Abstract
The centrosome, which consists of two centrioles surrounded by pericentriolar material, is a unique structure that has retained its main features in organisms of various taxonomic groups from unicellular algae to mammals over one billion years of evolution. In addition to the most noticeable function of organizing the microtubule system in mitosis and interphase, the centrosome performs many other cell functions. In particular, centrioles are the basis for the formation of sensitive primary cilia and motile cilia and flagella. Another principal function of centrosomes is the concentration in one place of regulatory proteins responsible for the cell’s progression along the cell cycle. Despite the existing exceptions, the functioning of the centrosome is subject to general principles, which are discussed in this review.
Keywords: centrosome, centriole, cilia, flagella, microtubules
1. Introduction
Nearly 150 years ago, almost simultaneously, three researchers described in dividing cells two symmetrically located structures that looked like a “radiance” and were called the centrosphere [1,2,3]. At the centrosphere’s focus, granules were sometimes visible, which were originally called “polar corpuscles” [3]. Van Beneden and Nate [4] and independently Boveri [5] found that the polar corpuscles do not entirely disappear after mitosis, but remain in interphase, often located near the geometric center of the cell.
Later, these granules were named centrioles [6]. Shortly after, Henneguy and von Lenhossék showed that centrioles and basal bodies are the same structure at distinct functional stages. They proposed a “hypothesis about the homology of centrioles and basal bodies of flagella” [7,8]. This idea was overlooked for many years but ultimately is correct. After the ultrastructure of the centrosome was studied using transmission electron microscopy, centrioles (and basal bodies) were shown to have a conserved structure in many types of cells—hollow cylinders containing triplets of microtubules (MTs) in their walls [9,10,11]. The unique centrally symmetric structure of centrioles has always generated amazing, and sometimes fantastic, hypotheses about the centrosome’s origin and functions. Fundamental advances in molecular biology, immunocytochemistry, and high-resolution light microscopy have allowed us to advance the understanding of the fundamental functions of this organelle that is small in size, but most important for many functions in the cells.
Summing up the many years of centrosome research, we formulated 12 postulates of centrosomal biology in 2007 [12]. A few years later, these postulates were somewhat supplemented and developed in a book on the centrosome [13]. The discoveries of recent years make it necessary once again to return to these postulates and update them.
2. Postulates of Centrosomal Biology
2.1. The Centriole and the Basal Body Are Two Forms of the Same Organelle
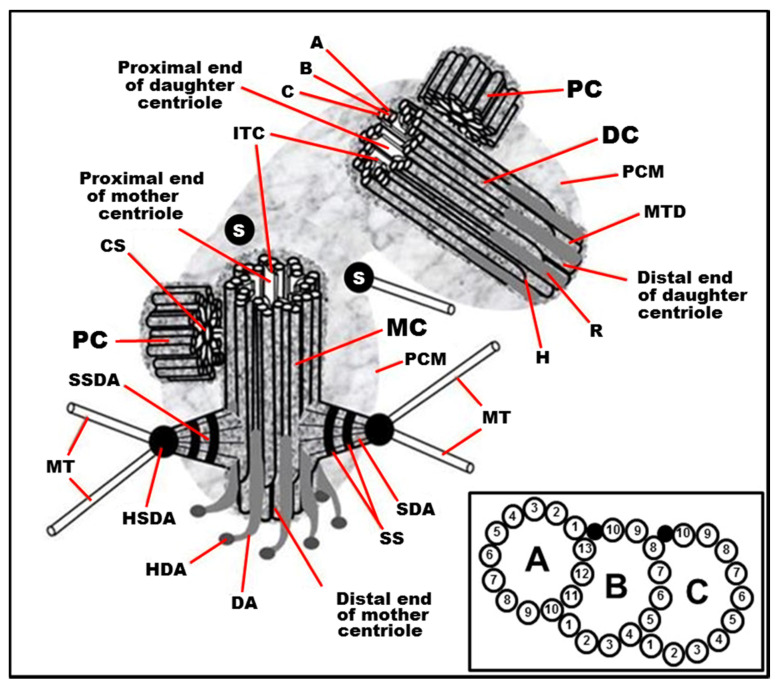
The centrosome usually organizes and orients the mitotic spindle. The basal body nucleates and anchors the cilium in interphase. The centrosome consists of two centrioles surrounded by pericentriolar material after mitosis in the G1 phase or the G0 phase of the cell cycle. At the end of the interphase before the next mitosis in the G2 phase of the cell cycle, the cell has two centrosomes, each having two centriolar cylinders in its composition. Each centriole consists of 9 MT triplets, and it is associated with subdistal appendages, distal appendages, striated roots, and satellites (Figure 1). In some cases or certain stages of the development of organisms, the walls of the centrioles may contain MT doublets [14,15,16], MT singlets [17,18,19], or no MT at all [20,21], while maintaining nine-beam symmetry.
Figure 1.
Typical centrosome structure in the S phase of the cell cycle in proliferating mammalian cells. MC: mother (mature) centriole; DC: daughter centriole; PC: procentriole; PCM: pericentriolar material (pericentriolar matrix); A: “A” MT of triplet; B: “B” MT of triplet; C: “C” MT of triplet; H: hook of “C” MT; MTD: A-B MT duplex (in the distal part of centriolar cylinder); ITC: internal triplets connections system (scaffold structure), which include A-C linkers; CS: cartwheel structure (an axis with spokes); SDA: sub-distal appendage; HSDA: head of sub-distal appendage; SSDA: the stem of sub-distal appendage (connected to three triplets in this case); S: satellites; SS: the striated structure of sub-distal appendage stem; MT: microtubule; DA: distal appendage; HAD: head of distal appendage; R: rib. From [12] with modifications. Insertion: The fine ultrastructure of the MT triplet, showing protofilaments of MTs (the data from [22,23] were used to make this drawing).
The structure of centrioles during the formation of cilia or flagella is conserved in the majority organisms and cell types—9 MT triplets (aka “9×3”) with no central MTs (aka “+0”) that is summarized by the formula “9×3 + 0”. In rare cases, cilia appear to form a basal body consisting of doublets MTs [24,25,26]. The MT triplets are made three tubules named the MT “A”, the MT “B”, and the MT C”. Only MT “A” has a closed rounded shape on a transverse section and consists of 13 protofilaments, as most cellular MTs [27,28]. MT “B” has the shape of an arc adjacent to MT “A” and having three or four common protofilaments with it. MT “C” also has the shape of an arc adjacent to MT “B” and has three or four common protofilaments with it. The number of protofilaments in MT “B” and MT “C” can be 10 or 11 in centrioles from different organisms, and the total number of protofilaments in one triplet can thus be from 33 to 35 [10,28,29,30,31,32]. There are suggestions that protofilaments number 11 of MT “B” and MT “C” may have a special biochemical composition [28].
The centriolar cylinders’ central nine-fold symmetry is established during the formation of the “cartwheel” structure [10,33]. This symmetry is generated by lateral interaction of the N-terminal domains of SAS-6 protein dimers [34,35]. The cartwheel structure disassembles in mature centrioles as cells exit mitosis of vertebrates [33,36] but remains in mature centrioles of insects [37].
2.2. The Centriole Is a Polar Structure with Two Morphologically and Functionally Different Ends
The centriole’s distal end (where the plus ends of the MT are) can serve as the site of cilia formation, and it associates with distal appendages. A complex of proteins, including CP110 and CEP97, is found at this end and supports cilium formation [38].
Subdistal appendages are also more often formed closer to the distal end of the centriole. The number of distal appendages (for centrioles of centrosome) and their homologous structures in basal bodies—alar sheets—are always equal to nine [39,40]. The number of subdistal appendages (the first original name of these structures was pericentriolar satellites [41]) is variable for different types of cells and various conditions and ranges from 0 to 13 [42,43,44].
The foot is the homologous structure of subdistal appendage in the basal body. One to two feet are found per basal body, and they are located closer to their distal end [40,45,46]. The foot base is connected to two or three triplets in a basal body [47]. In the basal bodies of functionally active cilia, feet are located in the plane of the cilia beat [48].
New centriolar cylinders (procentrioles) usually appear perpendicular to the mother centriole’s surface closer to its proximal end [33], where the minus ends of the MT triplets are located.
The pericentriolar material can also be located asymmetrically, surrounding only the centriole proximal part [36,49]. The MT “A” centriole’s proximal end is covered with a conical structure with a morphology similar to the γ-tubulin ring complex (γ-TuRC) [50].
The polarity of the centriole is manifested in the orientation of some of its components. When viewed from the proximal end of the centriole, the MT triplets are always twisted counterclockwise (in the direction from the inner MT “A” to the outer MT “C”), and the distal appendages are always twisted clockwise [12,51] (Figure 1).
2.3. The Outer Diameter of the Distal Part of the Centriolar Cylinder Is Smaller Than That of the Proximal End
There are two reasons for the diameter differences between the centriole ends. (i) The MT triplets transform into doublets of MT as MT “C” is usually shorter than MT “A” and MT “B”. (ii) The angle of inclination of the MT triplets (the line passing through the centers MT “A” and MT “B”) to the radius of the centrioles at the distal end of the centrioles is 80–90 degrees (the doublets lie almost in a circle). At the proximal end of the centriole, the triplets’ inclination to the radius is 50–55 degrees (the triplets are deployed like turbine blades). The inner radius of the lumen (the distance from the center of the centriole to MT “A”) does not change. In contrast, the outer radius (the distance from the center of the centriole to MT “C”) increases due to the unfolding of the triplets [36]. This twist’s angle appears to be controlled by the A-C linkers in the proximal segment and an inner scaffold at the distal portion [52].
2.4. The Centriolar Cylinder Length Is Highly Regulated
The centriolar cylinder’s length is precisely set and usually ranges from 200 to 700 nm depending on cell type and cell cycle phase [53,54]. However, in some cell types, extremely long centrioles can be observed [10,55,56]. The growth of centrioles in length is regulated by a complex of proteins, including SAS4/CPAP, POC1, and POC5 [57,58,59,60]. Overexpression of CPAP or its interaction partners, CEP120 and SPICE1 (Spindle and Centriole Associated Protein 1), lead to the assembly of excessively long centrioles. The protein CP110 is an antagonist to the CPAP; CP110 caps the distal end of the growing centriole [61,62,63,64].
2.5. In the Centrosome of Proliferating Cells, Two Centrioles Differ Structurally and Functionally
Only the older (mother) centriole has appendages at the distal end. Only the mother centriole has subdistal appendages. MT nucleating centers are located predominantly on or near the mother centriole. Gamma-tubulin [65,66] is the basis of two types of protein complexes that nucleate MTs—the large Gamma-TuRC [67] and small Gamma-TuSC [68,69]. There are other protein complexes on the centrosome that anchor the MTs. These complexes include CAP 350/FOP/EB1 [70], PCM/BBS4/ninein/centrin/pericentrin [71], and ninein/ODF2/Cep170/centriolin/epsilon-tubulin [72]. MT nucleation and MT attachment activities are located in the subdistal appendages’ heads, on the surface of the centrioles, and in the pericentriolar material. Centriolar satellites are dense rounded structures containing protein complexes of the pericentriolar material located near centrioles and undergo cell cycle-dependent assembly and disassembly [73,74,75,76]. They move towards the centrosome along MTs in a dynein-dependent manner; participate in targeting of centrin, pericentrin, and ninein to the centrosome; and are implicated in ciliogenesis [75,77,78].
2.6. The Proximal Ends of the Mother and Daughter Centrioles Are Connected via a Bundle of Thin Fibers
The Proximal Ends of the Mother and Daughter Centrioles are Connected Via A Bundle of Thin Fibers [79,80,81]. The composition of this ligament includes proteins rootletin, beta-catenin, and C-NAP1 [82,83,84]. Severing this connection can lead to centriole separation. The regulation of this separation is controlled by Nek2 kinase [84]. Separation of mother and daughter centrioles after mitosis is a necessary prerequisite for the start of centrioles duplication [85].
The separation of the mother and daughter centrioles in G1 phase of the cell cycle is a different process from the separation and divergence of the two centrosomes before mitosis. The last process differently depends on the intactness of MTs and actin microfilaments [86] and is controlled by AuroraA [87,88,89,90], p34cdc2 [91], and Plk1 [92,93,94] kinases. In the process of centrosome separation and movement during the formation of the two poles of the spindle, several types of motors are involved: the cytoplasmic dynein–dynactin–NUMA complex [95,96,97], Xklp2 [98], kinesin-related motors pEg5 [89,91,99], and XCTK2 [100].
2.7. The Centriole Lumen Helps in Stabilizing the Centriole
Centriolar MTs have much greater stability than spindle or interphase cytosolic MTs. They are not depolymerized either by anti-MT drugs or by exposure to cold [101,102,103]. However, MT triplets can be disassembled by high (1–2 M NaCl or KCl) salt concentrations once centrioles are isolated [104]. This stability of MT centrioles is associated with stabilizing proteins such as tektins between microtubules [105,106], post-translational polyglutamylation of centriolar tubulin [107,108], and presence of scaffold structures inside the centriole lumen.
Inside the centriolar cylinder, an interconnected system of ligaments connects the MT triplets [10,29,32,39,73]. The structure of these connections changes from the proximal end to the distal one. MT “A” has a connective with MT “C” of the neighboring triplet. In addition, inward directions from each MT “A” are strands of electron-dense material that are interconnected, with MT “A” and A–C bundles. At the ultrastructural level, the centriolar cylinder’s lumen looks “empty” near the proximal end and filled with electron-dense material at the distal lumen. The protein composition of the A–C linker is unknown. The distal lumen scaffold is made of POC1B, POC5, FAM161A, CETN, and WDR90 [52,109]. Mutating some of these protein results in destabilization of the centriole cylinder [110].
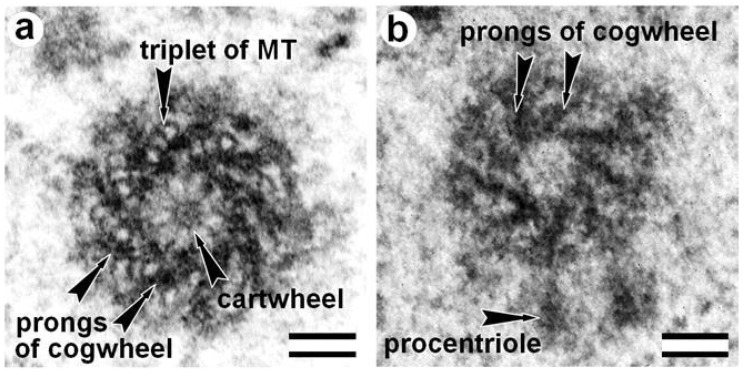
2.8. Maintaining a Cylindrical Shape of Centrioles Can Be Independent of MT Triplets
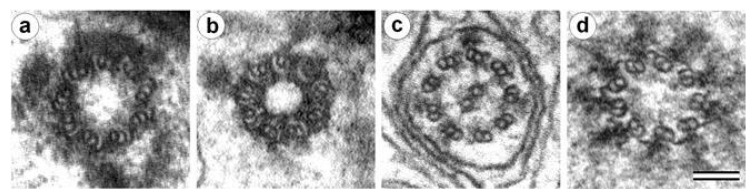
Centrioles do not have MTs in haploid male larvae trophocytes and hypodermal cells of wasps Anisopteromalus calandrae [20]. However, their MT-free centrioles’ shape and size are similar to canonical centrioles with MT triplets of adult (imago) insects or late larvae of different wasps’ types (Figure 2). The centriole’s symmetry is maintained, and they contain nine prongs of electron-dense material in their walls forming the structure of the “cogwheel” [20]. One possibility is that at this stage of development, the genes encoding the proteins responsible for the construction of MT triplets have not yet turned on. Alternatively, the MTs disassembled after the centriole formed. Practically identical cogwheel structure without MTs triplets was also found in the base of mature spermatozoa flagella of wasps Cotesia congregata. It replaced the “normal” centriole during spermiogenesis, which is present in spermatids [21]. Prongs of cogwheel structure are visible between triplets or doublets of MT in centrioles of other insects, particularly in Drosophila. It was shown that the protein SAS4 [111] is concentrated in these regions.
Figure 2.
Centriole structure in larvae of two wasps: (a) larvae of Nasonia vitripennis, where prongs of cogwheel structure are visible between triplets of MT; and (b) early larvae of Anisopteromalus calandrae, where the centriole has a cogwheel without MT. The cartwheel structure is not clearly visible in the centriole lumen. View from the distal end of the centriole. Scale bar: 100 nm. From [20] with modifications.
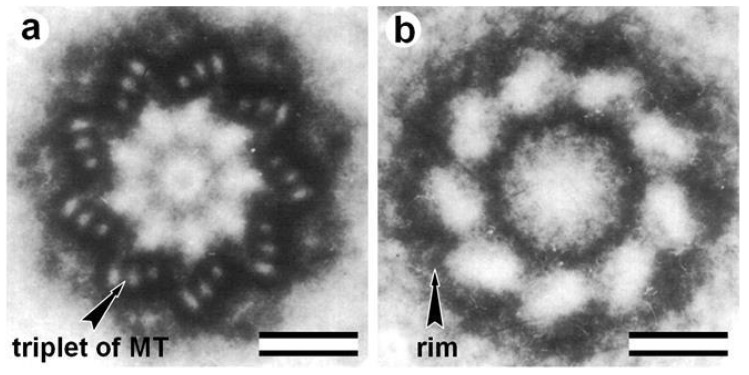
After processing the isolated centrioles from bovines spleen by 2 M salts, the triplets’ MTs are completely disassembled [104]. Holes were found at the MT sites (Figure 3); still, the centrioles’ cylindrical shape is preserved. The authors called the electron-dense structure surrounding triplets “centriolar rim” [104]. Later, was reported that the PCM is not homogeneous and that some centrosomal proteins are closely associated with the centriole to form a distinct PCM compartment called the “PCM tube”. In contrast, other proteins are more peripheral [112,113]. These MT surrounding protein complexes help maintain the cylindrical shape and provide mechanical strength in MT’s absence.
Figure 3.
Rotation images of centriole and centriolar rim. Nine photographs with a rotation angle of 40° were superimposed to obtain images: (a) centriole; and (b) centriolar rim after 1 M KC1 treatment. Scale bar: 100 nm. From [104] with modifications.
2.9. The Structure and Activity of Centrosomes Differ in Interphase and Mitotic Cells
The subdistal appendages and primary cilia disappear [36,114], and an amorphous mitotic halo surrounds the centrosome in mitosis. In addition, the centrosome-associated interphase MTs are completely depolymerized before mitosis. Finally, mitotic MT asters form around each of the two centrosomes during prophase. These MTs, together with other MTs nucleating activities, organize and form the mitotic spindle in metaphase [78]. All mitotic MTs have their minus ends near the centrosome but have three options for localizing their plus end: (1) MT plus end is directed in the opposite direction from the chromosomes and is localized near the cell membrane—astral MTs; (2) MT plus end is associated with the kinetochore of chromosomes—kinetochore MTs; and (3) MT plus end interacts with an MT coming from the opposite pole of the spindle—interzonal MTs. The halo also contains many short MTs, which probably later transform into one of three types. Mitotic MTs have dynamic and biochemical characteristics different from interphase MTs; in particular, they are less resistant to anti-microtubule drugs [115]. The ability of centrosomes to nucleate MT in mitosis increases several times during preparation to cell division (G2 phase of the cell cycle) in a process named centrosome maturation [36,116,117].
2.10. New Centrioles Are Usually Formed in Association with Mother Centrioles but Can Be Formed without Preexisting Centriole De Novo
Duplication of centrioles occurs only one time per cell cycle; on each mature centriole, only one procentriole appears (Figure 4). The accuracy of regulation of these processes is controlled by a complex of proteins that were first identified in Caenorhabditis elegans: ZYG-1 [118,119], SPD-2 [19,120,121], SAS-4 [122,123], SAS-5 [124], and SAS-6 [125,126]. In human cells, the ZYG-1 homolog is PLK4 kinase [127]; in Drosophila cells, it is SAK/PLK4 kinase [128]. More than 30 proteins have already been described that are somehow involved in the duplication of centrioles [129]. In mammals and insects, centriole duplication starts by CEP152/Asterless that recruits PLK4 [130,131,132]. CEP152 form a ring together with CEP63 and CEP57 around the proximal part of the mother centriole [133,134].
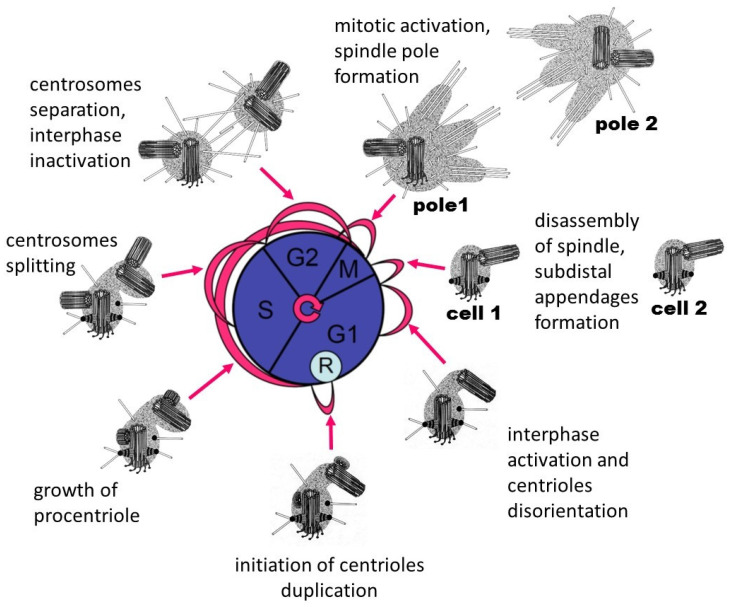
Figure 4.
The relationship of the cell (nuclear) cycle and centriolar cycle. R, restriction point.
Centriole duplication is independent of DNA replication [135,136,137] and starts near the end of the G1 phase (Figure 4) of the cell cycle [138,139]. At this time, a complex of biochemical reactions in the cell is launched dependent on the cell’s size, the presence of external growth factors, the growth conditions of the cell, and its interaction with surrounding cells [140,141,142]. It has been shown that the Cyclin D/CDK 4/6 complex phosphorylates the pRB protein, which loses its ability to bind the transcription activation factor EF2. The released transcription factor EF2 activates the synthesis of Cyclin E and Cyclin A, which starts the process of duplication of centrioles. Thus, DNA replication and centriole duplication are regulated by a single cytoplasmic mechanism. However, centriole duplication begins earlier than DNA replication [138], suggesting additional regulatory mechanisms.
In some cases, new centrioles form without preexisting centrioles. In the ciliary epithelium cells, which have hundreds of cilia, the centrioles form via deuterostomes [46,143]. Deuterosomes can be assembled autonomously from parental centrioles by de novo centriole amplification in multiciliated cells [144,145]. In murine early embryonic development, centrioles appear de novo, but the mechanism is unclear [146]. In cells with centrioles, centrioles elimination by micro-irradiation does not prevent procentriole formation at S phase. In contrast, in this case, many new centrioles are formed during the S phase [147]. In addition, centrioles can appear without progenitors during parthenogenetic development [148].
These observations suggest that centrioles can assemble independently of preexisting centrioles, and the role of centriole duplication is to restrict the number of assembling centrioles to only one new centriole.
2.11. The Centrosomes Have Four Types of MT Nucleating Activity
Many of the centrosome’s critical function is mediated by MTs that are nucleated by it at different cell types and cell cycle phases. This includes four main MTs nucleating activities:
-
(1)
Formation of two types of interphase MTs: (i) a radial MTs system around the centriole; and (ii) non-centrosomal (free) cytoplasmic MTs that were polymerized on the centrosome and later released to the cytoplasm.
-
(2)
Formation of three types of mitotic spindle microtubules: (i) astral MTs; (ii) kinetochore MTs; and (iii) interzonal MTs.
-
(3)
Formation of MT of procentrioles.
-
(4)
Formation MTs of cilia or flagellum, or a related structure, known as the centriolar adjunct, that is found in mammalian spermatids [149,150].
The more mature mother centrioles usually form the primary cilia with the formula 9×2 + 0 (Figure 5). The centrioles that arose in the current cell cycle and therefore are more immature form the motile cilia. Motile cilia have the formula 9×2 + 2 with the nine doublets MTs of the wall and two central MTs (Figure 4). The mature (proximal) centriole forms a centriolar adjunct in mammalian spermatids [150,151]. The centriolar adjunct has a formula similar to the primary cilium (9×2.5 + 0) (Figure 5). Simultaneously, the centriolar adjunct forms in the spermatid, the daughter (distal) centriole form a motile flagellum with the formula 9×2 + 2 (Figure 5).
Figure 5.
Four types of MT-contained structures: (a) proximal centriole in the early pig spermatid—formula “9×3 + 0”; (b) centriolar adjunct in the early pig spermatid—formula “9×2.5 + 0”; (c) flagellum in early pig spermatid—formula “9×2 + 2”; and (d) the cilia-like structure structurally similar to the primary cilium of mammals in the spermatid of the wasp Anisopteromalus calandrae—formula “9×2 + 0”. Scale bar: (a–d) 100 nm. (a–c) from [150]; and (d) from [20].
2.12. The Complete Process of Centrioles Maturation from Procentriole to Mother Centriole Takes More Than One and a Half Cell Cycles in Duration
The complete process of centrioles maturation from procentriole to mother centriole takes more than one and a half cell cycles in duration [36]. The exact timing of procentriole initiation is debatable [152]. The percentage of cells with procentrioles significantly exceeded the S-phase percentage in the cell cycle in synchronized HeLa cells. This difference may indicate that the process of procentriole formation began before DNA replication start [153]. It was shown later that the initiation of centriole duplication could occur even in the absence of a nucleus in cytoplasts and enucleated sea urchin zygotes [137,154]. Procentrioles were found near mother centrioles two hours before the start of DNA replication in pig kidney cell line, suggesting the procentrioles start to form before DNA replication [138,139]. However, whether this observation is universal in other cell types is unknown.
After its initial appearance, the centriole grows gradually to the mother’s size during the S phase and G2 phase of the cell cycle (Figure 4). It becomes a daughter centriole after mitosis in the newly formed cell. The centriole becomes a mature mother centriole, acquiring a complete set of its cell activities after the second mitosis in its life [36,152]. The cell (nuclear) cycle and the centriolar (centrosomal) cycle are mutually coordinated (Figure 4) at least at two critical points in the cell cycle: the end of the G1 and the end of the G2 phase [13,155]. In addition, some critical events take place in mitosis during a process termed “centriole to centrosome conversion” [156]. Moreover, many proteins involved in the regulation of the cell cycle are concentrated in the centrosome. Interference with this regulation leads to perturbation of cell cycle progression, ultimately leading to overproliferation or degeneration [157].
2.13. The Centrosome Is the Center of the Organization of Actin Microfilaments in the Cell
This novel function, “actin organization center”, is in addition to the classic centriole function in MT organizing [158]. The centrosome occupies a central position in the cytoplasm of many types of cells. This position is associated with other interconnected cytoskeleton elements, such as actin microfilaments. This position is regulated by the balance of the tension forces associated with the cytoplasmic dynein [159] and the repulsive forces caused by the growth of MT [160,161]. In addition, the centrosome position depends on the interaction of centrosomal MTs with the actomyosin complex, and on the activity of the actomyosin complex itself [162,163,164]. Besides this, the intermediate filaments’ architecture is indirectly dependent on the centrosome since the intermediate filaments system collapsed during the depolymerization of MT by colchicine or nocodazole [165,166].
2.14. Centrioles Can Gain an Atypical Structure and Composition and Become Undetected Using Standard Expectations and Techniques
Centrioles are remodeled and earn distinct novel structures in a species-specific manner that makes them difficult to detect [167,168]. In some insects, the sperm atypical centrioles are lacking MT [25,169,170]. In contrast, in human and bovine sperm, the MTs are present, but they are splayed around two novel rod structures [170]. Like canonical centrioles, these atypical centrioles recruit PCM, form centrosomes and asters, and participate in spindle formation in the zygote. In addition, canonical centrioles become undetected, including during myogenesis, oogenesis, and mice spermatogenesis [171,172,173]. In amoeboflagellate Naegleria, centriolar cylinders are formed de novo during the transition from centriole-less amoebae to flagellates with basal bodies from precursor complexes that act as radiometry centrioles [174,175]. Therefore, centrioles can gain novel structure and composition while performing many of their classical functions.
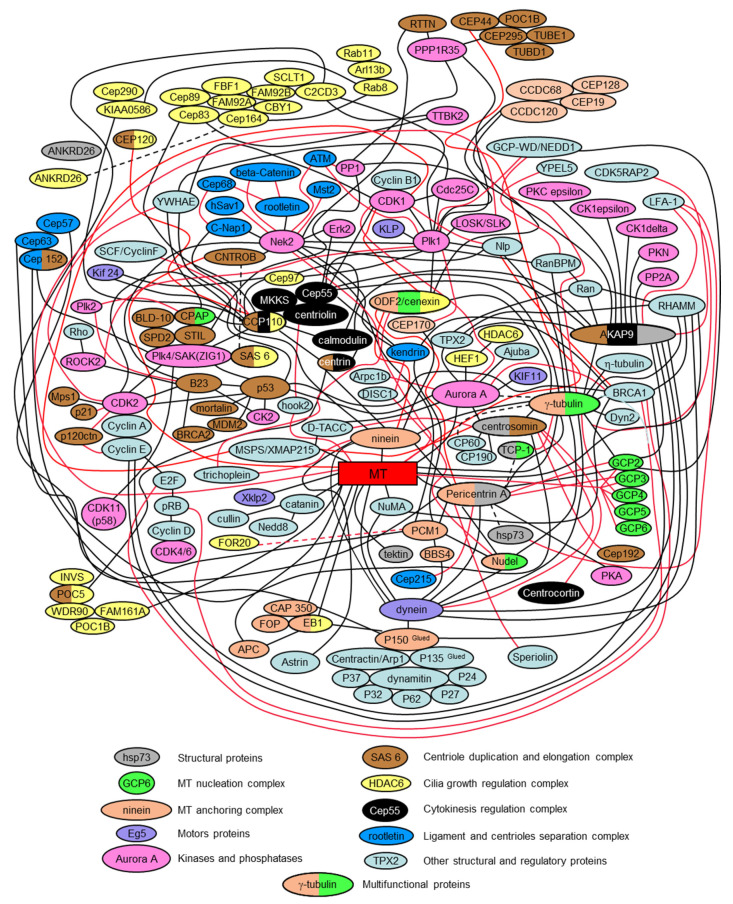
2.15. The Centrosome Is a Polyfunctional, Multi-Protein, and Cell Regulation Complex; Some of Its Proteins Simultaneously Regulate Several Intracellular Processes
The presented interactome scheme in Figure 6 is only a partial depiction of all centrosomal proteins and their interactions. It is intended to be a simplified diagram that helps to assess the complexity of the centrosome’s biochemical organization. Indeed, each year, more and more centrosomal proteins are characterized. These proteins include proteins that mediate or regulate processes such as signaling pathways, nucleation and anchoring of microtubules, centriole duplication, separation of the daughter centriole from the mother centriole, conversation of centrioles to basal bodies, and nucleating of cilia and flagella. It is often impossible to separate the functions performed by the same protein in different aspects of centrosome functioning.
Figure 6.
Scheme of protein interactions and functional protein complexes in centrosome (interactome) ([13] with modifications). Red and black lines mark centrosomal protein interactions.
A single universal classification for centrosomal proteins is unsubtle since they can be organized according to several parameters. Many of them have multiple characteristics for any parameters such as localization, timing, and activity. In the case of localization, some proteins, such as tubulins, are both in the centrioles and pericentriolar material, or the cilium axoneme. In terms of timing, some proteins always present in the centrosome. In contrast, other proteins appear in the centrosome only at specific periods of the cell cycle or at a particular cell differentiation stage. In terms of activity, the centrosomal proteins can be classified according to their biochemical activity—kinases, phosphatases, motors, and structural proteins.
Centrosomal proteins do not exist on their own, but form complex, often interconnected, functional complexes. Figure 6 shows how some of these complexes are related to each other.
The following publications were used to prepare the presented interactome version: [13,19,38,49,52,57,63,68,69,70,71,75,83,84,89,92,93,98,126,127,130,133,134,141,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355].
3. Questions and Perspectives
The centrosome continues to remain “the central enigma of cell biology” [30], although many data have been obtained in recent years on many of its various aspects. The exact time of the onset of centriole duplication in the cell cycle and the temporal relationship of this process with DNA replication remains unclear. It is also not entirely clear to what extent the principles of centriole biology are maintained in various cell types. For example, there is no certainty about the mechanism of formation of centrioles in the early development of mammals: How is the only proximal centriole of the spermatozoon transformed into four centrioles at two poles of mitotic division at later stages of development? What is the nature of the differences between this process in mice and other mammals [356]? What are the evolutionary and molecular mechanisms underlying these centriole specific properties [357]? Many of these aspects will be clarified in the near future, and the role of the centrosome, as the main regulatory center of the cell—a kind of “cell processor” [358]—will become more evident.
Acknowledgments
The authors are grateful to Elena Nadezhdina for helpful discussion and for providing Figure 3. Data were obtained with the assistance of the IBiSA Electron Microscopy Facility of Tours University and the University Hospital of Tours. Many thanks are given to Andreas Merdes, Greenfield Sluder, Pierre Gönczy, and Jadranka Loncarek for comments on the manuscript, which helped us to complement and better structure the review content.
Author Contributions
R.E.U. wrote the original draft of the manuscript; and R.E.U. and T.A.-R. wrote and edited the paper. Both authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant R03 HD087429 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Flemming W. Studien in der entwicklungsgeschichte der najaden. Sitz. Akad. Wissensch Wien. 1875;71:81–147. [Google Scholar]
- 2.Hertwig O. Beitrage zur kenntniss der bildung, befruchtung und theilung des thierischen eies. Morphol. Jb. 1875;1:347–434. doi: 10.2307/1411521. [DOI] [Google Scholar]
- 3.Van Beneden E. Recherches sur les dicyémides, survivants actuels d’un embranchement des mésozoaires. Bull. Acad. Roy. Méd. Belg. 1876;41:1160–1205. [Google Scholar]
- 4.Van Beneden E., Neyt A. Nouvelles recherches sur la fecondation et la division mitoshes l’ascaride megalocephale. [(accessed on 23 September 2020)];Bull. Acad. R. Méd. Belg. 1887 14:1–81. Available online: https://orbi.uliege.be/handle/2268/160175. [Google Scholar]
- 5.Boveri T. Die Bildung der Richtungskörper bei Ascaris megalocephala und Ascaris lumbricoides. Zellen-Studien Heft 1. Verlag von Gustav Fischer; Jena, Germany: 1887. [(accessed on 23 September 2020)]. Available online: https://www.biozentrum.uni-wuerzburg.de/zeb/research/topics/theodor-boveri/cell-studies/ [Google Scholar]
- 6.Boveri T. Ueber das Verhalten der Centrosomen bei der Befruchtung des Seeigel-Eies, nebst allgemeinen Bemerkungen über Centrosomen und Verwandtes. [(accessed on 23 September 2020)];Verhandl. Phys.-Med. Ges. Würzburg. 1895 29:1–75. Available online: https://www.biozentrum.uni-wuerzburg.de/fileadmin/07020100/2018/Downloads_PDF/Boveri_1895a.pdf. [Google Scholar]
- 7.Henneguy L.F. Sur les rapports des cils vibratiles avec les centrosomes. Arch. Microsc. Morph. Exp. 1898;1:481–496. [Google Scholar]
- 8.Von Lenhossék M. Uber flimmerzellen. Verh. Anat. Ges. Kiel. 1898;12:106–128. [Google Scholar]
- 9.Afzelius B. Electron microscopy of the sperm tail; results obtained with a new fixative. J. Biophys. Biochem. Cytol. 1959;5:269–278. doi: 10.1083/jcb.5.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbons I.R., Grimstone A.V. On flagellar structure in certain flagellates. J. Biophys. Biochem. Cytol. 1960;7:697–716. doi: 10.1083/jcb.7.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkley B.R., Stubblefield E. Ultrastructure and interaction of the kinetochore and centriole in mitosis and meiosis. In: Prescott D.M., Mc Conkey E., editors. Advances in Cell Biology. Volume 1. Appleton-Century Crofts; New York, NY, USA: 1970. pp. 119–184. [Google Scholar]
- 12.Uzbekov R., Prigent C. Clockwise or anticlockwise? Turning the centriole triplets in the right direction! FEBS Lett. 2007;581:1251–1254. doi: 10.1016/j.febslet.2007.02.069. [DOI] [PubMed] [Google Scholar]
- 13.Uzbekov R.E., Alieva I.B. Centrosome: History of Study and New Discoveries. From Cytoplasmic Granule to the Center of Intracellular Regulation. Moscow University Press; Moscow, Russia: 2013. [Google Scholar]
- 14.Sulston J.E., Albertson D.G., Thomson J.N. The caenorhabditis elegans male: Postembryonic development of nongonadal structures. Dev. Biol. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- 15.Riparbelli M.G., Dallai R., Callaini G. The insect centriole: A land of discovery. Tissue Cell. 2010;42:69–80. doi: 10.1016/j.tice.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Gottardo M., Callaini G., Riparbelli M.G. The drosophila centriole–conversion of doublets into triplets within the stem cell niche. J. Cell Sci. 2015;128:2437–2442. doi: 10.1242/jcs.172627. [DOI] [PubMed] [Google Scholar]
- 17.Favard P. Evolution des ultrastructures cellulaires au cours de la spermatogenese de l’ascaris (ascaris megalocephala, schrank = parascaris equorum, goerze) Ann. Des Sci. Nat. Zool. Et Biol. Anim. 1961;12:52–152. [Google Scholar]
- 18.Wolf N., Hirsh D., McIntosh J.R. Spermatogenesis in males of the free-living nematode, caenorhabditis elegans. J. Ultrastruct. Res. 1978;63:155–169. doi: 10.1016/S0022-5320(78)80071-9. [DOI] [PubMed] [Google Scholar]
- 19.Pelletier L., O’Toole E., Schwager A., Hyman A.A., Muller-Reichert T. Centriole assembly in caenorhabditis elegans. Nature. 2006;444:619–623. doi: 10.1038/nature05318. [DOI] [PubMed] [Google Scholar]
- 20.Uzbekov R., Garanina A., Bressac C. Centrioles without microtubules: A new morphological type of centriole. Biol. Open. 2018;7:bio036012. doi: 10.1242/bio.036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uzbekov R., Garanina A.S., Burlaud-Gaillard J., Bressac C. The flagellum of the shortest spermatozoon in the animal kingdom. Elongation and shortening of the axoneme in the process of spermiogenesis of the parasitic wasp cotesia congregata. In: Uzbekov R., editor. Flagella and Cilia: Types, Structure and Functions. Nova Science Publishers, Inc.; New York, NY, USA: 2018. pp. 83–108. [Google Scholar]
- 22.McNitt R. Centriole ultrastructure and its possible role in microtubule formation in an aquatic fungus. Protoplasma. 1974;80:91–108. doi: 10.1007/BF01666353. [DOI] [PubMed] [Google Scholar]
- 23.Greenan G.A., Keszthelyi B., Vale R.D., Agard D.A. Insights into centriole geometry revealed by cryotomography of doublet and triplet centrioles. ELife. 2018;7:e36851. doi: 10.7554/eLife.36851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jana S.C., Girotra M., Ray K. Heterotrimeric kinesin-ii is necessary and sufficient to promote different stepwise assembly of morphologically distinct bipartite cilia in drosophila antenna. Mol. Biol. Cell. 2011;22:769–781. doi: 10.1091/mbc.e10-08-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottardo M., Pollarolo G., Llamazares S., Reina J., Riparbelli M.G., Callaini G., Gonzalez C. Loss of centrobin enables daughter centrioles to form sensory cilia in drosophila. Curr. Biol. 2015;25:2319–2324. doi: 10.1016/j.cub.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Nechipurenko I.V., Berciu C., Sengupta P., Nicastro D. Centriolar remodeling underlies basal body maturation during ciliogenesis in caenorhabditis elegans. ELife. 2017;6:e25686. doi: 10.7554/eLife.25686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringo D.L. The arrangement of subunits in flagellar fibers. J. Ultrastruct. Res. 1967;17:266–277. doi: 10.1016/S0022-5320(67)80048-0. [DOI] [PubMed] [Google Scholar]
- 28.Tilney L.G., Bryan J., Bush D.J., Fujiwara K., Mooseker M.S., Murphy D.B., Snyder D.H. Microtubules: Evidence for 13 protofilaments. J. Cell Biol. 1973;59:267–275. doi: 10.1083/jcb.59.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.André J. Le centriole et la région centrosomienne. J. Microsc. 1964;3:1–23. [Google Scholar]
- 30.Wheatley D.N. The Centriole: A Central Enigma of Cell Biology. Elsevier Biomedical Press; Amsterdam, The Netherlands: New York, NY, USA: 1982. [Google Scholar]
- 31.Ou Y., Rattner J.B. The centrosome in higher organisms: Structure, composition, and duplication. Int. Rev. Cytol. 2004;238:119–182. doi: 10.1016/S0074-7696(04)38003-4. [DOI] [PubMed] [Google Scholar]
- 32.Guichard P., Hachet V., Majubu N., Neves A., Demurtas D., Olieric N., Fluckiger I., Yamada A., Kihara K., Nishida Y., et al. Native architecture of the centriole proximal region reveals features underlying its 9-fold radial symmetry. Curr. Biol. 2013;23:1620–1628. doi: 10.1016/j.cub.2013.06.061. [DOI] [PubMed] [Google Scholar]
- 33.Gall J.G. Centriole replication. A study of spermatogenesis in the snail viviparus. J. Biophys. Biochem. Cytol. 1961;10:163–193. doi: 10.1083/jcb.10.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitagawa D., Vakonakis I., Olieric N., Hilbert M., Keller D., Olieric V., Bortfeld M., Erat M.C., Fluckiger I., Gonczy P., et al. Structural basis of the 9-fold symmetry of centrioles. Cell. 2011;144:364–375. doi: 10.1016/j.cell.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Breugel M., Hirono M., Andreeva A., Yanagisawa H.A., Yamaguchi S., Nakazawa Y., Morgner N., Petrovich M., Ebong I.O., Robinson C.V., et al. Structures of sas-6 suggest its organization in centrioles. Science. 2011;331:1196–1199. doi: 10.1126/science.1199325. [DOI] [PubMed] [Google Scholar]
- 36.Vorobjev I.A., Chentsov Y.S. Centrioles in the cell cycle. I. Epithelial cells. J. Cell Biol. 1982;93:938–949. doi: 10.1083/jcb.93.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedlander M., Wahrman J. Giant centrioles in neuropteran meiosis. J. Cell Sci. 1966;1:129–144. doi: 10.1242/jcs.1.1.129. [DOI] [PubMed] [Google Scholar]
- 38.Spektor A., Tsang W.Y., Khoo D., Dynlacht B.D. Cep97 and cp110 suppress a cilia assembly program. Cell. 2007;130:678–690. doi: 10.1016/j.cell.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 39.Szollosi D. The structure and function of centrioles and their satellites in the jellyfish phialidium gregarium. J. Cell Biol. 1964;21:465–479. doi: 10.1083/jcb.21.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson R.G. The three-dimensional structure of the basal body from the rhesus monkey oviduct. J. Cell Biol. 1972;54:246–265. doi: 10.1083/jcb.54.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernhard W., de Harven E. L’ultrastructure du centriole et d’autres éléments de l’appareil achromatique. In: Bargmann W., Möllenstedt G., Niehrs H., Peters D., Ruska E., Wolpers C., editors. Vierter Internationaler Kongress fur Elektronenmikroskopie, 1958. Volume 2. Springer; Berlin, Germany: 1960. pp. 217–227. [Google Scholar]
- 42.Stubblefield E.B., Brinkley R. Architecture and function of the mammalian centriole. In: Warren K.B., editor. Formation and Fate of Cell Organelles. Academic Press Inc.; New York, NY, USA: 1967. pp. 175–218. [Google Scholar]
- 43.Vorobjev I.A., Chentsov Y.S. The ultrastructure of the centrosome in the cells of hematopoietic tissues of axolotl. Tsitologiia. 1977;19:598–603. [Google Scholar]
- 44.Uzbekov R., Alieva I. Who are you, subdistal appendages of centriole? Open Biol. 2018;8:180062. doi: 10.1098/rsob.180062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doolin P.F., Birge W.J. Ultrastructural organization of cilia and basal bodies of the epithelium of the choroid plexus in the chick embryo. J. Cell Biol. 1966;29:333–345. doi: 10.1083/jcb.29.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorokin S.P. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 1968;3:207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe J. Basal body fine structure and chemistry. Adv. Cell Mol. Biol. 1972;2:151–192. [Google Scholar]
- 48.Gibbons I.R. The relationship between the fine structure and direction of beat in gill cilia of a lamellibranch mollusc. J. Biophys. Biochem. Cytol. 1961;11:179–205. doi: 10.1083/jcb.11.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ou Y., Rattner J.B. A subset of centrosomal proteins are arranged in a tubular conformation that is reproduced during centrosome duplication. Cell Motil. Cytoskelet. 2000;47:13–24. doi: 10.1002/1097-0169(200009)47:1<13::AID-CM2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 50.Guichard P., Chretien D., Marco S., Tassin A.M. Procentriole assembly revealed by cryo-electron tomography. EMBO J. 2010;29:1565–1572. doi: 10.1038/emboj.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Hara P.T. Spiral tilt of triplet fibers in human leukocyte centrioles. J. Ultrastruct. Res. 1970;31:195–198. doi: 10.1016/S0022-5320(70)90154-1. [DOI] [PubMed] [Google Scholar]
- 52.Le Guennec M., Klena N., Gambarotto D., Laporte M.H., Tassin A.M., van den Hoek H., Erdmann P.S., Schaffer M., Kovacik L., Borgers S., et al. A helical inner scaffold provides a structural basis for centriole cohesion. Sci. Adv. 2020;6:eaaz4137. doi: 10.1126/sciadv.aaz4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avidor-Reiss T., Gopalakrishnan J. Cell cycle regulation of the centrosome and cilium. Drug Discov. Today. Dis. Mech. 2013;10:e119–e124. doi: 10.1016/j.ddmec.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basiri M.L., Blachon S., Chim Y.C., Avidor-Reiss T. Imaging centrosomes in fly testes. J. Vis. Exp. 2013:e50938. doi: 10.3791/50938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schreiner A., Schreiner K.E. Űber die entwicklung der männlichen geschlechtszellen von myxine glutinosa (l.) Arch. Biol. 1905;21:183–357. [Google Scholar]
- 56.Guichard P., Desfosses A., Maheshwari A., Hachet V., Dietrich C., Brune A., Ishikawa T., Sachse C., Gonczy P. Cartwheel architecture of trichonympha basal body. Science. 2012;337:553. doi: 10.1126/science.1222789. [DOI] [PubMed] [Google Scholar]
- 57.Azimzadeh J., Hergert P., Delouvee A., Euteneuer U., Formstecher E., Khodjakov A., Bornens M. Hpoc5 is a centrin-binding protein required for assembly of full-length centrioles. J. Cell Biol. 2009;185:101–114. doi: 10.1083/jcb.200808082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blachon S., Cai X., Roberts K.A., Yang K., Polyanovsky A., Church A., Avidor-Reiss T. A proximal centriole-like structure is present in drosophila spermatids and can serve as a model to study centriole duplication. Genetics. 2009;182:133–144. doi: 10.1534/genetics.109.101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohlmaier G., Loncarek J., Meng X., McEwen B.F., Mogensen M.M., Spektor A., Dynlacht B.D., Khodjakov A., Gonczy P. Overly long centrioles and defective cell division upon excess of the sas-4-related protein cpap. Curr. Biol. 2009;19:1012–1018. doi: 10.1016/j.cub.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang C.J., Fu R.H., Wu K.S., Hsu W.B., Tang T.K. Cpap is a cell-cycle regulated protein that controls centriole length. Nat. Cell Biol. 2009;11:825–831. doi: 10.1038/ncb1889. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt T.I., Kleylein-Sohn J., Westendorf J., Le Clech M., Lavoie S.B., Stierhof Y.D., Nigg E.A. Control of centriole length by cpap and cp110. Curr. Biol. 2009;19:1005–1011. doi: 10.1016/j.cub.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 62.Comartin D., Gupta G.D., Fussner E., Coyaud E., Hasegan M., Archinti M., Cheung S.W., Pinchev D., Lawo S., Raught B., et al. Cep120 and spice1 cooperate with cpap in centriole elongation. Curr. Biol. 2013;23:1360–1366. doi: 10.1016/j.cub.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Lin Y.N., Wu C.T., Lin Y.C., Hsu W.B., Tang C.J., Chang C.W., Tang T.K. Cep120 interacts with cpap and positively regulates centriole elongation. J. Cell Biol. 2013;202:211–219. doi: 10.1083/jcb.201212060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kong D., Sahabandu N., Sullenberger C., Vasquez-Limeta A., Luvsanjav D., Lukasik K., Loncarek J. Prolonged mitosis results in structurally aberrant and over-elongated centrioles. J. Cell Biol. 2020;219:e201910019. doi: 10.1083/jcb.201910019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oakley C.E., Oakley B.R. Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipa gene of aspergillus nidulans. Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- 66.Joshi H.C., Palacios M.J., McNamara L., Cleveland D.W. Gamma-tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 1992;356:80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- 67.Zheng Y., Wong M.L., Alberts B., Mitchison T. Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- 68.Moritz M., Zheng Y., Alberts B.M., Oegema K. Recruitment of the gamma-tubulin ring complex to drosophila salt-stripped centrosome scaffolds. J. Cell Biol. 1998;142:775–786. doi: 10.1083/jcb.142.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oegema K., Wiese C., Martin O.C., Milligan R.A., Iwamatsu A., Mitchison T.J., Zheng Y. Characterization of two related drosophila gamma-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 1999;144:721–733. doi: 10.1083/jcb.144.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan X., Habedanck R., Nigg E.A. A complex of two centrosomal proteins, cap350 and fop, cooperates with eb1 in microtubule anchoring. Mol. Biol. Cell. 2006;17:634–644. doi: 10.1091/mbc.e05-08-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J.C., Badano J.L., Sibold S., Esmail M.A., Hill J., Hoskins B.E., Leitch C.C., Venner K., Ansley S.J., Ross A.J., et al. The bardet-biedl protein bbs4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat. Genet. 2004;36:462–470. doi: 10.1038/ng1352. [DOI] [PubMed] [Google Scholar]
- 72.Azimzadeh J., Bornens M. Structure and duplication of the centrosome. J. Cell Sci. 2007;120:2139–2142. doi: 10.1242/jcs.005231. [DOI] [PubMed] [Google Scholar]
- 73.De Harven E. The centriole and the mitotic spindle. In: Dalton A.J., Haguenau F., editors. The Nucleus. Academic Press; New York, NY, USA: 1968. pp. 197–227. [Google Scholar]
- 74.Tilney L.G., Goddard J. Nucleated sites for the assembly of cytoplasmic microtubules in the ectodermal cells of blastulae of arbacia punctulata. J. Cell Biol. 1970;46:564–575. doi: 10.1083/jcb.46.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dammermann A., Merdes A. Assembly of centrosomal proteins and microtubule organization depends on pcm-1. J. Cell Biol. 2002;159:255–266. doi: 10.1083/jcb.200204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kubo A., Tsukita S. Non-membranous granular organelle consisting of pcm-1: Subcellular distribution and cell-cycle-dependent assembly/disassembly. J. Cell Sci. 2003;116:919–928. doi: 10.1242/jcs.00282. [DOI] [PubMed] [Google Scholar]
- 77.Conkar D., Bayraktar H., Firat-Karalar E.N. Centrosomal and ciliary targeting of ccdc66 requires cooperative action of centriolar satellites, microtubules and molecular motors. Sci. Rep. 2019;9:14250. doi: 10.1038/s41598-019-50530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prosser S.L., Pelletier L. Centriolar satellite biogenesis and function in vertebrate cells. J. Cell Sci. 2020;133:jcs239566. doi: 10.1242/jcs.239566. [DOI] [PubMed] [Google Scholar]
- 79.Bornens M., Paintrand M., Berges J., Marty M.C., Karsenti E. Structural and chemical characterization of isolated centrosomes. Cell Motil. Cytoskelet. 1987;8:238–249. doi: 10.1002/cm.970080305. [DOI] [PubMed] [Google Scholar]
- 80.Tournier F., Komesli S., Paintrand M., Job D., Bornens M. The intercentriolar linkage is critical for the ability of heterologous centrosomes to induce parthenogenesis in xenopus. J. Cell Biol. 1991;113:1361–1369. doi: 10.1083/jcb.113.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paintrand M., Moudjou M., Delacroix H., Bornens M. Centrosome organization and centriole architecture: Their sensitivity to divalent cations. J. Struct. Biol. 1992;108:107–128. doi: 10.1016/1047-8477(92)90011-X. [DOI] [PubMed] [Google Scholar]
- 82.Yang J., Liu X., Yue G., Adamian M., Bulgakov O., Li T. Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. J. Cell Biol. 2002;159:431–440. doi: 10.1083/jcb.200207153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bahe S., Stierhof Y.D., Wilkinson C.J., Leiss F., Nigg E.A. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J. Cell Biol. 2005;171:27–33. doi: 10.1083/jcb.200504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bahmanyar S., Kaplan D.D., Deluca J.G., Giddings T.H., Jr., O’Toole E.T., Winey M., Salmon E.D., Casey P.J., Nelson W.J., Barth A.I. Beta-catenin is a nek2 substrate involved in centrosome separation. Genes Dev. 2008;22:91–105. doi: 10.1101/gad.1596308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsou M.F., Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–951. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- 86.Uzbekov R., Kireyev I., Prigent C. Centrosome separation: Respective role of microtubules and actin filaments. Biol. Cell. 2002;94:275–288. doi: 10.1016/S0248-4900(02)01202-9. [DOI] [PubMed] [Google Scholar]
- 87.Glover D.M., Leibowitz M.H., McLean D.A., Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 88.Roghi C., Giet R., Uzbekov R., Morin N., Chartrain I., Le Guellec R., Couturier A., Doree M., Philippe M., Prigent C. The xenopus protein kinase peg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J. Cell Sci. 1998;111:557–572. doi: 10.1242/jcs.111.5.557. [DOI] [PubMed] [Google Scholar]
- 89.Giet R., Uzbekov R., Cubizolles F., Le Guellec K., Prigent C. The xenopus laevis aurora-related protein kinase peg2 associates with and phosphorylates the kinesin-related protein xleg5. J. Biol. Chem. 1999;274:15005–15013. doi: 10.1074/jbc.274.21.15005. [DOI] [PubMed] [Google Scholar]
- 90.Cowley D.O., Rivera-Perez J.A., Schliekelman M., He Y.J., Oliver T.G., Lu L., O’Quinn R., Salmon E.D., Magnuson T., Van Dyke T. Aurora-a kinase is essential for bipolar spindle formation and early development. Mol. Cell. Biol. 2009;29:1059–1071. doi: 10.1128/MCB.01062-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blangy A., Arnaud L., Nigg E.A. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin-related motor hseg5 to the dynactin subunit p150. J. Biol. Chem. 1997;272:19418–19424. doi: 10.1074/jbc.272.31.19418. [DOI] [PubMed] [Google Scholar]
- 92.Lane H.A., Nigg E.A. Cell-cycle control: Polo-like kinases join the outer circle. Trends Cell Biol. 1997;7:63–68. doi: 10.1016/S0962-8924(96)10051-9. [DOI] [PubMed] [Google Scholar]
- 93.Glover D.M., Hagan I.M., Tavares A.A. Polo-like kinases: A team that plays throughout mitosis. Genes Dev. 1998;12:3777–3787. doi: 10.1101/gad.12.24.3777. [DOI] [PubMed] [Google Scholar]
- 94.Golsteyn R.M., Lane H.A., Mundt K.E., Arnaud L., Nigg E.A. The family of polo-like kinases. Prog. Cell Cycle Res. 1996;2:107–114. doi: 10.1007/978-1-4615-5873-6_11. [DOI] [PubMed] [Google Scholar]
- 95.Gaglio T., Saredi A., Bingham J.B., Hasbani M.J., Gill S.R., Schroer T.A., Compton D.A. Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J. Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaglio T.D., Dionne M.A., Compton D.A. Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J. Cell Biol. 1997;138:1055–1066. doi: 10.1083/jcb.138.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Echeverri C.J.P., Paschal B.M., Vaughan K.T., Vallee R.B. Molecular characterization of the 50-kd subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boleti H.K., Karsenti E., Vernos I. Xklp2, a novel xenopus centrosomal kinesin-like protein required for centrosome sepqration during mitosis. Cell. 1996;84:49–59. doi: 10.1016/S0092-8674(00)80992-7. [DOI] [PubMed] [Google Scholar]
- 99.Sawin K.E., LeGuellec K., Philippe M., Mitchison T.J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- 100.Walczak C.E., Verma S., Mitchison T.J. Xctk2: A kinesin-related protein that promotes mitotic spindle assembly in xenopus laevis egg extracts. J. Cell Biol. 1997;136:859–870. doi: 10.1083/jcb.136.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Harven E., Bernhard W. Etude au microscope électronique de l’ultrastructure du centriole chez les vertébrés. Z. Zellforsch. U. Mokr. Anat. 1956;45:1–378. [PubMed] [Google Scholar]
- 102.Osborn M., Weber K. Cytoplasmic microtubules in tissue culture cells appear to grow from an organizing structure towards the plasma membrane. Proc. Natl. Acad. Sci. USA. 1976;73:867–871. doi: 10.1073/pnas.73.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jensen C.G., Jensen L.C., Rieder C.L. The occurrence and structure of primary cilia in a subline of potorous tridactylus. Exp. Cell Res. 1979;123:444–449. doi: 10.1016/0014-4827(79)90497-X. [DOI] [PubMed] [Google Scholar]
- 104.Fais D.A., Nadezhdina E.S., Chentsov Y.S. The centriolar rim. The structure that maintains the configuration of centrioles and basal bodies in the absence of their microtubules. Exp. Cell Res. 1986;164:27–34. doi: 10.1016/0014-4827(86)90451-9. [DOI] [PubMed] [Google Scholar]
- 105.Steffen W., Linck R.W. Evidence for tektins in centrioles and axonemal microtubules. Proc. Natl. Acad. Sci. USA. 1988;85:2643–2647. doi: 10.1073/pnas.85.8.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stephens R.E., Lemieux N.A. Tektins as structural determinants in basal bodies. Cell Motil. Cytoskelet. 1998;40:379–392. doi: 10.1002/(SICI)1097-0169(1998)40:4<379::AID-CM6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 107.Plessmann U., Weber K. Mammalian sperm tubulin: An exceptionally large number of variants based on several posttranslational modifications. J. Protein Chem. 1997;16:385–390. doi: 10.1023/A:1026332621215. [DOI] [PubMed] [Google Scholar]
- 108.Bobinnec Y., Khodjakov A., Mir L.M., Rieder C.L., Edde B., Bornens M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 1998;143:1575–1589. doi: 10.1083/jcb.143.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steib E., Gambarotto D., Laporte M.H., Olieric N., Zheng C., Borgers S., Olieric V., Le Guennec M., Koll F., Tassin A.-M., et al. The inner scaffold protects from centriole fracture. bioRxiv. 2020 doi: 10.1101/2020.02.15.950444. [DOI] [Google Scholar]
- 110.Pearson C.G., Osborn D.P., Giddings T.H., Jr., Beales P.L., Winey M. Basal body stability and ciliogenesis requires the conserved component poc1. J. Cell Biol. 2009;187:905–920. doi: 10.1083/jcb.200908019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lattao R., Kovacs L., Glover D.M. The centrioles, centrosomes, basal bodies, and cilia of drosophila melanogaster. Genetics. 2017;206:33–53. doi: 10.1534/genetics.116.198168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 2002;14:25–34. doi: 10.1016/S0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- 113.Ou Y.Y., Zhang M., Chi S., Matyas J.R., Rattner J.B. Higher order structure of the pcm adjacent to the centriole. Cell Motil. Cytoskelet. 2003;55:125–133. doi: 10.1002/cm.10115. [DOI] [PubMed] [Google Scholar]
- 114.Rieder C.L., Jensen C.G., Jensen L.C. The resorption of primary cilia during mitosis in a vertebrate (ptk1) cell line. J. Ultrastruct. Res. 1979;68:173–185. doi: 10.1016/S0022-5320(79)90152-7. [DOI] [PubMed] [Google Scholar]
- 115.De Brabander M., Geuens G., Nuydens R., Willebrords R., Aerts F., De Mey J. Microtubule dynamics during the cell cycle: The effects of taxol and nocodazole on the microtubule system of pt k2 cells at different stages of the mitotic cycle. Int. Rev. Cytol. 1986;101:215–274. doi: 10.1016/s0074-7696(08)60250-8. [DOI] [PubMed] [Google Scholar]
- 116.Kuriyama R., Borisy G.G. Centriole cycle in chinese hamster ovary cells as determined by whole-mount electron microscopy. J. Cell Biol. 1981;91:814–821. doi: 10.1083/jcb.91.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Palazzo R.E., Vogel J.M., Schnackenberg B.J., Hull D.R., Wu X. Centrosome maturation. Curr. Top. Dev. Biol. 2000;49:449–470. doi: 10.1016/s0070-2153(99)49021-0. [DOI] [PubMed] [Google Scholar]
- 118.O’Connell K.F., Caron C., Kopish K.R., Hurd D.D., Kemphues K.J., Li Y., White J.G. The c. Elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell. 2001;105:547–558. doi: 10.1016/S0092-8674(01)00338-5. [DOI] [PubMed] [Google Scholar]
- 119.O’Connell K.F. The zyg-1 kinase, a mitotic and meiotic regulator of centriole replication. Oncogene. 2002;21:6201–6208. doi: 10.1038/sj.onc.1205776. [DOI] [PubMed] [Google Scholar]
- 120.Kemp C.A., Kopish K.R., Zipperlen P., Ahringer J., O’Connell K.F. Centrosome maturation and duplication in c. Elegans require the coiled-coil protein spd-2. Dev. Cell. 2004;6:511–523. doi: 10.1016/S1534-5807(04)00066-8. [DOI] [PubMed] [Google Scholar]
- 121.Pelletier L., Ozlu N., Hannak E., Cowan C., Habermann B., Ruer M., Muller-Reichert T., Hyman A.A. The caenorhabditis elegans centrosomal protein spd-2 is required for both pericentriolar material recruitment and centriole duplication. Curr. Biol. 2004;14:863–873. doi: 10.1016/j.cub.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 122.Kirkham M., Muller-Reichert T., Oegema K., Grill S., Hyman A.A. Sas-4 is a c. Elegans centriolar protein that controls centrosome size. Cell. 2003;112:575–587. doi: 10.1016/S0092-8674(03)00117-X. [DOI] [PubMed] [Google Scholar]
- 123.Leidel S., Gonczy P. Sas-4 is essential for centrosome duplication in c elegans and is recruited to daughter centrioles once per cell cycle. Dev. Cell. 2003;4:431–439. doi: 10.1016/S1534-5807(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 124.Delattre M., Leidel S., Wani K., Baumer K., Bamat J., Schnabel H., Feichtinger R., Schnabel R., Gonczy P. Centriolar sas-5 is required for centrosome duplication in c. Elegans. Nat. Cell Biol. 2004;6:656–664. doi: 10.1038/ncb1146. [DOI] [PubMed] [Google Scholar]
- 125.Dammermann A., Muller-Reichert T., Pelletier L., Habermann B., Desai A., Oegema K. Centriole assembly requires both centriolar and pericentriolar material proteins. Dev. Cell. 2004;7:815–829. doi: 10.1016/j.devcel.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 126.Leidel S., Delattre M., Cerutti L., Baumer K., Gonczy P. Sas-6 defines a protein family required for centrosome duplication in c. Elegans and in human cells. Nat. Cell Biol. 2005;7:115–125. doi: 10.1038/ncb1220. [DOI] [PubMed] [Google Scholar]
- 127.Habedanck R., Stierhof Y.D., Wilkinson C.J., Nigg E.A. The polo kinase plk4 functions in centriole duplication. Nat. Cell Biol. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- 128.Bettencourt-Dias M., Rodrigues-Martins A., Carpenter L., Riparbelli M., Lehmann L., Gatt M.K., Carmo N., Balloux F., Callaini G., Glover D.M. Sak/plk4 is required for centriole duplication and flagella development. Curr. Biol. 2005;15:2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 129.Strnad P., Gonczy P. Mechanisms of procentriole formation. Trends Cell Biol. 2008;18:389–396. doi: 10.1016/j.tcb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 130.Blachon S., Gopalakrishnan J., Omori Y., Polyanovsky A., Church A., Nicastro D., Malicki J., Avidor-Reiss T. Drosophila asterless and vertebrate cep152 are orthologs essential for centriole duplication. Genetics. 2008;180:2081–2094. doi: 10.1534/genetics.108.095141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guernsey D.L., Jiang H., Hussin J., Arnold M., Bouyakdan K., Perry S., Babineau-Sturk T., Beis J., Dumas N., Evans S.C., et al. Mutations in centrosomal protein cep152 in primary microcephaly families linked to mcph4. Am. J. Hum. Genet. 2010;87:40–51. doi: 10.1016/j.ajhg.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hatch E., Stearns T. The life cycle of centrioles. Cold Spring Harb. Symp. Quant. Biol. 2010;75:425–431. doi: 10.1101/sqb.2010.75.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brown N.J., Marjanovic M., Luders J., Stracker T.H., Costanzo V. Cep63 and cep152 cooperate to ensure centriole duplication. PLoS ONE. 2013;8:e69986. doi: 10.1371/journal.pone.0069986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lukinavicius G., Lavogina D., Orpinell M., Umezawa K., Reymond L., Garin N., Gonczy P., Johnsson K. Selective chemical crosslinking reveals a cep57-cep63-cep152 centrosomal complex. Curr. Biol. 2013;23:265–270. doi: 10.1016/j.cub.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 135.Rattner J.B., Phillips S.G. Independence of centriole formation and DNA synthesis. J. Cell Biol. 1973;57:359–372. doi: 10.1083/jcb.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sluder G. Centrosomes and the cell cycle. J. Cell Sci. Suppl. 1989;12:253–275. doi: 10.1242/jcs.1989.Supplement_12.21. [DOI] [PubMed] [Google Scholar]
- 137.Gorgidze L.A., Vorobjev I.A. Centrosome and microtubules behavior in the cytoplasts. J. Submicrosc. Cytol. Pathol. 1995;27:381–389. [PubMed] [Google Scholar]
- 138.Uzbekov R.E. Centriole duplication in PE (SPEV) cells starts before beginning of DNA replication. Biochem. Moscow Suppl. Ser. A. 2007;1:206–211. doi: 10.1134/S1990747807030026. [DOI] [Google Scholar]
- 139.Uzbekov R.E., Alieva I.B. Centriole duplication or DNA replication—What starts earlier? In: Lansing S., Rousseau T., editors. Cytoskeleton: Cell Movement, Cytokinesis and Organelles Organization. Nova Science Publishers, Inc.; New York, NY, USA: 2010. pp. 127–137. [Google Scholar]
- 140.Hinchcliffe E.H., Li C., Thompson E.A., Maller J.L., Sluder G. Requirement of cdk2-cyclin e activity for repeated centrosome reproduction in xenopus egg extracts. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- 141.Lacey K.R., Jackson P.K., Stearns T. Cyclin-dependent kinase control of centrosome duplication. Proc. Natl. Acad. Sci. USA. 1999;96:2817–2822. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Meraldi P., Lukas J., Fry A.M., Bartek J., Nigg E.A. Centrosome duplication in mammalian somatic cells requires e2f and cdk2-cyclin a. Nat. Cell Biol. 1999;1:88–93. doi: 10.1038/10054. [DOI] [PubMed] [Google Scholar]
- 143.Dirksen E.R. Centriole morphogenesis in developing ciliated epithelium of the mouse oviduct. J. Cell Biol. 1971;51:286–302. doi: 10.1083/jcb.51.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nanjundappa R., Kong D., Shim K., Stearns T., Brody S.L., Loncarek J., Mahjoub M.R. Regulation of cilia abundance in multiciliated cells. ELife. 2019;8:e44039. doi: 10.7554/eLife.44039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao H., Chen Q., Fang C., Huang Q., Zhou J., Yan X., Zhu X. Parental centrioles are dispensable for deuterosome formation and function during basal body amplification. EMBO Rep. 2019;20:e46735. doi: 10.15252/embr.201846735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Szollosi D., Calarco P., Donahue R.P. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J. Cell Sci. 1972;11:521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- 147.Khodjakov A., Rieder C.L., Sluder G., Cassels G., Sibon O., Wang C.L. De novo formation of centrosomes in vertebrate cells arrested during s phase. J. Cell Biol. 2002;158:1171–1181. doi: 10.1083/jcb.200205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Callaini G., Riparbelli M.G., Dallai R. Centrosome inheritance in insects: Fertilization and parthenogenesis. Biol. Cell. 1999;91:355–366. doi: 10.1111/j.1768-322X.1999.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 149.Fawcett D.W., Phillips D.M. The fine structure and development of the neck region of the mammalian spermatozoon. Anat. Rec. 1969;165:153–164. doi: 10.1002/ar.1091650204. [DOI] [PubMed] [Google Scholar]
- 150.Garanina A.S., Alieva I.B., Bragina E.E., Blanchard E., Arbeille B., Guerif F., Uzbekova S., Uzbekov R.E. The centriolar adjunct-appearance and disassembly in spermiogenesis and the potential impact on fertility. Cells. 2019;8:180. doi: 10.3390/cells8020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alieva I.B., Staub C., Uzbekova S., Uzbekov R.E. A question of flagella origin for spermatids—Mother or daughter centriole? In: Uzbekov R.E., editor. Flagella and Cilia. Types Structure and Functions. Nova Science Publishers, Inc.; New York, NY, USA: 2018. pp. 109–126. [Google Scholar]
- 152.Sullenberger C., Vasquez-Limeta A., Kong D., Loncarek J. With age comes maturity: Biochemical and structural transformation of a human centriole in the making. Cells. 2020;9:1429. doi: 10.3390/cells9061429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Robbins E., Jentzsch G., Micali A. The centriole cycle in synchronized hela cells. J. Cell Biol. 1968;36:329–339. doi: 10.1083/jcb.36.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sluder G. Using sea urchin gametes and zygotes to investigate centrosome duplication. Cilia. 2016;5:20. doi: 10.1186/s13630-016-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nigg E.A., Stearns T. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat. Cell Biol. 2011;13:1154–1160. doi: 10.1038/ncb2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Izquierdo D., Wang W.J., Uryu K., Tsou M.F. Stabilization of cartwheel-less centrioles for duplication requires cep295-mediated centriole-to-centrosome conversion. Cell Rep. 2014;8:957–965. doi: 10.1016/j.celrep.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nigg E.A., Holland A.J. Once and only once: Mechanisms of centriole duplication and their deregulation in disease. Nat. Rev. Mol. Cell Biol. 2018;19:297–312. doi: 10.1038/nrm.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Inoue D., Obino D., Pineau J., Farina F., Gaillard J., Guerin C., Blanchoin L., Lennon-Dumenil A.M., Thery M. Actin filaments regulate microtubule growth at the centrosome. EMBO J. 2019;38:e99630. doi: 10.15252/embj.201899630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dujardin D.L., Vallee R.B. Dynein at the cortex. Curr. Opin. Cell Biol. 2002;14:44–49. doi: 10.1016/S0955-0674(01)00292-7. [DOI] [PubMed] [Google Scholar]
- 160.Inoue S., Salmon E.D. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol. Biol. Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Holy T.E., Dogterom M., Yurke B., Leibler S. Assembly and positioning of microtubule asters in microfabricated chambers. Proc. Natl. Acad. Sci. USA. 1997;94:6228–6231. doi: 10.1073/pnas.94.12.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Salmon W.C., Adams M.C., Waterman-Storer C.M. Dual-wavelength fluorescent speckle microscopy reveals coupling of microtubule and actin movements in migrating cells. J. Cell Biol. 2002;158:31–37. doi: 10.1083/jcb.200203022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Burakov A., Nadezhdina E., Slepchenko B., Rodionov V. Centrosome positioning in interphase cells. J. Cell Biol. 2003;162:963–969. doi: 10.1083/jcb.200305082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Burakov A.V., Nadezhdina E.S. Centering and shifting of centrosomes in cells. Cells. 2020;9:1351. doi: 10.3390/cells9061351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goldman R.D. The role of three cytoplasmic fibers in bhk-21 cell motility. I. Microtubules and the effects of colchicine. J. Cell Biol. 1971;51:752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alieva I.B., Vaisberg E.A., Nadezhdina E.S., Vorobjev I.A. Microtubule and intermediate filament patterns around the centrosome in interphase cells. In: Kalnins V.C., editor. The Centrosome. Academic Press; Cambridge, MA, USA: 1991. pp. 103–129. [Google Scholar]
- 167.Avidor-Reiss T. Rapid evolution of sperm produces diverse centriole structures that reveal the most rudimentary structure needed for function. Cells. 2018;7:67. doi: 10.3390/cells7070067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Avidor-Reiss T., Fishman E.L. It takes two (centrioles) to tango. Reproduction. 2019;157:R33–R51. doi: 10.1530/REP-18-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Khire A., Jo K.H., Kong D., Akhshi T., Blachon S., Cekic A.R., Hynek S., Ha A., Loncarek J., Mennella V., et al. Centriole remodeling during spermiogenesis in drosophila. Curr. Biol. 2016;26:3183–3189. doi: 10.1016/j.cub.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fishman E.L., Jo K., Ha A., Royfman R., Zinn A., Krishnamurthy M., Avidor-Reiss T. Atypical centrioles are present in tribolium sperm. Open Biol. 2017;7:160334. doi: 10.1098/rsob.160334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huettner A.F., Rabinowitz M. Demonstration of the central body in the living cell. Science. 1933;78:367–368. doi: 10.1126/science.78.2025.367-a. [DOI] [PubMed] [Google Scholar]
- 172.Tassin A.M., Maro B., Bornens M. Fate of microtubule-organizing centers during myogenesis in vitro. J. Cell Biol. 1985;100:35–46. doi: 10.1083/jcb.100.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Manandhar G., Sutovsky P., Joshi H.C., Stearns T., Schatten G. Centrosome reduction during mouse spermiogenesis. Dev. Biol. 1998;203:424–434. doi: 10.1006/dbio.1998.8947. [DOI] [PubMed] [Google Scholar]
- 174.Fulton C., Dingle A.D. Basal bodies, but not centrioles, in naegleria. J. Cell Biol. 1971;51:826–836. doi: 10.1083/jcb.51.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fritz-Laylin L.K., Fulton C. Naegleria: A classic model for de novo basal body assembly. Cilia. 2016;5:10. doi: 10.1186/s13630-016-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Aprelikova O.N., Fang B.S., Meissner E.G., Cotter S., Campbell M., Kuthiala A., Bessho M., Jensen R.A., Liu E.T. Brca1-associated growth arrest is rb-dependent. Proc. Natl. Acad. Sci. USA. 1999;96:11866–11871. doi: 10.1073/pnas.96.21.11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Arquint C., Nigg E.A. The plk4-stil-sas-6 module at the core of centriole duplication. Biochem. Soc. Trans. 2016;44:1253–1263. doi: 10.1042/BST20160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Askham J.M., Vaughan K.T., Goodson H.V., Morrison E.E. Evidence that an interaction between eb1 and p150(glued) is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol. Biol. Cell. 2002;13:3627–3645. doi: 10.1091/mbc.e02-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Astuti P., Boutros R., Ducommun B., Gabrielli B. Mitotic phosphorylation of cdc25b ser321 disrupts 14-3-3 binding to the high affinity ser323 site. J. Biol. Chem. 2010;285:34364–34370. doi: 10.1074/jbc.M110.138412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Atorino E.S., Hata S., Funaya C., Neuner A., Schiebel E. Cep44 ensures the formation of bona fide centriole wall, a requirement for the centriole-to-centrosome conversion. Nat. Commun. 2020;11:903. doi: 10.1038/s41467-020-14767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Barrera J.A., Kao L.R., Hammer R.E., Seemann J., Fuchs J.L., Megraw T.L. Cdk5rap2 regulates centriole engagement and cohesion in mice. Dev. Cell. 2010;18:913–926. doi: 10.1016/j.devcel.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Basto R., Lau J., Vinogradova T., Gardiol A., Woods C.G., Khodjakov A., Raff J.W. Flies without centrioles. Cell. 2006;125:1375–1386. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 183.Bastos R.N., Barr F.A. Plk1 negatively regulates cep55 recruitment to the midbody to ensure orderly abscission. J. Cell Biol. 2010;191:751–760. doi: 10.1083/jcb.201008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Berrueta L., Kraeft S.K., Tirnauer J.S., Schuyler S.C., Chen L.B., Hill D.E., Pellman D., Bierer B.E. The adenomatous polyposis coli-binding protein eb1 is associated with cytoplasmic and spindle microtubules. Proc. Natl. Acad. Sci. USA. 1998;95:10596–10601. doi: 10.1073/pnas.95.18.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bowler M., Kong D., Sun S., Nanjundappa R., Evans L., Farmer V., Holland A., Mahjoub M.R., Sui H., Loncarek J. High-resolution characterization of centriole distal appendage morphology and dynamics by correlative storm and electron microscopy. Nat. Commun. 2019;10:993. doi: 10.1038/s41467-018-08216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brown C.R., Doxsey S.J., Hong-Brown L.Q., Martin R.L., Welch W.J. Molecular chaperones and the centrosome. A role for tcp-1 in microtubule nucleation. J. Biol. Chem. 1996;271:824–832. doi: 10.1074/jbc.271.2.824. [DOI] [PubMed] [Google Scholar]
- 187.Brown C.R., Hong-Brown L.Q., Doxsey S.J., Welch W.J. Molecular chaperones and the centrosome. A role for hsp 73 in centrosomal repair following heat shock treatment. J. Biol. Chem. 1996;271:833–840. doi: 10.1074/jbc.271.2.833. [DOI] [PubMed] [Google Scholar]
- 188.Brueckner M. Cilia propel the embryo in the right direction. Am. J. Med Genet. 2001;101:339–344. doi: 10.1002/1096-8628(20010715)101:4<339::AID-AJMG1442>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 189.Burakov A., Kovalenko O., Semenova I., Zhapparova O., Nadezhdina E., Rodionov V. Cytoplasmic dynein is involved in the retention of microtubules at the centrosome in interphase cells. Traffic. 2008;9:472–480. doi: 10.1111/j.1600-0854.2007.00698.x. [DOI] [PubMed] [Google Scholar]
- 190.Cajanek L., Nigg E.A. Cep164 triggers ciliogenesis by recruiting tau tubulin kinase 2 to the mother centriole. Proc. Natl. Acad. Sci. USA. 2014;111:E2841–E2850. doi: 10.1073/pnas.1401777111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Carroll P.E., Okuda M., Horn H.F., Biddinger P., Stambrook P.J., Gleich L.L., Li Y.Q., Tarapore P., Fukasawa K. Centrosome hyperamplification in human cancer: Chromosome instability induced by p53 mutation and/or mdm2 overexpression. Oncogene. 1999;18:1935–1944. doi: 10.1038/sj.onc.1202515. [DOI] [PubMed] [Google Scholar]
- 192.Casenghi M., Meraldi P., Weinhart U., Duncan P.I., Korner R., Nigg E.A. Polo-like kinase 1 regulates nlp, a centrosome protein involved in microtubule nucleation. Dev. Cell. 2003;5:113–125. doi: 10.1016/S1534-5807(03)00193-X. [DOI] [PubMed] [Google Scholar]
- 193.Casenghi M., Barr F.A., Nigg E.A. Phosphorylation of nlp by plk1 negatively regulates its dynein-dynactin-dependent targeting to the centrosome. J. Cell Sci. 2005;118:5101–5108. doi: 10.1242/jcs.02622. [DOI] [PubMed] [Google Scholar]
- 194.Chang P., Stearns T. Delta-tubulin and epsilon-tubulin: Two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nat. Cell Biol. 2000;2:30–35. doi: 10.1038/71350. [DOI] [PubMed] [Google Scholar]
- 195.Chang J., Cizmecioglu O., Hoffmann I., Rhee K. Plk2 phosphorylation is critical for cpap function in procentriole formation during the centrosome cycle. EMBO J. 2010;29:2395–2406. doi: 10.1038/emboj.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chartier N.T., Oddou C.I., Laine M.G., Ducarouge B., Marie C.A., Block M.R., Jacquier-Sarlin M.R. Cyclin-dependent kinase 2/cyclin e complex is involved in p120 catenin (p120ctn)-dependent cell growth control: A new role for p120ctn in cancer. Cancer Res. 2007;67:9781–9790. doi: 10.1158/0008-5472.CAN-07-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen Z., Indjeian V.B., McManus M., Wang L., Dynlacht B.D. Cp110, a cell cycle-dependent cdk substrate, regulates centrosome duplication in human cells. Dev. Cell. 2002;3:339–350. doi: 10.1016/S1534-5807(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 198.Chen C.H., Howng S.L., Cheng T.S., Chou M.H., Huang C.Y., Hong Y.R. Molecular characterization of human ninein protein: Two distinct subdomains required for centrosomal targeting and regulating signals in cell cycle. Biochem. Biophys. Res. Commun. 2003;308:975–983. doi: 10.1016/S0006-291X(03)01510-9. [DOI] [PubMed] [Google Scholar]
- 199.Chen C.Y., Olayioye M.A., Lindeman G.J., Tang T.K. Cpap interacts with 14-3-3 in a cell cycle-dependent manner. Biochem. Biophys. Res. Commun. 2006;342:1203–1210. doi: 10.1016/j.bbrc.2006.02.089. [DOI] [PubMed] [Google Scholar]
- 200.Chen H.Y., Wu C.T., Tang C.C., Lin Y.N., Wang W.J., Tang T.K. Human microcephaly protein rttn interacts with stil and is required to build full-length centrioles. Nat. Commun. 2017;8:247. doi: 10.1038/s41467-017-00305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cheng T.S., Hsiao Y.L., Lin C.C., Hsu C.M., Chang M.S., Lee C.I., Yu R.C., Huang C.Y., Howng S.L., Hong Y.R. Hninein is required for targeting spindle-associated protein astrin to the centrosome during the s and g2 phases. Exp. Cell Res. 2007;313:1710–1721. doi: 10.1016/j.yexcr.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 202.Choi Y.K., Liu P., Sze S.K., Dai C., Qi R.Z. Cdk5rap2 stimulates microtubule nucleation by the gamma-tubulin ring complex. J. Cell Biol. 2010;191:1089–1095. doi: 10.1083/jcb.201007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cogswell J.P., Brown C.E., Bisi J.E., Neill S.D. Dominant-negative polo-like kinase 1 induces mitotic catastrophe independent of cdc25c function. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 2000;11:615–623. [PubMed] [Google Scholar]
- 204.Collins E.S., Hornick J.E., Durcan T.M., Collins N.S., Archer W., Karanjeet K.B., Vaughan K.T., Hinchcliffe E.H. Centrosome biogenesis continues in the absence of microtubules during prolonged s-phase arrest. J. Cell. Physiol. 2010;225:454–465. doi: 10.1002/jcp.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Colombo E., Marine J.C., Danovi D., Falini B., Pelicci P.G. Nucleophosmin regulates the stability and transcriptional activity of p53. Nat. Cell Biol. 2002;4:529–533. doi: 10.1038/ncb814. [DOI] [PubMed] [Google Scholar]
- 206.Conduit P.T., Brunk K., Dobbelaere J., Dix C.I., Lucas E.P., Raff J.W. Centrioles regulate centrosome size by controlling the rate of cnn incorporation into the pcm. Curr. Biol. 2010;20:2178–2186. doi: 10.1016/j.cub.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 207.D’Angiolella V., Donato V., Vijayakumar S., Saraf A., Florens L., Washburn M.P., Dynlacht B., Pagano M. Scf(cyclin f) controls centrosome homeostasis and mitotic fidelity through cp110 degradation. Nature. 2010;466:138–142. doi: 10.1038/nature09140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Delattre M., Canard C., Gonczy P. Sequential protein recruitment in c. Elegans centriole formation. Curr. Biol. 2006;16:1844–1849. doi: 10.1016/j.cub.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 209.Diviani D., Langeberg L.K., Doxsey S.J., Scott J.D. Pericentrin anchors protein kinase a at the centrosome through a newly identified rii-binding domain. Curr. Biol. 2000;10:417–420. doi: 10.1016/S0960-9822(00)00422-X. [DOI] [PubMed] [Google Scholar]
- 210.Doxsey S.J., Stein P., Evans L., Calarco P.D., Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- 211.El Din El Homasany B.S., Volkov Y., Takahashi M., Ono Y., Keryer G., Delouvee A., Looby E., Long A., Kelleher D. The scaffolding protein cg-nap/akap450 is a critical integrating component of the lfa-1-induced signaling complex in migratory t cells. J. Immunol. 2005;175:7811–7818. doi: 10.4049/jimmunol.175.12.7811. [DOI] [PubMed] [Google Scholar]
- 212.Ellinger-Ziegelbauer H., Karasuyama H., Yamada E., Tsujikawa K., Todokoro K., Nishida E. Ste20-like kinase (slk), a regulatory kinase for polo-like kinase (plk) during the g2/m transition in somatic cells. Genes Cells. 2000;5:491–498. doi: 10.1046/j.1365-2443.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 213.Eyers P.A., Erikson E., Chen L.G., Maller J.L. A novel mechanism for activation of the protein kinase aurora a. Curr. Biol. 2003;13:691–697. doi: 10.1016/S0960-9822(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 214.Fabbro M., Zhou B.B., Takahashi M., Sarcevic B., Lal P., Graham M.E., Gabrielli B.G., Robinson P.J., Nigg E.A., Ono Y., et al. Cdk1/erk2- and plk1-dependent phosphorylation of a centrosome protein, cep55, is required for its recruitment to midbody and cytokinesis. Dev. Cell. 2005;9:477–488. doi: 10.1016/j.devcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 215.Feng Y., Yuan J.H., Maloid S.C., Fisher R., Copeland T.D., Longo D.L., Conrads T.P., Veenstra T.D., Ferris A., Hughes S., et al. Polo-like kinase 1-mediated phosphorylation of the gtp-binding protein ran is important for bipolar spindle formation. Biochem. Biophys. Res. Commun. 2006;349:144–152. doi: 10.1016/j.bbrc.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 216.Fong K.W., Choi Y.K., Rattner J.B., Qi R.Z. Cdk5rap2 is a pericentriolar protein that functions in centrosomal attachment of the gamma-tubulin ring complex. Mol. Biol. Cell. 2008;19:115–125. doi: 10.1091/mbc.e07-04-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fong C.S., Ozaki K., Tsou M.B. Ppp1r35 ensures centriole homeostasis by promoting centriole-to-centrosome conversion. Mol. Biol. Cell. 2018;29:2801–2808. doi: 10.1091/mbc.E18-08-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Franck N., Montembault E., Rome P., Pascal A., Cremet J.Y., Giet R. Cdk11(p58) is required for centriole duplication and plk4 recruitment to mitotic centrosomes. PLoS ONE. 2011;6:e14600. doi: 10.1371/journal.pone.0014600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Frank-Vaillant M., Haccard O., Thibier C., Ozon R., Arlot-Bonnemains Y., Prigent C., Jessus C. Progesterone regulates the accumulation and the activation of eg2 kinase in xenopus oocytes. J. Cell Sci. 2000;113:1127–1138. doi: 10.1242/jcs.113.7.1127. [DOI] [PubMed] [Google Scholar]
- 220.Fry A.M., Mayor T., Meraldi P., Stierhof Y.D., Tanaka K., Nigg E.A. C-nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase nek2. J. Cell Biol. 1998;141:1563–1574. doi: 10.1083/jcb.141.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fry A.M., Meraldi P., Nigg E.A. A centrosomal function for the human nek2 protein kinase, a member of the nima family of cell cycle regulators. EMBO J. 1998;17:470–481. doi: 10.1093/emboj/17.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fu J., Hagan I.M., Glover D.M. The centrosome and its duplication cycle. Cold Spring Harb. Perspect. Biol. 2015;7:a015800. doi: 10.1101/cshperspect.a015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fukasawa K. P53, cyclin-dependent kinase and abnormal amplification of centrosomes. Biochim. Biophys. Acta. 2008;1786:15–23. doi: 10.1016/j.bbcan.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Giet R., Uzbekov R., Kireev I., Prigent C. The xenopus laevis centrosome aurora/ipl1-related kinase. Biol. Cell. 1999;91:461–470. doi: 10.1111/j.1768-322X.1999.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 225.Gluenz E., Hoog J.L., Smith A.E., Dawe H.R., Shaw M.K., Gull K. Beyond 9+0: Noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J. 2010;24:3117–3121. doi: 10.1096/fj.09-151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gopalakrishnan J., Mennella V., Blachon S., Zhai B., Smith A.H., Megraw T.L., Nicastro D., Gygi S.P., Agard D.A., Avidor-Reiss T. Sas-4 provides a scaffold for cytoplasmic complexes and tethers them in a centrosome. Nat. Commun. 2011;2:359. doi: 10.1038/ncomms1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Goto M., O’Brien D.A., Eddy E.M. Speriolin is a novel human and mouse sperm centrosome protein. Hum. Reprod. 2010;25:1884–1894. doi: 10.1093/humrep/deq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Graser S., Stierhof Y.D., Lavoie S.B., Gassner O.S., Lamla S., Le Clech M., Nigg E.A. Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 2007;179:321–330. doi: 10.1083/jcb.200707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Groen A.C., Cameron L.A., Coughlin M., Miyamoto D.T., Mitchison T.J., Ohi R. Xrhamm functions in ran-dependent microtubule nucleation and pole formation during anastral spindle assembly. Curr. Biol. 2004;14:1801–1811. doi: 10.1016/j.cub.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 230.Gromley A., Yeaman C., Rosa J., Redick S., Chen C.T., Mirabelle S., Guha M., Sillibourne J., Doxsey S.J. Centriolin anchoring of exocyst and snare complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 231.Guarguaglini G., Duncan P.I., Stierhof Y.D., Holmstrom T., Duensing S., Nigg E.A. The forkhead-associated domain protein cep170 interacts with polo-like kinase 1 and serves as a marker for mature centrioles. Mol. Biol. Cell. 2005;16:1095–1107. doi: 10.1091/mbc.e04-10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gudi R., Zou C., Li J., Gao Q. Centrobin-tubulin interaction is required for centriole elongation and stability. J. Cell Biol. 2011;193:711–725. doi: 10.1083/jcb.201006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Guillet V., Knibiehler M., Gregory-Pauron L., Remy M.H., Chemin C., Raynaud-Messina B., Bon C., Kollman J.M., Agard D.A., Merdes A., et al. Crystal structure of gamma-tubulin complex protein gcp4 provides insight into microtubule nucleation. Nat. Struct. Mol. Biol. 2011;18:915–919. doi: 10.1038/nsmb.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Guo J., Yang Z., Song W., Chen Q., Wang F., Zhang Q., Zhu X. Nudel contributes to microtubule anchoring at the mother centriole and is involved in both dynein-dependent and -independent centrosomal protein assembly. Mol. Biol. Cell. 2006;17:680–689. doi: 10.1091/mbc.e05-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gurkaslar H.K., Culfa E., Arslanhan M.D., Lince-Faria M., Firat-Karalar E.N. Ccdc57 cooperates with microtubules and microcephaly protein cep63 and regulates centriole duplication and mitotic progression. Cell Rep. 2020;31:107630. doi: 10.1016/j.celrep.2020.107630. [DOI] [PubMed] [Google Scholar]
- 236.Hamel V., Steib E., Hamelin R., Armand F., Borgers S., Fluckiger I., Busso C., Olieric N., Sorzano C.O.S., Steinmetz M.O., et al. Identification of chlamydomonas central core centriolar proteins reveals a role for human wdr90 in ciliogenesis. Curr. Biol. 2017;27:2486–2498.e6. doi: 10.1016/j.cub.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hames R.S., Crookes R.E., Straatman K.R., Merdes A., Hayes M.J., Faragher A.J., Fry A.M. Dynamic recruitment of nek2 kinase to the centrosome involves microtubules, pcm-1, and localized proteasomal degradation. Mol. Biol. Cell. 2005;16:1711–1724. doi: 10.1091/mbc.e04-08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Harbour J.W., Luo R.X., Dei Santi A., Postigo A.A., Dean D.C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block rb functions as cells move through g1. Cell. 1999;98:859–869. doi: 10.1016/S0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 239.Haren L., Stearns T., Luders J. Plk1-dependent recruitment of gamma-tubulin complexes to mitotic centrosomes involves multiple pcm components. PLoS ONE. 2009;4:e5976. doi: 10.1371/journal.pone.0005976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hirayama S., Yamazaki Y., Kitamura A., Oda Y., Morito D., Okawa K., Kimura H., Cyr D.M., Kubota H., Nagata K. Mkks is a centrosome-shuttling protein degraded by disease-causing mutations via chip-mediated ubiquitination. Mol. Biol. Cell. 2008;19:899–911. doi: 10.1091/mbc.e07-07-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hirota T., Kunitoku N., Sasayama T., Marumoto T., Zhang D., Nitta M., Hatakeyama K., Saya H. Aurora-a and an interacting activator, the lim protein ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–598. doi: 10.1016/S0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- 242.Hosono K., Noda S., Shimizu A., Nakanishi N., Ohtsubo M., Shimizu N., Minoshima S. Ypel5 protein of the ypel gene family is involved in the cell cycle progression by interacting with two distinct proteins ranbpm and ranbp10. Genomics. 2010;96:102–111. doi: 10.1016/j.ygeno.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 243.Hsu L.C., White R.L. Brca1 is associated with the centrosome during mitosis. Proc. Natl. Acad. Sci. USA. 1998;95:12983–12988. doi: 10.1073/pnas.95.22.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Huang N., Xia Y., Zhang D., Wang S., Bao Y., He R., Teng J., Chen J. Hierarchical assembly of centriole subdistal appendages via centrosome binding proteins ccdc120 and ccdc68. Nat. Commun. 2017;8:15057. doi: 10.1038/ncomms15057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Huang Y.H., Wu C.C., Chou C.K., Huang C.Y. A translational regulator, pum2, promotes both protein stability and kinase activity of aurora-a. PLoS ONE. 2011;6:e19718. doi: 10.1371/journal.pone.0019718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ibi M., Zou P., Inoko A., Shiromizu T., Matsuyama M., Hayashi Y., Enomoto M., Mori D., Hirotsune S., Kiyono T., et al. Trichoplein controls microtubule anchoring at the centrosome by binding to odf2 and ninein. J. Cell Sci. 2011;124:857–864. doi: 10.1242/jcs.075705. [DOI] [PubMed] [Google Scholar]
- 247.Iwamori T., Iwamori N., Ma L., Edson M.A., Greenbaum M.P., Matzuk M.M. Tex14 interacts with cep55 to block cell abscission. Mol. Cell. Biol. 2010;30:2280–2292. doi: 10.1128/MCB.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jackman M., Lindon C., Nigg E.A., Pines J. Active cyclin b1-cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- 249.Jakobsen L., Vanselow K., Skogs M., Toyoda Y., Lundberg E., Poser I., Falkenby L.G., Bennetzen M., Westendorf J., Nigg E.A., et al. Novel asymmetrically localizing components of human centrosomes identified by complementary proteomics methods. EMBO J. 2011;30:1520–1535. doi: 10.1038/emboj.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jang Y.J., Ji J.H., Ahn J.H., Hoe K.L., Won M., Im D.S., Chae S.K., Song S., Yoo H.S. Polo-box motif targets a centrosome regulator, rangtpase. Biochem. Biophys. Res. Commun. 2004;325:257–264. doi: 10.1016/j.bbrc.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 251.Jin S., Gao H., Mazzacurati L., Wang Y., Fan W., Chen Q., Yu W., Wang M., Zhu X., Zhang C., et al. Brca1 interaction of centrosomal protein nlp is required for successful mitotic progression. J. Biol. Chem. 2009;284:22970–22977. doi: 10.1074/jbc.M109.009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Jo K.H., Jaiswal A., Khanal S., Fishman E.L., Curry A.N., Avidor-Reiss T. Poc1b and sas-6 function together during the atypical centriole formation in drosophila melanogaster. Cells. 2019;8:841. doi: 10.3390/cells8080841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Joukov V., Groen A.C., Prokhorova T., Gerson R., White E., Rodriguez A., Walter J.C., Livingston D.M. The brca1/bard1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127:539–552. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 254.Kao L.R., Megraw T.L. Centrocortin cooperates with centrosomin to organize drosophila embryonic cleavage furrows. Curr. Biol. 2009;19:937–942. doi: 10.1016/j.cub.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Katayama H., Sasai K., Kawai H., Yuan Z.M., Bondaruk J., Suzuki F., Fujii S., Arlinghaus R.B., Czerniak B.A., Sen S. Phosphorylation by aurora kinase a induces mdm2-mediated destabilization and inhibition of p53. Nat. Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 256.Kellogg D.R., Moritz M., Alberts B.M. The centrosome and cellular organization. Annu. Rev. Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- 257.Keryer G., Di Fiore B., Celati C., Lechtreck K.F., Mogensen M., Delouvee A., Lavia P., Bornens M., Tassin A.M. Part of ran is associated with akap450 at the centrosome: Involvement in microtubule-organizing activity. Mol. Biol. Cell. 2003;14:4260–4271. doi: 10.1091/mbc.e02-11-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kim H.S., Takahashi M., Matsuo K., Ono Y. Recruitment of cg-nap to the golgi apparatus through interaction with dynein-dynactin complex. Genes Cells. 2007;12:421–434. doi: 10.1111/j.1365-2443.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 259.Kim J.C., Ou Y.Y., Badano J.L., Esmail M.A., Leitch C.C., Fiedrich E., Beales P.L., Archibald J.M., Katsanis N., Rattner J.B., et al. Mkks/bbs6, a divergent chaperonin-like protein linked to the obesity disorder bardet-biedl syndrome, is a novel centrosomal component required for cytokinesis. J. Cell Sci. 2005;118:1007–1020. doi: 10.1242/jcs.01676. [DOI] [PubMed] [Google Scholar]
- 260.Kleylein-Sohn J., Westendorf J., Le Clech M., Habedanck R., Stierhof Y.D., Nigg E.A. Plk4-induced centriole biogenesis in human cells. Dev. Cell. 2007;13:190–202. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 261.Kobayashi T., Kim S., Lin Y.C., Inoue T., Dynlacht B.D. The cp110-interacting proteins talpid3 and cep290 play overlapping and distinct roles in cilia assembly. J. Cell Biol. 2014;204:215–229. doi: 10.1083/jcb.201304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kobayashi T., Tsang W.Y., Li J., Lane W., Dynlacht B.D. Centriolar kinesin kif24 interacts with cp110 to remodel microtubules and regulate ciliogenesis. Cell. 2011;145:914–925. doi: 10.1016/j.cell.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 263.Kotani T., Yamashita M. Overexpression of truncated gamma-tubulins disrupts mitotic aster formation in xenopus oocyte extracts. Biochem. J. 2005;389:611–617. doi: 10.1042/BJ20050243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Krause A., Hoffmann I. Polo-like kinase 2-dependent phosphorylation of npm/b23 on serine 4 triggers centriole duplication. PLoS ONE. 2010;5:e9849. doi: 10.1371/journal.pone.0009849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kurz T., Pintard L., Willis J.H., Hamill D.R., Gonczy P., Peter M., Bowerman B. Cytoskeletal regulation by the nedd8 ubiquitin-like protein modification pathway. Science. 2002;295:1294–1298. doi: 10.1126/science.1067765. [DOI] [PubMed] [Google Scholar]
- 266.Lee M.J., Gergely F., Jeffers K., Peak-Chew S.Y., Raff J.W. Msps/xmap215 interacts with the centrosomal protein d-tacc to regulate microtubule behaviour. Nat. Cell Biol. 2001;3:643–649. doi: 10.1038/35083033. [DOI] [PubMed] [Google Scholar]
- 267.Lee S., Rhee K. Cep215 is involved in the dynein-dependent accumulation of pericentriolar matrix proteins for spindle pole formation. Cell Cycle. 2010;9:774–783. doi: 10.4161/cc.9.4.10667. [DOI] [PubMed] [Google Scholar]
- 268.Li J., Wang J., Jiao H., Liao J., Xu X. Cytokinesis and cancer: Polo loves rock’n’ rho(a) J. Genet. Genom. = Yi Chuan Xue Bao. 2010;37:159–172. doi: 10.1016/S1673-8527(09)60034-5. [DOI] [PubMed] [Google Scholar]
- 269.Li J., Zhan Q. The role of centrosomal nlp in the control of mitotic progression and tumourigenesis. Br. J. Cancer. 2011;104:1523–1528. doi: 10.1038/bjc.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Li Q., Hansen D., Killilea A., Joshi H.C., Palazzo R.E., Balczon R. Kendrin/pericentrin-b, a centrosome protein with homology to pericentrin that complexes with pcm-1. J. Cell Sci. 2001;114:797–809. doi: 10.1242/jcs.114.4.797. [DOI] [PubMed] [Google Scholar]
- 271.Lin C.C., Cheng T.S., Hsu C.M., Wu C.H., Chang L.S., Shen Z.S., Yeh H.M., Chang L.K., Howng S.L., Hong Y.R. Characterization and functional aspects of human ninein isoforms that regulated by centrosomal targeting signals and evidence for docking sites to direct gamma-tubulin. Cell Cycle. 2006;5:2517–2527. doi: 10.4161/cc.5.21.3404. [DOI] [PubMed] [Google Scholar]
- 272.Linck R.W., Albertini D.F., Kenney D.M., Langevin G.L. Tektin filaments: Chemically unique filaments of sperm flagellar microtubules. Prog. Clin. Biol. Res. 1982;80:127–132. doi: 10.1002/cm.970020724. [DOI] [PubMed] [Google Scholar]
- 273.Lou Y., Xie W., Zhang D.F., Yao J.H., Luo Z.F., Wang Y.Z., Shi Y.Y., Yao X.B. Nek2a specifies the centrosomal localization of erk2. Biochem. Biophys. Res. Commun. 2004;321:495–501. doi: 10.1016/j.bbrc.2004.06.171. [DOI] [PubMed] [Google Scholar]
- 274.Ma S., Trivinos-Lagos L., Graf R., Chisholm R.L. Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in dictyostelium. J. Cell Biol. 1999;147:1261–1274. doi: 10.1083/jcb.147.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ma Z., Kanai M., Kawamura K., Kaibuchi K., Ye K., Fukasawa K. Interaction between rock ii and nucleophosmin/b23 in the regulation of centrosome duplication. Mol. Cell. Biol. 2006;26:9016–9034. doi: 10.1128/MCB.01383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mardin B.R., Lange C., Baxter J.E., Hardy T., Scholz S.R., Fry A.M., Schiebel E. Components of the hippo pathway cooperate with nek2 kinase to regulate centrosome disjunction. Nat. Cell Biol. 2010;12:1166–1176. doi: 10.1038/ncb2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mariappan A., Soni K., Schorpp K., Zhao F., Minakar A., Zheng X., Mandad S., Macheleidt I., Ramani A., Kubelka T., et al. Inhibition of cpap-tubulin interaction prevents proliferation of centrosome-amplified cancer cells. EMBO J. 2019;38:e99876. doi: 10.15252/embj.201899876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Matsuo K., Nishimura T., Hayakawa A., Ono Y., Takahashi M. Involvement of a centrosomal protein kendrin in the maintenance of centrosome cohesion by modulating nek2a kinase activity. Biochem. Biophys. Res. Commun. 2010;398:217–223. doi: 10.1016/j.bbrc.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 279.Maxwell C.A., Keats J.J., Belch A.R., Pilarski L.M., Reiman T. Receptor for hyaluronan-mediated motility correlates with centrosome abnormalities in multiple myeloma and maintains mitotic integrity. Cancer Res. 2005;65:850–860. [PubMed] [Google Scholar]
- 280.May-Simera H.L., Ross A., Rix S., Forge A., Beales P.L., Jagger D.J. Patterns of expression of bardet-biedl syndrome proteins in the mammalian cochlea suggest noncentrosomal functions. J. Comp. Neurol. 2009;514:174–188. doi: 10.1002/cne.22001. [DOI] [PubMed] [Google Scholar]
- 281.McKendrick L., Milne D., Meek D. Protein kinase ck2-dependent regulation of p53 function: Evidence that the phosphorylation status of the serine 386 (ck2) site of p53 is constitutive and stable. Mol. Cell. Biochem. 1999;191:187–199. doi: 10.1023/A:1006854109926. [DOI] [PubMed] [Google Scholar]
- 282.McNally F.J., Vale R.D. Identification of katanin, an atpase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- 283.Megraw T.L., Li K., Kao L.R., Kaufman T.C. The centrosomin protein is required for centrosome assembly and function during cleavage in drosophila. Development. 1999;126:2829–2839. doi: 10.1242/dev.126.13.2829. [DOI] [PubMed] [Google Scholar]
- 284.Merdes A., Cleveland D.W. Pathways of spindle pole formation: Different mechanisms; conserved components. J. Cell Biol. 1997;138:953–956. doi: 10.1083/jcb.138.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Merdes A., Ramyar K., Vechio J.D., Cleveland D.W. A complex of numa and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/S0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- 286.Mi J., Guo C., Brautigan D.L., Larner J.M. Protein phosphatase-1alpha regulates centrosome splitting through nek2. Cancer Res. 2007;67:1082–1089. doi: 10.1158/0008-5472.CAN-06-3071. [DOI] [PubMed] [Google Scholar]
- 287.Miyoshi K., Asanuma M., Miyazaki I., Diaz-Corrales F.J., Katayama T., Tohyama M., Ogawa N. Disc1 localizes to the centrosome by binding to kendrin. Biochem. Biophys. Res. Commun. 2004;317:1195–1199. doi: 10.1016/j.bbrc.2004.03.163. [DOI] [PubMed] [Google Scholar]
- 288.Mogensen M.M. Microtubule organizing centers in polarized epithelial cells. In: Nigg E.A., editor. Centrosomes in Development and Disease. Wiley-VCH Verlag GmbH & Co., KGaA; Weinheim, Germany: 2004. [Google Scholar]
- 289.Mogensen M.M., Malik A., Piel M., Bouckson-Castaing V., Bornens M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: The role of ninein. J. Cell Sci. 2000;113:3013–3023. doi: 10.1242/jcs.113.17.3013. [DOI] [PubMed] [Google Scholar]
- 290.Molli P.R., Li D.Q., Bagheri-Yarmand R., Pakala S.B., Katayama H., Sen S., Iyer J., Chernoff J., Tsai M.Y., Nair S.S., et al. Arpc1b, a centrosomal protein, is both an activator and substrate of aurora a. J. Cell Biol. 2010;190:101–114. doi: 10.1083/jcb.200908050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Morris J.A., Kandpal G., Ma L., Austin C.P. Disc1 (disrupted-in-schizophrenia 1) is a centrosome-associated protein that interacts with map1a, mipt3, atf4/5 and nudel: Regulation and loss of interaction with mutation. Hum. Mol. Genet. 2003;12:1591–1608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- 292.Moudjou M., Bordes N., Paintrand M., Bornens M. Gamma-tubulin in mammalian cells: The centrosomal and the cytosolic forms. J. Cell Sci. 1996;109:875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- 293.Munemitsu S., Souza B., Muller O., Albert I., Rubinfeld B., Polakis P. The apc gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–3681. [PubMed] [Google Scholar]
- 294.Murphy S.M., Preble A.M., Patel U.K., O’Connell K.L., Dias D.P., Moritz M., Agard D., Stults J.T., Stearns T. Gcp5 and gcp6: Two new members of the human gamma-tubulin complex. Mol. Biol. Cell. 2001;12:3340–3352. doi: 10.1091/mbc.12.11.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Nakamura M., Masuda H., Horii J., Kuma K., Yokoyama N., Ohba T., Nishitani H., Miyata T., Tanaka M., Nishimoto T. When overexpressed, a novel centrosomal protein, ranbpm, causes ectopic microtubule nucleation similar to gamma-tubulin. J. Cell Biol. 1998;143:1041–1052. doi: 10.1083/jcb.143.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Negi S.S., Olson M.O. Effects of interphase and mitotic phosphorylation on the mobility and location of nucleolar protein b23. J. Cell Sci. 2006;119:3676–3685. doi: 10.1242/jcs.03090. [DOI] [PubMed] [Google Scholar]
- 297.Niethammer M., Smith D.S., Ayala R., Peng J., Ko J., Lee M.S., Morabito M., Tsai L.H. Nudel is a novel cdk5 substrate that associates with lis1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/S0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 298.Nishimura T., Takahashi M., Kim H.S., Mukai H., Ono Y. Centrosome-targeting region of cg-nap causes centrosome amplification by recruiting cyclin e-cdk2 complex. Genes Cells. 2005;10:75–86. doi: 10.1111/j.1365-2443.2005.00816.x. [DOI] [PubMed] [Google Scholar]
- 299.Okuda M., Horn H.F., Tarapore P., Tokuyama Y., Smulian A.G., Chan P.K., Knudsen E.S., Hofmann I.A., Snyder J.D., Bove K.E., et al. Nucleophosmin/b23 is a target of cdk2/cyclin e in centrosome duplication. Cell. 2000;103:127–140. doi: 10.1016/S0092-8674(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 300.Ou Y.Y., Mack G.J., Zhang M., Rattner J.B. Cep110 and ninein are located in a specific domain of the centrosome associated with centrosome maturation. J. Cell Sci. 2002;115:1825–1835. doi: 10.1242/jcs.115.9.1825. [DOI] [PubMed] [Google Scholar]
- 301.Ouchi M., Fujiuchi N., Sasai K., Katayama H., Minamishima Y.A., Ongusaha P.P., Deng C., Sen S., Lee S.W., Ouchi T. Brca1 phosphorylation by aurora-a in the regulation of g2 to m transition. J. Biol. Chem. 2004;279:19643–19648. doi: 10.1074/jbc.M311780200. [DOI] [PubMed] [Google Scholar]
- 302.Piehl M., Cassimeris L. Organization and dynamics of growing microtubule plus ends during early mitosis. Mol. Biol. Cell. 2003;14:916–925. doi: 10.1091/mbc.e02-09-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Pietromonaco S.F., Seluja G.A., Elias L. Identification of enzymatically active ca2+/calmodulin-dependent protein kinase in centrosomes of hemopoietic cells. Blood Cells Mol. Dis. 1995;21:34–41. doi: 10.1006/bcmd.1995.0006. [DOI] [PubMed] [Google Scholar]
- 304.Pockwinse S.M., Krockmalnic G., Doxsey S.J., Nickerson J., Lian J.B., van Wijnen A.J., Stein J.L., Stein G.S., Penman S. Cell cycle independent interaction of cdc2 with the centrosome, which is associated with the nuclear matrix-intermediate filament scaffold. Proc. Natl. Acad. Sci. USA. 1997;94:3022–3027. doi: 10.1073/pnas.94.7.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Pugacheva E.N., Jablonski S.A., Hartman T.R., Henske E.P., Golemis E.A. Hef1-dependent aurora a activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Purohit A., Tynan S.H., Vallee R., Doxsey S.J. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 1999;147:481–492. doi: 10.1083/jcb.147.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Rapley J., Baxter J.E., Blot J., Wattam S.L., Casenghi M., Meraldi P., Nigg E.A., Fry A.M. Coordinate regulation of the mother centriole component nlp by nek2 and plk1 protein kinases. Mol. Cell. Biol. 2005;25:1309–1324. doi: 10.1128/MCB.25.4.1309-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rodrigues-Martins A., Riparbelli M., Callaini G., Glover D.M., Bettencourt-Dias M. Revisiting the role of the mother centriole in centriole biogenesis. Science. 2007;316:1046–1050. doi: 10.1126/science.1142950. [DOI] [PubMed] [Google Scholar]
- 309.Rosales J.L., Rattner J.B., Lee K.Y. Cdk5 in the centriolar appendages mediates cenexin1 localization and primary cilia formation. Cell Cycle. 2010;9:2037–2039. doi: 10.4161/cc.9.10.11600. [DOI] [PubMed] [Google Scholar]
- 310.Ruffner H., Jiang W., Craig A.G., Hunter T., Verma I.M. Brca1 is phosphorylated at serine 1497 in vivo at a cyclin-dependent kinase 2 phosphorylation site. Mol. Cell. Biol. 1999;19:4843–4854. doi: 10.1128/MCB.19.7.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ruiz F., Krzywicka A., Klotz C., Keller A., Cohen J., Koll F., Balavoine G., Beisson J. The sm19 gene, required for duplication of basal bodies in paramecium, encodes a novel tubulin, eta-tubulin. Curr. Biol. 2000;10:1451–1454. doi: 10.1016/S0960-9822(00)00804-6. [DOI] [PubMed] [Google Scholar]
- 312.Saavedra H.I., Maiti B., Timmers C., Altura R., Tokuyama Y., Fukasawa K., Leone G. Inactivation of e2f3 results in centrosome amplification. Cancer Cell. 2003;3:333–346. doi: 10.1016/S1535-6108(03)00083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Sasaki S., Shionoya A., Ishida M., Gambello M.J., Yingling J., Wynshaw-Boris A., Hirotsune S. A lis1/nudel/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/S0896-6273(00)00146-X. [DOI] [PubMed] [Google Scholar]
- 314.Schafer D.A., Gill S.R., Cooper J.A., Heuser J.E., Schroer T.A. Ultrastructural analysis of the dynactin complex: An actin-related protein is a component of a filament that resembles f-actin. J. Cell Biol. 1994;126:403–412. doi: 10.1083/jcb.126.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Schroder J.M., Schneider L., Christensen S.T., Pedersen L.B. Eb1 is required for primary cilia assembly in fibroblasts. Curr. Biol. 2007;17:1134–1139. doi: 10.1016/j.cub.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 316.Sedjai F., Acquaviva C., Chevrier V., Chauvin J.P., Coppin E., Aouane A., Coulier F., Tolun A., Pierres M., Birnbaum D., et al. Control of ciliogenesis by for20, a novel centrosome and pericentriolar satellite protein. J. Cell Sci. 2010;123:2391–2401. doi: 10.1242/jcs.065045. [DOI] [PubMed] [Google Scholar]
- 317.Shinmura K., Bennett R.A., Tarapore P., Fukasawa K. Direct evidence for the role of centrosomally localized p53 in the regulation of centrosome duplication. Oncogene. 2007;26:2939–2944. doi: 10.1038/sj.onc.1210085. [DOI] [PubMed] [Google Scholar]
- 318.Siller S.S., Sharma H., Li S., Yang J., Zhang Y., Holtzman M.J., Winuthayanon W., Colognato H., Holdener B.C., Li F.Q., et al. Conditional knockout mice for the distal appendage protein cep164 reveal its essential roles in airway multiciliated cell differentiation. PLoS Genet. 2017;13:e1007128. doi: 10.1371/journal.pgen.1007128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sillibourne J.E., Milne D.M., Takahashi M., Ono Y., Meek D.W. Centrosomal anchoring of the protein kinase ck1delta mediated by attachment to the large, coiled-coil scaffolding protein cg-nap/akap450. J. Mol. Biol. 2002;322:785–797. doi: 10.1016/S0022-2836(02)00857-4. [DOI] [PubMed] [Google Scholar]
- 320.Sir J.H., Barr A.R., Nicholas A.K., Carvalho O.P., Khurshid M., Sossick A., Reichelt S., D’Santos C., Woods C.G., Gergely F. A primary microcephaly protein complex forms a ring around parental centrioles. Nat. Genet. 2011;43:1147–1153. doi: 10.1038/ng.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sonn S., Oh G.T., Rhee K. Nek2 and its substrate, centrobin/nip2, are required for proper meiotic spindle formation of the mouse oocytes. Zygote. 2011;19:15–20. doi: 10.1017/S0967199410000183. [DOI] [PubMed] [Google Scholar]
- 322.Soung N.K., Park J.E., Yu L.R., Lee K.H., Lee J.M., Bang J.K., Veenstra T.D., Rhee K., Lee K.S. Plk1-dependent and -independent roles of an odf2 splice variant, hcenexin1, at the centrosome of somatic cells. Dev. Cell. 2009;16:539–550. doi: 10.1016/j.devcel.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Sun Y. P53 and its downstream proteins as molecular targets of cancer. Mol. Carcinog. 2006;45:409–415. doi: 10.1002/mc.20231. [DOI] [PubMed] [Google Scholar]
- 324.Sydor A.M., Coyaud E., Rovelli C., Laurent E., Liu H., Raught B., Mennella V. PPP1R35 is a novel centrosomal protein that regulates centriole length in concert with the microcephaly protein rttn. eLife. 2018;7:e37846. doi: 10.7554/eLife.37846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Szebenyi G., Hall B., Yu R., Hashim A.I., Kramer H. Hook2 localizes to the centrosome, binds directly to centriolin/cep110 and contributes to centrosomal function. Traffic. 2007;8:32–46. doi: 10.1111/j.1600-0854.2006.00511.x. [DOI] [PubMed] [Google Scholar]
- 326.Takahashi M., Mukai H., Oishi K., Isagawa T., Ono Y. Association of immature hypophosphorylated protein kinase cepsilon with an anchoring protein cg-nap. J. Biol. Chem. 2000;275:34592–34596. doi: 10.1074/jbc.M005285200. [DOI] [PubMed] [Google Scholar]
- 327.Takahashi M., Shibata H., Shimakawa M., Miyamoto M., Mukai H., Ono Y. Characterization of a novel giant scaffolding protein, cg-nap, that anchors multiple signaling enzymes to centrosome and the golgi apparatus. J. Biol. Chem. 1999;274:17267–17274. doi: 10.1074/jbc.274.24.17267. [DOI] [PubMed] [Google Scholar]
- 328.Takahashi M., Yamagiwa A., Nishimura T., Mukai H., Ono Y. Centrosomal proteins cg-nap and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol. Biol. Cell. 2002;13:3235–3245. doi: 10.1091/mbc.e02-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Tanos B.E., Yang H.J., Soni R., Wang W.J., Macaluso F.P., Asara J.M., Tsou M.F. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 2013;27:163–168. doi: 10.1101/gad.207043.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Terada Y., Uetake Y., Kuriyama R. Interaction of aurora-a and centrosomin at the microtubule-nucleating site in drosophila and mammalian cells. J. Cell Biol. 2003;162:757–763. doi: 10.1083/jcb.200305048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Toya M., Terasawa M., Nagata K., Iida Y., Sugimoto A. A kinase-independent role for aurora a in the assembly of mitotic spindle microtubules in caenorhabditis elegans embryos. Nat. Cell Biol. 2011;13:708–714. doi: 10.1038/ncb2242. [DOI] [PubMed] [Google Scholar]
- 332.Tsai J.J., Hsu W.B., Liu J.H., Chang C.W., Tang T.K. Author correction: Cep120 interacts with c2cd3 and talpid3 and is required for centriole appendage assembly and ciliogenesis. Sci. Rep. 2020;10:1265. doi: 10.1038/s41598-020-58151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Tsang W.Y., Spektor A., Luciano D.J., Indjeian V.B., Chen Z., Salisbury J.L., Sanchez I., Dynlacht B.D. Cp110 cooperates with two calcium-binding proteins to regulate cytokinesis and genome stability. Mol. Biol. Cell. 2006;17:3423–3434. doi: 10.1091/mbc.e06-04-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Tzivion G., Shen Y.H., Zhu J. 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene. 2001;20:6331–6338. doi: 10.1038/sj.onc.1204777. [DOI] [PubMed] [Google Scholar]
- 335.Uzbekov R., Prigent C., Arlot-Bonnemains Y. Cell cycle analysis and synchronization of the xenopus laevis xl2 cell line: Study of the kinesin related protein xleg5. Microsc. Res. Tech. 1999;45:31–42. doi: 10.1002/(SICI)1097-0029(19990401)45:1<31::AID-JEMT3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 336.Uzbekov R.E. Ph.D. Thesis. Moscow State University; Moscow, Russia: 2004. Dynamics of Functional Activity of the Centrosome during Cell Cycle. [Google Scholar]
- 337.Vasquez R.J., Gard D.L., Cassimeris L. Phosphorylation by cdk1 regulates xmap215 function in vitro. Cell Motil. Cytoskelet. 1999;43:310–321. doi: 10.1002/(SICI)1097-0169(1999)43:4<310::AID-CM4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 338.Vaughan K.T., Vallee R.B. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150glued. J. Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wang H., Shao N., Ding Q.M., Cui J., Reddy E.S., Rao V.N. Brca1 proteins are transported to the nucleus in the absence of serum and splice variants brca1a, brca1b are tyrosine phosphoproteins that associate with e2f, cyclins and cyclin dependent kinases. Oncogene. 1997;15:143–157. doi: 10.1038/sj.onc.1201252. [DOI] [PubMed] [Google Scholar]
- 340.Wang H.F., Takenaka K., Nakanishi A., Miki Y. Brca2 and nucleophosmin coregulate centrosome amplification and form a complex with the rho effector kinase rock2. Cancer Res. 2011;71:68–77. doi: 10.1158/0008-5472.CAN-10-0030. [DOI] [PubMed] [Google Scholar]
- 341.Wang L., Failler M., Fu W., Dynlacht B.D. A distal centriolar protein network controls organelle maturation and asymmetry. Nat. Commun. 2018;9:3938. doi: 10.1038/s41467-018-06286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wang L.H., Mayer B., Stemmann O., Nigg E.A. Centromere DNA decatenation depends on cohesin removal and is required for mammalian cell division. J. Cell Sci. 2010;123:806–813. doi: 10.1242/jcs.058255. [DOI] [PubMed] [Google Scholar]
- 343.Watanabe K., Takao D., Ito K.K., Takahashi M., Kitagawa D. The cep57-pericentrin module organizes pcm expansion and centriole engagement. Nat. Commun. 2019;10:931. doi: 10.1038/s41467-019-08862-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wei Q., Xu Q., Zhang Y., Li Y., Zhang Q., Hu Z., Harris P.C., Torres V.E., Ling K., Hu J. Transition fibre protein fbf1 is required for the ciliary entry of assembled intraflagellar transport complexes. Nat. Commun. 2013;4:2750. doi: 10.1038/ncomms3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wei Z., Kim T.S., Ahn J.I., Meng L., Chen Y., Ryu E.K., Ku B., Zhou M., Kim S.J., Bang J.K., et al. Requirement of the cep57-cep63 interaction for proper cep152 recruitment and centriole duplication. Mol. Cell. Biol. 2020;40 doi: 10.1128/MCB.00535-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wu L., Timmers C., Maiti B., Saavedra H.I., Sang L., Chong G.T., Nuckolls F., Giangrande P., Wright F.A., Field S.J., et al. The e2f1-3 transcription factors are essential for cellular proliferation. Nature. 2001;414:457–462. doi: 10.1038/35106593. [DOI] [PubMed] [Google Scholar]
- 347.Yao J., Fu C., Ding X., Guo Z., Zenreski A., Chen Y., Ahmed K., Liao J., Dou Z., Yao X. Nek2a kinase regulates the localization of numatrin to centrosome in mitosis. FEBS Lett. 2004;575:112–118. doi: 10.1016/j.febslet.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 348.Yarden R.I., Brody L.C. Brca1 interacts with components of the histone deacetylase complex. Proc. Natl. Acad. Sci. USA. 1999;96:4983–4988. doi: 10.1073/pnas.96.9.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ye X., Zeng H., Ning G., Reiter J.F., Liu A. C2cd3 is critical for centriolar distal appendage assembly and ciliary vesicle docking in mammals. Proc. Natl. Acad. Sci. USA. 2014;111:2164–2169. doi: 10.1073/pnas.1318737111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Zach F., Grassmann F., Langmann T., Sorusch N., Wolfrum U., Stohr H. The retinitis pigmentosa 28 protein fam161a is a novel ciliary protein involved in intermolecular protein interaction and microtubule association. Hum. Mol. Genet. 2012;21:4573–4586. doi: 10.1093/hmg/dds268. [DOI] [PubMed] [Google Scholar]
- 351.Zhang H., Somasundaram K., Peng Y., Tian H., Zhang H., Bi D., Weber B.L., El-Deiry W.S. Brca1 physically associates with p53 and stimulates its transcriptional activity. Oncogene. 1998;16:1713–1721. doi: 10.1038/sj.onc.1201932. [DOI] [PubMed] [Google Scholar]
- 352.Zhang J., Megraw T.L. Proper recruitment of gamma-tubulin and d-tacc/msps to embryonic drosophila centrosomes requires centrosomin motif 1. Mol. Biol. Cell. 2007;18:4037–4049. doi: 10.1091/mbc.e07-05-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Zhang W., Fletcher L., Muschel R.J. The role of polo-like kinase 1 in the inhibition of centrosome separation after ionizing radiation. J. Biol. Chem. 2005;280:42994–42999. doi: 10.1074/jbc.M505450200. [DOI] [PubMed] [Google Scholar]
- 354.Zhao X., Jin S., Song Y., Zhan Q. Cdc2/cyclin b1 regulates centrosomal nlp proteolysis and subcellular localization. Cancer Biol. Ther. 2010;10:945–952. doi: 10.4161/cbt.10.9.13368. [DOI] [PubMed] [Google Scholar]
- 355.Zimmerman W.C., Sillibourne J., Rosa J., Doxsey S.J. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell. 2004;15:3642–3657. doi: 10.1091/mbc.e03-11-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Avidor-Reiss T., Mazur M., Fishman E.L., Sindhwani P. The role of sperm centrioles in human reproduction—The known and the unknown. Front. Cell Dev. Biol. 2019;7:188. doi: 10.3389/fcell.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Avidor-Reiss T., Turner K. The evolution of centriole structure: Heterochrony, neoteny, and hypermorphosis. Results Probl. Cell Differ. 2019;67:3–15. doi: 10.1007/978-3-030-23173-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Uzbekov R.E., Alieva I.B. The centrosome—A riddle of the “cell processor”. Tsitologiia. 2008;50:91–112. [PubMed] [Google Scholar]