Abstract
Background: A large number of idiosyncratic drug induced liver injury (iDILI) and herb induced liver injury(HILI) cases of variable quality has been published but some are a matter of concern if the cases were not evaluated for causality using a robust causality assessment method (CAM) such as RUCAM (Roussel Uclaf Causality Assessment Method) as diagnostiinjuryc algorithm. The purpose of this analysis was to evaluate the worldwide use of RUCAM in iDILI and HILI cases. Methods: The PubMed database (1993–30 June 2020) was searched for articles by using the following key terms: Roussel Uclaf Causality Assessment Method; RUCAM; Idiosyncratic drug induced liver injury; iDILI; Herb induced liver injury; HILI. Results: Considering reports published worldwide since 1993, our analysis showed the use of RUCAM for causality assessment in 95,885 cases of liver injury including 81,856 cases of idiosyncratic DILI and 14,029 cases of HILI. Among the top countries providing RUCAM based DILI cases were, in decreasing order, China, the US, Germany, Korea, and Italy, with China, Korea, Germany, India, and the US as the top countries for HILI. Conclusions: Since 1993 RUCAM is certainly the most widely used method to assess causality in IDILI and HILI. This should encourage practitioner, experts, and regulatory agencies to use it in order to reinforce their diagnosis and to take sound decisions.
Keywords: RUCAM, Roussel Uclaf Causality Assessment Method, diagnostic algorithm, iDILI, iDrug induced liver injury, DILI, HILI, herb induced liver injury
1. Introduction
Idiosyncratic drug induced liver injury (DILI), in short also termed iDILI, and herb induced liver injury (HILI) are complex diseases and received much attention in recent years [1,2,3,4,5,6,7,8,9]. The present scientometric study comprehensively analyzed the global knowledge base and specific emerging topics of DILI derived from 1995 publications in 79 countries and regions, with an impressive annual growth of reports between 2010 and 2019 and almost 340 studies published in 2020 [1]. In parallel, more and more publications on DILI and HILI cases refer to RUCAM (Roussel Uclaf Causality Assessment Method) for causality assessment [10,11,12,13]. The original RUCAM was first published in 1993 [14] and updated in 2016 [15] with additional information on its use and perspectives [16,17], which is now the preferred version to be used in future cases of DILI and HILI [15]. It is widely recognized that causality assessment in DILI and HILI is a multifaceted approach [7,8,9,15], a real medical challenge, for which a diagnostic quantitative algorithm such as RUCAM is an easy tool for case evaluation [10,11,12,13,14,15,16,17,18] to solve complex conditions [18].
The RUCAM algorithm is a structured, standardized, transparent, liver specific and quantitative diagnostic clinical scale based on key elements of liver injury, which are individually scored and provide a score for five-degree causality grading from unrelated up to highly probable causality levels [15]. Since key elements are specifically described and scored, assessments are objective with little risk of subjectivity [15,16,17] commonly observed if the approach to assess causality lacks scored key elements [19]. RUCAM can help expand our knowledge by enlarging population analysis with prospective and scored causality assessment, allowing for harmonized interpretation of data across populations [20]. In this context, RUCAM should be viewed as a cornerstone approach assessing causality of liver injury cases [15,16,17,21], because robust diagnostic biomarkers are rarely available due to misconducted studies as outlined by EMA (European Medicines Agency: Formerly London, UK, now Amsterdam, Netherlands) [21].
In this review article, current conditions of DILI and HILI cases assessed worldwide using RUCAM were critically analyzed. For the first time, the focus is on reports published from1993 to mid 2020 and the discussion of their potential use to describe specific features of DILI and HILI cases.
2. Literature Search and Source
The PubMed database (1993–30 June 2020) was searched for articles by using the following key terms: Roussel Uclaf Causality Assessment Method; RUCAM; Idiosyncratic drug induced liver injury (iDILI); Herb induced liver injury (HILI). Key terms were used alone or in combination. Limited to the English language, publications from each search terms were analyzed for suitability of this review article. The electronic search was completed on 30 June 2020 and supplemented by a manual literature search, using also the large private archive of the authors when the publication was not yet referenced in PubMed. The final compilation consisted of original papers including individual case reports and case series, consensus reports, and review articles with the most relevant publications included in the reference list of this review.
3. Definitions
RUCAM is presented as an algorithm that requires a few criteria allowing for a final quantitative evaluation. In particular, establishing RUCAM based criteria of liver test thresholds and liver injury patterns was revolutionary at the time of first publication issued from an international consensus meeting of experts, without the requirement of a liver biopsy [14] with same principles preserved in the updated RUCAM [15].
3.1. RUCAM Based Liver Injury
3.1.1. Liver Test Thresholds
A liver injury caused by exogenous compounds such as drugs and herbs is defined by specific threshold values established for the liver tests (LTs) alanine aminotransferase (ALT) and alkaline phosphatase (ALP), with current serum activities considered as relevant for ALT ≥ 5 × ULN (upper limit of normal) and ALP ≥ 2 × ULN [15] provided that ALP is of hepatic origin. The original RUCAM was the first causality assessment method (CAM) ever considering threshold criteria although initially with lower values for ALT [15] as compared to currently used criteria [16]. Of note, serum bilirubin is not part of the diagnostic RUCAM algorithm that uses ALT or ALP as diagnostic liver test. In this context, conjugated bilirubin is a sign of the severity of the liver injury.
3.1.2. Liver Injury Pattern
RUCAM was also the first CAM proposing different patterns of liver injury based on LTs [14] and are included also in the updated RUCAM [15]. To determine the liver injury pattern, the ratio R is to be calculated using the multiple of the ULN of serum ALT divided by the multiple of the ULN of serum ALP, provided the ALP increase is of hepatic origin. For causality assessment purposes, two types of liver injury are defined (independently from histological findings): first a hepatocellular injury with R > 5, and second, a cholestatic/mixed liver injury with R ≤ 5.
3.2. Idiosyncratic Versus Intrinsic Liver Injury
Liver injury is either idiosyncratic, due to the interaction between the exogenous synthetic chemical or phytochemical and a susceptible individual with some genetic factor(s), or it is intrinsic due to chemical overdose [11,12,13]. In the present analysis, idiosyncratic injury is considered, as opposed to intrinsic liver injury most commonly observed with overdosed drugs such as acetaminophen [22].
4. Worldwide Publications of DILI
The current scientometric report from China on knowledge mapping confirmed the high worldwide interest in DILI publications and identified a total of 1995 DILI studies published between 2010 and 2019, although information on the applied method of causality assessment was not provided and will need further clarification [1]. This Chinese analysis on the top 10 countries involved in DILI research listed the US, China, Japan, Germany, UK, Spain, France, the Netherlands, Sweden, and Canada. In addition, many interesting details on DILI were comprehensively discussed with focus on definition, incidence rate, clinical characteristics, etiology or pathogenesis such as the character of the innate immune system, the regulation of cell-death pathways, susceptible HLA (Human Leukocyte Antigen) identification, or criteria and methods of causality assessment, all topics were considered as the knowledge base for DILI research [1].
5. Worldwide Publications of RUCAM Based Idiosyncratic DILI
The worldwide impact of DILI can best be quantified by using liver injury cases assessed for causality with a robust method that allows for establishing causality gradings for each implicated drug and to exclude alternative causes unrelated to drug administration.
5.1. Countries and Regions
In the current analysis, authors from 31 countries worldwide reported on cases of idiosyncratic DILI caused by multiple drugs published from 1993 up to mid 2020 and applied in all cases RUCAM to assess causality (Table 1) [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180]. Such a table with a comprehensive list of publications over a long period has never been reported before and will facilitate the search for RUCAM based DILI cases caused by individual drugs, considering that databases such as LiverTox may have problems providing real DILI cases [10,74].
Table 1.
Worldwide countries with a selection of published DILI cases assessed for causality using RUCAM.
| Country/ DILI Cases, n |
First Author/Year | DILI Cases, n |
Drugs | Comments on RUCAM Based DILI Cases |
|---|---|---|---|---|
|
Argentina n = 625 |
Bessone, 2016 [23] | 197 | Various drugs | DILI caused by a variety of drugs, not allowing individual description of features |
| Bessone, 2019 [24] | 114 | Various drugs | Individual drugs not available for DILI feature characterization | |
| Colaci, 2019 [25] | 311 | Various drugs | DILI features for single drugs were not presented | |
| García, 2019 [26] | 3 | Methotrexate | Feature details provided for DILI by methotrexate | |
|
Australia n = 106 |
Lin, 2014 [27] | 47 | Various volatile anaesthetics | DILI by anesthetics without individual features of isoflurane, desflurane, or sevoflurane |
| Ahmed, 2015 [28] | 1 | Ipilimumab | Detailed features of the DILI case | |
| Laube, 2019 [29] | 1 | Atorvastatin | Good feature presentation of this DILI case | |
| Worland, 2020 [30] | 57 | Infliximab | Feature presentation of DILI by the drug | |
|
Bahrain n = 25 |
Sridharan, 2020 [31] | 25 | Various antiepileptic drugs | No feature details provided of DILI due to individual drugs |
|
Brazil n = 4 |
Becker, 2019 [32] | 4 | Various drugs | Features of DILI caused by some drugs |
|
Canada n = 4 |
Yan, 2006 [33] | 2 | Rofecoxib | Two well described case features of DILI caused by rofecoxib |
| Nhean, 2019 [34] | 2 | Dolutegravir | Careful described features of DILI | |
|
China n = 35,825 |
Hou, 2012 [35] | 300 | Various drugs | No feature details available for DILI by individual drugs |
| Lv, 2012 [36] | 89 | Various drugs | Specific features of DILI by individual drugs were not presented | |
| Hao, 2014 [37] | 140 | Anti-Tuberculotics | Lacking specific DILI features of any drug | |
| Ou, 2015 [38] | 231 | Various drugs | No feature specifics of DILI are available for individual drugs | |
| Zhu, 2015 [39] | 39 | Various drugs | Specific features of DILI caused by individual drugs were not provided | |
| Lu, 2016 [40] | 513 | Various drugs | Missing specific features of DILI caused by individual drugs | |
| Yang, 2016 [41] | 124 | Various drugs | Feature specifics of DILI caused by individual drugs were not provided | |
| Zhu, 2016 [42] | 870 | Various drugs | No specific features of DILI by individual drugs were presented | |
| Naqiong, 2017 [43] | 157 | Various statins | Cohort consisted of patients with DILI caused by atorvastatin, simvastatin, and rosuvastatin, but specific features were not provided for individual statins | |
| Li, 2018 [44] | 1 | Iguratmod | Detailed feature description of DILI | |
| Song, 2018 [45] | 1 | Posaconazole | Careful feature presentation of DILI by this drug | |
| Tao, 2018, [46] | 290 | Anti-Tuberculotics | Cohort included patients with DILI caused by isoniazid, rifampin, pyrazinamide, ethambutol, and streptomycin, but specific features were not presented for individual drugs | |
| Liao, 2019 [47] | 1 | Cefepime | Well described features of DILI by this drug | |
| Shen, 2019 [48] | 18,956 | Various drugs | Cohort comprized patients with DILI, but special DILI features related to individual drugs were not published. | |
| Xing, 2019 [49] | 133 | Various drugs | No specific feature presentation of DILI by individual drugs | |
| Ma, 2020 [50] | 1 | Fenofibrate | Specific feature of DILI by this drug presented | |
| Tao, 2020 [51] | 146 | Anti-Tuberculotics | Lacking feature data of DILI caused by individual drugs | |
| Wang, 2020 [52] | 155 | Anti-Tuberculotics | Cohort included patients with DILI due to not further identified anti-TB regimens, hence attributing specific DILI features to individual drugs was not possible | |
| Yang, 2020 [53] | 13,678 | Various drugs | No feature details of DILI by individual drugs | |
|
Colombia n = 19 |
Ríos, 2013 [54] | 1 | Albendazole | Detailed feature description of DILI caused by albendazole |
| Cano-Paniagua, 2019 [55] | 18 | Various drugs | Perfect feature description of DILI by drugs in this excellent prospective epidemiology study using the updated RUCAM for causality assessment | |
|
Egypt n = 75 |
Alhaddad, 2020 [56] | 75 | Various drugs | Feature details of DILI by individual drugs incompletely provided |
|
France n = 170 |
Bénichou, 1993 [57] | 94 | Various drugs | No detailed feature description of DILI by the drugs |
| Arotcarena, 2004 [58] | 1 | Pioglitazone | Feature description of the case | |
| Moch, 2012 [59] | 18 | Etifoxine | Detailed features of DILI due to etifoxine treatment | |
| Carrier, 2013 [60] | 1 | Methyl-prednisolone | Features of DILI well described for the drug | |
| Ripault, 2013 [61] | 1 | Crizotinib | Good feature details provided for DILI by this drug | |
| Dumortier, 2017 [62] | 5 | Methyl-prednisolone | Careful feature description of DILI caused by the drug | |
| Meunier, 2018 [63] | 50 | Nimesulide | No feature description of DILI by this drug | |
|
Germany n = 10,907 |
Stammschulte, 2012 [64] | 37 | Flupirtine | Carefully presented features of DILI caused by flupirtine |
| Douros, 2014 [65] | 7 | Flupirtine | Comprehensive feature presentation of DILI due to flupirtine | |
| Douros, 2014 [66] | 198 | Various drugs | Cohort of patients with DILI associated with the use of various drugs, but special features of DILI by individual drugs were not provided | |
| Buechter, 2018 [67] | 15 | Various drugs | No detailed feature presentation of DILI caused by individual drugs | |
| Dragoi, 2018 [68] | 16 | Diclofenac | Cohort of DILI patients with presentation of limited specific DILI features | |
| Teschke, 2018 [69] | 7278 | Various drugs | Cohort of DILI patients without feature specification for individual drugs | |
| Teschke, 2018 [70] | 3312 | Various drugs | Cohort of DILI cases not providing special features of DILI by individual drugs | |
| Weber, 2019 [71] | 44 | Various drugs | No specific features of DILI caused by individual drugs provided | |
|
Iceland n = 367 |
Björnsson, 2012 [72] | 73 | Statins | Specific feature details provided of DILI by statins |
| Björnsson, 2013 [73] | 72 | Various drugs | Cohort of DILI cases without providing typical features of DILI by single drugs | |
| Björnsson, 2016 [74] | 222 | Various drugs | The two assessed cohorts provided no typical features of DILI caused by the evaluated drugs | |
|
India n = 424 |
Harugeri, 2009 [75] | 1 | Montelukast | Good feature presentation of a patient with DILI caused by montelukast |
| Devarbhavi, 2010 [76] | 313 | Various drugs | No feature description of DILI due to individual drugs | |
| Rathi, 2017 [77] | 82 | Various drugs | Cohort of DILI cases but features of DILI by indivual drugs were not presented | |
| Taneja, 2017 [78] | 2 | Etodolac | Detailed feature presentation of DILI | |
| Das, 2018 [79] | 24 | Various drugs | Cohort with limited feature description of few patients with DILI caused by drugs assessed for causality by RUCAM or other CAMs | |
| Dutta, 2020 [80] | 1 | Haloperidol | Perfect presented feature details of DILI caused by this drug | |
| Kulkarni, 2020 [81] | 1 | Vitamin A | Perfect feature details of this DILI case | |
|
Israel n = 1 |
Gluck, 2011 [82] | 1 | Amiodarone | Careful feature description of a patient with DILI caused by a single drug |
|
Italy n = 1562 |
Rigato, 2007 [83] | 1 | Flavoxate | Good feature presentation of a patient with DILI caused by flavoxate |
| Licata, 2010 [84] | 46 | Various drugs including Nimesulide | Feature description of patients with DILI by nimesulide but no description for DILI by other drugs | |
| Abenavoli, 2013 [85] | 1 | Cyproterone acetate | Detailed feature description of a patient with DILI | |
| Ferrajolo, 2017 [86] | 938 | Various antibiotics | Combined feature presentation of all antibiotics causing DILI in paediatric patients | |
| Licata, 2017 [87] | 185 | Various drugs | Epidemiology study, hence no feature description of patients with DILI by any drug | |
| Giacomelli, 2018 [88] | 362 | Nevirapine | Detailed feature description of DILI by nevirapine observed in all patients | |
| Licata, 2018 [89] | 28 | Rivaroxaban | Perfect feature description of this DILI cohort | |
| Lovero, 2018 [90] | 1 | Ustekinumab | Careful feature description of DILI caused by this drug | |
|
Japan n = 939 |
Masumoto, 2003 [91] | 85 | Various drugs | No detailed feature description of DILI caused by drugs |
| Hanatani, 2014 [92] | 182 | Various drugs | Detailed features of DILI by individual drugs not provided | |
| Niijima, 2017 [93] | 1 | Ipragliflozin | Provided case features of DILI | |
| Ji, 2017 [94] | 1 | Methimazole | Perfect feature presentation of DILI caused by this drug | |
| Aiso, 2019 [95] | 270 | Various drugs | Global feature description of DILI by all drugs | |
| Kishimoto, 2019 [96] | 1 | Clonazepam | Detailed feature of DILI by this drug | |
| Hiraki, 2019 [97] | 1 | Tegafur-Uracil | Good feature presentation of DILI caused by the drug | |
| Kakisaki, 2019 [98] | 398 | Various drugs | Perfect feature description of the cohort | |
|
Korea n = 6528 |
Choi, 2008 [99] | 1 | Albendazole | Detailed feature description of DILI by albendazole in a case report of a single patient |
| Suk, 2012 [100] | 101 | Various drugs | Lacking detailed feature description of DILI by individual drugs | |
| Son, 2015 [101] | 1 | Various drugs | No specific feature description of DILI due to comedication | |
| Woo, 2016 [102] | 1 | Various comedicated drugs | Lacking specific feature description of DILI due to comedication | |
| Byeon, 2019 [103] | 6391 | Various drugs | Missing specific feature of DILI caused by individual drugs | |
| Kwon, 2019 [104] | 33 | Nimesulide | Detailed feature description of DILI caused by nimesulide, using a prospective study design in this perfect analysis | |
|
Malaysia n = 1 |
Thalha, 2018 [105] | 1 | Kombiglyze | Perfect feature description of DILI caused by the combination of metformin and saxagliptin |
|
Mexico n = 1 |
Lammel-Lindemann, 2018 [106] | 1 | Candesartan | Well described features of DILI by this drug |
|
Morocco n = 1 |
Essaid, 2010 [107] | 1 | Tadalafil | Feature description of DILI due to the drug |
|
Pakistan n = 264 |
Abid, 2020 [108] | 264 | Various drugs | No specific feature details presented for DILI caused by individual drugs |
|
Portugal n = 53 |
Costa-Moreira, 2020 [109] | 53 | Various drugs | Specific feature details of DILI caused by individual drugs were not provided |
|
Saudi Arabia n = 1 |
Alqrinawi, 2020 [110] | 1 | Menotropin | Perfect feature details of DILI by this specific drug |
|
Serbia n = 99 |
Miljkovic, 2010 [111] | 80 | Various drugs | No detailed feature description of DILI by individual drugs |
| Miljkovic, 2011 [112] | 19 | Various drugs | Lacking detailed feature presentation of DILI caused by individual drugs | |
|
Singapore n = 14 |
Wai, 2006 [113] | 14 | Various drugs | Limited feature description of DILI |
|
Spain n = 1181 |
Rodríguez, 1996 [114] | 35 | Various drugs | No feature details of DILI cases provided for individual drugs |
| Andrade, 2005 [115] | 461 | Various drugs | Limited feature description of DILI case details | |
| Andrade, 2006 [116] | 28 | Various drugs | Partial feature description of DILI by few drugs | |
| García-Cortés, 2008 [117] | 225 | Various drugs | Limited feature description of DILI by few drug groups | |
| Lucena, 2011 [118] | 78 | Amoxicillin Clavulanate | Careful feature description of DILI by the drug combination | |
| Lucena, 2011 [119] | 9 | Various drugs | Feature description of DILI caused by individual drugs | |
| Robles-Diaz, 2015 [120] | 25 | Anabolic and androgenetic steroids | Limited feature description of DILI | |
| Tong, 2015 [121] | 1 | Methylphenidate | Careful evaluation of feature details provided for this DILI case | |
| Medina-Calitz, 2016 [122] | 298 | Various drugs | No specific feature description for DILI by any drug | |
| López-Riera, 2018 [123] | 17 | Various drugs | Lack of specific feature presentation of DILI by any drug | |
| Machlab, 2019 [124] | 1 | Apixabam | Careful feature description of the DILI case caused by the drug | |
| Zoubek, 2019 [125] | 3 | Methyl-prednisolone | Perfect feature presentation of DILI | |
|
Sweden n = 1508 |
Björnsson, 2005 [126] | 784 | Various drugs | Detailed feature description of DILI by few drugs |
| De Valle, 2006 [127] | 77 | Various drugs | Limited feature description of DILI by a few drugs | |
| Björnsson, 2007 [128] | 77 | Various drugs | Lacking substantial feature description of DILI caused by few drugs | |
| Björnsson, 2007 [129] | 570 | Various drugs | Feature details of DILI presented | |
|
Switzerland n = 68 |
Goossens, 2013 [130] | 1 | Ibandronate | Detailed description of immune DILI features by ibandronate, a biphosphonate |
| Russmann, 2014 [131] | 14 | Rivaroxaban | Perfect individual feature presentation of each DILI case | |
| Scalfaro, 2017 [132] | 49 | Sacubitril Valsartan |
No feature details of DILI caused by the drugs | |
| Schneider, 2017 [133] | 1 | Zoledronic acid | Feature details provided for DILI by this drug | |
| Terziroli Beretta-Piccoli, 2018 [134] | 1 | Atovaquon/Proguanil | Detailed feature description of DILI | |
| Visentin, 2018 [135] | 2 | NSAID Amoxicillin/Clavulanate |
Lack of feature details of DILI by individual drugs | |
|
Thailand n = 509 |
Treeprasertsuk, 2010 [136] | 80 | Various antibiotics | No feature details provided for DILI caused by individual drugs in the context of this epidemiology study |
| Sobhonslidsuk, 2016 [137] | 383 | Various drugs | Missing feature details of DILI caused by individual drugs | |
| Chayanupatkul, 2020 [138] | 46 | Various drugs | Feature details of DILI due to individual drugs not provided | |
| Duzenli, 2019 [139] | 1 | Phenprobamate | Detailed feature description of DILI by this drug | |
| Hussaini, 2007 [140] | 43 | Various antibiotics | No feature details of DILI caused by individual drugs | |
| Daly, 2009 [141] | 51 | Flucloxacillin | Excellent feature details presented for DILI cases | |
|
Turkey n = 1 |
Spraggs, 2011 [142] | 61 | Laptinib | Excellent feature description of DILI case |
|
United Kingdom n = 263 |
Islam, 2014 [143] | 1 | Anastrazole | Feature presentation of a patient with DILI due to anastrazole |
| Dyson, 2016 [144] | 1 | Sofosbuvir | Features of DILI by this drug provided | |
| Abbara, 2017 [145] | 105 | Various anti-Tuberculotics | Feature presentation of all DILI cases due to various anti-tuberculosis drugs | |
| Vliegenthart, 2017 [146] | 1 | Nitrofurantoin | Feature description of DILI by the drug | |
|
United States n = 20,311 |
Fontana, 2005 [147] | 2 | Amoxicillin, Amoxicillin/Clavulanate | Well described features of DILI caused by the drugs |
| Lee, 2005 [148] | 6448 | Ximelagatran | Perfect presentation of DILI features | |
| Stojanovski, 2007 [149] | 1 | Atomoxetine | Good DILI case feature presentation | |
| Lammert, 2008 [150] | 598 | Various drugs | No feature presentation of DILI by individual drugs | |
| Singla, 2010 [151] | 1 | Cephalexin | DILI features of a single case | |
| Nabha, 2012 [152] | 1 | Etravirine | Presentation of DILI feature | |
| Sprague, 2012 [153] | 1 | Varenicline | Careful DILI feature description | |
| Markova, 2013 [154] | 56 | Bosentan | Some global feature description | |
| Marumoto, 2013 [155] | 4 | NSAID | Limited feature details of DILI cases | |
| Bohm, 2014 [156] | 1 | Daptomycin | DILI feature description in this case | |
| Cheetham, 2014 [157] | 11,109 | Various drugs | No specific feature presentation of any drug under consideration | |
| Lim, 2014 [158] | 1 | Various drugs | No presentation of specific features of DILI by 4 drugs used concomitantly or sequentially | |
| Russo, 2014 [159] | 22 | Statins | No individual feature description for DILI caused by various statins | |
| Veluswamy, 2014 [160] | 1 | Polamidomide | Feature presentation of DILI | |
| Baig, 2015 [161] | 1 | Rivaroxaban | Good feature decription of this DILI case | |
| Hammerstrom, 2015 [162] | 1 | Amblodipine | Feature presentation of DILI caused by this drug | |
| Stine, 2015 [163] | 2 | Simeprevir | Detailed feature presentation of the DILI cases | |
| Tang, 2015 [164] | 1 | Bupropion, doxycycline | Complex feature presentation of DILI due to comedication | |
| Unger, 2016 [165] | 1 | Ciprofloxacin | Detailed feature description of DILI by this single drug | |
| Gharia, 2017 [166] | 1 | Letrozole | Perfect feature presentation of DILI | |
| Nicoletti, 2017 [167] | 339 | Various drugs | No specific feature details of DILI caused by individual drugs | |
| Gayam, 2018 [168] | 3 | Various drugs | Feature details of DILI by the drugs | |
| Hayashi, 2018 [169] | 493 | Various drugs | Lacking specific feature details of DILI by individual drugs | |
| Patel, 2018 [170] | 1 | Everolimus | Specific features described for DILI by this drug | |
| Shamberg, 2018 [171] | 34 | Various drugs | No specific features of DILI caused by individual drugs presented | |
| Cirulli, 2019 [172] | 268 | Various drugs | Feature details of DILI by individual drugs not provided | |
| Nicoletti, 2019 [173] | 197 | Flucloxacillin | Specific feature details of DILI by flucloxacillin were not provided | |
| Sandritter, 2019 [174] | 1 | Various drugs | No feature description of DILI by individual drugs | |
| Shumar, 2019 [175] | 1 | Memantine | Detailed feature description of DILI by this drug | |
| Tsung, 2019 [176] | 70 | Pembrolizumab | Features described in detail for DILI caused by this drug | |
| Xie, 2019 [177] | 1 | Anastrozole | Feature details of DILI presented | |
| Ghabril, 2020 [178] | 551 | Various drugs | No feature details of DILI by individual drugs | |
| Mullins, 2020 [179] | 99 | Micafungin | Feature details presented of DILI by this drug |
Abbreviations: CAMs, Causality Assessment Methods; DILI, Drug induced liver injury; NSAID, Non-steroidal anti-inflammatory drug; RUCAM, Roussel Uclaf Causality Assessment Method.
5.2. Hospital and Other Sources
RUCAM based DILI cases were mostly published by authors from university hospitals and their affiliated teaching hospitals known for their high reputation (Table 1). Among these were a broad range of departments, which in most cases include departments of Hepatology and Gastroenterology, ensuring careful clinical evaluation of patients with suspected DILI and associated causality assessment for the offending drug(s). To a lesser degree, other departments were contributors, for instance, Pharmacology, or Pharmacy and Pharmaceutical sciences [170].
In addition to hospitals, other sources provided RUCAM based DILI cases (Table 1). Among these were National Institutes of Health from Japan [92] and the US [165], consortia from Spain [115,141], the adverse drug reactions advisory committee (ADRAC) from Sweden [126], regulatory pharmacovigilance and pharmacoepidemiology centers from France [58,59] and Italy [86], drug commission of medical association from Germany [64], committee for drug induced liver injury from China [42]; also, drug reaction reporting database from Spain [65], regulatory agency from Spain [114] health insurance from the US [157], and drug safety departments of drug companies from France [57], Sweden {148], and Switzerland [132]. Some of these played an eminent role in promoting the use of RUCAM in prospective studies, particularly those from Spain [115], Sweden [126], and the US with France and Sweden [148].
5.3. Top Ranking Countries
Among the top 10 countries were in decreasing order China, the US, Germany, Korea, Italy, Sweden, Spain, Japan, Argentina, and Thailand, whereby the top 5 countries provided most of the DILI cases (Table 2). Authors from these 5 countries contributed together 75,133 DILI cases out of a total 81,856 worldwide DILI cases, corresponding to 91.8%. On the lower part of the list ranked the 6 countries Israel, Malaysia, Mexico, Morocco, Saudi Arabia, and Turkey, authors from these low ranking countries provided each one single DILI case assessed for causality using RUCAM, corresponding to 6 cases altogether out of a total of 81,856 DILI cases. Authors from the remaining 20 countries with a ranking from 6 down to 25 contributed 6.723 DILI cases out of overall 81,856 cases corresponding to 8.2%. In essence, RUCAM based DILI cases were mostly published in English language journals, raising the question how DILI cases were assessed and published by the other countries in local journals in languages other than English. Currently, overall 81,856 cases of idiosyncratic DILI assessed for causality by RUCAM have been retrieved via PubMed, all published 1993–June 2020 (Table 1) [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180].
Table 2.
Top ranking of countries providing DILI cases assessed for causality by RUCAM.
| Top Ranking Countries |
Cases, n |
References |
|---|---|---|
| 1. China | 35,825 | [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] |
| 2. United States | 20,311 | [147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179] |
| 3. Germany | 10,907 | [64,65,66,67,68,69,70,71] |
| 4. Korea | 6528 | [99,100,101,102,103,104] |
| 5. Italy | 1562 | [83,84,85,86,87,88,89,90] |
| 6. Sweden | 1508 | [126,127,128,129] |
| 7. Spain | 1181 | [114,115,116,117,118,119,120,121,122,123,124,125] |
| 8. Japan | 939 | [91,92,93,94,95,96,97,98] |
| 9. Argentina | 625 | [23,24,25,26] |
| 10.Thailand | 509 | [136,137,138] |
| 11. India | 424 | [75,76,77,78,79,80,81] |
| 12. Iceland | 367 | [72,73,74] |
| 13. Pakistan | 264 | [108] |
| 14. UK | 263 | [140,141,142,143,144,145,146] |
| 15. France | 170 | [57,58,59,60,61,62,63] |
| 16. Australia | 106 | [27,28,29,30] |
| 17. Serbia | 99 | [111,112] |
| 18. Egypt | 75 | [56] |
| 19. Switzerland | 68 | [130,131,132,133,134,135] |
| 20. Portugal | 53 | [109] |
| 21. Bahrain | 25 | [31] |
| 22. Colombia | 19 | [54] |
| 23. Singapore | 14 | [113] |
| 24. Brazil | 4 | [32] |
| 25. Canada | 4 | [33,34] |
| 26. Israel | 1 | [82] |
| 27. Malaysia | 1 | [105] |
| 28. Mexico | 1 | [106] |
| 29. Morocco | 1 | [107] |
| 30. Saudi Arabia | 1 | [110] |
| 31. Turkey | 1 | [139] |
Abbreviations: DILI, Drug induced liver injury; RUCAM, Roussel Uclaf Causality Assessment Method.
5.4. Annual Growth Trends of RUCAM Based DILI Case Publications
Analyses of growth trends provided additional information after identification of a total 1995 DILI studies, published between 2010 and 2019 but not stratified for causality assessment using RUCAM [1]. In the frame of the present analysis, only publications of idiosyncratic DILI cases were included if they had been assessed for causality using RUCAM, providing a more homogenous series with established DILI diagnoses.
5.4.1. Published Annual RUCAM Based DILI Cases
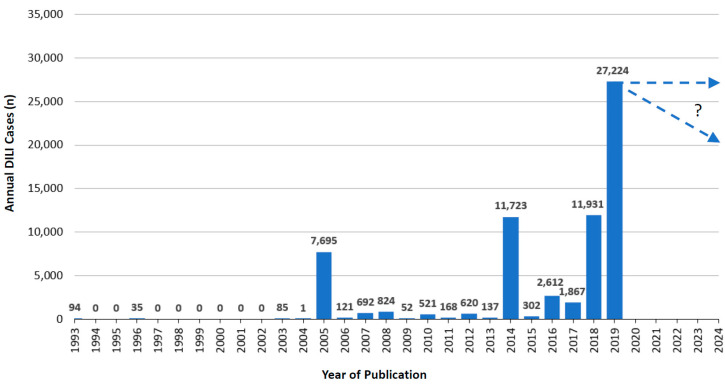
Considering the period from 1993 to 2019, annually published cases of RUCAM based idiosyncratic DILI ranged between 0 and 27,224 in 2019, but data of 2020 were not included because case counting stopped by end of June in this particular year (Figure 1). Three phases of trends appeared with respect to published RUCAM based DILI cases: (1) phase 1 with clinical field testing from 1996 to 2004 (2) phase 2 with promotion from 2005 to 2013, and (3) phase 3 of worldwide use from 2014 to 2019.
Figure 1.
Annual cases of DILI assessed for causality by RUCAM and published since 1993.
Phase 1 started after the launch of RUCAM in 1993 [16,47] and the analysis of 94 DILI cases [47], the number of subsequent annual published DILI cases remained small until 2004, reaching 121 cases (Figure 1). This was the period of initial testing the RUCAM algorithm under clinical field conditions with interesting early information provided by 3 reports [58,91,114]. The first report came from Spain, was published in 1996, analyzed a major study cohort of DILI due to amoxicillin and clavulanate, and described their typical clinical features, with Rodríguez as first author and Zimmerman as senior author [114] who actually was involved as an expert from the US in the international consensus meetings [14] but did not promote RUCAM in DILI evaluations in his own country. Of interest was also the retrospective design of this analysis, suggesting that this particular study approach is feasible [114] although a prospective approach is recommended [15]. The second report was from Japan with Japanese patients, published in 2003 by Masumoto et al. [91]. This study favored RUCAM over other CAMs, provided evidence that the performance of the lymphocyte transformation test was poor in line with previous reports, and the RUCAM criteria were viewed as useful for diagnosing DILI in Japanese patients. The third publication came from France, reported in 2004 on details of a patient with DILI by pioglitazone, and showed the feasibility of a good case report to be assessed by RUCAM, evaluated by Arotcarena et al. [58]. All three reports were hallmarks of the first phase of RUCAM based DILI case series devoted to clinical field evaluation that ended in 2004 (Figure 1).
Phase 2 started in 2005 with overall 7695 annually published RUCAM based DILI cases (Figure 1) [115,126,147,148]. Among these were 461 cases provided by Andrade et al. from Spain retrieved from a prospective study involving various drugs [115], additional 784 cases from Sweden were published by Björnsson and Olsson retrieved from a prospective study of DILI by various drugs [126], whereas from the US 2 case reports of DILI by amoxicillin and clavulanate were presented by Fontana et al. [147] as well as a large cohort of DILI caused by ximelagatran occurred in clinical trials was published by Lee et al. [148]. These 4 studies promoted the usefulness of RUCAM evaluating DILI cases [115,126,147,148] by preferring a prospective study design [115,126], evaluating single DILI case reports [147], and correctly assessing suspected DILI cases in clinical trials [148]. Whereas RUCAM had already a firm place among DILI experts in Europe, it seems that experts in the US became more familiar with the use and practicability of RUCAM.
Phase 3 is characterized by the worldwide use of RUCAM for DILI started in 2014 with 11,525 DILI cases (Figure 1), mostly attributed to one study with 11,109 DILI cases provided by Cheetham et al. [157]. Starting in 2015, there was a continuous rise of published RUCAM based DILI cases (Figure 1), likely driven also by the updated RUCAM available online 2015 and published in 2016 [15]. With 27,224 published DILI cases, the maximum level on an annual base was achieved in 2019 (Figure 1). Until end of June 2020, additional 15,153 published DILI cases were counted but not included in Figure 1, corresponding already to more than half of the cases counted in 2019 and representing a good base for 2020 and further years.
5.4.2. Annual RUCAM Based DILI Publications and Growth Trend
Over the years starting from 1993, when RUCAM was launched [14,57], and until 2019 an upward trend of annual RUCAM based DILI publications can be observed with some dips in between (Figure 2). In 2019, 26 publications were counted, and 15 publications from January 2020 until end of June 2020 that were not included in the listing (Figure 2). Overall 158 publications with RUCAM based DILI cases were counted from 1993 until mid 2020 (Table 1).
Figure 2.
Annual publications of DILI cases assessed for causality by RUCAM as reported since 1993.
5.5. Specificities of DILI Case Evaluation
Large study cohorts of RUCAM based DILI cases accumulated many different drugs and provided as expected a global information of the DILI cases due to various drugs without a detailed description of clinical features drug by drug (Table 1). Consequently, typical clinical features of a DILI by a single drug cannot be obtained from large cohorts as opposed to single DILI case reports or case series that included DILI cases due to a single drug (Table 1). In general, studies with a single DILI case or a few cases are more informative because they provide an exhaustive past medical history with clinical details required for a sound case evaluation. In search for typical DILI features by specific drugs, therefore, assistance may be provided by the drug listing (Table 1). In addition, details can be retrieved via the internet, using the search terms drug induced liver injury and the name of the suspected drug, combined with RUCAM or the updated RUCAM.
5.6. Worldwide Top Ranking of Drugs Causing DILI
There is concern how best to establish a top ranking of drugs most commonly implicated in DILI [70,74]. A recent study presented a list with top ranking drugs out of overall 3312 DILI cases evaluated by RUCAM (Table 3) [70]. The RUCAM based DILI cases were retrievd from 15 reports by six national databases of DILI registries and three large medical centers worldwide, which provided the DILI cases under consideration. Contributing countries and regions were in alphabetical order China, Germany, Latin America, Iceland, India, Singapore, Spain, Sweden, and the US. It was found that the databases of national registries and large medical centers are the best sources of drugs implicated in DILI cases. There is also the note that presently DILI cases of the LiverTox database are less suitable for clinical or regulatory purposes as presented on its website because many suspected DILI cases were derived from published cases of poor quality, lacking a robust CAM such as RUCAM [70,74]. Consequently, the majority of LiverTox based cases of assumed DILI could previously not be classified as real DILI [74]. To overcome these diagnostic shortcomings, LiverTox attempted a top ranking of drugs by counting the published DILI cases for each individual drug [74]. It was assumed that the degree of causality probability increases with the number of published DILI reports: the higher the case number the higher the probability. This special approach explains the variability of the top listing presented by liverTox [74] as compared to RUCAM based cohorts [70].
Table 3.
Worlwide top ranking of drugs causing DILI cases with causality assessment by RUCAM.
| Drug | RUCAM Based DILI Cases (n) |
|---|---|
| 1. Amoxicillin-clavulanate | 333 |
| 2. Flucloxacilllin | 130 |
| 3. Atorvastatin | 50 |
| 4. Disulfiram | 48 |
| 5. Diclofenac | 46 |
| 6. Simvastatin | 41 |
| 7. Carbamazepine | 38 |
| 8. Ibuprofen | 37 |
| 9. Erythromycin | 27 |
| 10. Anabolic steroids | 26 |
| 11. Phenytoin | 22 |
| 12. Sulfamethoxazole/Trimethoprim | 21 |
| 13. Isoniazid | 19 |
| 14. Ticlopidine | 19 |
| 15. Azathioprine/6-Mercaptopurine | 17 |
| 16. Contraceptives | 17 |
| 17. Flutamide | 17 |
| 18. Halothane | 15 |
| 19. Nimesulide | 13 |
| 20. Valproate | 13 |
| 21. Chlorpromazine | 11 |
| 22. Nitrofurantoin | 11 |
| 23. Methotrexate | 8 |
| 24. Rifampicin | 7 |
| 25. Sulfazalazine | 7 |
| 26. Pyrazinamide | 6 |
| 27. Gold salts | 5 |
| 28. Sulindac | 5 |
| 29. Amiodarone | 4 |
| 30. Interferon beta | 3 |
| 31. Propylthiouracil | 2 |
| 32. Allopurinol | 1 |
| 33. Hydralazine | 1 |
| 34. Infliximab | 1 |
| 35. Interferon alpha/Peginterferon | 1 |
| 36. Ketaconazole | 1 |
| 37. Busulfan | 0 |
| 38. Dantrolene | 0 |
| 39. Didanosine | 0 |
| 40. Efavirenz | 0 |
| 41. Floxuridine | 0 |
| 42. Methyldopa | 0 |
| 43. Minocycline | 0 |
| 44. Telithromycin | 0 |
| 45. Nevirapine | 0 |
| 46. Quinidine | 0 |
| 47. Sulfonamides | 0 |
| 48. Thioguanine | 0 |
Substantially modified from a previous report [70], which provides references for each implicated drug.
6. Worldwide Publications of HILI Cases Assessed for Causality Using RUCAM
Highlights of liver injury cases have been reported not only for DILI but with increasing frequency also for HILI cases questionable due to lack of a robust CAM [7,8,9]. The problems associated with HILI are specifically addressed in the current analysis, which considers for the first time worldwide HILI cases using RUCAM as a robust algorithm for assessing causality.
6.1. Countries and Regions
Authors from many countries around the world reported on cases of HILI in connection with the consumption of various herbs, all published since 1993 (Table 4) [29,37,38,42,48,100,101,102,103,113,115,116,117,118,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255]. Specifically considered were patients, who experienced HILI with established causality using RUCAM. Such a table with a comprehensive list of publications over a long period of time will help the search for RUCAM based HILI cases caused by specific herbs or herbal products containing a mixture of several herbs. This list is unique as compared to databases that may have problems providing real HILI cases not confounded by alternative causes or lack of a robust causality assessment.
Table 4.
Worldwide countries with a selection of published HILI cases assessed for causality using RUCAM.
| Country/ HILI Cases, n |
First Author/ Year |
HILI Cases, n |
Herbal Products | Comments on RUCAM Based HILI Cases |
|---|---|---|---|---|
|
Australia n = 2 |
Smith, 2016 [181] | 1 | Garcinia Cambogia | Careful feature detail description of HILI by this herb |
| Laube, 2019 [29] | 1 | Ginseng | Feature presentation of this single HILI case | |
|
Austria n = 2 |
Stadlbauer, 2005 [182] | 2 | Various herbs contained in Tahitian NONI juice | Features described for these HILI cases |
|
Brazil n = 1 |
Barcelos, 2019 [183] | 1 | Senecio brasiliensis | Complete feature description of HSOS caused by this herb |
|
China n = 10,914 |
Yuen, 2006 [184] | 7 | Various herbs | No specific feature description of HILI by individual herbs |
| Cheung, 2009 [185] | 3 | Psoralea corylifolia | Well described features of HILI cases | |
| Chau, 2011 [186] | 27 | Various herbs | Lacking feature presentation of HILI by individual herbs | |
| Lin, 2011 [187] | 1 | Gynura segetum | Perfect feature presentation of HSOS | |
| Gao, 2012 [188] | 5 | Gynura segetum | Excellent feature description of HSOS | |
| Lai, 2012 [189] | 74 | Various herbs Polygonum multiflorum |
Missing feature presentation of HILI by individual herbs | |
| Dong, 2014 [190] | 18 | Various herbs | Good feature presentation of HILI | |
| Hao, 2014 [37] | 8 | PA containing herbs | Lacking feature presentation of HILI by individual herbs | |
| Gao, 2015 [191] | 23 | Various herbs | Perfect feature presentation of HSOS cases | |
| Ou, 2015 [38] | 130 | Polygonum multiflorum | No feature description of HILI caused by the herb | |
| Wang, 2015 [192] | 40 | Polygonum multiflorum | Comprehensive feature description of HILI cases | |
| Zhu, 2015 [193] | 158 | Polygonum multiflorum | Detailed feature presentation of HILI cases | |
| Zhang, 2016 [194] | 54 | Various herbs | No feature details described of HILI by individual herbs | |
| Zhu, 2016 [42] | 866 | Various herbs | Missing feature details of HILI caused by individual herbs | |
| Li, 2017 [195] | 1 | Polygonum multiflorum | Excellent description of feature details provided for HILI case | |
| Chow, 2019 [196] | 1552 | Various herbs | No feature presentation of HILI by individual herbs but excellent listings | |
| Jing, 2019 [197] | 145 | Polygonum multiflorum | Missing feature presentation of HILI caused by individual herbs | |
| Li, 2019 [198] | 1 | Psoralea corylifolia | Perfect feature presentation of HILI | |
| Ni, 2019 [199] | 331 | Polygonum multiflorum | Feature description of HILI cases | |
| Shen, 2019 [48] | 6971 | Various herbs | No feature details presented for HILI by individual herbs | |
| Tan, 2019 [200] | 3 | Swietenia macrophylla | Perfect presentation of feature details for these HILI cases | |
| Zhu, 2019 [201] | 488 | Various herbs | Lacking feature details of HILI caused by individual herbs | |
| Gao, 2020 [202] | 1 | Psoralea | Feature description of this HILI case | |
| Xia, 2020 [203] | 7 | Swietenia macrophylla, syn skyfruit | Careful description of HILI features | |
|
Colombia n = 1 |
Cárdenas, 2006 [204] | 1 | Polygomun multiflorum | Features well described for this HILI case |
|
France n = 10 |
Parlati, 2017 [205] | 10 | Aloe vera | Excellent feature presentation of the HILI cases |
|
Germany n = 170 |
Teschke, 2009 [206] | 1 | Ayurveda herbs | Complete feature presentation of the HILI case |
| Teschke, 2011 [207] | 22 | Chelidonium majus syn. Greater Celandine | Complete feature description provided for HILI cases | |
| Teschke, 2012 [208] | 21 | Chelidonium majus syn. Greater Celandine | Thorough features presented of HILI cases | |
| Douros, 2015 [209] | 10 | Various herbs | No detailed features reported of HILI caused by individual herbs | |
| Teschke, 2015 [210] | 12 | Camellia sinensis, syn. Green tea, or Lu Cha | Feature details presented of HILI cases | |
| Melchart, 2017 [211] | 26 | Herbal TCMs | Well described features of HILI caused by individual TCM herbs | |
| Diener, 2018 [212] | 10 | Petasites hybridus | Provided features of HILI by this herb | |
| Anderson, 2019 [213] | 48 | Petasites hybridus | Description of HILI features | |
| Gerhardt, 2019 [214] | 1 | Chelidonium majus, syn. Greater Celandine | HILI features described | |
| Teschke, 2019 [215] | 19 | Camellia sinensis | Careful feature presentation of HILI cases | |
|
India n = 117 |
Philips, 2018 [216] | 94 | Ayurvedic and other herbs | Features not individually described for HILI by various herbs |
| Philips, 2019 [217] | 17 | Various herbs | No detailed features presented for HILI cases by individual herbs | |
|
Italy n = 77 |
Lapi, 2010 [218] | 1 | Serenoa repens | Detailed feature presentation of HILI |
| Mazzanti, 2015 [219] | 19 | Camellia sinensis, syn. green tea | Careful feature description of HILI cases | |
| Sáez-González, 2016 [220] | 1 | Chelidonium majus | Feature details of the HILI case | |
| Mazzanti, 2017 [221] | 55 | Red yeast rice | Thorough feature presentation of HILI | |
| Osborne, 2019 [222] | 1 | Mitragyna speciosa, syn. Kraton | Individual feature details not provided for the HILI case |
|
|
Japan n = 3 |
Tsuda, 2010 [223] | 1 | Saireito | Perfect feature details presented for the HILI case |
| Hisamochi, 2013 [224] | 2 | Agaricus blazei Murill | Excellent presentation of HILI features | |
|
Korea n = 2507 |
Ahn, 2004 [225] | 64 | Various herbs | Missing feature presentation of HILI by individual herbs |
| Seo, 2006 [226] | 17 | Various herbs | No individual feature description of HILI by specific herbs | |
| Kang, 2008 [227] | 66 | Various herbs | Lacking feature details of HILI by individual herbs | |
| Sohn, 2008 [228] | 24 | Various herbs | Feature details of HILI by individual herbs not provided | |
| Kang, 2009 [229] | 1 | Corydalis speciosa | Perfect feature details provided for this single HILI | |
| Kim, 2009 [230] | 2 | Arrowroot, syn. ge Gen | Excellent presentation of features for these HILI cases | |
| Bae, 2010 [231] | 1 | Polygonum multiflorum | Careful feature details presented for this HILI case | |
| Yang, 2010 [232] | 3 | Aloe vera or arborescens | Thorough description of features of these HILI cases | |
| Jung, 2011 [233] | 25 | Polygonum | Excellent feature presentation of the HILI cases | |
| Kim, 2012 [234] | 1 | multiflorum | Perfect feature description for this HILI case | |
| Suk, 2012 [100] | 149 | Hovenia dulcis, syn. Juguju | No feature description of HILI caused by individual herbs | |
| Lee, 2015 [235] | 27 | Various herbs | Lacking feature details of HILI caused by individual herbs | |
| Lee, 2015 [236] | 97 | Various herbs | Feature details of HILI cases caused by individual herbs were not provided | |
| Woo, 2015 [102] | 5 | Various herbs | No feature details presented for HILI by individual herbs | |
| Cho, 2017 [237] | 6 | Various herbs | Missing feature details of HILI cases by individual herbs | |
| Byeon, 2019 [103] | 2019 | Various herbs | No detailed feature description of HILI by individual herbs | |
|
Singapore n = 25 |
Wai, 2006 [113] | 15 | Various herbs | No detailed features presented for HILI cases by individual herbs |
| Teo, 2016 [238] | 10 | Various herbs | Missing feature details of HILI cases | |
|
South Africa n = 47 |
Awortwe, 2018 [239] | 47 | Various herbs | Features were not provided for cases of HILI caused by individual herbs |
|
Spain n = 46 |
Andrade, 2005 [115] | 9 | Various herbs | No feature details of HILI by individual herbs |
| Jimenez-Saenz, 2006 [240] | 1 | Camellia sinensis | Feature details presented for this HILI case | |
| García-Cortés, 2008 [241] | 13 | Various herbs | Lacking feature details of HILI caused by individual herbs | |
| García-Cortés, 2008 [117] | 5 | Various herbs | Specific feature details of HILI by individual herbs not provided | |
| Medina-Caliz, 2018 [242] | 18 | Camellia sinensis and other herbs | No specific feature details provided for HILI | |
|
Sweden n = 5 |
Björnsson, 2007 [243] | 5 | Camellia sinensis | Feature details provided for HILI cases |
|
Switzerland n = 1 |
Ruperti-Repilado, 2019 [244] | 1 | Artemisia annua | Careful feature presentation of this HILI case |
|
Turkey n = 1 |
Yilmaz, 2015 [245] | 1 | Lesser Celandine, syn. Pilewort | Excellent feature presentation of the HILI case |
|
United States n = 100 |
Papafragkakis, 2016 [246] | 1 | Chinese skullcap plus Black catechu | Perfect feature presentation of this HILI case |
| Dalal, 2017 [247] | 1 | Ayurvedic herb | Specific case features described | |
| Kesavarapu, 2017 [248] | 1 | Yogi Detox tea with multiple herbs | Individual specific features not provided for this HILI case caused specifically by a single herb | |
| Kothadia, 2018 [249] | 19 | Garcinia Cambogia | Careful feature presentation of HILI cases | |
| Surapaneni, 2018 [250] | 19 | Camellia sinensis | Feature details provided for the HILI case | |
| Imam, 2019 [251] | 1 | Curcumin | Thorough feature description of the HILI case | |
| Yousaf, 2019 [252] | 9 | Garcinia Cambogia | Excellent feature description of the HILI case | |
| Oketch-Rabah, 2020 [253] | 29 | Camellia sinensis extract | Perfect feature presentation of HILI by this herb | |
| Schimmel, 2020 [254] | 20 | Mitragyna speciosa, syn. Kraton | Feature details provided for HILI cases |
Abbreviations: DILI, Drug induced liver injury; HILI, Herb induced liver injury; HSOS, Hepatic sinusoidal obstruction syndrome; RUCAM, Roussel Uclaf Causality Assessment Method, TCM, Traditional Chinese Medicines; USP, United States Pharmacopeia; WHO, World Health Organizations.
6.2. Hospital and Other Sources
Most RUCAM based HILI cases were provided by authors from university hospitals and their affiliated teaching hospitals with their departments of Hepatology and Gastroenterology, Medicine or Internal Medicine (Table 3). Rare contributors were other departments like those with focus on Emergency Medicine [255], Clinical Pharmacology and Toxicology in Berlin [209], Pharmacology and Toxicology in Hannover [213], Pharmacy in Singapore [238], Physiology and Pharmacology in Rome [219,221], Anatomical, Histological, Forensic and Orthopedic Sciences in Rome [222], Pediatrics in Seoul [234], and among the contributors were even the Neurology and Headache Center in Essen [212] and Spine and Joint Research Institute in Seoul [235].
Other sources providing RUCAM based HILI cases include the Chinese Academy of Medical Sciences in Beijing [195], School of Chinese Materia Medica in Beijing [199,202], Competence Centre for Complementary Medicine and Naturopathy in Munich [211], Biomedical Research and Innovation Platform South African Medical Research Council in Tygerberg [239], United States Pharmacopeia in Rockville [254], and Center of Pharmacovigilance of Florence [218].
6.3. Top Ranking Countries
Among the countries presenting RUCAM based HILI cases were on top in descending order China and Korea, followed by Germany, India and the US, whereby the top 5 countries provided most of the HILI cases (Table 5). Authors from these 5 countries contributed together 13,808 HILI cases out of a total 14,029 worldwide HILI cases, corresponding to 98.4%. On the lower part of the list ranked the 4 countries Brazil, Colombia, Switzerland, and Turkey, authors from these low ranking countries provided each one HILI case assessed for causality using RUCAM, corresponding to 4 cases altogether out of a total of 14,029 HILI cases. Authors from the remaining 20 countries with a ranking from 6 down to 14 contributed 217 HILI cases out of overall 14,029 cases corresponding to almost 1.6%.
Table 5.
Top ranking of countries providing HILI cases assessed for causality by RUCAM.
| Top Ranking Countries |
Cases, n |
References |
|---|---|---|
| 1. China | 10,914 | [37,38,42,48,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203] |
| 2. Korea | 2507 | [100,101,102,103,225,226,227,228,229,230,231,232,233,234,235,236,237] |
| 3. Germany | 170 | [206,207,208,209,210,211,212,213,214,215] |
| 4. India | 117 | [216,217] |
| 5. US | 100 | [247,248,249,250,251,252,253,254,255] |
| 6. Italy | 77 | [218,219,220,221,222] |
| 7. South Africa | 47 | [239] |
| 8. Spain | 46 | [115,117,240,241,242] |
| 9. Singapore | 25 | [113,238] |
| 10. France | 10 | [205] |
| 11. Sweden | 5 | [244] |
| 12. Japan | 3 | [223,224] |
| 13. Australia | 2 | [29,181] |
| 14. Austria | 2 | [182] |
| 15. Brazil | 1 | [183] |
| 16. Colombia | 1 | [204] |
| 17. Switzerland | 1 | [245] |
| 18. Turkey | 1 | [246] |
Abbreviations: HILI, Herb induced liver injury; RUCAM, Roussel Uclaf Causality Assessment Method.
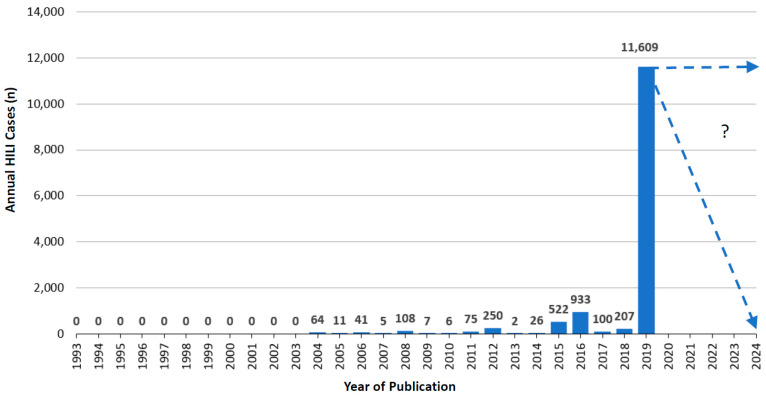
6.3.1. Published Annual RUCAM Based HILI Cases
From 1993 to 2019, published annual cases of RUCAM based HILI ranged between 0 and 11,609 in 2019, while 57 HILI cases of 2020 were not included because case counting stopped by end of June in this particular year (Figure 3). Three phases of trends appeared with respect to published RUCAM based HILI cases: (1) phase 1 with lack of any clinical field testing from 1993 to 2003, (2) phase 2 with slow promotion from 2004 to 2016, and (3) phase 3 of worldwide use from 2017 to 2019.
Figure 3.
Annual cases of HILI cases assessed for causality by RUCAM and published since 1993.
Phase 1 started after the launch of RUCAM in 1993 [16,47] but without a single published HILI case until 2003 (Figure 3). The lack of published RUCAM based HILI cases during this period might be due to the fact that the value of RUCAM was not yet sufficiently known or to uncertainties whether herbs have the potential to cause liver injury. In addition, the term of herb induced liver injury or its acronym HILI was unknown at that time and therefore not in common use.
During the subsequent phase 2, the number of annual published HILI cases remained small with cases ranging from 2 to 933, considering the years from 2004 until 2016 (Figure 3). In 2008, there were 108 HILI cases, with 18 Spanish cases published by García-Cortés et al. [117,241] and 90 Korean cases published by Kang et al. [227] and Sohn et al. [228]. During 2016, there was a sharp increase with 933 HILI cases, mostly attributed to 866 cases from China published by Zhu et al. [42]. As a reminder and outlined recently, herb induced liver injury with HILI as its acronym was first introduced and proposed as a specific term in the scientific literature only in 2011 [12]. This may explain retarded publications on HILI cases (Figure 3).
Phase 3 started with low HILI case numbers in 2017 and 2018 (Figure 3), considering that the updated RUCAM applicable also to HILI cases was published only in 2016 [15]. With 11,609 the largest HILI case number was published in 2019 (Figure 3) as a consequence of the ongoing worldwide use of RUCAM for assessing causality in suspected HILI cases (Table 4). In particular, contributing countries were in alphabetical order Australia [29], Brazil [183], China [48,196,197,198,199,200,201], Germany [213,214,215], India [217], Italy [222], Korea [103], Spain [115,117,240,241,242], Switzerland [244], and the US [245,246,247,248,249,250,251,252,253,254]. Most of the 11,619 HILI cases published in 2019 were from China [48,196] and Korea [103], with 6971 cases published by Shen et al. [48], 2019 cases reported by Byeon et al. [103], and 1552 cases provided by Chow et al. [196]. However, until mid 2020 only 57 HILI cases were published (Table 4) [202,203,253,254], suggesting for the whole year 2020 at best 100 cases (Figure 3).
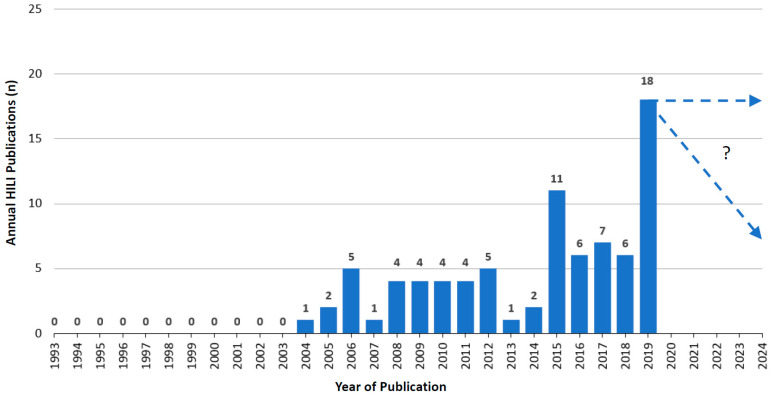
6.3.2. Annual RUCAM Based HILI Publications and Growth Trend
Over the years starting from 1993, when RUCAM was launched [14,57] and until 2019, an upward trend of annual RUCAM based HILI publications can be observed with some dips in between (Figure 4). In 2019, 18 publications were counted and 4 publications until end of June 2020 that were not included (Figure 4). For the whole year 2020, therefore, at best perhaps 8 publications can be anticipated (Figure 4). These figures show that a total of 85 publications with RUCAM based HILI cases were reported from 1993 until mid 2020 (Table 2).
Figure 4.
Annual publications of HILI cases assessed for causality by RUCAM as reported since 1993.
6.4. Specificities of HILI Cases
Large study cohorts of RUCAM based HILI cases accumulate many different herbs and provide as expected a global information of many HILI cases without a detailed description of clinical features for specific herbs (Table 4). Consequently, studies with a single or a few HILI cases have many advantages because they focus on a single herb or herbal product causing the liver injury and usually provide an exhaustive past medical history with clinical details required for a sound case evaluation. For interested physicians, regulators, and manufacturers, this listing provides individual cases with herbs causing HILI.
7. Utility of RUCAM
The utility of RUCAM has been confirmed in in many liver injury cases of DILI (Table 1) and HILI (Table 4) published from countries and regions around the world, as outlined in various reports [5,11,15,16,17,18] and briefly summarized (Table 6). In short, the high qualification of RUCAM as an objective diagnostic algoritm to assess causality in liver injury cases of DILI and HILI is the clue of its increasing use (Figure 1, Figure 2, Figure 3 and Figure 4). RUCAM is smoothly applied by clinicians or regulators and obviously without problems (Table 1 and Table 4). The worldwide use allows data comparison among different countries, a unique condition for multifacetted diseases as DILI and HILI are. RUCAM is also applied in epidemiology studies. Finally and most importantly, each individual DILI and HILI case report contain important details of liver injury cases that may be helpful for physicians in care of patients with suspected DILI and HILI.
Table 6.
Characteristics of RUCAM.
| RUCAM Specificities |
|---|
| Basic features |
|
|
|
|
|
|
|
|
|
|
|
| Clearly defined and scored key elements |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other important specificities |
|
|
|
|
|
Abbreviations: AI: Artificial Intelligence; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; CAM: Causality assessment method; CMV: Cytomegalovirus; DILI: Drug induced liver injury; EBV: Epstein Barr virus; HAV: Hepatitis A virus; HBV: Hepatitis B virus; HCV: Hepatitis C virus; HEV: Hepatitis E virus; HILI: herb induced liver injury; HSV: Herpes simplex virus; RUCAM: Roussel Uclaf Causality Assessment Method; VZV: Varicella zoster virus.
8. Other CAMs
Apart from the objective diagnostic RUCAM algorithm, a few non-RUCAM based CAMs are known, critically discussed elsewhere in detail [5,15]. In short, they are less accurate than RUCAM, not quantitative as not based on specific elements to be scored individually, not specific for liver injury cases, not structured, not validated, or based on individual arbitrary subjective opinions. In fact, other CAMs are still caught up in the pre-RUCAM and pre-AI era [18] and thereby neglecting the use of diagnostic algorithms such as the original RUCAM [14] or the now preferred updated version [18].
9. Limitation of the Analysis
The current analysis is based on published data of DILI and HILI reports in English, or at least an abstract in English, rather than on unpublished data contained in the original data sets that were not available to the authors of the analysis for re-analysis. Although most of the published DILI and HILI cases provide excellent data, some authors forgot presenting RUCAM based causality gradings or included cases with a possible causality grading in their final evaluations of cases together with a probable or highly probable causality level. Nevertheless, a broad range of different causality gradings was commonly provided in most published cases, respective references allow for detailed information. As being outside the scope of this article, causality gradings for individual reports were not provided (Table 1 and Table 5), but some details of 46,266 DILI cases assessed by RUCAM were published earlier [11]. Problematic are study cohorts with inclusion of both DILI and HILI cases, unless both groups were separately evaluated [48]. As expected, not all of the patients were commonly confirmed as being DILI by RUCAM scoring, but the number of published cases remained accurate. For instance, special conditions are evident in the randomized clinical trial of ximelagatran [148]. In this prospective, report, hepatic findings were analyzed in all suspected cases with regard to causal relationship to ximelagatran by using RUCAM, considered as the most reliable tool to assess causality [148]. Applying RUCAM based on ALT thresholds only is insufficient since 92% of the ximelagatran group did not meet this criterion missing then a final robust causality grading, as opposed to 8% of the study group receiving partially high causality gradings. This study reaffirms the utility of RUCAM to identify cases with real DILI cases in cohorts under real world conditions.
10. Outlook
The perspectives using the updated RUCAM in future DILI and HILI cases are favorable because many authors including those from the US become more familiar with RUCAM and are ready to use this diagnostic algorithm (Table 1, Table 2, Table 3 and Table 4), in line with principles of Artificial Intelligence to solve difficult processes [18]. Moreover, as in the US and many other countries RUCAM was successfully used to assess causality in cases of DILI, there is no need to invent another instrument specifically designed for drug development [255]. The issue of overlooked alternative causes remains a clinical problem and was described already in 1999 by Aithal et al. [256] and guided by RUCAM subsequently confirmed [69,257].
Future DILI and HILI studies should adhere on a prospective study design as strongly recommended in the RUCAM updated in 2016 because a retrospective approach may create concern on the validity of the published results due to incomplete information [15]. Neglecting this recommendation and using instead a retrospective design could be problematic [48]. In addition, attempts to lift RUCAM based causality gradings from possible to probable must be resisted [48]. Discouraged is in particular the use of a non-RUCAM based CAM in addition to RUCAM, because such a combination causes uncertainty due to disputable results of causality gradings. It is not recommended to mix in the same cohort patients with DILI or HILI [48] because this situation will complicate a separate evaluation of DILI or HILI features. However, it is clear that in individual cases RUCAM allows for a distinction between a drug and a medicinal herb when causality gradings are different.
11. Conclusions
The current analysis showed a favorable run of the RUCAM algorithm globally used since its launch in 1993, considering the annually published DILI and HILI cases. Overall 95,885 liver injury cases were published using RUCAM for causality assessment, namely 81,856 iDILI cases and 14,029 HILI cases. The global use of RUCAM assessing causality in cases of DILI and HILI helps compare study results among various countries and facilitates description of typical clinical features, best derived from case reports or small case series. RUCAM solves complex conditions as an algorithm in line with principles of Artificial Intelligence. Top ranking countries providing RUCAM based DILI cases were China, the United States, Germany, Korea, and Italy, whereas most RUCAM based HILI cases were published by authors from China, Korea, Germany, India, and the United States. In term of number of cases published, there is no other causality assessment method that could outperform RUCAM evaluating DILI and HILI cases. This should encourage all the stakeholders involved in DILI and HILI to systematically use RUCAM in order to reinforce their diagnosis and take the right decisions for the benefit of the patients.
Acknowledgments
The authors are grateful to Sabine Veltens for her professional providing of the figures.
Author Contributions
Conceptualization and methodology, R.T. and G.D.; formal analysis and draft preparation, R.T.; review and editing, G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declared that they have no conflict of interests regarding this invited article.
References
- 1.Ke L., Lu C., Shen R., Lu T., Ma B., Hua Y. Knowledge mapping of drug-induced liver injury: A scientometric investigation (2010–2019) Front. Pharmacol. 2020 doi: 10.3389/fphar.2020.00842. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uetrecht J. Mechanistic studies of idiosyncratic DILI: Clinical implications. Front. Pharmacol. 2019;10:837. doi: 10.3389/fphar.2019.00837. In: Special issue: Clinical drug induced liver injury: Current diagnostic and mechanistic challenges, guest editors: Rolf Teschke, Gaby Danan & James H. Lewis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teschke R., Uetrecht J. Mechanism of idiosyncratic drug induced liver injury (DILI): Unresolved basic issues. Ann. Transl. Med. 2020 doi: 10.21037/atm-2020-ubih-05. In special issue: Unresolved basic issues in hepatology, guest editors Ralf Weiskirchen & Wolfgang Stremmel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M., Will Y. In: Drug-Induced Liver Toxicity. James K., David C., editors. Springer Protocols, Springer Nature; Berlin, Germany: 2018. (Series: Methods in Pharmacology and Toxicology). [Google Scholar]
- 5.Teschke R., Danan G. Causality assessment methods in drug-induced liver injury. In: James K., David C.C., Minjun C., Yvonne W., editors. Drug-Induced Liver Toxicity. Springer Nature; Berlin, Germany: 2018. pp. 555–594. (Series: Methods in Pharmacology and Toxicology/Y). Chapter 27. [DOI] [Google Scholar]
- 6.Teschke R., Danan G., Lewis J.H. Special issue: Clinical drug induced liver injury: Current diagnostic and mechanistic challenges, guest editors: Rolf Teschke, Gaby Danan & James H. Lewis. [(accessed on 30 June 2020)];Front. Pharmacol. 2019 Available online: https://www.frontiersin.org/research-topics/9104/clinical-drug-induced-liver-injury-current-diagnostic-and-mechanistic-challenges. [Google Scholar]
- 7.Sarges P., Steinberg J.M., Lewis J.H. Drug-induced liver injury: Highlights from a review of the 2015 literature. Drug Saf. 2016;39:561–575. doi: 10.1007/s40264-016-0427-8. [DOI] [PubMed] [Google Scholar]
- 8.Shahbaz O., Mahajan S., Lewis J.H. Highlights of drug- and herb-induced liver injury in the literature from 2016, How best to translate new information into clinical practice? Exp. Opin. Drug Metab. Toxicol. 2017;13:935–951. doi: 10.1080/17425255.2017.1362391. [DOI] [PubMed] [Google Scholar]
- 9.Real M., Barnhill M.S., Higley C., Rosenberg J., Lewis J.H. Drug-induced liver injury: Highlights of the recent literature. Drug Saf. 2019;42:365–387. doi: 10.1007/s40264-018-0743-2. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg J.J., Higley C., Lewis J.H. Selected highlights from the recent literature of newly reported herbal and dietary supplement-induced liver injury. Adv. Res. Gastroenterol. Hepatol. 2020;15:555904. doi: 10.19080/ARGH.2020.15.555904. [DOI] [Google Scholar]
- 11.Teschke R. Idiosyncratic DILI: Analysis of 46, 266 cases assessed for causality by RUCAM and published from 2014 to early 2019. Front. Pharmacol. 2019;10:730. doi: 10.389/fphar.2019.00730. In: Special issue: Clinical drug induced liver injury: Current diagnostic and mechanistic challenges. Guest editors: Rolf Teschke, Gaby Danan & James H. Lewis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teschke R., Zhu Y., Jing J. Herb induced liver injury (HILI) in the Asian region and current role of RUCAM for causality assessment in 11, 160 published cases: Analysis and outlook. J. Clin. Transl. Hepatol. 2020;8:1–15. doi: 10.14218/JCTH.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teschke R., Eickhoff A., Schulze J., Danan G. Herb-induced liver injury (HILI) with 12,068 worldwide cases published with causality assessments by Roussel Uclaf Causality Assessment Method (RUCAM): An overview. Transl. Gastroenterol. Hepatol. 2020 doi: 10.21037/tgh-20-149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danan G., Bénichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 15.Danan G., Teschke R. RUCAM in drug and herb induced liver injury: The update. Int. J. Mol. Sci. 2016;17:14. doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danan G., Teschke R. Drug-induced liver injury: Why is the Roussel Uclaf Causality Assessment Method (RUCAM) still used 25 years after its launch? Drug Saf. 2018;41:735–743. doi: 10.1007/s40264-018-0654-2. [DOI] [PubMed] [Google Scholar]
- 17.Danan G., Teschke R. Roussel Uclaf Causality Assessment Method for drug-induced liver injury: Present and Future. Front. Pharmacol. 2019;10:853. doi: 10.3389/fphar.2019.00853. In: Special issue “Clinical drug induced liver injury: Current diagnostic and mechanistic challenges”, guest editors: Rolf Teschke, Gaby Danan & James H. Lewis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teschke R. Editorial. DILI, HILI, RUCAM algorithm, and AI, the Artificial Intelligence: Provocative issues, progress, and proposals. Arch. Gastroenterol. Res. 2020;1:4–11. [Google Scholar]
- 19.Hayashi P.H. Drug-Induced Liver Injury Network causality assessment: Criteria and experience in the United States. Int. J. Mol. Sci. 2016;17:201. doi: 10.3390/ijms17020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teschke R., Andrade R.J. Drug-induced liver injury: Expanding our knowledge by enlarging population analysis with prospective and scoring causality assessment. Gastroenterology. 2015;148:1271–1273. doi: 10.1053/j.gastro.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Teschke R., Eickhoff A., Brown A.C., Neuman M.G., Schulze J. Diagnostic biomarkers in liver injury by drugs, herbs, and alcohol: Tricky dilemma after EMA correctly and officially retracted Letter of Support. Int. J. Mol. Sci. 2020;21:212. doi: 10.3390/ijms21010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teschke R. Acetaminophen syn. paracetamol: Acute liver injury and acute on chronic liver failure with case analysis and causality assessment using RUCAM. In: Nikolaos T., editor. Liver Failure: Acute and Acute on Chronic. Springer Nature; Cham, Switzerland: 2020. [Google Scholar]
- 23.Bessone F., Hermandez N., Lucena M.I., Andrade R.J. The Latin American DILI registry experience: A successful ongoing collaborative strategic initiative. Int. J. Mol. Sci. 2016;17:313. doi: 10.3390/ijms17030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bessone F., Hernandez N., Mendizabal M., Sanchez A., Paraná R., Arrese M., Tagle M., Girala M., Lizarzabal M., Carrera E., et al. When the creation of a consortium provides useful answers: Experience of The Latin American DILI Network (LATINDILIN) Clin. Liver Dis. 2019;13:51–57. doi: 10.1002/cld.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colaci C.S., Mendizaba M., Bessone F. Idiosyncratic drug-induced acute liver failure: A challenging and distressing scenario. Curr. Drug Saf. 2019;14:94–101. doi: 10.2174/1574886314666190215115434. [DOI] [PubMed] [Google Scholar]
- 26.García D.S., Saturansky E.I., Poncino D., Martínez-Artola Y., Rosenberg S., Abritta G., Ascimani-Peña C., Cravero A. Hepatic toxicity by methotrexate with weekly single doses associated with folic acid in rheumatoid and psoriatic arthritis. What is its real frequency? Ann. Hepatol. 2019;18:765–769. doi: 10.1016/j.aohep.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Lin J., Moore D., Hockey B., Di Lernia R., Gorelik A., Liew D., Nicoll A. Drug-induced hepatotoxicity: Incidence of abnormal liver function tests consistent with volatile anaesthetic hepatitis in trauma patients. Liver Int. 2014;34:576–586. doi: 10.1111/liv.12278. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed T., Pandey R., Shah R., Black J. Resolution of ipilimumab induced severe hepatotoxicity with triple immunosuppressants therapy. BMJ Case Rep. 2015;2015:bcr2014208102. doi: 10.1136/bcr-2014-208102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laube R., Liu K. An unwanted complement: Rare case of potential liver injury induced by an interaction between ginseng and atorvastatin. Br. J. Clin. Pharm. 2019;85:1612–1613. doi: 10.1111/bcp.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worland T., Chin K.L., van Langenberg D., Garg M., Nicoll A. Retrospective study of idiosyncratic drug-induced liver injury from infliximab in an inflammatory bowel disease cohort: The IDLE study. Ann. Gastroenterol. 2020;33:162–169. doi: 10.20524/aog.2020.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sridharan K., Al Daylami A., Ajjawi R., Al Ajooz H.A.M. Drug-induced liver injury in critically ill children taking antiepileptic drugs: A retrospective study. Curr. Ther. Res. 2020 doi: 10.1016/j.curtheres.2020.100580. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker M.W., Muller Lunardelli M.J., Valle Tovo C. Drug and herb-induced liver injury: A critical review of Brazilian cases with proposals for the improvement of causality assessment using RUCAM. Ann. Hepatol. 2019;18:742–750. doi: 10.1016/j.aohep.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Yan B., Leung Y., Urbanski S.J., Myers R.P. Rofecoxib-induced hepatotoxicity: A forgotten complication of the coxibs. Can. J. Gastroenterol. 2006;20:351–355. doi: 10.1155/2006/356434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nhean S., Yoong D., Wong D.K., Gough K., Tseng A.L. Probable hepatotoxicity with dolutegravir: Report of two cases and review of the literature. Aids. 2019;33:1261–1263. doi: 10.1097/QAD.0000000000002191. [DOI] [PubMed] [Google Scholar]
- 35.Hou F.Q., Zeng Z., Wang G.Q. Hospital admissions for drug-induced liver injury: Clinical features, therapy, and outcomes. Cell Biochem. Biophys. 2012;64:77–83. doi: 10.1007/s12013-012-9373-y. [DOI] [PubMed] [Google Scholar]
- 36.Lv X., Tang S., Xia Y., Zhang Y., Wu S., Yang Z., Li X., Tu D., Chen Y., Deng P., et al. NAT2 genetic polymorphisms and anti-tuberculosis drug-induced hepatotoxicity in Chinese community population. Ann. Hepatol. 2012;11:700–707. doi: 10.1016/S1665-2681(19)31446-2. [DOI] [PubMed] [Google Scholar]
- 37.Hao K., Yu Y., He C., Wang M., Wang S., Li X. RUCAM scale-based diagnosis, clinical features and prognosis of 140 cases of drug-induced liver injury. Chin. J. Hepatol. 2014;22:938–941. doi: 10.3760/ma.j.issn.1007-3418.2014.12.012. (Abstract in English, Article in Chinese) [DOI] [PubMed] [Google Scholar]
- 38.Ou P., Chen Y., Li B., Zhang M., Liu X., Li F., Li Y., Chen C., Mao Y., Chen J. Causes, clinical features and outcomes of drug-induced liver injury in hospitalized patients in a Chinese tertiary care hospital. SpringerPlus. 2015;4:802. doi: 10.1186/s40064-015-1600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y., Li Y.G., Wang J.B., Liu S.H., Wang L.F., Zhao Y.L., Bai Y.F., Wang Z.X., Li J.Y., Xiao X.H. Causes, features, and outcomes of drug-induced liver injury in 69 children from China. Gut Liver. 2015;9:525–533. doi: 10.5009/gnl14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu R.J., Zhang Y., Tang F.L., Zheng Z.W., Fan Z.D., Zhu S.M., Qian X.F., Liu N.N. Clinical characteristics of drug-induced liver injury and related risk factors. Exp. Med. 2016;12:2606–2616. doi: 10.3892/etm.2016.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J., Yu Y.L., Jin Y., Zhang Y., Zheng C.Q. Clinical characteristics of drug-induced liver injury and primary biliary cirrhosis. World J. Gastroenterol. 2016;22:7579–7586. doi: 10.3748/wjg.v22.i33.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y., Niu M., Chen J., Zou Z.S., Ma Z.J., Liu S.H., Wang R.L., He T.T., Song H.B., Wang Z.X., et al. Comparison between Chinese herbal medicine and Western medicine-induced liver injury of 1985 patients. J. Gastroenterol. Hepatol. 2016;31:1476–1482. doi: 10.1111/jgh.13323. [DOI] [PubMed] [Google Scholar]
- 43.Naiqiong W., Liansheng W., Zhanying H., Yuanlin G., Chenggang Z., Ying G., Qian D., Dongchen L., Yanjun Z., Jianjun L. A multicenter and randomized controlled trial of Bicyclol in the treatment of statin-induced liver injury. Med. Sci. Monit. 2017;23:5760–5766. doi: 10.12659/MSM.904090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X.L., Liu X., Song Y., Hong R.T., Shi H. Suspected drug-induced liver injury associated with iguratimod: A case report and review of the literature. BMC Gastroenterol. 2018;18:130. doi: 10.1186/s12876-018-0858-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song Z.W., Panx Y.C., Huang Z.C., Liu W.X., Zhao R.S., Jing H.M., Dong F. Posaconazole-associated severe hyperbilirubinemia in acute myeloid leukemia following chemotherapy: A case report. World J. Clin. Cases. 2018;6:1206–1209. doi: 10.12998/wjcc.v6.i16.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao B., Chen S., Lin G., Yang M., Lu L., He X., Pan H., Tang S. Genetic polymorphisms of UGT 1A1 and susceptibility to anti-tuberculosis drug-induced liver injury: A RUCAM-based case-control study. Int. J. Immunopathol. Pharm. 2018;32:1–6. doi: 10.1177/2058738418816288. [DOI] [Google Scholar]
- 47.Liao P.F., Wu Y.K., Huang K.L., Chen H.Y. A rare case of cefapime-induced cholestatic liver injury. Tzu Chi Med. J. 2019;31:124–128. doi: 10.4103/tcmj.tcmj_151_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen T., Liu Y., Shang J., Xie Q., Li J., Yan M., Xu J., Niu J., Liu J., Watkins P.B., et al. Incidence and etiology of drug-induced liver injury in Mainland China. Gastroenterology. 2019;156:2230–2241. doi: 10.1053/j.gastro.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Xing M., Zhai L., Li J., Li Q., Gao M., Wen J., Xu Z. Assessment of cholestasis in drug-induced liver injury by different methods. Medicine. 2019;98:e14399. doi: 10.1097/MD.0000000000014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma S., Liu S., Wang Q., Chen L., Yang P., Sun H. Fenofibrate-induced hepatotoxicity: A case with a special feature that is different from those in the LiverTox database. J. Clin. Pharm. 2020;45:204–207. doi: 10.1111/jcpt.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tao B., Yang M., Chen H., Pan H., Liu W., Yi H., Tang S. Association of ABO blood group and antituberculosis drug-induced liver injury: A case-control study from a Chinese Han population. J. Clin. Pharm. 2020 doi: 10.1111/jcpt.13139. [DOI] [PubMed] [Google Scholar]
- 52.Wang S., Shangguan Y., Ding C., Li P., Ji Z., Shao J., Fang H., Yang M., Shi P., Wu J., et al. Risk factors for acute liver failure among inpatients with anti-tuberculosis drug-induced liver injury. J. Int. Med. Res. 2020;48 doi: 10.1177/0300060518811512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang H., Guo D., Xu Y., Zhu M., Yao C., Chen C., Jia W. Comparison of different liver test thresholds for drug-induced liver injury: Updated RUCAM versus other methods. Front. Pharmacol. 2019;10:816. doi: 10.3389/fphar.2019.00816. In: Special issue: Clinical drug induced liver injury: Current diagnostic and mechanistic challenges, guest editors Rolf Teschke, Gaby Danan, James H. Lewis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ríos D., Restrepo J.C. Abendazole-induced liver injury: A case report. Colomb. Med. 2013;44:118–120. doi: 10.25100/cm.v44i2.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cano-Paniagua A., Amariles P., Angulo N. Epidemiology of drug-induced liver injury in a university hospital from Colombia: Updated RUCAM being used for prospective causality assessment. Ann. Hepatol. 2019;18:501–507. doi: 10.1016/j.aohep.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Alhaddad O., Elsabaawy M., Abdelsameea E., Abdalla A., Shabaan A., Ehsan N., Elrefaey A., Elsabaawy D., Salama M. Presentations, causes and outcomes of drug-induced liver injury in Egypt. Sci. Rep. 2020;10:5124. doi: 10.1038/s41598-020-61872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bénichou C., Danan G., Flahault A. Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: Case reports with positive rechallenge. J. Clin. Epidemiol. 1993;46:1331–1336. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 58.Arotcarena R., Bigué J.P., Etcharry F., Pariente A. Pioglitazone-induced acute severe hepatitis. Gastroenterol. Clin. Biol. 2004;28:609–618. doi: 10.1016/s0399-8320(04)95021-x. (In French) [DOI] [PubMed] [Google Scholar]
- 59.Moch C., Rocher F., Lainé P., Lacotte J., Biour M., Gouraud A., Bernard N., Descotes J., Vial T. Etifoxine-induced acute hepatitis: A case series. Clin. Res. Hepatol. Gastroenterol. 2012;36:e85–e88. doi: 10.1016/j.clinre.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Carrier P., Godet B., Crepin S., Magy L., Debette-Gratien M., Pillegand B., Jacques J., Sautereau D., Vidal E., Labrousse F., et al. Acute liver toxicity due to methylprednisolone: Consider this diagnosis in the context of autoimmunity. Clin. Res. Hepatol. Gastroenterol. 2013;37:100–104. doi: 10.1016/j.clinre.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 61.Ripault M.P., Pinzani V., Fayolle V., Pageaux G.P., Larrey D. Crizotinib-induced acute hepatitis: First case with relaps after reintroduction with reduced dose. Clin. Res. Hepatol. Gastroenterol. 2013;37:e21–e23. doi: 10.1016/j.clinre.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Dumortier J., Cottin J., Lavie C., Guillaud O., Hervieu V., Chambon-Augoyard C., Scoazec J.Y., Vukusic S., Vital T. Methylprednisolone liver toxicity: A new case and a French regional pharmacovigilance survey. Clin. Res. Hepatol. Gastroenterol. 2017;41:497–501. doi: 10.1016/j.clinre.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 63.Meunier L., Larrey D. Recent advances in hepatotoxicity of non steroidal anti-inflammatory drugs. Ann. Hepatol. 2018;17:187–191. doi: 10.5604/01.3001.0010.8633. [DOI] [PubMed] [Google Scholar]
- 64.Stammschulte T., Treichel U., Pachl H., Gundert-Remy U. Cases of Liver Failure in Association with Flupirtine in the German Spontaneous Reporting System. [(accessed on 30 June 2020)]; Available online: http://www.akdae.de/Kommission/Organisation/Aufgaben/Publikationen/PDF/Stammschulte2012.pdf.
- 65.Douros A., Bronder E., Andersohn F., Klimpel A., Thomae M., Orzechowski H.D., Kreutz R., Garbe E. Flupirtine-induced liver injury-Seven cases from the Berlin Case-control Surveillance Study and review of the German spontaneous adverse drug reaction reporting database. Eur. J. Clin. Pharm. 2014;70:453–459. doi: 10.1007/s00228-013-1631-4. [DOI] [PubMed] [Google Scholar]
- 66.Douros A., Bronder E., Andersohn F., Klimpel A., Thomae M., Sarganas G., Kreutz R., Garbe E. Drug-induced liver injury: Results from the hospital-based Berlin Case-Control Surveillance Study. Br. J. Clin. Pharm. 2014;79:988–999. doi: 10.1111/bcp.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buechter M., Manka P., Heinemann F.M., Lindemann M., Baba H.A., Schlattjan M., Canbay A., Gerken G., Kahraman A. Potential triggering factors of acute liver failure as a first manifestation of autoimmune hepatitis-a single center experience of 52 adult patients. World J. Gastroenterol. 2018;24:1410–1418. doi: 10.3748/wjg.v24.i13.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dragoi D., Benesic A., Pichler G., Kulak N.A., Bartsch H.S., Gerbes A.L. Proteomics analysis of monocyte-derived hepatocyte-like cells identifies integrin beta 3 as a specific biomarker for drug-induced liver injury by diclofenac. Front. Pharmacol. 2018;9:699. doi: 10.3389/fphar.2018.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teschke R., Danan G. Review: Drug induced liver injury with analysis of alternative causes as confounding variables. Br. J. Clin. Pharm. 2018;84:1467–1477. doi: 10.1111/bcp.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teschke R. Review. Top-ranking drugs out of 3312 drug-induced liver injury cases evaluated by the Roussel Uclaf Causality Assessment Method. Expert Opin. Drug Metab. Toxicol. 2018;14:1169–1187. doi: 10.1080/17425255.2018.1539077. [DOI] [PubMed] [Google Scholar]
- 71.Weber S., Benesic A., Rotter I., Gerbes A.L. Early ALT response to corticosteroid treatment distinguishes idiosyncratic drug-induced liver injury from autoimmune hepatitis. Liver Int. 2019;39:1906–1917. doi: 10.1111/liv.14195. [DOI] [PubMed] [Google Scholar]
- 72.Björnsson E., Jacobsen E.I., Kalaitzakis E. Hepatotoxicity associated with statins: Reports of idiosyncratic liver injury post-marketing. J. Hepatol. 2012;56:374–380. doi: 10.1016/j.jhep.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 73.Björnsson E.S., Bergmann O.M., Björnsson H.K., Kvaran R.B., Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 74.Björnsson E.S., Hoofnagle J.H. Categorization of drugs implicated in causing liver injury: Critical assessment based on published case reports. Hepatology. 2016;63:590–603. doi: 10.1002/hep.28323. [DOI] [PubMed] [Google Scholar]
- 75.Harugeri A., Parthasarathi G., Sharma J., D’Souza G.A., Ramesh M. Montelukast induced acute hepatocellular injury. J. Postgrad. Med. 2009;55:141–142. doi: 10.4103/0022-3859.52850. [DOI] [PubMed] [Google Scholar]
- 76.Devarbhavi H., Dierkhising R., Kremers W.K., Sandeep M.S., Karanth D., Adarsh C.K. Single center experience with drug-induced liver injury from India: Causes, outcome, prognosis, and predictors of mortality. Am. J. Gastroenterol. 2010;105:2396–2404. doi: 10.1038/ajg.2010.287. [DOI] [PubMed] [Google Scholar]
- 77.Rathi C., Pipaliya N., Patel R., Ingle M., Phadke A., Sawant P. Drug induced liver injury at a tertiary hospital in India: Etiology, clinical features and predictors of mortality. Ann. Hepatol. 2017;16:442–450. doi: 10.5604/01.3001.0009.8600. [DOI] [PubMed] [Google Scholar]
- 78.Taneja S., Kumar P., Rathi S., Duseja A., Singh V., Dhiman R.K., Chawla Y.K. Acute liver failure due to Etodolac, a selective cycloxygenase- 2 (COX-2) inhibitor non-steroidal anti-inflammatory drug established by RUCAM-based causality assessment. Ann. Hepatol. 2017;16:818–821. doi: 10.5604/01.3001.0010.2829. [DOI] [PubMed] [Google Scholar]
- 79.Das S., Behera S.K., Xavier A.S., Velupula S., Dkkar S.A., Selvarajan S. Agreement among different scales for causality assessment in drug-induced liver injury. Clin. Drug Investig. 2018;38:211–218. doi: 10.1007/s40261-017-0601-5. [DOI] [PubMed] [Google Scholar]
- 80.Dutta S.K., Chakravarty P.J., Borah A.J. Haloperidol induced hepatotoxicity: A case report. Ann. Clin. Case Rep. 2020;5:1827. [Google Scholar]
- 81.Kulkarni A.V., Kumar P., Talukdar R., Rao N. Steroids as rescue therapy for vitamin A-induced acute liver failure. BMJ Case Rep. CP. 2020;13:e233902. doi: 10.1136/bcr-2019-233902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gluck N., Fried M., Porat R. Acute amiodarone liver toxicity likely due to ischemic hepatitis. Isr. Med. Assoc. J. 2011;13:748–752. [PubMed] [Google Scholar]
- 83.Rigato I., Cravatari M., Avellini C., Ponte E., Crocè S.L., Tiribelli C. Drug-induced acute cholestatic liver damage in a patient with mutation of UGT1A1. Nat. Clin. Pr. Gastroenterol. Hepatol. 2007;4:43–408. doi: 10.1038/ncpgasthep0871. [DOI] [PubMed] [Google Scholar]
- 84.Licata A., Calvaruso V., Capello M., Craxi A., Almasio P.L. Clinical course and outcomes of drug-induced liver injury: Nimesulide as the first implicated medication. Dig. Liver Dis. 2010;42:143–148. doi: 10.1016/j.dld.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 85.Abenavoli L., Milic N., Beaugrand M. Severe hepatitis by cyproterone acetate: Role of corticosteroids. A case report. Ann. Hepatol. 2013;1:152–155. doi: 10.1016/S1665-2681(19)31399-7. [DOI] [PubMed] [Google Scholar]
- 86.Ferrajolo C., Verhamme K.M., Trifirò G., W‘t Jong G., Picelli G., Giaquinto C., Mazzaglia G., Stricker B.H., Rossi F., Capuano A., et al. Antibiotic-induced liver injury in paediatric outpatients: A case-control study in primary care databases. Drug Saf. 2017;40:305–315. doi: 10.1007/s40264-016-0493-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Licata A., Minissale M.G., Calvaruso V., Craxi A. A focus on epidemiology of drug-induced liver injury; analysis of a prospective cohort. Eur. Rev. Pharm. Sci. 2017;21:112–121. [PubMed] [Google Scholar]
- 88.Giacomelli A., Rusconi S., Falvella F.S., Oreni M.L., Cattaneo D., Cozzi V., Renisi G., Monge E., Cheli S., Clementi E., et al. Clinical and genetic determinants of nevirapine plasma through concentration. SAGE Open Med. 2018;6 doi: 10.1177/2050312118780861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Licata A., Puccia F., Lombardo V., Serruto A., Minissale M.G., Morreale I., Giannitrapani L., Soresi M., Montalto G., Almasio P.L. Rivaroxaban-induced hepatotoxicity: Review of the literature and report of new cases. Eur. J. Gastroenterol. Hepatol. 2018;30:226–232. doi: 10.1097/MEG.0000000000001030. [DOI] [PubMed] [Google Scholar]
- 90.Lovero R., Losurdo G., Mastromauro M., Castellaneta N.M., Mongelli A., Gentile A., Di Leo A., Principi M. A case of severe transaminase elevation following a single Ustekinumab dose with remission after drug withdrawal. Curr. Drug Saf. 2018;13:221–223. doi: 10.2174/1574886313666180719165212. [DOI] [PubMed] [Google Scholar]
- 91.Masumoto T., Horiike N., Abe M., Kumaki T., Matsubara H., Fazle Akbar S.M., Michitaka K., Hyodo I., Onji M. Diagnosis of drug-induced liver injury in Japanese patients by criteria of the Consensus Meetings in Europe. Hepatol. Res. 2003;25:1–7. doi: 10.1016/S1386-6346(02)00148-1. [DOI] [PubMed] [Google Scholar]
- 92.Hanatani T., Sai K., Tohkin M., Segawa K., Kimura M., Hori K., Kawakami J., Saito Y. A detection algorithm for drug-induced liver injury in medical information databases using the Japanese diagnostic scale and its comparison with the Council for International Organizations of Medical Sciences/the Roussel Uclaf Causality Assessment Method scale. Pharm. Drug Saf. 2014;23:984–988. doi: 10.1002/pds.3603. [DOI] [PubMed] [Google Scholar]
- 93.Niijima K., Niijima Y., Okada S., Yamada M. Drug-induced liver injury caused by Ipragliflozin administration with causality established by a positive lymphocyte transformation test (LTT) and the Roussel Uclaf Causality Assessment Method (RUCAM): A case report. Ann. Hepatol. 2017;16:308–311. doi: 10.5604/16652681.1231594. [DOI] [PubMed] [Google Scholar]
- 94.Ji H., Yue F., Song J., Zhou X. A rare case of methimazole-induced cholestatic jaundice in an elderly man of Asian ethnicity with hyperthyroidism: A case report. Medicine. 2017;96:e9093. doi: 10.1097/MD.0000000000009093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aiso M., Takikawa H., Tsuji K., Kagawa T., Watanabe M., Tanaka A., Sato K., Sakisaka S., Hiasa Y., Takei Y., et al. Analysis of 307 cases with drug-induced liver injury between 2010 and 2018 in Japan. Hepatol. Res. 2019;49:105–110. doi: 10.1111/hepr.13288. [DOI] [PubMed] [Google Scholar]
- 96.Kishimoto M., Adachi M., Takahashi K., Washizaki K. Clonazepam-induced liver dysfunction, severe hyperlipidaemia, and hyperglycaemic crisis: A case report. SAGE Open Med. Cases Rep. 2019;7:1–5. doi: 10.1177/2050313X19842976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hiraki M., Tanaka T., Koga F., Saito A., Oza N., Ikeda O., Manabe T., Miyoshi A., Kitahara K., Sato S., et al. An unusual case of acute liver failure caused by adjuvant oral Tegafur-Uracil with folinate therapy for colon cancer patient: A case report. J. Gastrointest. Cancer. 2020;51:296–299. doi: 10.1007/s12029-019-00228-7. [DOI] [PubMed] [Google Scholar]
- 98.Kakisaka K., Suzuki Y., Jinnouchi Y., Kanazawa J., Sasaki T., Yonezawa T., Yoshida Y., Kuroda H., Takikawa Y. Unfavorable prognosis of patients with acute liver injury due to drug-induced liver injury and acute exacerbation of hepatitis B virus infection. Hepatol. Res. 2019;49:1286–1293. doi: 10.1111/hepr.13397. [DOI] [PubMed] [Google Scholar]
- 99.Choi G.Y., Yang H.W., Cho S.H., Kang D.W., Go H., Lee W.C., Lee Y.J., Jung S.H., Kim A.N., Cha S.W. Acute drug-induced hepatitis caused by albendazole. J. Korean Med. Sci. 2008;23:903–905. doi: 10.3346/jkms.2008.23.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suk K.T., Kim D.J., Kim C.H., Park S.H., Yoon J.H., Kim Y.S., Baik G.H., Kim J.B., Kweon Y.O., Kim B.I., et al. A prospective nationwide study of drug-induced liver injury in Korea. Am. J. Gastroenterol. 2012;107:1380–1387. doi: 10.1038/ajg.2012.138. [DOI] [PubMed] [Google Scholar]
- 101.Son C.G. Drug-induced liver injury by Western medication. J. Int. Korean Med. 2015;36:69–75. [Google Scholar]
- 102.Woo H.J., Kim H.Y., Choi E.S., Cho Y., Kim Y., Lee J.H., Jang E. Drug-induced liver injury: A 2-year retrospective study of 1169 hospitalized patients in a single medical center. Phytomedicine. 2015;22:1201–1205. doi: 10.1016/j.phymed.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 103.Byeon J.H., Kil J.H., Ahn Y.C., Son C.S. Systematic review of published data on herb induced liver injury. J. EthnoPharmacol. 2019;233:190–196. doi: 10.1016/j.jep.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 104.Kwon J., Kim S., Yoo H., Lee E. Nimesulide-induced hepatotoxicity: A systemic review and meta-analysis. PLoS ONE. 2019;14:e0209264. doi: 10.1371/journal.pone.0209264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thalha A.M., Mahadeva S., Tan A.T.B., Mun K.S. Kombiglyze (metformin and saxagliptin)-induced hepatotoxicity in a patient with non-alcoholic fatty liver disease. JGH Open. 2018;2:242–245. doi: 10.1002/jgh3.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lammel-Lindemann J.A., Clores-Villalba E., Martagón A.J., DeObeso-Gonzalez E., Puente-Gallegos F. Non-cholestatic acute hepatitis following Candesartan administration. Br. J. Clin. Pharm. 2018;84:204–207. doi: 10.1111/bcp.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Essaid A., Timraz A. Cholestatic acute hepatitis induced by tadalafil (Cialis®) Gastroenterol. Clin. Biol. 2010;34:e1–e2. doi: 10.1016/j.gcb.2010.01.001. (In French) [DOI] [PubMed] [Google Scholar]
- 108.Abid A., Subhani F., Kayani F., Awan S., Abid S. Drug induced liver injury is associated with high mortality-A study from a tertiary care hospital in Pakistan. PLoS ONE. 2020;15:e0231398. doi: 10.1371/journal.pone.0231398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Costa-Moreira P., Gaspar R., Pereira P., Lopes S., Canao P., Lopes J., Carneiro F., Macedo G. Role of liver biopsy in the era of clinical prediction scores for “drug-induced liver injury” (DILI): Experience of a tertiary referral hospital. Virchows Arch. 2020 doi: 10.1007/s00428-020-02824-6. [DOI] [PubMed] [Google Scholar]
- 110.Alqrinawi S.H., Akbar N., AlFaddag H., Akbar S., Akbar L., Butt S.A., Aljawad M. Menotrophin induced autoimmune hepatitis. Case Rep. Gastrointest. Med. 2019 doi: 10.1155/2019/7343805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miljkovic M.M., Dobric S., Dragojevic-Simic V. Consistency between causality assessments obtained with two scales and their agreement with clinical judgments in hepatotoxicity. Pharm. Drug Saf. 2011;20:272–285. doi: 10.1002/pds.2081. [DOI] [PubMed] [Google Scholar]
- 112.Miljkovic M.M., Dobric S., Dragojevic-Simic V. Accuracy and reproducibility of two scales in causality assessment of unexpected hepatotoxicity. J. Clin. Pharm. 2012;37:196–203. doi: 10.1111/j.1365-2710.2011.01282.x. [DOI] [PubMed] [Google Scholar]
- 113.Wai C.T. Presentation of drug-induced liver injury in Singapore. Singap. Med. J. 2006;47:116–120. [PubMed] [Google Scholar]
- 114.Rodríguez L.A., Stricker B.H., Zimmerman H.J. Risk of acute liver injury associated with the combination of amoxicillin and clavulanic acid. Arch. Int. Med. 1996;156:1327–1332. doi: 10.1001/archinte.1996.00440110099013. [DOI] [PubMed] [Google Scholar]
- 115.Andrade R.J., Lucena M.I., Fernández M.C., Pelaez G., Pachkoria K., García-Ruiz E., García-Muñoz B., Gonzalez-Grande R., Pizarro A., Durán J.A., et al. Spanish Group for the Study of Drug-induced Liver Disease. Drug-induced liver injury: An analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 116.Andrade R.J., Lucena M.I., Kaplowitz N., García-Muñoz B., Borraz Y., Pachkoria K., García-Cortés M., Fernández M.C., Pelaez G., Rodrigo L., et al. Outcome of acute idiosyncratic drug-induced liver injury: Long term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–1588. doi: 10.1002/hep.21424. [DOI] [PubMed] [Google Scholar]
- 117.García-Cortés M., Lucena M.I., Pachkoria K., Borraz Y., Hidalgo R., Andrade R.J. Evaluation of Naranjo Adverse Drug Reactions Probability Scale in causality assessment of drug-induced liver injury. Aliment. Pharm. 2008;27:780–789. doi: 10.1111/j.1365-2036.2008.03655.x. [DOI] [PubMed] [Google Scholar]
- 118.Lucena M.I., Molokhia M., Shen Y., Urban T.J., Aithal G.P., Andrade R.J., Day C.P., Ruiz-Cabello F., Donaldson P.T., Stephens C., et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lucena M.I., Kaplowitz N., Hallal H., Castiella A., García-Bengoechea M., Otazua P., Berenguer M., Fernandez M.C., Planas R., Andrade R.J. Recurrent drug-induced liver injury (DILI) with different drugs in the Spanish Registry: The dilemma of the relationship to autoimmune hepatitis. J. Hepatol. 2011;55:820–827. doi: 10.1016/j.jhep.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 120.Robles-Diaz M., Gonzalez-Jimenez A., Medina-Caliz I., Stephens C., García-Cortes M., García-Muñoz B., Ortega-Alonso A., Blanco-Reina E., Gonzalez-Grande R., Jimenez-Perez M., et al. on behalf of the Spanish DILI Registry and the SLatinDILI Network. Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment. Pharm. 2015;41:116–125. doi: 10.1111/apt.13023. [DOI] [PubMed] [Google Scholar]
- 121.Tong H.Y., Díaz C., Collantes E., Medrano N., Borobia A.M., Jara P., Ramírez E. Liver transplant in a patient under methylphenidate therapy: A case report and review of the literature. Case Rep. Pediatr. 2015;2015:437298. doi: 10.1155/2015/437298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Medina-Caliz I., Robles-Diaz M., Garcia-Muñoz B., Stephens C., Ortega-Alonso A., Garcia-Cortes M., González-Jimenez A., Sanabria-Cabrera J.A., Moreno I., Fernandez M.C., et al. Spanish DILI registry. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J. Hepatol. 2016;65:532–542. doi: 10.1016/j.jhep.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.López-Riera M., Conde I., Quitas G., Pedrola L., Zaragoza Á., Perez-Rojas J., Saledo M., Benlloch S., Castell J.V., Jover R. Non-invasive prediction of NAFLD severity: A comprehensive, independent validation of previously postulated serum microRNA biomarkers. Sci. Rep. 2018;8:10606. doi: 10.1038/s41598-018-28854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Machlab S., Mireia M., Vergara M., Escoda M.R., Casas M. Apixaban-induced liver injury. Rev. Esp. Enferm. Dig. 2019;111:161–163. doi: 10.17235/reed.2018.5877/2018. [DOI] [PubMed] [Google Scholar]
- 125.Zoubek M.E., Pinazo-Bandera J., Ortega-Alonso A., Hernández N., Crespo J., Contreras F., Medina-Cáliz I., Sanabria-Cabrera J., Sanjuan-Jiménez R., González-Jiménez A., et al. Liver injury after methylprednisolone pulses: A disputable cause of hepatotoxicity. A case series and literature review. United Eur. Gastroenterol. J. 2019;7:825–837. doi: 10.1177/2050640619840147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Björnsson E., Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–489. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 127.De Valle M.B., Klinteberg A.V., Alem N., Olsson R., Björnsson E. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment. Pharm. 2006;24:1187–1195. doi: 10.1111/j.1365-2036.2006.03117.x. [DOI] [PubMed] [Google Scholar]
- 128.Björnsson E., Kalaitzakis E., Klinteberg V.A.V., Alem E., Olsson R. Long-term follow-up of patients with mild to moderate drug-induced liver injury. Aliment. Pharm. 2007;26:79–85. doi: 10.1111/j.1365-2036.2007.03355.x. [DOI] [PubMed] [Google Scholar]
- 129.Björnsson E.S. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34:115–122. doi: 10.1055/s-0034-1375953. [DOI] [PubMed] [Google Scholar]
- 130.Goossens N., Spahr L., Rubbia-Brandt L. Severe immune-mediated drug-induced liver injury linked to ibandronate: A case report. J. Hepatol. 2013;59:1139–1142. doi: 10.1016/j.jhep.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 131.Russmann S., Niedrig D.F., Budmiger M., Schmidt C., Stieger B., Hürlimann S., Kullak-Ublick G.A. Rivaroxaban postmarketing risk of liver injury. J. Hepatol. 2014;61:293–300. doi: 10.1016/j.jhep.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 132.Scalfaro E., Streefkerk H.J., Merz M., Meier C., Lewis D. Preliminary results of a novel algorithmic method aiming to support initial causality assessment of routine pharmacovigilance case reports for medication-induced liver injury: The PV-RUCAM. Drug Saf. 2017;40:715–727. doi: 10.1007/s40264-017-0541-2. [DOI] [PubMed] [Google Scholar]
- 133.Schneider J.S., Montani M., Stickel F. Drug-induced autoimmune hepatitis following treatment with Zoledronic acid. Case Rep. Gastroenterol. 2017;11:440–445. doi: 10.1159/000479314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Terziroli Beretta-Piccoli B., Mieli-Vergani G., Bertoli R., Mazzucchelli L., Nofziger C., Paulmichl M., Vergani D. Atovaquone/proguanil-induced autoimmune-like hepatitis. Hepatol. Commun. 2017;1:293–298. doi: 10.1002/hep4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Visentin M., Lenggenhager D., Gai Z., Kullak-Ublick G.A. Drug-induced bile duct injury. BBA Mol. Basis Dis. 2018;1864:1498–1506. doi: 10.1016/j.bbadis.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 136.Treeprasertsuk S., Huntrakul J., Ridtitid W., Kullavanijaya P., Björnsson E.S. The predictors of complications in patients with drug-induced liver injury caused by antimicrobial agents. Aliment. Pharm. 2010;11:1200–1207. doi: 10.1111/j.1365-2036.2010.04292.x. [DOI] [PubMed] [Google Scholar]
- 137.Sobhonslidsuk A., Poovorawan K., Soonthornworasiri N., Pangnum W., Phaosawasdi K. The incidence, presentation, outcomes, risk of mortality and economic data of drug-induced liver injury from a national database in Thailand: A population-base study. BMC Gastroenterol. 2016;16:135. doi: 10.1186/s12876-016-0550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chayanupatkul M., Schiano T.D. Acute liver failure secondary to drug-induced liver injury. Clin. Liver Dis. 2020;24:75–87. doi: 10.1016/j.cld.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 139.Duzenli T., Tanoglu A., Akyol T., Kara M., Yazgan Y. Drug-induced liver injury caused by Phenprobamate: Strong probability due to repeated toxicity. Eur. J. HepatoGastroenterol. 2019;9:49–51. doi: 10.5005/jp-journals-10018-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hussaini S.H., O’Brien C.S., Despott E.J., Dalton H.R. Antibiotic therapy: A major cause of drug-induced jaundice in southwest England. Eur. J. Gastroenterol. Hepatol. 2007;19:15–20. doi: 10.1097/01.meg.0000250581.77865.68. [DOI] [PubMed] [Google Scholar]
- 141.Daly A.K., Donaldson P.T., Bhatnagar P., Shen Y., Pe’er I., Floratos A., Daly M.J., Goldstein D.B., John S., Nelson M.R., et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat. Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 142.Spraggs C.F., Budde L.R., Briley L.P., Bing N., Cox C.J., King K.S., Whittaker J.C., Mooser V.E., Preston A.J., Stein S.H., et al. HLA-DQA1*02, 01 is a major risk factor for Lapatinib-induced hepatotoxicity in women with advanced breast cancer. J. Clin. Oncol. 2011;29:667–673. doi: 10.1200/JCO.2010.31.3197. [DOI] [PubMed] [Google Scholar]
- 143.Islam M., Wright G., Tanner P., Lucas R. A case of anastrazole-related drug-induced autoimmune hepatitis. Clin. J. Gastroenterol. 2014;7:414–417. doi: 10.1007/s12328-014-0512-4. [DOI] [PubMed] [Google Scholar]
- 144.Dyson J.K., Hutchinson J., Harrison L., Rotimi O., Tiniakos D., Foster G.R., Aldersley M.A., McPherson S. Liver toxicity associated with sofosbuvir, an NS5A inhibitor and ribavirin use. J. Hepatol. 2016;64:234–238. doi: 10.1016/j.jhep.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 145.Abbara A., Chitty S., Roe J.K., Ghani R., Collin S.M., Ritchie A., Kon O.M., Dzvova J., Davidson H., Edwards T.E., et al. Drug-induced liver injury from antituberculous treatment: A retrospective study from a large TB centre in the UK. BMC Infect. Dis. 2017;17:231. doi: 10.1186/s12879-017-2330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vliegenthart A.D.B., Berends C., Potter C.M.J., Kersaudy-Kerhoas M., Dear J.W. MicroRNA-122 can be measured in capillary blood which facilitates point-of-care testing for drug-induced liver injury. Br. J. Clin. Pharm. 2017;83:2027–2033. doi: 10.1111/bcp.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fontana R.J., Shakil O., Greenson J.K., Boyd I., Lee W.M. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig. Dis. Sci. 2005;10:1785–1790. doi: 10.1007/s10620-005-2938-5. [DOI] [PubMed] [Google Scholar]
- 148.Lee W.M., Larrey D., Olsson R., Lewis J.H., Keisu M., Auclert L., Sheth S. Hepatic findings in long-term clinical trials of ximelagatran. Drug Saf. 2005;28:351–370. doi: 10.2165/00002018-200528040-00006. [DOI] [PubMed] [Google Scholar]
- 149.Stojanovski S.D., Casavant M.J., Mousa H.M., Baker P., Nahata M.C. Atomoxetine-induced hepatitis in a child. Clin. Toxicol. 2007;45:51–55. doi: 10.1080/15563650600795644. [DOI] [PubMed] [Google Scholar]
- 150.Lammert C., Einarsson S., Saha C., Niklasson A., Bjornsson E., Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: Search for signals. J. Hepatol. 2008;47:2003–2009. doi: 10.1002/hep.22272. [DOI] [PubMed] [Google Scholar]
- 151.Singla A., Hammad H.T., Hammoud G.M. Uncommon cause of acute drug-induced liver injury following mammoplasty. Gastroenterol. Res. 2010;3:171–172. doi: 10.4021/gr2010.06.210w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nabha L., Balba G.P., Tuanzon C., Kumar P.N. Etravirine induced severe hpersensitivity reaction and fulminant hepatitis: A case report and review of the literature. J. Aids Clin. Res. 2012;3:005. doi: 10.4172/2155-6113.S2-005. [DOI] [Google Scholar]
- 153.Sprague D., Bamha K. Drug-induced liver injury due to varenicline. BMC Gastroenterol. 2012;12:65. doi: 10.1186/1471-230X-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Markova S.M., De Marco T., Bendjilali N., Kobashigawa E.A., Mefford J., Sodhi J., Le H., Zhang C., Halladay J., Rettie A.E., et al. Association of CYP2C9*2 with bosentan-induced liver injury. Clin. Pharm. 2013;94:678–686. doi: 10.1038/clpt.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marumoto A., Roytman M.M., Tsai N.C.S. Trial and error: Investigational drug induced liver injury, a case series report. Hawaii J. Med. Public Health. 2013;72:30–33. [PMC free article] [PubMed] [Google Scholar]
- 156.Bohm N., Bohm N., Makowski C., Machado M., Davie A., Seabrook N., Wheless L., Bevill B., Clark B., Kyle T.R., III Case report and cohort analysis of drug-induced liver injury associated with daptomycin. Antimicrob. Agents Chemother. 2014;58:4902–4903. doi: 10.1128/AAC.03157-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cheetham T.C., Lee J., Hunt C.M., Niu F., Reisinger S., Murray R., Powell G., Papay J. An automated causality assessment algorithm to detect drug-induced liver injury in electronic medical record data. Pharm. Drug Saf. 2014;23:601–608. doi: 10.1002/pds.3531. [DOI] [PubMed] [Google Scholar]
- 158.Lim R., Choundry H., Conner K., Karnsakul W. A challenge for diagnosing acute liver injury with concomitant/sequential exposure to multiple drugs: Can causality assessment scales be utilized to identify the offending drug? Case Rep. Pediatr. 2014;2014:156389. doi: 10.1155/2014/156389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Russo M.W., Hoofnagle J.H., Gu J., Fontana R.J., Barnhart H., Kleiner D.E., Chalasani N., Bonkovsky H.L. Spectrum of statin hepatotoxicity: Experience of the drug-induced liver injury network. Hepatology. 2014;60:679–686. doi: 10.1002/hep.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Veluswamy R.R., Ward S.C., Yum K., Abramovitz R.B., Isola L.M., Jagannath S., Parekh S. Adverse drug reaction: Pomalidomide-induced liver injury. Lancet. 2014;383:2125–2126. doi: 10.1016/S0140-6736(14)61030-8. [DOI] [PubMed] [Google Scholar]
- 161.Baig M., Wool K.J., Halalnych J.H., Sarmad R.A. Acute liver failure after initiation of rivaroxaban: A case report and review of the literature. N. Am. J. Med. Sci. 2015;7:407–410. doi: 10.4103/1947-2714.166221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hammerstrom A.E. Possible Amlodipine-induced hepatotoxicity after stem cell transplant. Ann. Pharm. 2015;49:135–139. doi: 10.1177/1060028014552820. [DOI] [PubMed] [Google Scholar]
- 163.Stine J.G., Intagliata N., Sha N.L., Argo C.K., Caldwell S.H., Lewis J.H., Northup P.G. Hepatic decompensation likely attributable to Simeprevir in patients with advanced cirrhosis. Dig. Dis. Sci. 2015;60:1031–1035. doi: 10.1007/s10620-014-3422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tang D.M., Koh C., Twaddell W.S., von Rosenvinge E.C., Han H. Acute hepatocellular drug-induced liver injury from bupropion and doxycycline. ACG Case Rep. J. 2015;3:66–68. doi: 10.14309/crj.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Unger C., Al-Jashaami L. Ciprofloxacin exposure leading to fatal hepatotoxicity: An unusual correlation. Am. J. Case Rep. 2016;17:676–681. doi: 10.12659/AJCR.899080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gharia B., Seegobin K., Maharaj S., Marji N., Deutch A., Zuberi L. Letrozole-induced hepatitis with autoimmune features: A rare adverse drug reaction with review of the relevant literature. Oxf. Med. Case Rep. 2017;2017:omx074. doi: 10.1093/omcr/omx074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nicoletti P., Aithal G.P., Bjornsson E.S., Andrade R.J., Sawle A., Arrese M., Barnhart H.X., Bondon-Guitton E., Hayashi P.H., Bessone F., et al. International Drug-Induced Liver Injury Consortium, Drug-Induced Liver Injury Network Investigators, and International Serious Adverse Events Consortium. Association of liver injury from specific drugs or group of drugs with polymorphisms in HLA and other genes in a Genome-wide Association Study. Gastroenterology. 2017;152:1078–1089. doi: 10.1053/j.gastro.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gayam V., Khalid M., Shrestha B., Rajib Hossain M., Dahal S., Garlapati Gill P.A., Kumar Mandal A., Sangha R. Drug-Induced Liver Injury: An institutional case series and review of literature. J. Investig. Med. High Impact Case Rep. 2018;6:1–7. doi: 10.1177/2324709618761754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hayashi P.H., Björnsson E.S. Long-term outcomes after drug-induced liver injury. Curr. Hepatol. Rep. 2018;17:292–299. doi: 10.1007/s11901-018-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Patel S., Mendler M.H., Valasek M.A., Tsunoda S.M. Drug-induced liver injury associated with the use of Everolimus in a liver transplantant patient. Case Rep. Transpl. 2018 doi: 10.1155/2018/7410508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shamberg L., Vaziri H. Hepatotoxicity of inflammatory bowel disease medications. J. Clin. Gastroenterol. 2018;52:674–684. doi: 10.1097/MCG.0000000000001084. [DOI] [PubMed] [Google Scholar]
- 172.Cirulli E.T., Nicoletti P., Abramson K., Andrade R.J., Bjornsson E.S., Chalasani N., Fontana R.J., Hallberg P., Li Y.J., Lucena M.I., et al. Drug-Induced Liver Injury Network (DILIN) investigators; International DILI consortium (iDILIC). A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;56:1707–1716. doi: 10.1053/j.gastro.2019.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nicoletti P., Aithal G.P., Chamberlain T.C., Coulthard S., Alshabeeb M., Grove J.I., Andrade R.J., Björnsson E., Dillon J.F., Hallberg P., et al. International Drug-Induced Liver Injury Consortium (iDILIC). Drug-induced liver injury due to Flucloxacillin: Relevance of multiple human leukocyte antigen alleles. Clin. Pharm. 2019;106:245–253. doi: 10.1002/cpt.1375. [DOI] [PubMed] [Google Scholar]
- 174.Sandritter T.L., Goldman J.L., Habiger C.J., Daniel J.F., Lowry J., Fisher R.T. An electronic medical records-based approach to identify idiosyncratic drug-induced liver injury in children. Sci. Rep. 2019;9:18090. doi: 10.1038/s41598-019-54075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shumar J., Ordway S., Junga Z., Sadowski B., Torres D. Memantine-induced liver injury with probable causality as assessed using the Roussel Uclaf Causality Assessment Method (RUCAM) ACG Case Rep. J. 2019;6:e00184. doi: 10.14309/crj.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tsung I., Dolan R., Lao C.D., Fecher L., Riggenbach K., Yeboah-Korang A., Fontana R.J. Liver injury is most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment. Pharm. 2019;50:800–808. doi: 10.1111/apt.15413. [DOI] [PubMed] [Google Scholar]
- 177.Xie C., Abdullah H., Abdallah M., Quist E., Niazi M. Anastrozole-induced liver injury after a prolonged latency: A very rare complication of a commonly prescribed medication. BMJ Case Rep. 2019;12:e231741. doi: 10.1136/bcr-2019-231741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ghabril M., Gu J., Yoder L., Corbito L., Dakhoul L., Ringel A., Beyer C.D., Vuppalanchi R., Barnhart H., Hayashi P.H., et al. Significant medical comorbidities are associated with lower causality scores in patients presenting with suspected drug-induced liver injury. Clin. Transl. Gastroenterol. 2020;11:e00141. doi: 10.14309/ctg.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mehershahi S., Mantri N., Kumar A., Danial S., Harish P. Enoxaparin-induced liver injury. Case Rep. Gastroenterol. 2020;14:315–319. doi: 10.1159/000508471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mullins C., Beaulac K., Sylvia L. Drug-induced liver injury (DILI) with Micafungin: The importance of causality assessment. Ann. Pharm. 2020;54:526–532. doi: 10.1177/1060028019892587. [DOI] [PubMed] [Google Scholar]
- 181.Smith R.J., Bertilone C., Robertson A.G. Fulminant liver failure and transplantation after use of dietary supplements. Med. J. Aust. 2016;204:30–32. doi: 10.5694/mja15.00816. [DOI] [PubMed] [Google Scholar]
- 182.Stadlbauer V., Fickert P., Lackner C., Schmerlaib J., Krisper P., Trauner M., Stauber E.R. Hepatotoxicity of NONI juice: Report of two cases. World J. Gastroenterol. 2005;11:4758–4760. doi: 10.3748/wjg.v11.i30.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Barcelos S.T.A., Dall’Oglio V.M., de Araújo A., Schmidt Cerski C.T. Sinusoidal obstruction syndrome secondary the intake of Senecio brasiliensis: A case report. Ann. Hepatol. 2019 doi: 10.1016/j.aohep.2019.08.009. in press. [DOI] [PubMed] [Google Scholar]
- 184.Yuen M.F., Tam S., Fung J., Wong D.K.H., Wong B.C.Y., Lai C.L. Traditional Chinese Medicine causing hepatotoxicity in patients with chronic hepatitis B infection: A 1-year prospective study. Aliment. Pharm. 2006;24:1179–1186. doi: 10.1111/j.1365-2036.2006.03111.x. [DOI] [PubMed] [Google Scholar]
- 185.Cheung W.I., Tse M.L., Ngan T., Lin J., Lee W.K., Poon W.T., Mak T.W., Leung V.K.S., Chau T.N. Liver injury associated with the use of Fructus Psoraleae (Bol-gol-zhee or Bu-gu-zhi) and its related proprietary medicine. Clin. Toxicol. 2009;47:683–685. doi: 10.1080/15563650903059136. [DOI] [PubMed] [Google Scholar]
- 186.Chau T.N., Cheung W.I., Ngan T., Lin J., Lee K.W.S., Poon W.T., Leung V.K.S., Mak T., Tse M.L., the Hong Kong Herb-Induced Liver Injury Network (HK-HILIN) Causality assessment of herb-induced liver injury using multidisciplinary approach and the Roussel Uclaf Causality Assessment Method (RUCAM) Clin. Toxicol. 2011;49:34–39. doi: 10.3109/15563650.2010.537662. [DOI] [PubMed] [Google Scholar]
- 187.Lin G., Wang J.Y., Li N., Li M., Gao H., Ji Y., Zhang F., Wang H., Zhou Y., Ye Y., et al. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J. Hepatol. 2011;54:666–673. doi: 10.1016/j.jhep.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 188.Gao H., Li N., Wang J.Y., Zhang S.C., Lin D. Definitive diagnosis of hepatic sinusoidal obstruction syndrome induced by pyrrolizidine alkaloids. J. Dig. Dis. 2012;13:33–39. doi: 10.1111/j.1751-2980.2011.00552.x. [DOI] [PubMed] [Google Scholar]
- 189.Lai R.T., Wang H., Gui H.L., Ye M.Z., Dai W.J., Xiang X., Zhao G.D., Wang W.J., Xie Q. Clinical and pathological features in 138 cases of drug-induced liver injury. Zhonghua Gan Zang Bing Za ZhiZhonghua Ganzangbing Zazhi Chin. J. Hepatol. 2012;20:185–189. doi: 10.3760/cma.j.issn.1007-3418.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 190.Dong H., Slain D., Cheng J., Ma W., Liang W. Eighteen cases of liver injury following ingestion of Polygonum multiflorum. Complement. Med. 2014;22:70–74. doi: 10.1016/j.ctim.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 191.Gao H., Ruan J.Q., Chen J., Li N., Ke C.Q., Ye Y., Lin G., Wang J.Y. Blood pyrrole-protein adducts as diagnostic and prognostic index in pyrrolizidine alkaloid-hepatic sinusoidal obstruction syndrome. Drug Des. Dev. 2015;9:4861–4868. doi: 10.2147/DDDT.S87858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang J., Ma Z., Niu M., Li N., Meng Y., Li Q., Qin L., Teng G., Cao J., Li B., et al. Evidence chain-based causality identification in herb-induced liver injury: Exemplification of a well-known liver-restorative herb Polygonum multiflorum. Front. Med. 2015;9:457–467. doi: 10.1007/s11684-015-0417-8. [DOI] [PubMed] [Google Scholar]
- 193.Zhu Y., Liu S.H., Wang J.B., Song H.B., Li Y.G., He T.T., Ma X., Wang Z.X., Wang L.P., Zhou K., et al. Clinical analysis of drug-induced liver injury caused by Polygonum multiflorum and its preparations. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35:1442–1447. (Abstract in English, Article in Chinese) [PubMed] [Google Scholar]
- 194.Zhang P., Ye Y., Yang X., Jiao Y. Systematic review on Chinese herbal medicine induced liver injury. Evid.-Based Complement. Altern. Med. 2016 doi: 10.1155/2016/3560812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li C.Y., He Q., Gao D., Li R.Y., Zhu Y., Li H.F., Feng W.W., Yang M.H., Xiao X.H., Wang J.B. Idiosyncratic drug-induced liver injury linked to Polygonum multiflorum: A case study by pharmacognosy. Chin. Integr. Med. 2017;23:625–630. doi: 10.1007/s11655-017-2543-9. [DOI] [PubMed] [Google Scholar]
- 196.Chow H.C., So T.H., Choi H.C.W., Lam K.O. Medicine herbs-induced liver injury from an oncological perspective with RUCAM. Integr. Cancer. 2019;18:1–13. [Google Scholar]
- 197.Jing J., Wang R.L., Zhao X.Y., Zhu Y., Niu M., Wang L.F., Song X.A., He T.T., Sun Y.Q., Xu W.-T., et al. Association between the concurrence of pre-existing chronic liver disease and worse prognosis in patients with an herb-Polygonum multiflorum thunbinduced liver injury: A case-control study from a specialised liver disease center in China. BMJ Open. 2019;9:e023567. doi: 10.1136/bmjopen-2018-023567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li A., Gao M., Zhao N., Li P., Zhu J., Li W. Acute liver failure associated with Fructus Psoraleae: A case report and literature review. BMC Complement. Altern. Med. 2019;19:84. doi: 10.1186/s12906-019-2493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ni J., Liu Y., Wang W., Sun M., Ma B., Pang L., Du Y., Dong X., Yin X. Polygonum multiflorum-induced liver injury: Clinical characteristics, risk factors, material basis, action mechanism and current challenges. Front. Pharmacol. 2019;10:1467. doi: 10.3389/fphar.2019.01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tan Y., Chen H., Zhou X., Sun L. RUCAM-based assessment of liver injury by xian-tian-guo (Swietenia macrophylla) seeds, a plant used for treatment of hypertension and diabetes. Ann. Hepatol. 2019;18:403–407. doi: 10.1016/j.aohep.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 201.Zhu Y., Niu M., Wang J.B., Wang R.L., Li J.Y., Ma Y., Zhao Y.L., Zhang Y.F., He T.T., Yu S.M., et al. Predictors of poor outcomes in 488 patients with herb-induced liver injury. Turk. J. Gastroenterol. 2019;30:47–58. doi: 10.5152/tjg.2018.17847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gao Y., Wang Z., Tang J., Liu X., Shi W., Qin N., Wang X., Pang Y., Li R., Zhang Y., et al. New incompatible pair of TCM: Epimedii Folium combined with Psoraleae Fructus induces idiosyncratic hepatotoxicity under immunological stress conditions. Front. Med. 2020;28 doi: 10.1007/s11684-019-0690-z. [DOI] [PubMed] [Google Scholar]
- 203.Xia C., Liu Y., Yao H., Zhu W., Ding J., Jin J. Causality assessment of skyfruit-induced liver injury using the updated RUCAM: A case report and review of the literature. J. Int. Med. Res. 2020;48:300060520917569. doi: 10.1177/0300060520917569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cárdenas A., Restrepo J.C., Sierra F., Correa G. Acute hepatitis due to shen-min: A herbal product derived from Polygonum multiflorum. J. Clin. Gastroenterol. 2006;40:629–632. doi: 10.1097/00004836-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 205.Parlati L., Voican C.S., Perlemuter K., Perlemuter G. Aloe vera-induced acute liver injury: A case report and literature review. Clin. Res. Hepatol. Gastroenterol. 2017;41:e39–e42. doi: 10.1016/j.clinre.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 206.Teschke R., Bahre R. Severe hepatotoxicity by Indian Ayurvedic herbal products: A structured causality assessment. Ann. Hepatol. 2009;8:258–266. doi: 10.1016/S1665-2681(19)31777-6. [DOI] [PubMed] [Google Scholar]
- 207.Teschke R., Glass X., Schulze J. Herbal hepatotoxicity by Greater Celandine (Chelidonium majus): Causality assessment of 22 spontaneous reports. Regul. Toxicol. Pharm. 2011;61:282–291. doi: 10.1016/j.yrtph.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 208.Teschke R., Glass X., Schulze J., Eickhoff A. Suspected Greater Celandine hepatotoxicity: Liver specific causality evaluation of published case reports from Europe. Eur. J. Gastroenterol. Hepatol. 2012;24:270–280. doi: 10.1097/MEG.0b013e32834f993f. [DOI] [PubMed] [Google Scholar]
- 209.Douros A., Bronder E., Andersohn F., Klimpel A., Kreutz R., Garbe E., Bolbrinker J. Herb-induced liver injury in the Berlin Case-Control Surveillance Study. Int. J. Mol. Sci. 2016;17:114. doi: 10.3390/ijms17010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Teschke R., Zhang L., Long H., Schwarzenboeck A., Schmidt-Taenzer W., Genthner A., Wolff A., Frenzel C., Schulze J., Eickhoff A. Traditional Chinese Medicine and herbal hepatotoxicity: A tabular compilation of reported cases. Ann. Hepatol. 2015;14:7–19. doi: 10.1016/S1665-2681(19)30796-3. [DOI] [PubMed] [Google Scholar]
- 211.Melchart D., Hager S., Albrecht S., Dai J., Weidenhammer W., Teschke R. Herbal Traditional Chinese Medicine and suspected liver injury: A prospective study. World J. Hepatol. 2017;18:1141–1157. doi: 10.4254/wjh.v9.i29.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Diener H.C., Freitag F.G., Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Ceph. Rep. 2018;1:1–8. doi: 10.1177/2515816318759304. [DOI] [Google Scholar]
- 213.Anderson N., Borlak J. Hepatobiliary events in migraine therapy with herbs—The case of Petadolex, a Petasites hybridus extract. J. Clin. Med. 2019;8:652. doi: 10.3390/jcm8050652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gerhardt F., Benesic A., Tillmann H.L., Rademacher S., Wittekind C., Gerbes A.L., Henker R., Berg T., Maidhof H., Trauer H., et al. Iberogast-induced acute liver failure-reexposure and in vitro assay support causality. Am. J. Gastroenterol. 2019;114:1358–1359. doi: 10.14309/ajg.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 215.Teschke R., Xuan T.D. Suspected herb induced liver injury by green tea extracts: Critical review and case analysis applying RUCAM for causality assessment. Jpn. J. Gastroenterol. Hepatol. 2019;1:1–16. [Google Scholar]
- 216.Philips C.A., Paramaguru R., Joy A.K., Anthony K.L., Augustine P. Clinical outcomes, histopathological patterns, and chemical analysis of Ayurveda and herbal medicine associated with severe liver injury—A single-center experience from southern India. Indian J. Gastroenterol. 2018;37:9–17. doi: 10.1007/s12664-017-0815-8. [DOI] [PubMed] [Google Scholar]
- 217.Philips C.A., Augustine P., Rajesh S., Kumar P., Madhu D. Complementary and alternative medicine-related drug-induced liver injury in Asia. J. Clin. Transl. Hepatol. 2019;7:263. doi: 10.14218/JCTH.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lapi F., Gallo E., Giocalliere E., Vietri M., Baronti R., Pieraccini G., Tafi A., Menniti-Ippolito F., Mugelli A., Firenzuoli F., et al. Acute liver damage due to Serenoa repens: A case report. Br. J. Clin. Pharm. 2010;69:558–560. doi: 10.1111/j.1365-2125.2010.03618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mazzanti G., Di Soto A., Vitalone A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015;89:1175–1191. doi: 10.1007/s00204-015-1521-x. [DOI] [PubMed] [Google Scholar]
- 220.Sáez-González E., Conde I., Díaz-Jaime F.C., Benlloch S., Prieto M., Berenguer M. Iberogast-induced severe hepatotoxicity leading to liver transplantation. Am. J. Gastroenterol. 2016;111:1364–1365. doi: 10.1038/ajg.2016.260. [DOI] [PubMed] [Google Scholar]
- 221.Mazzanti G., Moro P.A., Raschi E., Da Cas R., Menniti-Ippolito F. Adverse reactions to dietary supplements containing red yeast rice: Assessment of cases from the Italian surveillance system: Safety of red yeast rice dietary supplements. Br. J. Clin. Pharm. 2017;83:894–908. doi: 10.1111/bcp.13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Osborne C.S., Overstreet A.N., Rockey D.C., Shreiner A.D. Drug-induced liver injury caused by Kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J. Investig. Med. High Impact Case Rep. 2019;7 doi: 10.1177/2324709619826167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tsuda T., Yashiro S., Gamo Y., Watanabe K., Hoshino T., Oikawa T., Hanawa T. Discrepancy between clinical course and drug-induced lymphocyte stimulation tests in a case of Saireito-induced liver injury accompanied by Sjögren syndrome. J. Altern. Complement. Med. 2010;16:501–505. doi: 10.1089/acm.2009.0183. [DOI] [PubMed] [Google Scholar]
- 224.Hisamochi A., Kage M., Arinaga T., Ide T., Miyajima I., Ogata K., Kuwhara T., Koga Y., Kumashiro R., Sata M. Drug-induced liver injury associated with Agaricus blazei Murill which is very similar to autoimmune hepatitis. Clin. J. Gastroenterol. 2013;6:139–144. doi: 10.1007/s12328-013-0359-0. [DOI] [PubMed] [Google Scholar]
- 225.Ahn B.M. Herbal preparation-induced liver injury. Korean J. Gastroenterol. 2004;44:113–125. [PubMed] [Google Scholar]
- 226.Seo J.C., Jeon W.J., Park S.S., Kim S.H., Lee K.M., Chae H.B., Park S.M., Youn S.J. Clinical experience of 48 acute toxic hepatitis patients. Korean J. Hepatol. 2006;12:74–81. (Abstract in English, Article in Korean) [PubMed] [Google Scholar]
- 227.Kang S.H., Kim J.I., Jeong K.H., Ko K.H., Ko P.G., Hwang S.W., Kim E.M., Kim S.H., Lee H.Y., Lee B.S. Clinical characteristics of 159 cases of acute toxic hepatitis. Korean J. Hepatol. 2008;14:483–492. doi: 10.3350/kjhep.2008.14.4.483. (Abstract in English, Article in Korean) [DOI] [PubMed] [Google Scholar]
- 228.Sohn C.H., Cha M.I., Oh B.J., Yeo W.H., Lee J.H., Kim W., Lim K.S. Liver transplantation for acute toxic hepatitis due to herbal medicines and preparations. J. Korean Soc. Clin. Toxicol. 2008;6:110–116. (Abstract in English, Article in Korean) [Google Scholar]
- 229.Kang H.S., Choi H.S., Yun T.J., Lee K.G., Seo Y.S., Yeon J.E., Byun K.S., Um H.S., Kim C.D., Ryu H.S. A case of acute cholestatic hepatitis induced by Corydalis speciosa Max. Korean Hepatol. 2009;15:517–523. doi: 10.3350/kjhep.2009.15.4.517. (Abstract in English, article in Korean) [DOI] [PubMed] [Google Scholar]
- 230.Kim S.Y., Yim H.J., Ahn J.H., Kim J.H., Kim J.N., Yoon I., Kim D.I., Lee H.S., Lee S.W., Choi J.H. Two cases of toxic hepatitis caused by arrowroot juice. Korean J. Hepatol. 2009;15:504–509. doi: 10.3350/kjhep.2009.15.4.504. (Abstract in English, Article in Korean) [DOI] [PubMed] [Google Scholar]
- 231.Bae S.H., Kim D.H., Bae Y.S., Lee K.J., Kim D.W., Yoon J.B., Hong J.H., Kim S.H. Toxic hepatitis associated with Polygoni multiflori. Korean J. Hepatol. 2010;16:182–186. doi: 10.3350/kjhep.2010.16.2.182. (Abstract in English, Article in Korean) [DOI] [PubMed] [Google Scholar]
- 232.Yang H., Kim D.J., Kim Y.M., Kim B.H., Sohn K.M., Choi M.J., Choi Y.H. Aloe-induced toxic hepatitis. J. Korean Med. Sci. 2010;25:492–495. doi: 10.3346/jkms.2010.25.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jung K.A., Min H.J., Yoo S.S., Kim H.J., Choi S.N., Ha C.Y., Kim H.J., Kim T.H., Jung W.T., Lee O.J. Drug-induced liver injury: Twenty five cases of acute hepatitis following ingestion of Polygonum multiflorum Thun. Gut Liver. 2011;5:493–499. doi: 10.5009/gnl.2011.5.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kim Y.J., Ryu S.L., Shim J.W., Kim D.S., Shim J.Y., Park M.S., Jung H.L. A pediatric case of toxic hepatitis induced by Hovenia dulcis. Pediatr. Gastroenterol. Hepatol. Nutr. 2012;15:111–116. doi: 10.5223/pghn.2012.15.2.111. [DOI] [Google Scholar]
- 235.Lee J., Shin J.S., Kim M.R., Byon J.H., Lee S.Y., Shin Y.S., Kim H., Park K.B., Shin B.C., Lee M.S., et al. Liver enzyme abnormalities in taking traditional herbal medicine in Korea: A retrospective large sample cohort study of musculoskeletal disorder patients. J. Ethnopharmacol. 2015;169:407–412. doi: 10.1016/j.jep.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 236.Lee W.J., Kim H.W., Lee H.Y., Son C.G. Systematic review on herb-induced liver injury in Korea. Food Chem. Toxicol. 2015;84:47–54. doi: 10.1016/j.fct.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 237.Cho J.H., Oh D.S., Hong S.H., Ko H., Lee N.H., Park S.E., Han C.W., Kim S.M., Kim Y.C., Kim K.S. A nationwide study of the incidence rate of herb-induced liver injury in Korea. Arch. Toxicol. 2017;91:4009–4015. doi: 10.1007/s00204-017-2007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Teo D.C.H., Ng P.S.L., Tan S.H., Lim A.T., Toh D.S.L., Chan S.Y., Cheong H.H. Drug-induced liver injury associated with Complementary and Alternative Medicine: A review of adverse event reports in an Asian community from 2009 to 2014. BMC Complement. Altern. Med. 2016;16:192. doi: 10.1186/s12906-016-1168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Awortwe C., Makiwane M., Reuter H., Muller C., Louw J., Rosenkranz B. Critical evaluation of causality assessment of herb-drug interactions in patients. Br. J. Clin. Pharm. 2018;84:679–693. doi: 10.1111/bcp.13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jimenez-Saenz M., Martinez-Sanchez C. Acute hepatitis associated with the use of green tea infusions. J. Hepatol. 2006;44:616–617. doi: 10.1016/j.jhep.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 241.García-Cortés M., Borraz Y., Lucena M.I., Paláez G., Salmerón J., Diago M., Martínez-Sierra J.M., Navarro J.M., Planas M.J., Bruguera M., et al. Liver injury induced by “natural remedies”: An analysis of cases submitted to the Spanish Liver Toxicity Registry. Rev. Esp. Enferm. Dig. 2008;100:688–695. doi: 10.4321/s1130-01082008001100004. [DOI] [PubMed] [Google Scholar]
- 242.Medina-Caliz I., Garcia-Cortes M., Gonzalez-Jimenez A., Cabello M.R., Robles-Diaz M., Sanabria-Cabrera J., Sanjuan-Jimenez R., Ortega-Alonso A., Garcia-Munoz B., Moreno I., et al. Herbal and dietary supplement-induced liver injuries in the Spanish DILI Registry. Clin. Gastroenterol. Hepatol. 2018;16:1495–1502. doi: 10.1016/j.cgh.2017.12.051. [DOI] [PubMed] [Google Scholar]
- 243.Björnsson E., Olsen R. Serious adverse liver reactions associated with herbal weight-loss supplements. J. Hepatol. 2007;47:295–302. doi: 10.1016/j.jhep.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 244.Ruperti-Repilado F.J., Haefliger S., Rehm S., Zweier M., Rentsch K.M., Blum J., Jetter A., Heim M., Leuppi-Taegtmeyer A., Terracciano L., et al. Danger of herbal tea: A case of acute cholestatic hepatitis due to Artemisia annua tea. Front. Med. 2019;6:221. doi: 10.3389/fmed.2019.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yilmaz B., Yilmaz B., Aktaş B., Unlu O., Roach E.C. Lesser celandine (pilewort) induced acute toxic liver injury: The first case report worldwide. World J. Hepatol. 2015;7:285–288. doi: 10.4254/wjh.v7.i2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Papafragkakis C., Ona M.A., Reddy M., Anand S. Acute hepatitis after ingestion of a preparation of Chinese Skullcap and Black Catechu for joint pains. Case Rep. Hepatol. 2016 doi: 10.1155/2016/4356749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dalal K.K., Holdbrook T., Peikin S.R. Ayurvedic drug induced liver injury. World J. Hepatol. 2017;9:1205–1209. doi: 10.4254/wjh.v9.i31.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kesavarapu K., Kang M., Shin J.J., Rothstein K. Yogi Detox Tea: A potential cause of acute liver failure. Case Rep. Gastrointest. Med. 2017;2017:3540756. doi: 10.1155/2017/3540756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kothadia J.P., Kaminski M., Samant H., Olivera-Martinez M. Hepatotoxicity associated with use of the weight loss supplement Garcinia cambogia: A case report and review of the literature. Case Rep. Hepatol. 2018;2018:6483605. doi: 10.1155/2018/6483605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Surapaneni B.K., Le M., Jakobovits J., Vinayek R., Dutta S. A case of acute severe hepatotoxicity and mild constriction of common bile duct associated with ingestion of green tea extract: A clinical challenge. Clin. Med. Insights Gastroenterol. 2018;11:1–4. doi: 10.1177/1179552218779970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Imam Z., Khasawneh M., Jomaa D., Iftikhar H., Sayedahmad Z. Drug induced liver injury attributed to a curcumin supplement. Case Rep. Gastrointest. Med. 2019;2019:6029403. doi: 10.1155/2019/6029403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yousaf M.N., Chaudhary F.S., Hodanazari S.M., Sittambalam C.D. Hepatotoxicity associated with with Garcinia cambogia: A case report. World J. Hepatol. 2019;11:735–742. doi: 10.4254/wjh.v11.i11.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Oketch-Rabah H.A., Roe A.L., Rider C.V., Bonkovsky H.L., Giancaspro G.I., Navarro V., Paine M.F., Betz J.M., Marles R.J., Casper S., et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol. Rep. 2020;7:386–402. doi: 10.1016/j.toxrep.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Schimmel J., Dart R.C. Kratom (Mitrogyna speciosa) Liver Injury: A Comprehensive Review. Drugs. 2020 doi: 10.1007/s40265-019-01242-6. [DOI] [PubMed] [Google Scholar]
- 255.Le Louet H. Drug-Induced Liver Injury (Dili): Current Status and Future Directions for Drug Development and the Post-Marketing Setting. [(accessed on 30 June 2020)]; Available online: https://cioms.ch/wp-content/uploads/2020/06/CIOMS_DILI_Web_16Jun2020.pdf.
- 256.Aithal G.P., Rawlins M.D., Day C.P. Accuracy of hepatic adverse drug reaction reporting in one English health region. Br. Med. J. 1999;319:154. doi: 10.1136/bmj.319.7224.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Teschke R., Frenzel C., Wolff A., Eickhoff A., Schulze J. Drug induced liver injury: Accuracy of diagnosis in published reports. Ann. Hepatol. 2014;13:248–255. doi: 10.1016/S1665-2681(19)30888-9. [DOI] [PubMed] [Google Scholar]