Abstract
Background
Chronic kidney disease (CKD) measures (estimated glomerular filtration rate [eGFR] and albuminuria) are frequently assessed in clinical practice and improve the prediction of incident cardiovascular disease (CVD), yet most major clinical guidelines do not have a standardized approach for incorporating these measures into CVD risk prediction. “CKD Patch” is a validated method to calibrate and improve the predicted risk from established equations according to CKD measures.
Methods
Utilizing data from 4,143,535 adults from 35 datasets, we developed several “CKD Patches” incorporating eGFR and albuminuria, to enhance prediction of risk of atherosclerotic CVD (ASCVD) by the Pooled Cohort Equation (PCE) and CVD mortality by Systematic COronary Risk Evaluation (SCORE). The risk enhancement by CKD Patch was determined by the deviation between individual CKD measures and the values expected from their traditional CVD risk factors and the hazard ratios for eGFR and albuminuria. We then validated this approach among 4,932,824 adults from 37 independent datasets, comparing the original PCE and SCORE equations (recalibrated in each dataset) to those with addition of CKD Patch.
Findings
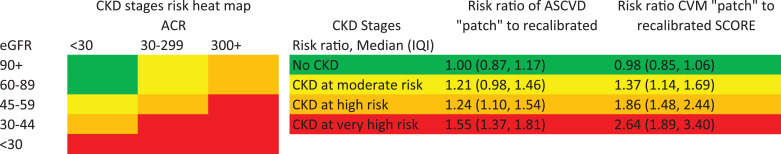
We confirmed the prediction improvement with the CKD Patch for CVD mortality beyond SCORE and ASCVD beyond PCE in validation datasets (Δc-statistic 0.027 [95% CI 0.018–0.036] and 0.010 [0.007–0.013] and categorical net reclassification improvement 0.080 [0.032–0.127] and 0.056 [0.044–0.067], respectively). The median (IQI) of the ratio of predicted risk for CVD mortality with CKD Patch vs. the original prediction with SCORE was 2.64 (1.89–3.40) in very high-risk CKD (e.g., eGFR 30–44 ml/min/1.73m2 with albuminuria ≥30 mg/g), 1.86 (1.48–2.44) in high-risk CKD (e.g., eGFR 45–59 ml/min/1.73m2 with albuminuria 30–299 mg/g), and 1.37 (1.14–1.69) in moderate risk CKD (e.g., eGFR 60–89 ml/min/1.73m2 with albuminuria 30–299 mg/g), indicating considerable risk underestimation in CKD with SCORE. The corresponding estimates for ASCVD with PCE were 1.55 (1.37–1.81), 1.24 (1.10–1.54), and 1.21 (0.98–1.46).
Interpretation
The “CKD Patch” can be used to quantitatively enhance ASCVD and CVD mortality risk prediction equations recommended in major US and European guidelines according to CKD measures, when available.
Funding
US National Kidney Foundation and the NIDDK.
Keywords: Chronic kidney disease, cardiovascular disease, risk prediction, meta-analysis
Research in context.
Evidence before this study
We searched PubMed on January 22, 2020 for articles relating to the two key chronic kidney disease (CKD) measures (estimated glomerular filtration rate [eGFR] and albuminuria) using the following terms: ("glomerular filtration rate" or “GFR” or "kidney function") and (“albuminuria” or “proteinuria” or “ACR” or “PCR” or “dipstick”) and ("cardiovascular events" or "cardiovascular outcomes" or "cardiovascular mortality" or "myocardial infarction" or “stroke” or "atherosclerotic cardiovascular disease") and (“prediction” or “discrimination” or “calibration” or “c-statistic” or “net reclassification”). Also, we sought feedback on relevant articles form co-authors. Although we found several studies reporting that these CKD measures improved cardiovascular risk prediction, we did not find any studies displaying a specific approach to incorporate CKD measures into established risk prediction models in major clinical guidelines (i.e., the Pooled Cohort Equation [PCE] and SCORE).
Added value of this study
Utilizing data from 4,143,535 adults from 35 datasets, we developed several CKD Patches (tools to enhance predicted risk according to the deviation between an individual's CKD measures and the values expected from their traditional CVD risk factors and the hazard ratios for eGFR and albuminuria) incorporating eGFR and albuminuria, to enhance prediction of risk of atherosclerotic cardiovascular disease (ASCVD) by PCE and CVD mortality by SCORE. In 37 validation datasets including 4,932,824 adults, CKD Patch improved the prediction for CVD mortality beyond SCORE and ASCVD beyond PCE (Δc-statistic 0.027 [95% CI 0.018–0.036] and 0.010 [0.007–0.013] and categorical net reclassification improvement 0.080 [0.032–0.127] and 0.056 [0.044–0.067], respectively). In very high risk CKD (e.g., eGFR 30–44 ml/min/1.73m2 with urine albumin-to-creatinine ratio ≥30 mg/g), the median (IQI) ratio of risk prediction according to the CKD Patch compared to the original equations was 1.55 (1.37–1.81) for ASCVD and 2.64 (1.89–3.40) for CVD mortality.
Implications of all the available evidence
The CKD Patch approach to incorporating eGFR and albuminuria into CVD risk prediction can be used to quantitatively enhance ASCVD and CVD mortality risk prediction equations recommended in major US and European guidelines. Risk prediction incorporating CKD measures is available online for PCE (http://ckdpcrisk.org/ckdpatchpce/) and SCORE (http://ckdpcrisk.org/ckdpatchscore/) and can guide clinical decision making for CVD prevention therapies and physician-patient discussion of CVD predicted risk when these CKD measures are readily available.
Alt-text: Unlabelled box
1. Introduction
Chronic kidney disease (CKD) affects more than 10% of adults worldwide and increases the risk of many adverse outcomes [1]. Among these, cardiovascular disease (CVD) is particularly important as the leading cause of death in persons with CKD [2]. A number of studies have shown that the key measures of CKD, estimated glomerular filtration rate (eGFR) and albuminuria, are strongly associated with CVD outcomes and can statistically significantly improve the risk prediction of incident CVD beyond traditional CVD risk factors [3,4]. Importantly, eGFR and albuminuria are readily available in many patients.
Despite a body of evidence, major clinical guidelines do not include uniform recommendations for incorporating CKD measures into CVD risk prediction. The American Heart Association (AHA) and the American College of Cardiology (ACC) 2018 Cholesterol Guideline recognizes eGFR <60 ml/min/1.73m2, but not albuminuria, as a “risk enhancer” but does not specify how to quantitatively enhance the CVD risk estimate. The European Society of Cardiology (ESC) 2016 CVD Prevention Guideline categorizes eGFR <30 ml/min/1.73m2 in general, or albuminuria in diabetes, as “very high-risk,” and eGFR 30–59 ml/min/1.73m2 as “high-risk;” these designations are equivalent to 10-year risk of CVD mortality of ≥10% and 5 to <10%, respectively [5]. This approach does not account for other risk factors and therefore may misclassify the risk. Furthermore, this ESC Guideline does not address albuminuria in those without diabetes as a predictor of CVD risk [6].
Importantly, both AHA/ACC and ESC Guidelines have their own risk prediction equations (the Pooled Cohort Equation [PCE] and Systematic COronary Risk Evaluation [SCORE], respectively), which are widely used in primary care settings to guide CVD preventive therapies (e.g., statins). Because CKD measures were not evaluated in the dataset from which PCE and SCORE were derived, these measures cannot be simply incorporated into these risk prediction equations.
To overcome this limitation and enable evidence-based inclusion of eGFR and albuminuria into established CVD risk prediction equations, we meta-analyzed datasets in the CKD Prognosis Consortium (CKD-PC). By applying our previously reported “Predictor Patch” method [7], we developed and validated several “CKD patches” to enhance the predicted CVD risk calculated from PCE and SCORE according to CKD measures. Developing CKD Patches in this meta-analysis which includes ∼9 million adults from 72 datasets from various countries has the key advantage of improved generalizability.
2. Methods
This study was approved for use of de-identified data by the institutional review board at the Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA (#IRB00003324). The need for informed consent was waived by the institutional review board.
2.1. Study populations
We included 72 cohorts in the CKD-PC with available data in the present study. The details of CKD-PC are described elsewhere [8], but in brief, this consortium included both research cohorts and health system datasets, with participants from 41 countries from North America, Europe, the Middle East, Asia, and Australia. These cohorts included general population, high-risk (specifically selected for clinical conditions, such as diabetes), and CKD (exclusively enrolling individuals with CKD) cohorts. We studied participants aged 30 years or older without prevalent CVD at baseline. Each cohort was required to be informative, defined as having at least four years of follow-up among 75% of participants and at least 50 incident CVD outcomes of interest.
2.2. CKD measures
We explored the two key measures of CKD used in nephrology clinical guidelines, and readily available in most clinical settings—eGFR and albuminuria [9]. eGFR was calculated by the CKD Epidemiology Collaboration creatinine equation [10]. Albuminuria was primarily measured as spot urine albumin-to-creatinine ratio (ACR), as recommended in clinical guidelines [9], with secondary analyses utilizing dipstick proteinuria as an alternative measure.
2.3. Traditional CVD predictors
We considered those factors included in either of PCE or SCORE as traditional predictors: age, sex, race, smoking status (current vs. non-current), diabetes, systolic blood pressure, antihypertensive medication use, total cholesterol, and high-density lipoprotein cholesterol [11,12].
2.4. CVD outcomes
CVD outcomes of interest were incident atherosclerotic CVD (ASCVD) and CVD mortality, as evaluated by PCE and SCORE, respectively [11,12]. ASCVD included coronary heart disease (CHD) (myocardial infarction and fatal CHD) and stroke as a composite outcome [11]. Consistent with the SCORE model, we analyzed CHD mortality and non-CHD CVD mortality separately [12]. Details about how each cohort defined ASCVD and CVD mortality are summarized in Web Appendix 1.
2.5. Statistical analysis
All analyses were performed using STATA 14 (College Station, TX) and based on complete data. Cohort characteristics were descriptively compared. As in prior CKD-PC studies [4,13], we analyzed each cohort separately and then pooled the estimates using random-effects models.
Among the 72 cohorts, 35 cohorts were selected as development datasets because they were able to share de-identified individual-level data with the CKD-PC Data Coordinating Center and represented a broad range of populations, including the general population. The remaining 37 cohorts were either unable to share individual-level data or included highly selected samples (e.g., persons with CKD), and were thus considered validation datasets. One exception was the OptumLabs® Data Warehouse (OLDW) datasets; half were randomly selected to be validation datasets in order to have good representation of health system databases in validation.
We first evaluated the performance of the original PCE and SCORE (both versions of low-risk countries and high-risk countries) in our development datasets. We then developed the “CKD Patch,” which contains both eGFR and albuminuria, in the development datasets using a published method [7]. Briefly, there are three steps in the development of the CKD Patch: 1) a linear regression equation was developed to estimate “expected” values of eGFR and log-ACR conditional on the traditional CVD predictors defined above; 2) hazard ratios for the CVD outcomes of interest were estimated for eGFR (with linear spline terms and knots at 60 and 90 ml/min/1.73m2 [major thresholds of CKD vs. no CKD and reduced vs. normal eGFR, respectively]) [9] and log-ACR, adjusted for the traditional CVD predictors; and 3) the CVD risk estimate was multiplied by the deviation between observed and expected eGFR and log-ACR and their hazard ratios for each individual. In the second step, log hazard ratios for the traditional CVD predictors were fixed according to the original PCE [11] or SCORE [12] coefficients. To match the method used in each original equation, we used Cox models with follow-up time as a time scale for the analysis of ASCVD as in PCE [11] and Weibull models with age as a time scale for the analysis of CVD mortality as in SCORE [12].
The original idea of the “CKD Patch” was to incorporate eGFR and ACR simultaneously [7]. However, to reflect current clinical settings where eGFR is more commonly available than albuminuria, we first developed the GFR Patch. Subsequently, the ACR Patch was added to the GFR Patch, comprising a “CKD Patch.” As a sensitivity analysis, we also developed CKD Patch including eGFR and dipstick proteinuria.
The improvement of an established risk equation through the use of additional predictors was predicated on the assumption that the original equation is well calibrated in the cohort of interest (namely, additional predictors generally cannot fix poorly calibrated prediction models). Thus, we evaluated the addition of the various “Patches” after recalibration in each cohort (i.e., calibrating the baseline risk at average levels of predictors and accounting for different average levels among relevant populations) [14]. In CKD cohorts, since expected values from non-CKD cohorts at given levels of traditional predictors were found to overestimate eGFR and underestimate albuminuria, instead of intercept from the linear regression model from the development datasets, we centered expected eGFR and albuminuria at the cohort-specific average.
To evaluate prediction performance in the validation cohorts, we assessed the following: a calibration plot (predicted vs. observed risk) [15], Harrel's c-statistic (a measure of risk discrimination accounting for censoring) [16], and categorical net reclassification improvement (NRI) [17]. The 95% confidence intervals of c-statistics and NRI were calculated using a normal approximation.
2.6. Role of the funding source
The funders had no role in the study design, data collection, analysis, data interpretation, or writing of the report. KM and JC had full access to all analyses and all authors had final responsibility for the decision to submit for publication, informed by discussions with collaborators.
3. Results
3.1. Study characteristics
The present study included 9,076,359 adults from 72 datasets (4,143,535 adults from 35 development datasets and 4,932,824 adults from 37 validation datasets) (Table 1 and Web Table 1). Mean age within datasets ranged from 44 to 80 years, and most cohorts included 50–60% women. The majority (78%) were White adults, but there were 790,095 (8.7%) Black adults (predominantly from US), 613,727 (6.8%) Asian adults (mainly from Asia), and 319,214 (3.5%) Hispanic adults. Of these 72 datasets, 58 contributed to the analysis of ASCVD and 34 contributed to the analysis of CVD mortality.
Table 1.
Baseline characteristics for development and validation datasets.
| Study | N | Age (SD), y | Female,% | eGFR (SD), ml/min/1.73m2 | N, ACR | ACR (IDI), mg/g* | N, Urine Dipstick | Dipstick ≥1+, % |
|---|---|---|---|---|---|---|---|---|
| Development datasets | ||||||||
| Aichi | 4701 | 49 (7) | 21 | 100 (13) | 4543 (97%) | 2.25 | ||
| ARIC | 10,056 | 63 (6) | 59 | 87 (16) | 9969 (99%) | 4 (2–7) | ||
| AusDiab | 8234 | 52 (13) | 56 | 86 (15) | 8229 (100%) | 5 (4–8) | ||
| BIS | 1625 | 80 (7) | 54 | 65 (17) | 1622 (100%) | 10 (5–30) | 1611 (99%) | 15.99 |
| China NS | 33,448 | 50 (12) | 59 | 99 (16) | 33,448 (100%) | 7 (3–15) | 32,946 (98%) | 4.58 |
| CIRCS | 4083 | 53 (9) | 47 | 93 (14) | 4083 (100%) | 3.23 | ||
| COBRA | 1008 | 53 (11) | 63 | 98 (20) | 1006 (100%) | 6 (4–15) | ||
| ESTHER | 4908 | 62 (7) | 57 | 84 (20) | 4806 (98%) | 10.24 | ||
| Framingham | 2837 | 59 (10) | 55 | 89 (19) | 2837 (100%) | 6 (3–15) | ||
| Geisinger | 313,550 | 53 (14) | 55 | 88 (20) | 67,068 (21%) | 9 (4–25) | ||
| Gubbio | 4246 | 53 (14) | 56 | 85 (15) | 1620 (38%) | 9 (4–14) | ||
| Maccabi | 1088,168 | 49 (14) | 55 | 98 (18) | 280,759 (26%) | 15 (9–32) | ||
| MESA | 6757 | 62 (10) | 53 | 83 (17) | 6747 (100%) | 5 (3–11) | ||
| Mt Sinai BioMe | 14,380 | 54 (13) | 61 | 82 (24) | 4903 (34%) | 11 (4–51) | ||
| NHANESIII | 10,889 | 53 (16) | 54 | 95 (22) | 10,666 (98%) | 6 (4–13) | ||
| NHANEScon | 27,277 | 53 (15) | 52 | 90 (22) | 27,047 (99%) | 7 (4–13) | ||
| Ohasama | 1486 | 63 (9) | 66 | 96 (13) | 1479 (100%) | 7.30 | ||
| OLDW cohort 1 | 210,841 | 54 (14) | 59 | 86 (19) | 35,008 (17%) | 11 (6–30) | 63,701 (30%) | 8.94 |
| OLDW cohort 2 | 171,715 | 57 (14) | 55 | 84 (19) | 26,458 (15%) | 12 (6–30) | 48,289 (28%) | 10.20 |
| OLDW cohort 3 | 153,271 | 54 (14) | 58 | 89 (18) | 28,061 (18%) | 8 (4–19) | 103,707 (68%) | 9.30 |
| OLDW cohort 4 | 466,471 | 55 (14) | 55 | 86 (20) | 88,129 (19%) | 12 (7–30) | 162,156 (35%) | 9.41 |
| OLDW cohort 5 | 33,817 | 55 (14) | 59 | 84 (20) | 3786 (11%) | 9 (4–27) | 13,676 (40%) | 5.09 |
| OLDW cohort 6 | 86,466 | 50 (11) | 60 | 95 (21) | 27,277 (32%) | 12 (6–37) | 20,556 (24%) | 11.98 |
| OLDW cohort 7 | 95,085 | 57 (15) | 58 | 82 (21) | 14,124 (15%) | 9 (5–23) | 58,470 (61%) | 7.07 |
| OLDW cohort 8 | 113,743 | 53 (13) | 59 | 90 (20) | 16,208 (14%) | 12 (6–34) | 24,658 (22%) | 6.08 |
| OLDW cohort 9 | 206,645 | 56 (14) | 56 | 86 (20) | 40,149 (19%) | 9 (5–25) | 68,465 (33%) | 9.26 |
| OLDW cohort 10 | 101,483 | 56 (14) | 58 | 85 (20) | 17,601 (17%) | 9 (5–25) | 26,954 (27%) | 6.10 |
| OLDW cohort 11 | 36,724 | 53 (13) | 60 | 88 (20) | 5631 (15%) | 12 (6–28) | 11,573 (32%) | 7.97 |
| OLDW cohort 12 | 125,067 | 53 (13) | 55 | 87 (20) | 18,885 (15%) | 11 (6–29) | 31,132 (25%) | 10.66 |
| OLDW cohort 13 | 782,375 | 54 (13) | 57 | 87 (20) | 107,390 (14%) | 9 (5–26) | 251,414 (32%) | 10.72 |
| PREVEND | 6105 | 50 (12) | 55 | 96 (16) | 6101 (100%) | 7 (5–13) | ||
| Rancho Bernardo | 1305 | 70 (12) | 62 | 66 (16) | 1301 (100%) | 6 (3–13) | ||
| Takahata | 3262 | 62 (10) | 56 | 99 (12) | 3246 (100%) | 9 (6–18) | 3257 (100%) | 4.48 |
| Tromso | 10,525 | 60 (8) | 58 | 92 (12) | 10,277 (98%) | 4 (3–7) | 10,252 (97%) | 0.86 |
| ULSAM | 982 | 71 (1) | 0 | 76 (11) | 975 (99%) | 8 (5–17) | ||
| Total | 4143,535 | 53 (14) | 56 | 90 (20) | 906,528 (22%) | 15 (9–32) | 947,728 (23%) | 9.28 |
| Validation datasets | ||||||||
| ADVANCE | 8412 | 66 (6) | 46 | 78 (17) | 8070 (96%) | 15 (7–38) | ||
| CARDIA | 4409 | 37 (5) | 55 | 108 (23) | 4364 (99%) | 4 (3–7) | ||
| CHS | 2399 | 78 (5) | 64 | 67 (16) | 2105 (88%) | 9 (5–20) | ||
| CRIC | 2757 | 57 (11) | 47 | 46 (16) | 2631 (95%) | 42 (8–419) | ||
| GCKD | 3687 | 60 (11) | 44 | 50 (18) | 3670 (100%) | 54 (10–425) | ||
| Hong Kong CKD | 326 | 60 (12) | 46 | 18 (7) | ||||
| IPHS | 92,345 | 59 (10) | 66 | 86 (14) | 92,060 (100%) | 2.32 | ||
| JHS | 2652 | 50 (11) | 63 | 99 (20) | 1831 (69%) | 6 (4–10) | ||
| LCC | 10,248 | 76 (10) | 65 | 52 (13) | 4792 (47%) | 9 (4–31) | ||
| NEFRONA | 1259 | 60 (11) | 40 | 33 (17) | 864 (69%) | 91 (12–409) | ||
| NIPPON DATA80 | 8826 | 50 (13) | 56 | 88 (17) | 8815 (100%) | 2.64 | ||
| NIPPON DATA90 | 7497 | 52 (14) | 59 | 98 (16) | 7396 (99%) | 2.50 | ||
| OLDW cohort 14 | 84,265 | 56 (13) | 59 | 82 (19) | 11,334 (13%) | 14 (7–34) | 20,286 (24%) | 10.27 |
| OLDW cohort 15 | 90,051 | 56 (14) | 60 | 87 (21) | 15,170 (17%) | 10 (5–28) | 39,295 (44%) | 10.00 |
| OLDW cohort 16 | 468,725 | 53 (13) | 58 | 90 (21) | 49,449 (11%) | 13 (6–30) | 186,746 (40%) | 8.52 |
| OLDW cohort 17 | 24,549 | 56 (13) | 59 | 84 (20) | 3271 (13%) | 13 (7–36) | 10,388 (42%) | 11.09 |
| OLDW cohort 18 | 95,738 | 53 (13) | 59 | 88 (18) | 15,948 (17%) | 8 (4–22) | 29,246 (31%) | 10.61 |
| OLDW cohort 19 | 360,879 | 54 (13) | 55 | 86 (19) | 53,235 (15%) | 10 (5–27) | 93,155 (26%) | 9.65 |
| OLDW cohort 20 | 94,596 | 55 (13) | 52 | 83 (19) | 12,709 (13%) | 12 (6–32) | 30,997 (33%) | 8.86 |
| OLDW cohort 21 | 204,861 | 55 (14) | 57 | 85 (19) | 23,498 (11%) | 11 (6–28) | 72,462 (35%) | 10.25 |
| OLDW cohort 22 | 136,301 | 54 (14) | 51 | 86 (19) | 20,044 (15%) | 10 (5–29) | 44,190 (32%) | 5.75 |
| OLDW cohort 23 | 90,989 | 54 (13) | 56 | 88 (19) | 11,269 (12%) | 13 (7–32) | 18,561 (20%) | 8.32 |
| OLDW cohort 24 | 95,652 | 52 (12) | 56 | 88 (18) | 11,002 (12%) | 8 (4–23) | 34,707 (36%) | 11.65 |
| OLDW cohort 25 | 749,323 | 55 (14) | 57 | 85 (19) | 92,450 (12%) | 13 (6–37) | 195,854 (26%) | 9.09 |
| OLDW cohort 26 | 84,918 | 54 (14) | 58 | 89 (22) | 17,014 (20%) | 9 (4–28) | 25,666 (30%) | 10.32 |
| OLDW cohort 27 | 32,485 | 51 (14) | 55 | 90 (18) | 5038 (16%) | 8 (4–20) | 6839 (21%) | 10.51 |
| RCAV | 1425,737 | 61 (13) | 7.3 | 82 (17) | 386,160 (27%) | 9 (4–29) | ||
| REGARDS | 21,773 | 65 (9) | 58 | 86 (19) | 1146 (100%) | 7 (4–14) | ||
| RENAAL | 1146 | 60 (8) | 39 | 41 (13) | 21,270 (98%) | 1283 (568–2631) | ||
| SEED | 8390 | 58 (10) | 52 | 85 (19) | 6050 (72%) | 13 (7–27) | ||
| SKS | 1585 | 64 (14) | 40 | 34 (17) | ||||
| SMART | 5427 | 54 (12) | 45 | 87 (19) | 2975 (55%) | 10 (5–25) | ||
| Sunnybrook | 1727 | 64 (16) | 43 | 52 (28) | 1149 (67%) | 80 (17–346) | 722 (42%) | |
| TaiwanMJ | 319,400 | 45 (12) | 50 | 91 (16) | 315,680 (99%) | 6.94 | ||
| TLGS | 10,148 | 44 (12) | 56 | 80 (15) | 5797 (57%) | 2.73 | ||
| UK Biobank | 378,133 | 57 (8) | 55 | 91 (13) | 367,315 (97%) | 6 (4–10) | ||
| ZODIAC | 1209 | 67 (12) | 60 | 68 (17) | 1183 (98%) | 2 (1–6) | ||
| Total | 4932,824 | 56 (14) | 42 | 86 (19) | 1,157,006 (23%) | 9 (4–29) | 1,238,862 (25%) | 7.92 |
* N for ACR or dipstick are a subset of the cohorts. ACR: urine albumin to creatinine ratio; eGFR: estimated glomerular filtration rate.
Predictor profiles varied considerably across cohorts among the development datasets. For example, the prevalence of antihypertensive medication use ranged from 17% to 77%, which was related to cohort mean age (Pearson correlation 0.76). Datasets from Asia and some from Europe had higher proportions of current smokers than other datasets. Although several validation datasets had a high burden of risk factors by design (e.g., 100% diabetes in a few datasets), the summary characteristics were similar between development datasets and validation datasets.
3.2. Performance of the PCE and score
Baseline survival free of ASCVD across the development datasets is summarized in Web Table 2. Almost all cohorts had higher baseline ASCVD-free survival than the original baseline survival from the PCE, indicating overestimation of ASCVD risk by PCE in these datasets. Indeed, calibration plots confirmed overestimation of ASCVD by PCE in most datasets (Web Fig. 1). Generally, a similar pattern was observed for CVD mortality with SCORE high-risk country calibration (Web Table 3 and Web Fig. 2). On the other hand, SCORE for low-risk countries tended to underestimate CVD mortality in our datasets. For both ASCVD and CVD mortality, baseline survival varied across baseline calendar years (Web Fig. 3).
Table 2.
Meta-analyzed hazard ratios (95% CI) in development datasets.
| Variables | ASCVD | Fatal CHD | non-CHD CVD mortality |
| eGFR patch | |||
| eGFR <60, −15 ml | 1.30 (1.26, 1.35) | 1.72 (1.46, 2.04) | 1.61 (1.31, 1.98) |
| eGFR 60–90, −15 ml | 0.91 (0.88, 0.94) | 1.08 (0.96, 1.22) | 1.09 (1.01, 1.17) |
| eGFR 90+, −15 ml | 0.71 (0.66, 0.75) | 0.75 (0.67, 0.83) | 0.80 (0.66, 0.95) |
| ACR patch on top of eGFR patch | |||
| ACR, 8 fold | 1.34 (1.28, 1.41) | 1.60 (1.47, 1.74) | 1.67 (1.51, 1.86) |
| Dipstick patch on top of eGFR patch | |||
| Dipstick trace | 1.28 (1.22, 1.34) | 0.80 (0.55, 1.18) | 1.33 (0.87, 2.01) |
| Dipstick + | 1.50 (1.38, 1.63) | 2.16 (1.17, 3.98) | 1.51 (1.13, 2.03) |
| Dipstick ++ | 1.93 (1.74, 2.13) | 1.91 (0.99, 3.67) | 3.26 (1.98, 5.39) |
| Dipstick +++ | 2.18 (1.98, 2.41) | 4.03 (1.44, 11.29) | 5.07 (0.71, 36.02) |
ACR: urine albumin to creatinine ratio; ASCVD: atherosclerotic cardiovascular disease; CHD: coronary heart disease; CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate.
Bold indicates statistical significance at p<0.05.
Table 3.
C-statistics and NRI for ASCVD and CVM in validation datasets.
| ASCVD |
CVM |
||||
| eGFR patch | CKD patch | eGFR patch | CKD patch | ||
| N | 4,489,273 | 1,153,790 | 875,693 | 419,732 | |
| Base C-statistic (IQI) | 0.755 (0.698, 0.772) | 0.687 (0.665, 0.726) | 0.711 (0.621, 0.790) | 0.680 (0.569, 0.732) | |
| ΔC-statistic (95% CI) | 0.002 (0.001, 0.002) | 0.010 (0.007, 0.013) | 0.008 (0.005, 0.011) | 0.027 (0.018, 0.036) | |
| Categorical NRI (95% CI) cut point at 7.5%, 20% for ASCVD, 5% and 10% for CVM | Overall | 0.039 (0.031, 0.047) | 0.056 (0.044, 0.067) | 0.035 (0.013, 0.056) | 0.080 (0.032, 0.127) |
| Event | 0.059 (0.050, 0.068) | 0.084 (0.066, 0.102) | 0.070 (0.046, 0.094) | 0.065 (0.007, 0.123) | |
| Non-event | −0.020 (−0.023, −0.017) | −0.016 (−0.027, −0.005) | −0.028 (−0.033, −0.023) | 0.037 (−0.007, 0.080) | |
ASCVD: atherosclerotic cardiovascular disease; CKD: chronic kidney disease; CVM: cardiovascular mortality; eGFR: estimated glomerular filtration rate; NRI: net reclassification improvement.
Once we had recalibrated each equation to each of our datasets, both PCE and SCORE were relatively well calibrated (Web Figs. 1A-C and 2A-C). The pooled c-statistic of PCE was 0.759 (IQI 0.737–0.787) and of SCORE was 0.795 (0.687–0.836), in the development datasets (Web Tables 4 and 5).
Fig. 1.
Enhancement of ASCVD and CVM risk by CKD status. ACR: urine albumin to creatinine ratio; ASCVD: atherosclerotic cardiovascular disease; CKD: chronic kidney disease; CVM: cardiovascular disease mortality; eGFR: estimated glomerular filtration rate. eGFR in ml/min/1.73m2 and ACR in mg/g.
3.3. Development of CKD patch
Based on spline models, lower eGFR levels below 60 ml/min/1.73 m2 were independently associated with increased risk of ASCVD and CVD mortality in the development datasets (Table 2 and Web Fig. 4). However, lower eGFR levels in the range of eGFR ≥90 ml/min/1.73m2 were associated with decreased risk for both ASCVD and CVD mortality, indicating a known reverse J-shaped association between eGFR and these CVD outcomes likely resulting from an association between frailty and low muscle mass [3]. Higher ACR was linearly associated with both ASCVD and CVD mortality. Elevated dipstick proteinuria categories were associated with higher ASCVD risk, but were less consistent for CVD mortality. Overall, as reported previously [3], both eGFR and ACR demonstrated stronger associations with CVD mortality than with ASCVD.
In the linear regression models to estimate “expected” levels of CKD measures, age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, the use of antihypertensive medication, current smoking, diabetes, and black race were all statistically significantly associated with eGFR levels (Web Table 6). ACR was also associated with all of these factors except black race. Gender was associated with ACR but not eGFR. The models for estimating “expected” eGFR and log-ACR were similar in datasets used for developing the “CKD Patch” for ASCVD and CVD mortality (Web Table 6).
Using these estimates, we constructed the “CKD Patch” for ASCVD and CVD mortality separately.
3.4. Performance of CKD patch in validation datasets
Among 29 validation datasets (n = 4489,273) with ASCVD data, the GFR Patch did not alter model calibration (Web Fig. 1B-C) but slightly improved the c-statistic by 0.002 (95% CI 0.001–0.002) compared to recalibrated PCE (Table 3 and Web Table 7). The improvement was more evident with the CKD Patch (including eGFR and ACR) (Δc-statistic 0.010 [0.007–0.013]). Of 29 datasets, only four showed a lower c-statistic with CKD Patch, but none of these reached statistical significance. On the other hand, 16 datasets showed a statistically significant improvement of risk discrimination. NRI was statistically significantly positive (indicating improved reclassification) for both the GFR Patch (0.039 [0.031, 0.047]) and the CKD Patch (0.056 [0.044, 0.067]) (Web Table 8).
The improvement in risk prediction with the GFR Patch and the CKD Patch (with eGFR and ACR) was also observed in the 17 validation datasets (n = 875,693) for CVD mortality data (Table 3 and Web Table 7). Δc-statistic was 0.008 (0.005–0.011) for the GFR Patch and 0.027 (0.018–0.036) for the CKD Patch. NRI was 0.035 (0.013–0.056) for GFR Patch and 0.080 (0.032–0.127) for CKD Patch. (Web Table 8).
The CKD Patch with eGFR and dipstick proteinuria also improved prediction of ASCVD and CVD mortality in the validation datasets (Web Tables 9 and 10). Improvements in validation cohorts were similar to those in the development cohorts (Web Tables 11 and 12).
3.5. Absolute risk estimates using the CKD patch with the PCE or score equations
We compared predicted risk with and without the CKD Patch (available at http://ckdpcrisk.org/ckdpatchpce/ and http://ckdpcrisk.org/ckdpatchscore/) (equations in Web Table 13) for recalibrated risk estimates by PCE for ASCVD and by SCORE for CVD mortality in the validation datasets (Fig. 1). The CKD Patch enhanced the predicted CVD risk in participants with lower eGFR and higher albuminuria. For example, across cohorts, the median ratio (IQI) of the ASCVD risk by PCE with the CKD Patch to ASCVD risk by PCE without the CKD Patch was 1.55 (1.37–1.81) in CKD at very high risk (e.g., eGFR 30–44 ml/min/1.73 m2 with albuminuria ≥30 mg/g), 1.24 (1.10–1.54) in CKD at high risk (e.g., eGFR 45–59 ml/min/1.73 m2 with albuminuria 30–299 mg/g), and 1.21 (0.98–1.46) in CKD at moderate risk (e.g., eGFR 60–89 ml/min/1.73 m2 with albuminuria 30–299 mg/g) (Fig. 1) [9], indicating considerable ASCVD risk underestimation in CKD by PCE. The corresponding ratios were even greater for CVD mortality by SCORE, with a median of 2.64 (1.89–3.40) for very high, 1.86 (1.48–2.44) for high, and 1.37 (1.14–1.69) for moderate risk CKD. The percentage of individuals with eGFR <30 ml/min/1.73 m2 classified at very high risk for CVD mortality (>10% in 10 years) increased from 30.9% to 53.5% by adding the eGFR patch to the recalibrated SCORE, compared to 14.2% and 29.0% for the original SCORE for low- and high-risk countries (Web Table 14).
4. Discussion
There are several key findings from this study. First, after recalibration, PCE and SCORE showed good discrimination across the cohorts in our global Consortium. Second, the “CKD Patch” improved discrimination and CVD risk classification beyond recalibrated PCE for ASCVD and recalibrated SCORE for CVD mortality. Third, the improvement by the CKD Patch was generally more evident for CVD mortality prediction than for ASCVD prediction. Fourth, as expected and now quantified, the impact on CVD risk was larger at lower eGFR and higher ACR (defined by KDIGO as higher risk CKD categories). Finally, the calibration of the original PCE and SCORE equations varied markedly across a broad range of international datasets.
Whether the changes in c-statistic with addition of the CKD Patch in our study (e.g., 0.010 for ASCVD and 0.027 for CVD mortality) are clinically meaningful deserves some discussion. These values may look small but are actually a magnitude ∼5–10 times larger than what was reported for the addition of high-sensitivity C-reactive protein or fibrinogen for ASCVD in an international meta-analysis [18]. Importantly, unlike most non-traditional predictors, eGFR is routinely assessed in clinical practice (e.g., hundreds of millions of tests of serum creatinine are conducted annually in the USA), and the assessment of albuminuria is a non-invasive test recommended for individuals with diabetes, hypertension, and CKD by major clinical guidelines. Thus, instead of a typical question of whether it is worth additionally measuring non-traditional predictors, the question for CKD measures is whether healthcare providers should ignore readily available information on CKD measures in CVD risk prediction. Our results clearly indicate that the answer is no.
The fundamental concept of a “CKD Patch” is consistent with the new concept of “risk enhancers” in the AHA/ACC 2018 Cholesterol Guideline. However, the AHA/ACC Guideline does not specify how to quantitatively enhance predicted risk based on kidney dysfunction. Our approach of the “CKD Patch” provides an objective method for enhancing predicted ASCVD risk by incorporating quantitative values of both CKD measures into PCE (http://ckdpcrisk.org/ckdpatchpce/). As shown in Fig. 1, not incorporating CKD measures leads to underestimation of ASCVD risk in a majority of individuals with very high-risk CKD (e.g., eGFR 30–44 ml/min/1.73m2 with ACR 30–299 mg/g) and high-risk CKD (e.g., eGFR 45–59 ml/min/1.73m2 with ACR 30–299 mg/g) by ∼55% and ∼25%, respectively.
The “CKD Patch” improved risk prediction of CVD mortality more than that of ASCVD. This is consistent with our previous report which demonstrated that CKD measures were more strongly associated with CVD mortality and heart failure compared to ASCVD [3]. These observations have biological plausibility since left ventricular hypertrophy [19] and accompanying diastolic dysfunction have been recognized as the most common cardiac phenotype related to CKD [2], and these conditions can lead to development of heart failure, a condition with high mortality.
This risk enhancement, quantified by the CKD Patch (http://ckdpcrisk.org/ckdpatchscore/), in the prediction of CVD mortality has important implications for the ESC CVD Prevention Guideline, which has focused on risk of CVD mortality to guide preventive approaches. The ESC Guideline provides general estimates of CVD mortality risk by CKD status while our CKD Patch refines CVD mortality risk prediction by adding CKD measures to traditional risk factors. For example, our risk tool predicts CVD mortality in persons with CKD at very high risk (red categories in Fig. 1) as ∼2.5 times higher than that predicted by SCORE with appropriate calibration. Therefore, some individuals with eGFR 30–59 ml/min/1.73m2 will have very high risk, likely requiring preventive medications, while the current European guideline classified all as having high risk (10-y CVD mortality risk of 5–9%) and emphasized intensive lifestyle advice.
We demonstrated heterogeneity across datasets in baseline survival free of CVD beyond what is explained by the traditional predictors, indicating that one size would not fit all [20]. This observation is not surprising since the incidence rate of CVD varies substantially by factors beyond traditional predictors, such as socioeconomic status, lifestyle, region/country, and calendar year. Different methods have been proposed to optimize calibration, e.g., recalibrating an existing equation [14] or developing a unique equation to specific regions/countries [21] or clinical groups (e.g., diabetes) [22]. Alternatively, a few groups have proposed a method to utilize national data to tailor risk prediction for each country [20,23]. A limitation of all approaches is that incidence rates often change over time due to various reasons (e.g., the development of novel therapies).
There are several limitations of this study. The assessment of CKD measures and traditional risk factors was not fully standardized across cohorts. Similarly, the ascertainment and definitions of CVD were not identical across cohorts. We relied on an assessment of eGFR and albuminuria at a single timepoint. Also, we did not have information on primary causes of CKD. In addition, the validation datasets were not necessarily randomly selected. However, our validation datasets with varying study characteristics seem actually conservative and advantageous in terms of generalizability. Although our cohorts represent 41 countries, we have only a few cohorts that include participants from South America, the Middle East, and Australia, and no cohorts from Africa. The complete case data analysis can be also viewed as a limitation. However, the results were largely consistent in research cohorts and clinical database studies; mechanisms of missing data can be considerably different in these two study types (typically sicker populations tend to have missing data in research cohorts, whereas clinical databases will oversample sicker populations who are more likely to have more laboratory measurements).
In conclusion, eGFR and albuminuria enhance CVD risk prediction. The “CKD Patch” developed in this study enables objective calibration of CVD risk in CKD at higher risk, defined by lower eGFR and higher albuminuria, and improvement of two major existing prediction models, the PCE for ASCVD and SCORE for CVD mortality.
Data sharing statement
Under agreement with the participating cohorts, CKD-PC cannot share individual data with third parties. Inquiries regarding specific analyses should be made to ckdpc@jhmi.edu. Investigators may approach the original cohorts regarding their own policies for data sharing (e.g., https://sites.cscc.unc.edu/aric/distribution-agreements for the Atherosclerosis Risk in Communities Study).
Contributors
K.M. and J.C. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. K.M. and J.C. were responsible for the study concept and design. K.M., Y.S., S.H.B., M.E.G., A.S., and J.C with the CKD-PC investigators/collaborators listed below were involved in the acquisition of data. All the authors contributed to the analysis and interpretation of data and to the critical revision of the manuscript for important intellectual content. K.M., S.K.J., Y.S., S.H.B., E.S., and J.C. drafted the manuscript. K.M. and J.C. guarantee the integrity of the work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
The CKD-PC Data Coordinating Center is funded in part by a program grant from the US National Kidney Foundation (NKF funding sources include Janssen and Boehringer Ingelheim) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100446). A variety of sources have supported enrollment and data collection including laboratory measurements, and follow-up in the collaborating cohorts of the CKD-PC (eAppendix 3). These funding sources include government agencies such as national institutes of health and medical research councils as well as foundations and industry sponsors. The funders of the study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. In addition, the funders had no right to veto publication or to control the decision regarding to which journal the paper would be submitted.
Declaration of Competing Interest
Dr. Matsushita reports grants from NIH during the conduct of the study; grants and personal fees from Kyowa Kirin and personal fees from Akebia outside the submitted work. Dr. Grams reports grants from NKF and grants from NIH during the conduct of the study and received travel funds to speak at DCI Director's meeting. Dr. Ärnlöv reports personal fees from AstraZeneca outside the submitted work. Dr. Ebert reports personal fees from Bayer AG, personal fees from Siemens Healthineers, and personal fees from Roche Diagnostics outside the submitted work. Dr. Eckardt reports grants from Astra Zeneca, grants from Bayer, grants from FMC, and grants from Vifor during the conduct of the study; personal fees from Akebia, personal fees from Astellas, personal fees from Bayer, and personal fees from Vifor outside the submitted work. Dr. Gutierrez reports grants and personal fees from Akebia, grants and personal fees from Amgen, grants from GSK, personal fees from QED, grants from National Institutes of Health, and grants from American Heart Association outside the submitted work. Dr. Kovesdy reports personal fees from Amgen, personal fees from Sanofi-Aventis, personal fees from Fresenius Medical Care, personal fees from Keryx, grants from Shire, personal fees from Bayer, personal fees from Abbott, personal fees from Abbvie, personal fees from Dr. Schar, personal fees from Astra-Zeneca, personal fees from Takeda, personal fees from Tricida, and personal fees from Reata outside the submitted work. Dr. Levey reports grants from NIDDK during the conduct of the study. Dr. Lloyd-Jones reports grants from NIH during the conduct of the study. Dr. Muntner reports grant support and consulting fees unrelated to this project. Dr. Nadkarni reports grants, personal fees, non-financial support and other from Renalytix AI, non-financial support and other from Pensieve Health, personal fees from Reata, personal fees from AstraZeneca, and personal fees from GLG Consulting outside the submitted work. Dr. Ohkubo reports grants from Omron Healthcare Co. Ltd. outside the submitted work. Dr. Shlipak reports consultancy fees from Cricket Health and Intercept Pharmaceuticals and stocks/stock options from TAI Diagnostics where he is a Scientific Advisor outside the submitted work. Dr. Woodward reports personal fees from Amgen and personal fees from Kirin outside the submitted work. Dr. Zhang reports grants from National Natural Science Foundation of China, grants from Beijing Nova Program Interdisciplinary Cooperation Project, grants from University of Michigan Health System-Peking University Health Science Center Joint Institute for Translational and Clinical Research, grants from PKU-Baidu Fund, grants from Peking University, and grants from AstraZeneca during the conduct of the study. Dr. Schaeffner reports other from Siemens Healthineers, other support from Fresenius Kabi and other support from Fresenius Medical Care outside the submitted work. Dr. Coresh reports grants from NIH and grants from National Kidney Foundation during the conduct of the study; personal fees and other support from Healthy.io outside the submitted work. All other coauthors have nothing to disclose.
Acknowledgments
CKD-PC investigators/collaborators (cohort acronyms/abbreviations are listed in eAppendix 2 in the Supplement:
ADVANCE: John Chalmers, Mark Woodward; Aichi: Hiroshi Yatsuya, Koji Tamakoshi, Yuanying Li, Yoshihisa Hirakawa; ARIC: Josef Coresh, Kunihiro Matsushita, Jung-Im Shin, Junichi Ishigami; AusDiab: Kevan Polkinghorne, Steven Chadban, Robert Atkins; BIS: Elke Schaeffner, Natalie Ebert, Dörte Huscher; CARDIA: Donald Lloyd-Jones, Orlando M. Gutierrez; China NS: Luxia Zhang, Minghui Zhao, Fang Wang, Bixia Gao, Jinwei Wang; CHS: Michael Shlipak, Nisha Bansal; CIRCS: Hiroyasu Iso, Kazumasa Yamagishi, Isao Muraki, Yasuhiko Kubota; COBRA: Tazeen Jafar, Imtiaz Jehan, Neil Poulter, Nish Chaturvedi; CRIC: Jiang He, Wei Yang, Matthew Weir, Stephanie Toth-Manikowski, Christopher Jepson; ESTHER: Hermann Brenner, Dietrich Rothenbacher, Ben Schöttker, Bernd Holleczek; Framingham: Daniel Levy, Shih-Jen Hwang; GCKD: Markus P. Schneider, Anna Köttgen, Heike Meiselbach, Kai-Uwe Eckardt; Geisinger: Alex R. Chang, Gurmukteshwar Singh, Jamie Green, H. Lester Kirchner; Gubbio: Massimo Cirillo; Hong Kong CKD: Angela Yee-Moon Wang, Hoi Ching Cheung, Hailey Yee Tsui, Victoria Ngai; IPHS: Fujiko Irie, Toshimi Sairenchi; JHS: Adolfo Correa, Casey M. Rebholz, Bessie Young, L. Ebony Boulware; LCC: Nigel Brunskill, Laura Gray, Rupert W. Major, James Medcalf; Maccabi: Varda Shalev, Gabriel Chodick; MESA: Michael Shlipak; Mt Sinai BioMe: Girish N. Nadkarni, Erwin P. Bottinger, Ruth J.F. Loos, Stephen B. Ellis; NEFRONA: José M. Valdivielso, Marcelino Bermúdez-López, Milica Bozic, Serafí Cambray; NHANES: Yingying Sang; NIPPON DATA80 & NIPPON DATA90: Hirotsugu Ueshima, Akira Okayama, Tomonori Okamura, Katsuyuki Miura; Ohasama: Takayoshi Ohkubo, Hirohito Metoki, Michihiro Satoh, Masahiro Kikuya; OLDW: John Cuddeback, Elizabeth Ciemins, Emily Carbonara, Stephan Dunning; PREVEND: Ron T. Gansevoort, Lyane M. Kieneker, Stephan J.L. Bakker, Hans L. Hillege, Pim van der Harst; Rancho Bernardo: Simerjot K. Jassal, Jacklyn Bergstrom, Joachim Ix; RCAV: Csaba P. Kovesdy, Keiichi Sumida, Miklos Z. Molnar, Praveen Potukuchi; REGARDS: Orlando M. Gutierrez, Paul Muntner, David Warnock; RENAAL: Dick de Zeeuw, Michelle J. Pena, Hiddo J.L. Heerspink; SEED: Tien Yin Wong, Charumathi Sabanayagam, Ching-Yu Cheng, Rehena Sultana; SKS: Philip Kalra, Rajkumar Chinnadurai, James Tollitt, Darren Green; SMART: Frank Visseren, Joep van der Leeuw; Sunnybrook: David Naimark, Navdeep Tangri; Taiwan MJ: Chi-Pang Wen, Min-Kuang Tsai; Takahata: Takamasa Kayama, Tsuneo Konta; TLGS: Mohammadhassan Mirbolouk, Fereidoun Azizi, Farzad Hadaegh, Farhad Hosseinpanah; Tromso: Marit Dahl Solbu, Bjørn Odvar Eriksen, Trond Geir Jenssen, Anne Elise Eggen; UK Biobank: Christoph Nowak, Johan Ärnlöv; ULSAM: Lars Lannfelt, Anders Larsson, Johan Ärnlöv; ZODIAC: Henk J.G. Bilo, Gijs W.D. Landman, Kornelis J.J. van Hateren, Nanne Kleefstra
CKD-PC Steering Committee: Josef Coresh (Chair), Shoshana H. Ballew, Alex R. Chang, Ron T. Gansevoort, Morgan E. Grams, Orlando M. Gutierrez, Tsuneo Konta, Anna Köttgen, Andrew S. Levey, Kunihiro Matsushita, Kevan Polkinghorne, Elke Schäffner, Mark Woodward, Luxia Zhang
CKD-PC Data Coordinating Center: Shoshana H. Ballew (Assistant Project Director), Jingsha Chen (Programmer), Josef Coresh (Principal Investigator), Morgan E. Grams (Director of Nephrology Initiatives), Kunihiro Matsushita (Director), Yingying Sang (Lead Programmer), Aditya Surapeneni (Programmer), Mark Woodward (Senior Statistician).
Footnotes
For the Chronic Kidney Disease Prognosis Consortium
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100552.
Contributor Information
Shoshana H Ballew, Email: sballew1@jhmi.edu.
Josef Coresh, Email: ckdpc@jhmi.edu.
Appendix. Supplementary materials
References
- 1.Eckardt K.U., Coresh J., Devuyst O., Johnson R.J., Kottgen A., Levey A.S. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 2.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R., Jafar T.H., Heerspink H.J., Mann J.F. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 3.Matsushita K., Coresh J., Sang Y., Chalmers J., Fox C., Guallar E. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushita K., van der Velde M., Astor B.C., Woodward M., Levey A.S., de Jong P.E. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox C.S., Matsushita K., Woodward M., Bilo H.J.G., Chalmers J., Heerspink H.J.L. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsushita K., Sang Y., Chen J., Ballew S.H., Shlipak M., Coresh J. Novel "predictor patch" method for adding predictors using estimates from outside datasets- a proof-of-concept study adding kidney measures to cardiovascular mortality prediction. Circ J. 2019;83(9):1876–1882. doi: 10.1253/circj.CJ-19-0320. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita K., Ballew S.H., Astor B.C., Jong P.E., Gansevoort R.T., Hemmelgarn B.R. Cohort profile: the chronic kidney disease prognosis consortium. Int J Epidemiol. 2013;42:1660–1668. doi: 10.1093/ije/dys173. [DOI] [PubMed] [Google Scholar]
- 9.Kidney disease: improving global outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter, Suppl. 2013;3(1):1–150. [Google Scholar]
- 10.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd Feldman HI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goff D.C., Jr., Lloyd-Jones D.M., Bennett G., Coady S., D'Agostino R.B., Gibbons R. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 12.Conroy R. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur. Heart J. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita K., Coresh J., Sang Y., Chalmers J., Fox C., Guallar E. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Agostino R.B., Sr., Grundy S., Sullivan L.M., Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 15.Woodward M. 2nd ed. Chapman & Hall/CRC; Boca Raton, FL: 2005. Epidemiology: study design and data analysis. [Google Scholar]
- 16.Pencina M.J., D'Agostino R.B. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 17.Pencina M.J., D'Agostino R.B., Sr., Steyerberg E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaptoge S., Di Angelantonio E., Pennells L., Wood A.M., White I.R., Gao P. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367(14):1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai Q.Z., Lu X.Z., Lu Y., Wang A.Y. Longitudinal changes of cardiac structure and function in CKD (CASCADE study) J Am Soc Nephrol. 2014;25(7):1599–1608. doi: 10.1681/ASN.2013080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risk Chart Working Group WHO CVD. World health organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332–e1e45. doi: 10.1016/S2214-109X(19)30318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hippisley-Cox J., Coupland C., Vinogradova Y., Robson J., May M., Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007;335(7611):136. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodward M., Hirakawa Y., Kengne A.P., Matthews D.R., Zoungas S., Patel A. Prediction of 10-year vascular risk in patients with diabetes: the AD-ON risk score. Diabetes Obes Metab. 2016;18(3):289–294. doi: 10.1111/dom.12614. [DOI] [PubMed] [Google Scholar]
- 23.Hajifathalian K., Ueda P., Lu Y., Woodward M., Ahmadvand A., Aguilar-Salinas C.A. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): a pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol. 2015;3(5):339–355. doi: 10.1016/S2213-8587(15)00081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.