Abstract
We compared a mixed exercise program (i.e., balance exercise plus resistance exercise) with resistance exercise in a single-blind, randomized controlled trial in a post-acute care unit. In total, 60 sarcopenic patients were randomly assigned to an intervention group (12-week mixed exercise) and a control group (12-week resistance exercise). The primary outcomes were the change of the Barthel Index and the number of fallers. The intervention group showed a mean increase of 9.5 points on the Barthel Index (95% confidence interval (CI) 3.9–15.1), while the control group showed a mean increase of 6.3 points (95% CI 2.3–10.4). The mixed exercise program provided a significant benefit over resistance exercise (adjusted mean difference of the change of Barthel Index: 6.8 points; 95% CI 1.4–12.1). The number of fallers was 13.3% and 23.3% in the intervention and control groups, respectively, but the difference was not significant (risk ratio (RR) 0.89, 95% CI 0.69–1.13, p = 0.506). In conclusion, compared with resistance exercise, the mixed exercise program appears to further improve the activities of daily living and physical performance in our study population. Under the monitoring of experienced physiotherapists, both exercise programs are feasible and safe for this population.
Subject terms: Health care, Medical research, Clinical trial design, Clinical trials, Randomized controlled trials
Introduction
Sarcopenia is a disease defined by loss of muscle mass and muscle function1. It has been proven to be associated with numerous adverse health outcomes, such as functional impairment, falls, poor quality of life, and even death1,2. The prevalence of sarcopenia varies significantly in different populations due to the lack of unique diagnostic criteria and different clinical features of study populations3. However, there is a consensus that the prevalence of sarcopenia increases with advancing age1,2,4. Meanwhile, older adults in acute care settings or post-acute care settings are more prone to sarcopenia than their community-dwelling counterparts1,4,5.
At present, the only candidate with robust evidence for treating sarcopenia in older adults is exercise6–8. Unfortunately, the best approach to exercise sarcopenic patients has not yet been established9,10. Resistance exercise is the most commonly suggested exercise regimen for sarcopenia by recent consensuses2,4. However, a recent overview of systematic reviews indicated that the majority of exercise interventions for sarcopenia were mixed exercise programs including aerobic, balance, and resistance exercise (instead of resistance exercise alone)11. Whether mixed exercise programs are better than resistance exercise alone for sarcopenic patients has not been reported.
Additionally, with an aging population has become the most rapidly increasing population segment in many countries7. Old people are generally frailer and sicker than their younger counterparts7; therefore, it is important to research the effectiveness, feasibility, and safety of exercise for older populations. At present, there is limited evidence regarding best-practice exercise for old people in post-acute settings.
Post-acute care refers to medical services that support patients’ recovery from acute illness or management of chronic conditions or functional disabilities. Older patients in post-acute care settings differ considerably from community-dwelling older adults with regard to their physical performances and comorbidities12. A recent systematic review demonstrated that exercise interventions are effective for preventing falls in community-dwelling older adults, but they might not have the same efficacy when transferred to post-acute care settings13. Therefore, the recommendations regarding exercise interventions for sarcopenic older patients in post-acute care settings should not be established only based on studies conducted in community-dwelling older adults. We systematically searched PubMed and Embase databases to identify four studies that addressed exercise programs for sarcopenic patients in post-acute care settings14–17. Of them, three were conducted in nursing homes14–16, while the other was conducted in a subacute care unit17. However, most of these studies had no control group and none was designed as a randomized controlled trial (RCT).
To address this important clinical question, we conducted an RCT to compare the effectiveness and feasibility of a mixed exercise program (i.e., balance exercise plus resistance exercise) with resistance exercise for treating sarcopenia in older patients in a post-acute care unit.
Methods
Study design
We conducted a single-center, two-arm, parallel-group and single-blind RCT according to the CONSORT statement of transparent reporting18 and SPIRIT 201319. We conducted this RCT in the post-acute care unit of the center of gerontology and geriatrics in a tertiary public hospital in Chengdu, China.
The study protocol was registered in ClinicalTrials.gov (ID: NCT04216368, the date of registration: 02/01/2020). The study protocol was approved by our hospital’s Biomedical Ethics Committee. We conducted the study in accordance with the ethical standards set by the 1964 Declaration of Helsinki and its later amendments. All participants (or their legal representatives) signed written informed consent.
Participants and randomization
In this study, we focused on older patients with sarcopenia, as well as stable comorbidities with a level of functional reserve and cognitive capacity high enough to allow them to follow a physiotherapist and perform exercise programs. Therefore, a physiotherapist (R.W.) conducted a screening interview within the first 48 h of admission from acute care units in our hospital to determine eligible patients using the following inclusion criteria: aged 80 years or older with sarcopenia defined by the recommendation from the Asian Working Group for Sarcopenia (AWGS)20; ambulate capabilities (assistance was allowed if necessary); and ability to communicate and collaborate with medical staff. The exclusion criteria were as follows: terminal illness, acute lower respiratory infection, uncontrolled arrhythmias, uncontrolled heart failure, recent myocardial infarction, uncontrolled respiratory failure, acute pulmonary embolism, recent major surgery, recent dialysis, a bone fracture in the past 3 months, or expected length of stay less than 12 weeks.
All the included patients received a baseline assessment, including history of falls in the past year and current nutrition status evaluated using the mini nutritional assessment (MNA)21. The total score of the MNA ranged from 0 (worst) to 30 (best) points. An MNA score of < 17 points indicated malnutrition, while a score between 17 and 23.5 points indicated “malnutrition risk”21. We also estimated the appendicular skeletal muscle mass (ASM) of each patient using a bioimpedance analysis (BIA) device (InBody 230, Biospace Co.Ltd., Korea). The appendicular skeletal muscle mass index (ASMI) was then calculated with the equation: ASMI (kg/m2) = ASM/ height220. In addition, the following data were collected from the local hospital information system: age, sex, education level, body mass index (BMI), and comorbidities (i.e., diabetes, hypertension, stroke, chronic obstructive pulmonary disease, and coronary heart disease). Moreover, the outcomes described below were also evaluated at baseline.
After the baseline assessment was performed, participants were randomly assigned with a 1:1 ratio into either the intervention group or control group. The random assignment was performed using a Researcher Randomizer. The outcome assessment staff and statistical analyzer were blind to the study design and group allocation.
Intervention
Before the start of intervention, participants in both groups and their caregivers were educated and familiarized with the training procedures. An experienced physiotherapist (Y. L.) provided instructions and encouragement, supervising each patient session during their exercise. In both groups, the exercise interventions were individually designed with twice-weekly sessions for 12 weeks and closely supervised by a physiotherapist.
The intervention group received a mixed exercise program including balance and resistance exercise. In each session, the participants received a light 5-min warm-up followed by 20 min of targeted balance training. Next, they were allotted a five-minute rest before another 20 min of resistance training. Each session ended with a 5-min cool-down that incorporated stretching. To be specific, the balance exercise program included: heel and toe raise and static balance in weeks 1–3; varied directional quick stepping in weeks 4–6; reaching and single-leg standing in weeks 7–9; heel to toe walking and complex cross-over stepping activities in weeks 10–12. The resistance exercise included leg press, leg extension and flexion, leg abduction and adduction, chest press, and seated row. The individual loads of resistance training were determined based on the strength test at the first intervention and at the 13th session. Resistance exercise was performed at 70–80% of one-repetition maximum, 3 sets of 8–12 repetitions each (with a 2-min rest between sets).
The control group engaged in a resistance exercise program. In each session, participants received a light 5-min warm-up first followed by 20 min of resistance training. Next, they were allotted a five-minute rest before another 20 min of resistance training. Each session also ended with a 5-min cool-down. The resistance exercise was the same as the intervention group.
Furthermore, patients in both groups received standard care for their chronic comorbidities by the geriatricians.
Outcomes
The primary outcomes were the improvement of activities of daily living (ADL) and the number of fallers during the 12-week period. The improvement of ADL was evaluated by the change in the Barthel Index from baseline to the end of the interventions. The Barthel Index is a 10-item scale of 0 (worst) to 100 (best) points, which has been widely used to evaluate ADL in both research and clinical practice22. The clinically significant change was 5 points for the Barthel Index score23.
The secondary outcomes were as follows. First, the change of the short physical performance battery (SPPB) score, which is a valid tool for assessing lower extremity function. The total score of SPPB ranged from 0 (worst) to 12 (best) points24. Second, the change of a 4-m usual gait speed was scored. Third, the change of handgrip strength was measured using a digital grip dynamometer (EH101, Xiangshan Inc., Guangdong, China) to the nearest of 0.1 kg. The recommendations from the Chinese National Physical Fitness Evaluation Standard were used as the handgrip strength measurement protocol25. Fourth, the change of Berg balance score was scored, which is a valid tool for assessing balance function in clinical practice26. The score of Berg balance ranged from 0 (worst) to 56 (best) points. Fifth, the change of the timed “UP and GO” test (TUG) score was used, which is a widely used tool for assessing functional mobility27. The patients were asked to rise from an armchair, walk three meters at a comfortable pace, turn, walk back, and sit down again27. The time to complete the whole process was recorded to the nearest of 0.1 s. Lower timed scores indicate better functional mobility. Sixth, any adverse event related to the interventions during the study period were noted, including falls, pain, injury, bone fracture, cerebrovascular accident, stroke, and the transfer to the acute unit.
Statistical analysis
To achieve 80% power at an α level of 0.05 (2-sided), we needed 25 patients in each group to detect a mean difference in change for the Barthel Index of 5 points during the 12-week period. This was based on a previous study that showed a significant improvement of the Barthel Index (20.4 ± 18.3 points) after 3 months of physical rehabilitation among sarcopenic older patients17. Based on our previous experience; we estimated an attrition rate of 10%. Therefore, at least 55 patients were required to ensure that there were sufficient participants at the end of the intervention.
We used the intention-to-treat approach for all analyses. Missing data were imputed by using “the conservative assumption that patients with missing data in the intervention group had the same rate of falls as observed in the control group and vice versa”28 (for the outcome of fallers) and by setting the change across time to be zero (for the other outcomes)29. The normality of the distribution of continuous data was evaluated using the Kolmogorov–Smirnoff test. Group differences at baseline were compared using the χ2 test for categorical data and the ANOVA for continuous data. Group differences for all outcomes (except for the number of fallers) were analyzed using analyses of covariance30. The change of these outcomes from the baseline to the end of the interventions were the dependent variables in these analyses, while the group assignment was the independent variables. We adjusted these analyses for age, sex, baseline scores, education level, comorbidities, and nutrition status. The group difference in the number of fallers was compared using the χ2 test.
All statistical analyses were performed in SPSS version 25 (IBM Corp., Armonk, NY, US) and R software, version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria). All comparisons were 2-sided with an α level of 0.05 indicating statistical significance.
Results
Trial participants
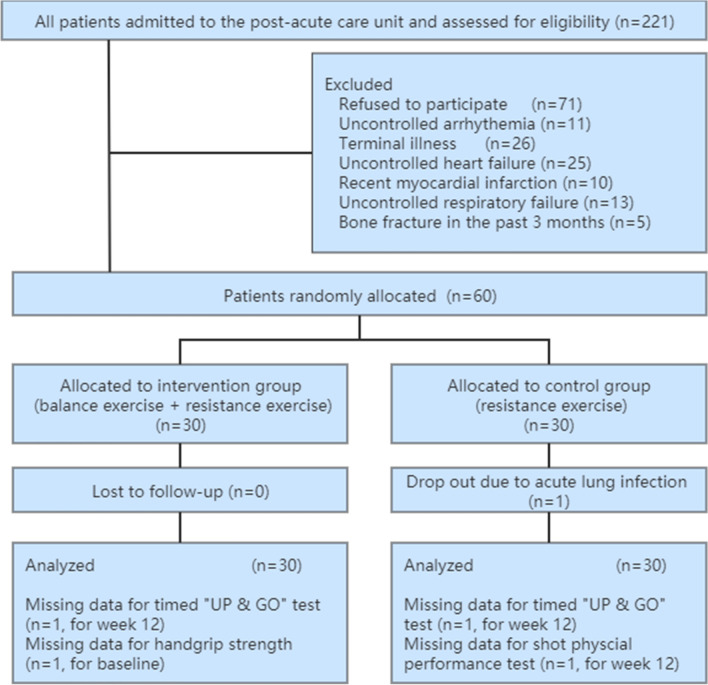
Figure 1 shows the study flow diagram. From June 2017 to January 2019, a total of 221 patients were screened, of whom 60 were included in the trial and underwent randomization. Next, 30 participants were assigned to the intervention group (receiving a mixed exercise program including balance and resistance exercise) and 30 to the control group (receiving a resistance exercise program). The participants in both groups also received standard care. One participant in the control group dropped out at the 13 weeks of intervention due to an acute lung infection. All remaining participants finished the 24 exercise sessions.
Figure 1.
Flowchart of enrolment and randomization. An intention-to-treat approach was performed.
Patients characteristics at baseline are presented in Table 1. The mean age of the study cohort was 87.3 ± 5.4 years (range, 80–99 years, with 24 patients (40%) were nonagenarians). In total, 26 (43.3%) patients were women. There were no significant differences between groups at baseline for demographic and medical characteristics or for the study outcomes except for the TUG score, which was higher in the control group.
Table 1.
Baseline characteristics of the study population.
| Intervention n = 30 | Control N = 30 | p | |
|---|---|---|---|
| Age (years) | 87.3 (6.0) | 86.8 (4.7) | 0.490 |
| Women (%) | 15 (50.0) | 11 (36.7) | 0.297 |
| BMI (kg/m2) | 21.6 (2.1) | 21.0 (2.7) | 0.367 |
| Education level (%) | |||
| ≤ 6 years | 9 (30.0) | 9 (30.0) | 0.301 |
| 7–12 years | 14 (46.7) | 9 (30.0) | |
| ≥ 13 years | 7 (23.3) | 12 (40.0) | |
| Comorbidities (%) | |||
| Diabetes | 4 (13.3) | 8 (26.7) | 0.197 |
| Hypertension | 18 (60.0) | 15 (50.0) | 0.436 |
| Stroke | 7 (23.3) | 5 (16.7) | 0.519 |
| Chronic obstructive pulmonary disease | 8 (26.7) | 6 (20.0) | 0.542 |
| Coronary heart disease | 4 (13.3) | 10 (33.3) | 0.067 |
| Patients with at least a fall in the past year (%) | 9 (30.0) | 10 (33.3) | 0.781 |
| Nutrition status (%) | |||
| Malnutrition | 13 (43.3) | 17 (56.7) | 0.579 |
| Malnutrition risk | 14 (46.7) | 11 (36.7) | |
| Barthel index score | 72.3 (17.3) | 64.5 (15.4) | 0.069 |
| ASMI | 5.9 (1.4) | 6.2 (1.1) | 0.179 |
| Gait speed (m/s) | 0.43 (0.15) | 0.35 (0.17) | 0.063 |
| Handgrip strength (kg) | 16.3 (4.8) | 14.9 (6.1) | 0.328 |
| SPPB score | 4.3 (1.4) | 3.8 (1.8) | 0.215 |
| Berg balance score | 20.8 (15.1) | 17.7 (10.2) | 0.345 |
| TUG score | 23.4 (11.2) | 40.4 (42.4) | 0.042 |
ASMI appendicular skeletal muscle mass index, SPPB short physical performance test, TUG timed up to go test.
Patients in the intervention group received mixed exercise program (balance exercise plus resistance exercise); while patients in the control group received resistance exercise alone. Patients in both groups also received standard care. Data are presented as mean (standard deviation) or n (percentage).
Primary outcome: improvement of ADL
At the end of the interventions, the mixed exercise group showed a mean increase of 9.5 points on the Barthel Index (95% CI 3.9–15.1 points), while the resistance exercise group showed a mean increase of 6.3 points (95% CI 2.3–10.4 points). The mixed exercise program provided a significant benefit over the resistance exercise program (adjusted mean difference of the change of Barthel Index: 6.8 points, 95% CI 1.4–12.1 points, Table 2).
Table 2.
Comparison of the primary and secondary outcomes by groups.
| Outcomes | After 12 weeks | Change from the baseline | Adjusted group difference in mean change over 12 weeks† | F† | P† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control* n = 30 | Intervention* n = 30 | Control* n = 30 | Intervention* n = 30 | |||||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
| Primary outcome | ||||||||||||
| Barthel Index score | 70.8 | 66.1–72.7 | 81.8 | 78.4–85.3 | 6.3 | 2.3–10.4 | 9.5 | 3.9–15.1 | 6.8 | 1.4–12.1 | 6.440 | 0.014 |
| Secondary outcomes | ||||||||||||
| Gait speed (m/s) | 0.40 | 0.35–0.46 | 0.54 | 0.49–0.61 | 0.05 | 0.02–0.07 | 0.12 | 0.06–0.18 | 0.07 | 0.01–0.14 | 4.347 | 0.042 |
| Handgrip strength (kg) | 16.3 | 14.0–18.7 | 20.5 | 18.1–23.0 | 1.5 | 0.8–2.2 | 4.3 | 2.1–6.4 | 3.3 | 0.7–5.9 | 6.626 | 0.013 |
| SPPB score | 4.6 | 4.0–5.2 | 6.6 | 5.7–7.5 | 0.8 | 0.4–1.2 | 2.3 | 1.3–3.3 | 1.6 | 0.5–2.8 | 8.396 | 0.006 |
| Berg balance score | 22.0 | 18.1–25.9 | 31.3 | 26.0–36.5 | 4.3 | 2.0–6.6 | 10.4 | 4.9–16.0 | 6.7 | − 0.4–14.0 | 3.649 | 0.062 |
| TUG score | 31.7 | 25.4–37.9 | 18.5 | 16.1–20.9 | − 8.5 | − 22.1–5.2 | − 4.7 | − 8.7–− 0.8 | 2.0 | − 14.8–18.8 | 0.056 | 0.813 |
| MRMI score | 29.4 | 27.2–31.7 | 34.6 | 33.0–36.2 | 0.8 | − 0.2–1.8 | 4.2 | 1.9–6.5 | 3.1 | 0.6–5.7 | 6.310 | 0.015 |
CI confidential interval, MRMI modified Rivermead Mobility Index, SPPB short physical performance test, TUG timed up to go test.
*Patients in the intervention group received balance and resistance training; while patients in the control group received resistance training. Patients in both groups also received personalized oral nutrition supplements and standard care.
†Between group change by analysis of covariates (adjusted for baseline, age, sex, education level, comorbidities, and nutrition status).
Primary outcome: number of fallers
During the 12-week period, the number of fallers was 13.3% (4/30) in the intervention group and 23.3% (7/30) in the control group, but the difference between groups was not statistically significant (risk ratio (RR) 0.89, 95% CI 0.69–1.13, p = 0.506).
Secondary outcomes
Both the mixed exercise program and resistance exercise program improved gait speed, handgrip strength, SPPB score, and Berg balance score, but not the TUG score (Table 2). Compared with resistance exercise, the mixed exercise program significantly improved usual gait speed, handgrip strength, and the SPPB score, but not the Berg balance score and TUG score (Table 2).
In addition, no advance events related to the interventions were recorded during the study process.
Discussion
To our knowledge, our study is the first RCT to compare a mixed exercise program with a resistance exercise program among older patients with sarcopenia in post-acute care settings.
Although the key role of resistance exercise for treating sarcopenia has been established7, the current evidence is mainly based on clinical trials conducted in community-dwelling older adults31. After systematically searching PubMed and Embase, we failed to identify any previous RCT to address this issue in post-acute care settings. However, two longitudinal intervention studies (without control arms) indicated that resistance exercise might be effective for improving muscle strength and physical performance in older adults living in post-acute care settings14,15. Chiu et al.14 observed that 12-week resistance exercise improved handgrip strength and functional independence in 64 older adults with sarcopenic obesity in long-term care facilities. Moreover, del Campo Cervantes et al.15 reported that the 12-week resistance exercise program improved handgrip strength and physical performance in 19 sarcopenic older adults living in a nursing home. Our study provides new evidence to support resistance exercise to be used in older adults living in post-acute care settings.
Our study found that both resistance exercise and mixed exercise programs improve ADL in older sarcopenic patients in post-acute care units, but the latter is better than the former. This finding supports the recent recommendation on exercise for older adults living in long-term care facilities: “The best exercise type is multicomponent training. . .other exercise types, particularly flexibility and balance, should be added to the exercise program whenever possible”32. This recommendation, however, was not especially designed for sarcopenic patients. No previous study directly compared the effect of two exercise programs on sarcopenia in this special population. However, a recent prospective study found that the rehabilitation program (including mobility training and endurance training) was effective for improving ADL in older sarcopenic patients in a subacute care unit17. In addition, another study showed that resistance and balance exercise improved muscle strength and reduced body fat in 42 very old residents living in nursing homes16.
Our study showed that the number of fallers was not significantly different between groups, although it appeared to be lower in the mixed exercise group than in the resistance exercise group. One possible reason is that the sample size of our study was too small to achieve the statistical power to detect differences between the two groups. In addition, we only assessed the number of fallers within 12 weeks. The effects of the two exercise programs for preventing falls in a longer period needs to be addressed in the future.
Other major concerns of exercise for older adults who commonly have multiple comorbidities and functional decline are feasibility and safety. We found no adverse events related to the exercise programs during the study period. Moreover, most of our participants completed all exercise sessions except for one who dropped out due to an acute lung infection. There is growing evidence to support the feasibility and safety of well-designed exercise programs for older adults. For example, a recent RCT indicated that resistance, balance, and walking exercises (2 daily sessions) was safe and effective to reverse the functional decline in older inpatients during acute hospitalization33. In addition, Hassan et al.16 reported that resistance and balance exercise programs were safe and feasible for older adults (approximately 1/3 having sarcopenia) living in nursing homes. On the other hand, based on our experience and findings from the literature1,2, older patients generally have difficulty or lack of motivation to participate in exercise programs. Therefore, encouragement and monitoring during exercise appears to be essential, especially for those in functional decline.
Our study has some limitations. First, we only assessed the post-intervention effects of the two exercise programs; however, the long-term outcomes are supposed to be more important in older patients with sarcopenia. According to a recent overview of systematic reviews, this was a common limitation shared across the relevant literature11. Therefore, the long-term effectiveness of different exercise programs needs to be evaluated in the future. Second, because our participants were very old, a physiotherapist in our team monitored the whole process of exercise to ensure the patients’ safety. Thus, these exercise programs are time-consuming and labor-intensive. As a result, the feasibility of transferring these interventions in real-world post-acute care settings may be compromised due to the lack of adequate physiotherapists or relevant medical staff. An individual exercise program may not be as cost-effective as a group-based exercise program, which has been proven to be effective for improving muscle function in institutionalized older adults34,35. Further studies are required to compare the two types of exercise for older sarcopenic patients in long-term care settings. Third, we did not assess some important outcomes, such as the change of lower limb muscle strength and muscle mass. Therefore, we could not provide a possible explanation for improved ADL and its relation to the interventions. Fourth, the follow-up duration of our study was relatively short and the sample size was small. Fifth, we performed ITT analyses and imputed missing data using previous reported methods28,29. These methods might have introduced bias into our results. However, as shown in Fig. 1, there were only four cases with a few missing data for the three variables. Therefore, our results are not significantly influenced. Lastly, although we randomly allocated the participants into two groups, their clinical characteristics were not fully balanced between the groups at the baseline, which would be expected from a simple randomization protocol with less than 200 cases. For example, the TUG score was significantly lower in the intervention group than in the controlled group (p = 0.042). Thus, our results might have been influenced by possible selection bias.
Conclusions
Compared with resistance exercise, the mixed exercise program (balance exercise plus resistance exercise) appeared to have improved the activities of daily living, strength, and physical performance among older sarcopenic patients in post-acute care settings. Thus, we conclude that both exercise programs are feasible and safe for this population. Based on our experience, the exercise programs should be personally modulated and performed under the guide and monitoring of experienced physiotherapists.
Some essential questions regarding exercise interventions for older sarcopenic patients are still pending. For example, a recent systematic review of 25 RCTs revealed a dose–response relationship of resistance training to improve muscle strength and morphology in healthy old adults36; however, the optimal dose (e.g., intensity, volume, rest, frequency, repetition) and type (e.g., aerobic, balance, resistance, Tai Chi) of exercise for treating or preventing sarcopenia remains unclear. Large, multicenter, and well-designed RCTs are therefore warranted to address these issues in different clinical settings.
Acknowledgements
This work is supported by the National Key R&D Program of China [2018YFC2002104]. The sponsors played no role in the design, methods, data collection, analysis, or preparation of this work.
Author contributions
Y.L. and M.Y. wrote the manuscript; M.Y. revised the manuscript; M.Y. conceived and designed the study; Y.L., R.W., L.T., and J.J. performed the study and collected the data; Y.L. and M.Y. analyzed the data. All authors reviewed the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. The Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 2.Chen L-K, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020;21:300–307. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Shafiee G, Keshtkar A, Soltani A, et al. Prevalence of sarcopenia in the world: A systematic review and meta-analysis of general population studies. J. Diabetes Metab. Disord. 2017;16:21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drew L. Fighting the inevitability of ageing. Nature. 2018;555:S15–S17. doi: 10.1038/d41586-018-02479-z. [DOI] [PubMed] [Google Scholar]
- 5.Shen Y, Chen J, Chen X, et al. Prevalence and associated factors of sarcopenia in nursing home residents: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2019;20:5–13. doi: 10.1016/j.jamda.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenzuela PL, Castillo-García A, Morales JS, et al. Physical exercise in the oldest old. Compr. Physiol. 2019;9:1281–1304. doi: 10.1002/cphy.c190002. [DOI] [PubMed] [Google Scholar]
- 8.Morley JE. Treatment of sarcopenia: The road to the future. J. Cachexia Sarcopenia Muscle. 2018;9:1196–1199. doi: 10.1002/jcsm.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anton SD, Hida A, Mankowski R, et al. Nutrition and exercise in sarcopenia. Curr. Protein Pept. Sci. 2018;19:649–667. doi: 10.2174/1389203717666161227144349. [DOI] [PubMed] [Google Scholar]
- 10.Keogh J, Henwood T, Senior H, Hewitt J. Prevalence, consequences and effects of exercise on sarcopenia in aged care. Aust. J. Ageing. 2017;36:32–33. doi: 10.1111/ajag.12354. [DOI] [Google Scholar]
- 11.Moore SA, Hrisos N, Errington L, et al. Exercise as a treatment for sarcopenia: An umbrella review of systematic review evidence. Physiotherapy. 2019;107:189–201. doi: 10.1016/j.physio.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Onder G, Carpenter I, Finne-Soveri H, et al. Assessment of nursing home residents in Europe: The Services and Health for Elderly in Long TERm care (SHELTER) study. BMC Health Serv. Res. 2012;12:5. doi: 10.1186/1472-6963-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst. Rev. 2018;9:CD005465. doi: 10.1002/14651858.CD005465.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu S-C, Yang R-S, Yang R-J, Chang S-F. Effects of resistance training on body composition and functional capacity among sarcopenic obese residents in long-term care facilities: a preliminary study. BMC Geriatr. 2018;18:21. doi: 10.1186/s12877-018-0714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Campo Cervantes JM, Macías Cervantes MH, Monroy TR. Effect of a resistance training program on sarcopenia and functionality of the older adults living in a nursing home. J. Nutr. Health Aging. 2019;23:829–836. doi: 10.1007/s12603-019-1261-3. [DOI] [PubMed] [Google Scholar]
- 16.Hassan BH, Hewitt J, Keogh JWL, et al. Impact of resistance training on sarcopenia in nursing care facilities: A pilot study. Geriatr. Nurs. 2016;37:116–121. doi: 10.1016/j.gerinurse.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Rodríguez D, Marco E, Miralles R, et al. Sarcopenia, physical rehabilitation and functional outcomes of patients in a subacute geriatric care unit. Arch. Gerontol. Geriat. 2014;59:39–43. doi: 10.1016/j.archger.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Schulz KF, Altman D, Group C The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 19.Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ. 2013;346:7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L-K, Liu L-K, Woo J, et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for sarcopenia. J. Am. Med. Dir. Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Vellas B, Villars H, Abellan G, et al. Overview of the MNA–Its history and challenges. J. Nutr. Health Aging. 2006;10:456–463. [PubMed] [Google Scholar]
- 22.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 23.van Bennekom CA, Jelles F, Lankhorst GJ, Bouter LM. Responsiveness of the rehabilitation activities profile and the Barthel index. J. Clin. Epidemiol. 1996;49:39–44. doi: 10.1016/0895-4356(95)00559-5. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994;49:85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 25.General Administration of Sport of China . Chinese National Physical Fitness Evaluation Standard (CNPFES) Beijing: People's Sports Press; 2003. [Google Scholar]
- 26.Bogle Thorbahn LD, Newton RA. Use of the Berg balance test to predict falls in elderly persons. Phys. Ther. 1996;76:576–583. doi: 10.1093/ptj/76.6.576. [DOI] [PubMed] [Google Scholar]
- 27.Podsiadlo D, Richardson S. The timed “up and go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 28.El-Khoury F, Cassou B, Latouche A, et al. Effectiveness of two year balance training programme on prevention of fall induced injuries in at risk women aged 75–85 living in community: Ossebo randomised controlled trial. BMJ. 2015;351:h3830. doi: 10.1136/bmj.h3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galvao DA, Taaffe DR, Spry N, et al. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial. J. Clin. Oncol. 2010;28:340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 30.Philippas D. Analysis of covariance (ANCOVA) In: Michalos AC, editor. Encyclopedia of Quality of Life and Well-Being Research. Dordrecht: Springer; 2014. pp. 157–161. [Google Scholar]
- 31.Yoshimura Y, Wakabayashi H, Yamada M, et al. Interventions for treating sarcopenia: A systematic review and meta-analysis of randomized controlled studies. J. Am. Med. Dir. Assoc. 2017;18(553):e1–e16. doi: 10.1016/j.jamda.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 32.de Souto BP, Morley JE, Chodzko-Zajko W, et al. Recommendations on physical activity and exercise for older adults living in long-term care facilities: A taskforce report. J. Am. Med. Dir. Assoc. 2016;17:381–392. doi: 10.1016/j.jamda.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Velilla N, Casas-Herrero A, Zambom-Ferraresi F, et al. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization: A randomized clinical trial. JAMA Intern. Med. 2019;179:28–36. doi: 10.1001/jamainternmed.2018.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baum EE, Jarjoura D, Polen AE, Faur D, Rutecki G. Effectiveness of a group exercise program in a long-term care facility: A randomized pilot trial. J. Am. Med. Dir. Assoc. 2003;4:74–80. doi: 10.1016/S1525-8610(04)70279-0. [DOI] [PubMed] [Google Scholar]
- 35.Fien S, Henwood T, Climstein M, Keogh JW. Feasibility and benefits of group-based exercise in residential aged care adults: A pilot study for the GrACE programme. PeerJ. 2016;4:e2018. doi: 10.7717/peerj.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borde R, Hortobágyi T, Granacher U. Dose–response relationships of resistance training in healthy old adults: A systematic review and meta-analysis. Sports Med. 2015;45:1693–1720. doi: 10.1007/s40279-015-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.