Abstract
The present study aimed to evaluate the effect of aerobic exercise training (AET) on the performance of mice with Parkinson’s disease (PD) and to explore the molecular mechanism of AET-associated long noncoding RNAs (lncRNAs) in PD treatment. The results showed that the behaviors of PD mice were significantly improved after 4 weeks of AET. The substantia nigra pars compacta of PD mice showed scattered large multipolar cells and surrounding neutrophils after AET. In addition, a total of 62 differentially expressed lncRNAs (DE-lncRNAs) were identified between the AET group and the PD group, including 55 up-regulated and 7 down-regulated DE-lncRNAs in the AET group. Furthermore, the target genes of DE-lncRNAs, including LOC102633466, LOC102637865, and LOC102638670, were mainly involved in ECM-receptor interaction, the Wnt pathway and the PI3K/AKT/mTOR pathway. Quantitative real-time polymerase chain reaction showed that these three DE-lncRNAs were significantly up-regulated in the AET group than in the PD group. The lncRNA–miRNA–mRNA ceRNA network suggested that these 3 DE-lncRNAs may improve PD via the ceRNA mechanism. In conclusion, this study suggests that aerobic exercise improves motor performance of PD mice and provides a foundation for further studies on the molecular mechanism of lncRNAs in treating PD.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02483-z) contains supplementary material, which is available to authorized users.
Keywords: Parkinson’s disease, Aerobic exercise, lncRNA, Transcriptome sequencing, Motor function
Introduction
Parkinson’s disease (PD) is a chronic progressive neurodegenerative disease, commonly occurs in elderly individuals, and has a prevalence of 1% in adults above the age of 60 years (McGregor and Nelson 2019). PD is characterized by the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the accumulation of Lewy bodies in the brain as well as motor dysfunction (Klemann et al. 2018). According to The Global Burden of Disease Study, there are approximately 6.2 million people with PD worldwide, and this number is increasing annually (Tysnes and Storstein 2017). In most PD cases, pain emerges before motor and cognitive symptoms and intensifies with disease progression, resulting in increasing disability and reduced quality of life (Buhmann et al. 2017). Many patients with PD develop cognitive impairments. Patients with PD who have cognitive impairments often present mood and neuropsychiatric symptoms (Emre 2003). Notably, as many as 90% of patients with PD experience depressive symptoms (Slaughter et al. 2001).
Currently, there is no cure for PD or treatment that slows its progression. However, some pharmacological and non-pharmacological therapies have been proven to improve PD symptoms (Riekkinen et al. 1998; Petzinger et al. 2013). Among non-pharmacological therapies, physical exercise may be a promising method. Many studies have demonstrated that aerobic exercise, as a rehabilitation intervention, can enhance the motor and cognitive functions of patients with PD and reduce the severity of depression (Duchesne et al. 2015, 2016; Ahlskog 2018). Silveira et al. (2018) investigated the effects of aerobic and goal-based exercises on cognitive domains in patients with PD and suggested that aerobic exercise is more effective for improving cognition in PD than goal-based exercise. Altmann et al. (2016) reported that aerobic exercise can positively affect mood and executive function in patients with PD. Additionally, Uc et al. (2014) demonstrated that aerobic exercise can improve aerobic fitness, motor function, mood, and cognition in these patients. Although these clinical effects of aerobic exercise on PD symptoms are obvious, little is known about how aerobic exercise influences brain function and consequently improves cognition in PD.
Long noncoding RNAs (lncRNAs) are a type of noncoding RNAs that consist of more than 200 nucleotides (Li et al. 2013). LncRNAs can interact with DNA, RNA, or protein molecules to regulate gene expression and mediate effects through various mechanisms (Lyu et al. 2019). LncRNAs have been proven to play a crucial role in neurodegenerative diseases, including PD (Majidinia et al. 2016). For example, lncRNA HOTAIR was increased in PD mice, and the knockdown of HOTAIR significantly promoted cell proliferation and inhibited apoptosis in PD cells (Lin et al. 2019). Additionally, Lu et al. (2018) found that lncRNA UCA1 promotes the progression of PD by upregulating SNCA expression. However, little is known about the mechanism underlying lncRNAs following aerobic exercise for improving PD.
In this study, PD mice models were established, and then, the mice underwent aerobic exercise training (AET). A swim test, pole test, and hematoxylin–eosin (HE) staining were used to assess the cognitive function and pathological changes in mice for ultimately evaluating the effect of aerobic exercise on PD. RNA sequencing (RNA-seq) technology was used to explore the role of lncRNAs in PD treatment. Six differentially expressed lncRNAs (DE-lncRNAs) were selected to verify the RNA-seq results using quantitative real-time polymerase chain reaction (qRT-PCR). Our study could provide valuable data for further studies on the molecular mechanism underlying lncRNAs following aerobic exercises for treating PD.
Methods
Animals
A total of 15 male C57BL/6 J mice (age 10–12 weeks) were randomly divided into three groups: sham group (n = 5), PD group (n = 5), and AET group (n = 5). All animals were housed in an air-conditioned room maintained at a room temperature of 20–24 °C under a 12-h light/dark cycle. They were provided free access to food and water and acclimated to laboratory conditions for 1 week before the start of the experiment. In the sham group, 0.9% sodium chloride (30 mg/kg) was injected into the mice abdomen for 7 days. Mice in the PD and AET groups were intraperitoneally injected with 0.33% 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (30 mg/kg) for 7 days to establish PD models. The sham and PD groups did not receive any training. The AET group received rehabilitative training for 4 weeks.
All experimental procedures were approved by the Ethics Committee and the Animal Experimental Committee of Hainan Medical College. Animal disposal was performed in accordance with “The Guidance on the Care of Laboratory Animals.”
Rehabilitative training
PD mice were trained to grasp, rotate, walk, and balance for 4 weeks using a circular mesh instrument, which was 100-cm long and 60 cm in diameter. All mice were trained for 30 min once a day, six times a week. In addition, the ability of PD mice to maintain balance was monitored via balance beam training, which lasted for 4 weeks. The balance beam was a 1-m long and 2-cm wide wooden beam suspended 7 cm above the ground. All mice were trained for 30 min once a day, six times a week.
Behavioral assessment
Mice behaviors in each group were observed after weeks 0, 2, and 4 of training.
The swim test process was as follows: mice were placed in a 20 × 30 × 20 cm tank containing water at a temperature of 22–25 °C, and the maximum evaluation time was 1 min. The scoring criteria were as follows: three points, mice could swim continuously; two points, mice swam occasionally; one point, mice floated on one side and only occasionally swam with their hind legs; zero point, only the head floated and the hind legs sank. Finally, the total score for each mouse was calculated.
The pole test was conducted as follows: a small foamed plastic ball with a diameter of 2.5 cm was fixed to the top of a wooden pole of 50-cm length and 1-cm diameter. The mice were placed upside down on the ball. The times that mice spent climbing from the top of the pole to its base (50 cm), climbing the top half of the pole, and climbing the lower half of the pole were recorded. The scoring criteria were as follows: three points, mice completed any of the above climbs in 3 s; two points, micee completed any of the above climbs in 6 s; one point: mice completed any of the above climbs in more than 6 s or mice fell from the pole. Finally, the average score was used as the final score for the experiment. If a mouse stopped or crawled in reverse, the trial was not recorded, and the measurement was performed again.
HE staining
Brain tissue samples were collected from each group after 0, 2, and 4 weeks of training for HE staining. The tissues were fixed in 10% formaldehyde solution, placed in an embedding box, and rinsed under running water for 30 min. Then, the tissues were embedded in paraffin. The staining process was performed as follows: hematoxylin for 3 min, water wash, hydrochloric ethanol for 10 s, water wash, 1% ammonia for 10 s, water wash, eosin for 1 min and 30 s, water wash, 75% alcohol for 10 s, anhydrous alcohol for 10 s, xylene for 2 s, and neutral resin mount. Finally, images were obtained and analyzed.
Total RNA extraction, cDNA library construction, and sequencing
Total RNA was isolated from the striatal tissues of mice from the PD group and the AET group using TRIzol reagent (Invitrogen Life Technologies, Inc.) according to the manufacturer’s instruction and was then immediately stored in the refrigerator at − 80 °C. Total RNA concentration was detected using a Nanodrop 2000 spectrophotometer (Invitrogen, Carlsbad, CA, USA), and RNA integrity was evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA). Ribosomal RNA was removed using a Ribo-zero Gold rRNA Removal kit (Illumina, USA). Subsequently, the cDNA library was generated from the rRNA-depleted RNA pools. Library quality was evaluated using the Agilent Bioanalyzer 2100 system according to manufacturer instructions. High-throughput lncRNA sequencing was conducted using a Hiseq TM 2500 platform (Illumina, USA). The raw reads obtained were evaluated using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The retrieved filtering data were mapped to the mouse reference genome (GRCm38) using the HISTA software (https://ccb.jhu.edu/software/hisat2/index.shtml).
Analysis of DE-lncRNAs
For lncRNA sequencing, raw reads were qualified using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The expression of each sample was normalized using the fragments per kilobase of exon per million fragments mapped method. The differential expression was assessed using the DESeq algorithm. LncRNAs with screening criteria of log2FC (fold change) > 1 or < − 1 and a false discovery rate of < 0.05 were identified as DE-lncRNAs.
Functional enrichment analysis of lncRNA target genes
Gene Ontology (GO) enrichment analysis of predicted DE-lncRNA target genes was performed using the Database for Annotation, Visualization, and Integrated Discovery. The pathway analysis of predicted DE-lncRNA targets was performed via the Kyoto Encyclopedia of Genes and Genomes (KEGG) online website (https://www.genome.jp/kegg/). Significant correlations between DE-lncRNA target genes and their related functions and pathways were evaluated based on a threshold of p < 0.05.
Analysis of lncRNA–miRNA–mRNA ceRNA networks
To determine relationships between lncRNA expression and mRNA co-expression regulation, a network of lncRNA–miRNA–mRNA was constructed. We selected 6 DE-lncRNAs with high expression associated with the development of PD. Besides, the predictive target genes of the 6 DE-lncRNAs were used for network construction. For a given lncRNA–mRNA pair relationship, these DE-lncRNAs were targeted by a common miRNA to construct an lncRNA–miRNA–mRNA ceRNA network. The network was visualized using vision 3.6.1 of Cytoscape.
qRT-PCR validation of DE-lncRNAs
We selected 6 DE-lncRNAs to verify the reliability of sequencing data. TRIzol (Invitrogen Life Technologies, Inc.) was used to isolate total RNA, and a microspectrophotometer (Tiangen Biotech Co., Ltd.) was used to detect the concentration and purity of RNA. Briefly, first strand cDNA was synthesized using the RevertAid First Strand cDNA synthesis kit (Thermo Fisher Scientific, MA, USA). Subsequently, qRT-PCR was performed with an ABI Q6 real-time PCR machine (Applied Biosystem Inc., MA, USA) using the QuantiFast SYBR Green PCR Kit (Qiagen, Hilden, Germany) according to the manufacturer instructions. The PCR cycling parameters were as follows: 95 °C for 10 min, 45 cycles of 95 °C for 15 s, and 60 °C for 60 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The relative expression of lncRNAs was determined by the 2−ΔΔCt method. All primer sequences of lncRNAs are listed in Supplementary Table S1.
Statistics
Statistical analysis was performed using SPSS 16 (Chicago, IL, USA). Each experiment was repeated at least three times, and the data are expressed as means ± standard deviation. One-way analysis of variance followed by Tukey’s test was used to assess statistically significant differences among the groups. Results with a value of p < 0.05 were defined as statistically significant.
Results
Behavior test
To investigate the effect of aerobic exercise on motor function in PD mice, swim and pole tests were performed. Mice in the sham group achieved high scores in the swim and pole tests, and there was a significant difference between the sham group and the other two groups (Fig. 1a, b). Mice in the AET group showed no significant difference in the performance on the swim test compared with the PD group at weeks 0 and 2. At week 4, the swimming ability of mice in the AET group improved significantly compared with that of mice in the PD group (Fig. 1a). In addition, there was no significant difference in the performance on the pole test between the AET group and PD group at weeks 0 and 2. The performance of mice in the AET group was remarkably improved compared with that in the PD group at week 4 (Fig. 1b). These results suggested that aerobic exercise can improve the motor function of PD mice.
Fig. 1.
Effects of aerobic exercise training (AET) on the behavior of mice with Parkinson’s disease (PD). a A swim test was conducted at weeks 0, 2, and 4 after the PD mice model was successfully established. b A pole test was conducted at weeks 0, 2, and 4 after the PD mice model was successfully established. The Y-axis represents the average score of mice in swim and pole tests. *Represents p < 0.05, **represents p < 0.01, ***represents p < 0.001
HE staining
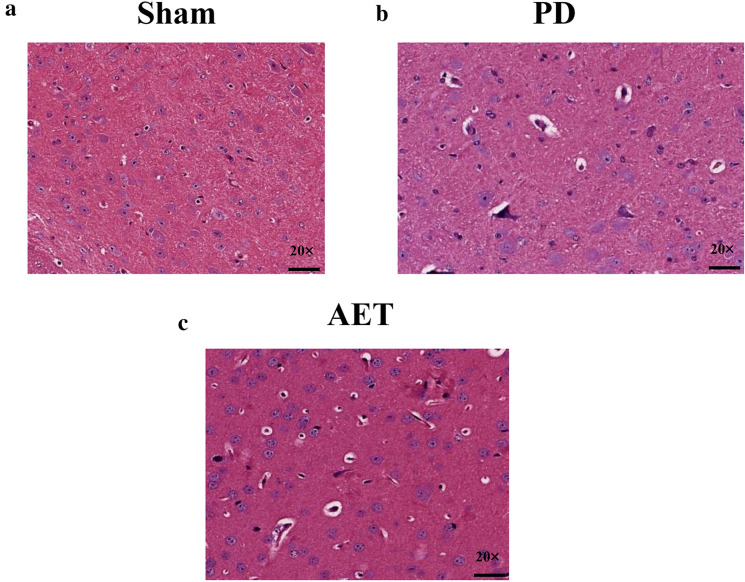
To verify the effect of AET on the brain tissue morphology of PD mice, we used HE staining to observe the pathological differences in the brain tissues in all groups. Under a light microscope, the SNpc in the sham group showed scattered, large multipolar neurons with surrounding neutrophils and no pathological changes (Fig. 2a). The morphology of SNpc cells in the PD group was altered, and the number of SNpc cells was decreased. SNpc cells showed a deeply stained cytoplasm and nucleus (Fig. 2b). Compared with those in the PD group, SNpc cells in the AET group showed fewer changes, and most SNpc cells had normal structures. In addition, the SNpc showed scattered large multipolar cells and surrounding neutrophils (Fig. 2c). These results indicated that aerobic exercise improves the pathological features of PD mice.
Fig. 2.
Hematoxylin-eosin staining of mice brain tissues in the sham, Parkinson’s disease (PD), and aerobic exercise training (AET) group (20 ×). a The substantia nigra pars compacta (SNpc) of mice in the sham group showed scattered large multipolar neurons with surrounding neutrophils. b SNpc cells of mice in the PD group showed a deeply stained cytoplasm and nucleus. c SNpc of mice in the AET group exhibited scattered large multipolar cells and surrounding neutrophils
Overall review of sequencing data
Statistics and quality evaluation were conducted on the sequencing data, and the results are shown in Table 1. In total, 106,516,647 and 76,147,871 raw reads were generated from the PD group and the AET group, respectively. Furthermore, 101,082,158 and 72,744,862 clean reads were obtained from the PD and AET groups, respectively. In addition, 92.1% clean reads in the PD group and 93.5% clean reads in the AET group were perfectly mapped to the reference genome (Table 1).
Table 1.
Sequencing and data analysis of the transcriptome data
| Sample | Raw reads | Clean reads | Mapped | Mapped rate (%) |
|---|---|---|---|---|
| PD1 | 104,305,434 | 98,777,574 | 90,425,707 | 91.5 |
| PD2 | 122,697,584 | 116,864,136 | 108,109,661 | 92.5 |
| PD3 | 92,546,924 | 87,604,768 | 80,893,471 | 92.3 |
| AET1 | 99,067,446 | 94,325,600 | 87,077,956 | 92.3 |
| AET2 | 58,217,482 | 55,639,460 | 52,127,929 | 93.7 |
| AET3 | 71,158,686 | 68,269,528 | 64,613,500 | 94.6 |
Analysis of DE-lncRNAs in striatal tissues
To search for lncRNAs associated with the development of PD, we identified DE-lncRNAs between the PD and AET groups. Analysis of DE-lncRNAs revealed 62 DE-lncRNAs between the AET and PD groups. Among these DE-lncRNAs, 55 were up-regulated and 7 were down-regulated in the AET group (Fig. 3a). All DE-lncRNAs and their expression are illustrated in Fig. 3b. As expected, DE-lncRNAs in the PD and AET groups were significantly clustered into two branches. The expression trend of DE-lncRNAs in the AET group was opposite to that in the PD group.
Fig. 3.
Analysis of the expression of differentially expressed lncRNAs (DE-lncRNAs) in striatal tissues of the aerobic exercise training (AET) and Parkinson’s disease (PD) groups. a Volcano plots of DE-lncRNAs between the AET and PD groups. The red and blue dots represent up- and down-regulated lncRNAs, respectively. The gray dots represent the lncRNAs without significant differential expression. b Hierarchical clustering of DE-lncRNAs in the striatal tissues of the AET and PD groups. The expression of these DE-lncRNAs is illustrated in this heatmap as the mean normalized number of reads. Red indicates high expression, and blue means low expression
Functional and pathway analysis of DE-lncRNAs
To identify the functional clusters and biochemical pathways of the identified DE-lncRNAs, GO and KEGG enrichment analyses were performed for the target genes of DE-lncRNAs. GO analysis revealed that the target genes of DE-lncRNAs were mainly involved in dopamine biosynthetic process, response to immobilization stress, and response to hypoxia (Fig. S1A). KEGG analysis revealed that the target genes of DE-lncRNAs mainly participated in the ECM-receptor interaction, dopaminergic synapse, oxytocin signaling pathway, and calcium signaling pathway (Fig. S1B).
Construction of lncRNA–miRNA–mRNA ceRNA network
It has been reported that the ECM-receptor interaction, Wnt signaling pathway and PI3K/AKT pathway are involved in the development of PD (Botta-Orfila et al. 2014; Wu et al. 2019; Nakaso et al. 2008). Therefore, we selected six DE-lncRNAs that had the high expression and were involved in these 3 pathways. To further investigate the molecular mechanism of these 6 DE-lncRNAs, we constructed the lncRNA–miRNA–mRNA network using Cytoscape 3.6.1. The ceRNA network is shown in Figure S2. It includes 6 lncRNAs, 60 miRNAs, and 3 mRNAs. Interestingly, except for LOC102634932 targeting miR-6925-5p, miR-7226-5p, miR-7229-5p, and miR-6901-5p, the remaining 5 lncRNAs had more than 10 target miRNAs. LOC102637640 predicted 12 target miRNAs, including miR-702-5p, miR-370-3p, and miR-3103-5p. LOC102633466 predicted 12 target miRNAs, including miR-17-3p, miR-505-5p, and miR-3095-3p. LOC102638670 predicted 16 target miRNAs, including miR-370-3p, miR-3103-5p, and miR-1966-5p. LOC102633419 predicted 18 target miRNAs, including miR-777, miR-17-3p, and miR-702-5p. LOC102637865 predicted 27 target miRNAs, including miR-127-5p, miR-17-3p, and miR-188-3p. Collagen type VI alpha 1 (Col6a1), integrin alpha-11 (Itga11), and protein Wnt-6 (Wnt6) were the common target genes of these 6 DE-lncRNAs, which were associated with the ECM-receptor interaction, Wnt signaling pathway, and PI3K/AKT pathway (Table S2). Therefore, we speculated that these 6 DE-lncRNAs might be involved in mediating the beneficial effects of aerobic exercise for improving PD via the ceRNA mechanism.
qRT-PCR analysis of DE-lncRNAs
The 6 DE-lncRNAs with high expression between the PD and AET groups were chosen to validate the RNA-seq results. As shown in Fig. 4, LOC102633466, LOC102637865, and LOC102638670 were significantly up-regulated in the AET group than in the PD group, which was in agreement with the RNA-seq results (Table S3). In addition, the expression of LOC102633419, LOC102634932, and LOC102637640 showed no significant difference between the AET and PD groups.
Fig. 4.

Quantitative real-time polymerase chain reaction (qRT-PCR) of DE-lncRNAs. *represents p < 0.05, **represents p < 0.01. The relative expression of lncRNAs was determined by the 2−ΔΔCt method
Discussion
PD is the second most common neurodegenerative disease after Alzheimer’s disease. The prevalence of PD is increasing with the increase in aging population and has great effects on the quality of life of patients (Tang et al. 2017). Changes in dopaminergic neurons in the SNpc are the leading cause of PD progression (Essawy et al. 2017). In recent years, aerobic exercise has been reported to have beneficial effects on PD (Klemann et al. 2018). Exercise can extensively activate a series of biological processes, including cerebral vascular regeneration and brain function improvement, which can enhance the plasticity of the brain and improve the state of patients with PD (Cotman and Berchtold 2002; Araki et al. 2001). Maurus et al. (2019) reviewed that aerobic exercise was effective in improving negative symptoms and cognition in patients with schizophrenia. Another study has demonstrated that physical exercise improves motor ability in PD mice (Klemann et al. 2018). In accordance with this result, aerobic exercises, including the swim and pole tests, in our study improved the motor function of PD mice. In addition, the SNpc of PD mice after aerobic exercises showed scattered large multipolar cells with surrounding neutrophils. Therefore, aerobic exercise improved motor and neurological functions in PD mice.
In recent years, several studies have suggested that lncRNAs are involved in the development of PD and can be used as targets for PD treatment (Carrieri et al. 2015; Chi et al. 2019). Li et al. (2019) found that 407 lncRNAs were up-regulated and 51 lncRNAs were down-regulated in the PD rat model than in controls. Han et al. (2020) reported that 79 lncRNAs were decreased in PD rats than in sham controls. However, the differences in the expression of lncRNAs between PD mice and PD mice receiving aerobic exercise remain unknown. In the present study, 55 DE-lncRNAs were up-regulated and 7 DE-lncRNAs were down-regulated in the AET group compared with that in the PD group. This finding provides valuable resources for further study on the role of lncRNAs in the beneficial effect of aerobic exercise on PD progression.
To further research the functions of DE-lncRNAs, we focused on KEGG pathways. We noted that the target genes of the 6 identified DE-lncRNAs (LOC102634932, LOC102637640, LOC102633466, LOC102638670, LOC102633419, and LOC102637865) with high expression were associated with ECM-receptor interaction, the Wnt pathway and the PI3K/AKT/mTOR pathway. ECM-receptor interaction plays an important role in maintaining the micro-environmental pathways of the structure and function of cells and tissues (Wang et al. 2020). A previous study reported miRNAs related to PD and found that miRNA target genes were associated with ECM-receptor interaction (Botta-Orfila et al. 2014). Consequently, the ECM-receptor interaction pathway might be involved in the progression of PD. In addition, certain cellular signaling pathways, such as the Wnt pathway and PI3K/AKT/mTOR pathway, are involved in the progression of PD. Wu et al. (2019) found that the activation of the Wnt pathway could reduce apoptosis of dopaminergic neurons, thereby inhibiting PD progression. The PI3K/AKT/mTOR pathway plays a crucial role in neuronal protection (Heras-Sandoval et al. 2014). Dysregulation of the PI3K/Akt/mTOR pathway could cause loss of dopaminergic neurons in the development of PD (Wang et al. 2012). Furthermore, Nakaso et al. (2008) revealed that the activation of the PI3K/AKT pathway could regulate the neuroprotective effect of caffeine in a cellular model of PD. Therefore, we speculated that aerobic exercise improve PD through these pathways by regulating the expression of the aforementioned 6 DE-lncRNAs.
Emerging evidence has confirmed that lncRNAs target miRNAs to regulate the expression of related genes, activate signaling pathways, and thus participate in PD progression. Peng et al. (2019) discovered that upregulation of the lncRNA HAGLROS promotes the development of PD by regulating the miR-100/ATG10 axis. Cao et al. (2018) indicated that lncRNA SNHG1 contributes to neuroinflammation in the pathogenesis of PD via regulating the miR-7/NLRP3 pathway. In our study, verification using qRT-PCR revealed that LOC102633466, LOC102637865, and LOC102638670 were significantly up-regulated in the AET group compared with that in the PD group. Col6a1 and Wnt6 were the common target genes of these 3 DE-lncRNAs. The overexpression of Wnt6 could reduce locomotor impairment and social behavior deficits in a mouse model of Rett syndrome (Hsu et al. 2020). Col6a1 was found to be involved in the development of PD and Alzheimer’s disease (Grozdanov et al. 2014; Cheng et al. 2009). A study revealed that the inhibition of Col6a1 could promote apoptosis and autophagy in neural cells (Cescon et al. 2016). Therefore, these 3 DE-lncRNAs might be involved in the progression of PD by targeting Wnt6 and Co16a1. In addition, LOC102637865 was predicted to target miR-188-3p or miR-873a-5p. Zhang et al. (2014) discovered that the overexpression of miR-188-3p could inhibit neuroinflammation and improve synaptic and cognitive function in Alzheimer’s disease mice. Long et al. (2020) found that miR-873a-5p could inhibit neuroinflammation and reduce neurological deficits after traumatic brain injury. Furthermore, miR-505-5p was found to be related to cognitive function (Mengel-From et al. 2018). A study has reported that miR-505-5p is highly expressed in the cerebrospinal fluid of patients with Alzheimer’s disease (Denk et al. 2015). Similarly, miR-505-5p was predicted to be a potential target of LOC102633466 in the present study. Therefore, AET-associated lncRNAs, including LOC102633466, LOC102637865, and LOC102638670, might improve PD via a ceRNA mechanism.
Conclusion
In summary, our study demonstrated that aerobic exercise could improve the behaviors and pathological characteristics of PD mice. A total of 62 DE-lncRNAs were identified between the AET group and the PD group. Additionally, LOC102633466, LOC102637865, and LOC102638670 were significantly up-regulated in the AET group compared with that in the PD group. These 3 lncRNAs might be involved in aerobic exercise to improve PD via the ceRNA mechanism. This study provides a foundation for understanding the molecular mechanism involving lncRNA regulation underlying the effects of aerobic exercise in improving PD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1 The top 20 enrichment of Gene Ontology (GO) terms and Kyoto encyclopedia of genes and genomes (KEGG) pathways of DE-lncRNAs target genes. (A) The top 20 enriched GO terms of the DE-lncRNAs target genes. Y-axis represents GO name, and X-axis represents rich factor. Size of each bubble represent the number of differentially expressed genes enriched in GO terms, and color represents -log10 (P-value). (B) The top 20 enriched KEGG pathways of DE-lncRNAs target genes. Y-axis represents pathway name, and X-axis represents rich factor. Size and color of each bubble represent the number of differentially expressed genes enriched in the pathway and -log10 (P-value), respectively (TIF 489 kb)
Figure S2 The lncRNA-miRNA-mRNA network of six DE-lncRNAs. The results were visualized using Cytoscape 3.6.1. Red represents DE-lncRNAs, blue represents miRNAs, and green represents mRNAs (TIF 1760 kb)
Abbreviations
- PD
Parkinson’s disease
- lncRNAs
Long noncoding RNAs
- AET
Aerobic exercise training
- DE-lncRNAs
Differentially expressed lncRNAs
- HE
Hematoxylin–eosin
- RNA-seq
RNA sequencing
- GO
Gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- qRT-PCR
Quantitative real-time polymerase chain reaction
- GAPDH
Glyceraldehyde-3-Phosphate Dehydrogenase
- SNpc
Substantia nigra pars compacta
- Col6a1
Collagen type VI alpha 1
- Wnt6
Protein Wnt-6
Author contributions
Conceptualization and funding acquisition: WL and XL. Data curation: XZ and YW. Formal analysis: ZZ and XC. Experimental studies: XZ and XC. Software: YW and ZZ. Writing: original draft and review and editing: all authors. All authors read and approved the final manuscript.
Funding
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
All experimental procedures and animal care performed in the present study were approved according to the recommendations of the First Affiliated Hospital of Hainan Medical College.
Footnotes
Xiang Zhang and Yachun Wang are equal contributors.
Zhenqiang Zhao and Xinxu Chen are equal contributors.
Contributor Information
Wen Li, Email: 13617541828@163.com.
Xiating Li, Email: 253575732@qq.com.
References
- Ahlskog JE. Aerobic exercise: evidence for a direct brain effect to slow parkinson disease progression. Mayo Clin Proc. 2018;93(3):360–372. doi: 10.1016/j.mayocp.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Altmann LJ, Stegemoller E, Hazamy AA, Wilson JP, Bowers D, Okun MS, Hass CJ. Aerobic exercise improves mood, cognition, and language function in Parkinson’s disease: results of a controlled study. J Int Neuropsychol Soc. 2016;22(9):878–889. doi: 10.1017/S135561771600076X. [DOI] [PubMed] [Google Scholar]
- Araki T, Mikami T, Tanji H, Matsubara M, Imai Y, Mizugaki M, Itoyama Y. Biochemical and immunohistological changes in the brain of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mouse. Eur J Pharm Sci. 2001;12(3):231–238. doi: 10.1016/s0928-0987(00)00170-6. [DOI] [PubMed] [Google Scholar]
- Botta-Orfila T, Morato X, Compta Y, Lozano JJ, Falgas N, Valldeoriola F, Pont-Sunyer C, Vilas D, Mengual L, Fernandez M, Molinuevo JL, Antonell A, Marti MJ, Fernandez-Santiago R, Ezquerra M. Identification of blood serum micro-RNAs associated with idiopathic and LRRK2 Parkinson’s disease. J Neurosci Res. 2014;92(8):1071–1077. doi: 10.1002/jnr.23377. [DOI] [PubMed] [Google Scholar]
- Buhmann C, Wrobel N, Grashorn W, Fruendt O, Wesemann K, Diedrich S, Bingel U. Pain in Parkinson disease: a cross-sectional survey of its prevalence, specifics, and therapy. J Neurol. 2017;264(4):758–769. doi: 10.1007/s00415-017-8426-y. [DOI] [PubMed] [Google Scholar]
- Cao B, Wang T, Qu Q, Kang T, Yang Q. Long noncoding RNA SNHG1 promotes neuroinflammation in Parkinson’s Disease via regulating miR-7/NLRP3 Pathway. Neuroscience. 2018;388:118–127. doi: 10.1016/j.neuroscience.2018.07.019. [DOI] [PubMed] [Google Scholar]
- Carrieri C, Forrest AR, Santoro C, Persichetti F, Carninci P, Zucchelli S, Gustincich S. Expression analysis of the long non-coding RNA antisense to Uchl1 (AS Uchl1) during dopaminergic cells’ differentiation in vitro and in neurochemical models of Parkinson’s disease. Front Cell Neurosci. 2015;9:114. doi: 10.3389/fncel.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cescon M, Chen P, Castagnaro S, Gregorio I, Bonaldo P. Lack of collagen VI promotes neurodegeneration by impairing autophagy and inducing apoptosis during aging. Aging (Albany NY) 2016;8(5):1083–1101. doi: 10.18632/aging.100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JS, Dubal DB, Kim DH, Legleiter J, Cheng IH, Yu GQ, Tesseur I, Wyss-Coray T, Bonaldo P, Mucke L. Collagen VI protects neurons against Abeta toxicity. Nat Neurosci. 2009;12(2):119–121. doi: 10.1038/nn.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi LM, Wang LP, Jiao D. Identification of differentially expressed genes and long noncoding RNAs associated with Parkinson’s Disease. Parkinsons Dis. 2019;2019:6078251. doi: 10.1155/2019/6078251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Denk J, Boelmans K, Siegismund C, Lassner D, Arlt S, Jahn H. MicroRNA profiling of CSF reveals potential biomarkers to detect Alzheimer’s Disease. PLoS ONE. 2015;10(5):e0126423. doi: 10.1371/journal.pone.0126423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne C, Lungu O, Nadeau A, Robillard ME, Bore A, Bobeuf F, Lafontaine AL, Gheysen F, Bherer L, Doyon J. Enhancing both motor and cognitive functioning in Parkinson’s disease: aerobic exercise as a rehabilitative intervention. Brain Cogn. 2015;99:68–77. doi: 10.1016/j.bandc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Duchesne C, Gheysen F, Bore A, Albouy G, Nadeau A, Robillard ME, Bobeuf F, Lafontaine AL, Lungu O, Bherer L, Doyon J. Influence of aerobic exercise training on the neural correlates of motor learning in Parkinson’s disease individuals. Neuroimage Clin. 2016;12:559–569. doi: 10.1016/j.nicl.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M. What causes mental dysfunction in Parkinson’s disease? Mov Disord. 2003;18(Suppl 6):S63–71. doi: 10.1002/mds.10565. [DOI] [PubMed] [Google Scholar]
- Essawy SS, Tawfik MK, Korayem HE. Effects of adenosine receptor antagonists in MPTP mouse model of Parkinson’s disease: mitochondrial DNA integrity. Arch Med Sci. 2017;13(3):659–669. doi: 10.5114/aoms.2017.67284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov V, Bliederhaeuser C, Ruf WP, Roth V, Fundel-Clemens K, Zondler L, Brenner D, Martin-Villalba A, Hengerer B, Kassubek J, Ludolph AC, Weishaupt JH, Danzer KM (2014) Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol 128(5):651-663. 10.1007/s00401-014-1345-4 [DOI] [PMC free article] [PubMed]
- Han CL, Liu YP, Sui YP, Chen N, Du TT, Jiang Y, Guo CJ, Wang KL, Wang Q, Fan SY, Shimabukuro M, Meng FG, Yuan F, Zhang JG. Integrated transcriptome expression profiling reveals a novel lncRNA associated with L-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Aging (Albany NY) 2020;12(1):718–739. doi: 10.18632/aging.102652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heras-Sandoval D, Pérez-Rojas J, Hernández-Damián J, Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell signal. 2014;26(12):2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Hsu WL, Ma YL, Liu YC, Tai DJC, Lee EHY. Restoring Wnt6 signaling ameliorates behavioral deficits in MeCP2 T158A mouse model of Rett syndrome. Sci Rep. 2020;10(1):1074. doi: 10.1038/s41598-020-57745-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemann C, Xicoy H, Poelmans G, Bloem BR, Martens GJM, Visser JE. Physical exercise modulates L-DOPA-regulated molecular pathways in the MPTP mouse model of Parkinson’s Disease. Mol Neurobiol. 2018;55(7):5639–5657. doi: 10.1007/s12035-017-0775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wu Z, Fu X, Han W. Long noncoding RNAs: insights from biological features and functions to diseases. Med Res Rev. 2013;33(3):517–553. doi: 10.1002/med.21254. [DOI] [PubMed] [Google Scholar]
- Li J, Sun Y, Chen J. Transcriptome sequencing in a 6-hydroxydopamine rat model of Parkinson’s disease. Genes Genet Syst. 2019;94(2):61–69. doi: 10.1266/ggs.18-00036. [DOI] [PubMed] [Google Scholar]
- Lin Q, Hou S, Dai Y, Jiang N, Lin Y. LncRNA HOTAIR targets miR-126-5p to promote the progression of Parkinson’s disease through RAB3IP. Biol Chem. 2019;400(9):1217–1228. doi: 10.1515/hsz-2018-0431. [DOI] [PubMed] [Google Scholar]
- Long X, Yao X, Jiang Q, Yang Y, He X, Tian W, Zhao K, Zhang H. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J Neuroinflammation. 2020;17(1):89. doi: 10.1186/s12974-020-01761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Sun WL, Shen J, Wei M, Chen B, Qi YJ, Xu CS. LncRNA-UCA1 promotes PD development by upregulating SNCA. Eur Rev Med Pharmacol Sci. 2018;22(22):7908–7915. doi: 10.26355/eurrev_201811_16417. [DOI] [PubMed] [Google Scholar]
- Lyu Y, Bai L, Qin C. Long noncoding RNAs in neurodevelopment and Parkinson’s disease. Animal Model Exp Med. 2019;2(4):239–251. doi: 10.1002/ame2.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidinia M, Mihanfar A, Rahbarghazi R, Nourazarian A, Bagca B, Avci CB. The roles of non-coding RNAs in Parkinson’s disease. Mol Biol Rep. 2016;43(11):1193–1204. doi: 10.1007/s11033-016-4054-3. [DOI] [PubMed] [Google Scholar]
- Maurus I, Hasan A, Roh A, Takahashi S, Rauchmann B, Keeser D, Malchow B, Schmitt A, Falkai P. Neurobiological effects of aerobic exercise, with a focus on patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2019;269(5):499–515. doi: 10.1007/s00406-019-01025-w. [DOI] [PubMed] [Google Scholar]
- McGregor MM, Nelson AB. Circuit mechanisms of Parkinson’s Disease. Neuron. 2019;101(6):1042–1056. doi: 10.1016/j.neuron.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Mengel-From J, Feddersen S, Halekoh U, Heegaard NHH, McGue M, Christensen K, Tan Q, Christiansen L. Circulating microRNAs disclose biology of normal cognitive function in healthy elderly people—a discovery twin study. Eur J Hum Genet. 2018;26(9):1378–1387. doi: 10.1038/s41431-018-0157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaso K, Ito S, Nakashima K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neurosci Lett. 2008;432(2):146–150. doi: 10.1016/j.neulet.2007.12.034. [DOI] [PubMed] [Google Scholar]
- Peng T, Liu X, Wang J, Liu Y, Fu Z, Ma X, Li J, Sun G, Ji Y, Lu J, Wan W, Lu H. Long noncoding RNA HAGLROS regulates apoptosis and autophagy in Parkinson’s disease via regulating miR-100/ATG10 axis and PI3K/Akt/mTOR pathway activation. Artif Cells Nanomed Biotechnol. 2019;47(1):2764–2774. doi: 10.1080/21691401.2019.1636805. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12(7):716–726. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riekkinen M, Kejonen K, Jakala P, Soininen H, Riekkinen P., Jr Reduction of noradrenaline impairs attention and dopamine depletion slows responses in Parkinson’s disease. Eur J Neurosci. 1998;10(4):1429–1435. doi: 10.1046/j.1460-9568.1998.00145.x. [DOI] [PubMed] [Google Scholar]
- Silveira C, Roy E, Intzandt B, Almeida Q. Aerobic exercise is more effective than goal-based exercise for the treatment of cognition in Parkinson’s disease. Brain Cogn. 2018;122:1–8. doi: 10.1016/j.bandc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Slaughter JR, Slaughter KA, Nichols D, Holmes SE, Martens MP. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2001;13(2):187–196. doi: 10.1176/jnp.13.2.187. [DOI] [PubMed] [Google Scholar]
- Tang Y, Meng L, Wan CM, Liu ZH, Liao WH, Yan XX, Wang XY, Tang BS, Guo JF. Identifying the presence of Parkinson’s disease using low-frequency fluctuations in BOLD signals. Neurosci Lett. 2017;645:1–6. doi: 10.1016/j.neulet.2017.02.056. [DOI] [PubMed] [Google Scholar]
- Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 2017;124(8):901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- Uc EY, Doerschug KC, Magnotta V, Dawson JD, Thomsen TR, Kline JN, Rizzo M, Newman SR, Mehta S, Grabowski TJ, Bruss J, Blanchette DR, Anderson SW, Voss MW, Kramer AF, Darling WG. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology. 2014;83(5):413–425. doi: 10.1212/WNL.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Pan J, Chen S. Kinases and kinase signaling pathways: potential therapeutic targets in Parkinson’s disease. Prog Neurobiol. 2012;98(2):207–221. doi: 10.1016/j.pneurobio.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Q, Li S, Chen Z, Tan J, Yao J, Duan D. Low molecular weight fucoidan alleviates diabetic nephropathy by binding fibronectin and inhibiting ECM-receptor interaction in human renal mesangial cells. Int J Biol Macromol. 2020;150:304–314. doi: 10.1016/j.ijbiomac.2020.02.087. [DOI] [PubMed] [Google Scholar]
- Wu DM, Wang S, Wen X, Han XR, Wang YJ, Shen M, Fan SH, Zhuang J, Zhang ZF, Shan Q, Li MQ, Hu B, Sun CH, Lu J, Chen GQ, Zheng YL. Suppression of microRNA-342-3p increases glutamate transporters and prevents dopaminergic neuron loss through activating the Wnt signaling pathway via p21-activated kinase 1 in mice with Parkinson’s disease. J Cell Physiol. 2019;234(6):9033–9044. doi: 10.1002/jcp.27577. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hu M, Teng Z, Tang YP, Chen C. Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer’s disease. J Neurosci. 2014;34(45):14919–14933. doi: 10.1523/JNEUROSCI.1165-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The top 20 enrichment of Gene Ontology (GO) terms and Kyoto encyclopedia of genes and genomes (KEGG) pathways of DE-lncRNAs target genes. (A) The top 20 enriched GO terms of the DE-lncRNAs target genes. Y-axis represents GO name, and X-axis represents rich factor. Size of each bubble represent the number of differentially expressed genes enriched in GO terms, and color represents -log10 (P-value). (B) The top 20 enriched KEGG pathways of DE-lncRNAs target genes. Y-axis represents pathway name, and X-axis represents rich factor. Size and color of each bubble represent the number of differentially expressed genes enriched in the pathway and -log10 (P-value), respectively (TIF 489 kb)
Figure S2 The lncRNA-miRNA-mRNA network of six DE-lncRNAs. The results were visualized using Cytoscape 3.6.1. Red represents DE-lncRNAs, blue represents miRNAs, and green represents mRNAs (TIF 1760 kb)