Abstract
Simple Summary
The diamondback moth, Plutella xylostella, is a destructive pest of cruciferous crops worldwide. Integrated pest management (IPM) strategies, largely involve the use chemical pesticides which are harmful for the environment and human health. In this study, the virulence of three species of entomopathogenic fungi were tested. Metarhizium anisopliae proved to be the most effective by killing more than 90% of the population. Based on which the fungus was selected to study the host-pathogen immune interactions. More precisely, after infection, superoxide dismutase (SOD) and phenoloxidase (PO), two major enzymes involved in immune response, were studied at different time points. The fungus gradually weakened the enzyme activities as the time progressed, indicating that physiological attributes of host were adversely affected. The expression of immune-related genes (Defensin, Spaetzle, Cecropin, Lysozyme, and Hemolin) varied on different time points. Moreover, the fungus negatively impacted the development of the host by reducing the life span and egg laying ability. Thus, M. anisopliae can become a potent prospect for the control of this pest. This information will also reinforce the development of policies for biocontrol-based pest management.
Abstract
Entomopathogenic fungi are naturally existing microbes, that can serve as a key regulator of insect pests in integrated pest management strategies. Besides having no hazardous effects on the environment, these entomopathogens are alternatives to synthetic insecticides that can control notorious insect-like Plutella xylostella, a destructive pest of cruciferous crops. Three different species of entomopathogenic fungi were evaluated before the selection (high larval mortality and least LC50) of Metarhizum anisopliae. The study was designed to investigate the mortality, development, and immune responses of P. xylostella when challenged with M. anisopliae, a naturally existing soil-borne entomopathogenic fungus. M. anisopliae resulted in high pest mortality by killing 93% of larvae. However, no statistically significant effect on hemocyte concentration was observed. The activity of enzymes (Phenoloxidase and Superoxide dismutase) and immune genes (Defensin, Spaetzle, Cecropin, Lysozyme, and Hemolin) did vary at different time points (24, 48, 72 and 96 h) after exposure to M. anisopliae. Disturbance in the biological cycles of P. xylostella was also detected, significantly shorter adult life span (8.11:6.87, M:F) and reduced fecundity (101 eggs/female) were observed along with disturbed larval and pupal duration. Results suggest that M. anisopliae can efficiently hinder the P. xylostella defense and developmental system, resulting in mortality and disturbed demography.
Keywords: entomopathogenic fungi, genes, mortality, pathogenicity, demography
1. Introduction
Entomopathogenic fungi are naturally existing microbial control agents that effectively regulate the insect pest populations [1,2]. Several entomopathogenic fungi have been used to control insect pests from different orders such as Diptera [3,4], Hemiptera [5], Coleoptera [6], Homoptera [7], and Lepidoptera [8]. The excessive and injudicious use of synthetic insecticides has resulted in pest resurgence, environment degradation, resistance development while also causing harmful effects to human health [9,10,11]. Such detrimental effects of chemical insecticides stressed the need to develop alternative control strategies. Furthermore, the resistance to insecticide curbed the control strategies, whereas insect pests are unable to develop resistance against entomopathogenic fungi making them an effective weapon against resilient pests [12,13,14,15,16].
Plutella xylostella, one of the most destructive lepidopteran pests of cruciferous crops, has caused severe economic damages (quantitative and qualitative), with an annual cost estimated to be USD 4–5 billion [17,18,19,20]. Over the years the pest has developed resistance against many control agents including dichloro-diphenyl-trichloroethane (DTT) and Bacillus thuringiensis (Bt) [21,22,23] making it difficult to control while also emphasizing the need to develop alternative control strategies. Among all the current strategies, biological control represents an eco-friendly approach with no hazardous effects on human health. Entomopathogenic fungi are biological control agents which are cosmopolitan in nature [1,24,25,26,27,28]. Metarhizium anisopliae, a soil-borne entomopathogenic fungus, represents an ecologically safe alternative to chemical pesticides [29]. The entomopathogen has proven to be effective against lepidopteran pests such as Helicoverpa armigera [30] and Spodoptera exigua [31]. M. anisopliae produces secondary toxins such as Destruxin A and E to repress the host immune responses while also deploying evasion protein such as Mcl1 protein (Metarhizium collagen-like protein) to avoid detection [32,33]. In response to the invasion of microbes, an array of recognition molecules detects the pathogen, resulting in the initiation of Toll and immune deficiency (Imd) pathways, that regulate anti-fungal and antibacterial defenses respectively [34,35]. Spaetzle, a gene encoding toll activating protease, initiates the immune pathway. Defensins are antimicrobial peptides responding to pathogenic challenges or injury. Similarly, cecropins constitute a major part of the insect innate immune system. Antioxidant enzymes such as superoxide dismutase (SOD), a key factor in host defense system, function in melanization and phagocytosis [36,37]. Likewise, Phenoloxidase (PO) is a key enzyme in the melanization cascade that also participates in cuticle sclerotization and wound healing [38,39]. In this study, we investigated the efficacy of M. anisopliae against P. xylostella. The present study aims to explore the interaction of entomopathogenic fungi with its insect host and elaborate the immune and developmental changes after the infection.
2. Materials and Methods
2.1. Insect and Fungi Culture
The population of P. xylostella was taken from the Institute of Plant Protection, Guangdong Academy of Agricultural Sciences, China by the Engineering Research Centre of Biological Control Ministry of Education, South China Agricultural University, Guangzhou, Guangdong Province, P. R. China. The colony was maintained in a pathogen-free environment. Larvae were kept at 25 ± 1 °C with a light: dark cycle of 16:8 h and 60–70% relative humidity [34]. Three different entomopathogenic fungi were obtained from the Laboratory of Insect Microbiology and Biotechnology, Bahauddin Zakariya University, Multan, Pakistan (Table 1), and screened against P. xylostella. To prevent aging, isolates were passage through the host [40]. Monoconidial culture (14 days) grown on potato dextrose agar (PDA) was harvested with a disinfected spatula in 0.05% Tween-80 (Sigma-P1754) solution [10,41]. The calculation of spores was done by using a hemocytometer [42,43]. Stock solutions were kept at 4 °C and used in serial dilution for making the desired concentration of entomopathogenic fungi.
Table 1.
Isolates of entomopathogenic fungi from Pakistan.
| Fungi | Source | Location (Pakistan) | Coordinates |
|---|---|---|---|
| Metarhizium anisopliae | Soil | Multan, Punjab | 30°05′11.65″ N 71°39′15.65″ E |
| Beauveria bassiana | Soil | Multan, Punjab | 30°05′11.65″ N 71°39′15.65″ E |
| Isaria fumosorosea | Soil | Multan, Punjab, | 30°05′11.65″ N 71°39′15.65″ E |
2.2. Screening of Entomopathogenic Fungi
Three different entomopathogenic fungi were screened out against P. xylostella. Five concentrations (4 × 10⁸, 4 × 107, 4 × 106, 4 × 105, 4 × 104 spores/mL) were prepared (hit and trial method) while aqueous 0.05% Tween-80 (Sigma-P1754) was taken as control [41]. The application of entomopathogenic fungi was done by dipping the larvae in desired concentrations. After dipping, larvae were placed on filter paper for drying and then placed in disinfected plastic dishes (5 cm diameter) [34]. Fifteen larvae (3rd instar neonates) were exposed to each concentration. The experiment was repeated four times. A sufficient amount of diet was provided throughout the experimentation. Larval mortality was recorded every 24 h for seven days. Larvae without movements were considered dead.
2.3. Isolate Selection
Lethal and sublethal doses were calculated from the pre experimentation data. Isolate having the least LC50 with maximum mortality was selected for the downstream application.
2.4. Experimental Validation of Lethal (LC50) and Sublethal (LC20) Concentrations
Calculated lethal and sub-lethal concentrations were validated. Experimentation was carried out by following similar methodology described above. Each treatment included 15 larvae (3rd instar neonates) of P. xylostella. The experiment was replicated four times.
2.5. Entomopathogenic Fungi Effect on Hemocyte Concentration of P. xylostella
The hemocyte concentration in P. xylostella larvae was calculated on lethal (LC50) concentration of M. anisopliae at different time points (24, 48, 72, and 96 h). Larvae were surface sterilized with ethanol (70%) and rinsed with double distilled water. Hemolymph was collected by dissection through proleg (30 larvae) using a sterilized blade and collected via glass capillary. Hemolymph was mixed with an equal amount of anticoagulant (98 mM NaOH, 186 mM NaCl, 17 mM Na2 EDTA, and 41 mM citric acid, pH 4.5). Hemocyte concentrations were quantified using a hemocytometer with 10 μL under a microscope [44]. The experiment was replicated four times.
2.6. PO and SOD Activity in P. xylostella Larvae
Hemolymph was collected from 30 treated larvae. The collection was done after 24, 48, 72, and 96 h post-infection. Collected hemolymph was diluted ten times and studied under a microplate reader (BIO-RAD). PO activity was checked using L-dihydroxyphenylalanine (L-DOPA) as the substrate on the initial linear increase in absorbance at 490 nm [23]. SOD activities were observed using respective kits following the manufacturer’s instructions (Suzhou Comin Biotech Co., Ltd., Suzhou, China). SOD activity was checked at a wavelength of 560 nm via a light reduction of nitro blue tetrazolium (NBT). NBT reduction (50%) is the quantity of enzyme for each unit of SOD. Units/mg protein was used for both enzyme activities.
2.7. Effect of M. anisopliae on the Expression of Immune-Related Genes in P. xylostella
After infection of M. anisopliae, quantitative real-time PCR (qRT-PCR) was used to investigate the expression of immune-related genes (Cecropin, Defensin, Attacin, and Spaetzle). Total RNA was extracted from hemolymph and reverse-transcribed in a 25-uL reaction according to the manufacturer guideline (TaKaRa, Beijing, China). After reverse transcription, qRT-PCR was done by using (Bio-Rad iQ2 optical system (BioRad) with SsoFast Eva Green Supermix (Bio-Rad, Hercules, CA, USA). The working program was set as 95 °C for 2 min, and 40 cycles of 95 °C for 5 s, and 60 °C for 10 s, melting curve from 65 to 95 °C [45]. The expression of β-actin was selected to normalize the expression of the immune-related genes according to the 2−ΔΔCt method by Pfaffl, 2001 [46]. Three replicates were used in all experiments. Gene-specific primers were designed using Primer Premier 5. The gene sequences were subjected to Primer BLAST (www.ncbi.com) to check them for specificity The primers used are given in Table S1 [47].
2.8. Effects of Lethal Concentration of M. anisopliae on Biological Parameters of P. xylostella
The effects of lethal concentration of M. anisopliae was evaluated against P. xylostella. Each replication consists of 30 larvae of 3rd instar. For the treatment of fungi, the dip method was used. Petri dishes (diameter 5 cm) cleaned (70% ethanol) and air-dried for treating the larvae. Newly emerged cabbage leaves which were gently washed with double distilled water and air-dried served as larval diet. On emergence, the adults were paired (1 pair/cage) in plastic cages (cleaned with 70% ethanol and air-dried) for egg-laying. Sugar solution (10%) was provided as an adult diet. Whatman filter tape was used as an egg-laying pad. Eggs were counted under a microscope and were placed in plastic boxes (15 cm × 10 cm × 5 cm) for hatching. Data were collected every 12 h until the end of the experiment. Immobile larvae were considered dead and placed in a humid chamber for conidial growth observation [48].
2.9. Statistical Analysis
Mortality data were analyzed by using Probit analysis [49]. Abbott formula was used for the correction of mortality [50,51]. Lethal and sublethal concentrations for all entomopathogenic fungi were calculated by using SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and Polo Pc (Petaluma, CA, Canada) [52]. One-way ANOVA was used for mortality data, means were separated by Tukey’s HSD test with a 5% level of significance (p < 0.05) [53]. Demographic data were analyzed by using Statistics 8.01 [53].
The hemocytes concentration and enzymatic activities (SOD and PO) after the treatment of M. anisopliae were analyzed by t-test. The relative expression of the selected immune genes was also analyzed via a t-test with a significance level set as p < 0.05.
3. Results
3.1. Screening of Entomopathogenic Fungi
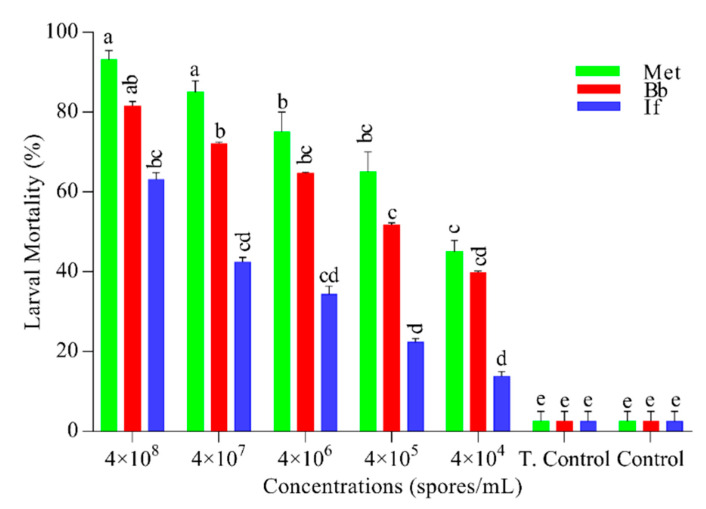
The results were highly concentration-dependent (Figure 1). M. anisopliae showed the highest mortality (93.13%) at 4 × 10⁸ (spores/mL) followed by B. bassiana (81.51%) while the least mortality (77.52%) was observed in I. fumosorosea (F = 89, DF = 4, p = 0.001). Mortality in control and tween control was insignificant. For confirmation of fungal pathogenicity, the carcasses were placed in a humid chamber for the growth of conidia (Figure 2).
Figure 1.
Larvicidal activity of entomopathogenic fungi. Mortality was recorded until seven days after every 24 h. Green, red, and blue bars represent the larval mortality of Plutella xylostella, after exposure to different concentrations of Metarhizium anisopliae (Met), Beauveria bassiana (Bb), and Isaria fumosorosea (If) respectively. While T. Control is tween control (0.05% aqueous diluted) and in Control, distilled water was used. Error bars show 95% confidence intervals (CI). Letters indicate significant differences at p < 0.05.
Figure 2.
Conidial growth of Metarhizium anisopliae on larvae of Plutella xylostella. Larvae were placed in a humid chamber for confirmation of death due to fungi. (A) Conidial growth over the full body of the dead larvae. (B) Zoomed in the image of the conidia growing on the head of the dead larvae. (C) Conidial growth around the head and thorax of larvae. (D) Zoomed in the image of conidial growth adjacent to the head.
3.2. Selection of Entomopathogenic Fungi
Based on pre experimentation data lethal (LC50) and sublethal (LC20) doses were calculated with (95% CL). M. anisopliae was selected on the basis of designed criteria (Table 2) as it showed the highest percent mortality and least LC50.
Table 2.
Lethal and sublethal concentrations of fungi against Plutella xylostella.
| Fungi | LC50 | LC20 | Slop ± SE | χ2 | p-Value | df |
|---|---|---|---|---|---|---|
| Metarhizium anisopliae | 6.2 × 104 | 2.3 × 102 | 0.29 ± 0.044 | 2.1 | 0.001 | 4 |
| Beauveria bassiana | 9.3 × 105 | 3.1 × 103 | 0.38 ± 0.044 | 1.1 | 0.003 | 4 |
| Isaria fumosorosea | 7.9 × 106 | 4.6 × 104 | 0.42 ± 0.046 | 1.8 | 0.002 | 4 |
3.3. Experimental Validation of Lethal (LC50) and Sublethal (LC20) Concentrations of M. anisopliae
Lethal and sublethal concentrations of M. anisopliae were validated, showing 51.74% and 32.11% larval mortality respectively (Supplementary Figure S1).
3.4. Effects of M. anisopliae on Hemocyte Concentration of P. xylostella
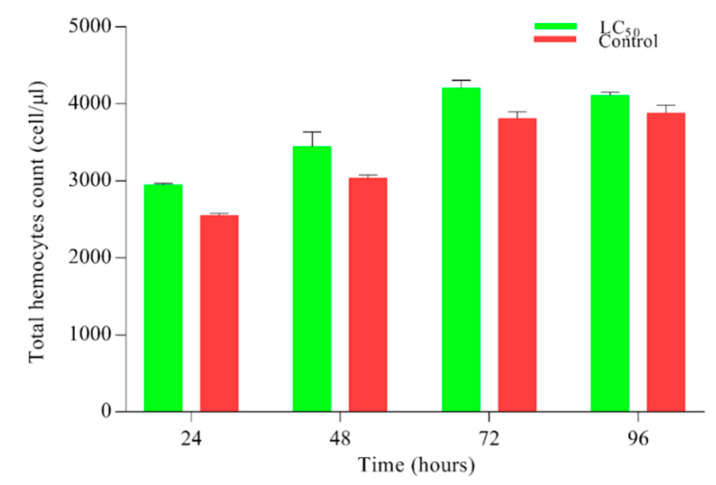
After infecting the larvae with lethal (LC50) concentration of M. anisopliae, results for hemocyte count are shown (Figure 3). Non-significant (0.071) results were observed regarding the hemocytes count on all time points.
Figure 3.
Hemocytes count in Plutella xylostella larvae after treatment with lethal (LC50) concentration of Metarhizium anisopliae. Green bars represent the hemocytes count of larvae under the pressure of LC50 while red bars show tween control (0.05% aqueous diluted) hemocytes count. Results were statistically non-significant p < 0. 071. Error bars show 95% confidence intervals (CI).
3.5. Effects of M. anisopliae (LC50) on the Activity of PO and SOD in Larvae of P. xylostella
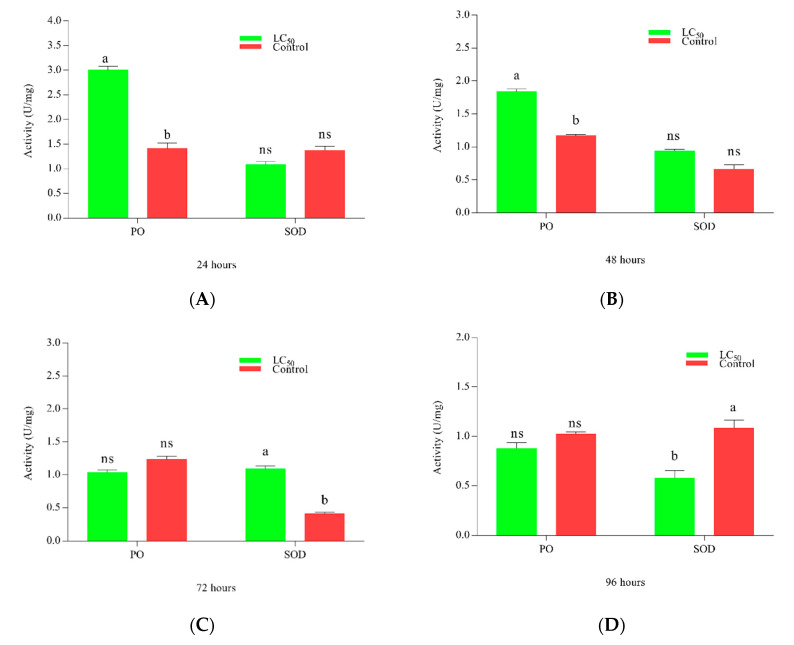
The activities of immune enzymes PO and SOD against LC50 of M. anisopliae are shown in Figure 4. PO activity peaked at 24 h, equaling control at 48 h while decreasing later on 48 and 72 h. SOD activity amplified around 72 h post-treatment. Figure 4A–D shows the activity of enzymes at 24, 48, 72, and 96 h respectively.
Figure 4.
Activity of phenoloxidase (PO) and superoxide dismutase (SOD) activity in Plutella xylostella against LC50 of Metarhizium anisopliae. Time-dependent activity is shown in the figure, the green color bar shows the activity of enzymes whereas the red bar represents the control. (A–D) shows the activities of enzymes after 24, 48, 72, and 96 h respectively. Error bars show 95% confidence intervals (CI). Letters indicate significant differences at p < 0.005. (ns = non-significant).
3.6. Effect of Lethal Concentration of M. anisopliae on Immune Genes of P. xylostella
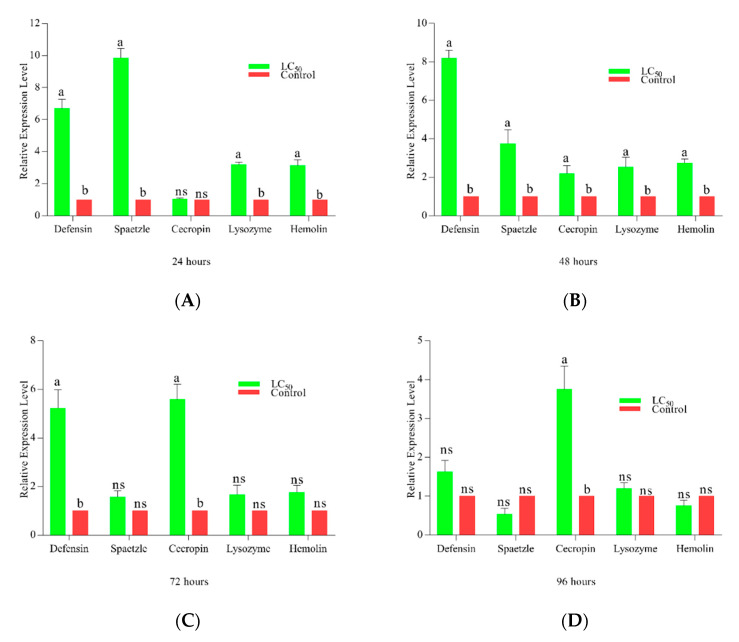
The expression of all immune genes was predominantly time-dependent (Figure 5), with a significance level of 0.001. Defensin showed a substantial increase in expression after 24 h of treatment compared to control followed by a decrease at 72 h ultimately approaching non-significant expression at 96 h. Spaetzle showed the highest expression at 24 h post-treatment among all the genes, gradually decreasing after 48 h and keep on decreasing until 96 h. Cecropin expression level increased significantly until 72 h while decreasing later at 96 h. Hemolin and lysozyme, both genes showed similar trends at 24 and 48, but both showed non-significant activity after 72- and 96-h post-treatment
Figure 5.
Immune genes expression in Plutella xylostella against LC50 of Metarhizium anisopliae. Time-dependent activity is shown in the figure, the green color bar shows the activity of gene at LC50 of M. anisopliae while the red bar shows the control. (A–D) shows the activities of enzymes after 24, 48, 72, and 96 h respectively. Error bars show 95% confidence intervals (CI). Letters indicate significant differences at p < 0.001. (ns = non-significant).
3.7. The Lethal Concentration of M. anisopliae Affects the Biological Parameters of P. xylostella
M. anisopliae (LC50) disturbs the biological parameters of P. xylostella (Table 3). After the application of lethal concentration on 3rd instar, a significant reduction in larval duration (1.10 days) was observed compared to control (2.4 days) (p > 0.05). A similar trend was followed by 4th instar. A considerable decrease in percent pupation was also reported in the infected population with only 64.21% larvae arriving at the pupal stage compared to 93.51% in control. Prolonged pupal duration (5.22 days) was followed by reduced adult emergence (61.47%) with a ratio of 1:2 sex ratio (M: F). The pre-oviposition period (APOP) in adult females increased to 1.92 days compared to 1.01 days in control. Fecundity was also significantly affected in treated females where the mean number of eggs laid was reduced to 101.55 eggs/female in comparison to 192.55 eggs/female control. A noteworthy effect was also seen on egg hatching (first filial generation). The daily egg production rate is shown in Figure 6, where the number of eggs in control is significantly higher than the treated group.
Table 3.
Biological parameters of Plutella xylostella after treatment of Metarhizium anisopliae.
| Parameters | M. anisopliae (LC50) | Control |
|---|---|---|
| Means ± SE | Means ± SE | |
| Mortality (%) | 51.34 ± 1.25 a | 5.4 ± 0.10 b |
| L3 (days) | 1.10 ± 0.21 b | 2.14 ± 0.17 a |
| L4 (days) | 1.72 ± 0.11 b | 2.59 ± 0.12 a |
| Percent pupation | 64.21 ± 2.46 b | 93.51 ± 3.11 a |
| Pupal duration (days) | 5.22 ± 0.71 a | 3.80 ± 0.27 b |
| Adult emergence (%) | 61.47 ± 3.41 b | 92.11 ± 3.57 a |
| Female longevity (days) | 6.87 ± 0.98 b | 10.11 ± 1.98 a |
| Male longevity (days) | 8.11 ± 1.27 b | 12.38 ± 2.8 a |
| Sex ratio (M:F) | 1:2 ns | 1:2 ns |
| APOP | 1.92 ± 0.04 ns | 1.01 ± 0.07 ns |
| Fecundity (eggs/female) | 101.55 ± 2.54 b | 192.55 ± 3.21 a |
| Egg hatching (%) | 60.2 ± 3.44 b | 94.22 ± 2.98 a |
L3 = 3rd instar larval duration; L4 = 4th instar larval duration; APOP = adult pre-oviposition period of female adult; means in the same row followed by the same letter are not significantly different (p > 0.05).
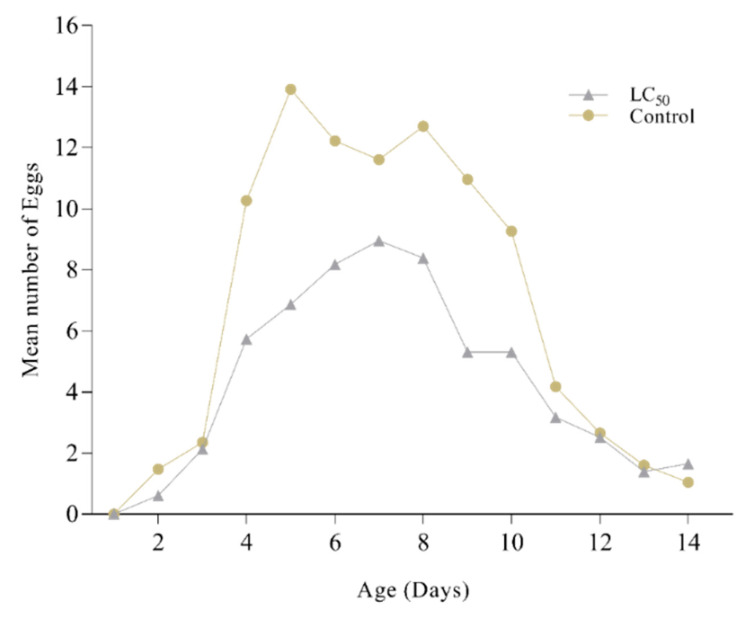
Figure 6.
Mean number of eggs from Plutella xylostella after treatment with Metarhizium anisopliae. Grey dots show the mean numbers of eggs laid by P. xylostella after treatment by M. anisopliae while yellow dots show the control group.
4. Discussion
P. xylostella is a notorious pest of cruciferous crops [20]. IPM strategies employed to control this pest has been focused primarily on the use of chemical insecticides [8]. Compared to synthetic insecticides, pathogenic fungi are promising biocontrol agents for various insect pests and exhibit efficient capabilities for insecticide-resistant pests with fewer environmental hazards [2,16]. The study presented here evaluated three different types of entomopathogenic fungi for larval mortality in P. xylostella. Our results found that M. anisopliae could efficiently infect P. xylostella, and cause significant mortality compared to the rest, suggesting the potential of this entomopathogenic fungus for the pest control [3]. The difference in pathogenicity could be due to the fact that some fungal species germinate and penetrate more rapidly compared to others. Besides, an increase in the production of secondary metabolites to resist antifungal compounds is also the primary adaptive behavior of a potent entomopathogenic fungus [54,55,56]. Conversely, insect cuticle, a hydrophobic surface, acts as the first line of defense against invading fungi [33,57]. Entomopathogenic fungi encounter this barrier by producing hydrophobin proteins that synergies with enzymes, leading to infection and causing the death of the insect [33,57]. The mortality caused by M. anisopliae was highly concentration-dependent as previously described in other insect pests [10,43,58]. Insects possess an innate immune system, a dynamic and instantaneous mechanism against pathogenic infections [59]. The suppression of immune responses is one of the major mechanisms which govern the outcome of an interaction between pathogen and host [60,61].
Hemocytes, upon invasion, play a vital role to defuse the pathogen activities by employing different biological processes such as phagocytosis and encapsulation [62,63]. Arthropods can produce various types of hemocytes depending upon the type of infection they face [64]. Studies have revealed that the number and types of hemocytes varied when infected with different strains and strengths of Metarhizium spp. [65].The ability of a pathogenic fungi to overcome host hemolymph also represents its virulence to the host [66]. The study presented here showed a change in hemocyte numbers under pressure from the lethal concentration of M. anisopliae, which could be due to evasion and overcoming hemolymph defense systems by entomopathogenic fungus. However, this change was not statistically significant, supported by similar findings in previous studies [67].
The enzymatic responses are a key constituent of the insect immune system under various stressful environments, reflecting physiological changes in the host. Enzymes such as PO play a crucial role in wound deposition, encapsulation of pathogens, and most importantly activation of immune pathways [68]. The increased PO activity strengthens the ability of the immune system to counter xenobiotics [69,70], while its inhibition suggests pathogenic fungi may overcome the immunity of PO. The current study depicted a time-based trend in PO activity rising to a maximum between 24 and 48 h while gradually decreasing at 72 and 96 h, indicating that M. anisopliae steadily get the better of the host defense system [69]. Similarly, SOD an antioxidant defense enzyme, also reported varying trends demonstrating that the physiological activities of P. xylostella larvae were distressed following the infection by fungi [71,72]. Insect immune responses are governed by various immune genes expressed at different time points. Scientists have been able to identify 1000 immune-related genes in P. xylostella when targeted by entomopathogenic fungi [34]. Likewise, our study found out key genes (spaetzle, defensin, cecropin) involved in immune responses against entomopathogenic fungi were exceedingly time-dependent, showing varying expression levels.
M. anisopliae produces secondary metabolite destruxin, a compound capable of evading insect cellular and humoral immune responses, troubling the demographic parameters of the pest before its eventual death [73,74,75,76]. Disturbances in biological parameters of various insect pests have been reported when infected with entomopathogenic fungi [10,11,26,61,62], which strengthens our findings where a distress in biological cycles are reported. During the invasion of pathogens (M. anisopliae) the body temperature of insects rises to encounter the infection but this, in turn, affects the larval stadium [77] supporting the current findings where reduced larval duration was observed. M. anisopliae absorbs hemolymph sugar content from tracheoles, reducing nutrients and leading toward early pupation, prolonged pupal duration [35,78], reduced percent pupation, and percent emergence in different insect pests [2,79,80] supporting the findings reported in a study presented here. Besides intrusion in immature stages, the adults are also affected in the form of shorter life span, an observation supported by previous findings where reduced adult age and fecundity were reported [43,57,80,81].
5. Conclusions
The study conducted demonstrates the interaction of fungal pathogen M. anisopliae with immune responses of P. xylostella and potentially overcoming it by causing the disturbance in its demography eventually killing the host. Hence, M. anisopliae can become a potent prospect for the control of this pest. The study will provide a basic hence important information for further field and semi field experimentation.
Acknowledgments
We are very grateful to Laboratory of Bio-Pesticide Creation and Application of Guangdong Province, Guangzhou, China for support. We also thank editors and anonymous referees for their invaluable comments and suggestion.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/11/10/694/s1, Figure S1: Experimental validation of lethal (LC50) and sublethal (LC20) concentrations of Metarhizium anisopliae. Error bars show 95% confidence intervals (CI), Table S1: Primers used in this study.
Author Contributions
Conceptualization, J.Z.; data curation, J.Z. and R.F.S.; formal analysis, J.Z.; funding acquisition, X.X. and F.J.; investigation J.Z. and Y.Z.; methodology, J.Z.; project administration, S.F. and F.J.; resources, X.X. and F.J.; supervision, F.J.; writing—original draft, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Key-Area Research and Development Program of Guangdong Province (2018B020205003), Natural Science Foundation of Guangdong, China (2018A030313402, 2019A1515011221) and State Key Laboratory of Biocontrol (Sun Yat-sen University) (2019SKLBC-KF02).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Freed S., Feng Liang J., Shun Xiang R. Intraspecific Variability among the Isolates of Metarhizium anisopliae var. anisopliae by RAPD Markers. Int. J. Agric. Biol. 2014;16:899–904. [Google Scholar]
- 2.Shoukat R.F., Hassan B., Shakeel M., Zafar J., Li S., Freed S., Xu X., Jin F. Pathogenicity and Transgenerational Effects of Metarhizium anisopliae on the Demographic Parameters of Aedes albopictus (Culicidae: Diptera) J. Med. Entomol. 2020;57:677–685. doi: 10.1093/jme/tjz236. [DOI] [PubMed] [Google Scholar]
- 3.Shoukat R.F., Freed S., Ahmad K.W. Evaluation of binary mixtures of entomogenous fungi and botanicals on biological parameters of Culex pipiens (Diptera: Culicidae) under laboratory and field conditions. Int. J. Mosq. Res. 2016;3:17–24. [Google Scholar]
- 4.Shoukat R.F., Freed S., Ahmad K.W., Rehman A.-U. Assessment of Binary Mixtures of Entomopathogenic Fungi and Chemical Insecticides on Biological Parameters of Culex pipiens (Diptera: Culicidae) under Laboratory and Field Conditions. Pak. J. Zool. 2018;50:299–309. doi: 10.17582/journal.pjz/2018.50.1.299.309. [DOI] [Google Scholar]
- 5.Zafar J., Freed S., Khan B.A., Farooq M. Effectiveness of Beauveria bassiana Against Cotton Whitefly, Bemisia tabaci (Gennadius) (Aleyrodidae: Homoptera) on Different Host Plants. Pak. J. Zool. 2016;48:91–99. [Google Scholar]
- 6.Khan B.A., Freed S., Zafar J., Farooq M., Shoukat R.F., Ahmad K.W., Li S., Zhang Y., Hua Y., Shoukat R.F. Efficacy of different entomopathogenic fungi on biological parameters of pulse beetle Callosobruchus chinensis L. (Coleoptera: Bruchidae) J. Entomol. Zool. Stud. 2018;6:1972–1976. [Google Scholar]
- 7.Khan B.A., Freed S., Zafar J., Farooq M. Evaluation of three different insect pathogenic fungi for the control of Dysdercus koenigii and Oxycarenus hyalinipennis. Pak. J. Zool. 2014;46:1759–1766. [Google Scholar]
- 8.Duarte R., Gonçalves K., Espinosa D., Moreira L., De Bortoli S., Humber R., Polanczyk R. Potential of entomopathogenic fungi as biological control agents of diamondback moth (Lepidoptera: Plutellidae) and compatibility with chemical insecticides. J. Econ. Entomol. 2016;109:594–601. doi: 10.1093/jee/tow008. [DOI] [PubMed] [Google Scholar]
- 9.Rizvi S.A.H., Xie F., Ling S., Zeng X. Development and evaluation of emulsifiable concentrate formulation containing Sophora alopecuroides L. extract for the novel management of Asian citrus psyllid. Environ. Sci. Pollut. Res. 2019;21:21871–21881. doi: 10.1007/s11356-019-05418-1. [DOI] [PubMed] [Google Scholar]
- 10.Shoukat R.F., Zafar J., Shakeel M., Zhang Y., Freed S., Xu X., Jin F. Assessment of Lethal, Sublethal, and Transgenerational Effects of Beauveria Bassiana on the Demography of Aedes Albopictus (Culicidae: Diptera) Insects. 2020;11:178. doi: 10.3390/insects11030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoukat R.F., Shakeel M., Rizvi S.A.H., Zafar J., Zhang Y., Freed S., Xu X., Jin F. Larvicidal, Ovicidal, Synergistic, and Repellent Activities of Sophora alopecuroides and Its Dominant Constituents Against Aedes albopictus. Insects. 2020;11:246. doi: 10.3390/insects11040246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 13.Relyea R., Hoverman J. Assessing the ecology in ecotoxicology: A review and synthesis in freshwater systems. Ecol. Lett. 2006;9:1157–1171. doi: 10.1111/j.1461-0248.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 14.Sukumar K., Perich M.J., Boobar L.R. Botanical derivatives in mosquito control: A review. J. Am. Mosq. Control Assoc. 1991;7:210–237. [PubMed] [Google Scholar]
- 15.Thomas M.B., Read A.F. Can fungal biopesticides control malaria? Nat. Rev. Microbiol. 2007;5:377. doi: 10.1038/nrmicro1638. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 2007;17:553–596. doi: 10.1080/09583150701309006. [DOI] [Google Scholar]
- 17.Ahmed S.M., Saeed M., Nawaz A., Usman M., Shoukat R.F., Li S., Zhang Y., Zeng L., Zafar J., Akash A. Monitoring of quantitative and qualitative losses by lepidopteran, and homopteran pests in different crop production systems of Brassica oleracea L. J. Entomol. Zool. Stud. 2018;6:6–12. [Google Scholar]
- 18.Malik S.U., Zia K., Ajmal M., Shoukat R.F., Li S., Saeed M., Zafar J., Shoukat R.F. Comparative efficacy of different insecticides and estimation of yield losses on BT and non-BT cotton for thrips, red cotton bug, and dusky cotton bug. J. Entomol. Zool. Stud. 2018;6:505–512. [Google Scholar]
- 19.Saeed M., Shoukat R.F., Zafar J. Population dynamics of natural enemies and insect pest in different Brassica oleracea (cabbage) growing seasons with different production systems. J. Entomol. Zool. Stud. 2017;5:1669–1674. [Google Scholar]
- 20.Sarfraz M., Keddie A.B., Dosdall L.M. Biological control of the diamondback moth, Plutella xylostella: A review. Biocontrol Sci. Technol. 2005;15:763–789. [Google Scholar]
- 21.Zhang S., Zhang X., Shen J., Li D., Wan H., You H., Li J. Cross-resistance and biochemical mechanisms of resistance to indoxacarb in the diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2017;140:85–89. doi: 10.1016/j.pestbp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Ashfaq M., Sonoda S., Tsumuki H. Expression of two methionine-rich storage protein genes of Plutella xylostella (L.) in response to development, juvenile hormone-analog and pyrethroid. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007;148:84–92. doi: 10.1016/j.cbpb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Liu S., Niu H., Xiao T., Xue C., Liu Z., Luo W. Does phenoloxidase contributed to the resistance? Selection with butane-fipronil enhanced its activities from diamondback moths. Open Biochem. J. 2009;3:9. doi: 10.2174/1874091X00903010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad K.W., Freed S., Shoukat R.F. Efficacy of entomopathogenic fungi and botanicals on development of Musca domestica. J. Entomol. Zool. Stud. 2017;5:593–599. [Google Scholar]
- 25.Farooq M., Freed S. Mortality, Biological, and Biochemical Response of Musca domestica (Diptera: Muscidae) to Selected Insecticides 1. J. Entomol. Sci. 2018;53:27–45. doi: 10.18474/JES17-22.1. [DOI] [Google Scholar]
- 26.Farooq M., Freed S. Insecticidal activity of toxic crude proteins secreted by entomopathogenic fungi against musca domestica l. (Diptera: Muscidae) Kuwait J. Sci. 2018;45:64–74. [Google Scholar]
- 27.Farooq M., Steenberg T., Castberg D., Freed S., Kristensen M. Impact of sequential exposure of Beauveria bassiana and imidacloprid against susceptible and resistant strains of Musca domestica. BioControl. 2018;63:703–718. doi: 10.1007/s10526-018-9892-6. [DOI] [Google Scholar]
- 28.Freed S., Farooq M. Entomopatojen fungus ve sentetik insektisit karışımlarının Musca domestica L.’nın biyolojik dönemleri üzerinde lethal ve sublethal etkileri. Türkiye Entomoloji Derg. 2016;40:211–225. [Google Scholar]
- 29.Zimmermann G. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci. Technol. 2007;17:879–920. [Google Scholar]
- 30.Fite T., Tefera T., Negeri M., Damte T., Sori W. Evaluation of Beauveria bassiana, Metarhizium anisopliae, and Bacillus thuringiensis for the management of Helicoverpa armigera (Hubner)(Lepidoptera: Noctuidae) under laboratory and field conditions. Biocontrol Sci. Technol. 2020;30:278–295. [Google Scholar]
- 31.Freed S., Saleem M.A., Khan M., Naeem M. Prevalence and effectiveness of Metarhizium anisopliae against Spodoptera exigua (Lepidoptera: Noctuidae) in southern Punjab, Pakistan. Pak. J. Zool. 2012;44:753. [Google Scholar]
- 32.Lu H.-L., Leger R.S. Advances in Genetics. Volume 94. Elsevier; Amsterdam, The Netherlands: 2016. Insect immunity to entomopathogenic fungi; pp. 251–285. [DOI] [PubMed] [Google Scholar]
- 33.Butt T.M., Greenfield B.P.J., Greig C., Maffeis T.G.G., Taylor J.W.D., Piasecka J., Dudley E., Abdulla A., Dubovskiy I.M., Garrido-Jurado I., et al. Metarhizium anisopliae Pathogenesis of Mosquito Larvae: A Verdict of Accidental Death. PLoS ONE. 2013;8:e81686. doi: 10.1371/journal.pone.0081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J., Xu X., Shakeel M., Li S., Wang S., Zhou X., Yu J., Xu X., Yu X., Jin F. The entomopathogenic fungi Isaria fumosorosea plays a vital role in suppressing the immune system of Plutella xylostella: RNA-Seq and DGE analysis of immunity-related genes. Front. Microbiol. 2017;8:1421. doi: 10.3389/fmicb.2017.01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng G., Jin K., Liu Y., Xia Y. Enhancing the utilization of host trehalose by fungal trehalase improves the virulence of fungal insecticide. Appl. Microbiol. Biotechnol. 2015;99:8611–8618. doi: 10.1007/s00253-015-6767-y. [DOI] [PubMed] [Google Scholar]
- 36.Broxton C.N., Culotta V.C. SOD enzymes and microbial pathogens: Surviving the oxidative storm of infection. PLoS Pathog. 2016;12:e1005295. doi: 10.1371/journal.ppat.1005295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinett N.G., Peterson R.L., Culotta V.C. Eukaryotic copper-only superoxide dismutases (SODs): A new class of SOD enzymes and SOD-like protein domains. J. Biol. Chem. 2018;293:4636–4643. doi: 10.1074/jbc.TM117.000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu K., Yang B., Wang J., Luo Y., Ni Y., Huang W. Immunity in Insects. Springer; Berlin/Heidelberg, Germany: 2020. Detection of Enzyme Distribution, Expression, Activation, and Activity of Insect Prophenoloxidase; pp. 115–126. [Google Scholar]
- 39.González-Rete B., Salazar-Schettino P.M., Bucio-Torres M.I., Córdoba-Aguilar A., Cabrera-Bravo M. Activity of the prophenoloxidase system and survival of triatomines infected with different Trypanosoma cruzi strains under different temperatures: Understanding Chagas disease in the face of climate change. Parasites Vectors. 2019;12:219. doi: 10.1186/s13071-019-3477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butt T., Ibrahim L., Ball B., Clark S. Pathogenicity of the entomogenous fungi Metarhizium anisopliae and Beauveria bassiana against crucifer pests and the honey bee. Biocontrol Sci. Technol. 1994;4:207–214. doi: 10.1080/09583159409355328. [DOI] [Google Scholar]
- 41.Darbro J.M., Thomas M.B. Spore persistence and likelihood of aeroallergenicity of entomopathogenic fungi used for mosquito control. Am. J. Trop. Med. Hyg. 2009;80:992–997. doi: 10.4269/ajtmh.2009.80.992. [DOI] [PubMed] [Google Scholar]
- 42.Scholte E.-J., Knols B.G., Takken W. Infection of the malaria mosquito Anopheles gambiae with the entomopathogenic fungus Metarhizium anisopliae reduces blood feeding and fecundity. J. Invertebr. Pathol. 2006;91:43–49. doi: 10.1016/j.jip.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Scholte E.-J., Takken W., Knols B.G. Infection of adult Aedes aegypti and Ae. albopictus mosquitoes with the entomopathogenic fungus Metarhizium anisopliae. Acta Trop. 2007;102:151–158. doi: 10.1016/j.actatropica.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Ibrahim A.M., Kim Y. Parasitism by Cotesia plutellae alters the hemocyte population and immunological function of the diamondback moth, Plutella xylostella. J. Insect Physiol. 2006;52:943–950. doi: 10.1016/j.jinsphys.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Li S., Xu X., Zheng Z., Zheng J., Shakeel M., Jin F. MicroRNA expression profiling of Plutella xylostella after challenge with B. thuringiensis. Dev. Comp. Immunol. 2019;93:115–124. doi: 10.1016/j.dci.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye J., Coulouris G., Zaretskaya I., Cutcutache I., Rozen S., Madden T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Golizadeh A., Kamali K., Fathipour Y., Abbasipour H. Life table of the diamondback moth, Plutella xylostella (L.)(Lepidoptera: Plutellidae) on five cultivated brassicaceous host plants. J. Econ. Entomol. 2009;112:932–938. [Google Scholar]
- 49.Ai C., Norton E.C. Interaction terms in logit and probit models. Econ. Lett. 2003;80:123–129. doi: 10.1016/S0165-1765(03)00032-6. [DOI] [Google Scholar]
- 50.Abbott W. Abbott’s formula. J. Econ. Entomol. 1925;18:267–268. [Google Scholar]
- 51.Abbott W. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. (USA) 1987;3:302–303. [PubMed] [Google Scholar]
- 52.LeOra Software LLC . Poloplus, A User’s Guide to Probit or Logit Analysis. LeOra Software LLC; Berkeley, CA, USA: 2003. [Google Scholar]
- 53.Statistical Analysis System Institute . SAS/STAT User’s Guide: Statistics. Statistical Analysis System Institute; Cary, NC, USA: 2000. version 8.1. [Google Scholar]
- 54.Liu X., Wang H.-Y., Ning Y.-B., Qiao K., Wang K.-Y. Resistance selection and characterization of chlorantraniliprole resistance in Plutella xylostella (Lepidoptera: Plutellidae) J. Econ. Entomol. 2015;108:1978–1985. doi: 10.1093/jee/tov098. [DOI] [PubMed] [Google Scholar]
- 55.Ortiz-Urquiza A., Keyhani N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects. 2013;4:357–374. doi: 10.3390/insects4030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aw K.M.S., Hue S.M. Mode of infection of Metarhizium spp. fungus and their potential as biological control agents. J. Fungi. 2017;3:30. doi: 10.3390/jof3020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rozilawati H., Masri M., Tanaselvi K., TH M.Z., Zairi J., Nazni W., Lee H. Life Table Characteristics of Malaysian Strain Aedes albopictus (Skuse) Serangga. 2018;22:85–121. [Google Scholar]
- 58.O’Brien D., McVey J. Blood Coagulation, Inflammation, and Defense. IRL Press; New York, NY, USA: 1993. pp. 257–280. The Natural Immune System, Humoral Factors. [Google Scholar]
- 59.Wang C., Leger R.J.S. A collagenous protective coat enables Metarhizium anisopliae to evade insect immune responses. Proc. Natl. Acad. Sci. USA. 2006;103:6647–6652. doi: 10.1073/pnas.0601951103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang S., He Z., Zhang S., Keyhani N.O., Song Y., Yang Z., Jiang Y., Zhang W., Pei Y., Zhang Y. Interplay between calcineurin and the Slt2 MAP-kinase in mediating cell wall integrity, conidiation and virulence in the insect fungal pathogen Beauveria bassiana. Fungal Genet. Biol. 2015;83:78–91. doi: 10.1016/j.fgb.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Strand M.R. The insect cellular immune response. Insect Sci. 2008;15:1–14. [Google Scholar]
- 62.Kwon B., Kim Y. Transient expression of an EP1-like gene encoded in Cotesia plutellae bracovirus suppresses the hemocyte population in the diamondback moth, Plutella xylostella. Dev. Comp. Immunol. 2008;32:932–942. doi: 10.1016/j.dci.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Fiorotti J., Menna-Barreto R.F.S., Gôlo P.S., Coutinho-Rodrigues C.J.B., Bitencourt R.O.B., Spadacci-Morena D.D., Angelo I.d.C., Bittencourt V.R.E.P. Ultrastructural and cytotoxic effects of Metarhizium robertsii infection on Rhipicephalus microplus hemocytes. Front. Physiol. 2019;10:654. doi: 10.3389/fphys.2019.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao G., Jia M., Zhao X., Wang L., Tu X., Wang G., Nong X., Zhang Z. Correction: Different Effects of Metarhizium anisopliae Strains IMI330189 and IBC200614 on Enzymes Activities and Hemocytes of Locusta migratoria L. PLoS ONE. 2017;12:e0175219. doi: 10.1371/journal.pone.0175219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samson R.A., Evans H.C., Latgé J.-P. Atlas of Entomopathogenic Fungi. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2013. [Google Scholar]
- 66.Yu Y., Cao Y., Xia Y., Liu F. Wright–Giemsa staining to observe phagocytes in Locusta migratoria infected with Metarhizium acridum. J. Invertebr. Pathol. 2016;139:19–24. doi: 10.1016/j.jip.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 67.Cerenius L., Lee B.L., Söderhäll K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Cerenius L., Söderhäll K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 69.Chang C.-C., Rahmawaty A., Chang Z.-W. Molecular and immunological responses of the giant freshwater prawn, Macrobrachium rosenbergii, to the organophosphorus insecticide, trichlorfon. Aquat. Toxicol. 2013;130:18–26. doi: 10.1016/j.aquatox.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 70.Jiang W., Peng Y., Ye J., Wen Y., Liu G., Xie J. Effects of the Entomopathogenic Fungus Metarhizium anisopliae on the Mortality and Immune Response of Locusta migratoria. Insects. 2020;11:36. doi: 10.3390/insects11010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang H., Zhang H., Hao C., Zhang X. Effects of Isaria fumosorosea infection on different enzyme activities in the larvae of Plutella xylostella. Mycosystema. 2013;32:269–276. [Google Scholar]
- 72.Keppanan R., Krutmuang P., Sivaperumal S., Hussain M., Bamisile B.S., Aguila L.C.R., Dash C.K., Wang L. Synthesis of mycotoxin protein IF8 by the entomopathogenic fungus Isaria fumosorosea and its toxic effect against adult Diaphorina citri. Int. J. Biol. Macromol. 2019;125:1203–1211. doi: 10.1016/j.ijbiomac.2018.09.093. [DOI] [PubMed] [Google Scholar]
- 73.Alves S., Alves L., Lopes R., Pereira R., Vieira S. Potential of some Metarhizium anisopliae isolates for control of Culex quinquefasciatus (Dipt., Culicidae) J. Appl. Entomol. 2002;126:504–509. [Google Scholar]
- 74.Leles R.N., D’Alessandro W.B., Luz C. Effects of Metarhizium anisopliae conidia mixed with soil against the eggs of Aedes aegypti. Parasitol. Res. 2012;110:1579–1582. doi: 10.1007/s00436-011-2666-z. [DOI] [PubMed] [Google Scholar]
- 75.Luz C., Tai M., Santos A., Rocha L., Albernaz D., Silva H. Ovicidal activity of entomopathogenic hyphomycetes on Aedes aegypti (Diptera: Culicidae) under laboratory conditions. J. Med. Entomol. 2007;44:799–804. doi: 10.1093/jmedent/44.5.799. [DOI] [PubMed] [Google Scholar]
- 76.Mousseau T.A., Dingle H. Maternal Effects in Insect Life Histories. Annu. Rev. Entomol. 1991;36:511–534. doi: 10.1146/annurev.en.36.010191.002455. [DOI] [Google Scholar]
- 77.Jin K., Peng G., Liu Y., Xia Y. The acid trehalase, ATM1, contributes to the in vivo growth and virulence of the entomopathogenic fungus, Metarhizium acridum. Fungal Genet. Biol. 2015;77:61–67. doi: 10.1016/j.fgb.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 78.Malarvannan S., Murali P., Shanthakumar S., Prabavathy V., Nair S. Laboratory evaluation of the entomopathogenic fungi, Beauveria bassiana against the Tobacco caterpillar, Spodoptera litura Fabricius (Noctuidae: Lepidoptera) J. Biopestic. 2010;3:126. [Google Scholar]
- 79.Darbro J.M., Johnson P.H., Thomas M.B., Ritchie S.A., Kay B.H., Ryan P.A. Effects of Beauveria bassiana on survival, blood-feeding success, and fecundity of Aedes aegypti in laboratory and semi-field conditions. Am. J. Trop. Med. Hyg. 2012;86:656–664. doi: 10.4269/ajtmh.2012.11-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howard A.F., N’Guessan R., Koenraadt C.J., Asidi A., Farenhorst M., Akogbéto M., Thomas M.B., Knols B.G., Takken W. The entomopathogenic fungus Beauveria bassiana reduces instantaneous blood feeding in wild multi-insecticide-resistant Culex quinquefasciatus mosquitoes in Benin, West Africa. Parasites Vectors. 2010;3:87. doi: 10.1186/1756-3305-3-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Paula A.R., Brito E.S., Pereira C.R., Carrera M.P., Samuels R.I. Susceptibility of adult Aedes aegypti (Diptera: Culicidae) to infection by Metarhizium anisopliae and Beauveria bassiana: Prospects for Dengue vector control. Biocontrol Sci. Technol. 2008;18:1017–1025. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.