Abstract
Alpha-lipoic acid (ALA) is a natural short-chain fatty acid that has attracted great attention in recent years as an antioxidant molecule. However, some concerns have been recently raised regarding its safety profile. To address the issue, we aimed to assess ALA safety profile through a systematic review of the literature and a meta-analysis of the available randomized placebo-controlled clinical studies. The literature search included EMBASE, PubMed Medline, SCOPUS, Google Scholar, and ISI Web of Science by Clarivate databases up to 15th August 2020. Data were pooled from 71 clinical studies, comprising 155 treatment arms, which included 4749 subjects with 2558 subjects treated with ALA and 2294 assigned to placebo. A meta-analysis of extracted data suggested that supplementation with ALA was not associated with an increased risk of any treatment-emergent adverse event (all p > 0.05). ALA supplementation was safe, even in subsets of studies categorized according to smoking habit, cardiovascular disease, presence of diabetes, pregnancy status, neurological disorders, rheumatic affections, severe renal impairment, and status of children/adolescents at baseline.
Keywords: α-lipoic acid, thioctic acid, dietary supplement, safety, meta-analysis
1. Introduction
Alpha-lipoic acid (1, 2-dithiolane-3-pentanoic acid; ALA) or thioctic acid is a natural short-chain fatty acid that has attracted great attention in recent years as an antioxidant molecule, being largely used worldwide as a dietary supplement [1].
Previous investigations revealed that ALA can affect central and peripheral modulation of 5′-adenosine-monophosphate-activated protein kinase. Furthermore, it activates peroxisome proliferator-activated receptor (PPAR) alpha and gamma (PPAR-γ), modulates PPAR-regulated genes and upregulates the expression of PPAR-γ messenger ribonucleic acid (mRNA) and other proteins in the cardiac tissue and aorta smooth muscle [2,3]. Hence, ALA antioxidant activity is potentially able to promote weight loss and blood pressure control and ameliorate atherogenic dyslipidemia and insulin resistance [3]. For example, in obese patients with non-alcoholic fatty liver disease (NAFLD), ALA supplementation was shown to reduce adipokine concentrations and improve liver steatosis grade [4,5]. However, some concerns have been recently raised regarding ALA safety profile, after some reports suggesting a direct causal link between its use and insulin autoimmune syndrome (IAS, also known as Hirata’s disease) due to its sulfhydryl group [6]. Indeed, in about 50% of cases, IAS development is associated with drugs or dietary supplement containing a sulphur or sulfhydryl group. These cases are closely related to certain specific antigens of the major histocompatibility complex (MHC), which are more common in populations where IAS incidence is higher [7]. It is hypothesised that ALA might cause the development of antibodies to insulin and lead to a hypoglycaemic syndrome in predisposed subjects, even though evidence are inconclusive [8].
In a recent study that performed a preliminary analysis of spontaneous reports of suspected adverse reactions (ARs), ALA-containing natural products have also been associated with skin and gastrointestinal disorders, such as urticaria and abdominal pain [9].
To address safety issues related to ALA supplementation, we aimed to perform a systematic review of the literature and a meta-analysis of the available randomized placebo-controlled clinical trials.
2. Materials and Methods
The study was designed according to guidelines of the 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement [10], and was registered in the PROSPERO database (Registration number CRD42020159028).
Due to the study design, neither Institutional Review Board (IRB) approval, nor patient informed consent were required. PRISMA Checklist was reported in supplementary file A.
2.1. Search Strategy
EMBASE, PubMed Medline, SCOPUS, Google Scholar and ISI Web of Science by Clarivate databases were searched, with no language restriction, using the following search terms: (“Alpha-lipoic acid” OR “Alpha lipoic acid” OR “α-lipoic acid” OR “α lipoic acid” OR “ALA” OR “A-LA” OR “Lipoic acid” OR “Thioctic acid” OR “Tioctic acid” OR “Thioctacid”) AND (“Clinical trial” OR “Clinical study”). The wild-card term “*” was used to increase the sensitivity of the search strategy, which was limited to studies in humans. The reference list of identified papers was manually checked for additional relevant articles. Additional searches included references of review articles on that issue, and abstracts from selected congresses on the subject of the meta-analysis. Literature was searched from inception to 15th August 2020.
All paper abstracts were firstly screened by two independent reviewers (F.F. and M.R.) to remove ineligible articles. The remaining articles were obtained in full-text and assessed again by the same two researchers who evaluated each article independently and carried out data extraction and quality assessment. Disagreements were resolved by discussion with a third party (A.F.G.C.).
2.2. Study Selection Criteria
Original studies were included if they met the following criteria: (i) being a clinical trial with either parallel or cross-over design, (ii) having an appropriate controlled design for ALA supplementation, (iii) blinding participants to intervention, (iv) testing the safety of ALA, (v) reporting treatment-emergent adverse events.
Exclusion criteria were: (i) lack of randomisation for treatment allocation, (ii) lack of a control group receiving placebo (iii) lack of sufficient information about the prevalence and nature of the adverse events. Studies were also excluded if they contained overlapping subjects with other studies.
2.3. Data Extraction
Data abstracted from eligible studies were: (i) first author’s name; (ii) year of publication; (iii) study location; (iv) study design; (v) follow-up; (vi) main inclusion criteria and underlying disease; (vii) study groups; (viii) number of participants in the active and control group; (ix) age and sex of study participants; (x) treatment-emergent adverse events occurred during the trials. Missing or unpublished data were sought by trying to contact authors via e-mail and repeated messages were sent in case of no response. Extracted data were reviewed by the principal investigator before the final analysis, and doubts were resolved by mutual agreement among the authors.
2.4. Quality Assessment
A systematic assessment of risk of bias in the included studies was performed using the Cochrane criteria [11]. The following items were used: adequacy of sequence generation, allocation concealment, blinding addressing of dropouts (incomplete outcome data), selective outcome reporting, and other probable sources of bias [12]. Overall evidence was qualified using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system [13]. Risk-of-bias assessment was performed independently by two reviewers; disagreements were resolved by a consensus-based discussion.
2.5. Data Synthesis
Meta-analysis was conducted using Comprehensive Meta-Analysis (CMA) V3 software (Biostat, NJ) [14].
Outcomes were treatment-emergent adverse events (AEs) occurring during the trials. In particular, data extracted from the studies included hypoglycaemic episodes, gastrointestinal AEs (e.g., heartburn, gastric complaints, nausea, gastrointestinal complications, duodenitis, and abdominal bloating), neurological AEs (e.g., headache, foggy thinking, drowsiness, leg weakness, legs periodic numbness and tingling, tingling in toe and fingers and intermittent bilateral toe numbness), psychiatric disorders (e.g., bipolar disorders, irritability, poor sleeping), musculoskeletal AEs (e.g., neck pain, lower back pain, and spasms), skin AEs (e.g., skin rash, disseminated maculopapular rash, itching sensation and urticaria), infections (e.g., laryngitis, pneumonia and yeast infections), cardiovascular (CV) system AEs (e.g., increase in arterial blood pressure, palpitations, myocardial infarction, heart rate and rhythm disorders, and heart valve disorders), hospitalisation and death.
The analysis was performed by excluding studies with zero events in both arms. If one or more outcomes could not be extracted from a study, the study was removed only from the analysis involving those outcomes. To avoid a double-counting problem, in trials comparing multiple treatment arms versus a single control group, the number of subjects in the control group was divided by the required comparisons [15].
To reduce the risk of bias due to effect dilution, the meta-analysis was performed considering per-protocol (PP) population.
Studies’ findings were combined using a fixed-effect model since the low level of inter-study heterogeneity, which was quantitatively assessed using the Higgins index (I2) [16]. Effect sizes were expressed as odds ratio (OR) and 95% confidence interval (95% CI) [17]. Finally, sensitivity analysis was conducted to account for the risk of bias. A leave-one-out method was used (i.e., one study was removed at a time and the analysis was repeated) [18].
Two-sided p-values < 0.05 were considered as statistically significant for all tests.
2.6. Additional Analysis
Subgroup analyses were carried out by presence of smoking habit, pregnancy, CV disease, diabetes, rheumatic disorders, neurological disorders, severe renal impairment, and status of children/adolescent at baseline.
2.7. Publication Biases
Potential publication biases were explored using visual inspection of Begg’s funnel plot asymmetry, Begg’s rank correlation test, and Egger’s weighted regression test [19]. Two-sided p-values < 0.05 were considered statistically significant for the tests.
3. Results
3.1. Flow and Characteristics of the Included Studies
After database searches performed strictly according to inclusion and exclusion criteria, 962 published articles were identified, and their abstracts reviewed. Of these, 359 did not report original data. Furthermore, 393 articles were excluded because they did not meet the inclusion criteria. Thus, 210 articles were carefully assessed and reviewed. Additional 139 papers were excluded due to being pre-print papers (n = 2), study protocols (n = 6), reporting data from studies lacking of an appropriate placebo-controlled design for the supplementation (n = 64), lacking of randomisation (n = 5), testing the acute effect of ALA supplementation (n = 7), testing ALA supplementation combined in nutraceutical compounds (n = 27), testing intravenous treatment with ALA (n = 11), testing topical treatment with ALA (n = 4), lacking sufficient information about the nature of the adverse events (n = 9), or reporting data overlapped with other publications (n = 4) (Supplementary file B). Finally, 71 studies were eligible and included in the systematic review [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90]. The study selection process is shown in Figure 1.
Figure 1.
Flow chart of the number of studies identified and included in the systematic review.
Data were pooled from 71 randomized placebo-controlled clinical studies, comprising 155 treatment arms (82 active arms and 73 control arms). The studies included 4749 subjects, with 2558 receiving treatment with ALA and 2294 subjects assigned to placebo. For reasons independent of the tested supplementation (i.e., withdrawal of informed consent and personal problems), 510 subjects prematurely terminated the trials in which they were enrolled. Then, the meta-analysis was performed considering the other subjects (i.e., PP population).
Eligible studies were published between 1982 and 2020 and were conducted in different locations across all continents. Follow-up periods ranged between 8 days and 4 years and several ALA regimens were tested. Selected clinical trials were designed with cross-over or parallel-group and enrolled pregnant women with gestational diabetes, children and/or adolescent, overall healthy subjects or subjects with minor or major underlying diseases (e.g., diabetes, CVD, rheumatic affections, neurological disorders, severe renal impairment).
Included clinical studies were fully or partially carried out independently and funded by the National Institutes of Health (n = 7), Health Ministries (n = 2), University Institutes (n = 42), Research Hospitals (n = 2), Private Research Institutes (n = 2), Scientific Societies (n = 3), Private Foundations (n = 8), or were financially supported by industries (n = 7).
The main characteristics of the evaluated studies are summarized in Table 1.
Table 1.
Main characteristics of the clinical trials testing safety of treatment with α-lipoic acid.
| Author, Year | Location | Study Design | Treatment Duration | Main Inclusion Criteria and Underlying Disease | Study Group | Enrolled Subjects (n) |
Age (years; mean ± SD) |
Male [n (%)] |
|---|---|---|---|---|---|---|---|---|
| Ahmadi, 2013 [20] | Iran | Randomized, single-blind, placebo-controlled, parallel-group, clinical study | 2 months | End-stage renal disease on haemodialysis (≥2 times/week for ≥1 year) | 600 mg/day α-lipoic acid | 20 | 48.8 ± 11.2 | 14 (70) |
| Placebo | 24 | 48.9 ± 12.5 | 9 (38) | |||||
| Ansar, 2011 [21] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 8 weeks | Type 2 diabetes mellitus FPG > 126 mg/dL |
300 mg/day α-lipoic acid | 29 | 49 ± 9.1 | 6 (21) |
| Placebo | 28 | 51.8 ± 8.3 | 8 (29) | |||||
| Aslfalah, 2019a [22] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 8 weeks | Gestational diabetes mellitus | 100 mg/day α-lipoic acid | 30 | 30.96 ± 0.93 | 0 (0) |
| Placebo | 30 | 31.1 ± 0.92 | 0 (0) | |||||
| Aslfalah, 2019b [23] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 8 weeks | Gestational diabetes mellitus | 100 mg/day α-lipoic acid | 30 | 30.96 ± 0.93 | 0 (0) |
| Placebo | 30 | 31.1 ± 0.92 | 0 (0) | |||||
| Baumgartner, 2017 [24] | The Netherlands | Randomized, double-blind, placebo-controlled, crossover, clinical study | 4 weeks | Impaired glucose tolerance or non-insulin-dependent type 2 diabetes BMI ≥ 20 kg/m2 and ≤35 kg/m2 |
600 mg/day α-lipoic acid | 20 | 63.1 ± 5.8 | 16 (80) |
| Placebo | ||||||||
| Baziar, 2020 [25] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 8 weeks | Non-insulin-dependent diabetes mellitus HbA1c < 7% BMI ≥ 18.5 kg/m2 and ≤29.9 kg/m2 |
1200 mg/day α-lipoic acid | 35 | 52.66 ± 4.81 | 15 (43) |
| Placebo | 35 | 53.34 ± 4.45 | 16 (46) | |||||
| Bobe, 2020 [26] | United States of America | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 24 weeks | Sedentary lifestyle BMI ≥ 27 kg/m2 TG ≥ 150 mg/dL FPG < 125 mg/dL |
600 mg/day α-lipoic acid | 40 | 38 ± 10 * | 12 (39) * |
| Placebo | 41 | 40 ± 8 | 16 (48) * | |||||
| Boriani, 2017 [27] | Italy | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 40 days | Primary tunnel carpal syndrome at least one of the following findings: anaesthesia or paraesthesia in the median nerve territory, positive Tinel sign, Phalen or reverse Phalen manoeuvres, and positive nerve conduction studies irrespective of severity |
800 mg/day α-lipoic acid | 32 | 57.3 ± 12 | 13 (41) |
| Placebo | 32 | 58.5 ± 11 | 9 (28) | |||||
| Carbone, 2009 [28] | Italy | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 8 weeks | Burning mouth syndrome | 800 mg/day α-lipoic acid | 22 | NA | NA |
| Placebo | 22 | NA | NA | |||||
| Cavalcanti, 2009 [29] | Brazil | Randomized, double-blind, placebo-controlled, crossover, clinical study | 30 days | Burning mouth syndrome | 600 mg/day α-lipoic acid | 38 | 63.1 (36–78) § | 4 (11) |
| Placebo | ||||||||
| Durastanti, 2016 [30] | Italy | Randomized, double-blind, placebo-controlled, parallel-group, pilot clinical study | 2 years | Relapsing-remitting multiple sclerosis EDSS score ≤ 3.5 |
800 mg/day α-lipoic acid during the first year and 400 mg/day α-lipoic acid during the second year | 7 | 33 (26–43) ° | 2 (29) |
| Placebo | 6 | 28.5 (22.5–44.3) ° | 1 (17) | |||||
| El Amrousy, 2020 [31] | Egypt | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 3 months | Obese healthy children and adolescents BMI > 95th percentile for age and sex |
600 mg/day α-lipoic acid | 40 | 12.3 ± 1.5 | 16 (40) |
| Placebo | 40 | 12.4 ± 1.4 | 18 (45) | |||||
| Falardeau, 2019 [32] | United States of America | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 6 weeks | Unilateral acute optic neuritis | 1200 mg/day α-lipoic acid | 15 | 41.2 ± 10.51 | 7 (47) |
| Placebo | 16 | 36.1 ± 9.84 | 4 (25) | |||||
| Femiano, 2002 [33] | Spain | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 2 months | Burning mouth syndrome | 600 mg/day α-lipoic acid | 30 | 45 (22–68) § | 18 (30) |
| Placebo | 30 | |||||||
| Georgakouli, 2018 [34] | Greece | Randomized, double-blind, placebo-controlled, crossover, clinical study | 4 weeks | Healthy status | 600 mg/day α-lipoic acid | 8 | 38.4 ± 5.6 | 8 (100) |
| Placebo | ||||||||
| Gianturco, 2009 [35] | Italy | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 4 weeks | Diabetes mellitus HbA1c < 7% |
400 mg/day α-lipoic acid | 7 | 61 ± 7 | 4 (57) |
| Placebo | 7 | 58 ± 16 | 4 (57) | |||||
| Gilron, 2020 [36] | Canada | Randomized, double-blind, placebo-controlled, crossover, clinical study | 5 weeks | Fibromyalgia daily moderate pain (≥4/10 on a NRS) for ≥3 months |
600 mg/day α-lipoic acid during the first week; 1200 mg/day α-lipoic acid during the second week; 1800 mg/day α-lipoic acid during the third and the fourth weeks | 27 | 57 (25–74) § | 5 (19) |
| Placebo | ||||||||
| Gosselin, 2019 [37] | United States of America | Randomized, double-blind, placebo-controlled, crossover, clinical study | 1 month | Sedentary lifestyle FPG ≥ 100 mg/dL and ≤125 mg/dL BMI ≥ 25 kg/m2 and ≤40 kg/m2 |
600 mg/day α-lipoic acid | 12 | 47.1 ± 2.9 | 4 (33) |
| Placebo | ||||||||
| Guo, 2014 [38] | United States of America | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 24 weeks | Cancer patients receiving chemotherapy with cisplatin or oxaliplatin | 1800 mg/day α-lipoic acid | 122 | 55 ± 11 | 66 (54) |
| Placebo | 121 | 57 ± 12 | 63 (52) | |||||
| Haghighian, 2015 [39] | Iran | Randomized, triple-blind, placebo-controlled, parallel-group, clinical study | 12 weeks | Idiopathic asthenozoospermia BMI < 30 kg/m2 |
600 mg/day α-lipoic acid | 24 | 32.98 ± 5.35 * | 24 (100) |
| Placebo | 24 | 34.12 ± 4.79 * | 24 (100) | |||||
| Hejazi, 2018 [40] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 10 days | Candidates for enteral feeding and expected to stay in the intensive care unit for ≥7 days | 2700 mg/day α-lipoic acid | 40 | 51.2 ± 17 | 17 (43) |
| Placebo | 40 | 57.4 ± 19 | 25 (63) | |||||
| Huang, 2008 [41] | United States of America | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 3 months | Pubertal or postpubertal adolescents with type 1 diabetes | 600–1200 mg/day (14–21 mg/kg/day) α-lipoic acid | 30 | 14 ± 2.4 | 13 (43) |
| Placebo | 10 | 15 ± 1.9 | 7 (70) | |||||
| Huerta, 2016 [42] | Spain | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 10 weeks | Sedentary lifestyle BMI ≥ 27.5 kg/m2 and ≤40 kg/m2 |
300 mg/day α-lipoic acid | 6 | 35.5 ± 8.4 | 0 (0) |
| Placebo | 6 | 41.8 ± 6.6 | 0 (0) | |||||
| Huerta, 2015 [43] | Spain | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 10 weeks | Healthy status regular menstrual cycles BMI ≥ 27.5 kg/m2 and ≤40 kg/m2 |
300 mg/day α-lipoic acid | 26 | 39 ± 8 * | 0 (0) |
| Placebo | 31 | 38 ± 7 * | 0 (0) | |||||
| Jacob, 1999 [44] | Germany | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 4 weeks | Well-controlled type 2 diabetes mellitus | 1800 mg/day α-lipoic acid | 18 | 62.1 ± 3 | 10 (56) |
| 1200 mg/day α-lipoic acid | 18 | 60.9 ± 2.2 | 11 (61) | |||||
| 600 mg/day α-lipoic acid | 19 | 58.1 ± 2.8 | 10 (53) | |||||
| Placebo | 19 | 60.4 ± 2.4 | 12 (63) | |||||
| Jamshidi, 2020 [45] | Iran | Randomized, double-blind, placebo-controlled, crossover, clinical study | 8 weeks | β-thalassemia major | 600 mg/day α-lipoic acid | 20 | 23.5 ± 5.47 | 13 (65) |
| Placebo | ||||||||
| Jariwalla, 2008 [46] | United States of America | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 6 months | HIV infection HIV-RNA viral load > 10.000 copies/cm3 despite HAART CD4+ cell count ≥ 50 cells/mm3 |
900 mg/day α-lipoic acid | 18 | 47.2 ± 6.8 | 29 (88) |
| Placebo | 15 | 43.7 ± 7.6 | ||||||
| Khabbazi, 2012 [47] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 8 weeks | Patients with end-stage renal disease on haemodialysis | 600 mg/day α-lipoic acid | 31 | 53.83 ± 13.29 | 16 (52) |
| Placebo | 32 | 54.04 ± 13.96 | 18 (56) | |||||
| Khalili, 2017 [48] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 12 weeks | Relapsing-remitting multiple sclerosis | 1200 mg/day α-lipoic acid | 15 | 32.3 ± 6.2 * | 5 (42) * |
| Placebo | 16 | 32.2 ± 10.5 * | 1 (8) * | |||||
| Khalili, 2014 [49] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 12 weeks | Relapsing-remitting multiple sclerosis | 1200 mg/day α-lipoic acid | 26 | 31.4 ± 6.2 * | 7 (27) |
| Placebo | 34 | 28.7 ± 9 * | 9 (26) | |||||
| Kim, 2020 [50] | South Korea | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 18 months | Geographic atrophy | 1200 mg/day α-lipoic acid | 26 | 80.6 ± 6.5 | 8 (31) |
| Placebo | 27 | 79 ± 7 | 11 (41) | |||||
| Kim, 2016 [51] | South Korea | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 12 weeks | Chronic schizophrenia in rehabilitation significant weight gain after starting treatment with atypical antipsychotics |
600–1800 mg/day α-lipoic acid | 10 | 40.5 ± 6.65 | 4 (40) |
| Placebo | 12 | 40.08 ± 9.14 | 7 (58) | |||||
| Koh, 2011 [52] | Republic of Korea | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 20 weeks | BMI ≥ 30 kg/m2 or BMI ≥ 27.5 kg/m2 and ≤40 kg/m2 if hypertension, diabetes mellitus and/or hypercholesterolemia coexisted | 1800 mg/day α-lipoic acid | 120 | 41.4 ± 1 | 82 (68) |
| 1200 mg/day α-lipoic acid | 120 | 41.6 ± 1.1 | 79 (66) | |||||
| Placebo | 120 | 40.7 ± 1.1 | 74 (62) | |||||
| Lampitella, 2005 [53] | Italy | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 6 months | Type 2 diabetes mellitus | 600 mg/day α-lipoic acid | 20 | NA | NA |
| Placebo | 20 | NA | NA | |||||
| Lee, 2017 [54] | Republic of Korea | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 24 weeks | Diabetic cardiac autonomic neuropathy | 600-1200 mg/day α-lipoic acid | 46 | 64.37 ± 7.8 | 27 (59) |
| Placebo | 45 | 62.4 ± 9.1 | 20 (44) | |||||
| Loy, 2018 [55] | United States of America | Randomized, double-blind, placebo-controlled, parallel-group, pilot clinical study | 2 years | Multiple sclerosis disability progression in absence of clinical relapse for 5 years EDSS ≤ 6.0 ability to walk ≥ 25 feet without aid |
1200 mg/day α-lipoic acid | 11 | 55.8 ± 5.7 | 5 (45) |
| Placebo | 10 | 55.7 ± 4.1 | 5 (50) | |||||
| López-D’alessandro, 2011 [56] | Argentina | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 2 months | Burning mouth syndrome | 600 g/day α-lipoic acid | 20 | NA | NA |
| Placebo | 60 | NA | NA | |||||
| López-Jornet, 2009 [57] | Spain | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 8 weeks | Burning mouth syndrome | 800 mg/day α-lipoic acid | 30 | 64.37 ± 11.61 | 6 (10) |
| Placebo | 30 | |||||||
| Magis, 2007 [58] | Belgium | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 3 months | Migraine with or without aura | 600 mg/day α-lipoic acid | 26 | 37.46 ± 13.43 | 4 (15) |
| Placebo | 18 | 38.94 ± 8.05 | 2 (11) | |||||
| Manning, 2013 [59] | New Zeland | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 1 year | Metabolic syndrome | 600 mg/day α-lipoic acid | 34 | 55 ± 10 | 14 (41) |
| Placebo | 40 | 57 ± 9 | 15 (38) | |||||
| Marfella, 2016 [60] | Italy | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 12 months | Takotsubo cadiomyopathy | 600 mg/day α-lipoic acid | 24 | 63.7 ± 6.5 | 0 (0) |
| Placebo | 24 | 63.9 ± 5.2 | 0 (0) | |||||
| Marshall, 1982 [61] | United Kingdom | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 24 weeks | Alcohol related liver disease | 300 mg/day α-lipoic acid | 20 | 50.7 ± 1.9 | 17 (85) |
| Placebo | 20 | 46.4 ± 2.7 | 15 (75) | |||||
| Martins, 2009 [62] | Brazil | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 3 months | Sickle cell disease | 200 mg/day α-lipoic acid | 10 | 17.7 ± 9.6 | 6 (60) |
| Placebo | 10 | 17 ± 11 | 5 (50) | |||||
| Sickle cell trait | 200 mg/day α-lipoic acid | 10 | 31.3 ± 15.4 | 2 (20) | ||||
| Placebo | 10 | 29.7 ± 10.8 | 2 (20) | |||||
| Healthy status | 200 mg/day α-lipoic acid | 10 | 23.5 ± 11 | 4 (40) | ||||
| Placebo | 10 | 23.3 ± 11 | 3 (30) | |||||
| Mendes, 2014 [63] | Brazil | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 12 weeks | Arterial hypertension | 600 mg/day α-lipoic acid | 32 | NA | NA |
| Placebo | 28 | NA | NA | |||||
| Mendoza-Núñez, 2019 [64] | Mexico | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 6 months | Type 2 diabetes mellitus without complications or comorbidity, treated with two tablets of glibenclamide/metformin (5/500 mg) per day BMI < 35 kg/m2 sedentary lifestyle |
600 mg/day α-lipoic acid | 50 | 63 ± 1 * | NA |
| Placebo | 50 | 64 ± 1 * | NA | |||||
| Mirtaheri, 2014 [65] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 8 weeks | Rheumatoid arthritis | 1200 mg/day α-lipoic acid | 35 | 36.09 ± 8.77 * | 0 (0) |
| Placebo | 35 | 38.28 ± 8.63 * | 0 (0) | |||||
| Mohammadi, 2018 [66] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 12 weeks | Previous thrombotic or embolic stroke BMI ≥ 18.5 kg/m2 and ≤35 kg/m2 |
600 mg/day α-lipoic acid | 40 | 62.33 ± 6.19 | NA |
| Placebo | 40 | 64.23 ± 8.01 | NA | |||||
| Mohammadi, 2015 [67] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 12 weeks | Spinal cord injury since ≥ 1 year BMI ≥ 18.5 kg/m2 |
600 mg/day α-lipoic acid | 28 | 39 ± 6.44 | 28 (100) |
| Placebo | 30 | 36.8 ± 7.48 | 30 (100) | |||||
| Mollo, 2012 [68] | Italy | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 5 weeks | Type 1 diabetes | 600 mg/day α-lipoic acid | 26 | 43 ± 9 | 15 (58) |
| Placebo | 25 | 46 ± 11 | 12 (48) | |||||
| Monroy Guízar, 2018 [69] | Mexico | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 3 months | Idiopathic carpal tunnel syndrome | 600 mg/day α-lipoic acid | 10 | 45.3 † | 1 (10) |
| Placebo | 10 | 48.4 † | 1 (10) | |||||
| Palacios-Sánchez, 2015 [70] | Spain | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 2 months | Burning mouth syndrome | 600 mg/day α-lipoic acid | 30 | 62.13 (36–86) § | 5 (8) |
| Placebo | 30 | |||||||
| Porasuphatana, 2012 [71] | Thailand | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 6 months | Type 2 diabetes mellitus with microalbuminuria | 1200 mg/day α-lipoic acid | 7 | 47.07 ± 2.18 | 1 (14) |
| 900 mg/day α-lipoic acid | 7 | 44 ± 2 | 1 (14) | |||||
| 600 mg/day α-lipoic acid | 8 | 45.7 ± 1.68 | 3 (38) | |||||
| 300 mg/day α-lipoic acid | 8 | 42.5 ± 1.12 | 4 (50) | |||||
| Placebo | 8 | 42.9 ± 2.52 | 1 (13) | |||||
| Pourghasem Gargari, 2014 [72] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 8 weeks | Rheumatoid arthritis DAS28 < 5.1 BMI < 40 kg/m2 |
1200 mg/day α-lipoic acid | 35 | 36.1 ± 8.8 | 0 (0) |
| Placebo | 35 | 38.3 ± 8.6 | 0 (0) | |||||
| Rahmanabadi, 2019 [4] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 12 weeks | Non-alcoholic fatty liver disease BMI ≥ 30 kg/m2 and ≤40 kg/m2 |
1200 mg/day α-lipoic acid | 25 | 40.28 ± 5.5 | 13 (52) |
| Placebo | 25 | 37.52 ± 9.67 | 14 (56) | |||||
| Ruhnau, 1999 [73] | Germany | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 3 weeks | Type 2 diabetes mellitus with distal symmetrical polyneuropathy | 1800 mg/day α-lipoic acid | 12 | 60.5 ± 6.9 | 6 (50) |
| Placebo | 12 | 62.1 ± 4.5 | 6 (50) | |||||
| Safa, 2014 [74] | Iran | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 12 months | End-stage renal disease on haemodialysis ≥ 6 months | 600 mg/day α-lipoic acid | 30 | 59.3 ± 10.47 | 21 (70) |
| Placebo | 31 | 55.2 ± 13.43 | 21 (68) | |||||
| Sammour, 2019 [75] | Egypt | Randomized, triple-blind, placebo-controlled, parallel-group, clinical study | 6 weeks | Primary caesarean section in singleton term pregnancy | 1200 mg/day α-lipoic acid | 51 | 25.3 ± 5.1 | 0 (0) |
| Placebo | 51 | 25.1 ± 5.4 | 0 (0) | |||||
| Sardu, 2017 [76] | Italy | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 12 months | Paroxysmal, symptomatic atrial fibrillation ≥ 6 months refractory to ≥1 class 1–3 antiarrhythmic drugs and treated with catheter ablation | 600 mg/day α-lipoic acid | 33 | 58.8 ± 6.7 | 15 (45) |
| Placebo | 40 | 61.5 ± 8.1 | 23 (58) | |||||
| Scaramuzza, 2015 [77] | Italy | Randomized, double-blind, placebo-controlled, parallel-group, pilot clinical study | 6 months | Type 1 diabetes endothelial dysfunction |
800 mg/day α-lipoic acid | 25 | 16.1 ± 3.1 | 15 (60) |
| Placebo | 27 | 16 ± 3.4 | 16 (59) | |||||
| Sola, 2005 [78] | United Stated of America | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 4 weeks | Metabolic syndrome | 300 mg/day α-lipoic acid | 15 | 46 ± 15 | 5 (33) |
| Placebo | 14 | 44 ± 13 | 6 (43) | |||||
| Spain, 2017 [79] | United Stated of America | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 2 years | Multiple sclerosis disability progression in absence of clinical relapse for 5 years | 1200 mg/day α-lipoic acid | 27 | 57.9 ± 6.7 | 11 (41) |
| Placebo | 24 | 59.7 ± 6 | 9 (38) | |||||
| Sun, 2012 [80] | China | Randomized, blind, placebo-controlled, parallel-group, clinical study | 3 months | Dry form of age-related macular degeneration | 600 mg/day α-lipoic acid | 32 | 65.8 ± 7.9 | 11 (35) |
| Placebo | 30 | 64.5 ± 8.1 | 10 (33) | |||||
| Tromba, 2019 [81] | Italy | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 12 weeks | BMI ≥ 85th percentile for age and sex | 800 mg/day α-lipoic acid | 34 | 11.5 ± 1.9 * | 16 (50) * |
| Placebo | 33 | 11.1 ± 2.1 * | 20 (63) * | |||||
| Udupa, 2013 [82] | India | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 90 days | Type 2 diabetes mellitus FGP ≥ 110 mg/dL and ≤250 mg/dL |
300 mg/day α-lipoic acid | 25 | 53.5 ± 1.4 | 12 (48) |
| Placebo | 25 | 53.8 ± 2.1 | 15 (60) | |||||
| Vincent, 2007 [83] | United States of America | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 3 months | ABI ≥ 0.3 and ≤0.9 claudication pain with walking |
600 mg/day α-lipoic acid | 16 | 75.1 ± 8.2 | 9 (56) |
| Placebo | 12 | 70.7 ± 18.9 | 6 (50) | |||||
| Yadav, 2005 [84] | United States of America | Randomized, double-blind, placebo-controlled, parallel-group, pilot clinical study | 14 days | Multiple sclerosis EDSS score ≤ 7.5 |
2400 mg/day α-lipoic acid | 8 | 44.5 (34–56) § | 0 (0) |
| 1200 mg/day α-lipoic acid | 16 | NA | 2 (13) | |||||
| Placebo | 9 | 50 (36–66) § | 2 (22) | |||||
| Yan, 2013 [85] | China | Randomized, double-blind, placebo-controlled, crossover, clinical study | 8 weeks | BMI ≥ 25 kg/m2 ≥1 of borderline hypertension, dyslipidemia, or impaired FPG |
1200 mg/day α-lipoic acid | 103 | NA | NA |
| Placebo | ||||||||
| Zembron-Lacny, 2013 [86] | Poland | Randomized, double-blind, placebo-controlled, crossover, clinical study | 10 days | Healthy status | 1200 mg/day α-lipoic acid | 16 | 20.7 ± 0.9 | 16 (100) |
| Placebo | ||||||||
| Zembron-Lacny, 2009 [87] | Poland | Randomized, double-blind, placebo-controlled, crossover, clinical study | 8 days | Physical education students healthy status forced training experience ≥3 years |
1200 mg/day α-lipoic acid | 13 | 25.5 ± 6 | 13 (100) |
| Placebo | ||||||||
| Ziegler, 2011 [88] | Canada, Croatia, Denmark, France, Italy, Spain, The Netherlands, United Kingdom, United States of America | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 4 years | Type 1 or 2 diabetes (duration ≥1 year) stage 1 or 2a distal symmetric sensorimotor polyneuropathy due to diabetes stable insulin regimen NIS[LL]+7 ≥ 2 one of the following abnormalities: abnormal nerve conduction attributes in two separate nerves ≥ 99th percentile for distal latency or ≤1st percentile for nerve conduction velocity or amplitude OR HRBD ≥ 1st percentile or TSS in the feet< 5 |
600 mg/day α-lipoic acid | 231 | 53.3 ± 8.3 | 152 (66) |
| Placebo | 225 | 53.9 ± 7.6 | 154 (67) | |||||
| Ziegler, 2006 [89] | Israel and Russia | Randomized, double-blind, placebo-controlled, parallel-group, clinical study | 5 weeks | Type 1 or 2 diabetes HbA1c < 10% symptomatic distal symmetric polyneuropathy due to diabetes TSS > 7.5 NIS[LL] ≥ 2 absent or decreased pain sensation according to pin-prick test |
1800 mg/day α-lipoic acid | 46 | 59 ± 9 | 19 (41) |
| 1200 mg/day α-lipoic acid | 47 | 59 ± 12 | 19 (40) | |||||
| 600 mg/day α-lipoic acid | 45 | 56 ± 12 | 20 (44) | |||||
| Placebo | 43 | 57 ± 11 | 15 (35) |
* data refer to safety population; § data reported as median (variation range); ° data reported as median (interquartile range); † data reported as mean; ABI = Ankle brachial index; BMI = Body mass index; CVD = Cardiovascular disease; DAS28 = Disease activity score in 28 joints; EDSS = Expanded disability status scale; HIV = Human immunodeficiency virus; HRBD = Heart rate during deep breathing; NA = Not available; NIS[LL] = Neuropathy impairment score — subscore for lower limbs; NIS[LL]+7 = Neuropathy impairment score—subscore for lower limbs and seven nerve conduction tests score; NRS = Numerical rating scale; FPG = Fasting plasma glucose; TSS = Total symptom score.
3.2. Risk of Bias Assessment
Almost all of the included studies were characterized by sufficient information regarding sequence generation, allocation concealment, personal and outcome assessments, incomplete outcome data, and selective outcome reporting. Details of the quality of bias assessment are reported in Table 2.
Table 2.
Quality of bias assessment of the included studies according to Cochrane guidelines.
| Author, Year | Sequence Generation | Allocation Concealment | Blinding to Participants, Personnel and Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Other Potential Threats to Validity |
|---|---|---|---|---|---|---|
| Ahmadi, 2013 [20] | L | L | H | L | L | U |
| Ansar, 2011 [21] | L | L | L | L | U | L |
| Aslfalah, 2019a [22] | L | L | L | L | L | L |
| Aslfalah, 2019b [23] | L | L | L | L | L | L |
| Baumgartner, 2017 [24] | L | L | L | L | L | L |
| Baziar, 2020 [25] | L | L | L | L | L | L |
| Bobe, 2020 [26] | L | L | L | L | L | L |
| Boriani, 2017 [27] | L | L | L | L | L | L |
| Carbone, 2009 [28] | L | L | L | L | L | L |
| Cavalcanti, 2009 [29] | L | L | L | L | L | L |
| Durastanti, 2016 [30] | L | L | L | U | U | U |
| El Amrousy, 2020 [31] | L | L | L | L | L | L |
| Falardeau, 2019 [32] | L | L | L | L | L | L |
| Femiano, 2002 [33] | U | L | L | L | U | U |
| Georgakouli, 2018 [34] | L | L | L | L | L | L |
| Gianturco, 2009 [35] | L | L | L | L | U | L |
| Gilron, 2020 [36] | L | L | L | L | L | L |
| Gosselin, 2019 [37] | L | L | L | L | L | L |
| Guo, 2014 [38] | L | L | L | L | L | L |
| Haghighian, 2015 [39] | L | L | L | L | L | L |
| Hejazi, 2018 [40] | L | L | L | L | L | L |
| Huang, 2008 [41] | L | L | L | L | L | L |
| Huerta, 2016 [42] | L | L | L | L | L | L |
| Huerta, 2015 [43] | L | L | L | L | L | L |
| Jacob, 1999 [44] | L | L | L | L | U | H |
| Jamshidi, 2020 [45] | L | L | L | L | L | L |
| Jariwalla, 2008 [46] | L | L | L | L | U | H |
| Khabbazi, 2012 [47] | L | L | L | L | L | L |
| Khalili, 2017 [48] | L | L | L | L | L | L |
| Khalili, 2014 [49] | L | L | L | L | L | L |
| Kim, 2020 [50] | L | L | L | L | L | L |
| Kim, 2016 [51] | L | L | L | L | L | L |
| Koh, 2011 [52] | L | L | L | L | L | L |
| Lampitella, 2005 [53] | L | U | U | L | L | U |
| Lee, 2017 [54] | L | L | L | L | L | L |
| Loy, 2018 [55] | L | L | L | L | L | L |
| López- D’Alessandro, 2011 [56] | L | L | L | H | H | U |
| López-Jornet, 2009 [57] | L | L | L | L | L | L |
| Magis, 2007 [58] | L | L | L | L | L | L |
| Manning, 2013 [59] | L | L | L | L | L | L |
| Marfella, 2016 [60] | L | L | U | L | L | U |
| Marshall, 1982 [61] | L | L | L | L | L | L |
| Martins, 2009 [62] | L | L | U | L | L | U |
| Mendes, 2014 [63] | L | L | L | L | H | U |
| Mendoza- Núñez, 2019 [64] |
L | L | L | L | L | L |
| Mirtaheri, 2014 [65] | L | L | L | L | L | L |
| Mohammadi, 2018 [66] | L | L | L | L | L | L |
| Mohammadi, 2015 [67] | L | L | L | L | L | L |
| Mollo, 2012 [68] | L | L | L | L | L | L |
| Monroy Guízar, 2018 [69] | L | L | L | L | L | L |
| Palacios- Sánchez, 2015 [70] |
L | L | L | L | L | L |
| Porasuphatana, 2012 [71] | L | L | L | L | L | H |
| Pourghasem Gargari, 2014 [72] | L | L | L | L | L | L |
| Rahmanabadi, 2019 [4] | L | L | L | L | L | L |
| Ruhnau, 1999 [73] | L | L | L | L | L | L |
| Safa, 2014 [74] | L | L | L | L | L | L |
| Sammour, 2019 [75] | L | L | L | L | L | L |
| Sardu, 2017 [76] | L | L | L | L | L | L |
| Scaramuzza, 2015 [77] | L | L | L | L | L | L |
| Sola, 2005 [78] | L | L | L | L | L | L |
| Spain, 2017 [79] | L | L | L | L | L | L |
| Sun, 2012 [80] | L | U | U | L | L | U |
| Tromba, 2019 [81] | L | L | L | L | L | L |
| Udupa, 2013 [82] | L | L | L | L | L | L |
| Vincent, 2007 [83] | L | L | L | L | L | L |
| Yadav, 2005 [84] | L | L | L | L | L | L |
| Yan, 2013 [85] | L | L | L | L | L | L |
| Zembron- Lacny, 2013 [86] |
L | L | L | L | L | L |
| Zembron- Lacny, 2009 [87] |
L | L | L | L | L | L |
| Ziegler, 2011 [88] | L | L | L | L | L | L |
| Ziegler, 2006 [89] | L | L | L | L | L | L |
H = High risk of bias; L = Low risk of bias; U = Unclear risk of bias.
The quality of evidence for each outcome across all the studies was considered high in accordance with the GRADE approach.
3.3. Primary Outcomes
3.3.1. Hypoglycaemic Episodes
Symptoms defined as ‘similar to hypoglycaemic episodes’ were reported only by Jacob et al. and were exclusively experienced by subjects randomized to placebo. Authors did not report if an attempt for treatment rechallenging was made during the trial [44].
3.3.2. Gastrointestinal AEs
Meta-analysis of extracted data suggested that supplementation with ALA was not associated with an increased risk of gastrointestinal AEs (OR = 1.32, 95% CI 0.97 to 1.78; p = 0.073; I2 = 0%) (Figure 2). The finding was robust in the leave-one-out sensitivity analysis (Figure S1).
Figure 2.
Forest plot for the risk of gastrointestinal adverse events (AEs) following alpha-lipoic acid (ALA) supplementation versus placebo.
Visually, the funnel plot of standard error by log OR was slightly asymmetric (Figure S2). This asymmetry was imputed to eight potentially missing studies on the left-side of the plot, which reduced the estimated effect size to 1.12 (95% CI 0.84 to 1.49). Egger’s linear regression and Begg’s rank correlation confirmed the presence of publication bias for the analysis (p < 0.05).
3.3.3. Neurological AEs
Meta-analysis of extracted data suggested that supplementation with ALA was not associated with an increased risk of neurological AEs (OR = 1.53, 95% CI 0.88 to 2.63; p = 0.129; I2 = 0%) (Figure 3). The finding was robust in the leave-one-out sensitivity analysis (Figure S3).
Figure 3.
Forest plot for the risk of neurological AEs following ALA supplementation versus placebo.
Visually, the funnel plot of standard error by log OR was slightly asymmetric (Figure S4). This asymmetry was imputed to 4 potentially missing studies on the left-side of the plot, which reduced the estimated effect size to 1.26 (95% CI 0.76 to 2.10). However, neither Egger’s linear regression nor Begg’s rank correlation confirmed the presence of publication bias for the analysis (p > 0.05 for both tests).
3.3.4. Psychiatric Disorders
Meta-analysis of extracted data suggested that supplementation with ALA was not associated with an increased risk of psychiatric disorders (OR = 1.13, 95% CI 0.64 to 1.99; p = 0.668; I2 = 0%) (Figure 4). The finding was robust in the leave-one-out sensitivity analysis (Figure S5).
Figure 4.
Forest plot for the risk of psychiatric AEs following ALA supplementation versus placebo.
Visually, the funnel plot of standard error by log OR was slightly asymmetric (Figure S6). This asymmetry was imputed to two potentially missing studies on the left-side of the plot, which reduced the estimated effect size to 1.01 (95% CI 0.59 to 1.75). Egger’s linear regression confirmed the presence of publication bias for the analysis (p < 0.01), though Begg’s rank correlation did not.
3.3.5. Musculoskeletal AEs
Meta-analysis of extracted data suggested that supplementation with ALA was not associated with an increased risk of musculoskeletal AEs (OR = 0.76, 95% CI 0.22 to 2.64; p = 0.666; I2 = 0%) (Figure 5). The finding was robust in the leave-one-out sensitivity analysis (Figure S7).
Figure 5.
Forest plot for the risk of musculoskeletal AEs following ALA supplementation versus placebo.
Visually, the funnel plot of standard error by log OR was slightly asymmetric (Figure S8). This asymmetry was imputed to 2 potentially missing studies on the left-side of the plot, which reduced the estimated effect size to 0.50 (95% CI 0.17 to 1.51). However, neither Egger’s linear regression nor Begg’s rank correlation confirmed the presence of publication bias for the analysis (p > 0.05 for both tests).
3.3.6. Skin AEs
Meta-analysis of extracted data suggested that supplementation with ALA was not associated with an increased risk of skin AEs (OR = 1.13, 95% CI 0.82 to 1.56; p = 0.469; I2 = 33.6%) (Figure 6). The finding was robust in the leave-one-out sensitivity analysis (Figure S9).
Figure 6.
Forest plot for the risk of skin AEs following ALA supplementation versus placebo.
Visually, the funnel plot of standard error by log OR was slightly asymmetric (Figure S10). This asymmetry was imputed to four potentially missing studies on the left-side of the plot, which reduced the estimated effect size to 0.92 (95% CI 0.68 to 1.24). However, neither Egger’s linear regression nor Begg’s rank correlation confirmed the presence of publication bias for the analysis (p > 0.05 for both tests).
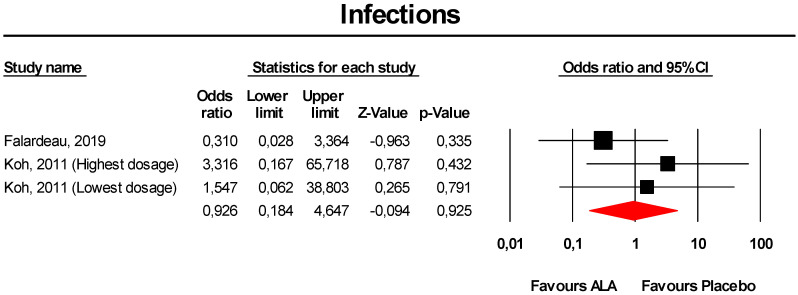
3.3.7. Infections
Meta-analysis of extracted data suggested that supplementation with ALA was not associated with an increased risk of infections (OR = 0.93, 95% CI 0.18 to 4.65; p = 0.925; I2 = 0%) (Figure 7). The finding was robust in the leave-one-out sensitivity analysis (Figure S11).
Figure 7.
Forest plot for the risk of infections following ALA supplementation versus placebo.
Visually, the funnel plot of standard error by log OR was slightly asymmetric (Figure S12). This asymmetry was imputed to two potentially missing studies on the left-side of the plot, which reduced the estimated effect size to 0.31 (95% CI 0.08 to 1.13). However, neither Egger’s linear regression nor Begg’s rank correlation confirmed the presence of publication bias for the analysis (p > 0.05 for both tests).
3.3.8. CV System AEs
Meta-analysis of extracted data suggested that supplementation with ALA was not associated with an increased risk of CV system AEs (OR = 1.25, 95% CI 0.84 to 1.85; p = 0.276; I2 = 15.8%) (Figure 8). The finding was robust in the leave-one-out sensitivity analysis (Figure S13).
Figure 8.
Forest plot for the risk of CV system AEs following ALA supplementation versus placebo.
Visually, the funnel plot of standard error by log OR was slightly asymmetric (Figure S14). This asymmetry was imputed to three potentially missing studies on the right-side of the plot, which increased the estimated effect size to 1.40 (95% CI 0.95 to 2.05). Egger’s linear regression confirmed the presence of publication bias for the analysis (p < 0.01), though Begg’s rank correlation did not.
3.3.9. Hospitalisation
Meta-analysis of extracted data suggested that supplementation with ALA was not associated with an increased risk of hospitalisation (OR = 5.66, 95% CI 0.64 to 49.85; p = 0.119; I2 = 0%) (Figure 9). The finding was robust in the leave-one-out sensitivity analysis (Figure S15).
Figure 9.
Forest plot for the risk of hospitalisation following ALA supplementation versus placebo.
3.3.10. Death
Meta-analysis of extracted data suggested that supplementation with ALA was not associated with an increased risk of death (OR = 0.56, 95% CI 0.21 to 1.48; p = 0.242; I2 = 0%) (Figure 10). The finding was robust in the leave-one-out sensitivity analysis (Figure S16).
Figure 10.
Forest plot for the risk of death following ALA supplementation versus placebo.
Visually, the funnel plot of standard error by log OR was slightly asymmetric (Figure S17). This asymmetry was imputed to three potentially missing studies on the right-side of the plot, which increased the estimated effect size to 0.71 (95% CI 0.31 to 1.64). Egger’s linear regression correlation confirmed the presence of publication bias for the analysis (p = 0.03), though Begg’s rank correlation did not.
3.4. Additional Analyses
Supplementation with ALA was not associated with a significant increased risk of any AE in subsets of studies classified by smoking habit, CV disease, diabetes, pregnancy, neurological disorders, rheumatic affections, and severe renal impairment at baseline (Table 3). Furthermore, ALA supplementation was safe in children (Table 3). The findings were robust in the leave-one-out sensitivity analysis.
Table 3.
Subgroup analyses for the risk of treatment-emergent AEs, stratified by smoking habit, cardiovascular disease, presence of diabetes, pregnancy, neurological disorders, rheumatic affections, age, and severe renal impairment at baseline.
| AEs | Smoking Habit | Cardiovascular Disease | Diabetes | Pregnancy | Neurological Disorders | Rheumatic Affections | Children and/or Adolescents | Severe Renal Impairment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||
| Gastrointestinal AEs | Number of reported AEs (active arm/placebo arm) | -/- | 4/2 | 2/0 | 97/88 | 137/77 | 17/14 | 3/2 | 180/97 | 144/76 | -/- | 5/2 | 4/3 | 3/2 | 180/97 | -/- | 94/81 |
| Odd ratio | - | 1.192 | 2.734 | 1.103 | 1.267 | 1.155 | 1.531 | 1.313 | 1.295 | - | 2.841 | 1.433 | 1.705 | 1.309 | - | 1.158 | |
| 95% CI (lower limit; upper limit) | - | 0.265; 5.361 | 0.273; 27.383 | 0.781; 1.558 | 0.879; 1.827 | 0.540; 2.468 | 0.245; 9.574 | 0.966; 1.784 | 0.897; 1.869 | - | 0.500; 16.138 | 0.300; 6.833 | 0.260; 11.156 | 0.964; 1.779 | - | 0.811; 1.653 |
|
| Z-value | - | 0.229 | 0.856 | 0.556 | 1.268 | 0.371 | 0.456 | 1.740 | 1.382 | - | 1.178 | 0.451 | 0.556 | 1.724 | - | 0.809 | |
| I2 (%) | - | 0 | 0 | 0 | 50 | 0 | 0 | 0 | 48 | - | 0 | 0 | 0 | 0 | - | 0 | |
| P-value | - | 0.819 | 0.392 | 0.578 | 0.205 | 0.711 | 0.649 | 0.082 | 0.167 | - | 0.239 | 0.652 | 0.578 | 0.085 | - | 0.418 | |
| Neurological AEs | Number of reported AEs (active arm/placebo arm) | -/- | 6/2 | 1/0 | 19/18 | 10/0 | 18/14 | -/- | 50/23 | 25/9 | -/- | 8/6 | 0/1 | -/- | 50/23 | -/- | 22/16 |
| Odd ratio | - | 1.024 | 3.078 | 1.153 | 2.368 | 1.268 | - | 1.526 | 1.718 | - | 1.474 | 0.315 | - | 1.526 | - | 3.078 | |
| 95% CI (lower limit; upper limit) | - | 0.236; 4.442 | 0.122; 77.905 | 0.544; 2.442 | 0.884; 2.634 | 0.552; 2.914 | - | 0.884; 2.634 | 0.742; 3.977 | - | 0.432; 5.027 | 0.012; 7.999 | - | 0.884; 2.634 | - | 0.122; 77.905 |
|
| Z-value | - | 0.032 | 0.682 | 0.371 | 1.517 | 0.560 | - | 1.517 | 1.264 | - | 0.619 | −0.700 | - | 1.517 | - | 0.682 | |
| I2 (%) | - | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - | 0 | - | 0 | |
| P-value | - | 0.974 | 0.495 | 0.711 | 0.129 | 0.575 | - | 0.129 | 0.206 | - | 0.536 | 0.484 | - | 0.129 | - | 0.495 | |
| Psychiatric AEs | Number of reported AEs (active arm/placebo arm) | -/- | 2/0 | -/- | 30/25 | 26/25 | 4/0 | -/- | 30/25 | 26/25 | -/- | -/- | 2/0 | -/- | 30/25 | -/- | 28/25 |
| Odd ratio | - | 5.145 | - | 1.131 | 1.014 | 5.071 | - | 1.131 | 1.014 | - | - | 5.145 | - | 1.131 | - | 1.073 | |
| 95% CI (lower limit; upper limit) | - | 0.238; 111.087 | - | 0.644; 1.986 |
0.566; 1.817 |
0.582; 44.174 |
- | 0.644; 1.986 |
0.566; 1.817 |
- | - | 0.238; 111.087 | - | 0.644; 1.986 | - | 0.605; 1.903 | |
| Z-value | - | 1.045 | - | 0.429 | 0.048 | 1.470 | - | 0.429 | 0.048 | - | - | 1.045 | - | 0.429 | - | 0.242 | |
| I2 (%) | - | 0 | - | 0 | 0 | 0 | - | 0 | 0 | - | - | 0 | - | 0 | - | 0 | |
| P-value | - | 0.296 | - | 0.668 | 0.962 | 0.142 | - | 0.668 | 0.962 | - | - | 0.296 | - | 0.668 | - | 0.809 | |
| Musculoskeletal AEs | Number of reported AEs (active arm/placebo arm) | -/- | 1/0 | -/- | 3/5 | -/- | 3/4 | -/- | 5/5 | 4/4 | -/- | 0/1 | 1/0 | -/- | 5/5 | -/- | 3/5 |
| Odd ratio | - | 3.000 | - | 0.625 | - | 0.738 | - | 0.761 | 0.683 | - | 0.321 | 3.000 | - | 0.761 | - | 0.625 | |
| 95% CI (lower limit; upper limit) | - | 0.118; 76.161 |
- | 0.147; 2.661 |
- | 0.146; 3.723 |
- | 0.220; 2.635 |
0.156; 2.997 |
- | 0.013; 8.241 |
0.118; 76.161 |
- | 0.220; 2.635 |
- | 0.147; 2.661 | |
| Z-value | - | 0.666 | - | −0.636 | - | −0.368 | - | −0.431 | −0.505 | - | −0.686 | 0.666 | - | −0.431 | - | −0.636 | |
| I2 (%) | - | 0 | - | 0 | - | 0 | - | 0 | 0 | - | 0 | 0 | - | 0 | - | 0 | |
| P-value | - | 0.506 | - | 0.525 | - | 0.713 | - | 0.666 | 0.614 | - | 0.493 | 0.506 | - | 0.666 | - | 0.525 | |
| Skin AEs | Number of reported AEs (active arm/placebo arm) | -/- | 21/4 | -/- | 92/94 | 83/90 | 14/6 | -/- | 139/103 | 83/91 | 1/0 | -/- | -/- | -/- | 139/103 | 2/0 | 104/95 |
| Odd ratio | - | 2.821 | - | 0.912 | 0.816 | 2.258 | - | 1.127 | 0.819 | 3.353 | - | - | - | 1.127 | 1.545 | 0.932 | |
| 95% CI (lower limit; upper limit) | - | 0.899; 8.850 | - | 0.635; 1.308 | 0.559; 1.191 | 0.851; 5.992 |
- | 0.815; 1.559 | 0.563; 1.192 | 0.120; 93.835 | - | - | - | 0.815; 1.559 | 0.067; 35.431 | 0.653; 1.331 | |
| Z-value | - | 1.778 | - | −0.502 | −1.052 | 1.636 | - | 0.724 | −1.041 | 0.712 | - | - | - | 0.724 | 0.272 | −0.387 | |
| I2 (%) | - | 0 | - | 29 | 0 | 0 | - | 34 | 0 | 0 | - | - | - | 34 | 0 | 36 | |
| P-value | - | 0.075 | - | 0.616 | 0.293 | 0.102 | - | 0.469 | 0.298 | 0.477 | - | - | - | 0.469 | 0.785 | 0.699 | |
| Infections | Number of reported AEs (active arm/placebo arm) | -/- | 3/0 | -/- | 1/3 | -/- | 1/3 | -/- | 5/3 | 1/3 | -/- | -/- | -/- | -/- | 5/3 | -/- | 4/3 |
| Odd ratio | - | 3.316 | - | 0.310 | - | 0.310 | - | 0.926 | 0.310 | - | - | - | - | 0.926 | - | 0.780 | |
| 95% CI (lower limit; upper limit) | - | 0.167; 65.718 | - | 0.028; 3.364 | - | 0.028; 3.364 | - | 0.184; 4.647 | 0.028; 3.364 | - | - | - | - | 0.184; 4.647 | - | 0.121; 5.028 | |
| Z-value | - | 0.787 | - | −0.963 | - | −0.963 | - | −0.094 | −0.963 | - | - | - | - | −0.094 | - | −0.262 | |
| I2 (%) | - | 0 | - | 0 | - | 0 | - | 0 | 0 | - | - | - | - | 0 | - | 32 | |
| P-value | - | 0.432 | - | 0.335 | - | 0.335 | - | 0.925 | 0.335 | - | - | - | - | 0.925 | - | 0.793 | |
| CV system AEs | Number of reported AEs (active arm/placebo arm) | -/- | 0/1 | 0/2 | 71/53 | 71/54 | 1/3 | -/- | 73/60 | 71/54 | -/- | -/- | 0/1 | -/- | 73/60 | -/- | 71/57 |
| Odd ratio | - | 0.149 | 0.191 | 1.441 | 1.409 | 0.450 | - | 1.247 | 1.409 | - | - | 0.333 | - | 1.247 | - | 1.313 | |
| 95% CI (lower limit; upper limit) | - | 0.006; 3.733 | 0.009; 4.214 | 0.950; 2.186 | 0.932; 2.130 | 0.056; 3.608 | - | 0.838; 1.854 | 0.932; 2.130 | - | - | 0.012; 9.068 | - | 0.838; 1.854 | - | 0.875; 1.972 | |
| Z-value | - | −1.159 | −1.049 | 1.720 | 1.625 | −0.752 | - | 1.089 | 1.625 | - | - | −0.652 | - | 1.089 | - | 1.314 | |
| I2 (%) | - | 0 | 0 | 0 | 0 | 0 | - | 16 | 0 | - | - | 0 | - | 16 | - | 27 | |
| P-value | - | 0.247 | 0.294 | 0.085 | 0.104 | 0.452 | - | 0.276 | 0.104 | - | - | 0.515 | - | 0.276 | - | 0.189 | |
| Hospitalisation | Number of reported AEs (active arm/placebo arm) | -/- | 4/0 | -/- | 2/0 | -/- | 2/0 | -/- | 4/0 | -/- | -/- | -/- | 2/0 | -/- | 4/0 | 2/0 | 2/0 |
| Odd ratio | - | 5.657 | - | 5.145 | - | 5.145 | - | 5.657 | - | - | - | 5.145 | - | 5.657 | 6.224 | 5.145 | |
| 95% CI (lower limit; upper limit) | - | 0.642; 49.849 | - | 0.238; 111.087 | - | 0.238; 111.087 | - | 0.642; 49.849 | - | - | - | 0.238; 111.087 | - | 0.642; 49.849 | 0.285; 135.784 | 0.238; 111.087 | |
| Z-value | - | 1.561 | - | 1.045 | - | 1.045 | - | 1.561 | - | - | - | 1.045 | - | 1.561 | 1.163 | 1.045 | |
| I2 (%) | - | 0 | - | 0 | - | 0 | - | 0 | - | - | - | 0 | - | 0 | 0 | 0 | |
| P-value | - | 0.119 | - | 0.296 | - | 0.296 | - | 0.119 | - | - | - | 0.296 | - | 0.119 | 0.245 | 0.296 | |
| Death | Number of reported AEs (active arm/placebo arm) | -/- | 0/2 | 4/5 | -/- | -/- | 1/2 | -/- | 6/12 | 1/3 | -/- | -/- | -/- | -/- | 6/12 | 0/2 | 6/9 |
| Odd ratio | - | 0.215 | 0.777 | - | - | 0.529 | - | 0.558 | 0.468 | - | - | - | - | 0.558 | 0.215 | 0.657 | |
| 95% CI (lower limit; upper limit) | - | 0.010; 4.690 | 0.192; 3.142 | - | - | 0.046; 6.109 | - | 0.210; 1.483 | 0.066; 3.300 | - | - | - | - | 0.210; 1.483 | 0.010; 4.690 | 0.222; 1.947 | |
| Z-value | - | −0.977 | −0.354 | - | - | −0.510 | - | −1.169 | −0.762 | - | - | - | - | −1.169 | −0.977 | −0.758 | |
| I2 (%) | - | 0 | 0 | - | - | 0 | - | 0 | 0 | - | - | - | - | 0 | 0 | 0 | |
| P-value | - | 0.328 | 0.724 | - | - | 0.610 | - | 0.242 | 0.446 | - | - | - | - | 0.242 | 0.328 | 0.448 | |
AEs = Adverse events; CI = Confidence Intervals.
4. Discussion
In the last years, the number of individuals assuming dietary supplements has been steadily increased worldwide [90,91]. Reasons for dietary supplements’ use widely varies across the countries: in Europe, it is just limited to general health and well-being, while other countries permit use for medicinal purposes [92].
Considering that dietary supplement production and marketing are usually not strictly subjected to rigid rules as drugs are, there is a need for more data in order to confirm their safe use in the general population and frail subjects.
Pooling data from 71 randomized placebo-controlled clinical studies, this meta-analysis suggests that antioxidant supplementation with ALA was not associated with an increased risk of any treatment-emergent AE. Of note, statistical significance was not even achieved in subsets of studies categorized according to smoking habit, CV disease, presence of diabetes, pregnancy status, neurological disorders, rheumatic affections, renal impairment, and status of children/adolescent.
From a certain point of view, the current analysis strengthens findings from a large observational study considering outcomes data of 610 expectant mothers and their newborns that concluded ALA supplementation is safe in pregnancy even when administered at high doses [93].
These findings are particularly important because they encourage ALA use in a number of conditions in which ALA is actually proven to be effective. As a matter of fact, even though ALA supplementation has already been demonstrated to influence a broad spectrum of metabolic pathways including inflammation and glucose homeostasis [94,95,96], to the best of our knowledge this is the first time that ALA safety profile has been comprehensively evaluated through a pooled analysis of randomized placebo-controlled clinical studies.
Once ALA safety has been established, clinical factors for predicting treatment response should be an objective for future investigations, in order to identify the patient group that might benefit from ALA supplementation the most.
In the past, several meta-analyses showed that ALA supplementation significantly improves both positive neuropathic symptoms and neuropathic deficits to a clinically meaningful degree in diabetic patients with symptomatic polyneuropathy [97,98,99]. Furthermore, ALA was shown to promote weight loss in adults and obese children and adolescents [100,101].
Despite its strengths, this systematic review and meta-analysis has some limitations that mostly inherits from the included clinical studies. First, the effect size on the risk of hypoglycaemic episodes may be affected by variations in the underlying hypoglycaemic therapy in clinical trials enrolling diabetic patients. In fact, the well-recognized euglycaemic effect of ALA may require the adjustment of antidiabetic agents and insulin doses in patients taking antidiabetic drugs [101]. Second, gastrointestinal and CV system AEs included several nosological entities, justifying the probable presence of publication biases for the analysis. However, this limitation is strongly conditioned by the way the AEs were reported in the individual clinical trials. Indeed, most of the studies included in the meta-analysis report the cumulative incidence of gastrointestinal and CV system AEs, without regard to specific type of AEs. Third, AEs were difficult to identify when they were represented by exacerbations of the underlying disease for which ALA was tested (e.g., leg cramps in patients with peripheral polyneuropathy). Moreover, clinical trials testing different ALA regimens often reported the cumulative number of AEs for the supplementation versus placebo. As a result, a sub-analysis by ALA daily dose was not provided. Furthermore, different ALA formulations were tested across the included clinical studies. Despite this, heterogeneity was low for all assessed outcomes, proving that the results were reliable for the whole population and the considered sub-groups [102]. Finally, as per other dietary supplements, a relatively large number of studies have been carried out with open design and/or without a control group, so that they could not be included in a well-carried out meta-analysis.
Future research is needed to understand if sporadic adverse events associated with ALA use are related to the production quality of the used supplements, to other components of mixed supplements and/or to concomitant treatments or diseases, while long-term safety has been already assessed in the NATHAN (Neurological Assessment of Thioctic Acid in Diabetic Neuropathy) 1 trial [84].
5. Conclusions
Pooling data from the available randomized placebo-controlled clinical studies, the current meta-analysis provides data in support of the safety of the use of ALA to improve health outcomes in overall healthy individuals and in patients affected by other diseases.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/9/10/1011/s1, Figure S1: Plots showing leave-one-out sensitivity analysis for the risk of gastrointestinal AEs following ALA supplementation versus placebo, Figure S2: Funnel plot detailing publication bias for the risk of gastrointestinal AEs following ALA supplementation versus placebo, Figure S3: Plot showing leave-one-out sensitivity analysis for the risk of neurological AEs following ALA supplementation versus placebo, Figure S4: Funnel plot detailing publication bias for the risk of neurological AEs following ALA supplementation versus placebo, Figure S5: Plot showing leave-one-out sensitivity analysis for the risk of psychiatric disorders following ALA supplementation versus placebo, Figure S6: Plot showing leave-one-out sensitivity analysis for the risk of musculoskeletal AEs following ALA supplementation versus placebo, Figure S7: Funnel plot detailing publication bias for the risk of musculoskeletal AEs following ALA supplementation versus placebo, Figure S8: Plot showing leave-one-out sensitivity analysis for the risk of skin AEs following ALA supplementation versus placebo, Figure S9: Funnel plot detailing publication bias for the risk of skin AEs following ALA supplementation versus placebo, Figure S10: Plot showing leave-one-out sensitivity analysis for the risk of infections following ALA supplementation versus placebo, Figure S11: Funnel plot detailing publication bias for the risk of infections following ALA supplementation versus placebo, Figure S12: Plot showing leave-one-out sensitivity analysis for the risk of CV system AEs following ALA supplementation versus placebo, Figure S13: Funnel plot detailing publication bias for the risk of CV system AEs following ALA supplementation versus placebo, Figure S14: Plot showing leave-one-out sensitivity analysis for the risk of hospitalisation following ALA supplementation versus placebo, Figure S15: Plot showing leave-one-out sensitivity analysis for the risk of death following ALA supplementation versus placebo, Figure S16: Funnel plot detailing publication bias for the risk of death following ALA supplementation versus placebo, File A: PRISMA Checklist, File B: Studies excluded from the systematic review after assessment.
Author Contributions
Conceptualization, F.F. and A.F.G.C.; methodology, F.F. and A.F.G.C.; software, F.F.; validation, F.F., M.R. and A.F.G.C.; formal analysis, F.F.; investigation, F.F., M.R., C.K. (Christoffer Krogager), C.K. (Cormac Kennedy), C.M.G.G., T.K., E.L., A.V., P.P.-M., E.F.E.W., A.Š., M.V. and A.F.G.C.; resources, F.F. and A.F.G.C.; data curation, F.F. and A.F.G.C.; writing—original draft preparation, F.F., M.R. and A.F.G.C.; writing—review and editing, C.K. (Christoffer Krogager), C.K. (Cormac Kennedy), C.M.G.G., T.K., E.L., A.V., P.P.-M., E.F.E.W., A.Š. and M.V.; visualization, F.F. and A.F.G.C.; supervision, A.F.G.C.; project administration, F.F. and A.F.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
MR is currently Chief Medical and Scientific Advisor, Novo Nordisk South East Europe, Middle East and Africa (SEEMEA). The other authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tibullo D., Li Volti G., Giallongo C., Grasso S., Tomassoni D., Anfuso C.D., Lupo G., Amenta F., Avola R., Bramanti V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017;66:947–959. doi: 10.1007/s00011-017-1079-6. [DOI] [PubMed] [Google Scholar]
- 2.Biewenga G.P., Haenen G.R., Bast A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997;29:315–331. doi: 10.1016/S0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 3.Pershadsingh H.A. Alpha-lipoic acid: Physiologic mechanisms and indications for the treatment of metabolic syndrome. Expert Opin. Investig. Drugs. 2007;16:291–302. doi: 10.1517/13543784.16.3.291. [DOI] [PubMed] [Google Scholar]
- 4.Rahmanabadi A., Mahboob S., Amirkhizi F., Hosseinpour-Arjmand S., Ebrahimi-Mameghani M. Oral α-lipoic acid supplementation in patients with non-alcoholic fatty liver disease: Effects on adipokines and liver histology features. Food Funct. 2019;10:4941–4952. doi: 10.1039/C9FO00449A. [DOI] [PubMed] [Google Scholar]
- 5.Hosseinpour-Arjmand S., Amirkhizi F., Ebrahimi-Mameghani M. The effect of alpha-lipoic acid on inflammatory markers and body composition in obese patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J. Clin. Pharm. Ther. 2019;44:258–267. doi: 10.1111/jcpt.12784. [DOI] [PubMed] [Google Scholar]
- 6.Yukina M., Nuralieva N., Solovyev M., Troshina E., Vasilyev E. Insulin autoimmune syndrome. Endocrinol. Diabetes Metab. Case Rep. 2020;2020:19–0159. doi: 10.1530/EDM-19-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izzo V., Greco C., Corradini D., Infante M., Staltari M.T., Romano M., Bellia A., Lauro D., Uccioli L. Insulin autoimmune syndrome in an Argentine woman taking α-lipoic acid: A case report and review of the literature. SAGE Open Med. Case Rep. 2018;6:2050313X18819601. doi: 10.1177/2050313X18819601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bresciani E., Bussi A., Bazzigaluppi E., Balestrieri G. Insulin autoimmune syndrome induced by α-lipoic acid in a Caucasian woman: Case report. Diabetes Care. 2011;34:e146. doi: 10.2337/dc11-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatti M., Ippoliti I., Poluzzi E., Antonazzo I.C., Moro P.A., Moretti U., Menniti-Ippolito F., Mazzanti G., De Ponti F., Raschi E. Assessment of adverse reactions to α-lipoic acid containing dietary supplements through spontaneous reporting systems. Clin. Nutr. 2020 doi: 10.1016/j.clnu.2020.07.028. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J., Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley and Sons Ltd; Chichester, UK: 2010. Version 5.0. 2. 2009. [Google Scholar]
- 12.Fogacci F., Ferri N., Toth P.P., Ruscica M., Corsini A., Cicero A.F.G. Efficacy and Safety of Mipomersen: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Drugs. 2019;79:751–766. doi: 10.1007/s40265-019-01114-z. [DOI] [PubMed] [Google Scholar]
- 13.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., Schünemann H.J., GRADE Working Group GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borenstein M., Hedges L., Higgins J., Rothstein H. Comprehensive Meta-Analysis Version 3. Volume 104 Biostat; Englewood, NJ, USA: 2005. [Google Scholar]
- 15.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; London, UK: 2019. [(accessed on 15 September 2020)]. Version 6.0 (updated July 2019) Available online: www.training.cochrane.org/handbook. [Google Scholar]
- 16.Melsen W.G., Bootsma M.C., Rovers M.M., Bonten M.J. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 2004;20:123–129. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- 17.Haenszel W., Hon N.B. Statistical approaches to the study of cancer with particular reference to case registers. J. Chronic. Dis. 1956;4:589–599. doi: 10.1016/0021-9681(56)90049-2. [DOI] [PubMed] [Google Scholar]
- 18.Fogacci S., Fogacci F., Banach M., Michos E.D., Hernandez A.V., Lip G.Y.H., Blaha M.J., Toth P.P., Borghi C., Cicero A.F.G. Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2020;39:1742–1752. doi: 10.1016/j.clnu.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Fogacci F., Banach M., Mikhailidis D.P., Bruckert E., Toth P.P., Watts G.F., Reiner Ž., Mancini J., Rizzo M., Mitchenko O., et al. Safety of red yeast rice supplementation: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019;143:1–16. doi: 10.1016/j.phrs.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadi A., Mazooji N., Roozbeh J., Mazloom Z., Hasanzade J. Effect of alpha-lipoic acid and vitamin E supplementation on oxidative stress, inflammation, and malnutrition in hemodialysis patients. Iran J. Kidney Dis. 2013;7:461–467. [PubMed] [Google Scholar]
- 21.Ansar H., Mazloom Z., Kazemi F., Hejazi N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med. J. 2011;32:584–588. [PubMed] [Google Scholar]
- 22.Aslfalah H., Jamilian M., Rafiei F., Khosrowbeygi A. Reduction in maternal serum values of glucose and gamma-glutamyltransferase after supplementation with alpha-lipoic acid in women with gestational diabetes mellitus. J. Obstet. Gynaecol. Res. 2019;45:313–317. doi: 10.1111/jog.13842. [DOI] [PubMed] [Google Scholar]
- 23.Aslfalah H., Jamilian M., Khosrowbeygi A. Elevation of the adiponectin/leptin ratio in women with gestational diabetes mellitus after supplementation with alpha-lipoic acid. Gynecol. Endocrinol. 2019;35:271–275. doi: 10.1080/09513590.2018.1519795. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner S., Mensink R.P., Haenen G.R., Bast A., Binder C.J., Bekers O., Husche C., Lütjohann D., Plat J. The effects of vitamin E or lipoic acid supplementation on oxyphytosterols in subjects with elevated oxidative stress: A randomized trial. Sci. Rep. 2017;7:15288. doi: 10.1038/s41598-017-15615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baziar N., Nasli-Esfahani E., Djafarian K., Qorbani M., Hedayati M., Mishani M.A., Faghfoori Z., Ahmaripour N., Hosseini S. The Beneficial Effects of Alpha Lipoic Acid Supplementation on Lp-PLA2 Mass and Its Distribution between HDL and apoB-Containing Lipoproteins in Type 2 Diabetic Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Oxid. Med. Cell Longev. 2020;2020:5850865. doi: 10.1155/2020/5850865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bobe G., Michels A.J., Zhang W.J., Purnell J.Q., Woffendin C., Pereira C., Vita J.A., Thomas N.O., Traber M.G., Frei B., et al. A Randomized Controlled Trial of Long-Term (R)-α-Lipoic Acid Supplementation Promotes Weight Loss in Overweight or Obese Adults without Altering Baseline Elevated Plasma Triglyceride Concentrations. J. Nutr. 2020;150:2336–2345. doi: 10.1093/jn/nxaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boriani F., Granchi D., Roatti G., Merlini L., Sabattini T., Baldini N. Alpha-lipoic Acid After Median Nerve Decompression at the Carpal Tunnel: A Randomized Controlled Trial. J. Hand Surg. Am. 2017;42:236–242. doi: 10.1016/j.jhsa.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Carbone M., Pentenero M., Carrozzo M., Ippolito A., Gandolfo S. Lack of efficacy of alpha-lipoic acid in burning mouth syndrome: A double-blind, randomized, placebo-controlled study. Eur. J. Pain. 2009;13:492–496. doi: 10.1016/j.ejpain.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Cavalcanti D.R., da Silveira F.R. Alpha lipoic acid in burning mouth syndrome—A randomized double-blind placebo-controlled trial. J. Oral Pathol. Med. 2009;38:254–261. doi: 10.1111/j.1600-0714.2008.00735.x. [DOI] [PubMed] [Google Scholar]
- 30.Durastanti V., Tinelli E., Di Rezze S., Berardelli A., Millefiorini E. Alpha lipoic acid as add-on therapy to subcutaneous interferon β-1a for relapsing-remitting multiple sclerosis: A pilot study. IJABPT. 2016;7:336–341. [Google Scholar]
- 31.El Amrousy D., El-Afify D. Effects of alpha lipoic acid as a supplement in obese children and adolescents. Cytokine. 2020;130:155084. doi: 10.1016/j.cyto.2020.155084. [DOI] [PubMed] [Google Scholar]
- 32.Falardeau J., Fryman A., Wanchu R., Marracci G.H., Mass M., Wooliscroft L., Bourdette D.N., Murchison C.F., Hills W.L., Yadav V. Oral lipoic acid as a treatment for acute optic neuritis: A blinded, placebo controlled randomized trial. Mult. Scler. J. Exp. Transl. Clin. 2019;5:2055217319850193. doi: 10.1177/2055217319850193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Femiano F., Scully C. Burning mouth syndrome (BMS): Double blind controlled study of alpha-lipoic acid (thioctic acid) therapy. J. Oral Pathol. Med. 2002;31:267–269. doi: 10.1034/j.1600-0714.2002.310503.x. [DOI] [PubMed] [Google Scholar]
- 34.Georgakouli K., Fatouros I.G., Fragkos A., Tzatzakis T., Deli C.K., Papanikolaou K., Koutedakis Y., Jamurtas A.Z. Exercise and Redox Status Responses Following Alpha-Lipoic Acid Supplementation in G6PD Deficient Individuals. Antioxidants. 2018;7:162. doi: 10.3390/antiox7110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gianturco V., Bellomo A., D’Ottavio E., Formosa V., Iori A., Mancinella M., Troisi G., Marigliano V. Impact of therapy with alpha-lipoic acid (ALA) on the oxidative stress in the controlled NIDDM: A possible preventive way against the organ dysfunction? Arch. Gerontol. Geriatr. 2009;49:129–133. doi: 10.1016/j.archger.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 36.Gilron I., Robb S., Tu D., Holden R., Towheed T., Ziegler D., Wang L., Milev R., Gray C. Double-blind, randomized, placebo-controlled crossover trial of alpha-lipoic acid for the treatment of fibromyalgia pain: The IMPALA trial. Pain. 2020 doi: 10.1097/j.pain.0000000000002028. [DOI] [PubMed] [Google Scholar]
- 37.Gosselin L.E., Chrapowitzky L., Rideout T.C. Metabolic effects of α-lipoic acid supplementation in pre-diabetics: A randomized, placebo-controlled pilot study. Food Funct. 2019;10:5732–5738. doi: 10.1039/C9FO00390H. [DOI] [PubMed] [Google Scholar]
- 38.Guo Y., Jones D., Palmer J.L., Forman A., Dakhil S.R., Velasco M.R., Weiss M., Gilman P., Mills G.M., Noga S.J., et al. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: A randomized, double-blind, placebo-controlled trial. Support. Care Cancer. 2014;22:1223–1231. doi: 10.1007/s00520-013-2075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haghighian H.K., Haidari F., Mohammadi-Asl J., Dadfar M. Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil. Steril. 2015;104:318–324. doi: 10.1016/j.fertnstert.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Hejazi N., Mazloom Z., Zand F., Rezaianzadeh A., Nikandish R. The Beneficial Effects of α-Lipoic Acid in Critically Ill Patients: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Asian J. Anesthesiol. 2018;56:45–55. doi: 10.6859/aja.201806_56(2).0002. [DOI] [PubMed] [Google Scholar]
- 41.Huang E.A., Gitelman S.E. The effect of oral alpha-lipoic acid on oxidative stress in adolescents with type 1 diabetes mellitus. Pediatr. Diabetes. 2008;9:69–73. doi: 10.1111/j.1399-5448.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 42.Huerta A.E., Prieto-Hontoria P.L., Sáinz N., Martínez J.A., Moreno-Aliaga M.J. Supplementation with α-Lipoic Acid Alone or in Combination with Eicosapentaenoic Acid Modulates the Inflammatory Status of Healthy Overweight or Obese Women Consuming an Energy-Restricted Diet. J. Nutr. 2016;146 doi: 10.3945/jn.115.224105. [DOI] [PubMed] [Google Scholar]
- 43.Huerta A.E., Navas-Carretero S., Prieto-Hontoria P.L., Martínez J.A., Moreno-Aliaga M.J. Effects of α-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity. 2015;23:313–321. doi: 10.1002/oby.20966. [DOI] [PubMed] [Google Scholar]
- 44.Jacob S., Ruus P., Hermann R., Tritschler H.J., Maerker E., Renn W., Augustin H.J., Dietze G.J., Rett K. Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: A placebo-controlled pilot trial. Free Radic. Biol. Med. 1999;27:309–314. doi: 10.1016/S0891-5849(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 45.Jamshidi K., Abdollahzad H., Nachvak M., Rezaei M., Golpayegani M.R., Sharifi Zahabi E. Effects of Alpha-Lipoic Acid Supplementation on Cardiovascular Disease Risk Factors in β-Thalassemia Major Patients: A Clinical Trial Crossover Study. J. Blood Med. 2020;11:131–139. doi: 10.2147/JBM.S252105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jariwalla R.J., Lalezari J., Cenko D., Mansour S.E., Kumar A., Gangapurkar B., Nakamura D. Restoration of blood total glutathione status and lymphocyte function following alpha-lipoic acid supplementation in patients with HIV infection. J. Altern. Complement Med. 2008;14:139–146. doi: 10.1089/acm.2006.6397. [DOI] [PubMed] [Google Scholar]
- 47.Khabbazi T., Mahdavi R., Safa J., Pour-Abdollahi P. Effects of alpha-lipoic acid supplementation on inflammation, oxidative stress, and serum lipid profile levels in patients with end-stage renal disease on hemodialysis. J. Ren. Nutr. 2012;22:244–250. doi: 10.1053/j.jrn.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Khalili M., Soltani M., Moghadam S.A., Dehghan P., Azimi A., Abbaszadeh O. Effect of alpha-lipoic acid on asymmetric dimethylarginine and disability in multiple sclerosis patients: A randomized clinical trial. Electron. Physician. 2017;9:4899–4905. doi: 10.19082/4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalili M., Azimi A., Izadi V., Eghtesadi S., Mirshafiey A., Sahraian M.A., Motevalian A., Norouzi A., Sanoobar M., Eskandari G., et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: A double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21:291–296. doi: 10.1159/000356145. [DOI] [PubMed] [Google Scholar]
- 50.Kim B.J., Hunter A., Brucker A.J., Hahn P., Gehrs K., Patel A., Edwards A.O., Li Y., Khurana R.N., Nissim I., et al. Orally Administered Alpha Lipoic Acid as a Treatment for Geographic Atrophy: A Randomized Clinical Trial. Ophthalmol. Retin. 2020;4:889–898. doi: 10.1016/j.oret.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim N.W., Song Y.M., Kim E., Cho H.S., Cheon K.A., Kim S.J., Park J.Y. Adjunctive α-lipoic acid reduces weight gain compared with placebo at 12 weeks in schizophrenic patients treated with atypical antipsychotics: A double-blind randomized placebo-controlled study. Int. Clin. Psychopharmacol. 2016;31:265–274. doi: 10.1097/YIC.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 52.Koh E.H., Lee W.J., Lee S.A., Kim E.H., Cho E.H., Jeong E., Kim D.W., Kim M.S., Park J.Y., Park K.G., et al. Effects of alpha-lipoic Acid on body weight in obese subjects. Am. J. Med. 2011;124:85.e1–85.e8. doi: 10.1016/j.amjmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Lampitella A., Rossi E., Carrino F., Griffo P., Carrino R. Effetto dell’acido lipoico e della vitamina E sulla polineuropatia diabetica: Analisi statistica. Prog. Nutr. 2005;7:116–134. (In Italian) [Google Scholar]
- 54.Lee S.J., Jeong S.J., Lee Y.C., Lee Y.H., Lee J.E., Kim C.H., Min K.W., Cha B.Y. Effects of High-Dose α-Lipoic Acid on Heart Rate Variability of Type 2 Diabetes Mellitus Patients with Cardiac Autonomic Neuropathy in Korea. Diabetes Metab. J. 2017;41:275–283. doi: 10.4093/dmj.2017.41.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loy B.D., Fling B.W., Horak F.B., Bourdette D.N., Spain R.I. Effects of lipoic acid on walking performance, gait, and balance in secondary progressive multiple sclerosis. Complement Ther. Med. 2018;41:169–174. doi: 10.1016/j.ctim.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.López-D’alessandro E., Escovich L. Combination of alpha lipoic acid and gabapentin, its efficacy in the treatment of Burning Mouth Syndrome: A randomized, double-blind, placebo controlled trial. Med. Oral Patol. Oral Cir. Bucal. 2011;16:e635–e640. doi: 10.4317/medoral.16942. [DOI] [PubMed] [Google Scholar]
- 57.López-Jornet P., Camacho-Alonso F., Leon-Espinosa S. Efficacy of alpha lipoic acid in burning mouth syndrome: A randomized, placebo-treatment study. J. Oral Rehabil. 2009;36:52–57. doi: 10.1111/j.1365-2842.2008.01914.x. [DOI] [PubMed] [Google Scholar]
- 58.Magis D., Ambrosini A., Sándor P., Jacquy J., Laloux P., Schoenen J. A randomized double-blind placebo-controlled trial of thioctic acid in migraine prophylaxis. Headache. 2007;47:52–57. doi: 10.1111/j.1526-4610.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- 59.Manning P.J., Sutherland W.H., Williams S.M., Walker R.J., Berry E.A., De Jong S.A., Ryalls A.R. The effect of lipoic acid and vitamin E therapies in individuals with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2013;23:543–549. doi: 10.1016/j.numecd.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Marfella R., Barbieri M., Sardu C., Rizzo M.R., Siniscalchi M., Paolisso P., Ambrosino M., Fava I., Materazzi C., Cinquegrana G., et al. Effects of α-lipoic acid therapy on sympathetic heart innervation in patients with previous experience of transient takotsubo cardiomyopathy. J. Cardiol. 2016;67:153–161. doi: 10.1016/j.jjcc.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 61.Marshall A.W., Graul R.S., Morgan M.Y., Sherlock S. Treatment of alcohol-related liver disease with thioctic acid: A six month randomised double-blind trial. Gut. 1982;23:1088–1093. doi: 10.1136/gut.23.12.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martins V.D., Manfredini V., Peralba M.C., Benfato M.S. Alpha-lipoic acid modifies oxidative stress parameters in sickle cell trait subjects and sickle cell patients. Clin. Nutr. 2009;28:192–197. doi: 10.1016/j.clnu.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Mendes P.R., Félix Ddos S., Silva P.C., Pereira G.H., Simões M.O. Effect of alpha lipoic acid on the blood cell count and iron kinetics in hypertensive patients. Nutr. Hosp. 2014;31:883–889. doi: 10.3305/nh.2015.31.2.7403. [DOI] [PubMed] [Google Scholar]
- 64.Mendoza-Núñez V.M., García-Martínez B.I., Rosado-Pérez J., Santiago-Osorio E., Pedraza-Chaverri J., Hernández-Abad V.J. The Effect of 600 mg Alpha-lipoic Acid Supplementation on Oxidative Stress, Inflammation, and RAGE in Older Adults with Type 2 Diabetes Mellitus. Oxid. Med. Cell Longev. 2019;2019:3276958. doi: 10.1155/2019/3276958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mirtaheri E., Pourghassem Gargari B., Kolahi S., Asghari-Jafarabadi M., Hajaliloo M. Effect of alpha-lipoic acid supplementation on serum lipid profile in women with rheumatoid arthritis. NFSR. 2014;1:11–18. [Google Scholar]
- 66.Mohammadi V., Khorvash F., Feizi A., Askari G. Does Alpha-lipoic Acid Supplementation Modulate Cardiovascular Risk Factors in Patients with Stroke? A Randomized, Double-blind Clinical Trial. Int. J. Prev. Med. 2018;9:34. doi: 10.4103/ijpvm.IJPVM_32_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohammadi V., Khalili M., Eghtesadi S., Dehghani S., Jazayeri S., Aghababaee S.K., Sabour H., Saberi H., Eghtesadi M., Gohari M.R. The effect of alpha-lipoic acid (ALA) supplementation on cardiovascular risk factors in men with chronic spinal cord injury: A clinical trial. Spinal Cord. 2015;53:621–624. doi: 10.1038/sc.2015.35. [DOI] [PubMed] [Google Scholar]
- 68.Mollo R., Zaccardi F., Scalone G., Scavone G., Rizzo P., Navarese E.P., Manto A., Pitocco D., Lanza G.A., Ghirlanda G., et al. Effect of α-lipoic acid on platelet reactivity in type 1 diabetic patients. Diabetes Care. 2012;35:196–197. doi: 10.2337/dc11-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monroy Guízar E.A., García Benavides L., Ambriz Plascencia A.R., Pascoe González S., Totsuka Sutto S.E., Cardona Muñoz E.G., Méndez-Del Villar M. Effect of Alpha-Lipoic Acid on Clinical and Neurophysiologic Recovery of Carpal Tunnel Syndrome: A Double-Blind, Randomized Clinical Trial. J. Med. Food. 2018;21:521–526. doi: 10.1089/jmf.2017.0056. [DOI] [PubMed] [Google Scholar]
- 70.Palacios-Sánchez B., Moreno-López L.A., Cerero-Lapiedra R., Llamas-Martínez S., Esparza-Gómez G. Alpha lipoic acid efficacy in burning mouth syndrome. A controlled clinical trial. Med. Oral Patol. Oral Cir. Bucal. 2015;20:e435–e440. doi: 10.4317/medoral.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Porasuphatana S., Suddee S., Nartnampong A., Konsil J., Harnwong B., Santaweesuk A. Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alpha-lipoic acid: A randomized double-blinded placebo-controlled study. Asia Pac. J. Clin. Nutr. 2012;21:12–21. [PubMed] [Google Scholar]
- 72.Pourghasem Gargari B., Kolahi S., Dehghan P., Khabbazi A., Mirtaheri E. Effects of alpha-lipoic acid supplementation on clinical status and anthropometric indices in women with rheumatoid arthritis. Curr. Top. Nutraceutical Res. 2015;13:33–40. [Google Scholar]
- 73.Ruhnau K.J., Meissner H.P., Finn J.R., Reljanovic M., Lobisch M., Schütte K., Nehrdich D., Tritschler H.J., Mehnert H., Ziegler D. Effects of 3-week oral treatment with the antioxidant thioctic acid (alpha-lipoic acid) in symptomatic diabetic polyneuropathy. Diabetic Med. 1999;16:1040–1043. doi: 10.1046/j.1464-5491.1999.00190.x. [DOI] [PubMed] [Google Scholar]
- 74.Safa J., Ardalan M.R., Rezazadehsaatlou M., Mesgari M., Mahdavi R., Jadid M.P. Effects of alpha lipoic acid supplementation on serum levels of IL-8 and TNF-α in patient with ESRD undergoing hemodialysis. Int. Urol. Nephrol. 2014;46:1633–1638. doi: 10.1007/s11255-014-0688-z. [DOI] [PubMed] [Google Scholar]
- 75.Sammour H., Elkholy A., Rasheedy R., Fadel E. The effect of alpha lipoic acid on uterine wound healing after primary cesarean section: A triple-blind placebo-controlled parallel-group randomized clinical trial. Arch. Gynecol. Obstet. 2019;299:665–673. doi: 10.1007/s00404-018-5011-2. [DOI] [PubMed] [Google Scholar]
- 76.Sardu C., Santulli G., Santamaria M., Barbieri M., Sacra C., Paolisso P., D’Amico F., Testa N., Caporaso I., Paolisso G., et al. Effects of Alpha Lipoic Acid on Multiple Cytokines and Biomarkers and Recurrence of Atrial Fibrillation Within 1 Year of Catheter Ablation. Am. J. Cardiol. 2017;119:1382–1386. doi: 10.1016/j.amjcard.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scaramuzza A., Giani E., Redaelli F., Ungheri S., Macedoni M., Giudici V., Bosetti A., Ferrari M., Zuccotti G.V. Alpha-Lipoic Acid and Antioxidant Diet Help to Improve Endothelial Dysfunction in Adolescents with Type 1 Diabetes: A Pilot Trial. J. Diabetes Res. 2015;2015:474561. doi: 10.1155/2015/474561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sola S., Mir M.Q., Cheema F.A., Khan-Merchant N., Menon R.G., Parthasarathy S., Khan B.V. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: Results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111:343–348. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- 79.Spain R., Powers K., Murchison C., Heriza E., Winges K., Yadav V., Cameron M., Kim E., Horak F., Simon J., et al. Lipoic acid in secondary progressive MS: A randomized controlled pilot trial. Neurol. Neuroimmunol. Neuroinflamm. 2017;4:e374. doi: 10.1212/NXI.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun Y.D., Dong Y.D., Fan R., Zhai L.L., Bai Y.L., Jia L.H. Effect of (R)-α-lipoic acid supplementation on serum lipids and antioxidative ability in patients with age-related macular degeneration. Ann. Nutr. Metab. 2012;60:293–297. doi: 10.1159/000338444. [DOI] [PubMed] [Google Scholar]
- 81.Tromba L., Perla F.M., Carbotta G., Chiesa C., Pacifico L. Effect of Alpha-Lipoic Acid Supplementation on Endothelial Function and Cardiovascular Risk Factors in Overweight/Obese Youths: A Double-Blind, Placebo-Controlled Randomized Trial. Nutrients. 2019;11:375. doi: 10.3390/nu11020375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Udupa A., Nahar P., Shah S., Kshirsagar M., Ghongane B. A comparative study of effects of omega-3 Fatty acids, alpha lipoic Acid and vitamin e in type 2 diabetes mellitus. Ann. Med. Health Sci. Res. 2013;3:442–446. doi: 10.4103/2141-9248.117954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vincent H.K., Bourguignon C.M., Vincent K.R., Taylor A.G. Effects of alpha-lipoic acid supplementation in peripheral arterial disease: A pilot study. J. Altern. Complement Med. 2007;13:577–584. doi: 10.1089/acm.2007.6177. [DOI] [PubMed] [Google Scholar]
- 84.Yadav V., Marracci G., Lovera J., Woodward W., Bogardus K., Marquardt W., Shinto L., Morris C., Bourdette D. Lipoic acid in multiple sclerosis: A pilot study. Mult. Scler. 2005;11:159–165. doi: 10.1191/1352458505ms1143oa. [DOI] [PubMed] [Google Scholar]
- 85.Yan W., Li N., Hu X., Huang Y., Zhang W., Wang Q., Wang F., Wang C., Zhai X., Xu R., et al. Effect of oral ALA supplementation on oxidative stress and insulin sensitivity among overweight/obese adults: A double-blinded, randomized, controlled, cross-over intervention trial. Int. J. Cardiol. 2013;167:602–603. doi: 10.1016/j.ijcard.2012.09.232. [DOI] [PubMed] [Google Scholar]
- 86.Zembron-Lacny A., Gajewski M., Naczk M., Dziewiecka H., Siatkowski I. Physical activity and alpha-lipoic acid modulate inflammatory response through changes in thiol redox status. J. Physiol. Biochem. 2013;69:397–404. doi: 10.1007/s13105-012-0221-8. [DOI] [PubMed] [Google Scholar]
- 87.Zembron-Lacny A., Slowinska-Lisowska M., Szygula Z., Witkowski K., Szyszka K. The comparison of antioxidant and hematological properties of N-acetylcysteine and alpha-lipoic acid in physically active males. Physiol. Res. 2009;58:855–861. doi: 10.33549/physiolres.931590. [DOI] [PubMed] [Google Scholar]
- 88.Ziegler D., Low P.A., Litchy W.J., Boulton A.J., Vinik A.I., Freeman R., Samigullin R., Tritschler H., Munzel U., Maus J., et al. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: The NATHAN 1 trial. Diabetes Care. 2011;34:2054–2060. doi: 10.2337/dc11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ziegler D., Ametov A., Barinov A., Dyck P.J., Gurieva I., Low P.A., Munzel U., Yakhno N., Raz I., Novosadova M., et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: The SYDNEY 2 trial. Diabetes Care. 2006;29:2365–2370. doi: 10.2337/dc06-1216. [DOI] [PubMed] [Google Scholar]
- 90.Mahady G.B. Global harmonization of herbal health claims. J. Nutr. 2001;131:1120S–1123S. doi: 10.1093/jn/131.3.1120S. [DOI] [PubMed] [Google Scholar]
- 91.Thakkar S., Anklam E., Xu A., Ulberth F., Li J., Li B., Hugas M., Sarma N., Crerar S., Swift S., et al. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul. Toxicol. Pharmacol. 2020;114:104647. doi: 10.1016/j.yrtph.2020.104647. [DOI] [PubMed] [Google Scholar]
- 92.Han T., Bai J., Liu W., Hu Y. A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur. J. Endocrinol. 2012;167:465–471. doi: 10.1530/EJE-12-0555. [DOI] [PubMed] [Google Scholar]
- 93.Parente E., Colannino G., Picconi O., Monastra G. Safety of oral alpha-lipoic acid treatment in pregnant women: A retrospective observational study. Eur. Rev. Med. Pharmacol. Sci. 2017;21:4219–4227. [PubMed] [Google Scholar]
- 94.Rahimlou M., Asadi M., Banaei Jahromi N., Mansoori A. Alpha-lipoic acid (ALA) supplementation effect on glycemic and inflammatory biomarkers: A Systematic Review and meta- analysis. Clin. Nutr. ESPEN. 2019;32:16–28. doi: 10.1016/j.clnesp.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 95.Tabrizi R., Borhani-Haghighi A., Mirhosseini N., Lankarani K.B., Naghibzadeh-Tahami A., Akbari M., Heydari S.T., Sangari M., Kolahdooz F., Raygan F., et al. The effects of alpha-lipoic acid supplementation on fasting glucose and lipid profiles among patients with stroke: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2019;18:585–595. doi: 10.1007/s40200-019-00423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akbari M., Ostadmohammadi V., Lankarani K.B., Tabrizi R., Kolahdooz F., Khatibi S.R., Asemi Z. The effects of alpha-lipoic acid supplementation on glucose control and lipid profiles among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Metabolism. 2018;87:56–69. doi: 10.1016/j.metabol.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Eisenberg D.M., Davis R.B., Ettner S.L., Appel S., Wilkey S., Van Rompay M., Kessler R.C. Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 98.Mijnhout G.S., Kollen B.J., Alkhalaf A., Kleefstra N., Bilo H.J. Alpha lipoic Acid for symptomatic peripheral neuropathy in patients with diabetes: A meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2012;2012:456279. doi: 10.1155/2012/456279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ziegler D., Nowak H., Kempler P., Vargha P., Low P.A. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: A meta-analysis. Diabet Med. 2004;21:114–121. doi: 10.1111/j.1464-5491.2004.01109.x. [DOI] [PubMed] [Google Scholar]
- 100.Vajdi M., Abbasalizad Farhangi M. Alpha-lipoic acid supplementation significantly reduces the risk of obesity in an updated systematic review and dose response meta-analysis of randomised placebo-controlled clinical trials. Int. J. Clin. Pract. 2020;74:e13493. doi: 10.1111/ijcp.13493. [DOI] [PubMed] [Google Scholar]
- 101.Ebada M.A., Fayed N., Fayed L., Alkanj S., Abdelkarim A., Farwati H., Hanafy A., Negida A., Ebada M., Noser Y. Efficacy of Alpha-lipoic Acid in The Management of Diabetes Mellitus: A Systematic Review and Meta-analysis. Iran J. Pharm. Res. 2019;18:2144–2156. doi: 10.22037/ijpr.2019.1100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sandercock P. The authors say: ‘The data are not so robust because of heterogeneity’—So, how should I deal with this systematic review? Meta-analysis and the clinician. Cerebrovasc. Dis. 2011;31:615–620. doi: 10.1159/000326068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.