Abstract
Background
Alzheimer’s disease (AD), being a complex disorder, is affected either by genetic or environmental factors or both. It is observed that there is an excessive accumulation of amyloid β (Aβ) in the extracellular space of the brain. AD is the first neurodegenerative disease in the elderly, and so far there is no effective treatment. In recent years, many studies have reported that Alzheimer’s disease has a relationship with gut microflora, indicating that regulating gut microbiota could offer therapeutic intervention for AD. This study explored the effect Bifidobacteria has in averting AD.
Methods
WT and APP/PS1 mice were used for the experiments. The mice were randomly assigned to four groups: WT group, WT + Bi group, AD group (APP/PS1 mouse) and AD + Bi group (Bifidobacteria-treated APP/PS1 mouse). Treatment with Bifidobacteria lasted for 6 months and mice were prepared for immunohistochemistry, immunofluorescence, Thioflavin S staining, Western blotting, PCR and Elisa quantitative assay.
Results
The results show that after 6 months of treatment with Bifidobacteria signiis to be lesficantly reduces Aβ deposition in cortex and hippocampus of AD mice. The level of insoluble Aβ in the hippocampus and cortex of AD+Bi mice was decreased compared with AD mice. Meanwhile, a significant decrease in the level of soluble Aβ in the cortex of AD+Bi mice but not in the hippocampus was observed. The activation of microglia and the release of inflammatory factors were also determined in this study. From the results, Bifidobacteria inhibited microglial activation and reduced IL-1β, TNF-α, IL-4, IL-6 and INF-γ release. Altogether, these results implied that Bifidobacteria can alleviate the pathological changes of AD through various effects.
Keywords: Bifidobacteria, AD, Aβ, Gut microflora
Introduction
Alzheimer’s disease (AD) is a disorder described by progressive cognition and memory impairments (Alzheimer’s Association, 2015). Its pathological features include extracellular amyloid plaque, intracellular nerve fiber bundle tangle and neuron loss, which mainly occur in the elderly with slow memory and cognitive function but progressive loss. In the past few decades, people have conducted extensive research on AD, but its pathogenesis is not clear, and no effective treatment has yet been found. Previous AD studies have focused on brain-related aspects such as the production and degradation of Aβ and abnormal phosphorylation of tau (Wang et al., 2019a; Wang et al., 2019b). In recent years, the gut microbiota has been implicated in the occurrence and progression of AD has become a research hotspot (Vogt et al., 2017).
The gut-brain axis is a multimodal communication system that is connected by both sympathetic and parasympathetic nervous systems, together with hormones that circulate as well as other molecules that regulate the nervous system (Mayer, 2011; Marrone & R, 2019). Recent studies have shown that the function of the gut-brain axis involves intestinal flora and plays a major role in brain and gut communication. It has therefore been reported that the gut-brain axis by extension with microorganism interaction, regulates the immune, gut and central nervous system functions (Cryan & Dinan, 2012). As a result, the microbial communities have become potential diagnostic and therapeutic targets for treating a variety of disorders such as stroke, Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, autism, stroke, depression, and drug addiction (Yarandi et al., 2016; Dong, Xie & Wang, 2019). The enteric nervous system (ENS) and the central nervous system (CNS) even though they are far apart, share many morphological, physiological and pharmacological features. Since bacteria can affect ENS, if they or their messengers can reach the CNS, they will have the same effect on the CNS. A study reported a change in the gut microflora of a bacterial free amyloid β-precursor protein (APP) double transgenic mice model. The amyloid protein deposition in the brains of mice was significantly reduced (Harach et al., 2017). Changes in microbial flora have also been found in other AD mice models and have become more pronounced with increasing age (Shen, Liu & Ji, 2017; Brandscheid et al., 2017). Compared with children/adults and young mice, the composition of gut microbiota in the aged persons and in mice differed. The population of Bifidobacteria in the elderly is shown to be less (Lee et al., 2019). Recent studies have also shown that the change of intestinal flora significantly correlates with cognitive behavior. Intestinal flora regulation can affect the cognitive behavior of the host when studied, by using sterile animals, probiotics or antibiotics and fecal flora transplantation (Gareau et al., 2011). Probiotics are active microorganisms that can improve the microecological balance of host and play a beneficial role. Bifidobacteria is an anaerobe isolated from the feces of breast-feeding infants by Tissier, a French scholar, in 1899. It has many functions such as antibacterial, anti-aging, immune enhancement, anti-cancer, etc (O’Callaghan & van Sinderen, 2016). It has been reported that the number of Bifidobacteria in the gut microbiota is decreased in patients with AD (Cheng et al., 2019).
Based on the above, APP/PS1 double transgenic mice were used as the AD model in this experiment. Bifidobacteria were given to mice at 4 months until 10 months of age. The changes in Aβ spot deposition and inflammation in the brain of mice were determined and analyzed. This study provided evidence on the effect of Bifidobacteria in AD mice and could provide a new target for the AD treatment.
Materials & Methods
Animals
WT and APP/PS1 mice were obtained from Dalian Medical University. The mice were housed in groups (3–5 mice per cage) in polypropylene cages with woodchip bedding, freely had access to food and water and were kept under standard conditions (room temperature of 24 °C, 12 h light/dark cycle and relative humidity of 50–60%). All procedures were subjected to the Institutional Animal Care and Use Committee guidelines and were approved by the Institutional Ethics Committee of Dalian Medical University (L2013011).
Animal grouping and treatment
The mice were randomly assigned to four groups: WT group, WT + Bi group, AD group (APP/PS1 mouse) and AD + Bi group (Bifidobacteria- treated APP/PS1 mouse). .Bifidobacteria (B. longum 1714), was dissolved in water (final concentration: 1 ×109 CFU/mL) right before the treatment and given once a day at 0.2 ml/10 g of body mass (Savignac et al., 2015; Fukuda et al., 2011; Wall et al., 2010). The mice in WT + Bi group and AD + Bi group were given Bifidobacteria by gavage. The mice in the control group were given the same volume of water. Samples were taken at 10 months old.
Euthanization
Mice were deeply anesthesized with pentobarbital (50 mg/kg, i.p) and ensured that it was effective by pinching the tail with tweezers. Any movement in the mice showed that pain could still be felt so enough time was allowed for the anesthesia to fully work before sacrificing the mice. Mice that were not anesthesized were injected with half of the original dose. Mice that survived the study or were excluded were bred for other experiments.
Immunohistochemistry
Fully anesthesized mice were perfused with cold 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer(PB) with a pH of 7.4. The brains were then removed and post-fixed in 4% PFA overnight at 4 °C. Brains were dehydrated in 30% sucrose in PB, embedded in paraffin, and then sliced into sections of 10-µm thickness. The sections were washed three times with PBS and then incubated with 3% H2O2 for 10 min to block all endogenous peroxidases. After incubation, sections were washed three times with PBS and blocked with 5% bovine serum albumin for 1 h at room temperature (RT). Slides were then incubated with a primary antibody 6E10 (Covance, SIG39320) overnight at 4 °C. Negative control was included by adding PBS instead of the primary antibody. The sections were again washed and incubated with mouse IgG secondary antibody at room temperature for 1 h, then stained with diaminobiphenylamine (DAB) to develop the color. The reaction of the DAB solution was terminated by submerging the slide in PSB. The slides were then dehydrated in graded ethanol series (50, 75, 90, 100%) for 5 min each and then cleared in xylene for 2 min. Finally, they were mounted with coverslips and imaged with a Leica microscope.
Immunofluorescence
For the immunofluorescence staining, slices were washed with PBST (0.01 M sodium phosphate buffer of pH 7.4, containing 0.3% (v/v) Triton X-100) and blocked with 5% bovine serum albumin for an hour at room temperature (RT). After that slices were incubated overnight at 4 °C using the following primary antibodies: Iba-1(Wako,019-19741). Negative control was included by adding PBS instead of the primary antibody. Slices were then washed in PBS followed by the addition of DAPI and the respective secondary antibody: goat anti-rabbit Alexa Fluor 488 (1:500). Slices were incubated in the dark for 2 h at RT and then washed with PBS. Images were collected using a Leica Microscope.
Thioflavin S staining
After the sections were washed three times with PBS, they were immersed in the ThT solution (1 g of Th S in 100 mL distilled water) for 20 min in the dark. Images were collected using a Leica Microscope.
Western blot analysis
Protein was extracted with a RIPA lysis solution and the protein concentration was determined by BCA protein assay(KeyGEN BioTECH, Jiangsu, China). Protein separation was done on 10% SDS-PAGE gel and transblotted onto PVDF membranes (Millipore, Billerica, MA, USA). The membranes were then blocked in 5% nonfat dried milk for 1 h at RT and were incubated with primary antibodies overnight at 4 °C: Iba-1(Wako,016-20001), β-actin (Abcam,ab179467). Membranes were washed 3 times with TBST and then incubated with the HRP-conjugated secondary antibodies for 1 h at room temperature. Protein bands were detected using enhanced chemiluminescence (ECL, Biotool). Semiquantitative analysis was conducted using ImageJ software.
PCR
Total RNA was extracted with TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). The RNA extracts were converted to cDNA using the One-Step RT-PCR System with the qualified total RNA as the template. The primers used were as follows:
IL-1β Forward 5′-CAGGACAGGTATAGATTCTTTCCTTT-3′
IL-1β Reverse 5′-ATGGCAACTGTTCCTGAACTCAACT-3′
TNF-α Forward 5′-CGTCAGCCGATTTGCTATCT-3′
TNF-α Reverse 5′-CGGACTCCGCAAAGTCTAAG-3′
IFN-γ Forward 5′-AGCGGCTGACTGAACTCAGATTGT-3′
IFN-γ Reverse 5′-GTCACAGTTTTCAGCTGTATAGGG-3′
IL-4 Forward 5′-ACAGGAGAAGGGACGCCAT-3′
IL-4 Reverse 5′-GAAGCCCTACAGACGAGCTCA-3′
IL-6 Forward 5′-TAGTCCTTCCTACCCCAATTTCC-3′
IL-6 Reverse 5′-TTGGTCCTTAGCCACTCCTTC-3′
GAPDHForward 5′-TCACCACCATGGAGAAGGC-3′
GAPDH Reverse 5′-GCTAAGCAGTTGGTGGTGCA-3′
Amplification proceeded as follows:
IL-1β 94 °C.45′, 60 °C.45′, 70 °C.60′, 33 Cycles;
TNF-α 95 °C.30′, 58 ° C.40′, 72 °C.40′, 37 Cycles;
IFN-γ 95 °C.20′, 60 ° C.30′, 72 °C.60′, 30 Cycles;
IL-4 95 °C.20′, 55 ° C.30′, 72 °C.60′, 35 Cycles;
IL-6 95 °C.20′, 60 ° C.30′, 72 °C.60′, 35Cycles;
GAPDH 94 °C.45′, 60 ° C.45′, 70 °C. 60′, 24 Cycles
ELISA quantitative assays for Aβ42
Aβ1-42 in brain tissue was quantitatively estimated using the ELISA kit. The procedure was done in accordance with the protocol provided by the manufacturer (KHB3441, Invitrogen).
Statiscal analysis
Data are presented as mean ±SEM. Differences between groups were determined by student t-test or a two-way analysis of variance (ANOVA), significant effects were evaluated with least signifificant difference (LSD) post hoc test. p-Value <0.05 was considered as the significance level for all analyses (* p < 0.05, ** p < 0.01;# p < 0.05, ## p < 0.01;### p < 0.001)
Results
Bifidobacteria reduces Aβ deposition in the brain of AD mice
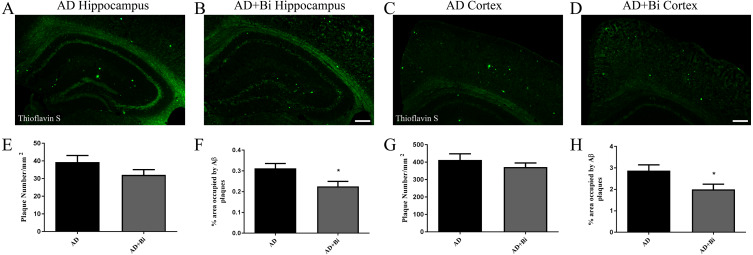
The deposition of β-amyloid (Aβ) in the brain has always been a key factor in the study of the pathological mechanism of AD (Honjo, Black & Verhoeff, 2012). As the disease progresses, Aβ aggregates into insoluble clusters or plaques in the AD brain. The extent of plaque formation in the cortical and hippocampus has a direct relation with learning and memory disorders in AD (Jahn, 2013). To examine how Bifidobacteria affect the formation of plaque in the brain, the levels of Aβ plaques were observed in mice. Brain sections were stained with Thioflavin S (ThS) and the insoluble β-sheet-rich Aβ plaques were observed. As there was no Aβ deposition in the brain of WT and WT+Bi mice, the size and number of Aβ plaques in AD and AD+Bi mice were quantified by stereological analyses in the cortex (Figs. 1C–1D) and hippocampus (Figs. 1A–1B). The results of immunohistochemistry showed that the percentage of Thioflavin-S positive Aβ deposition in the hippocampus (P = 0.0270) and cortex (P = 0.0360) was significantly reduced in AD+Bi mice compared with the AD mice (Figs. 1H, 1F). Interestingly, there was no significant change in the number of Aβ in the hippocampus (P = 0.1638) and cortex (P = 0.3557) in AD+Bi mice compared with AD mice (Figs. 1E, 1G). This trend is consistent in the cortex and hippocampus, which means that the administration of Bifidobacteria will affect the formation of plaque in different brain regions.
Figure 1. Bifidobacteria reduces insoluble Aβ plaques in the brains of AD mice.
Representative images of the hippocampal (A–B) and cortical (C–D) regions of the brains showing stained insoluble Aβ in Bifidobacteria-treated AD mice and AD mice. Scale bar = 100 µm. Quantification and analysis of insoluble Aβ plaques in the hippocampal (E–F) and cortical (G–H) regions of indicated groups. ∗P < 0.05; n = 6.
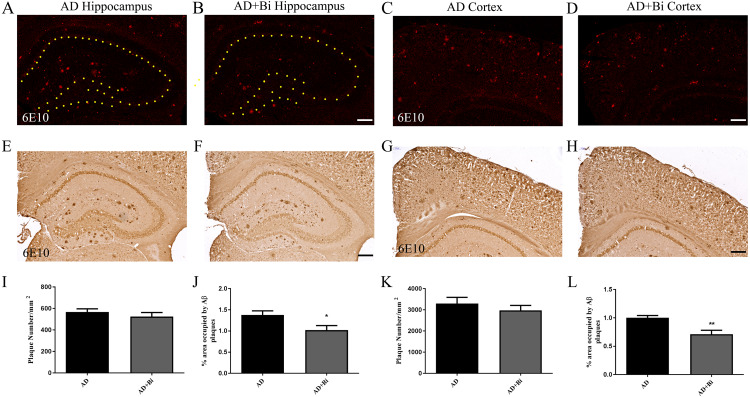
Aβ plaques occur either as dense-core plaques or diffuse plaques. There is a difference between the dense-core plaques and the diffuse plaques, in that ThS is only able to stain dense-core plaques, and this is as a result of their aggregated β-sheet structure (Rak et al., 2007; Yang et al., 2017). Previous clinical studies have shown that an early pathological sign of AD is depicted by ThS-negatively diffused A β plaques(Price & Morris, 1999). 6E10 was used to detect Aβ (both dense core and diffuse plaques) in the cortex and hippocampus (Figs. 2C–2D; 2G–2H and Figs. 2A–2B; 2E–2F). The results of immunohistochemistry showed that compared with AD mice, the percentage of 6E10 positive Aβ deposition was significantly reduced in the hippocampus (P = 0.0326) and cortex (P = 0.006) of AD+Bi mice (Figs. 2J, 2L). Similarly, the number of Aβ did not change significantly in the hippocampus (P = 0.4044) and cortex (P = 0.4145) in AD+Bi mice compared with AD mice (Figs. 2I, 2K).
Figure 2. Bifidobacteria reduces total Aβ plaques in the brains of AD mice.
Coronal sections of brains showing 6E10-positive Aβ plaques in the hippocampus (A–B; E–F) and cortex (C–D; G–H) of each group. Scale bar = 200 µm.Quantified 6E10-positive Aβ plaques in the hippocampus (I–J) and cortex (K–L) of AD mice. ∗P < 0.05; n = 6.
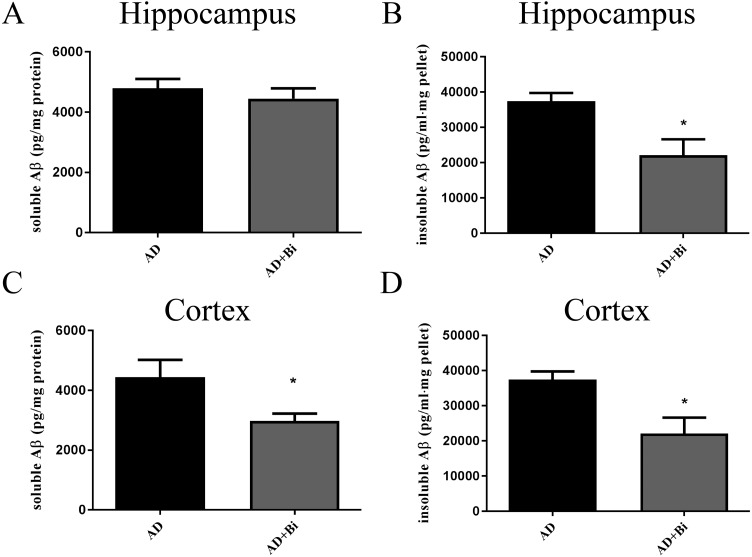
Levels of insoluble and soluble Aβ in the cortex and hippocampus were analyzed separately. The use of sandwich enzyme-linked immunosorbent assay (ELISA) was employed to measure Aβ levels. Brain tissues from the cortex and hippocampus were dissolved in RIPA (soluble fraction) and guanidine (insoluble fraction) lysis buffers. As we can see in the results, we found that the level of insoluble Aβ in the hippocampus (P = 0.0238) and cortex (P = 0.0128) of AD+Bi mice decreased compared with AD mice which are similar observations in the ThS-staining results (Fig. 3 B and D). Meanwhile, a significant decrease was observed in the level of soluble Aβ in the cortex (P = 0.0414) of AD+Bi mice (Fig. 3C). On the contrary, the level of soluble Aβ in the hippocampus (P = 0.4718) of AD+Bi mice did not decrease compared with AD mice (Fig. 3A).
Figure 3. Bifidobacteria decrease insoluble and soluble Aβ in the brains of AD mice.
Sandwich ELISA of the soluble Aβ and insoluble Aβ fractions from the hippocampal (A–B) and cortical (C–D) lysates of AD and Bifidobacteria-treated AD mice. Six brain lysates per group were analyzed. ∗P < 0.05.
Bifidobacteria inhibit the activation of microglia in AD mice
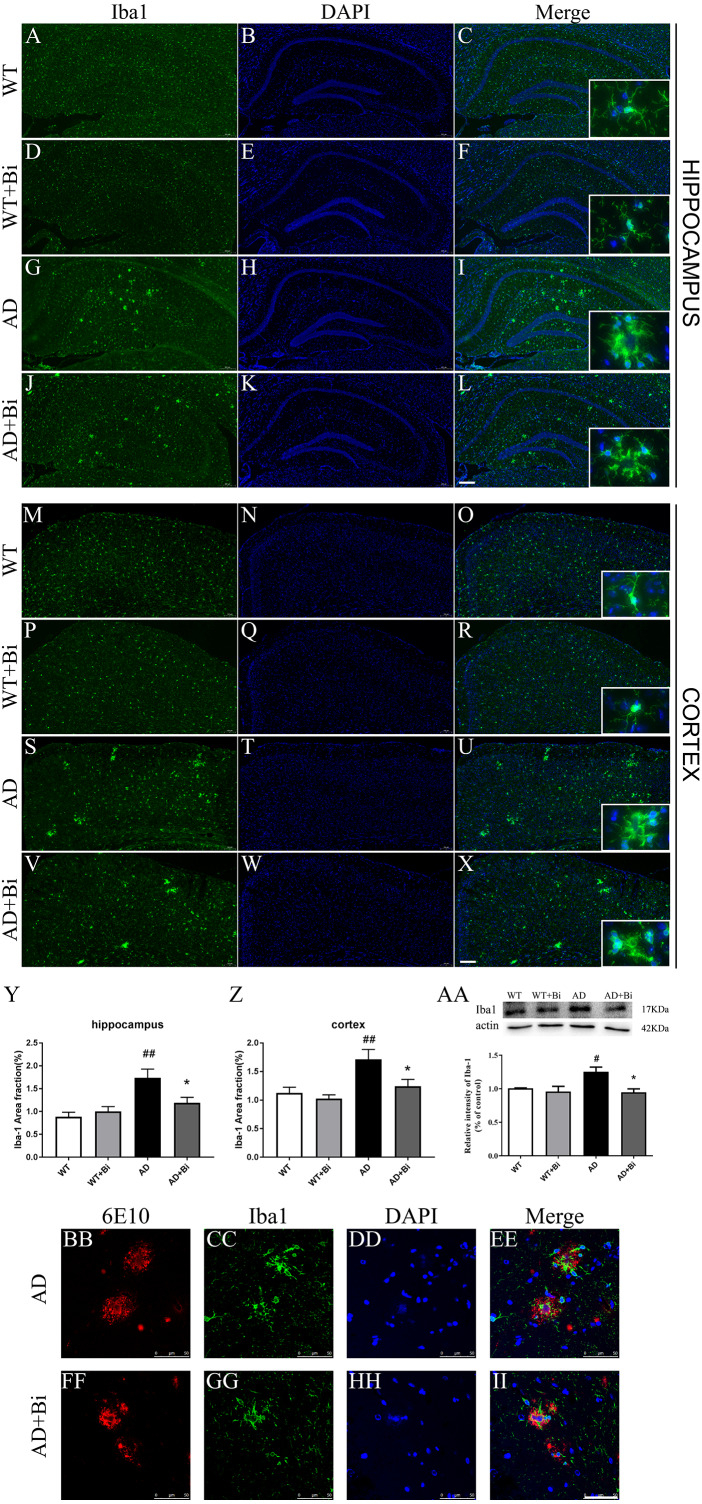
Microglia plays an important role in the neuroinflammatory pathogenesis of AD(Lee et al., 2014). When microglia are activated they can induce and maintain chronic inflammatory states, and lead to neuronal damage as well as neurodegeneration (Zhang et al., 2017). Therefore, we sought to measure whether the Bifidobacteria can inhibit the activation of microglia. The expression of Iba1 (one of the markers for microglia) was determined. As represented in Figs. 4A–4Z, treatment with Bifidobacteria reduced the percentage of the area immunostained with Iba-1 in the hippocampus (P = 0.0026; Fig. 4Y) and cortex (P = 0.0016; Fig. 4Z) of AD mice. To further confirm the IF findings, the protein expression level of Iba1 in the hippocampus was determined with WB. Consistent with the IF results, the protein level of Iba1 decreased in Bifidobacteria treated AD mice (P = 0.0144; Fig. 4AA). Immunostaining also revealed that Bifidobacteria treatment alleviate the activation of microglia around Aβ plaques (Figs. 4BB–4II). It is suggested that inhibition of microglial activation may be one of the protective mechanisms of Bifidobacteria on memory impairment in AD mice.
Figure 4. Bifidobacteria inhibit the activation of microglia in AD mice.
Coronal sections of brains showing Iba1-positive immuno-stained microglia in the hippocampus (A–L) and cortex (M–X). Scale bar = 200 µm. (Y–Z) Positive area % of Iba-1 immunostaining detected by immunofluorescence. (AA) Representative immunoblot and densitometric analysis showing Iba1 protein expression level. Here, the bands were normalized to β-actin. (BB–II) Bifidobacteria treatment alleviated the activations of microglia around Aβ plaque. Scale bar = 50 µm. *compared with AD group, ∗P < 0.05; # compared with WT group; n = 6.
Bifidobacteria inhibit neuroinflammation in AD mice
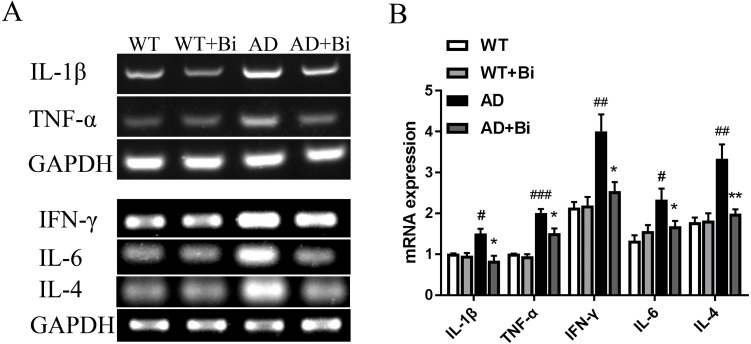
Activated microglia can mediate neuroinflammation in various neurological disorders via producing proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-4 (IL-4) and interferon-γ (IFN-γ) (Yan et al., 2019; Na, Jung & Kim, 2012). Therefore, the mRNA expression levels of the pro-inflammatory factors (TNF-α, IL-1β, IL-4, IL-6, IFN-γ) in the hippocampus were detected in each group by PCR. The mRNA levels of these pro-inflammatory factors were increased significantly in AD mice compared to the WT mice. On the other hand, the mRNA levels were reduced in Bifidobacteria-treated AD mice compared with the AD mice (IL-1 β, P = 0.0395; TNF-α, P = 0.0498; IL-4, P = 0.0099; IL-6, P = 0.0353; IFN-γ, P = 0.0212; Fig. 5). These findings suggested that Bifidobacteria may potentially have an anti-inflammatory effect in the AD condition.
Figure 5. Bifidobacteria inhibit neuroinflammation in AD mice.
Representative micrographs (A) and densitometric analysis (B) of IL-1β, TNF-α, IFN-γ , IL-4 and IL-6 mRNA expression levels. Here, bands were normalized to GAPDH. *compared with AD group, ∗P < 0.05, ∗∗P < 0.01; # compared with WT group, #P < 0.05, ##P < 0.01, ###P < 0.001; n = 6.
Discussion
A large number of bacteria colonize the human colon. The most popular among them are the sclerenchyma and Bacteroides, which account for 51% and 48% of the total microbial population respectively (Westfall et al., 2017; Qin et al., 2010). Gut microflora is very important for health. Originally, it was thought that all microbes in the gut were only restricted to colon-specific activities, such as fermentation of carbohydrates, synthesis of vitamins (such as vitamin B and K), and prevent other disease-causing bacteria from attacking the gastrointestinal tract (Salminen et al., 1998; Simon & Gorbach, 1986). Recently, probiotics have shown great potential in preventing and treating many diseases, and their role in diseases of central nervous system diseases have been gradually discovered. The gut-brain axis offers such a dynamic bidirectional neuroendocrine system composed of uninterrupted neural contacts, endocrine indicators as well as immune factors (Burokas et al., 2015; Forsythe, Bienenstock & Kunze, 2014; Bauer, Huus & Finlay, 2016). Many linked hormones and biochemical pathways exist that link the condition of gut microflora to some brain functions, giving probiotics a great potential to be used as therapeutic agents for neurodegenerative disorders. It has recently been reported that changes in gut microbial content can modify normal brain function, resulting in conditions such as anxiety, depression, and cognitive impairment (Zhu et al., 2017a; Zhu et al., 2017b; Foster & McVey Neufeld, 2013). In addition, there are reports showing that the make-up of gut microflora is significantly associated with aging. Hence, the diversity of gut microflora will be reduced in an aging intestinal tract (Biagi et al., 2010; Cheng et al., 2013; Claesson et al., 2011).
AD is the most common form of dementia in the elderly, found in about 60%–80% of all patients with dementia (Beckman et al., 2019). Some epidemiological and preclinical pieces of evidence show that changes in intestinal microflora are linked to AD pathogenesis and development. For instance, the composition of microbial colonies in AD patients was different from that in the control group (Vogt et al., 2017). A study reported that bacteria phyla or strains in feces of 5xFAD Alzheimer’s mice can be distinguished from that of wild-type littermate (Brandscheid et al., 2017). Several gut bacteria, such as E. coli, Salmonella and Citrobacter, produce Aβ (O’Toole, Kaplan & Kolter, 2000; Zhou et al., 2012). Exposure to microbial amyloid may cause misfolding of amyloid in the brain (Cerovic, Forloni & Balducci, 2019). Gv-971, a new anti-AD drug recently launched in China, can remodel the balance of gut microflora, normalize disordered metabolites to reduce Aβ deposition, and ultimately improve cognitive function (Wang et al., 2019a; Wang et al., 2019b). In view of this, probiotics and prebiotics may effectively fight against dementia, which is considered as an important progress in the field of AD.
Alzheimer’s disease has been categorized by four distinct hallmarks. These include Aβ plaque, tangled nerve fibers, loss of nerve and synapse, and cognitive impairment. At present, the treatment of AD has been the emphasis of much research. Some of these studies are Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) and Dominantly Inherited Alzheimer Network (DIAN), all of which had the aim to prevent or delay the AD onset and progression and other associated outcomes (Yang et al., 2017). In this present study, we hypothesize that Bifidobacteria ingestion can regulate a variety of pathological factors, that are critical in AD development. Aβ deposition in the brain is one of the pathological hallmarks well-known in AD and is strongly linked with synaptic plasticity impairment in the hippocampus, leading to behavioral abnormalities (Meng, Wang & Li, 2019). Our results confirmed the effect of Bifidobacteria on the Aβ burden in APP/PS1 double transgenic mice. After 6 months of treatment with Bifidobacteria, the pathological brains of Bifidobacteria treated APP/PS1 mice were evaluated. A significant reduction in Aβ plaque burden was observed in the hippocampus and cortex (Figs. 1 and 2). Also, the Aβ lowering properties of Bifidobacteria were assessed by measuring soluble and insoluble Aβ species in the Bifidobacteria-treated and -untreated APP/PS1 mice. Aβ (a 4 kDa peptide with 40- and 42-amino acid residue peptides as the predominant species) is produced via sequential amyloid precursor protein (APP) cleavage by the enzymes β-and γ-secretase (Katayama, 2019; Ferreira et al., 2015). Biochemical studies have shown that Aβ1-42 is more likely to aggregate into amyloid fibers and other combinations than Aβ1-40 (Jarrett & Lansbury, 1993). Therefore, using ELISA, soluble Aβ1-42 and insoluble Aβ1-42 were detected in the brain. As reported earlier, treatment with Bifidobacteria in APP/PS1 mice decreased the levels of insoluble and soluble A β1-42 in the cortex, while Bifidobacteria showed a significant reduction in the insoluble Aβ1-42 in the hippocampus and no effect on soluble Aβ1-42 (Fig. 3).
Taken together, we can see that Bifidobacteria reduce significantly, the pathological deposition of Aβ in APP/PS1 mice. Although we did not conduct behavioral tests, we have reason to believe that Bifidobacteria could recover learning and memory impairments in APP/PS1 mice, given the important role of Aβ in AD. Bifidobacteria breve A1, when orally administered to AD model mice (intracerebroventricularly administered Aβ25-35) daily by gavaging 1 ×109 organisms in 0.2ml can repress the expression of inflammation-related and immune-reactive genes (Kobayashi et al., 2017) and improved the cognitive function of participants with mild cognitive impairment (MCI) (Kobayashi et al., 2018). In a clinical trial of 52 subjects in Iran, the subjects took 200 ml/day of probiotic milk containing Lactobacilli and Bifidobacteria (2 ×109 CFU/g for each) every day for 12 consecutive weeks. Their results showed that consuming the probiotics for 12 weeks had a positive effect on some metabolic states of AD patients as well as improved their cognitive function. These results conform to our hypothesis (Akbari et al., 2016).
Inflammation in the brain is one of the hallmarks of AD and is initiated in the central nervous system (Zeng et al., 2018). Numerous studies have reported that Aβ can trigger oxidative stress in the brain, and also activate microglia leading to neuroinflammation and cognitive dysfunction (Kobayashi et al., 2017). Microglia are the resident immune cells in the central nervous system (CNS) (Wang et al., 2016) and are considered to be the important modulators in brain immunity and homeostasis. They play an important role in various nervous system diseases including AD. Microglia activation exerts effects that could be toxic or beneficial depending on their phenotype in the progression of AD. Activated microglia can internalize and degrade Aβ, suggesting that the initial response of microglia may contribute to the elimination of Aβ. However, as the disease progresses, their ability to clear Aβ is considered to be much limited or even reduced (Zhu et al., 2017a; Zhu et al., 2017b). At the same time, the continuous uncontrolled activation of glial cells induced by Aβ plaques still maintains its ability to produce a variety of cytokines and chemokines which lead to neurodegeneration, and thus even hasten the progression of AD (Zhao et al., 2018; Kiyota et al., 2018). The present findings showed that Bifidobacteria treatment inhibits the activation of microglial (Fig. 4). Also, examining the expression of IL-1 β, TNF-α, IL-4, IL-6 and IFN-γ to see whether Bifidobacteria treatment reduces the release of proinflammatory cytokines, the results obtained a significant reduction in the mRNA expression levels of these pro-inflammatory cytokines in Bifidobacteria treated APP/PS1 mice compared with the control (Fig. 5).
Conclusions
In conclusion, this study showed the therapeutic potential in Bifidobacteria. Treatment with Bifidobacteria can suppress Aβ accumulation and neuroinflammation in APP/PS1 mice. Further studies are therefore needed to elucidate the effect of Bifidobacteria on learning and memory and the possible mechanisms involved. However, with Bifidobacteria being an inherent bacterium in the intestine, it can be used as a safe and long-term means to fight AD.
Supplemental Information
Funding Statement
This work was supported by grants from the Liaoning Provincial Key R&D Program (2019020048-JH2/103), the Liaoning Revitalization Talents Program (XLYC1902044, XLYC1808031), the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2019zx09301102) and the National Natural Sciences Foundation of China (81571061, 81671061). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Jie Zhao, Email: zhaoj@dmu.edu.cn.
Shao Li, Email: lishao89@dmu.edu.cn.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Qiong Wu and Michael Ntim performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Qifa Li performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Xuan Zhang performed the experiments, prepared figures and/or tables, and approved the final draft.
Ming Li performed the experiments, prepared figures and/or tables, and approved the final draft.
Xuefei Wu conceived and designed the experiments, prepared figures and/or tables, and approved the final draft.
Li Wang analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Jie Zhao and Shao Li conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All animal care and experimental protocols were complied with the Animal Management Guidelines of China and approved by the Animal Use and Care Committee of Dalian Medical University (2002-03, L2013011).
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.
References
- Akbari et al. (2016).Akbari E, Asemi Z, DaneshvarKakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, Hamidi GA, Salami M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Frontiers in Aging Neuroscience. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association (2015).Alzheimer’s Association Alzheimer’s disease facts and figures. Alzheimer’s Dementia. 2015;2015(11):332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Bauer, Huus & Finlay (2016).Bauer KC, Huus KE, Finlay BB. Microbes and the mind: emerging hallmarks of the gut microbiota-brain axis. Cellular Microbiology. 2016;18(5):632–44. doi: 10.1111/cmi.12585. [DOI] [PubMed] [Google Scholar]
- Beckman et al. (2019).Beckman D, Ott S, Donis-Cox K, Janssen WG, Bliss-Moreau E, Rudebeck PH, Baxter MG, Morrison JH. Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(52):26239–26246. doi: 10.1073/pnas.1902301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi et al. (2010).Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLOS ONE. 2010;5(5):e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandscheid et al. (2017).Brandscheid C, Schuck F, Reinhardt S, Schäfer KH, Pietrzik CU, Grimm M, Hartmann T, Schwiertz A, Endres K. Altered gut microbiome composition and tryptic activity of the 5xFAD Alzheimer’s mouse model. Journal of Alzheimers Disease. 2017;56:775–788. doi: 10.3233/JAD-160926. [DOI] [PubMed] [Google Scholar]
- Burokas et al. (2015).Burokas A, Moloney RD, Dinan TG, Cryan JF. Microbiota regulation of the mammalian gut-brain axis. Advances in Applied Microbiology. 2015;91:1–62. doi: 10.1016/bs.aambs.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Cerovic, Forloni & Balducci (2019).Cerovic M, Forloni G, Balducci C. Neuroinflammation and the gut microbiota: possible alternative therapeutic targets to counteract Alzheimer’s disease? Frontiers in Aging Neuroscience. 2019;11:284. doi: 10.3389/fnagi.2019.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng et al. (2013).Cheng J, Palva AM, De Vos WM, Satokari R. Contribution of the intestinal microbiota to human health: from birth to 100 years of age. Current Topics in Microbiology and Immunology. 2013;358:323–346. doi: 10.1007/82_2011_189. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2019).Cheng LH, Liu YW, Wu CC, Wang S, Tsai YC. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. Journal of Food and Drug Analysis. 2019;27(3):632–648. doi: 10.1016/j.jfda.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson et al. (2011).Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O’Connor M, Harnedy N, O’Connor K, Henry C, O’Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Paul Ross R, O’Toole PW. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan & Dinan (2012).Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Dong, Xie & Wang (2019).Dong D, Xie J, Wang J. Neuroprotective effects of brain-gut peptides: a potential therapy for Parkinson’s disease. Neuroscience Bulletin. 2019;35:1085–1096. doi: 10.1007/s12264-019-00407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira et al. (2015).Ferreira ST, Lourenco MV, Oliveira MM, DeFelice FG. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Frontiers in Cellular Neuroscience. 2015;9:191. doi: 10.3389/fncel.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe, Bienenstock & Kunze (2014).Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Microbial endocrinology: the microbiota-gut-brain axis in health and disease. Advances in Experimental Medicine and Biology. 2014;817:115–133. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- Foster & McVey Neufeld (2013).Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends in Neurosciences. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Fukuda et al. (2011).Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- Gareau et al. (2011).Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, MacQueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- Harach et al. (2017).Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, Neher JJ, Fåk F, Jucker M, Lasser T, Bolmont T. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Scientific Reports. 2017;7:41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo, Black & Verhoeff (2012).Honjo K, Black SE, Verhoeff NP. Alzheimer’s disease, cerebrovascular disease, and the β-amyloid cascade. Canadian Journal of Neurological Sciences. 2012;39(6):712–728. doi: 10.1017/s0317167100015547. [DOI] [PubMed] [Google Scholar]
- Jahn (2013).Jahn H. Memory loss in Alzheimer’s disease. Dialogues in Clinical Neuroscience. 2013;15(4):445–454. doi: 10.31887/DCNS.2013.15.4/hjahn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett & Lansbury (1993).Jarrett JT, Lansbury PT. Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Katayama (2019).Katayama H. Anti-interleukin-17A and anti-interleukin-23 antibodies may be effective against Alzheimer’s disease: role of neutrophils in the pathogenesis. Brain and Behavior. 2019;17:e01504. doi: 10.1002/brb3.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota et al. (2018).Kiyota T, Machhi J, Lu Y, Dyavarshetty B, Nemati M, Zhang G, Mosley RL, Gelbard HA, Gendelman HE. URMC-099 facilitates amyloid- β clearance in a murine model of Alzheimer’s disease. Journal of Neuroinflammation. 2018;15(1):137. doi: 10.1186/s12974-018-1172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi et al. (2018).Kobayashi Y, Kinoshita T, Matsumoto A, Yoshino K, Saito I, Xiao JZ. Bifidobacterium Breve A1 supplementation improved cognitive decline in older adults with mild cognitive impairment: an open-label, single-arm study. Journal of Prevention of Alzheimer’s Disease. 2018;6:70–75. doi: 10.14283/jpad.2018.32. [DOI] [PubMed] [Google Scholar]
- Kobayashi et al. (2017).Kobayashi Y, Sugahara H, Shimada K, Mitsuyama E, Kuhara T, Yasuoka A, Kondo T, K Abe, Xiao JZ. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Scientific Reports. 2017;7:13510. doi: 10.1038/s41598-017-13368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2019).Lee HJ, Lee KE, Kim JK, Kim DH. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Scientific Reports. 2019;9(1):11814. doi: 10.1038/s41598-019-48342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2014).Li Y, Tan MS, Jiang T, Tan L. Microglia in Alzheimer’s disease. Biomed Research International; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone & R (2019).Marrone MC, R Coccurello. Dietary fatty acids and microbiota-brain communication in neuropsychiatric diseases. Biomolecules. 2019;10(1):12. doi: 10.3390/biom10010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer (2011).Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nature Reviews Neuroscience. 2011;12(8):453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, Wang & Li (2019).Meng SX, Wang B, Li WT. Intermittent hypoxia improves cognition and reduces anxiety-related behavior in APP/PS1 mice. Brain and Behavior. 2019;26:e01513. doi: 10.1002/brb3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na, Jung & Kim (2012).Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of Schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;48:277–286. doi: 10.1016/j.pnpbp.2012.10.022. [DOI] [PubMed] [Google Scholar]
- O’Callaghan & van Sinderen (2016).O’Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Frontiers in Microbiology. 2016;7:925. doi: 10.3389/fmicb.2016.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole, Kaplan & Kolter (2000).O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annual Review of Microbiology. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- Price & Morris (1999).Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Annals of Neurology. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3¡358::aid-ana12¿3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Qin et al. (2010).Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto J-M, Hansen T, LePaslier D, Linneberg A, Bjørn Nielsen H, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, MetaHITConsortium, Bork P, Dusko Ehrlich S, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak et al. (2007).Rak M, Del Bigio MR, Mai S, Westaway D, Gough K. Dense-core and diffuse Abeta plaques in TgCRND8 mice studied with synchrotron FTIR microspectroscopy. Biopolymers. 2007;87:207–217. doi: 10.1002/bip.20820. [DOI] [PubMed] [Google Scholar]
- Salminen et al. (1998).Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, Isolauri E, Moreau MC, Roberfroid M, Rowl I. Functional food science and gastrointestinal physiology and function. British Journal of Nutrition. 1998;80:S147–S171. doi: 10.1079/bjn19980108. [DOI] [PubMed] [Google Scholar]
- Savignac et al. (2015).Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF. Bifidobacteriamodulate cognitive processes in an anxious mouse strain. Behavioural Brain Research. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- Shen, Liu & Ji (2017).Shen L, Liu L, Ji HF. Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. Journal of Alzheimers Disease. 2017;56:385–390. doi: 10.3233/JAD-160884. [DOI] [PubMed] [Google Scholar]
- Simon & Gorbach (1986).Simon GL, Gorbach SL. The human intestinal microflora. Digestive Diseases and Sciences. 1986;31:147S–162S. doi: 10.1007/bf01295996. [DOI] [PubMed] [Google Scholar]
- Vogt et al. (2017).Vogt NM, Kerby RL, Dill-McFarl KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE. Gut microbiome alterations in Alzheimer’s disease. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall et al. (2010).Wall R, Ross RP, Shanahan F, O’Mahony L, Kiely B, Quigley E, Dinan TG, Fitzgerald G, Stanton C. Impact of administered bifidobacterium on murine host fatty acid composition. Impact of administered bifidobacterium on murine host fatty acid composition. Lipids. 2010;45(5):429–436. doi: 10.1007/s11745-010-3410-7. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2019b).Wang S, Jiang W, Ouyang T, Shen XY, Wang F, Qu YH, Zhang M, Luo T, Wang HQ. Jatrorrhizine balances the gut microbiota and reverses learning and memory deficits in APP/PS1 transgenic mice. Scientific Reports. 2019b;9(1):19575. doi: 10.1038/s41598-019-56149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2019a).Wang X, Sun G, Feng T, Zhang J, Huang X, Wang T, Xie Z, Chu X, Yang J, Wang H, Chang S, Gong Y, Ruan L, Zhang G, Yan S, Lian W, Du C, Yang D, Zhang Q, Lin F, Liu J, Zhang H, Ge C, Xiao S, Ding J, Geng M. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Research. 2019a;29(10):787–803. doi: 10.1038/s41422-019-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2016).Wang Y, Ulland TK, Ulrich JD, Song W, Tzaferis JA, Hole JT, Yuan P, Mahan TE, Shi Y, Gilfillan S, Cella M, Grutzendler J, DeMattos RB, Cirrito JR, Holtzman DM, Colonna M. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. Journal of Experimental Medicine. 2016;213:667–675. doi: 10.1084/jem.20151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall et al. (2017).Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S. PrakashMicrobiome, probiotics and neurodegenerative diseases: deciphering the gut-brain axis. Cellular and Molecular Life Sciences. 2017;74(20):3769–3787. doi: 10.1007/s00018-017-2550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al. (2019).Yan L, Xie Y, Satyanarayanan SK, Zeng H, Liu Q, Huang M, Ma Y, Wan JB, Yao X, Su KP, Su H. Omega-3 polyunsaturated fatty acids promote brain-to-blood clearance of β-Amyloid in a mouse model with Alzheimer’s disease. Brain Behavior and Immunity. 2019;85:35–45. doi: 10.1016/j.bbi.2019.05.033. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2017).Yang SH, Lee DK, Shin J, Lee S, Baek S, Kim J, Jung H, Hah JM, Kim Y. Nec-1 alleviates cognitive impairment with reduction of Aβ and tau abnormalities in APP/PS1 mice. EMBO Molecular Medicine. 2017;9(1):61–77. doi: 10.15252/emmm.201606566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarandi et al. (2016).Yarandi SS, Peterson DA, Treisman GJ, Moran TH, Pasricha PJ. Modulatory effects of gut microbiota on the central nervous system: how the gut could play a role in neuropsychiatric health and disease. Journal of Neurogastroenterology and Motility. 2016;22:201–212. doi: 10.5056/jnm15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng et al. (2018).Zeng YQ, Cui YB, Gu JH, Liang C, Zhou XF. Scutellarin mitigates Aβ-induced neurotoxicity and improves behavior impairments in AD mice. Molecules. 2018;23(4):869. doi: 10.3390/molecules23040869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang F, Zhong R, Li S, Fu Z, Cheng C, Cai H, Le W. Acute hypoxia induced an imbalanced M1/M2 activation of microglia through NF-kappaB signaling in Alzheimer’s disease mice and wild-type littermates. Frontiers in Aging Neuroscience. 2017;9:282. doi: 10.3389/fnagi.2017.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2018).Zhao Y, Chen X, Wu Y, Wang Y, Li Y, Xiang C. Transplantation of human menstrual blood-derived mesenchymal stem cells alleviates alzheimer’s disease-like pathology in APP/PS1 transgenic mice. Frontiers in Molecular Neuroscience. 2018;11:140. doi: 10.3389/fnmol.2018.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2012).Zhou Y, Smith D, Leong BJ, Brännström K, Almqvist F, Chapman MR. Promiscuous cross-seeding between bacterial amyloids promotes interspecies biofilms. Journal of Biological Chemistry. 2012;287(42):35092–103. doi: 10.1074/jbc.M112.383737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2017a).Zhu S, Wang J, Zhang Y, He J, Kong J, Wang JF, Li XM. The role of neuroinflammation and amyloid in cognitive impairment in an APP/PS1 transgenic mouse model of Alzheimer’s disease. CNS Neuroscience & Therapeutics. 2017a;23(4):310–320. doi: 10.1111/cns.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2017b).Zhu X, Han Y, Du J, Liu R, Jin K, Yi W. Microbiota-gut-brain axis and the central nervous system. Oncotarget. 2017b;8:53829–53838. doi: 10.18632/oncotarget.17754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.