Abstract
A number of different defects in the process of ribosome production can lead to a diversified spectrum of disorders that are collectively identified as ribosomopathies. The specific factors involved may either play a role only in ribosome biogenesis or have additional extra-ribosomal functions, making it difficult to ascribe the pathogenesis of the disease specifically to an altered ribosome biogenesis, even if the latter is clearly affected. We reviewed the available literature in the field from this point of view with the aim of distinguishing, among ribosomopathies, the ones due to specific alterations in the process of ribosome production from those characterized by a multifactorial pathogenesis.
Keywords: ribosome biogenesis, rare diseases, Diamond Blackfan anemia, X-linked dyskeratosis congenita, 5q− syndrome, Shwachman-Diamond syndrome, Treacher Collins syndrome, cartilage hair hypoplasia, cancer
1. Introduction
Specific defects in the process of ribosome production lead to a heterogeneous group of human disorders that are well known today as ribosomopathies [1,2]. The term ribosomopathy, with its correspondent meaning, was first suggested [3] for the skin and bone marrow failure syndrome X-linked dyskeratosis congenita (X-DC), in which the human pseudouridine synthase dyskerin is mutated [4]. Among its functions, dyskerin mediates the modification of approximately 100 specific uridine residues to pseudouridines in rRNA, an essential step of ribosomal biogenesis. Soon after, it was discovered that approximately one fourth of Diamond Blackfan anemia (DBA) patients harbor a mutation in the gene encoding the ribosomal protein (RP) S19 (or eS19 according to the new nomenclature [5]) [6], suggesting that the term ribosomopathy could be shared by more than a single disease. The list of recognized ribosomopathies then grew rapidly to include Schwachman-Diamond syndrome (SDS), cartilage hair hypoplasia (CHH), and Treacher Collins syndrome (TCS) [7,8,9]. Ever since the earliest classification attempts, these five disorders have been considered examples of known or suspected inherited ribosomopathies [10]. Their number continued to grow further, coming to include a list of other less characterized inherited disorders, as well as acquired conditions such as the 5q− myelodysplastic syndrome [11] and cancer [12,13]. To understand the molecular mechanism underlying most of these disorders, it may be helpful to quickly review the fundamental steps in ribosome production in human cells.
Ribosomes are ribonucleoprotein complexes dedicated to messenger RNA translation and protein synthesis. Human cytoplasmic ribosomes are made of four ribosomal RNA molecules and approximately 80 proteins divided into two subunits: the large subunit (60S) and the small subunit (40S). The first accounts for three rRNAs, 5S, 5.8S, and 28S together with 47 ribosomal proteins (RPs). The small subunit is made up of 18S rRNA and 33 RPs.
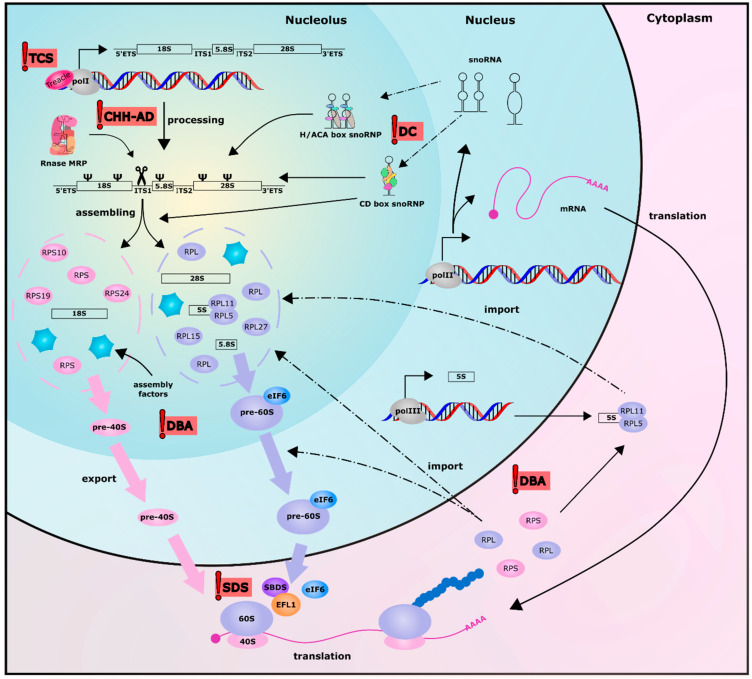
Ribosome biogenesis is an intricate and coordinated process (reviewed in [14,15]) that occurs in the nucleolus and later in the cytoplasm (Figure 1); it involves more than 200 trans-acting factors [16] that are required during the numerous steps of ribosomal subunits maturation. All three RNA polymerases are required in this process: RNA polymerase I is needed for the transcription of 28S, 5.8S, and 18S rRNAs [17], RNA pol II produces the mRNAs of the 80 ribosomal proteins and numerous ribosomal processing factors, and RNA pol III synthetizes 5S rRNA [18]. In the nucleolus, RNA polymerase I, complexed with different transcription initiation factors, synthetizes a large polycistronic transcript (47S) from the rDNA genes present in hundreds of copies within the cell [19]. The pre-rRNA 47S obtained is made up of two external transcribed spacers (ETS) positioned at 5’ and 3′ of the molecule, while the sequence of 18S, 5.8S, and 28S are separated by two internal transcribed spacers (ITS1 and ITS2).
Figure 1.
Schematic representation of the ribosome biogenesis process in human cells. The red flags highlight the steps where mutations in genes encoding for ribosomal proteins or for factors involved in ribosome biogenesis give rise to the five ribosomopathies originally identified: Treacher Collins syndrome (TCS), X-linked dyskeratosis congenita, cartilage hair hypoplasia-anauxetic dysplasia (CHH-AD), Diamond Blackfan anemia (DBA) and Schwachman-Diamond syndrome (SDS).
The maturation of this polycistronic transcript occurs in the nucleolus and starts with the action of a series of small nucleolar ribonucleoprotein complexes, namely C/D and H/ACA box snoRNPs [20]. C/D box snoRNPs are made up of fibrillarin and accessory proteins such as Nop56, Nop58, 15.5K/NHPX, and the small nucleolar RNAs (snoRNA) characterized by box C and D. This complex, guided by the complementary hybridization with the sequence of the snoRNA, catalyzes the site-specific 2′-O-methylation of the ribose of rRNA [21]. Moreover, the complex is also involved in rRNA processing and folding [20]. Recently, a new class of specialized C/D box snoRNA, which are able to guide cytosine rRNA acetylation has been reported [21]. On the other hand, the H/ACA snoRNP complex is made up of dyskerin, Nhp2, Nop10 and Gar1, and the H/ACA snoRNA component. This complex acts similarly to the C/D box snoRNPs: the H/ACA box snoRNA guides the complex to a specific uridine in rRNA, while dyskerin catalyzes the conversion of this uridine into pseudouridine [22]. These modifications can be found in functionally important regions of the ribosome, some of them being essential to regulate translational efficiency [23] and fidelity [24]. In addition, numerous C/D boxes or H/ACA boxes snoRNA not involved in ribose 2′-O-methylation or in rRNA pseudouridylation, like U3, U8, U14, U22, U17, and some long non-coding RNAs like RMRP are involved in rRNA processing and maturation [25].
The ribosomal RNA also undergoes a series of processes and assembly events that give rise to ribosome subunits. Some alternative processing pathways are described, although the most common one starts with the cleavage at the 5′ETS end (at the so-called site 01), removal of 3′ETS and, subsequentially, cleavage at site 2 of ITS1 (as extensively described in [15]). This last cleavage by RNAse MRP has the important function of separating the maturation of the two ribosome subunits: the small subunit containing 18S rRNA, and the large subunit containing 5.8S and 28S rRNA. After exonucleolytic and endonucleolytic cleavages at the 3′ of pre-18S rRNA, this RNA and the ribosomal proteins of the small subunit constituting pre-40S particles are exported to the cytoplasm to complete their maturation. On the other hand, the maturation of the large subunit rRNA continues in the nucleolus. It has been reported that, in mammals, two different forms of 5.8S are present: a short (5.8Ss) and a long (5.8Sl) form both originating after the cleavage at site 2 of ITS1 operated by RNase MRP, as is the case with yeast. However, the alternative pathway leading to the formation of 5.8Sl is still unclear, while the trimming of the 5′ end of 5.8S sequence operated by XRN2 leads to the formation of the short form. A second endonucleolytic cleavage occurs in ITS2 and leads to the maturation of 28S rRNA. After the cytoplasmic assembly and nucleolar import of 5S RNP, pre-60S subunits also containing ribosomal proteins can be exported to the cytoplasm to fully complete their maturation process. After this important step, the missing ribosomal proteins are added to the two subunits while completing their maturation [26]. The activation for translation of the nascent 60S subunit occurs when the anti-association factor eIF6 is removed from the large subunit thanks to the activity of EFL1 (elongation factor-like GTPase1) and its cofactor SBDS (Shwachman Bodian Diamond Syndrome) [27]. The correct dissociation of the different assembly factors from both 60S and 40S subunits consist of the final structural quality control and allows the formation of the complete ribosome in presence of a messenger RNA and the translation initiation complex [27,28].
A few years ago, in an attempt to provide a definition, De Keersmaecker, Sulima and Dinman suggested that a ribosomopathy is “any disease associated with a mutation in a ribosomal protein or biogenesis factor impairing ribosome biogenesis in which a defect in ribosome biogenesis or function can be clearly linked to disease causality” [29]. The intention of the Authors was to provide a conservative definition that would not include disorders in which ribosomal defects were not causative. Still, this definition, in addition to “pure” ribosomal disorders, leaves room for disorders in which the defect in ribosome biogenesis only concurs to the pathophysiology of the disease. According to this definition, ribosomopathies can be further classified as disorders whose pathogenesis could be fully ascribed to the defect in ribosome biogenesis and/or ribosomal functions on the one hand, and disorders deriving from defects in multiple cellular functions including ribosome biogenesis on the other. Table 1 shows examples of ribosomopathies following this classification.
Table 1.
Congenital/Inherited ribosomopathies.
| Congenital/Inherited Ribosomopathies | ||||
|---|---|---|---|---|
| Disease | Gene Mutated | Role in Ribosome Biogenesis | Clinical Manifestations | Type of Ribosomopathy |
| Diamond Blackfan anemia |
RPS19, RPS26, RPS17, RPS29 [30] RPS28 [31] RPS24, RPL5, RPL11 [32] RPL35, RPL18 [33] RPL26 [34] RPL15 [35] RPS27, RPL27 [36] |
40S and 60S subunits protein | Macrocytic anemia, skeletal abnormalities, short stature, cardiac and genitourinary malformations, cancer predisposition | Pure |
| Shwachman-Diamond syndrome |
SBDS [7] DNAJC21 [37] EFL1 [38] SRP54 [39] |
Assembly of 60S and 40S subunits in active 80S ribosomes | Bone marrow failure, skeletal dysplasia, cognitive impairment, and risk of developing myelodysplastic syndrome | Pure |
| Treacher Collins syndrome |
TCOF1 [40] POLR1C, POLR1D [41] POLR1B [42] |
Ribosomal RNA transcription | Severe craniofacial defects and mental retardation | Pure |
| Cartilage Hair Hypoplasia-Anauxetic dysplasia spectrum |
RMRP [8] POP1 [43] |
Ribosomal RNA processing | Short-limbed dwarfism, sparse hypoplastic hair, immunodeficiency, hypoplastic anemia, and predisposition to cancer | Mixed |
| Dyskeratosis Congenita |
DKC1 [4,44] PARN [45,46] NHP2, NOP10 [47,48,49] NPM1 [50] |
Ribosomal RNA pseudouridylation and processing | Abnormal skin pigmentation, dystrophy of the nails, oral leukoplakia, bone marrow failure, and cancer predisposition | Mixed |
The present review aims to classify the disorders due to a defect in ribosome production, highlighting their features as pure or mixed ribosomopathies.
2. Pure Ribosomopathies
2.1. Diamond Blackfan Anemia
Diamond Black anemia (DBA) is frequently present in early childhood as a red cell failure, defined by macrocytic anemia with low reticulocytes and decreased red cell precursors in the bone marrow [51]. Patients may also display a series of skeletal abnormalities and cardiac and genitourinary malformations, together with an increased cancer susceptibility. A very large proportion of patients diagnosed with DBA harbors heterozygous mutations of genes encoding for ribosomal proteins either of the small ribosomal subunit or of the large ribosomal subunit (see Table 1) [33]. The presence of mutated ribosomal proteins could impair ribosome biogenesis at different levels during ribosome assembly, this depends on the single ribosomal protein and the step in which it is involved. In general, these mutations lead to haploinsufficiency for ribosomal protein function, affecting the maturation of the ribosomal subunit containing the protein and ultimately reducing the amount of available functional 80S ribosomes within the cells [52,53,54]. In red cell precursors, this lack of ribosomes impinges the translation of mRNAs encoding key regulators of erythropoiesis such as, for instance, GATA1 [52]. The definition of the pathogenetic mechanisms underlying DBA clearly identifies this disorder as a pure ribosomopathy, in which the defect in a ribosome component clearly causes a misfunction, which can be considered responsible for most of the clinical features of the disease.
Interestingly, a few ribosomal proteins involved in DBA also have extra-ribosomal functions, in addition to their function in ribosome biogenesis. This is the case of RPL11 and RPL5 which play an important role in p53 stabilization [55,56,57,58] and appear to be mutated in approximately 40% of all DBA cases [32,59]. The presentation and severity of the anemia observed in these patients is not clearly different from that due to mutations in genes encoding other ribosomal proteins, confirming that this aspect can be ascribed to the ribosomal defect. Skeletal abnormalities, however, are more frequently associated with RPL5 and RPL11 mutations, which may suggest that the extra-ribosomal functions of these proteins may be involved [32,59] in these traits. On the other hand, skeletal development is known to require high protein synthesis. Therefore, mutations in RPL5 and RPL11 could provoke a severe impairment of ribosome biogenesis such as to cause the skeletal abnormalities associated with these mutations.
2.2. Shwachman-Diamond Syndrome
Shwachman-Diamond syndrome (SDS) is a rare autosomal recessive disease characterized by bone marrow failure and multiple developmental abnormalities, such as short stature, skeletal dysplasia, and cognitive impairment. Patients diagnosed with SDS can present an increased risk of transformation to myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) accurately reviewed in [60,61]. The disease was first identified by Shwachman, Bodian and Diamond in 1964 [62]. In 2003 it was reported that the biallelic mutation of the Shwachman Bodian Diamond Syndrome (SBDS) gene is the molecular cause for SDS [7]. The protein encoded by this gene is reported to be a cofactor of the elongation factor-like GTPase1 (EFL1). In the final step of the cytoplasmic ribosome maturation, EFL1 removes the anti-association factor eIF6 from the large ribosomal subunit, thus permitting the entry of 60S subunit in the actively translating pool and in the end, the association with the small ribosomal subunit and the formation of an active ribosome [63]. Therefore, mutations in the SBDS gene can impair ribosome assembly, indicating that SDS may be classified as a ribosomopathy. Recently, mutations in other genes associated with SBDS have been reported to cause SDS-like diseases. In particular, these genes are DNAJC21, EFL1, and SRP54, which are all involved, together with SBDS, in the removal of eIF6 from the ribosome large subunit [37,38,39].
As we have seen, mutations in SBDS and related genes cause reduced ribosome assembly, which, similarly to what occurs in DBA, could affect the global translation but also reduce the tissue-specific translation of selected mRNAs contributing to the development of the disease. This could be the case of highly proliferative tissues in embryonic development. Moreover, the impaired ribosome maturation can induce the activation of the ribosomal stress pathway and p53 stabilization, resulting in different tissue-specific outcomes. For example, loss of the Sbds gene in murine pancreas induced p53 activation and loss of digestive enzymes, resulting in an atrophy of post-natal acinar cells due to the induction of cell senescence [64].
2.3. Treacher Collins Syndrome
Treacher Collins syndrome (TCS) is an extremely rare congenital disease occurring in 1 out of 50,000 live births. It presents as an autosomal dominant disorder and is characterized by severe craniofacial defects. The symptoms include hypoplasia of the jaw and cheek bones, downward slant of palpebral fissures, cleft palate, and deformity of the ear [9]. The first gene reported to be responsible when mutated for the development of TCS was TCOF1, encoding for the protein Treacle. Treacle is a nucleolar protein that co-localizes with the Upstream Binding Factor (UBF) and Pol I and is involved in the transcription of ribosomal DNA [65]. All the different types of mutations reported result in the expression of a truncated protein. However, no correlations between genotype and severity of the disease have been reported [40]. In addition, in 2011, mutations in two genes encoding for Pol I subunits (POLR1D and POLR1C) were found mutated in a small number of TCS patients [41]. More recently, the work of Sanchez et al. [42] reported mutations connected with TCS also in the POLR1B gene. Therefore, all these genes are involved in ribosome biogenesis, and especially in rRNA transcription, so Treacher Collins syndrome may be rightly classified as a pure ribosomopathy. With the help of mice and zebrafish carrying mutations for the genes involved, researchers showed that deficient ribosome biogenesis caused a reduced proliferation of the progenitors of the craniofacial skeleton cells, called neural crest cells (NCC) [42,66,67,68]. It is well known that impaired ribosome biogenesis triggers nucleolar stress with the stabilization of p53 and consequent apoptosis. Therefore, the reduced proliferation and apoptosis of NCC could be caused by nucleolar stress, as demonstrated by the improvement of symptoms in Tcof1+/− mice embryos upon treatment with p53 inhibitor or p53 knockdown [69].
In recent years, however, new studies have reported that Treacle is involved in other cellular functions that may contribute to the development of TCS. In particular, Treacle seems to be involved in DNA damage response (DDR) pathway [70,71]. In NCC cells, the haploinsufficiency of Treacle could also impair the repair of DNA damage caused by high ROS production and activate p53-mediated apoptosis, thus contributing to the development of craniofacial abnormalities in TCS [72]. Nevertheless, despite the important role played by Treacle in DNA damage response, the fact that mutations in Pol I subunits cause symptoms similar to TCOF1 mutations enables us to say that Treacher Collins syndrome mainly develops due to impaired ribosome biogenesis.
3. Mixed Ribosomopathies
3.1. Dyskeratosis Congenita
On the opposite side of the spectrum of ribosomopathies lies dyskeratosis congenita (DC).
DC is a rare and severe inherited multisystemic syndrome known since the beginning of the last century [73,74,75]. The disorder initially came to the attention of dermatologists because it is characterized by a typical muco-cutaneous triad: abnormal skin pigmentation, dystrophy of the nails, and oral leukoplakia. However, the most severe problem occurring in almost all the DC patients is a progressive bone marrow failure [76]. In addition to these skin and blood defects, one additional relevant aspect of DC is an increased susceptibility to cancer. The overall incidence of malignant tumors in DC patients can reach 50% by the age of 50 [77].
The most frequent form of DC is the X-linked variant, caused by mutations of the DKC1 gene encoding dyskerin. Dyskerin (aka rat NAP57, Drosophila minifly, yeast Cbf5) is a nucleolar protein endowed with pleiotropic functions, all fundamental to basic cellular events including growth, proliferation, and gene expression control. The functions of dyskerin can be explained considering its ability to bind to H/ACA box snoRNAs [44]. Together with the three other core proteins (namely GAR1, NHP2, and NOP10), dyskerin binds to each snoRNA, guiding the complex to the specific uridine residue for its isomerization to pseudouridine. Most of these modifications occur in ribosomal RNA (rRNA) and small nuclear RNA (snRNA) [78,79,80]. On the other hand, always in association with core proteins, dyskerin binds to the telomerase RNA component (TERC), which also hosts a H/ACA sequence element. This enables TERC stabilization and proper telomerase complex function. DC-associated DKC1 mutations strongly reduce TERC levels and impair telomerase activity [81]. Importantly, other bone marrow failure syndromes classified as DC are due either to mutations of genes encoding for exclusive components of the telomerase complex such as TERC [82] and TERT [83] or to telomere binding proteins [84]. For this reason, most of the clinical features of DC are ascribed to a defect in telomerase function and the disorder is often considered more a telomeropathy than a ribosomopathy [85].
While accepting the fact that the impairment in telomerase activity and the consequent telomere attrition are clearly well-established effects of X-DC-associated DKC1 mutations, this view may, however, appear too simplistic after a careful look at the available evidence. In fact, in all the experimentally generated in vivo models based on DKC1 gene targeting, a clear defect in the rate of rRNA processing and/or rRNA pseudouridylation was observed [86,87]. In particular, the Dkc1 hypomorphic mouse recapitulates the signs of DC that have been reported in humans, while Terc homozygous deletion in mice induces a clearly much milder phenotype [88]. This indicates that the telomerase-independent effects consequent to a DKC1 defect are sufficient to raise a spectrum of signs consistent with DC in mice. Also, data from DC patients indicate that their cells harbor altered snoRNA regulation and rRNA modification. Although there are forms of DC due to mutations of genes encoding exclusive telomerase complex components or telomere binding proteins, what is reported for X-DC appears to be true also for autosomal recessive forms of DC such as those due to mutations of genes encoding the pseudouridylation core proteins NHP2 and NOP10 [47,48,49]. In addition, DC has been also reported to develop in consequence of NPM1 gene mutations [50]. In further support of the fact that alterations in rRNA modification are causative of DC, NPM1 mutations found in DC patients cause altered rRNA 2′-O-methylation [50]. In summary, the defect in ribosome biogenesis should be considered to contribute, at least to some extent, to the pathogenesis of DC.
3.2. Cartilage Hair Hypoplasia-Anauxetic Dysplasia (CHH-AD) Spectrum
Cartilage hair hypoplasia (CHH) is an autosomal recessive inherited disease reported first by Victor McKusick in 1965 in a population of Old Order Amish [89]. This pleiotropic disorder is characterized by short-limbed dwarfism, sparse hypoplastic hair, defective T-cell immunity, hypoplastic anemia, increased risk of developing malignancies [90,91], and other symptoms [92,93,94,95]. Many of these symptoms, including immunodeficiency and cancer predisposition, are considered responsible for a shorter life expectancy in these patients [96]. In 2001, the study by Ridanpaa et al. [8] first described how mutations in the RMRP (RNA component of RNase MRP) gene are the molecular cause for CHH. Mutations in this gene are also responsible for other disorders connected with skeletal abnormalities known as the CHH-AD spectrum. Recently a second form of anauxetic dysplasia (AD) not caused by mutations in RMRP but by mutations in POP1, a protein included in the RNase MRP complex [43], has been reported.
RMRP is a long non-coding RNA (lncRNA) that contributes to the formation of the RNase MRP complex with at least seven different proteins, some of which (e.g., POP1) shared with the RNase P complex and implied in tRNA maturation [97]. This complex has many functions in cell nucleus, cytoplasm, and mitochondria that were originally identified in yeast but also described in human cells. In fact, the RMRP complex is an endoribonuclease responsible for the cleavage of mitochondrial RNA, which functions as a primer for mitochondrial DNA replication [98], the cleavage of the 5′UTR of the cyclin B2 mRNA, which leads to a reduction in cyclin B2 synthesis, and cell cycle progression [99]. Recently, viperin (RSAD2) has been described as a novel mRNA substrate for RMRP cleavage [100]. An additional important function of RMRP lncRNA consists of the formation of a complex with the telomerase-associated reverse transcriptase (TERT), which produces a double-stranded RNA with RMRP sequence. These RNA duplexes are cleaved in siRNA by Dicer with the outcome of downregulating the level of RMRP [101]. At this regard, although this latter mechanism does not appear to affect telomerase function, it is worth noting that in two mixed ribosomopathies such as DC and in CHH components of the telomerase complex are involved. Lastly, RMRP is reported to cleave the precursor ribosomal RNA in the ITS1 sequence, leading to the formation of the 5′ end of 5.8S rRNA (as described previously) [102]. This has been confirmed in a recent study in which the disruption of RMRP by CRISPR/Cas9 in HeLa cells led to the accumulation of uncleaved ITS1 [103]. The impairment of this function can cause an altered ribosome biogenesis that makes possible to classify CHH as a ribosomopathy.
Although it is clear that CHH is caused by mutations in the RMRP gene, the molecular mechanism underlying the development of the disease is still unclear. A significant number of mutations in the RMRP gene have been identified in patients with CHH [104,105], which can involve, in particular, the promoter of RMRP or the transcribed region [8]. The first consists of insertions or duplications in the region between the transcription starting site and the TATA box, with the outcome of altering the correct distance between the promoter and the transcription starting site, while reducing the transcription level of RMRP. These types of mutations were found mainly in compound heterozygous patients [102], suggesting that at least a minimum level of RMRP is essential for cell life [106]. On the other hand, point mutations or the insertion/deletion of a few nucleotides in the RMRP sequence, in evolutionarily conserved regions important for RNA-protein interaction [107] or for the catalytic activity, result in either a reduced efficiency of the complex or an alteration of RMRP stability [108]. For instance, it has been reported that the most common mutation among the Amish and Finnish patients, but also the most prevalent in the European population, 70A > G, can be found in the putative catalytic pocket, thus reducing the RNase activity [99,109]. In addition, the 70A > G mutation found in CHH patients introduced in the yeast ortholog NME1 resulted in a 5.8Ss/l ratio of 2:3 in comparison with the 10:1 ratio of wild type strains [109].
In the attempt to find a potential causative relationship between the different functions of RMRP and CHH development, it has been reported that RMRP is expressed during the hypertrophic phase of chondrocyte differentiation in mice, and that the trans-differentiation in the chondrocytes from CHH patients’ fibroblasts is impaired in comparison with control fibroblasts. Moreover, CHH fibroblasts show an increase in the ITS1 pre-rRNA processing intermediate, suggesting a reduced ribosome biogenesis [110]. On the other hand, other functions of RMRP can contribute to the development of the pathology. As it was previously described, viperin is a substrate for the endoribonucleolytic activity of RMRP. This protein appears to be involved in the activation of a chondrogenesis regulatory pathway accountable for the reduced chondrocyte differentiation of fibroblasts from CHH patients with mutated RMRP [111]. Moreover, the sequencing analysis of RNA from fibroblasts of CHH patients compared to controls revealed a downregulation in the genes involved in the cell cycle, resulting in a delay in the progression from phase G2 to G1 [112]. Lastly, a recent study reported that the processing of RMRP by Dicer originates two small RNAs with silencing activity on the genes associated with CHH-phenotype [113].
The studies reported on so far highlight the numerous aspects in which RMRP is involved, and all of them can contribute to the pathophysiology of the CHH-AD spectrum: therefore, CHH can rightly be considered a mixed ribosomopathy.
4. Recently Identified Ribosomopathies
In recent years, there has been an increase in the number of diseases identified as novel congenital ribosomopathies. These extremely rare diseases are characterized by mutations in ribosomal proteins or in factors involved in ribosome biogenesis, but further studies are necessary to fully understand the contribution of altered ribosome production in their pathophysiology. These ribosomopathies are heterogeneous diseases showing generalized multisystemic symptoms or, alternatively, more specific manifestations selective for one tissue or organ. An example of multisystemic disease is Bowen-Conradi syndrome, a rare autosomal recessive disorder first described by Bowen and Conradi [114] in the Hutterite population, which is characterized by mental retardation, microcephaly, micrognathia, prominent nose, rocker bottom feet, and flexion contractures of the joints [115]. This severe disease is the cause of early death in children; in fact, the average death age is 13 months. Recently, it has been reported that the cause of Bowen-Conradi syndrome is a mutation in the gene EMG1, coding for a ribosome assembly protein, thus including this disease in the list of ribosomopathies [116]. The mutation in EMG1 causes 18S rRNA processing delay, with the result of reduced cell proliferation rates and G2/M arrest [117].
A more limited and specific effect is that caused by mutations in a ribosomal protein of the small subunit, RPS20. The mutation in RPS20 has been associated with a subtype of hereditary colorectal cancer (CRC) called Familial colorectal cancer type X (FCCTX), in which no mutation in mismatch repair genes was reported, but several pathogenic variants of predisposing genes were observed [118,119]. Two different heterozygous mutations of RPS20 were reported in a CRC-affected family and in a patient with hereditary CRC. The experiment conducted on samples from the CRC-affected family showed that the haploinsufficiency of RPS20 caused a reduced ribosome biogenesis and consequent stabilization of p53, which is probably responsible for the selection of cells that escape p53 regulation [118,119]. Since, for the two examples mentioned above at present, no additional extra-ribosomal functions of the products of the genes involved have been reported, they may be considered putative pure ribosomopathies although further research is necessary to confirm this definition.
A further example of the tissue-specific effect of mutations in ribosomal protein is represented by the outcome of mutations in RPL10. In fact, it has been reported that missense mutations causing an alteration in protein sequence can lead to a rare form of autism [120] or microcephaly [121,122]. A defective nervous system development can be caused by a decreased translational capacity of the cell coupled with an increased apoptosis due to the activation of ribosomal stress response. For RPL10, however, extra-ribosomal functions have also been reported [123]. Ribosomal protein L10 in mitochondria serves as a regulator for the ROS level in pancreatic cancer cells [123,124,125]; therefore, pending a more detailed characterization of the molecular pathogenesis of this disorder, it may be considered a putative mixed ribosomopathy.
In addition to the above-mentioned disorders, other recently identified ribosomopathies have been described. These conditions are listed in Table 2.
Table 2.
Recently identified ribosomopathies.
| Recently Identified Ribosomopathies | |||
|---|---|---|---|
| Disease | Gene Mutated | Role in Ribosome Biogenesis | Clinical Manifestations |
| Alopecia, neurologic defects, and endocrinopathy syndrome | RBM28 [126] | Ribosomal RNA processing | Alopecia, mental retardation, progressive motor deterioration, central hypogonadotropic hypogonadism and short stature, microcephaly, gynecomastia, and hypodontia |
| North American Indian Childhood Cirrhosis |
CIRHIN [127] NOL11 [128] |
18S rRNA processing | Transient neonatal jaundice that evolves into biliary cirrhosis requiring hepatic transplantation |
| Bowen-Conradi syndrome | EMG1 [116] | Ribosome assembly | Mental retardation, microcephaly, micrognathia, rocker bottom feet, and flexion contractures of the joints; causes early death |
| Familial colorectal cancer type X | RPS20 [118] | 40S subunit protein | Hereditary colorectal cancer without mutations in mismatch repair genes |
| Congenital asplenia | RPSA [129] | 40S subunit protein | Absence of spleen |
| Aplasia cutis congenita | BMS1 [130] | Ribosomal GTPase, 18S rRNA processing | Skin defect and alopecia of the scalp |
| RPS23-related ribosomopathy | RPS23 [131] | 40S subunit protein | Microcephaly, hearing loss, dysmorphic features, intellectual disability, and autism spectrum disorder |
| Leukoencephalopathy, intracranial calcifications, and cysts (LCC) | SNORD118 [132] | C/D box snoRNA U8 involved in ribosome biogenesis | Neurological disorder with leukoencephalopathy, intracranial calcifications, and cysts |
| Autism | RPL10 [120] | 60S subunit protein | Autism spectrum disorder |
| Microcephaly | RPL10 [121,122] | 60S subunit protein | Microcephaly, intellectual disability, epilepsy, and growth retardation |
5. Acquired Ribosomopathies
In addition to inherited ribosomopathies, a defect in the gene encoding for a ribosomal protein is also an underlying factor in an acquired myelodysplastic disorder termed 5q deletion (or 5q−) syndrome. This disorder, which is more frequently found in women over 75 years of age, is due to the somatic deletion of the short arm of chromosome 5, leading to macrocytic anemia and erythroid hypoplasia, which may subsequently progress to AML in some patients [133]. The haploinsufficiency of the RPS14 gene has been identified by means of an RNA interference-based screening as the predominant cause of the myelodysplastic phenotype in 5q− syndrome [11], indicating that the alteration in the ribosome biogenesis process may also be at the root of acquired disorders. Since for RPS14 no additional extra-ribosomal functions have been reported, 5q− syndrome may be considered a pure acquired ribosomopathy.
It has long been known that the process of ribosome biogenesis is highly deregulated in cancer [12,13], suggesting that a subset of human tumors may also be considered acquired ribosomopathies. Mutations of NPM1 gene encoding the multifunctional ribosome processing factor nucleophosmin have been described as the most frequent mutation in acute myeloid leukemia [133,134]. Whereas mutations of genes encoding for ribosomal proteins have been reported for the first time in pediatric acute lymphoblastic leukemia, where recurrent mutations of RPL10 and RPL5 genes have been found in approximately 10% of all cases [135]. Very interestingly, a large-scale study on more than 10,000 genomes from human tumors of different origins indicated that the hemizygous deletions of ribosomal protein genes occur in more than 40% of the cases [136]. In addition, a growing amount of data has become available on snoRNA mutations and expression alterations in human multiple cancer types [137,138]. All these studies indicate that as ribosome biogenesis deregulation is a frequent feature in cancer. In many cases, cancer itself may be considered, at least to some extent, an acquired ribosomopathy. The exact role of most of these ribosome biogenesis alterations, however, still remains to be determined.
A list of acquired ribosomopathies is also shown in Table 3.
Table 3.
Acquired ribosomopathies.
| Acquired Ribosomopathies | |||
|---|---|---|---|
| Disease | Gene Mutated | Role in Ribosome Biogenesis | Clinical Manifestations |
| 5q− syndrome | RPS14 [11] | 40S subunit protein | Macrocytic anemia and erythroid hypoplasia; may progress to AML |
| Acute myeloid leukemia (AML) | NPM1 [139] | Ribosome processing | AML with normal karyotype |
| Pediatric acute lymphoblastic leukemia (T-ALL) |
RPL5, RPL10, RPL22 [135] |
60S subunit proteins | T-ALL |
| Relapsed CLL | RPS15, RPSA, RPS20 [140] | 40S subunit proteins | Relapse after first-line treatment |
Acknowledgments
The authors are grateful to the members of the Montanaro’s Lab for helpful discussion.
Funding
This research was funded by Roberto and Cornelia Pallotti Legacy for Cancer Research.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kampen K.R., Sulima S.O., Vereecke S., De Keersmaecker K. Hallmarks of ribosomopathies. Nucleic Acids Res. 2019;48:1013–1028. doi: 10.1093/nar/gkz637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farley-Barnes K.I., Ogawa L.M., Baserga S.J. Ribosomopathies: Old Concepts, New Controversies. Trends Genet. 2019;35:754–767. doi: 10.1016/j.tig.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luzzatto L., Karadimitris A. Dyskeratosis and ribosomal rebellion. Nat. Genet. 1998;19:6–7. doi: 10.1038/ng0598-6. [DOI] [PubMed] [Google Scholar]
- 4.Heiss N.S., Knight S.W., Vulliamy T.J., Klauck S.M., Wiemann S., Mason P.J., Poustka A., Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat. Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 5.Ban N., Beckmann R., Cate J.H., Dinman J.D., Dragon F., Ellis S.R., Lafontaine D.L., Lindahl L., Liljas A., Lipton J.M., et al. A new system for naming ribosomal proteins. Curr. Opin. Struct. Biol. 2014;24:165–169. doi: 10.1016/j.sbi.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Draptchinskaia N., Gustavsson P., Andersson B., Pettersson M., Willig T.N., Dianzani I., Ball S., Tchernia G., Klar J., Matsson H., et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anemia. Nat. Genet. 1999;21:169–175. doi: 10.1038/5951. [DOI] [PubMed] [Google Scholar]
- 7.Boocock G.R., Morrison J.A., Popovic M., Richards N., Ellis L., Durie P.R., Rommens J.M. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat. Genet. 2003;33:97–101. doi: 10.1038/ng1062. [DOI] [PubMed] [Google Scholar]
- 8.Ridanpaa M., van Eenennaam H., Pelin K., Chadwick R., Johnson C., Yuan B., van Venrooij W., Pruijn G., Salmela R., Rockas S., et al. Mutations in the RNA component of RNase MRP cause a pleiotropic human disease, cartilage-hair hypoplasia. Cell. 2001;104:195–203. doi: 10.1016/S0092-8674(01)00205-7. [DOI] [PubMed] [Google Scholar]
- 9.Group T.T.C.S.C., Dixon J., Edwards S.J., Gladwin A.J., Dixon M.J., Loftus S.K., Bonner C.A., Koprivnikar K., Wasmuth J.J. Positional cloning of a gene involved in the pathogenesis of Treacher Collins syndrome. The Treacher Collins Syndrome Collaborative Group. Nat. Genet. 1996;12:130–136. doi: 10.1038/ng0296-130. [DOI] [PubMed] [Google Scholar]
- 10.Narla A., Ebert B.L. Ribosomopathies: Human disorders of ribosome dysfunction. Blood. 2010;115:3196–3205. doi: 10.1182/blood-2009-10-178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebert B.L., Pretz J., Bosco J., Chang C.Y., Tamayo P., Galili N., Raza A., Root D.E., Attar E., Ellis S.R., et al. Identification of RPS14 as a 5q− syndrome gene by RNA interference screen. Nature. 2008;451:335–339. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montanaro L., Treré D., Derenzini M. Nucleolus, ribosomes, and cancer. Am. J. Pathol. 2008;173:301–310. doi: 10.2353/ajpath.2008.070752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penzo M., Montanaro L., Trere D., Derenzini M. The Ribosome Biogenesis-Cancer Connection. Cells. 2019;8:55. doi: 10.3390/cells8010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henras A.K., Plisson-Chastang C., O’Donohue M.F., Chakraborty A., Gleizes P.E. An overview of pre-ribosomal RNA processing in eukaryotes. Wiley Interdiscip. Rev. RNA. 2015;6:225–242. doi: 10.1002/wrna.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullineux S.T., Lafontaine D.L. Mapping the cleavage sites on mammalian pre-rRNAs: Where do we stand? Biochimie. 2012;94:1521–1532. doi: 10.1016/j.biochi.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Henras A.K., Soudet J., Gerus M., Lebaron S., Caizergues-Ferrer M., Mougin A., Henry Y. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell Mol. Life Sci. 2008;65:2334–2359. doi: 10.1007/s00018-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McStay B. Nucleolar organizer regions: Genomic’dark matter’ requiring illumination. Genes. Dev. 2016;30:1598–1610. doi: 10.1101/gad.283838.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paule M.R., White R.J. Survey and summary: Transcription by RNA polymerases I and III. Nucleic Acids Res. 2000;28:1283–1298. doi: 10.1093/nar/28.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bersaglieri C., Santoro R. Genome Organization in and around the Nucleolus. Cells. 2019;8:579. doi: 10.3390/cells8060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watkins N.J., Bohnsack M.T. The box C/D and H/ACA snoRNPs: Key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip. Rev. RNA. 2012;3:397–414. doi: 10.1002/wrna.117. [DOI] [PubMed] [Google Scholar]
- 21.Sharma S., Yang J., van Nues R., Watzinger P., Kötter P., Lafontaine D.L.J., Granneman S., Entian K.D. Specialized box C/D snoRNPs act as antisense guides to target RNA base acetylation. PLoS Genet. 2017;13:e1006804. doi: 10.1371/journal.pgen.1006804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massenet S., Bertrand E., Verheggen C. Assembly and trafficking of box C/D and H/ACA snoRNPs. RNA Biol. 2017;14:680–692. doi: 10.1080/15476286.2016.1243646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang X.H., Liu Q., Fournier M.J. Loss of rRNA modifications in the decoding center of the ribosome impairs translation and strongly delays pre-rRNA processing. RNA. 2009;15:1716–1728. doi: 10.1261/rna.1724409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack K., Bellodi C., Landry D.M., Niederer R.O., Meskauskas A., Musalgaonkar S., Kopmar N., Krasnykh O., Dean A.M., Thompson S.R., et al. RNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol. Cell. 2011;44:660–666. doi: 10.1016/j.molcel.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojha S., Malla S., Lyons S.M. SnoRNPs: Functions in Ribosome Biogenesis. Biomolecules. 2020;10:783. doi: 10.3390/biom10050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panse V.G., Johnson A.W. Maturation of eukaryotic ribosomes: Acquisition of functionality. Trends Biochem. Sci. 2010;35:260–266. doi: 10.1016/j.tibs.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weis F., Giudice E., Churcher M., Jin L., Hilcenko C., Wong C.C., Traynor D., Kay R.R., Warren A.J. Mechanism of eIF6 release from the nascent 60S ribosomal subunit. Nat. Struct. Mol. Biol. 2015;22:914–919. doi: 10.1038/nsmb.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebaron S., Schneider C., van Nues R.W., Swiatkowska A., Walsh D., Böttcher B., Granneman S., Watkins N.J., Tollervey D. Proofreading of pre-40S ribosome maturation by a translation initiation factor and 60S subunits. Nat. Struct. Mol. Biol. 2012;19:744–753. doi: 10.1038/nsmb.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Keersmaecker K., Sulima S.O., Dinman J.D. Ribosomopathies and the paradox of cellular hypo- to hyperproliferation. Blood. 2015;125:1377–1382. doi: 10.1182/blood-2014-10-569616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirabello L., Macari E.R., Jessop L., Ellis S.R., Myers T., Giri N., Taylor A.M., McGrath K.E., Humphries J.M., Ballew B.J., et al. Whole-exome sequencing and functional studies identify RPS29 as a novel gene mutated in multicase Diamond-Blackfan anemia families. Blood. 2014;124:24–32. doi: 10.1182/blood-2013-11-540278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gripp K.W., Curry C., Olney A.H., Sandoval C., Fisher J., Chong J.X., Genomics UWC f M., Pilchman L., Sahraoui R., Stabley D.L., et al. Diamond-Blackfan anemia with mandibulofacial dysostosis is heterogeneous, including the novel DBA genes TSR2 and RPS28. Am. J. Med. Genet. 2014;164A:2240–2249. doi: 10.1002/ajmg.a.36633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boria I., Garelli E., Gazda H.T., Aspesi A., Quarello P., Pavesi E., Ferrante D., Meerpohl J.J., Kartal M., Da Costa L., et al. The ribosomal basis of Diamond-Blackfan Anemia: Mutation and database update. Hum. Mutat. 2010;31:1269–1279. doi: 10.1002/humu.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirabello L., Khincha P.P., Ellis S.R., Giri N., Brodie S., Chandrasekharappa S.C., Donovan F.X., Zhou W., Hicks B.D., Boland J.F., et al. Novel and known ribosomal causes of Diamond-Blackfan anaemia identified through comprehensive genomic characterisation. J. Med. Genet. 2017;54:417–425. doi: 10.1136/jmedgenet-2016-104346. [DOI] [PubMed] [Google Scholar]
- 34.Gazda H.T., Preti M., Sheen M.R., O’Donohue M.F., Vlachos A., Davies S.M., Kattamis A., Doherty L., Landowski M., Buros C., et al. Frameshift mutation in p53 regulator RPL26 is associated with multiple physical abnormalities and a specific pre-ribosomal RNA processing defect in diamond-blackfan anemia. Hum. Mutat. 2012;33:1037–1044. doi: 10.1002/humu.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landowski M., O’Donohue M.F., Buros C., Ghazvinian R., Montel-Lehry N., Vlachos A., Sieff C.A., Newburger P.E., Niewiadomska E., Matysiak M., et al. Novel deletion of RPL15 identified by array-comparative genomic hybridization in Diamond-Blackfan anemia. Hum. Genet. 2013;132:1265–1274. doi: 10.1007/s00439-013-1326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang R., Yoshida K., Toki T., Sawada T., Uechi T., Okuno Y., Sato-Otsubo A., Kudo K., Kamimaki I., Kanezaki R., et al. Loss of function mutations in RPL27 and RPS27 identified by whole-exome sequencing in Diamond-Blackfan anaemia. Br. J. Haematol. 2015;168:854–864. doi: 10.1111/bjh.13229. [DOI] [PubMed] [Google Scholar]
- 37.Tummala H., Walne A.J., Williams M., Bockett N., Collopy L., Cardoso S., Ellison A., Wynn R., Leblanc T., Fitzgibbon J., et al. DNAJC21 Mutations Link a Cancer-Prone Bone Marrow Failure Syndrome to Corruption in 60S Ribosome Subunit Maturation. Am. J. Hum. Genet. 2016;99:115–124. doi: 10.1016/j.ajhg.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stepensky P., Chacon-Flores M., Kim K.H., Abuzaitoun O., Bautista-Santos A., Simanovsky N., Siliqi D., Altamura D., Mendez-Godoy A., Gijsbers A., et al. Mutations in EFL1, an SBDS partner, are associated with infantile pancytopenia, exocrine pancreatic insufficiency and skeletal anomalies in a Shwachman-Diamond like syndrome. J. Med. Genet. 2017;54:558–566. doi: 10.1136/jmedgenet-2016-104366. [DOI] [PubMed] [Google Scholar]
- 39.Carapito R., Konantz M., Paillard C., Miao Z., Pichot A., Leduc M.S., Yang Y., Bergstrom K.L., Mahoney D.H., Shardy D.L., et al. Mutations in signal recognition particle SRP54 cause syndromic neutropenia with Shwachman-Diamond-like features. J. Clin. Investig. 2017;127:4090–4103. doi: 10.1172/JCI92876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teber O.A., Gillessen-Kaesbach G., Fischer S., Bohringer S., Albrecht B., Albert A., Arslan-Kirchner M., Haan E., Hagedorn-Greiwe M., Hammans C., et al. Genotyping in 46 patients with tentative diagnosis of Treacher Collins syndrome revealed unexpected phenotypic variation. Eur. J. Hum. Genet. 2004;12:879–890. doi: 10.1038/sj.ejhg.5201260. [DOI] [PubMed] [Google Scholar]
- 41.Dauwerse J.G., Dixon J., Seland S., Ruivenkamp C.A., van Haeringen A., Hoefsloot L.H., Peters D.J., Boers A.C., Daumer-Haas C., Maiwald R., et al. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat. Genet. 2011;43:20–22. doi: 10.1038/ng.724. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez E., Laplace-Builhe B., Mau-Them F.T., Richard E., Goldenberg A., Toler T.L., Guignard T., Gatinois V., Vincent M., Blanchet C., et al. POLR1B and neural crest cell anomalies in Treacher Collins syndrome type 4. Genet. Med. 2020;22:547–556. doi: 10.1038/s41436-019-0669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elalaoui S.C., Laarabi F.Z., Mansouri M., Mrani N.A., Nishimura G., Sefiani A. Further evidence of POP1 mutations as the cause of anauxetic dysplasia. Am. J. Med. Genet. A. 2016;170:2462–2465. doi: 10.1002/ajmg.a.37839. [DOI] [PubMed] [Google Scholar]
- 44.Lafontaine D.L., Bousquet-Antonelli C., Henry Y., Caizergues-Ferrer M., Tollervey D. The box H + ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase. Genes. Dev. 1998;12:527–537. doi: 10.1101/gad.12.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishikawa H., Yoshikawa H., Izumikawa K., Miura Y., Taoka M., Nobe Y., Yamauchi Y., Nakayama H., Simpson R.J., Isobe T., et al. Poly(A)-specific ribonuclease regulates the processing of small-subunit rRNAs in human cells. Nucleic Acids Res. 2017;45:3437–3447. doi: 10.1093/nar/gkw1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montellese C., Montel-Lehry N., Henras A.K., Kutay U., Gleizes P.E., O’Donohue M.F. Poly(A)-specific ribonuclease is a nuclear ribosome biogenesis factor involved in human 18S rRNA maturation. Nucleic Acids Res. 2017;45:6822–6836. doi: 10.1093/nar/gkx253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vulliamy T., Beswick R., Kirwan M., Marrone A., Digweed M., Walne A., Dokal I. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc. Natl. Acad. Sci. USA. 2008;105:8073–8078. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walne A.J., Vulliamy T., Marrone A., Beswick R., Kirwan M., Masunari Y., Al-Qurashi F.H., Aljurf M., Dokal I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum. Mol. Genet. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trahan C., Martel C., Dragon F. Effects of dyskeratosis congenita mutations in dyskerin, NHP2 and NOP10 on assembly of H/ACA pre-RNPs. Hum. Mol. Genet. 2010;19:825–836. doi: 10.1093/hmg/ddp551. [DOI] [PubMed] [Google Scholar]
- 50.Nachmani D., Bothmer A.H., Grisendi S., Mele A., Bothmer D., Lee J.D., Monteleone E., Cheng K., Zhang Y., Bester A.C., et al. Germline NPM1 mutations lead to altered rRNA 2’-O-methylation and cause dyskeratosis congenita. Nat. Genet. 2019;51:1518–1529. doi: 10.1038/s41588-019-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vlachos A., Ball S., Dahl N., Alter B.P., Sheth S., Ramenghi U., Meerpohl J., Karlsson S., Liu J.M., Leblanc T., et al. Participants of Sixth Annual Daniella Maria Arturi International Consensus, C. Diagnosing and treating Diamond Blackfan anaemia: Results of an international clinical consensus conference. Br. J. Haematol. 2008;142:859–876. doi: 10.1111/j.1365-2141.2008.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khajuria R.K., Munschauer M., Ulirsch J.C., Fiorini C., Ludwig L.S., McFarland S.K., Abdulhay N.J., Specht H., Keshishian H., Mani D.R., et al. Ribosome Levels Selectively Regulate Translation and Lineage Commitment in Human Hematopoiesis. Cell. 2018;173:90–103. doi: 10.1016/j.cell.2018.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flygare J., Aspesi A., Bailey J.C., Miyake K., Caffrey J.M., Karlsson S., Ellis S.R. Human RPS19, the gene mutated in Diamond-Blackfan anemia, encodes a ribosomal protein required for the maturation of 40S ribosomal subunits. Blood. 2007;109:980–986. doi: 10.1182/blood-2006-07-038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choesmel V., Bacqueville D., Rouquette J., Noaillac-Depeyre J., Fribourg S., Cretien A., Leblanc T., Tchernia G., Da Costa L., Gleizes P.E. Impaired ribosome biogenesis in Diamond-Blackfan anemia. Blood. 2007;109:1275–1283. doi: 10.1182/blood-2006-07-038372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marechal V., Elenbaas B., Piette J., Nicolas J.C., Levine A.J. The ribosomal L5 protein is associated with mdm-2 and mdm-2-p53 complexes. Mol. Cell. Biol. 1994;14:7414–7420. doi: 10.1128/MCB.14.11.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai M.S., Lu H. Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J. Biol. Chem. 2004;279:44475–44482. doi: 10.1074/jbc.M403722200. [DOI] [PubMed] [Google Scholar]
- 57.Lohrum M.A., Ludwig R.L., Kubbutat M.H., Hanlon M., Vousden K.H. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell. 2003;3:577–587. doi: 10.1016/S1535-6108(03)00134-X. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Wolf G.W., Bhat K., Jin A., Allio T., Burkhart W.A., Xiong Y. Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol. Cell. Biol. 2003;23:8902–8912. doi: 10.1128/MCB.23.23.8902-8912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulirsch J.C., Verboon J.M., Kazerounian S., Guo M.H., Yuan D., Ludwig L.S., Handsaker R.E., Abdulhay N.J., Fiorini C., Genovese G., et al. The Genetic Landscape of Diamond-Blackfan Anemia. Am. J. Hum. Genet. 2018;103:930–947. doi: 10.1016/j.ajhg.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bezzerri V., Cipolli M. Shwachman-Diamond Syndrome: Molecular Mechanisms and Current Perspectives. Mol. Diagn. 2019;23:281–290. doi: 10.1007/s40291-018-0368-2. [DOI] [PubMed] [Google Scholar]
- 61.Warren A.J. Molecular basis of the human ribosomopathy Shwachman-Diamond syndrome. Adv. Biol. Regul. 2018;67:109–127. doi: 10.1016/j.jbior.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shwachman H., Diamond L.K., Oski F.A., Khaw K.T. The Syndrome of Pancreatic Insufficiency and Bone Marrow Dysfunction. J. Pediatr. 1964;65:645–663. doi: 10.1016/S0022-3476(64)80150-5. [DOI] [PubMed] [Google Scholar]
- 63.Finch A.J., Hilcenko C., Basse N., Drynan L.F., Goyenechea B., Menne T.F., Gonzalez Fernandez A., Simpson P., D’Santos C.S., Arends M.J., et al. Uncoupling of GTP hydrolysis from eIF6 release on the ribosome causes Shwachman-Diamond syndrome. Genes. Dev. 2011;25:917–929. doi: 10.1101/gad.623011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tourlakis M.E., Zhang S., Ball H.L., Gandhi R., Liu H., Zhong J., Yuan J.S., Guidos C.J., Durie P.R., Rommens J.M. In Vivo Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency. PLoS Genet. 2015;11:e1005288. doi: 10.1371/journal.pgen.1005288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valdez B.C., Henning D., So R.B., Dixon J., Dixon M.J. The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor. Proc. Natl. Acad. Sci. USA. 2004;101:10709–10714. doi: 10.1073/pnas.0402492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dixon J., Dixon M.J. Genetic background has a major effect on the penetrance and severity of craniofacial defects in mice heterozygous for the gene encoding the nucleolar protein Treacle. Dev. DYN. 2004;229:907–914. doi: 10.1002/dvdy.20004. [DOI] [PubMed] [Google Scholar]
- 67.Noack Watt K.E., Achilleos A., Neben C.L., Merrill A.E., Trainor P.A. The Roles of RNA Polymerase I and III Subunits Polr1c and Polr1d in Craniofacial Development and in Zebrafish Models of Treacher Collins Syndrome. PLoS Genet. 2016;12:e1006187. doi: 10.1371/journal.pgen.1006187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dixon J., Jones N.C., Sandell L.L., Jayasinghe S.M., Crane J., Rey J.P., Dixon M.J., Trainor P.A. Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc. Natl. Acad. Sci. USA. 2006;103:13403–13408. doi: 10.1073/pnas.0603730103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones N.C., Lynn M.L., Gaudenz K., Sakai D., Aoto K., Rey J.P., Glynn E.F., Ellington L., Du C., Dixon J., et al. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat. Med. 2008;14:125–133. doi: 10.1038/nm1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ciccia A., Huang J.W., Izhar L., Sowa M.E., Harper J.W., Elledge S.J. Treacher Collins syndrome TCOF1 protein cooperates with NBS1 in the DNA damage response. Proc. Natl. Acad. Sci. USA. 2014;111:18631–18636. doi: 10.1073/pnas.1422488112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larsen D.H., Hari F., Clapperton J.A., Gwerder M., Gutsche K., Altmeyer M., Jungmichel S., Toledo L.I., Fink D., Rask M.B., et al. The NBS1-Treacle complex controls ribosomal RNA transcription in response to DNA damage. Nat. Cell. Biol. 2014;16:792–803. doi: 10.1038/ncb3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakai D., Dixon J., Achilleos A., Dixon M., Trainor P.A. Prevention of Treacher Collins syndrome craniofacial anomalies in mouse models via maternal antioxidant supplementation. Nat. Commun. 2016;7:10328. doi: 10.1038/ncomms10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zinsser F. Atropha cutis reticularis pigmentation, dystrophia ungiumet leukoplakia oris. Ikonogr. Derm. 1906;5:219–223. [Google Scholar]
- 74.Engman M.F. A unique case of reticular pigmentation of the skin with atrophy. Arch. Bleg. Derm. Syphil. 1926;13:685–687. [Google Scholar]
- 75.Cole H.N., Rauschkolb J.E., Toomey J. Dyskeratosis congenita with pigmentation, dystrophia unguis and leukokeratosis oris. Arch. Dermatol. Syphilol. 1930;21:71–95. doi: 10.1001/archderm.1930.01440070079008. [DOI] [PubMed] [Google Scholar]
- 76.Dokal I. Dyskeratosis congenita. Hematol. Am. Soc. Hematol. Educ. Program. 2011;2011:480–486. doi: 10.1182/asheducation-2011.1.480. [DOI] [PubMed] [Google Scholar]
- 77.Alter B.P., Giri N., Savage S.A., Rosenberg P.S. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ganot P., Bortolin M.L., Kiss T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell. 1997;89:799–809. doi: 10.1016/S0092-8674(00)80263-9. [DOI] [PubMed] [Google Scholar]
- 79.Wang C., Meier U.T. Architecture and assembly of mammalian H/ACA small nucleolar and telomerase ribonucleoproteins. EMBO J. 2004;23:1857–1867. doi: 10.1038/sj.emboj.7600181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu Y.T., Meier U.T. RNA-guided isomerization of uridine to pseudouridine—Pseudouridylation. RNA Biol. 2014;11:1483–1494. doi: 10.4161/15476286.2014.972855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitchell J.R., Wood E., Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 82.Vulliamy T., Marrone A., Goldman F., Dearlove A., Bessler M., Mason P.J., Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 83.Marrone A., Walne A., Tamary H., Masunari Y., Kirwan M., Beswick R., Vulliamy T., Dokal I. Telomerase reverse-transcriptase homozygous mutations in autosomal recessive dyskeratosis congenita and Hoyeraal-Hreidarsson syndrome. Blood. 2007;110:4198–4205. doi: 10.1182/blood-2006-12-062851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Savage S.A., Giri N., Baerlocher G.M., Orr N., Lansdorp P.M., Alter B.P. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am. J. Hum. Genet. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Savage S.A. In: Gene Reviews. Adam M.P., editor. University of Washington; Seattle, WA, USA: 1993. [Google Scholar]
- 86.Giordano E., Peluso I., Senger S., Furia M. minifly, a Drosophila gene required for ribosome biogenesis. J. Cell Biol. 1999;144:1123–1133. doi: 10.1083/jcb.144.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruggero D., Grisendi S., Piazza F., Rego E., Mari F., Rao P.H., Cordon-Cardo C., Pandolfi P.P. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- 88.Blasco M.A., Lee H.W., Hande M.P., Samper E., Lansdorp P.M., DePinho R.A., Greider C.W. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/S0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 89.McKusick V.A., Eldridge R., Hostetler J.A., Ruangwit U., Egeland J.A. Dwarfism in the Amish. Ii. Cartilage-Hair Hypoplasia. Bull. Johns Hopkins Hosp. 1965;116:285–326. [PubMed] [Google Scholar]
- 90.Makitie O., Kaitila I. Cartilage-hair hypoplasia—clinical manifestations in 108 Finnish patients. Eur. J. Pediatr. 1993;152:211–217. doi: 10.1007/BF01956147. [DOI] [PubMed] [Google Scholar]
- 91.Taskinen M., Ranki A., Pukkala E., Jeskanen L., Kaitila I., Makitie O. Extended follow-up of the Finnish cartilage-hair hypoplasia cohort confirms high incidence of non-Hodgkin lymphoma and basal cell carcinoma. Am. J. Med. Genet. A. 2008;146:2370–2375. doi: 10.1002/ajmg.a.32478. [DOI] [PubMed] [Google Scholar]
- 92.Makitie O., Heikkinen M., Kaitila I., Rintala R. Hirschsprung’s disease in cartilage-hair hypoplasia has poor prognosis. J. Pediatr. Surg. 2002;37:1585–1588. doi: 10.1053/jpsu.2002.36189. [DOI] [PubMed] [Google Scholar]
- 93.Kostjukovits S., Klemetti P., Fohr A., Kajosaari M., Valta H., Taskinen M., Toiviainen-Salo S., Makitie O. High prevalence of bronchiectasis in patients with cartilage-hair hypoplasia. J. Allergy Clin. Immunol. 2017;139:375–378. doi: 10.1016/j.jaci.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 94.Makitie O.M., Tapanainen P.J., Dunkel L., Siimes M.A. Impaired spermatogenesis: An unrecognized feature of cartilage-hair hypoplasia. Ann. Med. 2001;33:201–205. doi: 10.3109/07853890109002078. [DOI] [PubMed] [Google Scholar]
- 95.Vakkilainen S., Makitie R., Klemetti P., Valta H., Taskinen M., Husebye E.S., Makitie O. A Wide Spectrum of Autoimmune Manifestations and Other Symptoms Suggesting Immune Dysregulation in Patients with Cartilage-Hair Hypoplasia. Front. Immunol. 2018;9:2468. doi: 10.3389/fimmu.2018.02468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vakkilainen S., Taskinen M., Klemetti P., Pukkala E., Makitie O. A 30-Year Prospective Follow-Up Study Reveals Risk Factors for Early Death in Cartilage-Hair Hypoplasia. Front. Immunol. 2019;10:1581. doi: 10.3389/fimmu.2019.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Welting T.J., van Venrooij W.J., Pruijn G.J. Mutual interactions between subunits of the human RNase MRP ribonucleoprotein complex. Nucleic Acids Res. 2004;32:2138–2146. doi: 10.1093/nar/gkh539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang D.D., Clayton D.A. A mammalian mitochondrial RNA processing activity contains nucleus-encoded RNA. Science. 1987;235:1178–1184. doi: 10.1126/science.2434997. [DOI] [PubMed] [Google Scholar]
- 99.Thiel C.T., Horn D., Zabel B., Ekici A.B., Salinas K., Gebhart E., Ruschendorf F., Sticht H., Spranger J., Muller D., et al. Severely incapacitating mutations in patients with extreme short stature identify RNA-processing endoribonuclease RMRP as an essential cell growth regulator. Am. J. Hum. Genet. 2005;77:795–806. doi: 10.1086/497708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mattijssen S., Hinson E.R., Onnekink C., Hermanns P., Zabel B., Cresswell P., Pruijn G.J. Viperin mRNA is a novel target for the human RNase MRP/RNase P endoribonuclease. Cell Mol. Life Sci. 2011;68:2469–2480. doi: 10.1007/s00018-010-0568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maida Y., Yasukawa M., Furuuchi M., Lassmann T., Possemato R., Okamoto N., Kasim V., Hayashizaki Y., Hahn W.C., Masutomi K. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature. 2009;461:230–235. doi: 10.1038/nature08283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thiel C.T., Mortier G., Kaitila I., Reis A., Rauch A. Type and level of RMRP functional impairment predicts phenotype in the cartilage hair hypoplasia-anauxetic dysplasia spectrum. Am. J. Hum. Genet. 2007;81:519–529. doi: 10.1086/521034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goldfarb K.C., Cech T.R. Targeted CRISPR disruption reveals a role for RNase MRP RNA in human preribosomal RNA processing. Genes Dev. 2017;31:59–71. doi: 10.1101/gad.286963.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thiel C.T., Rauch A. The molecular basis of the cartilage-hair hypoplasia-anauxetic dysplasia spectrum. Best Pr. Res. Clin. Endocrinol. Metab. 2011;25:131–142. doi: 10.1016/j.beem.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 105.Mattijssen S., Welting T.J., Pruijn G.J. RNase MRP and disease. Wiley Interdiscip. Rev. RNA. 2010;1:102–116. doi: 10.1002/wrna.9. [DOI] [PubMed] [Google Scholar]
- 106.Kavadas F.D., Giliani S., Gu Y., Mazzolari E., Bates A., Pegoiani E., Roifman C.M., Notarangelo L.D. Variability of clinical and laboratory features among patients with ribonuclease mitochondrial RNA processing endoribonuclease gene mutations. J. Allergy Clin. Immunol. 2008;122:1178–1184. doi: 10.1016/j.jaci.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 107.Welting T.J., Mattijssen S., Peters F.M., van Doorn N.L., Dekkers L., van Venrooij W.J., Heus H.A., Bonafé L., Pruijn G.J. Cartilage-hair hypoplasia-associated mutations in the RNase MRP P3 domain affect RNA folding and ribonucleoprotein assembly. Biochim. Biophys. Acta. 2008;1783:455–466. doi: 10.1016/j.bbamcr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 108.Nakashima E., Tran J.R., Welting T.J., Pruijn G.J., Hirose Y., Nishimura G., Ohashi H., Schurman S.H., Cheng J., Candotti F., et al. Cartilage hair hypoplasia mutations that lead to RMRP promoter inefficiency or RNA transcript instability. Am. J. Med. Genet. A. 2007;143:2675–2681. doi: 10.1002/ajmg.a.32053. [DOI] [PubMed] [Google Scholar]
- 109.Hermanns P., Bertuch A.A., Bertin T.K., Dawson B., Schmitt M.E., Shaw C., Zabel B., Lee B. Consequences of mutations in the non-coding RMRP RNA in cartilage-hair hypoplasia. Hum. Mol. Genet. 2005;14:3723–3740. doi: 10.1093/hmg/ddi403. [DOI] [PubMed] [Google Scholar]
- 110.Steinbusch M.M.F., Caron M.M.J., Surtel D.A.M., Friedrich F., Lausch E., Pruijn G.J.M., Verhesen W., Schroen B.L.M., van Rhijn L.W., Zabel B., et al. Expression of RMRP RNA is regulated in chondrocyte hypertrophy and determines chondrogenic differentiation. Sci. Rep. 2017;7:6440. doi: 10.1038/s41598-017-06809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steinbusch M.M.F., Caron M.M.J., Surtel D.A.M., van den Akker G.G.H., van Dijk P.J., Friedrich F., Zabel B., van Rhijn L.W., Peffers M.J., Welting T.J.M. The antiviral protein viperin regulates chondrogenic differentiation via CXCL10 protein secretion. J. Biol. Chem. 2019;294:5121–5136. doi: 10.1074/jbc.RA119.007356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vakkilainen S., Skoog T., Einarsdottir E., Middleton A., Pekkinen M., Ohman T., Katayama S., Krjutskov K., Kovanen P.E., Varjosalo M., et al. The human long non-coding RNA gene RMRP has pleiotropic effects and regulates cell-cycle progression at G2. Sci. Rep. 2019;9:13758. doi: 10.1038/s41598-019-50334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rogler L.E., Kosmyna B., Moskowitz D., Bebawee R., Rahimzadeh J., Kutchko K., Laederach A., Notarangelo L.D., Giliani S., Bouhassira E., et al. Small RNAs derived from lncRNA RNase MRP have gene-silencing activity relevant to human cartilage-hair hypoplasia. Hum. Mol. Genet. 2014;23:368–382. doi: 10.1093/hmg/ddt427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bowen P., Conradi G.J. Syndrome of skeletal and genitourinary anomalies with unusual facies and failure to thrive in Hutterite sibs. Birth Defects Orig. Artic. Ser. 1976;12:101–108. [PubMed] [Google Scholar]
- 115.Lowry R.B., Innes A.M., Bernier F.P., McLeod D.R., Greenberg C.R., Chudley A.E., Chodirker B., Marles S.L., Crumley M.J., Loredo-Osti J.C., et al. Bowen-Conradi syndrome: A clinical and genetic study. Am. J. Med. Genet. A. 2003;120a:423–428. doi: 10.1002/ajmg.a.20059. [DOI] [PubMed] [Google Scholar]
- 116.Armistead J., Khatkar S., Meyer B., Mark B.L., Patel N., Coghlan G., Lamont R.E., Liu S., Wiechert J., Cattini P.A., et al. Mutation of a gene essential for ribosome biogenesis, EMG1, causes Bowen-Conradi syndrome. Am. J. Hum. Genet. 2009;84:728–739. doi: 10.1016/j.ajhg.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Armistead J., Hemming R., Patel N., Triggs-Raine B. Mutation of EMG1 causing Bowen-Conradi syndrome results in reduced cell proliferation rates concomitant with G2/M arrest and 18S rRNA processing delay. BBA Clin. 2014;1:33–43. doi: 10.1016/j.bbacli.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nieminen T.T., O’Donohue M.F., Wu Y., Lohi H., Scherer S.W., Paterson A.D., Ellonen P., Abdel-Rahman W.M., Valo S., Mecklin J.P., et al. Germline mutation of RPS20, encoding a ribosomal protein, causes predisposition to hereditary nonpolyposis colorectal carcinoma without DNA mismatch repair deficiency. Gastroenterology. 2014;147:595–598. doi: 10.1053/j.gastro.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Broderick P., Dobbins S.E., Chubb D., Kinnersley B., Dunlop M.G., Tomlinson I., Houlston R.S. Validation of Recently Proposed Colorectal Cancer Susceptibility Gene Variants in an Analysis of Families and Patients—A Systematic Review. Gastroenterology. 2017;152:75–77. doi: 10.1053/j.gastro.2016.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klauck S.M., Felder B., Kolb-Kokocinski A., Schuster C., Chiocchetti A., Schupp I., Wellenreuther R., Schmotzer G., Poustka F., Breitenbach-Koller L., et al. Mutations in the ribosomal protein gene RPL10 suggest a novel modulating disease mechanism for autism. Mol. Psychiatry. 2006;11:1073–1084. doi: 10.1038/sj.mp.4001883. [DOI] [PubMed] [Google Scholar]
- 121.Brooks S.S., Wall A.L., Golzio C., Reid D.W., Kondyles A., Willer J.R., Botti C., Nicchitta C.V., Katsanis N., Davis E.E. A novel ribosomopathy caused by dysfunction of RPL10 disrupts neurodevelopment and causes X-linked microcephaly in humans. Genetics. 2014;198:723–733. doi: 10.1534/genetics.114.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bourque D.K., Hartley T., Nikkel S.M., Pohl D., Tetreault M., Kernohan K.D., Care4Rare Canada C., Dyment D.A. A de novo mutation in RPL10 causes a rare X-linked ribosomopathy characterized by syndromic intellectual disability and epilepsy: A new case and review of the literature. Eur. J. Med. Genet. 2018;61:89–93. doi: 10.1016/j.ejmg.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 123.Yang J., Chen Z., Liu N., Chen Y. Ribosomal protein L10 in mitochondria serves as a regulator for ROS level in pancreatic cancer cells. Redox. Biol. 2018;19:158–165. doi: 10.1016/j.redox.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Monteclaro F.S., Vogt P.K. A Jun-binding protein related to a putative tumor suppressor. Proc. Natl. Acad. Sci. USA. 1993;90:6726–6730. doi: 10.1073/pnas.90.14.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chan Y.L., Diaz J.J., Denoroy L., Madjar J.J., Wool I.G. The primary structure of rat ribosomal protein L10: Relationship to a Jun-binding protein and to a putative Wilms’ tumor suppressor. Biochem. Biophys. Res. Commun. 1996;225:952–956. doi: 10.1006/bbrc.1996.1277. [DOI] [PubMed] [Google Scholar]
- 126.Damianov A., Kann M., Lane W.S., Bindereif A. Human RBM28 protein is a specific nucleolar component of the spliceosomal snRNPs. Biol. Chem. 2006;387:1455–1460. doi: 10.1515/BC.2006.182. [DOI] [PubMed] [Google Scholar]
- 127.Chagnon P., Michaud J., Mitchell G., Mercier J., Marion J.F., Drouin E., Rasquin-Weber A., Hudson T.J., Richter A. A missense mutation (R565W) in cirhin (FLJ14728) in North American Indian childhood cirrhosis. Am. J. Hum. Genet. 2002;71:1443–1449. doi: 10.1086/344580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Freed E.F., Prieto J.L., McCann K.L., McStay B., Baserga S.J. NOL11, implicated in the pathogenesis of North American Indian childhood cirrhosis, is required for pre-rRNA transcription and processing. PLoS Genet. 2012;8:e1002892. doi: 10.1371/journal.pgen.1002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bolze A., Mahlaoui N., Byun M., Turner B., Trede N., Ellis S.R., Abhyankar A., Itan Y., Patin E., Brebner S., et al. Ribosomal protein SA haploinsufficiency in humans with isolated congenital asplenia. Science. 2013;340:976–978. doi: 10.1126/science.1234864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marneros A.G. BMS1 is mutated in aplasia cutis congenita. PLoS Genet. 2013;9:e1003573. doi: 10.1371/journal.pgen.1003573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paolini N.A., Attwood M., Sondalle S.B., Vieira C., van Adrichem A.M., di Summa F.M., O’Donohue M.F., Gleizes P.E., Rachuri S., Briggs J.W., et al. A Ribosomopathy Reveals Decoding Defective Ribosomes Driving Human Dysmorphism. Am. J. Hum. Genet. 2017;100:506–522. doi: 10.1016/j.ajhg.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jenkinson E.M., Rodero M.P., Kasher P.R., Uggenti C., Oojageer A., Goosey L.C., Rose Y., Kershaw C.J., Urquhart J.E., Williams S.G., et al. Mutations in SNORD118 cause the cerebral microangiopathy leukoencephalopathy with calcifications and cysts. Nat. Genet. 2016;48:1185–1192. doi: 10.1038/ng.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arancio W., Genovese S.I., Bongiovanni L., Tripodo C. A ceRNA approach may unveil unexpected contributors to deletion syndromes, the model of 5q− syndrome. Oncoscience. 2015;2:872–879. doi: 10.18632/oncoscience.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Falini B., Nicoletti I., Martelli M.F., Mecucci C. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc + AML): Biologic and clinical features. Blood. 2007;109:874–885. doi: 10.1182/blood-2006-07-012252. [DOI] [PubMed] [Google Scholar]
- 135.De Keersmaecker K., Atak Z.K., Li N., Vicente C., Patchett S., Girardi T., Gianfelici V., Geerdens E., Clappier E., Porcu M., et al. Exome sequencing identifies mutation in CNOT3 and ribosomal genes RPL5 and RPL10 in T-cell acute lymphoblastic leukemia. Nat. Genet. 2013;45:186–190. doi: 10.1038/ng.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ajore R., Raiser D., McConkey M., Jöud M., Boidol B., Mar B., Saksena G., Weinstock D.M., Armstrong S., Ellis S.R., et al. Deletion of ribosomal protein genes is a common vulnerability in human cancer, especially in concert with TP53 mutations. EMBO Mol. Med. 2017;9:498–507. doi: 10.15252/emmm.201606660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gong J., Li Y., Liu C.J., Xiang Y., Li C., Ye Y., Zhang Z., Hawke D.H., Park P.K., Diao L., et al. A Pan-cancer Analysis of the Expression and Clinical Relevance of Small Nucleolar RNAs in Human Cancer. Cell Rep. 2017;21:1968–1981. doi: 10.1016/j.celrep.2017.10.070. [DOI] [PubMed] [Google Scholar]
- 138.Penzo M., Clima R., Trerè D., Montanaro L. Separated Siamese Twins: Intronic Small Nucleolar RNAs and Matched Host Genes May be Altered in Conjunction or Separately in Multiple Cancer Types. Cells. 2020;9:387. doi: 10.3390/cells9020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Falini B., Mecucci C., Tiacci E., Alcalay M., Rosati R., Pasqualucci L., La Starza R., Diverio D., Colombo E., Santucci A., et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 140.Ljungström V., Cortese D., Young E., Pandzic T., Mansouri L., Plevova K., Ntoufa S., Baliakas P., Clifford R., Sutton L.A., et al. Whole-exome sequencing in relapsing chronic lymphocytic leukemia: Clinical impact of recurrent RPS15 mutations. Blood. 2016;127:1007–1016. doi: 10.1182/blood-2015-10-674572. [DOI] [PMC free article] [PubMed] [Google Scholar]