., 2020). Then, the number of tubules has increased significantly in adult testes, and the tubular epithelium has been divided by junctional complexes formed by SC into two compartments, of which the basal compartment containing the spermatogonia and preleptotene spermatocytes, and the adluminal compartment with the more advanced stages of spermatogenesis (Guo et al., 2014). Specifically, SC in this stage have completed their morphological differentiation and lost their proliferation capacity. More importantly, the final number of SC in adult testis determines daily sperm production. It is of great interest to unveil the molecular mechanisms behind the processes of SC growth.
MicroRNAs are small noncoding RNAs that participate in many biological processes by downregulating target genes expression. Recent studies reported that miRNAs play an essential role in regulating SC proliferation and apoptosis. For example, in porcine, miR-196a promotes the proliferation and inhibits the apoptosis of immature SC by directly binding the 3′UTR of RCC2 and ABCB9 genes (Zhang et al., 2019); miR-362 targets and regulates the expression of RecQ-mediated genome instability protein 1(RMI1), and the knockdown of miR-362 promoted the entrance of cells into S phase and inhibited apoptosis (Ran et al., 2020). In human SC, miR-630 mimic treatment significantly decreased SC proliferation and migration (Qin et al., 2018); proliferation assay with miRNA mimics and inhibitors showed that miR-133b enhanced SC proliferation (Yao et al., 2016). Although many miRNAs have been implicated in the regulation of SC proliferation and apoptosis, their function and molecular mechanisms in bovine testes have been barely understood.
In this study, testes tissues from MB (24-mo old) and IB (neonatal) were used as the research objects, and miRNAs with significant differences were discovered through the sequence analysis. Results showed that miR-34b was differentially expressed in mature and immature testes, which indicated that miR-34b might play an important role in testicular development. Moreover, miR-34b has been reported to be expressed in granulosa cells, the homolog to SC (Yu et al., 2016). Thus, we decided to investigate its role in bovine PSC in our current study. miR-34b belongs to a miRNA family, which consists of six miRNA, including miR-34a/b/c and miR-449a/b/c. Consistent with our present data, in mouse testes, the miR-449 cluster was drastically upregulated during testicular development and in adult spermatogenesis, which indicated that the function of miR-34 family in testes might be highly conserved (Bouhallier et al., 2010). In double KO mice, lacking both miR-449 cluster and miR-34b/c leads to partial fertility disorder, and genes involved in cell-fate control have been significantly dysregulated (Comazzetto et al., 2014; Wu et al., 2014). Moreover, in somatic cells, miR-34 is known as an integral component of the P53 signaling pathway, and its ectopic expression induces cell cycle arrest and apoptosis in both primary and tumor-derived cell lines (Corney et al., 2007; He et al., 2007; Feng et al., 2019). miR-449a/b has also been reported to inhibit cell progression at the G1 phase through downregulating the expression of CDK6 and CDC25A (Yang et al., 2009). Taken together, all these results suggested that miR-34 plays a vital role in the regulation of cell proliferation and apoptosis. As expected, in our present study, miR-34b overexpression in PSC inhibited cell proliferation and induced apoptosis. Contrasted with the effects of miR-449a/b, the overexpression of miR-34b inhibited positive cell cycle regulators, such as CCNB1, CDK1, CDC25C, and C-MYC, leading to cell cycle arrest at G2 phase. With regard to another study, the ectopic expression of miR-34a and miR-34b/c in IMR90 cells (Human Caucasian fetal lung fibroblast) induces cell cycle arrest, leading to the fraction of cells in G1 and G2 phases increased (He et al., 2007). It is reasonable to deduce that the effect of miR-34 on cell progression might be cell context-dependent. Moreover, it is worth noting that the knockdown of miR-34b in PSC only partially reversed the effects observed in miR-34b mimic-treated cells. In accord with this, miR-449-null mice exhibited normal spermatogenesis and fertility, and the levels of miR-34b/c were significantly upregulated in miR-449-null mice (Bao et al., 2012; Wu et al., 2014). Thus, the miR-449 cluster and miR-34 cluster might function redundantly in the regulation of SC development in bovine testes. In summary, all these results revealed that miR-34b could inhibit PSC proliferation, induce cell cycle arrest at the G2 phase, and induce apoptosis.
To further investigate the molecular mechanisms of miR-34b on cell proliferation and apoptosis in PSC, the genome-wide mRNA expression of PSC with or without miR-34b overexpression was performed by mRNA sequencing. Combined with the results using TargetScan, qPCR, Western blot, and dual-luciferase reporter assay, MAP2K1 was selected as a target gene for miR-34b. Furthermore, the expression levels of P-ERK1/2 were also decreased in the miR-34b mimic-treated group. MAP2K1 is a threonine/tyrosine kinase that directly phosphorylates and thereby activates ERK1 and ERK2, constituting the MEK-ERK signaling transduction cascade (Tidyman and Rauen, 2009). It has been widely reported that MEK/ERK signaling pathway plays an essential role in regulating cell survival and proliferation in various human tumor cell lines (Ni et al., 2020; Zhou et al., 2020). Moreover, in rat immature SC, FSH treatment upregulated the expression of cyclin D1 and promoted the proliferation of neonatal SC, with the stimulated MAP2K1 activation and the phosphorylation level of ERK1/2 increased (Crépieux et al., 2001). Similar trends were also detected in mice, where the phosphorylation level of ERK increased until puberty, followed by a decrease during adulthood (Yao et al., 2015). In addition, the positive role of the ERK1/2 pathway in mediating the effect of insulin-like growth factor I on embryonic SC proliferation had also been detected (Villalpando et al., 2008). E2 plays an important role in male reproductive function and fertility; one study showed that E2 triggered the upregulation of CCND1 and increased the expression of antiapoptotic protein BCL2 and decreased the expression of proapoptotic protein BAX in SC, and all these effects were blocked by pretreatment with MAP2K1/2 inhibitor (Ni et al., 2019). In the present study, MAP2K1 knockdown led to PSC proliferation inhibition and induced cell cycle arrest at the G2 phase, and the apoptosis ratio was also significantly elevated, followed with downregulated P-ERK expression. Furthermore, MAP2K1 overexpression markedly reversed the effects of miR-34b on cell proliferation and apoptosis. These results suggest that MAP2K1 may play an essential role in mediating the inhibitory effect of miR-34b on PSC proliferation.
In the present study, through miRNA sequencing analysis, the miRNA expression in two different development stages was profiled. Then, the underlying mechanism of the miR-34b-mediated inhibition of PSC proliferation and induction of apoptosis was fully clarified. However, there are limitations to this study. First, no in situ experiments were conducted to validate the higher expression of miR-34b in SC of mature testes. Second, the upstream factors that activate the upregulation of miR-34b in mature testes remain unclear. Thus, further studies are warranted to resolve these issues.
In summary, our experimental data showed that miR-34b could target and repress MAP2K1, which could regulate the MEK/ERK signaling pathway to regulate testes development and PSC growth. Therefore, these results would be beneficial to further explore the action of miRNA in regulating the biological functions and development of bovine testes.
Supplementary Material
Acknowledgments
This project was supported by grants from China Postdoctoral Science Foundation (2019 M653775) and the Fundamental Research Funds for the Central Universities (2452020160).
Glossary
Abbreviations
- 3′UTR
3′-untranslated region
- DEGs
differentially expressed genes
- FBS
fetal bovine serum
- HRP
horseradish peroxidase
- KEGG
Kyoto encyclopedia of genes and genomes
- MAP2K1
mitogen-activated protein kinase kinase1
- miRNAs
microRNAs
- PSC
primary Sertoli cells
- SC
Sertoli cells
- TBST
Tris-buffered saline
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Literature Cited
- Aitken R J, and Baker M A. . 2013. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int. J. Dev. Biol. 57:265–272. doi: 10.1387/ijdb.130146ja [DOI] [PubMed] [Google Scholar]
- An J, Cai T, Che H, Yu T, Cao Z, Liu X, Zhao F, Jing J, Shen X, Liu M, . et al. 2014. The changes of miRNA expression in rat hippocampus following chronic lead exposure. Toxicol. Lett. 229:158–166. doi: 10.1016/j.toxlet.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Bai C, Gao Y, Zhang X, Yang W, and Guan W. . 2017. MicroRNA-34c acts as a bidirectional switch in the maturation of insulin-producing cells derived from mesenchymal stem cells. Oncotarget 8:106844–106857. doi: 10.18632/oncotarget.21883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Li D, Wang L, Wu J, Hu Y, Wang Z, Chen Y, Cao X, Jiang C, Yan W, . et al. 2012. MicroRNA-449 and microRNA-34b/c function redundantly in murine testes by targeting E2F transcription factor-retinoblastoma protein (E2F-pRb) pathway. J. Biol. Chem. 287:21686–21698. doi: 10.1074/jbc.M111.328054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhallier F, Allioli N, Lavial F, Chalmel F, Perrard M H, Durand P, Samarut J, Pain B, and Rouault J P. . 2010. Role of miR-34c microRNA in the late steps of spermatogenesis. RNA 16:720–731. doi: 10.1261/rna.1963810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comazzetto S, Di Giacomo M, Rasmussen K D, Much C, Azzi C, Perlas E, Morgan M, and O’Carroll D. . 2014. Oligoasthenoteratozoospermia and infertility in mice deficient for miR-34b/c and miR-449 loci. PLoS Genet. 10:e1004597. doi: 10.1371/journal.pgen.1004597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corney D C, Flesken-Nikitin A, Godwin A K, Wang W, and Nikitin A Y. . 2007. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 67:8433–8438. doi: 10.1158/0008-5472.CAN-07-1585 [DOI] [PubMed] [Google Scholar]
- Crépieux P, Marion S, Martinat N, Fafeur V, Vern Y L, Kerboeuf D, Guillou F, and Reiter E. . 2001. The ERK-dependent signalling is stage-specifically modulated by FSH, during primary Sertoli cell maturation. Oncogene 20:4696–4709. doi: 10.1038/sj.onc.1204632 [DOI] [PubMed] [Google Scholar]
- Feng H, Ge F, Du L, Zhang Z, and Liu D. . 2019. MiR-34b-3p represses cell proliferation, cell cycle progression and cell apoptosis in non-small-cell lung cancer (NSCLC) by targeting CDK4. J. Cell. Mol. Med. 23:5282–5291. doi: 10.1111/jcmm.14404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlaender M R, Mackowiak S D, Li N, Chen W, and Rajewsky N. . 2012. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 40(1):37–52. doi: 10.1093/nar/gkr688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Chen B, Luo H, Weng B, Tang X, Chen Y, Yang A, and Ran M. . 2020. miR-499 promotes immature porcine Sertoli cell growth through the PI3K/AKT pathway by targeting the PTEN gene. Reproduction 159(2):145–157. doi: 10.1530/rep-19-0303 [DOI] [PubMed] [Google Scholar]
- Greco S, De Simone M, Colussi C, Zaccagnini G, Fasanaro P, Pescatori M, Cardani R, Perbellini R, Isaia E, Sale P, . et al. 2009. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 23:3335–3346. doi: 10.1096/fj.08-128579 [DOI] [PubMed] [Google Scholar]
- Guo J, Liu X, Yang Y, Liang M, Bai C, Zhao Z, and Sun B. . 2018. miR-375 down-regulation of the rearranged L-myc fusion and hypoxia-induced gene domain protein 1A genes and effects on Sertoli cell proliferation. Asian-Australas. J. Anim. Sci. 31:1103–1109. doi: 10.5713/ajas.17.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Yang B, Ju Z H, Wang X G, Qi C, Zhang Y, Wang C F, Liu H D, Feng M Y, Chen Y, . et al. 2014. Alternative splicing, promoter methylation, and functional SNPs of sperm flagella 2 gene in testis and mature spermatozoa of Holstein bulls. Reproduction 147:241–252. doi: 10.1530/REP-13-0343 [DOI] [PubMed] [Google Scholar]
- He L, He X, Lim L P, De Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, . et al. 2007. A microRNA component of the p53 tumour suppressor network. Nature 447(7148):1130-U1116. doi: 10.1038/nature05939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heninger N L, Staub C, Johnson L, Blanchard T L, Varner D, Ing N H, and Forrest D W. . 2006. Testicular germ cell apoptosis and formation of the Sertoli cell barrier during the initiation of spermatogenesis in pubertal stallions. Anim. Reprod. Sci. 94(1–4):127–131. doi: 10.1016/j.anireprosci.2006.03.104 [DOI] [Google Scholar]
- Hu P, Guan K, Feng Y, Ma C, Song H, Li Y, Xia X, Li J, and Li F. . 2017. miR-638 inhibits immature Sertoli cell growth by indirectly inactivating PI3K/AKT pathway via SPAG1 gene. Cell Cycle 16:2290–2300. doi: 10.1080/15384101.2017.1380130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zhu W Q, Zhu X C, Cai N N, Yang R, Cai H, and Zhang X M. . 2020. Cryopreservation of calf testicular tissues with knockout serum replacement. Cryobiology 92:255–257. doi: 10.1016/j.cryobiol.2020.01.010 [DOI] [PubMed] [Google Scholar]
- Jiao Z J, Yi W, Rong Y W, Kee J D, and Zhong W X. . 2015. MicroRNA-1285 regulates 17β-estradiol-inhibited immature boar Sertoli cell proliferation via adenosine monophosphate-activated protein kinase activation. Endocrinology 156: 4059–4070. doi: 10.1210/en.2014-1982 [DOI] [PubMed] [Google Scholar]
- Kao E, Villalon R, Ribeiro S, and Berger T. . 2012. Role for endogenous estrogen in prepubertal Sertoli cell maturation. Anim. Reprod. Sci. 135:106–112. doi: 10.1016/j.anireprosci.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, and Salzberg S L. . 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14:R36. doi: 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, and Salzberg S L. . 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10(3):R25. doi: 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu H, Li Y, Yang M, Qu C, Zhang Y, Liu Y, and Zhang X. . 2014. Identification of suitable endogenous control genes for quantitative RT-PCR analysis of miRNA in bovine solid tissues. Mol. Biol. Rep. 41:6475–6480. doi: 10.1007/s11033-014-3530-x [DOI] [PubMed] [Google Scholar]
- Li B, Zhuang M, Wu C, Niu B, Zhang Z, Li X, Wei Z, Li G, and Hua J. . 2016. Bovine male germline stem-like cells cultured in serum- and feeder-free medium. Cytotechnology 68:2145–2157. doi: 10.1007/s10616-015-9933-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Song H, Yu L, Guan K, Hu P, Li Y, Xia X, Li J, Jiang S, and Li F. . 2016. miR-762 promotes porcine immature Sertoli cell growth via the ring finger protein 4 (RNF4) gene. Sci. Rep. 6:32783. doi: 10.1038/srep32783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet B, Chevalier B, Luxardi G, Coraux C, Zaragosi L E, Cibois M, Robbe-Sermesant K, Jolly T, Cardinaud B, Moreilhon C, . et al. 2011. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat. Cell Biol. 13:693–699. doi: 10.1038/ncb2241 [DOI] [PubMed] [Google Scholar]
- Ni F D, Hao S L, and Yang W X. . 2019. Multiple signaling pathways in Sertoli cells: recent findings in spermatogenesis. Cell Death Dis. 10:541. doi: 10.1038/s41419-019-1782-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni D, Yi Q, Liu J, Hu Y, Lv T, Tan G, Liu Y, Xu L, Xia H, Zhou Q, . et al. 2020. A1CF-promoted colony formation and proliferation of RCC depends on DKK1-MEK/ERK signal axis. Gene 730:144299. doi: 10.1016/j.gene.2019.144299 [DOI] [PubMed] [Google Scholar]
- Oghbaei H, Alipour M R, Hamidian G, Ahmadi M, Ghorbanzadeh V, and Keyhanmanesh R. . 2018. Two months sodium nitrate supplementation alleviates testicular injury in streptozotocin-induced diabetic male rats. Exp. Physiol. 103:1603–1617. doi: 10.1113/EP087198 [DOI] [PubMed] [Google Scholar]
- Oghbaei H, Hamidian G, Alipour M R, Alipour S, and Keyhanmanesh R. . 2020. The effect of prolonged dietary sodium nitrate treatment on the hypothalamus-pituitary-gonadal axis and testicular structure and function in streptozotocin-induced diabetic male rats. Food Funct. 11:2451–2465. doi: 10.1039/c9fo00974d [DOI] [PubMed] [Google Scholar]
- O’Rourke J R, Swanson M S, and Harfe B D. . 2006. MicroRNAs in mammalian development and tumorigenesis. Birth Defects Res. C. Embryo Today 78:172–179. doi: 10.1002/bdrc.20071 [DOI] [PubMed] [Google Scholar]
- Orth J M, Gunsalus G L, and Lamperti A A. . 1988. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 122:787–794. doi: 10.1210/endo-122-3-787 [DOI] [PubMed] [Google Scholar]
- Qin L, Zhang G, Zhu C, Wu J, Zhao Z, and Zhao Y. . 2018. The predicted target genes of miR-122/449a validation and effects on Sertoli cells proliferation. Pak. J. Zool. 50(1):273–281. doi: 10.17582/journal.pjz/2018.50.1.273.281 [DOI] [Google Scholar]
- Ran M, Luo H, Gao H, Tang X, Chen Y, Zeng X, Weng B, and Chen B. . 2020. miR-362 knock-down promotes proliferation and inhibits apoptosis in porcine immature Sertoli cells by targeting the RMI1 gene. Reprod. Domest. Anim. 55:547–558. doi: 10.1111/rda.13626 [DOI] [PubMed] [Google Scholar]
- Ran M, Weng B, Cao R, Li Z, Peng F, Luo H, Gao H, and Chen B. . 2018. miR-26a inhibits proliferation and promotes apoptosis in porcine immature Sertoli cells by targeting the PAK2 gene. Reprod. Domest. Anim. 53:1375–1385. doi: 10.1111/rda.13254 [DOI] [PubMed] [Google Scholar]
- Ran M-L, Weng B, Cao R, Peng F-Z, Luo H, Gao H, and Chen B. . 2019. miR-34c inhibits proliferation and enhances apoptosis in immature porcine Sertoli cells by targeting the SMAD7 gene. J. Integr. Agric. 18(2):449–459. doi: 10.1016/s2095-3119(19)62612-2 [DOI] [Google Scholar]
- Rebourcet D, Darbey A, Monteiro A, Soffientini U, Tsai Y T, Handel I, Pitetti J L, Nef S, Smith L B, and O’Shaughnessy P J. . 2017. Sertoli cell number defines and predicts germ and Leydig cell population sizes in the adult mouse testis. Endocrinology 158:2955–2969. doi: 10.1210/en.2017-00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R M, McKinnell C, Kivlin C, and Fisher J S. . 2003. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125:769–784. doi: 10.1530/rep.0.1250769 [DOI] [PubMed] [Google Scholar]
- Sinowatz F, and Amselgruber W. . 1986. Postnatal development of bovine Sertoli cells. Anat. Embryol. (Berl). 174:413–423. doi: 10.1007/BF00698792 [DOI] [PubMed] [Google Scholar]
- Song L, and Tuan R S. . 2006. MicroRNAs and cell differentiation in mammalian development. Birth Defects Res. C. Embryo Today 78:140–149. doi: 10.1002/bdrc.20070 [DOI] [PubMed] [Google Scholar]
- Tang K, Jin Y, Chen F, and Wang L. . 2016. Overexpression of C/EBP affects the cell cycle regulators and spermatogenesis related genes expression and function of bovine Sertoli cells. Reprod. Domest. Anim. 51(4):591–596. doi: 10.1111/rda.12724 [DOI] [PubMed] [Google Scholar]
- Tidyman W E, and Rauen K A. . 2009. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr. Opin. Genet. Dev. 19:230–236. doi: 10.1016/j.gde.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscherner A, Gilchrist G, Smith N, Blondin P, Gillis D, and LaMarre J. . 2014. MicroRNA-34 family expression in bovine gametes and preimplantation embryos. Reprod. Biol. Endocrinol. 12:85. doi: 10.1186/1477-7827-12-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-Sanchez M A, Liu J, Hannon G J, and Parker R. . 2006. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 20:515–524. doi: 10.1101/gad.1399806 [DOI] [PubMed] [Google Scholar]
- Villalpando I, Lira E, Medina G, Garcia-Garcia E, and Echeverria O. . 2008. Insulin-like growth factor 1 is expressed in mouse developing testis and regulates somatic cell proliferation. Exp. Biol. Med. (Maywood). 233:419–426. doi: 10.3181/0708-RM-212 [DOI] [PubMed] [Google Scholar]
- Wen M, Shen Y, Shi S, and Tang T. . 2012. miREvo: an integrative microRNA evolutionary analysis platform for next-generation sequencing experiments. BMC Bioinformatics 13:140. doi: 10.1186/1471-2105-13-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng B, Ran M-L, Cao R, Peng F-Z, Luo H, Gao H, Tang X-W, Yang A-Q, and Chen B. . 2019. miR-10b promotes porcine immature Sertoli cell proliferation by targeting the DAZAP1 gene. J. Integr. Agric. 18(8):1924–1935. doi: 10.1016/s2095-3119(19)62564-5 [DOI] [Google Scholar]
- Wu J, Bao J, Kim M, Yuan S, Tang C, Zheng H, Mastick G S, Xu C, and Yan W. . 2014. Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc. Natl. Acad. Sci. U. S. A. 111:E2851–E2857. doi: 10.1073/pnas.1407777111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Feng M, Jiang X, Wu Z, Li Z, Aau M, and Yu Q. . 2009. miR-449a and miR-449b are direct transcriptional targets of E2F1 and negatively regulate pRb-E2F1 activity through a feedback loop by targeting CDK6 and CDC25A. Genes Dev. 23:2388–2393. doi: 10.1101/gad.1819009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W C, Tang K Q, Fu C Z, Riaz H, Zhang Q, and Zan L S. . 2014. Melatonin regulates the development and function of bovine Sertoli cells via its receptors MT1 and MT2. Anim. Reprod. Sci. 147:10–16. doi: 10.1016/j.anireprosci.2014.03.017 [DOI] [PubMed] [Google Scholar]
- Yao P L, Chen L, Hess R A, Müller R, Gonzalez F J, and Peters J M. . 2015. Peroxisome proliferator-activated receptor-D (PPARD) coordinates mouse spermatogenesis by modulating extracellular signal-regulated kinase (ERK)-dependent signaling. J. Biol. Chem. 290:23416–23431. doi: 10.1074/jbc.M115.664508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Sun M, Yuan Q, Niu M, Chen Z, Hou J, Wang H, Wen L, Liu Y, Li Z, . et al. 2016. MiRNA-133b promotes the proliferation of human Sertoli cells through targeting GLI3. Oncotarget 7(3):2201–2219. doi: 10.18632/oncotarget.6876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Lou Y, He K, Yang S, Yu W, Han L, and Zhao A. . 2016. Goose broodiness is involved in granulosa cell autophagy and homeostatic imbalance of follicular hormones. Poult. Sci. 95(5):1156–1164. doi: 10.3382/ps/pew006 [DOI] [PubMed] [Google Scholar]
- Zhang S X, Guo J, Liang M D, Qi J J, Wang Z B, Jian X R, Zhang Z B, Sun B X, and Li Z H. . 2019. miR-196a promotes proliferation and inhibits apoptosis of immature porcine Sertoli cells. DNA Cell Biol. 38(1):41–48. doi: 10.1089/dna.2018.4387 [DOI] [PubMed] [Google Scholar]
- Zhou C, Wang P, Tu M, Huang Y, Xiong F, and Wu Y. . 2020. Long non-coding RNA PART I promotes proliferation, migration and invasion of hepatocellular carcinoma cells via miR-149-5p/MAP2K1 axis. Cancer Manag. Res. 12:3771–3782. doi: 10.2147/cmar.S246311 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.